Abstract
Angiotensin II exacerbates oxidative stress in part by increasing superoxide () production by many renal tissues. However, whether it does so in proximal tubules and the source of in this segment are unknown. Dietary fructose enhances the stimulatory effect of angiotensin II on proximal tubule Na+ reabsorption, but whether this is true for oxidative stress is unknown. We hypothesized that angiotensin II causes proximal nephron oxidative stress in part by stimulating NADPH oxidase (NOX)4-dependent production and decreasing the amount of the antioxidant glutathione, and this is exacerbated by dietary fructose. We measured basal and angiotensin II-stimulated production with and without inhibitors, NOX1 and NOX4 expression, and total and reduced glutathione (GSH) in proximal tubules from rats drinking either tap water (control) or 20% fructose. Angiotensin II (10 nM) increased production by 113 ± 42 relative light units·mg protein−1·s−1 in controls and 401 ± 74 relative light units·mg protein−1·s−1 with 20% fructose (n = 11 for each group, P < 0.05 vs. control). Apocynin and the Nox1/4 inhibitor GKT136901 prevented angiotensin II-induced increases in both groups. NOX4 expression was not different between groups. NOX1 expression was undetectable. Angiotensin II decreased GSH by 1.8 ± 0.8 nmol/mg protein in controls and by 4.2 ± 0.9 nmol/mg protein with 20% fructose (n = 18 for each group, P < 0.047 vs. control). We conclude that 1) angiotensin II causes oxidative stress in proximal tubules by increasing production by NOX4 and decreasing GSH and 2) dietary fructose enhances the ability of angiotensin II to stimulate and diminish GSH, thereby exacerbating oxidative stress in this segment.
Keywords: kidney, hypertension, salt, NADPH oxidase
INTRODUCTION
The proximal nephron is critical in maintaining Na+ homeostasis because it reabsorbs ≈65% of the filtered load of water and Na+ (11, 19, 43, 69). Oxidative stress in this segment has been reported in renal injury (7, 32, 86, 87) and hypertension (48, 83). Many factors and hormones regulate proximal nephron function; chief among these is angiotensin II (ANG II).
ANG II stimulates the production of reactive oxygen species (ROS) such as superoxide () and H2O2 and thus oxidative stress in many tissues (34, 46, 73, 79, 84, 90), including those of the kidney (21, 55, 57, 84). A role for ANG II-induced oxidative stress has been implicated in proximal nephron injury (32). Oxidative stress has also been reported to both decrease and increase proximal nephron Na+ reabsorption in models of hypertension (61) and diabetes (14, 63), respectively. ANG II directly enhances production by collecting ducts (77), thick ascending limbs (33, 49), and cultured cells of proximal tubule origin (20); however, whether this is true for native proximal tubules is unclear.
Many enzymes are capable of producing and H2O2, and thus oxidative stress. These include the family of NADPH oxidases (NOXs). Three family members are expressed along the nephron, including NOX1 (31, 74), NOX2 (9, 44, 74), and NOX4 (44, 74, 75). NOX4 has been reported to be a source of ROS in collecting ducts (47) and thick ascending limbs (36, 71, 72), whereas NOX2 is the primary source of ROS in the macula densa (18). Although NOX generates ROS in proximal tubules (61, 63), the isoform responsible has not been identified. Uncoupled nitric oxide synthase (27) and xanthine oxidase (52) also produce , and these enzymes are expressed in thick ascending limbs (62) and proximal tubules (29). ANG II has been reported to stimulate ROS production by both NOXs (33, 49, 68, 77) and nitric oxide synthase (27); however, the source(s) of ROS production in the proximal nephron and the direct actions of ANG II on them have not been studied in detail.
Cells have several defense mechanisms to prevent or repair the deleterious effects of oxidative stress (16, 50). Chief among these is glutathione (16, 22, 50, 80). The reduced form of glutathione (GSH) is a three-amino acid peptide that undergoes cyclic oxidation and reduction of the sulfhydryl group on the terminal cysteine. During oxidation, two GSH molecules donate their electrons to reduce the target product, and a disulfhydryl linkage is formed between them, generating oxidized glutathione (GSSG) (40, 64). Oxidative stress can decrease both total glutathione and GSH. The effects of ANG II on the glutathione system in the kidney in general, and the proximal nephron specifically, have not been thoroughly investigated.
Since its introduction in the early 1970s, the use of low-cost high-fructose corn syrup has become widespread. This has increased consumption of fructose in the United States from <2 to >40 lb·yr−1·person−1 (41, 82). The average American consumes >11% of their daily caloric intake as fructose (53, 82), and the caloric composition of the diets of >17 million people in the United States is 20% or more fructose (82). Fructose-rich diets are also present in other industrialized countries (37, 60, 91). Dietary fructose increases renal oxidative stress (8, 12), particularly when combined with a high-salt diet (12, 26). Fructose-induced oxidative stress has been linked to both renal injury (2) and hypertension (12, 78). Increased oxidative stress can result from increased production of ROS such as and/or H2O2, decreased degradation of ROS, and/or reductions in other defense mechanisms (12). However, the mechanisms by which dietary fructose increases renal oxidative stress and the renal structures involved are poorly understood.
The proximal nephron is thought to be key in the hypertension (6, 25, 59) and renal injury (9, 42) caused by dietary fructose. Proximal tubules also reabsorb (23) and metabolize fructose (9a). Genetic deletion of fructokinase C from proximal tubules protects against renal injury (1), likely by reducing oxidative stress. Fructose alone increases Na+ reabsorption by this segment (6), and dietary fructose enhances the stimulatory effect of ANG II on transport (25, 26). Whether this is also true for production and oxidative stress as indicated by changes in GSH is unclear.
We hypothesized that ANG II induces proximal nephron oxidative stress in part by stimulating NOX4-dependent production and decreasing the amount of the antioxidant glutathione and that this is exacerbated by dietary fructose.
METHODS
Reagents.
Unless specified, all drugs and reagents were obtained from Sigma-Aldrich (St. Louis, MO). A Pierce Coomassie (Bradford) Protein Assay Kit was obtained from Thermo Scientific (Rockford, IL). Bicarbonate-buffered physiological saline contained (in mmol/L) 25 NaHCO3, 114 NaCl, 4 KCl, 0.4 NaH2PO4, 2.5 Na2HPO4, 1.2 MgSO4, 5.5 glucose, 6.0 dl-alanine, 2.0 Ca(lactate)2, and 1.0 Na3 citrate, pH 7.4 when gassed with 95% O2-5% CO2. HEPES-buffered physiological saline contained (in mmol/L) 10 HEPES (pH 7.4), 130 NaCl, 4 KCl, 0.4 NaH2PO4, 2.1 Na2HPO4, 1.2 MgSO4, 5.5 glucose, 6.0 dl-alanine, 2.0 Ca(lactate)2, and 1.0 Na3 citrate. The osmolality of bicarbonate-buffered and HEPES-buffered physiological saline was adjusted to 300 ± 5 mosmol/L with mannitol. The glutathione kit (catalog no. 703002) was obtained from Cayman Chemical (Ann Arbor, MI).
Animals.
Male Sprague-Dawley rats (Charles River Breeding Laboratories, Wilmington, MA) weighing between 101 and 125 g were randomly assigned to one of two experimental groups. One group received a 20% fructose solution as their source of fluid (Fruct group), whereas the other group received tap water (control group). Twenty percent fructose was prepared fresh every other day. Both groups were on a purified diet (no. 5876, TestDiet, St. Louis, MO) containing 18.6% protein, 4.3% fiber, 59.3% carbohydrates, and 10% fat, providing 4.02 kcal/g and ~100 meq/kg Na+. The metabolic characteristics of this model have been previously published (24).
Animals in both experimental groups were housed in pairs under normal rat housing conditions with a 12:12-h light-dark cycle and ad libitum provision of food and fluids. After 6–8 days of dietary treatment, the animals underwent terminal surgery. Rats were anesthetized with ketamine (100 mg/kg body wt ip) and xylazine (20 mg/kg body wt ip) and given 100 units heparin (ip).
Male Dahl salt-sensitive rats in which NOX4 was knocked out were treated in a manner similar to control rats. These rats were only used to validate the NOX4 antibody.
This study was approved by the Case Western Reserve University Institutional Animal Care and Use Committee. All experiments were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Proximal tubule suspension.
Proximal tubule suspensions were generated using methods similar to those we have previously used (24). Briefly, rats were anesthetized and an abdominal U-shaped incision was made. The kidneys were retroperfused from the abdominal aorta with 80 mL bicarbonate-buffered physiological saline at 37°C. Physiological saline contained 1 mg/mL collagenase type I and 2 U/mL heparin and was infused at 8 mL/min for 10 min. Digested kidneys were excised before flow was stopped and immediately cooled by immersion in physiological saline at 4°C. They were then transferred to a cold Lucite plate, and their cortexes were gently scraped with a razor blade to collect proximal tubules. This tissue was minced and transferred to a 5-mL conical tube. Tissue was disrupted by passing it through a pipette tip and stirring on ice for 5 min. The resulting suspension was filtered through a 390-µm mesh, and tubules were recovered by centrifugation at 100 g for 2 min at 4°C. The tubules were then rinsed, filtered through a 250-µm mesh, and recovered by centrifugation. The final pellet was resuspended in 3–5 mL physiological saline.
production.
was measured using methods similar to those we have previously used (27). Fruct and control suspensions were diluted to 200 µg protein/mL, and 200-µL aliquots were added to two plastic tubes containing bis-N-methylacridinium nitrate (Lucigenin) at a final concentration of 5 µmol/L and kept on ice. One tube was designated “basal.” The other tube was designated “ANG II stimulated.” production by each sample was measured sequentially. The order of the tubes was randomized to account for effects of time. The process was as follows. Eight hundred microliters of warm, oxygenated physiological saline alone or containing 10−8 mol/l ANG II was added to the tube and incubated at 37°C for 6 min. The tube was transferred to a luminometer (FB12/Sirius, Zylux Oak Ridge, TN). Two luminometers were used in this study which required the application of a small correction factor to normalize the data. Total luminescence in relative light units (RLU)/s was recorded at 4.8-s intervals for 7 min. The scavenger 4,5-dihydroxy-1,3-benzenedisulfonic acid (Tiron) was then added to a final concentration of 10 mmol/L, and luminescence was measured for 5 additional min. production was calculated as the steady-state luminescence without Tiron minus the luminescence of the last 2 min after Tiron addition. Protein content of the sample was measured using the Pierce Coomassie (Bradford) Protein Assay, and production was normalized for protein content. Differences in luminescence between the samples with and without ANG II were considered ANG II-stimulated production.
For the experiments involving apocynin and GKT136901, nonselective and NOX1/4 inhibitors, respectively, the chemical was added during the initial dilution of the suspensions.
Western blot analysis.
Proximal tubules from Fruct and control suspensions were dissolved in CelLytic M cell lysis reagent. Duplicates of 5 and 10 µg protein were loaded onto a 10% Criterion TGX Stain-Free polyacrylamide gels (Bio-Rad Laboratories, Hercules, CA). Samples from Fruct and control groups were run in pairs on the same gel. Electrophoresis was performed to separate proteins, which were transferred onto polyvinylidene difluoride membranes via the iBlot 2 Dry Blotting System (ThermoFisher Scientific, Waltham, MA). Membranes were exposed in an Azure c600 system (Azure Biosystems, Dublin, CA) at 507 nm for 30 s, and images of total protein fluorescence were captured to use as loading controls. Membranes were incubated in blocking buffer composed of 5% nonfat milk in PBS with Tween 20 (PBS-T; 137 mM NaCl, 2.7 mM KCl, 10 mM NaH2PO4, and 0.1% Tween 20) for 60 min. They were then incubated with a primary rabbit monoclonal anti-NOX4 antibody (ab133303, Abcam) at 1:4,000 dilution in 5% nonfat milk in PBS-T for 120 min at room temperature. Membranes were washed with PBS-T for 15 min and incubated at 1:4,000 dilution in 5% BSA with horseradish peroxidase (HRP)-conjugated anti-rabbit IgG (Amersham Pharmacia Biotech) for 60 min. Membranes were washed with PBS-T for 15 min and incubated with a luminol-based chemiluminescent HRP substrate (Pierce Biotechnology, Rockford, IL) and exposed in the Azure c600 system for 100 s. An image was captured, and densitometry was performed using ImageJ. The anti-NOX4 antibody was validated using renal tissue from NOX4 knockout mice.
The method to test for NOX1 expression was similar except that the primary antibody was directed against NOX1 and several dilutions were tested (ab131088, Abcam).
Glutathione.
Two aliquots of 1,000 µg protein from Fruct and control suspensions were diluted to 5 mL with HEPES-buffered physiological saline in separate incubation chambers at 37°C with stirring. ANG II was added to one of the chambers at a final concentration of 10−8 mol/L and incubated for 7 or 15 min. The 7- and 15-min incubations gave similar results, so the data were pooled. After incubation, the suspension was spun at 100 g for 2 min, and the supernatant was removed. Tubules were collected and resuspended in 2 mL MES buffer [0.2 M 2-(N-morpholino)ethanesulphonic acid, 0.05 M phosphate, and 1 mM EDTA (pH 6.0)] and deproteinated with the same volume of metaphosphoric acid (1 g/10 mL water). The mixed solution was incubated for 5 min at room temperature and then centrifuged at 2,000 g for 2 min. The supernatant was collected and frozen at −80°C. Standards were treated in the same manner. Before the GSH assay, samples and standards were thawed on ice. Total glutathione and GSSG were measured using a Glutathione Assay Kit (catalog no. 703002, Cayman Chemical). GSH was calculated by subtracting the amount of GSSG from the amount of total glutathione.
Statistical analysis.
Results are expressed as means ± SE. P values were calculated using an unpaired t test or two-way ANOVA as appropriate. When two-way ANOVA was used, post hoc testing was performed using paired or unpaired t tests as appropriate. Corrections for multiple testing were made using Hochberg’s method (35). Corrected P values are reported in the text.
RESULTS
ANG II stimulates ROS production in many tissues (34, 46, 73, 79, 84, 90), but whether it does so in the proximal nephron, and the effects of dietary fructose, are unknown. We first tested the effect of ANG II on production in control tubules. ANG II (10−8 mol/L) stimulated production from 772 ± 57 to 885 ± 49 RLU·mg protein−1·s−1 (n = 11, P < 0.022; Fig. 1A). Dietary fructose increases renal oxidative stress and augments the ability of ANG II to stimulate proximal nephron Na+ reabsorption. Therefore, we next studied the effect of ANG II on Fruct tubules. We found that ANG II augmented production from 682 ± 48 to 1,083 ± 86 RLU·mg protein−1·s−1 (n = 11, P < 0.0009; Fig. 1B). The effect of dietary fructose alone was not significant, whereas both the effects of ANG II alone (P < 0.002) and the interaction were significant (P < 0.025).
Fig. 1.
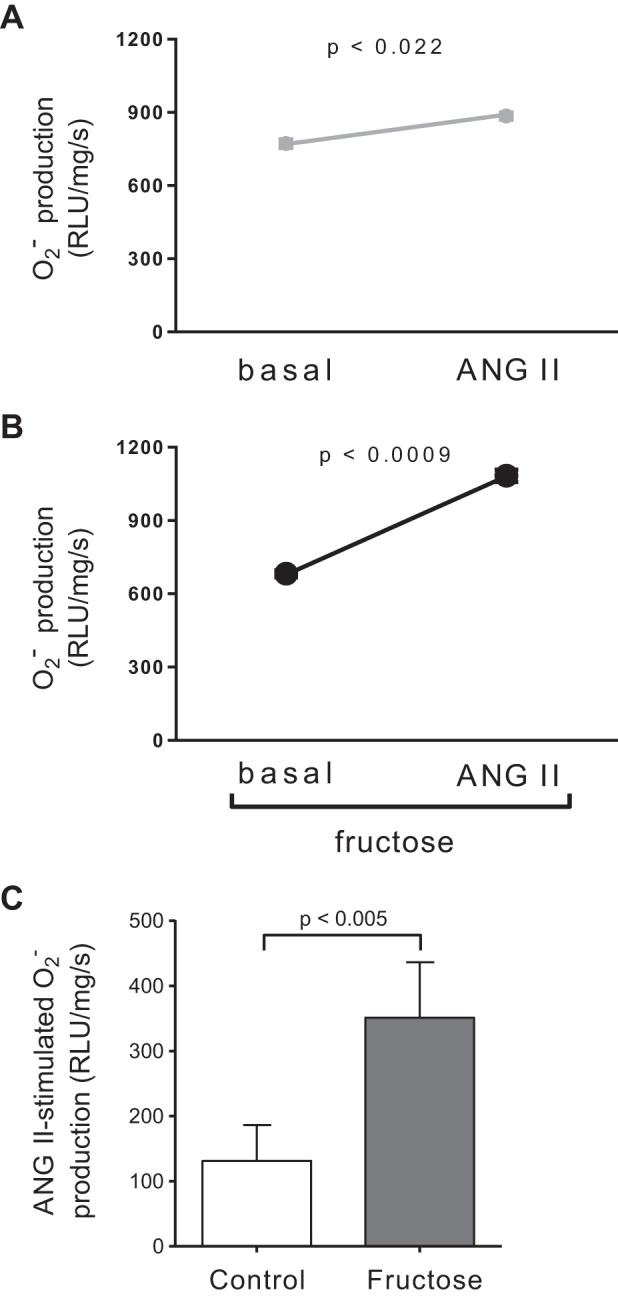
Effect of 10−8 mol/L angiotensin II (ANG II) on production by proximal tubule suspensions. A: control tubules. Error bars are covered by symbols. B: tubules from rats that consumed 20% fructose for 6–8 days. Error bars are covered by symbols. n = 11 for each group. The increase in both groups combined was significant by ANOVA (P < 0.0002). Post hoc testing using a paired a t test showed that the effect of ANG II was significant in each group, as indicated in the figure. C: ANG II-induced production by proximal tubule suspensions from control rats and rats that consumed 20% fructose for 6–8 days. ANOVA indicated a significant interaction between ANG II and fructose (P < 0.025). Post hoc testing using a paired t test indicated that the effect of ANG II was greater in rats on the fructose diet, as indicated in the figure. RLU, relative light units.
Since ANOVA showed that the effect of ANG II was significant when the groups were combined and there was a significant interaction between ANG II and fructose, we next performed post hoc testing to study whether the stimulation caused by ANG II was significant in each group separately and whether there was a difference in the effect of ANG II between groups. In control tubules, 10−8 mol/L ANG II enhanced production by 112 ± 42 RLU·mg protein−1·s−1 (n = 11, P < 0.022; Fig. 1C), while in Fruct tubules, ANG II increased production by 401 ± 74 RLU·mg protein−1·s−1 (n = 11, P < 0.0009; Fig. 1C). These data also show that ANG II stimulated production to a greater extent in Fruct tubules than in control tubules (P < 0.005; Fig. 1C). This difference was not due to dietary fructose affecting basal synthesis. Basal production by proximal tubules was not different between groups.
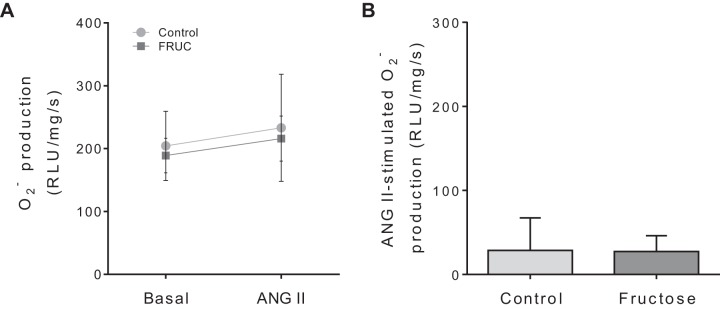
NOXs are a major source of in the kidney (84, 92). Therefore, we next tested whether this family of enzymes was the source of the ANG II-stimulated . In the presence of apocynin, production was 204 ± 55 RLU·mg protein−1·s−1 in the absence of ANG II and 233 ± 85 RLU·mg protein−1·s−1 in its presence in control tubules (n = 5; Fig. 2A). Similarly, in Fruct tubules, production was 189 ± 27 RLU·mg protein−1·s−1 in the absence of ANG II and 216 ± 36 RLU·mg protein−1·s−1 in its presence (n = 5; Fig. 2A). These data show that in the presence of apocynin, dietary fructose alone did not alter basal production. Additionally, apocynin blunted the ability of ANG II to stimulate production in both groups and eliminated the difference between them (Fig. 2B).
Fig. 2.
Effect of 10−8 mol/L angiotensin II (ANG II) on production by proximal tubule suspensions from control rats and rats that consumed 20% fructose (FRUC) for 6–8 days in the presence of apocynin, a nonselective NADPH oxidase inhibitor. A: basal and ANG II-stimulated production. B: ANG II-induced production. RLU, relative light units. n = 5 for each group.
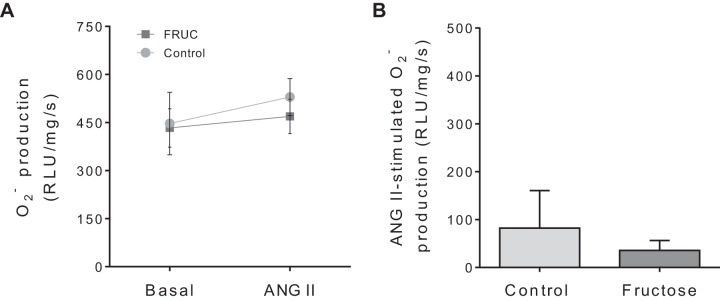
NOX4 is thought to be a primary contributor to oxidative stress in the kidney. Recently, a NOX1/4 inhibitor, GKT136901, has been reported (74, 81). Consequently, we tested the effect of GKT136901 on production (Fig. 3). In the presence of GKT136901, production was 447 ± 98 RLU·mg protein−1·s−1 in the absence of ANG II and 529 ± 58 RLU·mg protein−1·s−1 in its presence in control tubules (n = 5; Fig. 3A). Similarly, production was 433 ± 60 RLU·mg protein−1·s−1 in the absence of ANG II and 469 ± 54 RLU·mg protein−1·s−1 in its presence in Fruct tubules (n = 5; Fig. 3A). These data show that in the presence of GKT136901, dietary fructose alone did not alter basal production. Additionally, GKT136901 blunted the ability of ANG II to stimulate production in both groups and eliminated the difference between them (Fig. 3B).
Fig. 3.
Effect of 10−8 mol/L angiotensin II (ANG II) on production by proximal tubule suspensions from control rats and rats that consumed 20% fructose (FRUC) for 6–8 days in the presence of GKT136901, a NADPH oxidase 1/4 inhibitor. A: basal and ANG II-stimulated production. B: ANG II-induced production. RLU, relative light units. n = 5 for each group.
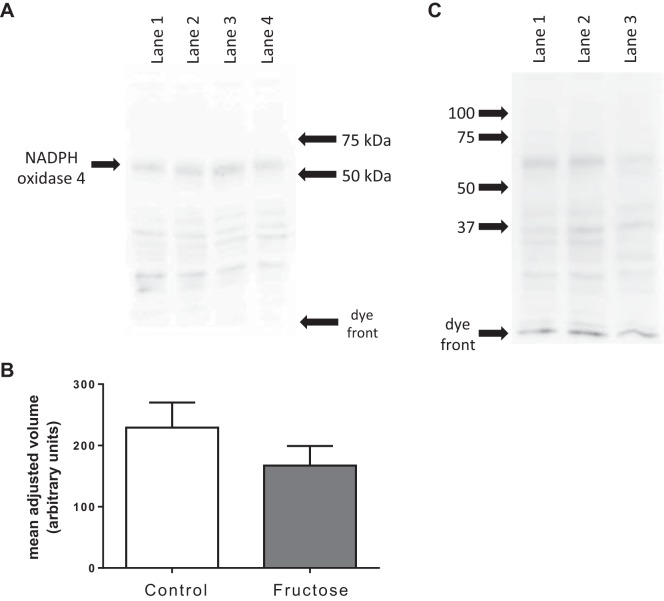
Since GKT136901 inhibits both NOX1 and NOX4, we measured their expression and the effect of dietary fructose. We found that proximal tubules expressed NOX4 but we could not detect NOX1. Figure 4A shows a representative Western blot for NOX4. A major band corresponding to the molecular weight of NOX4 was detected at ≈65 kDa. Figure 4B shows mean data from six such blots normalized for total protein loaded as measured by fluorescence of each lane in the gel at 507 nm. Expression of NOX4 by proximal tubules was 229 ± 41 arbitrary units in control tubules, whereas it was 167 ± 32 arbitrary units in those from Fruct tubules. Figure 4C shows the major band observed at 65 kDa was NOX4 because it was not detectable in renal homogenates from NOX4 knockout rats.
Fig. 4.
NADPH oxidase expression measured by Western blot analysis. A: representative membrane showing the entire gel. A major band was observed at ~65 kDa. Lanes 1 and 2, 10 µg of proximal tubule protein from controls; lanes 3 and 4, 10 µg of proximal tubule protein from rats that consumed 20% fructose for 6–8 days. B: quantification of 6 blots normalized by the total protein in each lane of the gel. C: validation of antibody. Lanes 1 and 2, 10 µg of proximal tubule protein from control rats; lane 3, 10 µg of proximal tubule protein from a NADPH oxidase 4 knockout rat on a Dahl salt-sensitive background.
Reactions involving glutathione are major intracellular antioxidant defense mechanisms that neutralize ROS including , thereby preventing tissue oxidative damage (16, 22, 80). Reductions in total glutathione and GSH thus reflect oxidative stress. Two-way ANOVA indicated that fructose alone did not significantly affect total glutathione but that 10−8 mol/L ANG II did (P < 0.0001). Thus, we next analyzed whether ANG II decreased total glutathione separately in tubules from control and Fruct groups. ANG II diminished total glutathione by 3.7 ± 1.1 ng/mg protein in control suspensions (n = 18, P < 0.001) and by 6.0 ± 0.8 ng/mg protein in Fruct suspensions (n = 18, P < 0.002). However, the difference between groups did not reach statistical significance (P < 0.10; Fig. 5).
Fig. 5.
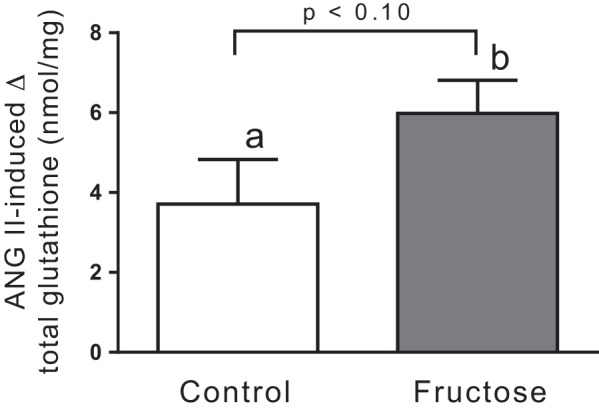
Angiotensin II (ANG II)-induced changes in total glutathione in proximal tubules from control rats and rats that consumed 20% fructose for 6–8 days. n = 18 in each group. ANOVA indicated a significant effect of 10−8 mol/L ANG II on total glutathione (P < 0.0001). Post hoc testing using paired t tests showed the effect was significant in each group (a: P < 0.001; b: P < 0.002). The difference between groups was not significant, as indicated in the figure.
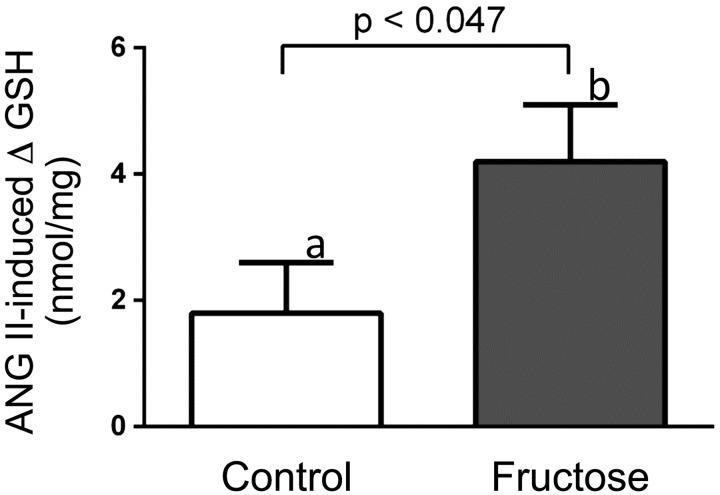
GSH is a more sensitive indicator of oxidative stress than total glutathione, so we next measured GSH. Two-way ANOVA indicated that 10−8 mol/L ANG II alone significantly altered GSH (P < 0.0001) and there was a significant interaction between ANG II and fructose (P < 0.030). Fructose alone did not significantly affect GSH. Consequently, we analyzed the effect of ANG II on GSH by group and whether fructose augmented its actions. ANG II (10−8 mol/L) decreased GSH by 1.8 ± 0.8 ng/mg protein in control tubules (n = 18, P < 0.032) while diminishing it by 4.2 ± 0.9 ng/mg protein in Fruct tubules (n = 18, P < 0.002). The decrease in GSH caused by ANG II was greater in the Fruct group than in the control group (P < 0.047; Fig. 6).
Fig. 6.
Angiotensin II (ANG II)-induced changes in GSH in proximal tubules from control rats and rats that consumed 20% fructose for 6–8 days. n = 18 in each group. ANOVA indicated a significant effect of 10−8 mol/L ANG II on GSH (P < 0.001). Post hoc testing using paired t tests showed the effect was significant in each group (a: P < 0.032; b: P < 0.002). The difference between groups was significant, as indicated in the figure.
DISCUSSION
The first part of our hypothesis was that ANG II causes proximal nephron oxidative stress in part by stimulating NOX4-dependent production and decreasing the amount of the antioxidant glutathione. We found that 1) acute ANG II treatment stimulated production in control tubules; 2) this could be blunted by apocynin and GKT136901, nonselective and selective NOX1/4 inhibitors, respectively; 3) proximal tubules express NOX4 but not NOX1; and 4) acute ANG II treatment decreased both total glutathione and GSH.
Our results showing that ANG II stimulates production by native proximal tubules are novel and have not been previously reported. They are consistent with data showing that ANG II augments ROS production by cultured cells of proximal tubule origin (20). They are also similar to data showing that ANG II enhances and/or H2O2 production by outer medullary tissue (54, 56), thick ascending limbs (49, 85), macula densa (89), collecting ducts (77), and mesangial cells (4, 21). We show here that the source of ANG II-induced was most likely NOX4 because a selective NOX1/4 inhibitor blocked the actions of ANG II and NOX4 but not NOX1 protein expression was detected in control suspensions. Again, these data are similar to those reported for thick ascending limbs (49) and mesangial cells (4, 21). However, in the macula densa, ANG II enhances ROS production by both NOX2 and NOX4 (89), although NOX2 is the primary source (18) and ANG II-induced ROS production by NOX1 has been reported in vascular smooth muscle cells (5).
The glutathione system is a cellular defense mechanism against oxidative stress. Consequently, we studied the effects of ANG II on total glutathione and GSH. We found that acute ANG II treatment decreased GSH levels in proximal tubules consistent with it causing oxidative stress in this segment. These are the first data showing such an effect although similar results have been reported for other tissues. ANG II has been previously reported to diminish GSH in endothelial progenitor cells (30). In addition, a previous study (3) has reported that ANG II reduced glutathione peroxidase activity in the rat hippocampus, which can also indicate the levels of reduced GSH.
The family of superoxide dismutases catalyze the degradation of to H2O2. As such, they also represent a defense mechanism against oxidative stress produced specifically by . However, while they reduce , they simultaneously increase H2O2, another ROS capable of causing oxidative stress. Consequently, we did not assess superoxide dismutase activity, favoring GSH instead.
Dietary fructose increases renal oxidative stress, particularly when combined with a high-salt diet (12); however, the mechanisms by which dietary fructose increases renal oxidative stress and the nephron segments involved are unclear. The second part of our hypothesis was that dietary fructose enhanced ANG II-induced ROS production by proximal tubules and diminished glutathione. We found that dietary fructose significantly enhanced the ability of ANG II to 1) stimulate production by NOX and 2) decrease GSH, thereby exacerbating oxidative stress in the proximal nephron. However, dietary fructose did not change basal, unstimulated production by rat proximal tubules or NOX4 expression, total glutathione, or GSH.
Our data showing that 20% fructose in the drinking water did not alter basal production by proximal tubules is novel, not having been reported before. This finding would seem to be at odds with reports that dietary fructose increases oxidative stress in the kidney (12), heart (10), skeletal muscle cells (38), and peripheral blood mononuclear cells (65). The explanation for the disparate results is likely straightforward. In the reports referenced above, preparations were used in which the effects of ANG II were likely still at least partially intact, reflecting the in vivo situation more closely. In contrast, generation of proximal tubule suspensions takes ~45 min. In this time, one would expect the effects of plasma ANG II to wane such that they were no longer observable. We chose to use this preparation precisely for this reason. This allowed us to study both potential basal effects of dietary fructose and whether the response to ANG II was altered. If we had used a preparation in which endogenous ANG II and its actions were intact, they would have confounded our ability to investigate whether dietary fructose altered these effects.
We show here that dietary fructose augmented the ability of ANG II to stimulate production by proximal tubules. This has not been previously reported. Our results are consistent with those previously reported for other tissues. Ten percent fructose in the drinking water causes increased oxidative stress and nitric oxide synthase activity and expression in the kidney, which are reversed by dietary supplementation of (−)-epicatechin (66). In vitro, fructose added to the culture media of skeletal muscle cells provoked mitochondrial ROS formation, mitochondrial dysfunction, and progressive apoptosis (38, 39). In vivo, fructose ingestion before aerobic exercise in male athletes resulted in a significant rise in oxidative stress, not seen with glucose ingestion (15). Rats drinking 10% fructose present systemic oxidative stress (65). About 20 wk of 20% high fructose and 8% high sodium has been reported to elevate oxidative stress due to a decrease in superoxide dismutase activity and induce salt-sensitive hypertension. Both were blunted by tempol (12). The increase in systolic blood pressure caused by concomitant administration of salt and fructose (6, 28) is completely reversed when rats are chronically given tempol in the drinking water (88). In addition, dietary fructose causes tubular hyperplasia and proliferation in proximal tubules and renal injury (58, 70).
We next tested whether NOXs were the source of fructose-induced ANG II-stimulated production. Our data show that both apocynin and GKT136901 prevented the increase. Together, these data indicate that the source of was NOX1 or NOX4. Since NOX1 was undetectable in our samples (data not shown) and NOX4 expression levels were not changed by dietary fructose, it is most likely that the increased activation of NOX4 was the source of the increased production of . These are the first data showing that dietary fructose augments the ability of ANG II to activate NOX4.
On the surface, our data appear to be at odds with those reporting that xanthine oxidase is likely the primary source of fructose-induced ROS in the proximal nephron (8). However, those authors used a diet that contained much more fructose than the diet used here. Additionally, it may be that ROS produced by xanthine oxidase stimulates NOX4 activity or the other way around. ROS-induced ROS production has been reported in the vasculature (17). NOX4-produced ROS has been reported to induce an increase in NOX2 mRNA (17), and, although it has not been reported, the reversed induction may also occur. Finally, NOX4 may not be the only source of ROS. NOX4-mediated oxidative stress has been reported to induce endothelial nitric oxide synthase uncoupling in the kidney, which leads to decreased nitric oxide production and increased production (13, 27); a similar mechanism has also been reported in the vasculature (45, 51). Our data do not eliminate such a possibility.
Finally, we studied the effect of dietary fructose and acute ANG II treatment on total glutathione and GSH. Our data show that acute ANG II treatment caused a greater reduction in GSH in Fruct tubules than in control tubules. These are the first data showing that dietary fructose enhances the ability of ANG II to diminish GSH in proximal tubules. These results are consistent with dietary fructose enhancing oxidative stress caused by ANG II. Although ANG II decreased total glutathione, this effect was not augmented by dietary fructose. Although our data showing that dietary fructose alone did not have a statistically significant effect on GSH seem to be at odds with studies reporting that dietary fructose causes systemic depletion of reduced GSH (15, 67), the likely explanation is the same as that described above, i.e., waning of the effects of ANG II in an in vitro preparation.
The physiological significance of increased oxidative stress caused by dietary fructose in the proximal nephron is likely to be profound. Previously, we reported that dietary fructose enhances the ability of ANG II to stimulate Na+ reabsorption in proximal tubules via increases in both Na+/H+ exchange activity (24, 26) and expression (24). We also have shown that dietary fructose stimulates fructose reabsorption by this segment (23). These physiological effects are likely to be at least partly dependent on ANG II-induced production.
In summary, we have shown that acute ANG II treatment stimulates production by NOX4 and reduces both total glutathione and GSH in the proximal nephron and that dietary fructose enhances the effects of ANG II on these parameters augmenting oxidative stress.
GRANTS
This work was supported in part by National Heart, Lung, and Blood Institute Grant HL-128053 (to J. L. Garvin).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.G.-V. and J.L.G. conceived and designed research; N.Y., A.G.-V., and J.L.G. performed experiments; N.Y. and J.L.G. analyzed data; N.Y., A.G.-V., and J.L.G. interpreted results of experiments; N.Y. and J.L.G. prepared figures; N.Y. drafted manuscript; A.G.-V. and J.L.G. edited and revised manuscript; N.Y., A.G.-V., and J.L.G. approved final version of manuscript.
ACKNOWLEDGMENTS
Present address of A. Gonzalez-Vicente: Dept. of Inflammation and Immunity, Cleveland Clinic, Lerner Research Institute, NB2-137, 9500 Euclid Ave., Cleveland, OH 44195.
REFERENCES
- 1.Andres-Hernando A, Li N, Cicerchi C, Inaba S, Chen W, Roncal-Jimenez C, Le MT, Wempe MF, Milagres T, Ishimoto T, Fini M, Nakagawa T, Johnson RJ, Lanaspa MA. Protective role of fructokinase blockade in the pathogenesis of acute kidney injury in mice. Nat Commun 8: 14181, 2017. doi: 10.1038/ncomms14181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aoyama M, Isshiki K, Kume S, Chin-Kanasaki M, Araki H, Araki S, Koya D, Haneda M, Kashiwagi A, Maegawa H, Uzu T. Fructose induces tubulointerstitial injury in the kidney of mice. Biochem Biophys Res Commun 419: 244–249, 2012. doi: 10.1016/j.bbrc.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 3.Bild W, Hritcu L, Stefanescu C, Ciobica A. Inhibition of central angiotensin II enhances memory function and reduces oxidative stress status in rat hippocampus. Prog Neuropsychopharmacol Biol Psychiatry 43: 79–88, 2013. doi: 10.1016/j.pnpbp.2012.12.009. [DOI] [PubMed] [Google Scholar]
- 4.Block K, Eid A, Griendling KK, Lee DY, Wittrant Y, Gorin Y. Nox4 NAD(P)H oxidase mediates Src-dependent tyrosine phosphorylation of PDK-1 in response to angiotensin II: role in mesangial cell hypertrophy and fibronectin expression. J Biol Chem 283: 24061–24076, 2008. doi: 10.1074/jbc.M803964200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Briones AM, Tabet F, Callera GE, Montezano AC, Yogi A, He Y, Quinn MT, Salaices M, Touyz RM. Differential regulation of Nox1, Nox2 and Nox4 in vascular smooth muscle cells from WKY and SHR. J Am Soc Hypertens 5: 137–153, 2011. doi: 10.1016/j.jash.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 6.Cabral PD, Hong NJ, Hye Khan MA, Ortiz PA, Beierwaltes WH, Imig JD, Garvin JL. Fructose stimulates Na/H exchange activity and sensitizes the proximal tubule to angiotensin II. Hypertension 63: e68–e73, 2014. doi: 10.1161/HYPERTENSIONAHA.113.02564. [DOI] [PubMed] [Google Scholar]
- 7.Chatterjee PK, Cuzzocrea S, Brown PA, Zacharowski K, Stewart KN, Mota-Filipe H, Thiemermann C. Tempol, a membrane-permeable radical scavenger, reduces oxidant stress-mediated renal dysfunction and injury in the rat. Kidney Int 58: 658–673, 2000. doi: 10.1046/j.1523-1755.2000.00212.x. [DOI] [PubMed] [Google Scholar]
- 8.Cirillo P, Gersch MS, Mu W, Scherer PM, Kim KM, Gesualdo L, Henderson GN, Johnson RJ, Sautin YY. Ketohexokinase-dependent metabolism of fructose induces proinflammatory mediators in proximal tubular cells. J Am Soc Nephrol 20: 545–553, 2009. doi: 10.1681/ASN.2008060576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Declèves AE, Jadot I, Colombaro V, Martin B, Voisin V, Nortier J, Caron N. Protective effect of nitric oxide in aristolochic acid-induced toxic acute kidney injury: an old friend with new assets. Exp Physiol 101: 193–206, 2016. doi: 10.1113/EP085333. [DOI] [PubMed] [Google Scholar]
- 9a.del Mar Sola M, Salto R, Oliver J, Vargas AM. Kinetic characterization of phosphofructokinase isolated from rat kidney cortex. Comp Biochem Physiol B 98: 495–500, 1991. doi: 10.1016/0305-0491(91)90243-7. [DOI] [PubMed] [Google Scholar]
- 10.Delbosc S, Paizanis E, Magous R, Araiz C, Dimo T, Cristol JP, Cros G, Azay J. Involvement of oxidative stress and NADPH oxidase activation in the development of cardiovascular complications in a model of insulin resistance, the fructose-fed rat. Atherosclerosis 179: 43–49, 2005. doi: 10.1016/j.atherosclerosis.2004.10.018. [DOI] [PubMed] [Google Scholar]
- 11.Dixit MP, Xu L, Xu H, Bai L, Collins JF, Ghishan FK. Effect of angiotensin-II on renal Na+/H+ exchanger-NHE3 and NHE2. Biochim Biophys Acta 1664: 38–44, 2004. doi: 10.1016/j.bbamem.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 12.Dornas WC, Cardoso LM, Silva M, Machado NL, Chianca DA Jr, Alzamora AC, Lima WG, Lagente V, Silva ME. Oxidative stress causes hypertension and activation of nuclear factor-κB after high-fructose and salt treatments. Sci Rep 7: 46051, 2017. doi: 10.1038/srep46051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eid AA, Lee DY, Roman LJ, Khazim K, Gorin Y. Sestrin 2 and AMPK connect hyperglycemia to Nox4-dependent endothelial nitric oxide synthase uncoupling and matrix protein expression. Mol Cell Biol 33: 3439–3460, 2013. doi: 10.1128/MCB.00217-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fakhruddin S, Alanazi WA, Alhamami HN, Briski KP, Jackson KE. Hyperglycaemia induced by chronic i.p. and oral glucose loading leads to hypertension through increased Na+ retention in proximal tubule. Exp Physiol 103: 236–249, 2018. doi: 10.1113/EP086604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fernández JM, Da Silva-Grigoletto ME, Gómez-Puerto JR, Viana-Montaner BH, Tasset-Cuevas I, Túnez I, López-Miranda J, Pérez-Jiménez F. A dose of fructose induces oxidative stress during endurance and strength exercise. J Sports Sci 27: 1323–1334, 2009. doi: 10.1080/02640410903266966. [DOI] [PubMed] [Google Scholar]
- 16.Förstermann U. Oxidative stress in vascular disease: causes, defense mechanisms and potential therapies. Nat Clin Pract Cardiovasc Med 5: 338–349, 2008. doi: 10.1038/ncpcardio1211. [DOI] [PubMed] [Google Scholar]
- 17.Frazziano G, Al Ghouleh I, Baust J, Shiva S, Champion HC, Pagano PJ. Nox-derived ROS are acutely activated in pressure overload pulmonary hypertension: indications for a seminal role for mitochondrial Nox4. Am J Physiol Heart Circ Physiol 306: H197–H205, 2014. doi: 10.1152/ajpheart.00977.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fu Y, Zhang R, Lu D, Liu H, Chandrashekar K, Juncos LA, Liu R. NOX2 is the primary source of angiotensin II-induced superoxide in the macula densa. Am J Physiol Regul Integr Comp Physiol 298: R707–R712, 2010. doi: 10.1152/ajpregu.00762.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garvin JL. Angiotensin stimulates bicarbonate transport and Na+/K+ ATPase in rat proximal straight tubules. J Am Soc Nephrol 1: 1146–1152, 1991. [DOI] [PubMed] [Google Scholar]
- 20.Gil Lorenzo AF, Costantino VV, Appiolaza ML, Cacciamani V, Benardon ME, Bocanegra V, Vallés PG. Heat shock protein 70 and CHIP promote Nox4 ubiquitination and degradation within the losartan antioxidative effect in proximal tubule cells. Cell Physiol Biochem 36: 2183–2197, 2015. doi: 10.1159/000430184. [DOI] [PubMed] [Google Scholar]
- 21.Gill PS, Wilcox CS. NADPH oxidases in the kidney. Antioxid Redox Signal 8: 1597–1607, 2006. doi: 10.1089/ars.2006.8.1597. [DOI] [PubMed] [Google Scholar]
- 22.Giustarini D, Colombo G, Garavaglia ML, Astori E, Portinaro NM, Reggiani F, Badalamenti S, Aloisi AM, Santucci A, Rossi R, Milzani A, Dalle-Donne I. Assessment of glutathione/glutathione disulphide ratio and S-glutathionylated proteins in human blood, solid tissues, and cultured cells. Free Radic Biol Med 112: 360–375, 2017. doi: 10.1016/j.freeradbiomed.2017.08.008. [DOI] [PubMed] [Google Scholar]
- 23.Gonzalez-Vicente A, Cabral PD, Hong NJ, Asirwatham J, Saez F, Garvin JL. Fructose reabsorption by rat proximal tubules: role of Na+-linked cotransporters and the effect of dietary fructose. Am J Physiol Renal Physiol 316: F473–F480, 2019. doi: 10.1152/ajprenal.00247.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gonzalez-Vicente A, Cabral PD, Hong NJ, Asirwatham J, Yang N, Berthiaume JM, Dominici FP, Garvin JL. Dietary fructose enhances the ability of low concentrations of angiotensin ii to stimulate proximal tubule Na+ reabsorption. Nutrients 9: 885, 2017. doi: 10.3390/nu9080885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gonzalez-Vicente A, Hong NJ, Cabral PD, Garvin JL. A moderately-enriched fructose diet increases the sensitivity of the proximal nephron to angiotensin II. Hypertension 64, Suppl 1: A304, 2014. [Google Scholar]
- 26.Gonzalez-Vicente A, Hong NJ, Yang N, Cabral PD, Berthiaume JM, Dominici FP, Garvin JL. Dietary fructose increases the sensitivity of proximal tubules to angiotensin ii in rats fed high-salt diets. Nutrients 10: 1244, 2018. doi: 10.3390/nu10091244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gonzalez-Vicente A, Saikumar JH, Massey KJ, Hong NJ, Dominici FP, Carretero OA, Garvin JL. Angiotensin II stimulates superoxide production by nitric oxide synthase in thick ascending limbs. Physiol Rep 4: e12697, 2016. doi: 10.14814/phy2.12697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gordish KL, Kassem KM, Ortiz PA, Beierwaltes WH. Moderate (20%) fructose-enriched diet stimulates salt-sensitive hypertension with increased salt retention and decreased renal nitric oxide. Physiol Rep 5: e13162, 2017. doi: 10.14814/phy2.13162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Greene EL, Paller MS. Xanthine oxidase produces O2-. in posthypoxic injury of renal epithelial cells. Am J Physiol 263: F251–F255, 1992. doi: 10.1152/ajprenal.1992.263.2.F251. [DOI] [PubMed] [Google Scholar]
- 30.Han T, Liu M, Yang S. DJ-1 alleviates angiotensin ii-induced endothelial progenitor cell damage by activating the PPARγ/HO-1 pathway. J Cell Biochem 119: 392–400, 2018. doi: 10.1002/jcb.26191. [DOI] [PubMed] [Google Scholar]
- 31.Han W, Li H, Villar VA, Pascua AM, Dajani MI, Wang X, Natarajan A, Quinn MT, Felder RA, Jose PA, Yu P. Lipid rafts keep NADPH oxidase in the inactive state in human renal proximal tubule cells. Hypertension 51: 481–487, 2008. doi: 10.1161/HYPERTENSIONAHA.107.103275. [DOI] [PubMed] [Google Scholar]
- 32.Haugen EN, Croatt AJ, Nath KA. Angiotensin II induces renal oxidant stress in vivo and heme oxygenase-1 in vivo and in vitro. Kidney Int 58: 144–152, 2000. doi: 10.1046/j.1523-1755.2000.00150.x. [DOI] [PubMed] [Google Scholar]
- 33.Herrera M, Silva GB, Garvin JL. Angiotensin II stimulates thick ascending limb superoxide production via protein kinase C(α)-dependent NADPH oxidase activation. J Biol Chem 285: 21323–21328, 2010. doi: 10.1074/jbc.M110.109157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hitomi H, Kiyomoto H, Nishiyama A. Angiotensin II and oxidative stress. Curr Opin Cardiol 22: 311–315, 2007. doi: 10.1097/HCO.0b013e3281532b53. [DOI] [PubMed] [Google Scholar]
- 35.Hochberg Y. A sharper Bonferroni procedure for multiple tests of significance. Biometrika 75: 800–802, 1988. doi: 10.1093/biomet/75.4.800. [DOI] [Google Scholar]
- 36.Hong NJ, Garvin JL. NADPH oxidase 4 mediates flow-induced superoxide production in thick ascending limbs. Am J Physiol Renal Physiol 303: F1151–F1156, 2012. doi: 10.1152/ajprenal.00181.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Iwai N, Inagami T. Isolation of preferentially expressed genes in the kidneys of hypertensive rats. Hypertension 17: 161–169, 1991. doi: 10.1161/01.HYP.17.2.161. [DOI] [PubMed] [Google Scholar]
- 38.Jaiswal N, Maurya CK, Arha D, Avisetti DR, Prathapan A, Raj PS, Raghu KG, Kalivendi SV, Tamrakar AK. Fructose induces mitochondrial dysfunction and triggers apoptosis in skeletal muscle cells by provoking oxidative stress. Apoptosis 20: 930–947, 2015. doi: 10.1007/s10495-015-1128-y. [DOI] [PubMed] [Google Scholar]
- 39.Jaiswal N, Maurya CK, Pandey J, Rai AK, Tamrakar AK. Fructose-induced ROS generation impairs glucose utilization in L6 skeletal muscle cells. Free Radic Res 49: 1055–1068, 2015. doi: 10.3109/10715762.2015.1031662. [DOI] [PubMed] [Google Scholar]
- 40.Kaplowitz N. The importance and regulation of hepatic glutathione. Yale J Biol Med 54: 497–502, 1981. [PMC free article] [PubMed] [Google Scholar]
- 41.Kelishadi R, Mansourian M, Heidari-Beni M. Association of fructose consumption and components of metabolic syndrome in human studies: a systematic review and meta-analysis. Nutrition 30: 503–510, 2014. doi: 10.1016/j.nut.2013.08.014. [DOI] [PubMed] [Google Scholar]
- 42.Kobori H, Nangaku M, Navar LG, Nishiyama A. The intrarenal renin-angiotensin system: from physiology to the pathobiology of hypertension and kidney disease. Pharmacol Rev 59: 251–287, 2007. doi: 10.1124/pr.59.3.3. [DOI] [PubMed] [Google Scholar]
- 43.Kokko JP, Burg MB, Orloff J. Characteristics of NaCl and water transport in the renal proximal tubule. J Clin Invest 50: 69–76, 1971. doi: 10.1172/JCI106485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li H, Han W, Villar VA, Keever LB, Lu Q, Hopfer U, Quinn MT, Felder RA, Jose PA, Yu P. D1-like receptors regulate NADPH oxidase activity and subunit expression in lipid raft microdomains of renal proximal tubule cells. Hypertension 53: 1054–1061, 2009. doi: 10.1161/HYPERTENSIONAHA.108.120642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li H, Horke S, Förstermann U. Vascular oxidative stress, nitric oxide and atherosclerosis. Atherosclerosis 237: 208–219, 2014. doi: 10.1016/j.atherosclerosis.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 46.Li Y, Lappas G, Anand-Srivastava MB. Role of oxidative stress in angiotensin II-induced enhanced expression of Giα proteins and adenylyl cyclase signaling in A10 vascular smooth muscle cells. Am J Physiol Heart Circ Physiol 292: H1922–H1930, 2007. doi: 10.1152/ajpheart.01166.2006. [DOI] [PubMed] [Google Scholar]
- 47.Lu X, Wang F, Liu M, Yang KT, Nau A, Kohan DE, Reese V, Richardson RS, Yang T. Activation of ENaC in collecting duct cells by prorenin and its receptor PRR: involvement of Nox4-derived hydrogen peroxide. Am J Physiol Renal Physiol 310: F1243–F1250, 2016. doi: 10.1152/ajprenal.00492.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Luo H, Wang X, Chen C, Wang J, Zou X, Li C, Xu Z, Yang X, Shi W, Zeng C. Oxidative stress causes imbalance of renal renin angiotensin system (RAS) components and hypertension in obese Zucker rats. J Am Heart Assoc 4: e001559, 2015. doi: 10.1161/JAHA.114.001559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Massey KJ, Hong NJ, Garvin JL. Angiotensin II stimulates superoxide production in the thick ascending limb by activating NOX4. Am J Physiol Cell Physiol 303: C781–C789, 2012. doi: 10.1152/ajpcell.00457.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Modlinger PS, Wilcox CS, Aslam S. Nitric oxide, oxidative stress, and progression of chronic renal failure. Semin Nephrol 24: 354–365, 2004. doi: 10.1016/j.semnephrol.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 51.Mollnau H, Wendt M, Szöcs K, Lassègue B, Schulz E, Oelze M, Li H, Bodenschatz M, August M, Kleschyov AL, Tsilimingas N, Walter U, Förstermann U, Meinertz T, Griendling K, Münzel T. Effects of angiotensin II infusion on the expression and function of NAD(P)H oxidase and components of nitric oxide/cGMP signaling. Circ Res 90: E58–E65, 2002. doi: 10.1161/01.RES.0000012569.55432.02. [DOI] [PubMed] [Google Scholar]
- 52.Mondal MS, Mitra S. Kinetics and thermodynamics of the molecular mechanism of the reductive half-reaction of xanthine oxidase. Biochemistry 33: 10305–10312, 1994. doi: 10.1021/bi00200a010. [DOI] [PubMed] [Google Scholar]
- 53.Montonen J, Järvinen R, Knekt P, Heliövaara M, Reunanen A. Consumption of sweetened beverages and intakes of fructose and glucose predict type 2 diabetes occurrence. J Nutr 137: 1447–1454, 2007. doi: 10.1093/jn/137.6.1447. [DOI] [PubMed] [Google Scholar]
- 54.Mori T, Cowley AW Jr. Angiotensin II-NAD(P)H oxidase-stimulated superoxide modifies tubulovascular nitric oxide cross-talk in renal outer medulla. Hypertension 42: 588–593, 2003. doi: 10.1161/01.HYP.0000091821.39824.09. [DOI] [PubMed] [Google Scholar]
- 55.Mori T, Cowley AW Jr, Ito S. Molecular mechanisms and therapeutic strategies of chronic renal injury: physiological role of angiotensin II-induced oxidative stress in renal medulla. J Pharmacol Sci 100: 2–8, 2006. doi: 10.1254/jphs.FMJ05003X2. [DOI] [PubMed] [Google Scholar]
- 56.Mori T, O’Connor PM, Abe M, Cowley AW Jr. Enhanced superoxide production in renal outer medulla of Dahl salt-sensitive rats reduces nitric oxide tubular-vascular cross-talk. Hypertension 49: 1336–1341, 2007. doi: 10.1161/HYPERTENSIONAHA.106.085811. [DOI] [PubMed] [Google Scholar]
- 57.Mori T, Ogawa S, Cowely AW Jr, Ito S. Role of renal medullary oxidative and/or carbonyl stress in salt-sensitive hypertension and diabetes. Clin Exp Pharmacol Physiol 39: 125–131, 2012. doi: 10.1111/j.1440-1681.2011.05653.x. [DOI] [PubMed] [Google Scholar]
- 58.Nakayama T, Kosugi T, Gersch M, Connor T, Sanchez-Lozada LG, Lanaspa MA, Roncal C, Perez-Pozo SE, Johnson RJ, Nakagawa T. Dietary fructose causes tubulointerstitial injury in the normal rat kidney. Am J Physiol Renal Physiol 298: F712–F720, 2010. doi: 10.1152/ajprenal.00433.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nguyen MT, Lee DH, Delpire E, McDonough AA. Differential regulation of Na+ transporters along nephron during ANG II-dependent hypertension: distal stimulation counteracted by proximal inhibition. Am J Physiol Renal Physiol 305: F510–F519, 2013. doi: 10.1152/ajprenal.00183.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pagano PJ. Vascular gp91phox: beyond the endothelium. Circ Res 87: 1–3, 2000. doi: 10.1161/01.RES.87.1.1. [DOI] [PubMed] [Google Scholar]
- 61.Panico C, Luo Z, Damiano S, Artigiano F, Gill P, Welch WJ. Renal proximal tubular reabsorption is reduced in adult spontaneously hypertensive rats: roles of superoxide and Na+/H+ exchanger 3. Hypertension 54: 1291–1297, 2009. doi: 10.1161/HYPERTENSIONAHA.109.134783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pawelczyk T, Bizon D, Angielski S. The distribution of enzymes involved in purine metabolism in rat kidney. Biochim Biophys Acta 1116: 309–314, 1992. doi: 10.1016/0304-4165(92)90045-V. [DOI] [PubMed] [Google Scholar]
- 63.Persson P, Hansell P, Palm F. NADPH oxidase inhibition reduces tubular sodium transport and improves kidney oxygenation in diabetes. Am J Physiol Regul Integr Comp Physiol 302: R1443–R1449, 2012. doi: 10.1152/ajpregu.00502.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pompella A, Visvikis A, Paolicchi A, De Tata V, Casini AF. The changing faces of glutathione, a cellular protagonist. Biochem Pharmacol 66: 1499–1503, 2003. doi: 10.1016/S0006-2952(03)00504-5. [DOI] [PubMed] [Google Scholar]
- 65.Porto ML, Lírio LM, Dias AT, Batista AT, Campagnaro BP, Mill JG, Meyrelles SS, Baldo MP. Increased oxidative stress and apoptosis in peripheral blood mononuclear cells of fructose-fed rats. Toxicol In Vitro 29: 1977–1981, 2015. doi: 10.1016/j.tiv.2015.08.006. [DOI] [PubMed] [Google Scholar]
- 66.Prince PD, Lanzi CR, Toblli JE, Elesgaray R, Oteiza PI, Fraga CG, Galleano M. Dietary (−)-epicatechin mitigates oxidative stress, NO metabolism alterations, and inflammation in renal cortex from fructose-fed rats. Free Radic Biol Med 90: 35–46, 2016. doi: 10.1016/j.freeradbiomed.2015.11.009. [DOI] [PubMed] [Google Scholar]
- 67.Rabie EM, Heeba GH, Abouzied MM, Khalifa MM. Comparative effects of aliskiren and telmisartan in high fructose diet-induced metabolic syndrome in rats. Eur J Pharmacol 760: 145–153, 2015. doi: 10.1016/j.ejphar.2015.04.019. [DOI] [PubMed] [Google Scholar]
- 68.Rajagopalan S, Kurz S, Münzel T, Tarpey M, Freeman BA, Griendling KK, Harrison DG. Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation. Contribution to alterations of vasomotor tone. J Clin Invest 97: 1916–1923, 1996. doi: 10.1172/JCI118623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Reilly AM, Harris PJ, Williams DA. Biphasic effect of angiotensin II on intracellular sodium concentration in rat proximal tubules. Am J Physiol 269: F374–F380, 1995. doi: 10.1152/ajprenal.1995.269.3.F374. [DOI] [PubMed] [Google Scholar]
- 70.Roncal-Jimenez CA, Ishimoto T, Lanaspa MA, Milagres T, Hernando AA, Jensen T, Miyazaki M, Doke T, Hayasaki T, Nakagawa T, Marumaya S, Long DA, Garcia GE, Kuwabara M, Sánchez-Lozada LG, Kang DH, Johnson RJ. Aging-associated renal disease in mice is fructokinase dependent. Am J Physiol Renal Physiol 311: F722–F730, 2016. doi: 10.1152/ajprenal.00306.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Saez F, Hong NJ, Garvin JL. Luminal flow induces NADPH oxidase 4 translocation to the nuclei of thick ascending limbs. Physiol Rep 4: e12724, 2016. doi: 10.14814/phy2.12724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Saez F, Hong NJ, Garvin JL. NADPH oxidase 4-derived superoxide mediates flow-stimulated NKCC2 activity in thick ascending limbs. Am J Physiol Renal Physiol 314: F934–F941, 2018. doi: 10.1152/ajprenal.00631.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Saha S, Li Y, Anand-Srivastava MB. Reduced levels of cyclic AMP contribute to the enhanced oxidative stress in vascular smooth muscle cells from spontaneously hypertensive rats. Can J Physiol Pharmacol 86: 190–198, 2008. doi: 10.1139/Y08-012. [DOI] [PubMed] [Google Scholar]
- 74.Sedeek M, Callera G, Montezano A, Gutsol A, Heitz F, Szyndralewiez C, Page P, Kennedy CR, Burns KD, Touyz RM, Hébert RL. Critical role of Nox4-based NADPH oxidase in glucose-induced oxidative stress in the kidney: implications in type 2 diabetic nephropathy. Am J Physiol Renal Physiol 299: F1348–F1358, 2010. doi: 10.1152/ajprenal.00028.2010. [DOI] [PubMed] [Google Scholar]
- 75.Shiose A, Kuroda J, Tsuruya K, Hirai M, Hirakata H, Naito S, Hattori M, Sakaki Y, Sumimoto H. A novel superoxide-producing NAD(P)H oxidase in kidney. J Biol Chem 276: 1417–1423, 2001. doi: 10.1074/jbc.M007597200. [DOI] [PubMed] [Google Scholar]
- 77.Sun P, Yue P, Wang WH. Angiotensin II stimulates epithelial sodium channels in the cortical collecting duct of the rat kidney. Am J Physiol Renal Physiol 302: F679–F687, 2012. doi: 10.1152/ajprenal.00368.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tapia E, Cristóbal M, García-Arroyo FE, Soto V, Monroy-Sánchez F, Pacheco U, Lanaspa MA, Roncal-Jiménez CA, Cruz-Robles D, Ishimoto T, Madero M, Johnson RJ, Sánchez-Lozada LG. Synergistic effect of uricase blockade plus physiological amounts of fructose-glucose on glomerular hypertension and oxidative stress in rats. Am J Physiol Renal Physiol 304: F727–F736, 2013. doi: 10.1152/ajprenal.00485.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Touyz RM, Schiffrin EL. Increased generation of superoxide by angiotensin II in smooth muscle cells from resistance arteries of hypertensive patients: role of phospholipase D-dependent NAD(P)H oxidase-sensitive pathways. J Hypertens 19: 1245–1254, 2001. doi: 10.1097/00004872-200107000-00009. [DOI] [PubMed] [Google Scholar]
- 80.Vaziri ND, Wang XQ, Oveisi F, Rad B. Induction of oxidative stress by glutathione depletion causes severe hypertension in normal rats. Hypertension 36: 142–146, 2000. doi: 10.1161/01.HYP.36.1.142. [DOI] [PubMed] [Google Scholar]
- 81.Vendrov AE, Madamanchi NR, Niu XL, Molnar KC, Runge M, Szyndralewiez C, Page P, Runge MS. NADPH oxidases regulate CD44 and hyaluronic acid expression in thrombin-treated vascular smooth muscle cells and in atherosclerosis. J Biol Chem 285: 26545–26557, 2010. doi: 10.1074/jbc.M110.143917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Vos MB, Kimmons JE, Gillespie C, Welsh J, Blanck HM. Dietary fructose consumption among US children and adults: the Third National Health and Nutrition Examination Survey. Medscape J Med 10: 160, 2008. [PMC free article] [PubMed] [Google Scholar]
- 83.Welch WJ, Blau J, Xie H, Chabrashvili T, Wilcox CS. Angiotensin-induced defects in renal oxygenation: role of oxidative stress. Am J Physiol Heart Circ Physiol 288: H22–H28, 2005. doi: 10.1152/ajpheart.00626.2004. [DOI] [PubMed] [Google Scholar]
- 84.Wilcox CS. Reactive oxygen species: roles in blood pressure and kidney function. Curr Hypertens Rep 4: 160–166, 2002. doi: 10.1007/s11906-002-0041-2. [DOI] [PubMed] [Google Scholar]
- 85.Yang J, Lane PH, Pollock JS, Carmines PK. Protein kinase C-dependent NAD(P)H oxidase activation induced by type 1 diabetes in renal medullary thick ascending limb. Hypertension 55: 468–473, 2010. doi: 10.1161/HYPERTENSIONAHA.109.145714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zager RA, Conrad DS, Burkhart K. Phospholipase A2: a potentially important determinant of adenosine triphosphate levels during hypoxic-reoxygenation tubular injury. J Am Soc Nephrol 7: 2327–2339, 1996. [DOI] [PubMed] [Google Scholar]
- 87.Zager RA, Johnson AC, Hanson SY, Lund S. Ischemic proximal tubular injury primes mice to endotoxin-induced TNF-α generation and systemic release. Am J Physiol Renal Physiol 289: F289–F297, 2005. doi: 10.1152/ajprenal.00023.2005. [DOI] [PubMed] [Google Scholar]
- 88.Zenner ZP, Gordish KL, Beierwaltes WH. Free radical scavenging reverses fructose-induced salt-sensitive hypertension. Integr Blood Press Control 11: 1–9, 2017. doi: 10.2147/IBPC.S147674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhang J, Chandrashekar K, Lu Y, Duan Y, Qu P, Wei J, Juncos LA, Liu R. Enhanced expression and activity of Nox2 and Nox4 in the macula densa in ANG II-induced hypertensive mice. Am J Physiol Renal Physiol 306: F344–F350, 2014. doi: 10.1152/ajprenal.00515.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zimmerman MC, Lazartigues E, Sharma RV, Davisson RL. Hypertension caused by angiotensin II infusion involves increased superoxide production in the central nervous system. Circ Res 95: 210–216, 2004. doi: 10.1161/01.RES.0000135483.12297.e4. [DOI] [PubMed] [Google Scholar]
- 91.Zou AP, Cowley AW Jr. Nitric oxide in renal cortex and medulla. An in vivo microdialysis study. Hypertension 29: 194–198, 1997. doi: 10.1161/01.HYP.29.1.194. [DOI] [PubMed] [Google Scholar]
- 92.Zou AP, Li N, Cowley AW Jr. Production and actions of superoxide in the renal medulla. Hypertension 37: 547–553, 2001. doi: 10.1161/01.HYP.37.2.547. [DOI] [PubMed] [Google Scholar]