Abstract
Significance: The skin undergoes an inevitable degeneration as an individual ages. As intrinsic and extrinsic factors degrade the structural integrity of the skin, it experiences a critical loss of function and homeostatic stability. Thus, aged skin becomes increasingly susceptible to injury and displays a prolonged healing process.
Recent Advances: Several studies have found significant differences during wound healing between younger and older individuals. The hypoxia-inducible factor 1-alpha (HIF-1α) signaling pathway has recently been identified as a major player in wound healing. Hypoxia-inducible factors (HIFs) are pleiotropic key regulators of oxygen homeostasis. HIF-1α is essential to neovascularization through its regulation of cytokines, such as SDF-1α (stromal cell-derived factor 1-alpha) and has been shown to upregulate the expression of genes important for a hypoxic response. Prolyl hydroxylase domain proteins (PHDs) and factor inhibiting HIF effectively block HIF-1α signaling in normoxia through hydroxylation, preventing the signaling cascade from activating, leading to impaired tissue survival.
Critical Issues: Aged wounds are a major clinical burden, resisting modern treatment and costing millions in health care each year. At the molecular level, aging has been shown to interfere with PHD regulation, which in turn prevents HIF-1α from activating gene expression, ultimately leading to impaired healing. Other studies have identified loss of function in cells during aging, impeding processes such as angiogenesis.
Future Directions: An improved understanding of the regulation of molecular mediators, such as HIF-1α and PHD, will allow for manipulation of the various factors underlying delayed wound healing in the aged. The findings highlighted in this may facilitate the development of potential therapeutic approaches involved in the alteration of cellular dynamics and aging.
Keywords: HIF-1α, PHD, aging, neovascularization, skin

Geoffrey C. Gurtner MD, FACS
Scope and Significance
This review focuses on the effects of aging on the hypoxia-inducible factor 1-alpha (HIF-1α) signaling pathway and the molecules that regulate it. HIF-1α is integral to neovascularization and has been shown to upregulate the expression of genes involved in a hypoxic response. Aging engenders impediments to HIF-1α signaling, slowing the wound healing process. Developments toward regenerative medicine in wound healing are detailed, with a focus on targeting the age-associated defects in the HIF-1α pathway.
Translational Relevance
Multiple treatments are available that attempt to prevent skin from aging and accelerate the different phases of healing in aged wounds. With an understanding of the pathophysiology of aging skin, therapies can be developed to specifically target the pathways that are altered with age, leading to the development of new treatments.
Clinical Relevance
The aged population is disproportionately affected by chronic wounds. Aged wounds can be highly resistant to treatment, persisting indefinitely. Many patients require multiple rounds of treatment, costing the health care system millions each year. The methods discussed in this review are aimed to give an updated summary of wound healing in aged skin and the signaling pathways affected by aging. With a better understanding of these subjects, new treatments may be developed to improve clinical outcomes.
Discussion of Findings and Recent Literature
Introduction
The skin is the largest organ within the human body, serving several purposes, including the provision of a barrier between the internal organs and the outside environment, homeostatic and temperature regulation, and fluid retention.1,2 Under normal conditions, cells within the skin exist as a complex and balanced mosaic with continuous turnover. As humans age, the skin undergoes a visible degeneration, with a reduced ability to perform these functions. The physical structure of skin changes with age, leading to an increased susceptibility to injury and morbidity, with a majority of those over 65 years of age having at least one skin disorder.3–5 Such disorders and changes in the skin can exacerbate the process of healing following injury through their influences on the microenvironment. As the elderly population continues to rise, efforts have been made to attain a better understanding of the effects of aging on various processes associated with wound healing.
Overview of aging
Intrinsic and extrinsic aging factors are two commonly accepted categories associated with deleterious changes to the skin and can lead to accelerated aging.6 Intrinsic aging consists of the physiological changes over time that are determined by genetics, whereas extrinsic aging is the result of controllable, external factors such as exposure to ultraviolet (UV) radiation, pollution and nicotine, excessive and repetitive use of muscles, and a lack of proper nutrition and sleep.7,8 UV radiation is likely the most important of these factors and is split into two major components: UVA [ultraviolet A (long-wave)], which penetrates deeply into the dermis and generates several reactive oxygen species (ROS) which damage DNA, and UVB [ultraviolet B (shortwave)], which affects the epidermis and is absorbed by DNA, leading to harmful, carcinogenic mutations.9 UV generated ROS downregulate several factors and processes that are essential to maintaining skin integrity, such as transforming growth factor beta (TGF-β) and collagen synthesis.10,11 Furthermore, these ROS cause excessive oxidative stress that brings about an inflammatory response and subsequent cascading effects that lead to collagen degradation.12 UVB can also induce inflammation and various signaling cascades, leading to irregular keratinocyte function and lifespan.13–15
While both intrinsic and extrinsic factors lead to unhealthy alterations in the skin, the exact changes brought about by one or the other often differ.16 For instance, skin largely protected from harmful UV radiation typically displays a dermal thinning along with a flattening of the dermal–epidermal junction (DEJ) as it ages.17–19 Conversely, skin subjected to high amounts of UV radiation displays a dermal thickening, suggesting that differing pathways likely govern the various ways skin can age.20 These changes, while different, yield a loss of function within the skin, leading to a higher susceptibility to disease.21
Structure of the skin, how it differs with age
The skin comprises roughly 12–16% of the human body weight and consists of three separate layers: the epidermis, dermis, and hypodermis.9,19 The epidermis is the outermost layer and is between 50 to 100 μm thick in a healthy individual, depending on the location.22 The epidermis primarily consists of keratinocytes that are constantly sloughed off and replaced with younger, healthier cells. This turnover rate is reduced within older individuals, with the time it takes for keratinocytes to migrate from the basal layer to the epidermis increased by up to 50%.23 The stratum corneum is the most superficial region of the epidermis and provides the skin with its water-resistant properties.24 A complex matrix of hydrophobic lipids and “natural moisturization factors,” including cholesterol, amino acids, and fatty acids, regulate skin moisturization in normal, healthy skin.24–26 Aged skin displays significantly lower levels of lipids and amino acids essential to water retention, thereby contributing to higher levels of xerosis (dry skin) and sensitivity to irritants.27,28 The stratum granulosum, stratum spinosum, and stratum basale make up the rest of the epidermis, with keratinocytes within the stratum basale proliferating and migrating to the more superficial layers.29
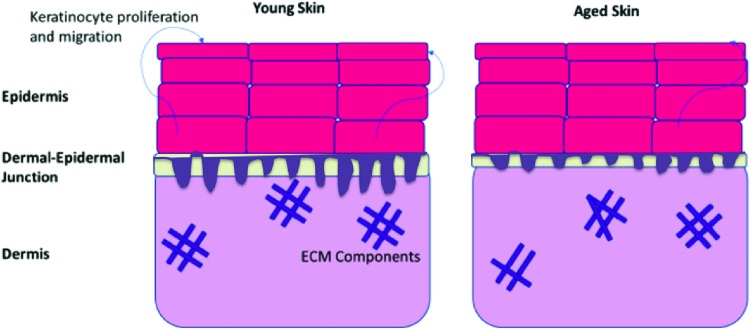
Immediately underlying the epidermis is the DEJ, joining the keratinocyte layers of the epidermis to the infrastructure of the dermis.30 Several studies have observed a notable thinning of the DEJ as a result of intrinsic aging, making aged skin more susceptible to shearing forces, and thus, injury.30–32 Furthermore, studies have found that the protein composition of the DEJ changes with age, which could yield lesser adhesion between the epidermis and the dermis in addition to a loss of structural integrity.30,33 The deeper dermal layer displays fewer cellular constituents than the epidermis and is primarily composed of extracellular matrix (ECM) components. The ECM provides structure for an array of nerves and vasculature that support the integrity of the skin. Fewer cells responsible for ECM deposition and tissue remodeling are found within the aged dermis, leading to a reduction in vasculature and collagen.23 Additionally, collagen and elastin deposition become less organized, resulting in weaker and less elastic skin23 (Fig. 1).
Figure 1.
Structure of unwounded, young skin and aged skin. Keratinocytes display a loss of function, proliferating, and migrating at lesser rates than those found in younger, healthy skin. The epidermis in aged skin thus lacks the same cellular upkeep found in young skin. Aged skin displays a significantly thinner dermal-epidermal junction, making aged skin more susceptible to shearing forces and injury. The dermis of aged skin is characterized by lesser organization of ECM components, making it less stable and sturdy than the dermis in young skin. ECM, extracellular matrix. Color images are available online.
These age-associated, structural and etiological changes are only some of many that bring about increased susceptibility to injury. This altered microenvironment of aged skin leads to an irregular progression through the stages of regeneration: hemostasis, inflammation, proliferation, and tissue remodeling. Often times, these changes are deleterious and yield slow-healing, chronic wounds (Table 1).
Table 1.
The major characteristics of the phases of wound healing59
| Inflammatory |
Proliferative |
Remodeling |
|---|---|---|
| 2–5 Days | 5 Days to 3 Weeks | 3 Weeks to 2 Years |
|
Clotting cascade Extensive clotting occurs to attempt to obtain hemostasis Inflammatory response Macrophages release signaling molecules/inflammatory factors (serotonin, histamine, FGF, IL-1) to enhance vasodilation Release of proinflammatory cytokines and growth factors (TGF-β, PDGF, FGF, EGF) causing leukocyte migration Polymorphonuclear neutrophils and macrophages phagocytize debris, bacteria, and breakdown damaged tissue |
Angiogenesis Provides adequate supply of oxygen, nutrients, and growth factors, and promotes tissue granulation Granulation Fibroblasts synthesize collagen type III and fibronectin to form a provisional ECM Re-ephitheliazation Re-epithelialization of the dermis bridges and resurfaces the wound Contraction Wound contraction begins |
Scar formation Collagen production and degradation are equalized Collagen remodeled from type III to type I Collagen crosslinked structure forms |
ECM, extracellular matrix; EGF, epidermal growth factor; FGF, fibroblast growth factor; IL, interleukin; PDGF, platelet-derived growth factor; TGF-β, transforming growth factor beta.
Hemostasis
Immediately following injury to the skin, the regeneration process begins with hemostasis. Blood vessels vasoconstrict and platelets adhere to the endothelium and accumulate, forming blood clots that prevent excessive blood loss.34 These platelet clots are responsible for the secretion of various growth factors and signaling molecules, such as platelet-derived growth factor (PDGF), transforming growth factor alpha (TGF-α), and TGF-β.23,35,36 Such signals are produced to recruit inflammatory cells to the wound bed and stimulate their activity. Thus, platelets are essential to progression into the inflammatory phase of wound healing. In vivo studies have found that aged individuals display an increase in protein aggregation compared with younger ones.37,38 Additionally, the release of signaling molecules PDGF, TGF-α, and TGF-β is increased with age as well.38
Inflammation
Following the release of proinflammatory cytokines by platelets and mast cells, circulating neutrophils are recruited to the wound bed. Monocytes are also recruited to the wound, where they subsequently differentiate into phagocytic macrophages. Macrophages and neutrophils work together to debride the wound bed of any harmful biotics and detritus, while also secreting chemokines to recruit other cells to aid with wound resolution. Moreover, ROS are produced by neutrophils to assist with cleaning up the wound. ROS also serve as inflammatory mediators, and are beneficial to wound healing in low, contained concentrations. ROS also have a low specificity, which can become problematic, as an excess of ROS can induce a chronic inflammatory state and degrade a variety of beneficial molecules, including many protein and lipid constituents and even DNA.39 ROS production is higher in the elderly, leading to higher levels of oxidative stress and thus, greater DNA degradation.40
Aged individuals display alterations in cell function, cellular adherence, and cell migration, thereby affecting wound healing in a multitude of ways.41,42 In vivo mouse studies have found that neutrophils are likely essential to regular wound healing in aged mice, as neutrophil depletion significantly decreased the rate of wound closure in mice over 20 months of age.43 This is likely due to a loss of function with age, as in vitro studies have found that neutrophils from the elderly display a lesser ability to phagocytose bacteria.44 This could potentially lead to wound infection and subsequent chronic inflammation.
In addition to the alterations in neutrophil recruitment, circulating monocytes have been found to be recruited to the wound at a lesser rate in aged individuals. This coincides with a higher percentage of mature macrophages that are active in the wound. Such macrophages have been shown to have a loss in function, leading to reductions in angiogenesis, re-epithelialization, and tissue remodeling.23 This was confirmed by in vivo studies, where injection of macrophages from aged mice into younger mice did not accelerate wound healing, whereas injection of macrophages from young mice did.45 The exact mechanisms by which these observed changes act to influence wound healing in the aged have yet to be elucidated but provide several implications. Any drastic change in the number of these cell types could affect the rates of phagocytosis, chemokine production, and growth factor production, not only changing the outcome of the inflammatory phase, but also the subsequent wound repair phases as well.
Proliferation
Following a successful immune response, endothelial cells, fibroblasts, and keratinocytes are recruited to the wound bed to stimulate re-epithelialization and angiogenesis. This is typically after 3 days in a healthy individual with acute wounds.46 Fibroblasts are recruited to the wound bed by factors secreted by platelets and inflammatory cells, such as PDGF and TGF-β.47 Following migration to the wound, fibroblasts proliferate at a high rate and begin to synthesize ECM components, such as fibronectin and collagen, which are deposited into a provisional matrix.42 This matrix brings about further cell migration to aid with the healing process, although the ratio of collagen types differs in a repaired dermis from that of the unwounded dermis. Specifically, deposition of collagen I decreases significantly, leading to a higher ratio of collagen III to collagen I.48 Furthermore, collagen content in the skin has been found to decrease by roughly 1% per year after reaching adulthood as a result of age-associated, waning fibroblast function.49
As they continue proliferating, fibroblasts differentiate into myofibroblasts, characterized by their expression of α-smooth muscle actin (α-SMA).50 These actin bundles give myofibroblasts contractile properties that they use to contract the wound, an essential process to wound closure.
Concurrently, local endothelial cells are recruited to aid in angiogenesis. Tissue disruption causes the wound to experience hypoxic conditions, activating several signaling pathways, such as the HIF-1α pathway, which stimulates endothelial cell proliferation and growth. Studies have examined the expression of endothelial cell adhesion molecule (CAM), taking samples from human wounds. Samples from aged individuals displayed a significant delay in appearance and peak in both intracellular CAM-1 and vascular CAM-1.41
Hypoxic conditions also have been shown to impair keratinocyte motility in aged individuals, whereas keratinocyte motility was increased in younger individuals.51 Matrix metalloproteinase (MMP)-1 and -9 expressions were found to be reduced as well in response to hypoxia in aged, whereas both were upregulated in younger control groups.51 Both have been implicated to have a significant role in keratinocyte migration.51 Moreover, vascular endothelial growth factor (VEGF) expression has been positively correlated with hypoxia in younger, healthy individuals, and studies have found that older individuals display lower levels of VEGF expression along with lower HIF-1α expression.52
Thus, several factors underlie delayed wound regeneration during the proliferation phase, with several of these factors working in concert to exacerbate many of the issues. These alterations in the healing process hold an influence over the final phase of tissue remodeling.
Tissue remodeling
Following re-epithelialization and angiogenesis, cells work to remodel the tissue in a way that restores the skin as closely to its original integrity as possible. The end result of wound healing in nonfetal mammals most often is a scar that is characterized by collagen composition and organization that differ from healthy skin.53 This causes the recovered skin to have a weaker mechanical strength than normal skin.54 Interestingly, old subjects display a faster scar maturation rate than younger individuals.55 Yet, hypertrophic scars and keloids are rarely found in elderly individuals, possibly due to a reduction of molecules that enhance collagen deposition, such as TGF-β. Studies have found that TGF-β inhibition has been shown to impede excessive scar formation in adults.56,57
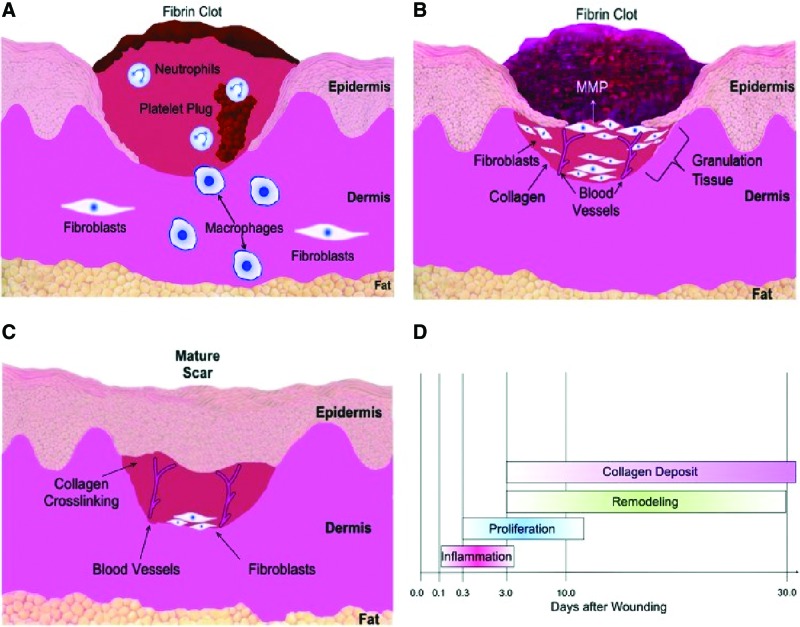
With an increase in MMP production and a concordant downregulation of tissue inhibitors of metalloproteinases (TIMPs), aged wounds seem primed for tissue breakdown as well.23,54 Animal studies have noted an expedited rate of wound healing following the application of growth factors to wounds in aged subjects.58 The exact mechanism by which TGF-β1 acts to improve wound healing is unknown, although topical delivery was shown to increase fibroblast migration and collagen deposition, decrease MMP-1 production, and increase TIMP-1 expression58 (Fig. 2).
Figure 2.
(A) Inflammation. Immediately after injury hemostasis and inflammation occur leading to vasoconstriction. Platelets adhere and form blood clots with a wealth of signaling molecules, recruiting monocytes and neutrophils to the site of the wound. Monocytes then differentiate into mature macrophages at the wound site. Macrophages and neutrophils phagocytize the wound bed and release additional factors to stimulate migration of fibroblasts to the wound site. (B) Proliferation and remodeling. Fibroblasts secrete ECM to form granulation tissue during proliferation. Endothelial cells migrate to the wound and engender neovascularization. Remodeling begins with degradation of collagen and ECM through MMPs. (C) Remodeling. During the remodeling phase, collagen production and degradation equalize. The wound gains its tensile strength by disorganized collagen fibers that are crosslinked and aligned along tension lines. (D) Time course of several wound healing phases.59 MMPs, matrix metalloproteinases. Color images are available online.
Impaired neovascularization in aging
Oxygen is key to many processes of life and is involved in all stages of wound healing in the skin, with many cells and pathways being reactive to changes in oxygen concentration.59 Following injury to the skin, disruption of the vasculature results in a hypoxic environment, which is further exacerbated by high oxygen consumption through the cells present at the edge of the wound and in the granulation tissue.60,61 Hypoxia has been found to have myriad effects on cells and their function, such as inducing greater dermal fibroblast proliferation and production of TGF-β1.62,63 Adipose-derived stromal cells (ASCs) are proangiogenic in hypoxic conditions, producing high levels of VEGF.64 In vitro ASCs display an inverse relationship between oxygen concentration and VEGF production, with significantly higher levels of VEGF being produced in lower levels of oxygen.65 Studies revealed that higher levels of HIF-1α expression are correlated with decreasing oxygen levels. Furthermore, hypoxia has been shown to promote in vitro keratinocyte motility66 and leads to the secretion of several growth factors. These are but a few of the many roles acute hypoxia plays in the induction of skin healing, and although hypoxia is necessary for regeneration, a return to normoxic conditions is eventually required. Fibroblasts, for example, require sufficient amounts of oxygen to adequately produce the collagen necessary for wound reconstruction. With insufficient oxygen, protocollagen strands are produced instead, which lack the tensile strength that is characteristic of mature collagen.59,67 Thus, an understanding of the pathways governed by oxygen is critical to developing effective treatments for wound regeneration.
HIF-1's role in wound healing
With hypoxia being of such importance to regeneration of the skin, the HIF pathways have drawn much attention. Hypoxia-inducible factors (HIFs) are pleiotropic key regulators of oxygen homeostasis. Hypoxia-inducible factor 1 (HIF-1) is a heterodimeric transcription factor and is found in the nucleated cells of animals.59 HIF-1 consists of two subunits: HIF-1α (or its analogs HIF-2α and HIF-3α) and HIF-1β, which bind to acquire the transcriptional capabilities that promote cell survival during hypoxia. Specifically, HIF-1 regulates the expression of almost 200 genes involved in biological processes such as glycolysis, angiogenesis, apoptosis, adhesion, migration, invasion, and metastasis.68–70 Additionally, HIF-1 serves as a crucial modulator in the homeostatic processes during hypoxia by increasing vascularization and regulating anaerobic respiration.71,72 The HIF-1 transcription factor activates genes to direct migration of mature endothelial cells toward sites of hypoxia through VEGF transcription.73–75 These endothelial cells ultimately help to form new vessels, enhancing delivery of oxygenated blood to the hypoxic tissues.76
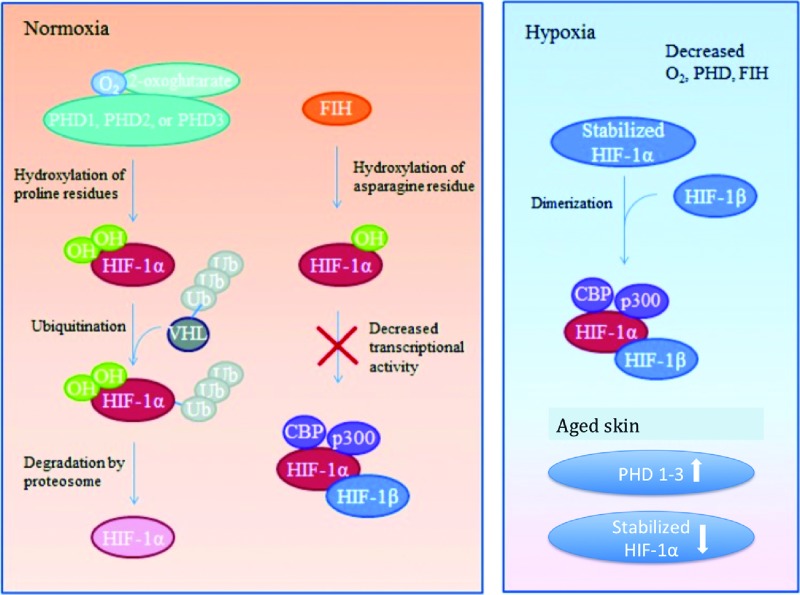
HIF-1 is integral to many homeostatic processes that occur throughout the body, prompting studies to further investigate the intricacies of the HIF pathways. HIF-1α is an oxygen-sensitive subunit, whereby its expression is dependent on hypoxic conditions.77,78 Under hypoxic conditions, HIF-1α expression is higher and sustained, whereas under normoxic conditions, HIF-1α is quickly degraded following translation.78 This is due to oxygen-dependent hydroxylation of HIF-1α amino acid residues by prolyl hydroxylase domain proteins (PHDs), which causes conformational changes in the HIF-1α protein. These changes make the protein susceptible to ubiquitination through von Hippel–Lindau (VHL) complexes, marking them for proteosomal destruction.79 Furthermore, HIF-1α is regulated by factor inhibiting HIF, which blocks HIF-1α interaction with its coactivators through oxygen-dependent hydroxylation.80 These inhibitory processes prevent excessive accumulation of HIF-1, which through its signaling pathways could cause fibroproliferative healing, characterized by fibrotic scarring.52 Under hypoxic conditions, the requisite oxygen for hydroxylation and degradation is unavailable, allowing HIF-1α levels to increase.71,81 The HIF-1 complex then stabilizes as the HIF-1α and HIF-1β subunits bind, allowing it to bind to coactivators, CBP and p300, activating its transcriptional properties (Fig. 3).
Figure 3.
HIF-1 regulation during normoxia, hypoxia. Under normoxic conditions HIF-1α is inhibited from binding with its coactivators by hydroxylation through PHD-1, -2, or -3 or FIH. PHD hydroxylates select proline residues on HIF-1α causing a conformational change that allows ubiquitination by a VHL ubiquitin–protein ligase complex followed by proteosomal degradation. FIH hydroxylates asparagine residues within HIF-1α, preventing interaction between HIF-1α and its coactivators. The hydroxylation described requires available oxygen. Under reduced oxygen conditions (hypoxia) HIF-1α is stabilized. With a dearth of available oxygen, hydroxylation of the HIF-1α subunit cannot occur and it readily binds with its coactivators. The stabilized HIF-1α subunit binds to HIF-1β subunit to form an active transcription factor. Active HIF-1 α/HIF-1β then bind to coactivators CBP and p300 to promote expression of downstream target genes. Aged skin PHD 1–3 levels are unregulated, leading to an increase in their prevalence and subsequent increase in HIF-1α degradation.59 FIH, factor inhibiting HIF; HIF-1α, hypoxia-inducible factor 1-alpha; PHDs, prolyl hydroxylase domain proteins; VHL, von Hippel–Lindau. Color images are available online.
The HIF-1 pathway is responsible for transcriptionally upregulating many genes that amplify wound healing. As inflammatory cells initially arrive at a site of injury, there is a dearth of oxygen required for cellular respiration. These hypoxic conditions not only make adenosine triphosphate (ATP) generation more difficult for early responders such as macrophages and neutrophils, but they also drive HIF-1-associated gene expression changes as well. Among these changes is an upregulation of glycolytic genes, which allows inflammatory cells to adapt to the hypoxic microenvironment of a wound and change their metabolic production to that of glycolysis.82 Thus, macrophages and neutrophils gain the ability to generate higher amounts of energy even with little oxygen available. This energy is required for virtually any and all functions carried out by these cells, including motility, chemotaxis, phagocytic activity, and aggregation.
Furthermore, HIF-1 has been shown to concurrently repress mitochondrial function, preventing cells from consuming what little oxygen is available for energy production purposes. This is a result of HIF-1 induction of pyruvate dehydrogenase kinase 1 (PDK1), which acts to block a crucial step in the mitochondrial tricarboxylic acid cycle.82 Ultimately, this yields a significant drop in mitochondrial oxygen consumption, leading to higher levels of available oxygen found within the wound microenvironment and lesser total cell death.82 These changes allow for inflammatory cells to better accumulate in the wound bed and perform their duties. Of note, in vivo studies with HIF knockout mice have observed a loss in macrophage motility, highlighting that HIF-1-induced upregulation of glycolysis is crucial to successful resolution of wound healing.83 Moreover, in vitro studies observed that HIF-1 was effective in inducing myeloid cell differentiation into macrophages and monocytes.84
HIF-1 has also been heavily implicated in the stimulation of angiogenesis, targeting a variety of genes that promote the growth reorganization of already extant vessels.59,85 Factors such as VEGF, stromal cell-derived factor 1 (SDF-1), and angiopoietin 2 are among those that are upregulated and are necessary for neovascularization.52,86 These vessels are responsible for returning surrounding tissue to normoxic conditions, which are required during the later stages of regeneration in the skin, promoting cellular activity and survival. Notably, studies have shown that fibroblasts lose the ability to carry out many of their functions when exposed to chronic ischemia. Thus, proper functioning of the HIF-1 pathway is essential to the growth of vasculature and successful wound healing.
Finally, HIF-1 promotes the upregulation of other genes recognized as integral to proper wound healing, including those that encode molecules, such as integrins, growth factors, and ECM components among others. As such, HIF-1 is clearly among the pathways that are paramount to regeneration of the skin.
While HIF-1 is undoubtedly important for wound healing, excessive expression of HIF-1 can lead to unwanted fibroproliferation in the form of keloids and hypertrophic scarring. Such conditions are characterized by unrestrained angiogenesis, as well as collagen and ECM deposition. Keloids and hypertrophic scars are both difficult to treat in a clinical setting, often requiring surgical correction. Biopsies of said conditions have revealed higher levels of growth factors and their respective receptors, such as TGF- β1 and VEGF.87 Such increases in expression likely drive the development of fibrotic diseases in the skin and are associated with unchecked HIF-1 signaling.
Conversely, deficits in HIF-1 signaling can lead to inadequate wound healing. Chronic wounds are often characterized by a constant, nonresolving inflammatory phase, causing proinflammatory signaling cascades to persist. This results in higher levels of proteases that work to destroy ECM components and other molecules beneficial to wound healing, preventing the proliferation and tissue remodeling phases from advancing normally.59,88 Studies have identified HIF-1 signaling as one of the underlying causes behind many nonhealing wounds. Common examples of chronic wounds include diabetic wounds, pressure ulcers, and aged wounds.89
HIF signaling in the aged and potential treatments
As stated before, the structure and pathophysiology of aged skin differs greatly from the skin in younger individuals. These discrepancies are responsible for the delayed healing found in aged skin, many of which are the result of irregular HIF-1 signaling. Over the past decade, extensive studies have examined the expression levels of HIF-1 in the aged compared with the young, and how regeneration was affected.
One of the major observations of aged wound healing is that there is significantly lesser neovascularization following ischemia, impeding recovery.90 Neovascularization in humans occurs through both angiogenesis, the sprouting of new vessels from old ones, and vasculogenesis, the formation of new vessels by migration and aggregation of endothelial progenitor cells.91,92 HIF-1α signaling has been shown to regulate both of these processes through transcription of cytokines such as SDF-1α.52 Interleukin-1β (IL-1β) is another important cytokine involved in wound healing and was posited to regulate SDF-1α expression independently of the HIF pathway. Thus, murine studies were performed to investigate the role HIF-1α signaling played in neovascularization in both aged and young mice. Aged mice displayed delayed wound healing and lesser granulation tissue when compared with their younger counterparts, as expected. Aged mice displayed lesser CD31+ expression following immunofluorescence (IF) staining as well, indicative of lesser neovascularization. Real-time polymerase chain reaction (qPCR) exhibited significantly reduced SDF-1α prevalence in aged wounds, complementing the CD31+ staining results. Western blot analysis found decreased HIF-1α levels in aged wounds, whereas neither aged mice nor young mice displayed major changes in IL-1β expression. Moreover, the only cells that displayed a significant response to IL-1β were fibroblasts found in young mice, and this response was nullified following mutation of HIF-binding sites.52 Thus, HIF-1α was found to be more critical with regard to SDF-1α transactivation following skin injury. As such, HIF-1α is integral to neovascularization in wound healing, and reduced HIF-1α expression is likely a major hindrance to regeneration in the aged.
With disturbances in HIF signaling having been correlated with delayed wound healing in the aged, several studies have attempted to manipulate its expression. As such, Zimmerman et al. examined the role of PHD-2 in aged wounds. Prior studies had found that PHD-2 inhibition brought about several responses to hypoxia, such as stimulation of angiogenesis, protection from metabolic stress, HIF-1α stabilization, and the induction of hypoxia-inducible genes.93 Furthermore, PHD-2 has been implicated in the regulation of angiogenesis independent of HIF signaling as well.94 With PHD-2 being unregulated and found in higher proportion in aged animals, it became a target of interest.
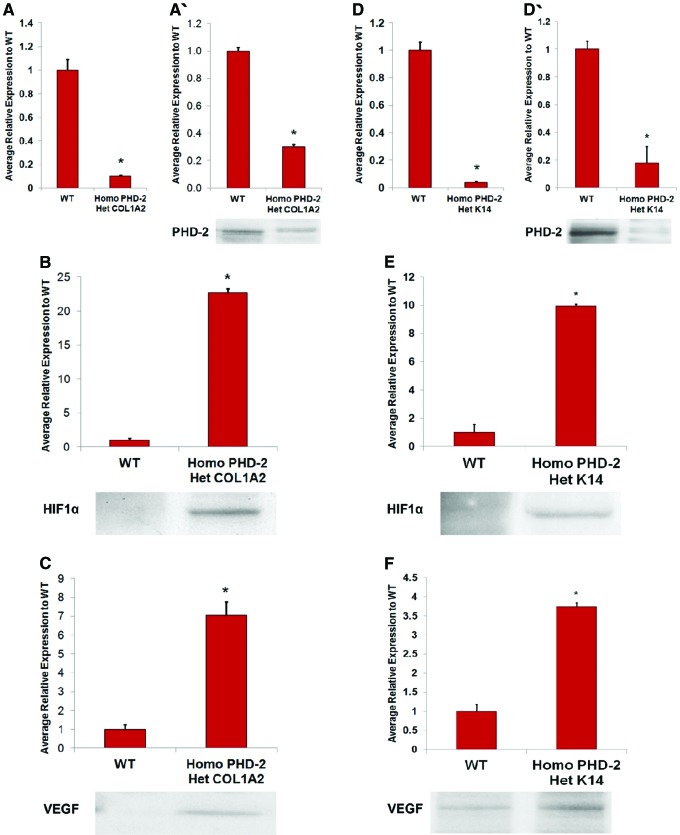
Thus, epidermal- and dermal-specific PHD-2 knockout mice were split into several groups to further investigate the role of PHD-2. Heterozygous K14-Cre/homozygous floxed PHD-2 and heterozygous Col1α2-Cre-ER/homozygous floxed PHD-2 mice were bred and had fibroblasts and keratinocytes harvested for analysis. These cells were analyzed with quantitative reverse transcriptase–polymerase chain reaction (qRT-PCR) and western blotting. Results displayed notably reduced mRNA levels of PHD-2 in knockout mice, with normal levels found in wild-type animals. Both knockout groups also displayed significantly reduced levels of PHD-2 and increased levels of VEGF and HIF-1α when compared with the wild type following western blot analysis. Thus, animals separated into groups were members from both knockout groups that were wounded with an excisional wound model or an ischemic pedicle flap model.95
Both of the PHD-2 knockout mice groups displayed significantly shorter time to wound closure when compared with wild-type mice in the excisional wound model, with appreciable differences being observed as early as day 4 postinjury. In addition, the ischemic pedicle flap model was used to observe tissue perfusion. Both knockout groups displayed significantly less necrosis and ischemia when compared with wild-type mice. Furthermore, IF staining for PHD-2, HIF-1α, VEGF, and CD31 were performed on tissue taken from animals used in the excisional wound model, with both knockout groups displaying significantly less PHD-2 expression. Concurrently, HIF-1α, VEGF, and CD31 were all higher in the knockout groups95 (Fig. 4).
Figure 4.
(A) qRT-PCR for PHD-2 KO in heterozygous Col1α2-Cre-ER/homozygous floxed PHD-2 fibroblasts compared with wild-type fibroblasts (*p < 0.05). (A′) Western blot data indicating lesser PHD-2 expression in fibroblasts of heterozygous Col1α2-Cre-ER/homozygous floxed PHD-2 mice than wild type. (B) Western blot data displaying greater HIF-1α expression in fibroblasts of heterozygous Col1α2-Cre-ER/homozygous floxed PHD-2 mice than wild type. (C) Western blot data displaying greater VEGF expression in fibroblasts of heterozygous Col1α2-Cre-ER/homozygous floxed PHD-2 mice than wild type. (D) qRT-PCR for PHD-2 KO in heterozygous Col1α2-Cre-ER/homozygous floxed PHD-2 keratinocytes compared with wild-type fibroblasts (*p < 0.05). (D′) Western blot data indicating lesser PHD-2 expression in keratinocytes of heterozygous Col1α2-Cre-ER/homozygous floxed PHD-2 mice than wild type. (E) Western blot data displaying greater HIF-1α expression in keratinocytes of heterozygous Col1α2-Cre-ER/homozygous floxed PHD-2 mice than wild type. (F) Western blot data displaying greater VEGF expression in keratinocytes of heterozygous Col1α2-Cre-ER/homozygous floxed PHD-2 mice than wild type.95 KO, knockout; qRT-PCR, quantitative reverse transcriptase–polymerase chain reaction; VEGF, vascular endothelial growth factor. Color images are available online.
Duscher et al. found similar findings when carrying out a study examining the effects of fibroblast-specific HIF-1α knockout mice, specifically that new vessel formation was hindered in knockout animals, further suggesting that HIF-1α deficits may be particularly deleterious in chronic wounds.96 Altogether, these results implicate PHD-2 as a strong inhibitor of HIF-1α as well as confirming that HIF-1α accelerates wound closure and improves local tissue perfusion. Targeting specific genes and molecules involved in the HIF pathways, such as PHD-2, may provide a solid foundation for future treatment aged wounds.
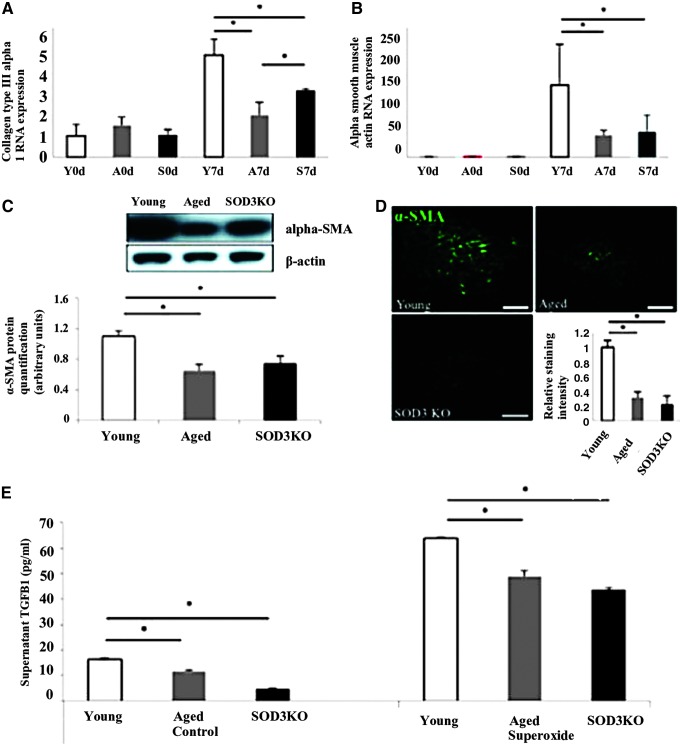
Other studies have investigated the role of ROS in the exacerbation of delayed wound healing in aged individuals. Among the molecules that has garnered interest is superoxide dismutase 3 (SOD3), an extracellular enzyme that contributes to oxygen homeostasis by consuming superoxide anions and has been shown to encourage neovascularization in response to ischemia.97,98 With decreased neovascularization underlying many of the complications found in aged wounds, Fujiwara et al. examined the effects of SOD3 activity on regeneration in the aged. SOD3 knockout mice were wounded using an excisional wound model and their healing rate was found to be comparable to aged, wild-type mice. Oxidative stress levels were found to be similar between the knockout group and aged group, both of which were significantly higher than the young, wild-type group. Furthermore, protein analysis of the wounds found significantly less SOD3 expression in the aged mice as early as day 3 postwounding. This ultimately led to lesser growth of vasculature in both the aged mice and knockout groups when compared with the young mice.98
Previous studies have demonstrated that many cell types are responsive to ROS, including neutrophils and fibroblasts. Specifically, ROS have been shown to incite an inflammatory response, which serves to recruit neutrophils to the site of injury.99 Furthermore, studies have displayed that fibroblast function can be influenced through ROS activity as well.100,101 Both the aged and knockout groups in the Fujiwara et al. study displayed increased neutrophil infiltration and reduced fibroblast proliferation when compared with the young mice. Furthermore, reductions in TGF-β1 and α-SMA were found to correspond with SOD3, suggesting myofibroblast differentiation is affected by high levels of ROS due to reduced SOD3 activity98 (Fig. 5).
Figure 5.
(A, B) Reduced fibroblast to myofibroblast transition in aged and SOD3 KO mice. (C, D) At the baseline, collagen type-III α-I and α-SMA RNA expressions are similar on the day of wounding. Compared with young control wounds their expression decreased in aged and SOD3 KO wounds at 7 days after. Y = young mice, A = aged mice, S = SOD3 KO mice. (E) Significant reduction of α-SMA in aged and SOD3 KO wounds on a protein level (western blot and immunohistochemistry). In both control conditions and when exposed to xanthine oxidase, fibroblasts from aged and SOD3 KO mice release significantly lower levels of TGF-β1, compared with fibroblasts from young mice. *p < 0.05. Scale bar = 100 μm.98 α-SMA, α-smooth muscle actin; SOD3, superoxide dismutase 3; TGF-β, transforming growth factor beta. Color images are available online.
Duscher et al. performed another study investigating the effects of several drugs on both diabetic and aged wounds. Previous studies had found that the FDA-approved iron chelator, deferoxamine (DFO), stabilized HIF-1α by chelating free radicals and preventing hydroxylation.102 Thus, DFO was applied to the wounds of diabetic and aged mice and resulted in accelerated wound healing. This was correlated with increased HIF-1α binding under hypoxic conditions in addition to greater neovascularization.103 Bonham et al. had similar findings when applying DFO to the wounds of aged mice as well as pressure ulcers through a newly developed transdermal delivery system (DFO-TDDS).104 Thus, DFO provides another means to correct HIF-1α deficiencies in the aged.
While there are certainly many aspects in molecular signaling that underlie the problems associated with wound healing in the aged, there are also discrepancies with regard to the cellular behavior and function. These alterations in cellular function impact regeneration of the skin in various ways.
Effects of aging on stem/progenitor cell populations
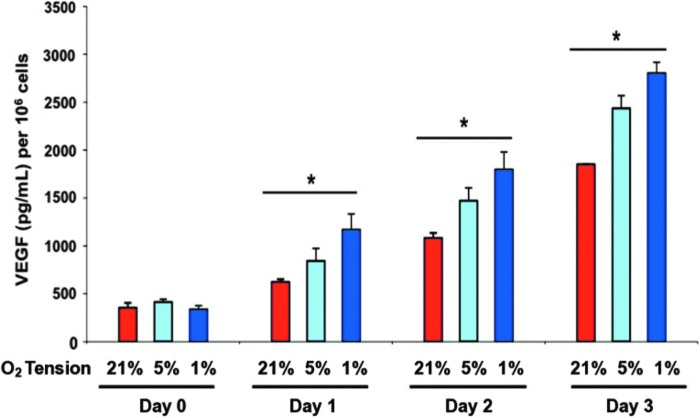
Studies have shown that ASCs are proangiogenic in hypoxic conditions, producing high levels of VEGF.64 Thangarajah et al. found that in vitro ASCs displayed an inverse relationship between oxygen concentration and VEGF production, with significantly higher levels of VEGF being produced in lower levels of oxygen.65 Western blotting also found higher levels of HIF-1α expression correlated with decreasing oxygen levels. Furthermore, hypoxia-treated ASCs were found to have a greater response to SDF-1 than normoxic ASCs, suggesting that hypoxia enhances their migratory capabilities. SDF-1 has been shown to mediate the migration of circulating stem cells to sites of neovascularization.105 Additionally, ASCs displayed higher levels of proliferation under progressively lower levels of oxygen concentration. ASCs were also found to adopt endothelial cell characteristics, such as the ability to promote tubule formation when subjected to hypoxia in the presence of VEGF.65 Altogether, these findings suggest that ASCs are integral to the reconstruction of blood vessels following injury (Fig. 6).
Figure 6.
VEGF quantification by ELISA that displays ASC production of VEGF has an inverse relationship with oxygen present. Lower levels of oxygen lead to progressively higher output of VEGF by ASCs (modified from Thangarajah et al.65). ASCs, adipose-derived stromal cells. Color images are available online.
Building upon these findings, El-ftesi et al. examined ASCs in both aged and diabetic mice to determine how they differed from their younger counterparts. Using the surface markers Sca-1, CD90 and CD44 as positive identifiers, ASCs were harvested from old, young, and diabetic mice and subjected to several analyses. ASCs in younger mice displayed significantly higher VEGF secretion under in vitro, normoxic conditions when compared with older and diabetic mice. While cells from each group displayed higher VEGF secretion under hypoxia than normoxia, ASCs from the younger mice produced VEGF at much higher levels under hypoxia than the other groups. ASC proliferation was also stunted under hypoxic conditions in aged and diabetic mice. Furthermore, a reduction in endothelial cell proliferation in the aged and diabetic groups correlated with lesser ASC proliferation, further implicating that ASCs are crucial to stimulating endothelial cell proliferation. Tubulogenesis was also found to be reduced in the aged and diabetic groups when compared with ASCs from younger animals.106 These findings display significant impediments in ASCs as they age, with a notable loss in their ability to stimulate neovascularization through VEGF secretion. With how accessible ASCs are and given their role in facilitating wound healing, they are an attractive potential cornerstone for cell therapies and tissue engineering (Fig. 7).
Figure 7.
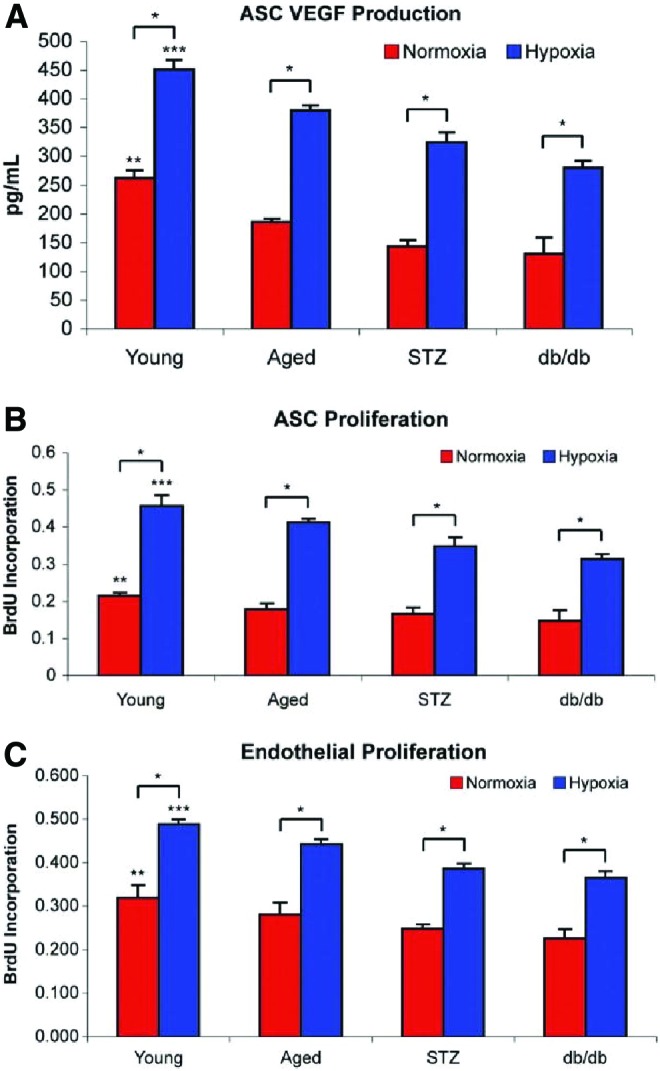
(A) VEGF quantification by ELISA displays lesser VEGF production by diabetic and aged mice in both normoxic and hypoxic conditions when compared with their younger counterparts. (B) ASC proliferation measured with bromodeoxyuridine colorimetric cell proliferation assay is lower in aged and diabetic mice when compared with young mice. (C) Endothelial cell proliferation measured with bromodeoxyuridine colorimetric cell proliferation assay is lower in aged and diabetic mice when compared with young mice.106 Color images are available online.
Mesenchymal stem cells (MSCs) have also been noted to contribute to neovascularization and tissue repair throughout the body.107 Studies have shown that MSCs, like all other cells, begin to lose function as a result of aging.108 Duscher et al. performed a study observing how aging affects MSCs, finding that frequency within adipose tissue did not differ between aged animals and young animals. This was consistent with previous studies performed by other groups.109,110 Subsequent in vitro seeding of these MSCs displayed that the aged MSCs did not have reduced proliferative capacity. However, single cell analysis found that a particular subpopulation of ASC, characterized by various genes related to stemness, tissue repair, and neovascularization, including HIF-1α, was depleted in aged mice. This subset may be more pluripotent than other subsets and was implicated in several pathways, such as those involved with hypoxic signaling and cell differentiation. Furthermore, when hydrogels were seeded with aged ASCs and applied to the wounds of aged mice, there was no observable difference in wound healing, whereas application of young ASC seeded hydrogels to aged wounds improved the healing rate. Finally, aged ASC-treated wounds displayed lower levels of SOD3, VEGF, and neovascularization when compared with young ASC-treated wounds.111
Summary
With so many variables it is difficult to ascertain which aspect of wound healing is of the greatest importance. Age-associated defects in both cellular function and molecular signaling have been shown to impede various processes in the aged. Recent research however, shows that targeting the signaling pathways may in fact ameliorate some of the problems in aged cells and normalize wound healing to that found in younger animals. Treatments such as genetic knockout and drugs such as DFO have proven to be useful and pave the way for optimal treatments that may reduce the burden of chronic and aged wounds in the future.
Abbreviations and Acronyms
- α-SMA
α-smooth muscle actin
- ASCs
adipose-derived stromal cells
- CAM
cell adhesion molecule
- DEJ
dermal–epidermal junction
- DFO
deferoxamine
- ECM
extracellular matrix
- HIF-1
hypoxia-inducible factor 1
- HIF-1α
hypoxia-inducible factor 1-alpha
- HIFs
hypoxia-inducible factors
- IF
immunofluorescence
- IL-1β
interleukin-1β
- MMP
matrix metalloproteinase
- MSCs
mesenchymal stem cells
- PDGF
platelet-derived growth factor
- PHD
prolyl hydroxylase domain
- ROS
reactive oxygen species
- SDF-1α
stromal cell-derived factor 1-alpha
- SOD3
superoxide dismutase 3
- TGF-α
transforming growth factor alpha
- TGF-β
transforming growth factor beta
- TIMPs
tissue inhibitors of metalloproteinases
- UV
ultraviolet
- UVA
ultraviolet A (long-wave)
- UVB
ultraviolet B (shortwave)
- VEGF
vascular endothelial growth factor
Acknowledgments and Funding Sources
None.
Author Disclosure and Ghostwriting
The content of this article was expressly written by the authors listed. No ghostwriters were involved in the writing of this article. No competing financial interest exists. G.C.G. has no disclosures relevant to this topic. C.A.B. and B.K. have no potential conflicts of interest, affiliations, or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in this study.
About the Authors
Clark A. Bonham, BS, is a research fellow at Stanford University studying wound regeneration in the skin. Britta Kuehlmann, MD, PhD, is a plastic and reconstructive surgeon and postdoctoral research fellow at Stanford University. Her research interests include foreign body responses, capsular fibrosis around implants, and skin regeneration. Geoffrey C. Gurtner, MD, is the Johnson and Johnson Professor of Surgery and Materials Science Engineering at Stanford University. He also serves as the Associate Chairman for Research in the Department of Surgery. Dr. Gurtner runs an NIH- and DoD-funded laboratory that seeks to elucidate the human response to injury for the promotion of tissue repair and regeneration.
References
- 1. Monteiro-Riviere NA. Introduction to histological aspects of dermatotoxicology. Microsc Res Tech 1997;37:171. [DOI] [PubMed] [Google Scholar]
- 2. Casarrett L, Doull J. Toxic responses of the skin. In: Klassen CD, ed. Casarrett and Doull's Toxicology, 8th ed. New York, NY: McGraw-Hill Education, 2013:839. [Google Scholar]
- 3. Montagna W, Carlisle K. Structural changes in aging human skin. J Invest Dermatol 1979;73:47–53 [DOI] [PubMed] [Google Scholar]
- 4. Kligman AM, Koblenzer C. Demographics and psychological implications for the aging population. Dermatol Clin 1997;15:549–553 [DOI] [PubMed] [Google Scholar]
- 5. Martini FH, Nath JL, Bartholomew EF. Fundamentals of Anatomy and Physiology, 11th edition. London, UK: Pearson, 2017 [Google Scholar]
- 6. Farage MA, Miller KW, Elsner P, Maibach HI. Intrinsic and extrinsic factors in skin ageing: a review. Int J Cosmet Sci 2008;30:87–95 [DOI] [PubMed] [Google Scholar]
- 7. Bergfeld WF. The aging skin. Int J Fertil Womens Med 1997;42:57–66 [PubMed] [Google Scholar]
- 8. Papakonstantinou E, Roth M, Karakiulakis G. Hyaluronic acid: a key molecule in skin aging. Dermatoendocrinol 2012;4:253–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. D'Orazio J, Jarrett S, Amaro-Ortiz A, Scott T. UV radiation and the skin. Int J Mol Sci 2013;14:12222–12248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Park K. Role of micronutrients in skin health and function. Biomol Ther (Seoul) 2015;23:207–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Walraven M, Gouverneur M, Middelkoop E, Beelen RH, Ulrich MM. Altered TGF-beta signaling in fetal fibroblasts: what is known about the underlying mechanisms? Wound Repair Regen 2014;22:3–13 [DOI] [PubMed] [Google Scholar]
- 12. Chen L, Hu JY, Wang SQ. The role of antioxidants in photoprotection: a critical review. J Am Acad Dermatol 2012;67:1013–1024 [DOI] [PubMed] [Google Scholar]
- 13. Skobowiat C, Sayre RM, Dowdy JC, Slominski AT. Ultraviolet radiation regulates cortisol activity in a waveband-dependent manner in human skin ex vivo. Br J Dermatol 2013;168:595–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Clydesdale GJ, Dandie GW, Muller HK. Ultraviolet light induced injury: immunological and inflammatory effects. Immunol Cell Biol 2001;79:547–568 [DOI] [PubMed] [Google Scholar]
- 15. Bayerl C, Taake S, Moll I, Jung EG. Characterization of sunburn cells after exposure to ultraviolet light. Photodermatol Photoimmunol Photomed 1995;11:149–154 [DOI] [PubMed] [Google Scholar]
- 16. Makrantonaki E, Zouboulis CC. Molecular mechanisms of skin aging: state of the art. Ann N Y Acad Sci 2007;1119:40–50 [DOI] [PubMed] [Google Scholar]
- 17. Lavker RM. Structural alterations in exposed and unexposed aged skin. J Invest Dermatol 1979;73:59–66 [DOI] [PubMed] [Google Scholar]
- 18. Moragas A, Castells C, Sans M. Mathematical morphologic analysis of aging-related epidermal changes. Anal Quant Cytol Histol 1993;15:75–82 [PubMed] [Google Scholar]
- 19. Makrantonaki E, Zouboulis CC, William J. Cunliffe Scientific Awards. Characteristics and pathomechanisms of endogenously aged skin. Dermatology 2007;214:352–360 [DOI] [PubMed] [Google Scholar]
- 20. Kligman LH. Photoaging. Manifestations, prevention, and treatment. Clin Geriatr Med 1989;5:235–251 [PubMed] [Google Scholar]
- 21. Blume-Peytavi U, Kottner J, Sterry W, et al. Age-associated skin conditions and diseases: current perspectives and future options. Gerontologist 2016;56 Suppl 2:S230–S242 [DOI] [PubMed] [Google Scholar]
- 22. Sandby-Moller J, Poulsen T, Wulf HC. Epidermal thickness at different body sites: relationship to age, gender, pigmentation, blood content, skin type and smoking habits. Acta Derm Venereol 2003;83:410–413 [DOI] [PubMed] [Google Scholar]
- 23. Sgonc R, Gruber J. Age-related aspects of cutaneous wound healing: a mini-review. Gerontology 2013;59:159–164 [DOI] [PubMed] [Google Scholar]
- 24. Thomas DR, Burkemper NM. Aging skin and wound healing. Clin Geriatr Med 2013;29:xi–xx [DOI] [PubMed] [Google Scholar]
- 25. Prausnitz MR, Elias PM, Franz TJ, Schmuth M, et al. Skin barrier and transdermal drug delivery. In: Bolognia JL, Schaffer JV, Lorenzo C, eds. Dermatology, 4th ed. Philadelphia, PA: Elsevier, 2018:2065–2067 [Google Scholar]
- 26. Rawlings AV, Matts PJ. Stratum corneum moisturization at the molecular level: an update in relation to the dry skin cycle. J Invest Dermatol 2005;124:1099–1110 [DOI] [PubMed] [Google Scholar]
- 27. Garibyan L, Chiou AS, Elmariah SB. Advanced aging skin and itch: addressing an unmet need. Dermatol Ther 2013;26:92–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Engelke M, Jensen JM, Ekanayake-Mudiyanselage S, Proksch E. Effects of xerosis and ageing on epidermal proliferation and differentiation. Br J Dermatol 1997;137:219–225 [DOI] [PubMed] [Google Scholar]
- 29. Simpson CL, Patel DM, Green KJ. Deconstructing the skin: cytoarchitectural determinants of epidermal morphogenesis. Nat Rev Mol Cell Biol 2011;12:565–580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Langton AK, Halai P, Griffiths CE, Sherratt MJ, Watson RE. The impact of intrinsic ageing on the protein composition of the dermal-epidermal junction. Mech Ageing Dev 2016;156:14–16 [DOI] [PubMed] [Google Scholar]
- 31. Vazquez F, Palacios S, Aleman N, Guerrero F. Changes of the basement membrane and type IV collagen in human skin during aging. Maturitas 1996;25:209–215 [DOI] [PubMed] [Google Scholar]
- 32. Kurban RS, Bhawan J. Histologic changes in skin associated with aging. J Dermatol Surg Oncol 1990;16:908–914 [DOI] [PubMed] [Google Scholar]
- 33. Hatje LK, Richter C, Blume-Peytavi U, Kottner J. Blistering time as a parameter for the strength of dermoepidermal adhesion: a systematic review and meta-analysis. Br J Dermatol 2015;172:323–330 [DOI] [PubMed] [Google Scholar]
- 34. Nurden AT, Nurden P, Sanchez M, Andia I, Anitua E. Platelets and wound healing. Front Biosci 2008;13:3532–3548 [DOI] [PubMed] [Google Scholar]
- 35. Weyrich AS, Zimmerman GA. Platelets: signaling cells in the immune continuum. Trends Immunol 2004;25:489–495 [DOI] [PubMed] [Google Scholar]
- 36. Singer AJ, Clark RA. Cutaneous wound healing. N Engl J Med 1999;341:738–746 [DOI] [PubMed] [Google Scholar]
- 37. Grigorova-Borsos AM, Bara L, Aberer E, et al. Aging and diabetes increase the aggregating potency of rat skin collagen towards normal platelets. Thromb Haemost 1988;60:75–78 [PubMed] [Google Scholar]
- 38. Silverman EM, Silverman AG. Granulocyte adherence in the elderly. Am J Clin Pathol 1977;67:49–52 [DOI] [PubMed] [Google Scholar]
- 39. Mittal M, Siddiqui MR, Tran K, Reddy SP, Malik AB. Reactive oxygen species in inflammation and tissue injury. Antioxid Redox Signal 2014;20:1126–1167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Poljsak B, Dahmane RG, Godic A. Intrinsic skin aging: the role of oxidative stress. Acta Dermatovenerol Alp Pannonica Adriat 2012;21:33–36 [PubMed] [Google Scholar]
- 41. Ashcroft GS, Horan MA, Ferguson MW. Aging alters the inflammatory and endothelial cell adhesion molecule profiles during human cutaneous wound healing. Lab Invest 1998;78:47–58 [PubMed] [Google Scholar]
- 42. Gosain A, DiPietro LA. Aging and wound healing. World J Surg 2004;28:321–326 [DOI] [PubMed] [Google Scholar]
- 43. Nishio N, Okawa Y, Sakurai H, Isobe K. Neutrophil depletion delays wound repair in aged mice. Age (Dordr) 2008;30:11–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Butcher SK, Chahal H, Nayak L, et al. Senescence in innate immune responses: reduced neutrophil phagocytic capacity and CD16 expression in elderly humans. J Leukoc Biol 2001;70:881–886 [PubMed] [Google Scholar]
- 45. Danon D, Kowatch MA, Roth GS. Promotion of wound repair in old mice by local injection of macrophages. Proc Natl Acad Sci U S A 1989;86:2018–2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Velnar T, Bailey T, Smrkolj V. The wound healing process: an overview of the cellular and molecular mechanisms. J Int Med Res 2009;37:1528–1542 [DOI] [PubMed] [Google Scholar]
- 47. Goldman R. Growth factors and chronic wound healing: past, present, and future. Adv Skin Wound Care 2004;17:24–35 [DOI] [PubMed] [Google Scholar]
- 48. Oikarinen A. The aging of skin: chronoaging versus photoaging. Photodermatol Photoimmunol Photomed 1990;7:3–4 [PubMed] [Google Scholar]
- 49. Shuster S, Black MM, McVitie E. The influence of age and sex on skin thickness, skin collagen and density. Br J Dermatol 1975;93:639–643 [DOI] [PubMed] [Google Scholar]
- 50. Masur SK, Dewal HS, Dinh TT, Erenburg I, Petridou S. Myofibroblasts differentiate from fibroblasts when plated at low density. Proc Natl Acad Sci U S A 1996;93:4219–4223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Xia YP, Zhao Y, Tyrone JW, Chen A, Mustoe TA. Differential activation of migration by hypoxia in keratinocytes isolated from donors of increasing age: implication for chronic wounds in the elderly. J Invest Dermatol 2001;116:50–56 [DOI] [PubMed] [Google Scholar]
- 52. Loh SA, Chang EI, Galvez MG, et al. SDF-1 alpha expression during wound healing in the aged is HIF dependent. Plast Reconstr Surg 2009;123:65S–75S [DOI] [PubMed] [Google Scholar]
- 53. van Zuijlen PP, Ruurda JJ, van Veen HA, et al. Collagen morphology in human skin and scar tissue: no adaptations in response to mechanical loading at joints. Burns 2003;29:423–431 [DOI] [PubMed] [Google Scholar]
- 54. Ashcroft GS, Mills SJ, Ashworth JJ. Ageing and wound healing. Biogerontology 2002;3:337–345 [DOI] [PubMed] [Google Scholar]
- 55. Bond JS, Duncan JA, Sattar A, et al. Maturation of the human scar: an observational study. Plast Reconstr Surg 2008;121:1650–1658 [DOI] [PubMed] [Google Scholar]
- 56. Younai S, Nichter LS, Wellisz T, Reinisch J, Nimni ME, Tuan TL. Modulation of collagen synthesis by transforming growth factor-beta in keloid and hypertrophic scar fibroblasts. Ann Plast Surg 1994;33:148–151 [DOI] [PubMed] [Google Scholar]
- 57. Shah M, Foreman DM, Ferguson MW. Control of scarring in adult wounds by neutralising antibody to transforming growth factor beta. Lancet 1992;339:213–214 [DOI] [PubMed] [Google Scholar]
- 58. Puolakkainen PA, Twardzik DR, Ranchalis JE, Pankey SC, Reed MJ, Gombotz WR. The enhancement in wound healing by transforming growth factor-beta 1 (TGF-beta 1) depends on the topical delivery system. J Surg Res 1995;58:321–329 [DOI] [PubMed] [Google Scholar]
- 59. Hong WX, Hu MS, Esquivel M, et al. The role of hypoxia-inducible factor in wound healing. Adv Wound Care (New Rochelle) 2014;3:390–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Varghese MC, Balin AK, Carter DM, Caldwell D. Local environment of chronic wounds under synthetic dressings. Arch Dermatol 1986;122:52–57 [PubMed] [Google Scholar]
- 61. Ninikoski J, Heughan C, Hunt TK. Oxygen tensions in human wounds. J Surg Res 1972;12:77–82 [DOI] [PubMed] [Google Scholar]
- 62. Tandara AA, Mustoe TA. Oxygen in wound healing—more than a nutrient. World J Surg 2004;28:294–300 [DOI] [PubMed] [Google Scholar]
- 63. Falanga V, Kirsner RS. Low oxygen stimulates proliferation of fibroblasts seeded as single cells. J Cell Physiol 1993;154:506–510 [DOI] [PubMed] [Google Scholar]
- 64. Rehman J, Traktuev D, Li J, et al. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation 2004;109:1292–1298 [DOI] [PubMed] [Google Scholar]
- 65. Thangarajah H, Vial IN, Chang E, et al. IFATS collection: adipose stromal cells adopt a proangiogenic phenotype under the influence of hypoxia. Stem Cells 2009;27:266–274 [DOI] [PubMed] [Google Scholar]
- 66. O'Toole EA, Marinkovich MP, Hoeffler WK, Furthmayr H, Woodley DT. Laminin-5 inhibits human keratinocyte migration. Exp Cell Res 1997;233:330–339 [DOI] [PubMed] [Google Scholar]
- 67. Chambers AC, Leaper DJ. Role of oxygen in wound healing: a review of evidence. J Wound Care 2011;20:160–164 [DOI] [PubMed] [Google Scholar]
- 68. Pouyssegur J, Dayan F, Mazure NM. Hypoxia signalling in cancer and approaches to enforce tumour regression. Nature 2006;441:437–443 [DOI] [PubMed] [Google Scholar]
- 69. Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer 2003;3:721–732 [DOI] [PubMed] [Google Scholar]
- 70. Rezvani HR, Ali N, Serrano-Sanchez M, et al. Loss of epidermal hypoxia-inducible factor-1alpha accelerates epidermal aging and affects re-epithelialization in human and mouse. J Cell Sci 2011;124:4172–4183 [DOI] [PubMed] [Google Scholar]
- 71. Semenza GL. Regulation of metabolism by hypoxia-inducible factor 1. Cold Spring Harb Symp Quant Biol 2011;76:347–353 [DOI] [PubMed] [Google Scholar]
- 72. Shay JE, Celeste Simon M. Hypoxia-inducible factors: crosstalk between inflammation and metabolism. Semin Cell Dev Biol 2012;23:389–394 [DOI] [PubMed] [Google Scholar]
- 73. Ebersole JL, Novak MJ, Orraca L, et al. Hypoxia-inducible transcription factors, HIF1A and HIF2A, increase in aging mucosal tissues. Immunology 2018;154:452–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Carmeliet P, Dor Y, Herbert JM, et al. Role of HIF-1alpha in hypoxia-mediated apoptosis, cell proliferation and tumour angiogenesis. Nature 1998;394:485–490 [DOI] [PubMed] [Google Scholar]
- 75. Dery MA, Michaud MD, Richard DE. Hypoxia-inducible factor 1: regulation by hypoxic and non-hypoxic activators. Int J Biochem Cell Biol 2005;37:535–540 [DOI] [PubMed] [Google Scholar]
- 76. Richard DE, Berra E, Pouyssegur J. Nonhypoxic pathway mediates the induction of hypoxia-inducible factor 1alpha in vascular smooth muscle cells. J Biol Chem 2000;275:26765–26771 [DOI] [PubMed] [Google Scholar]
- 77. Semenza GL. Hypoxia. Cross talk between oxygen sensing and the cell cycle machinery. Am J Physiol Cell Physiol 2011;301:C550–C552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Tamama K, Kawasaki H, Kerpedjieva SS, Guan J, Ganju RK, Sen CK. Differential roles of hypoxia inducible factor subunits in multipotential stromal cells under hypoxic condition. J Cell Biochem 2011;112:804–817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Semenza GL. Involvement of oxygen-sensing pathways in physiologic and pathologic erythropoiesis. Blood 2009;114:2015–2019 [DOI] [PubMed] [Google Scholar]
- 80. Lisy K, Peet DJ. Turn me on: regulating HIF transcriptional activity. Cell Death Differ 2008;15:642–649 [DOI] [PubMed] [Google Scholar]
- 81. Lee JW, Bae SH, Jeong JW, Kim SH, Kim KW. Hypoxia-inducible factor (HIF-1)alpha: its protein stability and biological functions. Exp Mol Med 2004;36:1–12 [DOI] [PubMed] [Google Scholar]
- 82. Papandreou I, Cairns RA, Fontana L, Lim AL, Denko NC. HIF-1 mediates adaptation to hypoxia by actively downregulating mitochondrial oxygen consumption. Cell Metab 2006;3:187–197 [DOI] [PubMed] [Google Scholar]
- 83. Roy S, Khanna S, Bickerstaff AA, et al. Oxygen sensing by primary cardiac fibroblasts: a key role of p21(Waf1/Cip1/Sdi1). Circ Res 2003;92:264–271 [DOI] [PubMed] [Google Scholar]
- 84. Corzo CA, Condamine T, Lu L, et al. HIF-1alpha regulates function and differentiation of myeloid-derived suppressor cells in the tumor microenvironment. J Exp Med 2010;207:2439–2453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Koch S, Claesson-Welsh L. Signal transduction by vascular endothelial growth factor receptors. Cold Spring Harb Perspect Med 2012;2:a006502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Ahluwalia A, Tarnawski AS. Critical role of hypoxia sensor—HIF-1alpha in VEGF gene activation. Implications for angiogenesis and tissue injury healing. Curr Med Chem 2012;19:90–97 [DOI] [PubMed] [Google Scholar]
- 87. Schmid P, Itin P, Cherry G, Bi C, Cox DA. Enhanced expression of transforming growth factor-beta type I and type II receptors in wound granulation tissue and hypertrophic scar. Am J Pathol 1998;152:485–493 [PMC free article] [PubMed] [Google Scholar]
- 88. McCarty SM, Percival SL. Proteases and delayed wound healing. Adv Wound Care (New Rochelle) 2013;2:438–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Mustoe T. Understanding chronic wounds: a unifying hypothesis on their pathogenesis and implications for therapy. Am J Surg 2004;187:65S–70S [DOI] [PubMed] [Google Scholar]
- 90. Edelberg JM, Reed MJ. Aging and angiogenesis. Front Biosci 2003;8:s1199–s1209 [DOI] [PubMed] [Google Scholar]
- 91. Asahara T, Murohara T, Sullivan A, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science 1997;275:964–967 [DOI] [PubMed] [Google Scholar]
- 92. Chang EI, Loh SA, Ceradini DJ, et al. Age decreases endothelial progenitor cell recruitment through decreases in hypoxia-inducible factor 1alpha stabilization during ischemia. Circulation 2007;116:2818–2829 [DOI] [PubMed] [Google Scholar]
- 93. Hirota K, Semenza GL. Regulation of hypoxia-inducible factor 1 by prolyl and asparaginyl hydroxylases. Biochem Biophys Res Commun 2005;338:610–616 [DOI] [PubMed] [Google Scholar]
- 94. Chan DA, Kawahara TL, Sutphin PD, Chang HY, Chi JT, Giaccia AJ. Tumor vasculature is regulated by PHD2-mediated angiogenesis and bone marrow-derived cell recruitment. Cancer Cell 2009;15:527–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Zimmermann AS, Morrison SD, Hu MS, et al. Epidermal or dermal specific knockout of PHD-2 enhances wound healing and minimizes ischemic injury. PLoS One 2014;9:e93373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Duscher D, Maan ZN, Whittam AJ, et al. Fibroblast-specific deletion of hypoxia inducible factor-1 critically impairs murine cutaneous neovascularization and wound healing. Plast Reconstr Surg 2015;136:1004–1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Kim HW, Lin A, Guldberg RE, Ushio-Fukai M, Fukai T. Essential role of extracellular SOD in reparative neovascularization induced by hindlimb ischemia. Circ Res 2007;101:409–419 [DOI] [PubMed] [Google Scholar]
- 98. Fujiwara T, Duscher D, Rustad KC, et al. Extracellular superoxide dismutase deficiency impairs wound healing in advanced age by reducing neovascularization and fibroblast function. Exp Dermatol 2016;25:206–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Laurila JP, Laatikainen LE, Castellone MD, Laukkanen MO. SOD3 reduces inflammatory cell migration by regulating adhesion molecule and cytokine expression. PLoS One 2009;4:e5786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Vile GF, Basu-Modak S, Waltner C, Tyrrell RM. Heme oxygenase 1 mediates an adaptive response to oxidative stress in human skin fibroblasts. Proc Natl Acad Sci U S A 1994;91:2607–2610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Siwik DA, Pagano PJ, Colucci WS. Oxidative stress regulates collagen synthesis and matrix metalloproteinase activity in cardiac fibroblasts. Am J Physiol Cell Physiol 2001;280:C53–C60 [DOI] [PubMed] [Google Scholar]
- 102. Thangarajah H, Yao D, Chang EI, et al. The molecular basis for impaired hypoxia-induced VEGF expression in diabetic tissues. Proc Natl Acad Sci U S A 2009;106:13505–13510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Duscher D, Januszyk M, Maan ZN, et al. Comparison of the hydroxylase inhibitor dimethyloxalylglycine and the iron chelator deferoxamine in diabetic and aged wound healing. Plast Reconstr Surg 2017;139:695e–706e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Bonham CA, Rodrigues M, Galvez M, et al. Deferoxamine can prevent pressure ulcers and accelerate healing in aged mice. Wound Repair Regen 2018;26:300–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Ceradini DJ, Kulkarni AR, Callaghan MJ, et al. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med 2004;10:858–864 [DOI] [PubMed] [Google Scholar]
- 106. El-Ftesi S, Chang EI, Longaker MT, Gurtner GC. Aging and diabetes impair the neovascular potential of adipose-derived stromal cells. Plast Reconstr Surg 2009;123:475–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Rustad KC, Wong VW, Sorkin M, et al. Enhancement of mesenchymal stem cell angiogenic capacity and stemness by a biomimetic hydrogel scaffold. Biomaterials 2012;33:80–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Alt EU, Senst C, Murthy SN, et al. Aging alters tissue resident mesenchymal stem cell properties. Stem Cell Res 2012;8:215–225 [DOI] [PubMed] [Google Scholar]
- 109. Aust L, Devlin B, Foster SJ, et al. Yield of human adipose-derived adult stem cells from liposuction aspirates. Cytotherapy 2004;6:7–14 [DOI] [PubMed] [Google Scholar]
- 110. Jurgens WJ, Oedayrajsingh-Varma MJ, Helder MN, et al. Effect of tissue-harvesting site on yield of stem cells derived from adipose tissue: implications for cell-based therapies. Cell Tissue Res 2008;332:415–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Duscher D, Rennert RC, Januszyk M, et al. Aging disrupts cell subpopulation dynamics and diminishes the function of mesenchymal stem cells. Sci Rep 2014;4:7144. [DOI] [PMC free article] [PubMed] [Google Scholar]