Abstract
Cerebral blood flow during exercise is impaired in patients with heart failure implanted with left ventricular assist devices (LVADs). Our aim was to determine whether a 3-mo exercise training program could mitigate cerebrovascular dysfunction. Internal carotid artery (ICA) blood flow and intracranial middle (MCAv) and posterior cerebral (PCAv) artery velocities were measured continuously using Doppler ultrasound, alongside cardiorespiratory measures at rest and in response to an incremental cycle ergometer exercise protocol in 12 LVAD participants (5 female, 53.6 ± 11.8 yr; 84.2 ± 15.7 kg; 1.73 ± 0.08) pre- (PreTR) and post- (PostTR) completion of a 3-mo supervised exercise rehabilitation program. At rest, only PCAv was different PostTR (38.1 ± 10.4 cm/s) compared with PreTR (43.0 ± 10.8 cm/s; P < 0.05). PreTR, the reduction in PCAv observed from rest to exercise (5.2 ± 1.8%) was mitigated PostTR (P < 0.001). Similarly, exercise training enhanced ICA flow during submaximal exercise (~8.6 ± 13.7%), resulting in increased ICA flow PostTR compared with a reduced flow PreTR (P < 0.001). Although both end-tidal partial pressure of carbon dioxide and mean arterial pressure responses during incremental exercise were greater PostTR than PreTR, only the improved was related to the improved ICA flow (R2 = 0.14; P < 0.05). Our findings suggest that short-term exercise training improves cerebrovascular function during exercise in patients with LVADs. This finding should encourage future studies investigating long-term exercise training and cerebral and peripheral vascular adaptation.
NEW & NOTEWORTHY Left ventricular assist devices, now used as destination therapy in end-stage heart failure, enable patients to undertake rehabilitative exercise training. We show, for the first time in humans, that training improves cerebrovascular function during exercise in patients with left ventricular assist devices. This finding may have implications for cerebrovascular health in patients with heart failure.
Keywords: blood flow, cardiac rehabilitation, exercise, heart failure, LVAD
INTRODUCTION
Left ventricular assist device (LVAD) implantation is an increasingly common approach for the treatment of advanced heart failure (HF) (2). Augmented cardiac output and peripheral blood flow are factors commonly proposed to underpin enhanced exercise capacity following LVAD implantation (21). However, relatively little is known about the impact of LVAD implantation on cerebral blood flow during exercise in this group. Despite the observation that LVADs normalize cerebral blood flow (CBF) at rest (11, 34, 37), we recently reported no observable increases during incremental exercise (37). This impairment may be ameliorated if pump speed is progressively increased in conjunction with exercise intensity (8). The relative lack of cerebral hyperemia during exercise may be related to a lack of reflex increase in cardiac output (8) as well as the absence of an increase in intracranial vasodilation due to impaired shear stress and the lack of pulsatility (40). Cerebrovascular dysfunction during aerobic exercise in HF that persists despite LVAD implantation (7, 8, 37) may have potential long-term implications for brain function and health.
Exercise rehabilitation programs are now recommended as usual care to improve functional capacity and quality of life in patients with LVADs (2). We have demonstrated that arterial function can be enhanced, including in patients with heart failure, as a result of repetitive and episodic increases in intra-arterial shear stress during exercise (17, 27, 28). However, a paucity of studies have investigated the impacts of exercise training (18, 23, 24) in patients with LVADs, and none to our knowledge have evaluated training effects on cerebrovascular responses during exercise. Therefore, the aim of the present study was to evaluate the effects of a 12-wk supervised and center-based exercise training program on CBF responses during exercise in patients implanted with an LVAD. We hypothesized that exercise training would enhance cerebrovascular function during exercise.
METHODS
Participants.
Participants (n = 15) were recruited after being implanted with an LVAD for clinical reasons. Twelve participants completed the intervention, and 11 subjects had sufficient ICA windows for preassessments and postassessments (55% male participants; 45% female participants; age: 53.6 ± 11.8 yr; weight: 84.2 ± 15.7 kg; height: 1.73 ± 0.08 m). Participants were recruited from the Advanced Heart Failure and Cardiac Transplant Service at Fiona Stanley Hospital (Perth, Western Australia) and were on average 68 ± 99 wk post-LVAD implantation (range: 12–311 wk) at the time of enrollment. All participants had been hemodynamically stable for more than 4 wk before participating in the study. Routine transthoracic echocardiography was performed at intervals in these patients to characterize ejection fraction.
The study protocol was approved by the Royal Perth Hospital Human Research Ethics Committee and Fiona Stanley Hospital Governance Unit (REG15-164). Reciprocal ethics approval was granted by Curtin University (HR13/2016) and by the University of Western Australia Human Research Ethics Committee. The study was prospectively registered with the Australian New Zealand Clinical Trials Registry (ACTRN12616001596493). All aspects of the study complied with the ethical principles outlined by the Declaration of Helsinki. The participants were informed of all experimental procedures and associated risks. They provided written informed consent before the commencement of the study.
Procedures.
Participants underwent a series of baseline assessments that were repeated at the completion of the 12-wk exercise training program. All assessments were conducted in a temperature and humidity-controlled exercise laboratory. Participants visited the laboratory after fasting for at least 6 h and having refrained from alcohol, caffeine, and vigorous exercise for at least 24 h. Upon arrival, participants were instrumented and underwent a 10-min resting period in a semirecumbent position before resting measures were collected during the final minute of this period. Subjects subsequently performed a cardiopulmonary exercise test (CPET) on a semirecumbent cycle ergometer (Angio imaging ergometer, Lode, the Netherlands) with a 15-watt starting workload, followed by 15-W increments every 2 min until volitional exhaustion (20). Peak V̇o2 (V̇o2peak) was calculated as the highest V̇o2 recorded during the exercise test averaged over a 30-s period. Cerebrovascular (transcranial Doppler and duplex vascular ultrasound) assessments were collected continuously. Post-training evaluations were performed between 3–5 days after training ceased.
Cerebrovascular assessment.
Noninvasive insonation via 2 MHz transcranial Doppler ultrasound (Spencer Technologies, Seattle, WA) was used to assess blood velocity in the middle and posterior cerebral arteries (MCAv and PCAv, respectively). The cerebral arteries were identified and signals optimized according to their depth, waveform, and velocities, in keeping with previously published guidelines (46). The MCAv and PCAv were continuously recorded at baseline and throughout the CPET.
Blood velocity and diameter of the internal carotid artery (ICA) and vertebral arteries (VA) were measured using a 10–15 MHz multifrequency linear array vascular ultrasound (Terason T3200, Teratech, Burlington, MA) (43, 44). The ICA and VA recordings were captured at baseline, and ICA recordings were continuously captured throughout the incremental exercise test using identical techniques to those previously outlined by our research group (10, 19, 36). Briefly, blood velocity and diameter of both arteries was measured using a 10–15-MHz multifrequency linear array vascular ultrasound. B-mode imaging was used to measure arterial diameter, whereas simultaneously captured pulse-wave mode was used to concurrently measure peak blood flow velocity (43, 47). Diameter and velocity were measured at least 1.5 cm distal to arterial bifurcations to eliminate recordings of turbulent and retrograde flow and nonuniform shear. Care was taken to ensure that the insonation angle (60°) was unchanged throughout each test, and there were no alterations of B-mode settings after acquisition of the resting baseline to avoid any artificial changes in arterial wall brightness/thickness. All recordings were made in accordance with our published guidelines (43, 44), with screen capture and storage of video files for offline analysis (47). This analysis involved concurrent determination of arterial diameter and peak blood velocity at 30 Hz using our customized edge detection and wall tracking software, which is automated and designed to mitigate observer bias. We have published extensive reproducibility data using this approach (47), and our within-day coefficient of variation for the assessment of ICA diameter is 1.5% using this technique (38). Blood flow and shear rate were calculated as previously described (5, 10, 19, 36). It was not technically possible to capture VA responses during exercise because of the challenges of imaging these vessels during movement in the exercising subjects.
Twelve-lead electrocardiogram (PC-ECG 1200, Norav Medical Ltd., Yokneam, Israel) and pulse oximetry (Oxypleth 520A, Novametrix Medical Systems Inc., Wallingford, CT) were measured continuously at rest and throughout the CPET. Mean arterial pressure (MAP) was measured using brachial Doppler ultrasound (Dopplex MD2, Huntleigh Healthcare Ltd., Cardiff, UK), which is considered an adequate surrogate measure in patients with LVADs (4, 37), who do not have a pulse that can be detected for conventional blood pressure measurement. Ratings of perceived exertion (Borg Category Scale) were recorded during the final 30 s of each 2-min stage and at peak exercise (6). This Doppler assessment of blood pressure was recorded for the last 30–40 s of each stage at the radial artery. All cardiorespiratory variables were sampled continuously throughout the protocol at 1,000 Hz via an analog-to-digital converter (Powerlab, 16/30; ADInstruments, Colorado Springs, CO). Breath by breath end-tidal partial pressure of oxygen () and carbon dioxide (), V̇o2, and minute ventilation were measured via indirect calorimetry (Vyntus CPX, Jaeger, CareFusion, Germany).
The CPET workloads relative to individual maximum achieved workload (Wmax) were compared at 20%, 40%, 60%, 80% and 100% of Wmax for all variables except for ICA, which was only recorded until 60% of Wmax. All of the ICA recordings were screen-captured and stored as video files for offline analysis (47).
Exercise training intervention.
The exercise training program consisted of 3 1-h sessions weekly for a period of 12 wk. Sessions were supervised by experienced exercise physiologists and physiotherapists in a dedicated cardiac rehabilitation facility.
Each exercise session commenced and concluded with a warmup/cooldown involving 5 min of low-intensity continuous aerobic exercise followed by 5 min of muscular stretching. Participants undertook aerobic exercise on a treadmill for 28 min at an intensity ranging from 50% to 90% of V̇o2 reserve; this was refined based on rating of perceived exertion from 11 (“fairly light”) to 15 (“hard”) on the Borg Category Scale (32). Exercise training involved both interval and continuous modes of exercise and was progressed across the 12-wk training period according to individual adaptation. The exercise program was reviewed every fortnight during the course of the intervention to adapt for cardiopulmonary changes by progressively increasing the intensity as tolerated. Following the aerobic exercise component, participants undertook a set of resistance exercises involving 3 lower-body exercises (leg press, hamstring curl, and leg extension) and 3 upper-body exercises (incline bench press, lat pull down, and biceps curl) at 50%–60% of the baseline 1-repetition maximum test. Although participants commenced lower-body exercises at the outset of the exercise intervention, upper-body exercise was restricted until 12 wk post-LVAD implantation as per the clinical protocol to avoid postsurgical sternal injury (15). As a consequence, two participants avoided just the upper-body resistance exercises for the first 2 wk of training, and another missed the initial 3 wk.
We chose our exercise training intervention based on our previous extensive experience of combined aerobic and resistance training in patients with end-stage heart failure (27–29) and other comorbidities. The rationale is to include an aerobic stimulus for cardiopulmonary conditioning alongside a resistance stimulus to enhance skeletal muscle adaptation. We have previously demonstrated improvements in aerobic capacity, skeletal muscle strength, and vascular function using this approach, which is routinely applied clinically in our advanced heart failure and heart transplantation service (27–29). Although we used treadmill exercise in the training program to maximize the training stimulus, we used recumbent cycling for outcome testing since it allowed us to optimize the imaging we performed.
Statistical analysis.
Statistical analysis was performed using SPSS 25 software (IBM, Chicago, IL) and GraphPad PRISM 6.01 software (GraphPad Software, La Jolla, CA). Changes from baseline during exercise were assessed using two-way repeated-measures ANOVA. Post hoc corrections for multiple comparisons were also performed for main effect and interaction effects between pretraining and post-training measures. To measure the strength of association between variables, either Pearson’s or Spearman’s rank correlation coefficient was used depending on normality assumptions as appropriate. All statistical testing was two-sided with a level of significance set at P < 0.05. All data are presented as mean ± SD unless otherwise stated.
RESULTS
Participants had a mean ejection fraction of 24.8 ± 7.6% at baseline. Several different continuous-flow LVAD systems were implanted in participants [2 Heartmate II (Thoratec Corporation), 2 HVAD (HeartWare), and 7 Heartmate 3 (Thoratec Corporation)], and no changes in pump speed (6,275 ± 2,741 revolutions/min) were made between pretraining and post-training. The drug regimens of participants are provided in Table 1. Compliance with the exercise training sessions was 95% (sessions attended).
Table 1.
Frequency of medication use for participants with LVADs
| Medical Therapy | Pretraining (n = 11) |
|---|---|
| ACE inhibitors | 10 (91%) |
| Angiotensin II receptor blockers | 7 (64%) |
| β-blockers | 8 (72%) |
| Clopidogrel | 7 (64%) |
| Warfarin | 11 (100%) |
| Diuretics | 7 (64%) |
| Digoxin | 3 (27%) |
| Aspirin | 5 (45%) |
| Sildenafil | 4 (36%) |
| Amiodarone | 4 (36%) |
ACE, angiotensin-converting enzyme; LVAD, left ventricular assist device.
Effect of training on V̇o2 peak and workload maximum.
Based on data derived from the recumbent bicycle ergometer CPET, V̇o2peak changed from 11.7 ± 3.1 to 14.6 ± 3.7 mL·kg−1·min−1 (P < 0.005). Mean respiratory exchange ratio at pretraining (PreTR) assessment was 1.2 ± 0.1 and 1.3 ± 0.1 V̇co2/V̇o2 post-training (PostTR). Workload maximum increased from 64 ± 19 to 85 ± 19 W (P < 0.001).
Effect of training on cerebral hemodynamics at rest.
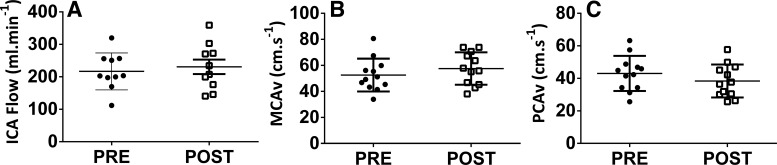
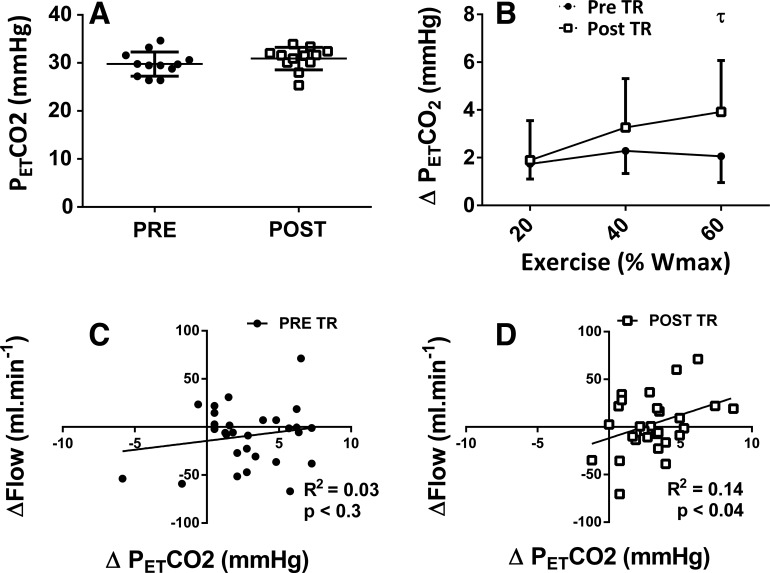
Resting ICA blood flow and MCAv were unchanged post-training, whereas post-training PCAv (P < 0.05) was lower than the pretraining value (Table 2 and Fig. 1). Similarly, velocity and shear rate were not significantly altered following training. In contrast, ICA diameter (4.9 ± 0.5 mm vs. 5.2 ± 0.8 mm; P < 0.05) increased following training. Resting (29.8 ± 2.5 mmHg vs. 30.9 ± 2.3 mmHg; P = 0.26) and MAPs were both unaltered by training (85.8 ± 9.8 mmHg vs. 92.5 ± 9.0 mmHg; P = 0.43).
Table 2.
Cardiorespiratory and cerebrovascular parameters at rest and during exercise (%workload maximum), pre and post 3 mo of exercise training
| BL | 20 | 40 | 60 | 80 | 100 | Significance | ||
|---|---|---|---|---|---|---|---|---|
| MCAv, cm/s | Pre | 55.0 ± 14.1 | 53.0 ± 13.7 | 53.4 ± 13.4 | 53.7 ± 13.8 | 53.1 ± 14.8 | 51.8 ± 14.3 | |
| Post | 57.6 ± 12.5 | 53.1 ± 10.3 | 53.8 ± 10.5 | 54.7 ± 10.1 | 54.4 ± 10.2 | 52.0 ± 10.3 | ||
| PCAv, cm/s | Pre | 43.0 ± 10.8 | 43.0 ± 9.9 | 43.1 ± 10.5 | 43.0 ± 10.7 | 42.1 ± 10.8 | 40.6 ± 10.0* | ME: *P < 0.05; INT: †P < 0.001 |
| Post | 38.1 ± 10.4† | 39.5 ± 11.2† | 39.6 ± 11.5† | 39.7 ± 12.0† | 39.7 ± 11.6† | 38.5 ± 11.6 | ||
| ICA flow, mL/min | Pre | 216.8 ± 56.8 | 220.5 ± 62.2 | 209.8 ± 75.9 | 192.2 ± 48.5 | INT: †P < 0.001 | ||
| Post | 222.6 ± 62.5 | 220.4 ± 59.9 | 230.4 ± 60.0† | 225.4 ± 56.0† | ||||
| ICA diameter, mm | Pre | 4.9 ± 0.5 | 4.8 ± 0.5 | 4.8 ± 0.5 | 4.7 ± 0.5 | ME: *P < 0.05 | ||
| Post | 5.2 ± 0.8† | 5.1 ± 0.8† | 5.1 ± 0.7† | 5.0 ± 0.7† | ||||
| ICA velocity, cm/s | Pre | 37.4 ± 6.4 | 39.1 ± 9.0 | 37.2 ± 9.5 | 36.3 ± 7.5 | INT: †P < 0.001 | ||
| Post | 36.1 ± 7.3 | 37.4 ± 9.6 | 38.6 ± 8.7† | 38.7 ± 9.8† | ||||
| , mmHg | Pre | 29.8 ± 2.5 | 31.5 ± 3.1* | 32.1 ± 3.0* | 31.8 ± 2.9* | 31.5 ± 3.3* | 30.9 ± 3.4* | ME: *P < 0.05; INT: †P < 0.001 |
| Post | 30.9 ± 2.3 | 32.8 ± 2.0*† | 34.2 ± 2.3*† | 34.8 ± 2.3*† | 34.4 ± 2.4*† | 33.4 ± 3.4*† | ||
| MAP, mmHg | Pre | 85.8 ± 9.8 | 93.2 ± 10.5 | 94.6 ± 8.8 | 97.3 ± 8.7 | 99.5 ± 7.9 | 102.6 ± 8.7 | ME: *P < 0.05; INT: †P < 0.001 |
| Post | 82.5 ± 9.0 | 89.6 ± 8.7 | 93.0 ± 10.3 | 97.5 ± 9.6 | 102.5 ± 8.3 | 108.3 ± 8.4† | ||
| Heart rate, beats/min | Pre | 83 ± 8 | 95 ± 13* | 100 ± 17* | 105 ± 21* | 110 ± 20* | 117 ± 23* | ME: *P < 0.05 |
| Post | 78 ± 9 | 91 ± 6* | 96 ± 7* | 101 ± 9* | 109 ± 12* | 119 ± 14* |
Values are means ± SD. BL, baseline; ICA, internal carotid artery; MAP, mean arterial pressure; MCAv, middle cerebral artery velocity; PCAv, posterior cerebral artery velocity; , end-tidal partial pressure of carbon dioxide; Pre, pretraining; Post, post-training; ME, main effect; INT, interaction effects.
Statistical difference from baseline (P < 0.05);
Statistical difference from pretraining (P < 0.001).
Fig. 1.
Pretraining (PRE) and post-training (POST) internal carotid artery flow [ICA; n = 10 (5 women: 5 men)] (A), middle cerebral artery velocity [MCAv; n = 12 (5 women: 7 men)] (B), and posterior cerebral artery velocity [PCAv; n = 12 (5 women: 7 men)] (C) at rest in participants implanted with left ventricular assist devices.
Effect of training on cerebral hemodynamics during exercise.
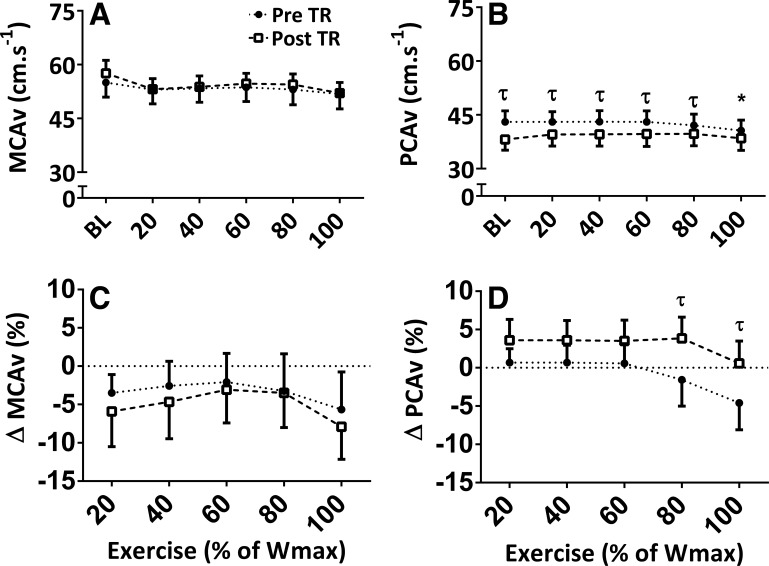
Table 2 presents cerebral hemodynamic responses measured during exercise, both pretraining and post-training. A main effect for exercise intensity and an interaction between exercise and intensity (P < 0.001) were observed for PCAv. Post hoc analysis revealed that only PreTR PCAv at 100% Wmax was reduced from baseline (2.4 ± 0.8 cm/s) (Fig. 2B; P < 0.01), with greater reductions in PCAv from baseline at 80 (5.4 ± 1.8%) and 100 (5.2 ± 1.8%) %Wmax pretraining than post-training (Fig. 2, B and D; P < 0.001). No post hoc differences were observed for MCAv (Fig. 2, A and C).
Fig. 2.
Absolute middle cerebral artery velocity (MCAv) and posterior cerebral artery velocity (PCAv) (A and B) at rest (BL) and during exercise at 20%, 40%, 60%, 80%, and 100% of the maximum achieved workload (% of Wmax) in participants implanted with left ventricular assist devices [n = 12 (5 women: 7 men]. Relative percent changes from baseline are shown (C and D). *Main effect difference from BL, P < 0.05. τ signifies interaction and post-hoc-revealed differences between pretraining (Pre TR) and post-training (Post TR); P < 0.001.
A main effect for exercise intensity was observed for ICA diameter (Fig. 3A; P < 0.05). No main effects of group or exercise intensity were observed for ICA velocity or flow. However, there were significant interaction effects for ICA velocity and flow (Figs. 3 and 4; P < 0.001). Post hoc analysis revealed that pretraining, but not post-training, ICA flows were reduced from baseline (−24.6 ± 8.1 mL/min) at 60% Wmax (P = 0.02). Post hoc assessment of the interactions between relative changes (i.e., Δ %) from baseline revealed divergent pretraining and post-training ICA velocity and flow responses to incremental exercise (Fig. 4). Mean differences between pretraining and post-training changes from baseline in ICA velocity at 40% and 60% Wmax were 8.6 ± 2.7% and 10.6 ± 2.7% (Fig. 4 B; P = 0.02), respectively. Similarly, mean differences between pretraining and post-training ICA flow at 40% and 60% Wmax were 8.6 ± 2.9% and 13.7 ± 2.9% (Fig. 4D; P = 0.004), respectively. No main effect or interactions were observed for ICA shear rate (Fig. 4).
Fig. 3.
Internal carotid artery (ICA) diameter (A), velocity (B), shear stress (C), and flow at rest (BL) (D) and during 20%, 40%, and 60% of the maximum achieved workload (% of Wmax) in participants implanted with left ventricular assist devices [n = 12 (5 women: 7 men)]. τ signifies interaction and post hoc-revealed differences between pretraining (Pre TR) and post-training (Post TR); P < 0.001.
Fig. 4.
Relative change from resting internal carotid artery diameter (A), velocity (B), shear stress (C), and flow (D) during 20%, 40%, and 60% of the maximum achieved workload (Wmax) in participants implanted with left ventricular assist devices [n = 10 (5 women: 5 men)]. τ signifies interaction and post hoc-revealed differences between pretraining (Pre TR) and post-training (Post TR); P < 0.001.
Effect of training on cardiorespiratory measures during exercise.
Elevated was identified at 40%, 60%, 80%, and 100% Wmax for both pretraining and post-training (Table 2; P < 0.001). A main effect was observed between pretraining and post-training, revealing a greater post-training PETCO2 during 40%, 60%, 80%, and 100% Wmax (Fig. 5B; mean difference = 2.6 ± 0.4 mmHg, P = 0.03). No correlation between the change in ICA flow and PETCO2 was observed pretraining, but a significant correlation was observed post-training (r = 0.37, R2 = 0.14; P < 0.05).
Fig. 5.
Resting (A) partial pressure of end-tidal carbon dioxide () and the relative percent change (B) from rest at 20%, 40%, and 60% of the maximum achieved workload (% of Wmax) in participants implanted with left ventricular assist devices [(n = 12 (5 women: 7 men)]. Pretraining (Pre TR) (C) and post-training (Post TR) (D) relationship between the change in internal carotid artery flow and during exercise. τ signifies interaction and post hoc-revealed differences between pretraining and post-training; P < 0.001.
Similar MAP values were observed at each intensity except during 100% Wmax for pretraining (102 ± 8.7 mmHg) and post-training (108 ± 8.4 mmHg) groups (Fig. 6B; P < 0.01). Post hoc analysis following interaction revealed for the MAP increases from baseline (Fig. 6; P = 0.0006) indicated that pretraining changes were lower than post-training at 80 (mean difference between pretraining and post-training Δ from baseline = −5.8 ± 1.7 mmHg) and 100% Wmax (mean difference between pretraining and post-training Δ from baseline = −8.2 ± 1.7 mmHg). Post hoc interaction analysis revealed a higher ICA vascular conductance at 60% Wmax post-training (Fig. 6; P = 0.0003). Exercise progressively increased heart rate similarly for both pre (12–34 beats/min) and post (13–40 beats/min) training (Table 2; P < 0.05). No difference between conditions was observed.
Fig. 6.
Mean arterial pressure (MAP) at rest (A) and the absolute change in MAP [20–100 of the maximum achieved workload (% of Wmax)] (B) and internal carotid conductance (ICA CVCi; 20–60% Wmax) (C) in participants implanted with left ventricular assist devices [n = 12 (5 women: 7 men)]. *Main effect difference from rest (BL); P < 0.05. τ signifies interaction and post hoc-revealed differences between pretraining (PreTR) and post-training (PostTR); P < 0.001.
Correlations between variables.
At rest, V̇o2peak correlated with ICA velocity (r = 0.596; P < 0.05) and ICA flow (r = 0.695; P < 0.01). Regarding pretraining data collected during exercise, specifically at 60% Wmax, V̇o2peak was correlated with PCA (r = 0.551; P < 0.05), ICA velocity (r = 0.747; P < 0.01), and ICA flow (r = 0.673; P < 0.05). There were no correlations between the change in cerebral blood flow parameters and the change of V̇o2peak. This may relate to the relatively modest sample size in this study.
DISCUSSION
This is the first study, to our knowledge, to measure cerebrovascular function during incremental exercise in patients with LVADs before and after an exercise training intervention. Our principal finding is that a 12-wk exercise training program significantly enhanced CBF responses to incremental exercise despite a reduction in absolute intracranial PCAv.
The improved durability of third-generation LVADs and the continuing scarcity of donor organs has led to an increase in the number of LVAD implantations worldwide for “destination therapy” (1). Although functional capacity improves from preimplantation status, recipients of LVADs still experience impaired aerobic capacity and cerebrovascular function during exercise (7, 8, 37). Exercise training is an evidence-based adjunct treatment for patients with HF (35, 41, 42). Although exercise training is currently being delivered in cardiac rehabilitation settings to improve functional capacity and health (2), there is a paucity of evidence regarding its specific effects in patients with LVADs (1, 13, 25). We have found that a comprehensive exercise training program involving aerobic and resistance exercise is associated with improvements in cerebrovascular function.
We recently demonstrated that LVAD implantation normalizes CBF at rest but fails to compensate during exercise compared with age-matched healthy controls and patients with HF (37). In healthy individuals, the exercise CBF-response follows a biphasic pattern of initial vasodilation during submaximal exercise, followed by a plateau (9, 31, 38). During submaximal exercise (20%–60% V̇o2peak), there is typically a 10%–20% vasodilatory increase in CBF, which is positively correlated with increases in , MAP, and intra-arterial shear rate (36). We did not observe such increases during exercise in LVAD individuals in our other recent study (37) or in our pretraining LVAD individuals in this study. A novel study by Brassard et al. (8) found that, to increase CBF during exercise in untrained LVAD patients with axial-flow pumps, increases in pump speed were required. Other studies investigating the effect of pump speed manipulation during exercise have resulted in trivial (22, 30) or no improvement (16, 33) in aerobic capacity. These findings suggest that peripheral factors (e.g., cardiorespiratory and musculoskeletal) are likely to play an important role in the ongoing exercise intolerance experienced by patients with LVADs (2). Our post-training participants with LVADs improved aerobic capacity, peak workload, and cardiorespiratory (MAP and ) responses during incremental exercise. Noncardiac (musculoskeletal and respiratory) improvements following exercise training have the capacity to enhance both aerobic capacity and peak achievable workload (2, 7). We did not design or power the present study to identify the relative contribution of specific cardiorespiratory and/or musculoskeletal variables to the changes in cerebral blood flows we observed. Nevertheless, our findings suggest that training-induced improvements occur in parallel with systemic cardiorespiratory responses (i.e., and MAP; Figs. 5 and 6, respectively) associated with healthy cerebral hemodynamics during exercise. Therefore, the improved aerobic capacity, peak workload, and cardiorespiratory responses in the study may be linked to the improved cerebrovascular function in patients with LVADs, keeping with the suggestion of Brassard et al. (7), who postulated links between exercise intolerance and cerebral perfusion in CHF.
The enhanced capacity of the brain to respond to physiological and regulatory stimuli is important to maintaining brain health across the lifespan (3, 4). Although a few cross-sectional studies indicate overall heightened cerebrovascular function in exercise-trained individuals, a more recent study demonstrated that dynamic cerebrovascular autoregulation is modestly attenuated in athletes following 6 wk of high-intensity interval training (HIIT) despite no change in resting CBF (14). It is unknown what impact our interval and continuous training intervention had on cerebral autoregulation in our clinical population. However, the same group who observed attenuated autoregulation also observed that 6 wk of HIIT enhanced cardiovascular function (i.e., blood pressure and heart rate) while triggering potentially dysfunctional cardiac remodeling (26). Additionally, Lewis et al. (25), despite finding significant improvement in V̇o2, observed no significant adaptations to cerebral autoregulation in patients with chronic obstructive pulmonary disease following an 8-wk exercise intervention that included 3 wk of HITT. The benefits of HIIT training in other clinical populations are not fully established, particularly with respect to changes in brain perfusion. Future research investigating different forms of exercise training on cerebrovascular function in clinical populations, including those with heart failure, are warranted.
Cerebrovascular endothelial function is quantified via hypercapnic shear-mediated vasodilation in the internal and common carotid arteries (10, 19). Shear-mediated vasodilation in the extracranial arteries also occurs during exercise in healthy individuals (36) but not in patients with LVADs (37). Our findings, including those related to resting carotid diameters, suggest that exercise training in patients with LVADs may improve cerebrovascular vasodilation in extracranial arteries. The observed mismatch between relative ICA flow and MCA responses during exercise suggests that intracranial dilation may be present in patients with LVADs in the current study. Because the 5%–10% change in ICA flow was not observed in MCA velocity, MCA artery dilation must occur (~2%–3%) to achieve conservation of blood flow. Unfortunately, because of technological limitations associated with measuring diameter and velocity in intracranial vessels (i.e., MCA), it is unknown if intracranial blood flow, shear stress, and endothelial function can be enhanced by exercise training in patients with LVADs. Future studies are needed to directly assess cerebrovascular endothelial in patients with LVADs during exercise and resting conditions.
Few studies have assessed the posterior cerebral circulation in patients with LVADs. Our findings indicate that during exercise, PCAv decreased significantly below baseline levels before training, but this did not occur after training. The lack of volumetric blood flow data through the vertebral artery during exercise (technically difficult to obtain) makes it difficult to discern the impact that a lower posterior cerebrovascular perfusion may have on LVAD cerebrovascular health. However, it would appear that exercise training improves PCAv responses to exercise, potentially via an elevation in cardiorespiratory responses (e.g., greater increase in MAP and responses at maximal exercise). Warnert et al. (45) suggested, via the “selfish brain” hypothesis, that maintaining adequate posterior cerebral perfusion is a critical regulatory factor. Although speculative, our data suggest that training may act to maintain adequate perfusion to autonomic centers of the brain. Unfortunately, our data are unable to fully address this speculation, and future studies investigating exercise that control for the impacts of CO2 and MAP and utilize volumetric posterior CBF measures in patients with LVADs will be required.
Recently, Stohr et al. (39, 40) and Cromwell et al. (12, 13) discussed the importance of pulsatile flow in maintaining normal cerebral perfusion in patients with LVADs. Both authors stated that resting cerebral perfusion was relatively normalized by LVADs. Although Cromwell et al. (12, 13) suggested that evidence does not indicate adverse impacts on cerebral processes in nonpulsatile systems, Stohr et al. (39, 40) stated that microbleeds and endothelial dysfunction may be higher in individuals implanted with nonpulsatile systems compared with pulsatile systems. Our experimental findings indicate that cerebral perfusion can be enhanced in patients implanted with LVADs during exercise and that training generally enhances cerebrovascular perfusion variables. We cannot speculate about the impact of pulsatile versus nonpulsatile LVADs on endothelial function or other outcomes such as cognition or infarction, but the relationships between perfusion and these variables deserve a larger dedicated trial. Since one outcome of LVAD implantation is that is enables many patients to return to the gym and undertake more robust and targeted exercise therapy, our findings are encouraging for the positive effects observed in cerebrovascular function.
Limitations.
There are several limitations of the current study. The most important relates to the lack of a control group, which restricts the interpretation of our results to distinguish between the effects of exercise training versus spontaneous effects occurring over time. However, participants were recruited to the study, on average, 68 wk post-LVAD implantation while receiving optimal medical therapy, and pump speed was not changed throughout the intervention. It seems unlikely that our findings were due to spontaneous recovery in such a group or to the short-term impacts of device implantation. Furthermore, in a previous study of exercise training in patients with LVADs commencing after a similar period of recovery to the current study (>12 wk), control participants did not show spontaneous changes in aerobic capacity, pulmonary function, or quality of life (24), suggesting that, like our cohort, they were physiologically stable. Another limitation relates to the use of transcranial Doppler ultrasound to assess intracranial cerebral blood flow, which has some technical limitations. As mentioned above, it does not elicit measures of intracranial arterial diameters and thus provides only an index of blood flow via velocity profiling. Typically, MCAv and ICA flow measures follow similar responses, and measurement of ICA caliber is an appropriate surrogate for changes in MCA caliber. However, our findings indicate divergent responses, with ICA flow increasing, largely because of an enhanced velocity, in contrast to no observable changes in MCA velocity. Downstream vasodilation would reduce resistance or inversely increase conductance, which might explain the lack of velocity change in the MCA. Nonetheless, the lack of direct intracranial diameter assessment in the study remains a limitation and hinders quantification of the contribution of intracranial diameter change during exercise. We have previously mentioned the potential confounding of the contralateral differences that may exist in cerebral artery responses (37); however, we applied standardized ultrasound imaging techniques to screen for differences in all intracranial (46) and extracranial (44) vessels. No differences were observed; thus, we interpreted our ICA, MCA, and PCA measures to be valid surrogates or global cerebrovascular hemodynamic responses to exercise. Disparate contralateral flow patterns during exercise in patients with LVADs have not been previously observed, and no evidence suggests that contralateral flow patterns would differ in vessels that have been screened to rule out any divergent patterns at rest.
Conclusion.
This pilot intervention study found that CBF responses to exercise were increased following a 12-wk exercise training program in patients with LVADs. Our findings therefore support the role of short-term exercise training to improve cerebrovascular function in LVAD patients. Future studies investigating long-term exercise training and cerebral and peripheral vascular adaptation are needed.
GRANTS
K. J. Smith was supported by a Natural Sciences and Engineering Council of Canada Post-Doctoral Fellowship. I. Moreno-Suarez was supported by a scholarship from Curtin University and the Heart and Lung Transplant Foundation of Western Australia. D. J. Green is a National Health and Medical Research Council Principal Research Fellow (1080914). This project was supported by a Vanguard Grant from the Heart Foundation of Australia (101040) and by a National Health and Medical Research Council project (APP1143660).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
D.J.G. conceived and designed research; K.J.S. and I.M.-S. performed experiments; K.J.S. and I.M.-S. analyzed data; I.M.-S., A.S., L.D., L.H.N., A.J.M., and D.J.G. interpreted results of experiments; K.J.S. prepared figures; K.J.S. and I.M.-S. drafted manuscript; L.D., L.H.N., A.J.M., and D.J.G. edited and revised manuscript; I.M.-S., A.S., L.D., L.H.N., A.J.M., and D.J.G. approved final version of manuscript.
REFERENCES
- 1.Acharya D, Loyaga-Rendon R, Morgan CJ, Sands KA, Pamboukian SV, Rajapreyar I, Holman WL, Kirklin JK, Tallaj JA. INTERMACS analysis of stroke during support with continuous-flow left ventricular assist devices: risk factors and outcomes. JACC Heart Fail 5: 703–711, 2017. doi: 10.1016/j.jchf.2017.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adamopoulos S, Corrà U, Laoutaris ID, Pistono M, Agostoni PG, Coats AJS, Crespo Leiro MG, Cornelis J, Davos CH, Filippatos G, Lund LH, Jaarsma T, Ruschitzka F, Seferovic PM, Schmid JP, Volterrani M, Piepoli MF. Exercise training in patients with ventricular assist devices: a review of the evidence and practical advice. A position paper from the Committee on Exercise Physiology and Training and the Committee of Advanced Heart Failure of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 21: 3–13, 2019. doi: 10.1002/ejhf.1352. [DOI] [PubMed] [Google Scholar]
- 3.Ainslie PN, Cotter JD, George KP, Lucas S, Murrell C, Shave R, Thomas KN, Williams MJ, Atkinson G. Elevation in cerebral blood flow velocity with aerobic fitness throughout healthy human ageing. J Physiol 586: 4005–4010, 2008. doi: 10.1113/jphysiol.2008.158279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bennett MK, Roberts CA, Dordunoo D, Shah A, Russell SD. Ideal methodology to assess systemic blood pressure in patients with continuous-flow left ventricular assist devices. J Heart Lung Transplant 29: 593–594, 2010. doi: 10.1016/j.healun.2009.11.604. [DOI] [PubMed] [Google Scholar]
- 5.Black MA, Cable NT, Thijssen DHJ, Green DJ. Importance of measuring the time course of flow-mediated dilatation in humans. Hypertension 51: 203–210, 2008. doi: 10.1161/HYPERTENSIONAHA.107.101014. [DOI] [PubMed] [Google Scholar]
- 6.Borg G. Measuring perceived exertion and pain. In: Borg’s Perceived Exertion and Pain Scales. Chicago, IL: Human Kinetics, 1998, p. 26. [Google Scholar]
- 7.Brassard P, Gustafsson F. Exercise intolerance in heart failure: did we forget the brain? Can J Cardiol 32: 475–484, 2016. doi: 10.1016/j.cjca.2015.12.021. [DOI] [PubMed] [Google Scholar]
- 8.Brassard P, Jensen AS, Nordsborg N, Gustafsson F, Møller JE, Hassager C, Boesgaard S, Hansen PB, Olsen PS, Sander K, Secher NH, Madsen PL. Central and peripheral blood flow during exercise with a continuous-flow left ventricular assist device: constant versus increasing pump speed: a pilot study. Circ Heart Fail 4: 554–560, 2011. doi: 10.1161/CIRCHEARTFAILURE.110.958041. [DOI] [PubMed] [Google Scholar]
- 9.Brugniaux JV, Marley CJ, Hodson DA, New KJ, Bailey DM. Acute exercise stress reveals cerebrovascular benefits associated with moderate gains in cardiorespiratory fitness. J Cereb Blood Flow Metab 34: 1873–1876, 2014. doi: 10.1038/jcbfm.2014.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carter HH, Atkinson CL, Heinonen IHA, Haynes A, Robey E, Smith KJ, Ainslie PN, Hoiland RL, Green DJ. Evidence for shear stress-mediated dilation of the internal carotid artery in humans. Hypertension 68: 1217–1224, 2016. doi: 10.1161/HYPERTENSIONAHA.116.07698. [DOI] [PubMed] [Google Scholar]
- 11.Cornwell WK III, Tarumi T, Aengevaeren VL, Ayers C, Divanji P, Fu Q, Palmer D, Drazner MH, Meyer DM, Bethea BT, Hastings JL, Fujimoto N, Shibata S, Zhang R, Markham DW, Levine BD. Effect of pulsatile and nonpulsatile flow on cerebral perfusion in patients with left ventricular assist devices. J Heart Lung Transplant 33: 1295–1303, 2014. doi: 10.1016/j.healun.2014.08.013. [DOI] [PubMed] [Google Scholar]
- 12.Cornwell WK III, Tarumi T, Lawley J, Ambardekar AV. CrossTalk opposing view: blood flow pulsatility in left ventricular assist device patients is not essential to maintain normal brain physiology. J Physiol 597: 357–359, 2019. doi: 10.1113/JP276730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cornwell WK III, Tarumi T, Lawley J, Ambardekar AV. Rebuttal from William K. Cornwell III, Takashi Tarumi, Justin Lawley and Amrut V. Ambardekar. J Physiol 597: 363–364, 2019. doi: 10.1113/JP277244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Drapeau A, Labrecque L, Imhoff S, Paquette M, Le Blanc O, Malenfant S, Brassard P. Six weeks of high-intensity interval training to exhaustion attenuates dynamic cerebral autoregulation without influencing resting cerebral blood velocity in young fit men. Physiol Rep 7: e14185, 2019. doi: 10.14814/phy2.14185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eickmeyer SM, Barker KD, Sayyad A, Rydberg L. The rehabilitation of patients with advanced heart failure after left ventricular assist device placement: a narrative review. PM R 11: 64–75, 2019. doi: 10.1016/j.pmrj.2018.05.027. [DOI] [PubMed] [Google Scholar]
- 16.Fresiello L, Buys R, Timmermans P, Vandersmissen K, Jacobs S, Droogne W, Ferrari G, Rega F, Meyns B. Exercise capacity in ventricular assist device patients: clinical relevance of pump speed and power. Eur J Cardiothorac Surg 50: 752–757, 2016. doi: 10.1093/ejcts/ezw147. [DOI] [PubMed] [Google Scholar]
- 17.Green DJ, Hopman MT, Padilla J, Laughlin MH, Thijssen DH. Vascular adaptation to exercise in humans: role of hemodynamic stimuli. Physiol Rev 97: 495–528, 2017. doi: 10.1152/physrev.00014.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hayes K, Leet AS, Bradley SJ, Holland AE. Effects of exercise training on exercise capacity and quality of life in patients with a left ventricular assist device: a preliminary randomized controlled trial. J Heart Lung Transplant 31: 729–734, 2012. doi: 10.1016/j.healun.2012.02.021. [DOI] [PubMed] [Google Scholar]
- 19.Hoiland RL, Smith KJ, Carter HH, Lewis NCS, Tymko MM, Wildfong KW, Bain AR, Green DJ, Ainslie PN. Shear-mediated dilation of the internal carotid artery occurs independent of hypercapnia. Am J Physiol Heart Circ Physiol 313: H24–H31, 2017. doi: 10.1152/ajpheart.00119.2017. [DOI] [PubMed] [Google Scholar]
- 20.Ingle L. Theoretical rationale and practical recommendations for cardiopulmonary exercise testing in patients with chronic heart failure. Heart Fail Rev 12: 12–22, 2007. doi: 10.1007/s10741-007-9000-y. [DOI] [PubMed] [Google Scholar]
- 21.Jung MH, Gustafsson F. Exercise in heart failure patients supported with a left ventricular assist device. J Heart Lung Transplant 34: 489–496, 2015. doi: 10.1016/j.healun.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 22.Jung MH, Hansen PB, Sander K, Olsen PS, Rossing K, Boesgaard S, Russell SD, Gustafsson F. Effect of increasing pump speed during exercise on peak oxygen uptake in heart failure patients supported with a continuous-flow left ventricular assist device. A double-blind randomized study. Eur J Heart Fail 16: 403–408, 2014. doi: 10.1002/ejhf.52. [DOI] [PubMed] [Google Scholar]
- 23.Kerrigan DJ, Williams CT, Ehrman JK, Saval MA, Bronsteen K, Schairer JR, Swaffer M, Brawner CA, Lanfear DE, Selektor Y, Velez M, Tita C, Keteyian SJ. Cardiac rehabilitation improves functional capacity and patient-reported health status in patients with continuous-flow left ventricular assist devices: the Rehab-VAD randomized controlled trial. JACC Heart Fail 2: 653–659, 2014. doi: 10.1016/j.jchf.2014.06.011. [DOI] [PubMed] [Google Scholar]
- 24.Laoutaris ID, Dritsas A, Adamopoulos S, Manginas A, Gouziouta A, Kallistratos MS, Koulopoulou M, Voudris V, Cokkinos DV, Sfirakis P. Benefits of physical training on exercise capacity, inspiratory muscle function, and quality of life in patients with ventricular assist devices long-term postimplantation. Eur J Cardiovasc Prev Rehabil 18: 33–40, 2011. doi: 10.1097/HJR.0b013e32833c0320. [DOI] [PubMed] [Google Scholar]
- 25.Lewis N, Gelinas JCM, Ainslie PN, Smirl JD, Agar G, Melzer B, Rolf JD, Eves ND. Cerebrovascular function in patients with chronic obstructive pulmonary disease: the impact of exercise training. Am J Physiol Heart Circ Physiol 316: H380–H391, 2019. doi: 10.1152/ajpheart.00348.2018. [DOI] [PubMed] [Google Scholar]
- 26.Mahjoub H, Le Blanc O, Paquette M, Imhoff S, Labrecque L, Drapeau A, Poirier P, Bédard É, Pibarot P, Brassard P. Cardiac remodeling after six weeks of high-intensity interval training to exhaustion in endurance-trained men. Am J Physiol Heart Circ Physiol 317: H685–H694, 2019. doi: 10.1152/ajpheart.00196.2019. [DOI] [PubMed] [Google Scholar]
- 27.Maiorana A, O’Driscoll G, Cheetham C, Collis J, Goodman C, Rankin S, Taylor R, Green D. Combined aerobic and resistance exercise training improves functional capacity and strength in CHF. J Appl Physiol (1985) 88: 1565–1570, 2000. doi: 10.1152/jappl.2000.88.5.1565. [DOI] [PubMed] [Google Scholar]
- 28.Maiorana A, O’Driscoll G, Cheetham C, Dembo L, Stanton K, Goodman C, Taylor R, Green D. The effect of combined aerobic and resistance exercise training on vascular function in type 2 diabetes. J Am Coll Cardiol 38: 860–866, 2001. doi: 10.1016/S0735-1097(01)01439-5. [DOI] [PubMed] [Google Scholar]
- 29.Maiorana A, O’Driscoll G, Dembo L, Cheetham C, Goodman C, Taylor R, Green D. Effect of aerobic and resistance exercise training on vascular function in heart failure. Am J Physiol Heart Circ Physiol 279: H1999–H2005, 2000. doi: 10.1152/ajpheart.2000.279.4.H1999. [DOI] [PubMed] [Google Scholar]
- 30.Mezzani A, Pistono M, Corrà U, Giordano A, Gnemmi M, Imparato A, Centofanti P, Rinaldi M, Colombo S, Canal E, Giannuzzi P. Systemic perfusion at peak incremental exercise in left ventricular assist device recipients: Partitioning pump and native left ventricle relative contribution. Int J Cardiol Heart Vessel 4: 40–45, 2014. doi: 10.1016/j.ijchv.2014.07.004. [DOI] [Google Scholar]
- 31.Moraine JJ, Lamotte M, Berré J, Niset G, Leduc A, Naeije R. Relationship of middle cerebral artery blood flow velocity to intensity during dynamic exercise in normal subjects. Eur J Appl Physiol Occup Physiol 67: 35–38, 1993. doi: 10.1007/BF00377701. [DOI] [PubMed] [Google Scholar]
- 32.Noble BJ, Borg GA, Jacobs I, Ceci R, Kaiser P. A category-ratio perceived exertion scale: relationship to blood and muscle lactates and heart rate. Med Sci Sports Exerc 15: 523–528, 1983. doi: 10.1249/00005768-198315060-00015. [DOI] [PubMed] [Google Scholar]
- 33.Noor MR, Bowles C, Banner NR. Relationship between pump speed and exercise capacity during HeartMate II left ventricular assist device support: influence of residual left ventricular function. Eur J Heart Fail 14: 613–620, 2012. doi: 10.1093/eurjhf/hfs042. [DOI] [PubMed] [Google Scholar]
- 34.Ono M, Joshi B, Brady K, Easley RB, Kibler K, Conte J, Shah A, Russell SD, Hogue CW. Cerebral blood flow autoregulation is preserved after continuous-flow left ventricular assist device implantation. J Cardiothorac Vasc Anesth 26: 1022–1028, 2012. doi: 10.1053/j.jvca.2012.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, González-Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P; Authors/Task Force Members; Document Reviewers . 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur J Heart Fail 18: 891–975, 2016. doi: 10.1002/ejhf.592. [DOI] [PubMed] [Google Scholar]
- 36.Smith KJ, Hoiland RL, Grove R, McKirdy H, Naylor L, Ainslie PN, Green DJ. Matched increases in cerebral artery shear stress, irrespective of stimulus, induce similar changes in extra-cranial arterial diameter in humans. J Cereb Blood Flow Metab 39: 849–858, 2019. doi: 10.1177/0271678X17739220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smith KJ, Suarez IM, Scheer A, Chasland LC, Thomas HJ, Correia MA, Dembo LG, Naylor LH, Maiorana AJ, Green DJ. Cerebral blood flow during exercise in heart failure: effect of ventricular assist devices. Med Sci Sports Exerc 51: 1372–1379, 2019. doi: 10.1249/MSS.0000000000001904. [DOI] [PubMed] [Google Scholar]
- 38.Smith KJ, Wildfong KW, Hoiland RL, Harper M, Lewis NC, Pool A, Smith SL, Kuca T, Foster GE, Ainslie PN. Role of CO2 in the cerebral hyperemic response to incremental normoxic and hyperoxic exercise. J Appl Physiol (1985) 120: 843–854, 2016. doi: 10.1152/japplphysiol.00490.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stöhr EJ, McDonnell BJ, Colombo PC, Willey JZ. Rebuttal from Eric J. Stöhr, Barry J. McDonnell, Paolo C. Colombo and Joshua Z. Willey. J Physiol 597: 361–362, 2019. doi: 10.1113/JP277243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stöhr EJ, McDonnell BJ, Colombo PC, Willey JZ. CrossTalk proposal: Blood flow pulsatility in left ventricular assist device patients is essential to maintain normal brain physiology. J Physiol 597: 353–356, 2019. doi: 10.1113/JP276729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Swank AM, Horton J, Fleg JL, Fonarow GC, Keteyian S, Goldberg L, Wolfel G, Handberg EM, Bensimhon D, Illiou M-C, Vest M, Ewald G, Blackburn G, Leifer E, Cooper L, Kraus WE; HF-ACTION Investigators . Modest increase in peak VO2 is related to better clinical outcomes in chronic heart failure patients: results from heart failure and a controlled trial to investigate outcomes of exercise training. Circ Heart Fail 5: 579–585, 2012. doi: 10.1161/CIRCHEARTFAILURE.111.965186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Taylor RS, Sagar VA, Davies EJ, Briscoe S, Coats AJS, Dalal H, Lough F, Rees K, Singh S. Exercise-based rehabilitation for heart failure. Cochrane Database Syst Rev 4: CD003331, 2014. doi: 10.1002/14651858.CD003331.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thijssen DH, Black MA, Pyke KE, Padilla J, Atkinson G, Harris RA, Parker B, Widlansky ME, Tschakovsky ME, Green DJ. Assessment of flow-mediated dilation in humans: a methodological and physiological guideline. Am J Physiol Heart Circ Physiol 300: H2–H12, 2011. doi: 10.1152/ajpheart.00471.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thomas KN, Lewis NC, Hill BG, Ainslie PN. Technical recommendations for the use of carotid duplex ultrasound for the assessment of extracranial blood flow. Am J Physiol Regul Integr Comp Physiol 309: R707–R720, 2015. doi: 10.1152/ajpregu.00211.2015. [DOI] [PubMed] [Google Scholar]
- 45.Warnert EAH, Hart EC, Hall JE, Murphy K, Wise RG. The major cerebral arteries proximal to the Circle of Willis contribute to cerebrovascular resistance in humans. J Cereb Blood Flow Metab 36: 1384–1395, 2016. doi: 10.1177/0271678X15617952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Willie CK, Colino FL, Bailey DM, Tzeng YC, Binsted G, Jones LW, Haykowsky MJ, Bellapart J, Ogoh S, Smith KJ, Smirl JD, Day TA, Lucas SJ, Eller LK, Ainslie PN. Utility of transcranial Doppler ultrasound for the integrative assessment of cerebrovascular function. J Neurosci Methods 196: 221–237, 2011. doi: 10.1016/j.jneumeth.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 47.Woodman RJ, Playford DA, Watts GF, Cheetham C, Reed C, Taylor RR, Puddey IB, Beilin LJ, Burke V, Mori TA, Green D. Improved analysis of brachial artery ultrasound using a novel edge-detection software system. J Appl Physiol (1985) 91: 929–937, 2001. doi: 10.1152/jappl.2001.91.2.929. [DOI] [PubMed] [Google Scholar]