Abstract
High dietary salt increases arterial blood pressure variability (BPV) in salt-resistant, normotensive rodents and is thought to result from elevated plasma [Na+] sensitizing central sympathetic networks. Our purpose was to test the hypothesis that water deprivation (WD)-induced elevations in serum [Na+] augment BPV via changes in baroreflex function and sympathetic vascular transduction in humans. In a randomized crossover fashion, 35 adults [17 female/18 male, age: 25 ± 4 yr, systolic/diastolic blood pressure (BP): 107 ± 11/60 ± 7 mmHg, body mass index: 23 ± 3 kg/m2] completed two hydration protocols: a euhydration control condition (CON) and a stepwise reduction in water intake over 3 days, concluding with 16 h of WD. We assessed blood and urine electrolyte concentrations and osmolality, resting muscle sympathetic nerve activity (MSNA; peroneal microneurography; 18 paired recordings), beat-to-beat BP (photoplethysmography), common femoral artery blood flow (Doppler ultrasound), and heart rate (single-lead ECG). A subset of participants (n = 25) underwent ambulatory BP monitoring during day 3 of each protocol. We calculated average real variability as an index of BPV. WD increased serum [Na+] (141.0 ± 2.3 vs. 142.1 ± 1.7 mmol/L, P < 0.01) and plasma osmolality (288 ± 4 vs. 292 ± 5 mosmol/kg H2O, P < 0.01). However, WD did not increase beat-to-beat (1.9 ± 0.4 vs. 1.8 ± 0.4 mmHg, P = 0.24) or ambulatory daytime (9.6 ± 2.1 vs. 9.4 ± 3.3 mmHg, P = 0.76) systolic BPV. Additionally, sympathetic baroreflex sensitivity (P = 0.20) and sympathetic vascular transduction were not different after WD (P = 0.17 for peak Δmean BP following spontaneous MSNA bursts). These findings suggest that, despite modestly increasing serum [Na+], WD does not affect BPV, arterial baroreflex function, or sympathetic vascular transduction in healthy young adults.
Keywords: arterial baroreflex function, blood pressure variability, hypohydration, serum sodium concentrations, sympathetic vascular transduction
INTRODUCTION
Inadequate water intake is highly prevalent in the United Stated (21) and is predictive of future incidence of cardiovascular disease (14). Acute reductions in water intake are characterized by plasma volume contraction and elevations in blood sodium concentrations ([Na+]) (64). Elevations in blood [Na+] are detected centrally by neurons in forebrain circumventricular organs (8), which can influence neurovascular function. For example, dietary salt-induced elevations in blood [Na+] produce increased resting pulse pressure (24), resting muscle sympathetic nerve activity (MSNA) (24, 27), ambulatory daytime systolic blood pressure (BP) (34), and BP during exercise (9) in humans. In rodents, a high-salt diet increases the responsiveness of rostral ventrolateral medulla (RVLM) neurons to exogenous l-glutamate or γ-aminobutyric acid (GABA) and exaggerates several sympathetic reflexes (1, 59, 62). Water deprivation (WD)-induced elevations blood [Na+] increase sympathetic outflow and BP in rodents (11, 57). Additionally, water deprivation may increase RVLM responsiveness to exogenous l-glutamate and elicit greater excitatory amino acid receptor activation to support BP in rodents (10, 12). However, it remains unclear whether elevated blood [Na+] induced by acute WD affects BP regulation in humans. This is important because identifying a link between acute WD and altered BP regulation may lend insight into the potential mechanisms underlying the association between underhydration (chronic inadequate water consumption; see Ref. 33) and increased cardiovascular disease incidence (14).
High dietary salt increases blood pressure variability (BPV) in salt-resistant, normotensive rodents and is thought to result from elevated plasma [Na+] sensitizing central sympathetic networks (59). BPV represents the differences in blood pressure values from one reading to the next. BPV is assessed in humans at the clinic (between routine health assessments or between seated measures recorded every minute), at home (between ambulatory monitoring measures every 15–30 min or between seated measures recorded every minute), or in the laboratory (between measures recorded every minute or as precisely as on a beat-to-beat basis) (7, 16, 25, 35–38, 40, 43, 44, 47, 50, 53). Importantly, high BPV in humans is associated with several cardiovascular morbidities such as cerebral small vessel disease (25), target organ damage (35, 65), and hypertensive status (50), in addition to higher cardiovascular mortality (38, 53). Although several factors influence BPV, two major determinants of BPV are arterial baroreflex function (i.e., the central arc of BP regulation) (45) and sympathetic vascular transduction (i.e., the peripheral arc of BP regulation) (5, 55, 68), defined as vasoconstrictor responses following spontaneous MSNA bursts (49). Sympathetic baroreflex function has been reported to be impaired in rodents after 11 days of hypertonic NaCl cerebroventricular infusion (13), suggesting central actions of NaCl. Cardiac vagal baroreflex function has been reported to be impaired in humans after 5 days of high-NaCl feeding (17). Dietary salt-induced reductions in blood [Na+] have been demonstrated to reduce sympathetic vascular transduction in humans (5). Specific to WD, previous findings in humans suggest that mild hypohydration impairs endothelial-dependent dilation (3). Intact endothelial function is important for blunting α1-adrenergic vasoconstriction (32), the primary mediator of sympathetic vascular transduction (23). Taken together, these data suggest that elevated blood [Na+] induced by acute WD may lead to increased (i.e., worsened) BPV via reductions in arterial baroreflex function and increases in sympathetic vascular transduction in humans.
To address the gaps in knowledge discussed above, the overall intent of the present study was to determine whether WD in humans augments BPV and impairs mechanisms related to central and peripheral BP regulation, thus providing mechanistic insight into the association between underhydration and cardiovascular disease. We hypothesized that WD-induced increases in serum [Na+] would augment BPV in humans. As secondary hypotheses, we speculated that WD would impair arterial baroreflex function and augment resting sympathetic vascular transduction. Finally, given that our sample included male and female participants, we sought to explore whether there was preliminary evidence of sex differences in BPV following WD.
MATERIALS AND METHODS
Participants.
The Institutional Review Board at the University of Delaware approved this protocol, and the study conformed with the Declaration of Helsinki. The data reported here are part of a completed registered protocol (ClinicalTrials.gov Identifier: NCT03560869). All participants provided verbal and written consent before enrollment in the study. During the initial screening visit, participants completed a physical activity readiness questionnaire and medical history questionnaire and underwent measurements of body height and mass. Resting brachial BP measurements were performed in triplicate with participants in the seated position following greater than or equal to 5 min of quiet rest (Dash 2000; GE Medical Systems, Milwaukee, WI). Inclusion criteria for this study included the following: age between 20 and 40 yr, resting systolic BP <140 mmHg, resting diastolic BP <90 mmHg, and body mass index <30 kg/m2 at screening. Study participants were nonsmokers free of any known cardiovascular disease and had no evidence of metabolic, neurological, renal, or pulmonary disease.
Hydration conditions.
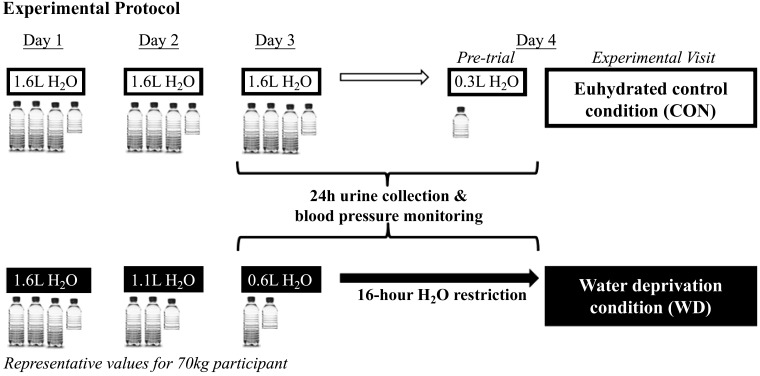
A timeline of the experimental protocol is presented in Fig. 1. Participants completed two 3-day-long hydration conditions in random order separated by ≥1 wk for males and by ∼1 mo for females. Female participants were tested in the early follicular phase of the menstrual cycle or placebo phase of oral contraceptives (self-report). During the normal hydration condition (CON), participants were asked to consume 23 mL H2O·kg body mass−1·day−1 for days 1–3 and to consume 250 mL of H2O before arriving to the laboratory for testing on day 4. The WD condition required participants to consume 23 mL H2O/kg body mass on day 1, 17 mL H2O/kg body mass on day 2, and 10 mL H2O/kg body mass on day 3, followed by 16 h of water abstention before testing on day 4. This intervention was chosen to maximize ecological validity (voluntary dehydration) and to minimize confounding effects from exercise‐, heat‐, or diuretic‐induced dehydration methods. Participants were asked to refrain from caffeine, alcohol, vigorous exercise, and exercise in the heat on days 2 and 3 during both 3-day hydration protocols. Participants were given guidance on estimating food portion sizes and keeping a diet log to maintain recommended (19) daily sodium intake (2,300 mg/day). They were also instructed to consume the same foods and maintain the same physical activity during the second 3-day hydration condition as reported in their diet log during the first 3-day hydration condition. A research dietitian analyzed participants’ 3-day diet records for total calories, macronutrients, sodium, and water content from foods content using Nutrient Data System for Research. Finally, participants collected their urine in a sterile, light-protected collection container for 24 h preceding the laboratory on the fourth day of each condition following a 4-h fast.
Fig. 1.
In randomized crossover fashion, participants completed a euhydrated control condition (CON) and a water deprivation condition (WD) before laboratory testing on day 4. In the laboratory, we assessed heart rate, beat-to-beat blood pressure, common femoral artery diameter and blood flow, and muscle sympathetic nerve activity during 10 minutes of quiet rest in the supine position.
Experimental visit.
Upon arrival to the laboratory, participants provided a spot urine sample, were weighed (Tanita Body Composition Analyzer, model TBF-300A), and provided a subjective rating of their thirst and mouth dryness using a Likert scale. The overall thirst rating was generated by calculating the mean value from the subjective rating of 1) thirst and 2) dryness of mouth on a 10-cm line with anchor points of “not at all” (left) to “very” (right). An intravenous catheter was placed in the antecubital space of the dominant arm for blood sampling. Participants were then outfitted with the required equipment for measurement of MSNA, beat-to-beat BP, brachial BP, heart rate, leg blood flow (described below), and respiratory excursions (via a strain-gauge pneumograph). Data were collected during a 10-min baseline period as participants rested quietly in a dimly lit, temperature-controlled room (22–24°C). The full duration of the baseline period was used for all baroreflex and sympathetic vascular transduction analyses.
Blood and urine analysis.
Participants laid in the supine position for ≥20 min before venous blood sample collection. Venous blood samples and 24-h urine samples were analyzed for serum electrolyte concentrations in triplicate and urine electrolyte concentrations in triplicate from two separate aliquots (EasyElectrolyte Analyzer; Medica, Beford, MA) as well as plasma osmolality in triplicate and urine osmolality in quintuplicate (3D3 Osmometer; Advanced Instruments, Norwood, MA). Venous blood samples were also analyzed for Hb (Hb 201+; Hemocue, Lake Forest, CA) and Hct (Precalibrated Clay Adams, Readacrit Centrifuge; Becton Dickinson, Sparks, MD) in triplicate. Change in plasma volume (expressed as a percentage) was calculated using the following equation (20): plasma volume (%) = {(100 × [(100 × (HbCON/HbWD)] × (1 − (HctWD/100)/(1 − HctCON/100)} − 100. Additionally, urine-specific gravity was determined from the spot and 24-h urine samples. Female participants’ spot urine samples were also used to confirm that they were not pregnant (hCG cassettes; Moore Medical).
Arterial blood pressure assessment.
Beat-to-beat BP was measured at the finger of participants’ nondominant hand using photoplethysmography (Finometer; Finapres Medical Systems) and calibrated to brachial BP according to the manufacturer’s recommended calibration procedures (29). Brachial BP was measured using an automated oscillometric sphygmomanometer (Dash 2000; GE Medical Systems) and used to verify absolute beat-to-beat BP values. Simultaneously, heart rate was continuously recorded using a standard single-lead ECG (Dash 2000; GE Medical Systems).
Arterial blood pressure variability.
BPV was calculated using standard deviation and using the average real variability index of BP values. The average real variability index calculates the average of absolute differences between consecutive BP measurements and is thought to provide further prognostic value compared with traditional measures of BPV, such as standard deviation of BP (7, 43). BPV was assessed during 10 min of quiet rest using the beat-to-beat BP signal derived from the Finometer (described above). Additionally, ambulatory BP was assessed in a subset of participants (n = 25) during the 24-h periods preceding each experimental visit (Oscar 2 with Sphygmacor; SunTech Medical). The monitor measured BP on the nondominant arm every 20 min from 0601 to 2200 and every 30 min from 2201 to 0600. Participants’ self-reported sleep and wake times in the laboratory noted the start of “daytime” and “nighttime” for the BP values. Data were included for analysis only if participants had at least 15 measurements during the daytime and at least eight measurements during the nighttime (47).
Muscle sympathetic nerve activity.
MSNA was directly assessed via microneurography (n = 18 pairs of recordings), as previously described by our laboratory (5, 9, 26, 41, 55). Briefly, a tungsten recording microelectrode was inserted percutaneously near the fibular head or in the popliteal fossa, and a reference microelectrode was inserted ≤3 cm from the recording electrode. The nerve recording was amplified (80–90,000×), band-pass filtered (700–2,000 Hz), rectified, and integrated (time constant 0.1 s) using a nerve traffic analyzer (Nerve Traffic Analyzer, model 662c-4; University of Iowa Bioengineering, Iowa City, IA). Bursts were identified in accordance with recent guidelines (58, 70) via the following criteria 1) >3:1 signal-to-noise ratio, 2) burst morphology consistent with MSNA bursts, and 3) a pulse-synchronous signal. MSNA was quantified as burst frequency (bursts/min), burst incidence (bursts/100 heartbeats), and total activity (burst frequency·burst amplitude−1·min−1, AU/min). The sympathetic neurogram was analyzed on a beat-to-beat basis to determine the presence/absence of MSNA bursts using custom LabView software and visually inspected by a laboratory member blinded to condition and trained in MSNA data analysis and processing (J. C. Watso).
Femoral artery blood flow.
Continuous measures of common femoral artery diameter and velocity were obtained via duplex Doppler ultrasound (GE Logiq P5; GE, Milwaukee, WI), as previously described (5, 22). Briefly, a 10- or 12-MHz linear array transducer was selected for optimal image quality and positioned at the common femoral artery via a custom-designed clamp, ∼2–3 cm proximal to the bifurcation of the superficial and deep femoral arteries. Blood velocity was simultaneously obtained with diameter in pulsed-wave mode at an insonation angle of ≤60° and operating at a linear frequency of 5 MHz. The sample volume encompassed the entire vessel lumen without extending beyond the walls.
Hemodynamic data analysis.
Data were collected continuously at a sampling rate of 20,000 Hz using LabChart (LabChart 8.0 Pro; ADInstruments, Colorado Springs, CO). Common femoral artery diameter and blood velocity were analyzed and synchronized with beat-to-beat ECG, BP, and MSNA signals using custom LabVIEW programs. Femoral blood flow was determined from the continuous recordings of common femoral artery diameter and blood velocity and calculated as blood flow [mL/min] = (mean diameter [cm]/2)2 × mean blood velocity [cm/s] × 60 [s/min] × π. Shear rate was calculated as shear rate [s−1] = 4 (mean blood velocity [cm/s]/mean diameter [cm])
Limb vascular conductance was determined by dividing femoral artery blood flow by mean BP, which was calculated as the integral of the BP waveform. Because of technical difficulties, paired limb vascular conductance was obtained in 13 of the participants.
Sympathetic baroreflex gain analysis.
Sympathetic baroreflex gain was assessed in the resting state during spontaneous BP oscillations, as previously described (28, 42, 63). This method quantifies baroreflex gain around the operating point and is well correlated to the modified Oxford technique (31). Briefly, each cardiac cycle was assigned to a bin (3 mmHg) based on diastolic BP on the corresponding cardiac cycle. Burst incidence and total activity regressed over diastolic BP bins. All data were weighted to account for the number of cardiac cycles within each bin (28, 42). Bins without MSNA bursts were excluded from the analysis. Slopes of the linear regressions were used as an index of sympathetic baroreflex gain if they had an r value of ≥0.5 (28, 42, 49).
Cardiac vagal baroreflex gain analysis.
Beat-to-beat time series of systolic BP and R-R interval were analyzed using the sequence method for estimating spontaneous cardiac vagal baroreflex gain (HemoLab version 8.9; Harald Stauss Scientific, Iowa City, IA). A detailed description of this method has been published previously (6). As previously performed in our laboratory (4), sequences of four or more consecutive cardiac cycles in which systolic BP and R-R interval change in the same direction were identified as baroreflex sequences. Sequences were detected only when the variation in R-R interval was >0.5 ms and systolic BP changes were >1 mmHg. A linear regression was applied to each individual sequence, and only those sequences in which r2 was >0.80 were accepted. Values of cardiac vagal baroreflex gain were accepted when the number of sequences was ≥3 for both up and down sequences. The slopes of those individual linear regressions were then calculated and averaged for a measure of spontaneous cardiac vagal baroreflex gain. Cardiac vagal baroreflex gain was determined for all sequences combined and separately for up (increase in both systolic BP and R-R interval) and down (decrease in both systolic BP and R-R interval) sequences.
Sympathetic vascular transduction analysis.
To quantify indices of sympathetic vascular transduction (i.e., the functional effect of individual bursts of MSNA on BP and blood flow), a spike-triggered averaging methodology was used that has been described previously (61, 67, 68, 72). Briefly, each cardiac cycle containing a burst of MSNA was identified and set as cardiac cycle 0 whether or not the burst was a singlet (bursts directly bordered by >1 heartbeat lacking MSNA) or part of a cluster [bursts adjacent to other burst(s) of MSNA]. Both mean BP and limb vascular conductance of cardiac cycle 0 were determined and followed for 10 subsequent cardiac cycles. The absolute change in mean BP and percent change in limb vascular conductance was determined in each of these 10 cardiac cycles. These methods reliably quantify beat-to-beat changes in BP and blood flow induced spontaneous MSNA bursts (22).
Statistical analysis.
An a priori power analysis was performed based on pilot data collected in our laboratory (effect size: Cohen’s dz = 0.5), and it was determined that a sample size of 34 adults would be sufficient to detect a condition effect for beat-to-beat systolic BPV with power at 80% and α set at 0.05 using a paired, two-tailed t test. Biochemical, MSNA, hemodynamic, and BPV measures were compared between the hydration conditions using paired, two-tailed t tests. The effects of the hydration condition on sympathetic vascular transduction were compared using two-way repeated-measures ANOVAs (condition × cardiac cycle number following spontaneous MSNA bursts). Tukey’s HSD were used to correct for multiple comparisons (post hoc analyses for repeated-measures ANOVAs). Additionally, peak changes in mean BP and limb vascular conductance were compared between the hydration conditions using paired, two-tailed t tests. The estimated plasma volume change between conditions was compared using a one-sample t test. On an exploratory basis, we examined the effect of sex on our main outcome variables using two-way independent groups ANOVAs (sex × condition). All data are presented as means ± SD. All data were analyzed using GraphPad Prism 8.0 (GraphPad Software, La Jolla, CA), and significance was set a priori at P < 0.05.
RESULTS
Participant screening characteristics are provided in Table 1. Caloric intake (CON: 1,738 ± 574 vs. WD: 1,771 ± 568 kcal/day, P = 0.72), dietary carbohydrate intake (CON: 194 ± 92 vs. WD: 213 ± 86 g/day, P = 0.28), dietary fat intake (CON: 75 ± 40 vs. WD: 71 ± 37 g/day, P = 0.67), dietary protein intake (CON: 84 ± 38 vs. WD: 82 ± 36 g/day, P = 0.62), dietary sodium intake (CON: 2,151 ± 372 vs. WD: 2,184 ± 400 mg/day, P = 0.72), and water content from food (CON: 919 ± 535 vs. WD: 940 ± 505 mL/day, P = 0.77) were not different between experimental conditions.
Table 1.
Participants
| Screening Characteristics | Value |
|---|---|
| No. (female/male) | 35 (17/18) |
| Age, yr | 25 ± 4 |
| Body mass, kg | 70 ± 14 |
| Body mass index, kg/m2 | 23 ± 3 |
| Systolic BP, mmHg | 107 ± 11 |
| Diastolic BP, mmHg | 60 ± 7 |
Data are presented as means ± SD. BP, arterial blood pressure.
Responses to water deprivation.
Plasma osmolality, serum [Na+], urine osmolality, urine-specific gravity, and thirst were all significantly elevated during WD compared with CON (Table 2). Despite significant changes in several hydration markers during WD, all resting hemodynamic measures reported were not different between conditions (Table 3). Plasma volume, estimated by changes in Hb (CON: 13.5 ± 1.5 vs. WD: 13.7 ± 1.4 g/dL, P = 0.11) and Hct (CON: 40.5 ± 4.0 vs. WD: 40.9 ± 4.1%, P = 0.84), was not significantly lower during WD (−1.1 ± 8.2%, P = 0.49). Twenty-four-hour urinary sodium excretion values (CON: 3,438 ± 2,065 vs. WD: 3,030 ± 1,571 mg/day, P = 0.76) were similar between each 3-day protocol. Model flow-derived estimates of cardiac output (CON: 5.1 ± 1.0 vs. WD: 5.3 ± 0.9 L/min, P = 0.28) and total peripheral resistance (CON: 1,614 ± 389 vs. WD: 1,512 ± 373 dynes·s−1·cm−5, P = 0.27) were not different between conditions. We did not observe reductions in body mass during WD compared with CON (CON: 68.5 ± 13.5 vs. WD: 67.8 ± 13.1 kg, P = 0.84).
Table 2.
Biochemical measures
| Control | Water Deprivation | P Value | |
|---|---|---|---|
| Plasma osmolality, mosmol/kg H2O | 288 ± 4 | 292 ± 5* | <0.01 |
| Serum sodium, mmol/L | 141.0 ± 2.3 | 142.1 ± 1.7* | <0.01 |
| Serum chloride, mmol/L | 103.7 ± 3.0 | 105.3 ± 2.6* | <0.01 |
| Serum potassium, mmol/L | 4.07 ± 0.30 | 4.14 ± 0.41 | 0.33 |
| Urine osmolality, mosmol/kg H2O | 480 ± 161 | 711 ± 199* | <0.01 |
| Spot urine-specific gravity | 1.016 ± 0.007 | 1.022 ± 0.004* | <0.01 |
| 24-h Urine-specific gravity | 1.013 ± 0.005 | 1.019 ± 0.005* | <0.01 |
| Urine rate, L/24 h | 1.3 ± 0.6 | 0.8 ± 0.3* | <0.01 |
| Thirst rating | 3 ± 3 | 7 ± 2* | <0.01 |
Data were compared using paired, 2-tailed t tests and are presented as means ± SD.
P < 0.05.
Table 3.
Baseline cardiovascular measures
| Control | Water Deprivation | P Value | |
|---|---|---|---|
| Systolic BP, mmHg | 110 ± 9 | 112 ± 9 | 0.18 |
| Mean BP, mmHg | 80 ± 6 | 80 ± 6 | 0.46 |
| Diastolic BP, mmHg | 64 ± 6 | 64 ± 6 | 0.88 |
| Heart rate, beats/min | 58 ± 7 | 58 ± 8 | 0.96 |
| Burst frequency, bursts/min | 15 ± 7 | 14 ± 7 | 0.55 |
| Burst incidence, bursts/100 heartbeats | 26 ± 14 | 25 ± 13 | 0.54 |
| Total activity, AU/min | 298 ± 387 | 210 ± 163 | 0.26 |
| Femoral artery diameter, mm | 7.8 ± 1.1 | 8.1 ± 1.3 | 0.28 |
| Mean femoral blood velocity, cm/s | 7.2 ± 2.2 | 6.6 ± 2.0 | 0.45 |
| Mean femoral blood flow, mL/min | 410 ± 228 | 456 ± 213 | 0.28 |
| Shear rate, s−1 | 48 ± 17 | 52 ± 12 | 0.37 |
Data were compared using paired, 2-tailed t tests and are presented as means ± SD. BP, arterial blood pressure. Arterial BP was measured at the brachial artery.
Blood pressure variability.
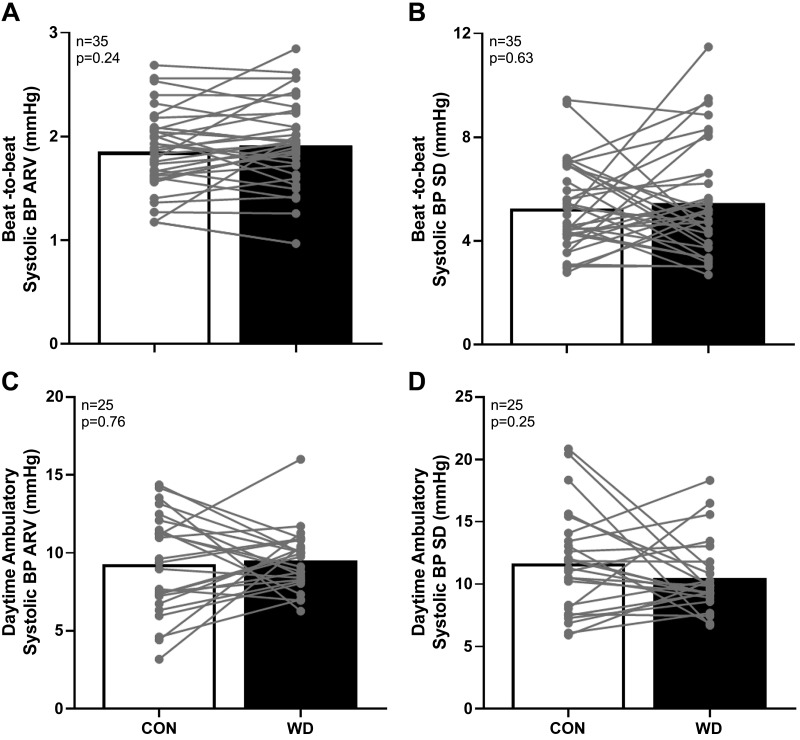
Resting beat-to-beat and ambulatory daytime systolic BPV (assessed via average real variability and standard deviation) were not different between conditions (Fig. 2). Similarly, we did not observe differences in beat-to-beat diastolic BP average real variability (CON: 1.5 ± 0.6 vs. WD: 1.5 ± 0.6 mmHg, P = 0.76) and standard deviation (CON: 3.9 ± 1.2 vs. WD: 3.7 ± 1.2 mmHg, P = 0.58) or ambulatory daytime diastolic BP average real variability (CON: 8.9 ± 2.5 vs.WD: 8.1 ± 1.7 mmHg, P = 0.19). Ambulatory daytime diastolic BP standard deviation was moderately higher during CON (CON: 10.5 ± 3.4 vs.WD: 9.0 ± 2.3 mmHg, P = 0.04). All indices of nighttime BPV were not different between conditions (P > 0.12 for all systolic and diastolic BP average real variability and standard deviation values). Twenty-four-hour, daytime, and nighttime BP, BP dipping, and heart rate during ambulatory monitoring were not different between conditions (Table 4). Finally, we found no relation between changes in serum [Na+] and changes in resting beat-to-beat systolic BP average real variability between conditions (r = 0.002, P = 0.99).
Fig. 2.
Summary data with individual data points are presented for beat-to-beat systolic arterial blood pressure (BP) average real variability (ARV) (A) and standard deviation (SD; B) during quiet rest in the supine position. Additionally, summary data with individual data points are presented for daytime ambulatory systolic BP ARV (C) and SD (D). Open bars indicate the normally hydrated control condition (CON), and filled bars indicate the water-deprived condition (WD). Data were compared with paired, 2-tailed t tests, with significance set at P < 0.05.
Table 4.
Ambulatory cardiovascular measures
| Control | Water Deprivation | P Value | |
|---|---|---|---|
| 24-h Systolic BP, mmHg | 117 ± 11 | 116 ± 11 | 0.75 |
| 24-h Diastolic BP, mmHg | 66 ± 6 | 66 ± 6 | 0.41 |
| Daytime systolic BP, mmHg | 121 ± 12 | 120 ± 11 | 0.84 |
| Daytime diastolic BP, mmHg | 70 ± 6 | 69 ± 6 | 0.38 |
| Nighttime systolic BP, mmHg | 105 ± 10 | 105 ± 12 | 0.87 |
| Nighttime diastolic BP, mmHg | 56 ± 6 | 56 ± 7 | 0.84 |
| 24-h Heart rate, beats/min | 67 ± 8 | 66 ± 7 | 0.70 |
| Daytime heart rate, beats/min | 83 ± 7 | 83 ± 8 | 0.92 |
| Nighttime heart rate, beats/min | 57 ± 7 | 56 ± 6 | 0.29 |
| Nighttime systolic BP dipping, % | 12 ± 5 | 12 ± 5 | 0.93 |
| Nighttime diastolic BP dipping, % | 19 ± 7 | 18 ± 6 | 0.27 |
Data were compared using paired, 2-tailed t tests and are presented as means ± SD. BP, arterial blood pressure. Arterial BP was measured at the brachial artery.
Arterial baroreflex function.
Resting sympathetic baroreflex gain (assessed as changes in both burst incidence and total activity) was not different between conditions (Fig. 3). Overall cardiac vagal baroreflex gain (CON: 23 ± 8 vs. WD: 25 ± 10 ms/mmHg, P = 0.43), gain from up sequences (CON: 26 ± 12 vs. WD: 26 ± 12 ms/mmHg, P = 0.74), and gain from down sequences (CON: 21 ± 9 vs. WD: 26 ± 10 ms/mmHg, P = 0.46) were not different between experimental conditions. Beat-to-beat systolic BP average real variability was not related to sympathetic baroreflex gain (r = 0.03, P = 0.71) or overall cardiac vagal baroreflex gain (r = 0.007, P = 0.96). Ambulatory systolic blood pressure average real variability was also not related to sympathetic baroreflex gain (r = 0.16, P = 0.53) or overall cardiac vagal baroreflex gain (r = 0.28, P = 0.20).
Fig. 3.
Summary data with individual data points are presented for the slopes of the linear regressions between muscle sympathetic nerve activity (MSNA) burst incidence and diastolic arterial blood pressure (BP; A) and total activity and diastolic BP (B) during quiet rest in the supine position. Open bars indicate the normally hydrated control condition (CON), and filled bars indicate the water-deprived condition (WD). Data were compared with paired, 2-tailed t tests, with significance set at P < 0.05. AU, arbitrary units. BRS, baroreflex sensitivity.
Sympathetic vascular transduction.
Mean BP changes and limb vascular conductance percent changes over the 10 cardiac cycles following spontaneous MSNA bursts were not different between conditions (Fig. 4). Additionally, peak mean BP and peak limb vascular conductance changes following spontaneous MSNA bursts were not different between conditions (Fig. 4). We observed nonsignificant relations between peak mean BP changes attained over the 10 cardiac cycles following spontaneous MSNA bursts and beat-to-beat mean BP average real variability within the CON condition (r = 0.22, P = 0.36) and within the WD condition (r = 0.03, P = 0.91). Additionally, there was no significant relation observed between changes in sympathetic vascular transduction of mean BP following spontaneous MSNA bursts (WD-CON) and changes in beat-to-beat mean BP average real variability (WD-CON) between conditions (r = 0.12, P = 0.64).
Fig. 4.
Summary data (means ± SD) are presented, showing the effect of spontaneous muscle sympathetic nerve activity (MSNA) bursts on mean arterial blood pressure (BP; A) and limb vascular conductance (LVC; C) over 10 cardiac cycles during quiet rest in the supine position. Additionally, summary data with individual data points are presented for peak absolute increases in mean BP (B) and peak %decreases in LVC (D) over the 10 cardiac cycles immediately following spontaneous MSNA bursts. ○ and open bars, normally hydrated control condition (CON); ● and filled bars, water-deprived condition (WD). Data were compared with 2-way (time × condition) repeated-measures ANOVAs (B and D) and paired, 2-tailed t tests (A and C), with significance set at P < 0.05. *Significant time effect.
Exploratory sex differences.
Blood pressure variability was not different between male and female adults (beat-to-beat systolic BP average real variability: sex, P = 0.35; condition, P = 0.24; interaction, P = 0.78; ambulatory systolic BP average real variability: sex, P = 0.54; condition, P = 0.77; interaction, P = 0.66). Resting arterial baroreflex gain values were also not different between sexes (spontaneous sympathetic baroreflex gain: sex, P = 0.55; condition, P = 0.29; interaction, P = 0.41; cardiac vagal baroreflex gain: sex, P = 0.33; condition, P = 0.35; interaction, P = 0.29). Finally, peak increases in mean BP following spontaneous bursts of MSNA were not different between sexes (sex: P = 0.90; condition: P = 0.21; interaction: P = 0.60).
DISCUSSION
The primary novel finding of this study was that short-term WD did not affect resting beat-to-beat or daytime ambulatory systolic BPV. Both spontaneous sympathetic and cardiac vagal baroreflex gain were unaffected by short-term WD. There were also no differences observed between conditions for indices of sympathetic vascular transduction following spontaneous MSNA bursts. Together, these findings suggest that short-term WD elicits modest increases in serum [Na+] and plasma osmolality but may not adversely affect resting or ambulatory BPV in healthy young adults. Finally, our exploratory analyses suggest that biological sex does not influence the effect of acute WD on BPV, arterial baroreflex function, or sympathetic vascular transduction.
Our hypothesis was developed in part by prior observations from rodent studies suggesting that hypernatremia induced by high-salt feeding increases BPV through central sensitization of sympathetic circuits (59). Thus, we reasoned that short-term WD-induced increases in serum [Na+] in humans would augment BPV through similar mechanisms. One key difference between this previous rodent study (59) and our current human study is length of time for the exposure to elevated serum/plasma [Na+]. In the previous study, rats were exposed to high-salt diets for 14–17 days, and it is likely that plasma [Na+] increased and remained elevated for several days before testing. In contrast, the current study design likely caused increases in serum [Na+] only during the final 24-h period before testing. Additionally, species differences could have potentially contributed to the discrepancy in findings related to BPV. Separately, there is also published evidence suggesting that WD impairs vasodilatory function (3), which could contribute to increases in BPV through reduced vasodilatory signaling to blunt α1-adrenergic vasoconstriction (32). However, one difference between our current study and the previous study (3) is that our study design included a WD-induced hypohydration that increased plasma osmolality by ∼4 mosmol/kg H2O, whereas the previous study (3) used exercise in the heat and fluid restriction to elicit a 9 mosmol/kg H2O increase in serum osmolality. Thus, more severe methods of hypohydration may be necessary to observe alterations in sympathetic vascular transduction and BPV. Together, these reasons may explain why, contrary to our hypothesis, we did not observe short-term WD-induced increases in serum [Na+] to increase BPV in healthy adults.
The Fadel laboratory has described the methodology for quantification of mean BP and limb vascular conductance changes following spontaneous MSNA bursts and prospectively applied it to gain additional insight into the peripheral arc of the arterial baroreflex (22, 23, 68). Our group recently observed reductions in sympathetic vascular transduction following a low-sodium (1,000 mg/day) diet compared with a recommended-sodium (2,300 mg/day) diet (5), which partially informed our hypothesis here. Additionally, mild hypohydration in humans has been demonstrated to impair endothelial-dependent dilation (2). Because intact endothelial function is important for blunting α1-adrenergic vasoconstriction (28) (i.e., the primary mediator of sympathetic vascular transduction; see Ref. 19), we reasoned that mild hypohydration would augment sympathetic vascular transduction as a result of reduced vasodilatory signaling. Within each condition, we did not observe a significant relation between sympathetic vascular transduction of mean BP changes following spontaneous MSNA bursts and beat-to-beat mean BP average real variability. This is in contrast to previous studies (5, 55, 68) that reported a significant relation between transduction of mean BP and BPV. The reasons for this discrepancy are currently unclear.
Although changes in BPV were not observed in the present study, our findings are consistent with a previous study in humans that reported no change in the power spectral density of mean BP, a measure of BPV in the frequency domain, following a furosemide-induced hypohydration protocol (48). However, a key difference is that in the present study WD increased plasma osmolality (∼4 mosmol/kg H2O) but did not significantly change plasma volume (∼1% reduction during WD), whereas in the previous study there were no reports of plasma osmolality or blood electrolyte concentrations, but there was moderate plasma volume contraction (−10% following furosemide administration) (48). Thus, because furosemide elicits iso-osmotic hypovolemia, the previous study does not necessarily inform our primary hypothesis regarding WD-induced elevations in serum [Na+] and BPV.
Regarding resting BP values following WD, one study in rodents reported that sympathetic blockade (via α1- and β1-adrenergic receptor antagonists) significantly attenuated the increases in BP brought about by 48 h of WD (66), suggesting that changes in BP following WD are mediated by the sympathetic nervous system. Additionally, work from Brooks and colleagues (10, 11) has reported that following 48 h of WD, BP is supported by increases in sympathetic outflow via increased excitatory amino acid neurotransmission in the RVLM. However, our recent work demonstrated that WD does not enhance responsiveness of RVLM neurons and does not exaggerate the somato-sympathetic reflex (69). One previous study in our laboratory suggested that an acute increase in serum [Na+] can increase resting BP and MSNA burst incidence in humans (9). However, this study employed rapid intravenous infusion of hypertonic saline and increased serum [Na+] of ∼4 mM compared with an increase in serum [Na+] of ∼1 mM reported here. Also, the previous study elicited plasma volume expansion (+4%), whereas in the current study we observed a nonsignificant change in plasma volume (−1%). Although currently unclear, it is possible that participants in the current study (increase in plasma osmolality of ∼1% during WD) did not reach their osmotic threshold (a change in plasma osmolality of 1–2%; see Ref. 18), which could be why resting BP and MSNA burst incidence were similar between conditions. Together, the physiological differences resulting from a water restriction protocol versus rapid infusion of hypertonic saline may explain why their effects on resting BP and MSNA differ.
Although changes in arterial baroreflex function were not observed in the present study, our findings are consistent with a previous study in humans that reported no change in sympathetic baroreflex gain following a water restriction protocol (54). However, a key difference is that in the present study WD increased plasma osmolality (∼4 mosmol/kg H2O), whereas in the previous study there was no observed change in plasma osmolality (euhydration: 286 ± 1 vs. dehydration: 286 ± 1 mosmol/kg H2O). This is likely because in the previous study, fluid intake was limited to 10 mL fluid/kg body wt for the 24-h period preceding the dehydrated visit, whereas in the present study participants reduced their water intake over 3 days and then completed a 16-h water abstention before testing. As discussed above, the relatively short time frame of elevated serum [Na+] or the difference in magnitude of changes in serum [Na+] may explain why no changes in sympathetic baroreflex gain were observed in the present study. Another study investigating the effects of dehydration on baroreflex function noted a trend for lower sympathetic baroreflex gain following dehydration induced by 90 min of acute aerobic exercise compared with intravenous rehydration ∼30 min later (15). Although insightful, these data (collected ∼1–2 h postexercise) could have potentially been influenced by the prior bout of exercise. A consideration in the present study design was to impose mild hypohydration without the potential confounding effects of heat stress and/or exercise, as performed in other previous studies (15, 52). Although several health factors have been demonstrated to affect BPV among healthy young adults (40), our data suggest that short-term WD does not increase (i.e., worsen) BPV among healthy young male and female adults.
Our strategy to achieve a mild, not severe, form of hypohydration in humans involved short-term WD. These data demonstrate that we were successful in experimentally causing modest increases in serum [Na+], plasma osmolality, urine osmolality, thirst, and urine-specific gravity. The increases in plasma osmolality (∼4 mosmol/kg H2O) represent physiologically relevant changes and are similar to those observed in previous studies examining the effects of mild hypohydration (15, 60). Based on published reference values for hydration biomarkers in humans, plasma osmolality and 24-h urine volume values in the present study suggest that participants were on average “well-hydrated/euhydrated” during CON and at least “slightly dehydrated” during WD (2). The change in hydration status achieved here increases relevance to the general public, as mild hypohydration is a contributing factor in numerous acute and chronic diseases (39), with underhydration predicting future incidence of diabetes (56) and heart disease (14). Other investigations have demonstrated that mild hypohydration affects cerebrovascular responses during the cold pressor test (51), impairs exercise performance (46), and reduces overall cognitive performance (71) and executive function (60). Our focus to examine the effect of mild hypohydration on BPV is highly clinically relevant given that high BPV is predictive of several disease states and mortality in humans (35, 36, 38, 50, 53, 65).
Limitations.
The primary objective of this study was to determine whether mild hypohydration increases BPV in young adults; however, there were limitations. First, this study provides insight into the effects of acute WD and consequent modest increases in serum [Na+] on BPV, but the effects of severe WD (i.e., longer-term water deprivation) on BPV are unknown. However, the water abstention model used here imposes physiologically relevant changes in serum [Na+], informing us about the effects of mild hypohydration without the confounding effects of heat stress and exercise. Second, although plasma osmolality and 24-h urine volume values suggest that participants were “slightly dehydrated” during WD, urine osmolality and specific gravity values indicate that participants were “well-hydrated/euhydrated” during WD (2). Nevertheless, there were clear differences in hydration status between the two conditions among these participants (see Table 2). Third, we were not fully powered to determine sex differences in the present analysis (given Cohen’s f of 0.25, a priori power analysis determined a need for 49 female and 49 male adults to complete testing). However, as reported in results, our exploratory analyses suggest that sex did not influence our findings for BPV, arterial baroreflex function, or sympathetic vascular transduction following acute WD. Fourth, our conclusions are restricted to healthy young adults, and it is possible that WD in old adults may elicit alterations in BPV. Specifically, postmenopausal females lose the protective effects of estrogen and MSNA increases with age, with resting MSNA becoming more tightly coupled to BP (30). Thus, a future study comparing the effects of mild hypohydration on BPV in young and old (postmenopausal) adults is warranted. Fifth, we did not measure renin-angiotensin-aldosterone system hormones or arginine vasopressin, which are known to play a role in BP regulation during hydration manipulations. Finally, this study did not assess endothelial function, which would have further informed our hypothesis. Future studies are warranted to address the limitations discussed above.
Perspectives and Significance
These data suggest that acute WD does not augment resting beat-to-beat or ambulatory BPV in healthy young adults. Furthermore, our findings suggest that acute WD does not impact spontaneous sympathetic baroreflex function or resting sympathetic vascular transduction in healthy young adults.
GRANTS
This research was supported by the National Heart, Lung, and Blood Institute (R01-HL-128388; W. B. Farquhar and S. D. Stocker), the American Heart Association (18POST34060020; A. T. Robinson), and a University of Delaware Doctoral Fellowship (J. C. Watso). This publication was made possible by the Delaware COBRE program, supported by a grant from the National Institute of General Medical Sciences (5-P30-GM-113125) from the National Institutes of Health.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.C.W., A.T.R., M.C.B., M.M.W., S.D.S., and W.B.F. conceived and designed research; J.C.W., A.T.R., M.C.B., K.U.M., M.M.W., and W.B.F. performed experiments; J.C.W. analyzed data; J.C.W., A.T.R., M.C.B., K.U.M., M.M.W., S.D.S., and W.B.F. interpreted results of experiments; J.C.W. prepared figures; J.C.W. and A.T.R. drafted manuscript; J.C.W., A.T.R., M.C.B., K.U.M., M.M.W., S.D.S., and W.B.F. edited and revised manuscript; J.C.W., A.T.R., M.C.B., K.U.M., M.M.W., S.D.S., and W.B.F. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank all study volunteers for their participation. Additionally, we thank laboratory coordinator Liza Walker, research nurse Wendy Nichols, Dr. Michal Brian, research dietitian Sofia Sanchez, undergraduate research assistant Erin Ryan, the Cardiovascular Core Laboratory staff, and the University of Delaware Nurse Managed Primary Care Center for their assistance with the study.
REFERENCES
- 1.Adams JM, Madden CJ, Sved AF, Stocker SD. Increased dietary salt enhances sympathoexcitatory and sympathoinhibitory responses from the rostral ventrolateral medulla. Hypertension 50: 354–359, 2007. doi: 10.1161/HYPERTENSIONAHA.107.091843. [DOI] [PubMed] [Google Scholar]
- 2.Armstrong LE, Pumerantz AC, Fiala KA, Roti MW, Kavouras SA, Casa DJ, Maresh CM. Human hydration indices: acute and longitudinal reference values. Int J Sport Nutr Exerc Metab 20: 145–153, 2010. doi: 10.1123/ijsnem.20.2.145. [DOI] [PubMed] [Google Scholar]
- 3.Arnaoutis G, Kavouras SA, Stratakis N, Likka M, Mitrakou A, Papamichael C, Sidossis LS, Stamatelopoulos K. The effect of hypohydration on endothelial function in young healthy adults. Eur J Nutr 56: 1211–1217, 2017. doi: 10.1007/s00394-016-1170-8. [DOI] [PubMed] [Google Scholar]
- 4.Babcock MC, Brian MS, Watso JC, Edwards DG, Stocker SD, Wenner MM, Farquhar WB. Alterations in dietary sodium intake affect cardiovagal baroreflex sensitivity. Am J Physiol Regul Integr Comp Physiol 315: R688–R695, 2018. doi: 10.1152/ajpregu.00002.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Babcock MC, Robinson AT, Migdal KU, Watso JC, Wenner MM, Stocker SD, Farquhar WB. Reducing dietary sodium to 1000 mg per day reduces neurovascular transduction without stimulating sympathetic outflow. Hypertension 73: 587–593, 2019. doi: 10.1161/HYPERTENSIONAHA.118.12074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bertinieri G, di Rienzo M, Cavallazzi A, Ferrari AU, Pedotti A, Mancia G. A new approach to analysis of the arterial baroreflex. J Hypertens Suppl 3: S79–S81, 1985. [PubMed] [Google Scholar]
- 7.Boggia J, Asayama K, Li Y, Hansen TW, Mena L, Schutte R. Cardiovascular risk stratification and blood pressure variability on ambulatory and home blood pressure measurement. Curr Hypertens Rep 16: 470–480, 2014. doi: 10.1007/s11906-014-0470-8. [DOI] [PubMed] [Google Scholar]
- 8.Kinsman BJ, Nation HN, Stocker SD. Hypothalamic signaling in body fluid homeostasis and hypertension. Curr Hypertens Rep 19: 50, 2017. doi: 10.1007/s11906-017-0749-7. [DOI] [PubMed] [Google Scholar]
- 9.Brian MS, Matthews EL, Watso JC, Babcock MC, Wenner MM, Rose WC, Stocker SD, Farquhar WB. The influence of acute elevations in plasma osmolality and serum sodium on sympathetic outflow and blood pressure responses to exercise. J Neurophysiol 119: 1257–1265, 2018. doi: 10.1152/jn.00559.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brooks VL, Freeman KL, Clow KA. Excitatory amino acids in rostral ventrolateral medulla support blood pressure during water deprivation in rats. Am J Physiol Heart Circ Physiol 286: H1642–H1648, 2004. doi: 10.1152/ajpheart.01004.2003. [DOI] [PubMed] [Google Scholar]
- 11.Brooks VL, Qi Y, O’Donaughy TL. Increased osmolality of conscious water-deprived rats supports arterial pressure and sympathetic activity via a brain action. Am J Physiol Regul Integr Comp Physiol 288: R1248–R1255, 2005. doi: 10.1152/ajpregu.00638.2004. [DOI] [PubMed] [Google Scholar]
- 12.Brooks VL, Freeman KL, O’Donaughy TL. Acute and chronic increases in osmolality increase excitatory amino acid drive of the rostral ventrolateral medulla in rats. Am J Physiol Regul Integr Comp Physiol 287: R1359–R1368, 2004. doi: 10.1152/ajpregu.00104.2004. [DOI] [PubMed] [Google Scholar]
- 13.Buñag RD, Miyajima E. Baroreflex impairment precedes hypertension during chronic cerebroventricular infusion of hypertonic sodium chloride in rats. J Clin Invest 74: 2065–2073, 1984. doi: 10.1172/JCI111630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chan J, Knutsen SF, Blix GG, Lee JW, Fraser GE. Water, other fluids, and fatal coronary heart disease: the Adventist Health Study. Am J Epidemiol 155: 827–833, 2002. doi: 10.1093/aje/155.9.827. [DOI] [PubMed] [Google Scholar]
- 15.Charkoudian N, Halliwill JR, Morgan BJ, Eisenach JH, Joyner MJ. Influences of hydration on post-exercise cardiovascular control in humans. J Physiol 552: 635–644, 2003. doi: 10.1113/jphysiol.2003.048629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Conen D, Aeschbacher S, Thijs L, Li Y, Boggia J, Asayama K, Hansen TW, Kikuya M, Björklund-Bodegård K, Ohkubo T, Jeppesen J, Gu YM, Torp-Pedersen C, Dolan E, Kuznetsova T, Stolarz-Skrzypek K, Tikhonoff V, Schoen T, Malyutina S, Casiglia E, Nikitin Y, Lind L, Sandoya E, Kawecka-Jaszcz K, Mena L, Maestre GE, Filipovský J, Imai Y, O’Brien E, Wang JG, Risch L, Staessen JA. Age-specific differences between conventional and ambulatory daytime blood pressure values. Hypertension 64: 1073–1079, 2014. doi: 10.1161/HYPERTENSIONAHA.114.03957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Creager MA, Roddy MA, Holland KM, Hirsch AT, Dzau VJ. Sodium depresses arterial baroreceptor reflex function in normotensive humans. Hypertension 17: 989–996, 1991. doi: 10.1161/01.HYP.17.6.989. [DOI] [PubMed] [Google Scholar]
- 18.Danziger J, Zeidel ML. Osmotic homeostasis. Clin J Am Soc Nephrol 10: 852–862, 2015. doi: 10.2215/CJN.10741013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DeSalvo KB, Olson R, Casavale KO. Dietary Guidelines for Americans. JAMA 315: 457–458, 2016. doi: 10.1001/jama.2015.18396. [DOI] [PubMed] [Google Scholar]
- 20.Dill DB, Costill DL. Calculation of percentage changes in volumes of blood, plasma, and red cells in dehydration. J Appl Physiol 37: 247–248, 1974. doi: 10.1152/jappl.1974.37.2.247. [DOI] [PubMed] [Google Scholar]
- 21.Drewnowski A, Rehm CD, Constant F. Water and beverage consumption among adults in the United States: cross-sectional study using data from NHANES 2005-2010. BMC Public Health 13: 1068, 2013. doi: 10.1186/1471-2458-13-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fairfax ST, Padilla J, Vianna LC, Davis MJ, Fadel PJ. Spontaneous bursts of muscle sympathetic nerve activity decrease leg vascular conductance in resting humans. Am J Physiol Heart Circ Physiol 304: H759–H766, 2013. doi: 10.1152/ajpheart.00842.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fairfax ST, Holwerda SW, Credeur DP, Zuidema MY, Medley JH, Dyke PC II, Wray DW, Davis MJ, Fadel PJ. The role of α-adrenergic receptors in mediating beat-by-beat sympathetic vascular transduction in the forearm of resting man. J Physiol 591: 3637–3649, 2013. doi: 10.1113/jphysiol.2013.250894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Farquhar WB, Wenner MM, Delaney EP, Prettyman AV, Stillabower ME. Sympathetic neural responses to increased osmolality in humans. Am J Physiol Heart Circ Physiol 291: H2181–H2186, 2006. doi: 10.1152/ajpheart.00191.2006. [DOI] [PubMed] [Google Scholar]
- 25.Filomena J, Riba-Llena I, Vinyoles E, Tovar JL, Mundet X, Castañé X, Vilar A, López-Rueda A, Jiménez-Baladó J, Cartanyà A, Montaner J, Delgado P; ISSYS Investigators . Short-term blood pressure variability relates to the presence of subclinical brain small vessel disease in primary hypertension. Hypertension 66: 634–640, 2015. doi: 10.1161/HYPERTENSIONAHA.115.05440. [DOI] [PubMed] [Google Scholar]
- 26.Greaney JL, Edwards DG, Fadel PJ, Farquhar WB. Rapid onset pressor and sympathetic responses to static handgrip in older hypertensive adults. J Hum Hypertens 29: 402–408, 2015. doi: 10.1038/jhh.2014.106. [DOI] [PubMed] [Google Scholar]
- 27.Greaney JL, Ray CA, Prettyman AV, Edwards DG, Farquhar WB. Influence of increased plasma osmolality on sympathetic outflow during apnea. Am J Physiol Regul Integr Comp Physiol 299: R1091–R1096, 2010. doi: 10.1152/ajpregu.00341.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Greaney JL, Schwartz CE, Edwards DG, Fadel PJ, Farquhar WB. The neural interaction between the arterial baroreflex and muscle metaboreflex is preserved in older men. Exp Physiol 98: 1422–1431, 2013. doi: 10.1113/expphysiol.2013.073189. [DOI] [PubMed] [Google Scholar]
- 29.Guelen I, Westerhof BE, Van Der Sar GL, Van Montfrans GA, Kiemeneij F, Wesseling KH, Bos WJ. Finometer, finger pressure measurements with the possibility to reconstruct brachial pressure. Blood Press Monit 8: 27–30, 2003. doi: 10.1097/00126097-200302000-00006. [DOI] [PubMed] [Google Scholar]
- 30.Hart EC, Joyner MJ, Wallin BG, Charkoudian N. Sex, ageing and resting blood pressure: gaining insights from the integrated balance of neural and haemodynamic factors. J Physiol 590: 2069–2079, 2012. doi: 10.1113/jphysiol.2011.224642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hart EC, Joyner MJ, Wallin BG, Karlsson T, Curry TB, Charkoudian N. Baroreflex control of muscle sympathetic nerve activity: a nonpharmacological measure of baroreflex sensitivity. Am J Physiol Heart Circ Physiol 298: H816–H822, 2010. doi: 10.1152/ajpheart.00924.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hearon CM Jr, Kirby BS, Luckasen GJ, Larson DG, Dinenno FA. Endothelium-dependent vasodilatory signalling modulates α1 -adrenergic vasoconstriction in contracting skeletal muscle of humans. J Physiol 594: 7435–7453, 2016. doi: 10.1113/JP272829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kavouras SA. Hydration, dehydration, underhydration, optimal hydration: are we barking up the wrong tree? Eur J Nutr 58: 471–473, 2019. doi: 10.1007/s00394-018-01889-z. [DOI] [PubMed] [Google Scholar]
- 34.Brian MS, Dalpiaz A, Matthews EL, Lennon-Edwards S, Edwards DG, Farquhar WB. Dietary sodium and nocturnal blood pressure dipping in normotensive men and women. J Hum Hypertens 31: 145–150, 2017. doi: 10.1038/jhh.2016.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Madden JM, O’Flynn AM, Dolan E, Fitzgerald AP, Kearney PM. Short-term blood pressure variability over 24 h and target organ damage in middle-aged men and women. J Hum Hypertens 29: 719–725, 2015. doi: 10.1038/jhh.2015.18. [DOI] [PubMed] [Google Scholar]
- 36.Mancia G, Parati G, Hennig M, Flatau B, Omboni S, Glavina F, Costa B, Scherz R, Bond G, Zanchetti A; ELSA Investigators . Relation between blood pressure variability and carotid artery damage in hypertension: baseline data from the European Lacidipine Study on Atherosclerosis (ELSA). J Hypertens 19: 1981–1989, 2001. doi: 10.1097/00004872-200111000-00008. [DOI] [PubMed] [Google Scholar]
- 37.Mancia G, Parati G. Ambulatory blood pressure monitoring and organ damage. Hypertension 36: 894–900, 2000. doi: 10.1161/01.HYP.36.5.894. [DOI] [PubMed] [Google Scholar]
- 38.Mancia G, Bombelli M, Facchetti R, Madotto F, Corrao G, Trevano FQ, Grassi G, Sega R. Long-term prognostic value of blood pressure variability in the general population: results of the Pressioni Arteriose Monitorate e Loro Associazioni Study. Hypertension 49: 1265–1270, 2007. doi: 10.1161/HYPERTENSIONAHA.107.088708. [DOI] [PubMed] [Google Scholar]
- 39.Manz F. Hydration and disease. J Am Coll Nutr 26, Suppl: 535S–541S, 2007. doi: 10.1080/07315724.2007.10719655. [DOI] [PubMed] [Google Scholar]
- 40.Maseli A, Aeschbacher S, Schoen T, Fischer A, Jung M, Risch M, Risch L, Conen D. Healthy lifestyle and blood pressure variability in young adults. Am J Hypertens 30: 690–699, 2017. doi: 10.1093/ajh/hpx034. [DOI] [PubMed] [Google Scholar]
- 41.Matthews EL, Brian MS, Coyle DE, Edwards DG, Stocker SD, Wenner MM, Farquhar WB. Peripheral venous distension elicits a blood pressure raising reflex in young and middle-aged adults. Am J Physiol Regul Integr Comp Physiol 310: R1128–R1133, 2016. doi: 10.1152/ajpregu.00438.2015. [DOI] [PubMed] [Google Scholar]
- 42.Matthews EL, Brian MS, Edwards DG, Stocker SD, Wenner MM, Farquhar WB. Blood pressure responses to dietary sodium: Association with autonomic cardiovascular function in normotensive adults. Auton Neurosci 208: 51–56, 2017. doi: 10.1016/j.autneu.2017.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mena LJ, Felix VG, Melgarejo JD, Maestre GE. 24-Hour Blood Pressure Variability Assessed by Average Real Variability: A Systematic Review and Meta-Analysis. J Am Heart Assoc 6: 1–10, 2017. doi: 10.1161/JAHA.117.006895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mena L, Pintos S, Queipo NV, Aizpúrua JA, Maestre G, Sulbarán T. A reliable index for the prognostic significance of blood pressure variability. J Hypertens 23: 505–511, 2005. doi: 10.1097/01.hjh.0000160205.81652.5a. [DOI] [PubMed] [Google Scholar]
- 45.Monahan KD. Effect of aging on baroreflex function in humans. Am J Physiol Regul Integr Comp Physiol 293: R3–R12, 2007. doi: 10.1152/ajpregu.00031.2007. [DOI] [PubMed] [Google Scholar]
- 46.Murray B. Hydration and physical performance. J Am Coll Nutr 26, Suppl: 542S–548S, 2007. doi: 10.1080/07315724.2007.10719656. [DOI] [PubMed] [Google Scholar]
- 47.O’Brien E, Coats A, Owens P, Petrie J, Padfield PL, Littler WA, de Swiet M, Mee F. Use and interpretation of ambulatory blood pressure monitoring: recommendations of the British hypertension society. BMJ 320: 1128–1134, 2000. doi: 10.1136/bmj.320.7242.1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ogawa Y, Iwasaki K, Aoki K, Saitoh T, Kato J, Ogawa S. Dynamic cerebral autoregulation after mild dehydration to simulate microgravity effects. Aviat Space Environ Med 80: 443–447, 2009. doi: 10.3357/ASEM.2449.2009. [DOI] [PubMed] [Google Scholar]
- 49.Ogoh S, Fisher JP, Young CN, Raven PB, Fadel PJ. Transfer function characteristics of the neural and peripheral arterial baroreflex arcs at rest and during postexercise muscle ischemia in humans. Am J Physiol Heart Circ Physiol 296: H1416–H1424, 2009. doi: 10.1152/ajpheart.01223.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Palatini P, Penzo M, Racioppa A, Zugno E, Guzzardi G, Anaclerio M, Pessina AC. Clinical relevance of nighttime blood pressure and of daytime blood pressure variability. Arch Intern Med 152: 1855–1860, 1992. doi: 10.1001/archinte.1992.00400210081013. [DOI] [PubMed] [Google Scholar]
- 51.Perry BG, Bear TLK, Lucas SJE, Mündel T. Mild dehydration modifies the cerebrovascular response to the cold pressor test. Exp Physiol 101: 135–142, 2016. doi: 10.1113/EP085449. [DOI] [PubMed] [Google Scholar]
- 52.Posch AM, Luippold AJ, Mitchell KM, Bradbury KE, Kenefick RW, Cheuvront SN, Charkoudian N. Sympathetic neural and hemodynamic responses to head-up tilt during isoosmotic and hyperosmotic hypovolemia. J Neurophysiol 118: 2232–2237, 2017. doi: 10.1152/jn.00403.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pringle E, Phillips C, Thijs L, Davidson C, Staessen JA, de Leeuw PW, Jaaskivi M, Nachev C, Parati G, O’Brien ET, Tuomilehto J, Webster J, Bulpitt CJ, Fagard RH; Syst-Eur investigators . Systolic blood pressure variability as a risk factor for stroke and cardiovascular mortality in the elderly hypertensive population. J Hypertens 21: 2251–2257, 2003. doi: 10.1097/00004872-200312000-00012. [DOI] [PubMed] [Google Scholar]
- 54.Rabbitts JA, Strom NA, Sawyer JR, Curry TB, Dietz NM, Roberts SK, Kingsley-Berg SM, Charkoudian N. Influence of endogenous angiotensin II on control of sympathetic nerve activity in human dehydration. J Physiol 587: 5441–5449, 2009. doi: 10.1113/jphysiol.2009.176693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Robinson AT, Babcock MC, Watso JC, Brian MS, Migdal KU, Wenner MM, Farquhar WB. Relation between resting sympathetic outflow and vasoconstrictor responses to sympathetic nerve bursts: sex differences in healthy young adults. Am J Physiol Regul Integr Comp Physiol 316: R463–R471, 2019. doi: 10.1152/ajpregu.00305.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Roussel R, Fezeu L, Bouby N, Balkau B, Lantieri O, Alhenc-Gelas F, Marre M, Bankir L; D.E.S.I.R. Study Group . Low water intake and risk for new-onset hyperglycemia. Diabetes Care 34: 2551–2554, 2011. doi: 10.2337/dc11-0652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Scrogin KE, McKeogh DF, Brooks VL. Is osmolality a long-term regulator of renal sympathetic nerve activity in conscious water-deprived rats? Am J Physiol Regul Integr Comp Physiol 282: R560–R568, 2002. doi: 10.1152/ajpregu.00780.2000. [DOI] [PubMed] [Google Scholar]
- 58.Shoemaker JK, Klassen SA, Badrov MB, Fadel PJ. Fifty years of microneurography: learning the language of the peripheral sympathetic nervous system in humans. J Neurophysiol 119: 1731–1744, 2018. doi: 10.1152/jn.00841.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Simmonds SS, Lay J, Stocker SD. Dietary salt intake exaggerates sympathetic reflexes and increases blood pressure variability in normotensive rats. Hypertension 64: 583–589, 2014. doi: 10.1161/HYPERTENSIONAHA.114.03250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stachenfeld NS, Leone CA, Mitchell ES, Freese E, Harkness L. Water intake reverses dehydration associated impaired executive function in healthy young women. Physiol Behav 185: 103–111, 2018. doi: 10.1016/j.physbeh.2017.12.028. [DOI] [PubMed] [Google Scholar]
- 61.Steinback CD, Fraser GM, Usselman CW, Reyes LM, Julian CG, Stickland MK, Chari RS, Khurana R, Davidge ST, Davenport MH. Blunted sympathetic neurovascular transduction during normotensive pregnancy. J Physiol 597: 3687–3696, 2019. doi: 10.1113/JP277714. [DOI] [PubMed] [Google Scholar]
- 62.Stocker SD, Madden CJ, Sved AF. Excess dietary salt intake alters the excitability of central sympathetic networks. Physiol Behav 100: 519–524, 2010. doi: 10.1016/j.physbeh.2010.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sundlöf G, Wallin BG. Human muscle nerve sympathetic activity at rest. Relationship to blood pressure and age. J Physiol 274: 621–637, 1978. doi: 10.1113/jphysiol.1978.sp012170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Szinnai G, Morgenthaler NG, Berneis K, Struck J, Müller B, Keller U, Christ-Crain M. Changes in plasma copeptin, the C-terminal portion of arginine vasopressin during water deprivation and excess in healthy subjects. J Clin Endocrinol Metab 92: 3973–3978, 2007. doi: 10.1210/jc.2007-0232. [DOI] [PubMed] [Google Scholar]
- 65.Tatasciore A, Renda G, Zimarino M, Soccio M, Bilo G, Parati G, Schillaci G, De Caterina R. Awake systolic blood pressure variability correlates with target-organ damage in hypertensive subjects. Hypertension 50: 325–332, 2007. doi: 10.1161/HYPERTENSIONAHA.107.090084. [DOI] [PubMed] [Google Scholar]
- 66.Veitenheimer BJ, Engeland WC, Guzman PA, Fink GD, Osborn JW. Effect of global and regional sympathetic blockade on arterial pressure during water deprivation in conscious rats. Am J Physiol Heart Circ Physiol 303: H1022–H1034, 2012. doi: 10.1152/ajpheart.00413.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vianna LC, Hart EC, Fairfax ST, Charkoudian N, Joyner MJ, Fadel PJ. Influence of age and sex on the pressor response following a spontaneous burst of muscle sympathetic nerve activity. Am J Physiol Heart Circ Physiol 302: H2419–H2427, 2012. doi: 10.1152/ajpheart.01105.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vranish JR, Holwerda SW, Young BE, Credeur DP, Patik JC, Barbosa TC, Keller DM, Fadel PJ. Exaggerated Vasoconstriction to Spontaneous Bursts of Muscle Sympathetic Nerve Activity in Healthy Young Black Men. Hypertension 71: 192–198, 2018. doi: 10.1161/HYPERTENSIONAHA.117.10229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Watso JC, Babcock MC, Robinson AT, Migdal KU, Wenner MM, Stocker SD, Farquhar WB. Water deprivation does not augment sympathetic or pressor responses to sciatic afferent nerve stimulation in rats or to static exercise in humans. J Appl Physiol (1985) 127: 235–245, 2019. doi: 10.1152/japplphysiol.00005.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.White DW, Shoemaker JK, Raven PB. Methods and considerations for the analysis and standardization of assessing muscle sympathetic nerve activity in humans. Auton Neurosci 193: 12–21, 2015. doi: 10.1016/j.autneu.2015.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wilson MM, Morley JE. Impaired cognitive function and mental performance in mild dehydration. Eur J Clin Nutr 57, Suppl 2: S24–S29, 2003. doi: 10.1038/sj.ejcn.1601898. [DOI] [PubMed] [Google Scholar]
- 72.Young BE, Holwerda SW, Vranish JR, Keller DM, Fadel PJ. Sympathetic Transduction in Type 2 Diabetes Mellitus. Hypertension 74: 201–207, 2019. doi: 10.1161/HYPERTENSIONAHA.119.12928. [DOI] [PMC free article] [PubMed] [Google Scholar]