Abstract
Cancer cachexia—cancer-associated body weight and muscle loss—is a significant predictor of mortality and morbidity in cancer patients across a variety of cancer types. However, despite the negative prognosis associated with cachexia onset, there are no clinical therapies approved to treat or prevent cachexia. This lack of treatment may be partially due to the relative dearth of literature on mechanisms occurring within the muscle before the onset of muscle wasting. Therefore, the purpose of this review is to compile the current scientific literature on mechanisms contributing to the development and progression of cancer cachexia, including protein turnover, inflammatory signaling, and mitochondrial dysfunction. We define “development” as changes in cell function occurring before the onset of cachexia and “progression” as alterations to cell function that coincide with the exacerbation of muscle wasting. Overall, the current literature suggests that multiple aspects of cellular function, such as protein turnover, inflammatory signaling, and mitochondrial quality, are altered before the onset of muscle loss during cancer cachexia and clearly highlights the need to study more thoroughly the developmental stages of cachexia. The studying of these early aberrations will allow for the development of effective therapeutics to prevent the onset of cachexia and improve health outcomes in cancer patients.
Keywords: cachexia, inflammation, mitochondria, protein synthesis
INTRODUCTION
Cancer and its associated comorbidities remain a major public health crisis and concern for patients, their families, and the greater medical and scientific community. Although 5-yr survival rates have increased over the past 40 yr, many cancers are still associated with high morbidity and mortality (129, 174). One mechanism for this elevated mortality rate is the presence of cancer-induced muscle loss, termed cancer cachexia. Whereas multiple definitions exist in the clinical literature (63, 64, 196), broadly, cancer cachexia is a cluster of pathologies, including muscle loss and inflammation, that is often accompanied by total body weight loss. Cachexia prevalence ranges from 20% to 89%, depending on the cancer type, with lung, pancreas, esophageal, gastrointestinal tract, head, and neck cancers having the highest rates of cachexia compared with other cancers (58, 107, 120, 178). Cachexia development is also quantified using sarcopenic status as the primary criterion variable, measured by mid-upper-arm area (women < 18 cm2, men < 32 cm2), skeletal muscle index by dual-energy X-ray absorptiometry scan (men < 7.26 kg/cm2, women < 5.45 kg/cm2), lumbar skeletal muscle content using computed tomography scans (women < 39 cm2, men < 55 cm2), as well as bone and fat-free body mass index (BMI) measured using bioelectrical impedance (women < 11.4 kg/m2, men < 14.6 kg/m2) (2). However, general clinical recommendations for cachexia diagnosis are determined by body weight loss and are dichotomized into precachexia and cachexia (64). Precachexia is defined as <5% involuntary weight loss within the prior 6 mo, and cachexia is defined as >5% involuntary weight loss over the last 6 mo or a BMI <20 (64). For the purpose of this review, we define cachexia development as alterations that occur within the muscle during the presence of a cachectic stimulus but before muscle-mass loss has initiated. We define cachexia progression as alterations occurring within the muscle, as wasting has begun and continues to exacerbate. Since the 1980s, the scientific community has known that cancer is associated with clinically significant weight loss (58); however, within the past 10 yr, it has become abundantly clear that cachexia is strongly associated with mortality, and it is not effectively alleviated by nutritional interventions alone (161). Of concern, many physicians still do not consider cachexia clinically relevant until it has already developed (132), by which time, cachexia may be irreversible due to metastasis (13, 91, 162).
The mechanisms for the relationship between muscle loss and mortality are not fully understood. One of the leading hypotheses for this relationship is the propensity for chemotherapy-induced toxicity in cachectic patients. Chemotherapeutic treatments have traditionally been based on a patient’s body surface area (193); however, in the context of muscle wasting, this measurement overestimates overall lean mass and likely leads to chemotherapy-induced toxicity (148). Heart failure, as a result of cardiac muscle wasting, has also been suggested as a contributor to mortality (21). Additionally, muscle breakdown during cachexia releases byproducts into systemic circulation that may cause toxicity to other organs, such as the kidney and liver (3). Regardless of specific causes of death, data clearly demonstrate the importance of maintaining muscle mass in this population.
The bleak relationship between muscle atrophy and mortality strongly suggests that treatment for cachexia should be initiated before the development of significant muscle atrophy. However, accurate diagnostic tests to predict who will develop cachexia do not currently exist in the clinical literature (175, 190). This is likely due to limited information on muscle alterations during the initial development and progression of muscle atrophy. Therefore, the purpose of this review is to summarize current preclinical literature on muscle alterations during the development and progression of cancer-induced muscle atrophy. Herein, we first characterize well-described and emerging models of cancer cachexia. We then describe classical mediators of cancer-induced pathologies, inflammation, and protein turnover. Finally, we move on to the role of mitochondrial function and oxidative stress as emerging mechanisms for the development of muscle wasting. We finish with emerging evidence for other considerations, such as sex as a biological variable and the contribution of nonmuscle tissues in the development of cancer-induced muscle wasting. Overall, the current literature suggests multiple aberrations to cellular processes during the progression of cachexia. Conversely, the available literature in cachexia development appears to suggest that mitochondrial mechanisms may be the initiating factor for cachexia-induced muscle loss (35, 199, 203). This review will serve as the first compilation of muscle alterations occurring before the onset of muscle wasting and hopefully inform future research into this highly significant research area.
MODELS OF CANCER CACHEXIA
Whereas no rodent model will perfectly mimic the heterogeneity of human cancer, multiple preclinical models have been developed and used to examine cancer cachexia. Many of these models attempt to recapitulate the human inflammatory state inherent to many cancers, for example, IL-6-driven C26 and adenomatous polyposis coli (Apc)Min/+ (Min) models. However, inflammation varies greatly in human cancer patients depending on the type of tumor. The heterogenous inflammatory states across various types of cancers highlight the necessity of models with wide ranges of inflammatory phenotypes, from the highly inflamed C26 and Min models (84, 85, 184) to the less inflamed RXF model (67). To understand the current literature of cachexia and progression in context, we will briefly summarize some of the common cancer cachexia models in animals, including key hallmarks, as well as advantages and shortcomings of each model. We first begin with allograft models, then genetic models, and finish with emerging models to study the development and progression of cachexia. A condensed summary of these models can be found in Table 1.
Table 1.
A compilation of current models of cancer cachexia along with benefits and shortcomings for each model
| Model | Cancer Type | Method to Induce Cancer | Average Lifespan of Animals | Advantages | Drawbacks |
|---|---|---|---|---|---|
| Lewis lung carcinoma | Lung | Injection of cancer cells (~1 × 106) into flank |
~24–36 Days postinjection | ∙ Well characterized ∙ Commercially available |
∙ Large tumor burden ∙ May not recapitulate all features of human cachexia |
| C26 | Colorectal | Injection of cancer cells (~1 × 106) into flank |
~22 Days postinjection | ∙ Well characterized ∙ Commercially available |
∙ Large tumor burden ∙ May not recapitulate all features of human cachexia |
| Yoshida AH-ascites | Hepatic | Injection of cancer cells (4 × 107–1 × 108) into the intraperitoneal region |
~12–16 Days postinjection | ∙ Well characterized ∙ Allows for relatively quick studies |
∙ Unlikely interventions could act fast enough |
| RXF cancer cells | Renal | Injection of cancer cells (1.5 × 106) subcutaneously |
~23 Days postinjection | ∙ Only renal model of cachexia | ∙ Does not display classical inflammation present in most models of cancer cachexia |
| MAC16 colonic adenocarcinoma | Colorectal | Implantation of tumor (~1 × 2 mm) in flank | ~18–20 Days postimplantation | ∙ Small tumor burden ∙ No hypophagia |
∙ Not commercially available |
| Walker-256 | Breast/mammary | Injection of cancer cells (~2 × 107) in flank | ~14 Days postimplantation | ∙ Well characterized ∙ Commercially available |
∙ Highly aggressive |
| ApcMin/+ | Colorectal | Genetic mutation in Apc gene that results in spontaneous tumor development | ~120–160 Days from birth | ∙ Well characterized ∙ Longer duration of tumor development ∙ No hypophagia |
∙ Unclear if lifelong genetic manipulation has external validity |
| KPC | Pancreatic | Genetic mutations or injection of KPC cancer cells (1–5 × 105) into the pancreas | ~30 Days postinjection | ∙ Location of the tumor mimics human disease ∙ Recapitulates many features of pancreatic cancer |
∙ Less characterized |
| KPP | Pancreatic | Genetic mutations that are induced by tamoxifen injection | ~100-Day post-tamoxifen injection | ∙ Longer development mimics human disease progression ∙ Recapitulates many features of pancreatic cancer |
∙ Less characterized |
| PDX | Pancreatic | Insertion of pancreatic tumors from humans onto the pancreas of mice | ~80 Days post-tumor implantation | ∙ Directly investigates human pancreatic cancer | ∙ Less characterized ∙ Potentially more heterogenous |
Apc, adenomatous polyposis coli; PDX, patient-derived xenograft.
Allograft Models
Lewis lung carcinoma.
First developed as an immortalized cell line in 1980 (22), the Lewis lung carcinoma (LLC) model of cancer has been used for cachexia research since the 1990s. LLC models typically involve an injection of ~1 × 106 cells into the flank of a mouse with the tumor allowed to progress for ~28 days (28, 34, 35, 106, 151). Generally speaking, 24 days of tumor growth are sufficient to induce “early cachexia,” whereas 36 days of tumor growth induce “late cachexia” and a severe phenotype (179). In the LLC model, tumor sizes typically average ~2.5 g after 28 days of growth (35, 95, 205, 211). Because the tumor burden is so large for animals (~10% body weight is attributable to tumor mass), the validity of the application of this model directly to humans may be limited. Other works have injected LLC cells directly into the muscle tissue to induce cachexia (48, 189), which tends to result in accelerated muscle wasting compared with flank injections. Additionally, other injection sites noted in the literature also include the shoulder blade of the animal (198). Yet, the general validity of these models may warrant some skepticism, as cancer rarely develops in the muscle, as the muscle tissue itself is postmitotic. Additionally, recent works have questioned the validity of using this model as a surrogate for human cachexia, because there appears to be limited overlap in potential mechanisms of disease progression (181). Overall, this model has been utilized effectively as a model of cachexia with a longer duration compared with other allograft models; however, the size of the tumor that develops in relation to animal size may limit external validity.
C26 cancer cells.
C26 is a murine model of colorectal cancer that first became prominent in the literature in the 1990s (184). This particular type of tumor cell will only proliferate and develop cancer cachexia in the BALB/c mouse, a significant deviation from the typical C57BL/6J mouse used in a large proportion of biomedical studies (184). Often, this model utilizes injections of 0.5–5 × 105–1 × 106 of cancer cells to the flank (108, 142, 184). After tumor implantation, animals typically start losing body weight around 14–15 days postinjection (108, 142, 179, 184), with large, functional detriments occurring at ~22 days of tumor implantation (108, 209). Some works have noted decreases in grip strength compared with control mice in as little as 7 days postimplantation with 5 × 105 tumor cells (108), and others have noted decreased force production with longer duration of cancer development in multiple muscles (154, 155). However, mice can survive up to 45 days post-tumor implantation (184). This model is characterized by significant increases in circulating IL-6, hypoglycemia, and hypercorticism (184). Interestingly, this model of cancer appears to develop similarly in younger as well as older animals (182). Similar to the aforementioned LLC model, the C26 model produces relatively large tumors compared with animal size (range 0.8–3.0 g), which can limit its external validity in relation to human cancers (49, 108, 115, 138, 183, 184). Also similar to LLC, the mechanisms contributing to muscle atrophy in this model appear to differ from humans with cachexia (181).
Yoshida AH-ascites.
First developed as a model of cachexia in the late 1980s (186), this cancer cell line has been utilized in over 60 studies investigating cancer cachexia to date. The Yoshida AH-ascites (AH-130) line is a model of hepatic cancer (168). In this model, mice are injected with 4 × 107–1 × 108 cancer cells into the intraperitoneal region (12, 36, 38, 50, 51, 112, 121, 168), a much more aggressive amount of cancer cells compared with the 1 × 106 often seen in other models, such as LLC and C26 cancer lines. Fat mass is maintained 4 days postinjection; however, by 7 days, fat mass is ~25–70% less in experimental animals compared with controls (36, 38, 39, 51, 113). This loss in fat mass corresponds to increased circulating nonesterified fatty acids (NEFAs) and triglycerides (51, 57, 113). Additionally, muscle loss coincides with fat loss, with ~25% lower body and gastrocnemius weights in experimental mice compared with controls at this same time point (39, 112, 168). In most animals, locomotor activity is decreased ~10 days postinjection, with most animals dying 12–16 days postinjection (168). Overall, this model is highly aggressive, allowing for the investigation of cachexia relatively fast; however, the accelerated timeline of this model makes investigations of interventions against cachexia difficult, as it is unlikely that any intervention would act rapidly enough to neutralize cancer-associated muscle loss sufficiently, overall reducing translatability.
RXF cancer cells.
The RXF line is a model of renal carcinoma (67, 150). Prior works have established that 1–1.5 × 106 cancer cell subcutaneous injections yield 20–30% body weight loss in mice, with animals typically living ~23 days postinjection (67, 150). In vitro work using RXF conditioned media in primary human myotubes results in increased forkhead box O (FOXO) and autophagy signaling, as well as decreased RNA processing, decreased mammalian target of rapamycin (mTOR) signaling, and decreased transforming growth factor β (TGF-β) signaling (67). In animals, this model has been shown to result in increased muscle RING-finger protein-1 (MuRF1) content within the muscle (150). Yet, RXF-injected mice do not have increased inflammatory markers in their serum (67), a major difference of this model compared with classical inflammatory models of cancer, such as Min or LLC.
MAC16 colonic adenocarcinoma.
The MAC16 colonic adenocarcinoma was first developed as a model of cancer cachexia in the late 1980s (20, 23). In the original characterization of this tumor line, cancer was induced through administration of 1,2-dimethlyhydrazine (23, 53, 59). After the original tumor inoculation, tumors have been maintained in live animals and transplanted into experimental animals. In most studies using this model, tumors are removed from host mice, and small sections of the tumor (~1 × 2 mm) are implanted into the flank of experimental mice, presumably euthanizing the host mouse (20, 23, 25, 27, 127). This model is typically used in either BALB/c mice (20) or NIMRI mice (25). Compared with more aggressive tumor models, such as LLC, a relatively small tumor burden induces significant body weight loss, with 20–30% body weight loss with only 3% of body weight tumor burden (20). Similar to other models, tumor size is related to body weight and muscle weight, with larger tumor burden corresponding to smaller muscle size (20, 23). This tumor type is known to have a doubling time of 6 days, with 18 days of tumor growth resulting in 24% body weight loss (25). Additionally, the MAC16 model also does not appear to influence food intake dramatically (20). Overall, the small tumor load in relation to body weight loss may provide more external validity to human models. However, because this model has comparatively less commercial availability (as opposed to LLC, C26, AH-130, and Walker-256 cancer cells that are all commercially available to other researchers), the external utility of this model across the broader scientific community is likely limited.
Walker-256.
Compared with the aforementioned models, the Walker-256 model is typically injected into rats as opposed to mice (17, 109, 137, 206). Animals are typically injected with ~2 × 107 cells (17, 109, 137, 206), allowing for a much more aggressive cancer cachexia model compared with others. In this model, food consumption typically decreases ~7 days postinjection (109), with decreases in body weight also occurring ~7 days postinjection (17, 109, 137, 206). Severe body weight and muscle loss begin to develop rapidly after 7 days, with most studies lasting ~14 days post-tumor implantation (17, 109, 137, 206). Overall, whereas this model recapitulates many features of cachexia, the rapid development of the cachexia (compared with other models) may limit external validity when extrapolated to humans.
Genetic Models
Min mouse model of colorectal cancer.
Genetic mouse models of cancer cachexia are also common and useful in establishing mechanisms and therapeutic targets. The Apc gene is mutated in a large percentage of human colon cancer cases. As such, the studying of mice with this same mutation has become a common model for studying environmental factors that influence the genetic predisposition for colorectal cancer (9, 10, 29, 202). The Min mouse is heterozygous for a mutation in the APC gene and will spontaneously develop intestinal adenomas (10). Most Min mice will not live past 4–5 mo due to the development of severe cachexia and anemia (9, 10). However, this time course allows for the study of cachexia as a progression and not simply as an end-point measurement. Final tumor burden for these mice is typically set around 12 weeks of age, and by 15 weeks of age, the mice will begin to lose body weight and initiate cachexia, with severe cachexia occurring around 18–20 weeks of age (9). The advantage to the use of the Min mouse model is that these mice have little to no anorexia, are chronically inflamed, and have a slow rate of wasting, which makes them ideal for interventions and more closely mimics the human progression of cancer cachexia (9, 10, 136, 200, 202). However, a distinct disadvantage is that polyps are present in the small intestine in Min mice, whereas in human patients, they present in the large intestine (9, 10, 114).
Emerging Cancer Models
In addition to the aforementioned, relatively well-characterized models, newer models have also begun to be developed that should be mentioned. Specifically, models of pancreatic ductal adenocarcinoma (PDAC) were recently developed, including the KPC, KPP, and patient-derived xenograft (PDX) models (73, 126, 181). The first model, KPC, was developed from the genetic alteration of pancreas-specific alleles in mice, including mutant KrasG12D, through the Pdx promotor, inducing genetic development of PDAC (65, 90). In the new allograft KPC model, pancreatic cancer cells are removed from cachectic KPC mice and cultured in vitro and cells injected orthotopically into the pancreas of experimental mice (1–5 × 105 cancer cells) (117, 126, 213). In this model, body weight decreases ~14 days postinjection (213). Gastrocnemius muscle weight is maintained 5 days postinjection; however, by 10 days of cancer development, hindlimb muscle mass is ~25% smaller compared with control animals (126), with most animals euthanized ~30 days after tumor implantation (213).
The KPP model uses the same mutant KrasG12D allele used in the original generation of the KPC mouse (118, 126). However, the stop cassette is removed through the Ptfla promoter, and the Pten allele is inactivated, reducing overall Pten synthesis (92, 140, 181, 208). These genetic alterations were made to mirror more closely gene alterations seen in PDAC patients (181). Cancer is conditionally induced by intraperitoneal injections of tamoxifen in ~4-week-old mice (181). Body and muscle weights are maintained 75 days post-tamoxifen injection, with an average lifespan of ~100 days post-tamoxifen injection (181). Approximately 100 days of tumor development corresponds to 40% lower body, tibialis anterior, and gastrocnemius weights compared with controls (181). Interestingly, sequencing data suggest that this new model may more closely resemble human PDAC compared with previous cachexia models (181). Similar to the Min model, the extended timeline of tumor development inherent in this model may provide more validity to human clinical models, a benefit that cannot be understated.
Contrasting the aforementioned models, the PDX model utilizes tumor implantation as opposed to genetic manipulation. In this model, tumors are removed from human patients with pancreatic cancer and ~2 × 2 mm sections of the tumor are implanted in the pancreas of experimental animals (70, 73). Tumors develop for ~84 days, with animals’ muscle weight correlating with tumor burden and expression of atrophy-related genes within the muscle (70, 73). This model clearly has the benefit of utilizing human-derived tumors to study cachexia; however, because tumors are removed from human patients, the degree of cachexia is directly proportional to the patient from which the tumor was derived (70), which may increase heterogeneity of the data derived from this model. Additionally, for researchers who do not have direct access to patients consenting to participate in these types of studies, this model has limited applicability. Taken together, emerging KPC, KPP, and PDX models of cachexia have great promise for the future of cachexia research, due to their purported ability to recapitulate many critical facets of pancreatic cancer compared with prior models (126, 213), an important consideration for one of the most cachexia-prevalent and aggressive cancers (87). However, as these are very new models and have not been as extensively utilized in the literature, more research using these models is necessary to elucidate fully their potential as models for cancer cachexia.
PROTEIN TURNOVER IN THE DEVELOPMENT AND PROGRESSION OF CANCER CACHEXIA
A hallmark feature of cancer cachexia is the net loss of skeletal muscle protein through an imbalance of synthesis and degradation. This disruption of muscle proteostasis leads to a net loss of skeletal muscle protein leading to mass loss. Nearly all preclinical mouse models of cachexia, as well as human cancer patients, exhibit elevated muscle protein degradation with a suppression of muscle protein synthesis. How and when this negative balance of synthesis and degradation occurs are poorly understood. A multitude of cachexia studies have examined the progression of cachexia and stratified analysis based on the severity of muscle-mass loss at various stages of the disease (2, 64). However, a recent study from our laboratory, utilizing the LLC implantation model to characterize protein turnover over 4 weeks, demonstrated that a suppression myogenic signaling occurs within 1 week of tumor implantation, which precedes cross-sectional area loss that begins at 3 weeks postimplantation (34). These findings suggest that the suppression of myogenic signaling at the onset or development of cancer is necessary for the initiation of muscle wasting as time and tumor burden increase. Whereas cancer cachexia may be a multifactorial condition, clear evidence suggests that the ubiquitin proteasome system (UPS) for protein degradation is a major contributor of muscle-mass regulation. The following section will discuss the imbalance of muscle proteostasis in detail, highlighting important studies that have examined this system and its regulation.
Protein Degradation During the Development and Progression of Cancer Cachexia
The UPS is considered a contributor to muscle wasting, due to data from studies that observed inhibition of calpains, and lysosomal proteases only accounted for an ~10–20% reduction in total proteolysis (124). Classically, the UPS degrades proteins through its unique ability to tag specifically proteins for degradation in a very highly regulated manner (45). This process occurs through a hierarchy of enzymes, such as the activation enzymes (E1), ubiquitin- conjugating enzyme (E2), and finally, the ubiquitin (E3) ligases (24). The E3 ligases are considered critical to the specificity of the 26S proteasome, allowing for highly targeted turnover of tagged proteins (131). The induction of the UPS pathway has been demonstrated in many diseased catabolic states, such as cachexia, and can occur early in the disease and worsen through progression (6, 36, 47, 50, 52, 99, 105, 200).
Interestingly, the degree of activation of the UPS may vary by model and tumor type; however, activation of the system remains constant across nearly all preclinical models of cancer cachexia and is exacerbated as cachexia develops in severity (11, 61, 200, 204). Early cachexia studies using a hepatoma-bearing rat model demonstrated increased proteolytic activity of gastrocnemius mRNA and cleavage of fluorogenic substrates (185). Additionally, the UPS is robustly induced in mildly (5–10% body weight loss) cachectic Min mice, as well as in the LLC model of cancer cachexia (34, 151, 200, 201, 203). Previous studies using anti-inflammatory cytokine inhibitors have demonstrated a suppression of E3 ligases and suggest that the UPS may be partially mediated by inflammation during cancer cachexia (199, 203). Further adding to the importance of the E3 ligases in the regulation of muscle mass, studies utilizing overexpression of E3 ligases in vitro induce atrophy, independent of inflammation or disease (157, 165, 167). Recently, the use of a small molecule inhibitor of MuRF-1 was sufficient to counteract cancer-induced muscle atrophy (32). However, it is important to state that whereas the inhibition of inflammatory cytokines attenuated muscle-mass loss, the effects that these treatments pose on muscle function remain largely unknown. A large body of evidence exists suggesting that the UPS is a key factor in cachexia-induced muscle wasting; however, the exact mechanisms of activation vary greatly and may be related to the severity of cachexia and type of cancer.
Contrasting to the literature that exists on the skeletal muscle UPS in preclinical animal models, data that have been collected in human cancer patients have produced varying results. Increases in UPS activity have been linked to disease severity in gastric cancer patients, and inflammatory markers [IL-6, C-reactive protein (CRP)] are tightly correlated to ubiquitin expression and weight loss in cachectic pancreatic cancer patients (55). However, this study only reported elevations in the UPS in subjects that presented with >10% body weight loss, suggesting that the severity of cachexia may dictate the degree of UPS activation (55). In line with preclinical models of colorectal cancer, such as the Min mouse mentioned above, a study using muscle biopsies from human colorectal cancer patients demonstrated elevated proteasome mRNA, which was reduced to the control level following tumor resection (169). Interestingly, studies that examine lung cancer patients and even some gastric and colorectal cancer patients reported no alteration of E3 ligase expression in the skeletal muscle (169). However, it is important to state that the inconsistencies in human cancer patients and the UPS may be consequences of fasting state, physical activity levels, and even the severity of cachexia. Nonetheless, inconsistencies in animal modeling and a lack overlapping targets between preclinical models and human cancer patients leave the role of the UPS in the development and progression of cachexia undefined.
Protein Degradation During the Development and Progression of Cancer Cachexia: The LPS
The lysosomal proteasome system (LPS) is another critical protein degradation system that is non-ATP driven (19, 164). Commonly, this system of degradation is referred to as autophagy (191). The process of autophagy is critical for the turnover of damaged cellular components and debris and is activated in response to a host of stimuli (191). Within the umbrella of autophagy are three distinct types, known as macroautophagy (general autophagy), chaperone-mediated autophagy, and microautophagy (72). The process of autophagy is a conserved cellular process that is essential for degrading protein aggregates and damaged organelles, such as mitochondria, and has been reviewed extensively elsewhere (72, 141, 163, 212).
Skeletal muscle autophagy has become a very active area of investigation in cancer cachexia and is quickly becoming an intriguing target for researchers (141). LC3 and p62 protein expression were elevated in the IL-6-dependent C26 and Min models of cachexia, and serum from cancer patients containing elevated IL-6 induced these proteins in vitro (143, 187, 188, 200). Many researchers believe that cancer cachexia is an energy balance disorder that is linked to inflammation. Recent evidence also suggests that inflammation may be a critical driver of cachexia-induced autophagy (8, 143, 146). The IL-6 signaling pathway has been deemed a powerful regulator of systemic inflammation during cancer cachexia (82, 85, 152). Recently, a role for IL-6 has been suggested in the regulation of autophagy; serum collected from cachectic cancer patients containing elevated IL-6 was incubated on C2C12 myotubes, which induced p62 and LC3 (143). Previous research has also demonstrated that the administration of an IL-6 receptor antibody to Min mice after the initiation of cachexia suppressed skeletal muscle AMP-activated protein kinase (AMPK) phosphorylation and autophagy signaling (201, 203). Skeletal muscle biopsies taken from cachectic cancer patients present with elevated mRNA for Beclin-1 (BCL-2-interacting protein, critical for autophagosome formation) and protein expression of LC3II and p62 (8, 41). Collectively, these results suggest that the LPS system plays a contributing role in protein degradation during cachexia; however, the exact mechanisms of how this occurs remain to be elucidated.
A major area of autophagy investigation pertains to efficiency or the ability of the lysosome to clear the autophagosome and minimize accumulation. To improve the efficiency of autophagy and improve autophagic clearance, researchers have turned to exercise as their intervention of choice. Researchers have been utilizing exercise as a way to counteract cachexia-induced muscle-mass loss for some time (146, 170, 191, 210). Exercise is a potent regulator of autophagy; with the induction of acute energy stress through the activation of AMPK signaling, the autophagy cascade is initiated. Whereas acute exercise has been demonstrated to induce autophagy, other studies have demonstrated the ability to improve autophagosome clearance through training (110, 146, 170, 191, 210). Treatments utilizing electrical stimulation and aerobic exercise have been shown to reduce muscle-mass loss and improve indices of autophagy during the progression of cachexia in tumor-bearing mice (84, 86, 146).
Skeletal Muscle Protein Synthesis During the Development and Progression of Cancer Cachexia
A strongly debated issue among cachexia researchers is the degree to which protein synthesis is suppressed (75, 180). Inflammation, hypogonadism, and insulin resistance are systemic perturbations associated with cancer that can disrupt protein metabolism and proteostasis during cancer cachexia (134, 194, 202). Some researchers suggest that although protein synthesis is suppressed in cancer patients, it may only be a contributing factor and not the total solution to restoring mass (130).
Suppressed skeletal muscle protein synthesis in preclinical animal models of cachexia has been reported in multiple studies using various tumor models (25, 34, 86, 188, 201). Despite the consensus agreement that protein synthesis is suppressed, an exact mechanism of regulation still escapes the field (17). Recently, some researchers have suggested that dysfunctional oxidative metabolism is to blame, due to a lack of sufficient ATP production and the chronic activation of AMPK, which serves to suppress mTOR complex 1 (mTORC1) signaling (100, 201). Another potential mechanism growing in popularity is the concept of anabolic resistance (a lack of sensitivity to nutrients or feeding), which may contribute to the simple fact that feeding is not sufficient to reverse or attenuate muscle-mass loss during cachexia (44, 66). Interestingly, the ideas of decreased physical activity and inflammation have also been shown to regulate protein synthesis during the development and progression of cancer cachexia in Min mice (86, 188, 214). The decrease of physical activity in Min mice has been reported actually to precede the initiation of body weight loss (10). Overall, there are multiple lines of evidence, each demonstrating an effect on muscle protein synthesis during cancer cachexia. The majority of literature suggests that altered protein synthesis is an event that occurs in the latter stages of cachexia and may even be influenced by cancer type (34, 35, 86, 188, 201). It is important to note that many of these previous studies have utilized various methods of protein synthesis measurement, such as puromycin incorporation and flooding dose of isotopic tracers. Whereas these methods have their respective uses, recent advancements in deuterium oxide labeling may provide the opportunity for a more reliable assessment of basal protein synthesis over the course of a full day rather than time-point measures with flooding dose techniques. Furthermore, many of these studies either do not account for or utilize various fasting paradigms to control for feeding, which can directly influence the results.
Inactivity—A Role for Exercise Training
Many cancer patients suffer from chronic fatigue, either from the disease itself or its treatment, which is often a confounder that limits regular exercise practice (75). This lack of physical activity or exercise has been demonstrated to occur in both the clinic and preclinical models (75, 155). Preclinical models have demonstrated that a reduction in voluntary activity actually precedes weight loss, further establishing its importance in the development of the condition (10). Voluntary muscle strength and exercise capacity are also found to be reduced in preclinical models and in human cancer patients after the onset of cachexia (71, 176). Muscle contractile properties related to fatigue resistance are also disrupted during experimental cancer cachexia in the tibialis anterior (195). However, our mechanistic understanding of the role of exercise training to counteract these deficits and stimulate protein synthesis is not well described.
The field of exercise oncology is limited by a lack of studies that examine cachectic cancer patients. However, initial progress has been made to improve our understanding of exercise adaptations during the development and progression of cachexia in preclinical models (76, 86, 125). Aerobic treadmill training, either alone or with nutritional supplementation, has been demonstrated to reduce tumor growth and improve gastrocnemius muscle mass in the Min model of cancer cachexia (9). Furthermore, a role for aerobic exercise treadmill training during systemic IL-6 overexpression has been shown to enhance mTORC1 signaling and improve mitochondrial quality control in Min mice (152). The improvement of mitochondrial quality control by exercise can lead to a reduction in the activation of AMPK and improve the ability to activate mTORC1 and stimulate protein synthesis (85, 86). Resistance exercise using overload hypertrophy models and electrical stimulation has also demonstrated attenuated or preserved muscle mass (85, 86). Additionally, repeated high-frequency electrical stimulation is capable of inducing tibialis anterior myofiber hypertrophy in cachectic skeletal muscle (86). Finally, this eccentric contraction training paradigm suppressed the cachexia induction of AMPK phosphorylation and robustly stimulated mTORC1 signaling and protein synthesis when compared with the contralateral control leg (86). These findings further coincide with improved tibialis anterior muscle oxidative capacity in tumor-bearing mice (86). Whether these alterations could improve basal protein synthesis long term remains to be further investigated. Collectively, these studies suggest a role for exercise training to improve skeletal muscle mass during cancer cachexia. Further studies are required to determine exact mechanisms and if an interaction between feeding and exercise could provide beneficial effects on protein synthesis and aid in the treatment of cachectic cancer patients.
INFLAMMATORY SIGNALING IN THE DEVELOPMENT AND PROGRESSION OF CANCER CACHEXIA
Inflammatory signaling has become a very active area of investigation in cancer cachexia over the past two decades (134, 139, 214). Chronic inflammation has become a hallmark of cancer cachexia in both clinical patients and preclinical rodent models. Numerous preclinical mouse models of cachexia have demonstrated elevated circulating inflammatory cytokines, such as IL-6 and TNF-α (originally called cachectin), coinciding with the initiation of muscle-mass loss (134, 139, 214). IL-6 is a pleiotropic cytokine that has emerged as a critical regulator of skeletal muscle mass and fat wasting during cancer cachexia (69). IL-6 signals through the ubiquitously expressed glycoprotein 130 (gp130) (54). IL-6 has been extensively investigated in recent years and demonstrated to play a role in the regulation of both muscle protein synthesis and degradation (42, 85, 143, 151, 152, 199, 201, 203). Elevated circulating IL-6 can be observed in cachectic cancer patients upon the initiation of cachexia and is strongly correlated to body weight and muscle-mass loss (103).
Increased circulating IL-6 has been clearly demonstrated to activate immediate downstream target STAT3, as well as ERK (196) and AMPK signaling (30, 69, 85). IL-6 is induced early in the development of cachexia and is exacerbated as the disease progresses (30, 82, 85, 89, 152, 199, 201). The activation of these signaling pathways by IL-6 in tumor-bearing mice has been demonstrated to induce muscle-mass loss through the suppression of muscle protein synthesis (mTORC1) signaling and induction of muscle protein breakdown in the Min mouse model of cancer cachexia (201). This imbalance disrupts muscle proteostasis (balance between synthesis and degradation) and leads to not only decreased muscle mass but also a suppression of skeletal muscle function and metabolism (79, 134, 143, 173, 201). Overexpression of IL-6 accelerates cancer cachexia-induced skeletal muscle wasting in Min mice (201, 203). Additionally, the chronically elevated IL-6 has also been demonstrated to suppress basal protein synthesis signaling in tumor and non-tumor-bearing mice (85). Interestingly, this suppression of protein synthesis in non-tumor-bearing mice occurred independent of muscle-mass loss (85).
Pharmacological inhibition targeting IL-6 and its downstream signaling targets gp130, STAT3, and ERK has demonstrated an attenuation of skeletal muscle-mass loss; however, it was not sufficient to prevent cachexia completely (7, 30, 214). Recently, a role for the IL-6 signaling pathway has also been described in the regulation of muscle oxidative metabolism and mitochondrial quality control during cancer cachexia (194, 203). IL-6 is also capable of disrupting mitochondrial remodeling and suppressing mitochondrial biogenesis in tumor and non-tumor-bearing mice alike (152, 203). These findings suggest that there may be a link between muscle protein turnover and the muscle oxidative metabolism in the net regulation of muscle mass via IL-6-mediated inflammation. With many studies reporting the inflammation disruption of mitochondria, it is interesting to theorize whether the disrupted mitochondrial function is a consequence of decreased skeletal muscle protein synthesis or a driver of this process.
Furthermore, in human cancer cachexia studies, elevated serum IL-6 is consistently associated with weight loss and a reduced survival rate in various types of cancer patients, especially in those presenting with pancreatic and colorectal cancer (18). Clinical trials of IL-6 antibody therapies in cancer patients have yielded some promising results (130). A clinical trial of ALD518 (anti-IL-6 antibody originally developed to treat rheumatoid arthritis) in patients with non-small cell lung cancer showed that this treatment has the potential to improve anemia and reduce cachexia-related fatigue, while having minimal adverse effects (18, 116). Additionally, other IL-6-targeted therapies, such as Selumetinib, have also been shown to be well tolerated while improving fatigue and attenuating the loss of lean body mass (149). Whereas IL-6 has been studied extensively as a potential driver of cancer cachexia, there appears to be no definitive answer as to how it elicits such a detrimental effect on skeletal muscle. Further research is warranted to examine the IL-6 signaling pathway to establish further a concrete mechanism of action.
TNF-α is another proinflammatory cytokine commonly elevated in several preclinical models of cancer cachexia, and pharmacological inhibition of this cytokine demonstrated a reduction in weight loss due to cancer (40). However, TNF-α (like IL-6) appears to be only a major player in certain preclinical models, such as the Yoshida hepatoma and the LLC model of cancer cachexia, which are commonly viewed as IL-6-independent models (11). In humans, several studies have demonstrated correlations of elevated TNF-α serum levels with degree of cachexia (96). Pancreatic cancer patients often exhibit elevated serum TNF-α, which is inversely correlated with BMI, hematocrit, hemoglobin, and serum albumin levels (133, 145). Additionally, it has been shown that the expression of the TNF-α gene was elevated in pancreatic cancer patients and was normalized following surgical resection (4). Recently, TNF-α and ubiquitin were shown to be upregulated in the skeletal muscle of 102 gastric cancer patients and were strongly correlated to disease stage and degree of weight loss. Collectively, these findings suggest that the TNF-α is a potent inducer of weight and muscle-mass loss in both preclinical models and human patients during cancer cachexia (180). The amount of TNF-α appears to be directly related to the severity and degree of muscle wasting that occurs during cancer cachexia.
The TGF-β family of cytokines is a superfamily that consists of 34 different proteins that are capable of regulating multiple signaling pathways and cellular processes (77, 80). Activin A, TNF-like weak inducer of apoptosis (TWEAK), and myostatin are all members of this family and bind either to type I or type II activin receptors in skeletal muscle (122). Upon binding, these cytokines can activate the SMA mothers against decapentaplegic (SMAD) signaling proteins (60). These proteins are capable of regulating multiple catabolic signaling processes, such as the activation of the UPS and suppression of protein kinase B (AKT) (80). Activin A, in particular, has even been demonstrated to induce muscle wasting in non-tumor-bearing mice (43). Treatment of mice with a specific activin A antibody was able to prevent cachexia and death (122). Whereas the evidence for TGF-β signaling as a regulator of muscle mass in mouse models has shown great promise, studies that have examined this cytokine in human cancer patients are scarce.
OXIDATIVE AND MITOCHONDRIAL STRESS DURING CANCER CACHEXIA
Within the last decade, the influence of mitochondrial health and function in relation to various pathologies has become a prominent research topic. This is also true in the cancer cachexia literature. Broadly, mitochondrial health encompasses multiple aspects, including ATP production/respiratory function, reactive oxygen species (ROS) production, and mitochondrial quality-control mechanisms (encompassing mitochondrial biogenesis, fusion and fission, and mitophagy). These processes have been reviewed elsewhere (160, 177, 207), but broadly speaking, all of these processes can dramatically influence overall cellular health.
Mitochondrial Aberrations During Cachexia Development and Progression
Whereas the scientific literature has established mitochondrial aberrations after cachexia has been initiated (discussed below), relatively few works have specifically focused on alterations to mitochondrial function and quality during the initial development of cachexia. However, recent works have begun to establish the interplay between mitochondrial biogenesis and muscle differentiation. For example, recent work has established that myoblast determination protein 1 (MyoD) and peroxisome proliferator-activated receptor γ coactivator 1- β (PGC-1β) are tightly coordinated to facilitate muscle differentiation by increasing the oxidative capacity to meet the energetic needs of differentiating muscles (172). This tight regulation is also seen in myogenesis with a shift from the glycolytic to oxidative metabolism (101). As such, deteriorations in oxidative capacity are highly likely to influence muscle size and quality. More so, mitochondrial stress, such as a discoordination between ATP production and electron donor availability, can result in the generation of ROS, which have also been shown to influence muscle size and health (35, 62, 104, 197). As such, it is plausible that deteriorations in mitochondrial function and subsequent maladies may influence overall muscle size during the initial development and progression of cancer cachexia.
In vitro work has provided foundational data establishing the interplay of mitochondrial stress and muscle aberrations in the development of muscle atrophy. Early work examining inflammatory signaling in relation to metabolic alterations has found that 2 h of TNF-α incubation on 3T3-L1 fibroblasts dramatically increases lipolysis and decreases lipogenesis (97). As substrate oxidation for the generation of ATP is the primary role of the mitochondria, these works provide some of the initial evidence suggesting that mitochondrial aberrations may be the initiating factor for deteriorations in cellular health. Similar findings have also been found using RXF kidney cancer conditioned media on human primary myotubes, whereby 1 h of RXF media incubation resulted in an ~50–100% increase in fatty acid oxidation and ~50–400% increase in ROS generation (67). Importantly, 1 h of incubation with RXF media increased measures of mitochondrial fatty acid oxidation, but alterations in other metabolic processes did not occur until after the aberrations to mitochondrial oxidation (67).
Similar findings have also been found using other cancer media incubations, with 4 h of LLC media incubation resulting in increased mitochondrial oxidative stress measured by MitoSox fluorescent dye (123). This stress was then followed by a progressive decrease in basal respiration, ATP-related oxygen consumption, and oxidative mitochondrial proteins (123). We have seen similar results in our laboratory, with increased mitochondrial polarization (suggesting increased mitochondrial activity) preceding mitochondrial stress in myoblasts cultured with LLC media (unpublished observations) (158). Importantly, these aberrations appear conserved across mammalian species, as this same LLC treatment in human myoblasts provided results in decreased maximal respiration and reserve capacity, although no effect on total oxygen consumption was noted (162). Additionally, LLC media in those same myoblasts disrupted mitochondrial morphology (162). Taken together, these in vitro works provide mechanistic evidence to the role of mitochondrial aberrations in the early phases of cancer cachexia development.
Works in animal models have begun to examine more thoroughly the temporal relationships between mitochondrial alterations and phenotypic alterations during the development of cancer cachexia. Our laboratory recently established that in the LLC model of cancer cachexia, mitochondrial degeneration (measured by mitochondrial oxidative stress-sensitive reporter gene pMitoTimer and ROS generation) precedes any significant phenotypic alterations to muscle size (35). Other works in additional models have found similar complementary results. For example, weight-stable Min mice (<5% of body weight loss) have significant decreases in mitochondrial fusion markers Mfn1 and Mfn2, as well as increased mitochondrial fission marker Fis1 (35, 199, 203). More so, adiponectin, a major mediator of lipolysis, is increased in the serum of rats injected with Walker tumor cells, which precedes any body weight loss (17), further suggesting that increased mitochondrial activity and subsequent stress may initiate phenotypic alterations to muscle health. Taken together, the current available literature suggests that increased mitochondrial respiration and subsequent ROS production may be among the initial aberrations during tumor development and contribute to the initial development of cancer cachexia.
Across many models of cancer cachexia, alterations in many aspects of mitochondrial quality are clearly present. For example, in our laboratory, we noted progressive decreases in oxidative capacity and mitochondrial content with increased cancer duration in LLC-tumor-bearing mice (35). This tumor progression also corresponds to alterations in oxidative phosphorylation and mitochondrial-related genes, with 4 weeks of tumor progression corresponding to decreases in mRNAs related to oxidative phosphorylation (28). Other works using the LLC model have also found intramuscular inoculation of LLC cells sufficient to result in decreased ATP synthesis, decreased TCA flux, and decreased mitochondrial coupling within 14 days of tumor injection (189). These alterations appeared to correspond with losses in muscle size (189). This same model of injection was also sufficient to alter lipid catabolism, as well as decrease defense mechanisms to superoxide and oxidative stress, such as SOD2, as well as increase uncoupling proteins (UCPs) (48). It should be noted that the generalizability of intramuscular injections of tumor agents likely represents a hyperphysiological state, as rarely are tumors initially developed in skeletal muscle, as it is generally considered postmitotic.
In addition to the LLC model of cancer cachexia, multiple other animal and human models have established mitochondrial deteriorations at minimum, coinciding with muscle wasting and poorer prognosis during cancer cachexia progression. In C26 models, decreased mitochondrial quality coincides with diaphragm fiber atrophy and decreased breathing capacity (154). Works using the Min mouse have found reduced mitochondrial quality control, aerobic metabolism, and aerobic capacity in mice with 6–19% body weight loss (10, 199, 203). These deteriorations are exacerbated with greater weight loss (10, 199, 203). However, these alterations appear to coincide with weight loss, as no differences were noted in mice with <5% body weight loss (10). Finally, in rats treated with Toshida AH-130 cancer cells, measures of mitochondrial oxidative stress and oxidative stress markers [total 4-hydroxynonenal (HNE) protein and 3-nitrotyrosine] corresponded to reduced body and muscle weights following 7 days of tumor growth (12). Overall, the animal literature strongly suggests that mitochondrial quality continually decreases during the progression of weight loss during cancer cachexia. Importantly, these findings have been extended into human models of cancer cachexia. In cachectic humans with adenocarcinoma, UCP-3 mRNA is increased (46). This result appears specific to cachexia, as weight-stable patients with adenocarcinoma had similar amounts of UCP-3 compared with controls without any cancer (46).
Mitochondrial Dysfunction in Relation to Protein Synthesis
These mitochondrial aberrations in both the development and progression of cancer cachexia likely have significant implications for the maintenance of muscle mass. Protein synthesis and associated kinase activity are energy-demanding processes accounting for ~20–30% of mammalian ATP consumption during basal resting conditions (102, 156). More so, ATP synthesis and mitochondrial respiration are tightly linked processes (37). Specifically, decreases in mitochondrial quality control have been associated with increased AMPK activation (157). AMPK regulates many different metabolic processes, including protein synthesis and degradation, primarily through phosphorylation of signaling intermediates TSC2 and Raptor (100, 171). TSC2 phosphorylation maintains Rheb in the GDP-bound inactive state, effectively inhibiting mTORC1 signaling (81, 171). Altered mitochondrial function and subsequent ATP production can activate the AMPK signaling cascade, suppressing the metabolically expensive process of protein synthesis through mTORC1 inhibition (157, 199).
Taken together in the aggregate, the current basic science and emerging clinical literature demonstrate that mitochondrial and oxidative metabolism alterations occur over the development and progression of cancer cachexia. These deteriorations and associated metabolic stress appear also to coincide with the degree of weight loss, which may suggest mitochondrial quality as a potential therapeutic target for the treatment of cancer cachexia.
Interventions to Restore Mitochondrial Quality
Due to the abundance of research suggesting increased ROS production and subsequent mitochondrial inefficiency (94, 118), it has recently been suggested that interventions to improve mitochondrial quality and/or antioxidant capacities may help mitigate maladies to muscle size. Interventions known to improve mitochondrial quality, such as exercise, have already begun to be investigated as treatments for mitigating cancer-associated cachexia and have shown promising outcomes (9, 10, 68, 151). However, as exercise participation may not necessarily be feasible for all patients, other interventions have also been investigated, such as artificially increasing mitochondrial content and antioxidant supplementation, although results have overall been less promising. In a study using female mice overexpressing PGC-1α through the muscle creatine kinase (MCK) promoter in the skeletal muscle and the LLC model, no protective effect of increased mitochondrial content was noted (198). MCK-PGC-1α animals had increased body weight compared with controls, but this may have been attributed to larger tumors, as muscle weight was comparable across groups, and this was despite increased mitochondria content and greater TCA activity (198). As PGC-1α expression primarily serves to enhance mitochondrial content, this may suggest that targeting of mitochondrial content itself could be insufficient to alleviate cachexia. Additionally, recent work has strongly suggested that an increase of global antioxidant consumption does not mitigate cachexia and may, in fact, accelerate the progression of the cancer (5). Other works using dehydroepiandrosterone (DHEA) have been found to reduce ROS in the gastrocnemius muscle; however, this was insufficient to maintain muscle mass in tumor-bearing mice (121). To our knowledge, two studies have found pharmacological interventions to restore mitochondrial aberrations and maintain muscle quality and morphology (67, 162). Therefore, whereas current literature strongly suggests that mitochondrial alterations may underlie initial development and progression of muscle wasting, more work remains to find efficacious treatments for these deteriorations and associated morbidities.
EMERGING AREAS OF RESEARCH AND OTHER CONSIDERATIONS
In addition to cancer-induced cachexia, recent works have clearly demonstrated that chemotherapy itself is sufficient to induce muscle wasting (15, 33, 44, 78, 111). Due to ethical considerations regarding the administration of chemotherapy to healthy human participants, most of our knowledge on chemotherapy-induced muscle wasting is in preclinical murine models. However, prior works have found that using Folfiri (a common chemotherapy cocktail of 5-fluorouracil, leucovorin, and CPT-11) itself is sufficient to reduce muscle weights compared with nontreated controls (15, 111). In studies using other chemotherapeutic agents, similar results are found (33, 44, 78). More so, C26 and Folfiri activate similar degradative pathways in skeletal muscle (14, 15); however, these deteriorations to muscle size may not be additive in animals inoculated with both C26 and Folfiri (147). Due to variations in an experimental time frame and/or tumor burden in early experiments on these combinatory effects, further work on the combinatory condition of the tumor-bearing state with chemotherapeutic regimens is required to understand truly the interactions of these conditions. Regardless, as cancer rarely occurs in a vacuum, future works should incorporate chemotherapy treatment in experimental designs to recapitulate more appropriately cancer cachexia in humans.
Recently, the scientific community has begun investigations into nonmuscle tissue contributions to the development and progression in cancer cachexia. For example, within a few hours of RXF conditioned media, the fatty acid metabolism is dramatically altered, strongly suggesting that the adipose tissue metabolism may have significant implications for the development of cancer cachexia (67, 98). These alterations to the fatty acid metabolism appear to correspond to alterations in overall adipose tissue phenotype. For example, works in murine and human models have established that cancer cachexia induces alterations in the plasticity and morphology of adipose tissue with increased adipose tissue fibrosis, increased collagen I/III ratios, and decreased markers for adipogenesis (1, 16, 26). Importantly, adipose tissue deteriorations appear to occur early in cachexia development, with 7 days of tumor induction in Walker-256-inoculated rats, resulting in significant increases in adipose tissue fibrosis (66), overall, suggesting that adipose tissue fibrosis may be an important consideration for preventing cachexia development. More so, prior works have reported alterations to hepatic metabolism and function during the progression and development of cachexia (83, 93, 135, 136, 159). which may implicate hepatic dysfunction as a contributor to muscle atrophy during cancer progression. Extensive reviews on this particular topic have been described elsewhere (3, 192). Finally, bone physiology and loss have been investigated as contributing factors in the development of cancer cachexia (31). In aggregate, the emerging literature strongly suggests that cancer cachexia development is multifactorial, and future research should continue to investigate these muscle-tissue interactions and how they may be optimized for therapeutic interventions for the treatment of cancer cachexia.
Additionally, a recent concern regarding cancer cachexia literature is the primary use of male specimens, including both male mice and immortalized cell lines derived from male species (5, 10, 12, 17, 48, 56, 67, 74). (Please note that this is not meant to be an exhaustive list of references but a sampling of the discrepancy in biomedical research using male and female models.) Recent works have begun to establish abundant differences between males and females of many physiological processes, including cardiovascular and neurological processes (119, 128, 153). These are only a few of the processes where published sex differences appear in the literature. Importantly, emerging evidence is beginning to show that cachexia presents differently in female models of cachexia. For example, Hetzler et al. (89) have found that cachexia in female Min mice is not IL-6 dependent, a dramatic shift from years of research in the model of Min mice (84, 134, 151, 199, 201, 203). Females appear to exhibit less body and muscle loss compared with males (89), as well as different responses to tumor development. For example, recent work has shown that females with halted estrous cycles exhibit body and muscle weight loss during cancer development, whereas females with functional estrous cycles maintain body weight and have reduced tumor burden (88, 89). Additionally, some literature has found improved outcomes of increasing mitochondrial content on muscle size in male mice (166), whereas studies in female mice have not necessarily had similar findings (198). Taken together, these data clearly implicate the role of sex in divergent responses to cachexia development and progression in animal models and warrant further exploration. The recent initiative by the National Institutes of Health to require the consideration of biological sex should help facilitate more research conducted in female models and allow for a more thorough understanding of the development and progression of cancer cachexia in both biological sexes and critical similarities and differences therein.
CONCLUSIONS
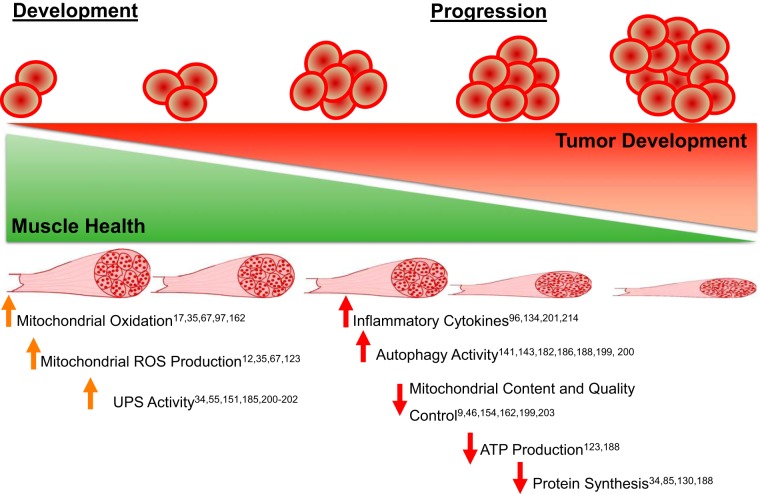
Cancer-associated muscle and body weight loss is a debilitating condition affecting thousands of cancer patients. Importantly, the development of this pathology is strongly tied to cancer-associated morbidity and mortality and therefore is a highly significant research line for “bench-top” scientists and clinical practitioners. The initial development of cancer cachexia is a multifaceted process that inevitably results in decreases to the muscle protein synthesis/degradation ratio. Emerging evidence is beginning to suggest that the mitochondria and its associated stress response may underlie some of the initial muscle pathologies that eventually lead to muscle loss and subsequent mortality (Fig. 1). However, more research investigating mechanisms in the development and progression of cancer cachexia using multiple models of cancer in both males and females is necessary to understand fully the temporal alterations that eventually lead to the development and progression of cancer cachexia.
Fig. 1.
A pictorial description of the current literature of cellular alterations during the development and progression of cancer cachexia. ROS, reactive oxygen species; UPS, ubiquitin proteasome system.
GRANTS
Support for this work was funded by the National Institute of Arthritis and Musculoskeletal and Skin Diseases (Grant R15 AR069913/AR/NIAMS).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
N.P.G. conceived and designed research; M.E.R.-C. and N.P.G. prepared figures; M.E.R.-C., D.K.F., T.A.W., and N.P.G. drafted manuscript; M.E.R.-C., D.K.F., T.A.W., and N.P.G. edited and revised manuscript; M.E.R.-C., D.K.F., T.A.W., and N.P.G. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank the many faculty, staff, and students at the Exercise Science Research Center for their consistent support of our research.
REFERENCES
- 1.Alves MJ, Figuerêdo RG, Azevedo FF, Cavallaro DA, Neto NI, Lima JD, Matos-Neto E, Radloff K, Riccardi DM, Camargo RG, De Alcântara PS, Otoch JP, Junior ML, Seelaender M. Adipose tissue fibrosis in human cancer cachexia: the role of TGFβ pathway. BMC Cancer 17: 190, 2017. doi: 10.1186/s12885-017-3178-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arends J, Bachmann P, Baracos V, Barthelemy N, Bertz H, Bozzetti F, Fearon K, Hütterer E, Isenring E, Kaasa S, Krznaric Z, Laird B, Larsson M, Laviano A, Mühlebach S, Muscaritoli M, Oldervoll L, Ravasco P, Solheim T, Strasser F, de van der Schueren M, Preiser JC. ESPEN guidelines on nutrition in cancer patients. Clin Nutr 36: 11–48, 2017. doi: 10.1016/j.clnu.2016.07.015. [DOI] [PubMed] [Google Scholar]
- 3.Argilés JM, Stemmler B, López-Soriano FJ, Busquets S. Nonmuscle tissues contribution to cancer cachexia. Mediators Inflamm 2015: 1–9, 2015. doi: 10.1155/2015/182872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ariapart P, Bergstedt-Lindqvist S, van Harmelen V, Permert J, Wang F, Lundkvist I. Resection of pancreatic cancer normalizes the preoperative increase of tumor necrosis factor alpha gene expression. Pancreatology 2: 491–494, 2002. doi: 10.1159/000064719. [DOI] [PubMed] [Google Scholar]
- 5.Assi M, Derbré F, Lefeuvre-Orfila L, Rébillard A. Antioxidant supplementation accelerates cachexia development by promoting tumor growth in C26 tumor-bearing mice. Free Radic Biol Med 91: 204–214, 2016. doi: 10.1016/j.freeradbiomed.2015.12.019. [DOI] [PubMed] [Google Scholar]
- 6.Attaix D, Combaret L, Tilignac T, Taillandier D. Adaptation of the ubiquitin-proteasome proteolytic pathway in cancer cachexia. Mol Biol Rep 26: 77–82, 1999. doi: 10.1023/A:1006961919775. [DOI] [PubMed] [Google Scholar]
- 7.Au ED, Desai AP, Koniaris LG, Zimmers TA. The MEK-inhibitor selumetinib attenuates tumor growth and reduces IL-6 expression but does not protect against muscle wasting in lewis lung cancer cachexia. Front Physiol 7: 682, 2017. doi: 10.3389/fphys.2016.00682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aversa Z, Pin F, Lucia S, Penna F, Verzaro R, Fazi M, Colasante G, Tirone A, Rossi Fanelli F, Ramaccini C, Costelli P, Muscaritoli M. Autophagy is induced in the skeletal muscle of cachectic cancer patients. Sci Rep 6: 30340, 2016. doi: 10.1038/srep30340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baltgalvis KA, Berger FG, Peña MM, Davis JM, Carson JA. Effect of exercise on biological pathways in ApcMin/+ mouse intestinal polyps. J Appl Physiol (1985) 104: 1137–1143, 2008. doi: 10.1152/japplphysiol.00955.2007. [DOI] [PubMed] [Google Scholar]
- 10.Baltgalvis KA, Berger FG, Peña MM, Mark Davis J, White JP, Carson JA. Activity level, apoptosis, and development of cachexia in Apc(Min/+) mice. J Appl Physiol (1985) 109: 1155–1161, 2010. doi: 10.1152/japplphysiol.00442.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baracos VE. Regulation of skeletal-muscle-protein turnover in cancer-associated cachexia. Nutrition 16: 1015–1018, 2000. doi: 10.1016/S0899-9007(00)00407-X. [DOI] [PubMed] [Google Scholar]
- 12.Barreiro E, de la Puente B, Busquets S, López-Soriano FJ, Gea J, Argilés JM. Both oxidative and nitrosative stress are associated with muscle wasting in tumour-bearing rats. FEBS Lett 579: 1646–1652, 2005. doi: 10.1016/j.febslet.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 13.Barret M, Antoun S, Dalban C, Malka D, Mansourbakht T, Zaanan A, Latko E, Taieb J. Sarcopenia is linked to treatment toxicity in patients with metastatic colorectal cancer. Nutr Cancer 66: 583–589, 2014. doi: 10.1080/01635581.2014.894103. [DOI] [PubMed] [Google Scholar]
- 14.Barreto R, Mandili G, Witzmann FA, Novelli F, Zimmers TA, Bonetto A. Cancer and chemotherapy contribute to muscle loss by activating common signaling pathways. Front Physiol 7: 472, 2016. doi: 10.3389/fphys.2016.00472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barreto R, Waning DL, Gao H, Liu Y, Zimmers TA, Bonetto A. Chemotherapy-related cachexia is associated with mitochondrial depletion and the activation of ERK1/2 and p38 MAPKs. Oncotarget 7: 43442–43460, 2016. doi: 10.18632/oncotarget.9779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Batista ML Jr, Henriques FS, Neves RX, Olivan MR, Matos-Neto EM, Alcântara PS, Maximiano LF, Otoch JP, Alves MJ, Seelaender M. Cachexia-associated adipose tissue morphological rearrangement in gastrointestinal cancer patients. J Cachexia Sarcopenia Muscle 7: 37–47, 2016. doi: 10.1002/jcsm.12037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Batista ML Jr, Neves RX, Peres SB, Yamashita AS, Shida CS, Farmer SR, Seelaender M. Heterogeneous time-dependent response of adipose tissue during the development of cancer cachexia. J Endocrinol 215: 363–373, 2012. doi: 10.1530/JOE-12-0307. [DOI] [PubMed] [Google Scholar]
- 18.Bayliss TJ, Smith JT, Schuster M, Dragnev KH, Rigas JR. A humanized anti-IL-6 antibody (ALD518) in non-small cell lung cancer. Expert Opin Biol Ther 11: 1663–1668, 2011. doi: 10.1517/14712598.2011.627850. [DOI] [PubMed] [Google Scholar]
- 19.Bechet D, Tassa A, Taillandier D, Combaret L, Attaix D. Lysosomal proteolysis in skeletal muscle. Int J Biochem Cell Biol 37: 2098–2114, 2005. doi: 10.1016/j.biocel.2005.02.029. [DOI] [PubMed] [Google Scholar]
- 20.Beck SA, Tisdale MJ. Production of lipolytic and proteolytic factors by a murine tumor-producing cachexia in the host. Cancer Res 47: 5919–5923, 1987. [PubMed] [Google Scholar]
- 21.Belloum Y, Rannou-Bekono F, Favier FB. Cancer-induced cardiac cachexia: pathogenesis and impact of physical activity (Review) Oncol Rep 37: 2543–2552, 2017. doi: 10.3892/or.2017.5542. [DOI] [PubMed] [Google Scholar]
- 22.Bertram JS, Janik P. Establishment of a cloned line of Lewis lung carcinoma cells adapted to cell culture. Cancer Lett 11: 63–73, 1980. doi: 10.1016/0304-3835(80)90130-5. [DOI] [PubMed] [Google Scholar]
- 23.Bibby MC, Double JA, Ali SA, Fearon KC, Brennan RA, Tisdale MJ. Characterization of a transplantable adenocarcinoma of the mouse colon producing cachexia in recipient animals. J Natl Cancer Inst 78: 539–546, 1987. [PubMed] [Google Scholar]
- 24.Bilodeau PA, Coyne ES, Wing SS. The ubiquitin proteasome system in atrophying skeletal muscle: roles and regulation. Am J Physiol Cell Physiol 311: C392–C403, 2016. doi: 10.1152/ajpcell.00125.2016. [DOI] [PubMed] [Google Scholar]
- 25.Bing C, Brown M, King P, Collins P, Tisdale MJ, Williams G. Increased gene expression of brown fat uncoupling protein (UCP)1 and skeletal muscle UCP2 and UCP3 in MAC16-induced cancer cachexia. Cancer Res 60: 2405–2410, 2000. [PubMed] [Google Scholar]
- 26.Bing C, Russell S, Becket E, Pope M, Tisdale MJ, Trayhurn P, Jenkins JR. Adipose atrophy in cancer cachexia: morphologic and molecular analysis of adipose tissue in tumour-bearing mice. Br J Cancer 95: 1028–1037, 2006. doi: 10.1038/sj.bjc.6603360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bing C, Taylor S, Tisdale MJ, Williams G. Cachexia in MAC16 adenocarcinoma: suppression of hunger despite normal regulation of leptin, insulin and hypothalamic neuropeptide Y. J Neurochem 79: 1004–1012, 2001. doi: 10.1046/j.1471-4159.2001.00639.x. [DOI] [PubMed] [Google Scholar]
- 28.Blackwell TA, Cervenka I, Khatri B, Brown JL, Rosa-Caldwell ME, Lee DE, Perry RA Jr, Brown LA, Haynie WS, Wiggs MP, Bottje WG, Washington TA, Kong BC, Ruas JL, Greene NP. Transcriptomic analysis of the development of skeletal muscle atrophy in cancer-cachexia in tumor-bearing mice. Physiol Genomics 50: 1071–1082, 2018. doi: 10.1152/physiolgenomics.00061.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blum D, Omlin A, Baracos VE, Solheim TS, Tan BH, Stone P, Kaasa S, Fearon K, Strasser F; European Palliative Care Research Collaborative . Cancer cachexia: a systematic literature review of items and domains associated with involuntary weight loss in cancer. Crit Rev Oncol Hematol 80: 114–144, 2011. doi: 10.1016/j.critrevonc.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 30.Bonetto A, Aydogdu T, Jin X, Zhang Z, Zhan R, Puzis L, Koniaris LG, Zimmers TA. JAK/STAT3 pathway inhibition blocks skeletal muscle wasting downstream of IL-6 and in experimental cancer cachexia. Am J Physiol Endocrinol Metab 303: E410–E421, 2012. doi: 10.1152/ajpendo.00039.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bonetto A, Kays JK, Parker VA, Matthews RR, Barreto R, Puppa MJ, Kang KS, Carson JA, Guise TA, Mohammad KS, Robling AG, Couch ME, Koniaris LG, Zimmers TA. Differential bone loss in mouse models of colon cancer cachexia. Front Physiol 7: 679, 2017. doi: 10.3389/fphys.2016.00679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bowen TS, Adams V, Werner S, Fischer T, Vinke P, Brogger MN, Mangner N, Linke A, Sehr P, Lewis J, Labeit D, Gasch A, Labeit S. Small-molecule inhibition of MuRF1 attenuates skeletal muscle atrophy and dysfunction in cardiac cachexia. J Cachexia Sarcopenia Muscle 8: 939–953, 2017. doi: 10.1002/jcsm.12233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Braun TP, Szumowski M, Levasseur PR, Grossberg AJ, Zhu X, Agarwal A, Marks DL. Muscle atrophy in response to cytotoxic chemotherapy is dependent on intact glucocorticoid signaling in skeletal muscle. PLoS One 9: e106489, 2014. doi: 10.1371/journal.pone.0106489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brown JL, Lee DE, Rosa-Caldwell ME, Brown LA, Perry RA, Haynie WS, Huseman K, Sataranatarajan K, Van Remmen H, Washington TA, Wiggs MP, Greene NP. Protein imbalance in the development of skeletal muscle wasting in tumour-bearing mice. J Cachexia Sarcopenia Muscle 9: 987–1002, 2018. doi: 10.1002/jcsm.12354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brown JL, Rosa-Caldwell ME, Lee DE, Blackwell TA, Brown LA, Perry RA, Haynie WS, Hardee JP, Carson JA, Wiggs MP, Washington TA, Greene NP. Mitochondrial degeneration precedes the development of muscle atrophy in progression of cancer cachexia in tumour-bearing mice. J Cachexia Sarcopenia Muscle 8: 926–938, 2017. doi: 10.1002/jcsm.12232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Busquets S, García-Martínez C, Alvarez B, Carbó N, López-Soriano FJ, Argilés JM. Calpain-3 gene expression is decreased during experimental cancer cachexia. Biochim Biophys Acta 1475: 5–9, 2000. doi: 10.1016/S0304-4165(00)00050-7. [DOI] [PubMed] [Google Scholar]
- 37.Buttgereit F, Brand MD. A hierarchy of ATP-consuming processes in mammalian cells. Biochem J 312: 163–167, 1995. doi: 10.1042/bj3120163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carbó N, Costelli P, Tessitore L, Bagby GJ, López-Soriano FJ, Baccino FM, Argilés JM. Anti-tumour necrosis factor-alpha treatment interferes with changes in lipid metabolism in a tumour cachexia model. Clin Sci (Lond) 87: 349–355, 1994. doi: 10.1042/cs0870349. [DOI] [PubMed] [Google Scholar]
- 39.Carbó N, López-Soriano J, Tarragó T, González O, Llovera M, López-Soriano FJ, Argilés JM. Comparative effects of beta2-adrenergic agonists on muscle waste associated with tumour growth. Cancer Lett 115: 113–118, 1997. doi: 10.1016/S0304-3835(97)04718-6. [DOI] [PubMed] [Google Scholar]
- 40.Catalano MG, Fortunati N, Arena K, Costelli P, Aragno M, Danni O, Boccuzzi G. Selective up-regulation of tumor necrosis factor receptor I in tumor-bearing rats with cancer-related cachexia. Int J Oncol 23: 429–436, 2003. doi: 10.3892/ijo.23.2.429. [DOI] [PubMed] [Google Scholar]
- 41.Chacon-Cabrera A, Fermoselle C, Urtreger AJ, Mateu-Jimenez M, Diament MJ, de Kier Joffé ED, Sandri M, Barreiro E. Pharmacological strategies in lung cancer-induced cachexia: effects on muscle proteolysis, autophagy, structure, and weakness. J Cell Physiol 229: 1660–1672, 2014. doi: 10.1002/jcp.24611. [DOI] [PubMed] [Google Scholar]
- 42.Chen JL, Walton KL, Qian H, Colgan TD, Hagg A, Watt MJ, Harrison CA, Gregorevic P. Differential effects of IL6 and activin A in the development of cancer-associated cachexia. Cancer Res 76: 5372–5382, 2016. doi: 10.1158/0008-5472.CAN-15-3152. [DOI] [PubMed] [Google Scholar]
- 43.Chen JL, Walton KL, Winbanks CE, Murphy KT, Thomson RE, Makanji Y, Qian H, Lynch GS, Harrison CA, Gregorevic P. Elevated expression of activins promotes muscle wasting and cachexia. FASEB J 28: 1711–1723, 2014. doi: 10.1096/fj.13-245894. [DOI] [PubMed] [Google Scholar]
- 44.Chen MC, Hsu WL, Hwang PA, Chen YL, Chou TC. Combined administration of fucoidan ameliorates tumor and chemotherapy-induced skeletal muscle atrophy in bladder cancer-bearing mice. Oncotarget 7: 51608–51618, 2016. doi: 10.18632/oncotarget.9958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cohen S, Brault JJ, Gygi SP, Glass DJ, Valenzuela DM, Gartner C, Latres E, Goldberg AL. During muscle atrophy, thick, but not thin, filament components are degraded by MuRF1-dependent ubiquitylation. J Cell Biol 185: 1083–1095, 2009. doi: 10.1083/jcb.200901052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Collins P, Bing C, McCulloch P, Williams G. Muscle UCP-3 mRNA levels are elevated in weight loss associated with gastrointestinal adenocarcinoma in humans. Br J Cancer 86: 372–375, 2002. doi: 10.1038/sj.bjc.6600074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Combaret L, Rallière C, Taillandier D, Tanaka K, Attaix D. Manipulation of the ubiquitin-proteasome pathway in cachexia: pentoxifylline suppresses the activation of 20S and 26S proteasomes in muscles from tumor-bearing rats. Mol Biol Rep 26: 95–101, 1999. doi: 10.1023/A:1006955832323. [DOI] [PubMed] [Google Scholar]
- 48.Constantinou C, Fontes de Oliveira CC, Mintzopoulos D, Busquets S, He J, Kesarwani M, Mindrinos M, Rahme LG, Argilés JM, Tzika AA. Nuclear magnetic resonance in conjunction with functional genomics suggests mitochondrial dysfunction in a murine model of cancer cachexia. Int J Mol Med 27: 15–24, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cornwell EW, Mirbod A, Wu CL, Kandarian SC, Jackman RW. C26 cancer-induced muscle wasting is IKKβ-dependent and NF-kappaB-independent. PLoS One 9: e87776, 2014. doi: 10.1371/journal.pone.0087776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Costelli P, García-Martínez C, Llovera M, Carbó N, López-Soriano FJ, Agell N, Tessitore L, Baccino FM, Argilés JM. Muscle protein waste in tumor-bearing rats is effectively antagonized by a beta 2-adrenergic agonist (clenbuterol). Role of the ATP-ubiquitin-dependent proteolytic pathway. J Clin Invest 95: 2367–2372, 1995. doi: 10.1172/JCI117929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Costelli P, Llovera M, García-Martínez C, Carbó N, López-Soriano FJ, Argilés JM. Enhanced leucine oxidation in rats bearing an ascites hepatoma (Yoshida AH-130) and its reversal by clenbuterol. Cancer Lett 91: 73–78, 1995. doi: 10.1016/0304-3835(94)03719-Y. [DOI] [PubMed] [Google Scholar]
- 52.Costelli P, Muscaritoli M, Bossola M, Penna F, Reffo P, Bonetto A, Busquets S, Bonelli G, Lopez-Soriano FJ, Doglietto GB, Argilés JM, Baccino FM, Rossi Fanelli F. IGF-1 is downregulated in experimental cancer cachexia. Am J Physiol Regul Integr Comp Physiol 291: R674–R683, 2006. doi: 10.1152/ajpregu.00104.2006. [DOI] [PubMed] [Google Scholar]
- 53.Cowen DM, Double JA, Cowen PN. Some biological characteristics of transplantable lines of mouse adenocarcinomas of the colon. J Natl Cancer Inst 64: 675–681, 1980. [PubMed] [Google Scholar]
- 54.Cron L, Allen T, Febbraio MA. The role of gp130 receptor cytokines in the regulation of metabolic homeostasis. J Exp Biol 219: 259–265, 2016. doi: 10.1242/jeb.129213. [DOI] [PubMed] [Google Scholar]
- 55.DeJong CH, Busquets S, Moses AG, Schrauwen P, Ross JA, Argiles JM, Fearon KC. Systemic inflammation correlates with increased expression of skeletal muscle ubiquitin but not uncoupling proteins in cancer cachexia. Oncol Rep 14: 257–263, 2005. [PubMed] [Google Scholar]
- 56.Deminice R, Cella PS, Padilha CS, Borges FH, da Silva LE, Campos-Ferraz PL, Jordao AA, Robinson JL, Bertolo RF, Cecchini R, Guarnier FA. Creatine supplementation prevents hyperhomocysteinemia, oxidative stress and cancer-induced cachexia progression in Walker-256 tumor-bearing rats. Amino Acids 48: 2015–2024, 2016. doi: 10.1007/s00726-016-2172-9. [DOI] [PubMed] [Google Scholar]
- 57.Dessì S, Batetta B, Spano O, Bagby GJ, Tessitore L, Costelli P, Baccino FM, Pani P, Argilès JM. Perturbations of triglycerides but not of cholesterol metabolism are prevented by anti-tumour necrosis factor treatment in rats bearing an ascites hepatoma (Yoshida AH-130). Br J Cancer 72: 1138–1143, 1995. doi: 10.1038/bjc.1995.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dewys WD, Begg C, Lavin PT, Band PR, Bennett JM, Bertino JR, Cohen MH, Douglass HO Jr, Engstrom PF, Ezdinli EZ, Horton J, Johnson GJ, Moertel CG, Oken MM, Perlia C, Rosenbaum C, Silverstein MN, Skeel RT, Sponzo RW, Tormey DC; Eastern Cooperative Oncology Group . Prognostic effect of weight loss prior to chemotherapy in cancer patients. Am J Med 69: 491–497, 1980. doi: 10.1016/S0149-2918(05)80001-3. [DOI] [PubMed] [Google Scholar]
- 59.Double JA, Ball CR. Chemotherapy of transplanted adenocarcinomas of the colon in mice. Cancer Chemother Rep 59: 1083–1089, 1975. [PubMed] [Google Scholar]
- 60.Dschietzig TB. Myostatin - from the mighty mouse to cardiovascular disease and cachexia. Clin Chim Acta 433: 216–224, 2014. doi: 10.1016/j.cca.2014.03.021. [DOI] [PubMed] [Google Scholar]
- 61.Dworzak F, Ferrari P, Gavazzi C, Maiorana C, Bozzetti F. Effects of cachexia due to cancer on whole body and skeletal muscle protein turnover. Cancer 82: 42–48, 1998. doi:. [DOI] [PubMed] [Google Scholar]
- 62.Espinosa A, Henríquez-Olguín C, Jaimovich E. Reactive oxygen species and calcium signals in skeletal muscle: a crosstalk involved in both normal signaling and disease. Cell Calcium 60: 172–179, 2016. doi: 10.1016/j.ceca.2016.02.010. [DOI] [PubMed] [Google Scholar]
- 63.Evans WJ. Skeletal muscle loss: cachexia, sarcopenia, and inactivity. Am J Clin Nutr 91: 1123S–1127S, 2010. doi: 10.3945/ajcn.2010.28608A. [DOI] [PubMed] [Google Scholar]
- 64.Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, Jatoi A, Loprinzi C, MacDonald N, Mantovani G, Davis M, Muscaritoli M, Ottery F, Radbruch L, Ravasco P, Walsh D, Wilcock A, Kaasa S, Baracos VE. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol 12: 489–495, 2011. doi: 10.1016/S1470-2045(10)70218-7. [DOI] [PubMed] [Google Scholar]
- 65.Foley K, Rucki AA, Xiao Q, Zhou D, Leubner A, Mo G, Kleponis J, Wu AA, Sharma R, Jiang Q, Anders RA, Iacobuzio-Donahue CA, Hajjar KA, Maitra A, Jaffee EM, Zheng L. Semaphorin 3D autocrine signaling mediates the metastatic role of annexin A2 in pancreatic cancer. Sci Signal 8: ra77, 2015. doi: 10.1126/scisignal.aaa5823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Franco FO, Lopes MA, Henriques FS, Neves RX, Bianchi Filho C, Batista ML Jr. Cancer cachexia differentially regulates visceral adipose tissue turnover. J Endocrinol 232: 493–500, 2017. doi: 10.1530/JOE-16-0305. [DOI] [PubMed] [Google Scholar]
- 67.Fukawa T, Yan-Jiang BC, Min-Wen JC, Jun-Hao ET, Huang D, Qian CN, Ong P, Li Z, Chen S, Mak SY, Lim WJ, Kanayama HO, Mohan RE, Wang RR, Lai JH, Chua C, Ong HS, Tan KK, Ho YS, Tan IB, Teh BT, Shyh-Chang N. Excessive fatty acid oxidation induces muscle atrophy in cancer cachexia. Nat Med 22: 666–671, 2016. doi: 10.1038/nm.4093. [DOI] [PubMed] [Google Scholar]
- 68.Gao S, Carson JA. Lewis lung carcinoma regulation of mechanical stretch-induced protein synthesis in cultured myotubes. Am J Physiol Cell Physiol 310: C66–C79, 2016. doi: 10.1152/ajpcell.00052.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Garbers C, Aparicio-Siegmund S, Rose-John S. The IL-6/gp130/STAT3 signaling axis: recent advances towards specific inhibition. Curr Opin Immunol 34: 75–82, 2015. doi: 10.1016/j.coi.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 70.Gerber MH, Underwood PW, Judge SM, Delitto D, Delitto AE, Nosacka RL, DiVita BB, Thomas RM, Permuth JB, Hughes SJ, Wallet SM, Judge AR, Trevino JG. Local and systemic cytokine profiling for pancreatic ductal adenocarcinoma to study cancer cachexia in an era of precision medicine. Int J Mol Sci 19: 3836, 2018. doi: 10.3390/ijms19123836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Glaus A. Fatigue and cachexia in cancer patients. Support Care Cancer 6: 77–78, 1998. doi: 10.1007/s005200050137. [DOI] [PubMed] [Google Scholar]
- 72.Glick D, Barth S, Macleod KF. Autophagy: cellular and molecular mechanisms. J Pathol 221: 3–12, 2010. doi: 10.1002/path.2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Go KL, Delitto D, Judge SM, Gerber MH, George TJ Jr, Behrns KE, Hughes SJ, Judge AR, Trevino JG. Orthotopic patient-derived pancreatic cancer xenografts engraft into the pancreatic parenchyma, metastasize, and induce muscle wasting to recapitulate the human disease. Pancreas 46: 813–819, 2017. doi: 10.1097/MPA.0000000000000843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gorselink M, Vaessen SF, van der Flier LG, Leenders I, Kegler D, Caldenhoven E, van der Beek E, van Helvoort A. Mass-dependent decline of skeletal muscle function in cancer cachexia. Muscle Nerve 33: 691–693, 2006. doi: 10.1002/mus.20467. [DOI] [PubMed] [Google Scholar]
- 75.Grande AJ, Silva V, Maddocks M. Exercise for cancer cachexia in adults: executive summary of a Cochrane collaboration systematic review. J Cachexia Sarcopenia Muscle 6: 208–211, 2015. doi: 10.1002/jcsm.12055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Grande AJ, Silva V, Riera R, Medeiros A, Vitoriano SG, Peccin MS, Maddocks M. Exercise for cancer cachexia in adults. Cochrane Database Syst Rev (11): CD010804, 2014. doi: 10.1002/14651858.CD010804.pub2. [DOI] [PubMed] [Google Scholar]
- 77.Greco SH, Tomkötter L, Vahle AK, Rokosh R, Avanzi A, Mahmood SK, Deutsch M, Alothman S, Alqunaibit D, Ochi A, Zambirinis C, Mohaimin T, Rendon M, Levie E, Pansari M, Torres-Hernandez A, Daley D, Barilla R, Pachter HL, Tippens D, Malik H, Boutajangout A, Wisniewski T, Miller G. TGF-β blockade reduces mortality and metabolic changes in a validated murine model of pancreatic cancer cachexia. PLoS One 10: e0132786, 2015. doi: 10.1371/journal.pone.0132786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Guigni BA, Fix DK, Bivona JJ III, Palmer BM, Carson JA, Toth MJ. Electrical stimulation prevents doxorubicin-induced atrophy and mitochondrial loss in cultured myotubes. Am J Physiol Cell Physiol ajpcell.00148.2019, 2019. doi: 10.1152/ajpcell.00148.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Guo D, Wang C, Wang Q, Qiao Z, Tang H. Pantoprazole blocks the JAK2/STAT3 pathway to alleviate skeletal muscle wasting in cancer cachexia by inhibiting inflammatory response. Oncotarget 8: 39640–39648, 2017. doi: 10.18632/oncotarget.17387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Guttridge DC. A TGF-β pathway associated with cancer cachexia. Nat Med 21: 1248–1249, 2015. doi: 10.1038/nm.3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gwinn DM, Shackelford DB, Egan DF, Mihaylova MM, Mery A, Vasquez DS, Turk BE, Shaw RJ. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell 30: 214–226, 2008. doi: 10.1016/j.molcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Haddad F, Zaldivar F, Cooper DM, Adams GR. IL-6-induced skeletal muscle atrophy. J Appl Physiol (1985) 98: 911–917, 2005. doi: 10.1152/japplphysiol.01026.2004. [DOI] [PubMed] [Google Scholar]
- 83.Halle JL, Pena GS, Paez HG, Castro AJ, Rossiter HB, Visavadiya NP, Whitehurst MA, Khamoui AV. Tissue-specific dysregulation of mitochondrial respiratory capacity and coupling control in colon-26 tumor-induced cachexia. Am J Physiol Regul Integr Comp Physiol 317: R68–R82, 2019. doi: 10.1152/ajpregu.00028.2019. [DOI] [PubMed] [Google Scholar]
- 84.Hardee JP, Counts BR, Gao S, VanderVeen BN, Fix DK, Koh HJ, Carson JA. Inflammatory signalling regulates eccentric contraction-induced protein synthesis in cachectic skeletal muscle. J Cachexia Sarcopenia Muscle 9: 369–383, 2018. doi: 10.1002/jcsm.12271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hardee JP, Fix DK, Wang X, Goldsmith EC, Koh HJ, Carson JA. Systemic IL-6 regulation of eccentric contraction-induced muscle protein synthesis. Am J Physiol Cell Physiol 315: C91–C103, 2018. doi: 10.1152/ajpcell.00063.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hardee JP, Mangum JE, Gao S, Sato S, Hetzler KL, Puppa MJ, Fix DK, Carson JA. Eccentric contraction-induced myofiber growth in tumor-bearing mice. J Appl Physiol (1985) 120: 29–37, 2016. doi: 10.1152/japplphysiol.00416.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hendifar AE, Petzel MQB, Zimmers TA, Denlinger CS, Matrisian LM, Picozzi VJ, Rahib L; Precision Promise Consortium . Pancreas cancer-associated weight loss. Oncologist 24: 691–701, 2019. doi: 10.1634/theoncologist.2018-0266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hetzler KL, Hardee JP, LaVoie HA, Murphy EA, Carson JA. Ovarian function’s role during cancer cachexia progression in the female mouse. Am J Physiol Endocrinol Metab 312: E447–E459, 2017. doi: 10.1152/ajpendo.00294.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hetzler KL, Hardee JP, Puppa MJ, Narsale AA, Sato S, Davis JM, Carson JA. Sex differences in the relationship of IL-6 signaling to cancer cachexia progression. Biochim Biophys Acta 1852: 816–825, 2015. doi: 10.1016/j.bbadis.2014.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hingorani SR, Wang L, Multani AS, Combs C, Deramaudt TB, Hruban RH, Rustgi AK, Chang S, Tuveson DA. Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer Cell 7: 469–483, 2005. doi: 10.1016/j.ccr.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 91.Huillard O, Mir O, Peyromaure M, Tlemsani C, Giroux J, Boudou-Rouquette P, Ropert S, Delongchamps NB, Zerbib M, Goldwasser F. Sarcopenia and body mass index predict sunitinib-induced early dose-limiting toxicities in renal cancer patients. Br J Cancer 108: 1034–1041, 2013. doi: 10.1038/bjc.2013.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jiang K, Lawson D, Cohen C, Siddiqui MT. Galectin-3 and PTEN expression in pancreatic ductal adenocarcinoma, pancreatic neuroendocrine neoplasms and gastrointestinal tumors on fine-needle aspiration cytology. Acta Cytol 58: 281–287, 2014. doi: 10.1159/000362221. [DOI] [PubMed] [Google Scholar]
- 93.Jones A, Friedrich K, Rohm M, Schäfer M, Algire C, Kulozik P, Seibert O, Müller-Decker K, Sijmonsma T, Strzoda D, Sticht C, Gretz N, Dallinga-Thie GM, Leuchs B, Kögl M, Stremmel W, Diaz MB, Herzig S. TSC22D4 is a molecular output of hepatic wasting metabolism. EMBO Mol Med 5: 294–308, 2013. doi: 10.1002/emmm.201201869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kaludercic N, Giorgio V. The dual function of reactive oxygen/nitrogen species in bioenergetics and cell death: the role of ATP synthase. Oxid Med Cell Longev 2016: 1–17, 2016. doi: 10.1155/2016/3869610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kanclerz A, Wiebe LI, Luu K, Knaus EE. The influence of tumor stage and metastasis on the biodistribution of gallium-67 citrate in the murine Lewis lung carcinoma model. J Nucl Med 29: 1252–1258, 1988. [PubMed] [Google Scholar]
- 96.Karayiannakis AJ, Syrigos KN, Polychronidis A, Pitiakoudis M, Bounovas A, Simopoulos K. Serum levels of tumor necrosis factor-alpha and nutritional status in pancreatic cancer patients. Anticancer Res 21: 1355–1358, 2001. [PubMed] [Google Scholar]
- 97.Kawakami M, Ishibashi S, Ogawa H, Murase T, Takaku F, Shibata S. Cachectin/TNF as well as interleukin-1 induces prostacyclin synthesis in cultured vascular endothelial cells. Biochem Biophys Res Commun 141: 482–487, 1986. doi: 10.1016/S0006-291X(86)80198-X. [DOI] [PubMed] [Google Scholar]
- 98.Kays JK, Shahda S, Stanley M, Bell TM, O’Neill BH, Kohli MD, Couch ME, Koniaris LG, Zimmers TA. Three cachexia phenotypes and the impact of fat-only loss on survival in FOLFIRINOX therapy for pancreatic cancer. J Cachexia Sarcopenia Muscle 9: 673–684, 2018. doi: 10.1002/jcsm.12307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Khal J, Wyke SM, Russell ST, Hine AV, Tisdale MJ. Expression of the ubiquitin-proteasome pathway and muscle loss in experimental cancer cachexia. Br J Cancer 93: 774–780, 2005. doi: 10.1038/sj.bjc.6602780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Koh HJ, Brandauer J, Goodyear LJ. LKB1 and AMPK and the regulation of skeletal muscle metabolism. Curr Opin Clin Nutr Metab Care 11: 227–232, 2008. doi: 10.1097/MCO.0b013e3282fb7b76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kraft CS, LeMoine CM, Lyons CN, Michaud D, Mueller CR, Moyes CD. Control of mitochondrial biogenesis during myogenesis. Am J Physiol Cell Physiol 290: C1119–C1127, 2006. doi: 10.1152/ajpcell.00463.2005. [DOI] [PubMed] [Google Scholar]
- 102.Kressler D, Hurt E, Baβler J. Driving ribosome assembly. Biochim Biophys Acta 1803: 673–683, 2010. doi: 10.1016/j.bbamcr.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 103.Kuroda N, Mizobuchi M, Shimamura Y, Daibata M, Miyoshi I, Ohara M, Hirouchi T, Mizuno K, Lee GH. Bridging necrosis and reticulin bridging fibrosis induced by intrahepatic involvement of acute biphenotypic leukemia. APMIS 114: 908–911, 2006. doi: 10.1111/j.1600-0463.2006.apm_540.x. [DOI] [PubMed] [Google Scholar]
- 104.Laviano A, Meguid MM, Preziosa I, Rossi Fanelli F. Oxidative stress and wasting in cancer. Curr Opin Clin Nutr Metab Care 10: 449–456, 2007. doi: 10.1097/MCO.0b013e328122db94. [DOI] [PubMed] [Google Scholar]
- 105.Lazarus DD, Destree AT, Mazzola LM, McCormack TA, Dick LR, Xu B, Huang JQ, Pierce JW, Read MA, Coggins MB, Solomon V, Goldberg AL, Brand SJ, Elliott PJ. A new model of cancer cachexia: contribution of the ubiquitin-proteasome pathway. Am J Physiol 277: E332–E341, 1999. doi: 10.1152/ajpendo.1999.277.2.E332. [DOI] [PubMed] [Google Scholar]
- 106.Lee DE, Brown JL, Rosa-Caldwell ME, Blackwell TA, Perry RA Jr, Brown LA, Khatri B, Seo D, Bottje WG, Washington TA, Wiggs MP, Kong BW, Greene NP. Cancer cachexia-induced muscle atrophy: evidence for alterations in microRNAs important for muscle size. Physiol Genomics 49: 253–260, 2017. doi: 10.1152/physiolgenomics.00006.2017. [DOI] [PubMed] [Google Scholar]
- 107.Leitner LM, Wilson RJ, Yan Z, Gödecke A. Reactive oxygen species/nitric oxide mediated inter-organ communication in skeletal muscle wasting diseases. Antioxid Redox Signal 26: 700–717, 2017. doi: 10.1089/ars.2016.6942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Levolger S, Wiemer EAC, van Vugt JLA, Huisman SA, van Vledder MG, van Damme-van Engel S, Ambagtsheer G, IJzermans JNM, de Bruin RWF. Inhibition of activin-like kinase 4/5 attenuates cancer cachexia associated muscle wasting. Sci Rep 9: 9826, 2019. doi: 10.1038/s41598-019-46178-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lira FS, Yamashita AS, Rosa JC, Tavares FL, Caperuto E, Carnevali LC Jr, Pimentel GD, Santos RV, Batista ML Jr, Laviano A, Rossi-Fanelli F, Seelaender M. Hypothalamic inflammation is reversed by endurance training in anorectic-cachectic rats. Nutr Metab (Lond) 8: 60, 2011. doi: 10.1186/1743-7075-8-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lira VA, Okutsu M, Zhang M, Greene NP, Laker RC, Breen DS, Hoehn KL, Yan Z. Autophagy is required for exercise training-induced skeletal muscle adaptation and improvement of physical performance. FASEB J 27: 4184–4193, 2013. doi: 10.1096/fj.13-228486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Liu J, Peng Y, Wang X, Fan Y, Qin C, Shi L, Tang Y, Cao K, Li H, Long J, Liu J. Mitochondrial dysfunction launches dexamethasone-induced skeletal muscle atrophy via AMPK/FOXO3 signaling. Mol Pharm 13: 73–84, 2016. doi: 10.1021/acs.molpharmaceut.5b00516. [DOI] [PubMed] [Google Scholar]
- 112.Llovera M, Garcia-Martinez C, Agell N, Lopez-Soriano FJ, Argiles JM. Muscle wasting associated with cancer cachexia is linked to an important activation of the ATP-dependent ubiquitin-mediated proteolysis. Int J Cancer 61: 138–141, 1995. doi: 10.1002/ijc.2910610123. [DOI] [PubMed] [Google Scholar]
- 113.López-Soriano J, Carbó N, Costelli P, López-Soriano FJ, Argilés JM. alpha-Adrenergic receptors may contribute to the hypertriglyceridemia associated with tumour growth. Cancer Lett 110: 213–216, 1996. doi: 10.1016/S0304-3835(96)04501-6. [DOI] [PubMed] [Google Scholar]
- 114.Luongo C, Moser AR, Gledhill S, Dove WF. Loss of Apc+ in intestinal adenomas from Min mice. Cancer Res 54: 5947–5952, 1994. [PubMed] [Google Scholar]
- 115.Luput L, Licarete E, Drotar DM, Nagy AL, Sesarman A, Patras L, Rauca VF, Porfire A, Muntean D, Achim M, Tomuta I, Vlase L, Catoi C, Dragos N, Banciu M. In vivo double targeting of C26 colon carcinoma cells and microenvironmental protumor processes using liposomal simvastatin. J Cancer 9: 440–449, 2018. doi: 10.7150/jca.21560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Macciò A, Madeddu C, Mantovani G. Current pharmacotherapy options for cancer anorexia and cachexia. Expert Opin Pharmacother 13: 2453–2472, 2012. doi: 10.1517/14656566.2012.734297. [DOI] [PubMed] [Google Scholar]
- 117.Mace TA, Shakya R, Pitarresi JR, Swanson B, McQuinn CW, Loftus S, Nordquist E, Cruz-Monserrate Z, Yu L, Young G, Zhong X, Zimmers TA, Ostrowski MC, Ludwig T, Bloomston M, Bekaii-Saab T, Lesinski GB. IL-6 and PD-L1 antibody blockade combination therapy reduces tumour progression in murine models of pancreatic cancer. Gut 67: 320–332, 2018. doi: 10.1136/gutjnl-2016-311585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mailloux RJ. Teaching the fundamentals of electron transfer reactions in mitochondria and the production and detection of reactive oxygen species. Redox Biol 4: 381–398, 2015. doi: 10.1016/j.redox.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Manwani B, Bentivegna K, Benashski SE, Venna VR, Xu Y, Arnold AP, McCullough LD. Sex differences in ischemic stroke sensitivity are influenced by gonadal hormones, not by sex chromosome complement. J Cereb Blood Flow Metab 35: 221–229, 2015. doi: 10.1038/jcbfm.2014.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Martin L, Senesse P, Gioulbasanis I, Antoun S, Bozzetti F, Deans C, Strasser F, Thoresen L, Jagoe RT, Chasen M, Lundholm K, Bosaeus I, Fearon KH, Baracos VE. Diagnostic criteria for the classification of cancer-associated weight loss. J Clin Oncol 33: 90–99, 2015. doi: 10.1200/JCO.2014.56.1894. [DOI] [PubMed] [Google Scholar]
- 121.Mastrocola R, Reffo P, Penna F, Tomasinelli CE, Boccuzzi G, Baccino FM, Aragno M, Costelli P. Muscle wasting in diabetic and in tumor-bearing rats: role of oxidative stress. Free Radic Biol Med 44: 584–593, 2008. doi: 10.1016/j.freeradbiomed.2007.10.047. [DOI] [PubMed] [Google Scholar]
- 122.Mathew SJ. InACTIVatINg cancer cachexia. Dis Model Mech 4: 283–285, 2011. doi: 10.1242/dmm.007658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.McLean JB, Moylan JS, Andrade FH. Mitochondria dysfunction in lung cancer-induced muscle wasting in C2C12 myotubes. Front Physiol 5: 503, 2014. doi: 10.3389/fphys.2014.00503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Medina R, Wing SS, Haas A, Goldberg AL. Activation of the ubiquitin-ATP-dependent proteolytic system in skeletal muscle during fasting and denervation atrophy. Biomed Biochim Acta 50: 347–356, 1991. [PubMed] [Google Scholar]
- 125.Mehl KA, Davis JM, Clements JM, Berger FG, Pena MM, Carson JA. Decreased intestinal polyp multiplicity is related to exercise mode and gender in ApcMin/+ mice. J Appl Physiol (1985) 98: 2219–2225, 2005. doi: 10.1152/japplphysiol.00975.2004. [DOI] [PubMed] [Google Scholar]
- 126.Michaelis KA, Zhu X, Burfeind KG, Krasnow SM, Levasseur PR, Morgan TK, Marks DL. Establishment and characterization of a novel murine model of pancreatic cancer cachexia. J Cachexia Sarcopenia Muscle 8: 824–838, 2017. doi: 10.1002/jcsm.12225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Mirza KA, Wyke SM, Tisdale MJ. Attenuation of muscle atrophy by an N-terminal peptide of the receptor for proteolysis-inducing factor (PIF). Br J Cancer 105: 83–88, 2011. doi: 10.1038/bjc.2011.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Mogil JS, Bailey AL. Sex and gender differences in pain and analgesia. Prog Brain Res 186: 140–157, 2010. doi: 10.1016/B978-0-444-53630-3.00009-9. [DOI] [PubMed] [Google Scholar]
- 129.Mokdad AH, Dwyer-Lindgren L, Fitzmaurice C, Stubbs RW, Bertozzi-Villa A, Morozoff C, Charara R, Allen C, Naghavi M, Murray CJ. Trends and patterns of disparities in cancer mortality among US counties, 1980–2014. JAMA 317: 388–406, 2017. doi: 10.1001/jama.2016.20324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Mueller TC, Bachmann J, Prokopchuk O, Friess H, Martignoni ME. Molecular pathways leading to loss of skeletal muscle mass in cancer cachexia–can findings from animal models be translated to humans? BMC Cancer 16: 75, 2016. doi: 10.1186/s12885-016-2121-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Murton AJ, Constantin D, Greenhaff PL. The involvement of the ubiquitin proteasome system in human skeletal muscle remodelling and atrophy. Biochim Biophys Acta 1782: 730–743, 2008. doi: 10.1016/j.bbadis.2008.10.011. [DOI] [PubMed] [Google Scholar]
- 132.Muscaritoli M, Rossi Fanelli F, Molfino A. Perspectives of health care professionals on cancer cachexia: results from three global surveys. Ann Oncol 27: 2230–2236, 2016. doi: 10.1093/annonc/mdw420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Nakashima J, Tachibana M, Ueno M, Miyajima A, Baba S, Murai M. Association between tumor necrosis factor in serum and cachexia in patients with prostate cancer. Clin Cancer Res 4: 1743–1748, 1998. [PubMed] [Google Scholar]
- 134.Narsale AA, Carson JA. Role of interleukin-6 in cachexia: therapeutic implications. Curr Opin Support Palliat Care 8: 321–327, 2014. doi: 10.1097/SPC.0000000000000091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Narsale AA, Enos RT, Puppa MJ, Chatterjee S, Murphy EA, Fayad R, Pena MO, Durstine JL, Carson JA. Liver inflammation and metabolic signaling in ApcMin/+ mice: the role of cachexia progression. PLoS One 10: e0119888, 2015. doi: 10.1371/journal.pone.0119888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Narsale AA, Puppa MJ, Hardee JP, VanderVeen BN, Enos RT, Murphy EA, Carson JA. Short-term pyrrolidine dithiocarbamate administration attenuates cachexia-induced alterations to muscle and liver in ApcMin/+ mice. Oncotarget 7: 59482–59502, 2016. doi: 10.18632/oncotarget.10699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Neves RX, Rosa-Neto JC, Yamashita AS, Matos-Neto EM, Riccardi DM, Lira FS, Batista ML Jr, Seelaender M. White adipose tissue cells and the progression of cachexia: inflammatory pathways. J Cachexia Sarcopenia Muscle 7: 193–203, 2016. doi: 10.1002/jcsm.12041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Neyroud D, Nosacka RL, Judge AR, Hepple RT. Colon 26 adenocarcinoma (C26)-induced cancer cachexia impairs skeletal muscle mitochondrial function and content. J Muscle Res Cell Motil 40: 59–65, 2019. doi: 10.1007/s10974-019-09510-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Onesti JK, Guttridge DC. Inflammation based regulation of cancer cachexia. BioMed Res Int 2014: 1–7, 2014. doi: 10.1155/2014/168407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Pan FC, Bankaitis ED, Boyer D, Xu X, Van de Casteele M, Magnuson MA, Heimberg H, Wright CV. Spatiotemporal patterns of multipotentiality in Ptf1a-expressing cells during pancreas organogenesis and injury-induced facultative restoration. Development 140: 751–764, 2013. doi: 10.1242/dev.090159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Penna F, Baccino FM, Costelli P. Coming back: autophagy in cachexia. Curr Opin Clin Nutr Metab Care 17: 241–246, 2014. doi: 10.1097/MCO.0000000000000048. [DOI] [PubMed] [Google Scholar]
- 142.Penna F, Ballarò R, Martinez-Cristobal P, Sala D, Sebastian D, Busquets S, Muscaritoli M, Argilés JM, Costelli P, Zorzano A. Autophagy exacerbates muscle wasting in cancer cachexia and impairs mitochondrial function. J Mol Biol 431: 2674–2686, 2019. doi: 10.1016/j.jmb.2019.05.032. [DOI] [PubMed] [Google Scholar]
- 143.Pettersen K, Andersen S, Degen S, Tadini V, Grosjean J, Hatakeyama S, Tesfahun AN, Moestue S, Kim J, Nonstad U, Romundstad PR, Skorpen F, Sørhaug S, Amundsen T, Grønberg BH, Strasser F, Stephens N, Hoem D, Molven A, Kaasa S, Fearon K, Jacobi C, Bjørkøy G. Cancer cachexia associates with a systemic autophagy-inducing activity mimicked by cancer cell-derived IL-6 trans-signaling. Sci Rep 7: 2046, 2017. doi: 10.1038/s41598-017-02088-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Pfitzenmaier J, Vessella R, Higano CS, Noteboom JL, Wallace D Jr, Corey E. Elevation of cytokine levels in cachectic patients with prostate carcinoma. Cancer 97: 1211–1216, 2003. doi: 10.1002/cncr.11178. [DOI] [PubMed] [Google Scholar]
- 146.Pigna E, Berardi E, Aulino P, Rizzuto E, Zampieri S, Carraro U, Kern H, Merigliano S, Gruppo M, Mericskay M, Li Z, Rocchi M, Barone R, Macaluso F, Di Felice V, Adamo S, Coletti D, Moresi V. Aerobic exercise and pharmacological treatments counteract cachexia by modulating autophagy in colon cancer. Sci Rep 6: 26991, 2016. doi: 10.1038/srep26991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Pin F, Barreto R, Couch ME, Bonetto A, O’Connell TM. Cachexia induced by cancer and chemotherapy yield distinct perturbations to energy metabolism. J Cachexia Sarcopenia Muscle 10: 140–154, 2019. doi: 10.1002/jcsm.12360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Prado CM, Baracos VE, McCargar LJ, Mourtzakis M, Mulder KE, Reiman T, Butts CA, Scarfe AG, Sawyer MB. Body composition as an independent determinant of 5-fluorouracil-based chemotherapy toxicity. Clin Cancer Res 13: 3264–3268, 2007. doi: 10.1158/1078-0432.CCR-06-3067. [DOI] [PubMed] [Google Scholar]
- 149.Prado CM, Bekaii-Saab T, Doyle LA, Shrestha S, Ghosh S, Baracos VE, Sawyer MB. Skeletal muscle anabolism is a side effect of therapy with the MEK inhibitor: selumetinib in patients with cholangiocarcinoma. Br J Cancer 106: 1583–1586, 2012. doi: 10.1038/bjc.2012.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Pretto F, Ghilardi C, Moschetta M, Bassi A, Rovida A, Scarlato V, Talamini L, Fiordaliso F, Bisighini C, Damia G, Bani MR, Piccirillo R, Giavazzi R. Sunitinib prevents cachexia and prolongs survival of mice bearing renal cancer by restraining STAT3 and MuRF-1 activation in muscle. Oncotarget 6: 3043–3054, 2015. doi: 10.18632/oncotarget.2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Puppa MJ, Gao S, Narsale AA, Carson JA. Skeletal muscle glycoprotein 130’s role in Lewis lung carcinoma-induced cachexia. FASEB J 28: 998–1009, 2014. doi: 10.1096/fj.13-240580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Puppa MJ, White JP, Velázquez KT, Baltgalvis KA, Sato S, Baynes JW, Carson JA. The effect of exercise on IL-6-induced cachexia in the Apc (Min/+) mouse. J Cachexia Sarcopenia Muscle 3: 117–137, 2012. doi: 10.1007/s13539-011-0047-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Riley JL III, Robinson ME, Wise EA, Myers CD, Fillingim RB. Sex differences in the perception of noxious experimental stimuli: a meta-analysis. Pain 74: 181–187, 1998. doi: 10.1016/S0304-3959(97)00199-1. [DOI] [PubMed] [Google Scholar]
- 154.Roberts BM, Ahn B, Smuder AJ, Al-Rajhi M, Gill LC, Beharry AW, Powers SK, Fuller DD, Ferreira LF, Judge AR. Diaphragm and ventilatory dysfunction during cancer cachexia. FASEB J 27: 2600–2610, 2013. doi: 10.1096/fj.12-222844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Roberts BM, Frye GS, Ahn B, Ferreira LF, Judge AR. Cancer cachexia decreases specific force and accelerates fatigue in limb muscle. Biochem Biophys Res Commun 435: 488–492, 2013. doi: 10.1016/j.bbrc.2013.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Rolfe DF, Brown GC. Cellular energy utilization and molecular origin of standard metabolic rate in mammals. Physiol Rev 77: 731–758, 1997. doi: 10.1152/physrev.1997.77.3.731. [DOI] [PubMed] [Google Scholar]
- 157.Romanello V, Sandri M. Mitochondrial quality control and muscle mass maintenance. Front Physiol 6: 422, 2016. doi: 10.3389/fphys.2015.00422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Rosa-Caldwell M, Brown J, Lee D, Washington T, Greene N.. Tumor derived factors induce muscle mitochondria hyperpolarization and subsequent superoxide production. Med Sci Sports Exerc 50 (5S): 148, 2018. [Google Scholar]
- 159.Rosa-Caldwell ME, Brown JL, Lee DE, Wiggs MP, Perry RA Jr, Haynie WS, Caldwell AR, Washington TA, Lo WJ, Greene NP. Hepatic alterations during the development and progression of cancer cachexia. Appl Physiol Nutr Metab. In press. doi: 10.1139/apnm-2019-0407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Russell AP, Foletta VC, Snow RJ, Wadley GD. Skeletal muscle mitochondria: a major player in exercise, health and disease. Biochim Biophys Acta 1840: 1276–1284, 2014. doi: 10.1016/j.bbagen.2013.11.016. [DOI] [PubMed] [Google Scholar]
- 161.Ryan AM, Power DG, Daly L, Cushen SJ, Ní Bhuachalla Ē, Prado CM. Cancer-associated malnutrition, cachexia and sarcopenia: the skeleton in the hospital closet 40 years later. Proc Nutr Soc 75: 199–211, 2016. doi: 10.1017/S002966511500419X. [DOI] [PubMed] [Google Scholar]
- 162.Ryan ZC, Craig TA, Wang X, Delmotte P, Salisbury JL, Lanza IR, Sieck GC, Kumar R. 1α,25-Dihydroxyvitamin D3 mitigates cancer cell mediated mitochondrial dysfunction in human skeletal muscle cells. Biochem Biophys Res Commun 496: 746–752, 2018. doi: 10.1016/j.bbrc.2018.01.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Sandri M. Autophagy in skeletal muscle. FEBS Lett 584: 1411–1416, 2010. doi: 10.1016/j.febslet.2010.01.056. [DOI] [PubMed] [Google Scholar]
- 164.Sandri M. New findings of lysosomal proteolysis in skeletal muscle. Curr Opin Clin Nutr Metab Care 14: 223–229, 2011. doi: 10.1097/MCO.0b013e3283457a75. [DOI] [PubMed] [Google Scholar]
- 165.Sandri M. Protein breakdown in cancer cachexia. Semin Cell Dev Biol 54: 11–19, 2016. doi: 10.1016/j.semcdb.2015.11.002. [DOI] [PubMed] [Google Scholar]
- 166.Sandri M, Lin J, Handschin C, Yang W, Arany ZP, Lecker SH, Goldberg AL, Spiegelman BM. PGC-1α protects skeletal muscle from atrophy by suppressing FoxO3 action and atrophy-specific gene transcription. Proc Natl Acad Sci USA 103: 16260–16265, 2006. doi: 10.1073/pnas.0607795103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Sandri M, Sandri C, Gilbert A, Skurk C, Calabria E, Picard A, Walsh K, Schiaffino S, Lecker SH, Goldberg AL. Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell 117: 399–412, 2004. doi: 10.1016/S0092-8674(04)00400-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Schmidt K, von Haehling S, Doehner W, Palus S, Anker SD, Springer J. IGF-1 treatment reduces weight loss and improves outcome in a rat model of cancer cachexia. J Cachexia Sarcopenia Muscle 2: 105–109, 2011. doi: 10.1007/s13539-011-0029-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Schmitt TL, Martignoni ME, Bachmann J, Fechtner K, Friess H, Kinscherf R, Hildebrandt W. Activity of the Akt-dependent anabolic and catabolic pathways in muscle and liver samples in cancer-related cachexia. J Mol Med (Berl) 85: 647–654, 2007. doi: 10.1007/s00109-007-0177-2. [DOI] [PubMed] [Google Scholar]
- 170.Schwalm C, Jamart C, Benoit N, Naslain D, Prémont C, Prévet J, Van Thienen R, Deldicque L, Francaux M. Activation of autophagy in human skeletal muscle is dependent on exercise intensity and AMPK activation. FASEB J 29: 3515–3526, 2015. doi: 10.1096/fj.14-267187. [DOI] [PubMed] [Google Scholar]
- 171.Shaw RJ. LKB1 and AMP-activated protein kinase control of mTOR signalling and growth. Acta Physiol (Oxf) 196: 65–80, 2009. doi: 10.1111/j.1748-1716.2009.01972.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Shintaku J, Peterson JM, Talbert EE, Gu JM, Ladner KJ, Williams DR, Mousavi K, Wang R, Sartorelli V, Guttridge DC. MyoD regulates skeletal muscle oxidative metabolism cooperatively with alternative NF-κB. Cell Reports 17: 514–526, 2016. doi: 10.1016/j.celrep.2016.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Shyh-Chang N. Metabolic changes during cancer cachexia pathogenesis. Adv Exp Med Biol 1026: 233–249, 2017. doi: 10.1007/978-981-10-6020-5_11. [DOI] [PubMed] [Google Scholar]
- 174.Siegel RL, Miller KD, Jemal A. Colorectal cancer mortality rates in adults aged 20 to 54 years in the United States, 1970–2014. JAMA 318: 572–574, 2017. doi: 10.1001/jama.2017.7630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Stegel P, Kozjek NR, Brumen BA, Strojan P. Bioelectrical impedance phase angle as indicator and predictor of cachexia in head and neck cancer patients treated with (chemo)radiotherapy. Eur J Clin Nutr 70: 602–606, 2016. doi: 10.1038/ejcn.2016.13. [DOI] [PubMed] [Google Scholar]
- 176.Stewart GD, Skipworth RJ, Fearon KC. Cancer cachexia and fatigue. Clin Med (Lond) 6: 140–143, 2006. doi: 10.7861/clinmedicine.6-2-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Suliman HB, Piantadosi CA. Mitochondrial quality control as a therapeutic target. Pharmacol Rev 68: 20–48, 2016. doi: 10.1124/pr.115.011502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Sun L, Quan XQ, Yu S. An epidemiological survey of cachexia in advanced cancer patients and analysis on its diagnostic and treatment status. Nutr Cancer 67: 1056–1062, 2015. doi: 10.1080/01635581.2015.1073753. [DOI] [PubMed] [Google Scholar]
- 179.Sun R, Zhang S, Lu X, Hu W, Lou N, Zhao Y, Zhou J, Zhang X, Yang H. Comparative molecular analysis of early and late cancer cachexia-induced muscle wasting in mouse models. Oncol Rep 36: 3291–3302, 2016. doi: 10.3892/or.2016.5165. [DOI] [PubMed] [Google Scholar]
- 180.Sun YS, Ye ZY, Qian ZY, Xu XD, Hu JF. Expression of TRAF6 and ubiquitin mRNA in skeletal muscle of gastric cancer patients. J Exp Clin Cancer Res 31: 81, 2012. doi: 10.1186/1756-9966-31-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Talbert EE, Cuitiño MC, Ladner KJ, Rajasekerea PV, Siebert M, Shakya R, Leone GW, Ostrowski MC, Paleo B, Weisleder N, Reiser PJ, Webb A, Timmers CD, Eiferman DS, Evans DC, Dillhoff ME, Schmidt CR, Guttridge DC. Modeling human cancer-induced cachexia. Cell Reports 28: 1612–1622.e4, 2019. doi: 10.1016/j.celrep.2019.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Talbert EE, Metzger GA, He WA, Guttridge DC. Modeling human cancer cachexia in colon 26 tumor-bearing adult mice. J Cachexia Sarcopenia Muscle 5: 321–328, 2014. doi: 10.1007/s13539-014-0141-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Talbert EE, Yang J, Mace TA, Farren MR, Farris AB, Young GS, Elnaggar O, Che Z, Timmers CD, Rajasekera P, Maskarinec JM, Bloomston M, Bekaii-Saab T, Guttridge DC, Lesinski GB. Dual inhibition of MEK and PI3K/Akt rescues cancer cachexia through both tumor-extrinsic and -intrinsic activities. Mol Cancer Ther 16: 344–356, 2017. doi: 10.1158/1535-7163.MCT-16-0337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Tanaka Y, Eda H, Tanaka T, Udagawa T, Ishikawa T, Horii I, Ishitsuka H, Kataoka T, Taguchi T. Experimental cancer cachexia induced by transplantable colon 26 adenocarcinoma in mice. Cancer Res 50: 2290–2295, 1990. [PubMed] [Google Scholar]
- 185.Temparis S, Asensi M, Taillandier D, Aurousseau E, Larbaud D, Obled A, Béchet D, Ferrara M, Estrela JM, Attaix D. Increased ATP-ubiquitin-dependent proteolysis in skeletal muscles of tumor-bearing rats. Cancer Res 54: 5568–5573, 1994. [PubMed] [Google Scholar]
- 186.Tessitore L, Bonelli G, Baccino FM. Early development of protein metabolic perturbations in the liver and skeletal muscle of tumour-bearing rats. A model system for cancer cachexia. Biochem J 241: 153–159, 1987. doi: 10.1042/bj2410153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Tessitore L, Costelli P, Baccino FM. Pharmacological interference with tissue hypercatabolism in tumour-bearing rats. Biochem J 299: 71–78, 1994. doi: 10.1042/bj2990071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Tessitore L, Costelli P, Bonetti G, Baccino FM. Cancer cachexia, malnutrition, and tissue protein turnover in experimental animals. Arch Biochem Biophys 306: 52–58, 1993. doi: 10.1006/abbi.1993.1479. [DOI] [PubMed] [Google Scholar]
- 189.Tzika AA, Fontes-Oliveira CC, Shestov AA, Constantinou C, Psychogios N, Righi V, Mintzopoulos D, Busquets S, Lopez-Soriano FJ, Milot S, Lepine F, Mindrinos MN, Rahme LG, Argiles JM. Skeletal muscle mitochondrial uncoupling in a murine cancer cachexia model. Int J Oncol 43: 886–894, 2013. doi: 10.3892/ijo.2013.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Vagnildhaug OM, Brunelli C, Hjermstad MJ, Strasser F, Baracos V, Wilcock A, Nabal M, Kaasa S, Laird B, Solheim TS. A prospective study examining cachexia predictors in patients with incurable cancer. BMC Palliat Care 18: 46, 2019. doi: 10.1186/s12904-019-0429-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Vainshtein A, Hood DA. The regulation of autophagy during exercise in skeletal muscle. J Appl Physiol (1985) 120: 664–673, 2016. doi: 10.1152/japplphysiol.00550.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Vaitkus JA, Celi FS. The role of adipose tissue in cancer-associated cachexia. Exp Biol Med (Maywood) 242: 473–481, 2017. doi: 10.1177/1535370216683282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.van Vledder MG, Levolger S, Ayez N, Verhoef C, Tran TC, Ijzermans JN. Body composition and outcome in patients undergoing resection of colorectal liver metastases. Br J Surg 99: 550–557, 2012. doi: 10.1002/bjs.7823. [DOI] [PubMed] [Google Scholar]
- 194.VanderVeen BN, Fix DK, Carson JA. Disrupted skeletal muscle mitochondrial dynamics, mitophagy, and biogenesis during cancer cachexia: a role for inflammation. Oxid Med Cell Longev 2017: 1–13, 2017. doi: 10.1155/2017/3292087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.VanderVeen BN, Hardee JP, Fix DK, Carson JA. Skeletal muscle function during the progression of cancer cachexia in the male ApcMin/+ mouse. J Appl Physiol (1985) 124: 684–695, 2018. doi: 10.1152/japplphysiol.00897.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Vanhoutte G, van de Wiel M, Wouters K, Sels M, Bartolomeeussen L, De Keersmaecker S, Verschueren C, De Vroey V, De Wilde A, Smits E, Cheung KJ, De Clerck L, Aerts P, Baert D, Vandoninck C, Kindt S, Schelfhaut S, Vankerkhoven M, Troch A, Ceulemans L, Vandenbergh H, Leys S, Rondou T, Dewitte E, Maes K, Pauwels P, De Winter B, Van Gaal L, Ysebaert D, Peeters M. Cachexia in cancer: what is in the definition? BMJ Open Gastroenterol 3: e000097, 2016. doi: 10.1136/bmjgast-2016-000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Vasilaki A, Richardson A, Van Remmen H, Brooks SV, Larkin L, McArdle A, Jackson MJ. Role of nerve-muscle interactions and reactive oxygen species in regulation of muscle proteostasis with ageing. J Physiol 595: 6409–6415, 2017. doi: 10.1113/JP274336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Wang X, Pickrell AM, Zimmers TA, Moraes CT. Increase in muscle mitochondrial biogenesis does not prevent muscle loss but increased tumor size in a mouse model of acute cancer-induced cachexia. PLoS One 7: e33426, 2012. doi: 10.1371/journal.pone.0033426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.White JP, Baltgalvis KA, Puppa MJ, Sato S, Baynes JW, Carson JA. Muscle oxidative capacity during IL-6-dependent cancer cachexia. Am J Physiol Regul Integr Comp Physiol 300: R201–R211, 2011. doi: 10.1152/ajpregu.00300.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.White JP, Baynes JW, Welle SL, Kostek MC, Matesic LE, Sato S, Carson JA. The regulation of skeletal muscle protein turnover during the progression of cancer cachexia in the Apc(Min/+) mouse. PLoS One 6: e24650, 2011. doi: 10.1371/journal.pone.0024650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.White JP, Puppa MJ, Gao S, Sato S, Welle SL, Carson JA. Muscle mTORC1 suppression by IL-6 during cancer cachexia: a role for AMPK. Am J Physiol Endocrinol Metab 304: E1042–E1052, 2013. doi: 10.1152/ajpendo.00410.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.White JP, Puppa MJ, Narsale A, Carson JA. Characterization of the male ApcMin/+ mouse as a hypogonadism model related to cancer cachexia. Biol Open 2: 1346–1353, 2013. doi: 10.1242/bio.20136544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.White JP, Puppa MJ, Sato S, Gao S, Price RL, Baynes JW, Kostek MC, Matesic LE, Carson JA. IL-6 regulation on skeletal muscle mitochondrial remodeling during cancer cachexia in the ApcMin/+ mouse. Skelet Muscle 2: 14, 2012. doi: 10.1186/2044-5040-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Williams JP, Phillips BE, Smith K, Atherton PJ, Rankin D, Selby AL, Liptrot S, Lund J, Larvin M, Rennie MJ. Effect of tumor burden and subsequent surgical resection on skeletal muscle mass and protein turnover in colorectal cancer patients. Am J Clin Nutr 96: 1064–1070, 2012. doi: 10.3945/ajcn.112.045708. [DOI] [PubMed] [Google Scholar]
- 205.Yamamura M, Hamada Y, Kogata M, Kiba M, Uetsuji S, Yamamoto M, Adachi M. Postoperative immunization with peanut agglutinin-binding glycoprotein from Lewis lung carcinoma in mice. J Surg Res 38: 39–44, 1985. doi: 10.1016/0022-4804(85)90008-3. [DOI] [PubMed] [Google Scholar]
- 206.Yamashita AS, das Neves RX, Rosa-Neto JC, Lira FD, Batista ML Jr, Alcantara PS, Otoch JP, Seelaender M. White adipose tissue IFN-γ expression and signalling along the progression of rodent cancer cachexia. Cytokine 89: 122–126, 2017. doi: 10.1016/j.cyto.2016.02.015. [DOI] [PubMed] [Google Scholar]
- 207.Yan Z, Lira VA, Greene NP. Exercise training-induced regulation of mitochondrial quality. Exerc Sport Sci Rev 40: 159–164, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Ying H, Elpek KG, Vinjamoori A, Zimmerman SM, Chu GC, Yan H, Fletcher-Sananikone E, Zhang H, Liu Y, Wang W, Ren X, Zheng H, Kimmelman AC, Paik JH, Lim C, Perry SR, Jiang S, Malinn B, Protopopov A, Colla S, Xiao Y, Hezel AF, Bardeesy N, Turley SJ, Wang YA, Chin L, Thayer SP, DePinho RA. PTEN is a major tumor suppressor in pancreatic ductal adenocarcinoma and regulates an NF-κB-cytokine network. Cancer Discov 1: 158–169, 2011. doi: 10.1158/2159-8290.CD-11-0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Yoshimura M, Shiomi Y, Ohira Y, Takei M, Tanaka T. Z-505 hydrochloride, an orally active ghrelin agonist, attenuates the progression of cancer cachexia via anabolic hormones in Colon 26 tumor-bearing mice. Eur J Pharmacol 811: 30–37, 2017. doi: 10.1016/j.ejphar.2017.05.036. [DOI] [PubMed] [Google Scholar]
- 210.Yu L, Chen Y, Tooze SA. Autophagy pathway: cellular and molecular mechanisms. Autophagy 14: 207–215, 2018. doi: 10.1080/15548627.2017.1378838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Zhang G, Liu Z, Ding H, Miao H, Garcia JM, Li YP. Toll-like receptor 4 mediates Lewis lung carcinoma-induced muscle wasting via coordinate activation of protein degradation pathways. Sci Rep 7: 2273, 2017. doi: 10.1038/s41598-017-02347-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Zhao M, Klionsky DJ. AMPK-dependent phosphorylation of ULK1 induces autophagy. Cell Metab 13: 119–120, 2011. doi: 10.1016/j.cmet.2011.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Zhong X, Pons M, Poirier C, Jiang Y, Liu J, Sandusky GE, Shahda S, Nakeeb A, Schmidt CM, House MG, Ceppa EP, Zyromski NJ, Liu Y, Jiang G, Couch ME, Koniaris LG, Zimmers TA. The systemic activin response to pancreatic cancer: implications for effective cancer cachexia therapy. J Cachexia Sarcopenia Muscle 10: 1083–1101, 2019. doi: 10.1002/jcsm.12461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Zimmers TA, Fishel ML, Bonetto A. STAT3 in the systemic inflammation of cancer cachexia. Semin Cell Dev Biol 54: 28–41, 2016. doi: 10.1016/j.semcdb.2016.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]