Abstract
Sexual dimorphism in mitochondrial respiratory function has been reported in young women and men without diabetes, which may have important implications for exercise. The purpose of this study was to determine if sexual dimorphism exists in skeletal muscle mitochondrial bioenergetics in people with type 1 diabetes (T1D). A resting muscle microbiopsy was obtained from women and men with T1D (n = 10/8, respectively) and without T1D (control; n = 8/7, respectively). High-resolution respirometry and spectrofluorometry were used to measure mitochondrial respiratory function, hydrogen peroxide (mH2O2) emission and calcium retention capacity (mCRC) in permeabilized myofiber bundles. The impact of T1D on mitochondrial bioenergetics between sexes was interrogated by comparing the change between women and men with T1D relative to the average values of their respective sex-matched controls (i.e., delta). These aforementioned analyses revealed that men with T1D have increased skeletal muscle mitochondrial complex I sensitivity but reduced complex II sensitivity and capacity in comparison to women with T1D. mH2O2 emission was lower in women compared with men with T1D at the level of complex I (succinate driven), whereas mCRC and mitochondrial protein content remained similar between sexes. In conclusion, women and men with T1D exhibit differential responses in skeletal muscle mitochondrial bioenergetics. Although larger cohort studies are certainly required, these early findings nonetheless highlight the importance of considering sex as a variable in the care and treatment of people with T1D (e.g., benefits of different exercise prescriptions).
Keywords: bioenergetics, female, mitochondria, sex differences, type 1 diabetes
INTRODUCTION
Sexual dimorphism in the development of type 1 diabetes (T1D) complications exists and evidence indicates a greater negative impact in women. Women with T1D have a twofold increased risk of developing and dying from cardiovascular disease as well as a near 40% greater excess risk for all-cause mortality compared with men with T1D (2, 5, 6). In addition, it is known that women with T1D typically have greater struggles with insulin management (5), have worse glycemic control (8), and suffer from increased adiposity (13) and a greater degree of insulin resistance (9) compared with men with T1D. This is striking considering the clinically reported cardioprotective effects of estrogen in the female sex as well as the beneficial effects of estrogen on insulin action and metabolism (3). Thus, with accumulating evidence of sex differences in diabetes complications development, it is becoming ever-more evident that a sex-specific approach to diabetes research and disease care/management is necessary.
Although the mechanisms for the metabolic effects of female sex hormones (e.g., estrogen) are still being unraveled, there is a growing appreciation that these hormones play a role in regulating insulin signaling and action, substrate oxidation, and mitochondrial bioenergetics (3, 15). With respect to estrogen, recent findings in healthy, recreationally active women and men suggest that sex may influence mitochondrial respiratory function in skeletal muscle (1, 19, 12). This is of particular interest in the context of T1D, considering the recent findings from our group (11) and others (4) of site-specific alterations in mitochondrial functions in the skeletal muscle of young adults with T1D and considering the fact that skeletal muscle is our largest metabolic organ, by mass, thus making it a major determinant of whole body insulin sensitivity and a critical player for glycemic control. Whether there is a differential response in skeletal muscle mitochondrial bioenergetics between women and men with T1D is not currently known. Therefore, to address this gap in knowledge, we systematically assessed mitochondrial bioenergetics and content in skeletal muscle biopsies from women and men with and without T1D.
RESEARCH DESIGN AND METHODS
Participants
Eighteen volunteers with T1D (women n = 10/men n = 8) and fifteen volunteers without T1D (controls; women n = 8/men n = 7) were recruited and closely matched for age, sex, and body mass index (Table 1). A subset of these volunteers (T1D, women n = 7/men n = 5; controls, women n = 7/men n = 5) was derived from our previously published cohort (11). Menstrual cycle phase was not controlled for in this study. All procedures were approved by the Research Ethics Board at York University (REB# e2013–032) and conformed to the Declaration of Helsinki.
Table 1.
Participant demographics
| Control |
Type 1 Diabetes (T1D) |
|||
|---|---|---|---|---|
| Women (n = 8) | Men (n = 7) | Women (n = 10) | Men (n = 8) | |
| Age, yr | 26 ± 2 | 25 ± 3 | 26 ± 5 | 25 ± 4 |
| Height, m | 1.65 ± 0.06 | 1.76 ± 0.02††† | 1.63 ± 0.08 | 1.79 ± 0.11** |
| Weight, kg | 57.9 ± 9.7 | 76.5 ± 4.8††† | 67.3 ± 15.2 | 81.6 ± 9.5* |
| BMI, kg/m2 | 21.1 ± 2.4 | 24.7 ± 1.7†† | 25.2 ± 4.5 | 25.4 ± 2.2 |
| HbA1c, % | 8.1 ± 1.5 | 6.9 ± 0.8* | ||
| Disease duration, yr | 13 ± 10 | 12 ± 5 | ||
| Disease onset, yr | 12 ± 7 | 13 ± 7 | ||
| Physical activity, min/wk | 321 ± 197 | 246 ± 150 | 240 ± 162 | 180 ± 100 |
Data are expressed as mean ± SD. BMI, body mass index; HbA1c, hemoglobin A1c. Physical activity was based on a 7-day activity recall record of moderate-to-vigorous activity levels. No difference was found between sexes in either the control group (P = 0.459) or T1D group (P = 0.430) using an unpaired, two-tail Student’s t test.
P < 0.01,
P < 0.001 vs control women; unpaired, two-tail Student’s t test;
P < 0.05,
P < 0.01 vs T1D women; unpaired, two-tail Student’s t test.
Study Design
The study design has been described in detail previously (11). Briefly, participants arrived at the laboratory at 8:30 AM or 2:30 PM and were instructed to consume a standardized meal 1.5–2 h before their visit and refrain from any form of exercise for at least 24 h. Prior to giving informed consent, all participants were given oral and written information about the experimental procedures. Body mass and height were recorded to determine body mass index, and blood was collected using venepuncture for hemoglobin A1c analysis. Skeletal muscle samples were then obtained from the vastus lateralis muscle by a micropunch percutaneous needle under local anesthetic and utilized for mitochondrial bioenergetic and Western blot analyses, as described below.
Mitochondrial Bioenergetic Analyses
Freshly obtained muscle samples were placed in ice-cold BIOPS and immediately used to prepare permeabilized muscle fibers for the assessment of in vitro mitochondrial oxygen consumption, mitochondrial H2O2 emission (mH2O2) and mitochondrial Ca2+ retention capacity (mCRC) as previously described in detail (11). Briefly, respiration assays were undertaken in MiR05 respiration medium supplemented with 20 mM creatine using the Oxygraph-2k (Oroboros Instruments, Innsbruck, Austria), and mH2O2 assays were measured spectrofluorometrically (QuantaMaster 40, HORIBA Scientific, Edison, NJ) and undertaken in buffer Z supplemented with 10 µM Amplex UltraRed reagent (#A36006, Life Technologies, Carlsbad, CA), 20 mM creatine, and 40 U/ml Cu/Zn superoxide dismutase (#S9697, Sigma Aldrich, St. Louis, MO). Fiber bundles used for complex I (pyruvate + malate)-, pyruvate dehydrogenase complex-, and mitochondrial glycerol-3-phosphate dehydrogenase-derived mH2O2 were also treated with 35 µM 1-chloro-2,4-dinitrobenzene (#237329, Sigma) during permeabilization. mCRC assays were measured spectrofluorometrically and performed in duplicate in buffer Y supplemented with 1 µM Calcium Green-5N (C3737; Life Technologies).
Western Blot Analyses
Frozen muscle samples were homogenized in 10 µL/mg muscle of ice-cold lysis buffer [in mM: 40 HEPES, 120 NaCl, 1 EDTA, 10 NaHP2O7·10H2O pyrophosphate, 10 β-glycerophosphate, 10 NaF and 0.3% CHAPS detergent (pH 7.1) supplemented with 1 mM sodium orthovanadate, 1 mM DTT, 1% Triton X-100, and ¼ tablet of SIGMAFAST protease inhibitor (SLBR7552V, Sigma)]. Homogenized samples were agitated for 1 h at 4°C followed by centrifuging for 10 min. The supernatant was aliquoted, analyzed for total protein using a bicinchoninic acid assay (Thermo Scientific, Waltham, MA), and diluted in 4× SDS loading buffer [62.5 mM Tris(HCl) pH 6.8, 2% SDS, 10% glycerol, 1 mM of 0.2 M EDTA, 100 mM DTT and 0.25% bromophenol blue]. Total proteins (50 µg equally loaded per lane) were separated by SDS-PAGE, transferred to a PVDF membrane, blocked for 1 h [Tris-buffered saline (TBS), 5% non-fat dry milk (wt/vl), 0.1% Tween 20 (TBS-T)] and incubated overnight at 4°C with the commercially available antibody human total oxidative phosphorylation cocktail (OXPHOS; 1:208 in block; cat. no. ab110411, Abcam, Cambridge, MA). Following this, the membrane was washed, incubated with secondary antibody mouse IgG conjugated to horseradish peroxidase (1:10,000 in TBS-T, CST#7076, Cell Signaling, Danvers, MA) for 1 h, and visualized by chemiluminescence (Gel Logic 6000 Pro Imager, Carestream, Rochester, NY). Bands were quantified by densitometry using Image J. Membranes were incubated with Ponceau S (cat. no. P7170, Sigma) for loading control.
Statistics
All data were analyzed using GraphPad Prism 8 software and were initially tested for normal distribution using the Shapiro–Wilk test of normality. The ROUT test was used to identify outliers. Data that did not follow normal distribution were log transformed.
Based on previously published work (1, 10, 12), an a priori hypothesis was established that control women and men would differ in mitochondrial respiratory function and thus we could not strictly compare absolute measures between men and women with T1D. To determine if T1D truly affected women and men differentially, the T1D data were also expressed as the change from the average control values of their respective sex (i.e., delta). A Student’s unpaired, two-tailed t test was used to compare differences between women and men within the control group, women and men within the T1D group, and the delta between T1D women and T1D men relative to their sex-matched control values (average value). Significance was established at P < 0.05.
RESULTS
Skeletal Muscle Mitochondrial Bioenergetics
ADP-stimulated mitochondrial respiration.
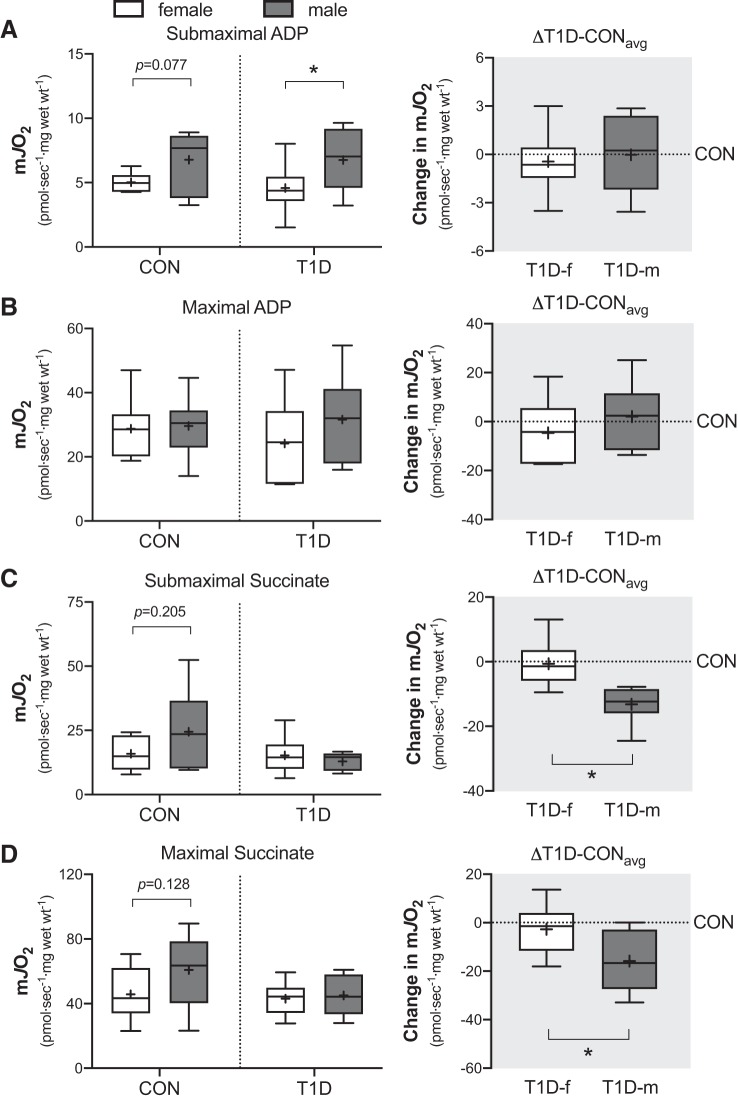
We first investigated the ability of the mitochondria to respond to low versus high metabolic demands (i.e., low and high ADP concentrations). As can be seen in Fig. 1A (left), in the presence of complex I substrates and submaximal ADP (25 µM), the absolute rate of mitochondrial respiration was trending lower in control women (P = 0.077) compared with control men, whereas in T1D, it was significantly lower in women (P = 0.043) compared with men. This indicates a lower sensitivity of the mitochondria to ADP in women and corroborates previous work that reported reductions in mitochondrial ADP sensitivity in control women compared with men (10). When the T1D data were expressed as the absolute change from the control (average values) of their respective sex (i.e., delta), no differences (P = 0.678) were observed between sexes Fig. 1A (right). In the presence of maximal ADP (i.e., oxidative capacity; >5 mM ADP), the absolute rate of mitochondrial respiration was not different between sexes in either the control (P = 0.871) or the T1D group (P = 0.242) as shown in Fig. 1B (left). In line with this, there was no difference (P = 0.295) in the delta (Fig. 1B, right). Together, these observations suggest that skeletal muscle mitochondrial oxidative capacity is similar between sexes in both control and T1D individuals.
Fig. 1.
Mitochondrial ADP-stimulated respiration supported by pyruvate (5 mM) and malate (2 mM) in the presence of either submaximal (25 µM) ADP (A) or maximal (varied 6 to 18 mM) ADP (B) in skeletal muscle biopsies from control (CON) and type 1 diabetes (T1D) women and men. Right panel of (A) and (B) is the absolute change in ADP-stimulated respiration from each T1D participant relative to the average of their sex-matched control (ΔT1D-CONavg). In (C) and (D) is mitochondrial complex II respiration supported by ADP (5 mM), rotenone (0.5 µM), and in the presence of either submaximal (2 mM) succinate (C) or maximal (varied 22 to 30 mM) succinate (D). Right panel of (C) and (D) is the absolute change in complex II-supported respiration from each T1D participant relative to the average of their sex-matched control (△T1D-CONavg). Boxes represent the interquartile range, whiskers show the maximum and minimum, the horizontal line indicates the median and the cross indicates the mean. *P < 0.05 versus women. f, female; m, male; mJO2, mitochondrial oxygen flux; Wt, weight. n = 7–8 for CON women; n = 7 for CON men; n = 9–10 for T1D women; n = 7–8 for T1D men.
Complex II-supported mitochondrial respiration.
We next investigated complex II function by measuring mitochondrial respiration in the presence of varying succinate concentrations, maximal ADP (5 mM) and rotenone (0.5 µM). As can be seen in Fig. 1 C and D (left), the absolute rate of mitochondrial respiration in the presence of either submaximal (2 mM) or maximal (>20 mM) succinate was observably higher in control men compared with women (P = 0.205 and P = 0.128, respectively). In contrast, in the T1D group, the absolute rate of mitochondrial respiration was comparable between sexes in the presence of both submaximal (P = 0.423) and maximal (P = 0.685) succinate (Fig. 1, C and D, left). Thus, when the delta was calculated, men with T1D displayed a greater change in mitochondrial respiration compared with women with T1D, where respiration was lower in the T1D men in the presence of both submaximal (P < 0.001) and maximal (P = 0.031) succinate (Fig. 1, C and D, right). Combined, this suggests that the T1D environment negatively alters mitochondrial complex II sensitivity and capacity in men compared with women.
Complex I-supported mitochondrial respiration.
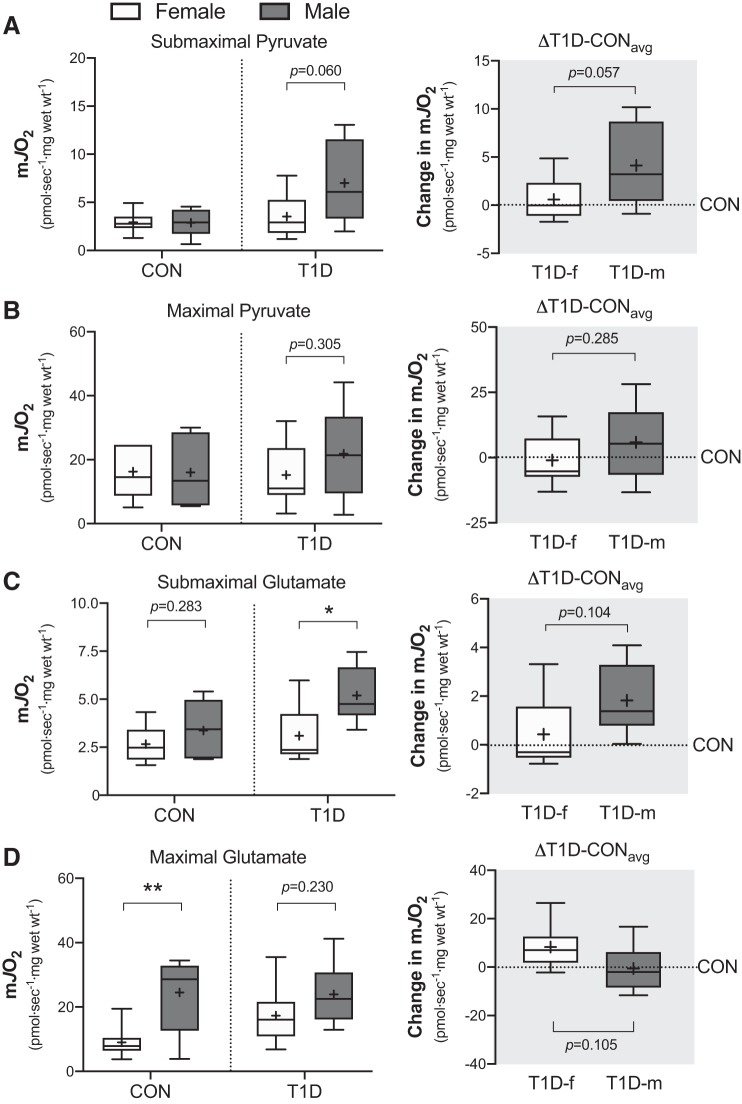
We next investigated complex I function by measuring mitochondrial respiration in the presence of varying pyruvate concentrations, maximal ADP (5 mM) and malate (2 mM). As shown in Fig. 2, A and B (left), the women and men in control group had similar absolute rates of mitochondrial respiration in the presence of submaximal (25 µM) and maximal (>6 mM) pyruvate concentrations (P = 0.961 and P = 0.955, respectively). In contrast, in the T1D group, the absolute rate of mitochondrial respiration was trending higher in men compared with women with submaximal pyruvate (P = 0.060) but not maximal pyruvate (P = 0.305) (Fig. 2, A and B, left). Given this, the change in submaximal pyruvate-supported respiration was trending greater in T1D men compared with T1D women (P = 0.057), whereas T1D men displayed greater pyruvate-supported respiration (Fig. 2A, right). The change in maximal pyruvate-supported respiration on the other hand was comparable between sexes (P = 0.285; Fig. 2B, right).
Fig. 2.
Mitochondrial complex I respiration supported by ADP (5 mM) and malate (2 mM) in the presence of either submaximal pyruvate (A; 25 µM), maximal pyruvate (B; varied 6 to 38 mM), submaximal glutamate (C; 50 µM) or maximal glutamate (D; varied 4 to 20 mM) in control (CON) and type 1 diabetes (T1D) women and men. Right panel of (A) through (D) is the absolute change from each T1D participant relative to the average of their sex-matched control (△T1D-CONavg). Boxes represent the interquartile range, whiskers show the maximum and minimum, the horizontal line indicates the median and the cross indicates the mean. *P < 0.05 versus women, **P < 0.01 versus women. f, female; m, male; mJO2, mitochondrial oxygen flux; Wt, weight. n = 6–8 for CON women; n = 6–7 for CON men; n = 8–9 for T1D women; n = 6 for T1D men.
However, to ascertain that these observations were specific to complex I and not upstream dehydrogenases, we also measured mitochondrial respiration in the presence of varying glutamate concentrations, maximal ADP (5 mM), and malate (2 mM). In the control group, the absolute rate of respiration was similar between sexes with submaximal glutamate (50 µM; P = 0.283), as shown in Fig. 2C (left). However, in the presence of maximal glutamate (>4 mM), the absolute rate of respiration was increased in control men compared with women (P = 0.007; Fig. 2D, left). Thus, this suggests that the capacity of the enzyme glutamate dehydrogenase, but not the capacity of complex I, is increased in control men. In the T1D group, the absolute rate of respiration with submaximal but not maximal glutamate was increased in men compared with women (P = 0.021 and P = 0.230, respectively; Fig. 2, C and D, left). The change in submaximal and maximal glutamate-supported respiration was observably greater in T1D men versus T1D women (P = 0.104 and P = 0.105, respectively; Fig. 2, C and D, right), whereas T1D men had higher respiration rates. Altogether, the greater change in both submaximal pyruvate- and glutamate-supported respiration in T1D men compared with women suggests that T1D increases the sensitivity of complex I in men.
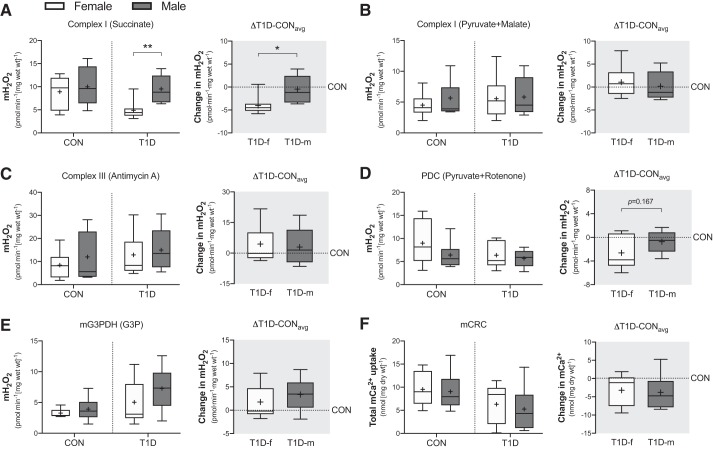
Mitochondrial H2O2 emission.
In the control group, mH2O2 emission potential was similar between women and men at all sites measured, including: complex I driven by succinate (P = 0.600; Fig. 3A, left), complex I driven by pyruvate and malate (P = 0.389; Fig. 3B, left), complex III driven by antimycin A (P = 0.412; Fig. 3C, left), pyruvate dehydrogenase complex (PDC) driven by rotenone and pyruvate (P = 0.242; Fig. 3D, left), and mitochondrial glycerol-3-phosphate dehydrogenase (mG3PDH) driven by glycerol-3-phosphate (P = 0.421; Fig. 3E, left). On the other hand, in the T1D group, mH2O2 emission potential was lower (P = 0.004) at complex I (succinate driven) in women compared with men (Fig. 3A, left) but remained similar at complex I when driven by pyruvate and malate (P = 0.867; Fig. 3B, left), complex III (P = 0.645; Fig. 3C, left), PDC (P = 0.606; Fig. 3D, left), and mG3PDH (P = 0.195; Fig. 3E, left). Similarly, the change in mH2O2 emission potential was comparable at all sites (P = 0.550 complex I pyruvate + malate; P = 0.740 complex III; P = 0.167 PDC; P = 0.344 mG3PDH) except for complex I (succinate driven), where T1D women displayed a greater change in emission potential compared men (P = 0.017); specifically, lower mH2O2 emission potential (Fig. 3, A–E, right).
Fig. 3.
Skeletal muscle site-specific mitochondrial hydrogen peroxide (mH2O2) emission and susceptibility to opening of the permeability transition pore in control (CON) and type 1 diabetes (T1D) women and men. (A) Complex I-derived mH2O2 (10 mM succinate). (B) Complex I-derived mH2O2 (10 mM pyruvate and 2 mM malate). (C) Complex III-derived mH2O2 (2.5 µM antimycin A). (D) Pyruvate dehydrogenase complex (PDC)-derived mH2O2 (10mM pyruvate and 0.5 µM rotenone). (E) Mitochondrial glycerol-3-phosphate dehydrogenase (mG3PDH)-derived mH2O2 (20mM glycerol-3-phosphate). (F) Mitochondrial Ca2+ retention capacity (mCRC). Right shaded panel in (A) through (F) is the absolute change from each T1D participant relative to the average of their sex-matched control (△T1D-CONavg). Boxes represent the interquartile range, whiskers show the maximum and minimum, the horizontal line indicates the median and the cross indicates the mean. *P < 0.05 versus women, **P < 0.01 versus women. f, female; m, male; Wt, weight. n = 7–8 for CON women; n = 6–7 for CON men; n = 8–10 for T1D women; n = 6–8 for T1D men.
Mitochondrial Ca2+ tolerance.
As can be seen in Fig. 3F (left), the ability of the mitochondria to retain Ca2+ (i.e., mCRC) before opening of the mitochondrial permeability transition pore (marker of susceptibility to cell death) was comparable between sexes in the control (P = 0.822) and the T1D group (P = 0.634). Similarly, the delta was not different between sexes (P = 0.802) (Fig. 3F, right).
Skeletal Muscle Mitochondrial Content
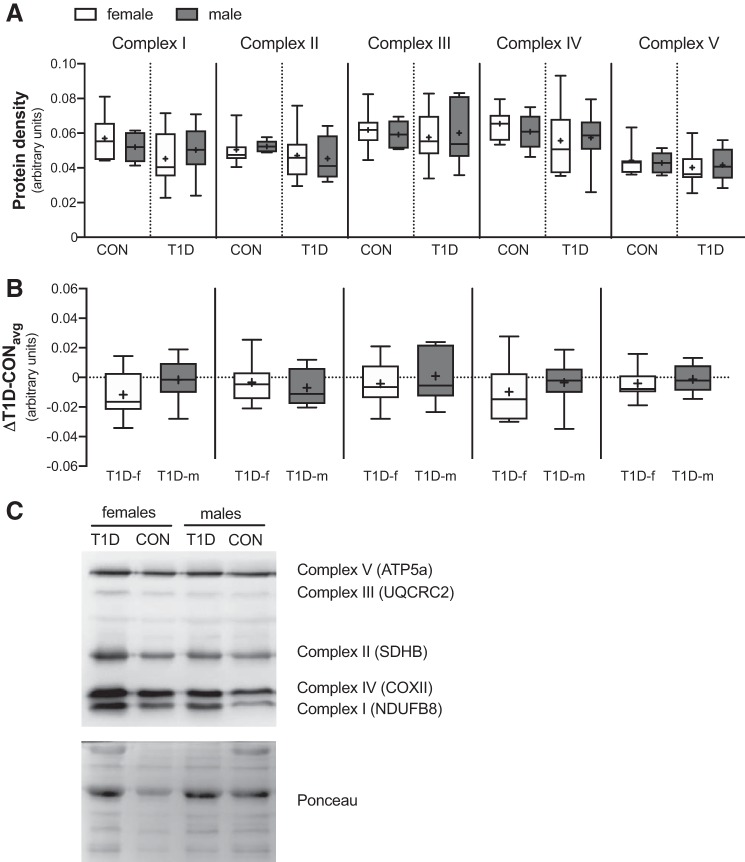
Protein expression of complexes I through V of the electron transport chain.
To ensure that the differences in mitochondrial bioenergetics were not due to changes to the content of the individual complexes of the electron transport chain, we next measured the protein expression of complexes I through V. As shown in Fig. 4A, in the control group, no differences were observed in complex I (P = 0.474), complex II (P = 0.689), complex III (P = 0.676), complex IV (P = 0.435), or complex V (P = 0.786). Similarly, in the T1D group, no differences were observed in complex I (P = 0.512), complex II (P = 0.774), complex III (P = 0.752), complex IV (P = 0.849), or complex V (P = 0.775). As expected, the delta was also not different between sexes (Fig. 4B) [complex I (P = 0.198), complex II (P = 0.564), complex III (P = 0.523), complex IV (P = 0.490), or complex V (P = 0.588)]. Combined, these data suggest that skeletal muscle mitochondrial content is similar between sexes irrespective of T1D status.
Fig. 4.
Skeletal muscle mitochondrial content in control (CON) and type 1 diabetes (T1D) women (f) and men (m). (A) Protein expression of the individual complexes of the electron transport chain (I through V) were individually quantified by densitometry. (B) Protein density absolute change from each T1D participant relative to the average of their sex-matched control (△T1D-CONavg). (C) Representative immunoblot of Complex I (~18 kDa), Complex II (~29 kDa), Complex III (~48 kDa), Complex IV (~22 kDa) and Complex V (~54 kDa) and Ponceau stain for loading control. Boxes represent the interquartile range, whiskers show the maximum and minimum, the horizontal line indicates the median and the cross indicates the mean. ATP5a, ATP synthase subunit alpha 5; COXII, cytochrome oxidase subunit II; NDUFB8, NADH:ubiquinone oxidoreductase subunit B8; SDHB, succinate dehydrogenase [ubiquinone] iron-sulfur subunit B; UQCRC2, ubiquinol-cytochrome c reductase core protein 2. n = 7 for CON women; n = 5 for CON men; n = 10 for T1D women; n = 8 for T1D men.
Conclusions
This study is the first to reveal site-specific differences in skeletal muscle mitochondrial respiratory function and mH2O2 emission between young adult women and men with T1D that have moderately well-controlled glycemia. Importantly, unexpectedly, these findings indicate that T1D alters skeletal muscle mitochondrial respiratory function to a greater extent in men compared with women despite women exhibiting a higher hemoglobin A1c (Table 1). This study also extends our current understanding of sexual dimorphism and similarities in mitochondrial bioenergetics in young, healthy people (control).
Sexual dimorphism in skeletal muscle metabolism has long been identified in the healthy (control) population, where women rely on fat oxidation to a greater extent than men during prolonged exercise (14). It was not until more recently that studies began exploring the possibility of specific differences between women and men in humans with respect to skeletal muscle mitochondrial phenotype and oxidative capacity. Although disparate results exist between these studies (1, 10, 12), owing in part to different methodologies and protocols employed, these reports nonetheless led us to reflect on our previous study that systematically assessed mitochondrial bioenergetics in both women and men with T1D compared with controls (11). Thus, by increasing subject number, we sought to identify whether T1D differentially impacts skeletal muscle mitochondria in women compared men. Our experimental approach was similar to that of Miotto et al. (10), and, consistent with this work, we observed lower mitochondrial ADP-stimulated respiration at submaximal ADP but not maximal ADP concentrations (oxidative capacity) in women compared with men in both the control and T1D groups. This indicates that 1) mitochondria are less sensitive to ADP at rest in women (control and T1D) and 2) T1D does not differentially impact ADP-stimulated respiration between sexes. We also observed significantly lower complex I-supported respiration in control women compared with control men when stimulated with maximal glutamate concentrations but not maximal pyruvate concentrations, suggesting sexual dimorphism in upstream dehydrogenase capacity. In the T1D group, when the delta was calculated from absolute rates of mitochondrial respiration, men displayed lower complex II-supported respiration (both submaximal and maximal succinate) but greater complex I-supported respiration in the presence of either submaximal glutamate or submaximal pyruvate concentrations compared with women. Combined, these observations suggest enhanced complex I sensitivity/respiratory function in men with T1D, potentially as compensation for deficits in complex II function.
Additionally, we demonstrate for the first time that in healthy controls, mCRC and site-specific mH2O2 emission are generally similar between women and men. In people with T1D, mCRC was also similar between sexes, whereas sex differences in mH2O2 emission rates were observed at complex I (succinate driven), where women demonstrated lower emission rates compared with men. These findings guide future investigations to examine the impact of these altered emission rates on antioxidant, cellular oxidative stress, and redox signaling networks related to metabolic homeostasis. For example, women may be particularly capable of preventing oxidative stress in T1D through the combined effects of lower H2O2 emission kinetics seen at complex I (succinate driven) in the present study and the greater and higher expression and content of antioxidants in women reported in other studies [e.g., increased glutathione levels and superoxide dismutase (7)].
Lastly, it is important to highlight that these sex-specific alterations in mitochondrial bioenergetics in T1D could impact how women and men with T1D respond and adapt to exercise and/or exercise training (e.g., metabolic responses to exercise), which, in turn, has the potential to greatly influence glucose homeostasis between the sexes during and after exercise (16). For example, the greater reliance on carbohydrate oxidation, and hence glycogen, reported in healthy and T1D men compared with women during exercise can increase the likelihood of postexercise hypoglycemia in T1D men. Therefore, alterations in mitochondrial respiratory function would not only reduce cellular ATP and hence, exercise efficiency, but also likely further increase reliance on glycolysis and, consequently, further depletion of glycogen stores. Ultimately, we expect this would amplify the risk of postexercise hypoglycemia in T1D men. Future studies are certainly warranted to address these important questions.
Altogether, our study revealed sexual dimorphism in mitochondrial bioenergetic adaptations to T1D that, to our surprise, were not worse in women compared with men and highlights the need for larger cohort studies with an expanded age range to fully tease out sex differences in skeletal muscle metabolism. Given the importance of skeletal muscle to our physical and metabolic wellbeing, maintaining the health of this organ system is of primary importance in this metabolic disease.
GRANTS
This project was funded by the Natural Sciences and Engineering Research Council of Canada (NSERC; Grants 2018-06324 and 2019-522456 to T.J.H. and Grant 2019-06687 to C.G.R.P.) and the James H. Cummings Foundation (to C.G.R.P.). C.M.F.M., C.A.B., and M.C.H. were recipients of NSERC graduate scholarships. C.M.F.M. was also a recipient of the DeGroote Doctoral Scholarship of Excellence. S.V.R. was a recipient of the Ontario Graduate Scholarship.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
C.M.F.M., R.L., C.G.P., and T.J.H. conceived and designed research; C.M.F.M., C.A.B., M.C.H., S.V.R., and C.G.P. performed experiments; C.M.F.M., C.A.B., C.G.P., and T.J.H. analyzed data; C.M.F.M., M.C.H., C.G.P., and T.J.H. interpreted results of experiments; C.M.F.M. prepared figures; C.M.F.M. and T.J.H. drafted manuscript; C.M.F.M., C.A.B., M.C.H., S.V.R., R.L., C.G.P., and T.J.H. edited and revised manuscript; C.M.F.M., C.A.B., M.C.H., S.V.R., R.L., C.G.P., and T.J.H. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors express their sincerest gratitude to the participants for their contributions (time and tissue) to this research study.
REFERENCES
- 1.Cardinale DA, Larsen FJ, Schiffer TA, Morales-Alamo D, Ekblom B, Calbet JAL, Holmberg HC, Boushel R. superior intrinsic mitochondrial respiration in women than in men. Front Physiol 9: 1133, 2018. doi: 10.3389/fphys.2018.01133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dabelea D, Kinney G, Snell-Bergeon JK, Hokanson JE, Eckel RH, Ehrlich J, Garg S, Hamman RF, Rewers M; Coronary Artery Calcification in Type 1 Diabetes Study . Effect of type 1 diabetes on the gender difference in coronary artery calcification: a role for insulin resistance? The Coronary Artery Calcification in Type 1 Diabetes (CACTI) Study. Diabetes 52: 2833–2839, 2003. doi: 10.2337/diabetes.52.11.2833. [DOI] [PubMed] [Google Scholar]
- 3.Gupte AA, Pownall HJ, Hamilton DJ. Estrogen: an emerging regulator of insulin action and mitochondrial function. J Diabetes Res 2015: 916585, 2015. doi: 10.1155/2015/916585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heyman E, Daussin F, Wieczorek V, Caiazzo R, Matran R, Berthon P, Aucouturier J, Berthoin S, Descatoire A, Leclair E, Marais G, Combes A, Fontaine P, Tagougui S. Muscle oxygen supply and use in type 1 diabetes, from ambient air to the mitochondrial respiratory chain: is there a limiting step? Diabetes Care, in press. doi: 10.2337/dc19-1125. [DOI] [PubMed] [Google Scholar]
- 5.Huxley R, Barzi F, Woodward M. Excess risk of fatal coronary heart disease associated with diabetes in men and women: meta-analysis of 37 prospective cohort studies. BMJ 332: 73–78, 2006. doi: 10.1136/bmj.38678.389583.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huxley RR, Peters SAE, Mishra GD, Woodward M. Risk of all-cause mortality and vascular events in women versus men with type 1 diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrinol 3: 198–206, 2015. doi: 10.1016/S2213-8587(14)70248-7. [DOI] [PubMed] [Google Scholar]
- 7.Kander MC, Cui Y, Liu Z. Gender difference in oxidative stress: a new look at the mechanisms for cardiovascular diseases. J Cell Mol Med 21: 1024–1032, 2017. doi: 10.1111/jcmm.13038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Manicardi V, Russo G, Napoli A, Torlone E, Li Volsi P, Giorda CB, Musacchio N, Nicolucci A, Suraci C, Lucisano G, Rossi MC; AMD Annals Study Group . Gender-disparities in adults with type 1 diabetes: more than a quality of care issue. a cross-sectional observational study from the AMD Annals Initiative. PLoS One 11: e0162960, 2016. doi: 10.1371/journal.pone.0162960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Millstein RJ, Pyle LL, Bergman BC, Eckel RH, Maahs DM, Rewers MJ, Schauer IE, Snell-Bergeon JK. Sex-specific differences in insulin resistance in type 1 diabetes: The CACTI cohort. J Diabetes Complications 32: 418–423, 2018. doi: 10.1016/j.jdiacomp.2018.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miotto PM, McGlory C, Holloway TM, Phillips SM, Holloway GP. Sex differences in mitochondrial respiratory function in human skeletal muscle. Am J Physiol Regul Integr Comp Physiol 314: R909–R915, 2018. doi: 10.1152/ajpregu.00025.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Monaco CMF, Hughes MC, Ramos SV, Varah NE, Lamberz C, Rahman FA, McGlory C, Tarnopolsky MA, Krause MP, Laham R, Hawke TJ, Perry CGR. Altered mitochondrial bioenergetics and ultrastructure in the skeletal muscle of young adults with type 1 diabetes. Diabetologia 61: 1411–1423, 2018. doi: 10.1007/s00125-018-4602-6. [DOI] [PubMed] [Google Scholar]
- 12.Montero D, Madsen K, Meinild-Lundby A-K, Edin F, Lundby C. Sexual dimorphism of substrate utilization: Differences in skeletal muscle mitochondrial volume density and function. Exp Physiol 103: 851–859, 2018. doi: 10.1113/EP087007. [DOI] [PubMed] [Google Scholar]
- 13.Szadkowska A, Madej A, Ziółkowska K, Szymańska M, Jeziorny K, Mianowska B, Pietrzak I. Gender and age-dependent effect of type 1 diabetes on obesity and altered body composition in young adults. Ann Agric Environ Med 22: 124–128, 2015. doi: 10.5604/12321966.1141381. [DOI] [PubMed] [Google Scholar]
- 14.Tarnopolsky LJ, MacDougall JD, Atkinson SA, Tarnopolsky MA, Sutton JR. Gender differences in substrate for endurance exercise. J Appl Physiol (1985) 68: 302–308, 1990. doi: 10.1152/jappl.1990.68.1.302. [DOI] [PubMed] [Google Scholar]
- 15.Ventura-Clapier R, Piquereau J, Veksler V, Garnier A. Estrogens, estrogen receptors effects on cardiac and skeletal muscle mitochondria. Front Endocrinol (Lausanne) 10: 557, 2019. doi: 10.3389/fendo.2019.00557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yardley JE, Brockman NK, Bracken RM. Could age, sex and physical fitness affect blood glucose responses to exercise in type 1 diabetes? Front Endocrinol (Lausanne) 9: 674, 2018. doi: 10.3389/fendo.2018.00674. [DOI] [PMC free article] [PubMed] [Google Scholar]