Abstract
Despite remission of clinical symptoms postpartum, women who have had preeclampsia demonstrate microvascular endothelial dysfunction, mediated in part by increased sensitivity to angiotensin II (ANG II). Angiotensin-(1–7) [Ang-(1–7)] is an endogenous inhibitor of the actions of ANG II and plausible druggable target in women who had preeclampsia. We therefore examined the therapeutic potential of Ang-(1–7) in the microvasculature of women with a history of preeclampsia (PrEC; n = 13) and parity-matched healthy control women (HC; n = 13) hypothesizing that administration of Ang-(1–7) would increase endothelium-dependent dilation and nitric oxide (NO)-dependent dilation and decrease ANG II-mediated constriction in PrEC. Using the cutaneous microcirculation, we assessed endothelium-dependent vasodilator function in response to graded infusion of acetylcholine (ACh; 10−7 to 102 mmol/L) in control sites and sites treated with 15 mmol/L NG-nitro-l-arginine methyl ester (l-NAME; NO-synthase inhibitor), 100 µmol/L Ang-(1–7), or 15 mmol/L l-NAME + 100 µmol/L Ang-(1–7). Vasoconstrictor function was measured in response to ANG II (10−20-10−4 mol/L) in control sites and sites treated with 100 µmol/L Ang-(1–7). PrEC had reduced endothelium-dependent dilation (P < 0.001) and NO-dependent dilation (P = 0.04 vs. HC). Ang-(1–7) coinfusion augmented endothelium-dependent dilation (P < 0.01) and NO-dependent dilation (P = 0.03) in PrEC but had no effect in HC. PrEC demonstrated augmented vasoconstrictor responses to ANG II (P < 0.01 vs. HC), which was attenuated by coinfusion of Ang-(1–7) (P < 0.001). Ang-(1–7) increased endothelium-dependent vasodilation via NO synthase-mediated pathways and attenuated ANG II-mediated constriction in women who have had preeclampsia, suggesting that Ang-(1–7) may be a viable therapeutic target for improved microvascular function in women who have had a preeclamptic pregnancy.
Keywords: angiotensin 1-7, angiotensin II, endothelial dysfunction, microvascular, preeclampsia
INTRODUCTION
Cardiovascular disease (CVD) is the leading cause of death among women worldwide and women who develop preeclampsia during pregnancy are at a significantly greater risk for the development of CVD (4, 37). These women suffer from CVD at a younger age and with greater frequency than women who have healthy pregnancies (3, 15, 21, 37) and are more likely to die of CVD (4, 6, 46a). Because of this association between preeclampsia history and CVD morbidity and mortality, women who have had preeclampsia represent an important at-risk cohort that require early mechanism-specific intervention strategies to prevent or mitigate the accelerated progression of CVD.
Preeclampsia, a hypertensive disorder of pregnancy, affects ~7–10% of pregnancies in the United States and ~8 million pregnancies worldwide. Preeclampsia is characterized by new onset hypertension with either proteinuria or, in the absence of proteinuria, new onset of thrombocytopenia, renal insufficiency, abnormal liver function, pulmonary edema, and/or cerebral or visual symptoms after 20 wk of gestation (5). While the association between preeclampsia during pregnancy and elevated lifetime CVD risk is apparent, the mechanism(s) responsible for this association remain unclear. We have recently demonstrated that otherwise healthy women with a history of preeclampsia have attenuated microvascular endothelium-dependent dilation, mediated in part by reductions in nitric oxide (NO)-dependent dilation and exaggerated vasoconstrictor sensitivity to angiotensin II (ANG II) (50). Furthermore, we found that localized inhibition of angiotensin type-1 receptors (AT1R) rescued endothelial function and improved NO-dependent dilation in these women. As such, strategies that inhibit or attenuate the actions of ANG II in the vasculature may be viable interventional approaches for the treatment of residual vascular dysfunction and the prevention of overt CVD in women who have had a preeclamptic pregnancy.
Recently, counterregulatory components of the renin-angiotensin system (RAS) have been identified as endogenously occurring inhibitors or competitors of the vasoconstrictor mechanisms of the classical RAS and have been put forward as viable druggable targets for hypertension and other forms of CVD (26, 40, 43). Specifically, the angiotensin 1–7 [Ang-(1–7]/Mas receptor pathway triggers vasodilation, inhibits cell growth, and has anti-inflammatory (33, 65) and antithrombotic (17, 18) effects. Ang-(1–7) inhibits ANG II-induced vasoconstriction in human arteries (35) and, collectively, is an endogenous inhibitor of the intracellular actions of ANG II/AT1R (42, 61, 64, 65). Consequently, interventions increasing bioavailable Ang-(1–7) have emerged as novel therapeutic strategies in conditions of endothelial dysfunction, including essential hypertension (38, 62, 64).
Given the role of increased AT1R signaling in persistent vascular dysfunction following preeclampsia, the actions of Ang-(1–7) may be an unexplored mechanistic target directed at improving endothelial function in women who have had a preeclamptic pregnancy. However, to date, no mechanistic studies exploring this potential have been performed. Therefore, the purpose of this study was to systematically examine the therapeutic potential of increasing Ang-(1–7) on endothelial function and ANG II-mediated constriction in the cutaneous microvasculature of women with a history of preeclampsia compared with control women with a history of healthy pregnancy. Given our previous findings that a history of preeclampsia (50) is associated with 1) reduced endothelium-dependent dilation, 2) reduced NO-mediated dilation, and 3) increased ANG II-mediated constriction, we hypothesized that localized Ang-(1–7) treatment would increase endothelium-dependent dilation in these women and that this increase would be mediated by an increase in NO-dependent dilation. We further hypothesized that localized Ang-(1–7) treatment would prevent the exaggerated ANG II-mediated constriction in women with a history of preeclampsia.
METHODS
Study Population
Twenty-six healthy normotensive postpartum women who had delivered within the past 17 mo (range: 1–17 mo) participated in the study. Thirteen of these women had a history of preeclampsia diagnosed by their obstetrician and confirmed according to the American College of Obstetricians and Gynecologists criteria for severe preeclampsia (PrEC) (1). Six of the 13 preeclamptic patients (46%) had early onset preeclampsia (diagnosed <34 wk of gestation) and 1 participant was diagnosed with HELLP syndrome. The other 13 subjects had a history of healthy pregnancy and served as controls (HC). Experimental protocols were approved by the Institutional Review Board of The Pennsylvania State University and the U.S. Food and Drug Administration. Written and verbal consent were obtained voluntarily from all subjects before participation according to the Declaration of Helsinki and the U.S. Code of Federal Regulations, Part 46 (NCT03482440). Subjects were screened for neurological, cardiovascular, and metabolic diseases and underwent a complete medical screening including physical examination, lipid profile, and blood chemistry (Quest Diagnostics, Pittsburgh, PA). All subjects were normally active, nonhypertensive at the time of testing, nondiabetic, and healthy nonsmokers who were not taking prescription medications with primary or secondary vascular effects (e.g., statins, antihypertensives, anticoagulants, etc.). Five women (3 HC/2 PrEC) were taking oral contraceptives. Not all women were normally cycling, and subjects were tested without consideration for phase of the menstrual cycle. All women met the inclusion criteria of no history of gestational diabetes and no history of hypertension [systolic blood pressure ≥140 mmHg and/or diastolic blood pressure ≥90 mmHg (25)] before pregnancy. Subjects were matched for time postpartum (months), body mass index (BMI), and blood chemistry. Subject characteristics are presented in Table 1.
Table 1.
Subject characteristics
| HC (n = 13) | PrEC (n = 13) | P Value | |
|---|---|---|---|
| Age, yr | 32 ± 1 | 26 ± 2 | 0.003 |
| Time postpartum, mo | 6 ± 1 | 7 ± 1 | 0.47 |
| MAP, mmHg | 88 ± 2 | 91 ± 2 | 0.43 |
| SBP, mmHg | 116 ± 2 | 121 ± 4 | 0.25 |
| DBP, mmHg | 74 ± 2 | 76 ± 2 | 0.70 |
| BMI, kg/m2 | 27 ± 1 | 33 ± 3 | 0.06 |
| Total cholesterol, mg/dL | 202 ± 10 | 197 ± 15 | 0.98 |
| HDL, mg/dL | 64 ± 4 | 50 ± 3 | 0.01 |
| LDL, mg/dL | 113 ± 9 | 118 ± 10 | 0.74 |
| BUN, mg/dL | 13 ± 1 | 14 ± 1 | 0.52 |
| Creatinine, mg/dL | 0.8 ± 0.1 | 0.7 ± 0.1 | 0.40 |
| BUN-to-creatinine ratio | 170.1 ± 1.1 | 19.1 ± 1.3 | 0.25 |
| HbA1c, % | 4.9 ± 0.1 | 5.1 ± 0.1 | 0.07 |
Values are means ± SE. MAP, mean arterial pressure; SBP, systolic blood pressure; DBP, diastolic blood pressure; BMI, body mass index; HC, women with a history of normal pregnancy; HDL, high-density lipoprotein; LDL, low-density lipoprotein; BUN, blood urea nitrogen; PrEC, women who have had preeclampsia.
In Vivo Microvascular Reactivity Studies
Each subject participated in one laboratory visit that lasted ~3.5 h. Intradermal microdialysis fibers (CMA, Holliston, MA) were placed into the dermal layer of the ventral forearm for the local delivery of pharmacological agents (9). All six microdialysis fibers were placed in the same arm. Fibers were placed ≥2.5-cm distance apart to ensure no cross reactivity of specific pharmacological agents. The human cutaneous microcirculation serves as a model for generalized microvascular function and is a validated in vivo bioassay of systemic microvascular function (11, 14, 22, 29, 31, 57). Pharmacological agents were mixed just before use, dissolved in lactated Ringer’s solution, sterilized using syringe microfilters (Acrodisc; Pall, Ann Arbor, MI), wrapped in foil to prevent degradation due to light exposure, and perfused through the microdialysis fibers at a rate of 2 µL/min (Bee Hive controller and Baby Bee microinfusion pumps; Bioanalytical Systems). The concentrations of the pharmacological agents utilized were determined based on previous work utilizing intradermal microdialysis and pilot studies to establish efficacy in our model. Each protocol commenced after an initial hyperemia-resolution period (~60–90 min). Cutaneous red blood cell flux was continually measured directly over each microdialysis site with an integrated laser-Doppler flowmetry probe placed in a local heating unit (Moor Instruments SHO2), which was set to thermoneutral (33°C) unless otherwise noted. Automated brachial blood pressure (Cardiocap; GE Healthcare, Milwaukee, WI) was measured every 5 min throughout each protocol.
Cutaneous vasodilator responsiveness to exogenous acetylcholine.
Four intradermal microdialysis fibers were placed and randomly assigned for the delivery of lactated Ringer (control), 15 mmol/L NG-nitro-l-arginine methyl ester (l-NAME; Calbiochem, EMD Millipore, Billerica, MA) for the inhibition of nitric oxide synthase (NOS) (50), 100 μmol/L Ang-(1–7) (52) (Tocris, Ellisville, MO), or 15 mmol/L l-NAME + 100 μmol/L Ang-(1–7).
Acetylcholine dose-response protocol.
After baseline measurements, ascending concentrations of acetylcholine (ACh; 10−7–102 mmol/L; USP, Rockville, MD), mixed with the site-specific treatment, were perfused sequentially for 5 min each to ensure a plateau. After the ACh doses, 28 mmol/L sodium nitroprusside (USP) was perfused and local temperature increased to 43°C to elicit maximal dilation (CVCmax) (27).
Cutaneous vasoconstrictor responsiveness to exogenous ANG II.
Two intradermal microdialysis fibers were placed and randomly assigned for the local delivery of lactated Ringer or 100 μmol/L Ang-(1–7). After the baseline measurements, sites received ascending concentrations of ANG II (10−20–10−4 mol/L; Tocris) mixed with the site-specific treatment. Doses were perfused sequentially for 5 min each to ensure a plateau.
Data and Statistical Analysis
All data collection and analysis procedures were standardized before testing. Blood flow data were digitized at 40 Hz, recorded, and stored for offline analysis using Windaq software and Dataq data acquisition system (Datq Instruments, Akron, OH). Cutaneous vascular conductance was calculated (CVC = laser Doppler flux/mean arterial pressure) and normalized to a percentage of site-specific maximum (% max) for dilation and site-specific baseline (% base) for vasoconstriction. Dose-response CVC data were analyzed using sigmoidal dose-response curve with variable slope as previously described (58). NO-dependent dilation was calculated for each subject at the control site [area under the curve (AUC) Ringer-AUC l-NAME] and the Ang-(1–7)-treated site [AUC Ang-(1–7)-AUC Ang-(1–7) + l-NAME].
Sample size was determined a priori by power analysis (power = 0.80, α = 0.05) using previously published data with similar primary outcomes in subjects with microvascular dysfunction (47, 53) and significance was set a priori at α = 0.05. CVC data were analyzed using a two-way repeated measures ANOVA (group × pharmacological site; SAS 9.4, Cary, IN) adjusted for BMI and HDL with post hoc Bonferroni corrections applied for specific planned comparisons when appropriate. Values are presented as means ± SE.
RESULTS
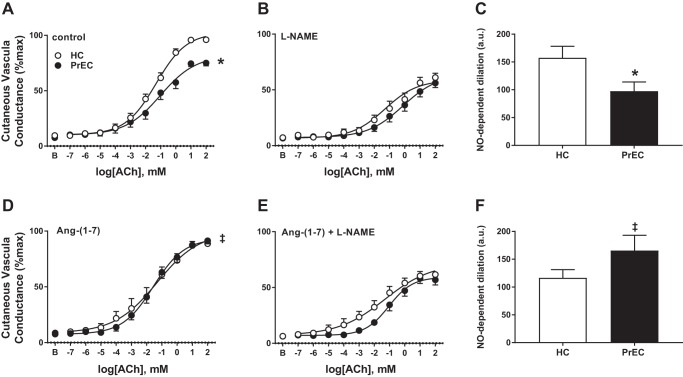
There were no group or site differences in baseline or maximal CVC (all P > 0.05). Figure 1 presents cutaneous vasodilation (cutaneous conductance, %maximal) responses to acetylcholine in control (Fig. 1A), NOS-inhibited (l-NAME, Fig 1B), Ang-(1–7)-treated (Fig. 1D), and NOS-inhibited + Ang-(1–7)-treated (Fig. 1E) sites, and NO-dependent dilation (arbitrary units) in control (Fig. 1C) and Ang-(1–7)-treated (Fig. 1F) sites in women who had a healthy pregnancy (HC) and women who had preeclampsia (PrEC). Table 2 presents the curve modeling parameters for these sites. PrEC had an attenuated vasodilation response to acetylcholine (P < 0.001 main effect of group) and attenuated NO-dependent dilation (P = 0.04) compared with HC in the control site. Ang-(1–7) treatment increased cutaneous vasodilation responses (P < 0.01 main effect of site within PrEC) and NO-dependent dilation (P = 0.03) compared with the control site in PrEC but had no effect in HC (P > 0.05 for both). There were no differences between groups or across sites at the NOS-inhibited or the NOS-inhibited + Ang-(1–7)-treated sites (all P > 0.05).
Fig. 1.
Data are means ± SE of vasodilation (cutaneous conductance, %max) responses to acetylcholine in control (A), NO synthase (NOS)-inhibited [NG-nitro-l-arginine methyl ester (l-NAME; B)], angiotensin-(1–7) [Ang-(1–7)]-treated (D), and NOS-inhibited + Ang-(1–7)-treated (E) sites in postpartum women who have had a healthy pregnancy (HC) and women who have had preeclampsia (PrEC). Data are means ± SE of nitric oxide (NO)-dependent dilation (Δarea under the curve) responses in control (C) and Ang-(1–7)-treated (F) sites; a.u., arbitrary units. *P < 0.05, PrEC vs. HC. ‡P < 0.05, vs. control within PrEC.
Table 2.
Parameters of acetylcholine dose-response curves in control, NOS-inhibited (l-NAME), Ang-(1–7)-treated, and Ang-(1–7)-treated + NOS-inhibited sites
| Control | l-NAME | Ang-(1–7) | Ang-(1–7) + l-NAME | |
|---|---|---|---|---|
| HC | ||||
| Minimum | 9.9 ± 1.7 | 10.9 ± 1.5 | 8.4 ± 3.9 | 11.8 ± 1.8 |
| logEC50 | −1.4 ± 0.1 | −1.0 ± 0.2 | −1.5 ± 0.4 | −1.9 ± 0.2 |
| Maximum | 101.3 ± 3.6 | 55.0 ± 2.9 | 96.7 ± 9.2 | 57.2 ± 2.3 |
| PrEC | ||||
| Minimum | 9.0 ± 2.4 | 9.4 ± 1.3 | 7.4 ± 2.0 | 8.3 ± 1.2 |
| logEC50 | −1.0 ± 0.3 | −0.7 ± 0.2 | −1.6 ± 0.2 | −1.1 ± 0.1 |
| Maximum | 82.6 ± 7.3* | 50.6 ± 2.3 | 93.4 ± 3.9‡ | 55.4 ± 2.0 |
Values are means ± SE. Ang-(1–7), angiotensin-(1–7); HC, women with a history of normal pregnancy; l-NAME, NG-nitro-l-arginine methyl ester; PrEC, women who have had preeclampsia.
P < 0.05, PrEC vs. HC within the control site.
P < 0.05, Ang-(1–7)-treated vs. control within group.
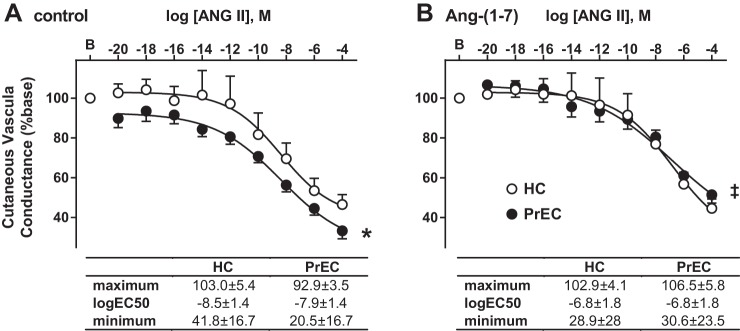
Figure 2 presents cutaneous vasoconstriction (cutaneous conductance, %baseline) responses, and the curve modeling parameters of these responses, to ANG II in control (Fig. 2A) and Ang-(1–7)-treated (Fig. 2B) sites in HC and PrEC. PrEC had greater vasoconstriction responses to ANG II at the control site compared with HC (P < 0.01 main effect of group). Ang-(1–7) treatment decreased vasoconstriction responses compared with the control site in PrEC (P < 0.001 main effect of site within PrEC) but had no effect in HC (P = 0.7).
Fig. 2.
Data are means ± SE vasoconstriction (cutaneous conductance, %base) responses to angiotensin II (ANG II) in control (A) and angiotensin-(1–7) [Ang-(1–7)]-treated (B) sites in postpartum women who have had a healthy pregnancy (HC) and women who have had preeclampsia (PrEC). *P < 0.05, PrEC vs HC. ‡P < 0.05, vs. control within PrEC.
In our participants, PrEC had significantly lower HDL and, although not different between groups, were in a higher BMI category (obese vs. overweight) compared with HC (Table 1). To examine the potential for BMI and HDL to influence endothelium-dependent dilation responses, these variables were included as covariates in our statistical model. There was no main effect of HDL on ACh-mediated CVC responses, total AUC, or NO-dependent dilation in the control sites suggesting that group differences in HDL did not contribute to attenuated endothelium-dependent dilation in PrEC. There was no main effect of BMI on CVC responses or total AUC in the control sites. BMI did significantly influence NO-dependent dilation responses (P = 0.03); however, after adjustment for BMI, the main effect of group remained (P = 0.004). Similar to endothelium-dependent dilation, adjustment for BMI and HDL in the statistical model did not yield main effects for either covariate for CVC responses to angiotensin II, and the main effect of group remained after adjustment for these variables.
DISCUSSION
The primary finding of this study was that locally administered Ang-(1–7) augmented endothelium-dependent dilation via NO-dependent mechanisms and attenuated ANG II-mediated constriction, in the microvasculature of women who have had a preeclamptic pregnancy. Together, these findings suggest that increasing Ang-(1–7) bioavailability mitigates residual microvascular dysfunction in postpartum women who have had preeclampsia and that interventional approaches that target Ang-(1–7)/Mas may be viable strategies for the treatment and/or prevention of peripheral vascular dysfunction in these women.
Despite the remission of the clinical symptoms of preeclampsia after delivery, women with a history of preeclamptic pregnancy are at a significantly (2–4 times) greater risk for the development of CVD (7). These women develop primary hypertension at a younger age (~30–40 yr. of age vs. ~50–60 yr. in women who have a normal pregnancy) and with greater frequency than women who have healthy pregnancies (3, 19, 21, 37), and they are significantly more likely to die of stroke, myocardial infarction, and end-stage renal disease (4, 6, 46a). This increased risk is likely explained, at least in part, by residual vascular dysfunction that persists postpartum (20, 48). Several studies demonstrate that subclinical indices of vascular dysfunction persist for weeks, months, and even years after the affected pregnancy, even in the absence of traditional CVD risk factors (2, 8, 15, 16, 34, 54). We recently demonstrated that endothelium-dependent dilation is attenuated in the microvasculature of otherwise healthy women who have had preeclampsia and that increased sensitivity to endogenous vasoconstrictors ANG II and endothelin-1 contributes to a proconstrictor milieu in this cohort (49, 50). As such, interventional approaches that target these mechanisms may prevent or slow the progression of overt CVD in this group of women with elevated risk. Our current results suggest that amplification of Ang-(1–7)/Mas signaling may represent a mechanism-specific strategy to achieve this goal.
Amplification of Ang-(1–7)/Mas signaling opposes the classical RAS, and systemic treatment with Ang-(1–7) lowers blood pressure, improves endothelial function, and attenuates the progression of atherosclerosis in animal models of hypertension and atherosclerotic vascular disease (13, 26). Few studies have examined these mechanisms in vivo in humans. However, those that have suggest that amplification of this axis may play a vasoprotective role in human hypertension and obesity-associated vascular dysfunction (44, 46). During healthy pregnancy plasma Ang-(1–7) concentrations increase (36, 39, 55). In contrast, plasma Ang-(1–7) concentrations are significantly decreased in preeclampsia (10, 36, 56), such that there is a negative correlation between plasma Ang-(1–7) and both systolic and diastolic blood pressure (36). These findings support an association for Ang-(1–7) in the vascular adjustments that accompany pregnancy and suggest that reductions in circulating Ang-(1–7) may contribute to vascular dysfunction during and persisting after a preeclamptic pregnancy. However, to date, no mechanistic studies examining Ang-(1–7) have been performed in postpartum women following preeclamptic pregnancy.
To examine the potential of increasing Ang-(1–7) bioavailability in improved endothelium-dependent dilation responses in women who have had preeclampsia, we locally administered Ang-(1–7) during assessment of endothelium- and NO-dependent dilation. We found that local administration of Ang-(1–7) improved endothelium-dependent dilation in women who have had preeclampsia via NO-dependent mechanisms but had no effect in our control group. These data agree with previous findings that Ang-(1–7)/Mas activation improves endothelial function in hypertensive humans and animal models of vascular disease and align with our prior study in which pharmaceutical inhibition of the classical RAS (local administration of losartan) improved endothelium- and NO-dependent dilation in the microvasculature of women with a history of preeclampsia (50).
Women who develop preeclampsia have an exaggerated pressor response to ANG II during pregnancy (12, 32, 59) and postpartum (45), implicating overactivation of the classical RAS in the vascular dysfunction during pregnancy and postpartum in women who develop preeclampsia. To assess the potential for increasing Ang-(1–7) bioavailability to mitigate this exaggerated responsiveness, we coinfused exogenous Ang-(1–7) during assessment of ANG II-mediated vasoconstrictor responses in the current study. We found that locally increasing Ang-(1–7) bioavailability attenuated the vasoconstrictor response to ANG II in women with a history of preeclampsia but did not affect the responses of women with a history of healthy pregnancy, such that there was no difference in responses between the groups when Ang-(1–7) was administered. These findings suggest that the opposing actions of Ang-(1–7) on ANG II-mediated constriction ameliorates the exaggerated constrictor responses following preeclampsia.
Previously we have shown that acute pharmacological inhibition of the AT1R augments endothelium-dependent dilation via improved NO-dependent dilation in women with a history of preeclampsia (50). These data, coupled with our finding that NO synthesis is not impaired in these women, suggest that tonic constrictor tone mediated by exaggerated responses to ANG II inhibits endothelium-dependent dilation following a preeclamptic pregnancy. In the present study, we utilized a dose of Ang-(1–7) that did not influence basal vascular tone to isolate the potential for Ang-(1–7) to oppose ANG II-mediated constriction, rather than induce NO-dependent dilation. Importantly, we did not observe differences in baseline blood flow between Ang-(1–7)-treated and Ang-(1–7) + l-NAME-treated sites, confirming that our exogenous delivery of Ang-(1–7) was not inducing NO-dependent dilation. We demonstrate that Ang-(1–7) restores endothelium-dependent dilation and ANG II-mediated constrictor responses similar to those observed in our control group, presumably by releasing exaggerated constrictor tone, similar to pharmacological AT1R blockade. However, Ang-(1–7)/Mas receptor binding does induce NO synthesis in vascular endothelial cells, and exogenous Ang-(1–7) administration attenuates blood pressure and prevents atherosclerotic plaque formation via NO-dependent mechanisms in murine models of vascular disease (60, 63). While further investigation of the potential for Ang-(1–7) to directly induce NO-dependent dilation in the microvasculature of women who have had preeclampsia is warranted, our in vivo data are the first to identify Ang-(1–7) as a viable interventional approach to mitigate microvascular dysfunction in this population.
Our data demonstrate that acute, local perfusion of Ang-(1–7) improves endothelium-dependent dilation and reduces ANG II-mediated constriction in the microvasculature of women who have had preeclampsia, suggesting that increased circulating Ang-(1–7) is clinically relevant in this population. However, native Ang-(1–7) has been limited in its pharmacological administration in chronic human studies because of its elimination by the first pass effect when administered orally and its short half-life in vivo. Alternatively, synthetic agonists of the Mas receptor have been developed and tested in animal models but have yet to be administered in controlled human trials. Similarly, activators of angiotensin-converting enzyme (ACE) 2 have been proposed and tested in animal models as a means to increase endogenously synthesized circulating Ang-(1–7) but have yet to be tested in human. As an alternative to directly administering Ang-(1–7) or Ang-(1–7) analogues, inhibitors of the classical RAS, including ACE inhibitors and angiotensin receptor blockers, increase circulating Ang-(1–7) (23, 24). Our previous findings suggest that angiotensin receptor blockers may represent a mechanisms-specific interventional approach to treat or prevent CVD in women who have had preeclampsia (50), and the findings of this current study imply that increased circulating Ang-(1–7) may be a synergistic mechanism by which this treatment may improve microvascular function in these women.
Limitations
We did not measure circulating Ang-(1–7) in this study. Circulating Ang-(1–7) is attenuated in preeclamptic pregnancy (10, 36, 56), but it is unknown whether this persists postpartum. Independent of basal Ang-(1–7) concentrations, our data suggest that increasing Ang-(1–7) bioavailability improves microvascular responses in women who have had preeclampsia. However, we are unable to address whether that represents a return to concentrations observed in women with a history of healthy pregnancy or an increase above that normally observed in healthy women.
We did not control for menstrual cycle status or oral contraceptive use, nor did we quantify ovarian hormone status, in this study. While recent data suggest that menstrual cycle phase does not influence acetylcholine-mediated responses in the microvasculature (30, 41), ovarian hormones do play a role in modulating the mechanisms contributing to vascular endothelial function (51) and it is possible that variation in hormone status, particularly in the postpartum period, may have influenced our results. To address this potential confounding variable, we matched our participant groups for time postpartum, but future work is required to fully elucidate how hormone status in the postpartum period may influence the mechanisms governing microvascular responses.
Traditional CVD risk factors, such as preexisting hypertension, diabetes, high BMI, and dyslipidemia, increase the risk of preeclampsia as well as negatively affect microvascular responses. We excluded potential participants who had been diagnosed with overt cardiovascular and/or metabolic disease(s) before pregnancy. However, we cannot rule out subclinical alterations in cardiovascular health (e.g., dyslipidemia, high BMI, elevated blood pressure) that may have preceded the pregnancy and contributed to our results. In this investigation women with a history of preeclampsia tended to have higher, although not statistically different, BMI, and significantly lower HDL cholesterol compared with our control group. Inclusion of HDL and BMI as covariates in our statistical model did not change our main effects, and it is unlikely that these factors drive the alterations in microvascular function observed in women who have had preeclampsia, or the efficacy of local Ang-(1–7) treatment in mitigating this dysfunction. However, longitudinal studies that examine vascular function before, during, and after pregnancy are needed to fully elucidate whether this dysfunction precedes preeclamptic pregnancy and what role CVD risk factors before pregnancy play in mediating the development of preeclampsia and subsequent elevated lifetime CVD risk.
Clinical Perspectives
The clinical symptoms of preeclampsia (high blood pressure, proteinuria, edema, etc.) typically resolve within 12 wk postpartum. However, otherwise healthy women who have had preeclampsia demonstrate attenuated endothelium-dependent dilation and augmented ANG II vasoconstrictor sensitivity postpartum, indicating the need for early intervention. Our findings suggest that locally increasing bioavailable Ang-(1–7) rescues microvascular endothelium-dependent vasodilation and attenuates exaggerated ANG II-mediated constriction in these women. As such, Ang-(1–7) mimetics and/or strategies to increase endogenous Ang-(1–7) production may be mechanism specific intervention strategies to improve microvascular function and slow or prevent the development of clinical CVD in this at-risk population.
GRANTS
This project was supported by National Heart, Lung, and Blood Institute Grants HL-129677-03 (to A. E. Stanhewicz), HL-138133-01 (to A. E. Stanhewicz), and HL-093238-07 (to L. M. Alexander).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.E.S. and L.M.A. conceived and designed research; A.E.S. performed experiments; A.E.S. analyzed data; A.E.S. and L.M.A. interpreted results of experiments; A.E.S. prepared figures; A.E.S. drafted manuscript; A.E.S. and L.M.A. edited and revised manuscript; A.E.S. and L.M.A. approved final version of manuscript.
ACKNOWLEDGMENTS
We acknowledge the participants for contributing time and effort. We also acknowledge Jane Pierzga and Susan Slimak for assistance throughout the project.
REFERENCES
- 1.ACOG Committee on Obstetric Practice; American College of Obstetricians and Gynecologists . ACOG practice bulletin. Diagnosis and management of preeclampsia and eclampsia. Number 33, January 2002. Int J Gynaecol Obstet 77: 67–75, 2002. [PubMed] [Google Scholar]
- 2.Agatisa PK, Ness RB, Roberts JM, Costantino JP, Kuller LH, McLaughlin MK. Impairment of endothelial function in women with a history of preeclampsia: an indicator of cardiovascular risk. Am J Physiol Heart Circ Physiol 286: H1389–H1393, 2004. doi: 10.1152/ajpheart.00298.2003. [DOI] [PubMed] [Google Scholar]
- 3.Ahmed R, Dunford J, Mehran R, Robson S, Kunadian V. Pre-eclampsia and future cardiovascular risk among women: a review. J Am Coll Cardiol 63: 1815–1822, 2014. doi: 10.1016/j.jacc.2014.02.529. [DOI] [PubMed] [Google Scholar]
- 4.Alvarez-Alvarez B, Martell-Claros N, Abad-Cardiel M, Garcia-Donaire JA. [Hypertensive disorders during pregnancy: Cardiovascular long-term outcomes] Hipertens Riesgo Vasc 34: 85–92, 2016. doi: 10.1016/j.hipert.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 5.American College of Gynecologists, and Task Force on Hypertension in Pregnancy Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet Gynecol 122: 1122–1131, 2013. doi: 10.1097/01.AOG.0000437382.03963.88. [DOI] [PubMed] [Google Scholar]
- 6.Arnadottir GA, Geirsson RT, Arngrimsson R, Jonsdottir LS, Olafsson O. Cardiovascular death in women who had hypertension in pregnancy: a case-control study. BJOG 112: 286–292, 2005. doi: 10.1111/j.1471-0528.2004.00396.x. [DOI] [PubMed] [Google Scholar]
- 7.Bellamy L, Casas JP, Hingorani AD, Williams DJ. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta-analysis. BMJ 335: 974, 2007. doi: 10.1136/bmj.39335.385301.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blaauw J, Souwer ET, Coffeng SM, Smit AJ, van Doormaal JJ, Faas MM, van Pampus MG. Follow up of intima-media thickness after severe early-onset preeclampsia. Acta Obstet Gynecol Scand 93: 1309–1316, 2014. doi: 10.1111/aogs.12499. [DOI] [PubMed] [Google Scholar]
- 9.Bruning RS, Santhanam L, Stanhewicz AE, Smith CJ, Berkowitz DE, Kenney WL, Holowatz LA. Endothelial nitric oxide synthase mediates cutaneous vasodilation during local heating and is attenuated in middle-aged human skin. J Appl Physiol (1985) 112: 2019–2026, 2012. doi: 10.1152/japplphysiol.01354.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen YP, Lu YP, Li J, Liu ZW, Chen WJ, Liang XJ, Chen X, Wen WR, Xiao XM, Reichetzeder C, Hocher B. Fetal and maternal angiotensin (1-7) are associated with preterm birth. J Hypertens 32: 1833–1841, 2014. doi: 10.1097/HJH.0000000000000251. [DOI] [PubMed] [Google Scholar]
- 11.Coulon P, Constans J, Gosse P. Impairment of skin blood flow during post-occlusive reactive hyperhemy assessed by laser Doppler flowmetry correlates with renal resistive index. J Hum Hypertens 26: 56–63, 2012. doi: 10.1038/jhh.2010.117. [DOI] [PubMed] [Google Scholar]
- 12.Cunningham MW Jr, Williams JM, Amaral L, Usry N, Wallukat G, Dechend R, LaMarca B. Agonistic autoantibodies to the angiotensin II type 1 receptor enhance angiotensin II-induced renal vascular sensitivity and reduce renal function during pregnancy. Hypertension 68: 1308–1313, 2016. doi: 10.1161/HYPERTENSIONAHA.116.07971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Souza-Neto FP, Carvalho Santuchi M, de Morais E Silva M, Campagnole-Santos MJ, da Silva RF. Angiotensin-(1–7) and alamandine on experimental models of hypertension and atherosclerosis. Curr Hypertens Rep 20: 17, 2018. doi: 10.1007/s11906-018-0798-6. [DOI] [PubMed] [Google Scholar]
- 14.Debbabi H, Bonnin P, Ducluzeau PH, Lefthériotis G, Levy BI. Noninvasive assessment of endothelial function in the skin microcirculation. Am J Hypertens 23: 541–546, 2010. doi: 10.1038/ajh.2010.10. [DOI] [PubMed] [Google Scholar]
- 15.Drost JT, Arpaci G, Ottervanger JP, de Boer MJ, van Eyck J, van der Schouw YT, Maas AH. Cardiovascular risk factors in women 10 years post early preeclampsia: the Preeclampsia Risk EValuation in FEMales study (PREVFEM). Eur J Prev Cardiol 19: 1138–1144, 2012. doi: 10.1177/1741826711421079. [DOI] [PubMed] [Google Scholar]
- 16.Evans CS, Gooch L, Flotta D, Lykins D, Powers RW, Landsittel D, Roberts JM, Shroff SG. Cardiovascular system during the postpartum state in women with a history of preeclampsia. Hypertension 58: 57–62, 2011. doi: 10.1161/HYPERTENSIONAHA.111.173278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fraga-Silva RA, Costa-Fraga FP, De Sousa FB, Alenina N, Bader M, Sinisterra RD, Santos RAS. An orally active formulation of angiotensin-(1-7) produces an antithrombotic effect. Clinics (São Paulo) 66: 837–841, 2011. doi: 10.1590/S1807-59322011000500021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fraga-Silva RA, Pinheiro SV, Gonçalves AC, Alenina N, Bader M, Santos RA. The antithrombotic effect of angiotensin-(1-7) involves mas-mediated NO release from platelets. Mol Med 14: 28–35, 2008. doi: 10.2119/2007-00073.Fraga-Silva. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ghossein-Doha C, van Neer J, Wissink B, Breetveld NM, de Windt LJ, van Dijk AP, van der Vlugt MJ, Janssen MC, Heidema WM, Scholten RR, Spaanderman ME. Pre-eclampsia: an important risk factor for asymptomatic heart failure. Ultrasound Obstet Gynecol 49: 143–149, 2017. doi: 10.1002/uog.17343. [DOI] [PubMed] [Google Scholar]
- 20.Grand’Maison S, Pilote L, Okano M, Landry T, Dayan N. Markers of vascular dysfunction after hypertensive disorders of pregnancy. A systematic review and meta-analysis. Hypertension 68: 1447–1458, 2016. doi: 10.1161/HYPERTENSIONAHA.116.07907. [DOI] [PubMed] [Google Scholar]
- 21.Haukkamaa L, Salminen M, Laivuori H, Leinonen H, Hiilesmaa V, Kaaja R. Risk for subsequent coronary artery disease after preeclampsia. Am J Cardiol 93: 805–808, 2004. doi: 10.1016/j.amjcard.2003.11.065. [DOI] [PubMed] [Google Scholar]
- 22.Holowatz LA, Thompson-Torgerson CS, Kenney WL. The human cutaneous circulation as a model of generalized microvascular function. J Appl Physiol (1985) 105: 370–372, 2008. doi: 10.1152/japplphysiol.00858.2007. [DOI] [PubMed] [Google Scholar]
- 23.Iyer SN, Chappell MC, Averill DB, Diz DI, Ferrario CM. Vasodepressor actions of angiotensin-(1-7) unmasked during combined treatment with lisinopril and losartan. Hypertension 31: 699–705, 1998. doi: 10.1161/01.HYP.31.2.699. [DOI] [PubMed] [Google Scholar]
- 24.Iyer SN, Ferrario CM, Chappell MC. Angiotensin-(1-7) contributes to the antihypertensive effects of blockade of the renin-angiotensin system. Hypertension 31: 356–361, 1998. doi: 10.1161/01.HYP.31.1.356. [DOI] [PubMed] [Google Scholar]
- 25.James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, Lackland DT, LeFevre ML, MacKenzie TD, Ogedegbe O, Smith SC Jr, Svetkey LP, Taler SJ, Townsend RR, Wright JT Jr, Narva AS, Ortiz E. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 311: 507–520, 2014. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- 26.Jiang F, Yang J, Zhang Y, Dong M, Wang S, Zhang Q, Liu FF, Zhang K, Zhang C. Angiotensin-converting enzyme 2 and angiotensin 1-7: novel therapeutic targets. Nat Rev Cardiol 11: 413–426, 2014. doi: 10.1038/nrcardio.2014.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johnson JM, O’Leary DS, Taylor WF, Kosiba W. Effect of local warming on forearm reactive hyperaemia. Clin Physiol 6: 337–346, 1986. doi: 10.1111/j.1475-097X.1986.tb00239.x. [DOI] [PubMed] [Google Scholar]
- 29.Kenney WL, Cannon JG, Alexander LM. Cutaneous microvascular dysfunction correlates with serum LDL and sLOX-1 receptor concentrations. Microvasc Res 85: 112–117, 2013. doi: 10.1016/j.mvr.2012.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ketel IJ, Stehouwer CD, Serné EH, Poel DM, Groot L, Kager C, Hompes PG, Homburg R, Twisk JW, Smulders YM, Lambalk CB. Microvascular function has no menstrual-cycle-dependent variation in healthy ovulatory women. Microcirculation 16: 714–724, 2009. doi: 10.3109/10739680903199186. [DOI] [PubMed] [Google Scholar]
- 31.Khan F, Patterson D, Belch JJ, Hirata K, Lang CC. Relationship between peripheral and coronary function using laser Doppler imaging and transthoracic echocardiography. Clin Sci (Lond) 115: 295–300, 2008. doi: 10.1042/CS20070431. [DOI] [PubMed] [Google Scholar]
- 32.Lamarca B. The role of immune activation in contributing to vascular dysfunction and the pathophysiology of hypertension during preeclampsia. Minerva Ginecol 62: 105–120, 2010. [PMC free article] [PubMed] [Google Scholar]
- 33.Liang B, Wang X, Zhang N, Yang H, Bai R, Liu M, Bian Y, Xiao C, Yang Z. Angiotensin-(1-7) attenuates angiotensin II-induced ICAM-1, VCAM-1, and MCP-1 expression via the MAS receptor through suppression of P38 and NF-κB pathways in HUVECs. Cell Physiol Biochem 35: 2472–2482, 2015. doi: 10.1159/000374047. [DOI] [PubMed] [Google Scholar]
- 34.McDonald SD, Ray J, Teo K, Jung H, Salehian O, Yusuf S, Lonn E. Measures of cardiovascular risk and subclinical atherosclerosis in a cohort of women with a remote history of preeclampsia. Atherosclerosis 229: 234–239, 2013. doi: 10.1016/j.atherosclerosis.2013.04.020. [DOI] [PubMed] [Google Scholar]
- 35.Mendonça L, Mendes-Ferreira P, Bento-Leite A, Cerqueira R, Amorim MJ, Pinho P, Brás-Silva C, Leite-Moreira AF, Castro-Chaves P. Angiotensin-(1-7) modulates angiotensin II-induced vasoconstriction in human mammary artery. Cardiovasc Drugs Ther 28: 513–522, 2014. doi: 10.1007/s10557-014-6555-4. [DOI] [PubMed] [Google Scholar]
- 36.Merrill DC, Karoly M, Chen K, Ferrario CM, Brosnihan KB. Angiotensin-(1-7) in normal and preeclamptic pregnancy. Endocrine 18: 239–245, 2002. doi: 10.1385/ENDO:18:3:239. [DOI] [PubMed] [Google Scholar]
- 37.Mongraw-Chaffin ML, Cirillo PM, Cohn BA. Preeclampsia and cardiovascular disease death: prospective evidence from the child health and development studies cohort. Hypertension 56: 166–171, 2010. doi: 10.1161/HYPERTENSIONAHA.110.150078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Murugan D, Lau YS, Lau CW, Mustafa MR, Huang Y. Angiotensin 1–7 protects against angiotensin II-induced endoplasmic reticulum stress and endothelial dysfunction via mas receptor. PLoS One 10: e0145413, 2015. [Erratum in PLos One 11: e0147892, 2016]. doi: 10.1371/journal.pone.0145413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nogueira AI, Souza Santos RA, Simões E Silva AC, Cabral AC, Vieira RL, Drumond TC, Machado LJ, Freire CM, Ribeiro-Oliveira A Jr. The pregnancy-induced increase of plasma angiotensin-(1-7) is blunted in gestational diabetes. Regul Pept 141: 55–60, 2007. doi: 10.1016/j.regpep.2006.12.014. [DOI] [PubMed] [Google Scholar]
- 40.Oparil S, Schmieder RE. New approaches in the treatment of hypertension. Circ Res 116: 1074–1095, 2015. doi: 10.1161/CIRCRESAHA.116.303603. [DOI] [PubMed] [Google Scholar]
- 41.Rossi M, Di Maria C, Erba P, Galetta F, Carpi A, Santoro G. Study of skin vasomotion during phollicular and luteal phase in young healthy women. Clin Hemorheol Microcirc 42: 107–115, 2009. doi: 10.3233/CH-2009-1189. [DOI] [PubMed] [Google Scholar]
- 42.Sampaio WO, Henrique de Castro C, Santos RA, Schiffrin EL, Touyz RM. Angiotensin-(1-7) counterregulates angiotensin II signaling in human endothelial cells. Hypertension 50: 1093–1098, 2007. doi: 10.1161/HYPERTENSIONAHA.106.084848. [DOI] [PubMed] [Google Scholar]
- 43.Santos RA, Ferreira AJ, Verano-Braga T, Bader M. Angiotensin-converting enzyme 2, angiotensin-(1-7) and Mas: new players of the renin-angiotensin system. J Endocrinol 216: R1–R17, 2013. doi: 10.1530/JOE-12-0341. [DOI] [PubMed] [Google Scholar]
- 44.Sasaki S, Higashi Y, Nakagawa K, Matsuura H, Kajiyama G, Oshima T. Effects of angiotensin-(1-7) on forearm circulation in normotensive subjects and patients with essential hypertension. Hypertension 38: 90–94, 2001. doi: 10.1161/01.HYP.38.1.90. [DOI] [PubMed] [Google Scholar]
- 45.Saxena AR, Karumanchi SA, Brown NJ, Royle CM, McElrath TF, Seely EW. Increased sensitivity to angiotensin II is present postpartum in women with a history of hypertensive pregnancy. Hypertension 55: 1239–1245, 2010. doi: 10.1161/HYPERTENSIONAHA.109.147595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schinzari F, Tesauro M, Veneziani A, Mores N, Di Daniele N, Cardillo C. Favorable vascular actions of angiotensin- (1–7) in human obesity. Hypertension 71: 185–191, 2018. doi: 10.1161/HYPERTENSIONAHA.117.10280. [DOI] [PubMed] [Google Scholar]
- 46a.Jónsdóttir LS, Arngrímsson R, Geirsson RT, Sigvaldason H, Sigfússon N. Death rates from ischemic heart disease in women with a history of hypertension in pregnancy. Acta Obstet Gynecol Scand 74: 772–776 1995. doi: 10.3109/00016349509021195. [DOI] [PubMed] [Google Scholar]
- 47.Smith CJ, Santhanam L, Bruning RS, Stanhewicz A, Berkowitz DE, Holowatz LA. Upregulation of inducible nitric oxide synthase contributes to attenuated cutaneous vasodilation in essential hypertensive humans. Hypertension 58: 935–942, 2011. doi: 10.1161/HYPERTENSIONAHA.111.178129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stanhewicz AE. Residual vascular dysfunction in women with a history of preeclampsia. Am J Physiol Regul Integr Comp Physiol 315: R1062–R1071, 2018. doi: 10.1152/ajpregu.00204.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stanhewicz AE, Jandu S, Santhanam L, Alexander LM. Alterations in endothelin type B receptor contribute to microvascular dysfunction in women who have had preeclampsia. Clin Sci (Lond) 131: 2777–2789, 2017. doi: 10.1042/CS20171292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stanhewicz AE, Jandu S, Santhanam L, Alexander LM. Increased angiotensin II sensitivity contributes to microvascular dysfunction in women who have had preeclampsia. Hypertension 70: 382–389, 2017. doi: 10.1161/HYPERTENSIONAHA.117.09386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stanhewicz AE, Wenner MM, Stachenfeld NS. Sex differences in endothelial function important to vascular health and overall cardiovascular disease risk across the lifespan. Am J Physiol Heart Circ Physiol 315: H1569–H1588, 2018. doi: 10.1152/ajpheart.00396.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stewart JM, Ocon AJ, Clarke D, Taneja I, Medow MS. Defects in cutaneous angiotensin-converting enzyme 2 and angiotensin-(1-7) production in postural tachycardia syndrome. Hypertension 53: 767–774, 2009. doi: 10.1161/HYPERTENSIONAHA.108.127357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stewart JM, Taneja I, Glover J, Medow MS. Angiotensin II type 1 receptor blockade corrects cutaneous nitric oxide deficit in postural tachycardia syndrome. Am J Physiol Heart Circ Physiol 294: H466–H473, 2008. doi: 10.1152/ajpheart.01139.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Timokhina E, Kuzmina T, Strizhakov A, Pitskhelauri E, Ignatko I, Belousova V. Maternal cardiac function after normal delivery, preeclampsia, and eclampsia: a prospective study. J Pregnancy 2019: 9795765, 2019. doi: 10.1155/2019/9795765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Valdés G, Germain AM, Corthorn J, Berrios C, Foradori AC, Ferrario CM, Brosnihan KB. Urinary vasodilator and vasoconstrictor angiotensins during menstrual cycle, pregnancy, and lactation. Endocrine 16: 117–122, 2001. doi: 10.1385/ENDO:16:2:117. [DOI] [PubMed] [Google Scholar]
- 56.Velloso EP, Vieira R, Cabral AC, Kalapothakis E, Santos RA. Reduced plasma levels of angiotensin-(1-7) and renin activity in preeclamptic patients are associated with the angiotensin I- converting enzyme deletion/deletion genotype. Braz J Med Biol Res 40: 583–590, 2007. doi: 10.1590/S0100-879X2007000400018. [DOI] [PubMed] [Google Scholar]
- 57.Walther G, Obert P, Dutheil F, Chapier R, Lesourd B, Naughton G, Courteix D, Vinet A. Metabolic syndrome individuals with and without type 2 diabetes mellitus present generalized vascular dysfunction: cross-sectional study. Arterioscler Thromb Vasc Biol 35: 1022–1029, 2015. doi: 10.1161/ATVBAHA.114.304591. [DOI] [PubMed] [Google Scholar]
- 58.Wenner MM, Wilson TE, Davis SL, Stachenfeld NS. Pharmacological curve fitting to analyze cutaneous adrenergic responses. J Appl Physiol (1985) 111: 1703–1709, 2011. doi: 10.1152/japplphysiol.00780.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wenzel K, Rajakumar A, Haase H, Geusens N, Hubner N, Schulz H, Brewer J, Roberts L, Hubel CA, Herse F, Hering L, Qadri F, Lindschau C, Wallukat G, Pijnenborg R, Heidecke H, Riemekasten G, Luft FC, Muller DN, Lamarca B, Dechend R. Angiotensin II type 1 receptor antibodies and increased angiotensin II sensitivity in pregnant rats. Hypertension 58: 77–84, 2011. doi: 10.1161/HYPERTENSIONAHA.111.171348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yang G, Istas G, Höges S, Yakoub M, Hendgen-Cotta U, Rassaf T, Rodriguez-Mateos A, Hering L, Grandoch M, Mergia E, Rump LC, Stegbauer J. Angiotensin-(1-7) -induced Mas receptor activation attenuates atherosclerosis through a nitric oxide-dependent mechanism in apolipoproteinE-KO mice. Pflugers Arch 470: 661–667, 2018. doi: 10.1007/s00424-018-2108-1. [DOI] [PubMed] [Google Scholar]
- 61.Zhang F, Hu Y, Xu Q, Ye S. Different effects of angiotensin II and angiotensin-(1-7) on vascular smooth muscle cell proliferation and migration. PLoS One 5: e12323, 2010. doi: 10.1371/journal.pone.0012323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang F, Liu J, Li SF, Song JX, Ren JY, Chen H. Angiotensin-(1-7): new perspectives in atherosclerosis treatment. J Geriatr Cardiol 12: 676–682, 2015. doi: 10.11909/j.issn.1671-5411.2015.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang F, Tang H, Sun S, Luo Y, Ren X, Chen A, Xu Y, Li P, Han Y. Angiotensin-(1–7) induced vascular relaxation in spontaneously hypertensive rats. Nitric Oxide 88: 1–9, 2019. doi: 10.1016/j.niox.2019.03.007. [DOI] [PubMed] [Google Scholar]
- 64.Zhang Y, Liu J, Luo JY, Tian XY, Cheang WS, Xu J, Lau CW, Wang L, Wong WT, Wong CM, Lan HY, Yao X, Raizada MK, Huang Y. Upregulation of angiotensin (1-7) -mediated signaling preserves endothelial function through reducing oxidative stress in diabetes. Antioxid Redox Signal 23: 880–892, 2015. doi: 10.1089/ars.2014.6070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang YH, Zhang YH, Dong XF, Hao QQ, Zhou XM, Yu QT, Li SY, Chen X, Tengbeh AF, Dong B, Zhang Y. ACE2 and Ang-(1-7) protect endothelial cell function and prevent early atherosclerosis by inhibiting inflammatory response. Inflamm Res 64: 253–260, 2015. doi: 10.1007/s00011-015-0805-1. [DOI] [PubMed] [Google Scholar]