Abstract
We examined the contribution of the carotid chemoreceptors to insulin-mediated increases in muscle sympathetic nerve activity (MSNA) in healthy humans. We hypothesized that reductions in carotid chemoreceptor activity would attenuate the sympathoexcitatory response to hyperinsulinemia. Young, healthy adults (9 male/9 female, 28 ± 1 yr, 24 ± 1 kg/m2) completed a 30-min euglycemic baseline followed by a 90-min hyperinsulinemic (1 mU·kg fat-free mass−1·min−1), euglycemic infusion. MSNA (microneurography of the peroneal nerve) was continuously measured. The role of the carotid chemoreceptors was assessed at baseline and during hyperinsulinemia via 1) acute hyperoxia, 2) low-dose dopamine (1–4 µg·kg−1·min−1), and 3) acute hyperoxia + low-dose dopamine. MSNA burst frequency increased from baseline during hyperinsulinemia (P < 0.01). Acute hyperoxia had no effect on MSNA burst frequency at rest (P = 0.74) or during hyperinsulinemia (P = 0.83). The insulin-mediated increase in MSNA burst frequency (P = 0.02) was unaffected by low-dose dopamine (P = 0.60). When combined with low-dose dopamine, acute hyperoxia had no effect on MSNA burst frequency at rest (P = 0.17) or during hyperinsulinemia (P = 0.85). Carotid chemoreceptor desensitization in young, healthy men and women does not attenuate the sympathoexcitatory response to hyperinsulinemia. Our data suggest that the carotid chemoreceptors do not contribute to acute insulin-mediated increases in MSNA in young, healthy adults.
Keywords: carotid body, hyperinsulinemia, muscle sympathetic nerve activity
INTRODUCTION
The carotid body (CB) chemoreceptors are small organs located bilaterally at the bifurcation of the common carotid artery in the neck. Type 1 glomus cells within the CBs sense and respond to a wide range of stimuli (e.g., hypoxia, hypercapnia). Activation of the CB chemoreceptors results in release of neurotransmitters, which increases afferent nerve activity and initiates autonomic and cardiorespiratory responses (i.e., increase in sympathetic nerve activity and ventilation) (28, 39). It is commonly accepted that even small increases in plasma insulin concentrations have marked sympathoexcitatory effects (1, 2, 42), and emerging evidence from animal models has uncovered a potential role for the carotid chemoreceptors in insulin-mediated activation of the sympathetic nervous system. For example, in anesthetized dogs, insulin injected into the carotid artery increased arterial blood pressure, and this effect was abolished with ganglionic blockade (38), supporting a role for a local sympathoexcitatory mechanism. More recently, using a rodent model, Ribeiro and colleagues identified 1) insulin receptors on the CBs and 2) an increase in CB activation (increased ventilation and neurotransmitter release) with insulin exposure (40). Despite evidence supporting an effect of insulin on carotid chemoreceptor-mediated increases in sympathetic nervous system activity, these findings have yet to be translated to humans. Thus we sought to determine the contribution of the carotid chemoreceptors to insulin-mediated sympathoexcitation in healthy humans. We hypothesized that 1) an increase in muscle sympathetic nerve activity (MSNA) would be observed during hyperinsulinemia and 2) the effect of hyperinsulinemia on MSNA would be attenuated by hyperoxia and/or low-dose dopamine, both of which are known to inhibit CB sensory nerve activity (29).
METHODS
Participants.
Data are presented from two protocols conducted in two groups of individuals. All participants (n = 18, 9 male/9 female) were young (≤35 yr of age), healthy, nonobese (<30 kg/m2), and nonsmokers and were without chronic diseases and took no medications known to affect endocrine, cardiovascular, or autonomic function. Women were not pregnant (confirmed by negative pregnancy test ≤48 h before participation) and were studied in the early follicular phase of the menstrual cycle or the placebo phase of oral contraceptive use (self-report) to minimize effects of reproductive hormone status on data interpretation. Subjects were asked to refrain from alcohol, caffeine, and exercise for 24 h and fast for 12 h before the screening and study visits. Informed consent was obtained from all subjects, and all experiments and procedures were approved by the Institutional Review Board at the Mayo Clinic (protocol 13-001237) and conformed to the Declaration of Helsinki. Data unrelated to present hypotheses were published previously from a subset of participants (3). Preliminary findings were published in abstract form (33).
Screening visit.
Participants completed a screening visit, which included medical history, fasting blood chemistries, body composition testing [dual-energy X-ray absorptiometry (Lunar iDXA software version 6.10, GE Healthcare Technologies, Madison, WI)], and hypoxic ventilatory response testing. In protocol 1 (n = 7, 4 male/3 female), the acute hypoxic ventilatory response was assessed using a variable inspired O2 method (35). Breath-by-breath ventilation and inspired/expired gasses were monitored using a free-standing metabolic cart (Ultima CardiO2, MCG Diagnostics, St. Paul, MN). During the test, subjects breathed through a two-way nonrebreathing valve connected to a switching valve and were exposed to hypoxia using inspired gas mixtures of 21%, 16%, and 10% O2-balance N2. Each level was maintained for 2 min, and CO2 was added to the inspired air to maintain end-tidal CO2 at baseline levels. Data were averaged every five to seven breaths, and the hypoxic ventilatory response was calculated as the slope of the linear regression line for average ventilation (L/min) and O2 saturation (%SpO2).
In protocol 2 (n = 11, 5 male/6 female), the acute hypoxic ventilatory response was assessed as part of a dopamine dose-response test. Methods and results from this test were published previously (31). Briefly, an intravenous catheter was placed for infusion of dopamine, and breath-by-breath ventilation and inspired/expired gasses were monitored (Ultima CardiO2). Subjects breathed through a two-way nonrebreathing valve. Each hypoxic response test began with two to six breaths of 100% N2 followed by room air for 2 min (36). This was repeated four times during each dopamine infusion trial, achieving levels of 70–99% SpO2 (pulse oximetry). The hypoxic ventilatory response was calculated as follows: 1) for each administration of 100% N2, the three largest consecutive breaths were averaged; 2) the nadir %SpO2 was recorded for each N2 administration; and 3) chemosensitivity to hypoxia was assessed as the slope of the linear regression line for average ventilation (L/min) and nadir O2 saturation (%SpO2) (36). The response during saline conditions was used as a measure of baseline chemosensitivity. The individualized dose of dopamine that resulted in the maximum reduction in the hypoxic ventilatory response was used for the subsequent study visit (31). The two methods for assessing the hypoxic ventilatory response (variable inspired O2 and N2 breathing) have been shown to provide comparable results (9, 16, 19).
Study visit.
The initial set of subjects (protocol 1, n = 7) completed a single visit under saline conditions only. An additional group of subjects (protocol 2, n = 11) participated in two identical study visits separated by ≥1 wk and randomized to intravenous dopamine or placebo (saline). Data from protocols 1 and 2 (saline visit) were pooled for analysis (n = 18). Study procedures for both protocols were as follows.
Subjects were admitted to the Clinical Research and Trials Unit at the Mayo Clinic at 1700 on the evening before the study day. A standard 10 kcal/kg evening meal (55% carbohydrate, 30% fat, 15% protein) was consumed between 1800 and 1830 on the night before the study visit, and participants fasted thereafter. The study day began with instrumentation at 0800. Subjects rested supine during instrumentation, which included a three-lead electrocardiogram and pulse oximetry (GE Datex-Ohmeda Cardiocap/5, GE Healthcare, Chicago, IL). An intravenous catheter was placed in the dominant arm for insulin and glucose infusion, and an arterial catheter was placed in the nondominant arm for blood sampling and blood pressure monitoring (20-gauge, 5 cm) using aseptic technique under local anesthesia (2% lidocaine).
A tungsten microelectrode and standard microneurography procedures and techniques (21, 50) were used to record multiunit postganglionic MSNA from the peroneal nerve, posterior to the fibular head. The peroneal nerve was localized with transcutaneous stimulation and two-dimensional ultrasonography. The microneurography electrode was placed through the skin and into the peroneal nerve under ultrasound guidance (13). A muscle sympathetic fascicle was identified when taps on the muscle belly or passive muscle stretch evoked mechanoreceptive impulses and no afferent neural response was evoked by skin stimuli. The recorded signal was amplified 100,000-fold, band-pass-filtered (700–2,000 Hz), rectified, and integrated (resistance-capacitance integrator circuit, time constant 0.1 s) using a nerve-traffic analyzer. Sympathetic bursts in the integrated neurogram were identified using a custom-manufactured semiautomated analysis program; burst identification was then corrected by visual inspection by a single investigator (M.T.M.). The program compensated for baroreflex latency and associated each sympathetic burst with the appropriate cardiac cycle (8, 22, 23). MSNA was expressed as burst frequency (bursts/min) and burst incidence (bursts/100 heartbeats).
Subjects completed 30 min of resting baseline followed by a 90-min hyperinsulinemic, euglycemic infusion (Fig. 1). Intravenous insulin was infused at a rate of 1.0 mU·kg fat-free mass−1·min−1 for 90 min. Exogenous glucose (50% dextrose) was infused intravenously to maintain plasma glucose at baseline levels. Blood glucose was measured every 5–10 min at bedside using the glucose oxidase method (GM9 Glucose Analyzer, Analox Instruments, Stourbridge, UK; 2900D Biochemistry Analyzer, Yellow Springs Instruments, Yellow Springs, OH). All additional blood samples were placed on ice and centrifuged at 4°C, plasma was removed, and samples were stored at −80°C until analysis (insulin, epinephrine, norepinephrine). Standard assays were performed by the Immunochemistry Core Laboratory of the Clinical Research and Trials Unit and the Department of Laboratory Medicine and Pathology at the Mayo Clinic. Plasma insulin was assessed using a two-site immunoenzymatic assay performed on the Dxl automated immunoassay system (Beckman Instruments, Chaska, MN). Plasma catecholamines were measured by reverse-phase high-performance liquid chromatography with electrochemical detection after extraction with activated alumina.
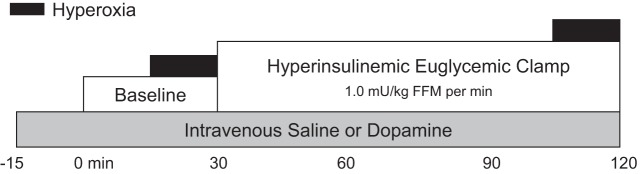
Fig. 1.
Study timeline. All subjects completed 30 min of resting baseline followed by a 90-min hyperinsulinemic, euglycemic infusion. Steady-state measures were collected at baseline and during the last 30 min of the insulin infusion. At this time, subjects breathed room air freely for 15 min and then were acutely exposed to hyperoxia. An initial group of subjects (protocol 1) completed a saline visit only. A second group of individuals (protocol 2) participated in 2 identical study visits randomized to placebo (saline) or intravenous dopamine. Dopamine/saline was infused for 15 min before collection of baseline measurements until the end of the 90-min insulin infusion. FFM, fat-free mass.
Steady-state measures were collected at baseline and after 60 min of the insulin infusion (Fig. 1). At this time, subjects were instrumented with a mouthpiece and noseclip connected to a two-way nonrebreathing valve, with care taken not to disrupt the MSNA signal. Breath-by-breath tidal volume (Universal Ventilation meter, VacuMed, Ventura, CA), respiratory rate, and inspired/expired gases (GE Datex-Ohmeda Cardiocap/5) were monitored and used to calculate minute ventilation. Subjects breathed room air [21% O2 (normoxia)] freely for 15 min followed by acute exposure to hyperoxia (14, 59). During hyperoxia, subjects were quietly switched to 100% O2 for ≥2 min to experimentally “turn off” the carotid chemoreceptors (14, 46). A 3-L meteorological balloon served as a volume reservoir. Arterial blood gases [arterial Po2 () and arterial Pco2] were collected at the end of the O2 exposure and analyzed immediately (model ABL700, Radiometer, Westlake, OH) in a subset of subjects (n = 10).
Intravenous low-dose dopamine.
As noted above, subjects in protocol 2 participated in two identical study visits separated by ≥1 wk and were randomized to intravenous dopamine or placebo (saline). Study procedures were identical between protocols, with the exception of placement of a second intravenous catheter in the dominant arm for dopamine/saline infusion. Dopamine/saline was infused for 15 min before collection of baseline measurements and continued until the end of the 90-min insulin infusion (31, 36, 58). Dopamine was infused at an individualized dose (average dose: 2.6 ± 0.3 μg⋅kg−1⋅min−1) that resulted in a maximum reduction in the hypoxic ventilatory response on the screening visit (saline: −0.31 ± 0.07 L·min−1·%−1; dopamine: −0.14 ± 0.07 L·min−1·%−1) (31). Subjects and research personnel were blinded to condition (saline, dopamine). The randomization scheme was determined by a single pharmacist within the Research Pharmacy at the Mayo Clinic. Only pharmacy and nursing staff were aware of study condition until each subject completed both visits and main outcome data were analyzed, at which time research personnel were unblinded (double-blind randomized crossover design).
Data analysis.
All data were collected using a PowerLab data acquisition system (analog-to-digital converter; ADinstruments, Colorado Springs, CO) with a sampling rate of 1,000 Hz. Because of the acute effects of hyperoxia on the carotid chemoreceptors, data were analyzed from the first 1 min of hyperoxia exposure and compared with the 1 min of normoxia immediately preceding the exposure (47). Main outcome variables included MSNA burst frequency and MSNA burst incidence. The effect of insulin (baseline, hyperinsulinemia) and chemoreceptor desensitization (normoxia/hyperoxia, saline/dopamine) on main outcome variables was assessed using a two-way repeated-measures analysis of variance, and pairwise multiple comparisons were made using Student-Newman-Keuls method. Statistical analysis was completed using SigmaPlot 14.0 (Systat Software, Inc.). An alpha of P < 0.05 was considered statistically significant. Data are reported as means ± SE.
RESULTS
Eighteen young, healthy men and women (9 male/9 female, 28 ± 1 yr, 24 ± 1 kg/m2) participated. Subject characteristics are reported in Table 1. Data from protocols 1 and 2 (saline visit) were pooled (n = 18). MSNA data were not available from all participants/visits. One female subject in protocol 2 did not complete the dopamine visit due to uncontrolled hypotension and tachycardia midway through the insulin infusion; therefore, data from protocol 2 (dopamine visit) are reported from 10 participants.
Table 1.
Subject demographics
| Value | |
|---|---|
| Sex, M/F | 9/9 |
| Age, yr | 28 ± 4 |
| Height, cm | 175 ± 13 |
| Weight, kg | 74 ± 16 |
| Body mass index, kg/m2 | 24 ± 3 |
| Body fat, % | 27 ± 6 |
| Glucose, mg/dL | 85 ± 9 |
| Total cholesterol, mg/dL | 164 ± 25 |
| Triglycerides, mg/dL | 83 ± 32a |
| LDL, mg/dL | 57 ± 24a |
| HDL, mg/dL | 85 ± 24a |
| Hypoxic ventilatory response, L·min−1·%−1 | −0.30 ± 0.20 |
Values are means ± SE; n = 18 unless otherwise noted.
n = 15.
Under control (saline) conditions (n = 18, protocols 1 and 2 pooled), intravenous insulin was infused at a constant rate (plasma insulin: 7 ± 1 to 51 ± 2 mg/dL), during which time exogenous glucose was infused (glucose infusion rate: 28 ± 2 mL/h) to maintain euglycemia (94 ± 2 to 95 ± 2 mg/dL). Within 15 s of exposure to hyperoxic gas, fraction of inspired O2 () was increased above baseline (Fig. 2) and resulted in a significant increase in : from 100 ± 3 mmHg at baseline to 487 ± 14 mmHg and from 112 ± 8 to 489 ± 14 mmHg during hyperinsulinemia (main effect of hyperoxia, P < 0.05). Heart rate increased from baseline during the hyperinsulinemic, euglycemic infusion period (main effect of insulin, P = 0.04). Acute hyperoxia resulted in a reduction in heart rate and breathing frequency that did not differ between baseline and hyperinsulinemia (main effect of insulin, P < 0.05; interaction of insulin and hyperoxia, P > 0.05; Table 2).
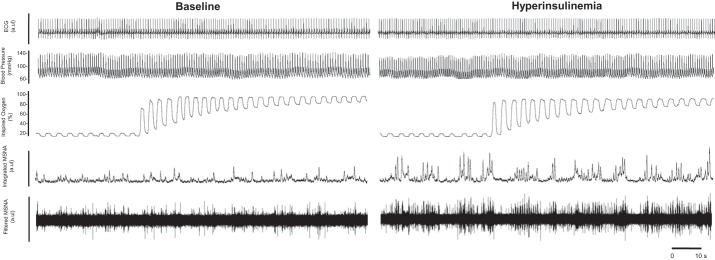
Fig. 2.
Representative data traces. Subjects breathed room air (21% O2, normoxia) and then quietly switched to 100% O2 to experimentally “turn off” the carotid chemoreceptors. Because of the acute effects of hyperoxia on the carotid chemoreceptors, data were analyzed from the first 1 min of hyperoxia exposure and compared with data from the first min of normoxia immediately preceding the exposure. MSNA, muscle sympathetic nerve activity; au, arbitrary units.
Table 2.
Effect of acute hyperoxia at baseline and during hyperinsulinemia
| n | Normoxia | Hyperoxia | |
|---|---|---|---|
| Heart rate, beats/min | 18 | ||
| Baseline | 64 ± 2 | 62 ± 2† | |
| Hyperinsulinemia | 66 ± 2* | 64 ± 2†* | |
| Mean blood pressure, mmHg | 17 | ||
| Baseline | 98 ± 2 | 98 ± 2 | |
| Hyperinsulinemia | 98 ± 2 | 98 ± 2 | |
| Tidal volume, L/breath | 16 | ||
| Baseline | 489 ± 28 | 532 ± 47 | |
| Hyperinsulinemia | 544 ± 38 | 551 ± 48 | |
| Breathing frequency, breaths/min | 18 | ||
| Baseline | 14.9 ± 0.9 | 13.3 ± 0.9† | |
| Hyperinsulinemia | 14.4 ± 0.8 | 13.6 ± 0.8† | |
| Minute ventilation, L/min | 16 | ||
| Baseline | 7.3 ± 0.5 | 7.4 ± 0.9 | |
| Hyperinsulinemia | 7.5 ± 0.3 | 7.2 ± 0.5 | |
| Inspired O2, % | 18 | ||
| Baseline | 21 ± 1 | 90 ± 2† | |
| Hyperinsulinemia | 21 ± 1 | 90 ± 2† | |
Values are means ± SE; n = 18 (9 male/9 female) unless otherwise noted. Data were analyzed by 2-way repeated-measures ANOVA, normality test (Shapiro-Wilk), equal variance test, and pairwise multiple comparison (Student-Newman-Keuls method):
P < 0.05 vs. baseline,
P < 0.05 vs. normoxia.
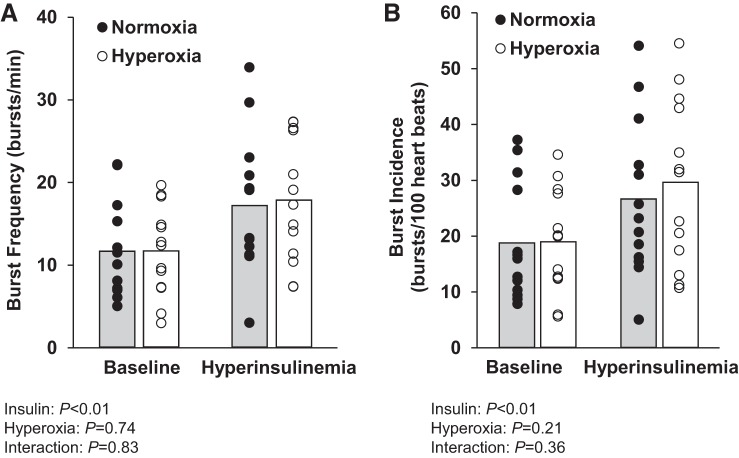
MSNA burst frequency and incidence (n = 13) increased from baseline during the hyperinsulinemic, euglycemic infusion period (main effect of insulin, P < 0.05; Fig. 3). Plasma catecholamines also increased from baseline during hyperinsulinemia (norepinephrine from 138 ± 11 to 188 ± 14 pg/mL, epinephrine from 43 ± 6 to 57 ± 7 pg/mL; main effect of insulin, P < 0.05). There was no effect of acute hyperoxia on MSNA burst frequency or burst incidence at baseline or during hyperinsulinemia (main effect of hyperoxia, P > 0.05; interaction of insulin and hyperoxia, P > 0.05; Fig. 3).
Fig. 3.
Effect of acute hyperoxia on muscle sympathetic nerve activity at baseline and during hyperinsulinemia. Individual (● and ○) and mean (open and shaded bars) values from 13 subjects are shown. A and B: average of 1 min before hyperoxia and during the first 1 min of hyperoxia for burst frequency and burst incidence. Data were analyzed by 2-way repeated-measures ANOVA and pairwise multiple comparison (Student-Newman-Keuls method).
For protocol 2, individuals (n = 10) completed two visits randomized to saline or low-dose dopamine. On both visits, insulin was infused at a constant rate (plasma insulin: 7 ± 1 to 46 ± 2 mg/dL during the saline visit and 13 ± 2 to 50 ± 4 mg/dL during the low-dose dopamine visit), and exogenous glucose was infused concomitantly (29 ± 3 mL/h during the saline visit and 29 ± 4 mL/h during the low-dose dopamine visit) to maintain blood glucose at fasted levels (93 ± 2 to 94 ± 2 mg/dL during the saline visit and 101 ± 3 to 101 ± 3 mg/dL during the low-dose dopamine visit). Heart rate, tidal volume, and minute ventilation increased from baseline during the hyperinsulinemic, euglycemic infusion period (main effect of insulin, P < 0.05). Dopamine resulted in an increase in resting heart rate (~5 beats/min) and a reduction in breathing frequency compared with saline conditions (main effect of dopamine, P < 0.05; Table 3).
Table 3.
Effect of low-dose dopamine at baseline and during hyperinsulinemia
| Saline | Dopamine | |
|---|---|---|
| Heart rate, beats/min | ||
| Baseline | 59 ± 2 | 64 ± 2† |
| Hyperinsulinemia | 63 ± 3* | 66 ± 2†* |
| Mean blood pressure, mmHg | ||
| Baseline | 95 ± 2 | 95 ± 2 |
| Hyperinsulinemia | 96 ± 2 | 94 ± 2 |
| Tidal volume, mL/breath | ||
| Baseline | 486 ± 77 | 438 ± 40 |
| Hyperinsulinemia | 535 ± 56* | 509 ± 39* |
| Breathing frequency, breaths/min | ||
| Baseline | 14.4 ± 1.0 | 13.8 ± 0.8† |
| Hyperinsulinemia | 15.0 ± 1.2 | 13.3 ± 1.2† |
| Minute ventilation, L/min | ||
| Baseline | 6.6 ± 0.7 | 5.8 ± 0.4 |
| Hyperinsulinemia | 7.6 ± 0.3* | 6.4 ± 0.4* |
Values are means ± SE; n = 10 (5 male/5 female). Data were analyzed by 2-way repeated-measures ANOVA, normality test (Shapiro-Wilk), equal variance test, and pairwise multiple comparison (Student-Newman-Keuls method):
P < 0.05 vs. baseline,
P < 0.05 vs. saline.
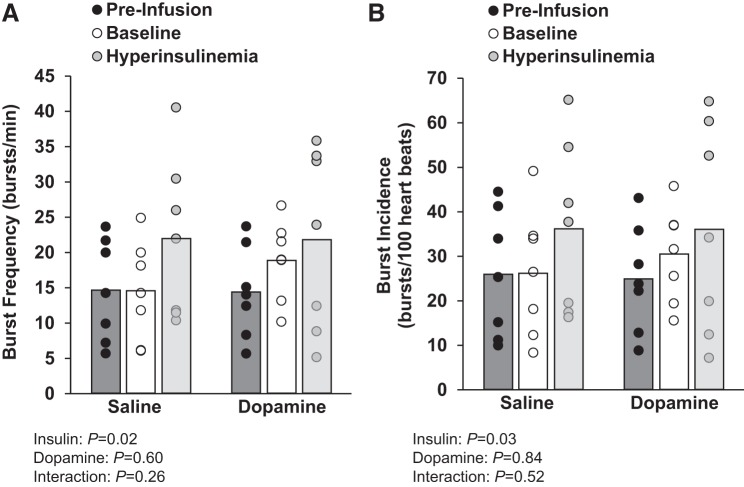
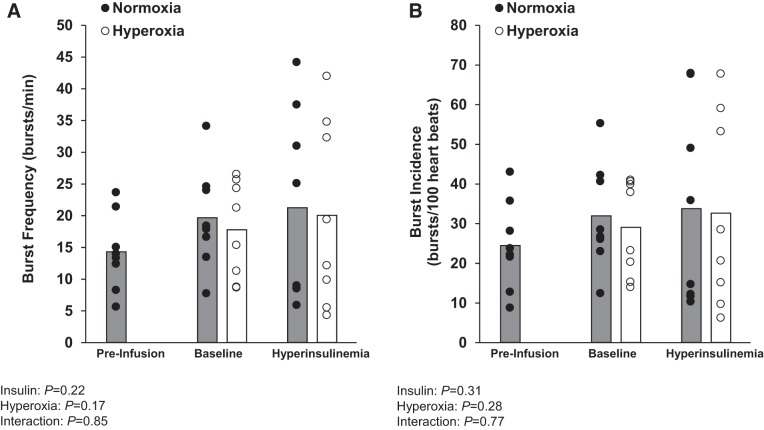
The increase in plasma norepinephrine (from 118 ± 13 to 176 ± 20 pg/mL during the saline visit and from 126 ± 8 to 176 ± 17 pg/mL during the low-dose dopamine visit) and epinephrine (saline: 37 ± 7 to 56 ± 12 pg/mL during the saline visit, dopamine: 42 ± 7 to 77 ± 18 pg/mL during the low-dose dopamine visit) with hyperinsulinemia was unaffected by dopamine infusion (main effect of insulin, P > 0.05; interaction of insulin and dopamine, P > 0.05). The insulin-mediated increase in MSNA burst frequency (n = 7) was unaffected by low-dose dopamine (main effect of insulin, P = 0.02; main effect of dopamine, P = 0.60; interaction of insulin and dopamine, P = 0.26). Similar observations were made when examining MSNA burst incidence (main effect of insulin, P = 0.03; main effect of dopamine, P = 0.84; interaction of insulin and dopamine, P = 0.52; Fig. 4).
Fig. 4.
Effect of low-dose dopamine at baseline and during hyperinsulinemia. Individual (● and ○) and mean (open and shaded bars) values from 7 subjects are shown. A and B: muscle sympathetic nerve activity burst frequency and burst incidence from an average of 5 min during quiet normoxic breathing before saline/dopamine infusion (preinfusion), during steady-state saline/dopamine infusion at baseline (baseline), and during steady-state hyperinsulinemia (hyperinsulinemia). Data were analyzed by 2-way repeated-measures ANOVA and pairwise multiple comparison (Student-Newman-Keuls method).
In the presence of low-dose dopamine (n = 10), acute hyperoxia resulted in a reduction in heart rate at baseline (main effect of hyperoxia, P < 0.01) and during hyperinsulinemia (interaction of insulin and hyperoxia, P = 0.01). There was no added effect of acute hyperoxia on tidal volume, breathing frequency, or minute ventilation during dopamine infusion (Table 4). There was no effect of dopamine + acute hyperoxia on MSNA burst frequency or burst incidence (n = 8) at baseline or during hyperinsulinemia (main effect of hyperoxia, P > 0.05; interaction of insulin and hyperoxia, P > 0.05; Fig. 5).
Table 4.
Effect of acute hyperoxia and low-dose dopamine at baseline and during hyperinsulinemia
| Normoxia + DA | Hyperoxia + DA | |
|---|---|---|
| Heart rate, beats/min | ||
| Baseline | 63 ± 1 | 62 ± 1† |
| Hyperinsulinemia | 66 ± 2 | 64 ± 2† |
| Mean blood pressure, mmHg | ||
| Baseline | 97 ± 2 | 96 ± 2 |
| Hyperinsulinemia | 95 ± 2 | 95 ± 2 |
| Tidal volume, L/breath | ||
| Baseline | 469 ± 40 | 542 ± 84 |
| Hyperinsulinemia | 527 ± 39 | 567 ± 75 |
| Breathing frequency, breaths/min | ||
| Baseline | 14.3 ± 0.7 | 13.9 ± 0.8 |
| Hyperinsulinemia | 13.6 ± 1.2 | 13.8 ± 1.1 |
| Minute ventilation, L/min | ||
| Baseline | 6.5 ± 0.4 | 7.1 ± 0.7 |
| Hyperinsulinemia | 6.8 ± 0.4 | 7.2 ± 0.6 |
| Inspired O2, % | ||
| Baseline | 21 ± 1 | 84 ± 2† |
| Hyperinsulinemia | 21 ± 1 | 86 ± 2† |
Values are means ± SE; n = 10 (5 male/5 female). DA, dopamine. Data were analyzed by 2-way repeated-measures ANOVA, normality test (Shapiro-Wilk), equal variance test, and pairwise multiple comparison (Student-Newman-Keuls method):
P < 0.05 vs. normoxia + DA.
Fig. 5.
Effect of acute hyperoxia and low-dose dopamine at baseline and during hyperinsulinemia. Individual (● and ○) and mean (open and shaded bars) values from 8 subjects are shown. A and B: muscle sympathetic nerve activity burst frequency and burst incidence before dopamine infusion (preinfusion), during steady-state dopamine infusion at baseline (baseline), and during steady-state hyperinsulinemia (hyperinsulinemia). Data are reported as an average of 1 min before and during hyperoxia. Data were analyzed by 2-way repeated-measures ANOVA.
DISCUSSION
Contrary to our hypotheses, carotid chemoreceptor desensitization did not attenuate the sympathoexcitatory response to hyperinsulinemia in the individuals studied. These findings appear consistent across three experimental paradigms, and MSNA data are corroborated by measures of plasma catecholamines. Together, these data suggest that the carotid chemoreceptors do not contribute to acute insulin-mediated increases in sympathetic nervous system activity in young, healthy adults.
Increases in plasma insulin increase MSNA in healthy humans (1, 2, 5, 41, 43). In the majority of work in this area, hyperinsulinemic, euglycemic clamps were used, and MSNA was measured using microneurography, similar to the present study design. Concentrations of insulin range from ~15 to 150 mIU/L to be consistent with concentrations observed after a meal or in insulin-resistant individuals, and significant increases in MSNA are observed even at the lowest doses (1, 2, 24, 43, 52–54, 60). A large body of preclinical work has identified a key role for the central nervous system, specifically, neurons in the arcuate nucleus and hypothalamic paraventricular nucleus, in insulin-mediated sympathoexcitation (4, 6, 7, 34, 48, 56). Although controversy exists, recent evidence from rodents suggests that the CB chemoreceptors may play an independent role in the physiological response to hyperinsulinemia (40). Despite growing interest in this area (10, 12, 37), studies implicating the CB in the sympathoexcitatory response to insulin employed only indirect measures; thus the relative contribution of the CB chemoreceptors to the robust increase in MSNA observed with hyperinsulinemia was previously unknown.
Using acute hyperoxia [modified Dejours test (14)], we observed a rapid (within 15 s) increase in , reaching values known to cause significant attenuation of CB chemoreceptor activity (17). Consistent with attenuation of CB chemoreceptor activity, we observed a reduction in heart rate and breathing frequency with hyperoxia. Hyperoxia, however, did not result in a significant change in MSNA burst frequency or burst incidence at baseline. This finding is consistent with previous work supporting a limited role for the CB chemoreceptors in basal sympathetic nervous system activity in healthy humans (18, 47). When the modified Dejours test was repeated during systemic hyperinsulinemia, we again observed reductions in heart rate and breathing frequency; however, the effect of hyperoxia did not differ from responses observed at baseline. In addition, there continued to be no consistent, measurable effect of acute hyperoxia on MSNA under conditions of high systemic insulin. Together, we interpret these results to mean that the CB chemoreceptors do not contribute to insulin-mediated increases in sympathetic nervous system activity in young, healthy adults.
To further explore these ideas, we recruited a second cohort of individuals to complete two visits randomized to intravenous saline or low-dose dopamine infusion. Dopamine in low doses has been shown repeatedly to blunt the ventilatory response to hypoxia (58), a measure of CB chemosensitivity. We continued to observe an increase in heart rate, MSNA, and plasma catecholamines with insulin infusion; this increase was not different between saline and dopamine conditions. Additionally, the glucose infusion rate required to maintain euglycemia was not different between saline and dopamine visits. These data further suggest that the CB chemoreceptors do not contribute to insulin-mediated increases in sympathetic nervous system activity and/or insulin sensitivity in young, healthy adults. The lack of an effect of dopamine on the glucose infusion rate is in contrast to previous work from our group during insulin-mediated hypoglycemia. Using systemic hyperoxia to attenuate CB afferent activity, Wehrwein and colleagues (57) showed that the normal rise in glucoregulatory hormones (e.g., epinephrine, cortisol, glucagon, growth hormone) during a hyperinsulinemic-hypoglycemic clamp is impaired in healthy adults. In combination, the results reported by Wehrwein and colleagues and our present findings lead us to speculate that the carotid chemoreceptors may play a role in the counterregulatory response to hypoglycemia in humans that is independent of any effect of systemic insulin (26, 30).
In our third experimental approach, we examined the combined effects of intravenous low-dose dopamine and acute hyperoxia (46). Consistent with the response to hyperoxia under saline conditions, there was no effect of hyperoxia on MSNA at baseline or during hyperinsulinemia when combined with low-dose dopamine. Furthermore, there was no effect of hyperoxia on breathing frequency when participants received dopamine simultaneously. These data suggest that any fall in breathing frequency with transient hyperoxia in our initial protocol was the result of direct CB chemoreceptor inhibition, and, therefore, no further response would be expected with combined hyperoxia and dopamine, providing strength to our initial experimental design. In contrast, we continued to observe a fall in heart rate during simultaneous hyperoxia and dopamine infusion that did not differ between baseline and hyperinsulinemia. We examined the early (1 min) response to acute hyperoxia to avoid potential confounding effects of secondary, time-dependent influences of hyperoxia on steady-state cardiovascular effects (such as an increase in peripheral vasoconstriction or a rise in blood pressure). With this, it is possible that the fall in heart rate toward the end of the acute hyperoxic exposure is a baroreflex-mediated response to a rise in blood pressure, rather than a response to an attenuated CB chemoreflex.
Perspectives and Significance
Despite present findings, there is a growing body of literature to support a role for the CB chemoreceptors in glucose regulation (10, 11, 20, 26). The majority of studies examining a role for the carotid chemoreceptors in glucose regulation in humans were carried out in the setting of high insulin (e.g., hyperinsulinemic clamp, mixed meal) (45, 55, 57). Because of combined changes in both systemic glucose and insulin, it is difficult to fully elucidate the mechanisms and/or factors that may be driving previous findings. Present data suggest that the CB chemoreceptors are not necessary for the sympathoexcitatory response to steady-state insulin infusion in healthy humans in the hyperinsulinemic range tested (~50 mg/dL). However, Smorschok et al. recently observed an increase in MSNA following glucose ingestion that was attenuated when individuals breathed 100% O2 acutely (45). Our data would suggest that any role for the CB chemoreceptors in the rise in MSNA is unrelated to their ability to sense and/or respond to increases in plasma insulin commonly seen following glucose ingestion. With this, there are recent data to suggest that the role of CB chemoreceptors in glucose regulation is likely indirect and may be facilitated by the glucoregulatory hormone epinephrine (25, 49). Future work in this area is warranted.
Strengths of the current study design include use of the gold-standard approach of microneurography in a relatively large cohort of healthy men and women to assess MSNA during concurrent systemic infusion of insulin while blood glucose is maintained at baseline levels. Repeat visits were conducted under identical study conditions (including controlled meals followed by an overnight fast), and research questions were examined using three well-accepted methods for attenuating CB chemoreceptor activity in humans (i.e., acute hyperoxia, dopamine, hyperoxia + dopamine). Furthermore, the dose of dopamine was individualized and combined with acute hyperoxia to achieve the greatest possible reduction in CB chemosensitivity. Despite these strengths, there are a number of experimental limitations to acknowledge and consider when interpreting the present findings and planning follow-up work.
First, we did not control respiratory rate or tidal volume at any time during the experiments. It is well known that MSNA is modulated by the respiratory cycle, with sympathetic bursts occurring primarily during late expiration. Thus, by not controlling respiratory rate or tidal volume, we may have added variability into the data, which could complicate interpretation. With this, we observed an independent effect of insulin on tidal volume that was shown previously to vary in magnitude by study cohort (3). Importantly, previous work has shown no effect of increases or decreases in breathing frequency and/or tidal volume on steady-state MSNA burst frequency (15, 32, 44). Therefore, we believe that this is a minor limitation. Second, despite careful dosing, we observed a small increase in heart rate (~5 beats/min) with dopamine infusion; this was unexpected, given that the dose of dopamine was relatively low (average dose: 2.6 ± 0.3 μg⋅kg−1⋅min−1) and was selected based on limited cardiovascular effects during a screening visit (31). These data may suggest that the use of dopamine as an experimental tool is limited; however, given that our conclusions are consistent across experimental approaches (i.e., hyperoxia, dopamine), this is likely a minor limitation. Third, we examined changes in MSNA burst frequency and incidence but are unable to compare burst amplitude between interventions due to the importance of a stable microneurography site for such comparisons and our inability to maintain a consistent site in all individuals due to the long protocol duration (~120 min). Given evidence that chemoreceptor activation may preferentially influence burst amplitude (27), this is an important limitation of the present investigation. Last, the present study included only relatively young, healthy men and women. Whether similar findings can be translated to patient populations known for exhibiting high circulating insulin has yet to be thoroughly addressed; however, there are strong data to suggest that, in disease populations, a role for the CBs in insulin-mediated sympathoexcitation may be significant (37, 40, 51). Consistent with this, through an exploratory post hoc analysis, we did observe significant relationships between the rise in MSNA burst frequency with insulin (Δ = insulin – baseline) and the difference between the fall in MSNA with hyperoxia at baseline and during insulin infusion (R = −0.69, P < 0.01). The difference between the fall in MSNA with hyperoxia at baseline and with insulin infusion also tended to be related to glucose infusion rate (R = 0.44, P = 0.13). Together, these data seem to suggest that, in some subjects, hyperoxia may have a greater effect on MSNA with insulin than at baseline. Where this occurred, the subjects were more likely to have the greatest increase in MSNA with insulin infusion and were the least insulin sensitive (lowest glucose infusion rate).
In conclusion, our data suggest that the carotid chemoreceptors do not contribute acutely to insulin-mediated increases in sympathetic nervous system activity in healthy humans. With this, additional work in disease populations with chronic insulin exposure is warranted.
GRANTS
This work was funded by National Institutes of Health Grants DK-090541 and HL-83947 (to M. J. Joyner) and National Institutes of Health Grant HL-130339 and American Heart Association Grant 15SDG 25080095 (to J. K. Limberg).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.K.L., T.B.C., N.R.P., and M.J.J. conceived and designed research; J.K.L., B.D.J., M.T.M., W.W.H., T.B.C., and M.J.J. performed experiments; J.K.L. analyzed data; J.K.L., B.D.J., M.T.M., W.W.H., T.B.C., N.R.P., and M.J.J. interpreted results of experiments; J.K.L. prepared figures; J.K.L. drafted manuscript; J.K.L., B.D.J., M.T.M., W.W.H., T.B.C., N.R.P., and M.J.J. edited and revised manuscript; J.K.L., B.D.J., M.T.M., W.W.H., T.B.C., N.R.P., and M.J.J. approved final version of manuscript.
REFERENCES
- 1.Anderson EA, Balon TW, Hoffman RP, Sinkey CA, Mark AL. Insulin increases sympathetic activity but not blood pressure in borderline hypertensive humans. Hypertension 19: 621–627, 1992. doi: 10.1161/01.HYP.19.6.621. [DOI] [PubMed] [Google Scholar]
- 2.Anderson EA, Hoffman RP, Balon TW, Sinkey CA, Mark AL. Hyperinsulinemia produces both sympathetic neural activation and vasodilation in normal humans. J Clin Invest 87: 2246–2252, 1991. doi: 10.1172/JCI115260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barbosa TC, Kaur J, Holwerda SW, Young CN, Curry TB, Thyfault JP, Joyner MJ, Limberg JK, Fadel PJ. Insulin increases ventilation during euglycemia in humans. Am J Physiol Regul Integr Comp Physiol 315: R84–R89, 2018. doi: 10.1152/ajpregu.00039.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bardgett ME, McCarthy JJ, Stocker SD. Glutamatergic receptor activation in the rostral ventrolateral medulla mediates the sympathoexcitatory response to hyperinsulinemia. Hypertension 55: 284–290, 2010. doi: 10.1161/HYPERTENSIONAHA.109.146605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berne C, Fagius J, Pollare T, Hjemdahl P. The sympathetic response to euglycaemic hyperinsulinaemia. Evidence from microelectrode nerve recordings in healthy subjects. Diabetologia 35: 873–879, 1992. doi: 10.1007/BF00399935. [DOI] [PubMed] [Google Scholar]
- 6.Cassaglia PA, Hermes SM, Aicher SA, Brooks VL. Insulin acts in the arcuate nucleus to increase lumbar sympathetic nerve activity and baroreflex function in rats. J Physiol 589: 1643–1662, 2011. doi: 10.1113/jphysiol.2011.205575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cassaglia PA, Shi Z, Brooks VL. Insulin increases sympathetic nerve activity in part by suppression of tonic inhibitory neuropeptide Y inputs into the paraventricular nucleus in female rats. Am J Physiol Regul Integr Comp Physiol 311: R97–R103, 2016. doi: 10.1152/ajpregu.00054.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Charkoudian N, Joyner MJ, Johnson CP, Eisenach JH, Dietz NM, Wallin BG. Balance between cardiac output and sympathetic nerve activity in resting humans: role in arterial pressure regulation. J Physiol 568: 315–321, 2005. doi: 10.1113/jphysiol.2005.090076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chua TP, Coats AJ. The reproducibility and comparability of tests of the peripheral chemoreflex: comparing the transient hypoxic ventilatory drive test and the single-breath carbon dioxide response test in healthy subjects. Eur J Clin Invest 25: 887–892, 1995. doi: 10.1111/j.1365-2362.1995.tb01962.x. [DOI] [PubMed] [Google Scholar]
- 10.Conde SV, Ribeiro MJ, Melo BF, Guarino MP, Sacramento JF. Insulin resistance: a new consequence of altered carotid body chemoreflex? J Physiol 595: 31–41, 2017. doi: 10.1113/JP271684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Conde SV, Sacramento JF, Guarino MP. Carotid body: a metabolic sensor implicated in insulin resistance. Physiol Genomics 50: 208–214, 2018. doi: 10.1152/physiolgenomics.00121.2017. [DOI] [PubMed] [Google Scholar]
- 12.Conde SV, Sacramento JF, Guarino MP, Gonzalez C, Obeso A, Diogo LN, Monteiro EC, Ribeiro MJ. Carotid body, insulin, and metabolic diseases: unraveling the links. Front Physiol 5: 418, 2014. doi: 10.3389/fphys.2014.00418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Curry TB, Charkoudian N. The use of real-time ultrasound in microneurography. Auton Neurosci 162: 89–93, 2011. doi: 10.1016/j.autneu.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dejours P. Control of respiration by arterial chemoreceptors. Ann NY Acad Sci 109: 682–695, 1963. doi: 10.1111/j.1749-6632.1963.tb13497.x. [DOI] [PubMed] [Google Scholar]
- 15.Derchak PA, Sheel AW, Morgan BJ, Dempsey JA. Effects of expiratory muscle work on muscle sympathetic nerve activity. J Appl Physiol (1985) 92: 1539–1552, 2002. doi: 10.1152/japplphysiol.00790.2001. [DOI] [PubMed] [Google Scholar]
- 16.Duffin J. Measuring the ventilatory response to hypoxia. J Physiol 584: 285–293, 2007. doi: 10.1113/jphysiol.2007.138883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eyzaguirre C, Lewin J. Effect of different oxygen tensions on the carotid body in vitro. J Physiol 159: 238–250, 1961. doi: 10.1113/jphysiol.1961.sp006805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fisher JP, Flück D, Hilty MP, Lundby C. Carotid chemoreceptor control of muscle sympathetic nerve activity in hypobaric hypoxia. Exp Physiol 103: 77–89, 2018. doi: 10.1113/EP086493. [DOI] [PubMed] [Google Scholar]
- 19.Gabel RA, Kronenberg RS, Severinghaus JW. Vital capacity breaths of 5 percent or 15 percent CO2 in N2 or O2 to test carotid chemosensitivity. Respir Physiol 17: 195–208, 1973. doi: 10.1016/0034-5687(73)90061-3. [DOI] [PubMed] [Google Scholar]
- 20.Gao L, Ortega-Sáenz P, García-Fernández M, González-Rodríguez P, Caballero-Eraso C, López-Barneo J. Glucose sensing by carotid body glomus cells: potential implications in disease. Front Physiol 5: 398, 2014. doi: 10.3389/fphys.2014.00398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hart EC, Head GA, Carter JR, Wallin BG, May CN, Hamza SM, Hall JE, Charkoudian N, Osborn JW. Recording sympathetic nerve activity in conscious humans and other mammals: guidelines and the road to standardization. Am J Physiol Heart Circ Physiol 312: H1031–H1051, 2017. doi: 10.1152/ajpheart.00703.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hart EC, Wallin BG, Barnes JN, Joyner MJ, Charkoudian N. Sympathetic nerve activity and peripheral vasodilator capacity in young and older men. Am J Physiol Heart Circ Physiol 306: H904–H909, 2014. doi: 10.1152/ajpheart.00181.2013. [DOI] [PubMed] [Google Scholar]
- 23.Harvey RE, Barnes JN, Hart EC, Nicholson WT, Joyner MJ, Casey DP. Influence of sympathetic nerve activity on aortic hemodynamics and pulse wave velocity in women. Am J Physiol Heart Circ Physiol 312: H340–H346, 2017. doi: 10.1152/ajpheart.00447.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoffman RP, Sinkey CA, Anderson EA. Hypoglycemic symptom variation is related to epinephrine and not peripheral muscle sympathetic nerve response. J Diabetes Complications 11: 15–20, 1997. doi: 10.1016/1056-8727(95)00082-8. [DOI] [PubMed] [Google Scholar]
- 25.Holmes AP, Ray CJ, Thompson EL, Alshehri Z, Coney AM, Kumar P. Adrenaline activation of the carotid body: key to CO2 and pH homeostasis in hypoglycaemia and potential pathological implications in cardiovascular disease. Respir Physiol Neurobiol 265: 92–99, 2019. doi: 10.1016/j.resp.2018.05.008. [DOI] [PubMed] [Google Scholar]
- 26.Joyner MJ, Limberg JK, Wehrwein EA, Johnson BD. Role of the carotid body chemoreceptors in glucose homeostasis and thermoregulation in humans. J Physiol 596: 3079–3085, 2018. doi: 10.1113/JP274354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kienbaum P, Karlssonn T, Sverrisdottir YB, Elam M, Wallin BG. Two sites for modulation of human sympathetic activity by arterial baroreceptors? J Physiol 531: 861–869, 2001. doi: 10.1111/j.1469-7793.2001.0861h.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kumar P, Bin-Jaliah I. Adequate stimuli of the carotid body: more than an oxygen sensor? Respir Physiol Neurobiol 157: 12–21, 2007. doi: 10.1016/j.resp.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 29.Kumar P, Prabhakar NR. Peripheral chemoreceptors: function and plasticity of the carotid body. Compr Physiol 2: 141–219, 2012. doi: 10.1002/cphy.c100069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Limberg JK. Glucose, insulin, and the carotid body chemoreceptors in humans. Physiol Genomics 50: 504–509, 2018. doi: 10.1152/physiolgenomics.00032.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Limberg JK, Johnson BD, Holbein WW, Ranadive SM, Mozer MT, Joyner MJ. Interindividual variability in the dose-specific effect of dopamine on carotid chemoreceptor sensitivity to hypoxia. J Appl Physiol (1985) 120: 138–147, 2016. doi: 10.1152/japplphysiol.00723.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Limberg JK, Morgan BJ, Schrage WG, Dempsey JA. Respiratory influences on muscle sympathetic nerve activity and vascular conductance in the steady state. Am J Physiol Heart Circ Physiol 304: H1615–H1623, 2013. doi: 10.1152/ajpheart.00112.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Limberg JK, Mozer MT, Holbein WW, Johnson BD, Prabhakar NR, Curry TB, Joyner MJ. Low-dose dopamine, but not acute hyperoxia, attenuates the sympathoexcitatory response to hyperinsulinemia in healthy humans. FASEB J 31: 31, 2017. [Google Scholar]
- 34.Luckett BS, Frielle JL, Wolfgang L, Stocker SD. Arcuate nucleus injection of an anti-insulin affibody prevents the sympathetic response to insulin. Am J Physiol Heart Circ Physiol 304: H1538–H1546, 2013. doi: 10.1152/ajpheart.00081.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mou XB, Howard LS, Robbins PA. A protocol for determining the shape of the ventilatory response to hypoxia in humans. Respir Physiol 101: 139–143, 1995. doi: 10.1016/0034-5687(95)00027-B. [DOI] [PubMed] [Google Scholar]
- 36.Niewinski P, Tubek S, Banasiak W, Paton JF, Ponikowski P. Consequences of peripheral chemoreflex inhibition with low-dose dopamine in humans. J Physiol 592: 1295–1308, 2014. doi: 10.1113/jphysiol.2013.266858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Paton JF, Sobotka PA, Fudim M, Engelman ZJ, Hart EC, McBryde FD, Abdala AP, Marina N, Gourine AV, Lobo M, Patel N, Burchell A, Ratcliffe L, Nightingale A. The carotid body as a therapeutic target for the treatment of sympathetically mediated diseases. Hypertension 61: 5–13, 2013. doi: 10.1161/HYPERTENSIONAHA.111.00064. [DOI] [PubMed] [Google Scholar]
- 38.Pereda SA, Eckstein JW, Abboud FM. Cardiovascular responses to insulin in the absence of hypoglycemia. Am J Physiol 202: 249–252, 1962. doi: 10.1152/ajplegacy.1962.202.2.249.14485087 [DOI] [Google Scholar]
- 39.Prabhakar NR, Peng YJ. Oxygen sensing by the carotid body: past and present. Adv Exp Med Biol 977: 3–8, 2017. doi: 10.1007/978-3-319-55231-6_1. [DOI] [PubMed] [Google Scholar]
- 40.Ribeiro MJ, Sacramento JF, Gonzalez C, Guarino MP, Monteiro EC, Conde SV. Carotid body denervation prevents the development of insulin resistance and hypertension induced by hypercaloric diets. Diabetes 62: 2905–2916, 2013. doi: 10.2337/db12-1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Scherrer U, Randin D, Tappy L, Vollenweider P, Jéquier E, Nicod P. Body fat and sympathetic nerve activity in healthy subjects. Circulation 89: 2634–2640, 1994. doi: 10.1161/01.CIR.89.6.2634. [DOI] [PubMed] [Google Scholar]
- 42.Scherrer U, Sartori C. Insulin as a vascular and sympathoexcitatory hormone: implications for blood pressure regulation, insulin sensitivity, and cardiovascular morbidity. Circulation 96: 4104–4113, 1997. doi: 10.1161/01.CIR.96.11.4104. [DOI] [PubMed] [Google Scholar]
- 43.Scherrer U, Vollenweider P, Randin D, Jéquier E, Nicod P, Tappy L. Suppression of insulin-induced sympathetic activation and vasodilation by dexamethasone in humans. Circulation 88: 388–394, 1993. doi: 10.1161/01.CIR.88.2.388. [DOI] [PubMed] [Google Scholar]
- 44.Seals DR, Suwarno NO, Dempsey JA. Influence of lung volume on sympathetic nerve discharge in normal humans. Circ Res 67: 130–141, 1990. doi: 10.1161/01.RES.67.1.130. [DOI] [PubMed] [Google Scholar]
- 45.Smorschok MP, Sobierajski FM, Purdy GM, Riske LA, Busch SA, Skow RJ, Matenchuk BA, Pfoh JR, Vanden Berg ER, Linares A, Borle K, Lavoie L, Saran G, Dyck R, Funk DR, Day TA, Boulé NG, Davenport MH, Steinback CD. Peripheral chemoreceptor deactivation attenuates the sympathetic response to glucose ingestion. Appl Physiol Nutr Metab 44: 389–396, 2019. doi: 10.1139/apnm-2018-0062. [DOI] [PubMed] [Google Scholar]
- 46.Stickland MK, Fuhr DP, Haykowsky MJ, Jones KE, Paterson DI, Ezekowitz JA, McMurtry MS. Carotid chemoreceptor modulation of blood flow during exercise in healthy humans. J Physiol 589: 6219–6230, 2011. doi: 10.1113/jphysiol.2011.218099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stickland MK, Morgan BJ, Dempsey JA. Carotid chemoreceptor modulation of sympathetic vasoconstrictor outflow during exercise in healthy humans. J Physiol 586: 1743–1754, 2008. doi: 10.1113/jphysiol.2007.147421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stocker SD, Gordon KW. Glutamate receptors in the hypothalamic paraventricular nucleus contribute to insulin-induced sympathoexcitation. J Neurophysiol 113: 1302–1309, 2015. doi: 10.1152/jn.00764.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thompson EL, Ray CJ, Holmes AP, Pye RL, Wyatt CN, Coney AM, Kumar P. Adrenaline release evokes hyperpnoea and an increase in ventilatory CO2 sensitivity during hypoglycaemia: a role for the carotid body. J Physiol 594: 4439–4452, 2016. doi: 10.1113/JP272191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vallbo AB, Hagbarth KE, Torebjörk HE, Wallin BG. Somatosensory, proprioceptive, and sympathetic activity in human peripheral nerves. Physiol Rev 59: 919–957, 1979. doi: 10.1152/physrev.1979.59.4.919. [DOI] [PubMed] [Google Scholar]
- 51.Vera-Cruz P, Guerreiro F, Ribeiro MJ, Guarino MP, Conde SV. Hyperbaric oxygen therapy improves glucose homeostasis in type 2 diabetes patients: a likely involvement of the carotid bodies. Adv Exp Med Biol 860: 221–225, 2015. doi: 10.1007/978-3-319-18440-1_24. [DOI] [PubMed] [Google Scholar]
- 52.Vollenweider L, Tappy L, Owlya R, Jéquier E, Nicod P, Scherrer U. Insulin-induced sympathetic activation and vasodilation in skeletal muscle. Effects of insulin resistance in lean subjects. Diabetes 44: 641–645, 1995. doi: 10.2337/diab.44.6.641. [DOI] [PubMed] [Google Scholar]
- 53.Vollenweider P, Randin D, Tappy L, Jéquier E, Nicod P, Scherrer U. Impaired insulin-induced sympathetic neural activation and vasodilation in skeletal muscle in obese humans. J Clin Invest 93: 2365–2371, 1994. doi: 10.1172/JCI117242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vollenweider P, Tappy L, Randin D, Schneiter P, Jéquier E, Nicod P, Scherrer U. Differential effects of hyperinsulinemia and carbohydrate metabolism on sympathetic nerve activity and muscle blood flow in humans. J Clin Invest 92: 147–154, 1993. doi: 10.1172/JCI116542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ward DS, Voter WA, Karan S. The effects of hypo- and hyperglycaemia on the hypoxic ventilatory response in humans. J Physiol 582: 859–869, 2007. doi: 10.1113/jphysiol.2007.130112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ward KR, Bardgett JF, Wolfgang L, Stocker SD. Sympathetic response to insulin is mediated by melanocortin 3/4 receptors in the hypothalamic paraventricular nucleus. Hypertension 57: 435–441, 2011. doi: 10.1161/HYPERTENSIONAHA.110.160671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wehrwein EA, Basu R, Basu A, Curry TB, Rizza RA, Joyner MJ. Hyperoxia blunts counterregulation during hypoglycaemia in humans: possible role for the carotid bodies? J Physiol 588: 4593–4601, 2010. doi: 10.1113/jphysiol.2010.197491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Welsh MJ, Heistad DD, Abboud FM. Depression of ventilation by dopamine in man. Evidence for an effect on the chemoreceptor reflex. J Clin Invest 61: 708–713, 1978. doi: 10.1172/JCI108983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Whipp BJ, Wasserman K. Carotid bodies and ventilatory control dynamics in man. Fed Proc 39: 2668–2673, 1980. [PubMed] [Google Scholar]
- 60.Young CN, Deo SH, Chaudhary K, Thyfault JP, Fadel PJ. Insulin enhances the gain of arterial baroreflex control of muscle sympathetic nerve activity in humans. J Physiol 588: 3593–3603, 2010. doi: 10.1113/jphysiol.2010.191866. [DOI] [PMC free article] [PubMed] [Google Scholar]