Abstract
We have previously shown that TNF-α produced by renal epithelial cells inhibits Na+-K+-2Cl− cotransporter (NKCC2) activity as part of a mechanism that attenuates increases in blood pressure in response to high NaCl intake. As the role of TNF-α in the kidney is still being defined, the effects of low salt intake on TNF-α and NKCC2B expression were determined. Mice given a low-salt (0.02% NaCl) diet (LSD) for 7 days exhibited a 62 ± 7.4% decrease in TNF-α mRNA accumulation in the renal cortex. Mice that ingested the LSD also exhibited an ~63% increase in phosphorylated NKCC2 expression in the cortical thick ascending limb of Henle’s loop and a concomitant threefold increase in NKCC2B mRNA abundance without a concurrent change in NKCC2A mRNA accumulation. NKCC2B mRNA levels increased fivefold in mice that ingested the LSD and also received an intrarenal injection of a lentivirus construct that specifically silenced TNF-α in the kidney (U6-TNF-ex4) compared with mice injected with control lentivirus. Administration of a single intrarenal injection of murine recombinant TNF-α (5 ng/g body wt) attenuated the increases of NKCC2B mRNA by ~50% and inhibited the increase in phosphorylated NKCC2 by ~54% in the renal cortex of mice given the LSD for 7 days. Renal silencing of TNF-α decreased urine volume and NaCl excretion in mice given the LSD, effects that were reversed when NKCC2B was silenced in the kidney. Collectively, these findings demonstrate that downregulation of renal TNF-α production in response to low-salt conditions contributes to the regulation of NaCl reabsorption via an NKCC2B-dependent mechanism.
Keywords: low salt, Na+-K+-2Cl− cotransporter isoform B, renal cortex, tumor necrosis factor-α
INTRODUCTION
Reabsorption of NaCl along the thick ascending limb of Henle’s loop (TAL) is mediated on the apical side by the Na+-K+-2Cl− cotransporter (NKCC2), which is also important for NaCl sensing by the macula densa (MD) (27, 30). The MD and TAL play important roles in the maintenance of blood pressure, as functional defects and mutations in these regions of the nephron have been linked to the development of salt-sensitive hypertension as well as salt-losing tubulopathies (9, 18, 22, 37). Three isoforms of NKCC2 (NKCC2A, NKCC2F, and NKCC2B) differ by alternative splicing of exon 4, which encodes part of the TM2 domain and the intracellular loop connecting TM2 and TM3 (8, 16). These isoforms differ in both their distribution along the nephron and affinity for Na+, K+, and Cl−. Accordingly, NKCC2F is present only in the renal medulla, NKCC2A is expressed in both the cortex and medulla, and NKCC2B is exclusively expressed in the cortex (16). NKCC2B has the highest affinity, NKCC2A has an intermediate affinity, and NKCC2F has the lowest affinity for Na+, K+, and Cl− (10). Seminal studies have shown that these characteristics help maintain the sensitivity to ions in tubular fluid that becomes diluted as it goes from the medulla to the cortex (7, 27, 28, 32).
The distinct transport characteristics and tubular localization of NKCC2A and NKCC2B are particularly well suited to help regulate renal function in response to changes in dietary salt intake (4, 8). Under low-salt (LS) conditions, NaCl reabsorption along the TAL is dependent on the high-Cl− affinity NKCC2B isoform, and tubuloglomerular responses were attenuated in NKCC2B-deficient mice subjected to low-flow rate/low-NaCl conditions (4, 27). Changes in NaCl intake also have been shown to regulate the relative expression of NKCC2A and NKCC2B isoforms (11, 35). For instance, injestion of a LS diet (LSD) differentially favors elevation of the NKCC2B isoform in the cortical TAL (cTAL) and MD and helps maintain salt and water balance by increasing TAL NaCl reabsorption (5, 18, 27). The mechanisms that regulate salt-induced changes in NKCC2 isoform expression and function are still being determined.
Multiple factors contribute to the ability of the kidneys to maintain plasma Na+ levels in a narrow range in response to high or low Na+ intake (25, 36). TNF-α is produced by several renal cell types, including proximal tubule, TAL, collecting duct, and mesangial cells as well as podocytes (17, 20, 23, 24, 34). We found that medullary TAL (mTAL) cells produce TNF-α, and genetic deletion of this cytokine in mice selectively increases expression of NKCC2A, which is increased in response to high-salt conditions (3, 14). Moreover, TNF-α is selectively released into the urine in response to high salt intake, and renal-specific silencing of TNF-α unmasks salt-dependent increases in blood pressure via a mechanism involving NKCC2A (11, 13). The effects of LS conditions on renal production of TNF-α and its ability to regulate NKCC2 isoforms is unknown. The present findings suggest that suppression of renal TNF-α by ingestion of a LSD facilitates the concurrent increase of NKCC2B expression and NKCC2 activity in the renal cortex.
METHODS
Materials and reagents.
Antibodies for β-actin were obtained from Abcam, and anti-phosphorylated (p)NKCC2 was a gift from Dr. Kerim Mutig (26). Lipofectamine reagents, TRIzol, and SDS polyacrylamide bis-Tris gels were from Invitrogen, and the pLKO.1, psPAX2, and pMD2.G plasmids were from Addgene. PCR for TNF-α silencing and nonsilencing shRNA controls used a common primer for the 5′-end, forward 5′-gcagaattcGATCCGACGCCGCCATCTCT-3′; the 3′-end for the scrambled nonsilencing shRNA was 5′-gcagctagcCTCGAGAAAAAAGAACGTTCGATAATGGATCCTACACAAAGATCCATTATCGAACGTTCAAACAAGGCTTTTCTCCAAGGGATA-3′. The inhibitory construct for TNF-α (U6-TNF-ex4), which was cloned into pcDNA3.1, was designed by targeting exon 4 of the TNF-α gene; the TNF-α reverse primer for the 3′-end was 5′-gcagctagcCTCGAGAAAAAACCCAGTGATAGAGGTTACCCTACACAAAGGTAACCTCTATCACTGGGAAACAAGGCTTTTCTCCAAGGGATA-3′. Silencing of TNF-α mRNA also was accomplished using the lentiviral vector psiLv-U6 (GeneCopoeia) and the lentivirus purification kit from Takara Bio USA. Recombinat mouse TNF-α was obtained from PharMingen. Polyvinylidene difluoride membranes were obtained from Amersham. The Nonidet P-40 lysis buffer for Western blots was prepared as 0.4 M NaCl, 0.5 mM EGTA, 1.5 mM MgCl2, 10 mM HEPES (pH 7.9), 5% (vol/vol) glycerol, and 0.5% (vol/vol) Nonidet P-40 and contained protease inhibitors (Roche Diagnostics) for anti-pNKCC2 analysis. All other chemical reagents were purchased from Sigma.
Cell culture and transfection.
Human embryonic kidney (HEK)-293T cells were maintained in Eagle's minimum essential medium supplemented with 10% heat-inactivated FBS and 4 mM l-glutamine. The target sequence of the inhibitory construct for TNF-α (U6-TNF-ex4) was 5′-GATGGGTTGTACCTTGTCT-3′, and the constructs were designed by targeting exon 4 of the TNF-α gene. The inhibitory construct for NKCC2B was designed using a shRNA-expressing construct targeting exon 4 of murine NKCC2B (U6-N2B-ex4) as previously described (15). The target sequence of the inhibitory construct for NKCC2B (U6-N2B ex4) was 5′-CTTAGCCGTGACAGTGACA-3′, and the constructs were designed by targeting exon 4 of the Slc12a1 gene. Scrambled U6 shRNA (U6) was used as a negative control. Subcloning of enhanced green fluorescent protein (EGFP) or EGFP-N2B-ex4 into a pLKO.1 vector and cotransfection of HEK-293T cells with pLKO.1 was performed to generate lentivirus encoding EGFP or EGFP-N2B-ex4. psPAX2 and pMD2.G plasmids were used for the preparation of lentivirus as previously described (11).
TNF-α treatment of an MD-like cell line.
A mouse MD cell line (MMDD1; a gift from Dr. J. Schnermann, National Institutes of Health, Bethesda, MD) was cultured as previously described (46). Briefly, MMDD1 cells were grown to >90 confluence in six-well plates in Eagle's minimum essential medium/nutrient mixture F-12 Ham, 10% FBS, and 1% penicillin-streptomycin in a humidified (95% air-5% CO2) incubator at 37°C. In the experiments, MMDD1 monolayers were exposed to normal-salt medium (NSM) or LS medium (LSM), which was made using serum-free DMEM in a 1:1 mixture with isotonic saline (control) or 300 mM raffinose (to reduce NaCl to half for the LS solution) as previously described (46). Confluent MMDD1 cells were treated with 1 nM TNF-α for 4 h to study its effects on NKCC2.
Lentivirus amplication and purification.
Lentivirus constructs were generated and purified as previously described (11, 15). Briefly, DNA for transfection was prepared by mixing psPAX2 and pMD2.G with pLKO.1 or psiLV plasmids. A mixture of plasmid with OptiMEM (GIBCO) and FUGENE (Roche) was then added to the 7 × 10 e5 packaging cells per 20-cm2 flask. Lentiviral supernatants were collected 48 h after transfection, and the supernatants were pooled and then filter purified with the Lenti-X Maxi purification kit. Transfection efficiency was evaluated by quantitative RT-PCR and flow cytometry as previously described (14). The titer of filter-purified lentivirus was ~5 × 108 transducing units.
Administration of lentivirus or TNF-α products in vivo.
The intrarenal gene transfer procedure was used and verified as previously described (11, 15). In vivo treatment with TNF-α may be accomplished by several routes of administration (2, 3, 11, 40, 41). In the present study, murine recombinant TNF-α (5 ng/g body wt) or saline control was administered by intrarenal injection. Briefly, a 31-gauge needle was inserted at the lower pole of the each kidney parallel to the long axis and then carefully pushed toward the upper pole after the animals had been anesthetized intraperitoneally using ketamine and xylazine. As the needle was slowly removed, 50 μL filter-purified lentivirus (U6 or U6-TNF-ex4, ~5 × 107 transducing units) was injected.
Mice and renal tissue preparation.
Male C57BL/6J mice (20–22 g) were obtained from The Jackson Laboratory and housed in pathogen-free housing. In these experiments, mice were fed a LSD (0.02% NaCl) or normal-salt (0.4% NaCl) diet (NSD) as well as tap water ad libitum, as indicated. Group size was determined to ensure an adequate statistical power base. Mice were given the LSD for 7 days after intrarenal injection for target gene silencing with various lentivirus constructs for 3 days. For administration of TNF-α, mice were fed the LSD for an additional 7 days after an intrarenal injection of TNF-α for 24 h. The renal cortex was isolated as previously described Briefly, kidneys were perfused, removed, and cut along the corticopapillary axis after mice had been anesthetized at the end of the treatment period. The renal cortex was excised, separated, and further washed three times and then transferred to the tubes for analyses (15). MD/cTAL tubules were microdissected and purified as previously described (12, 13, 15, 44). Briefly, cortical MD/cTAL segments were incubated with collagenase and oxygen. The suspension was sedimented on ice and mixed with HBSS containing 2% BSA, and the supernatant containing MD/cTAL tubules was filtered through a 52-μm nylon mesh membrane (Fisher Scientific, Springfield, NJ). The cortical MD/cTAL tubules retained on the mesh were resuspended in HBSS and centrifuged, and the pelleted MD/cTAL tubular fragments were further selected according to morphological appearance. Purified MD/cTAL tubules were verified by NKCC2 expression.
Isolation of total RNA and amplification of cDNA fragments.
The procedures for total RNA isolation, purification, and cDNA fragment amplification from mouse tissues and cells have been previously described (11). Briefly, total RNA was obtained using TRIzol reagent, chloroform, and isopropanol. The RNA pellet was washed with 75% ethanol, dried, and resuspended in RNase-free distilled H2O treated with deoxyribonuclease I. Finally, a 3-μg aliquot was used for cDNA synthesis using the Superscript Preamplification System (Life Technologies) with Superscript II reverse transcriptase and random hexamers. cDNA fragments were size fractionated on a 1% agarose gel and stained with ethidium bromide.
Quantitative RT-PCR analysis.
The measurement of mRNA abundance using quantitative RT-PCR with either SYBR green chemistry or Taqman (Applied Biosystems) was accomplished as previously described (11, 13, 14). Briefly, RNA was isolated and purified from renal tissues or cells, and a 0.5-μg aliquot of total RNA was converted to cDNA using random primers and PowerScript RT (Clontech). PCR of cDNA from each RNA sample was performed with Platinum Taq polymerase (Invitrogen), a FastStart DNA Master SYBR Green I kit (Roche), and MgCl2. Reactions were performed using specific primer pairs for murine TNF-α, NKCC2A, and NKCC2B (Table 1) in triplicate, and threshold cycle (CT) numbers were averaged. The mRNA results for specific target genes were calculated by normalization to β-actin mRNA. The efficiency of primer pair amplification was determined from a standard curve generated using previously described protocols (29, 31). The method was used to evaluate changes in mRNA accumulation (21).
Table 1.
Oligonucleotide-specific primers for PCR
| Primer | Sequence | PCR Size, bp | Position |
|---|---|---|---|
| TNF-α (m) | |||
| Forward | 5′-GAGAAGTTCCCAAATGGCCT-3′ | 242 | 351–370 |
| Reverse | 5′-GAGAACCTGGGAGTAGACAA-3′ | 593–574 | |
| NKCC2A (m) | |||
| Forward | 5′-GGTAACCTCTATCACTGGGT-3′ | 237 | 883–902 |
| Reverse | 5′-GTCATTGGTTGGATCCACCA-3′ | 1,120–1,101 | |
| NKCC2B (m) | |||
| Forward | 5′-GCCGTGACAGTGACAGCCAT-3′ | 235 | 873–892 |
| Reverse | 5′-GGATCCACCATCATTGAATCG-3′ | 1,108–1,088 |
NKCC, Na+-K+-2Cl− cotransporter; m, mouse.
Western blot analysis.
Analysis of pNKCC2 (13) expression was performed as previously described (14, 15). Briefly, renal tissue or cells were homogenized and solubilized with RIPA buffer (catalog no. 9806s, Cell Signaling Technology) or Nonidet P-40 lysis buffer for pNKCC2 (13) after protease inhibitors (Roche Diagnostics) were added. Protein content was measured in triplicate by a colorimetric assay using the modified Lowry assay. Lysates were heated at 100 or 60°C for 5 min, spun at 4°C for 5 min at 10,000 g, loaded into each lane of a 12% SDS-PAGE gel, and stained with Coomassie blue. Random bands were quantified by densitometry to assess equal loading. Equal amounts of protein were separated by SDS-PAGE and electrophoretically transferred to nitrocellulose membranes. Nonspecific protein-binding sites were blocked for 1 h by immersion in 5% nonfat dry milk in PBS. Membranes were subsequently incubated with primary antibody by an overnight incubation at 4°C. After incubation with primary antibodies, membranes were probed with secondary antibodies followed by treatment with enhanced chemiluminescence substrate (Pierce). Bands on the membrane were visualized and analyzed using the UVP BioImaging System, and the final reported data are the target protein band densities divided by β-actin density.
ELISA for the TNF-α level.
The TNF-α content in 100 μL urine was measured in duplicate by ELISA as previously described (43). MD/cTAL tubules were homogenized, and 100 μL of cell-free supernatants were used to determine TNF-α content. Data were normalized by protein amount, which was determined by a Bradford protein assay (Bio-Rad).
Radiotelemetric determination of blood pressure.
Direct measurement of blood pressure was accomplished using the Dataquest IV telemetry system and a radiotelemetry probe (PA-C10, Data Science International, St. Paul, MN) via a catheter that was inserted into the aorta through the right common carotid artery as previously described (13).
Assessment of renal excretory function.
Mice placed in metabolic cages (Harvard Apparatus, Holliston, MA) were used for experiments to evaluate the effects of renal TNF-α and NKCC2B silencing on Na+ and Cl− excretion as well as urine volume. Daily food intake, water intake, body weight, and urine excretion were measured. A 200-μL urine sample was diluted with 400 μL double-distilled H2O to obtain a diluted sample, from which 200 μL were used for the measurement of Na+ or Cl− concentration. Na+ and Cl− concentrations were determined using flame atomic absorption spectroscopy (Perkin-Elmer) or a SmartLyte Electrolyte ISE Analyzer (Diamond Diagnostics); duplicate measurements were made for each sample.
Statistical analysis.
GraphPad Prism 4 and Microsoft Excel were used for data analysis. Comparison between two groups was performed by an unpaired Student’s t test. Comparison between more than two groups was analyzed by one-way ANOVA. All data are expressed as means ± SE. P values of <0.05 were considered significant; P values are either shown directly in the figures or symbols for P values are described in the figure legends.
Study approval.
Animal experiments were performed in accordance with New York Medical College Institutional Animal Care and Use Committee approval and international guidelines for the welfare of animals (animal welfare assurance nos. A3362–01 or A5848–01, Office of Laboratory Animal Welfare, Public Health Service, National Institutes of Health).
RESULTS
LS intake decreases TNF-α in the renal cortex.
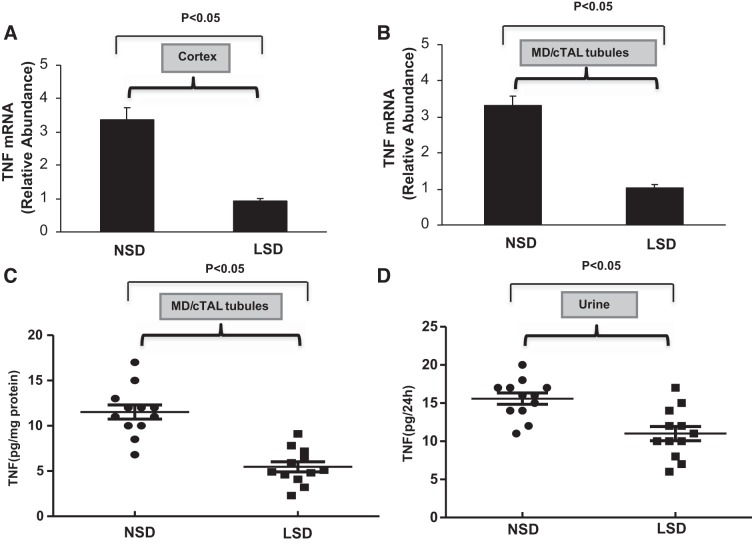
Quantitative RT-PCR was used to assess TNF-α mRNA expression in the renal cortex and MD/cTAL obtained from mice that ingested either a NSD or LSD for 7 days. TNF-α mRNA expression in the renal cortex (Fig. 1A) and MD/cTAL tubules (Fig. 1B) was significantly reduced after ingestion of the LSD compared with ingestion of the NSD. In control experiments, total RNA was amplified before cDNA synthesis to exclude possible contamination with genomic DNA; DNA sequences for the PCR fragments derived from renal tisuues were identical to those previously published for TNF-α (11, 13, 14). TNF-α protein expression in MD/cTAL tubules and urine was then determined by ELISA. Ingestion of the LSD reduced TNF-α levels in MD/cTAL tubules (Fig. 1C) and urine (Fig. 1D) compared with ingestion of the NSD for 7 days. Overall, the data suggest that, compared with basal TNF-α production under NSD conditions, LSD intake downregulates TNF-α synthesis by the MD/cTAL region in the renal cortex, an effect that is reflected by reduced urinary levels of TNF-α.
Fig. 1.
Effects of low salt on TNF-α expression in the kidney. Mice were given either a normal-salt diet (NSD) or low-salt diet (LSD) for 7 days. The relative abundance of TNF-α mRNA expression in the renal cortex (A) and microdissected macula densa (MD)/cortical thick ascending limb of Henle’s loop (cTAL) tubules (B) was measured by quantitative RT-PCR; n = 8. TNF-α content in MD/cTAL tubules (C) and urine (D) was determined in duplicate by ELISA (PharMingen); n = 12.
LS intake increases NKCC2B expression in the renal cortex.
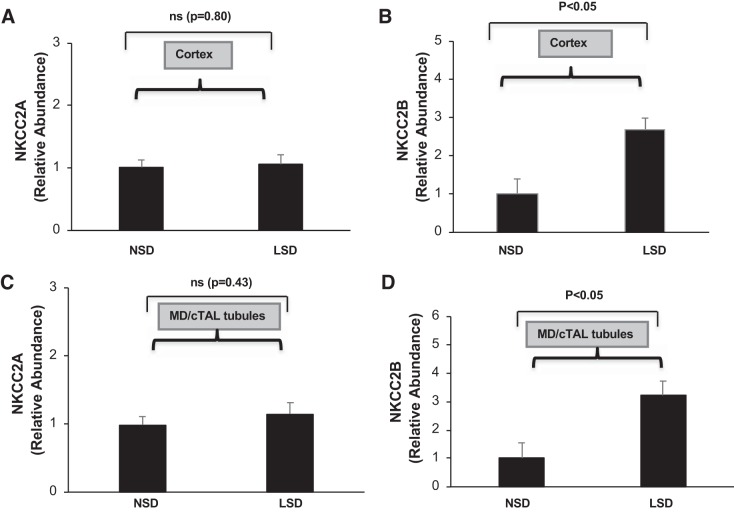
We have previously shown that the addition of 1% NaCl to the drinking water increases NKCC2A but not NKCC2F mRNA accumulation in the mTAL, suggesting that expression of NKCC2 isoforms is differentially affected by changes in NaCl intake (11, 13). Accordingly, specific primer pairs for murine NKCC2A and NKCC2B were designed using Epicentre software according to the cDNA sequences of mutually exclusive cassette exons A and B for mouse NKCC2 isoforms (Table 1) to evaluate the effects of a LSD on these isoforms in the cortex. Accumulation of NKCC2A mRNA did not change in mice that ingested the LSD compared with those given the NSD (Fig. 2A). In contrast, the relative abundance of NKCC2B mRNA increased approximately threefold in the renal cortex of mice given the LSD for 7 days compared with mice given the NSD (Fig. 2B). Similarly, NKCC2B but not NKCC2A mRNA accumulation increased in microdissected MD/cTAL tubules from mice that ingested the LSD for 7 days compared with mice given the NSD (Fig. 2, C and D). Collectively, these data indicate that low NaCl intake selectively increases NKCC2B expression in MD/cTAL tubules in the renal cortex.
Fig. 2.
Effects of low salt on Na+-K+-2Cl− cotransporter (NKCC2) isoforms (NKCC2A and NKCC2B) in the kidney. The relative abundance of mRNA levels for NKCC2A (A) and NKCC2B (B) in the renal cortex from mice given a normal-salt diet (NSD) or low-salt diet (LSD) for 7 days was determined by quantitative RT-PCR; n = 8. Similarly, NKCC2A (C) and NKCC2B (D) mRNA accumulation in freshly microdissected macula densa (MD)/cortical thick ascending limb of Henle’s loop (cTAL) tubules from mice that ingested the NSD or LSD for 7 days was determined by quantitative RT-PCR; n = 8. ns, Not significant.
Effects of renal-specific TNF-α silencing on NKCC2B expression.
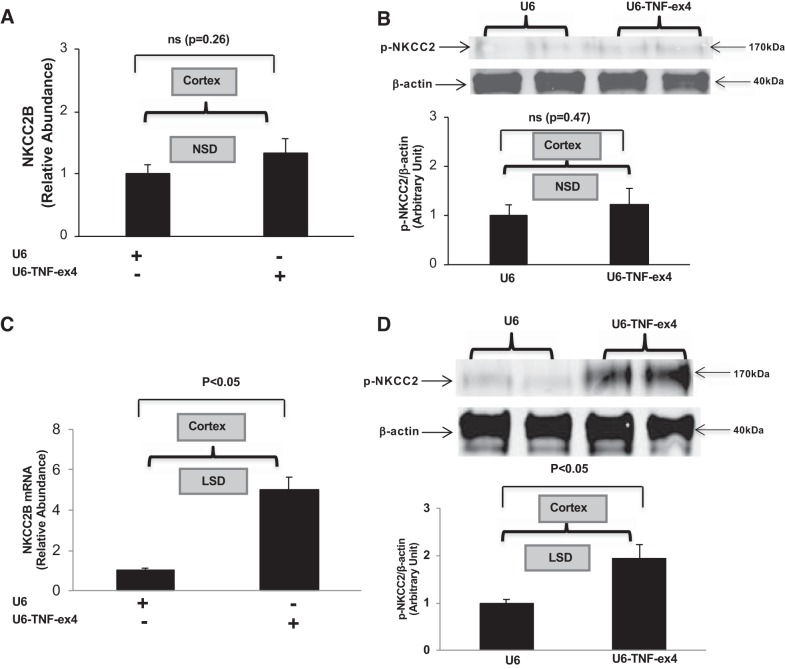
We achieved specific silencing of TNF-α in the kidney using a shRNA TNF-α lentivirus construct (U6-TNF-ex4) as previously described (13). In the present study, lentiviral transduction was used to evaluate a possible functional link between TNF-α and NKCC2B in vivo. Silencing of TNF-α with U6-TNF-ex4 did not significantly increase either NKCC2B mRNA accumulation (Fig. 3A) or pNKCC2 protein expression (Fig. 3B) in the renal cortex of mice that were given the NSD for 7 days. In contrast, silencing TNF-α greatly increased both NKCC2B mRNA levels (Fig. 3C) and pNKCC2 protein expression (Fig. 3D) in the renal cortex of mice that were given the LSD for 7 days. Collectively, these data suggest that the increases in NKCC2B mRNA and pNKCC2 expression induced by the LSD are facilitated in the absence of renal production of TNF-α.
Fig. 3.
Renal silencing of TNF-α contributes to Na+-K+-2Cl− cotransporter (NKCC2) isoform B (NKCC2B) and phosphorylated (p-)NKCC2 expression in the kidney. Mice were pretreated with control (U6) or TNF-α silencing (U6-TNF-ex4) lentivirus for 3 days and then given either a normal-salt diet (NSD) or low-salt diet (LSD) for 7 days. The relative abundance of NKCC2B mRNA expression (A) and pNKCC2 expression (B) in the renal cortex from mice given the NSD were determined by quantitative RT-PCR and Western blot analysis, respectively (n = 6), as were NKCC2B mRNA expression (C) and p-NKCC2 expression (D) in mice given the LSD (n = 4). ns, Not significant.
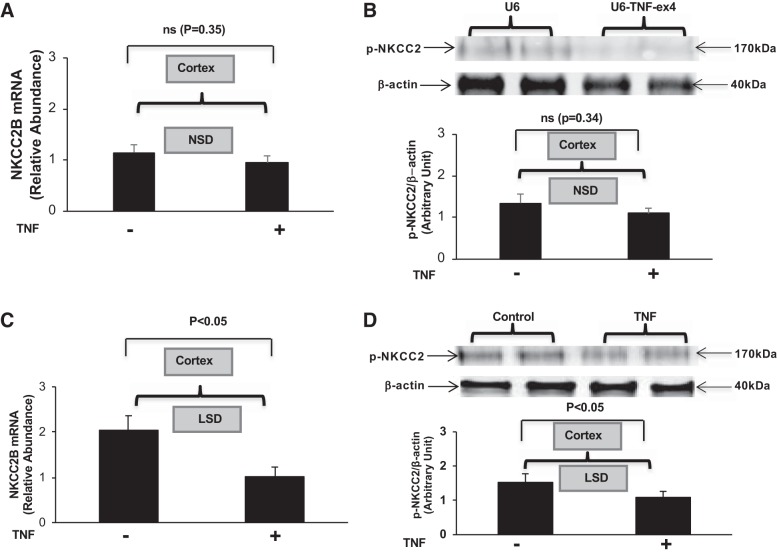
Intrarenal administration of TNF-α inhibits NKCC2B mRNA and pNKCC2 expression.
The effects of TNF-α on NKCC2B mRNA and pNKCC2 expression in mice given the NSD or LSD were determined in renal cortexes obtained from mice given a single intrarenal injection of murine recombinant TNF-α (5 ng/g body wt) or saline control into each kidney for 24 h and then given either diet for 7 days. Administration of TNF-α did not significantly affect NKCC2B mRNA (Fig. 4A) or pNKCC2 protein expression (Fig. 4B) in cortexes from mice given the NSD compared with the control group. However, TNF-α inhibited both NKCC2B mRNA (Fig. 4C) and pNKCC2 protein expression (Fig. 4D) in mice given the LSD for 7 days. Collectively, these data suggest that TNF-α acts as a negative regulator of NKCC2B expression and activity in the context of salt restriction.
Fig. 4.
Intrarenal administration of TNF-α inhibits Na+-K+-2Cl − cotransporter (NKCC2) isoform B (NKCC2B) mRNA and phosphorylated (p-)NKCC2 expression. NKCC2B mRNA (A) and p-NKCC2 (B) expression were determined in the cortex obtained from mice that ingested a normal-salt diet (NSD) for 7 days after a single intrarenal injection of murine recombinant TNF-α compared with the saline-injected control group; n = 6. NKCC2B mRNA (C) and p-NKCC2 (D) expression were determined in mice that ingested a low-salt diet (LSD) for 7 days after a single intrarenal injection of murine recombinant TNF-α compared with the control group; n = 4. ns, Not significant.
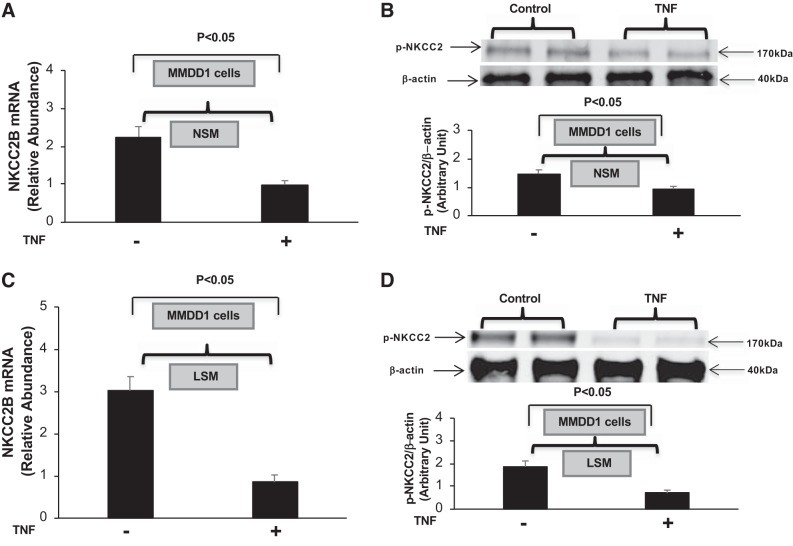
TNF-α inhibits NKCC2B mRNA and pNKCC2 expression in MMDD1 cells.
The MD, which expresses NKCC2B, plays a pivotal role in physiological feedback mechanisms related to sensing changes in tubular salt content. Accordingly, we examined whether the addition of exogenous TNF-α alters NKCC2B expression and pNKCC2 levels using a MD-like cell line (MMDD1 cells). Confluent MMDD1 cells were incubated in NSM or LSM for 4 h in the presence or absence of TNF-α (1 nM) or vehicle. TNF-α markedly inhibited both NKCC2B mRNA accumulation and pNKCC2 expression under both NSM and LSM conditions (Fig. 5, A–D). These data suggest that TNF-α-mediated inhibition of NKCC2B expression and NKCC2 activity in MMDD1 cells can occur independently of NaCl concentration.
Fig. 5.
TNF-α inhibits Na+-K+-2Cl− cotransporter (NKCC2) isoform B (NKCC2B) and phosphorylated (p-)NKCC2 expression in a mouse macula densa-like cell line (MMDD1 cells). NKCC2B mRNA (A) and p-NKCC2 (B) expression were determined in cultured MMDD1 cells treated for 4 h with normal-salt medium (NSM) containing either TNF-α (1 nM) or vehicle; n = 6. NKCC2B mRNA (C) and pNKCC2 (D) expression were determined in cultured MMDD1 cells treated for 4 h with low-salt medium (LSM) medium containing either TNF-α (1 nM) or vehicle; n = 6.
Effects of renal TNF-α and NKCC2B silencing on renal function during LS intake.
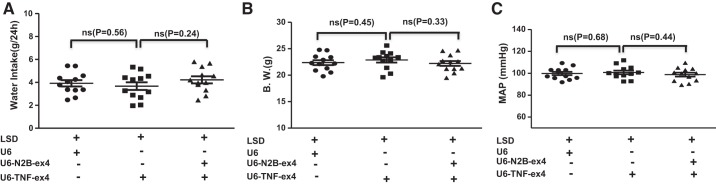
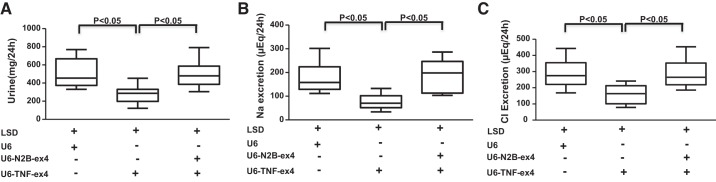
To determine the functional effects of renal TNF-α in the context of LS conditions, experiments were performed using mice that were placed in metabolic cages and that received intrarenal injections of control (U6), TNF-α silencing (U6-TNF-ex4), or a combination of U6-TNF-ex4 and NKCC2B-silencing (U6-N2B-ex4) lentivirus constructs. Mice were injected with lentivirus and then acclimatized for 3 days in metabolic cages and given the LSD and tap water ad libitum for 7 days. Urine was collected, and urine volume, Na+, Cl−, and water intake were determined. There were no significant changes in water intake, body weight, or blood pressure between groups (Fig. 6). However, under LS conditions, renal silencing of TNF-α elicited decreases in urine volume (Fig. 7A) and Na+ (Fig. 7B) and Cl− excretion (Fig. 7C) compared with mice injected with control lentivirus. Concomitant inhibition of TNF-α and NKCC2B reversed the inhibitory effects of TNF-α silencing on urine volume and Na+ and Cl− excretion. Taken together, the data suggest that TNF-α attenuates NKCC2B expression under LS conditions in a manner that maintains salt and water homeostasis.
Fig. 6.
Effects of silencing TNF-α and Na+-K+-2Cl− cotransporter isoform B on water intake, body weight, and blood pressure. Mice injected with U6, U6-TNF-ex4, or a combination of U6-TNF-ex4 and U6-N2B-ex4 were acclimatized for 3 days in metabolic cages and given tap water ad libitum. Water intake (A) and body weight (B.W.; B) were determined in mice that ingested a low-salt diet (LSD) for 7 days. Mean arterial pressure (MAP) values (C) obtained by radiotelemetry are shown for day 7; n = 12. ns, Not significant.
Fig. 7.
Effects of silencing TNF-α and Na+-K+-2Cl− cotransporter isoform B on renal excretory function. Mice injected with U6, U6-TNF-ex4, or a combination of U6-TNF-ex4 and U6-N2B-ex4 were acclimatized for 3 days in metabolic cages and given tap water ad libitum. Urine was collected for determinations of volume (A), Na+ (B), and Cl− (C) 24 h after mice were provided with access to a low-salt diet (LSD) for 7 days; n = 8.
DISCUSSION
We demonstrated that LS conditions suppress TNF-α mRNA and protein expression in the renal cortex and MD/cTAL tubules, an effect that is reflected by reduced urinary levels of TNF-α. Ingestion of a LSD also selectively increased expression of the NKCC2B isoform. Intrarenal silencing of TNF-α amplified the LS-mediated increase in NKCC2B mRNA accumulation in MD/cTAL tubules by more than fourfold and amplified pNKCC2B expression by approximately twofold. In contrast, intrarenal administration of recombinant TNF-α attenuated the LS-mediated increase in NKCC2B mRNA accumulation and pNKCC2 expression, effects that were mimicked in cultured MMDD1 cells that were exposed to exogenous TNF-α. Silencing TNF-α in the kidney reduced urine volume and NaCl excretion in mice that ingested the LSD, an effect that was reversed by concomitant silencing of NKCC2B. Thus, downregulation of TNF-α production in the MD/cTAL region in response to LS intake contributes to the regulation of NaCl reabsorption via a NKCC2B-dependent mechanism.
NKCC2 isoform expression differs along the corticomedullary axis of the TAL and MD regions and plays an important role in NaCl homeostasis (10, 16, 45). NKCC2B, which is the least abundant of all NKCC2 isoforms, is expressed primarily in the cTAL and MD (27). Thus, a reduction in luminal NaCl concentration is sensed by MD cells, which form a cluster of specialized sensor cells that detect changes in TAL fluid composition and contribute to tubuloglomerular feedback (4, 27), and results in the induction of NKCC2B expression and activation (35, 42, 45). The present data are consistent with this finding and suggest that TNF-α is part of a mechanism that contributes to the fine tuning of NaCl transport under LS conditions that are mediated by NKCC2B. Suppression of TNF-α production by LS intake was observed concomitantly with an increase in NKCC2B and pNKCC2 levels as well as a decrease in NaCl excretion, although body weight and blood pressure remained unchanged. Moreover, these responses were linked to the degree of TNF-α suppression, as lentivirus silencing of TNF-α, which reduced TNF-α levels more than LS conditions alone, yielded levels of NKCC2B and pNKCC2 expression that greatly exceded those induced by LS alone. In contrast, the intrarenal injection of TNF-α inhibited increases in NKCC2B and pNKCC2 induced by the LSD. Although this is, to our knowledge, the first report of TNF-α altering NKCC2B in response to restriction in salt intake, its ability to modulate NKCC2 isoform expression is neither limited to the cortex nor limited to LS intake since genetic deletion of this cytokine selectively increases expression of NKCC2A in the mTAL of mice that ingested a NSD (3). Moreover, renal-specific silencing of TNF-α unmasks an NKCC2A-dependent increase in blood pressure in mice given a high-salt diet (13). These data are consistent with the findings that the mTAL produces TNF-α in response to high NaCl intake and exogenous administration of recombinant TNF-α to mice elicits natriuretic responses (11, 38, 39). Collectively, the differential regulation of TNF-α production in the renal cortex and medulla in response to LS and high salt intake, respectively, support the emerging concept that TNF-α contributes to the regulation of NaCl homeostasis by modulating the expression of regulatory NKCC2 isoforms capable of responding to differences in NaCl delivery along the nephron.
During dietary Na+ restriction, multiple mechanisms, including those in the MD, act to increase NaCl reabsorption. Since high salt intake and hypertonicity in vitro increased renal TNF-α production (11), we hypothesized that LS conditions would decrease TNF-α production by the kidney. Indeed, mice placed on the LSD had lower levels of urinary TNF-α compared with those given the NSD. Moreover, TNF-α mRNA and protein was reduced in MD/cTAL isolated from mice given the LSD. Regulation of TNF-α production in response to changes in dietary salt intake may help modulate the expression of NKCC2 isoforms that are concurrently altered in response to changes in NaCl concentration along the nephron. Thus, the reduced excretion of NaCl and urine volume in response to LS intake are primarily the result of an increase in NKCC2B expression in the MD/cTAL region (5). However, as TNF-α has an inhibitory effect on NKCC2B expression, silencing of TNF-α in the kidney of mice given a LSD increases NKCC2B-mediated reabsorption of NaCl, an effect that was reversed by concomitant silencing of TNF-α and NKCC2B. These data suggest that TNF-α regulates the extent to which LS conditions increase NKCC2B as a means to limit NaCl reabsorption. We have recently shown that high NaCl intake increases TNF-α as part of an adaptive mechanism that inhibits NKCC2A and limits NaCl reabsorption and blood pressure (13). The results of the present study suggest that limiting NaCl reabsorption is a fundamental function of TNF-α in the kidney via its regulation of distinct NKCC2 isoforms. Collectively, these findings are consistent with the concept of an intrarenal regulatory system that uses TNF-α derived from renal epithelial cells to modulate the activity and expression of NKCC2 isoforms.
TNF-α is the quintessential pleiotropic cytokine, and it is produced not only by macrophages and T cells but also by many nonhemopoietic cell types. In the kidney, TNF-α is produced by several cell types, including proximal tubule, TAL, collecting duct, and mesangial cells as well as podocytes (17, 20, 23, 24, 34). The conditions that regulate TNF-α production by individual nephron segments and the intrinsic effects of this cytokine in each segment are still being defined despite seminal studies that have shown that cytokines induce changes in renal function (19). TNF-α exhibits regulatory effects in diverse epithelial cells, including those in the kidney, lung, and colon, in which active Na+ transport is important for fluid clearance and other homeostatic mechanisms (1, 6, 33). To the best of our knowledge, the present study is the first to show modulation by TNF-α of the increase in NKCC2B isoform expression in response to a LSD, and this study extends the functional effects of TNF-α along the nephron beyond the medullary segment of the TAL. These findings aid in the understanding of the intrinsic effects of TNF-α along the nephron under noninflammatory conditions and may help to explain a role for TNF-α in the regulation of genetic or acquired defects of distal tubular function, such as salt-losing tubulopathies involving basolateral extrusion defects, including those associated with type 3 Bartter’s syndrome as well as those in patients with arterial hypertension and congestive heart failure, whose retention of salt may benefit from lowering dietary salt intake.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant R01-HL-133077.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.H. and N.R.F. conceived and designed research; S.H., J.S., and M.H. performed experiments; S.H., J.S., M.H., and N.R.F. analyzed data; S.H., J.S., M.H., and N.R.F. interpreted results of experiments; S.H., M.H., and N.R.F. prepared figures; S.H. and N.R.F. drafted manuscript; S.H. and N.R.F. edited and revised manuscript; S.H., J.S., M.H., and N.R.F. approved final version of manuscript.
REFERENCES
- 1.Amasheh S, Barmeyer C, Koch CS, Tavalali S, Mankertz J, Epple HJ, Gehring MM, Florian P, Kroesen AJ, Zeitz M, Fromm M, Schulzke JD. Cytokine-dependent transcriptional down-regulation of epithelial sodium channel in ulcerative colitis. Gastroenterology 126: 1711–1720, 2004. doi: 10.1053/j.gastro.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 2.Anandam KY, Alwan OA, Subramanian VS, Srinivasan P, Kapadia R, Said HM. Effect of the proinflammatory cytokine TNF-α on intestinal riboflavin uptake: inhibition mediated via transcriptional mechanism(s). Am J Physiol Cell Physiol 315: C653–C663, 2018. doi: 10.1152/ajpcell.00295.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Battula S, Hao S, Pedraza PL, Stier CT, Ferreri NR. Tumor necrosis factor-α is an endogenous inhibitor of Na+-K+-2Cl− cotransporter (NKCC2) isoform A in the thick ascending limb. Am J Physiol Renal Physiol 301: F94–F100, 2011. doi: 10.1152/ajprenal.00650.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Castrop H, Schnermann J. Isoforms of renal Na-K-2Cl cotransporter NKCC2: expression and functional significance. Am J Physiol Renal Physiol 295: F859–F866, 2008. doi: 10.1152/ajprenal.00106.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Edwards A, Castrop H, Laghmani K, Vallon V, Layton AT. Effects of NKCC2 isoform regulation on NaCl transport in thick ascending limb and macula densa: a modeling study. Am J Physiol Renal Physiol 307: F137–F146, 2014. doi: 10.1152/ajprenal.00158.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Escalante BA, Ferreri NR, Dunn CE, McGiff JC. Cytokines affect ion transport in primary cultured thick ascending limb of Henle’s loop cells. Am J Physiol Cell Physiol 266: C1568–C1576, 1994. doi: 10.1152/ajpcell.1994.266.6.C1568. [DOI] [PubMed] [Google Scholar]
- 7.Gagnon E, Forbush B, Caron L, Isenring P. Functional comparison of renal Na-K-Cl cotransporters between distant species. Am J Physiol Cell Physiol 284: C365–C370, 2003. doi: 10.1152/ajpcell.00262.2002. [DOI] [PubMed] [Google Scholar]
- 8.Gamba G, Friedman PA. Thick ascending limb: the Na+:K+:2Cl− co-transporter, NKCC2, and the calcium-sensing receptor, CaSR. Pflugers Arch 458: 61–76, 2009. doi: 10.1007/s00424-008-0607-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.García NH, Plato CF, Stoos BA, Garvin JL. Nitric oxide-induced inhibition of transport by thick ascending limbs from Dahl salt-sensitive rats. Hypertension 34: 508–513, 1999. doi: 10.1161/01.HYP.34.3.508. [DOI] [PubMed] [Google Scholar]
- 10.Giménez I, Isenring P, Forbush B. Spatially distributed alternative splice variants of the renal Na-K-Cl cotransporter exhibit dramatically different affinities for the transported ions. J Biol Chem 277: 8767–8770, 2002. doi: 10.1074/jbc.C200021200. [DOI] [PubMed] [Google Scholar]
- 11.Hao S, Bellner L, Ferreri NR. NKCC2A and NFAT5 regulate renal TNF production induced by hypertonic NaCl intake. Am J Physiol Renal Physiol 304: F533–F542, 2013. doi: 10.1152/ajprenal.00243.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hao S, Bellner L, Zhao H, Ratliff BB, Darzynkiewicz Z, Vio CP, Ferreri NR. NFAT5 is protective against ischemic acute kidney injury. Hypertension 63: e46–e52, 2014. doi: 10.1161/HYPERTENSIONAHA.113.02476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hao S, Hao M, Ferreri NR. Renal-specific silencing of TNF (tumor necrosis factor) unmasks salt-dependent increases in blood pressure via an NKCC2A (Na+-K+-2Cl− cotransporter isoform A)-dependent mechanism. Hypertension 71: 1117–1125, 2018. doi: 10.1161/HYPERTENSIONAHA.117.10764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hao S, Zhao H, Darzynkiewicz Z, Battula S, Ferreri NR. Expression and function of NFAT5 in medullary thick ascending limb (mTAL) cells. Am J Physiol Renal Physiol 296: F1494–F1503, 2009. doi: 10.1152/ajprenal.90436.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hao S, Zhao H, Darzynkiewicz Z, Battula S, Ferreri NR. Differential regulation of NFAT5 by NKCC2 isoforms in medullary thick ascending limb (mTAL) cells. Am J Physiol Renal Physiol 300: F966–F975, 2011. doi: 10.1152/ajprenal.00408.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Igarashi P, Vanden Heuvel GB, Payne JA, Forbush B III. Cloning, embryonic expression, and alternative splicing of a murine kidney-specific Na-K-Cl cotransporter. Am J Physiol Renal Physiol 269: F405–F418, 1995. doi: 10.1152/ajprenal.1995.269.3.F405. [DOI] [PubMed] [Google Scholar]
- 17.Jevnikar AM, Brennan DC, Singer GG, Heng JE, Maslinski W, Wuthrich RP, Glimcher LH, Kelley VE. Stimulated kidney tubular epithelial cells express membrane associated and secreted TNF alpha. Kidney Int 40: 203–211, 1991. doi: 10.1038/ki.1991.201. [DOI] [PubMed] [Google Scholar]
- 18.Kleta R, Bockenhauer D. Salt-losing tubulopathies in children: what’s new, what’s controversial? J Am Soc Nephrol 29: 727–739, 2018. doi: 10.1681/ASN.2017060600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kohan DE, Merli CA, Simon EE. Micropuncture localization of the natriuretic effect of interleukin 1. Am J Physiol Renal Physiol 256: F810–F813, 1989. doi: 10.1152/ajprenal.1989.256.5.F810. [DOI] [PubMed] [Google Scholar]
- 20.Lai KN, Leung JC, Chan LY, Saleem MA, Mathieson PW, Lai FM, Tang SC. Activation of podocytes by mesangial-derived TNF-alpha: glomerulo-podocytic communication in IgA nephropathy. Am J Physiol Renal Physiol 294: F945–F955, 2008. doi: 10.1152/ajprenal.00423.2007. [DOI] [PubMed] [Google Scholar]
- 21.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−ΔΔCT) method. Methods 25: 402–408, 2001. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 22.Lu Y, Wei J, Stec DE, Roman RJ, Ge Y, Cheng L, Liu EY, Zhang J, Hansen PB, Fan F, Juncos LA, Wang L, Pollock J, Huang PL, Fu Y, Wang S, Liu R. Macula densa nitric oxide synthase 1β protects against salt-sensitive hypertension. J Am Soc Nephrol 27: 2346–2356, 2016. doi: 10.1681/ASN.2015050515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Macica CM, Escalante BA, Conners MS, Ferreri NR. TNF production by the medullary thick ascending limb of Henle’s loop. Kidney Int 46: 113–121, 1994. doi: 10.1038/ki.1994.250. [DOI] [PubMed] [Google Scholar]
- 24.Markewitz BA, Michael JR, Kohan DE. Cytokine-induced expression of a nitric oxide synthase in rat renal tubule cells. J Clin Invest 91: 2138–2143, 1993. doi: 10.1172/JCI116439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mederle K, Mutig K, Paliege A, Carota I, Bachmann S, Castrop H, Oppermann M. Loss of WNK3 is compensated for by the WNK1/SPAK axis in the kidney of the mouse. Am J Physiol Renal Physiol 304: F1198–F1209, 2013. doi: 10.1152/ajprenal.00288.2012. [DOI] [PubMed] [Google Scholar]
- 26.Mutig K, Paliege A, Kahl T, Jöns T, Müller-Esterl W, Bachmann S. Vasopressin V2 receptor expression along rat, mouse, and human renal epithelia with focus on TAL. Am J Physiol Renal Physiol 293: F1166–F1177, 2007. doi: 10.1152/ajprenal.00196.2007. [DOI] [PubMed] [Google Scholar]
- 27.Oppermann M, Mizel D, Huang G, Li C, Deng C, Theilig F, Bachmann S, Briggs J, Schnermann J, Castrop H. Macula densa control of renin secretion and preglomerular resistance in mice with selective deletion of the B isoform of the Na,K,2Cl co-transporter. J Am Soc Nephrol 17: 2143–2152, 2006. doi: 10.1681/ASN.2006040384. [DOI] [PubMed] [Google Scholar]
- 28.Oppermann M, Mizel D, Kim SM, Chen L, Faulhaber-Walter R, Huang Y, Li C, Deng C, Briggs J, Schnermann J, Castrop H. Renal function in mice with targeted disruption of the A isoform of the Na-K-2Cl co-transporter. J Am Soc Nephrol 18: 440–448, 2007. doi: 10.1681/ASN.2006091070. [DOI] [PubMed] [Google Scholar]
- 29.Overbergh L, Valckx D, Waer M, Mathieu C. Quantification of murine cytokine mRNAs using real time quantitative reverse transcriptase PCR. Cytokine 11: 305–312, 1999. doi: 10.1006/cyto.1998.0426. [DOI] [PubMed] [Google Scholar]
- 30.Peti-Peterdi J, Harris RC. Macula densa sensing and signaling mechanisms of renin release. J Am Soc Nephrol 21: 1093–1096, 2010. doi: 10.1681/ASN.2009070759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29: e45, 2001. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Plata C, Meade P, Vazquez N, Hebert SC, Gamba G. Functional properties of the apical Na+-K+-2Cl− cotransporter isoforms. J Biol Chem 277: 11004–11012, 2002. doi: 10.1074/jbc.M110442200. [DOI] [PubMed] [Google Scholar]
- 33.Rezaiguia S, Garat C, Delclaux C, Meignan M, Fleury J, Legrand P, Matthay MA, Jayr C. Acute bacterial pneumonia in rats increases alveolar epithelial fluid clearance by a tumor necrosis factor-alpha-dependent mechanism. J Clin Invest 99: 325–335, 1997. doi: 10.1172/JCI119161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rosa AC, Rattazzi L, Miglio G, Collino M, Fantozzi R. Angiotensin II induces tumor necrosis factor-α expression and release from cultured human podocytes. Inflamm Res 61: 311–317, 2012. doi: 10.1007/s00011-011-0412-8. [DOI] [PubMed] [Google Scholar]
- 35.Schießl IM, Rosenauer A, Kattler V, Minuth WW, Oppermann M, Castrop H. Dietary salt intake modulates differential splicing of the Na-K-2Cl cotransporter NKCC2. Am J Physiol Renal Physiol 305: F1139–F1148, 2013. doi: 10.1152/ajprenal.00259.2013. [DOI] [PubMed] [Google Scholar]
- 36.Schnermann J. Sodium transport deficiency and sodium balance in gene-targeted mice. Acta Physiol Scand 173: 59–66, 2001. doi: 10.1046/j.1365-201X.2001.00885.x. [DOI] [PubMed] [Google Scholar]
- 37.Seyberth HW, Schlingmann KP. Bartter- and Gitelman-like syndromes: salt-losing tubulopathies with loop or DCT defects. Pediatr Nephrol 26: 1789–1802, 2011. doi: 10.1007/s00467-011-1871-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shahid M, Francis J, Majid DS. Tumor necrosis factor-alpha induces renal vasoconstriction as well as natriuresis in mice. Am J Physiol Renal Physiol 295: F1836–F1844, 2008. doi: 10.1152/ajprenal.90297.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shahid M, Francis J, Matrougui K, Majid DS. Involvement of tumor necrosis factor-alpha in natriuretic response to systemic infusion of nitric oxide synthase inhibitor in anesthetized mice. Am J Physiol Renal Physiol 299: F217–F224, 2010. doi: 10.1152/ajprenal.00611.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sriramula S, Haque M, Majid DS, Francis J. Involvement of tumor necrosis factor-alpha in angiotensin II-mediated effects on salt appetite, hypertension, and cardiac hypertrophy. Hypertension 51: 1345–1351, 2008. doi: 10.1161/HYPERTENSIONAHA.107.102152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Staelens J, Wielockx B, Puimège L, Van Roy F, Guénet JL, Libert C. Hyporesponsiveness of SPRET/Ei mice to lethal shock induced by tumor necrosis factor and implications for a TNF-based antitumor therapy. Proc Natl Acad Sci USA 99: 9340–9345, 2002. doi: 10.1073/pnas.142293699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vargas-Poussou R, Feldmann D, Vollmer M, Konrad M, Kelly L, van den Heuvel LP, Tebourbi L, Brandis M, Karolyi L, Hebert SC, Lemmink HH, Deschênes G, Hildebrandt F, Seyberth HW, Guay-Woodford LM, Knoers NV, Antignac C. Novel molecular variants of the Na-K-2Cl cotransporter gene are responsible for antenatal Bartter syndrome. Am J Hum Genet 62: 1332–1340, 1998. doi: 10.1086/301872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang D, Pedraza PL, Abdullah HI, McGiff JC, Ferreri NR. Calcium-sensing receptor-mediated TNF production in medullary thick ascending limb cells. Am J Physiol Renal Physiol 283: F963–F970, 2002. doi: 10.1152/ajprenal.00108.2002. [DOI] [PubMed] [Google Scholar]
- 44.Wright PA, Burg MB, Knepper MA. Microdissection of kidney tubule segments. Methods Enzymol 191: 226–231, 1990. doi: 10.1016/0076-6879(90)91015-X. [DOI] [PubMed] [Google Scholar]
- 45.Yang T, Huang YG, Singh I, Schnermann J, Briggs JP. Localization of bumetanide- and thiazide-sensitive Na-K-Cl cotransporters along the rat nephron. Am J Physiol Renal Physiol 271: F931–F939, 1996. doi: 10.1152/ajprenal.1996.271.4.F931. [DOI] [PubMed] [Google Scholar]
- 46.Yang T, Park JM, Arend L, Huang Y, Topaloglu R, Pasumarthy A, Praetorius H, Spring K, Briggs JP, Schnermann J. Low chloride stimulation of prostaglandin E2 release and cyclooxygenase-2 expression in a mouse macula densa cell line. J Biol Chem 275: 37922–37929, 2000. doi: 10.1074/jbc.M006218200. [DOI] [PubMed] [Google Scholar]