Abstract
In humans, loss of central tolerance for the cardiac self-antigen α-myosin heavy chain (α-MHC) leads to circulation of cardiac autoreactive T cells and renders the heart susceptible to autoimmune attack after acute myocardial infarction (MI). MI triggers profound tissue damage, releasing danger signals and self-antigen by necrotic cardiomyocytes, which lead to recruitment of inflammatory monocytes. We hypothesized that excessive inflammation by monocytes contributes to the initiation of adaptive immune responses to cardiac self-antigen. Using an experimental model of MI in α-MHC-mCherry reporter mice, which specifically express mCherry in cardiomyocytes, we detected α-MHC antigen in myeloid cells in the heart-draining mediastinal lymph node (MLN) 7 days after MI. To test whether monocytes were required for cardiac self-antigen trafficking to the MLN, we blocked monocyte recruitment with a C-C motif chemokine receptor type 2 (CCR2) antagonist or immune modifying nanoparticles (IMP). Blockade of monocyte recruitment reduced α-MHC antigen detection in the MLN after MI. Intramyocardial injection of the model antigen ovalbumin into OT-II transgenic mice demonstrated the requirement for monocytes in antigen trafficking and T-cell activation in the MLN. Finally, in nonobese diabetic mice, which are prone to postinfarction autoimmunity, blockade of monocyte recruitment reduced α-MHC-specific responses leading to improved tissue repair and ventricular function 28 days after MI. Taken together, these data support a role for monocytes in the onset of pathological cardiac autoimmunity following MI and suggest that therapeutic targeting of monocytes may mitigate postinfarction autoimmunity in humans.
NEW & NOTEWORTHY Our study newly identifies a role for inflammatory monocytes in priming an autoimmune T-cell response after myocardial infarction. Select inhibition of monocyte recruitment to the infarct prevents trafficking of cardiac self-antigen and activation of cardiac myosin reactive T cells in the heart-draining lymph node. Therapeutic targeting of inflammatory monocytes may limit autoimmune responses to improve cardiac remodeling and preserve left ventricular function after myocardial infarction.
Keywords: autoimmunity, monocytes, myocardial infarction
INTRODUCTION
Following myocardial infarction (MI), interruption of blood flow leads to rapid ischemic death of cardiomyocytes. Release of damage-associated molecular patterns and debris by necrotic cardiomyocytes stimulate inflammation and recruitment of phagocytes, including Ly6Chi monocytes and dendritic cells. During phagocytic clearance of necrotic debris, cardiac self-antigen can be processed and then presented in the heart-draining lymph nodes to α-MHC-specific CD4+ T cells (22), which escape thymic negative selection (16), triggering an autoimmune response directed against the heart. In the general population, this presents as transient-cellular and humoral responses directed against cardiac myosin (17, 18). However, in patients with type 1 diabetes, this response can develop into a persistent autoimmune myocarditis (9), which may contribute to the worsened outcomes in patients with type 1 diabetes after MI (21). In both type 1 diabetes and postinfarction autoimmunity, autoreactive CD4+ T cells display Th1 polarization and are characterized by proinflammatory interferon (IFN)-γ production (1, 9). While conventional dendritic cells have been shown to prime α-MHC-specific CD4+ T-cell responses (22), in some instances they are not required for postinfarction autoimmunity, necessitating a better understanding of the role of alternative innate immune cells in cardiac self-antigen trafficking and antigen presentation after MI.
In both humans and mice, heightened levels of inflammatory Ly6Chi monocytes are associated with the pathogenesis of variety of diseases, including autoimmunity and MI (8, 10, 15). After MI, ischemic injury releases Ly6Chi monocytes from both the bone marrow and spleen into the circulation and recruits these cells through C-C motif chemokine receptor type 2 (CCR2)-dependent signaling from the periphery to the infarct. Inflammatory Ly6Chi monocytes dominate in the early hours after MI to phagocytose necrotic and apoptotic cardiomyocytes as evidenced by an increase in cardiomyocyte debris in infarct-associated monocytes (5). Ly6Chi monocytes peak around 3 days after MI and can differentiate into Ly6Clo macrophages and monocyte-derived dendritic cells (11, 22). Both monocyte-derived macrophages and dendritic cells are known to traffic and present antigen to T cells (13); however, whether inflammatory Ly6Chi monocytes contribute to priming of cardiac myosin-specific CD4+ T cells after MI remains unclear. Here, we show that inflammatory Ly6Chi monocyte recruitment to the heart after MI leads to accumulation of cardiac antigen in the MLN and initiation of a α-MHC-specific CD4+ T-cell immune responses. Inhibition of inflammatory Ly6Chi monocyte recruitment to the infarct abrogated these responses indicating that selective targeting of inflammatory Ly6Chi monocytes may be an effective therapeutic strategy to limit postinfarction autoimmunity.
MATERIALS AND METHODS
Mice.
Mice were bred and maintained in a pathogen-free environment, and all experiments used 2–4-mo-old female mice since postinfarction autoimmunity was first described in female mice within this age range (9). C57BL/6 mice were used as wild-type controls. α-MHC-mCherry (021577), OT-II (004194), and nonobese diabetic (NOD, 001976) mice were purchased from Jackson Laboratory. Blood glucose levels remained below 200 mg/dL in NOD mice for the duration of experiments. LysM-eGFP mice were provided by Dr. William Muller (Northwestern University). Animal studies were conducted in accordance with guidelines using a protocol approved by Institutional Animal Care and Use Committee at Northwestern University.
Myocardial infarction.
Myocardial infarction was induced by permanently ligating the left anterior descending (LAD) coronary artery as previously described (24). Blanching/pale discoloration and hypokinesis of the anterior wall verified LAD ligation. Monocyte recruitment was inhibited by daily subcutaneous injections of the CCR2 antagonist (2 mg/kg), RS504393 (Tocris), or intravenous injections of IMP in sterile PBS at 24, 48, 72, and 96 h after infarction as previously described (8). IMP were composed of biodegradable 500 nm poly(lactic-co-glycolic acid) microparticles with surface carboxylate groups (Phosphorex). For intramyocardial antigen delivery, OT-II mice were subjected to sham or MI surgery and intramyocardially injected with 50 μg ovalbumin 323–339 (OVA323–339) peptide (Anaspec) before ligation.
Flow cytometry.
Hearts were flushed with PBS to remove peripheral cells, and infarcted myocardium was then excised, weighed, minced with fine scissors, and digested with collagenase and DNase at 37°C for 30 min. Hearts were subsequently triturated through a 40-μm cell strainer. Whole spleens and mediastinal lymph node (MLN) were harvested and subsequently triturated through a 40-μm cell strainer to prepare single cell suspensions. Red blood cells were lysed, and total viable cell numbers were enumerated on a hemocytometer. Cells were labeled with Zombie Aqua Fixable Dye (BioLegend) following the manufacturer’s protocol and then incubated with TruStain FcX antibody (1:100, BioLegend). Cells were then labeled with fluorescently conjugated antibodies for CD45, CD3, CD4, CD8, CD44, CD69, CD11b, CD11c, MHCII, CD64, Ly6G, and Ly6C (all 1:200, all BioLegend). Flow cytometry was performed on a LSRFortessa cytometer (BD Biosciences) and data were analyzed by FlowJo software (Tree Star). All cells were gated on live, single cells (FSC-A vs. FSC-H and SSC-A vs. SSC-H). Ly6Chi monocytes were CD11b+Ly6G−Ly6ChiCD64−. Macrophages were CD11b+Ly6G−Ly6CloCD64+. CD4+ T cells were CD45+CD3+CD4+CD8− and activation status determined by CD69 and CD44 expression. CD8+ T cells were CD45+CD3+CD4−CD8+.
Infarct size and histology.
Triphenyltetrazolium chloride staining was used as previously described to differentiate between viable and infarcted tissue (24). Area at risk was assessed by fluorescent microsphere perfusion to ensure equal and consistent levels of surgically induced ischemia. For whole heart histology, hearts were excised, formalin fixed, and embedded in paraffin by the Mouse Histology and Phenotyping Laboratory at Northwestern University. Paraffin-embedded heart blocks were serially cut along the lateral axis, and sections were stained using routine Masson’s trichrome staining protocol.
Echocardiography.
At day 28 after permanent occlusion, cardiac function was assessed by two-dimensional M-mode echocardiography (Vevo 770; Visualsonics, Toronto, Canada). M-mode images were collected 1 mm before, at, and after the papillary muscles, and measurements were made in three consecutive cardiac cycles and averaged for analysis. In anesthetized animals, heart rate was maintained above 400 beats/min to ensure physiologically relevant measurements and no differences were observed between groups (data not shown).
Myosin-specific immune responses.
To measure anticardiac myosin titers, high-binding EIA plates (Corning) were coated with 5 μg/mL mouse cardiac myosin, blocked, and incubated with sera. IgG1 antibodies were detected by incubation with horseradish peroxidase-labeled goat anti-mouse IgG1 antibody (Jackson ImmunoResearch). 3,3′,5,5′-Tetramethylbenzidine was used as substrate, and absorbance was read at 405 nm. To measure anticardiac T-cell responses, MLN or splenic single cell suspensions were stimulated without or with 25 μg/mL mouse cardiac myosin for 72 h at 37°C. IFN-γ levels were measured by ELISA (BD Biosciences).
Statistics.
Statistical analyses were performed with GraphPad Prism. Statistical significance (P < 0.05) was determined between two groups by two-tailed, unpaired t-test and among more than two variables by ANOVA. Data are presented as means ± SE.
RESULTS
Cardiac antigen is present in myeloid cells in lymphoid organs after MI.
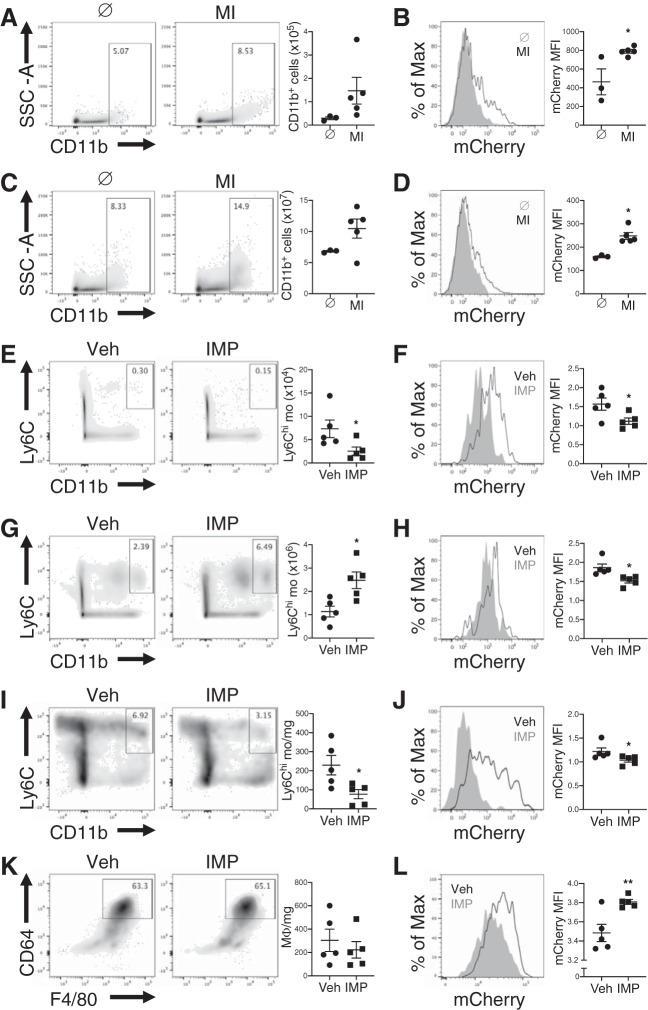
Immune responses directed against α-MHC are implicated in postinfarction autoimmunity (9). To determine whether myeloid cells trafficked cardiac self-antigen to heart-draining lymph nodes to prime autoimmunity, we subjected Myh6-mCherry reporter mice, which specifically express mCherry protein in cardiomyocytes, to a preclinical model of permanent-occlusion MI and tracked myeloid cells and cardiac self-antigen to the MLN. At steady state, flow cytometric analysis revealed few myeloid cells and minimal phagocytic-associated cardiac antigen (Fig. 1, A and B). One week after MI, when both antigen-presenting cells peak in numbers and CD4+ T-cell proliferation is detectable within the MLN (12, 22), there was an increase in both absolute number of myeloid cells and myeloid cells with engulfed cardiac antigen in the MLN (Fig. 1, A and B). We also observed an increase in myeloid cells with engulfed cardiac antigen in the spleen after MI (Fig. 1, C and D). These data suggest that myeloid cells with engulfed cardiac self-antigen accumulate in lymphoid organs after MI.
Fig. 1.
Recruitment of Ly6Chi monocytes to the infarct drives cardiac self-antigen accumulation in lymphoid organs after myocardial infarction (MI). Trafficking of cardiac self-antigen α-myosin heavy chain to the heart-draining mediastinal lymph node (MLN) after MI using Myh6-mCherry reporter mice, which specifically express mCherry in cardiomyocytes, is shown. A and C: total number of CD11b+ cells in the MLN (A) and spleen (C) 7 days after MI. B and D: cardiac self-antigen in CD11b+ cells in the MLN (B) and spleen (D) 7 days after MI. Myh6-mCherry reporter mice were subjected to MI surgery followed by treatment with vehicle (Veh) or immune modifying nanoparticles (IMP). E, G, and I: total Ly6Chi monocytes in MLN (E), spleen (G), and heart (I) 7 days after MI. K: total macrophages in heart 7 days after MI. F and H: cardiac self-antigen expression in CD11b+ cells in the MLN (F) and spleen (H) 7 days after MI. J and L: cardiac self-antigen expression in Ly6Chi monocytes (J) and macrophages (L) in the heart 7 days after MI. *P < 0.05, **P < 0.01 by unpaired t-test; n = 3–5 mice/group.
Ly6Chi monocyte recruitment drives cardiac self-antigen accumulation in lymphoid organs after MI.
After recruitment to the infarct, inflammatory Ly6Chi monocytes engage in activities that may increase cardiac self-antigen release and presentation, including phagocytosis of dying cardiomyocytes, proinflammatory cytokine secretion, and differentiation into macrophages and dendritic cells. To test whether inflammatory Ly6Chi monocyte recruitment to the infarct was required for the increase in cardiac antigen in lymphoid organs, we treated mice with immune-modifying microparticles (IMP), which redirect Ly6Chi monocytes from the sites of injury to the spleen for their clearance (8). Treatment of mice with IMP significantly reduced the absolute numbers of Ly6Chi monocytes in the MLN and heart after MI, while increasing their numbers in the spleen (Fig. 1, E, G, and I). Redirection of Ly6Chi monocytes to the spleen after MI resulted in a significant reduction in myeloid cells with engulfed cardiac self-antigen in the MLN and spleen (Fig. 1, F and H). There was also a significant reduction in phagocytosed cardiac self-antigen in Ly6Chi monocytes in the heart after MI (Fig. 1J). While treatment of mice with IMP did not alter absolute number of macrophages in the heart after MI (Fig. 1K), it increased macrophage phagocytosis of cardiac self-antigen (Fig. 1L). Together, these results suggest that recruitment of Ly6Chi monocytes to the infarct promotes cardiac self-antigen accumulation in lymphoid organs.
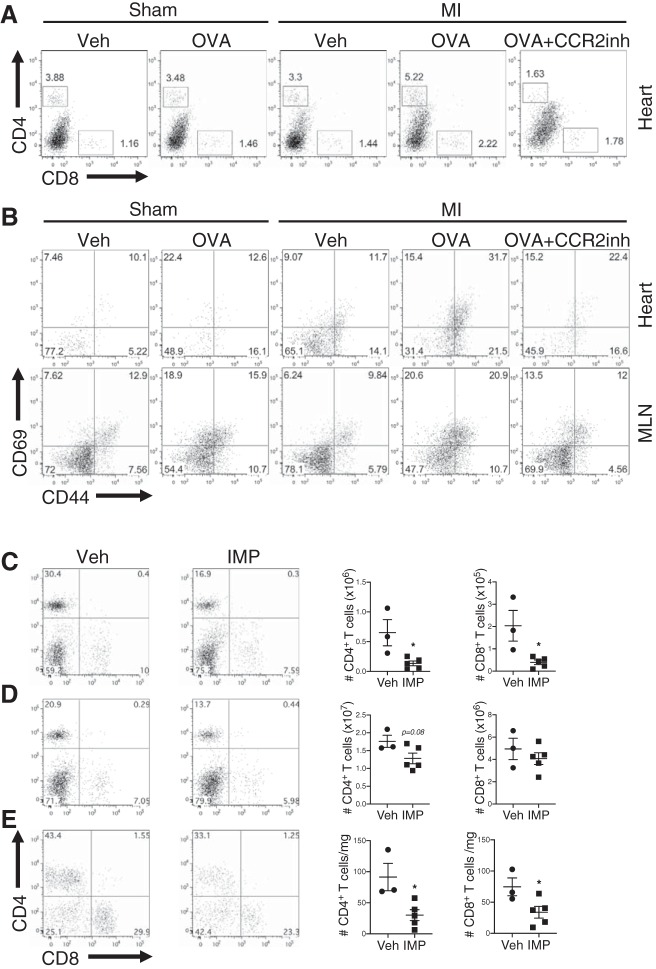
Ly6Chi monocyte recruitment promotes antigen-specific CD4+ T-cell responses after MI.
To determine whether inflammatory Ly6Chi monocyte-driven accumulation of cardiac self-antigen in lymphoid organs after MI was sufficient to prime a self-reactive T-cell response, we subjected transgenic OT-II mice, which have CD4+ T cells specific for the chicken OVA323–339 peptide, to sham operation or MI surgery and intramyocardially injected vehicle (PBS) or OVA323–339 peptide. Intramyocardial administration of a fixed amount of OVA323–339 peptide increased CD4+ T-cell activation in OT-II mice subjected to MI compared with sham or vehicle-treated controls (Fig. 2, A and B). Following MI and OVA323–339 peptide administration, inhibition of inflammatory Ly6Chi monocyte recruitment using IMP significantly decreased the absolute number of CD4+ T cells in the MLN, spleen, and heart of OT-II mice (Fig. 2, C–E). IMP treatment also significantly reduced the absolute number of CD8+ T cells in the MLN and heart (Fig. 2, C and E), which may contribute to postinfarction autoimmune responses through direct killing of cardiomyocytes (23). Similarly, treatment of mice with a CCR2-specific antagonist, which blocks inflammatory Ly6Chi monocyte migration to the infarct (4, 14), diminished activation and accumulation of CD4+ T cells in both the heart and MLN of OT-II mice after MI (Fig. 2, A and B). Taken together, these findings suggest that inflammatory Ly6Chi monocyte recruitment to the infarct primes an antigen-specific CD4+ T-cell response in the heart-draining lymphoid organs.
Fig. 2.
C-C motif chemokine receptor type 2 (CCR2)-dependent Ly6Chi monocyte recruitment to the infarct promotes antigen-specific CD4+ T-cell responses after myocardial infarction (MI). OT-II mice were subjected to sham operation or MI surgery and intramyocardially injected with either vehicle (Veh; PBS) or ovalbumin 323–339 (OVA323–339) peptide. To inhibit monocyte recruitment, mice were treated daily with subcutaneous injections of the CCR2 antagonist, RS504393, or vehicle control. A: flow cytometric analysis of CD8+ and OVA323–339-specific CD4+ T cells in the heart 7 days after sham operation or MI surgery. B: flow cytometric analysis of CD69 and CD44 expression on OVA323–339-specific CD4+ T cells in the heart and mediastinal lymph node (MLN) 7 days after sham operation or MI surgery. Alternatively, OT-II mice were subjected to MI surgery and intramyocardial injection of OVA323–339 peptide followed by treatment with vehicle or immune modifying nanoparticles (IMP). C–E: total OVA323–339-specific CD4+ T cells and CD8+ T cells in the MLN (C), spleen (D), and heart (E) 7 days after MI. *P < 0.05 by unpaired t-test; n = 3–5 mice/group.
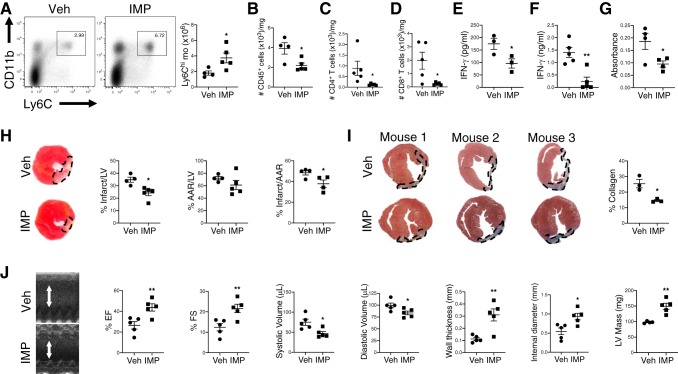
Ly6Chi monocyte recruitment drives postinfarction autoimmunity and adverse tissue remodeling.
Given that cardiac infiltration of inflammatory Ly6Chi monocytes promoted accumulation of cardiac self-antigen in lymphoid organs and was associated with the priming of antigen-specific CD4+ T-cell responses, we hypothesized that inflammatory Ly6Chi monocytes may induce pathological cardiac autoimmune responses after MI. To determine whether inflammatory Ly6Chi monocyte recruitment to the infarct induced autoimmune responses directed against cardiac myosin, we subjected NOD mice, which are prone to postinfarction autoimmunity, to MI and inhibited inflammatory Ly6Chi monocyte recruitment using IMP. Consistent with prior reports (9), postinfarction autoimmunity was only observed in NOD mice after MI but not in control C57BL/6 mice (data not shown). IMP treatment redirected Ly6Chi monocytes to the spleen (Fig. 3A) and significantly reduced the absolute number of CD45+ cells, CD4+ T cells, and CD8+ T cells in the hearts of NOD mice after MI (Fig. 3, B–D). To test whether these T-cell responses were directed against cardiac myosin, we cultured single cell preparations from spleen or MLN with purified mouse cardiac myosin and measured IFN-γ production. IMP treatment significantly reduced IFN-γ production (Fig. 3, E and F), suggesting that cardiac infiltration of inflammatory monocytes primed an anti-myosin-specific T-cell response after MI. Consistent with a role for inflammatory Ly6Chi monocytes in the onset of postinfarction autoimmunity, anticardiac myosin IgG titers were significantly reduced in IMP-treated NOD mice compared with vehicle-treated mice after MI (Fig. 3G). To determine whether the decrease in postinfarction autoimmunity was associated with improved tissue repair and preservation of left ventricular (LV) function, we compared LV remodeling and contractile function after MI in vehicle or IMP-treated NOD mice. Pathological measurements of infarct size and fibrosis after MI showed smaller-sized infarcts and less collagen deposition in IMP-treated NOD mice compared with controls (Fig. 3, H and I). Similar area-at-risk measurements suggested equal and consistent levels of surgically induced ischemia (Fig. 3H); however, initial injury was not examined and may have differed between groups. Echocardiography performed after MI revealed improved LV systolic function, reduced LV dilatation, and increased LV wall thickness in IMP-treated mice compared with controls (Fig. 3J). Taken together, these data suggest that inflammatory Ly6Chi monocyte recruitment contributes to the induction of postinfarction autoimmunity leading to worsened cardiac remodeling and function after MI.
Fig. 3.
Ly6Chi monocyte recruitment drives postinfarction autoimmunity and adverse cardiac remodeling after myocardial infarction (MI) in nonobese diabetic (NOD) mice. Prediabetic NOD mice were subjected to MI surgery followed by treatment with vehicle (Veh) or immune modifying nanoparticles (IMP). A: flow cytometric analysis of Ly6Chi monocytes in the spleen 7 days after MI with quantification of total Ly6Chi monocytes. B–F: total CD45+ cells (B), CD4+ T cells (C), and CD8+ T cells (D) in the hearts 28 days after MI. interferon-γ (IFN-γ) production by mediastinal lymph node (E) or splenic cells (F) stimulated with mouse myosin for 72 h ex vivo. G: anticardiac myosin IgG titers in sera 28 days after MI. H: infarct and area-at-risk measurements 7 days after MI. I: collagen deposition 28 days after MI. J: echocardiography quantification of percent ejection fraction (%EF), percent fractional shortening (%FS), systolic and diastolic volume, wall thickness, internal diameter, and left ventricular (LV) mass 28 days after MI. *P < 0.05, **P < 0.01 by unpaired t-test; n = 3–5 mice/group.
DISCUSSION
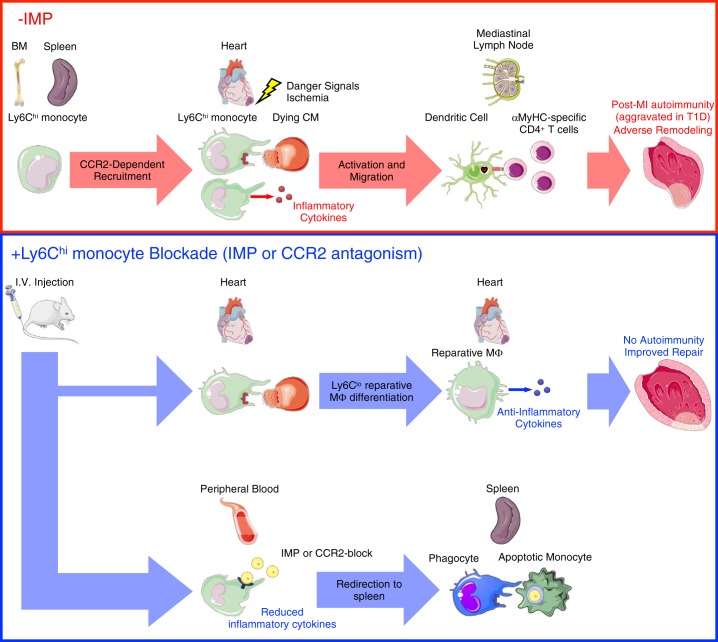
In summary, these findings support the conclusion that inflammatory Ly6Chi monocyte recruitment to the heart after MI leads to accumulation of cardiac antigen in lymphoid organs and the initiation of cardiac myosin-specific T- and B-cell responses. According to our working model (Fig. 4), MI causes release of danger signals and self-antigens from dying cardiomyocytes. Inflammatory Ly6Chi monocytes are then released from the bone marrow and spleen into the peripheral blood and are recruited to the heart. Within the infarct, inflammatory Ly6Chi monocytes phagocytose cardiac self-antigen and differentiate into dendritic cells and macrophages. Inflammatory Ly6Chi monocytes also release proteases and cytokines, which liberate additional cardiac self-antigen for direct phagocytosis by dendritic cells and macrophages. Migration of phagocytes with engulfed cardiac self-antigen to the MLN and spleen leads to priming of cardiac myosin-specific CD4+ T cells and a pathological autoimmune response directed against the heart. This culminates in adverse tissue remodeling and progression to heart failure.
Fig. 4.
Monocytes prime autoreactive T cells after myocardial infarction (MI). MI causes release of danger signals and self-antigens from dying cardiomyocytes (CM). Ly6Chi monocytes are then released from the bone marrow (BM) and spleen into the peripheral blood and are recruited to the heart. Within the infarct, Ly6Chi monocytes produce inflammatory cytokines and phagocytose-damaged tissue and dying CM. In autoimmune-susceptible individuals, including patients with type 1 diabetes (T1D), persistent inflammatory LyC6hi monocytosis promotes infiltration and activation of antigen-presenting cells with phagocytosed cardiac self-antigen. Migration of mature antigen-presenting cells (dendritic cells) with engulfed cardiac self-antigen to the mediastinal lymph node and spleen leads to priming of cardiac myosin-specific CD4+ T cells and a pathological autoimmune response directed against the heart. This culminates in adverse tissue remodeling and progression to heart failure. Targeting monocyte recruitment to the heart using immune modifying nanoparticles (IMP) redirects the majority of Ly6Chi monocytes to the spleen for their apoptotic removal, while permitting a minority of these cells to infiltrate the infarct, mediate clearance of tissue debris, and differentiate into Ly6Clo reparative macrophages. This limits inflammation and both the release of cardiac self-antigen and its presentation by antigen-presenting cells in lymphoid organs, leading to improved cardiac repair after MI. α-MHC, α-myosin heavy chain; CCR2, C-C motif chemokine receptor type 2; Mϕ, macrophage.
Our findings indicate that targeting inflammatory Ly6Chi monocyte recruitment to the heart limits the release of cardiac self-antigen and its presentation by antigen-presenting cells in lymphoid organs after MI. When compared with interferon regulatory factor (IRF)4- and IRF8-dependent conventional dendritic cells, inflammatory Ly6Chi monocytes and monocyte-derived dendritic cells are inferior in presenting antigen to α-MHC-specific CD4+ T cells (22), suggesting an indirect role for inflammatory Ly6Chi monocytes in cardiac myosin presentation. However, neither IRF4- nor IRF8-dependent conventional dendritic cells are specifically required for activation of α-MHC-specific CD4+ T cells after MI (22). While representing a small proportion of the total dendritic cells in the MLN, monocyte-derived dendritic cells are the predominant dendritic cell in the heart after MI (22). Phagocytosis of self-antigen from dying cardiomyocytes by inflammatory monocytes and differentiation into monocyte-derived dendritic cells may support cross-priming of α-MHC-specific CD4+ T cells through transfer of intact, functional MHC class I/peptide complexes to conventional dendritic cells (20). Inhibition of a component of inflammatory Ly6Chi monocyte recruitment would be expected to limit α-MHC-specific CD4+ T-cell activation through reduced transfer of antigen to other dendritic cells. Alternatively, blockade of inflammatory Ly6Chi monocyte recruitment may lead to preservation of cardioprotective functions by tissue phagocytes in some cases (4, 6). Engulfment of cardiac antigen and debris by tissue resident macrophages favors repair through production of anti-inflammatory IL-10 and induction of Foxp3+CD4+ regulatory T cells, which mediate wound healing after MI and promote tolerance toward cardiac self-antigen (25, 26).
In a therapeutic context, our findings indicate that treatment of patients with IMP after MI is a potential intervention to limit inflammation and postinfarction autoimmunity, which may contribute to pathological tissue remodeling and accelerate progression to heart failure (21). IMP also enable encapsulation of cardiac self-antigens to promote long-term tolerance through deletion of cardiac autoreactive T cells and induction of Foxp3+CD4+ regulatory T cells (7, 19). The greatest clinical benefit may be observed in patients with type I diabetes, where circulating inflammatory CD14+ monocytes display an inflammatory phenotype compared with that in healthy controls (2, 3), priming these patients for exaggerated, nonresolving inflammation after MI. Taken together, these data highlight a new concept where exaggerated inflammatory monocyte function contribute to autoimmune responses after MI, which can be therapeutically targeted to improve patient outcomes.
GRANTS
This work was supported by American Heart Association Grant CDA34110032 (to M. DeBerge) and National Heart, Lung, and Blood Institute Grants F32-HL-127958 (to M. DeBerge) and R01-HL-122309 and R01-HL-139812-01 (to E. B. Thorp and X. Luo).
DISCLOSURES
S. Miller is a cofounder, member of the scientific advisory board, and consultant of Cour Pharmaceutical Development, Co., which is partnering with Takeda Pharmaceutical, Co., for clinical translation of the immune modifying nanoparticle tolerance technology.
AUTHOR CONTRIBUTIONS
M.D., S.Y., S.D., S.M., and E.B.T. conceived and designed research; M.D., S.Y., S.D., I.I., and X.Y.Y. performed experiments; M.D., S.Y., S.D., M.F., A.B., S.M., and E.B.T. analyzed data; M.D., S.Y., S.D., M.F., A.B., S.M., and E.B.T. interpreted results of experiments; M.D., S.Y., and E.B.T. prepared figures; M.D., S.Y., X.L., and E.B.T. drafted manuscript; M.D., S.Y., M.F., A.B., X.L., S.M., and E.B.T. edited and revised manuscript; M.D., S.Y., S.D., I.I., X.Y.Y., M.F., A.B., X.L., S.M., and E.B.T. approved final version of manuscript.
REFERENCES
- 1.Arif S, Tree TI, Astill TP, Tremble JM, Bishop AJ, Dayan CM, Roep BO, Peakman M. Autoreactive T cell responses show proinflammatory polarization in diabetes but a regulatory phenotype in health. J Clin Invest 113: 451–463, 2004. doi: 10.1172/JCI19585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bradshaw EM, Raddassi K, Elyaman W, Orban T, Gottlieb PA, Kent SC, Hafler DA. Monocytes from patients with type 1 diabetes spontaneously secrete proinflammatory cytokines inducing Th17 cells. J Immunol 183: 4432–4439, 2009. doi: 10.4049/jimmunol.0900576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cipolletta C, Ryan KE, Hanna EV, Trimble ER. Activation of peripheral blood CD14+ monocytes occurs in diabetes. Diabetes 54: 2779–2786, 2005. doi: 10.2337/diabetes.54.9.2779. [DOI] [PubMed] [Google Scholar]
- 4.DeBerge M, Yeap XY, Dehn S, Zhang S, Grigoryeva L, Misener S, Procissi D, Zhou X, Lee DC, Muller WA, Luo X, Rothlin C, Tabas I, Thorp EB. MerTK cleavage on resident cardiac macrophages compromises repair after myocardial ischemia reperfusion injury. Circ Res 121: 930–940, 2017. doi: 10.1161/CIRCRESAHA.117.311327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dehn S, Thorp EB. Myeloid receptor CD36 is required for early phagocytosis of myocardial infarcts and induction of Nr4a1-dependent mechanisms of cardiac repair. FASEB J 32: 254–264, 2018. doi: 10.1096/fj.201700450R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dick SA, Macklin JA, Nejat S, Momen A, Clemente-Casares X, Althagafi MG, Chen J, Kantores C, Hosseinzadeh S, Aronoff L, Wong A, Zaman R, Barbu I, Besla R, Lavine KJ, Razani B, Ginhoux F, Husain M, Cybulsky MI, Robbins CS, Epelman S. Self-renewing resident cardiac macrophages limit adverse remodeling following myocardial infarction. Nat Immunol 20: 29–39, 2019. doi: 10.1038/s41590-018-0272-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Getts DR, Shea LD, Miller SD, King NJ. Harnessing nanoparticles for immune modulation. Trends Immunol 36: 419–427, 2015. doi: 10.1016/j.it.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Getts DR, Terry RL, Getts MT, Deffrasnes C, Müller M, van Vreden C, Ashhurst TM, Chami B, McCarthy D, Wu H, Ma J, Martin A, Shae LD, Witting P, Kansas GS, Kühn J, Hafezi W, Campbell IL, Reilly D, Say J, Brown L, White MY, Cordwell SJ, Chadban SJ, Thorp EB, Bao S, Miller SD, King NJ. Therapeutic inflammatory monocyte modulation using immune-modifying microparticles. Sci Transl Med 6: 219ra217, 2014. doi: 10.1126/scitranslmed.3007563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gottumukkala RV, Lv H, Cornivelli L, Wagers AJ, Kwong RY, Bronson R, Stewart GC, Schulze PC, Chutkow W, Wolpert HA, Lee RT, Lipes MA. Myocardial infarction triggers chronic cardiac autoimmunity in type 1 diabetes. Sci Transl Med 4: 138ra180, 2012. doi: 10.1126/scitranslmed.3003551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hayashidani S, Tsutsui H, Shiomi T, Ikeuchi M, Matsusaka H, Suematsu N, Wen J, Egashira K, Takeshita A. Anti-monocyte chemoattractant protein-1 gene therapy attenuates left ventricular remodeling and failure after experimental myocardial infarction. Circulation 108: 2134–2140, 2003. doi: 10.1161/01.CIR.0000092890.29552.22. [DOI] [PubMed] [Google Scholar]
- 11.Hilgendorf I, Gerhardt LM, Tan TC, Winter C, Holderried TA, Chousterman BG, Iwamoto Y, Liao R, Zirlik A, Scherer-Crosbie M, Hedrick CC, Libby P, Nahrendorf M, Weissleder R, Swirski FK. Ly-6Chigh monocytes depend on Nr4a1 to balance both inflammatory and reparative phases in the infarcted myocardium. Circ Res 114: 1611–1622, 2014. doi: 10.1161/CIRCRESAHA.114.303204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hofmann U, Beyersdorf N, Weirather J, Podolskaya A, Bauersachs J, Ertl G, Kerkau T, Frantz S. Activation of CD4+ T lymphocytes improves wound healing and survival after experimental myocardial infarction in mice. Circulation 125: 1652–1663, 2012. doi: 10.1161/CIRCULATIONAHA.111.044164. [DOI] [PubMed] [Google Scholar]
- 13.Jakubzick CV, Randolph GJ, Henson PM. Monocyte differentiation and antigen-presenting functions. Nat Rev Immunol 17: 349–362, 2017. doi: 10.1038/nri.2017.28. [DOI] [PubMed] [Google Scholar]
- 14.Lavine KJ, Epelman S, Uchida K, Weber KJ, Nichols CG, Schilling JD, Ornitz DM, Randolph GJ, Mann DL. Distinct macrophage lineages contribute to disparate patterns of cardiac recovery and remodeling in the neonatal and adult heart. Proc Natl Acad Sci USA 111: 16029–16034, 2014. doi: 10.1073/pnas.1406508111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liehn EA, Piccinini AM, Koenen RR, Soehnlein O, Adage T, Fatu R, Curaj A, Popescu A, Zernecke A, Kungl AJ, Weber C. A new monocyte chemotactic protein-1/chemokine CC motif ligand-2 competitor limiting neointima formation and myocardial ischemia/reperfusion injury in mice. J Am Coll Cardiol 56: 1847–1857, 2010. doi: 10.1016/j.jacc.2010.04.066. [DOI] [PubMed] [Google Scholar]
- 16.Lv H, Havari E, Pinto S, Gottumukkala RV, Cornivelli L, Raddassi K, Matsui T, Rosenzweig A, Bronson RT, Smith R, Fletcher AL, Turley SJ, Wucherpfennig K, Kyewski B, Lipes MA. Impaired thymic tolerance to α-myosin directs autoimmunity to the heart in mice and humans. J Clin Invest 121: 1561–1573, 2011. doi: 10.1172/JCI44583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moraru M, Roth A, Keren G, George J. Cellular autoimmunity to cardiac myosin in patients with a recent myocardial infarction. Int J Cardiol 107: 61–66, 2006. doi: 10.1016/j.ijcard.2005.02.036. [DOI] [PubMed] [Google Scholar]
- 18.O’Donohoe TJ, Schrale RG, Sikder S, Surve N, Rudd D, Ketheesan N. Significance of anti-myosin antibody formation in patients with myocardial infarction: a prospective observational study. Heart Lung Circ 28: 583–590, 2019. doi: 10.1016/j.hlc.2018.03.008. [DOI] [PubMed] [Google Scholar]
- 19.Prasad S, Neef T, Xu D, Podojil JR, Getts DR, Shea LD, Miller SD. Tolerogenic Ag-PLG nanoparticles induce tregs to suppress activated diabetogenic CD4 and CD8 T cells. J Autoimmun 89: 112–124, 2018. doi: 10.1016/j.jaut.2017.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qu C, Nguyen VA, Merad M, Randolph GJ. MHC class I/peptide transfer between dendritic cells overcomes poor cross-presentation by monocyte-derived APCs that engulf dying cells. J Immunol 182: 3650–3659, 2009. doi: 10.4049/jimmunol.0801532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sousa GR, Pober D, Galderisi A, Lv H, Yu L, Pereira AC, Doria A, Kosiborod M, Lipes MA. Glycemic control, cardiac autoimmunity, and long-term risk of cardiovascular disease in type 1 diabetes mellitus. Circulation 139: 730–743, 2019. doi: 10.1161/CIRCULATIONAHA.118.036068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Van der Borght K, Scott CL, Nindl V, Bouché A, Martens L, Sichien D, Van Moorleghem J, Vanheerswynghels M, De Prijck S, Saeys Y, Ludewig B, Gillebert T, Guilliams M, Carmeliet P, Lambrecht BN. Myocardial infarction primes autoreactive T cells through activation of dendritic cells. Cell Rep 18: 3005–3017, 2017. doi: 10.1016/j.celrep.2017.02.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Varda-Bloom N, Leor J, Ohad DG, Hasin Y, Amar M, Fixler R, Battler A, Eldar M, Hasin D. Cytotoxic T lymphocytes are activated following myocardial infarction and can recognize and kill healthy myocytes in vitro. J Mol Cell Cardiol 32: 2141–2149, 2000. doi: 10.1006/jmcc.2000.1261. [DOI] [PubMed] [Google Scholar]
- 24.Wan E, Yeap XY, Dehn S, Terry R, Novak M, Zhang S, Iwata S, Han X, Homma S, Drosatos K, Lomasney J, Engman DM, Miller SD, Vaughan DE, Morrow JP, Kishore R, Thorp EB. Enhanced efferocytosis of apoptotic cardiomyocytes through myeloid-epithelial-reproductive tyrosine kinase links acute inflammation resolution to cardiac repair after infarction. Circ Res 113: 1004–1012, 2013. doi: 10.1161/CIRCRESAHA.113.301198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weirather J, Hofmann UD, Beyersdorf N, Ramos GC, Vogel B, Frey A, Ertl G, Kerkau T, Frantz S. Foxp3+ CD4+ T cells improve healing after myocardial infarction by modulating monocyte/macrophage differentiation. Circ Res 115: 55–67, 2014. doi: 10.1161/CIRCRESAHA.115.303895. [DOI] [PubMed] [Google Scholar]
- 26.Zhang S, Weinberg S, DeBerge M, Gainullina A, Schipma M, Kinchen JM, Ben-Sahra I, Gius DR, Yvan-Charvet L, Chandel NS, Schumacker PT, Thorp EB. Efferocytosis fuels requirements of fatty acid oxidation and the electron transport chain to polarize macrophages for tissue repair. Cell Metab 29: 443–456, 2019. doi: 10.1016/j.cmet.2018.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]