Abstract
Given its potential for high-resolution, customizable, and waste-free fabrication of medical devices and in vitro biological models, 3-dimensional (3D) bioprinting has broad utility within the biomaterials field. Indeed, 3D bioprinting has to date been successfully used for the development of drug delivery systems, the recapitulation of hard biological tissues, and the fabrication of cellularized organ and tissue-mimics, among other applications. In this study, we highlight convergent efforts within engineering, cell biology, soft matter, and chemistry in an overview of the 3D bioprinting field, and we then conclude our work with outlooks toward the application of 3D bioprinting for ocular research in vitro and in vivo.
Keywords: 3-dimensional (3D) printing, biomaterials, tissue engineering, bioprinting, ocular research
Introduction
Modern medicine is trending toward personalization. Every day, advances in engineering, chemistry, biology, and soft matter create new tools for the study of human health and disease, and with each new advance comes a better understanding of the complex pathology and physiology that makes every patient unique.1 Indeed, with the emergence of the -omics era, it has been evidenced that each patient and each patient's disease is unique.2,3 Consequently, state-of-the-art treatment regimens still fail in a certain percentage of patients, independent of genetic homology across a patient population. In part, this finding is due to the fact that every person has a unique genetic and environmental make-up, and this individuality highlights some of the current limitations associated with a “one size fits all” therapeutic strategy as is most commonly practiced today for the treatment of human disease.
The difficulties associated with developing personalized medicines also arise from challenges in studying disease both inside and outside of the body. Although many animal models have been described that afford definitive results on the role that specific molecules play in disease progression and management, ethical limitations and inconsistent results between animal models and human clinical trials are still observed. Indeed, ∼50% of drugs that pass preclinical testing may be toxic for humans, yet, others have been described to be nontoxic in humans even if they failed in animal models.4 Efforts to understand these inconsistencies are conducted using cell culture experiments that aim to provide simplified models for studying, preventing, and treating human disease. However, many cell culture models provide an incomplete picture of drug delivery into the body, resulting in limitations associated with in vitro to in vivo predictive correlations. While there are many factors that govern this finding, one driver is that it is difficult to properly recapitulate processes occurring within the body [a 3-dimensional (3D) structure] on 2D cell culture plates. This is because it is difficult to model complex cell–cell and cell–matrix interactions or cellular differentiation within a flat environment because cells natively receive cues through the x, y, and z dimensions at specific times when in the body. Although 3D tissue and organ models can improve our ability to understand the function, formation, and pathology of healthy and diseased states within the body, challenges associated with their fabrication remain a major challenge to overcome. Scaffolds, sometimes made of biomaterials such as proteins from the extracellular matrix (ECM) may be used to culture cells, a strategy first proposed by Langer and Vacanti.5 However, populating an inert scaffold with cells triggers various issues, including the ability of cells to diffuse within the material. Many current 3D tissue models lack the complex architecture, sometimes at the single-cell scale, and tissue–tissue interfaces associated with endogenous human organs.6 As a consequence, many of these models predominantly focus on recapitulating the basic function of the respective tissue while ignoring essential features, including complex vasculatures and fluid mechanics within the system.
To overcome some of these limitations associated with personalized medicine and ex vivo disease modeling, 3D bioprinting has been developed (Fig. 1). Three-dimensional bioprinting is a modern manufacturing technique whereby a digital design for a 3D object is assembled in a layer-by-layer manner.4,6–9 Inspired by initial developments within the industrial sector, 3D bioprinting is similar to early work in classical STereoLithography (STL), in which it aims to rapidly construct readily customized 3D structures in a cost, time, and waste-effective manner.7 Where these processes differ, however, is that 3D bioprinting aims to create either acellular or cellularized 3D objects that can mimic, or directly interface with, the human body.7,10–12 Below, we describe the basics of 3D bioprinting, beginning with a description of different printing techniques. We then provide discussion surrounding the development and implementation of specific bioinks and biomaterial inks.13,14 Finally, we look to the future of 3D bioprinting, highlighting potential applications for this emerging technology in the development of advanced ocular models and for therapeutic implementation within human patients.
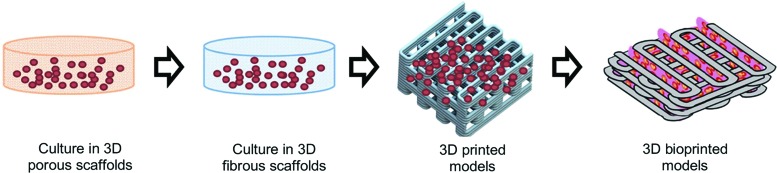
FIG. 1.
Evolution of 3D cell-culture models.4 3D, 3-dimensional. Color images are available online.
Bioprinting Techniques
From a conceptual standpoint, 3D bioprinting is a straightforward process—finite layers of a given material can be stacked one on top of the other, ultimately resulting in the formation of a 3D object of interest.10 In practice, however, 3D bioprinting is considerably more challenging. Indeed, several bioprinting strategies have been developed that confer specific advantages and disadvantages over the 3D bioprinting process (Table 1). Moreover, careful attention must be given to ensure that cellular viability is maintained during and after the 3D bioprinting process. Indeed, just as is the case of 2D cell culture, cells printed in 3-dimensions are sensitive to factors, including temperature, nutrient exchange, sterility, and cellular density; beyond this, however, cells must also survive differential shear forces incurred during the bioprinting process, a challenge that cells do not normally encounter in traditional 2D cell culture.15,16 In this study, we describe the basic strategies for 3D bioprinting, describing first the generation of computer-aided design (CAD) files, and then describing three overarching 3D bioprinting strategies referred to as extrusion-based bioprinting, inkjet-based bioprinting, and laser-assisted bioprinting.
Table 1.
Overview of 3-Dimensional Bioprinting Techniques
| Technique | Extrusion-based | Laser-assisted |
Inkjet printing | |
|---|---|---|---|---|
| Light-based | Laser-induced forward transfer | |||
| Principle6,8,11 | Material is driven through a nozzle onto a flat substrate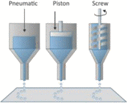
|
UV or visible light triggers polymerization of a thin layer or a small focal volume of a photocrosslinkable resin-containing solution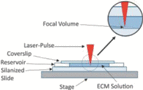
|
Forward transfer of the printed material/ink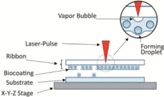
|
Successive drops of bioink are propelled onto a substrate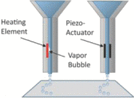
|
| Printing process15 | Serial (line by line) | Parallel and continuous (projection based) | Serial (dot by dot) | Serial (drop by drop) |
| Printing speed6,15,16 | Medium (10–1,000 μm/s) | Fast (mm3/s) | Medium (200–1,600 μm/s) | Medium (mm/s) |
| Spatial resolution8,15–17 | 25 μm in the x, y plane are achievable with line dimensions and layer thickness of 200 − 500 μm, determined by the nozzle diameter | Single micrometer range | <500 nm | 50 μm |
| Compatible ink materials18 | Polymers: hydrogels composed of agarose, alginate, collagen type I, and Pluronic F127 | UV-crosslinkable polymers | Polymers and biological materials | Polymers: photocurable solutions, colloidal suspensions (allowing high-molecular-weight polymers in low viscosity form), and polymer melts. Biological materials, including cells, can be incorporated into the ink formulation. |
| Ink form8 | Polymers that can be formed into a filament with sharp solid-to-melt transition | Viscous liquid | Solid (ribbon) | Viscosity <10 cP (mPa s), under high shear rates, between 1 × 105 and 1 × 106 s−1 |
| Surface tension from 28 to 350 mN m−1. | ||||
| Examples of printed tissues4 | Cartilage (ears),19 heart aortic valve,20 microvasculature,21 skin,16 brain-like structures22 | Skin16,23 | Cartilage,24 branched vasculature, liver | |
| Reported cell viability15,16,25 | 40%–80% | >85% | Near 100% | >85% |
| Main advantage(s)6 | High cell density can be achieved | Fast and precise | Single-cell manipulation, no clogging associated with nozzles, wide viscosity range | Low cost, simplicity of use |
| Main disadvantage(s)6 | Nozzle clogging, limited number of biomaterials used to date | Limited number of compatible bioinks | High cost, complexity to produce ribbon | Nozzle clogging, low droplet directionality, liquid state required for deposition, variable cell encapsulation |
CAD: developing models for 3D bioprinting
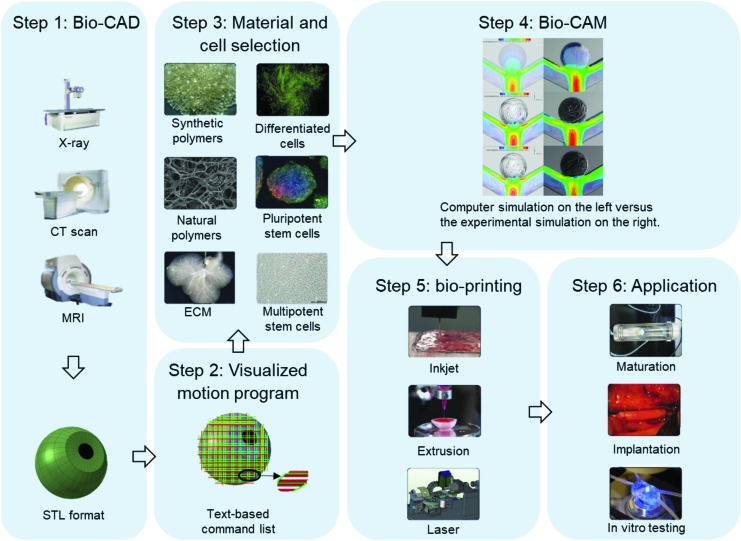
To successfully create bioprinted tissues, it is necessary to first generate a set of printing instructions, select appropriate bioinks and cells, control the bioprinter, and perform quality control after printing.7 Three-dimensional-bioprinting, just as general 3D-printing, occurs in several steps: First, the printing geometry is designed [bio-computer-aided design (Bio-CAD)] and the feasibility of its realization is verified [bio-computer-aided manufacturing (Bio-CAM)]. The introduction of Bio-CAD techniques has significantly improved the automation of bioprinting path generation. Bioprinting models are often converted to the STL file format. These files contain accurate surface information of complex 3D geometries. They can be created from clinical images, including those from magnetic resonance imaging, computed tomography, or confocal microscopy for smaller structures (Fig. 2, step 1)26–28 and modified via graphic user interfaces.
FIG. 2.
The 3D bioprinting process, from biocomputer-aided design to tissue culture.7,8 Color images are available online.
In a process analogous to histologic sectioning, printing paths are created by “slicing” these STL model into layers and creating bioprinter toolpaths that trace out the perimeter and interior features of each slice. A popular slicing software is the open source Slic3r, which automatically produces G-code printing program. The Slic3r slicing software calculates flow rate, that is, the amount of material to be extruded per distance unit, based on layer height and extrusion width. This flow rate is an estimation which needs to be calibrated for each bioink used and optimized according to the print geometry (Fig. 2, step 2).29
Consequently, appropriate cell types and bioinks are loaded into the system (Fig. 2, step 3). Bio-CAM plays an important role during and after bioprinting. Bio-CAM aims to predict the feasibility of the fabrication process by simulating relevant physical models, such as the laminar multiphase flow model. Bio-CAM is also used to simulate postprinting modeling of the cellular dynamics and the typical types of printed tissues, including tumors30 and soft tissues.31 Bio-CAM research not only provides a fast way to check design feasibility but also gives a chance to better understand the physical and chemical principles governing printing (Fig. 2, step 4).12,32 Through control language and protocols, such as RS 274 (G-Code; Massachusetts Institute of Technology, Cambridge, MA) and LabView (National Instruments, Austin, TX), the designed paths are sent to the bioprinting system. The bioprinter builds structures by depositing bioinks following the instructions sent by the software (Fig. 2, step 5). Finally, bioprinted tissues are checked manually (via microscopy) after bioprinting. Successfully printed constructs are transferred to an incubator for culturing (Fig. 2, step 6). The bioprinting process still requires many manual operations and verifications, which can result in slow processing speeds and increase the chance for mistakes. With the integration of Bio-CAD and Bio-CAM, an advanced design flow for bioprinting begins to take shape.
Extrusion-based bioprinting
Extrusion-based bioprinting strategies operate by driving a cell-laden material through a micronozzle onto a flat substrate using either air pressure or a motorized plunger as force (Table 1). Printing in a line by line (ie, serial) format, extrusion-based 3D bioprinting strategies have to date been successfully used in the printing of tissues ranging from cartilage (ears),19 cardiac (aortic valves),20,33 to various forms of microvasculature.21 As with all strategies for 3D bioprinting, the cellular viability of extrusion printed cells is, in part, dependent on the printed cell type; nevertheless, this printing strategy traditionally offers moderate to good viability ranging from ∼40%–80%.8 In addition to its moderate printing speed (ranging from ∼10–1,000 μm/s),6,8 extrusion-based 3D bioprinting strategies also confer spatial resolution on the order of ∼25 μm in the x and y planes with line dimensions and layer thicknesses of ∼200–500 μm in the z dimension.8,34
Beyond these properties, extrusion-based 3D bioprinting is also versatile in the sense that different architectures can be achieved by simply varying the nozzle—indeed, by attaching a nozzle with a smaller exit geometry, finer print resolution can be achieved. This process, however, is also accompanied by higher requirements for internal pressure within the system that can damage entrapped cells and reduce viability. Moreover, nozzle clogging is a predominant issue associated with extrusion-based 3D bioprinting technologies, a process which can slow or completely impede the capacity to print using this strategy.6,7,10 Nevertheless, extrusion-based methods are capable of printing tissues and organs of clinically relevant shape and size, a finding that has inspired the generation of several commercially available extrusion-based bioprinting systems, including the Bioplotter (Envision TEC GmbH, Gladbeck, Germany), the INKREDIBLE (Cellink, Gothenburg, Sweden), the RegenHU, the Allevi, and the Novogen MMX Bioprinter (Organovo, Inc., San Diego, CA).4,35 Home-based systems as well as open software and hardware bioprinters have also been developed that combine the ease and flexibility of commercial systems with customizable printing abilities for complex structure development.
Inkjet-based bioprinting
Inkjet-based strategies are a noncontact 3D printing methodology wherein the 3D architecture is formed by the successive layering of ink droplets onto a given surface (Table 1). For biomedical applications, inkjet-based bioprinting is most common.8 Both thermal and piezoelectric inkjet-based strategies are commonly used for 3D bioprinting36; while similar in that they both are useful strategies for depositing multiple proteins and cells in a targeted specific location, these two strategies predominantly differ in the manner in which ink droplets are formed. Indeed, while thermal inkjet 3D bioprinting generates vapor bubbles along the bioink stream, piezoelectric bioprinting uses a piezoelectric actuator to portion the bioink stream into the required ink droplets. To date, inkjet-based bioprinting has been utilized to create 3D replicas of cartilage,24 branched vasculatures, and liver, among others. Moreover, inkjet bioprinting could be beneficial in areas including wound healing, a process by which individual ink droplets containing cells or other factors could be used to fill empty wounds in a layer-by-layer manner with varied cell populations as a function of depth.37
From an operational standpoint, inkjet-based bioprinting is often lauded for its affordability and simplicity of use. Indeed, early research efforts using inkjet bioprinting indicated that a modified Hewlett-Packard printer could be used to template Chinese hamster ovary and embryonic motor neuron cells onto hydrogel substrates.4,12,38 Moreover, the effects of material strength, agitation, and flow rate on cellular viability have also been explored. Although viability does depend upon cell type, some reports using 3D inkjet printing indicate printed cell viabilities upwards of 85%.15,16 Photocurable solutions, colloidal suspensions, and polymer melts have been exploited as polymer inks using this printing strategy, with most viscosity values sitting below 10 cP at shear rates ranging from 105 to 106 s−1.8 The printing speed and resolution of the inkjet method is modest (ranging in the mm/s range and at ∼50 μm, respectively), and some limitations of the system include frequent nozzle clogging, limited directionality of droplet formation, and variable levels of cellular encapsulation, among others.8
Laser-assisted bioprinting
Much like inkjet bioprinting, laser-assisted bioprinting is an inkjet droplet methodology that can be used to arrange cellularized materials into specific 3D geometries for biomaterials application. Consisting of a pulsed laser source, an absorption layer, and a substrate on which cells and biomolecules can reside, laser-assisted bioprinters are traditionally considered more complex than extrusion and inkjet-based bioprinters. Nevertheless, laser-assisted bioprinting can be used to achieve high levels of printing resolution (<25 μm) with small volumes of cells (between 10 and 7,000 pL).4,8 An additional advantage of laser-assisted bioprinting is that it can be used to print at medium to high rates ranging from ∼200–1,600 μm/second.8 Moreover, laser-assisted bioprinting has been successfully adapted to print skin mimics containing fibroblasts and keratinocytes16,23; bone regeneration has also been explored as a potential application by incorporating human cells with inorganic components of bone. In addition, it has been shown that this process can be used to perform single-cell manipulations, and it is also beneficial from an operational standpoint in that there are no nozzles that can become clogged and bioinks with a wide range of viscosities can be seamlessly integrated into the printer.
Despite these advantages, laser-assisted bioprinting is costly and complex relative to extrusion and inkjet strategies. Indeed, to successfully layer 3D bioprinted architectures using laser-assisted bioprinting, the absorption layer is first coated with a cell- or biomolecule-laden bioink. A laser beam is then irradiated onto the surface of the absorption layer, and this process ultimately transfers enough heat into the system to project the cell suspension toward the printing substrate. Sequential iterations of this process can afford 3D bioprinted architectures of precise resolution within a reasonable time frame. Nevertheless, limitations in terms of the scalability of photocurable bioinks for laser-assisted bioprinting, as well as concerns over the cytotoxicity of photoinitiators and UV light, still must be addressed as a function of each individual cell type.6
Bioinks–Platform Materials for 3D Bioprinting
Just as ink is needed to print a document in 2-dimensions, so too is a bioink required for the successful rendering of a 3D-printed biological object. Indeed, proper selection of a bioink is particularly important when incorporating sensitive cells and biologics, primarily because this material platform can not only help cultivate the cells and molecules of interest but can also protect them from shear and impact forces encountered during the printing and deposition processes, respectively. Moreover, bioinks are important for the recapitulation of several important parameters for 3D printing, including biomimicry and tissue liquidity. From a theoretical standpoint, bioinks must be adjustable (so as to match complex biological architectures found in organs and tissues), must be tunable (so as to model specific concentration gradients throughout the tissue), and must be customizable (so as to properly mirror the biological forces associated with endogenous biological tissues). Beyond this, the proper design of a bioink must also consider the fact that certain cell populations can restructure and/or remodel their local environment. Although there are conflicting opinions as to the importance of cellular remodeling of a 3D-bioprinted network, it is nevertheless important to consider that bioinks must be accommodating to the fact that cells behave in a dynamic manner in real-time as they interact with their immediate environment. To date, numerous bioinks [including, but not limited to, agarose, alginate, chitosan, collagen type I, fibrin, gelatin, hyaluronic acid, Matrigel™, methacrylated gelatin, Pluronic® F-127, poly(ethylene glycol), decellularized extracellular matrices, and microcarriers, among others] have been developed that offer advantages and disadvantages that have been extensively reviewed elsewhere.39
Moving beyond theory, 3D bioinks must also satisfy a number of practical considerations to ensure facile and widespread use within the biomaterials and drug delivery communities. In this study, we describe three of the key attributes associated with bioink manufacture and function—printability, mechanical property requirements, and tolerability.
Bioink printability and mechanical properties
Materials used for 3D bioprinting must satisfy a number of requirements to ensure their successful printing to render 3D architectures. From a mechanical standpoint, two of the most important parameters associated with bioink printability are the viscosity and surface tension of the ink solution. Although variations for these parameters exist as a function of which printing strategy is being used (ie, extrusion based, inkjet based, or laser based), successfully implemented bioink solutions often have a suitably low (<10 cP) viscosity under high shear rates between 105 and 106 s−1. Moreover, the surface tension value (which determines the shape of the drop emerging from the nozzle and the shape of the drop on the substrate) typically ranges from ∼28 to 350 mNm−1.8,40 Beyond this, inkjet-based bioprinting benefits from a low viscosity, whereas it is important to have low surface tension for extrusion and laser-based bioprinting to optimize printing accuracy.35 Toward these ends, many successfully implemented bioinks have been described that consist of low concentrations of high-molecular-weight natural polymers. Hydrogel-based bioinks are also quite widely implemented—this is because their 3D rendering can be quite facile if the cross-linking mechanism can be readily incorporated into the layer-by-layer fabrication scheme.35 Indeed, 3D bioprinted hydrogel networks to date have been reinforced with double networks and interpenetrating networks, incorporated nanoparticles, nanotubes, and electrospun fibers, among other materials.40
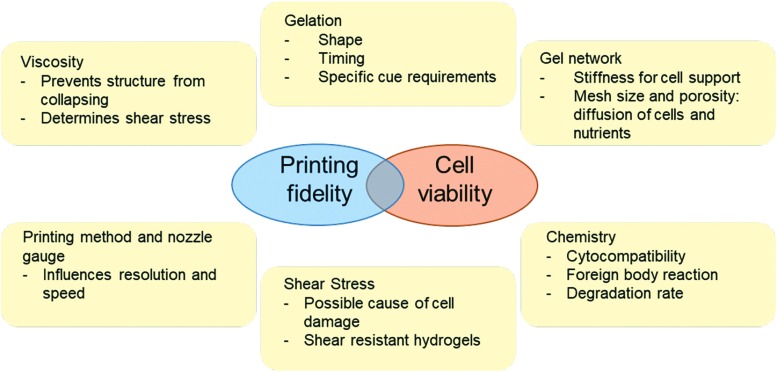
Beyond these mechanical properties, the bioink must also satisfy a number of practical considerations to be successfully printed (Fig. 3). Specifically, bioink materials must be able to cure with an appropriate volume within an appropriate amount of time to retain its shape after deposition. For example, if a bioink cures too slowly, diffusion away from the printed area can result in incorrectly shaped 3D-printed objects; by contrast, if a bioink cures too quickly, problems associated with nozzle clogging, high pressure, and variable cell encapsulation can occur. Moreover, bioinks should not impede the resolution of the printer itself. Indeed, one important area associated with 3D bioprinting involves the printing of intricate vasculature-like structures into the bioink network. Indeed, without a functional circulatory system, 3D-printed tissues currently rely on fluid diffusion for nutrition and excretion of metabolites, which in itself is limited to only a few hundred micrometers from the edges of the matrix.37 Strategies to achieve these goals have consisted in a postprinting dissolved lattice (that is populated with cells, or is populated with cells after printing).5 Additional efforts to print 3D vasculature networks directly without pre-/postfabrication have also been explored wherein direct bioprinting of vessel-like cellular microfluidic channels with hydrogels can be achieved.42–47
FIG. 3.
Parameters to consider for bioink selection.40,41 Color images are available online.
Bioink tolerability
Given that an overarching goal for many 3D bioprinting strategies involves housing living cells, the cytocompatibility of bioinks is particularly important in rendering a successfully printed bioobject. Toward this end, bioinks derived from natural and naturally derived materials are particularly common bioinks—indeed, bioinks composed of alginates, chitosan, collagen, and gelatin, among others, have all been successfully used in the development of 3D bioprinted scaffolds.40,48 To further enhance the cytocompatibility of some bioinks, ECM proteins are also often integrated into hydrogel networks to create materials with an enhanced propensity to host cells.30,34,49 Moreover, cells have also been encapsulated within microcarriers and further incorporated within bioinks for bioprinting. For example, cell spheroids generated by biofabrication approaches, including the hanging drop method, micromolding, microfluidics, and spinner flasks, have also been explored as one potential strategy for enhancing the cytocompatibility of a given substrate.6,44
Beyond maintaining cellular viability, ensuring that bioinks are biocompatible is important, particularly for applications where reintroduction into the human body is one of the end goals of the project. One of the most common ways to assess bioink biocompatibility involves understanding how the immune system interacts with the 3D-printed construct. Toward this end, histology and immunohistochemistry are often performed on the resected tissue and 3D bioprinted implant following a terminal study. The most common assays for this process are hematoxylin and eosin (H&E) or Masson's trichrome, each of which stains for immune cell populations and fibrosis, respectively. Beyond this, immunohistochemistry is used to more specifically identify specific cell populations that have infiltrated the surgical area. Indeed, these antibody–antigen-specific interactions have enabled researchers to analyze the presence of B and T cells, neutrophils, and fibroblasts within bioprinted implants, among others, ultimately highlighting the important role that the immune system plays in recognizing 3D bioprinted materials.
Outlook for 3D Bioprinting for Ocular Biomaterials Research
Successful construction of ocular components, or even the whole eyeball, represents an enticing application of 3D bioprinting techniques. In some cases, an accident or progression of a disease will permanently damage certain cells or parts of the eye, necessitating a replacement to restore vision to the patient. For instance, cells such as the retinal ganglion cells (RGCs) in glaucoma, the photoreceptor cells in retinitis pigmentosa, or larger sections of the retina (the macula) in age-related macular degeneration are all damaged in the case of their respective diseases. In the example of macular degeneration, the surgical implantation of a newly printed macula into the eye would represent an exciting new treatment option with the possibility of bioprinted ocular tissue. Currently, the mere feasibility of these implantations is under exploration, while in the future, the efficacy and responsiveness of these implanted tissues would need to be further evaluated. Following severe ocular trauma or retinoblastoma, the whole eyeball, including retina, may need to be replaced. In search of this potential goal, a noncellular, 3D model of a human eye has recently been printed.50 As both a research tool and a possible surgical replacement treatment, the bioprinting ocular components appears as an exciting new frontier in the development of 3D printing technology, with several promising examples reported so far in the literature.
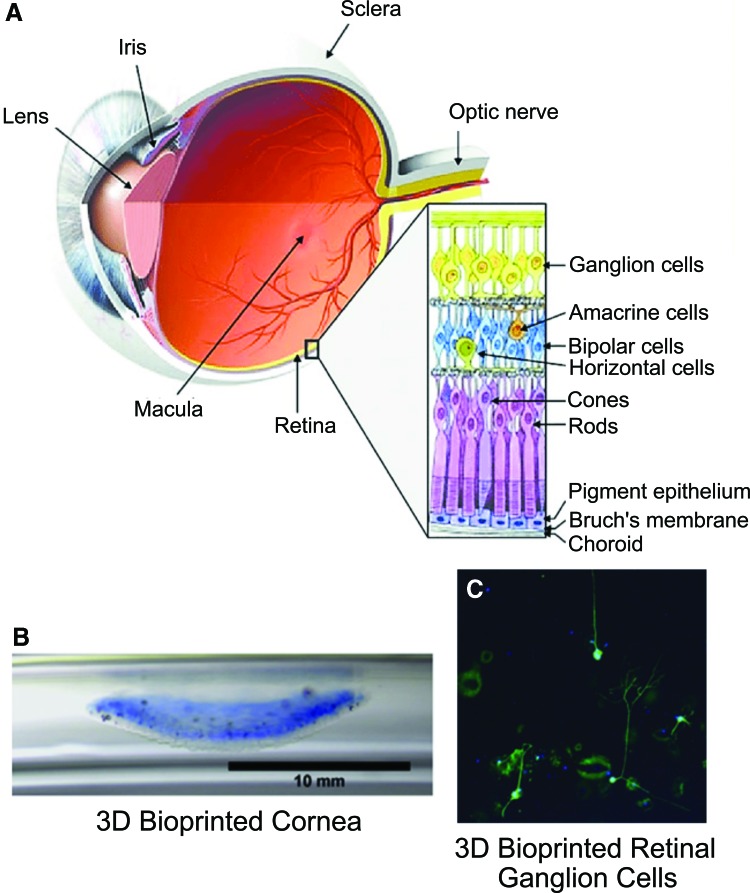
While 3D bioprinting techniques are constantly improving, the overall complexity of the eye creates a unique combination of cell types and material requirements that have thus far prevented complete construction of ocular models. Different ocular components have been evaluated separately, but the integration of different cell types and structures offers a new frontier in advancing the field toward printing of ocular models. However, the entire eye contains a variety of further components composed of a multitude of cell types or matrix proteins resulting in differing material properties. For example, the sclera (the whites of the eye) is composed of ∼50% collagen type I in an ECM, while the retina is composed of many types of neurons, including RGCs, rods, and cones.51,52 These differences in composition and properties lead to overall difficulty in the printing of a complete, cellularized eye model. Current investigations into 3D bioprinting components of ocular models for research or eventual tissue generation are primarily focused on the retina and the cornea (Fig. 4A).
FIG. 4.
(A) Anatomical structure of the human eye, and 3D bioprinted parts: (B) 3D bioprinted cornea, (C) 3D bioprinted retinal ganglion cells.53,54 Color images are available online.
Retina
The retina serves as a crucial target in the development of ocular models as the retina is composed of multiple different types of neurons, together converting visual signals and cues into neurological signals. Located at the back of the eye (opposite the pupil) the retina also contains the macula which itself includes the fovea, the area of the retina with the highest photoreceptor concentration. These features are therefore susceptible to ocular diseases and their effects can vary from visual impairment to complete blindness depending on the condition. For example, age-related macular degeneration is one of the leading causes of blindness in the elderly population.55 Similarly, retinitis pigmentosa leads to progressive vision loss that can also culminate in blindness.56 Furthermore, due to the complex network of interactions between the different retinal cells, it is difficult to target a certain cell population for specific treatment. For this reason, implantation of a complete retinal scaffold, although more complicated to construct, may offer a better option to treat certain retinal diseases since the viability and differentiation of cells is improved through 3D scaffolds.57
Since the retina requires intricate intercellular interactions to successfully conduct optical signals together to create a visual image, the bioprinting of multiple types of retinal cells offers clear benefit as potential research tool. A study by Shi et al. attempted 3D bioprinting of a retinal pigment epithelial cell (RPE) and photoreceptors, mimicking the functional interaction necessary for proper vision. An alginate/pluronic hybrid solution was used to print an ultrathin layer of ARPE-19 cells on a preformed membrane, which was then added to Y79 photoreceptor cells.58 These printed cells showed no reduction in viability compared to manually seeded controls and the Y79 cells were successfully printed into patterns upon the ARPE-19 membrane layer. While the technology currently requires an ultrathin membrane layer upon which to print the RPE layer, the overall morphology of the cells was not impacted by the printing process and these cells were capable of further culture. This hybrid 3D bioprinting approach offers the potential for introducing intercellular interactions to aid research into these retinal complexes.
RGCs are part of the central nervous system and, as such, they are unable to regenerate. They often die following injury or in disease (glaucoma), but they are needed for the transmission of signals from the eye to the brain. A key characteristic of RGCs is their organization. Their axons orient along axon bundles to reach the optic nerve head. Three-dimensional bioprinting techniques have been used to deposit RGCs in an organized manner and represent the structure of the RGC layer in the retina.36,54 Kador et al. showed that a combined 3D printing approach that utilized both electrospinning of a polylactic acid scaffold and thermal inkjet printing with multiple nozzles using optimized media conditions could lead to significantly improved RGC orientation compared to traditional 2D culturing. Their 3D-printed cells with axons were found to be 72% aligned with their radial scaffold with 49% of dendrites aligned as well. In both cases, this alignment was significantly improved compared to typical 2D culturing, which only showed 11% alignment.54 In addition, their printed cells did not see a decrease in overall viability or reduced function when monitored by electrophysiology. In a promising study, Kador et al. were able to achieve a printed density of about 30 cells/mm2 as compared to the overall cell density in the human retina of about 2100 cells/mm2.2,54
Other types of cells present in the retina, such as glial cells, have been printed along with RGC in an attempt to reproduce the whole retina.36,59 Lorber et al. published a study that attempted to print both RGCs and glial cells together to evaluate the effects of a piezoelectric printing on the cellular viability. Using a single nozzle piezoelectric inkjet printer, they were able to print both cell types and continue culturing the cells to monitor their health. Similar to the study of Kador et al., their results showed good viability of cells postprinting and that neither the vibration of the piezoelectric activator nor the shear forces present on the cells during printing irreversibly harmed retinal or glial cells.36 Although the viability was considered unaffected by the printing process, the total amount of cells printed was reduced compared to the traditional controls. In this study, 40% to 67% of glial and retinal cells, respectively, were present in the printed cultures compared to the control populations, possibly due to cell settlement in the nozzle during printing. Together, the Kador et al. and Lorber et al. studies show clear promise for the future of bioprinting of retinal cells. However, novel methods or improvements to current technology to improve the overall efficiency in maintaining a large cell population at higher density are needed to construct viable printed retinal tissue for research or surgical implantation.
Cornea
The cornea is the clear outer covering of the eye, refracting light as it enters the pupil and protecting the lens and vitreous body. Composed of five layers, the outermost epithelial cells and the keratocytes of the stroma, comprising the vast majority of the thickness of the cornea, are the main focuses of tissue engineering approaches.60 As a protective barrier and one of the most important components of light refraction and focus, any damage or disease affecting the cornea can lead to visual problems. The cornea is susceptible to keratitis and can develop ulceration or scarring.61 Keratoplasty, or corneal transplants, offer one of the only true remedies to restore vision in patients, however, there is a severe shortage of donor tissue relative to the total population suffering from corneal blindness.62 Bioprinting of corneal cells could therefore offer a future source of implantable tissue, mediating the need for larger donor pool.
Several groups have reported on the printing of human corneal epithelial cells (HCECs).48,63 Wu et al. utilized a hybrid 3D bioprinting platform containing alginate, collagen, and gelatin that was further cross-linked with calcium chloride (CaCl2) to suspend HCECs. They were able to achieve high viability (95%) after using an extrusion printing method at 37°C but deposited into a refrigerated scaffold.48 The study also focused on the role of sodium citrate in the degradation of the 3D-printed gel, providing insight toward development of a tunable and controlled degradation of the 3D-printed scaffold. Controlled degradation of the alginate network allowed Wu et al. to achieve high viability of HCECs while also improving cellular proliferation and expression of corneal epithelial-specific cytokeratin 3 (CK3), considered a specific marker of HCECs compared to other 3D-cultured HCECs.48 Although, the final constructs did not have the size nor shape of corneas, their experiments opened the path to other attempts to 3D bioprint corneas.
Another study by Gibney et al. reported on the use of thin collagen films in 3D printing to enable layer deposition coprinted with corneal mesenchymal stem cells (MSCs), which offer the capability of differentiating into functional stromal keratocytes.60,64 The researchers chose recombinant collagen type III (RHCIII) as their gel platform since a promising phase I clinical study by Griffith et al. showed that RHCIII-based biosynthetic cornea could be used successfully in keratoplasty.62 In the study by Gibney et al., the RHCIII was aerosolized and extruded by a pressurized stream of nitrogen to form 2 μm-thick layers of collagen that was further cross-linked by carbodiimide and NHS ester chemistry. Both corneal MSCs and corneal keratocytes were evaluated in the printed RHCIII scaffold and the MSCs were found to align better with the printed scaffold and exhibited better proliferation than the keratocytes.60 However, while studies of Wu et al. and Gibney et al. successfully demonstrated the viability of printed corneal cells, neither study fully evaluated the bioprinting of a complete cornea.
Very recently, Isaacson et al. reported their own 3D bioprinting of corneal stroma using a bioink laden with corneal keratocytes (Fig. 4C). Isaacson et al. utilized a CAD model with G code printing (using a Cellink INKREDIBLE) to develop their printed structure. Their ink was based on an alginate/methacrylated collagen solution that could be cross-linked with CaCl2 upon printing with an extrusion printer.53 After optimizing the printing process, human stromal keratocytes were introduced into the bioprinting process and their viability was assessed after 7 days postprinting, indicating no significant change in viability from culturing in the printed corneal structures. Isaacson et al. suggest that their printed corneal stroma could prove a valuable research tool, proving capable of replicating the geometry of the cornea to discern biologically relevant insights about cellular remodeling of the ECM as well as serving as a potential construct for epithelial cell seeding.53
Future Directions
The recent study by Isaacson et al. offers the most promising recent attempt of bioprinting ocular tissue. More studies in a similar vein will hopefully follow to further expand the field into exciting new advances. Successful printing of corneal tissue represents an achievable first step in pioneering the use of 3D printing to create ocular components and materials.53 Generating cellularized models of the different components or overall structure of the eye would prove an invaluable research tool, as therapies could be assessed on a model capable of replicating the complicated structure of the human eye. By creating an improved biomimetic methodology for culturing ocular cells, the translational impact of studies of potential therapeutics can be explored. Studies such as the one performed by Lorber et al. focused on the printing of multiple cell types that are also quite beneficial in achieving this goal.36 Cell–cell and cell–matrix interactions contain significantly more complexity in vivo than current 2D cell cultures can reproduce. While improving cellular density to biological levels remains an obstacle, cellular viability postprinting appears sufficient such that bioprinting of desired tissue architectures and ocular structures may be viable in the future.
Three-dimensional bioprinting represents a powerful tool in the developing world of tissue engineering. Bioprinting offers the user control over the material properties and architecture of a scaffold to maintain a target population of cells. Given the paucity of appropriate donor tissue, optimizing these techniques to utilize patient cells or ocular stem cells to generate biocompatible tissue for transplantation would be of tremendous benefit to patients.65 In addition, the use of 3D bioprinting to develop anatomically accurate models for the orbital bones may diminish the need for cadavers, providing better training opportunities for aspiring medical students and researchers.66 Engineered corneal tissue has previously been used to restore transparency to the corneal epithelia through tissue grown ex vivo from harvested patient cells.67 This strategy could conceivably be exploited on a larger scale for the more complicated tissue of the eye as well, taking stem cells and 3D bioprinting to create a functional macula or lens for example.
Outlook
Bioprinting of materials for ocular research is still a promising nascent field. While successful investigations into 3D printing of retinal and corneal cells show good viability of printed cells, the total efficiency and cellular density of printed tissue remain low. In total, the current state of research offers hope for more complicated ocular models and tissue engineering, and optimization of the technology will require significant efforts.
Acknowledgment
The authors would like to acknowledge from the NIH (Grant Number ROI-DE013023).
Author Disclosure Statement
The authors have relationships with Allevi and Cellink.
References
- 1. Fenton O.S., Olafson K.N., Pillai P.S., Mitchell M.J., and Langer R.. Advances in biomaterials for drug delivery. Adv. Mater. 1705328:1–29, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Groen N., Guvendiren M., Rabitz H., Welsh W.J., Kohn J., and De Boer J.. Stepping into the omics era: opportunities and challenges for biomaterials science and engineering. Acta Biomater. 34:133–142, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tibbitt M.W., and Langer R.. Living biomaterials. Acc. Chem. Res. 50:508–513, 2017 [DOI] [PubMed] [Google Scholar]
- 4. Pati F., Gantelius J., and Svahn H.A.. 3D bioprinting of tissue/organ models. Angew. Chem Int. Ed. 55:4650–4665, 2016 [DOI] [PubMed] [Google Scholar]
- 5. Langer R., and Vacanti J.. Tissue engineering. Science. 260:920–926, 1993 [DOI] [PubMed] [Google Scholar]
- 6. Borovjagin A.V., Ogle B.M., Berry J.L., and Zhang J.. From microscale devices to 3D printing: advances in fabrication of 3D cardiovascular tissues. Circ. Res. 120:150–165, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Murphy S.V., and Atala A.. 3D bioprinting of tissues and organs. Nat. Biotechnol. 32:773–785, 2014 [DOI] [PubMed] [Google Scholar]
- 8. Guvendiren M., Molde J., Soares R.M.D., and Kohn J.. Designing biomaterials for 3D printing. ACS Biomater. Sci. Eng. 2:1679–1693, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kyle S., and Whitaker I.S.. To print or not to print, that is the question: how close are we to clinical translation of contemporary bioinks? J. 3D Print. Med. 2:1–3, 2018 [Google Scholar]
- 10. Ozbolat I.T., and Yu Y.. Bioprinting toward organ fabrication: challenges and future trends. IEEE Trans. Biomed. Eng. 60:691–699, 2013 [DOI] [PubMed] [Google Scholar]
- 11. Bishop E.S., Mostafa S., Pakvasa M., Luu H.H., Lee M.J., Wolf J.M., Ameer G.A., He T.C., and Reid R.R.. 3-D bioprinting technologies in tissue engineering and regenerative medicine: current and future trends. Genes Dis. 4:185–195, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mandrycky C., Wang Z., Kim K., and Kim D.H.. 3D bioprinting for engineering complex tissues. Biotechnol. Adv. 34:422–434, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Groll J., Boland T., Blunk T., Burdick J.A., Cho D.-W., Dalton P.D., Derby B., Forgacs G., Li Q., Mironov V.A., et al. Biofabrication: reappraising the definition of an evolving field. Biofabrication. 8:013001, 2016 [DOI] [PubMed] [Google Scholar]
- 14. Groll J., Burdick J.A., Cho D.W., Derby B., Gelinsky M., Heilshorn S.C., Jüngst T., Malda J., Mironov V.A., Nakayama K., et al. A definition of bioinks and their distinction from biomaterial inks. Biofabrication. 11:013001, 2019 [DOI] [PubMed] [Google Scholar]
- 15. Zhu W., Ma X., Gou M., Mei D., Zhang K., and Chen S.. 3D printing of functional biomaterials for tissue engineering. Curr. Opin. Biotechnol. 40:103–112, 2016 [DOI] [PubMed] [Google Scholar]
- 16. He P., Zhao J., Zhang J., Li B., Gou Z., Gou M., and Li X.. Bioprinting of skin constructs for wound healing. Burn. Trauma. 6:5, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hopp B., Smausz T., Kresz N., Barna N., Bor Z., Kolozsvári L., Chrisey D.B., Szabó A., and Nógrádi A.. Survival and proliferative ability of various living cell types after laser-induced forward transfer. Tissue Eng. 11:1817–1823, 2005 [DOI] [PubMed] [Google Scholar]
- 18. Patra S., and Young V.. A review of 3D printing techniques and the future in biofabrication of bioprinted tissue. Cell Biochem. Biophys. 74:93–98, 2016 [DOI] [PubMed] [Google Scholar]
- 19. Mannoor M.S., Jiang Z., James T., Kong Y.L., Malatesta K.A., Soboyejo W.O., Verma N., Gracias D.H., and McAlpine M.C.. 3D printed bionic ears. Nano Lett. 13:2634–2639, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hockaday L.A., Kang K.H., Colangelo N.W., Cheung P.Y.C., Duan B., Malone E., Wu J., Girardi L.N., Bonassar L.J., Lipson H., et al. Rapid 3D printing of anatomically accurate and mechanically heterogeneous aortic valve hydrogel scaffolds. Biofabrication. 4:035005, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Skardal A., Zhang J., and Prestwich G.D.. Bioprinting vessel-like constructs using hyaluronan hydrogels crosslinked with tetrahedral polyethylene glycol tetracrylates. Biomaterials. 31:6173–6181, 2010 [DOI] [PubMed] [Google Scholar]
- 22. Lozano R., Stevens L., Thompson B.C., Gilmore K.J., Gorkin R., Stewart E.M., in het Panhuis M., Romero-Ortega M., and Wallace G.G.. 3D printing of layered brain-like structures using peptide modified gellan gum substrates. Biomaterials. 67:264–273, 2015 [DOI] [PubMed] [Google Scholar]
- 23. Koch L., Deiwick A., Schlie S., Michael S., Gruene M., Coger V., Zychlinski D., Schambach A., Reimers K., Vogt P.M., et al. Skin tissue generation by laser cell printing. Biotechnol. Bioeng. 109:1855–1863, 2012 [DOI] [PubMed] [Google Scholar]
- 24. Kwon H., Paschos N.K., Hu J.C., and Athanasiou K.. Articular cartilage tissue engineering: the role of signaling molecules. Cell. Mol. Life Sci. 73:1173–1194, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Barron J.A., Krizman D.B., and Ringeisen B.R.. Laser printing of single cells: statistical analysis, cell viability, and stress. Ann. Biomed. Eng. 33:121–130, 2005 [DOI] [PubMed] [Google Scholar]
- 26. Kislitsyn A., Savinkov R., Novkovic M., Onder L., and Bocharov G.. Computational approach to 3D modeling of the lymph node geometry. Computation. 3:222–234, 2015 [Google Scholar]
- 27. Keriquel V., Guillemot F., Arnault I., Guillotin B., Miraux S., Amédée J., Fricain J.C., and Catros S.. In vivo bioprinting for computer- and robotic-assisted medical intervention: preliminary study in mice. Biofabrication. 2:014101, 2010 [DOI] [PubMed] [Google Scholar]
- 28. Arai K., Iwanaga S., Toda H., Genci C., Nishiyama Y., and Nakamura M.. Three-dimensional inkjet biofabrication based on designed images. Biofabrication. 3:034113, 2011 [DOI] [PubMed] [Google Scholar]
- 29. Rodriguez M.J., Brown J., Giordano J., Lin S.J., Omenetto F.G., and Kaplan D.L.. Silk based bioinks for soft tissue reconstruction using 3-dimensional (3D) printing with in vitro and in vivo assessments. Biomaterials. 117:105–115, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhao Y., Yao R., Ouyang L., Ding H., Zhang T., Zhang K., Cheng S., and Sun W.. Three-dimensional printing of hela cells for cervical tumor model in vitro. Biofabrication. 6:035001, 2014 [DOI] [PubMed] [Google Scholar]
- 31. Zhang T., Yan K.C., Ouyang L., and Sun W.. Mechanical characterization of bioprinted in vitro soft tissue models. Biofabrication. 5:045010, 2013 [DOI] [PubMed] [Google Scholar]
- 32. Dernowsek J., Rezende R., and Lopes da Silva J.V. BioCAE: a new strategy of complex biological systems for biofabrication of tissues and organs. J. Tissue Sci. Eng. 08, 2017 [Google Scholar]
- 33. van der Valk D., van der Ven C., Blaser M., Grolman J., Wu P.-J., Fenton O., Lee L., Tibbitt M., Andresen J., Wen J., et al. Engineering a 3D-bioprinted model of human heart valve disease using nanoindentation-based biomechanics. Nanomaterials. 8:296, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kolesky D.B., Truby R.L., Gladman A.S., Busbee T.A., Homan K.A., and Lewis J.A.. 3D bioprinting of vascularized, heterogeneous cell-laden tissue constructs. Adv. Mater. 26:3124–3130, 2014 [DOI] [PubMed] [Google Scholar]
- 35. Hospodiuk M., Dey M., Sosnoski D., and Ozbolat I.T.. The bioink: a comprehensive review on bioprintable materials. Biotechnol. Adv. 35:217–239, 2017 [DOI] [PubMed] [Google Scholar]
- 36. Lorber B., Hsiao W.K., Hutchings I.M., and Martin K.R.. Adult rat retinal ganglion cells and glia can be printed by piezoelectric inkjet printing. Biofabrication. 6:15001, 2014 [DOI] [PubMed] [Google Scholar]
- 37. Chang C.C., Boland E.D., Williams S.K., and Hoying J.B.. Direct-write bioprinting three-dimensional biohybrid systems for future regenerative therapies. J. Biomed. Mater. Res. Part B Appl. Biomater. 98 B:160–170, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Boland T., Xu T., Damon B., and Cui X.. Application of inkjet printing to tissue engineering. Biotechnol. J. 1:910–917, 2006 [DOI] [PubMed] [Google Scholar]
- 39. Donderwinkel I., Van Hest J.C.M., and Cameron N.R.. Bio-inks for 3D bioprinting: recent advances and future prospects. Polym Chem. 8:4451–4471, 2017 [Google Scholar]
- 40. Malda J., Visser J., Melchels F.P., Jüngst T., Hennink W.E., Dhert W.J.A., Groll J., and Hutmacher D.W.. 25th anniversary article: engineering hydrogels for biofabrication. Adv. Mater. 25:5011–5028, 2013 [DOI] [PubMed] [Google Scholar]
- 41. Seal B., Otero T.C., and Panitch A.. Polymeric biomaterials for tissue and organ regeneration. Mater. Sci. Eng. R Rep. 34:147–230, 2001 [Google Scholar]
- 42. Borselli C., Ungaro F., Oliviero O., D'Angelo I., Quaglia F., La Rotonda M.I., and Netti P.A.. Bioactivation of collagen matrices through sustained VEGF release from PLGA microspheres. J. Biomed. Mater. Res. Part A. 92:94–102, 2010 [DOI] [PubMed] [Google Scholar]
- 43. Barralet J., Gbureck U., Habibovic P., Vorndran E., Gerard C., and Doillon C.J.. Angiogenesis in calcium phosphate scaffolds by inorganic copper ion release. Tissue Eng. Part A. 15:1601–1609, 2009 [DOI] [PubMed] [Google Scholar]
- 44. Norotte C., Marga F.S., Niklason L.E., and Forgacs G.. Scaffold-free vascular tissue engineering using bioprinting. Biomaterials. 30:5910–5917, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Miller J.S., Stevens K.R., Yang M.T., Baker B.M., Nguyen D.H.T., Cohen D.M., Toro E., Chen A.A., Galie P.A., Yu X., et al. Rapid casting of patterned vascular networks for perfusable engineered three-dimensional tissues. Nat. Mater. 11:768–774, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zhang Y., Yu Y., and Ozbolat I.T.. Direct bioprinting of vessel-like tubular microfluidic channels. J. Nanotechnol. Eng. Med. 4:020902, 2013 [Google Scholar]
- 47. Yu Y., Zhang Y., and Ozbolat I.T.. A hybrid bioprinting approach for scale-up tissue fabrication. J. Manuf. Sci. Eng. 136:061013, 2014 [Google Scholar]
- 48. Wu Z., Su X., Xu Y., Kong B., Sun W., and Mi S.. Bioprinting three-dimensional cell-laden tissue constructs with controllable degradation. Sci. Rep. 6:1–10, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Stanton M.M., Samitier J., and Sánchez S.. Bioprinting of 3D hydrogels. Lab Chip. 15:3111–3115, 2015 [DOI] [PubMed] [Google Scholar]
- 50. Xie P., Hu Z., Zhang X., Li X., Gao Z., Yuan D., and Liu Q.. Application of 3-dimensional printing technology to construct an eye model for fundus viewing study. PLoS One. 9:1–9, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Coudrillier B., Pijanka J., Jefferys J., Sorensen T., Quigley H.A., Boote C., and Nguyen T.D.. Collagen structure and mechanical properties of the human sclera: analysis for the effects of age. J. Biomech. Eng. 137:041006, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Keeley F.W., Morin J.D., Vesely S.. Characterization of collagen from normal human sclera. Exp. Eye Res. 39:533–542, 1984 [DOI] [PubMed] [Google Scholar]
- 53. Isaacson A., Swioklo S., and Connon C.J.. 3D bioprinting of a corneal stroma equivalent. Exp. Eye Res. 173:188–193, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kador K.E., Grogan S.P., Dorthé E.W., Venugopalan P., Malek M.F., Goldberg J.L., and D'lima D.D.. Control of retinal ganglion cell positioning and neurite growth: combining 3D printing with radial electrospun scaffolds. Tissue Eng. Part A. 22:286–294, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Jager R.D., Mieler W.F., and Miller J.W.. Age-related macular degeneration. N. Engl. J. Med. 358:2606–2617, 2008 [DOI] [PubMed] [Google Scholar]
- 56. Ferrari S., Di Iorio E., Barbaro V., Ponzin D., Sorrentino F.S., and Parmeggiani F.. Retinitis pigmentosa: genes and disease mechanisms. Curr. Genomics. 12:238–249, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hynes S.R., and Lavik E.B.. A tissue-engineered approach towards retinal repair: scaffolds for cell transplantation to the subretinal space. Graefes Arch Clin Exp Ophthalmol. 248:763–778, 2010 [DOI] [PubMed] [Google Scholar]
- 58. Shi P., Yong T., Edgar S., Yeong W.Y., and Laude A.. Hybrid three-dimensional (3D) bioprinting of retina equivalent for ocular research. Int. J. Bioprinting. 3:138–146, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lorber B., Hsiao W.K., and Martin K.R.. Three-dimensional printing of the retina. Curr. Opin. Ophthalmol. 27:262–267, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Gibney R., Matthyssen S., Patterson J., Ferraris E., and Zakaria N.. The human cornea as a model tissue for additive biomanufacturing: a review. Proc CIRP. 65:56–63, 2017 [Google Scholar]
- 61. Garg P., Krishna P.V., Stratis A.K., and Gopinathan U.. The value of corneal transplantation in reducing blindness. Eye. 19:1106–1114, 2005 [DOI] [PubMed] [Google Scholar]
- 62. Fagerholm P., Lagali N.S., Merrett K., Jackson W.B., Munger R., Liu Y., Polarek J.W., Söderqvist M., and Griffith M.. A biosynthetic alternative to human donor tissue for inducing corneal regeneration: 24-month follow-up of a phase 1 clinical study. Sci. Transl. Med. 2:46ra61, 2010 [DOI] [PubMed] [Google Scholar]
- 63. Islam M.M., Cepla V., He C., Edin J., Rakickas T., Kobuch K., Ružele, Ž., Jackson W.B., Rafat M., Lohmann C.P., et al. Functional fabrication of recombinant human collagen-phosphorylcholine hydrogels for regenerative medicine applications. Acta Biomater. 12:70–80, 2015 [DOI] [PubMed] [Google Scholar]
- 64. Matthyssen S., Van den Bogerd B., Dhubhghaill S.N., Koppen C., and Zakaria N.. Corneal regeneration: a review of stromal replacements. Acta Biomater. 69:31–41, 2018 [DOI] [PubMed] [Google Scholar]
- 65. Huang W., and Zhang X.. 3D printing: print the future of ophthalmology. Investig. Opthalmology Vis. Sci. 55:5380, 2014 [DOI] [PubMed] [Google Scholar]
- 66. Adams J.W., Paxton L., Dawes K., Burlak K., Quayle M., and McMenamin P.G.. 3D printed reproductions of orbital dissections: a novel mode of visualising anatomy for trainees in ophthalmology or optometry. Br. J. Ophthalmol. 99:1162–1167, 2015 [DOI] [PubMed] [Google Scholar]
- 67. Nishida K., Yamato M., Hayashida Y., Watanabe K., Yamamoto K., Adachi E., Nagai S., Kikuchi A., Maeda N., Watanabe H., et al. Corneal reconstruction with tissue-engineered cell sheets composed of autologous oral mucosal epithelium. N. Engl. J. Med. 351:1187–1196, 2004 [DOI] [PubMed] [Google Scholar]