Abstract
Dysfunction of the brain serotonergic system is implicated in the pathogenesis of major depressive disorder (MDD). Serotonin is also a vasoactive signaling molecule, the effects of which are modulated by both nitric oxide (NO) and the serotonin transporter [the primary target of selective serotonin reuptake inhibitors (SSRIs)]. Despite its role in the neurobiology of depression, serotoninergic signaling mechanisms in the microvasculature of adults with MDD are unknown. We hypothesized that 1) cutaneous microvascular responsiveness to serotonin would be attenuated in MDD and mediated by reductions in both 2) NO-dependent and 3) serotonin reuptake-dependent mechanisms. In 12 adults with MDD (nonmedicated) and 12 nondepressed adults, red cell flux (laser-Doppler flowmetry) was measured during graded intradermal microdialysis perfusion of 1) serotonin (10−10 to 10−1 mol/L) alone and in combination with a nonselective NO synthase inhibitor NG-nitro-l-arginine methyl ester (l-NAME; 15 mmol/L) and the SSRI paroxetine (10 μmol/L); and 2) paroxetine (n = 6; 10−9 to 10−2 M) alone and in combination with l-NAME. Serotonin-induced vasodilation was preserved in MDD. The NO-dependent component of serotonin-induced vasodilation was not different between groups. Paroxetine augmented vasodilatory responsiveness to serotonin via NO-dependent mechanisms in both groups; however, the magnitude was blunted in MDD. The NO contribution to direct paroxetine-induced vasodilation was also reduced in adults with MDD. Collectively, these preliminary data suggest that cutaneous microvascular serotoninergic signaling is dysregulated in adults with MDD and mediated by NO-dependent and serotonin reuptake-dependent mechanisms, providing initial mechanistic insight to the purported vasculoprotective effect of chronic SSRI treatment.
NEW & NOTEWORTHY Cutaneous microvascular vasodilatory responsiveness to serotonin was preserved in adults with major depressive disorder (MDD). However, the contribution of serotonin reuptake-dependent mechanisms to serotonin-induced dilation was reduced in MDD. Direct perfusion of the selective serotonin reuptake inhibitor (SSRI) paroxetine elicited vasodilation that is partially mediated by nitric oxide (NO)-dependent mechanisms, but these responses were blunted in MDD, reflective of a diminished contribution of NO to the direct effects of a SSRI on the cutaneous microvasculature.
Keywords: microdialysis, nitric oxide, selective serotonin reuptake inhibitor, vascular endothelial function
INTRODUCTION
Serotonin [5-hydroxytryptamine (5-HT)] is a biogenic monoamine that has an important role in a myriad of physiological functions, including blood coagulation, the maintenance of blood pressure, and gastrointestinal motility (2). Although initially discovered as a substance that modifies smooth muscle tone, serotonin is best known as a neurotransmitter that modulates neural activity and neuropsychological processes (2). Dysregulation of the brain serotonergic system is associated with the pathogenesis of many psychiatric disorders, including major depressive disorder (MDD) (1, 32), a mood disorder occurring in ~10–15% of the population (22).
Serotonin is primarily synthesized within the central nervous system; however, the vast majority (~90–95%) is located in the periphery, stored primarily in either the dense granules of platelets or in enterochromaffin cells, with very little circulating in plasma (17, 34). During platelet activation, serotonin is secreted and plays a critical role not only in promoting platelet aggregation but also in regulating vascular smooth muscle tone to facilitate hemostasis (2). Although serotonin is a potent vasoconstrictor, it can also elicit vascular smooth muscle relaxation, a response that is more prominent in arterioles and the microcirculation (51, 52). In the human forearm vasculature, exogenously administered serotonin causes an increase in blood flow, mediated in part through nitric oxide (NO)-dependent mechanisms (3, 4, 6, 7, 21, 46). Despite its clear role in the neural pathophysiology of MDD, to date, the peripheral mechanisms of serotonin-induced vasodilation in the microcirculation have not been examined in adults with depression, a cohort with noted microvascular endothelial dysfunction and reductions in NO bioavailability (9, 16, 37).
Additional regulation of serotoninergic signaling in the peripheral vasculature is provided by the serotonin transporter (SERT), which terminates the action of serotonin by its reuptake into platelets or presynaptic neurons (2, 38). In this way, platelet SERT tightly regulates plasma and localized tissue serotonin concentration, ensuring stable nutritive blood flow and preventing untoward platelet activation (24). Many widely used pharmacological treatments for the management of depressive symptoms target SERT, thereby inhibiting serotonin reuptake [i.e., selective serotonin reuptake inhibitors (SSRIs)] (40, 47). Interestingly, SSRIs also directly influence vascular reactivity, independent of their actions on SERT, in part by modulating the NO signaling pathway (18, 29, 39). However, despite the increasing prevalence of antidepressant use (28a), the direct effect of SSRIs on the peripheral microvasculature in humans, including adults with MDD, is unknown.
The purpose of this investigation was to examine microvascular serotoninergic reactivity in healthy nondepressed young adults and in nonmedicated otherwise healthy young adults with MDD. We hypothesized that 1) cutaneous microvascular responsiveness to serotonin would be blunted in MDD and mediated by reductions in both 2) NO-dependent and 3) serotonin reuptake-dependent mechanisms. To begin to understand the SERT-independent effects of SSRIs, we also assessed cutaneous microvascular responsiveness to SSRI administration. We hypothesized that direct administration of a SSRI to the microvasculature would elicit vasodilation and that this response would be attenuated in MDD.
METHODS
The Institutional Review Board at The Pennsylvania State University (no. 7927) and the Food and Drug Administration (IND 125,994) approved all experimental procedures. The investigation was conducted in accordance with the Declaration of Helsinki. The nature, risks, and benefits of all study procedures were explained to volunteers, and their verbal and written informed consent were obtained voluntarily before participation.
Diagnostic assessment of participants.
Participants were recruited from campus and the surrounding community, including the Counseling and Psychological Services clinics at The Pennsylvania State University, using common means of study advertisement (e.g., posting recruitment fliers, lecture hall presentations, etc.). Fourteen otherwise healthy nonmedicated adults with MDD were screened; of these, 12 patients (8 women) met the inclusion criteria for study enrollment (Table 1). Three of these adults with MDD participated in previous studies in our laboratory (15, 16). Twelve healthy adults (8 women) without any history of major psychiatric illness, also recruited from campus using standard recruitment strategies, served as the control group. All participants underwent the structured clinical Mini-International Neuropsychiatric Interview (41), and a diagnosis of MDD was confirmed by a psychiatrist according to DSM-5 criteria (15, 16). Exclusion criteria included comorbid current psychiatric disorders (e.g., psychosis, schizophrenia, bipolar disorder, panic disorder, etc.), the use of psychoactive or psychopharmacological drugs within 1 yr, and active suicidal ideation. Although two adults with MDD indicated previous use of a SSRI to manage their depressive symptoms, both had stopped ~2 yr before study entry. Depressive symptom severity was evaluated using the Patient Health Questionnaire-9 (PHQ-9), which provides a valid and sensitive index of symptomology based on the diagnostic criteria for DSM-5 depressive disorders (44). The PHQ-9 rates the nine symptoms of depression on a four-point scale, ranging from 0 = “not at all” to 3 = “nearly every day.” Response options are used to calculate a total score (maximum = 27) and symptom severity is quantified as none (0–4), mild (5–9), moderate (10–14), moderately severe (15–19), or severe (20–27).
Table 1.
Subject characteristics
| Characteristic | HA | MDD |
|---|---|---|
| n (Men/Women) | 12 (4/8) | 12 (4/8) |
| Age, yr | 23 ± 5 | 21 ± 3 |
| Height, cm | 170 ± 7 | 169 ± 10 |
| Mass, kg | 70 ± 9 | 70 ± 23 |
| BMI, kg/m2 | 24.3 ± 3.0 | 24.0 ± 5.4 |
| Heart rate, beats/min | 69 ± 11 | 71 ± 7 |
| Systolic BP, mmHg | 118 ± 9 | 120 ± 13 |
| Diastolic BP, mmHg | 74 ± 4 | 76 ± 6 |
| Blood biochemistry | ||
| HbA1c, % | 4.9 ± 0.3 | 5.0 ± 0.3 |
| Fasting total cholesterol, mg/dl | 170 ± 27 | 154 ± 29 |
| Fasting HDL, mg/dl | 58 ± 12 | 51 ± 17 |
| Fasting LDL, mg/dl | 96 ± 23 | 83 ± 17 |
| Fasting triglycerides, mg/dl | 92 ± 35 | 74 ± 47 |
| Depression assessment | ||
| PHQ-9 | 1 ± 0.9 | 6 ± 4.0* |
Values are means ± SD. HA, healthy adults; MDD, major depressive disorder; BMI, body mass index; BP, blood pressure; HDL, high-density lipoprotein; LDL, low-density lipoprotein; PHQ-9, Patient Health Questionnaire. PHQ-9 depressive symptom severity score ranges: 0–4 (minimal), 5–9 (mild), 10–14 (moderate), 15–19 (moderately severe), and 20–27 (severe).
P < 0.05 v. HA.
All participants underwent a complete medical screening, which included a diagnostic psychiatric interview, resting blood pressure and heart rate measurements, and 12-h fasting blood chemistry and lipid profile (Quest Diagnostics, Pittsburgh, PA). Additional blood samples were obtained in serum separator tubes at the screening, and serum was stored at −80°C for batched analysis of serotonin concentration (ELISA, quantified fluorometrically in triplicate; Abcam; Cambridge, MA). All participants were free of cardiovascular, metabolic, or renal disease, were recreationally active, were nonobese (body mass index: <30 kg/m2), did not use tobacco products, and were not taking prescription medications, with the exception of hormonal contraception [n = 4 healthy adults (HA); n = 4 MDD]. The timing of the experimental visit was not controlled for menstrual cycle phase, because, from an ethical perspective, every attempt was made to test MDD patients within ~1 wk of qualification and enrollment to facilitate expedient follow-up with a mental healthcare provider. This approach has been previously employed by our laboratory (15, 16). A urine pregnancy test confirmed the absence of pregnancy.
Assessment of cutaneous microvascular reactivity.
With the use of sterile technique, intradermal microdialysis probes (CMA Linear 30 probe, 6 kDa; Harvard Apparatus, Holliston, MA) were inserted into the dermal layer of the ventral forearm for the local delivery of pharmacological agents, as previously described (15, 16). Pharmacological agents were mixed immediately before use, dissolved in lactated Ringer solution, filtered using sterile syringe microfilters (Acrodisc; Pall, Ann Arbor, MI), and wrapped in foil to prevent photodegradation. Pharmacological agents were perfused through the microdialysis probes at 2 μL/min (Bee Hive controller and Baby Bee microinfusion pump; BASi, West Lafayette, IN). Each protocol commenced after an initial hyperemia-resolution period (~60–90 min), during which site-specific pharmacological agents were perfused. Red blood cell flux, an index of cutaneous blood flow, was continuously measured directly over each microdialysis site with an integrated laser Doppler flowmeter probe placed in a local heating unit (VP12 and VHP2; Moor Instruments, Wilmington, DE) set to thermoneutrality (33°C). Automated brachial blood pressure (Connex Spot Monitor; Welch Allyn, Skaneateles Falls, NY) was measured every 5 min throughout the protocol. At the conclusion of each dose-response protocol, sodium nitroprusside (SNP; 28 mmol/L); United States Pharmacopeia, Rockville, MD) was perfused and the local temperature was increased to 43°C to elicit maximal dilation. A purposefully wide range of dosage concentrations was used, based on pilot work conducted in our laboratory.
For the assessment of microvascular responsiveness to serotonin, four intradermal microdialysis probes were placed for the local delivery of lactated Ringer solution, the nonselective NO synthase inhibitor NG-nitro-l-arginine methyl ester (l-NAME; 15 mmol/L; Calbiochem, EMD Millipore, Billerica, MA), the SSRI paroxetine (PAX; 10 μmol/L; Sigma-Aldrich, St. Louis, MO), or PAX + l-NAME. Extensive pilot work was conducted in our laboratory to ensure that this concentration of paroxetine did not induce baseline vasodilation. After baseline measurements, ascending concentrations of serotonin (10−10 to 10−1 mol/L; Sigma-Aldrich) were coperfused with the site-specific pharmacological agent sequentially for 5 min each. Microvascular responsiveness to paroxetine was assessed in a subset of individuals (n = 6 HA; n = 6 MDD). In these individuals, two additional microdialysis probes were placed for the local delivery of lactated Ringer solution and the nonselective NO synthase inhibitor l-NAME (15 mmol/L; Calbiochem, EMD Millipore). After baseline measurements, ascending concentrations of paroxetine (10−9 to 10−2 mol/L; Sigma-Alrich) were coperfused with the site-specific pharmacological agent sequentially for 5 min each.
Data and statistical analysis.
Red blood cell flux was recorded at 40 Hz (PowerLab and LabChart; ADInstruments, Bella Vista, NSW, Australia). Vascular conductance was calculated as laser Doppler flux (perfusion units) divided by mean arterial pressure, normalized as a percentage of the maximum (%max), and averaged during 5 min of baseline and during the last min of each serotonin or paroxetine dose. Area under the dose-response curve (AUC) was calculated using the trapezoid rule (Prism v8.1; GraphPad Software, La Jolla, CA). Sample size estimates were based on an a priori power analysis (α = 0.05, β = 0.80) to detect a meaningful physiological difference of 15% between groups (average SD 15%). Student’s unpaired t tests were used to compare subject characteristics. Vascular conductance and AUC were analyzed using two-way (group × pharmacological agent) and three-way (group × pharmacological agent × dose) mixed-model ANOVA (SAS v9.4; Cary, NC), with post hoc Bonferroni corrections applied for specific planned comparisons when appropriate. Effect size was calculated as ƞ2. Significant was set at α < 0.05.
RESULTS
There were no differences in age, anthropometric characteristics, resting hemodynamics, and blood biochemistry between groups (Table 1; all P > 0.05). Serum serotonin concentration was not different between groups (182 ± 39 HA vs. 197 ± 51 ng/ml MDD; P = 0.62). At the time of the experimental visit, adults with MDD were experiencing a major depressive episode of mild severity (Table 1; P < 0.01).
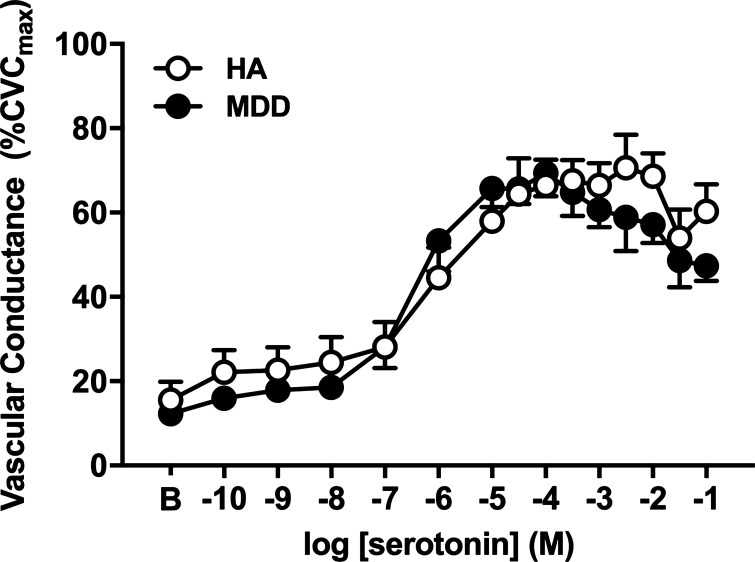
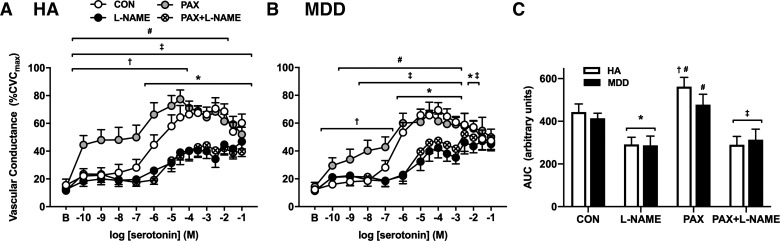
Neither baseline nor SNP-induced maximal vascular conductance (CVCmax) was different between groups or pharmacological treatments (Table 2; all P > 0.05). Exogenous serotonin elicited a vasodilatory response that peaked at ~10−4 mol/L in both groups. Cutaneous microvascular responsiveness to serotonin was preserved in MDD (Fig. 1; P = 0.09; ƞ2 = 0.028). NO synthase inhibition blunted serotonin-induced vasodilation in both groups; the magnitude of inhibition was modestly reduced at the highest concentrations of serotonin (>10−2 M) in MDD (CON: 47 ± 4 vs. l-NAME: 45 ± 4%CVCmax; P = 0.63) but not in HA (Fig. 2, A and B; CON: 60 ± 6 vs. l-NAME: 47 ± 5%CVCmax; P = 0.02). However, when quantifying the full profile of the NO-mediated component, calculated as the difference in AUC between control and l-NAME, there were no differences between groups (HA: 151 vs. MDD: 127 arbitrary units; P = 0.71).
Table 2.
Baseline and maximum vascular conductance
| Pharmacological Treatment | HA | MDD |
|---|---|---|
| 5-HT | ||
| Baseline | 0.27 ±. 0.11 | 0.19 ± 0.02 |
| Maximum | 1.58 ± 0.16 | 1.55 ± 0.14 |
| 5-HT + l-NAME | ||
| Baseline | 0.15 ± 0.02 | 0.11 ± 0.01 |
| Maximum | 1.48 ± 0.30 | 1.16 ± 0.13 |
| 5-HT + PAX | ||
| Baseline | 0.17 ± 0.03 | 0.20 ± 0.06 |
| Maximum | 1.60 ± 0.25 | 1.32 ± 0.16 |
| 5-HT + l-NAME + PAX | ||
| Baseline | 0.15 ± 0.02 | 0.13 ± 0.03 |
| Maximum | 1.45 ± 0.25 | 1.32 ± 0.26 |
| PAX | ||
| Baseline | 0.18 ± 0.03 | 0.15 ± 0.03 |
| Maximum | 1.53 ± 0.21 | 1.84 ± 0.27 |
| PAX + l-NAME | ||
| Baseline | 0.11 ± 0.02 | 0.13 ± 0.02 |
| Maximum | 1.25 ± 0.13 | 2.20 ± 0.46 |
Values are means ± SE. HA, healthy adults; MDD, Major Depressive Disorder; 5-HT, serotonin; l-NAME, NG-nitro-l-arginine methyl ester; PAX, paroxetine. Vascular conductance = flux × mean arterial pressure−1.
Fig. 1.
Vascular conductance in response to increasing doses of serotonin in healthy adults [HA, n = 12 (8 women); open symbols] and in those with major depressive disorder [MDD, n = 12 (8 women); filled symbols]. Microvascular responsiveness to serotonin was preserved in MDD (P = 0.09). CVCmax, maximal cutaneous vascular conductance. Data are means ± SE and were analyzed using two-way repeated-measures ANOVA.
Fig. 2.
Vascular conductance in response to increasing doses (A and B) and area under the curve (AUC; C) of serotonin alone (CON; open symbols) and during concurrent perfusion of the nitric oxide (NO) synthase inhibitor NG-nitro-l-arginine methyl ester (l-NAME; filled symbols) and the selective serotonin reuptake inhibitor paroxetine (PAX; gray symbols), both alone and in combination with l-NAME (PAX + l-NAME; hatched symbols), in healthy adults [HA, n = 12 (8 women); A and C] and in those with major depressive disorder [MDD, n = 12 (8 women); B and C]. At the highest doses of serotonin, the buffering capacity of NO to offset vasoconstriction was blunted in MDD. Acute treatment with PAX potentiated microvascular sensitivity to serotonin in both groups; however, this effect was greater in HA. CVCmax, maximal cutaneous vascular conductance. Data are means ± SE and were analyzed using two-way or three-way repeated-measures ANOVA. *P < 0.05 CON vs. l-NAME; †P < 0.05 CON vs. PAX; ‡P < 0.05 PAX vs. PAX + L-NAME; #P < 0.05 PAX vs. l-NAME.
In both groups, concurrent perfusion of the SSRI paroxetine augmented vasodilation in response to low concentrations of exogenous serotonin (Fig. 2, A and B; HA CON: 22 ± 5 vs. PAX: 44 ± 7%CVCmax at 10−10 M; P < 0.0001; MDD CON: 16 ± 1 vs. PAX: 30 ± 6%CVCmax at 10−10 M; P = 0.04). In both HA and MDD adults, concurrent NOS inhibition prevented the paroxetine-induced increase in vasodilatory responsiveness to serotonin at all doses, indicating that paroxetine amplifies serotonin-induced vasodilation primarily via NO-dependent mechanism (Fig. 2). However, the magnitude of paroxetine-mediated increases in serotonin-induced vasodilation was blunted in MDD (Fig. 2C), suggesting reductions in cutaneous microvascular sensitivity to acute serotonin administration in the presence of paroxetine in depressed adults.
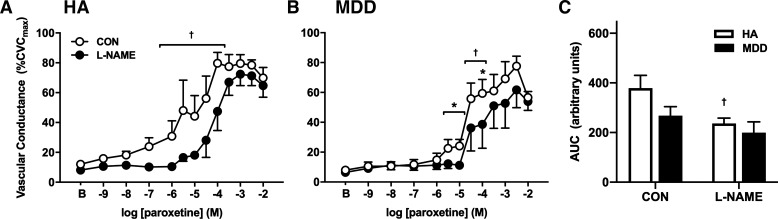
Exogenous perfusion of the SSRI paroxetine elicited vasodilation in both groups in a concentration-dependent manner; however, this vasodilatory response was modestly reduced in MDD (P = 0.076; Fig. 3; ƞ2 = 0.053). NO synthase inhibition blunted paroxetine-induced vasodilation in both groups at discrete concentrations (Fig. 3, A and B; HA CON: 80 ± 7 vs. l-NAME: 44 ± 13%CVCmax at 10−4 M; P < 0.0001; MDD CON: 59 ± 9 vs. l-NAME: 39 ± 16%CVCmax at 10−4 M; P = 0.02). However, when considering the full profile of the response as AUC, the degree to which l-NAME attenuated vasodilation was significantly diminished in MDD (Fig. 3C), indicative of reductions in the NO-mediated component of paroxetine-induced vasodilation in adults with MDD.
Fig. 3.
Vascular conductance in response to increasing doses (A and B) and area under the curve (AUC; C) of the selective serotonin reuptake inhibitor paroxetine alone (CON; open symbols) and during concurrent nitric oxide (NO) synthase inhibition [NG-nitro-l-arginine methyl ester (l-NAME), filled symbols] in healthy adults [HA, n = 6 (5 women)] and in those with major depressive disorder [MDD, n = 6 (4 women)]. The NO-mediated component of paroxetine-induced vasodilation was reduced in MDD. CVCmax, maximal cutaneous vascular conductance. Data are means ± SE and were analyzed using two-way or three-way repeated-measures ANOVA. *P < 0.05 HA vs. MDD; †P < 0.05 CON vs. l-NAME.
DISCUSSION
Our laboratory recently demonstrated significant impairments in NO-mediated endothelium-dependent dilation in young, otherwise healthy, adults with MDD (16). We further demonstrated that NO-dependent dilation was preserved in adults with MDD in remission and currently treated with a SSRI for their depressive symptoms (16). However, the potential for a direct influence of SSRIs on microvascular function independent of improvements in depressive symptoms was not addressed in our previous study. To begin to lend insight to this question, we conducted this small pilot study to directly assess potential alterations in the mechanisms mediating serotoninergic microvascular reactivity in adults with MDD using a targeted in vivo pharmacological approach. Our novel findings demonstrate that exogenous serotonin-induced cutaneous microvascular vasodilation was preserved in MDD. Consistent with the available literature (6, 7, 46), NO mechanistically contributed to serotonin-induced vasodilation in the microvasculature in MDD. Contrary to our hypothesis, the NO-dependent component of this response was largely intact in MDD, although it was modestly reduced at high concentrations. Exogenous coperfusion of the SSRI paroxetine increased vasodilatory responsiveness to serotonin, in part via NO signaling, in both groups, but the magnitude of this response was reduced in MDD. Furthermore, we demonstrated for the first time in humans that direct perfusion of the SSRI paroxetine elicited vasodilation that was partially mediated by NO-dependent mechanisms. These responses were blunted in MDD, reflective of a diminished contribution of NO to the direct effects of a SSRI on the microvasculature. Collectively, these data add to the growing body of literature examining altered mechanistic regulation of peripheral microvascular vasodilatory function in MDD (15, 16) and lend preliminary mechanistic insight to the purported vasculoprotective effect of chronic SSRI treatment for the management of depressive symptoms (8, 16, 19, 35).
Although serotonin has potent vasoconstrictor effects in most circulatory beds (51, 52), in the forearm vasculature it causes vascular smooth muscle relaxation and a subsequent increase in blood flow (4, 6, 7, 21, 46). In pulmonary and coronary arteries isolated from rodents, serotonin-induced vasodilation is largely mediated by the 5-HT2B and 5-HT7 receptor subtypes, located on the endothelium and smooth muscle cells, respectively (54). Attempts to identify the specific receptor mediating serotonin-induced vasodilation in the forearm have not been definitive, likely due to the methodological constraints surrounding the use of specific pharmacological serotonin agonists and antagonists in humans. A potential role for the 5-HT2 and 5-HT4 receptors in mediating the peripheral vascular effects of serotonin has been identified (4), but the potential involvement of the 5-HT1A, 5-HT2B, and 5-HT3 receptors in the microcirculation is less apparent (6, 7, 21). Nevertheless, serotonin-induced vasodilation appears to be mediated in large part by NO-dependent mechanisms (6, 46). In addition, the vasodilatory response to serotonin is blunted in adults with increased cardiovascular risk (46), likely due to impairments in the NO-mediated component of the serotonergic signal transduction pathway (42, 46). In both HA and MDD, perfusion of exogenous serotonin elicited robust cutaneous microvascular vasodilation that was partially NO dependent. Interestingly, serotonin elicited a biphasic response in the cutaneous microvasculature: vasodilation occurred at lower concentrations followed by a reduction in dilation and/or active constriction at higher doses. Contrary to our hypothesis, the vasodilatory response to exogenous serotonin was preserved in MDD. Furthermore, when considering the full profile of the cutaneous microvascular response to serotonin (i.e., quantified as AUC), we did not detect a reduction in the NO-mediated component in MDD. This was somewhat surprising, given that marked reductions in NO-dependent vasodilation have been reported in MDD (11, 16, 37). The reason(s) for these discrepant findings are not readily apparent but may potentially be related to the severity of depression. The adults in this pilot study were only experiencing mild depressive symptoms at the time of the experiment, and given the relation between symptom severity and magnitude of endothelial dysfunction (16), this possibility cannot be discounted and requires additional investigation.
An additional aspect of serotoninergic signaling in the peripheral vasculature is the regulation provided by SERT, which terminates the action of serotonin by its reuptake into platelets or presynaptic neurons, preventing against uncontrolled serotonin-induced constriction and platelet activation (3, 38). SERT is the primary target of SSRIs, which are well-established antidepressants (40, 47). SSRIs simultaneously block platelet serotonin reuptake and also prevent its release from the dense granules, both effects that may contribute to the increased risk of bleeding posed by chronic SSRI treatment (31). In an attempt to elucidate a mechanistic role for SERT in contributing to the vasodilatory effects of serotonin, we locally administered the SSRI paroxetine, alone and concurrently with NO synthase inhibition. With the use of this approach, paroxetine presumably locally increased the concentration of “free” serotonin exposed to the microvasculature during direct perfusion of exogenous serotonin, although the extent to which chronic oral SSRI administration influences plasma serotonin concentrations remains equivocal (1, 45). Here, locally administered paroxetine augmented the vasodilatory responses to serotonin at low concentrations, an effect mediated entirely by NO-dependent signaling, in both groups. However, the AUC data indicate that this effect was blunted in MDD adults, suggesting that cutaneous microvascular vasodilatory sensitivity to serotonin is diminished in the presence of SSRIs in adults with depression.
There are, however, several potential explanations and limitations to our approach that warrant consideration. First, acutely inhibiting serotonin reuptake during concurrent administration of exogenous serotonin may have unmasked a reduction in endogenous circulating serotonin in MDD, limiting the potentiation of serotonin-induced vasodilation. In an attempt to account for this possibility, we measured circulating serotonin concentration in serum and demonstrated no difference between groups. The vast majority of circulating serotonin is sequestered within the dense granules of platelets, with the remainder bound to plasmatic proteins and the free unconjugated form of serotonin (17, 34). This is an important caveat when interpreting our measurements of circulating serum serotonin concentration, which may not accurately reflect the “free” serotonin exposed to the vasculature. However, there is relatively little evidence that whole blood or cerebrospinal fluid concentrations of serotonin are important markers for depressive disorders (27, 30, 33), which further substantiates the notion that modulation of downstream signaling pathways triggered by serotonin are relatively more important for the regulation of vascular function than is the absolute concentration of serotonin in the microcirculation.
Second, alterations in platelet SERT density, activity, or sensitivity in MDD may also contribute to the noted responses. However, several studies have demonstrated no difference in [3H]paroxetine binding (an estimate of platelet SERT number and function) between depressed patients and controls (12, 13), suggesting that alterations in platelet SERT likely did not contribute to the present findings. Finally, SSRIs also have direct effects on vascular NO and muscarinic signaling, independent of their actions on SERT (18, 29, 39). Thus alterations in the SERT-independent effects of SSRIs may contribute to blunted cutaneous microvascular responsiveness to coperfusion of serotonin and paroxetine in MDD.
Therefore, as a necessary first step, we examined the direct effect of the SSRI paroxetine on cutaneous microvascular function in humans. Acute administration of paroxetine elicited substantial vasodilation mediated, in part, by NO. In MDD, paroxetine-induced dilation was modestly reduced when examining the full profile of the response. Moreover, the NO-dependent portion of paroxetine-induced dilation was significantly blunted in MDD adults, potentially reflective of MDD-associated reductions in vascular NO bioavailability and function (14, 16, 37). Although chronic treatment with a SSRI secondarily improves NO-mediated endothelial function in depression (8, 16, 19, 35), our novel data demonstrate impairments in cutaneous microvascular responsiveness to, and mechanistic control of, direct acute SSRI administration in MDD adults. Thus our preliminary findings suggest that direct paroxetine-induced vasodilation likely contributes to 1) the augmented vasodilatory response to serotonin in the presence of paroxetine and 2) the reduced magnitude of this effect noted in adults with MDD.
Limitations.
Importantly, although we validate for the first time direct paroxetine-induced vasodilation in humans, given the preliminary nature of this small pilot study, we did not probe the degree to which serotonin-dependent versus serotonin-independent mechanism(s) contribute to this dilatory response. As noted above, the complexity of serotonin receptor physiology (54), coupled with both the lack of consensus regarding the specific serotonin receptor(s) mediating cutaneous microvascular dilatory function (4, 6, 7, 21) and the methodological constraints surrounding the use of selective serotonin receptor antagonists imposed by in vivo approaches in humans, makes these studies challenging, if not impossible. Nevertheless, the present findings are a critical first step in designing future investigations to more fully elucidate the mechanistic regulation of cutaneous microvascular serotoninergic signaling in humans and its potential dysregulation in MDD.
The timing of the experimental visits for women was not controlled for menstrual cycle phase, because from an ethical perspective, patients with MDD were tested as soon as possible after qualification to facilitate timely follow-up with a mental healthcare provider. Although there is undoubtedly an important role for estrogen and progesterone in modulating NO bioavailability and vascular function (53), surprisingly few studies have assessed the influence of menstrual cycle phase, and thus female sex hormone exposure, on microvascular endothelial function in the cutaneous circulation, and to our knowledge, no studies have utilized intradermal microdialysis to pharmacologically target the NO or serotoninergic signaling pathways throughout the phases of the menstrual cycle. Given that the findings of the present investigation suggest dysregulated peripheral microvascular serotoninergic signaling in young men and women with MDD, coupled with evidence implicating sex-dependent pathophysiology in the link between depression and cardiovascular disease (CVD) (50), this is a clinically important line of inquiry and future studies are clearly necessary.
Perspectives and conclusions.
The effect of antidepressant pharmacotherapy on CVD outcomes is controversial, with studies suggesting both lower and higher cardiovascular risk with chronic treatment (25, 36, 43, 48). In line with this, the influence of SSRIs on peripheral endothelial function remains debatable, with studies indicating both vasodilatory and vasoconstrictor responses (10, 20), the balance of which may be related to the degree of underlying endothelial dysfunction (23). Because depression itself is a CVD risk factor (26, 28), interpretation of these conflicting results is made even more complex given the confounding bias of the salutary effects of chronic SSRI treatment on depressive symptomology. Increases in NO bioavailability and subsequent improvements in endothelial function have been demonstrated in depressed adults treated with a SSRI (8, 16, 35) and posited to be one potential mechanism underlying the reduction in cardiovascular risk with chronic SSRI treatment (36, 43). However, to our knowledge, the present study is the first attempt to characterize the regulation of serotoninergic signaling and to elucidate the direct effect of acute SSRI administration in the cutaneous microvasculature in MDD. Acute perfusion of the SSRI paroxetine, alone and in the presence of serotonin, induced substantial vasodilation in adults with MDD. Collectively, one interpretation is that the beneficial pleiotropic effects of SSRI treatment to improve peripheral microvascular function may be evident immediately upon administration, whereas their primary intended effect to mitigate depressive symptoms and improve mood in SSRI-sensitive MDD patients may not be apparent for weeks or months (49). Although these data lend important additional mechanistic insight to the potential mechanisms of vasoprotection due to SSRIs (8, 16, 19, 35), whether chronic SSRI treatment for the management of depressive symptoms further potentiates the synergistic interaction between serotoninergic and NO vasodilatory signaling pathways represents a critical area of future investigation.
GRANTS
This work was supported by National Institutes of Health Grants HL-093238 (to L. M. Alexander), HL-133414 (to J. L. Greaney), and UL1-TR-002014.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.L.G., E.F.S., and L.M.A. conceived and designed research; J.L.G. and G.A.D. performed experiments; J.L.G. and G.A.D. analyzed data; J.L.G., G.A.D., E.F.S., and L.M.A. interpreted results of experiments; J.L.G. prepared figures; J.L.G. drafted manuscript; J.L.G., G.A.D., E.F.S., and L.M.A. edited and revised manuscript; J.L.G., G.A.D., E.F.S., and L.M.A. approved final version of manuscript.
ACKNOWLEDGMENTS
We appreciate the effort expended by the volunteer participants. We thank Susan Slimak and Jane Pierzga for assistance.
REFERENCES
- 1.Alvarez JC, Gluck N, Fallet A, Grégoire A, Chevalier JF, Advenier C, Spreux-Varoquaux O. Plasma serotonin level after 1 day of fluoxetine treatment: a biological predictor for antidepressant response? Psychopharmacology (Berl) 143: 97–101, 1999. doi: 10.1007/s002130050924. [DOI] [PubMed] [Google Scholar]
- 2.Berger M, Gray JA, Roth BL. The expanded biology of serotonin. Annu Rev Med 60: 355–366, 2009. doi: 10.1146/annurev.med.60.042307.110802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blakely RD, Berson HE, Fremeau RT Jr, Caron MG, Peek MM, Prince HK, Bradley CC. Cloning and expression of a functional serotonin transporter from rat brain. Nature 354: 66–70, 1991. doi: 10.1038/354066a0. [DOI] [PubMed] [Google Scholar]
- 4.Blauw GJ, van Brummelen P, Chang PC, Vermeij P, van Zwieten PA. Regional vascular effects of serotonin and ketanserin in young, healthy subjects. Hypertension 11: 256–263, 1988. doi: 10.1161/01.HYP.11.3.256. [DOI] [PubMed] [Google Scholar]
- 6.Bruning TA, Chang PC, Blauw GJ, Vermeij P, van Zwieten PA. Serotonin-induced vasodilatation in the human forearm is mediated by the “nitric oxide-pathway”: no evidence for involvement of the 5-HT3-receptor. J Cardiovasc Pharmacol 22: 44–51, 1993. doi: 10.1097/00005344-199307000-00008. [DOI] [PubMed] [Google Scholar]
- 7.Bruning TA, van Zwieten PA, Blauw GJ, Chang PC. No functional involvement of 5-hydroxytryptamine1A receptors in nitric oxide-dependent dilatation caused by serotonin in the human forearm vascular bed. J Cardiovasc Pharmacol 24: 454–461, 1994. doi: 10.1097/00005344-199409000-00014. [DOI] [PubMed] [Google Scholar]
- 8.Chrapko W, Jurasz P, Radomski MW, Archer SL, Newman SC, Baker G, Lara N, Le Mellédo JM. Alteration of decreased plasma NO metabolites and platelet NO synthase activity by paroxetine in depressed patients. Neuropsychopharmacology 31: 1286–1293, 2006. doi: 10.1038/sj.npp.1300961. [DOI] [PubMed] [Google Scholar]
- 9.Chrapko WE, Jurasz P, Radomski MW, Lara N, Archer SL, Le Mellédo JM. Decreased platelet nitric oxide synthase activity and plasma nitric oxide metabolites in major depressive disorder. Biol Psychiatry 56: 129–134, 2004. doi: 10.1016/j.biopsych.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 10.Coleiro B, Marshall SE, Denton CP, Howell K, Blann A, Welsh KI, Black CM. Treatment of Raynaud’s phenomenon with the selective serotonin reuptake inhibitor fluoxetine. Rheumatology (Oxford) 40: 1038–1043, 2001. doi: 10.1093/rheumatology/40.9.1038. [DOI] [PubMed] [Google Scholar]
- 11.Cooper DC, Milic MS, Tafur JR, Mills PJ, Bardwell WA, Ziegler MG, Dimsdale JE. Adverse impact of mood on flow-mediated dilation. Psychosom Med 72: 122–127, 2010. doi: 10.1097/PSY.0b013e3181cdbfc0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.D’haenen H, De Waele M, Leysen JE. Platelet 3H-paroxetine binding in depressed patients. Psychiatry Res 26: 11–17, 1988. doi: 10.1016/0165-1781(88)90082-0. [DOI] [PubMed] [Google Scholar]
- 13.D’Hondt P, Maes M, Leysen JE, Gommeren W, Scharpé S, Cosyns P. Binding of [3H]paroxetine to platelets of depressed patients: seasonal differences and effects of diagnostic classification. J Affect Disord 32: 27–35, 1994. doi: 10.1016/0165-0327(94)90058-2. [DOI] [PubMed] [Google Scholar]
- 14.García RG, Zarruk JG, Barrera C, Pinzón A, Trillos E, Arenas WD, Luengas C, Tomaz C, López-Jaramillo P. Plasma nitrate levels and flow-mediated vasodilation in untreated major depression. Psychosom Med 73: 344–349, 2011. doi: 10.1097/PSY.0b013e31821566cf. [DOI] [PubMed] [Google Scholar]
- 15.Greaney JL, Koffer RE, Saunders EF, Almeida DM, Alexander LM. Self‐reported everyday psychosocial stressors are associated with greater impairments in endothelial function in young adults with major depressive disorder. J Am Heart Assoc 8: e010825, 2019. doi: 10.1161/JAHA.118.010825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Greaney JL, Saunders EF, Santhanam L, Alexander LM. Oxidative stress contributes to microvascular endothelial dysfunction in men and women with major depressive disorder. Circ Res 124: 564–574, 2019. doi: 10.1161/CIRCRESAHA.118.313764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hervé P, Launay JM, Scrobohaci ML, Brenot F, Simonneau G, Petitpretz P, Poubeau P, Cerrina J, Duroux P, Drouet L. Increased plasma serotonin in primary pulmonary hypertension. Am J Med 99: 249–254, 1995. doi: 10.1016/S0002-9343(99)80156-9. [DOI] [PubMed] [Google Scholar]
- 18.Hooper CW, Delaney C, Streeter T, Yarboro MT, Poole S, Brown N, Slaughter JC, Cotton RB, Reese J, Shelton EL. Selective serotonin reuptake inhibitor exposure constricts the mouse ductus arteriosus in utero. Am J Physiol Heart Circ Physiol 311: H572–H581, 2016. doi: 10.1152/ajpheart.00822.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Isingrini E, Belzung C, Freslon JL, Machet MC, Camus V. Fluoxetine effect on aortic nitric oxide-dependent vasorelaxation in the unpredictable chronic mild stress model of depression in mice. Psychosom Med 74: 63–72, 2012. doi: 10.1097/PSY.0b013e31823a43e0. [DOI] [PubMed] [Google Scholar]
- 20.Kahl KG, Westhoff-Bleck M, Krüger TH. Effects of psychopharmacological treatment with antidepressants on the vascular system. Vascul Pharmacol 96–98: 11–18, 2017. doi: 10.1016/j.vph.2017.07.004. [DOI] [PubMed] [Google Scholar]
- 21.Kemme MJ, Burggraaf J, Schoemaker RC, Cohen AF, Blauw GJ. No evidence for functional involvement of 5-HT2B receptors in serotonin-induced vasodilatation in the human forearm. J Cardiovasc Pharmacol 36: 699–703, 2000. doi: 10.1097/00005344-200012000-00003. [DOI] [PubMed] [Google Scholar]
- 22.Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry 62: 593–602, 2005. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- 23.Khouri C, Gailland T, Lepelley M, Roustit M, Cracowski JL. Fluoxetine and Raynaud’s phenomenon: friend or foe? Br J Clin Pharmacol 83: 2307–2309, 2017. doi: 10.1111/bcp.13314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim DK, Tolliver TJ, Huang SJ, Martin BJ, Andrews AM, Wichems C, Holmes A, Lesch KP, Murphy DL. Altered serotonin synthesis, turnover and dynamic regulation in multiple brain regions of mice lacking the serotonin transporter. Neuropharmacology 49: 798–810, 2005. doi: 10.1016/j.neuropharm.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 25.Kimmel SE, Schelleman H, Berlin JA, Oslin DW, Weinstein RB, Kinman JL, Sauer WH, Lewis JD. The effect of selective serotonin re-uptake inhibitors on the risk of myocardial infarction in a cohort of patients with depression. Br J Clin Pharmacol 72: 514–517, 2011. doi: 10.1111/j.1365-2125.2011.04008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lett HS, Blumenthal JA, Babyak MA, Sherwood A, Strauman T, Robins C, Newman MF. Depression as a risk factor for coronary artery disease: evidence, mechanisms, and treatment. Psychosom Med 66: 305–315, 2004. [DOI] [PubMed] [Google Scholar]
- 27.Mann JJ, McBride PA, Anderson GM, Mieczkowski TA. Platelet and whole blood serotonin content in depressed inpatients: correlations with acute and life-time psychopathology. Biol Psychiatry 32: 243–257, 1992. doi: 10.1016/0006-3223(92)90106-A. [DOI] [PubMed] [Google Scholar]
- 28.Musselman DL, Evans DL, Nemeroff CB. The relationship of depression to cardiovascular disease: epidemiology, biology, and treatment. Arch Gen Psychiatry 55: 580–592, 1998. doi: 10.1001/archpsyc.55.7.580. [DOI] [PubMed] [Google Scholar]
- 28a.National Center for Health Statistics Health, United States, 2010: With Special Feature on Death and Dying. Hyattsville, MD: US Department of Health and Human Services, 2011. [PubMed] [Google Scholar]
- 29.Ofek K, Schoknecht K, Melamed-Book N, Heinemann U, Friedman A, Soreq H. Fluoxetine induces vasodilatation of cerebral arterioles by co-modulating NO/muscarinic signalling. J Cell Mol Med 16: 2736–2744, 2012. doi: 10.1111/j.1582-4934.2012.01596.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ogawa S, Tsuchimine S, Kunugi H. Cerebrospinal fluid monoamine metabolite concentrations in depressive disorder: A meta-analysis of historic evidence. J Psychiatr Res 105: 137–146, 2018. doi: 10.1016/j.jpsychires.2018.08.028. [DOI] [PubMed] [Google Scholar]
- 31.Ottervanger JP, Stricker BH, Huls J, Weeda JN. Bleeding attributed to the intake of paroxetine. Am J Psychiatry 151: 781–782, 1994. doi: 10.1176/ajp.151.5.781. [DOI] [PubMed] [Google Scholar]
- 32.Owens MJ, Nemeroff CB. Role of serotonin in the pathophysiology of depression: focus on the serotonin transporter. Clin Chem 40: 288–295, 1994. [PubMed] [Google Scholar]
- 33.Pech J, Forman J, Kessing LV, Knorr U. Poor evidence for putative abnormalities in cerebrospinal fluid neurotransmitters in patients with depression versus healthy non-psychiatric individuals: A systematic review and meta-analyses of 23 studies. J Affect Disord 240: 6–16, 2018. doi: 10.1016/j.jad.2018.07.031. [DOI] [PubMed] [Google Scholar]
- 34.Pettersson G. The neural control of the serotonin content in mammalian enterochromaffin cells. Acta Physiol Scand Suppl 470: 1–30, 1979. [PubMed] [Google Scholar]
- 35.Pizzi C, Mancini S, Angeloni L, Fontana F, Manzoli L, Costa GM. Effects of selective serotonin reuptake inhibitor therapy on endothelial function and inflammatory markers in patients with coronary heart disease. Clin Pharmacol Ther 86: 527–532, 2009. doi: 10.1038/clpt.2009.121. [DOI] [PubMed] [Google Scholar]
- 36.Pizzi C, Rutjes AW, Costa GM, Fontana F, Mezzetti A, Manzoli L. Meta-analysis of selective serotonin reuptake inhibitors in patients with depression and coronary heart disease. Am J Cardiol 107: 972–979, 2011. doi: 10.1016/j.amjcard.2010.11.017. [DOI] [PubMed] [Google Scholar]
- 37.Rajagopalan S, Brook R, Rubenfire M, Pitt E, Young E, Pitt B. Abnormal brachial artery flow-mediated vasodilation in young adults with major depression. Am J Cardiol 88: 196–198, 2001. doi: 10.1016/S0002-9149(01)01623-X. [DOI] [PubMed] [Google Scholar]
- 38.Ramamoorthy S, Bauman AL, Moore KR, Han H, Yang-Feng T, Chang AS, Ganapathy V, Blakely RD. Antidepressant- and cocaine-sensitive human serotonin transporter: molecular cloning, expression, and chromosomal localization. Proc Natl Acad Sci USA 90: 2542–2546, 1993. doi: 10.1073/pnas.90.6.2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ribback S, Pavlovic D, Herbst D, Nedeljkov-Jancic R, Wendt M, Nedeljkov V, Bleich S, Frieling H. Effects of amitriptyline, fluoxetine, tranylcypromine and venlafaxine on rat vascular smooth muscle in vitro–the role of the endothelium. J Physiol Pharmacol 63: 119–125, 2012. [PubMed] [Google Scholar]
- 40.Serebruany VL, Gurbel PA, O’Connor CM. Platelet inhibition by sertraline and N-desmethylsertraline: a possible missing link between depression, coronary events, and mortality benefits of selective serotonin reuptake inhibitors. Pharmacol Res 43: 453–461, 2001. doi: 10.1006/phrs.2001.0817. [DOI] [PubMed] [Google Scholar]
- 41.Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 59, Suppl 20: 22–33, 1998. [PubMed] [Google Scholar]
- 42.Shimokawa H, Flavahan NA, Vanhoutte PM. Loss of endothelial pertussis toxin-sensitive G protein function in atherosclerotic porcine coronary arteries. Circulation 83: 652–660, 1991. doi: 10.1161/01.CIR.83.2.652. [DOI] [PubMed] [Google Scholar]
- 43.Spier SA, Frontera MA. Unexpected deaths in depressed medical inpatients treated with fluoxetine. J Clin Psychiatry 52: 377–382, 1991. [PubMed] [Google Scholar]
- 44.Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study JAMA 282: 1737–1744, 1999. doi: 10.1001/jama.282.18.1737. [DOI] [PubMed] [Google Scholar]
- 45.Spreux-Varoquaux O, Gailledreau J, Vanier B, Bothua D, Plas J, Chevalier JF, Advenier C, Pays M, Brion S. Initial increase of plasma serotonin: a biological predictor for the antidepressant response to clomipramine? Biol Psychiatry 40: 465–473, 1996. doi: 10.1016/0006-3223(95)00449-1. [DOI] [PubMed] [Google Scholar]
- 46.Stroes ES, Koomans HA, de Bruin TW, Rabelink TJ. Vascular function in the forearm of hypercholesterolaemic patients off and on lipid-lowering medication. Lancet 346: 467–471, 1995. doi: 10.1016/S0140-6736(95)91322-X. [DOI] [PubMed] [Google Scholar]
- 47.Taciak PP, Lysenko N, Mazurek AP. Drugs which influence serotonin transporter and serotonergic receptors: Pharmacological and clinical properties in the treatment of depression. Pharmacol Rep 70: 37–46, 2018. doi: 10.1016/j.pharep.2017.07.011. [DOI] [PubMed] [Google Scholar]
- 48.Tata LJ, West J, Smith C, Farrington P, Card T, Smeeth L, Hubbard R. General population based study of the impact of tricyclic and selective serotonin reuptake inhibitor antidepressants on the risk of acute myocardial infarction. Heart 91: 465–471, 2005. doi: 10.1136/hrt.2004.037457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Taylor MJ, Freemantle N, Geddes JR, Bhagwagar Z. Early onset of selective serotonin reuptake inhibitor antidepressant action: systematic review and meta-analysis. Arch Gen Psychiatry 63: 1217–1223, 2006. doi: 10.1001/archpsyc.63.11.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tobet SA, Handa RJ, Goldstein JM. Sex-dependent pathophysiology as predictors of comorbidity of major depressive disorder and cardiovascular disease. Pflugers Arch 465: 585–594, 2013. doi: 10.1007/s00424-013-1248-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vanhoutte PM. Cardiovascular effects of serotonin. J Cardiovasc Pharmacol 10, Suppl 3: S8–S11, 1987. doi: 10.1097/00005344-198706103-00004. [DOI] [PubMed] [Google Scholar]
- 52.Vanhoutte PM. Serotonin and the vascular wall. Int J Cardiol 14: 189–203, 1987. doi: 10.1016/0167-5273(87)90008-8. [DOI] [PubMed] [Google Scholar]
- 53.Vanhoutte PM, Zhao Y, Xu A, Leung SWS. Thirty years of saying NO Circ Res 119: 375–396, 2016. doi: 10.1161/CIRCRESAHA.116.306531. [DOI] [PubMed] [Google Scholar]
- 54.Watts SW, Morrison SF, Davis RP, Barman SM. Serotonin and blood pressure regulation. Pharmacol Rev 64: 359–388, 2012. doi: 10.1124/pr.111.004697. [DOI] [PMC free article] [PubMed] [Google Scholar]