Abstract
Preeclampsia is a hypertensive disorder of pregnancy characterized by systemic perturbations of nitric oxide function, reflective of generalized endothelial dysfunction. Therapies that target the nitric oxide pathway have shown promise in both clinical and preclinical studies of preeclampsia. The glucagon-like peptide 1 agonists have been shown to increase nitric oxide and lower blood pressure in patients with diabetes, in part, through activation of nitric oxide synthase (NOS). Therefore, we hypothesized that a direct acting glucagon-like peptide 1 receptor agonist would improve stigmata of the preeclampsia syndrome. Using the reduced uterine perfusion pressure rat model, we found that treatment with liraglutide significantly lowered blood pressure, improved renal function, and upregulated NOS3 protein expression in the mesenteric arterial bed. However, there were adverse effects on pup growth that were likely related to diminished food intake in the dams. Collectively, these data support the premise that the use of drugs that improve NOS abundance, including the glucagon-like peptide 1 agonists, is a rational therapeutic approach to the treatment of preeclampsia, but suggest cautious and careful study of their safety before potential clinical use in humans.
NEW & NOTEWORTHY Drugs that target the glucagon-like peptide-1 pathway such as liraglutide are already used clinically, and it has been shown to promote endothelial nitric oxide synthase (NOS3) expression. We demonstrate that liraglutide, a glucagon-like peptide 1 receptor (GLP-1R) agonist, lowers blood pressure, improves renal function, and upregulates NOS3 in a rat model of placental ischemia. These data suggest that drugs that target the nitric oxide system, including GLP-1R agonists, are a potential therapeutic option for preeclampsia.
Keywords: GLP-1, nitric oxide, preeclampsia, pregnancy
INTRODUCTION
Preeclampsia is a multisystem disorder of pregnancy characterized by impaired nitric oxide signaling and hypertension, reflecting systemic endothelial dysfunction. The only known cure for this devastating disorder is delivery of the infant and placenta. As such, preeclampsia is a significant cause of both maternal and perinatal morbidity and mortality; thus, novel treatment approaches are needed. Although the etiology and pathogenesis of preeclampsia remain largely enigmatic, large strides have been made by a host of studies in preclinical models of the disorder. Data from these studies suggest a paradigm of preeclampsia, whereby placental ischemia—whether due to impaired placentation, preexisting maternal cardiovascular dysfunction, or both—is the final common pathology of this syndrome, with subtleties in specific clinical features varying among these causes.
Placental ischemia is thought to result in the production of a variety of soluble factors from trophoblasts, which, upon entering the maternal circulation, cause multiple signs and symptoms to develop, which characterize the preeclampsia syndrome (23). For example, tumor necrosis factor-α, which is found in high levels in the circulation of women with preeclampsia, and a relative imbalance in the circulating levels of the proangiogenic factors vascular endothelial growth factor and placental growth factor with the antiangiogenic factor soluble fms-like tyrosine kinase 1 together are thought to contribute mechanistically to the disorder (8, 12, 13, 25). In turn, the physiological responses induced by these factors are thought to culminate in reduced levels of nitric oxide (9). Indeed, impaired nitric oxide is commonly observed in both patients with preeclampsia and experimental models (9, 20, 22). Thus, the nitric oxide pathway is an important target of potential therapeutic interventions.
Glucagon-like peptide 1 (GLP-1) is an incretin hormone (2)—a peptide that enhances endogenous insulin secretion. Drugs targeting the GLP-1 signaling axis have been extensively leveraged for the treatment of type 2 diabetes mellitus. GLP-1 itself is rapidly cleared from plasma, limiting its duration of action. Thus, two distinct pharmacological approaches to amplifying GLP-1 signaling have been developed: inhibition of the breakdown of endogenous GLP-1 via small molecule peptidase inhibitors and synthetic peptides, which are resistant to degradation. Both drug classes have been shown to effectively improve glycemic control. However, the latter class of drugs that act as direct GLP-1 receptor (GLP-1R) agonists also reduce cardiovascular mortality in patients with type 2 diabetes (15), a benefit attributed, at least in part, to improvements in nitric oxide signaling (18). Additionally, basic science studies have shown that GLP-1 agonism can lower blood pressure in several hypertensive models (10, 14, 24). By coupling to the cyclic AMP-dependent protein kinase A and PI3K-Akt pathways, GLP-1 receptor activation results in phosphorylation and activation of endothelial nitric oxide synthase (NOS3) (11), resulting in increased nitric oxide bioavailability. Collectively, these clinical and basic science data provide a strong rationale for examining the potential of GLP-1 agonists for the treatment of preeclampsia.
Therefore, we hypothesized that administration of liraglutide, a GLP-1 analog, would attenuate stigmata of the preeclampsia syndrome in a rat model of placental ischemia caused by reducing uterine perfusion pressure in the pregnant rat.
MATERIALS AND METHODS
Animal model.
Timed pregnant CD-001 rats were obtained from Charles River Laboratories. Day 1 of gestation was defined as the presence of a copulatory (sperm) plug. Animals arrived at the University of Mississippi Medical Center (UMMC) laboratory animal facility on gestation day 10–13 and were maintained on a 12-h:12-h light-dark cycle. On gestation day 15, animals were randomly assigned to undergo either sham laparotomy or the reduced uterine perfusion pressure (RUPP) procedure while under 2 to 3% isoflurane anesthesia. This operation consists of placing silver clips on the arterial systems that feed the growing uteroplacental unit to reduce blood flow and produce a model of placental ischemia. Thus, a single clip of 0.203-mm internal diameter is placed on the aorta at the iliac bifurcation. Second, two clips of 0.100-mm internal diameter are placed on the ovarian-uterine arterial arcade distal to the ovarian arterial supply and proximal to the first uteroplacental branch. This reduces blood flow to the uteroplacental unit by ~40% (1). This model has been shown to recapitulate many aspects of the human preeclampsia syndrome. For all surgical procedures, animals received 0.1 mg/kg carprofen injection intramuscularly for analgesia.
Animals were maintained on standard laboratory chow given in powdered form for accurate intake measurements (Envigo Laboratories, Diet 8640, 22% protein). Given the documented effects of GLP-1R agonism on appetite, daily food intake and body weights were recorded.
All studies involving animals were approved by the UMMC Institutional Animal Care and Use Committee and conformed to the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals.
Drug treatment.
Commercially available liraglutide was obtained (Novo-Nordisk) and diluted 1:10 into sterile saline. Vehicle was synthesized on the basis of the drug package insert and similarly diluted 1:10 into sterile saline, producing the following solution: 0.798 mM of disodium diphosphate, 18.4 mM of propylene glycol, and 584 mM of phenol. Animals were randomly assigned to receive either drug or vehicle treatment for the duration of the study. A daily subcutaneous injection at ~7:00 am of either drug (0.3 mg/kg) or an appropriately matched volume of vehicle on gestation days 15–20 (i.e., from the time of sham or RUPP surgery until the end of the study) was given. This dose was selected as it produces a plasma AUC approximating the maximum dose in humans (17). One animal was excluded from the RUPP + vehicle group due to severe cachexia, and one animal was excluded from the RUPP + liraglutide group as a statistical outlier (mean arterial pressure greater than 4 SDs from the mean).
Mean arterial pressure.
On gestation day 19, animals were instrumented with a carotid artery catheter placed in the left common carotid artery and advanced ~2.3 cm into the aortic arch with subsequent tunneling to the back of the animal. Catheters were made of vinyl tubing (Scientific Commodities; V/1 tubing, 0.64-mm outer diameter, telescoped to V/3 tubing, 0.99-mm outer diameter).
Mean arterial pressure was measured on gestation day 20 at ~8:00 am in conscious, restrained animals. The carotid catheter was connected to a Deltran pressure transducer (Utah Medical). Data were collected using an amplifier and PowerLab from ADInstruments. Animals were acclimated to the restraint for 30 min followed by 30 min of continuous blood pressure monitoring. Data were analyzed using LabChart software (ADInstruments).
Tissue collection.
Animals were anesthetized under 3% isoflurane at ~11:00 am. Blood was collected from the carotid catheters, and animals were then perfused with 60 mL ice-cold saline through the catheter. Tissues and organs were then collected, snap frozen in liquid nitrogen, and stored at −80°C for later analysis.
Plasma creatinine and blood urea nitrogen measurement.
Plasma creatinine and blood urea nitrogen levels were measured from stored, frozen plasma samples via a VetAxcel Chemistry Analyzer (Alfa Wassermann Diagnostic Technologies), according to the manufacturer’s instructions.
Western blot analysis.
The entire mesenteric arterial arcade was homogenized in standard lysis buffer, consisting of 20 mM Tris, 5 mM EDTA, 10 mM EGTA, 1 mM sodium orthovanadate, 1% Triton X-100, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 2 mM DTT, 0.1 mg/ml PMSF, 0.01 mg/ml leupeptin, and 0.01 mg/ml aprotinin. Homogenate was run on Bio-Rad mini-Protean TGX stain-free gels (4–15%) and transferred to a nitrocellulose membrane via Bio-Rad TransBlot Turbo protocol. Total protein per lane was determined via activation and imaging of Bio-Rad Stain-Free technology on a Chemidoc MP imager. Total NOS3 expression was determined with a BD Transduction Laboratory antibody (Cat. No. 610297) at a dilution of 1:1,000. Phosphorylation of NOS3 at serine residue 1177 (NOS3-pSer-1177) was determined with Cell Signaling antibody (Cat. No. 9571L) at a dilution of 1:1,000. Bio-Rad goat anti-mouse StarBright Blue 520 (Cat. No. 12005866) and Bio-Rad goat anti-rabbit StarBright Blue 700 (Cat. No. 12004161) were used as secondary antibodies, each at a dilution of 1:5,000. Protein quantification was performed in Bio-Rad ImageLab software in the following manner. Appropriate bands for NOS3 and phospho-NOS3 were selected on the basis of size (expected band at ~134 kDa). The band density for total NOS3 was then divided by the total protein density, as determined above to yield a protein-to-total protein-loaded ratio. The relative amount of NOS3-pSer-1177 phosphorylation was determined by dividing the band density for phospho-NOS3 by the band density for total NOS3. These values were then normalized to the sham + vehicle control group.
Statistical analysis.
All statistical analyses were performed in GraphPad Prism 8 software. Data were analyzed using two-way ANOVA with Holm-Sidak-corrected multiple comparison as appropriate. A P value of less than 0.05 was deemed to be statistically significant. All data are shown as means ± SE.
RESULTS
Liraglutide treatment attenuates placental-ischemia induced hypertension.
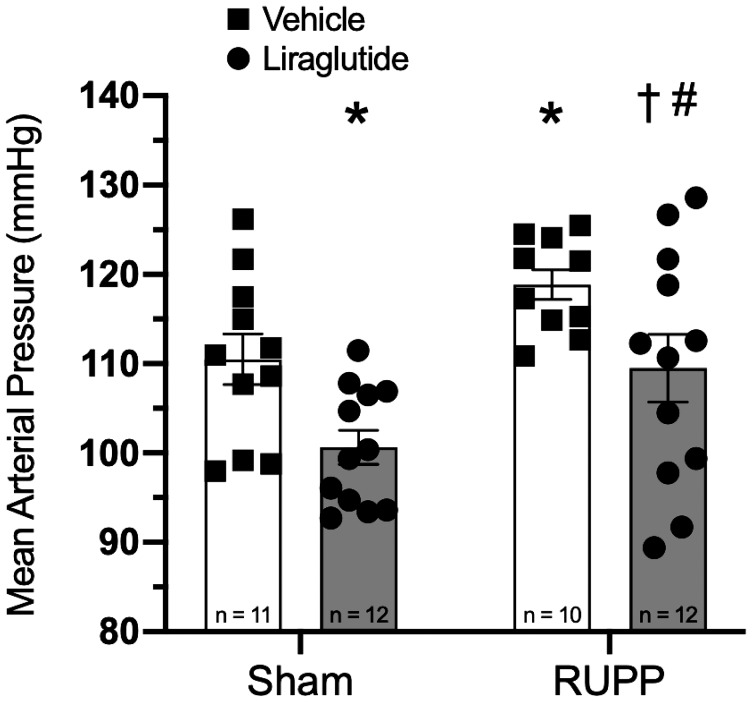
Treatment with liraglutide significantly lowered mean arterial pressure in RUPP animals (Fig. 1; 118.9 ± 1.6 vs. 109.5 ± 3.6 mmHg, P < 0.05). Moreover, this effect was not limited to the setting of placental ischemia, as sham-operated animals treated with liraglutide exhibited a similar decrease in mean arterial pressure as compared with their vehicle-treated counterparts (110.5 ± 2.7 vs. 100.6 ± 1.8 mmHg, P < 0.05).
Fig. 1.
Liraglutide lowers mean arterial pressure in both sham-operated and reduced uterine perfusion pressure (RUPP) animals; P < 0.01 for both surgery and treatment effect by two-way ANOVA. Holm-Sidak multiple-comparison tests with P < 0.05 are indicated by symbols: *Significant difference, vs. sham + vehicle; †Significant difference, vs. RUPP + vehicle; #Significant difference vs. sham + liraglutide.
Administration of liraglutide lowers serum creatinine.
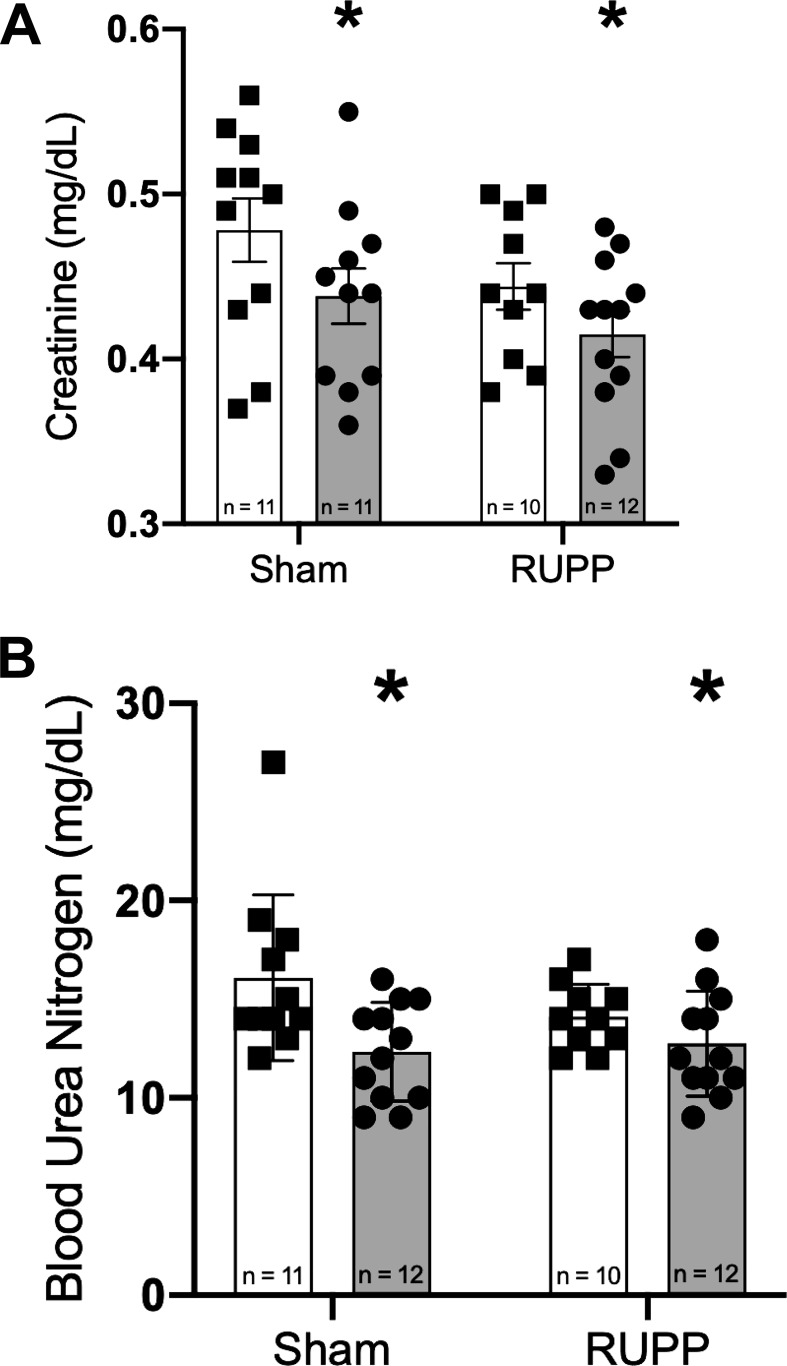
To assess the effects of GLP-1R agonism on renal function, we measured creatinine and blood urea nitrogen in plasma. As shown in Fig. 2, A and B, respectively, there was a statistically significant decrease in both of these measures in animals treated with liraglutide, as determined by two-way ANOVA.
Fig. 2.
Liraglutide treatment lowers creatinine (A) and blood urea nitrogen (B) in both sham-operated and reduced uterine perfusion pressure (RUPP) animals. *P < 0.05 for treatment effect by two-way ANOVA.
Liraglutide suppresses appetite without changes in body weight.
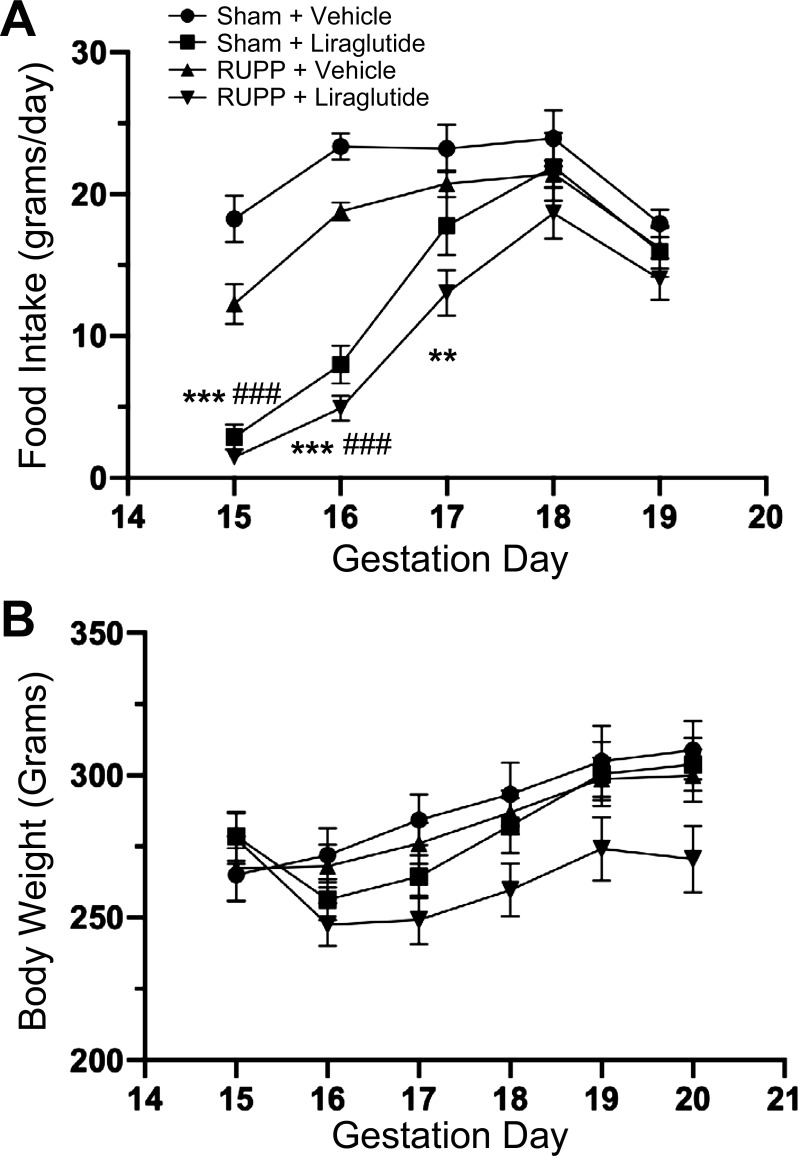
In keeping with the known appetite-suppressant effects of GLP-1R agonism, animals that received liraglutide exhibited a severely diminished appetite following initiation of treatment (Fig. 3A). However, this effect was not sustained for the entire duration of treatment. Daily food intake slowly recovered to normal levels, such that by the end of the study, there were no differences among the groups. Despite this transient appetite suppression, there was no significant drop in body weight in liraglutide-treated groups, as shown in Fig. 3B.
Fig. 3.
A: liraglutide suppresses appetite, but does not result in significant differences in maternal body weight (B). ***P < 0.001 vs. RUPP + vehicle; **P < 0.01 vs. RUPP + vehicle; ###P < 0.001 vs. sham + vehicle by multiple t tests. n = 10–12. RUPP, reduced uterine perfusion pressure.
Treatment with liraglutide affects fetal outcomes.
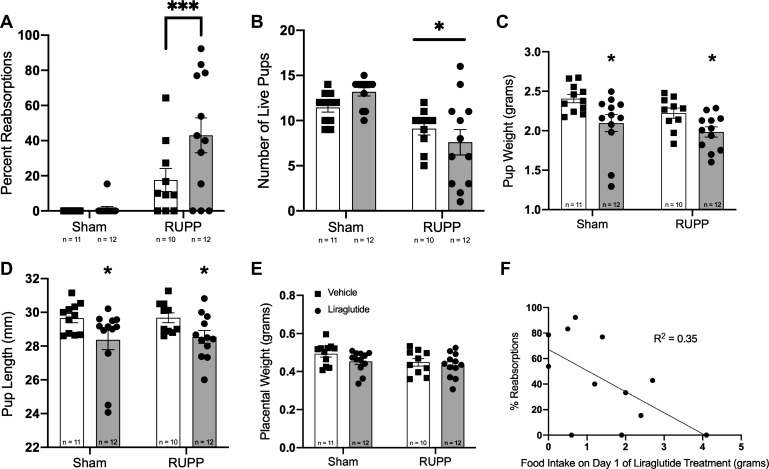
Figure 4 shows various measures of fetal outcomes among the groups. Beginning with pup reabsorptions, placental ischemia significantly increased instances of fetal reabsorption as expected (Fig. 4A). In subsequent multiple comparisons, it also became apparent that RUPP animals treated with liraglutide showed significantly more reabsorptions than those RUPP animals treated with vehicle. However, there was only a single animal out of 12 in the sham + liraglutide group, which exhibited a fetal reabsorption, suggesting that liraglutide itself may not have direct fetal toxicity. Only RUPP surgery exhibited a statistically significant effect on litter size (Fig. 4B).
Fig. 4.
Fetal biometrics. A: percentage of reabsorptions observed in each animal. P < 0.05 for treatment effect and P < 0.0001 for surgery effect by two-way ANOVA. Holm-Sidak multiple comparisons test indicated by line: ***P < 0.01 vs. RUPP + vehicle. B: number of live pups per litter. *P < 0.05 for surgery effect by two-way ANOVA. There was no statistically significant effect of liraglutide treatment by two-way ANOVA nor on Holm-Sidak multiple-comparison testing. C: average pup weight from each litter. *P < 0.05 for treatment effect by two-way ANOVA. D: average pup length from each litter. *P < 0.05 for treatment effect by two-way ANOVA. E: average placental weight. There was no statistically significant difference among groups. F: Percentage of pups reabsorbed versus food intake on day 1 of treatment for the reduced uterine perfusion pressure (RUPP) + liraglutide treatment group (n = 12). P < 0.05 by linear regression and Pearson correlation analysis.
Liraglutide treatment significantly lowered pup weight and crown-to-rump length (as measured on gestation day 20) when compared with vehicle-treated animals, as determined by two-way ANOVA for treatment effect (Fig. 4, C and D). No differences in placental weight were observed among the groups (Fig. 4E). To determine whether this was related to a direct effect of the drug on the fetus or was related to the aforementioned decrease in food intake by the pregnant dams, we correlated food intake on day 1 of treatment with percent reabsorptions in the RUPP + liraglutide group (Fig. 4F). Pearson correlation analysis identified a significant correlation between day 1 food intake and fetal reabsorption, suggesting that the increased incidence of fetal demise in the RUPP + liraglutide animals was primarily related to restrictions in food intake. However, we cannot definitively rule out a direct toxic effect of the drug, and further toxicity studies are strongly warranted.
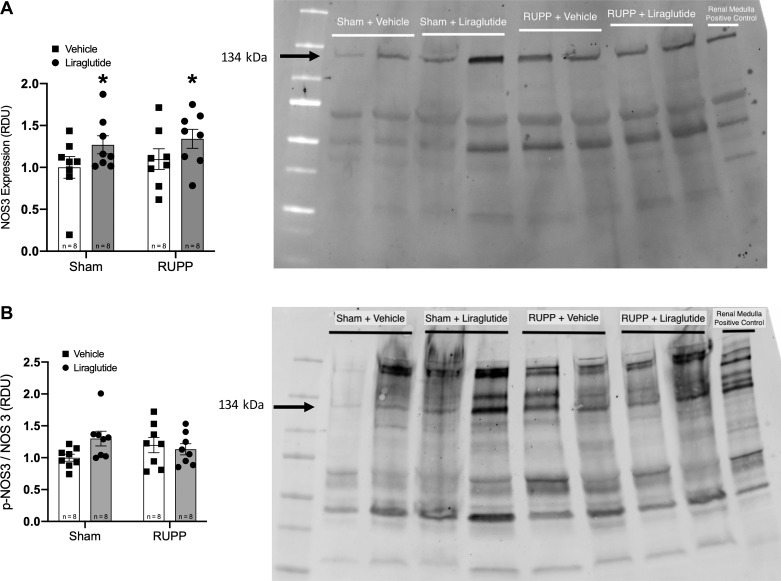
Liraglutide upregulates endothelial NOS expression in resistance vessels.
Because GLP-1R agonism has been shown to activate NOS3 in endothelial cells, we examined its expression in mesenteric vessels by Western blot analysis. Total NOS3 levels in these vessels was upregulated by 5-day treatment with liraglutide (Fig. 5A). The ratio of phospho(Ser-1177)-NOS3 to total NOS3 was not different among the groups (Fig. 5B).
Fig. 5.
A: liraglutide upregulates expression of endothelial nitric oxide synthase (NOS3) in mesenteric vessels. *P < 0.05 for treatment effect by two-way ANOVA. A representative blot is shown; note that differing amounts of total protein was loaded in each lane. B: ratio of phospho-Ser-1177 NOS3 to total NOS3 is not significantly different among groups. RDU, relative density units.
DISCUSSION
Novel treatment options and approaches are needed to alleviate the high maternal and infant morbidity and mortality associated with preeclamptic pregnancies. Given the importance of reduced nitric oxide to the disorder, we reasoned that pharmacological agents which have been shown to stimulate nitric oxide production would be rational therapeutic options for this disorder. The GLP-1R agonists have previously been shown to improve cardiovascular health and lower blood pressure through a nitric oxide-dependent mechanism, rendering these drugs potential candidates for the treatment of preeclampsia (2, 18, 21).
Using a placental ischemic rat model of preeclampsia, we showed that treatment with the direct-acting GLP-1R agonist liraglutide attenuates hypertension, a cardinal feature of the preeclampsia syndrome, suggesting that it provides therapeutic benefit.
The GLP-1R agonists have previously been shown to suppress appetite (19), and many agonists carry a Food and Drug Administration label for obesity treatment. Thus, we closely monitored food intake and body weight during our study. As expected, appetite was significantly repressed following initiation of therapy; however, this effect was only transient, as food intake recovered to normal levels within 3 days. There was no significant difference in body weight among groups by the end of the study. Nevertheless, liraglutide treatment negatively affected fetal weight. Whereas we cannot exclude a direct drug effect, as liraglutide does cross the placenta (17), it is likely that this decrease reflects some degree of maternal malnutrition caused by the two-day suppression of appetite. Such malnutrition may also explain the increased reabsorptions seen in the placental ischemic group. While this is not directly comparable to models of protein restriction that have been shown to impair fetal growth, this reduction in food intake would correspond to an 88 and 73% reduction in protein intake over this two-day period, respectively. In the setting of preexisting placental insufficiency induced by surgical restriction of blood flow, it is possible that this superimposed malnutrition exceeded any physiological reserve, resulting in fetal demise, a supposition supported by the correlation between food intake and fetal reabsorptions in this group (see Fig. 4F). Regardless, taken together, these data highlight potential adverse effects of GLP-1R agonism during pregnancy. Thus, further studies are needed to evaluate the safety of these drugs.
The GLP-1R is a Gs-coupled protein receptor that activates a signaling pathway culminating in the activation of cAMP-dependent PKA and the PI3K-Akt pathway. In turn, both of these kinase pathways have been shown to phosphorylate and activate NOS3 (4, 6, 16). Moreover, GLP-1R activation has been shown to upregulate total NOS3 protein expression in several different model systems through PKA and PI3-Akt-dependent mechanisms (3, 5, 7). These cellular mechanisms are believed to underlie the observed cardiovascular benefit of GLP-1R agonists in vivo. Therefore, we evaluated the Ser-1177 phosphorylation and total expression of NOS3 in a representative resistance vascular bed, the mesenteric arterial bed. We found that liraglutide treatment increased total NOS3 expression with preserved phosphorylation at the activating Ser-1177 site. Taken together with our data on serum creatinine and blood urea nitrogen (see Fig. 2, A and B), this suggests in vivo renal vasodilation produced by liraglutide-induced upregulation of NOS3, presumably through the aforementioned signaling pathways.
Our study has a few limitations. The transient appetite suppression presents a potential confounding factor. For example, we cannot distinguish between direct drug actions and the transient decrease in food intake as it relates to fetal outcomes. Future studies will consider adopting a paired-feeding approach to better discriminate between these two physiological effects. Finally, whereas GLP-1 has pleiotropic physiological effects, this study focused solely on the cardiovascular system. How GLP-1 agonism affects endocrine, metabolic, and neural physiology in the context of placental ischemia, as well as the potential benefit or risk associated with the changes thereof, remains largely unexplored.
There are many pharmacological agents other than the GLP-1R agonists with effects on the nitric oxide system and the ability to improve endothelial function, including phosphodiesterase-5 inhibitors, soluble guanylate cyclase activators and/or stimulators, statins, and many others. The relative efficacy and safety of these drugs in the context of preeclampsia are an ongoing area of research. This study adds to the body of evidence supporting the validity and potential efficacy of targeting the nitric oxide pathway and the endothelium for the treatment of preeclampsia.
In summary, we show that treatment with the GLP-1R agonist liraglutide attenuates hypertension in a placental ischemic model of preeclampsia and upregulates NOS3 expression in mesenteric vessels, offering a new potential therapeutic approach and mechanistic pathway for the treatment and further investigation of this disorder. However, the use of liraglutide must be considered in the context of the potential impact on fetal outcomes—a concern that strongly warrants future research. These data indicate the efficacy of drugs that target the nitric oxide pathway for the treatment of preeclampsia and underscore the importance of further development of such drugs for the treatment of this disorder.
GRANTS
This work was supported by National Institutes of Health Grants R01-HL-136684 and BX-002604-01A2 (to M. J. Ryan), R01-HL-134711 (to J. M. Sasser), P01-HL-051971 and U54-GM-115428 (to the University of Mississippi Medical Center Physiology Department), and T32-HL-105324 (to K. J. Maeda) and American Heart Association Grant AHA18PRE34050024 (to S. T. Younes).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.T.Y. and M.J.R. conceived and designed research; S.T.Y. and K.J.M. performed experiments; S.T.Y., K.J.M., and J.M.S. analyzed data; S.T.Y., K.J.M., J.M.S., and M.J.R. interpreted results of experiments; S.T.Y. prepared figures; S.T.Y. drafted manuscript; S.T.Y., K.J.M., J.M.S., and M.J.R. edited and revised manuscript; S.T.Y., K.J.M., J.M.S., and M.J.R. approved final version of manuscript.
REFERENCES
- 1.Alexander BT, Kassab SE, Miller MT, Abram SR, Reckelhoff JF, Bennett WA, Granger JP. Reduced uterine perfusion pressure during pregnancy in the rat is associated with increases in arterial pressure and changes in renal nitric oxide. Hypertension 37: 1191–1195, 2001. doi: 10.1161/01.HYP.37.4.1191. [DOI] [PubMed] [Google Scholar]
- 2.Andersen A, Lund A, Knop FK, Vilsbøll T. Glucagon-like peptide 1 in health and disease. Nat Rev Endocrinol 14: 390–403, 2018. doi: 10.1038/s41574-018-0016-2. [DOI] [PubMed] [Google Scholar]
- 3.Dai Y, Mehta JL, Chen M. Glucagon-like peptide-1 receptor agonist liraglutide inhibits endothelin-1 in endothelial cell by repressing nuclear factor-κB activation. Cardiovasc Drugs Ther 27: 371–380, 2013. doi: 10.1007/s10557-013-6463-z. [DOI] [PubMed] [Google Scholar]
- 4.Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature 399: 601–605, 1999. doi: 10.1038/21224. [DOI] [PubMed] [Google Scholar]
- 5.Erdogdu O, Nathanson D, Sjöholm A, Nyström T, Zhang Q. Exendin-4 stimulates proliferation of human coronary artery endothelial cells through eNOS-, PKA-, and PI3K/Akt-dependent pathways and requires GLP-1 receptor. Mol Cell Endocrinol 325: 26–35, 2010. doi: 10.1016/j.mce.2010.04.022. [DOI] [PubMed] [Google Scholar]
- 6.Fulton D, Gratton JP, McCabe TJ, Fontana J, Fujio Y, Walsh K, Franke TF, Papapetropoulos A, Sessa WC. Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature 399: 597–601, 1999. [Erratum in Nature 400: 792, 1990.] doi: 10.1038/21218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gaspari T, Liu H, Welungoda I, Hu Y, Widdop RE, Knudsen LB, Simpson RW, Dear AE. A GLP-1 receptor agonist liraglutide inhibits endothelial cell dysfunction and vascular adhesion molecule expression in an ApoE-/- mouse model. Diab Vasc Dis Res 8: 117–124, 2011. [Diab Vasc Dis Res 9: 79, 2012.]. doi: 10.1177/1479164111404257. [DOI] [PubMed] [Google Scholar]
- 8.Gilbert JS, Babcock SA, Granger JP. Hypertension produced by reduced uterine perfusion in pregnant rats is associated with increased soluble fms-like tyrosine kinase-1 expression. Hypertension 50: 1142–1147, 2007. doi: 10.1161/HYPERTENSIONAHA.107.096594. [DOI] [PubMed] [Google Scholar]
- 9.Granger JP, Alexander BT, Llinas MT, Bennett WA, Khalil RA. Pathophysiology of preeclampsia: linking placental ischemia/hypoxia with microvascular dysfunction. Microcirculation 9: 147–160, 2002. doi: 10.1038/sj.mn.7800137. [DOI] [PubMed] [Google Scholar]
- 10.Hirata K, Kume S, Araki S, Sakaguchi M, Chin-Kanasaki M, Isshiki K, Sugimoto T, Nishiyama A, Koya D, Haneda M, Kashiwagi A, Uzu T. Exendin-4 has an anti-hypertensive effect in salt-sensitive mice model. Biochem Biophys Res Commun 380: 44–49, 2009. doi: 10.1016/j.bbrc.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 11.Ishii M, Shibata R, Kondo K, Kambara T, Shimizu Y, Tanigawa T, Bando YK, Nishimura M, Ouchi N, Murohara T. Vildagliptin stimulates endothelial cell network formation and ischemia-induced revascularization via an endothelial nitric-oxide synthase-dependent mechanism. J Biol Chem 289: 27235–27245, 2014. doi: 10.1074/jbc.M114.557835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.LaMarca BB, Bennett WA, Alexander BT, Cockrell K, Granger JP. Hypertension produced by reductions in uterine perfusion in the pregnant rat: role of tumor necrosis factor-alpha. Hypertension 46: 1022–1025, 2005. doi: 10.1161/01.HYP.0000175476.26719.36. [DOI] [PubMed] [Google Scholar]
- 13.Levine RJ, Maynard SE, Qian C, Lim KH, England LJ, Yu KF, Schisterman EF, Thadhani R, Sachs BP, Epstein FH, Sibai BM, Sukhatme VP, Karumanchi SA. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med 350: 672–683, 2004. doi: 10.1056/NEJMoa031884. [DOI] [PubMed] [Google Scholar]
- 14.Liu L, Liu J, Wong WT, Tian XY, Lau CW, Wang YX, Xu G, Pu Y, Zhu Z, Xu A, Lam KS, Chen ZY, Ng CF, Yao X, Huang Y. Dipeptidyl peptidase 4 inhibitor sitagliptin protects endothelial function in hypertension through a glucagon-like peptide 1-dependent mechanism. Hypertension 60: 833–841, 2012. doi: 10.1161/HYPERTENSIONAHA.112.195115. [DOI] [PubMed] [Google Scholar]
- 15.Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JF, Nauck MA, Nissen SE, Pocock S, Poulter NR, Ravn LS, Steinberg WM, Stockner M, Zinman B, Bergenstal RM, Buse JB, Committee LS, Investigators LT; LEADER Steering Committee; LEADER Trial Investigators . Liraglutide and cardiovascular outcomes in Type 2 diabetes. N Engl J Med 375: 311–322, 2016. doi: 10.1056/NEJMoa1603827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Michell BJ, Chen Z, Tiganis T, Stapleton D, Katsis F, Power DA, Sim AT, Kemp BE. Coordinated control of endothelial nitric-oxide synthase phosphorylation by protein kinase C and the cAMP-dependent protein kinase. J Biol Chem 276: 17625–17628, 2001. doi: 10.1074/jbc.C100122200. [DOI] [PubMed] [Google Scholar]
- 17.Novo Nordisk . Victoza (liraglutide) [package insert]. Plansboro, NJ: Novo Nordisk, 2019. [Google Scholar]
- 18.Nyström T, Gutniak MK, Zhang Q, Zhang F, Holst JJ, Ahrén B, Sjöholm A. Effects of glucagon-like peptide-1 on endothelial function in type 2 diabetes patients with stable coronary artery disease. Am J Physiol Endocrinol Metab 287: E1209–E1215, 2004. doi: 10.1152/ajpendo.00237.2004. [DOI] [PubMed] [Google Scholar]
- 19.Robinson LE, Holt TA, Rees K, Randeva HS, O’Hare JP. Effects of exenatide and liraglutide on heart rate, blood pressure and body weight: systematic review and meta-analysis. BMJ Open 3: e001986, 2013. doi: 10.1136/bmjopen-2012-001986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seligman SP, Buyon JP, Clancy RM, Young BK, Abramson SB. The role of nitric oxide in the pathogenesis of preeclampsia. Am J Obstet Gynecol 171: 944–948, 1994. doi: 10.1016/S0002-9378(94)70064-8. [DOI] [PubMed] [Google Scholar]
- 21.Shiraki A, Oyama J, Komoda H, Asaka M, Komatsu A, Sakuma M, Kodama K, Sakamoto Y, Kotooka N, Hirase T, Node K. The glucagon-like peptide 1 analog liraglutide reduces TNF-α-induced oxidative stress and inflammation in endothelial cells. Atherosclerosis 221: 375–382, 2012. doi: 10.1016/j.atherosclerosis.2011.12.039. [DOI] [PubMed] [Google Scholar]
- 22.Walsh SK, English FA, Johns EJ, Kenny LC. Plasma-mediated vascular dysfunction in the reduced uterine perfusion pressure model of preeclampsia: a microvascular characterization. Hypertension 54: 345–351, 2009. doi: 10.1161/HYPERTENSIONAHA.109.132191. [DOI] [PubMed] [Google Scholar]
- 23.Warrington JP, George EM, Palei AC, Spradley FT, Granger JP. Recent advances in the understanding of the pathophysiology of preeclampsia. Hypertension 62: 666–673, 2013. doi: 10.1161/HYPERTENSIONAHA.113.00588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu M, Moreno C, Hoagland KM, Dahly A, Ditter K, Mistry M, Roman RJ. Antihypertensive effect of glucagon-like peptide 1 in Dahl salt-sensitive rats. J Hypertens 21: 1125–1135, 2003. doi: 10.1097/00004872-200306000-00012. [DOI] [PubMed] [Google Scholar]
- 25.Zeisler H, Llurba E, Chantraine F, Vatish M, Staff AC, Sennström M, Olovsson M, Brennecke SP, Stepan H, Allegranza D, Dilba P, Schoedl M, Hund M, Verlohren S. Predictive value of the sFlt-1:PlGF ratio in women with suspected preeclampsia. N Engl J Med 374: 13–22, 2016. doi: 10.1056/NEJMoa1414838. [DOI] [PubMed] [Google Scholar]