Abstract
Objective: Exposure to ultraviolet (UV) light from the sun is known to accelerate the skin aging process and leads to significant alterations in skin biomechanics; however, the molecular mechanisms by which chronic UVB affects biomechanical properties of the skin have not been well described.
Approach: A murine model for chronic UVB exposure was used to examine changes in epidermal barrier function, skin biomechanics, and miRNA expression as a result of UVB.
Results: UVB irradiation caused skin to be weaker, less elastic, stiffer, and less pliable. Notably, these changes were not reversed after a 5-week period of recovery. Following UVB exposure, dermal collagen fibrils were significantly smaller in diameter and expression of the miR-34 family was significantly increased.
Innovation: To our knowledge, this is the first study to concurrently examine alterations in skin function, miRNA expression, and tissue biomechanics in response to chronic UVB exposure.
Conclusion: The data suggest that UVB alters miR-34 family expression in skin, in addition to dysregulating collagen structure with subsequent reductions in strength and elasticity. miRNAs may play a pivotal role in regulating extracellular matrix deposition and skin biomechanics following chronic UVB exposure, and thus may be a possible target for therapeutic development. However, additional studies are needed to directly probe the link between UVB exposure, miRNA production, and skin biomechanics.
Keywords: UVB, skin, microRNA, biomechanics, collagen

Heather M. Powell, PhD
Introduction
Skin serves as the primary barrier between the internal and external environment, although its function extends much further with key roles in physical protection, thermal regulation, sensory perception, and the prevention of fluid, electrolyte, and protein loss.1,2 Mechanical properties of skin play a critical role in its protective functions. Exposure to ultraviolet (UV) light from the sun is known to accelerate the skin aging process and leads to significant alterations in skin biomechanics.3 UVB irradiation, in particular, has dramatic effects on skin anatomy and physiology, ranging from disruption of collagen structure to increased DNA damage, inflammation, and tumor development, and is thought to play an important role in photoaging.4,5
The molecular mechanisms by which chronic UVB affects biomechanical properties of the skin have not been well described. However, miRNAs recently have been proposed to be a key player in the DNA damage response system,6,7 and they have been shown to be involved in UVB-regulated apoptosis, inflammation, and cell cycle control. The effects of UVB radiation on miRNA expression were first published in 2009.8 UVB irradiation of murine fibroblasts (NIH3T3) led to a swift (within 4 h) upregulation of several miRNAs, including several known to regulate cell cycle and proliferation.8 UVB irradiation was also shown to slightly induce miR-203 (involved in epidermal proliferation) and reduce miR-205 (involved in keratinocyte migration and cell-cell adhesion) in primary human keratinocytes.9 In sun-exposed skin, miR-125-positive epidermal cells were less abundant than in nonexposed skin.10 In addition, miR-34a and miR-34b-5 were significantly upregulated in human dermis of elderly subjects compared to young subjects.11 When miRNA processing was downregulated by Dicer siRNA, HeLA cells were shown to be hypersensitive to UVB with a marked increase in cells in the S-phase of the cell cycle.12 Upregulation of miR-22 in HEK 293T and HaCaT keratinocytes also inhibited apoptosis in cells exposed to UV.6 The effect of UVB irradiation on Dicer, a key enzyme mediating miRNA maturation, is not known, but is hypothesized to control global miRNA levels in response to UVB irradiation.
The goal of this study was to examine changes in skin function, structure, and biomechanics as a result of chronic exposure to UVB and associated changes in Dicer production and miRNA expression. Using a murine model of UVB exposure, we found that UVB irradiation caused skin to be weaker and less pliable. Notably, these changes were not reversed after a 5-week period of recovery. Fibroblast proliferation was low in all groups, but was significantly reduced following UVB. A decrease in average collagen fibril size was also observed after UVB exposure along with significant decreases in Dicer expression and elevated expression of the miR-34 family at 15 weeks. These studies indicate that miRNAs may play a pivotal role in regulating skin biomechanics following chronic UVB exposure.
Clinical Problem Addressed
For example, aging-related reductions in skin thickness and mechanical integrity contribute to the increase in susceptibility to skin tears in the elderly, with an estimated incidence of nearly 2 million skin tears per year.2,3,13 By 2030, it is estimated that 20% of the U.S. population will be older than 6514; thus the number of defects associated with reduced skin mechanics in older individuals is anticipated to increase. As a result, understanding how extrinsic aging, including chronic UVB exposure, contributes to altered skin properties and function is needed.
Materials and Methods
UVB exposure model
Female SKH1 hairless mice (6–8 weeks old; Charles River, Wilmington, MA) were housed in the vivarium at the Ohio State University according to the requirements established by the American Association for Accreditation of Laboratory Animal Care. All procedures were approved by the Institutional Animal Care and Use Committee before beginning the studies. To mimic the photoaging process, mice were exposed dorsally three times per week to escalating doses of UVB light from Phillips FS40 UVB lamps (American Ultraviolet Company). Previous photoaging studies have used an escalating dose regimen with FS40 UVB lamps.15,16 The FS40 lamps are widely used as an experimental source of UVB, as they primarily emit light in the UVB range (290–320 nm) with a peak at ∼313 nm.17 However, some light in the UVA range is emitted. Mice received 745 J/m2 UVB three times per week in week 1; 2,240 J/m2 three times per week in week 2; 3,732 J/m2 three times per week in week 3; and 5,227 J/m2 three times per week in weeks 4–10. A subset of mice was maintained for an additional 5 weeks without UVB exposure. Age-matched, nonirradiated mice served as controls.
Photography
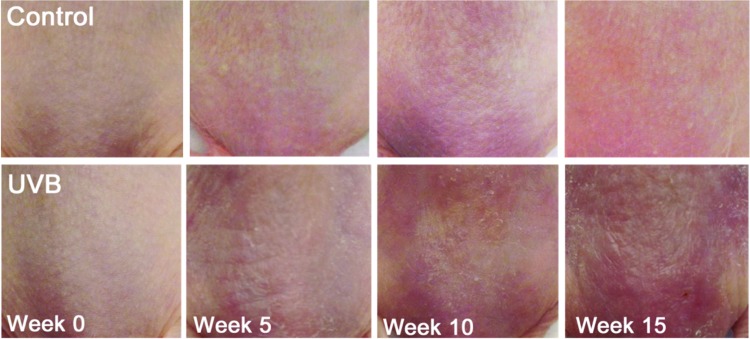
Photographs of the control and UVB groups were taken before the start of UVB irradiations and at 5, 10, and 15 weeks. Representative images from each group were shown at all time points.
Transepidermal water loss
Transepidermal water loss (TEWL) directly assesses epidermal barrier function of the skin by measuring the humidity of the air directly above the skin relative to the humidity in the ambient environment, with higher values indicating more water loss and less barrier function.18 TEWL (DermaLab® Combo; Cortex Technology ApS, Denmark) was measured in the control and UVB-treated mice in weeks 0, 2, 4, 5, 6, 8, 10, 12, 14, and 15 weeks and average TEWL (g/m2/h) ± standard error of the mean was reported.
Histological analysis
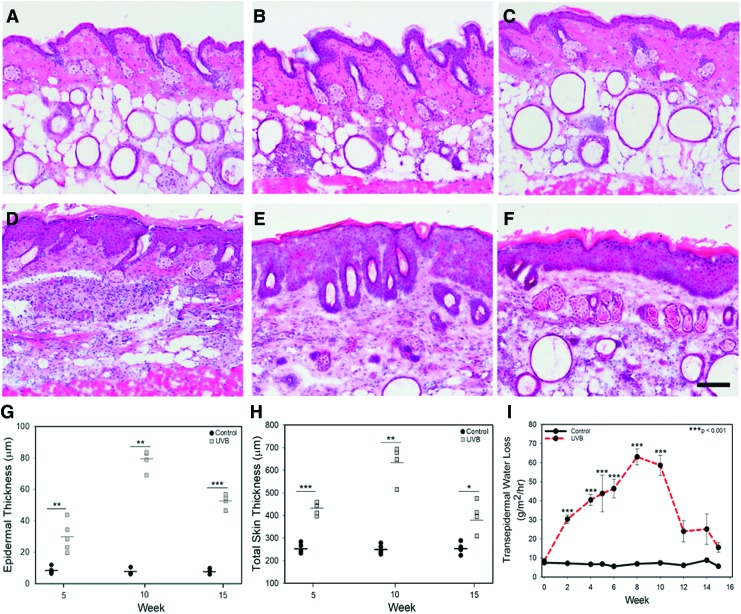
Biopsies (n = 5 per group per time point) were taken from control and UVB skin for histology. The biopsies were embedded in optimal cutting temperature compound and stored at −80°C until sectioning. Sections were cut at 7 μm thickness and stained with hematoxylin and eosin (H&E) or immunostained with Ki67 to assess cell proliferation. Epidermal area and total skin thickness were quantified on two unique sections for each group at each time point (n = 5/group). The entire epidermis within the field of view at 10 × was traced in ImageJ and the area quantified. Total skin thickness, measured from the top of the stratum corneum to the top of the adipose tissue, was assessed at three locations on two unique sections per group/time point. Average epidermal area and skin thickness were reported along with each data point.
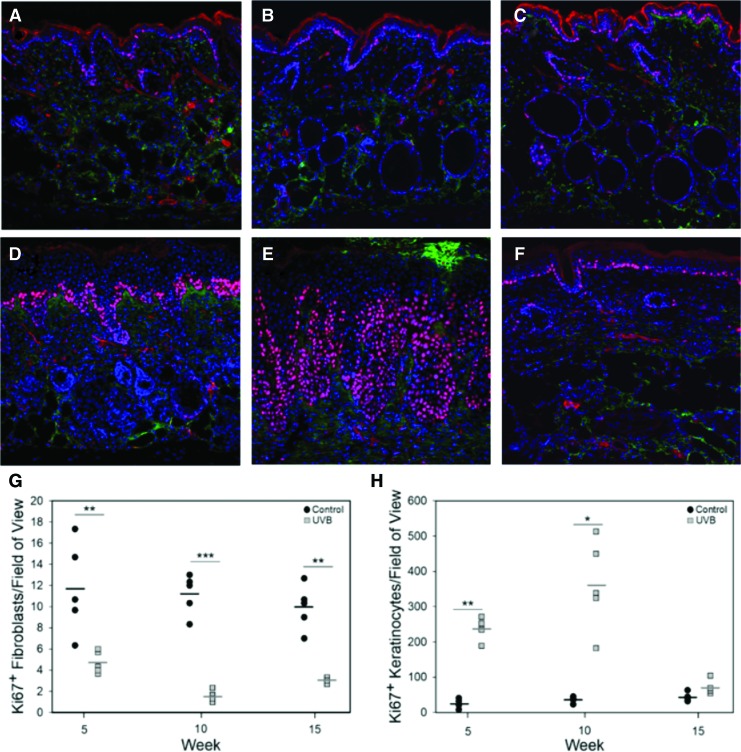
For immunostaining, slides were fixed in methanol for 8 min, rehydrated in phosphate-buffered saline (PBS), and incubated in primary antibodies for Ki67 (Abcam, Cambridge, MA) and anti-fibroblast TE-7 (Sigma). The following day, the sections were stained with the appropriate secondary antibody along with 4′,6-diamidino-2-phenylindole (DAPI) for 1 h at room temperature. Sections were rinsed with PBS, mounted with glycerin, and cover slipped. The stained sections were imaged using an Olympus FV1000 Multi-Photon confocal microscope. Quantitative analysis for Ki67-positive cells within the epidermis and dermis was performed using ImageJ with total number of positive cells per 20 × field of view. For each cell type, the images were collected such that the entirety of the dermis or epidermis was within the field of view. Ki67-positive fibroblasts were determined as cells where positive Ki67 and TE-7 staining were co-localized. Quantitation was performed on sections from five different samples per group at weeks 5, 10, and 15. Average Ki67-positive cells were plotted along with each individual data point.
Transmission electron microscopy
At week 15, control and UVB skin were fixed in 2.5% glutaraldehyde in 0.1 M PBS (pH = 7.4) overnight at 4°C (n = 3/group). Skin was postfixed with 1% osmium tetroxide in PBS for 1 h at room temperature. En block staining was performed using 2% uranyl acetate in 10% ethanol followed by dehydration in a graded series of ethanol and embedding in Eponate 12 epoxy resin (Ted Pella, Redding, CA). Ultrathin sections were cut on a Leica EM UC6 ultramicrotome (Leica Microsystems), collected on copper grids, and stained with lead citrate and uranyl acetate. Images were acquired with an FEI Technai G2 spirit (FEI; Hillsboro, OR) transmission electron microscope and Microfire digital camera (Optronics; Goleta, CA), and advanced microscopy techniques image capture software. Images for both the control and UVB-exposed skin were collected from three samples each. Images were thresholded, areas within the image to quantify were traced (to avoid areas where the fibers were parallel to the section), and cross-sections of fibers were measured using the particle measure function in ImageJ. Fibril diameter was calculated from the cross-sectional area assuming a circular cross-section. A minimum of 1,000 collagen fibril diameters were measured (ImageJ) from each sample and plotted as histogram for each sample type.
Protein analysis
Total Dicer protein was assessed using Western blotting. Control and UVB-treated skin were collected at weeks 5, 10, and 15 (n = 4 per time point). Briefly, tissue was pulverized and lysed in lysis buffer containing 10 mM Tris pH 7.4, 150 mM NaCl, 1% Triton X-100, 1% deoxycholic acid, 0.1% sodium dodecyl sulfate (SDS), and 5 mM ethylenediaminetetraacetic acid (EDTA). Tissue lysates were resolved in SDS polyacrylamide gel electrophoresis, transblotted to polyvinylidene difluoride membrane (Amersham), blocked in 10% skim milk, and incubated with primary antibody against Dicer1 (Sigma-Aldrich, St. Louis, MO) overnight at 4°C. Signal was visualized using corresponding horseradish peroxidase-conjugated secondary antibody (Amersham; 1:3,000) and ECL Plus™ Western Blotting Detection Reagents (Amersham). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH; Sigma-Aldrich) served as loading control. Average expression of Dicer1 normalized to GAPDH was plotted along with each individual data point.
NanoString assay
MiRNA expression profiling was performed using a multiplexed nanoString nCounter miR system (NanoString Technologies) following procedures previously described.19 Small RNA samples were prepared according to manufacturer's instruction (NanoString Technologies) and involved ligating a specific DNA tag onto the 3′ end of each mature miRNA, which provides identification for each miR species in the sample. The material hybridized, following removal of any excess tags, with a panel of miR:tag-specific nCounter capture and barcoded reporter probes. Hybridization reactions were incubated at 64°C for 18 h. Hybridized probes were purified and immobilized on a streptavidin-coated cartridge using the nCounter Prep Station (NanoString Technologies). nCounter Digital Analyzer was used to count individual fluorescent barcodes and quantify target RNA molecules present in each sample. For each assay, a high-density scan (600 fields of view) was performed. Average fold changes in the UVB group versus nonirradiated controls were reported for miRNAs with at least a 2.25-fold difference in expression.
To validate key findings of the miRNA profile, quantitative reverse transcription polymerase chain reaction (qRT-PCR) was utilized. Control and UVB-treated skin (n = 4/group) were collected and placed into RNA lysis solution (RNAqueous Microkit; Ambion). RNA was isolated using mirVANA PARIS miRNA isolation kits with miR-34a, b, and c quantified using TaqMan miRNA assays with β-actin as a control. Expression levels were quantified employing the 2−ΔΔct relative quantification method with average along with each data point reported.
Biomechanics
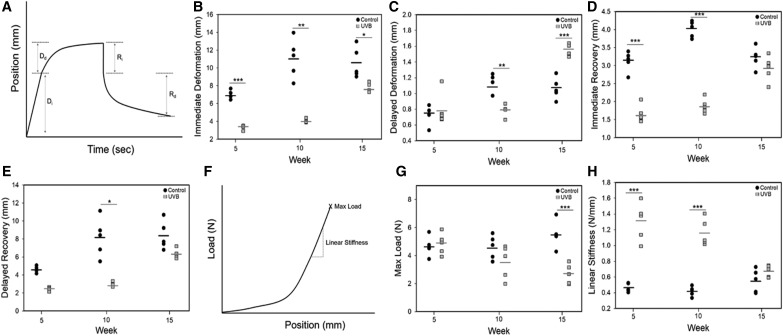
Biomechanics of the tissue were examined ex vivo. Tissue was removed from the dorsum of the mice immediately after euthanasia. The skin was maintained in hydrated state and cut into dogbone- shaped samples using a punch (gauge width 3 mm and gauge length 10 mm) with the long axis of the punch in the cranial-caudal direction. Care was taken to collect the samples from the same approximate location on each mouse and all skin was tested within 2 h of collection. To assess failure biomechanics, skin was strained at 2 mm/s until failure (TestResources 100R with a 2.2 lb load cell; Shakopee, MN) (n = 5/group). Maximum load at failure (N), linear stiffness (N/mm), and percent elongation at failure were reported as individual data points in addition to the average. A creep and recovery test was also performed on a subset of the samples where a 0.2N load was applied to the skin and held for 60 s, after which the load was removed and the skin assessed for an additional 60 s. As the load was applied by displacement of the grips (2 mm/s), the full load was not instantaneous; thus, the deformation in response to the initial load was reported as immediate deformation (Fig. 6A). Deformation of the tissue in response to the load over the 60-s dwell period was labeled “delayed deformation” with recovery immediately following load removal, “immediate recovery.” Data were plotted for each measurement using individual data points along with average.
Figure 6.
Ex vivo skin biomechanics. (A) A creep-recovery test was performed on skin samples loaded to 0.2N, held for 60 s, and then returned to 0N. Immediate deformation (B), delayed deformation (C), immediate recovery (D), and delayed recovery (E) were assessed. Control samples exhibited greater immediate deformation at all time points. All dynamic mechanical properties significantly changed with time (p < 0.01). Failure properties of control and UVB-treated skin were also assessed using tensile testing to failure at 2mm/s grip displacement. A toe-in region was present in all skin samples, followed by a linear region before failure (F). Maximum load at failure was significantly greater in control samples versus UVB treatment (G). Linear stiffness was increased in UVB samples at weeks 5 and 10 (H). Failure properties were not dependent on time in either group. *p < 0.05, **p < 0.01, ***p < 0.001.
Statistical analyses
Statistical analyses were performed using SigmaStat. Statistically significant differences were detected using either Student's t-test or a one-way ANOVA with a post hoc test of Tukey. Statistical significance was considered at p < 0.05.
Results
Skin morphology, TEWL, and cell proliferation
Before UVB exposure, murine skin was smooth, thin, and pink in color. With UVB exposure, skin became more erythematous, thicker, and rougher with redness increasing until 15 weeks (Fig. 1). Histological analysis showed that control unirradiated murine skin remained unchanged over the course of the study with a thin epidermis (Fig. 2A–C). In the UVB group, the epidermis was significantly thicker after 5 weeks of exposure and reached peak thickness at week 10 (Fig. 2D, E). Following cessation of UVB exposure at week 10, epidermal thickness was reduced, but did not return to baseline values by week 15 (Fig. 2F).
Figure 1.
Photographs of murine skin over a 15-week period. In the UVB group, mice received 734 J/m2 UVB at week 1; 2,240 J/m2 at week 2; 3,732 J/m2 at week 3; and 5,227 J/m2 at weeks 4–10. Neither group received UVB in weeks 11–15. UVB, ultraviolet B. Color images are available online.
Figure 2.
H&E-stained cryosections of control (A–C) and UVB-treated (D–F) mouse skin at week 5 (A, D), 10 (B, E), and 15 (C, F). Skin receiving UVB had a marked increase in epidermal thickness that plateaued at week 10. At week 15, epidermal thickness in the UVB group (F) had not returned to that of the control (C). Scale bar = 50 μm. Epidermal area (G) and total skin thickness (H) as a function of time and treatment. Neither epidermal area nor skin thickness changed with time in the control groups (p = 0.513 and p = 0.917, respectively). Both epidermal area and skin thickness changed as a function of time with a statistically significant increase in both at week 10 versus week 15 (p < 0.01 and p < 0.001, respectively). (I) Transepidermal water loss in control and UVB-exposed skin. Skin receiving UVB exhibited a marked increase in TEWL after 2 weeks of treatment, reaching peak water loss at week 8. After UVB exposure was stopped at week 10, TEWL values in the UVB group returned to baseline. *p < 0.05, **p < 0.01, ***p < 0.001. TEWL, transepidermal water loss. Color images are available online.
Two weeks following the first UVB exposure, epidermal barrier was significantly compromised as measured by TEWL (Fig. 2I). TEWL values continued to increase with time and UVB exposure, reaching a plateau at 8 weeks of UVB exposure with TEWL values approximately eightfold greater than controls. After UVB exposure was stopped, TEWL rapidly declined, reaching normal skin levels by week 15 (Fig. 2I).
Cell proliferation was significantly altered by UVB with the number of keratinocytes actively proliferating, increased by an order of magnitude at weeks 5 and 10 (Fig. 3A). By week 15, epidermal proliferation in the UVB group returned to baseline. Overall, the populations of proliferating fibroblasts were low in both groups; however, the number of proliferating fibroblasts was significantly lower in the UVB-exposed group (Fig. 3B).
Figure 3.
Representative images of control (A–C) and UVB-exposed (D–F) murine skin immunostained with Ki67 (red) and TE7 (green) and counterstained with DAPI (blue). The number of Ki67-positive fibroblasts (G) and keratinocytes (H) was quantified from separate sets of images (for fibroblasts, the field of view was shifted such that the bottom of the epidermis was at the top of the field of view). UVB exposure significantly increased the number of proliferating cells at all time points. In contrast, UVB exposure increased keratinocyte proliferation reaching a maximum at week 10 and returning to baseline at week 15. *p < 0.05, **p < 0.01, ***p < 0.001. DAPI, 4′,6-diamidino-2-phenylindole. Color images are available online.
Protein and gene expression
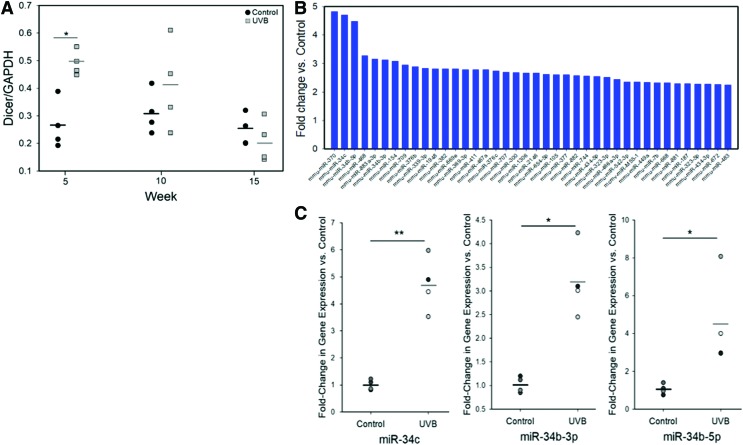
Dicer1 protein expression was increased with exposure to UVB, with an approximately twofold increase versus control at week 5. No significant differences between groups were observed at weeks 10 and 15; however, there was a significant reduction in the UVB group at week 15 versus week 5 (Fig. 4A). At week 15, 40 different miRNAs upregulated at least 2.25-fold, including miR-370 (4.82-fold increase). Three out of six of the highest upregulated miRNAs were members of the miR-34 family. The nanoString findings were validated using qRT-PCR where significant upregulation of miR-34c, 34b-5p, and 34b-3p was observed in the UVB group (Fig. 4C).
Figure 4.
(A) Dicer1 protein levels in mouse skin as a function of UVB exposure and time. A significant increase in Dicer1 expression was observed after 5 weeks of UVB irradiation. No differences between treatment groups were observed at weeks 10 and 15. Dicer levels did not significantly change with time in control samples, but significantly decreased with time in the UVB group (p < 0.01). (B) MiRNA expression profiling was performed on week-15 samples by nanoString analysis. All miRNAs with ≥2.25-fold change in expression are shown. Of the top six miRNAs differentially expressed, three were within the miR-34 family. (C) miR-34 family expression at week 15 was validated by quantitative reverse transcription polymerase chain reaction. Significant increases in miR-34c, miR-34b-5p, and miR-34b-3p were observed after chronic exposure to UVB. *p < 0.05, **p < 0.01. Color images are available online.
Transmission electron microscopy
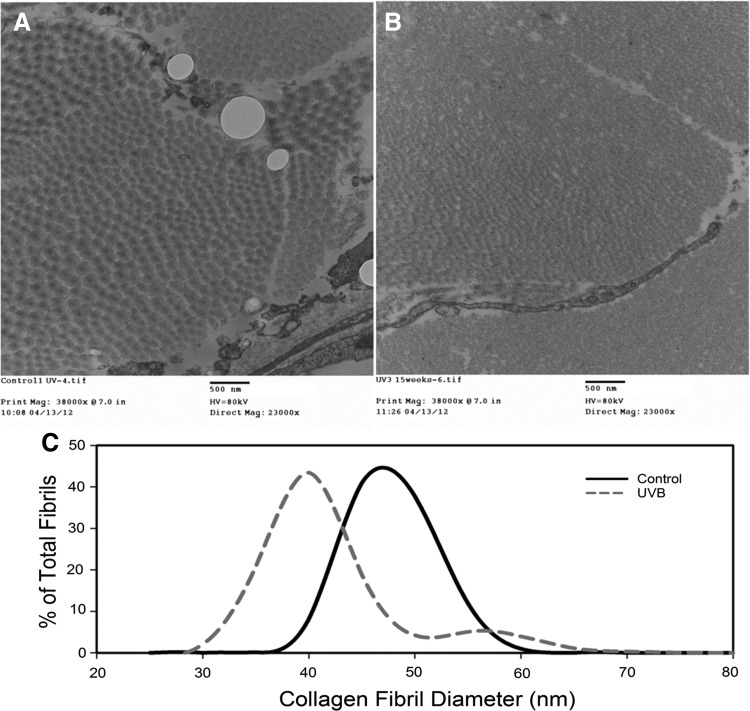
Collagen ultrastructure was significantly altered by UVB exposure with small, tightly packed collagen fibrils observed in the UVB group (Fig. 5B). Average collagen fibril diameter in the control group was 52.9 ± 0.34 nm versus 41.5 ± 0.2 nm in the UVB group (Fig. 5C). In addition, the fibril diameter distribution was Gaussian in the control group, but had a slight bimodal distribution in the UVB group with a smaller population (∼10–15% of the total) of larger fibril diameters (50–65 nm).
Figure 5.
Transmission electron micrographs of control (A) and UVB (B)-exposed skin at week 15. (C) Histogram of collagen fibril diameter in control and UVB groups at week 15. UVB decreases average fibril diameter versus controls.
Biomechanical properties
Skin biomechanics were measured ex vivo. Following exposure to UVB, skin exhibited significantly less immediate deformation in response to a 0.2N load at all time points (Fig. 6B). Delayed deformation was also significantly reduced following 10 weeks of UVB exposure and this was not regained following cessation of UVB. In addition, recovery of deformation both immediately and with time was reduced following 10 weeks of UVB exposure (Fig. 6D, E). Deformation and recovery, both immediate and delayed, were a function of time in both groups and increased with time. Failure mechanics were also evaluated by uniaxial tensile testing. Average maximum load at failure began to decrease after 10 weeks of exposure and was significantly lower in the UVB group at week 15 (Fig. 6G). In contrast, linear stiffness was rapidly increased by UVB exposure with stiffness in the UVB group, 2 times greater than the controls at 5 weeks, with this difference maintained at 10 weeks (Fig. 6H). By week 15, stiffness values returned to the level of the controls. Failure mechanics were not altered in either group as a function of time.
Discussion
Following UVB exposure, the epidermis became hyperproliferative, as evidenced by the significant increase in the Ki67-positive keratinocyte population, with reduced barrier function as measured by TEWL. These changes in epidermal function have been observed previously in mice and have been shown to scale with the magnitude of UVB dose, with higher doses resulting in more proliferation and greater loss of barrier function.20 In addition, epidermal hyperproliferation following UVB exposure was demonstrated in humans with populations of Ki67-positive epidermal cells scaling with the magnitude of UVB dose and repeated exposure.21
While the effect of UV irradiation on epidermal proliferation and differentiation has been widely studied, far less is known regarding the effect of UVB on miRNA processing and expression. In this study, a significant, yet transient, increase in Dicer1 was observed with UVB irradiation along with significant increases in the miR-34 family at the 15-week time point. UV irradiation has recently been shown to alter miRNA expression with the wavelength-specific responses.22–24 UVB irradiation of cultured keratinocytes showed dysregulation of many miRNAs, including members of the let-7 family, miR-23a, miR-22, miR-200b, and a number of other miRNAs depending on the magnitude of exposure and duration of exposure. Upregulation of expression in the miR-34 family was observed in only one study of fibroblasts in vitro with a modest increase in miR-34a at 4 and 24 h postexposure to UVB.22 While the fold-change differences observed in prior studies were significantly lower than those observed currently, it is likely that acute and chronic UVB exposure have differential effects on miRNA expression along with differences in vitro versus in vivo.
Although the response of the epidermis to UV exposure is most commonly studied, recent investigations have shown that many components of the skin are altered by UV irradiation. In this study, chronic exposure of murine skin to UVB resulted in reduced fibroblast proliferation, smaller collagen fibril diameter, and skin that was weaker, stiffer, less pliable, and less elastic than controls. Premature senescence of dermal fibroblasts can occur as a result of DNA damage and oxidative stress with senescence observed in cultured human dermal fibroblasts after repeated exposure to subcytotoxic doses of UVB.25 More recently, UVB-treated human dermal fibroblasts were shown to have a marked upregulation of miR-34c-5p and concomitant reduction in SA-β-gal activity. When miR-34c-5p was reduced by siRNA, fibroblast senescence was delayed.22 This reduction in fibroblast activity may have resulted in alterations in the quality and quantity of collagen within the dermis, which would impact tissue biomechanics. Exposure of human forearm skin to UVB has previously been shown to reduce immediate and delayed deformation, in addition to skin recovery following off-loading.3 In that study, changes in collagen bundles within the dermis were suggested to be the cause of the mechanical alterations.3 Prior studies have shown a marked decreased in collagen content in sun-exposed skin with a 20% decrease in total collagen content and a 40% decrease in intact propeptides for collagen type III.26 In addition to possible alterations in total collagen content, this study showed that collagen ultrastructure was altered by UVB exposure. UVB exposure reduced average fibril diameter, which has previously been linked to lower tensile strength,27, suggesting that chronic irradiation may functionally impair the dermis by altering the structural properties of collagen. While the dermis commonly dominates the mechanical properties of normal mouse skin as the epidermis is very thin, significant increases in epidermal thickness likely causes a stiffening of the skin.
In this study, several limitations exist, including the inability to quantify the mechanical properties of the dermis and epidermis individually. As the epidermis of normal mouse skin is thin and fragile, methods to remove the epidermis often results in damage and alteration of the mechanical properties. In addition, the expression of Dicer and the miRNA was assessed on whole tissue lysates; thus, the individual response of the epidermis and dermis could not be separated. Further experiments are required to directly probe the link between miRNA, specifically the miR-34 family, and photoaging.
Innovation
To our knowledge, this is the first study to concurrently examine alterations in epidermal function (TEWL), extracellular matrix structure, miRNA expression, and tissue biomechanics in response to chronic UVB exposure. As UVB led to a significant upregulation of many miRNAs, including the miR-34 family, these small molecules may play a role in regulating photoaging; however, further studies that directly assess the mechanism are needed.
Key Findings.
Chronic UVB exposure increased miR-34c, 34b-5p, and 34b-3p expression in skin
Fibroblast proliferation was significantly reduced following UVB exposure
UVB exposed skin comprised collagen fibrils with smaller average diameter
Chronic UVB exposure reduced deformation and recovery of skin, increased skin stiffness, and reduced maximum load at failure.
Acknowledgments and Funding Sources
The authors would like to thank the Campus Microscopy and Imaging Facility at The Ohio State University and the Histology and Pathology Core Facility at the Shriners Hospitals for Children in Cincinnati.
Abbreviations and Acronyms
- DAPI
4′,6-diamidino-2-phenylindole
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- H&E
hematoxylin and eosin
- miR
miRNA
- PBS
phosphate-buffered saline
- qRT-PCR
quantitative reverse transcription polymerase chain reaction
- RNA
ribonucleic acid
- SDS
sodium dodecyl sulfate
- TEWL
transepidermal water loss
- UV
ultraviolet
- UVB
ultraviolet B
Author Disclosure and Ghostwriting
No competing financial interests exist. The content of this article was expressly written by the authors listed. No ghostwriters were used to write this article.
About the Authors
Britani N. Blackstone, PhD, is a Research Scientist in the Department of Materials Science and Engineering working with Heather M. Powell, PhD, an Associate Professor of Materials Engineering and Biomedical Engineering, on wound healing, scarring, skin biomechanics, and engineered skin. Brian C. Wulff, PhD, was a postdoctoral researcher in the laboratory of Traci A. Wilgus, PhD, an Associate Professor of Pathology, working on the role of inflammation in wound healing, scar formation, and UV-induced skin carcinogenesis. Sashwati Roy, PhD, is a full professor in the Department of Surgery, specializing in wound healing and inflammation.
References
- 1. Farage MA, Miller KW, Elsner P, Maibach HI. Structural characteristics of the aging skin: a review. Cutan Ocul Toxicol 2007;26:343–357 [DOI] [PubMed] [Google Scholar]
- 2. Farage MA, Miller KW, Berardesca E, Maibach HI. Clinical implications of aging skin: cutaneous disorders in the elderly. Am J Clin Dermatol 2009;10:73–86 [DOI] [PubMed] [Google Scholar]
- 3. Nishimori Y, Edwards C, Pearse A, Matsumoto K, Kawai M, Marks R. Degenerative alterations of dermal collagen fiber bundles in photodamaged human skin and UV-irradiated hairless mouse skin: possible effect on decreasing skin mechanical properties and appearance of wrinkles. J Invest Dermatol 2001;117:1458–1463 [DOI] [PubMed] [Google Scholar]
- 4. Matsumura Y, Ananthaswamy HN. Toxic effects of ultraviolet radiation on the skin. Toxicol Appl Pharmacol 2004;195:288–297 [DOI] [PubMed] [Google Scholar]
- 5. Rabe JH, Mamelak AJ, McElgunn PJS, Morison WL, Sauder DN. Photoaging: mechanisms and repair. J Am Acad Dermatol 2006;55:1–19 [DOI] [PubMed] [Google Scholar]
- 6. Pothof J, Verkaik NS, van IJcken W, Wiemer EAC, Ta VTB, van der Horst GTJ, et al. MicroRNA-mediated gene silencing modulates the UV-induced DNA-damage response. EMBO J 2009;28:2090–2099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tan G, Shi Y, Wu Z-H. MicroRNA-22 promotes cell survival upon UV radiation by repressing PTEN. Biochem Biophys Res Commun 2012;417:546–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Guo L, Huang ZX, Chen XW, Deng QK, Yan W, Zhou MJ, et al. Differential expression profiles of microRNAs in NIH3T3 cells in response to UVB irradiation. Photochem Photobiol 2009;85:765–773 [DOI] [PubMed] [Google Scholar]
- 9. Dziunycz P, Iotzova-Weiss G, Eloranta JJ, Laeuchli S, Hafner J, French LE, et al. Squamous cell carcinoma of the skin shows a distinct MicroRNA profile modulated by UV radiation. J Invest Dermatol 2010;130:2686–2689 [DOI] [PubMed] [Google Scholar]
- 10. Toyokuni S, Jiang L, Wang S, Hirao A, Wada T, Soh C, et al. Aging rather than sun exposure is a major determining factor for the density of miR-125b-positive epidermal stem cells in human skin. Pathol Int 2015;65:415–419 [DOI] [PubMed] [Google Scholar]
- 11. Li T, Yan X, Jiang M, Xiang L. The comparison of microRNA profile of the dermis between the young and elderly. J Dermatol Sci 2016;82:75–83 [DOI] [PubMed] [Google Scholar]
- 12. Castelo-Branco C, Duran M, Gonzalez-Merlo J. Skin collagen changes related to age and hormone replacement therapy. Maturitas 1992;15:113–119 [DOI] [PubMed] [Google Scholar]
- 13. Baranoski S. Skin tears: the enemy of frail skin. Adv Ski Wound Care 2000;13:123–126 [PubMed] [Google Scholar]
- 14. Statistics FIF on AR. No Title. 2014. www.agingstats.gov (last accessed June25, 2019)
- 15. Schwartz E, Kligman LH. Topical tretinoin increases the tropoelastin and fibronectin content of photoaged hairless mouse skin. J Invest Dermatol 1995;104:518–522 [DOI] [PubMed] [Google Scholar]
- 16. Yano K, Oura H, Detmar M. Targeted overexpression of the angiogenesis inhibitor thrombospondin-1 in the epidermis of transgenic mice prevents ultraviolet-B-induced angiogenesis and cutaneous photo-damage. J Invest Dermatol 2002;118:800–805 [DOI] [PubMed] [Google Scholar]
- 17. Kligman LH, Murphy GF. Ultraviolet B radiation increases hairless mouse mast cells in a dose-dependent manner and alters distribution of UV-induced mast cell growth factor. Photochem Photobiol 1996;63:123–127 [DOI] [PubMed] [Google Scholar]
- 18. Gebhard KL, Effendy I, Löffler H. Artificial disruption of skin barrier prior to irritant patch testing does not improve test design. Br J Dermatol 2004;150:82–89 [DOI] [PubMed] [Google Scholar]
- 19. Dickerson R, Roy S, Zhang SW, Rink C, Sen CK, Powers CJ. Human cerebrospinal fluid microRNA: temporal changes following subarachnoid hemorrhage. Physiol Genomics 2016;48:361–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Haratake A, Uchida Y, Schmuth M, Tanno O, Yasuda R, Epstein JH, et al. UVB-induced alterations in permeability barrier function: roles for epidermal hyperproliferation and thymocyte-mediated response. J Invest Dermatol 1997;108:769–775 [DOI] [PubMed] [Google Scholar]
- 21. Lee JH, An HT, Chung JH, Kim KH, Eun HC, Cho KH. Acute effects of UVB radiation on the proliferation and differentiation of keratinocytes. Photodermatol Photoimmunol Photomed 2002;18:253–261 [DOI] [PubMed] [Google Scholar]
- 22. Zhou BR, Guo XF, Zhang JA, Xu Y, Li W, Wu D, et al. Elevated miR-34c-5p mediates dermal fibroblast senescence by ultraviolet irradiation. Int J Biol Sci 2013;9:743–752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Syed DN, Khan MI, Shabbir M, Mukhtar H. MicroRNAs in skin response to UV radiation. Curr Drug Targets 2013;14:1128–1134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kraemer A, Chen I-P, Henning S, Faust A, Volkmer B, Atkinson MJ, et al. UVA and UVB irradiation differentially regulate microRNA expression in human primary keratinocytes. PLoS One 2013;8:e83392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Debacq-Chainiaux F, Borlon C, De Hertogh B, Remacle J, Morvan P-Y, Vallée R, et al. Identification of potential anti-photoageing algal compounds using an in-vitro model of photoageing. J Pharm Pharmacol 2006;58:1577–1583 [DOI] [PubMed] [Google Scholar]
- 26. Schwartz E, Cruickshank FA, Christensen CC, Perlish JS, Lebwohl M. Collagen alterations in chronically sun-damaged human skin. Photochem Photobiol 1993;58:841–844 [DOI] [PubMed] [Google Scholar]
- 27. Parry DA, Barnes GR, Craig AS. A comparison of the size distribution of collagen fibrils in connective tissues as a function of age and a possible relation between fibril size distribution and mechanical properties. Proc R Soc London Ser B Biol Sci 1978;203:305–321 [DOI] [PubMed] [Google Scholar]