Abstract
The discovery of new genetic mutations that cause hypertension has illuminated previously unrecognized physiological pathways. One such regulatory pathway was identified when mutations in with no lysine kinase (WNK)4, Kelch-like 3 (KLHL3), and cullin 3 (CUL3) were shown to cause the disease familial hyperkalemic hypertension (FHHt). Mutations in all three genes upregulate the NaCl cotransporter (NCC) due to an impaired ability to degrade WNK protein through the cullin-RING-ligase (CRL) ubiquitin-proteasome system. The CUL3 FHHt mutations cause the most severe phenotype, yet the precise mechanism by which these mutations cause the disease has not been established and current proposed models are controversial. New data have identified a possible novel mechanism involving dysregulation of CUL3 activity by the COP9 signalosome (CSN). The CSN interaction with mutant CUL3 is diminished, causing hyperneddylation of the CRL. Recent work has shown that direct renal CSN impairment mimics some aspects of the CUL3 mutation, including lower KLHL3 abundance and activation of the WNK-NCC pathway. Furthermore, in vitro and in vivo studies of CSN inhibition have shown selective degradation of CRL substrate adaptors via auto-ubiquitination, allowing substrate accumulation. In this review, we will focus on recent research that highlights the role of the CSN role in CUL3 mutations that cause FHHt. We will also highlight how these results inform other recent studies of CSN dysfunction.
Keywords: COP9 signalosome, cullin 3, distal nephron, familial hyperkalemic hypertension
INTRODUCTION
E3 ubiquitin ligases covalently attach ubiquitin motifs to proteins, targeting them for degradation via the proteasome. The cullin-RING-ubiquitin ligase (CRL) family regulates ~20% of all proteasome-degraded proteins and represents ~30% of all E3 ligases (31). Since E3 ubiquitin ligases are the primary signaling pathway for the regulation of protein turnover, CRLs play an important role in multiple cellular processes, including DNA damage repair, cell cycle progression, development, and signal transduction. An important NaCl transport regulatory pathway was recently discovered to be regulated by CRLs. Mutations in with no lysine kinase 4 (WNK4) (37), Kelch-like 3 (KLHL3) (17), and cullin 3 (CUL3) (3) cause the Mendelian disease familial hyperkalemic hypertension (FHHt), the pathophysiology of which involves activation of the NaCl cotransporter (NCC) in the distal convoluted tubule (DCT) (20). Treatment with thiazide diuretics ameliorates the disease phenotype. Under normal conditions, WNK4 activates NCC through a kinase signaling cascade. The recently discovered mutations uncovered a regulatory pathway in which CUL3 ubiquitinates and degrades WNKs via the adaptor protein KLHL3 (22, 29). Ultimately, the major contributing factor in the mechanism for all FHHt-causing mutations is an increase in WNK4 protein. The WNK4 and KLHL3 mutations cause the disease by preventing interaction with the cullin-RING complex, allowing WNK4 to accumulate (34). Unlike WNK4 and KLHL3, where the mechanisms by which mutations increase WNK4 abundance are relatively clear, there are multiple competing hypotheses for mechanisms by which CUL3 mutations increase WNKs to cause the disease (12). All hypertension-causing CUL3 mutations reported to date result in skipping of exon 9, causing loss of amino acids 403–459 (referred to here as CUL3Δ9). Recent work has uncovered a possible novel mechanism by which the CUL3Δ9 mutant protein lacks the ability to interact normally with the COP9 signalosome (CSN). The CSN is a multisubunit enzyme that regulates all CRL activity by catalyzing the removal of the ubiquitin-like modifier NEDD8 (6). The CSN has been studied extensively since it was first discovered in plants in 1994 (36), although its role in regulating blood pressure has only recently been recognized. Here, we describe recent work focusing on the role of the CSN in the hypertension-causing CUL3Δ9 mutation and discuss other models of CSN impairment, which show similar effects seen in the mutant.
CSN REGULATION OF CRLs
The diverse function of CRLs results from their modular structure, consisting of one of eight cullin (CUL1, CUL2, CUL3, CUL4A, CUL4B, CUL5, CUL7, and CUL9) scaffold proteins (4). Attached to the COOH terminus is the RING subunit (Rbx1/Rbx2), which interacts with the ubiquitin-conjugating enzyme (E2) and facilitates ubiquitin transfer. The target substrate binds to the NH2 terminus of the cullin protein through variable adaptor and substrate receptor proteins. For most cullin proteins, the adaptor and substrate receptor are two separate proteins, but not for CUL3, which binds to adaptor/substrate receptor complexes called broad complex, tramtrack, bric-a-brac (BTB) proteins (for simplicity, we will refer to all adaptors and substrate receptors as substrate adaptors). Each substrate adaptor can bind a specific cullin protein and one or a few specific substrates (24). With more than 400 known adaptors, CRLs are the largest family of E3 ubiquitin ligases (5).
CRLs undergo an enzymatic activation step, in which the ubiquitin-like NEDD8 posttranslational modifier is covalently attached to the cullin protein (23). It is important here to indicate that NEDD8 is distinct from the ubiquitin ligase Nedd4-2, a protein that regulates the amiloride-sensitive epithelial Na+ channel. This neddylation process modifies the cullin-RING complex, allowing ubiquitin transfer to the substrate (26). To prevent anomalous autoubiquitylation when substrate abundance is low, the CRL is “turned off” via enzymatic NEDD8 removal by interacting with the deneddylase CSN (24). All CRL activity is regulated by the CSN; it prevents hyperneddylation, which can alter normal function. The CSN comprises eight unique subunits, CSN1–CSN8. All eight subunits are necessary for activity. The CSN5 subunit, also known as JAB1 (referred to here as CSN5/JAB1), however, contains the sole JAMM Zn2+-metalloprotease motif, which gives the CSN its catalytic activity (16). Since neddylation activates CRLs, CSN deneddylation was thought to inhibit ubiquitin ligase activity, and, indeed, in vitro analysis supports this idea (38). CSN dysfunction in vivo, however, paradoxically disrupts, rather than stimulates, CRL activity and inhibits substrate degradation (25). This is most likely because overactive CRLs often shift their action to facilitate degradation of their substrate adaptors instead of the substrate (11, 14, 18, 35). The current model posits that CSN deneddylation prevents auto-ubiquitination and allows dismantling of the CRL proteins for adaptor/substrate recycling (19).
CSN BINDING AND ACTIVITY TOWARD CUL3Δ9 IS DISRUPTED
The first evidence for diminished CSN function toward CUL3Δ9 was discovered by our laboratory in 2014 (21). When expressed in cultured cells, CUL3Δ9 was shown to be in a highly neddylated state, suggesting enhanced activity. Although CUL3Δ9 showed an impaired degradation of the substrate WNK4, the mutant had increased ubiquitin-ligase activity toward the substrate adaptor KLHL3. KLHL3 destruction was prevented by the neddylation inhibitor MLN4924, suggesting that the altered function of CUL3Δ9 was from hyperneddylation. These findings were confirmed by us and others (10, 13, 28, 39) and suggested that KLHL3 degradation contributes to WNK4 accumulation. Schumacher et al. (28) further demonstrated impaired CSN activity toward CUL3Δ9 by showing that the mutation had a decreased rate of deneddylation. They also, however, detected enhanced auto-ubiquitylation and degradation of the mutant CUL3 protein. Additionally, the study was the first to look directly at the interaction of the CSN with CUL3Δ9. Immunoprecipitation showed an almost complete loss of CUL3Δ9 binding to both CSN5/JAB1 and CSN8 subunits. Our group confirmed that CSN5/JAB1 binding was reduced and subsequently determined the precise location of interaction along the CUL3 protein (10). As mentioned above, CUL3Δ9 is missing amino acids 403–459, which contain the 4HB domain. Our results, however, showed that the CSN binds to an adjacent α/β1-domain. Thus, the CUL3Δ9 mutation causes changes to its protein structure, disturbing the downstream binding site for the CSN. This diminishes the interaction between the two proteins leading to hyperneddylation of CUL3Δ9. It may also cause auto-ubiquitylation of mutant CUL3, leading to auto-degradation.
FHHT CUL3 MUTATIONS CAUSE SELECTIVE DEGRADATION OF SUBSTRATE ADAPTORS
As mentioned above, the CUL3 substrate adaptor KLHL3 is degraded by CUL3Δ9 in vitro. Of note, whether a similar decrease in KLHL3 occurs in human FHHt, or in animal models, remains controversial; Schumacher et al. (28) could not detect a decrease in KLHL3 abundance in vivo, in C57BL/6J mice expressing the Cul3Δ9 mutation. Yoshida et al. (39), however, also generated a C57BL/6J knockin mouse containing the Cul3Δ9 mutation. The 8- to 16-wk-old heterozygotes displayed a FHHt phenotype and an ~75% decrease in KLHL3 abundance. Importantly, this group used antibodies validated in KLHL3 knockout tissue.
The possibility of selective KLHL3 degradation would explain a confusing feature of CUL3Δ9 FHHt. Although the CUL3Δ9 human mutation is heterozygous and expressed in all cells, and although deletion of Cul3 is embryonic lethal (30), the disease itself is a manifestation primarily of dysfunction within the DCT of the kidney [although evidence supports additional effects in vascular smooth muscle (1, 2)]. We postulate that the kidney (and likely vascular) specificity of the disease is a direct result of anomalous degradation of specific BTB substrate adaptor proteins, with highly restricted expression patterns. Cornelius et al. (10) demonstrated, in vitro, that another BTB protein, Kelch-like ECH-associated protein 1 (Keap1), was unaffected by the mutation. Its substrate, Nuclear factor erythroid 2-related factor 2 (Nrf2), however, showed increased abundance, similar to WNK4. Furthermore, Yoshida et al. (39) showed that the abundance of other BTB proteins, including KLHL2, KLHL16, and Keap1, was not altered in Cul3Δ9 heterozygous mice. Unfortunately, the substrates for these adaptors were not analyzed. In other experiments examining binding affinity, the CUL3Δ9 mutant displayed enhanced binding to multiple BTB proteins (15, 21), although the effects on protein expression were not identical; RhoBTB1 abundance was increased, but Bacurd1 was unaltered. The results demonstrate a unique mechanism of the CUL3Δ9 mutant to degrade KLHL3. Furthermore, the lack of degradation of other substrate adaptors does not seem to prevent an increase in substrate abundance, as shown with the substrate adaptor-substrate complexes Keap1-Nrf2 (10) and Bacurd1-RhoA (15), suggesting that ubiquitin ligase activity toward substrates is disrupted.
CSN DYSFUNCTION CAUSES SELECTIVE DEGRADATION OF CRL SUBSTRATE ADAPTORS
CUL3Δ9 showed enhanced neddylation and an impaired binding to the CSN. Therefore, we hypothesized that the main mechanism for the disease is decreased CSN activity toward CUL3. To test this, we analyzed the effects of CSN5/JAB1 inhibition on the KLHL3-WNK4-NCC pathway. In cultured cells, CSN5/JAB1 knockdown with siRNA caused a decrease in KLHL3 abundance (10), similar to CUL3Δ9. We then generated an inducible, kidney-specific CSN5/JAB1 knockout mouse with a C57BL/6J background (9), postulating that these mice would lose the ability to deneddylate cullin proteins along the nephron, once induced with doxycycline. The mice demonstrated increased activation of the WNK4-SPAK-NCC pathway, once induced with doxycycline, but the full phenotype appeared to be masked by off-target effects, resulting from deletion of CSN5/JAB1 all along the nephron. Blood pressure was not different compared with controls, and mice were hypokalemic as opposed to the hyperkalemia seen in the disease. We speculated that these off-target effects could also be a result of loss of CSN activity toward all CRLs, not just CUL3. As mentioned above, 20% of all proteasome degraded proteins are mediated by CRLs. Thus, CSN dysfunction would affect a multitude of proteins. More information on the effects of CSN dysfunction on other cullin substrates can be found in a recent review that explored the role of cullin proteins in kidney pathophysiology (8). Importantly, the mice showed that CSN5/JAB1 deletion caused a striking reduction in KLHL3 abundance. In addition, Keap1 (9) and Nrf2 (unpublished) protein levels were increased. This effect of CSN inhibition to impair substrate adaptor stability is supported by multiple studies (7, 11, 14, 18, 33, 35), but not all adaptors are targeted (32). This selective effect toward specific adaptors was demonstrated in a recent study; Schlierf et al. (27) used a specific CSN5/JAB1 inhibitor to show different effects on multiple CUL1 and CUL3 substrate adaptors. The substrate adaptors Skp2, Fbxo22, and Fbxo30 all showed lower abundance in the presence of the CSN5/JAB1 inhibitor. In contrast, the abundance of another set of CUL1 adaptors (βTrCP, Fbxo3, and Fbxw7) as well as the CUL3 adaptor Keap1, were not affected by CSN5/JAB1 inhibition. The work demonstrates how the selective effects of CSN inhibition on substrate adaptors parallels that of the CUL3Δ9 mutation, further validating our hypothesis.
CONCLUSIONS
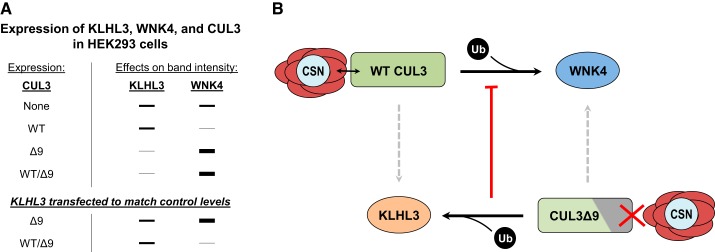
Great progress has been made in unraveling the complicated effects of the CUL3Δ9 mutation to cause FHHt. We surmised that CSN dysfunction plays an important role, based on data showing that the CUL3Δ9 mutant protein does not interact normally with the CSN, resulting in hyperneddylation. This alters the activity of the CRL complex leading to increased ubiquitin ligase activity directed toward the substrate adaptor KLHL3 (and likely to instability of cullin itself). Since KLHL3 is degraded, WNK4 accumulates along the DCT, activating NCC. Additionally, the results demonstrate that CUL3Δ9 loses normal function toward substrates. An important aspect of the human disease, however, is that the mutation is heterozygous so both wild type and mutant CUL3 protein are present. Although it was suggested that functional CUL3 haploinsufficiency might lead to the disease phenotype, this suggestion was convincingly disproven when Cul3 heterozygotes were shown to lack this phenotype (13). Instead, we suggest that the degradation of KLHL3 by CUL3Δ9 prevents the wild-type CUL3 protein from degrading WNKs in a segment-specific manner. This was emphasized in a study by Cornelius et al. (10), who coexpressed WNK4, KLHL3, and CUL3Δ9 together with and without wild-type CUL3. CUL3Δ9 expression reduced KLHL3 and increased WNK4 abundance. When KLHL3 abundance was adjusted to control levels, WNK4 abundance was attenuated only in the presence of both CUL3Δ9 and wild-type CUL3 (Fig. 1A). Thus, 1) CUL3Δ9 itself cannot degrade WNK4 and 2) the reduction in KLHL3 prevents WNK4 degradation by wild-type CUL3 (Fig. 1B). Another important feature of the disease is the selective degradation of KLHL3. Although, in vitro, multiple substrates accumulate when expressed with CUL3Δ9, this is in the absence of wild-type CUL3. We predict that, in vivo, the presence of wild-type CUL3 protein would allow for the normal degradation of substrates since most other adaptors are not degraded. Thus, the selective degradation of KLHL3 could explain the DCT-specific phenotype of CUL3Δ9-mediated FHHt.
Fig. 1.
Proposed mechanism for the CUL3Δ9 mutation in the pathogenesis of familial hyperkalemic hypertension (FHHt). A: summary of the results from Cornelius et al. (10), which determined the effects of wild-type (WT) cullin 3 (CUL3) and CUL3Δ9 expression on Kelch-like 3 (KLHL3) and with no lysine kinase (WNK)4 abundance in cultured cells. Importantly, normalization of KLHL3 abundance attenuated the increase in WNK4 abundance caused by CUL3Δ9 expression only in the presence of WT CUL3. B: our proposed mechanism for the CUL3Δ9 mutation. The disease mutation is heterozygous with one WT and one mutant CUL3 allele. WT CUL3 interacts normally with the CSN and has ubiquitin (Ub) ligase activity (black arrow) toward the substrate WNK4. There is no effect from WT CUL3 on the substrate adaptor KLHL3 (dashed arrow). The mutant CUL3Δ9 has impaired binding to the COP9 signalosome (CSN), which alters its ubiquitin ligase activity, targeting KLHL3 instead of WNK4. The loss of KLHL3 abundance will then prevent WT CUL3 from degrading WNK4 (red arrow), causing WNK4 to accumulate.
GRANTS
This work was supported by funding from the National Institutes of Health (Grants DK-51496 and F32-DK-112531) and the Department of Veteran Affairs (Grant I01BX002228).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
R.J.C., C.-L.Y., and D.H.E. conceived and designed research; R.J.C. performed experiments; R.J.C., C.-L.Y., and D.H.E. analyzed data; R.J.C., C.-L.Y., and D.H.E. interpreted results of experiments; R.J.C. and D.H.E. prepared figures; R.J.C. drafted manuscript; R.J.C., C.-L.Y., and D.H.E. edited and revised manuscript; R.J.C., C.-L.Y., and D.H.E. approved final version of manuscript.
REFERENCES
- 1.Abdel Khalek W, Rafael C, Loisel-Ferreira I, Kouranti I, Clauser E, Hadchouel J, Jeunemaitre X. Severe aterial hypertension from Cullin 3 mutations is caused by both renal and vascular effects. J Am Soc Nephrol 30: 811–823, 2019. doi: 10.1681/ASN.2017121307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agbor LN, Ibeawuchi SC, Hu C, Wu J, Davis DR, Keen HL, Quelle FW, Sigmund CD. Cullin-3 mutation causes arterial stiffness and hypertension through a vascular smooth muscle mechanism. JCI Insight 1: e91015, 2016. doi: 10.1172/jci.insight.91015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boyden LM, Choi M, Choate KA, Nelson-Williams CJ, Farhi A, Toka HR, Tikhonova IR, Bjornson R, Mane SM, Colussi G, Lebel M, Gordon RD, Semmekrot BA, Poujol A, Välimäki MJ, De Ferrari ME, Sanjad SA, Gutkin M, Karet FE, Tucci JR, Stockigt JR, Keppler-Noreuil KM, Porter CC, Anand SK, Whiteford ML, Davis ID, Dewar SB, Bettinelli A, Fadrowski JJ, Belsha CW, Hunley TE, Nelson RD, Trachtman H, Cole TRP, Pinsk M, Bockenhauer D, Shenoy M, Vaidyanathan P, Foreman JW, Rasoulpour M, Thameem F, Al-Shahrouri HZ, Radhakrishnan J, Gharavi AG, Goilav B, Lifton RP. Mutations in kelch-like 3 and cullin 3 cause hypertension and electrolyte abnormalities. Nature 482: 98–102, 2012. doi: 10.1038/nature10814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen H-Y, Chen R-H. Cullin 3 ubiquitin ligases in cancer biology: functions and therapeutic implications. Front Oncol 6: 113, 2016. doi: 10.3389/fonc.2016.00113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheng J, Guo J, Wang Z, North BJ, Tao K, Dai X, Wei W. Functional analysis of Cullin 3 E3 ligases in tumorigenesis. Biochim Biophys Acta Rev Cancer 1869: 11–28, 2018. doi: 10.1016/j.bbcan.2017.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chung D, Dellaire G. The role of the COP9 signalosome and neddylation in DNA damage signaling and repair. Biomolecules 5: 2388–2416, 2015. doi: 10.3390/biom5042388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cope GA, Deshaies RJ. Targeted silencing of Jab1/Csn5 in human cells downregulates SCF activity through reduction of F-box protein levels. BMC Biochem 7: 1, 2006. doi: 10.1186/1471-2091-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cornelius RJ, Ferdaus MZ, Nelson JW, McCormick JA. Cullin-Ring ubiquitin ligases in kidney health and disease. Curr Opin Nephrol Hypertens 28: 490–497, 2019. doi: 10.1097/MNH.0000000000000527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cornelius RJ, Si J, Cuevas CA, Nelson JW, Gratreak BDK, Pardi R, Yang C-L, Ellison DH. Renal COP9 signalosome deficiency alters CUL3-KLHL3-WNK signaling pathway. J Am Soc Nephrol 29: 2627–2640, 2018. doi: 10.1681/ASN.2018030333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cornelius RJ, Zhang C, Erspamer KJ, Agbor LN, Sigmund CD, Singer JD, Yang C-L, Ellison DH. Dual gain and loss of cullin 3 function mediates familial hyperkalemic hypertension. Am J Physiol Renal Physiol 315: F1006–F1018, 2018. doi: 10.1152/ajprenal.00602.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Denti S, Fernandez-Sanchez ME, Rogge L, Bianchi E. The COP9 signalosome regulates Skp2 levels and proliferation of human cells. J Biol Chem 281: 32188–32196, 2006. doi: 10.1074/jbc.M604746200. [DOI] [PubMed] [Google Scholar]
- 12.Ferdaus MZ, McCormick JA. Mechanisms and controversies in mutant Cul3-mediated familial hyperkalemic hypertension. Am J Physiol Renal Physiol 314: F915–F920, 2018. doi: 10.1152/ajprenal.00593.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferdaus MZ, Miller LN, Agbor LN, Saritas T, Singer JD, Sigmund CD, McCormick JA. Mutant Cullin 3 causes familial hyperkalemic hypertension via dominant effects. JCI Insight 2: e96700, 2017. doi: 10.1172/jci.insight.96700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.He Q, Cheng P, He Q, Liu Y. The COP9 signalosome regulates the Neurospora circadian clock by controlling the stability of the SCFFWD-1 complex. Genes Dev 19: 1518–1531, 2005. doi: 10.1101/gad.1322205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ibeawuchi SR, Agbor LN, Quelle FW, Sigmund CD. Hypertension-causing mutations in cullin3 protein impair RhoA protein ubiquitination and augment the association with substrate adaptors. J Biol Chem 290: 19,208–19,217, 2015. doi: 10.1074/jbc.M115.645358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lingaraju GM, Bunker RD, Cavadini S, Hess D, Hassiepen U, Renatus M, Fischer ES, Thomä NH. Crystal structure of the human COP9 signalosome. Nature 512: 161–165, 2014. doi: 10.1038/nature13566. [DOI] [PubMed] [Google Scholar]
- 17.Louis-Dit-Picard H, Barc J, Trujillano D, Miserey-Lenkei S, Bouatia-Naji N, Pylypenko O, Beaurain G, Bonnefond A, Sand O, Simian C, Vidal-Petiot E, Soukaseum C, Mandet C, Broux F, Chabre O, Delahousse M, Esnault V, Fiquet B, Houillier P, Bagnis CI, Koenig J, Konrad M, Landais P, Mourani C, Niaudet P, Probst V, Thauvin C, Unwin RJ, Soroka SD, Ehret G, Ossowski S, Caulfield M, Bruneval P, Estivill X, Froguel P, Hadchouel J, Schott J-J, Jeunemaitre X. KLHL3 mutations cause familial hyperkalemic hypertension by impairing ion transport in the distal nephron. Nat Genet 44: 456–460, 2012. [Erratum in Nat Genet 44: 609, 2012.] doi: 10.1038/ng.2218. [DOI] [PubMed] [Google Scholar]
- 18.Luke-Glaser S, Roy M, Larsen B, Le Bihan T, Metalnikov P, Tyers M, Peter M, Pintard L. CIF-1, a shared subunit of the COP9/signalosome and eukaryotic initiation factor 3 complexes, regulates MEL-26 levels in the Caenorhabditis elegans embryo. Mol Cell Biol 27: 4526–4540, 2007. doi: 10.1128/MCB.01724-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lydeard JR, Schulman BA, Harper JW. Building and remodelling Cullin-RING E3 ubiquitin ligases. EMBO Rep 14: 1050–1061, 2013. doi: 10.1038/embor.2013.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mayan H, Vered I, Mouallem M, Tzadok-Witkon M, Pauzner R, Farfel Z. Pseudohypoaldosteronism type II: marked sensitivity to thiazides, hypercalciuria, normomagnesemia, and low bone mineral density. J Clin Endocrinol Metab 87: 3248–3254, 2002. doi: 10.1210/jcem.87.7.8449. [DOI] [PubMed] [Google Scholar]
- 21.McCormick JA, Yang C-L, Zhang C, Davidge B, Blankenstein KI, Terker AS, Yarbrough B, Meermeier NP, Park HJ, McCully B, West M, Borschewski A, Himmerkus N, Bleich M, Bachmann S, Mutig K, Argaiz ER, Gamba G, Singer JD, Ellison DH. Hyperkalemic hypertension-associated cullin 3 promotes WNK signaling by degrading KLHL3. J Clin Invest 124: 4723–4736, 2014. doi: 10.1172/JCI76126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ohta A, Schumacher F-R, Mehellou Y, Johnson C, Knebel A, Macartney TJ, Wood NT, Alessi DR, Kurz T. The CUL3-KLHL3 E3 ligase complex mutated in Gordon’s hypertension syndrome interacts with and ubiquitylates WNK isoforms: disease-causing mutations in KLHL3 and WNK4 disrupt interaction. Biochem J 451: 111–122, 2013. doi: 10.1042/BJ20121903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pan Z-Q, Kentsis A, Dias DC, Yamoah K, Wu K. Nedd8 on cullin: building an expressway to protein destruction. Oncogene 23: 1985–1997, 2004. doi: 10.1038/sj.onc.1207414. [DOI] [PubMed] [Google Scholar]
- 24.Petroski MD, Deshaies RJ. Function and regulation of cullin-RING ubiquitin ligases. Nat Rev Mol Cell Biol 6: 9–20, 2005. doi: 10.1038/nrm1547. [DOI] [PubMed] [Google Scholar]
- 25.Pintard L, Kurz T, Glaser S, Willis JH, Peter M, Bowerman B. Neddylation and deneddylation of CUL-3 is required to target MEI-1/Katanin for degradation at the meiosis-to-mitosis transition in C. elegans. Curr Biol 13: 911–921, 2003. doi: 10.1016/S0960-9822(03)00336-1. [DOI] [PubMed] [Google Scholar]
- 26.Saha A, Deshaies RJ. Multimodal activation of the ubiquitin ligase SCF by Nedd8 conjugation. Mol Cell 32: 21–31, 2008. doi: 10.1016/j.molcel.2008.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schlierf A, Altmann E, Quancard J, Jefferson AB, Assenberg R, Renatus M, Jones M, Hassiepen U, Schaefer M, Kiffe M, Weiss A, Wiesmann C, Sedrani R, Eder J, Martoglio B. Targeted inhibition of the COP9 signalosome for treatment of cancer. Nat Commun 7: 13166, 2016. doi: 10.1038/ncomms13166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schumacher FR, Siew K, Zhang J, Johnson C, Wood N, Cleary SE, Al Maskari RS, Ferryman JT, Hardege I, Yasmin, Figg NL, Enchev R, Knebel A, O’Shaughnessy KM, Kurz T. Characterisation of the Cullin-3 mutation that causes a severe form of familial hypertension and hyperkalaemia. EMBO Mol Med 7: 1285–1306, 2015. doi: 10.15252/emmm.201505444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shibata S, Zhang J, Puthumana J, Stone KL, Lifton RP. Kelch-like 3 and Cullin 3 regulate electrolyte homeostasis via ubiquitination and degradation of WNK4. Proc Natl Acad Sci USA 110: 7838–7843, 2013. doi: 10.1073/pnas.1304592110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Singer JD, Gurian-West M, Clurman B, Roberts JM. Cullin-3 targets cyclin E for ubiquitination and controls S phase in mammalian cells. Genes Dev 13: 2375–2387, 1999. doi: 10.1101/gad.13.18.2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Soucy TA, Smith PG, Milhollen MA, Berger AJ, Gavin JM, Adhikari S, Brownell JE, Burke KE, Cardin DP, Critchley S, Cullis CA, Doucette A, Garnsey JJ, Gaulin JL, Gershman RE, Lublinsky AR, McDonald A, Mizutani H, Narayanan U, Olhava EJ, Peluso S, Rezaei M, Sintchak MD, Talreja T, Thomas MP, Traore T, Vyskocil S, Weatherhead GS, Yu J, Zhang J, Dick LR, Claiborne CF, Rolfe M, Bolen JB, Langston SP. An inhibitor of NEDD8-activating enzyme as a new approach to treat cancer. Nature 458: 732–736, 2009. doi: 10.1038/nature07884. [DOI] [PubMed] [Google Scholar]
- 32.Su H, Huang W, Wang X. The COP9 signalosome negatively regulates proteasome proteolytic function and is essential to transcription. Int J Biochem Cell Biol 41: 615–624, 2009. doi: 10.1016/j.biocel.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Su H, Li J, Menon S, Liu J, Kumarapeli AR, Wei N, Wang X. Perturbation of cullin deneddylation via conditional Csn8 ablation impairs the ubiquitin-proteasome system and causes cardiomyocyte necrosis and dilated cardiomyopathy in mice. Circ Res 108: 40–50, 2011. doi: 10.1161/CIRCRESAHA.110.230607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wakabayashi M, Mori T, Isobe K, Sohara E, Susa K, Araki Y, Chiga M, Kikuchi E, Nomura N, Mori Y, Matsuo H, Murata T, Nomura S, Asano T, Kawaguchi H, Nonoyama S, Rai T, Sasaki S, Uchida S. Impaired KLHL3-mediated ubiquitination of WNK4 causes human hypertension. Cell Reports 3: 858–868, 2013. doi: 10.1016/j.celrep.2013.02.024. [DOI] [PubMed] [Google Scholar]
- 35.Wee S, Geyer RK, Toda T, Wolf DA. CSN facilitates Cullin-RING ubiquitin ligase function by counteracting autocatalytic adapter instability. Nat Cell Biol 7: 387–391, 2005. doi: 10.1038/ncb1241. [DOI] [PubMed] [Google Scholar]
- 36.Wei N, Chamovitz DA, Deng XW. Arabidopsis COP9 is a component of a novel signaling complex mediating light control of development. Cell 78: 117–124, 1994. doi: 10.1016/0092-8674(94)90578-9. [DOI] [PubMed] [Google Scholar]
- 37.Wilson FH, Disse-Nicodème S, Choate KA, Ishikawa K, Nelson-Williams C, Desitter I, Gunel M, Milford DV, Lipkin GW, Achard JM, Feely MP, Dussol B, Berland Y, Unwin RJ, Mayan H, Simon DB, Farfel Z, Jeunemaitre X, Lifton RP. Human hypertension caused by mutations in WNK kinases. Science 293: 1107–1112, 2001. doi: 10.1126/science.1062844. [DOI] [PubMed] [Google Scholar]
- 38.Yang X, Menon S, Lykke-Andersen K, Tsuge T, Di Xiao, Wang X, Rodriguez-Suarez RJ, Zhang H, Wei N. The COP9 signalosome inhibits p27(kip1) degradation and impedes G1-S phase progression via deneddylation of SCF Cul1. Curr Biol 12: 667–672, 2002. doi: 10.1016/S0960-9822(02)00791-1. [DOI] [PubMed] [Google Scholar]
- 39.Yoshida S, Araki Y, Mori T, Sasaki E, Kasagi Y, Isobe K, Susa K, Inoue Y, Bomont P, Okado T, Rai T, Uchida S, Sohara E. Decreased KLHL3 expression is involved in the pathogenesis of pseudohypoaldosteronism type II caused by cullin 3 mutation in vivo. Clin Exp Nephrol 22: 1251–1257, 2018. doi: 10.1007/s10157-018-1593-z. [DOI] [PubMed] [Google Scholar]