Abstract
E-cigarettes are portrayed as safer relative to conventional tobacco. However, burgeoning evidence suggests that E-cigarettes may adversely affect host defenses. However, the precise mechanisms by which E-cigarette vapor alters innate immune cell function have not been fully elucidated. We determined the effects of E-cigarette exposure on the function and responses to infectious challenge of the most abundant innate immune cell, the neutrophil, using isolated human neutrophils and a mouse model of gram-negative infection. Our results revealed that human neutrophils exposed to E-cigarette vapor had 4.2-fold reductions in chemotaxis toward the bacterial cell-well component f-Met-Leu-Phe (P < 0.001). F-actin polarization and membrane fluidity were also adversely affected by E-cigarette vapor exposure. E-cigarette-exposed human neutrophils exhibited a 48% reduction in production of reactive oxygen species (ROS; P < 0.001). Given the central role of ROS in neutrophil extracellular trap (NET) production, NET production was quantified, and E-cigarette vapor exposure was found to reduce NETosis by 3.5-fold (P < 0.01); formulations with and without nicotine containing propylene glycol exhibiting significant suppressive effects. However, noncanonical NETosis was unaffected. In addition, exposure to E-cigarette vapor lowered the rate of phagocytosis of bacterial bioparticles by 47% (P < 0.05). In our physiological mouse model of chronic E-cigarette exposure and sepsis, E-cigarette vapor inhalation led to reduced neutrophil migration in infected spaces and a higher burden of Pseudomonas. These findings provide evidence that E-cigarette use adversely impacts the innate immune system and may place E-cigarette users at higher risk for dysregulated inflammatory responses and invasive bacterial infections.
Keywords: chemotaxis, E-cigarette, human neutrophils, neutrophil extracellular trap, Pseudomonas; reactive oxygen species; sepsis
INTRODUCTION
Neutrophils are key effector cells of the innate immune system that play a critical role in controlling and eliminating infections. Representing 50–70% of circulating white blood cells, neutrophils reach sites of infection by detecting and migrating in response to gradients of chemoattractants such as the bacterial cell wall component f-Met-Leu-Phe (fMLP). Upon encountering pathogens, neutrophils use a diverse molecular arsenal to neutralize the threat, including production of reactive oxygen species (ROS) and the formation of neutrophil extracellular traps (NETs), DNA-based structures coated with antimicrobial proteins/peptides. It is known that exposure to environmental toxins, such as cigarette smoke (10) and bisphenol A (3), can diminish the antimicrobial capacity of neutrophils. However, although increasing evidence from in vitro, in vivo, and human studies have linked electronic (E)-cigarette use with altered host defenses, cell damage, and inflammatory responses (4, 13, 31, 37), relatively little is known about the effects of E-cigarette use on neutrophil function.
It has been estimated that electronic nicotine delivery systems are being used by 7–12% of adults and up to 37% of adolescents and young adults (14, 20, 22, 28, 32, 34). E-cigarettes have been aggressively promoted as a safer less toxic alternative to smoking conventional cigarettes. E-cigarettes are composed of a lithium ion battery, atomizer, and coil to heat the e-liquid and tank filled with e-liquid composed of propylene glycol (PG), glycerin (Gly), and nicotine at varying concentrations. Despite their continued popularity, the safety profiles of E-cigarettes and their impact on human health are not well understood (7, 11). E-cigarette use has been associated with occurrences of acute eosinophilic pneumonia, lipoid pneumonia, hypersensitivity, and bronchiolitis (12, 24, 33, 35). Asthma, bronchitis, and impaired cough sensitivity and mucociliary clearance have also been associated with E-cigarette use (5, 16, 17, 21). More severe diseases, including acute lung injury and acute respiratory distress syndrome, also appear to be causally linked to inhalation of E-cigarette vapor. These cases provide clear evidence of E-cigarette toxicity on human health and make it imperative to understand the specific cells and functions impacted by E-cigarette vapor inhalation (4, 9, 19, 31).
Previously, we showed that direct exposure to electronic vapor extract (EVE) decreased bacterial killing of Pseudomonas aeruginosa by neutrophils isolated from the blood of healthy human volunteers; however, the mechanistic basis for this effect was unclear (10). P. aeruginosa is a Gram-negative pathogen, commonly causing bacteremia, intra-abdominal infections, and pneumonia (25). Here, we describe the impact of E-cigarette vapor exposure on key neutrophil functions, including chemotaxis, NET formation, and generation of ROS. In addition, using our murine model of chronic E-cigarette exposure, we describe the effects of E-cigarette use on extravasation and chemotaxis of neutrophils in an infected space, and bacterial burden, following intraperitoneal challenge with P. aeruginosa.
METHODS
Human neutrophil isolation.
Human subjects, approximately one-half female and one-half male, underwent written informed consent before sample collection as approved by the University of California San Diego (UCSD) Institutional Review Board (IRB) protocol. Circulating polymorphonuclear cells (neutrophils) were isolated from human peripheral blood as previously described (8). In brief, 25 mL of blood were layered on 20 mL Polymorphprep (Axis-Shield, Dundee, Scotland) before centrifugation at 630 g for 35 min at room temperature without braking. The granulocyte layer was transferred to a fresh tube, rinsed with 50 mL PBS, and centrifuged at 500 g for 10 min before red blood cell lysis with sterile water. Following enumeration, neutrophils were resuspended in PBS and kept at room temperature (RT) before use in functional assays. All studies were performed with UCSD IRB approval.
E-cigarette liquids and EVE.
Individual E-liquid chemicals were purchased from Xtreme Vapor, a popular online store. PG was mixed 1:1 with Gly to create a 50:50 solution of PG + Gly, with nicotine added at 24 mg/mL to create the E-liquid used for the majority of the studies: PG + Gly + 24 mg/mL nicotine. The E-cigarettes used for these studies were Kanger Mini-protank glassomizers with 1.5-Ohm coils, attached to a Kanger eVOD Variable Voltage 1,000-mAh battery, set at 4.8 volts. E-cigarettes were filled with E-liquid (1–1.5 mL) and attached to a three-way stopcock via rubber tubing attached to the mouthpiece, and 50-mL puffs of e-cigarette vapor were “inhaled” in a 60-mL sterile syringe containing 10 mL of HBSS. The syringe (now containing both HBSS and e-cigarette vapor) was shaken for 15 s to maximize exposure of the surface of the liquid to the e-cigarette vapor, before expulsion of the e-cigarette vapor. Following expulsion of the vapor, an additional puff was performed until each batch of media was exposed to 30 puffs of e-cigarette vapor to create 100% EVE. Thus, each batch of EVE was exposed to fresh e-cigarette vapor for 7.5 min total (30 puffs × 15 s each). For some assays, individual components were used to create different types of EVE, such as 100% PG vaped in media to create PG-EVE and 50% PG + 50% Gly to create PG + Gly-EVE (EVE without nicotine).
Transwell chemotaxis assay.
Neutrophils isolated from human subjects were incubated for 20 min at RT in control or EVE-infused HBSS. Neutrophils exposed to EVE were assessed for viability relative to unexposed neutrophils via annexin V and propidium iodide staining and FACS (Supplemental Fig. SA; all Supplemental material is available at https://doi.org/10.6084/m9.figshare.9922601.v1). Cells were also assessed for viability by trypan blue staining before seeding in 6-mm Transwell permeable supports (3-μm pore size; Corning) in 24-well plates. The lower chambers contained HBSS alone or 100 nM of the chemoattractant fMLP. Neutrophils were allowed to migrate for 45 min at 37°C with 5% CO2, after which inserts were removed, and cells that migrated in the lower well were lysed by addition of Triton X-100 (0.1% final, 10 min, 24°C). Samples of cell lysates (180 µL) were transferred to a fresh 96-well plate containing 20 µL of 100 mM N-methoxysuccinyl-Ala-Ala-Pro-Val-p-nitroanilide, a colorimetric elastase substrate. Following a 30-min incubation at 24°C, absorbance at 405 nm was measured using a SpectraMAX Gemini EM fluorescence reader (Molecular Devices, Sunnyvale, CA).
Visualization of F-actin distribution.
Human neutrophils were resuspended in control or EVE-infused HBSS and plated at 1 × 105 cells/well in Lab-Tek II chambered cover glass slides. fMLP (100 nM) was added to each well for 10 min at 37°C with 5% CO2, and cells were fixed in 4% paraformaldehyde. Cells were stained with rhodamine phalloidin (Thermo Fisher, Waltham, MA) as indicated by the manufacturer. Following three washes with PBS, images were obtained using a Zeiss AxioObserver D1 microscope equipped with a plan apochromat 63x/1.4 oil-immersion objective.
Neutrophil membrane fluidity.
Isolated human neutrophils were suspended in control or EVE-infused HBSS for 30 min before staining with the Abcam Membrane Fluidity Kit (according to the manufacturer’s instructions; Abcam). In brief, cells were stained with the fluorescent lipophilic reagent containing pyrenedecanoic acid at room temperature. The lipid reagent exists at baseline as a monomer but forms an excimer upon monomer spatial interaction, resulting in a red shift of the emission spectrum that was detected with ratiometric fluorescence measurements using an EnVision plate reader (PerkinElmer, Waltham, MA).
Tomographic microscopy.
Human neutrophils were seeded in fibronectin-coated 35-mm glass-bottom dishes in 75 µL HBSS with calcium and magnesium. An additional 75 µL HBSS or 100% EVE was added to the dishes, and neutrophils were imaged using a NanoLive 3D Cell Explorer (Ecublens, Switzerland).
ROS production.
Isolated human neutrophils exposed were incubated in calcium- and magnesium-free HBSS containing 10 μM 2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA) for 20 min at 37°C with gentle agitation. Following centrifugation at 400 g for 5 min, neutrophils were resuspended in control or EVE-infused HBSS with calcium and magnesium in 96-well plates at 2 × 105 cells/well. Following addition of 25 nM phorbol 12-myristate 13-acetate (PMA) or vehicle to applicable wells, fluorescence intensity (485 nm excitation, 530 nm emission) was measured using an EnVision plate reader (PerkinElmer, Waltham, MA), with reads collected every 15 min for 2 h. Between reads, plates were incubated (protected from light) at 37°C with 5% CO2.
Induction and quantification of NETs.
Human neutrophils were incubated in control or EVE-infused HBSS for 20 min in siliconized tubes. Neutrophils were seeded in 96-well plates at 2 × 105 cells/well and treated with 25 nM PMA or 15 μM nigericin for 2 h at 37°C with 5% CO2 to stimulate NET production via canonical and noncanonical pathways, respectively. Subsequently, 500 mU/mL micrococcal nuclease was added to each well, and plates were incubated for 10 min at 37°C. Following addition of 5 mM EDTA to stop the micrococcal nuclease reaction, plates were spun at 200 g for 8 min. Supernatants (100 μL) were collected from each well, and extracellular DNA content was quantified using a fluorescent Quant-IT PicoGreen dsDNA Assay kit (Life Technologies, Carlsbad, CA) according to the manufacturer’s instructions using an EnVision plate reader (PerkinElmer, Waltham, MA).
NET visualization and fluorescence microscopy.
To visualize NETs after ex vivo EVE exposure, human neutrophils were seeded at 1 × 105 cells/well in 96-well plates. Following stimulation of NET production (2 h, 37°C with 5% CO2 with and without PMA), cells were fixed in 4% paraformaldehyde for 10 min. After blocking for 45 min with 2% bovine serum albumin (BSA) and 2% goat serum in PBS, cells were immunostained via the following incubation steps at RT: 1 h with rabbit anti-human myeloperoxidase primary antibody (1:300 in 2% PBS-BSA, catalog no. A039829–2; Dako North America, Inc., Carpinteria, CA), 45 min with Alexa Fluor 488 goat anti-rabbit IgG secondary antibody (1:500 in 2% PBS-BSA, catalog no. A11070; Life Technologies, Carlsbad, CA), and 10 min with 1 μM Hoechst-33342-trihydrochloride. Three washes with PBS were performed after each staining step. Images were obtained using a Zeiss AxioObserver D1 microscope equipped with an LD A-Plan 20X/0.35 Ph1 objective.
Neutrophil phagocytosis.
Isolated human neutrophils were suspended in control or EVE-infused HBSS for 30 min before plating in 96-well plates and the addition of fluorescently labeled bacterial bioparticles (Staphylococcus aureus and Escherichia coli; Life Technologies). Fluorescence intensity at 560/585 nm was quantified using an EnVision plate reader (PerkinElmer, Waltham, MA) at time 0 and every 15 min thereafter for 2 h.
Mouse model of chronic E-cigarette use.
For abdominal infection experiments, C57BL/6 female mice, 6–8 wk old (Envigo), were exposed for 60 min daily to fresh E-cigarette vapor (EV) using the nose-only InExpose system (SciReq). EV was produced from 50% PG, 50% Gly, and 24 mg/mL nicotine, in GS-CE-4 2.4-Ω Clear Cartomizers (Xtreme Vaping) attached to 510 280-mAh Automatic Rechargeable E-Cigarette Batteries (FastTech). The E-cigarette is activated by the InExpose system, with application of pneumatic pressure at 2 L/s for 1 s, and 1 L/s for 3 s, followed by 16 s of room air. Mice were placed in individual mesh restraints and exposed via nose only for 1 h/day, 5 days/wk, for 4 wk, before infection. Mice were recovered in 37°C preheated cages for 30 min after each exposure to air or EV. For lung RNAseq studies, C57BL/6 and CD-1 (Envigo) 6- to 8-wk-old female mice were exposed to air or EV for 3 and 6 mo, respectively. All animal experiments were approved by the UCSD Institutional Animal Care and Use Committee.
Mouse pseudomonal infection model.
P. aeruginosa (PAO1) was streaked from a glycerol stock on Luria agar (LA) and incubated at 37°C overnight. The day before infection, one colony of P. aeruginosa was used to inoculate 3 mL of Luria broth (LB) and was incubated at 37°C with shaking overnight. The day of infection, the overnight culture was diluted 1:100 in LB and grown at 37°C with shaking to midlog phase. Bacteria were centrifuged at 2,800 rpm for 10 min, rinsed in 50 mL of 1× PBS, and spun again. Bacteria slurry was added to 3 mL PBS until absorbance = 0.6 and was diluted to 1 × 106 colony-forming units (CFU)/mL. Mice that had undergone 4 wk of 1 h daily EV inhalation were infected intraperitoneally with 200 μL of P. aeruginosa (2 × 105 CFU; n = 6/group). After 4 h, mice were euthanized with inhaled CO2 and underwent peritoneal lavage with 10 mL PBS three times. Peritoneal lavage fluid was assessed for total cell count via trypan blue quantification, cell differential via cytospin (800 rpm × 3 min) and Giemsa Wright stain, enumeration via light microscopy, and serially diluted in PBS and plated on LA overnight at 37°C for CFU enumeration.
RNAseq.
Lungs were isolated from female mice exposed to e-cigarette vapor for 3 (C57BL/6) and 6 (CD-1) mo. Total RNA was isolated (Qiagen), and mRNA stranded libraries were created and QC assessed, followed by sequencing on HiSeq4000 SR75 at the IGM Genomics Center, UCSD. The data set had 19,282 genes and, with a gene set size filter of minimum = 1 and maximum = 500, resulted in 0/4,872 gene sets filtered out, and thus 4,872 gene sets were used in the analysis. RNAseq unbiased pathway analysis was performed with Ingenuity Pathway Analysis (Qiagen), with limits of P adj < 0.10 and log2(fc) > 0.58, at the La Jolla Institute for Allergy and Immunology Core. Gene expression changes were only deemed significant if found in both strains of mice. Heat map analysis was performed on all 10 genes associated with leukocyte extravasation pathways, using Heatmapper with Centroid Linkage as the clustering method and Manhattan distance measurement (2).
Statistics.
All of the statistical analyses described in the legends for Figs. 1–7 were performed using Prism version 6.0 or 8.0. For neutrophil studies, unless otherwise noted, n values represent the number of independent replicates performed using individual human donors. For murine studies, the n value represents individual mice. Statistical analysis of chemotaxis, NET formation, ROS production, and membrane fluidity after ex vivo exposure of neutrophils to e-cigarette vapor was done using one-way analysis of variance (ANOVA) and post hoc Dunnett’s multiple-comparisons test. Data sets were evaluated for even distribution of values on histogram before ANOVA analyses. Microscopy studies of F-actin distribution used random selection of regions and production of fluorescence intensity plots based on the results of three independent experiments. Total peritoneal mammalian cell counts and bacterial cell counts from the peritoneal cavity were analyzed by paired one-tailed t test. Immune cell differential counts from the peritoneal cavity were assessed with two-way ANOVA and uncorrected Fisher’s test.
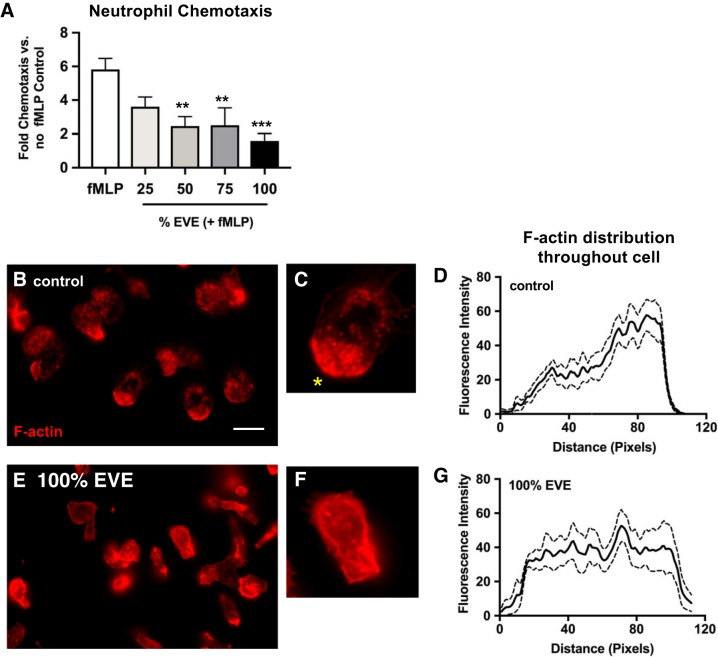
Fig. 1.
Electronic vapor extract (EVE) exposure inhibits f-Met-Leu-Phe (fMLP)-induced chemotaxis and alters F-actin distribution. A: neutrophils, preincubated in 0, 25, 50, 75, or 100% EVE solutions, were placed in the upper well of a Transwell chamber, with lower wells containing the chemoattractant fMLP (100 nM). Chemotaxis to the lower well was quantified by lysing cells and measuring elastase activity. Data are expressed as fold changes relative to neutrophils migrating in the absence of fMLP in the lower well (n = 7–8 using neutrophils isolated from 4 unique donors). The fluorescent F-actin probe rhodamine phalloidin was used to assess F-actin distribution in control (B) and EVE-treated (E) neutrophils in response to fMLP (100 nM) exposure (regions were randomly selected and represent the result of 7 independent experiments; scale bar represents 10 µm). The leading edge of untreated control neutrophils is clearly defined (yellow asterisk, C), whereas there is no identifiable leading or trailing edge in EVE-treated neutrophils (F). D and G: F-actin distribution was quantified (based on rhodamine phalloidin fluorescence intensity) along the length of fMLP-treated cells in the representative images. Transwell chemotaxis results are expressed as mean values ± SE; results were analyzed using 1-way analysis of variance and post hoc Dunnett’s multiple-comparisons test. **P < 0.01 and ***P < 0.001 versus control values.
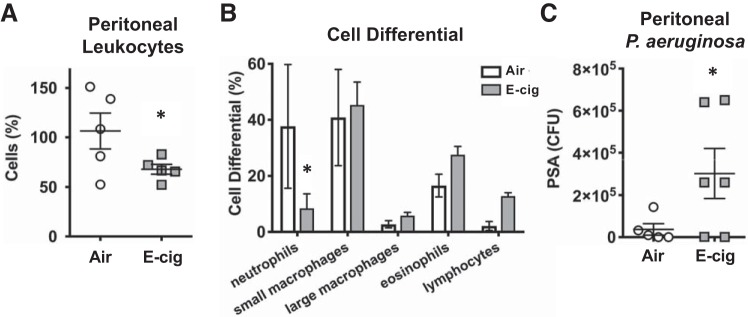
Fig. 7.
Daily inhalation of E-cigarette vapor decreases innate immune responses to ip challenge with Pseudomonas aeruginosa (PSA) in mice. A: total leukocytes recruited to the site of bacterial infection in mice exposed to E-cigarette (E-cig) vapor for 1 h daily for 1 mo relative to air controls (n = 5). B: cell differential of leukocytes recovered from the peritoneal space in response to pseudomonal challenge in E-cigarette mice versus controls. Data were analyzed with 2-way analysis of variance with Fisher’s test. C: colony-forming units (CFU) of P. aeruginosa recovered from the ip space at 4 h after infection (n = 6). Data shown are expressed as mean values ± SE. Results were analyzed by Student’s t test. *P < 0.05.
RESULTS
E-cigarette vapor inhibits human neutrophil chemotaxis and alters actin polarization.
To assess the effects of EVE exposure on neutrophil chemotaxis toward the bacterially derived chemoattractant fMLP, a Transwell chamber approach was used. EVE exposure (50, 75, and 100% solutions) significantly reduced migration of neutrophils, placed in the Transwell insert (upper well), toward the fMLP-containing lower well (Fig. 1A). Chemotaxis is highly dependent on coordinated rearrangement of the actin cytoskeleton, to form an F-actin-rich “leading edge” that drives forward movement; using rhodamine phalloidin as a probe for F-actin, we found that EVE-exposed neutrophils appeared to exhibit a reduced capacity to polarize following exposure to fMLP, as evidenced by microscopy (Fig. 1, B–G; full-size images provided in Supplemental Fig. SB).
E-cigarette vapor exposure alters neutrophil membrane fluidity.
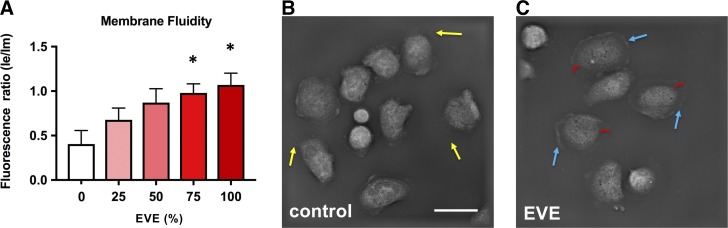
Neutrophil responses to fMLP are known to be dependent on membrane fluidity (38); given the effects of EVE exposure on chemotaxis, we assessed the effects of EVE exposure on human neutrophil membrane fluidity. Using a fluorescent lipophilic pyrene probe that exhibits fluorescent shifts between excimer (emission maximum 470 nm) and monomer (emission maximum 372 nm) forms, we found that EVE exposure significantly alters membrane fluidity (Fig. 2A). Probe-free tomographic microscopy using a NanoLive 3D Cell Explorer microscope revealed an altered morphology of EVE-exposed neutrophils (Fig. 2C). Compared with control neutrophils, EVE-treated neutrophils exhibited extended flattened membranous regions without apparent organelles.
Fig. 2.
Electronic vapor extract (EVE) exposure alters membrane fluidity and the morphology of activated neutrophils. A: membrane fluidity of control and EVE-exposed neutrophils (25, 50, 75, 100, according to the %EVE in the incubation solution) was quantified using a fluorescent lipophilic pyrene probe that exhibits fluorescent shifts between excimer (le; emission maximum 470 nm) and monomer (lm; emission maximum 372 nm) forms (n = 3). Tomographic images of live control (B) and EVE-exposed (C) neutrophils were obtained using a NanoLive 3D Cell Explorer system. Yellow arrows indicate normal membrane extensions seen when neutrophils are plated. Blue arrows indicate the smoother rounded edge of EVE-exposed neutrophils, in the absence of membrane extensions. Red calipers mark the distance between the main cell body and cell membrane, which is greater in EVE-exposed neutrophils because of increased membrane fluidity. Scale bar represents 10 µm. Membrane fluidity results are expressed as mean values ± SE; results were analyzed by 1-way analysis of variance and post hoc Dunnett’s multiple-comparisons test. *P < 0.05 versus control values.
Neutrophil ROS production is inhibited by E-cigarette vapor exposure.
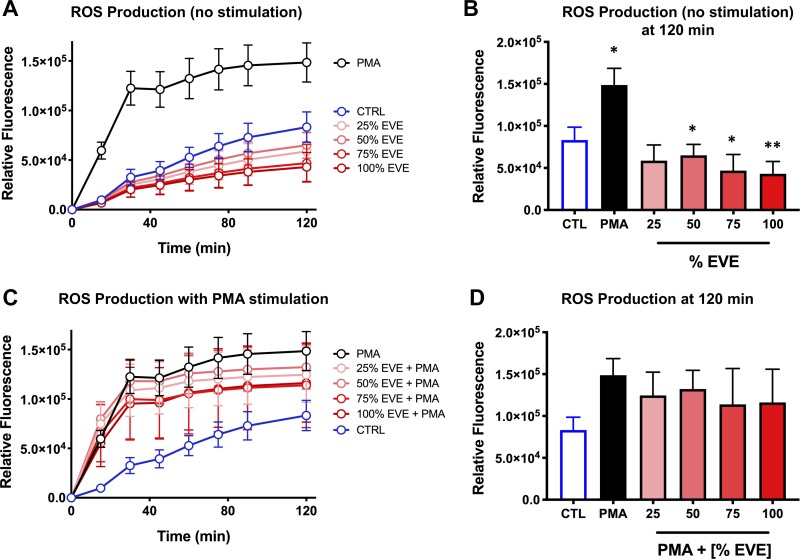
We next assessed the effects of EVE exposure on ROS production, a key driver of the antimicrobial activity of neutrophils, using the membrane-permeable fluorescent ROS probe H2DCFDA. Following incubation with H2DCFDA, neutrophils were exposed to varying concentrations of EVE, and ROS production was quantified over a 2-h window. Using this approach, we found that exposure to 50, 75, and 100% EVE significantly reduced baseline levels of ROS production by human neutrophils at 2 h (Fig. 3, A and B). Although a modest reduction in PMA-induced ROS production in EVE-exposed neutrophils was also observed (Fig. 3, C and D), this effect did not reach statistical significance.
Fig. 3.
Electronic vapor extract (EVE) exposure reduces reactive oxygen species (ROS) production by neutrophils. A: ROS production by nonstimulated control (CTRL) and EVE-exposed versus phorbol 12-myristate 13-acetate (PMA)-stimulated neutrophils was quantified over time using the membrane-permeable fluorescent ROS probe 2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA) (n = 3). B: ROS production at time (t) = 120 min demonstrates EVE-induced suppression of ROS production relative to non-EVE-exposed controls (blue) (n = 3). C and D: ROS production by EVE-exposed neutrophils stimulated with PMA was similar to control neutrophils stimulated with PMA (n = 3). Data shown are expressed as mean values ± SE; where applicable, results were analyzed by 1-way analysis of variance and post hoc Dunnett’s multiple-comparisons test. *P < 0.05 and **P < 0.01 versus control values.
Human NET production is inhibited by E-cigarette vapor exposure.
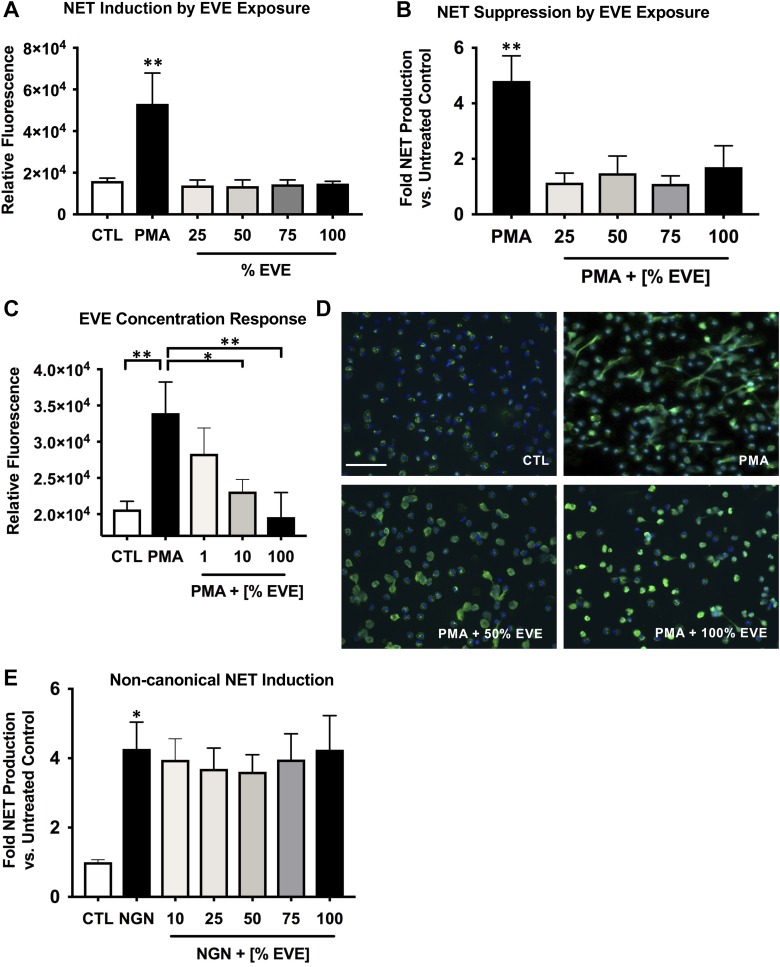
We next assessed the effects of EVE exposure on NET formation. Fluorescence-based quantification of extracellular DNA release demonstrated that exposure to EVE ex vivo does not induce NET formation (Fig. 4A). However, when the known NET inducer PMA was added, preexposure to EVE significantly inhibited PMA-induced NET production (P < 0.01 at all EVE concentrations; Fig. 4B). A concentration effect was seen with EVE doses of 1, 10 and 100%, with the highest EVE having the greatest NETosis suppressive effect (Fig. 4C). This effect was confirmed using an immunocytochemical approach where control/PMA-treated cells were fixed and stained using the nuclear stain Hoechst-33342 and an antibody against myeloperoxidase, a marker of NETs (Fig. 4D). Because the noncanonical NETosis pathway may play a distinct role in inflammatory diseases relative to canonical NETosis, we also tested the effect of EVE on this pathway via NET induction with nigericin, an inducer of noncanonical NETosis. Exposure to EVE did not suppress NETosis in the presence of nigericin (Fig. 4E).
Fig. 4.
Electronic vapor extract (EVE) exposure inhibits production of neutrophil extracellular traps (NETs). A: extracellular DNA/NET production in response to EVE was quantified using the fluorescent DNA dye PicoGreen (n = 5). B: the PicoGreen method was also used to quantify the effects of EVE exposure on NET production in response to a 2-h treatment with NET inducer phorbol 12-myristate 13-acetate (PMA) (n = 3). C: to assess the concentration dependence of NETosis inhibition by EVE, the effect of a range of concentrations between 1 and 100% on PMA-induced NETosis was tested (n = 7). D: an immunocytochemical approach was used to image NETs produced by untreated neutrophils [control (CTL)] or PMA-treated neutrophils (2 h treatment) incubated in control or EVE-containing media (green, myeloperoxidase; blue, Hoechst-33342; scale bar = 50 µm). Regions were randomly selected for imaging and are representative of 5 separate experiments. E: effect of EVE on noncanonical NETosis induced by nigericin (NGN) was assessed using the PicoGreen method (n = 8). Data shown are expressed as mean values ± SE; where applicable, results were analyzed by 1-way analysis of variance and post hoc Dunnett’s multiple-comparisons test. *P < 0.05 and **P < 0.01 versus PMA or nigericin controls.
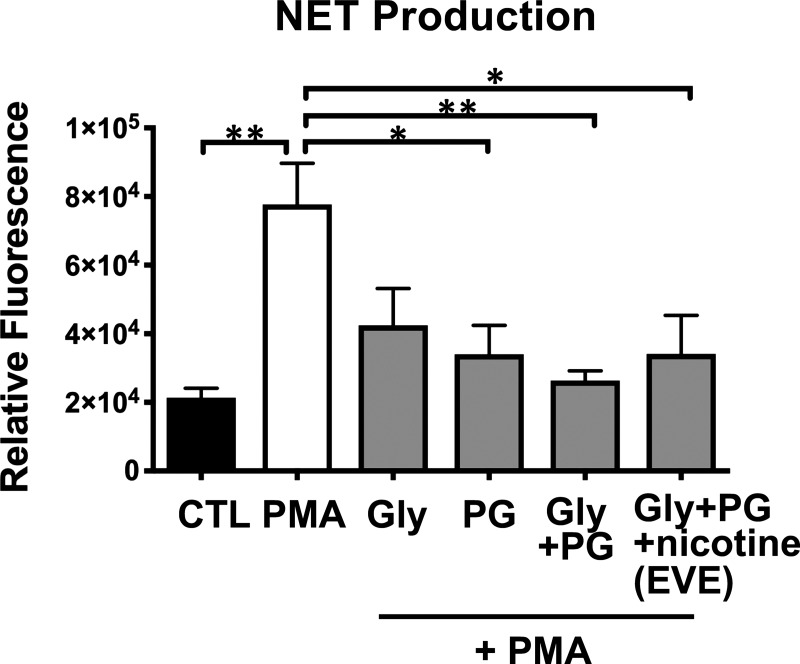
Nicotine-free E-liquid formulations inhibit NET production.
We next sought to determine whether the effects of EVE exposure were dependent on the effects of nicotine, which is known to affect neutrophil function via activation of α7-nicotinic acetylcholine receptors (18). With the use of fresh E-cigarette tanks that had not previously been exposed to E-cigarette liquid, EVE solutions were prepared using E-liquids composed of various chemical compositions, including propylene glycol alone (PG-EVE), glycerin alone (Gly-EVE), a combination of propylene glycol and glycerin (PG + Gly-EVE), or the same E-liquid used for all other studies (PG + Gly + nicotine; EVE, which contains 24 mg/mL nicotine). Significantly reduced NET production in response to PMA treatment was observed not only in the nicotine-containing EVE-treated group but also in groups treated with non-nicotine-containing vaped e-liquids (PG-EVE and PG + Gly-EVE; Fig. 5A).
Fig. 5.
Exposure to electronic vapor extract (EVE) produced using nicotine-free E-liquid formulations inhibits neutrophil extracellular trap (NET) production. PicoGreen DNA dye was used to quantify NET production in control (CTL) or phorbol 12-myristate 13-acetate (PMA)-treated neutrophils incubated in E-cigarette extracts produced using nicotine-free formulations containing propylene glycol (PG) or glycerin (Gly) alone and a combination of propylene glycol and glycerin (PG + Gly). The standard formulation containing 24 mg/mL nicotine, PG, and Gly (EVE) was included for comparison (n = 3). Data shown are expressed as mean values ± SE. Results were analyzed by 1-way analysis of variance and post hoc Dunnett’s multiple-comparisons test. *P < 0.05 and **P < 0.01 versus control.
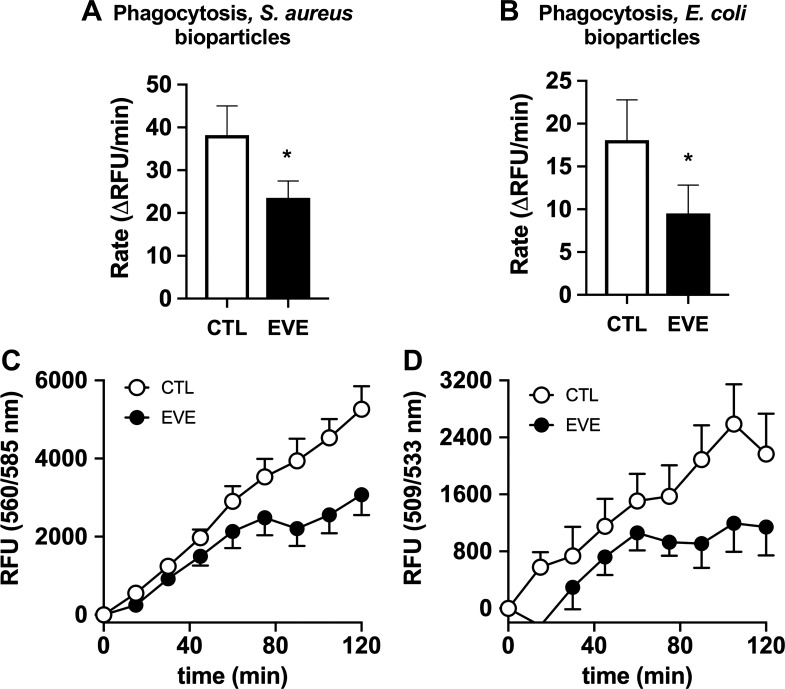
Exposure to e-cigarette vapor extract has modest effects on neutrophil phagocytosis.
Phagocytosis is a critical actin-dependent pathway the facilitates the antimicrobial function of neutrophils. To assess the effect of EVE exposure on phagocytosis by human neutrophils, we incubated isolated neutrophils with fluorescent bioparticles produced using components from both Gram-positive (S. aureus) and Gram-negative (E. coli) pathogens. The rate of phagocytosis of both bioparticle types was reduced by ~40% (Fig. 6, A and B) in 100% EVE-exposed neutrophils. Phagocytosis rates were quantified by monitoring the increase in whole well fluorescence, corresponding with total phagocytosis by neutrophils, in 15 min over a 2-h period (Fig. 6, C and D).
Fig. 6.
Electronic vapor extract (EVE) inhibits phagocytosis by human neutrophils. The effect of EVE exposure on phagocytosis by human neutrophils was assessed by monitoring uptake of fluorescently labeled Staphylococcus aureus and Escherichia coli bioparticles. Rates of phagocytosis were calculated over a 2-h period (A and B: both n = 5), with fluorescence intensity values corresponding to the bead phagocytosis measured at 15-min intervals (C and D; both n = 5). CTL, control; RFU, relative fluorescence units. Data shown are expressed as mean values ± SE; results were analyzed via Student’s t test. *P < 0.05 versus control values.
Inhalation of E-cigarette vapor daily for 1 mo leads to systemic alterations in host defenses with diminished recruitment of murine neutrophils to the site of bacterial infection.
To confirm adverse effects of E-cigarette vapor on the ability of circulating neutrophils to successfully participate in host defense, we exposed mice to inhaled E-cigarette vapor containing nicotine for 60 min/day for 1 mo. Because airways have both macrophages and epithelial cells as their front lines of defense, pneumonia models primarily assess the host defense capabilities of these cells instead of neutrophils. Therefore, we introduced a bacterial challenge in a site where recruitment of neutrophils is necessary for successful host defense. We used a Gram-negative sepsis model in which mice underwent intraperitoneal P. aeruginosa challenge. Fewer total leukocytes were found at the site of infection in E-cigarette mice versus air controls (68 versus 108%, P = 0.038, n = 5; Fig. 7A). Fewer neutrophils migrated out of circulating blood and in the infected peritoneal space in E-cigarette mice compared with air controls (7.9 × 104 versus 66 × 104 murine neutrophils, or 8 versus 38%, respectively; P = 0.042; Fig. 7B). These data suggest that inhalation of E-cigarette vapor daily impairs the ability of circulating neutrophils to chemotaxis into infected sites. Based on our ex vivo human neutrophil data, part of this effect could be the result of direct effects on circulating neutrophils.
We also performed RNAseq on lung tissue from mice chronically exposed to daily E-cigarette vapor and found significant downregulation of leukocyte extravasation signaling genes (ratio 0.047, P value = 2.95 × 10−6, Q value = 4.45 × 10−4; Supplemental Fig. SC), which suggests that E-cigarette effects on endothelial cells may also be impacting transmigration of neutrophils from the circulation into the site of infection. E-cigarette-exposed mice had a higher burden of Pseudomonas intraperitoneally compared with air controls (30.2 × 104 versus 3.7 × 104 CFU, respectively; P = 0.039; Fig. 7C). These data demonstrate that chronic use of E-cigarettes may have adverse effects on circulating neutrophils, decreasing their ability to travel to the site of infection and thus decreasing the host’s ability to defend against invasive pathogens.
DISCUSSION
Neutrophils are key effector cells of the innate immune response and are typically the first line of defense against infections. Central to this role is the ability of neutrophils to leave the circulation and migrate to the site of insult, where they kill invading pathogens via multiple mechanisms that include the production of ROS and the formation of NETs. Here, we have assessed the effect of E-cigarette vapor exposure on these critical neutrophil functions using primary human neutrophils. We found that exposure to E-cigarette vapor inhibits human neutrophil chemotaxis in response to the bacterial chemoattractant fMLP, potentially via disruption of the actin cytoskeleton. EVE exposure also significantly altered human neutrophil membrane fluidity, suggesting multiple mechanisms for the effects of EVE on neutrophil chemotaxis. EVE exposure also reduced baseline ROS production by human neutrophils, consistent with previously published studies describing effects of nicotine on neutrophil α7-nicotinic acetylcholine receptors (23). However, EVE may have the opposite effect on other cell types, such as human umbilical vein endothelial cells and human gingival fibroblasts, because of alternative pathway activation or other cell-specific effects (1, 27). In particular, neutrophils use ROS for antimicrobial activities while other cell types use ROS for very different mechanisms, which likely leads to different regulation of ROS within cell types. Formation of NETs in response to PMA treatment was also reduced by exposure to EVE, including, surprisingly, formulations that did not contain nicotine, suggesting that the non-nicotine ingredients (or compounds produced when such ingredients are exposed to the E-cigarette heating element) can also have deleterious effects on neutrophil function.
Our data from studies using primary human neutrophils suggest that EVE-exposed neutrophils are less capable of performing their dedicated antimicrobial functions. Indeed, in our physiological mouse model of E-cigarette vapor exposure, we found that E-cigarette mice had increased bacterial burdens compared with air only exposed controls. The higher number of surviving Pseudomonas may be because of both lower numbers of murine neutrophils recruited to the peritoneal cavity and diminished neutrophil antimicrobial function once at the site of infection. Specifically, inhalation of E-cigarette vapor may have a deleterious effect on circulating neutrophils, leading to decreased mobilization of these cells from circulating blood into a site of infection, leading to increased bacterial survival and burden. Ween et al. demonstrated reduced antimicrobial function of another powerful immune cell, the macrophage, after exposure to multiple types of E-cigarette vapor extracts in vitro (36). Scott et al. also showed diminished phagocytosis by alveolar macrophages after E-cigarette vapor condensate exposure (30). Thus, E-cigarette vapers may be at higher risk for both colonization by pathogens and invasive bacterial infections by both impaired neutrophil chemotaxis to sites of infection and by impaired ability of neutrophils and macrophages to kill bacterial pathogens at the site.
Prior studies on human neutrophils demonstrated that nonvaped flavored e-liquids added directly to primary human neutrophils could impact NET production (5). In particular, Clapp et al. found flavor-specific effects, with Kola flavored e-liquid enhancing and Hot Cinnamon Candies-flavored e-liquid blocking NET formation (6). Because heating and aerosolizing of the e-liquids, as occurs during human vaping of e-cigarettes, changes the chemical composition of e-liquid and likely health results, it is difficult to ascribe clinical relevance of these ex vivo findings. Interestingly, our findings on PMA-induced human NET production in this study are in contrast to the effects published by Reidel and colleagues showing that neutrophils isolated from peripheral blood of E-cigarette users were more sensitive to PMA-induced NET formation (26); however, these findings are not necessarily contradictory. For one, >99% of human users vape flavored e-liquids, and flavorings themselves may have more intense and even opposite effects on host cells than the base chemicals (PG, Gly, and nicotine) tested in our studies. Also, acute/direct exposure of human neutrophils to EVE may impede their ability to clear infections (e.g., as in bacterial pneumonia) while systemic exposure to E-cigarette components could promote an inflammatory state (through actions on other cell types) that promotes NET production, representing a possible “one-two punch” of E-cigarette use. Indeed, E-cigarette vapor has been shown to directly affect endothelial cells (29), and we have previously shown that e-cigarette vapor impacts macrophage function (13). Thus, the effects of E-cigarettes on neutrophil recruitment/function may be a function of decreased chemokine release by resident peritoneal macrophages and other cell types in addition to direct effects on the neutrophils themselves.
Taken together, our findings provide further evidence that E-cigarette vapor alters host innate immune responses and may lead to increased susceptibility to infections and/or increased severity of infections in human E-cigarette users. Our work has limitations, including exposure of primary human neutrophils ex vivo. This allowed us to study E-liquid components vaped in isolation (propylene glycol, glycerin, and a combination of both with nicotine), although >99% of E-cigarette users vape e-liquids containing all three in a multitude of combinations, in addition to added flavors. We used female mice for the bacterial abdominal infection model; thus, sex effect differences cannot be excluded. Further studies using methodology with the highest clinical relevance (e-cigarette vapor, e-cigarette vapor extract, and samples directly from e-cigarette users) are needed to fully elucidate potential short-term and long-term toxicities associated with these widely used products.
GRANTS
This work was funded by an American Heart Association Beginning Grant-in-Aid (L. E. Crotty Alexander 16BGIA27790079), National Heart, Lung, and Blood Institute Grant R01-HL-137052-01 (L. E. Crotty Alexander), and an American Thoracic Society Foundation Award for Outstanding Early Career Investigators (L. E. Crotty Alexander). L. E. Crotty Alexander and C. M. Bojanowski were partially supported by The Veterans Affairs San Diego Healthcare System.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
R.C. and L.E.C.A. conceived and designed research; R.C., A. Moshensky, C.M.B., A. Meier, J.C., and L.E.C.A. performed experiments; R.C., A. Moshensky, C.M.B., A. Meier, R.K.N., and L.E.C.A. analyzed data; R.C., A. Moshensky, C.M.B., A. Meier, J.C., R.K.N., and L.E.C.A. interpreted results of experiments; R.C., C.M.B., and L.E.C.A. prepared figures; R.C., C.M.B., and L.E.C.A. drafted manuscript; R.C., A. Moshensky, C.M.B., A. Meier, J.C., R.K.N., and L.E.C.A. edited and revised manuscript; R.C., A. Moshensky, C.M.B., A. Meier, J.C., R.K.N., and L.E.C.A. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Victor Nizet and Dr. Atul Malhotra for support and guidance of this work.
REFERENCES
- 1.Anderson C, Majeste A, Hanus J, Wang S. E-cigarette aerosol exposure induces reactive oxygen species, DNA damage, and cell death in vascular endothelial cells. Toxicol Sci 154: 332–340, 2016. doi: 10.1093/toxsci/kfw166. [DOI] [PubMed] [Google Scholar]
- 2.Babicki S, Arndt D, Marcu A, Liang Y, Grant JR, Maciejewski A, Wishart DS. Heatmapper: web-enabled heat mapping for all. Nucleic Acids Res 44, Suppl W1: W147– W153, 2016. doi: 10.1093/nar/gkw419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balistrieri A, Hobohm L, Srivastava T, Meier A, Corriden R. Alterations in human neutrophil function caused by bisphenol A. Am J Physiol Cell Physiol 315: C636–C642, 2018. doi: 10.1152/ajpcell.00242.2017. [DOI] [PubMed] [Google Scholar]
- 4.Chun LF, Moazed F, Calfee CS, Matthay MA, Gotts JE. Pulmonary toxicity of e-cigarettes. Am J Physiol Lung Cell Mol Physiol 313: L193–L206, 2017. doi: 10.1152/ajplung.00071.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clapp PW, Pawlak EA, Lackey JT, Keating JE, Reeber SL, Glish GL, Jaspers I. Flavored e-cigarette liquids and cinnamaldehyde impair respiratory innate immune cell function. Am J Physiol Lung Cell Mol Physiol 313: L278–L292, 2017. doi: 10.1152/ajplung.00452.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crotty Alexander L, Fuster M, Montgrain P, Malhotra A. The need for more E-cigarette data: a call to action. Am J Respir Crit Care Med 192: 275–276, 2015. doi: 10.1164/rccm.201505-0915ED. [DOI] [PubMed] [Google Scholar]
- 8.Crotty Alexander LE, Maisey HC, Timmer AM, Rooijakkers SH, Gallo RL, von Köckritz-Blickwede M, Nizet V. M1T1 group A streptococcal pili promote epithelial colonization but diminish systemic virulence through neutrophil extracellular entrapment. J Mol Med (Berl) 88: 371–381, 2010. doi: 10.1007/s00109-009-0566-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crotty Alexander LE, Malhotra A. The civil liberty of smoking cigarettes. Chest 148: 6–8, 2015. doi: 10.1378/chest.15-0340. [DOI] [PubMed] [Google Scholar]
- 10.Crotty Alexander LE, Shin S, Hwang JH. Inflammatory diseases of the lung induced by conventional cigarette smoke: a review. Chest 148: 1307–1322, 2015. doi: 10.1378/chest.15-0409. [DOI] [PubMed] [Google Scholar]
- 11.Crotty Alexander LE, Vyas A, Schraufnagel DE, Malhotra A. Electronic cigarettes: the new face of nicotine delivery and addiction. J Thorac Dis 7: E248–E251, 2015. doi: 10.3978/j.issn.2072-1439.2015.07.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Flower M, Nandakumar L, Singh M, Wyld D, Windsor M, Fielding D. Respiratory bronchiolitis-associated interstitial lung disease secondary to electronic nicotine delivery system use confirmed with open lung biopsy. Respirol Case Rep 5: e00230, 2017. doi: 10.1002/rcr2.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hwang JH, Lyes M, Sladewski K, Enany S, McEachern E, Mathew DP, Das S, Moshensky A, Bapat S, Pride DT, Ongkeko WM, Crotty Alexander LE. Electronic cigarette inhalation alters innate immunity and airway cytokines while increasing the virulence of colonizing bacteria. J Mol Med (Berl) 94: 667–679, 2016. doi: 10.1007/s00109-016-1378-3. [DOI] [PubMed] [Google Scholar]
- 14.Johnston LD, Miech RA, O’Malley PM, Bachman JG, Schulenberg JE, Patrick ME. Monitoring the Future National Survey Results on Drug Use, 1975–2017: Overview, Key Findings on Adolescent Drug Use. Ann Arbor, MI: Institute for Social Research, The University of Michigan, 2018. [Google Scholar]
- 15.Kirchner T, Möller S, Klinger M, Solbach W, Laskay T, Behnen M. The impact of various reactive oxygen species on the formation of neutrophil extracellular traps. Mediators Inflamm 2012: 849136, 2012. doi: 10.1155/2012/849136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Larcombe AN, Janka MA, Mullins BJ, Berry LJ, Bredin A, Franklin PJ. The effects of electronic cigarette aerosol exposure on inflammation and lung function in mice. Am J Physiol Lung Cell Mol Physiol 313: L67–L79, 2017. doi: 10.1152/ajplung.00203.2016. [DOI] [PubMed] [Google Scholar]
- 17.Laube BL, Afshar-Mohajer N, Koehler K, Chen G, Lazarus P, Collaco JM, McGrath-Morrow SA. Acute and chronic in vivo effects of exposure to nicotine and propylene glycol from an E-cigarette on mucociliary clearance in a murine model. Inhal Toxicol 29: 197–205, 2017. doi: 10.1080/08958378.2017.1336585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee J, Luria A, Rhodes C, Raghu H, Lingampalli N, Sharpe O, Rada B, Sohn DH, Robinson WH, Sokolove J. Nicotine drives neutrophil extracellular traps formation and accelerates collagen-induced arthritis. Rheumatology (Oxford) 56: 644–653, 2017. doi: 10.1093/rheumatology/kew449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lerner CA, Sundar IK, Yao H, Gerloff J, Ossip DJ, McIntosh S, Robinson R, Rahman I. Vapors produced by electronic cigarettes and e-juices with flavorings induce toxicity, oxidative stress, and inflammatory response in lung epithelial cells and in mouse lung. PLoS One 10: e0116732, 2015. doi: 10.1371/journal.pone.0116732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levy DT, Yuan Z, Li Y. The prevalence and characteristics of E-cigarette users in the U.S. Int J Environ Res Public Health 14: 1200, 2017. doi: 10.3390/ijerph14101200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lim HB, Kim SH. Inhallation of e-cigarette cartridge solution aggravates allergen-induced airway inflammation and hyper-responsiveness in mice. Toxicol Res 30: 13–18, 2014. doi: 10.5487/TR.2014.30.1.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marynak KL, Gammon DG, King BA, Loomis BR, Fulmer EB, Wang TW, Rogers T. National and state trends in sales of cigarettes and E-cigarettes, U.S., 2011-2015. Am J Prev Med 53: 96–101, 2017. doi: 10.1016/j.amepre.2017.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matthews JB, Chen FM, Milward MR, Wright HJ, Carter K, McDonagh A, Chapple IL. Effect of nicotine, cotinine and cigarette smoke extract on the neutrophil respiratory burst. J Clin Periodontol 38: 208–218, 2011. doi: 10.1111/j.1600-051X.2010.01676.x. [DOI] [PubMed] [Google Scholar]
- 24.McCauley L, Markin C, Hosmer D. An unexpected consequence of electronic cigarette use. Chest 141: 1110–1113, 2012. doi: 10.1378/chest.11-1334. [DOI] [PubMed] [Google Scholar]
- 25.Morrissey I, Hackel M, Badal R, Bouchillon S, Hawser S, Biedenbach D. A review of ten years of the Study for Monitoring Antimicrobial Resistance Trends (SMART) from 2002 to 2011. Pharmaceuticals (Basel) 6: 1335–1346, 2013. doi: 10.3390/ph6111335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reidel B, Radicioni G, Clapp P, Ford AA, Abdelwahab S, Rebuli ME, Haridass P, Alexis NE, Jaspers I, Kesimer M. E-cigarette use causes a unique innate immune response in the lung involving increased neutrophilic activation and altered mucin secretion. Am J Respir Crit Care Med 197: 492–501, 2018. doi: 10.1164/rccm.201708-1590OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sancilio S, Gallorini M, Cataldi A, di Giacomo V. Cytotoxicity and apoptosis induction by e-cigarette fluids in human gingival fibroblasts. Clin Oral Investig 20: 477–483, 2016. doi: 10.1007/s00784-015-1537-x. [DOI] [PubMed] [Google Scholar]
- 28.Schoenborn CA, Gindi RM. Electronic cigarette use among adults: United States, 2014. NCHS Data Brief 217: 1–8, 2015. [PubMed] [Google Scholar]
- 29.Schweitzer KS, Chen SX, Law S, Van Demark M, Poirier C, Justice MJ, Hubbard WC, Kim ES, Lai X, Wang M, Kranz WD, Carroll CJ, Ray BD, Bittman R, Goodpaster J, Petrache I. Endothelial disruptive proinflammatory effects of nicotine and e-cigarette vapor exposures. Am J Physiol Lung Cell Mol Physiol 309: L175–L187, 2015. doi: 10.1152/ajplung.00411.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scott A, Lugg ST, Aldridge K, Lewis KE, Bowden A, Mahida RY, Grudzinska FS, Dosanjh D, Parekh D, Foronjy R, Sapey E, Naidu B, Thickett DR. Pro-inflammatory effects of e-cigarette vapour condensate on human alveolar macrophages. Thorax 73: 1161–1169, 2018. doi: 10.1136/thoraxjnl-2018-211663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shields PG, Berman M, Brasky TM, Freudenheim JL, Mathe E, McElroy JP, Song MA, Wewers MD. A review of pulmonary toxicity of electronic cigarettes in the context of smoking: a focus on inflammation. Cancer Epidemiol Biomarkers Prev 26: 1175–1191, 2017. doi: 10.1158/1055-9965.EPI-17-0358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shin S, Crotty Alexander LE. Global state of tobacco use: summary from the American Thoracic Society International Conference 2016. J Thorac Dis 8, Suppl 7: S582–S585, 2016. doi: 10.21037/jtd.2016.07.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sommerfeld CG, Weiner DJ, Nowalk A, Larkin A. Hypersensitivity pneumonitis and acute respiratory distress syndrome from E-cigarette use. Pediatrics 141: e20163927, 2018. doi: 10.1542/peds.2016-3927. [DOI] [PubMed] [Google Scholar]
- 34.Syamlal G, King BA, Mazurek JM. Tobacco use among working adults - United States, 2014-2016. MMWR Morb Mortal Wkly Rep 66: 1130–1135, 2017. doi: 10.15585/mmwr.mm6642a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thota D, Latham E. Case report of electronic cigarettes possibly associated with eosinophilic pneumonitis in a previously healthy active-duty sailor. J Emerg Med 47: 15–17, 2014. doi: 10.1016/j.jemermed.2013.09.034. [DOI] [PubMed] [Google Scholar]
- 36.Ween MP, Whittall JJ, Hamon R, Reynolds PN, Hodge SJ. Phagocytosis and Inflammation: Exploring the effects of the components of E-cigarette vapor on macrophages. Physiol Rep 5: e13370, 2017. doi: 10.14814/phy2.13370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yu V, Rahimy M, Korrapati A, Xuan Y, Zou AE, Krishnan AR, Tsui T, Aguilera JA, Advani S, Crotty Alexander LE, Brumund KT, Wang-Rodriguez J, Ongkeko WM. Electronic cigarettes induce DNA strand breaks and cell death independently of nicotine in cell lines. Oral Oncol 52: 58–65, 2016. doi: 10.1016/j.oraloncology.2015.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yuli I, Tomonaga A, Synderman R. Chemoattractant receptor functions in human polymorphonuclear leukocytes are divergently altered by membrane fluidizers. Proc Natl Acad Sci USA 79: 5906–5910, 1982. doi: 10.1073/pnas.79.19.5906. [DOI] [PMC free article] [PubMed] [Google Scholar]