Abstract
Decreases in activity levels in children worldwide are feared to have long-term health repercussions. Yet, because of the difficulty of performing controlled long-term studies in humans, we do not yet understand how decreases in childhood activity influence adult functional capacity. Here, in an avian bipedal model, we evaluated the elimination of all high-intensity activity during growth on adult performance. We evaluated three alternative hypotheses: Elimination of high-intensity activity 1) does not influence adult function, 2) results in task-specific deficits in adulthood, or 3) results in deficits that generalize across locomotor tasks. We found that animals restricted from jumping and sprinting during growth showed detriments as adults in maximal jump performance in comparison to controls, but did not require more metabolic energy during steady-state running or standing. From this, we conclude that functional deficits from elimination of high-intensity exercise are task specific and do not generalize across all locomotor functions.
NEW & NOTEWORTHY Decreasing childhood activity levels are feared to have long-term health repercussions, but testing this hypothesis is hampered by restrictions of human experimentation. Here, in a bipedal animal model, we examine how the elimination of high-intensity activity during all of maturation influences adult locomotor capacity. We found restricted activity during growth reduced mechanical power capacity but not submaximal metabolic cost. This suggests that reduced childhood activity may result in task-specific, rather than generalized locomotor deficits.
Keywords: avian, disuse, energetics, jumping, plasticity
INTRODUCTION
Decreases in activity levels in children worldwide (49, 57) are feared to have long-term health repercussions (50, 74). This is, in part, because childhood activity levels have been shown to be a predictor of adult activity (66, 67), and there is strong experimental and epidemiological evidence correlating a wide range of health impairments with low levels of activity in adults (19, 71). While associations between youth and adult activity levels may result from behavioral habits (21, 66), several studies have shown that increases in childhood activity can change the musculoskeletal system (12, 15). This suggests that it is also possible that decreased activity during growth causes musculoskeletal and/or metabolic changes that make physical activity more difficult in adulthood. Although studies exploring morphological and functional plasticity in response to activity in adults abound (10, 27, 46, 72), controlled longitudinal studies exploring the effects of decreased activity during childhood in humans are lacking (34, 50). This paucity is partly because controlling and monitoring activity levels throughout childhood is practically difficult, but also because of ethical concerns related to potentially causing permanent musculoskeletal detriments. For instance, while twins have been studied to isolate the influence of varying degrees of activity during childhood on adult health, activity level was most often quantified with questionnaires rather than long-term monitoring (42, 70). Further, many studies have found functional and morphological plasticity to vary between adults and children (35, 41), suggesting that predictions of the plastic responses of children that are based on adult studies may be inaccurate (8, 34). Thus, we do not yet understand how decreases in childhood activity influence adult functional locomotor capacity.
To test whether and how decreased functional demand during growth influences adult performance, we chose to experimentally manipulate the locomotor demands of an animal model (the helmeted guinea fowl) throughout maturation. Guinea fowl have become a model species for bipedal locomotor studies (22, 25, 30, 60) because they are amenable to biomechanical analyses (e.g., they will run readily on a treadmill) and because they are ground-dwelling birds that run and walk bipedally with joint mechanics similar to those of humans (59, 60). In comparison, it has recently been demonstrated that the biomechanics of rodent models may impede their translational value in musculoskeletal research (33). Further, the activity profile of guinea fowl mirrors that of human children (6, 9), consisting primarily of low to moderate activity (standing and walking) interspersed with brief bouts of high-intensity activity (sprinting and jumping) (62). Guinea fowl mature rapidly, growing from 0.02-kg keets to 2-kg adults in just 6 mo, which facilitates close control of functional demand during the entire growth period, while minimizing confounding factors. We used this model to test whether lack of intense activity during growth hinders locomotor development. Specifically, we explored how decreased intense physical activity (elimination of jumping and sprinting) throughout maturation influences adult capacity across several locomotor tasks. We chose to study the elimination of high-intensity (burst) activity, as opposed to steady-state locomotion, because burst activity is most often performed when children engage in play (9, 57). As a first pass to evaluate the response to inactivity during growth, we examined maximal jumping performance and submaximal metabolic energetics because they provide a broad scale assessment of function. Maximal jumping provides a convenient measure of the upper limit to muscle performance, while submaximal economy provides a measure of the effort required to move under conditions that are more common in tasks of daily living. Submaximal economy can also serve as an indicator of submaximal muscle metabolic function and cardiopulmonary efficacy. We evaluated the influence of decreased activity on 1) a high-intensity task (jumping) that the restricted birds were impeded from performing during growth, 2) a task all birds performed regularly (standing), and 3) a task that none of the animals regularly participated in during growth (steady-state submaximal running).
Building on a framework put forth by Wakeling et al. (69), we tested three hypotheses. The “No-Advantage” hypothesis holds that decreases in activity levels during growth will have no effect on the performance of the adult. This implies that 1) changes to locomotor demands are nonadaptive, like most plastic responses to environmental variation (23, 29, 68), and 2) adult capacity is primarily determined by genetic factors rather than childhood conditions. Alternately, the “Beneficial Acclimation” hypothesis suggests that the adult phenotype will adapt to the conditions in which it was reared, implying that adaptations are specific to the particular functional demands. It suggests, for example, that children who run more will exhibit phenotypes better adapted for running and those who jump more will be better adapted for jumping. Lastly, the “Optimal Development” hypothesis holds that there are “optimal” movement conditions during childhood that will produce the most robust phenotype. This suggests that adults who were raised in conditions closer to optimal will outperform individuals raised in less optimal conditions across all locomotor tasks. This hypothesis predicts that deficits generalize across locomotor function. For instance, it suggests that adults who exercised less in childhood will be both less economical and jump with less power than individuals who exercised more.
If our results support the Optimal Development hypothesis, restricted birds would show detriments across tasks at adulthood, exhibiting decreased jump performance and requiring more energy to run and stand than controls. If, in the restricted birds, we see detriments only in jump performance (or detriments in jump performance and improvements in economy), our results would support the Beneficial Acclimation hypothesis and suggest that locomotor and biomechanical adaptations during growth are task-specific rather than generalized. Lastly, similar performance in all tasks across groups would support the No-Advantage hypothesis and imply that genetics is more influential on adult locomotor capacity than movement patterns during childhood.
METHODS
Experimental Protocol
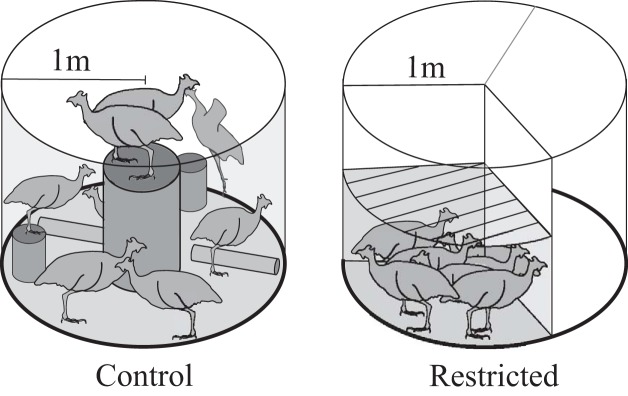
To study these questions, one-day-old guinea fowl keets (Numida meleagris) were obtained from a regional breeder (Guineafarm, IA) and, after a 2-wk brooding period, were pen reared to skeletal maturity (6 mo) in one of two conditions. An exercise control group (C; n = 8) was housed in two large, circular pens (3.14 m2) that allowed ample room for locomotion and objects for jumping/perching (Fig. 1). The restricted treatment group (R; n = 7) were raised in smaller pens (1 m2 at maturity) with low mesh ceilings that constrained movement. Here, we assume that optimal locomotor conditions during growth are those most closely resembling typical behavior in the wild (although natural behavior may not represent the absolute optimal condition). This assumption is based on observations in other species that locomotor deficits will scale with distance from natural conditions (36, 69). The wing feathers of birds in both treatments were regularly clipped. The restricted treatment group’s cage size was increased as birds grew such that the proportion of the pen area per bird to bird size was maintained throughout treatment. Food and water were available ad libitum (food intake did not differ between groups). Lights were programmed to be on a 12:12-h light-dark cycle. At skeletal maturity, the functional capacity of both groups was tested for jumping performance and energy cost of submaximal stead-state running and standing. The experimental protocol was approved by Institutional Animal Care and Use Committee at The Pennsylvania State University (IACUC; Ref. #46435).
Fig. 1.
Rearing conditions for control and restricted birds from 2 wk through skeletal maturity (6 mo old). Control birds were provided perches to jump to, hurdles to jump over, and enough room to sprint. Restricted birds were raised in pens with a low ceiling to restrict jumping less floor space of controls, effectively eliminating their ability to jump or sprint during maturation.
Movement Analysis
To quantify the influence of pen configuration on the movement patterns of both treatment groups, pens were filmed from above for 10 min, four times per day, across the growth period (Foscam; C2 1080p HD cameras; Houston, TX). A randomly chosen subset of 49 video records were analyzed with a custom MATLAB script that recorded a timestamp and observer keystrokes matched to activity. For each bird in the pen, behaviors were tracked and categorized into two states (standing or walking) and three events [short sprint (<2 s), hurdle jump, and perch jump (~2× body height)]. For each bird in each video (n = 271; 5-min observations), time spent in each state and the number of each type of event were computed, and mean values were calculated for each. Individual birds were not identifiable between videos, so movement data for individual birds were not extracted.
Functional Measures
Jumping task.
Wing feathers were clipped again just before testing to reduce their contribution to the jump. Birds were placed on a 6 in. × 6 in. force plate (AMTI 6×6; Watertown, MA) enclosed by a tapering vertical box (Fig. 2A) and encouraged to jump repeatedly during two trials lasting either 5 min or until the bird completed three jumps in which the feet left the ground. The jumps were constrained to the vertical direction with the animals landing back on the force plate. Data were collected via AD converter (CED Micro1401–3; Cambridge, UK) at a sample rate of 1,000 samples/s in Spike (Cambridge Analytics) and analyzed with a custom MATLAB script. We fit a piecewise polynomial to the force trace and differentiated the result to calculate the instantaneous slope. We applied a cubic smoothing spline (smoothing parameter set at 0.9999997) to the slope trace to minimize noise. The start and end of the jump were defined as the first time before and after the absolute value of the slope dropped below 50 N/s (Fig. 2C). We chose to use thresholds for the slope of the force trace as a metric of jump initiation and liftoff rather than body weight normalized thresholds because thresholds of slope were less sensitive to variations in baseline values due to bird defecation or drift of the force plate, which averaged less than 1% of peak force values. These variations resulted in differences in calculated velocities of 0.07 m/s, on average. Jumps included in the analysis had a peak vertical ground reaction force greater than 1.5 times body weight and were followed by an aerial phase in which force dropped below 0.5 body weights for at least 200 ms (blue shaded region in Fig. 2B). We used a threshold of 0.5 body weights because forces from the bird pushing off the box after liftoff could be transmitted to the force plate. Here, we simplified our analysis to include only vertical forces and movement of the center of mass. Peak forces in the horizontal direction were typically one-fifth of the peak forces in the vertical direction, and horizontal peak power has been shown to be an order of magnitude lower than vertical peak power even in jumps in which the animal’s goal is vertical and horizontal translation (32). Because we were comparing between groups, we do not believe the small contribution of horizontal forces would alter our results.
Fig. 2.
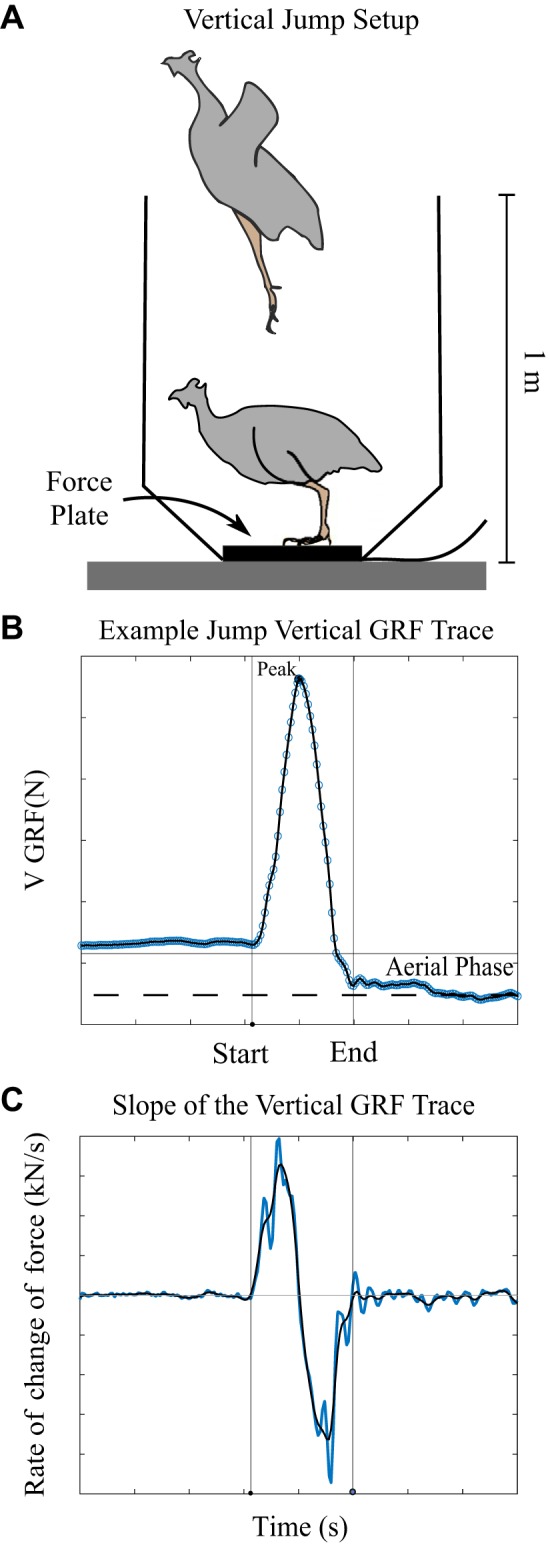
A: setup for the jump task at adulthood. B: typical jump trace with jump start and end identified. Blue circles are raw data. The black line is the piecewise polynomial fit. Horizontal gray line shows the weight of the bird. Vertical lines identify the start and end of the jump. C: slope of the force trace (in blue) and the smoothed trace (in black) was used to identify the time of jump initiation and liftoff. Only jumps that included an aerial phase (blue shaded region where the force drops below 0.5 body weights) post peak force production were included in the analysis.
We evaluated three metrics of jump performance. As a proxy for maximal jump height (44), we found the peak vertical velocity (analysis described below) in each jump and recorded the maximum value for each bird across all trials. Second, we calculated mass-specific maximum jump power to quantify the rate of force delivery. While most jump studies primarily focus on maximum jump height or distance as a metric of performance (3, 28, 38, 39), higher-power jumps can be completed in less time, allowing for a faster escape, which could be ecologically relevant in this species. Lastly, to quantify behavioral differences between groups, we computed the frequency of bird jumping during our jump trials.
The maximum vertical velocity of the center of mass of the bird was calculated by first finding the bird’s vertical acceleration. At each time point, i, the acceleration of the bird’s center of mass, ai, was found by dividing the instantaneous net vertical ground reaction force, Fi, by the animal’s mass, m.
Using the trapezoidal rule to integrate acceleration with respect to time, velocity at any time point was calculated as
where Δt is the inverse of the sample rate, and we assume the velocity of the center of mass of the bird was zero at jump initiation. We recorded maximum velocity for each bird from this array. Instantaneous power (Pi), then, is the product of velocity and force.
The maximum value for peak instantaneous power for each bird was used to calculate muscle mass-specific power output for comparison between groups. To allow for comparisons to earlier guinea fowl-jumping studies, we also recorded the peak body mass-normalized vertical ground reaction force and calculated the muscle mass-specific work done during each jump by multiplying the average power of any jump by its duration.
Mass of jump-contributor leg muscles.
The amount of extensor muscle mass is a key determinant of peak power in jumping (32). Therefore, at the end of functional data collection, birds were euthanized (intravenous pentobarbital, >160 mg/kg) and the muscles of one leg were dissected and individually weighed. To calculate muscle-mass specific power, we summed the masses of the lower limb muscles that could have powered jumping. Following Henry et al. (32), we excluded from this sum the masses of the iliotrochantericus medius and cranialis, the tibialis cranialis, the extensor digitorum longus, the iliotibialis cranialis, and the iliofibularis. Unlike Henry et al. (32), we did not distinctly weigh the anterior and posterior portions of the iliofibularis, so we did not include the mass of either in our summation. To estimate bilateral extensor muscle mass, masses from the individual limb were doubled assuming symmetry.
Energetic economy of running and standing.
Running and standing economy were evaluated by measuring the rate of oxygen consumption (V̇o2) with a flow-through metabolic system, while the birds either ran on a treadmill at 1.5 m/s (flow rate 80 L/min) or stood (flow rate of 25 L/min) for 6 min. A running speed of 1.5 m/s was chosen because it is a comfortable submaximal running speed and allows comparisons to earlier studies (25, 60). The animals ran or stood inside a clear Lucite metabolic chamber (length: 0.6 m, width: 0.3 m, height: 0.45 m) that fit snug to the treadmill belt using foam padding. The chamber permitted in-current air flow generated using two vacuum pumps (HiBlow, VP-7045S, Saline, MI) connected via impervious plastic hosing. To increase the mixing of the ex-current flow, we placed a small electric fan inside the chamber and also ran the excurrent flow through an external mixing chamber. The flow rate exiting the metabolic chamber was recorded continuously using a mass flow meter positioned behind the mixing chamber (Bronkhorst Mass-Stream MFM D-6370-DR for the large flow and Mass-Stream MFM D-6360-DR for the low flow, Bethlehem, PA). The flow rate was converted to standard temperature and pressure of dry air from a continuous measurement of water vapor pressure and temperature, as well as barometric pressure (model HMP110; Vaisala, Woburn, MA).
A sample of the ex-current air stream was taken from a manifold placed at the pump exhaust using a subsample pump (SS-4; Sable, Las Vegas, NV). The subsample gas was pushed through a chamber of magnesium perchlorate to dry it and was subsequently passed through one of the sample lines of a dual-channel oxygen analyzer (FC-2 Oxzilla; Sable). The second channel measured room air that had been similarly subsampled and dried and was used to compute a delta fractional concentration of O2 (drift corrected).
We computed rates of oxygen consumption using the mass balance equations from (73) from data recorded with a USB data acquisition system (50 Hz; CED Micro1401–3; Cambridge, UK). Because carbon dioxide was not removed from our air flow and subsamples, we solved for the fractional concentration of CO2 in our subsample, assuming a respiratory exchange ratio (RER) of 0.8 and using a rootsolver implemented in MATLAB (fmincon; The MathWorks, Natick MA) to satisfy the zero function:
where V̇o2 is the rate of oxygen consumption and V̇co2 is the rate of carbon dioxide production. We subsequently converted the rate of oxygen consumption to a rate of energy expenditure using the energy equivalent of O2 from the equation of Péronnett and Massicotte (14, 37, 52). Leaving the CO2 in the air flow and predicting its concentration using an RER of 0.8 permitted a calculation of energy expenditure that is insensitive to fluctuations in RER (73) (Withers PC, personal communication). This approach also mitigated the need to scrub the metered flow of CO2, which is impractical at high flow rates (43). Data were averaged over the last minute of the trial and normalized by body mass. Data from randomly ordered 2–4 standing trials and 1–3 running trials were averaged for each individual. Two birds were excluded from the analyses because they would not run on the treadmill and one because sufficient data were not available to correct for drift in the oxygen analyzers.
The oxygen analyzers were calibrated before each measurement trial using CO2-free dry air. The oxygen analyzers were also zeroed using pure nitrogen gas. The entire system was calibrated using a series of accurately metered nitrogen bleeds into the chamber. Nitrogen bleeds were set to within 1.0 ml/min using a flow controller (Bronkhorst El-Flow Select F-201CV-500-AAD-11-V, Bethlehem, PA). The deflection in the O2 fractional concentration predicted by the nitrogen displacement and the metered chamber flow were compared with the measured O2 fractional concentration. This procedure demonstrated the sensitivity of the metabolic system to be within 1% of the measured energy expenditure of the animals.
Statistical Analyses
We evaluated the influence of treatment group (restricted vs. control) on functional capacity with t-tests for data that passed the homogeneity of variance assumption and with a Kruskal-Wallis test by ranks, when this criterion was not met. Nonparametric analyses are indicated with an asterisk after the P value in Table 1. The influence of pen configuration on movement during growth (Table 1) was compared between treatments by the same methods. We adjusted the P values with a Benjamini-Hochberg (20) correction for multiple comparisons and used Cohen’s D analysis to calculate effect sizes. All analyses were performed in R (53).
Table 1.
Quantification of the influence of differences in locomotor demand during growth on functional performance and bird physical metrics at maturity
| Restricted | Control | pVal | Adjusted P Value | ES | |
|---|---|---|---|---|---|
| Total animals | 7 | 8 | |||
| Adult body mass, kg | 1.7 (0.11) | 1.7 (0.12) | 0.66 | 0.66 | 0.23 |
| Lower limb muscle masses, kg | 0.295 (0.027) | 0.318 (0.038) | 0.21 | 0.35 | 0.67 |
| Extensor muscle mass, kg | 0.239 (0.022) | 0.257 (0.029) | 0.18 | 0.34 | 0.71 |
| Percent time during growth | |||||
| Standing | 72 (20) | 74 (20) | 0.48 | 0.54 | 0.09 |
| Walking | 27 (20) | 25 (20) | 0.44 | 0.55 | 0.1 |
| Sprinting | 0.1 (0.43) | 1 (2.5) | 2.9e-05* | 1.4e-4 | 0.49 |
| Hurdling | 0.041 (0.13 | 0.4 (0.82) | 2e-6 | 1.5e-5 | 0.59 |
| Jumping | 0 | 0.29 (0.55) | 2.4e-12* | 3.6e-11 | 0.72 |
| Jumps/day | 0 | 194 (349) | |||
| Sprint min/week | 0.97 (4.7) | 92 (197) | |||
| Functional measures at maturity | |||||
| Maximum velocity m/s | 3.3 (0.43) | 4.0 (0.36) | 0.007 | 0.017 | 1.7 |
| Jump work J/kg extensor muscle mass | 37 (9.2) | 50 (8.4) | 0.013 | 0.028 | 1.5 |
| Peak power W/kg extensor muscle mass | 787 (165) | 1171 (117) | 3.5e-4 | 0.001 | 2.7 |
| Maximum force/body weight, N/N | 4.7 (0.54) | 6.7 (0.74) | 5e-5 | 1.875e-4 | 3.0 |
| Jumps/min | 1.3 (1.8) | 1.8 (2.0) | 0.61 | 0.65 | 0.27 |
| Running metabolic power, W/kg body mass | 19 (0.87) | 20 (2.0) | 0.35 | 0.53 | 0.58 |
| Standing metabolic power, W/kg body mass | 9.9 (2.1) | 9 (1.8) | 0.43 | 0.55 | 0.48 |
Bold type indicates significance under P value adjustment for multiple comparisons. Data are presented as means (SD). ES, effect size.
P values with an asterisk required nonparametric analysis.
RESULTS
Movement Analysis
Pen configuration eliminated jumping and constrained running for restricted birds in comparison to controls. Restricted birds were unable to jump during their entire growth period, while controls jumped at least two times their body height an average of 194 times/day or ~10 times/hour (assuming only jumping during waking hours), on average. Likewise, adult birds that were raised in restrictive conditions decreased sprinting by a factor of 100 (Table 1), while time standing and walking were unaffected by the treatment (Table 1 and Fig. 3).
Fig. 3.
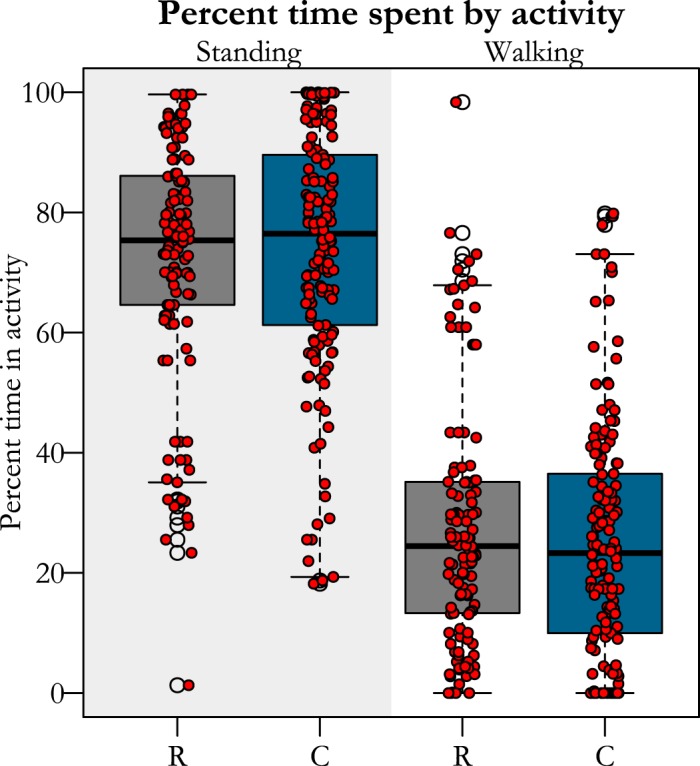
Percent of time birds from each treatment (R, restricted; C, controls) group spent either standing or walking. Red dots represent the average time in an activity for one bird during a 5-min window. There were no differences between groups in time spent in either activity.
Functional Measures
Birds raised in restrictive conditions jumped as frequently as controls, and both were regularly able to clear the top of the box. Restricted birds produced lower jump maximum velocities (Fig. 4A and Table 1) and lower peak power (Fig. 4B and Table 1) than controls. These detriments in jump performance occurred in the absence of changes in running (Fig. 4C) and standing economy (Fig. 4D and Table 1), in which there were no differences between groups.
Fig. 4.
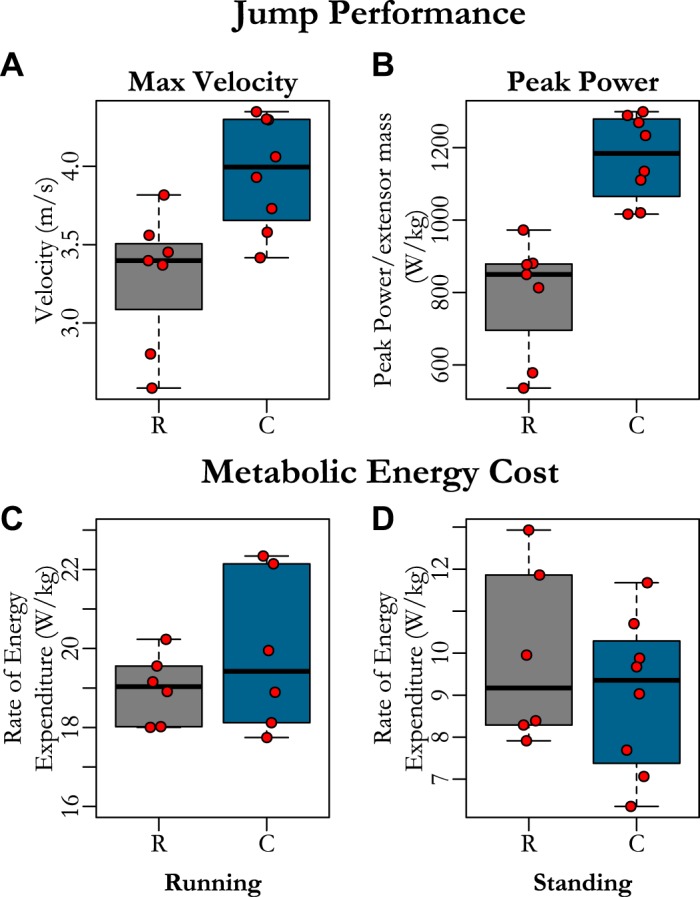
Comparison between functional performance of restricted birds and controls at skeletal maturity for jump performance [maximum velocity (A), maximum muscle mass-specific power (B)) and net rate of energy expenditure during running (C) and standing (D). Restricted birds generated greater maximum jump velocity (A) and peak powers (B) but had similar running (C) and standing (D) metabolic power as control birds. Each red dot represents the maximum value for each bird in A and B and the average value for each individual in C and D. Data from restricted birds (R) is presented with gray boxplots. Control birds (C) are represented by blue boxplots.
DISCUSSION
Evaluation of Hypotheses
The goal of this study was to evaluate how the locomotor functional capacity of adults is influenced by decreases in childhood activity levels. Specifically, we focused on the functional consequences of eliminating high-intensity exercise. We chose an animal model to study this question because it allowed us to carefully control the functional demands on individuals during the entire growth period. Such a study would require nearly two decades to perform on humans. While restricted birds rarely, if ever, jumped during the growth period, control birds jumped twice their body height nearly 200 times/day (~10 jumps/h), on average, for their entire growth period. In contrast, most controlled exercise studies in humans impose treatments over, at most, 6 to 12 wk (13, 65). Conservatively, this is equivalent to less than 2% of the duration of our study. Therefore, we would expect that if the body plastically adapts to variations in locomotor demand during growth, our treatment would result in a very strong effect.
We found that birds restricted from jumping and sprinting during growth, showed deficits in maximal jumping capacity. The difference in jump performance between groups implies that excluding jumping during growth does impede jumping ability as an adult. This suggests that the No-Advantage hypothesis is false for high-intensity locomotor activities in this species; activity patterns during growth do alter adult performance. Furthermore, we also found that the decrements in jump performance did not generalize to steady-state submaximal tasks. Decreased jumping capacity did not correlate with energy expenditure during standing or continuous running. In other words, elimination of high-intensity exercise during all of maturation did not produce adults for which steady-state submaximal movement required more effort. Thus, our results also do not support the Optimal Development hypothesis, which predicted that suboptimal rearing conditions would produce adults with a performance disadvantage that generalized across high-intensity and sub-maximal tasks. Instead, our results support the Beneficial Acclimation hypothesis, which predicts that functional plasticity during growth is task specific; deficits do not generalize across locomotor functions.
The Beneficial Acclimation hypothesis can also be supported in conditions with functional trade-offs, where the tasks involved are associated with incompatible physiological adaptations (36, 69). Such a trade-off could be expected here on the premise that muscle morphology diverges on the basis of requirements for power production versus economy (18). Specifically, muscles adapted specifically for power production are thought to be characterized by long, strap-like parallel fibers with fast myosin isoforms, compared with short, slow, pennate-fibered muscles for economy (16). In theory, reducing the need for high-power generation during all of ontogeny could remove the power constraints on the underlying musculature (5), allowing muscles to plastically adapt morphology to be more economical. If this were the case, one would expect birds restricted from high-power exercise to be more efficient while standing, and perhaps also during a steady-state activity like nonmaximal running. Our results, however, do not indicate that there is a power versus economy trade-off.
The lack of a power-energetics trade-off could be explained by several factors. First, given that we did not actually measure the muscle architecture or fiber type, it could be that individual muscles adapted, but their influence on the whole-body energetics of the system was not robust enough to be detectable within the large interindividual variation of metabolic power (40). An analysis of muscle morphology in these animals would shed light on muscle adaptations. Second, the trade-off between power and economy may exist for muscles with little to no tendon, but does not necessarily exist for muscles paired with a tendon, where the stretch and recoil of the tendon can increase power production over the muscle alone. Although muscle differentiation between functions (power vs. economy) are most easily identified across species or between muscles specialized for an individual task (17), in guinea fowl (as in humans and several other species), many of the muscles thought to be key for economical weight support during standing and gait are, in fact, the same as those that are key for work production and high power movements (58). These muscles, like the gastrocnemius, are associated with short pennate fibers but also tendons that store and release energy to overcome the inherent power constraints of the muscle fibers (2, 16, 56). The lack of tradeoff between power production and efficiency could suggest that a muscle-tendon unit appropriately activated is adept at multiple functional roles rather than being highly specialized for one role.
Influence on Jump Performance
Our results suggest that a reduced jumping performance is not dictated by a simple deficit in muscle mass, but instead may be largely driven by a change in neural control. This is supported, in particular, by the substantial difference in peak power that remains after accounting for the available mass of extensor muscle (muscle mass-specific peak power). The peak powers produced during jumps were well above the power capacity of muscle alone, estimated to be ~380 W/kg muscle (32). This supports the conclusions of Henry et al. (32) that guinea fowl decrease the duration of energy delivery by driving the jump, at least in part, with the release of strain energy stored in long distal tendons. Several studies have shown that muscle activation must be appropriately timed with tendon recoil to amplify rather than dissipate energy (55, 63). Increases in power without increases in muscle mass suggest that birds with jump experience may coordinate muscle excitations more efficiently to produce greater power. Furthermore, the fact that we found changes in jump performance, but not in standing or running metabolic power, is consistent with the observation that most plastic adaptations that result from changes in spinal circuits are task-specific (1, 26, 64), and can improve performance in the absence of muscular changes (26, 41). The combination of these aspects of our results suggests that restricted jumping during growth primarily results in neural rather than muscle morphological changes. We cannot rule out, however, that changes in muscle morphology, including muscle architecture, fiber type, or specific tension contributed to the decreased force capacity without changes in muscle mass. A previous study in guinea fowl, for instance, found longer muscle fascicles (sarcomerogenesis) in the absence of an increase in muscle mass in animals that underwent acceleration running training during growth (62). Longer muscles may provide an advantage for power production by reducing force-velocity constraints.
Also, of note is the very large effect size observed for peak muscle mass-specific power (ES = 3.1) in comparison to the effect of our treatment on maximum jump velocity (1.7). That our treatment had a larger influence on power than maximum jump velocity underscores the point that there may be several metrics of jump performance that could be ecologically relevant. Jump height may be important when traversing complex terrain, for instance, but power may be prioritized for organisms needing to escape predators quickly (54). Whether humans under comparable conditions would exhibit a larger effect in peak power compared with other jump metrics is not known and may depend on the specific goal of the jumping task (e.g., jump height or reaction time).
Implications for the Plasticity of Locomotor Economy
We were surprised that the elimination of high-intensity exercise during all of growth, including the use of a running gait, does not alter running (or standing) economy. While the causal mechanisms that determine an individual’s locomotor energy cost are complex and not fully understood (11, 47), we had expected that animals that were confined to walking and standing throughout growth would have morphological, physiological, or neural changes that altered running economy. In adult humans, for instance, endurance training (40), plyometric training (exercises in which muscles exert maximum force in short intervals of time) (11), or high-intensity interval training (13, 45, 48), have been shown to improve running economy, though the exact mechanism that explain these functional differences is still debated (11, 40, 47). For these reasons, we expected locomotor economy to change in response to variations in functional demand.
Multiple explanations are possible for why we found running economy unchanged while other studies show modifications. First, it is possible that the body responds differently to increases and decreases in functional demand; there may not be a linear dose-response relationship between exercise and economy. While increasing high-intensity exercise has been shown to improve running economy in adult humans (13, 45, 48), decreasing demand may not lead to higher cost of transport. An alternative possibility is that there are thresholds of increased or decreased demand that signal the body to adapt (10) and that those thresholds may change at maturation. This possibility suggests that there may be a wide swath of activity levels around what is “normal” where deviations are not great enough to induce metabolic or physiological changes in a growing animal, but that may result in changes in an adult. A third possibility is that our treatment may not have triggered the signals that induce metabolic changes. Since high-intensity burst running and jumping were a small fraction of the daily activity of the subjects in our study, the overall energy budget between groups was likely not that different. If adaptations for economy require an energy expenditure imbalance, or a difference in the amount of steady-state endurance running performed during growth, it is possible that our intervention did not impart a signal that led to physiological adaptations specific to energetics.
In summary, we tested the influence of eliminating all high-intensity activity during growth and found that restricted birds showed deficits in jump performance but that running and standing energy consumption did not vary between groups. It is important to take a step back and note that birds that rarely, if ever, jumped throughout all of maturation were still able to jump and produce ground reaction forces over four times their body weight and coordinate muscle-tendon dynamics to amplify muscle power twofold. Further, elimination of all high-intensity exercise resulted in no appreciative change in energy expenditure during steady state tasks. Therefore, while we did observe functional deficits, the influence of eliminating all high-power activity was not drastic. The data from this study, together with those of previous developmental studies (31, 35, 41, 51, 61, 62), provide evidence that in many cases, genetics may outweigh environmental factors during growth. These results could point to a tension, suggested by others (4, 24), between the ability of an organism to adapt to environmental conditions during development and the need for developmental stability to produce a viable adult. Finally, while it is premature to make any clear recommendations pertaining to activity during human growth, data from this study, nevertheless, highlight the importance of considering the specificity of exercise prescription in growing children.
Limitations
With a sample size of 6 to 8 per group, depending on the test, it may be that our experiment is underpowered to quantify the influence of the disuse treatment on certain quantities, for example, muscle properties or energetics. A greater sample size could alter our conclusions that deficits are not generalized, although our results do not indicate any clear trends toward differences in functional parameters other than jump performance. We also recognize the difficulties in eliciting maximal performance from nonhuman subjects (7). It is difficult to differentiate behavior and physiological differences between groups, and both may play a role in jumping performance here. Further analysis of morphological and muscle physiological changes between groups could shed light on their relative contribution to these results. Lastly, while the fast growth rate of guinea fowl was practical for this experimental design, it may limit the ability to infer from these results to the adaptations of other species to decreased high-intensity exercise. If the magnitude of plastic adaptations is dependent on the duration of exposure, a slower growing species may show greater response to differences in functional demand.
GRANTS
Research reported in this publication was supported in part through a seed grant from the Center for Human Evolution and Diversity, Penn State University and The National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under award number R21AR071588. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.M.C., S.J.P., and J.R. conceived and designed research; S.M.C. and M.Q.S. performed experiments; S.M.C. analyzed data; S.M.C., M.Q.S., S.J.P., and J.R. interpreted results of experiments; S.M.C. prepared figures; S.M.C. drafted manuscript; S.M.C., M.Q.S., S.J.P., and J.R. edited and revised manuscript; S.M.C., M.Q.S., S.J.P., and J.R. approved final version of manuscript.
ACKNOWLEDGMENTS
We would like to thank Dr. Kirsty McDonald and Adam DeBoef for help collecting this data. We would also like to thank Talayah Johnston and Kavyasree Katugam for their help with the analysis of animal movement. And finally, we would like to thank the staff at the Central Biological Laboratory at The Pennsylvania State University for their care of our animals during the study.
REFERENCES
- 1.Adkins DL, Boychuk J, Remple MS, Kleim JA. Motor training induces experience-specific patterns of plasticity across motor cortex and spinal cord. J Appl Physiol (1985) 101: 1776–1782, 2006. doi: 10.1152/japplphysiol.00515.2006. [DOI] [PubMed] [Google Scholar]
- 2.Alexander RM. Elastic Mechanisms in the Locomotion of Vertebrates. New York: Cambridge University Press, 1988. [Google Scholar]
- 3.Ali N, Rouhi G, Robertson G. Gender, vertical height and horizontal distance effects on single-leg landing kinematics: implications for risk of non-contact ACL injury. J Hum Kinet 37: 27–38, 2013. doi: 10.2478/hukin-2013-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Álvarez D, Nicieza AG. Effects of induced variation in anuran larval development on postmetamorphic energy reserves and locomotion. Oecologia 131: 186–195, 2002. doi: 10.1007/s00442-002-0876-x. [DOI] [PubMed] [Google Scholar]
- 5.Anderson PS, Renaud S, Rayfield EJ. Adaptive plasticity in the mouse mandible. BMC Evol Biol 14: 85, 2014. doi: 10.1186/1471-2148-14-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Armstrong N, Welsman JR. The physical activity patterns of European youth with reference to methods of assessment. Sports Med 36: 1067–1086, 2006. doi: 10.2165/00007256-200636120-00005. [DOI] [PubMed] [Google Scholar]
- 7.Astley HC, Abbott EM, Azizi E, Marsh RL, Roberts TJ. Chasing maximal performance: a cautionary tale from the celebrated jumping frogs of Calaveras County. J Exp Biol 216: 3947–3953, 2013. doi: 10.1242/jeb.090357. [DOI] [PubMed] [Google Scholar]
- 8.Aucouturier J, Baker JS, Duché P. Fat and carbohydrate metabolism during submaximal exercise in children. Sports Med 38: 213–238, 2008. doi: 10.2165/00007256-200838030-00003. [DOI] [PubMed] [Google Scholar]
- 9.Bailey RC, Olson J, Pepper SL, Porszasz J, Barstow TJ, Cooper DM. The level and tempo of children’s physical activities: an observational study. Med Sci Sports Exerc 27: 1033–1041, 1995. doi: 10.1249/00005768-199507000-00012. [DOI] [PubMed] [Google Scholar]
- 10.Baldwin KM, Haddad F. Skeletal muscle plasticity: cellular and molecular responses to altered physical activity paradigms. Am J Phys Med Rehabil 81, Suppl: S40–S51, 2002. doi: 10.1097/00002060-200211001-00006. [DOI] [PubMed] [Google Scholar]
- 11.Barnes KR, Kilding AE. Strategies to improve running economy. Sports Med 45: 37–56, 2015. doi: 10.1007/s40279-014-0246-y. [DOI] [PubMed] [Google Scholar]
- 12.Bass SL, Saxon L, Daly RM, Turner CH, Robling AG, Seeman E, Stuckey S. The effect of mechanical loading on the size and shape of bone in pre-, peri-, and postpubertal girls: a study in tennis players. J Bone Miner Res 17: 2274–2280, 2002. doi: 10.1359/jbmr.2002.17.12.2274. [DOI] [PubMed] [Google Scholar]
- 13.Batacan RB Jr, Duncan MJ, Dalbo VJ, Tucker PS, Fenning AS. Effects of high-intensity interval training on cardiometabolic health: a systematic review and meta-analysis of intervention studies. Br J Sports Med 51: 494–503, 2017. doi: 10.1136/bjsports-2015-095841. [DOI] [PubMed] [Google Scholar]
- 14.Beck ON, Kipp S, Byrnes WC, Kram R. Use aerobic energy expenditure instead of oxygen uptake to quantify exercise intensity and predict endurance performance. J Appl Physiol (1985) 125: 672–674, 2018. doi: 10.1152/japplphysiol.00940.2017. [DOI] [PubMed] [Google Scholar]
- 15.Behringer M, Vom Heede A, Matthews M, Mester J. Effects of strength training on motor performance skills in children and adolescents: a meta-analysis. Pediatr Exerc Sci 23: 186–206, 2011. doi: 10.1123/pes.23.2.186. [DOI] [PubMed] [Google Scholar]
- 16.Biewener AA. Muscle function in vivo: a comparison of muscles used for elastic energy savings versus muscles used to generate mechanical power. Am Zool 38: 703–717, 1998. doi: 10.1093/icb/38.4.703. [DOI] [Google Scholar]
- 17.Biewener AA. Locomotion as an emergent property of muscle contractile dynamics. J Exp Biol 219: 285–294, 2016. doi: 10.1242/jeb.123935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Biewener AA, Roberts TJ. Muscle and tendon contributions to force, work, and elastic energy savings: a comparative perspective. Exerc Sport Sci Rev 28: 99–107, 2000. [PubMed] [Google Scholar]
- 19.Blair SN, Morris JN. Healthy hearts—and the universal benefits of being physically active: physical activity and health. Ann Epidemiol 19: 253–256, 2009. doi: 10.1016/j.annepidem.2009.01.019. [DOI] [PubMed] [Google Scholar]
- 20.Chen SY, Feng Z, Yi X. A general introduction to adjustment for multiple comparisons. J Thorac Dis 9: 1725–1729, 2017. doi: 10.21037/jtd.2017.05.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cleland V, Dwyer T, Venn A. Which domains of childhood physical activity predict physical activity in adulthood? A 20-year prospective tracking study. Br J Sports Med 46: 595–602, 2012. doi: 10.1136/bjsports-2011-090508. [DOI] [PubMed] [Google Scholar]
- 22.Daley MA, Usherwood JR, Felix G, Biewener AA. Running over rough terrain: guinea fowl maintain dynamic stability despite a large unexpected change in substrate height. J Exp Biol 209: 171–187, 2006. doi: 10.1242/jeb.01986. [DOI] [PubMed] [Google Scholar]
- 23.de Jong G. Evolution of plasticity and the emergence patterns of ecotypes. New Phytol 166: 101–117, 2014. doi: 10.1111/j.1469-8137.2005.01322.x. [DOI] [PubMed] [Google Scholar]
- 24.DeWitt TJ, Sih A, Wilson DS. Costs and limits of phenotypic plasticity [Online]. Trends Ecol Evol 13: 77–81, 1998. doi: 10.1016/S0169-5347(97)01274-3. [DOI] [PubMed] [Google Scholar]
- 25.Ellerby DJ, Henry HT, Carr JA, Buchanan CI, Marsh RL. Blood flow in guinea fowl Numida meleagris as an indicator of energy expenditure by individual muscles during walking and running. J Physiol 564: 631–648, 2005. doi: 10.1113/jphysiol.2005.082974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Enoka RM. Neural adaptations with chronic physical activity [Online]. J Biomech 30: 447–455, 1997. doi: 10.1016/S0021-9290(96)00170-4. [DOI] [PubMed] [Google Scholar]
- 27.Fitts RH, Widrick JJ. Muscle mechanics: adaptations with exercise-training. Exerc Sport Sci Rev 24: 427–474, 1996. doi: 10.1249/00003677-199600240-00016. [DOI] [PubMed] [Google Scholar]
- 28.Floria P, Gómez-Landero LA, Harrison AJ. Variability in the application of force during the vertical jump in children and adults. J Appl Biomech 30: 679–684, 2014. doi: 10.1123/jab.2014-0043. [DOI] [PubMed] [Google Scholar]
- 29.Ghalambor CK, McKay JK, Carroll SP, Reznick DN. Adaptive versus non-adaptive phenotypic plasticity and the potential for contemporary adaptation in new environments. Funct Ecol 21: 394–407, 2007. doi: 10.1111/j.1365-2435.2007.01283.x. [DOI] [Google Scholar]
- 30.Gordon JC, Rankin JW, Daley MA. How do treadmill speed and terrain visibility influence neuromuscular control of guinea fowl locomotion? J Exp Biol 218: 3010–3022, 2015. doi: 10.1242/jeb.104646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heiss E, Handschuh S, Aerts P, Van Wassenbergh S. Musculoskeletal architecture of the prey capture apparatus in salamandrid newts with multiphasic lifestyle: does anatomy change during the seasonal habitat switches? J Anat 228: 757–770, 2016. doi: 10.1111/joa.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Henry HT, Ellerby DJ, Marsh RL. Performance of guinea fowl Numida meleagris during jumping requires storage and release of elastic energy. J Exp Biol 208: 3293–3302, 2005. doi: 10.1242/jeb.01764. [DOI] [PubMed] [Google Scholar]
- 33.Hu X, Charles JP, Akay T, Hutchinson JR, Blemker SS. Are mice good models for human neuromuscular disease? Comparing muscle excursions in walking between mice and humans. Skelet Muscle 7: 26, 2017. doi: 10.1186/s13395-017-0143-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Livingstone MB, Robson PJ, Wallace JM, McKinley MC. How active are we? Levels of routine physical activity in children and adults. Proc Nutr Soc 62: 681–701, 2003. doi: 10.1079/PNS2003291. [DOI] [PubMed] [Google Scholar]
- 35.Johnston IA. Environment and plasticity of myogenesis in teleost fish. J Exp Biol 209: 2249–2264, 2006. doi: 10.1242/jeb.02153. [DOI] [PubMed] [Google Scholar]
- 36.Johnston IA, Temple GK. Thermal plasticity of skeletal muscle phenotype in ectothermic vertebrates and its significance for locomotory behaviour. J Exp Biol 205: 2305–2322, 2002. [DOI] [PubMed] [Google Scholar]
- 37.Kipp S, Byrnes WC, Kram R. Calculating metabolic energy expenditure across a wide range of exercise intensities: the equation matters. Appl Physiol Nutr Metab 43: 639–642, 2018. doi: 10.1139/apnm-2017-0781. [DOI] [PubMed] [Google Scholar]
- 38.Kubo K, Kawakami Y, Fukunaga T. Influence of elastic properties of tendon structures on jump performance in humans. J Appl Physiol (1985) 87: 2090–2096, 1999. doi: 10.1152/jappl.1999.87.6.2090. [DOI] [PubMed] [Google Scholar]
- 39.Kubo K, Morimoto M, Komuro T, Yata H, Tsunoda N, Kanehisa H, Fukunaga T. Effects of plyometric and weight training on muscle-tendon complex and jump performance. Med Sci Sports Exerc 39: 1801–1810, 2007. doi: 10.1249/mss.0b013e31813e630a. [DOI] [PubMed] [Google Scholar]
- 40.Lacour JR, Bourdin M. Factors affecting the energy cost of level running at submaximal speed. Eur J Appl Physiol 115: 651–673, 2015. doi: 10.1007/s00421-015-3115-y. [DOI] [PubMed] [Google Scholar]
- 41.Legerlotz K, Marzilger R, Bohm S, Arampatzis A. Physiological adaptations following resistance training in youth athletes—a narrative review. Pediatr Exerc Sci 28: 501–520, 2016. doi: 10.1123/pes.2016-0023. [DOI] [PubMed] [Google Scholar]
- 42.Leskinen T, Waller K, Mutikainen S, Aaltonen S, Ronkainen PHA, Alén M, Sipilä S, Kovanen V, Perhonen M, Pietiläinen KH, Cheng S, Suominen H, Kainulainen H, Kaprio J, Kujala UM. Effects of 32-year leisure time physical activity discordance in twin pairs on health (TWINACTIVE study): aims, design and results for physical fitness. Twin Res Hum Genet 12: 108–117, 2009. doi: 10.1375/twin.12.1.108. [DOI] [PubMed] [Google Scholar]
- 43.Lighton JRB. Measuring Metabolic Rates: A Manual for Scientists. New York: Oxford University Press, 2008. [Google Scholar]
- 44.Linthorne N. Analysis of standing vertical jumps using a force platform. Am J Phys 69: 1198–1204, 2001. doi: 10.1119/1.1397460. [DOI] [Google Scholar]
- 45.McCann DJ, Higginson BK. Training to maximize economy of motion in running gait. Curr Sports Med Rep 7: 158–162, 2008. doi: 10.1097/01.CSMR.0000319711.63793.84. [DOI] [PubMed] [Google Scholar]
- 46.McDonagh MJN, Davies CTM. Adaptive response of mammalian skeletal muscle to exercise with high loads. Eur J Appl Physiol Occup Physiol 52: 139–155, 1984. doi: 10.1007/BF00433384. [DOI] [PubMed] [Google Scholar]
- 47.Morgan DW, Martin PE, Krahenbuhl GS. Factors affecting running economy. Sports Med 7: 310–330, 1989. doi: 10.2165/00007256-198907050-00003. [DOI] [PubMed] [Google Scholar]
- 48.Saunders PU, Pyne DB, Telford RD, Hawley JA. Factors affecting running economy in trained distance runners. Sports Med 34: 465–485, 2004. doi: 10.2165/00007256-200434070-00005. [DOI] [PubMed] [Google Scholar]
- 49.Ozemek C, Lavie CJ, Rognmo Ø. Global physical activity levels. Need for intervention. Prog Cardiovasc Dis 62: 102–107, 2019. doi: 10.1016/j.pcad.2019.02.004. [DOI] [PubMed] [Google Scholar]
- 50.Parikh T, Stratton G. Influence of intensity of physical activity on adiposity and cardiorespiratory fitness in 5–18 year olds. Sports Med 41: 477–488, 2011. doi: 10.2165/11588750-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 51.Park JC, Kim HS, Yamashita M, Choi I. Development of contractile and energetic capacity in anuran hindlimb muscle during metamorphosis. Physiol Biochem Zool 76: 533–543, 2003. doi: 10.1086/376422. [DOI] [PubMed] [Google Scholar]
- 52.Péronnet F, Massicotte D. Table of nonprotein respiratory quotient: an update. Can J Sport Sci 16: 23–29, 1991. [PubMed] [Google Scholar]
- 53.R Core Team R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing, 2017. [Google Scholar]
- 54.Rankin JW, Doney KM, McGowan CP. Functional capacity of kangaroo rat hindlimbs: adaptations for locomotor performance. J R Soc Interface 15: 20180303, 2018. doi: 10.1098/rsif.2018.0303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Richards CT, Biewener AA. Modulation of in vivo muscle power output during swimming in the African clawed frog (Xenopus laevis). J Exp Biol 210: 3147–3159, 2007. doi: 10.1242/jeb.005207. [DOI] [PubMed] [Google Scholar]
- 56.Roberts TJ. The integrated function of muscles and tendons during locomotion. Comp Biochem Physiol A Mol Integr Physiol 133: 1087–1099, 2002. doi: 10.1016/S1095-6433(02)00244-1. [DOI] [PubMed] [Google Scholar]
- 57.Rowlands AV, Eston RG, Ingledew DK. Measurement of physical activity in children with particular reference to the use of heart rate and pedometry. Sports Med 24: 258–272, 1997. doi: 10.2165/00007256-199724040-00004. [DOI] [PubMed] [Google Scholar]
- 58.Rubenson J, Henry HT, Dimoulas PM, Marsh RL. The cost of running uphill: linking organismal and muscle energy use in guinea fowl (Numida meleagris). J Exp Biol 209: 2395–2408, 2006. doi: 10.1242/jeb.02310. [DOI] [PubMed] [Google Scholar]
- 59.Rubenson J, Lloyd DG, Heliams DB, Besier TF, Fournier PA. Adaptations for economical bipedal running: the effect of limb structure on three-dimensional joint mechanics. J R Soc Interface 8: 740–755, 2011. doi: 10.1098/rsif.2010.0466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rubenson J, Marsh RL. Mechanical efficiency of limb swing during walking and running in guinea fowl (Numida meleagris). J Appl Physiol (1985) 106: 1618–1630, 2009. doi: 10.1152/japplphysiol.91115.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rupert JE, Joll JE, Elkhatib WY, Organ JM. Mouse hind limb skeletal muscle functional adaptation in a simulated fine branch arboreal habitat. Anat Rec (Hoboken) 301: 434–440, 2018. doi: 10.1002/ar.23744. [DOI] [PubMed] [Google Scholar]
- 62.Salzano MQ, Cox SM, Piazza SJ, Rubenson J. American Society of Biomechanics Journal of Biomechanics Award 2017: High-acceleration training during growth increases optimal muscle fascicle lengths in an avian bipedal model. J Biomech 80: 1–7, 2018. doi: 10.1016/j.jbiomech.2018.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sawicki GS, Robertson BD, Azizi E, Roberts TJ. Timing matters: tuning the mechanics of a muscle-tendon unit by adjusting stimulation phase during cyclic contractions. J Exp Biol 218: 3150–3159, 2015. doi: 10.1242/jeb.121673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schubert M, Beck S, Taube W, Amtage F, Faist M, Gruber M. Balance training and ballistic strength training are associated with task-specific corticospinal adaptations. Eur J Neurosci 27: 2007–2018, 2008. doi: 10.1111/j.1460-9568.2008.06186.x. [DOI] [PubMed] [Google Scholar]
- 65.Silva R, Damasceno M, Cruz R, Silva-Cavalcante MD, Lima-Silva AE, Bishop DJ, Bertuzzi R. Effects of a 4-week high-intensity interval training on pacing during 5-km running trial. Braz J Med Biol Res 50: e6335, 2017. doi: 10.1590/1414-431x20176335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Telama R, Yang X, Viikari J, Välimäki I, Wanne O, Raitakari O. Physical activity from childhood to adulthood: a 21-year tracking study. Am J Prev Med 28: 267–273, 2005. doi: 10.1016/j.amepre.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 67.van der Zee MD, van der Mee D, Bartels M, de Geus EJC. Tracking of voluntary exercise behaviour over the lifespan. Int J Behav Nutr Phys Act 16: 17, 2019. doi: 10.1186/s12966-019-0779-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Van Kleunen M, Fischer M. Constraints on the evolution of adaptive phenotypic plasticity in plants. New Phytol 166: 49–60, 2005. doi: 10.1111/j.1469-8137.2004.01296.x. [DOI] [PubMed] [Google Scholar]
- 69.Wakeling JM, Cole NJ, Kemp KM, Johnston IA. The biomechanics and evolutionary significance of thermal acclimation in the common carp Cyprinus carpio. Am J Physiol Regul Integr Comp Physiol 279: R657–R665, 2000. doi: 10.1152/ajpregu.2000.279.2.R657. [DOI] [PubMed] [Google Scholar]
- 70.Waller K, Kaprio J, Kujala UM. Associations between long-term physical activity, waist circumference and weight gain: a 30-year longitudinal twin study. Int J Obes 32: 353–361, 2008. doi: 10.1038/sj.ijo.0803692. [DOI] [PubMed] [Google Scholar]
- 71.Warburton DER, Nicol CW, Bredin SSD. Health benefits of physical activity: the evidence. CMAJ 174: 801–809, 2006. doi: 10.1503/cmaj.051351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wisdom KM, Delp SL, Kuhl E. Use it or lose it: multiscale skeletal muscle adaptation to mechanical stimuli. Biomech Model Mechanobiol 14: 195–215, 2015. doi: 10.1007/s10237-014-0607-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Withers PC. Design, calibration and calcuation for flow-through respirometry systems. Aust J Zool 49: 445–461, 2001. doi: 10.1071/ZO00057. [DOI] [Google Scholar]
- 74.Wu XY, Han LH, Zhang JH, Luo S, Hu JW, Sun K. The influence of physical activity, sedentary behavior on health-related quality of life among the general population of children and adolescents: A systematic review. PLoS One 12: e0187668, 2017. doi: 10.1371/journal.pone.0187668. [DOI] [PMC free article] [PubMed] [Google Scholar]