Abstract
Intermediate filaments (IFs) contribute to force transmission, cellular integrity, and signaling in skeletal muscle. We previously identified keratin 19 (Krt19) as a muscle IF protein. We now report the presence of a second type I muscle keratin, Krt18. Krt18 mRNA levels are about half those for Krt19 and only 1:1,000th those for desmin; the protein was nevertheless detectable in immunoblots. Muscle function, measured by maximal isometric force in vivo, was moderately compromised in Krt18-knockout (Krt18-KO) or dominant-negative mutant mice (Krt18 DN), but structure was unaltered. Exogenous Krt18, introduced by electroporation, was localized in a reticulum around the contractile apparatus in wild-type muscle and to a lesser extent in muscle lacking Krt19 or desmin or both proteins. Exogenous Krt19, which was either reticular or aggregated in controls, became reticular more frequently in Krt19-null than in Krt18-null, desmin-null, or double-null muscles. Desmin was assembled into the reticulum normally in all genotypes. Notably, all three IF proteins appeared in overlapping reticular structures. We assessed the effect of Krt18 on susceptibility to injury in vivo by electroporating siRNA into tibialis anterior (TA) muscles of control and Krt19-KO mice and testing 2 wk later. Results showed a 33% strength deficit (reduction in maximal torque after injury) compared with siRNA-treated controls. Conversely, electroporation of siRNA to Krt19 into Krt18-null TA yielded a strength deficit of 18% after injury compared with controls. Our results suggest that Krt18 plays a complementary role to Krt19 in skeletal muscle in both assembling keratin-based filaments and transducing contractile force.
Keywords: contraction, costamere, desmin, injury, intermediate filament, sarcomere
INTRODUCTION
Intermediate filaments (IF) are flexible, rod-shaped fibers averaging 10 nm in diameter, a size that is “intermediate” between microfilaments (7–8 nm) and microtubules (25 nm). IFs are classified into several major families, with types I–IV localized in the cytoplasm, type V comprising the lamins at the nuclear envelope, and type VI comprising neural and lens proteins (8, 14, 25, 29, 33, 43). IF networks organize and integrate the cytoplasm, providing mechanical integrity and a signaling scaffold that is important for tissue function (11, 18, 21, 23, 28, 31, 41, 46, 65, 66, 68, 77). Many human genetic diseases are caused by deficiencies in these networks, including skin fragility and epidermolytic disorders, laminopathies, myopathies, neuropathies, and premature aging (6, 9, 17, 26, 30, 37, 38, 52, 54, 55, 57, 75, 78).
IFs in skeletal muscle form a complex, integrated cytoskeletal network connecting myofibrillar Z-disks to the sarcolemma, nucleus, and cytoplasmic organelles (2, 9, 10, 19, 21, 34, 35, 46, 49, 56, 59, 72, 73, 75). The IF cytoskeleton of mature muscle is composed predominantly of type III IF proteins, particularly desmin, which is muscle specific. Also present are type IV IF proteins, such as synemin (also known as desmuslin) (2, 3, 15, 44, 53, 61) and paranemin (24, 60, 70), which copolymerize with desmin (3, 70), and syncoilin and nestin (36, 50, 71, 81). With few exceptions, the functions of these proteins and the filaments they form in muscle remain elusive.
In addition to the type III and IV IF proteins, adult striated muscle contains type I and II keratins. Predominantly expressed in epithelial cells, the keratin family comprises more than 25 members (keratin 1 to keratin 28) that form obligate, noncovalent heteropolymers of at least one type I (Krt9–Krt28) and one type II (Krt1–Krt8) keratin. In striated muscle, Krt8 and Krt19 associate with the periphery of Z-disks in the interior of muscle fibers and with the cytoplasmic surface of the sarcolemma, where they play a role in transmission of contractile force (73, 75, 79), similar to desmin and its associated proteins (9, 15, 19, 45, 46, 67, 73). Previous studies also identified Krt18 in developing skeletal muscle but suggested that its expression is lost as muscle matures (39). We, too, failed to identify Krt18 in mature muscle (79), although others reported it to be upregulated in cardiac muscle that expresses TNFα (58). As part of a comprehensive study of the role of intermediate filaments to the structure and function of striated muscle, we decided to re-examine the presence of Krt18 in mature skeletal muscle and explore its contributions to the assembly of the IF network and to force transmission. We tested the hypothesis that Kr18 plays a small but significant role in both.
MATERIALS AND METHODS
Animals.
Male and female FVB mice homozygous for the Krt18-null mutation (Krt18-KO) (26) carrying a Krt18 dominant-negative mutation (Krt18-DN) (40) and Krt19-KO (26) mice were the kind gift of Dr. M. Bishr Omary (Department of Molecular and Integrative Physiology, University of Michigan Medical School, Ann Arbor). Mice lacking both Krt19 and desmin were created at the University of Maryland, Baltimore, by breeding Des-KO mice (56) into the FVB background (46) and then breeding them with Krt19 KO-mice to produce the double knockout (DKO) (46). Mutants were identified by PCR of tail snips (Nucleon Genomic DNA kit; Tepnel Life Sciences, Manchester, UK) and bred to homogeneity for both traits. Control FVB mice were obtained from Jackson Laboratories (Bar Harbor, ME).
Mice (males and females) were studied at 3 mo of age. They were anesthetized with isoflurane and euthanized by cervical location or by perfusion with 2% paraformaldehyde in buffered saline. There were no apparent differences between sexes in any of our studies. All animal protocols were approved by the Institutional Animal Care and Use Committee of the University of Maryland School of Medicine.
Western blotting.
Western blots were used to validate the specificities of all antibodies used in these studies. Extracts of unfixed gastrocnemius muscles were snap-frozen in liquid nitrogen, pulverized, and homogenized with a TissueLyser homogenizer (Qiagen, Hilden, Germany) at a weight/volume ratio of 0.05 in solubilization buffer (M-PER Mammalian Protein Extraction Reagent; Pierce/Thermo, Rockford, IL) with protease inhibitors (Complete Protease Inhibitor Tablets; Roche Diagnostics, Indianapolis, IN). Samples were subjected to brief centrifugation at 18,000 g, and the protein concentration of the supernatants was determined with the Modified Lowry Protein Assay Kit (Thermo Scientific). Samples were then subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) in a 4–12% gradient gel and transferred to nitrocellulose membranes. Membranes were blocked in 3% milk in PBS with 0.1% Tween-20, washed, and then incubated for 3 h with a mouse monoclonal antibody against KRT18 (antibody DC-10; Santa Cruz Biotechnology, Dallas, TX). The blots were washed before incubation with secondary antibodies conjugated to horseradish peroxidase (Jackson Laboratories). After removal of unbound secondary antibodies by repeated washing, bands were visualized by chemiluminescence (Tropix, Bedford, MA).
PCR and siRNA.
Total RNA was extracted with TRIzol (Life Technologies, Carlsbad, CA), following the manufacturer’s recommendations. cDNA was synthesized from total RNA using the Quantitect Reverse Transcription kit (Qiagen). Primers and probes used for qPCR analysis were designed and synthesized by IDT Technologies (Coralville, IA). The following primers were used for semiquantitative RT-PCR: Krt18 forward, GGCCAGCTACCTAGACAAGGTGAAG; Krt18 reverse, GGATGTCCGCCATGATCTTGCTGAG; Gapdh forward, ACGACCCCTTCATTGACC; Gapdh reverse, ATCACGCCACAGCTTTCC. Upon electrophoresis in agarose, these yielded products of 488 and 496 bp, respectively.
siRNAs targeted to mRNAs encoding Krt18 or Krt19 were from Santa Cruz Biotechnology (Dallas, TX) and consisted of a proprietary mixture of three specific oligonucleotides for each at a concentration of 3 nM. This was resuspended in 330 μl of DEPC-treated distilled water to give final concentrations of 10 μM. Aliquots of 20 μl were injected into the tibialis anterior (TA) muscles of 2- to 3-mo-old mice, followed by electroporation with 5 pulses of 20 ms each at 180 V/cm, with 200-ms intervals between pulses. Tissue was collected 2 wk later.
Electroporation of plasmids encoding FLAG-tagged constructs was done identically, except that the DNA concentration was 1 µg/ml and the volume injected was 100 µl.
Immunofluorescent labeling.
For determining percentage of centrally nucleated fibers and minimal Feret’s diameter, tibialis anterior (TA) muscles from perfusion-fixed mice were snap-frozen, cryosectioned, and stained either with hematoxylin and eosin (H&E) or with rabbit anti-dystrophin antibody (Thermo), followed by incubation with Alexa-568 goat anti-rabbit IgG (Molecular Probes), and then mounted in Vectashield + DAPI (Vectorlabs). Additional cross sections were stained with monoclonal antibodies to myosin heavy chain slow (MHC-S; Sigma-Aldrich, St. Louis, MO; see Ref. 1) and rabbit antibodies to dystrophin, followed by Alexa-568 goat anti-rabbit IgG and Alexa-488 goat anti-mouse IgG (Molecular Probes). Sections were examined under a confocal microscope, and measurements were made from 250 to 300 fibers from at least three different mice of each genotype. To evaluate the organization of costameres, we labeled longitudinal sections of TA muscles with chicken antibodies to βI-spectrin and scored the patterns as either normal [i.e., with all 3 components of costameres present, overlying Z-disks (Z-domains) and M-bands (M-domains), and in longitudinal elements (longitudinal domains; score = 3)], partially organized (costameres with domains remaining only over Z disks; score = 2), or extensively disorganized (absence of costameres over large sarcolemmal areas; score = 1); (see Ref. 62).
Morphology and ultrastructure.
Cross sections and longitudinal sections of perfusion-fixed TA muscles were prepared and analyzed as described (14, 73). Light microscopic images were taken on a Nikon Eclipse 501 microscope with a ×20 objective. At least 500 fibers were analyzed per sample to determine fiber diameters, centrally nucleated fibers, and fiber types. Cross sections for immunofluorescence studies were obtained with a Zeiss Duo confocal microscope with ×40 or ×63 objectives. Costameric organization was determined by analysis of 50–100 fibers per sample with a Ziess LSM 510 confocal microscope and a ×63 objective.
For ultrastructural studies, perfusion-fixed TA muscles were removed and incubated overnight in 2% glutaraldehyde, 2 mg/ml tannic acid, and 0.2 M cacodylate, pH 7.2, post-fixed in 1% osmium tetroxide in 0.5 M acetate buffer, en bloc stained with 1% uranyl acetate, dehydrated, embedded in Araldite-Embed 812 resin (EM Sciences), sectioned for electron microscopy at ∼90 nm, and viewed with a Tecnai T12 transmission electron microscope. Images were digital.
Treadmill stress tests.
The treadmill stress tests were adapted from Haubold et al. (22). Before running, mice were acclimated to the treadmill (1055M-Exer 6M; Columbus Instruments, Columbus, OH) by placing them on the stationary treadmill belt for 10 min, followed by gentle running on the belt at 2 m/min for 10 min and then at 5 m/min for 5 min for 3 consecutive days. For testing, mice ran on the treadmill inclined at 7° at an initial speed of 10 m/min. The speed was then increased by 1.5 m/min every 2 min until the mouse was unable to avoid the shock grid for a full minute.
Muscle force measurements.
In vivo contractile function of isolated TA muscles was measured as described (64). We used six FVB mice of each strain (WT, Krt18-KO, Krt19-KO, Des-KO, and DKO mice) to compare contractile characteristics. The tendon of the TA muscle was released and attached to a load cell, and using subcutaneous electrodes at the deep fibula nerve we applied single twitches (rectangular pulse, 1 ms) at different muscle lengths to determine the optimal length (resting length, L0, measured with calipers as the distance between the tibial tuberosity and the myotendinous junction). With muscles set at L0, we gradually increased the stimulation frequency to establish a force-frequency relationship. A maximally fused tetanic contraction at 100 Hz (300-ms train duration of 1-ms pulses at a constant current of 5 mA) was used for all strains. We used 150% of the maximal stimulation intensity to induce maximal activation of contraction, P0. With muscles maintained at L0, fatigue was induced through tetanic contractions (100 Hz for 200 ms) delivered every 2 s for 5 min. Maximal tetanic tension was measured during continuous stimulation, and the final tension was expressed as a percentage of P0 to provide an index of fatigue. The cross-sectional area for each muscle was determined by dividing the mass of the muscle by the product of its fiber length (Lf) and density. Lf was determined by multiplying L0 of the TA by 0.6, the ratio of Lf to L0 (7). The density of mammalian skeletal muscle is 1.06 g/cm3 (51). Muscle output was expressed as specific force (g/mm2), which was determined by dividing the tension (P0) by the muscle cross-sectional area.
Measuring susceptibility to injury.
Additional in vivo measurements of torque before and after eccentric injury were performed as described (47, 64). Briefly, the mouse was anesthetized with isoflurane vapor and placed into a custom-designed rig. Using subcutaneous electrodes placed at the deep fibula nerve, maximal isometric torque for ankle dorsiflexion was recorded, and then injury was induced by lengthening contractions performed by stretching the maximally stimulated left-ankle dorsiflexors through an arc of 80° of plantarflexion at 1,200°/s. The lengthening contraction was repeated 15 times to yield a reproducible injury, quantified as loss of maximal torque compared with pre-injury. Maximal contractile torque was measured before, immediately after, and 3 days after injury to assess susceptibility to injury and recovery thereafter. The TA muscle accounts for ∼90% of the force generated by the ankle dorsiflexors (32).
Electroporation of siRNA and plasmids and analysis of FLAG-tagged proteins.
TA muscles of 2- to 4-mo-old mice were injected with 20 µl of 10 µM siRNA in DEPC water and electroporated at 180 V/cm (36V) five times for 20 ms each, with 200-ms intervals between pulses. Muscles were harvested and assayed physiologically and biochemically 1 wk later.
FDB muscles were electroporated (12, 13, 48) and dissociated and placed into culture 1 wk later. Immunolabeling of cultured fibers was done on day 1 or 2 after culture.
Electroporation of FDB muscles with FLAG (and in some cases) Myc constructs followed a similar protocol, but one week after electroporation muscles were removed, snap frozen, cryosectioned. For Fig. 6, sections were stained with rabbit anti-desmin (Invitrogen) and mouse anti-FLAG antibodies (Sigma). This was followed by Alexa-568 goat anti-rabbit IgG and Alexa-488 goat anti-mouse IgG (Molecular Probes). All fibers expressing FLAG-tagged constructs were counted, and the organization of the expressed protein was scored as reticular, punctate, or disrupted. For Fig. 7, muscles were electroporated with Myc-tagged Krt18 and FLAG-tagged Krt19. Samples were processed identically, but chicken anti-Myc antibodies (Abcam) were used in addition to anti-desmin and anti-FLAG. Secondary antibodies in this case were Alexa-568 goat anti-chicken IgG, Alexa-633 goat anti-rabbit IgG, and Alexa-488 goat anti-mouse IgG.
Fig. 6.
Mutual dependence among desmin, keratin 18 (Krt18), and Krt19 for incorporation into the intermediate filament reticulum. A–C: flexor digitorum brevis (FDB) muscles from wild-type (WT), Krt18-knockout (KO), Krt18-dominant-negative (DN), Krt19-KO, and Des-KO mice, as well as mice lacking both desmin and Krt19 [double knockout (DKO)], were electroporated to overexpress FLAG-tagged versions of Krt18, Krt19, or desmin. After freezing and cross-sectioning, samples were labeled with anti-FLAG (green) and anti-desmin (red). The distribution of the FLAG label in individual fibers was scored as reticular (1:A), punctate (2:B), or disrupted (3:C). Images obtained with FLAG-tagged Krt18. Scale bars in A-C, 5 µm. D–F: quantitation of the scoring determined above. n = 2–3 muscles each from male and female mice of each genotype per condition. The results show that desmin assembles into a reticulum independently of the keratins, whereas Krt19 requires desmin and Krt18 requires both Krt19 and desmin for assembly into a reticulum (see Table 2 for statistical analysis).
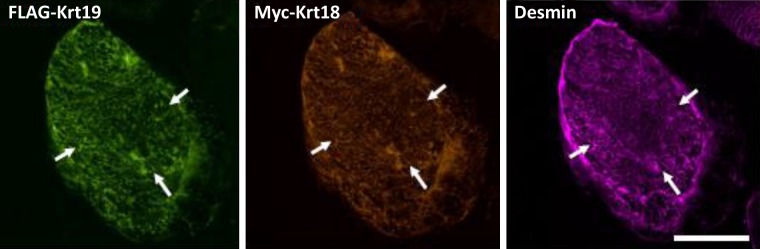
Fig. 7.
Keratin 18 (Krt19), Krt18, and desmin colocalize in flexor digitorum brevis (FDB) myofibers. FDB muscles were transfected by electroporation with plasmids encoding Myc-Krt18 and FLAG-Krt19 and were immunolabeled 1 wk later for Myc, FLAG, and desmin (see materials and methods). Labeling for the three proteins coincides (e.g., arrows). Bar, 50 µm.
Statistics.
Statistics were analyzed with Prism, SigmaPlot, VassarStats (http://vassarstats.net/anova1u.html and www.danielsoper.com/statcalc/calculator.aspx?id=58) as indicated in text and figures.
Materials.
Unless otherwise indicated, all materials were from VWR (Radnor, PA) or Sigma-Aldrich.
RESULTS
Keratin 18 is expressed in murine skeletal muscle.
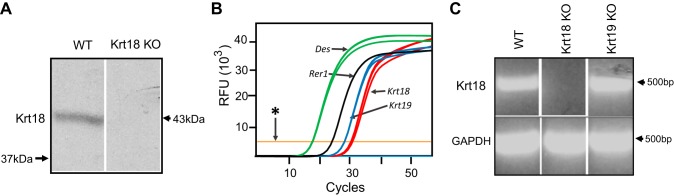
To analyze the possible roles of Krt18 in mature skeletal muscle, we characterized the skeletal muscle of mice lacking keratin 18 (Krt18-KO) and mice expressing a dominant-negative form of keratin 18 (Krt18-DN) and compared them with wild-type (WT) FVB mice. We identified Krt18 in muscle from control but not Krt18-KO mice by immunoblot (Fig. 1A). We then analyzed RNA extracts of tibialis anterior (TA) muscles by qRT-PCR to determine the relative amounts of mRNA encoding Krt18 compared with Krt19 and desmin. They showed that Krt18 mRNA was present at about half the level of Krt19 mRNA and only ∼0.1% the level of desmin mRNA (Fig. 1B). Because keratins in connective tissue may confound the results of PCR from whole muscle, we isolated individual muscle fibers from flexor digitorum brevis (FDB) muscles by enzymatic digestion and then pooled them and analyzed their mRNA. Semiquantitative qRT-PCR studies of these RNA fractions confirmed the present mRNA encoding Krt18 in WT and Krt19 KO muscle but not in Krt18-KO muscle (Fig. 1C). Thus, skeletal muscle expresses Krt18.
Fig. 1.
Keratin 18 (Krt18) mRNA and protein in adult skeletal muscle. mRNA or protein was extracted from tibialis anterior (TA) muscles of 3-mo-old wild-type FVB (WT) mice or FVB mice lacking either Krt18 (Krt18) or Krt19 [Krt19-knockout (KO)]. A: immunoblot of Krt18 shows Krt18 in WT but not Krt18-KO muscle. B: quantitative RT-PCR of mRNA prepared from TA muscle shows ∼500-fold less Krt19 mRNA (blue) and ∼1,000-fold less Krt18 mRNA (red) than Des mRNA (green). A reference gene, Rer1, is included (black). *Lowest detectable level of PCR product (orange line). C: RT-PCR of mRNA prepared from myofibers isolated from dissociated flexor digitorum brevis (FDB) muscles shows a band representing Krt18 mRNA in WT and Krt19-KO mice but not in Krt18-KO mice. Each experiment was repeated at least once to test reproducibility. RFU, relative fluorescence units.
In the course of our studies, we also identified high levels of several transcripts with significant homology to Krt18, but with stop codons interspersed in the open reading frames (ORFs), in agreement with the identification of many mammalian Krt18 pseudogenes (27, 80); www.ncbi.nlm.nih.gov/gene/?Term=related_functional_gene_16668%5Bgroup%5D). We confirmed the continuous ORF of Krt18 (www.ncbi.nlm.nih.gov/nuccore/NM_010664.2), however, and used this sequence in subsequent studies.
Morphology of muscle lacking functional KRT18.
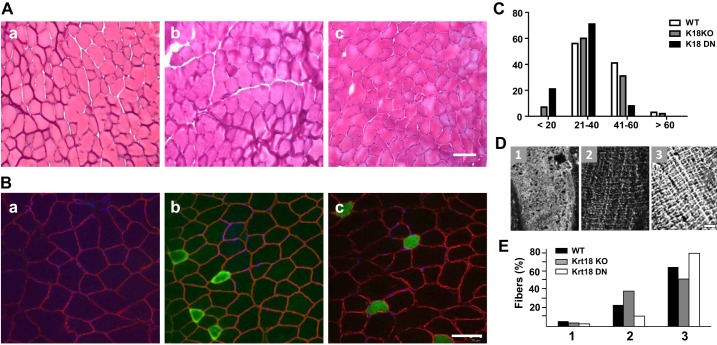
We studied TA muscles from the Krt18-KO and Krt18-DN mice histologically and by immunofluorescence (Fig. 2). Our results showed no significant difference in the number of centrally nucleated fibers (Table 1) or in myofiber size in either strain compared with WT FVB mice (Fig. 2A, images a–c, shown with hematoxylin/eosin; and Fig. 2B, images a–c, shown with DAPI, with fibers outlined in labeling with anti-dystrophin; see also Table 1). Fiber typing indicated a trend toward a greater number of slow fibers in Krt18-KO and Krt18-DN TA muscles than in controls (Fig. 2B), but there were no significant differences (Table 1). Fiber diameters of the TA muscles of Krt18-KO and Krt18-DN mice were also indistinguishable from those of wild-type mice (Fig. 2C and Table 1).
Fig. 2.
Morphological studies of muscles lacking functional keratin 18 (Krt18). A: hematoxylin and eosin (H&E) staining of cross sections of tibialis anterior (TA) muscles from wild-type (WT) mice (Aa), Krt18-knockout (KO) mice (Ab), and mice dominant-negative for Krt18 (Krt18-DN; Ac). B: cross sections were labeled for dystrophin at the sarcolemma (red) and for slow myosin (green). Scale bars in A and B= 50 µm. C: fiber sizes in WT, Krt18-KO, and Krt18-DN mice were measured from fluorescence micrographs like those in Aa–c from H&E staining by minimal Feret’s diameter and sorted by fiber diameter. The results show a range of myofiber sizes in all 3 samples, and a small increase in the number of slow fibers in the Krt18-KO and Krt18-DN samples. The differences were not significant, however. D and E: costameric organization was scored in longitudinal sections of WT, Krt18-KO, and Krt18-DN TA muscles as poorly organized (D, panel 1, and E), moderately organized (D, panel 2, and E), or fully organized (D, panel 3, and E). No significant differences were observed. n = 3–4 muscles from male mice per genotype were examined.
Table 1.
Morphological characteristics of TA muscle fibers from WT, Krt18-KO, and Krt18-DN mice
| Genotype | n | CNF, % | Slow Fibers, % | Average Fiber Size, μm | Variance, % |
|---|---|---|---|---|---|
| WT FVB | 4 | 1.2 ± 0.26 | 1.2 ± 0.34 | 36.6 ± 9.4 | 25.7 |
| K18-KO | 3 | 1.4 ± 0.23 | 6.3 ± 4 | 36.6 ± 10.8 | 29.5 |
| K18-DN | 3 | 1.0 ± 0.21 | 5.9 ± 2.4 | 27.7 ± 8.9 | 32.1 |
Values are means ± SD. No significant differences were found (analyzed with VassarStats). CNF, centrally nucleated fibers; DN, dominant negative; KO, knockout; TA, tibialis anterior; WT, wild type.
The absence of Krt18 also failed to affect the structure of costameres at the sarcolemma of skeletal myofibers. We labeled longitudinal sections of the TA muscles of WT, Krt18-KO, and Krt18-DN mice with antibodies to β-spectrin (Fig. 2D) and then scored for costameric organization (Fig. 2E). We found no significant difference among the different mouse strains. Similarly, ultrastructural studies of the Krt18-KO and WT fibers indicated that the various structures of the contractile apparatus and membrane systems that support excitation-contraction coupling were not obviously altered in the absence of Krt18 (Fig. 3).
Fig. 3.
Ultrastructure of muscles lacking keratin 18 (Krt18). Tibialis anterior (TA) muscles from wild-type (WT) FVB mice and FVB Krt18-knockout (KO) mice were processed for thin section electron microscopy (see materials and methods). Two Krt18-null (A) and two control (B) muscles were examined. No significant differences in 3 muscles from male mice were apparent in the contractile apparatus, lateral alignment of myofibrils, or membrane systems.
Thus, the morphology of fibers in TA muscles was not significantly affected by the absence of functional Krt18 in Krt18-KO or Krt18-DN mice.
Deficiency of Krt18 and Krt19 together significantly reduce muscle force.
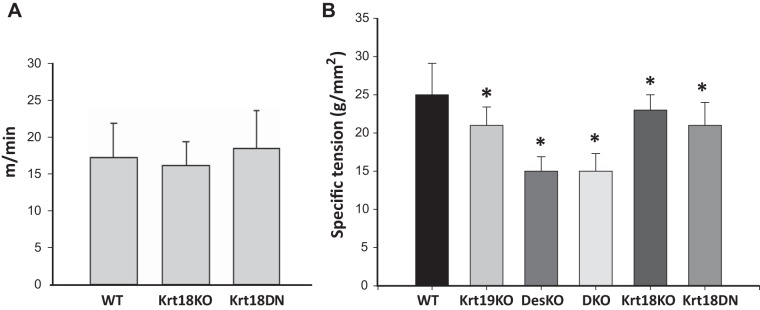
We next examined the functional roles of Krt18. When placed on a treadmill and induced to run uphill, Krt18-KO and Krt18-DN mice showed no differences in running ability compared with WT mice (Fig. 4A), suggesting that overall muscular function in running is not significantly altered by the absence of Krt18.
Fig. 4.
Functional studies of keratin 18 (Krt18)-knockout (KO) and Krt18-dominant-negative (DN) genotypes. A: wild-type (WT), Krt18-KO, and Krt18-DN mice were placed on a treadmill and their ability to run was assayed as the treadmill speed was increased. The bar graph shows the speed (y-axis) at which the mice in that group stopped running. No differences were apparent (ANOVA, with Prism, with Tukey’s post hoc multiple-comparisons test: WT, n = 7; Krt18-KO, n = 4; Krt18-DN, n = 4; results averaged over 3 separate experiments with each cohort). B: tibialis anterior (TA) muscles were analyzed for specific force in vivo by attaching their distal tendons to a force transducer, followed by stimulation via transcutaneous electrodes to the fibula nerve. n = 6 for each group. Krt18-KO and Krt18-DN muscles differed significantly in maximal force compared with WT (P < 0.05) at levels similar to those seen with the Krt19-KO. Muscles lacking desmin (Des KO, double knockout, DKO) showed a larger effect. *P < 0.001; n = 5–6/genotype compared with WT. Statistics determined by ANOVA followed by pairwise multiple comparison (Holm-Sidak), performed on SigmaPlot.
To examine the contractile force generated by isolated TA muscles in vivo, we attached the muscles to a load cell and recorded maximal isometric force normalized to muscle cross-sectional area (i.e., specific force). (Note that we used only TA muscles for physiological studies.) Muscles from the Krt18-KO or Krt18-DN mice showed an ∼15% decrease in specific force compared with WT (Fig. 4B), which was significant (P < 0.001; n = 6/group) and approximately the same as that seen in Krt19-KO muscles. Thus, muscle lacking Krt18 is weaker than in controls.
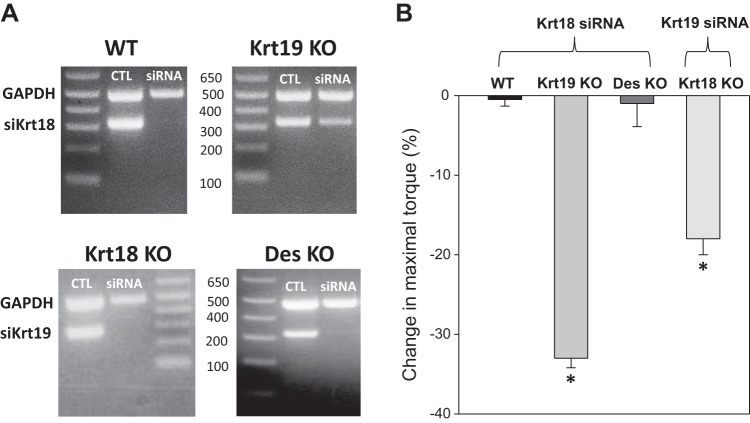
We next examined the susceptibility of Krt18-null TA muscles to injury in vivo. We reduced Krt18 expression by electroporating siRNA targeted to Krt18 mRNA into TA muscles of wild-type, Krt19-KO, and Des-KO mice (n = 5/group), following established methods (63), and measured the response to injury 2 wk later (Fig. 5). RT-PCR showed that the siRNA effectively reduced the levels of Krt18 mRNA (Fig. 5A). The maximal torque generated by the dorsiflexor muscles was reduced by 33% in the Krt19-KO muscles compared with pre-injury measures (P < 0.05; Fig. 5B), indicating that the reduced expression of Krt18 in this background rendered the muscle more susceptible to injury. By contrast, introduction of the siRNA has no additional effect on injury susceptibility in Des-KO or wild-type muscles (Fig. 5B). In a similar experiment, we electroporated siRNA targeting Krt19 mRNA into TA muscles of Krt18-KO mice. Again, siRNA treatment reduced the expression of Krt19 (Fig. 5A), and maximal torque was reduced by 18% after injury (P < 0.05; Fig. 5B). These results suggest that Krt18 and Krt19 complement each other in maintaining muscle force transduction when muscles are injured by eccentric contractions but that Krt18 does not contribute to force transduction in injured muscles that depends on the presence of desmin.
Fig. 5.
The reduction of keratin 18 (Krt18) and Krt19 increases susceptibility to injury. Wild-type (WT) tibialis anterior (TA) muscles and TA muscles lacking either Krt19 or desmin were electroporated with siRNA targeting Krt18 mRNA, and Krt18 knockout (KO) TA muscles were electroporated with siRNA targeting Krt19 mRNA. A: RT-PCR assays show that Krt18 and Krt19 mRNAs are significantly reduced by targeted siRNAs introduced 3 days prior. B: torque generated by the dorsiflexors of the hindlimb was assayed in vivo before and after eccentric injury (n = 5/group; see materials and methods). Results show that knockdown of either Krt18 in the Krt19-KO muscle or Krt19 in the Krt18-KO muscle results in a significant increase in injury (as measured by loss in maximal isometric force after injury) but that knockdown of Krt18 in WT or Des-KO muscle has no significant effect. [*P < 0.05, one-way ANOVA followed by pairwise multiple comparison procedures (Holm-Sidak method); n = 5–6 mice/condition]. Thus, both Krt18 and Krt19 are needed at approximately control levels to protect muscle against eccentric injury.
Localization of exogenous Krt18 depends in part on desmin and Krt19.
To examine the potential interactions of the keratins and desmin in skeletal muscle fibers, we prepared FLAG-tagged cDNAs encoding Krt18, Krt19 and desmin and then introduced them by electroporation into the FDB muscles of WT, Krt18-KO, Krt18-DN, Krt19-KO, Des-KO, and DKO mice. (Note that, like TA muscles, FDB muscles are primarily fast twitch.) We harvested the muscles 1 wk later and examined cross sections stained with anti-desmin and anti-FLAG antibodies. Any fibers that expressed the FLAG-tagged construct were counted and scored based on the organization of the FLAG immunolabeling as reticular (Fig. 6A), punctate (Fig. 6B), or disrupted (Fig. 6C). Our results are summarized in Fig. 6, D–F; statistical analysis of the results is in Table 2.
Table 2.
Statistical evaluation of data in Fig. 6
| Genotype | FLAG-Krt18 | FLAG-Des | FLAG-Krt19 |
|---|---|---|---|
| WT vs. Krt18-KO | 0.2560 | 1 | 0.225 |
| WT vs. Krt18-DN | 0 | 1 | 0.728 |
| WT vs. Krt19-KO | 0.00000021 | 1 | 0.476 |
| WT vs. Des-KO | 0.00007142 | 1 | 0.00000003 |
| WT vs. DKO | 0.00170 | 0.244 | 0.0000145 |
| Krt18-DN vs. Krt19-KO | 0.51 | NA | 0.503 |
| Krt19-KO vs. Des-KO | 0.000056 | NA | NA |
| Krt18-DN vs. Des-KO | 0.000058 | NA | NA |
| Krt18-DN vs. DKO | 0.0000041 | NA | NA |
| Des-KO vs. DKO | 0.596 | NA | NA |
| Krt19-KO vs. DKO | 0.000028 | NA | NA |
| Krt18-KO vs. Krt18-DN | NA | NA | 0.218 |
P values in Fig. 6 were calculated from 2 × 3 Fisher’s exact test, comparing the organization of electroporated FLAG-tagged Krt18, Krt19, and desmin constructs into multiple genotypes. Calculations were performed with https://www.danielsoper.com/statcalc/calculator.aspx?id=58. DKO, double knockout; DN, dominant negative; NA, not applicable; WT, wild type.
When we introduced FLAG-tagged Krt18 into FDB muscles, it assumed a reticular pattern in wild-type and Krt18-KO muscle, but its organization was partially inhibited by the absence of desmin, Krt19, or both proteins in the DKO. Formation of a reticulum by exogenous Krt18 was also partially inhibited by the Krt18-DN. Therefore, the reticular organization of Krt18 depends in part on Krt19 and in part on desmin. Notably, the dependence on Krt19 and desmin is additive, as the effect of the DKO is significantly different from the effects of each KO separately (for statistics, see Table 2). Thus, both Krt19 and desmin can contribute to the organization of Krt18 into a reticulum.
We also examined the incorporation of Krt19 and desmin into the filament reticulum using FLAG-tagged variants introduced into FDB muscles by electroporation. We found that the incorporation of Krt19 into the reticulum depends almost exclusively on desmin. The effect of the Krt18-KO is not significant, and that of the Krt18 DN is marginal (P = 0.052). Desmin assumes a reticular pattern independently of endogenous Krt18, Krt19, or desmin itself, and this is not affected by Krt18-DN. Therefore, the reticulum of IFs in skeletal muscle is formed primarily by desmin, with the organization of Krt18 and Krt19 being dependent on desmin partially or completely. This is congruent with our finding that Des mRNA levels are much higher than those of the keratins.
Finally, we electroporated FDB muscles with Myc-tagged Krt18 and FLAG-tagged Krt19 and prepared frozen sections and immunolabeled them for Myc, FLAG, and endogenous desmin.
The results showed that all three intermediate filament proteins colocalized in what appears to be the same reticulum in the myoplasm (Fig. 7). Using the criteria for Fig. 6, we found that ∼70% of the transfected fibers showed both Krt18 and Krt19 in a reticulum, with ∼20% in a punctate pattern and the remainder disrupted. The fact that these intermediate filament proteins colocalize is consistent with the results presented above and the idea that they are structurally and functionally interdependent.
DISCUSSION
Although the roles of actin microfilaments, myosin thick filaments, and their associated proteins have been intensively investigated in striated muscle, the intermediate filament systems are still poorly understood. Desmin has long been recognized as the major intermediate filament protein of muscle (see, e.g., Refs. 5, 8, 20, 42, 69), although its specific functions remain unclear. In addition, striated muscle also has keratin filaments, which have been studied by only a few laboratories (19, 39, 46, 58, 73, 79). Other than our early observation that the organization of keratins in mature muscle appears to be retained in the desmin-KO (56) and that Krt19 contributes to muscle health and mitochondrial organization (75), force transduction, and transverse alignment of sarcomeres (46), albeit to a lesser extent than desmin (46), the roles of the keratins and their relationship to desmin filaments have not been examined. Indeed, the full complement of keratin subunits in adult striated muscle has probably not yet been defined. Here, we show that Krt18 is expressed in adult skeletal muscle, that it organizes together with Krt19 and desmin in the myoplasm, and that together with Krt19 it contributes to force transduction and cytoskeletal organization.
Krt18 contributes somewhat less to muscle structure and function than Krt19 or desmin, however, perhaps because it is expressed at significantly lower levels. Our results indicate that, unlike Krt19, knockout of Krt18 does not affect the lateral alignment of sarcomeres (Fig. 3), although its absence or expression as the DN form does reduce contractile force by approximately the same extent as knockout of Krt19 (Fig. 4B). It also contributes to the susceptibility to injury of Krt19-null muscles or of muscles in which Krt19 expression is reduced by siRNA (Fig. 5B). Specifically, knockdown of Krt18 in Krt19-KO muscle results in an 18% loss of maximal torque after eccentric injury, and knockdown of Krt19 in Krt18-KO muscle results in a 30% loss of maximal torque after injury, considerably more than occurs in Krt19-KO muscle alone (46). Krt18 and Krt19 colocalize with each other and with desmin in what appears to be the same reticulum of intermediate filaments (Fig. 7), and moreover, the organization of Krt18 depends in part on the organization of Krt19 in muscle fibers. Our results suggest that Krt18 is an integral component of the intermediate filament network of skeletal muscle that contributes significantly to the network’s function in force transmission and resisting injury, whereas the network’s organization is controlled primarily by desmin and Krt19, which are expressed at higher levels. Thus, although the role of Krt18 may be subtle, it is distinctive.
Although our results identify Krt18, along with Krt19, as type 1 keratin subunits expressed in skeletal muscle, and we previously reported that Krt19 associates with the type 2 subunit, Krt8 (79), we cannot yet identify the type 2 subunit associated with Krt18. The nature of the intermediate filaments formed by Krt18 and Krt19 remains unknown. Krt8 could in principle interact with both to form either two distinct but overlapping filament arrays or a single array with a mixture of Krt18/Krt8 and Krt19/Krt8 heterodimers. Alternatively, muscle may contain additional keratin subunits that could contribute to filament assembly. Our results to date cannot distinguish between these possibilities. Addressing them further is a challenge, as the amounts of the keratin subunits in muscle are low, and antibodies specific for each of the keratins that may be present are not always available.
In addition to their presence at costameres, both the desmin and keratin filament systems in muscle surround each sarcomere at the level of the M-bands (Krt19) and Z-disks (Krt19 and desmin). As a result, when fibers are prepared in cross sections, these proteins appear in a reticulum (note that confocal microscopy cannot distinguish between the major structures that surround Z-disks and the minor structures around M-bands). Because of the absence of antibodies to Krt18 that are effective in immunofluorescence studies of muscle, we were unable to show that endogenous Krt18 is also present in this reticulum, but expression of FLAG-tagged Krt18 in wild-type muscle clearly shows that it concentrates there (Figs. 6 and 7). Unlike Krt19 (72), Krt18 when overexpressed does not aggregate significantly, but like Krt19 its presence in a reticulum is completely dependent on desmin, as both keratin subunits are disorganized when they are expressed in Des-KO muscle. This suggests that the assembly and distribution of keratin IFs in mature striated muscle occur along a template determined by desmin. This is not likely to be the case at early stages of muscle development, when desmin is not yet expressed (16), but it may well be a significant factor in initially establishing the alignment of keratin IFs with sarcomeres as myotubes transition to myofibers, where the organization of desmin at Z-disks is an early event (16).
As noted above, however, the organization of keratins in mature skeletal muscle does not appear to depend solely on desmin, as the effects of desmin on costameres and on sarcomeric alignment can leave sites of keratin localization unaffected in some myofibers (56). Thus, exogenously expressed keratins 18 and 19 require desmin to organize de novo, whereas endogenous keratins can remain normally assembled at costameres. This may be due to interactions that they maintain at the costameres through their association with the dystrophin-glycoprotein complex or synemin (15, 53, 79).
It is perhaps surprising that Krt18 and Krt19 play such a significant role in muscle when they are expressed at such low levels. Our qRT-PCR results show that their mRNAs together are present at levels ∼500-fold lower than mRNA encoding desmin. The very low level of expression of Krt18 mRNA, as well as the presence of many pseudogenes, may account for our inability to identify Krt18 in skeletal muscle earlier (77). Despite their low levels of expression, however, the keratins appear to mediate almost as much force (18–30%) as desmin (30%; Refs. 46, 67), even under conditions in which the expression of one subunit is reduced. This may be due in part to the costameric localization of keratins at the M- and longitudinal domains of costameres, as well as the Z-domains (56, 79). Desmin, by contrast, is present only at Z-domains (56), and although its elimination by homologous recombination can disrupt the Z-domains and reduce force by 30%, keratins can remain costameric (56). Additional factors that may influence the contributions of the keratins to muscle function include the relative stability of desmin versus keratin filaments, differences in their rates of polymerization and depolymerization, and their association with other proteins that could promote their respective activities. For example, dystrophin associates with Krt19 (74, 79) and could reinforce the stability of keratin filaments at costameres, where much of force transduction is likely to occur (4, 76). This is also consistent with the effect of the Krt19-KO on the passive biomechanical properties of muscle (73). Thus, if the IF systems of skeletal muscle act independently, together they undergird 50% or more of the force generated during the contraction of skeletal muscle. If they are interdependent, however, their contribution to force transduction may only be 30% (e.g. see Ref. 46). Further studies of muscle in which different subunits are absent or knocked down will be needed to address the quantitative contributions of the IFs to force and to determine the mechanisms through which they act.
GRANTS
This work was initially supported by grants from the NIH (National Institute of Arthritis and Musculoskeletal and Skin Diseases no. RO1-AR-055928) and the Muscular Dystrophy Association (no. 3771) and, more recently by the Kahlert Foundation and by funds made available by the University of Maryland School of Medicine.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.M.M., A.O., R.M.L., and R.J.B. conceived and designed research; J.M.M., A.O., J.P.K., E.K.-W., and R.M.L. performed experiments; J.M.M., A.O., R.M.L., and R.J.B. analyzed data; J.M.M., R.M.L., and R.J.B. interpreted results of experiments; J.M.M., A.O., R.M.L., and R.J.B. prepared figures; R.J.B. drafted manuscript; J.M.M., A.O., R.M.L., and R.J.B. edited and revised manuscript; J.M.M., A.O., J.P.K., E.K.-W., R.M.L., and R.J.B. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank John Strong and Ru-ching Hsia for assistance with electron micrography, Christian Kinney for advice about qRT-PCR, and Sankeerth Manne for assistance in maintaining the mouse colony.
Present address of J. P. Kerr: Muscle Metabolism Discovery Performance Unit, Future Pipelines Discovery, GlaxoSmithKline, Collegeville, PA 19426.
REFERENCES
- 1.Banks GB, Combs AC, Odom GL, Bloch RJ, Chamberlain JS. Muscle structure influences utrophin expression in mdx mice. PLoS Genet 10: e1004431, 2014. doi: 10.1371/journal.pgen.1004431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bellin RM, Huiatt TW, Critchley DR, Robson RM. Synemin may function to directly link muscle cell intermediate filaments to both myofibrillar Z-lines and costameres. J Biol Chem 276: 32330–32337, 2001. doi: 10.1074/jbc.M104005200. [DOI] [PubMed] [Google Scholar]
- 3.Bilak SR, Sernett SW, Bilak MM, Bellin RM, Stromer MH, Huiatt TW, Robson RM. Properties of the novel intermediate filament protein synemin and its identification in mammalian muscle. Arch Biochem Biophys 355: 63–76, 1998. doi: 10.1006/abbi.1998.0702. [DOI] [PubMed] [Google Scholar]
- 4.Bloch RJ, Gonzalez-Serratos H. Lateral force transmission across costameres in skeletal muscle. Exerc Sport Sci Rev 31: 73–78, 2003. doi: 10.1097/00003677-200304000-00004. [DOI] [PubMed] [Google Scholar]
- 5.Boriek AM, Capetanaki Y, Hwang W, Officer T, Badshah M, Rodarte J, Tidball JG. Desmin integrates the three-dimensional mechanical properties of muscles. Am J Physiol Cell Physiol 280: C46–C52, 2001. doi: 10.1152/ajpcell.2001.280.1.C46. [DOI] [PubMed] [Google Scholar]
- 6.Brodehl A, Gaertner-Rommel A, Milting H. Molecular insights into cardiomyopathies associated with desmin (DES) mutations. Biophys Rev 10: 983–1006, 2018. doi: 10.1007/s12551-018-0429-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burkholder TJ, Fingado B, Baron S, Lieber RL. Relationship between muscle fiber types and sizes and muscle architectural properties in the mouse hindlimb. J Morphol 221: 177–190, 1994. doi: 10.1002/jmor.1052210207. [DOI] [PubMed] [Google Scholar]
- 8.Capetanaki Y, Bloch RJ, Kouloumenta A, Mavroidis M, Psarras S. Muscle intermediate filaments and their links to membranes and membranous organelles. Exp Cell Res 313: 2063–2076, 2007. doi: 10.1016/j.yexcr.2007.03.033. [DOI] [PubMed] [Google Scholar]
- 9.Chapman MA, Zhang J, Banerjee I, Guo LT, Zhang Z, Shelton GD, Ouyang K, Lieber RL, Chen J. Disruption of both nesprin 1 and desmin results in nuclear anchorage defects and fibrosis in skeletal muscle. Hum Mol Genet 23: 5879–5892, 2014. doi: 10.1093/hmg/ddu310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheng F, Eriksson JE. Intermediate filaments and the regulation of cell motility during regeneration and wound healing. Cold Spring Harb Perspect Biol 9: a022046, 2017. doi: 10.1101/cshperspect.a022046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coulombe PA, Omary MB. ‘Hard’ and ‘soft’ principles defining the structure, function and regulation of keratin intermediate filaments. Curr Opin Cell Biol 14: 110–122, 2002. doi: 10.1016/S0955-0674(01)00301-5. [DOI] [PubMed] [Google Scholar]
- 12.DiFranco M, Neco P, Capote J, Meera P, Vergara JL. Quantitative evaluation of mammalian skeletal muscle as a heterologous protein expression system. Protein Expr Purif 47: 281–288, 2006. doi: 10.1016/j.pep.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 13.DiFranco M, Tran P, Quiñonez M, Vergara JL. Functional expression of transgenic α1sDHPR channels in adult mammalian skeletal muscle fibres. J Physiol 589: 1421–1442, 2011. doi: 10.1113/jphysiol.2010.202804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eriksson JE, Dechat T, Grin B, Helfand B, Mendez M, Pallari HM, Goldman RD. Introducing intermediate filaments: from discovery to disease. J Clin Invest 119: 1763–1771, 2009. doi: 10.1172/JCI38339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.García-Pelagio KP, Muriel J, O’Neill A, Desmond PF, Lovering RM, Lund L, Bond M, Bloch RJ. Myopathic changes in murine skeletal muscle lacking synemin. Am J Physiol Cell Physiol 308: C448–C462, 2015. doi: 10.1152/ajpcell.00331.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gard DL, Lazarides E. The synthesis and distribution of desmin and vimentin during myogenesis in vitro. Cell 19: 263–275, 1980. doi: 10.1016/0092-8674(80)90408-0. [DOI] [PubMed] [Google Scholar]
- 17.Gentil BJ, Tibshirani M, Durham HD. Neurofilament dynamics and involvement in neurological disorders. Cell Tissue Res 360: 609–620, 2015. doi: 10.1007/s00441-014-2082-7. [DOI] [PubMed] [Google Scholar]
- 18.Goldmann WH. Intermediate filaments and cellular mechanics. Cell Biol Int 42: 132–138, 2018. doi: 10.1002/cbin.10879. [DOI] [PubMed] [Google Scholar]
- 19.Goodall MH, Ward CW, Pratt SJP, Bloch RJ, Lovering RM. Structural and functional evaluation of branched myofibers lacking intermediate filaments. Am J Physiol Cell Physiol 303: C224–C232, 2012. doi: 10.1152/ajpcell.00136.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Granger BL, Lazarides E. Desmin and vimentin coexist at the periphery of the myofibril Z disc. Cell 18: 1053–1063, 1979. doi: 10.1016/0092-8674(79)90218-6. [DOI] [PubMed] [Google Scholar]
- 21.Hatzfeld M, Keil R, Magin TM. Desmosomes and Intermediate Filaments: Their Consequences for Tissue Mechanics. Cold Spring Harb Perspect Biol 9: a029157, 2017. doi: 10.1101/cshperspect.a029157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haubold KW, Allen DL, Capetanaki Y, Leinwand LA. Loss of desmin leads to impaired voluntary wheel running and treadmill exercise performance. J Appl Physiol (1985) 95: 1617–1622, 2003. doi: 10.1152/japplphysiol.00408.2003. [DOI] [PubMed] [Google Scholar]
- 23.Helfand BT, Chang L, Goldman RD. Intermediate filaments are dynamic and motile elements of cellular architecture. J Cell Sci 117: 133–141, 2004. doi: 10.1242/jcs.00936. [DOI] [PubMed] [Google Scholar]
- 24.Hemken PM, Bellin RM, Sernett SW, Becker B, Huiatt TW, Robson RM. Molecular characteristics of the novel intermediate filament protein paranemin. Sequence reveals EAP-300 and IFAPa-400 are highly homologous to paranemin. J Biol Chem 272: 32489–32499, 1997. doi: 10.1074/jbc.272.51.32489. [DOI] [PubMed] [Google Scholar]
- 25.Herrmann H, Aebi U. Intermediate filaments: structure and assembly. Cold Spring Harb Perspect Biol 8: a018242, 2016. doi: 10.1101/cshperspect.a018242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hesse M, Franz T, Tamai Y, Taketo MM, Magin TM. Targeted deletion of keratins 18 and 19 leads to trophoblast fragility and early embryonic lethality. EMBO J 19: 5060–5070, 2000. doi: 10.1093/emboj/19.19.5060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hesse M, Magin TM, Weber K. Genes for intermediate filament proteins and the draft sequence of the human genome: novel keratin genes and a surprisingly high number of pseudogenes related to keratin genes 8 and 18. J Cell Sci 114: 2569–2575, 2001. [DOI] [PubMed] [Google Scholar]
- 28.Hijikata T, Murakami T, Ishikawa H, Yorifuji H. Plectin tethers desmin intermediate filaments onto subsarcolemmal dense plaques containing dystrophin and vinculin. Histochem Cell Biol 119: 109–123, 2003. [DOI] [PubMed] [Google Scholar]
- 29.Hol EM, Capetanaki Y. Type III intermediate filaments desmin, glial fibrillary acidic protein (GFAP), vimentin, and peripherin. Cold Spring Harb Perspect Biol 9: a021642, 2017. doi: 10.1101/cshperspect.a021642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Holmgren A, Bouhy D, Timmerman V. Neurofilament phosphorylation and their proline-directed kinases in health and disease. J Peripher Nerv Syst 17: 365–376, 2012. doi: 10.1111/j.1529-8027.2012.00434.x. [DOI] [PubMed] [Google Scholar]
- 31.Houseweart MK, Cleveland DW. Intermediate filaments and their associated proteins: multiple dynamic personalities. Curr Opin Cell Biol 10: 93–101, 1998. doi: 10.1016/S0955-0674(98)80091-4. [DOI] [PubMed] [Google Scholar]
- 32.Ingalls CP, Warren GL, Zhang JZ, Hamilton SL, Armstrong RB. Dihydropyridine and ryanodine receptor binding after eccentric contractions in mouse skeletal muscle. J Appl Physiol (1985) 96: 1619–1625, 2004. doi: 10.1152/japplphysiol.00084.2003. [DOI] [PubMed] [Google Scholar]
- 33.Jacob JT, Coulombe PA, Kwan R, Omary MB. Types I and II keratin intermediate filaments. Cold Spring Harb Perspect Biol 10: a018275, 2018. doi: 10.1101/cshperspect.a018275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jones JC, Kam CY, Harmon RM, Woychek AV, Hopkinson SB, Green KJ. Intermediate filaments and the plasma membrane. Cold Spring Harb Perspect Biol 9: a025866, 2017. doi: 10.1101/cshperspect.a025866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Katsuma Y, Swierenga SHH, Marceau N, French SW. Connections of intermediate filaments with the nuclear lamina and the cell periphery. Biol Cell 59: 193–203, 1987. doi: 10.1111/j.1768-322X.1987.tb00531.x. [DOI] [PubMed] [Google Scholar]
- 36.Kemp MW, Edwards B, Burgess M, Clarke WT, Nicholson G, Parry DA, Davies KE. Syncoilin isoform organization and differential expression in murine striated muscle. J Struct Biol 165: 196–203, 2009. doi: 10.1016/j.jsb.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 37.Khadija SG, Chen F, Hadden T, Commissaris RL, Kowluru A. Biology and regulatory roles of nuclear lamins in cellular function and dysfunction. Recent Pat Endocr Metab Immune Drug Discov 9: 111–120, 2015. doi: 10.2174/1872214809666151009120402. [DOI] [PubMed] [Google Scholar]
- 38.Khani P, Ghazi F, Zekri A, Nasri F, Behrangi E, Aghdam AM, Mirzaei H. Keratins and epidermolysis bullosa simplex. J Cell Physiol 234: 289–297, 2019. doi: 10.1002/jcp.26898. [DOI] [PubMed] [Google Scholar]
- 39.Kosmehl H, Langbein L, Katenkamp D. Transient cytokeratin expression in skeletal muscle during murine embryogenesis. Anat Anz 171: 39–44, 1990. [PubMed] [Google Scholar]
- 40.Ku NO, Michie SA, Soetikno RM, Resurreccion EZ, Broome RL, Oshima RG, Omary MB. Susceptibility to hepatotoxicity in transgenic mice that express a dominant-negative human keratin 18 mutant. J Clin Invest 98: 1034–1046, 1996. doi: 10.1172/JCI118864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ku NO, Toivola DM, Strnad P, Omary MB. Cytoskeletal keratin glycosylation protects epithelial tissue from injury. Nat Cell Biol 12: 876–885, 2010. doi: 10.1038/ncb2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lazarides E. The distribution of desmin (100 A) filaments in primary cultures of embryonic chick cardiac cells. Exp Cell Res 112: 265–273, 1978. doi: 10.1016/0014-4827(78)90209-4. [DOI] [PubMed] [Google Scholar]
- 43.Lee MK, Cleveland DW. Neuronal intermediate filaments. Annu Rev Neurosci 19: 187–217, 1996. doi: 10.1146/annurev.ne.19.030196.001155. [DOI] [PubMed] [Google Scholar]
- 44.Li Z, Parlakian A, Coletti D, Alonso-Martin S, Hourdé C, Joanne P, Gao-Li J, Blanc J, Ferry A, Paulin D, Xue Z, Agbulut O. Synemin acts as a regulator of signalling molecules during skeletal muscle hypertrophy. J Cell Sci 127: 4589–4601, 2014. doi: 10.1242/jcs.143164. [DOI] [PubMed] [Google Scholar]
- 45.Lieber RL, Shah S, Fridén J. Cytoskeletal disruption after eccentric contraction-induced muscle injury. Clin Orthop Relat Res 403S, Suppl: S90–S99, 2002. doi: 10.1097/00003086-200210001-00011. [DOI] [PubMed] [Google Scholar]
- 46.Lovering RM, O’Neill A, Muriel JM, Prosser BL, Strong J, Bloch RJ. Physiology, structure, and susceptibility to injury of skeletal muscle in mice lacking keratin 19-based and desmin-based intermediate filaments. Am J Physiol Cell Physiol 300: C803–C813, 2011. doi: 10.1152/ajpcell.00394.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lovering RM, Roche JA, Bloch RJ, De Deyne PG. Recovery of function in skeletal muscle following 2 different contraction-induced injuries. Arch Phys Med Rehabil 88: 617–625, 2007. doi: 10.1016/j.apmr.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 48.Lukyanenko V, Muriel JM, Bloch RJ. Coupling of excitation to Ca2+ release is modulated by dysferlin. J Physiol 595: 5191–5207, 2017. doi: 10.1113/JP274515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lund LM, Kerr JP, Lupinetti J, Zhang Y, Russell MA, Bloch RJ, Bond M. Synemin isoforms differentially organize cell junctions and desmin filaments in neonatal cardiomyocytes. FASEB J 26: 137–148, 2012. doi: 10.1096/fj.10-179408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McCullagh KJ, Edwards B, Poon E, Lovering RM, Paulin D, Davies KE. Intermediate filament-like protein syncoilin in normal and myopathic striated muscle. Neuromuscul Disord 17: 970–979, 2007. doi: 10.1016/j.nmd.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 51.Mendez J, Keys A. Density and composition of mammalian muscle. Metabolism 9: 184–188, 1960. [Google Scholar]
- 52.Menko AS, Bleaken BM, Libowitz AA, Zhang L, Stepp MA, Walker JL. A central role for vimentin in regulating repair function during healing of the lens epithelium. Mol Biol Cell 25: 776–790, 2014. doi: 10.1091/mbc.e12-12-0900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mizuno Y, Thompson TG, Guyon JR, Lidov HG, Brosius M, Imamura M, Ozawa E, Watkins SC, Kunkel LM. Desmuslin, an intermediate filament protein that interacts with alpha -dystrobrevin and desmin. Proc Natl Acad Sci USA 98: 6156–6161, 2001. doi: 10.1073/pnas.111153298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Muñoz-Mármol AM, Strasser G, Isamat M, Coulombe PA, Yang Y, Roca X, Vela E, Mate JL, Coll J, Fernández-Figueras MT, Navas-Palacios JJ, Ariza A, Fuchs E. A dysfunctional desmin mutation in a patient with severe generalized myopathy. Proc Natl Acad Sci USA 95: 11312–11317, 1998. doi: 10.1073/pnas.95.19.11312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nicolas HA, Akimenko MA, Tesson F. Cellular and animal models of striated muscle laminopathies. Cells 8: 291, 2019. doi: 10.3390/cells8040291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.O’Neill A, Williams MW, Resneck WG, Milner DJ, Capetanaki Y, Bloch RJ. Sarcolemmal organization in skeletal muscle lacking desmin: evidence for cytokeratins associated with the membrane skeleton at costameres. Mol Biol Cell 13: 2347–2359, 2002. doi: 10.1091/mbc.01-12-0576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Omary MB. Intermediate filament proteins of digestive organs: physiology and pathophysiology. Am J Physiol Gastrointest Liver Physiol 312: G628–G634, 2017. doi: 10.1152/ajpgi.00455.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Papathanasiou S, Rickelt S, Soriano ME, Schips TG, Maier HJ, Davos CH, Varela A, Kaklamanis L, Mann DL, Capetanaki Y. Tumor necrosis factor-α confers cardioprotection through ectopic expression of keratins K8 and K18. Nat Med 21: 1076–1084, 2015. doi: 10.1038/nm.3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Price M, Sanger JW. Intermediate filaments connect z-discs in adult chicken muscle. J Exp Zool 208: 263–269, 1979. doi: 10.1002/jez.1402080214. [DOI] [PubMed] [Google Scholar]
- 60.Price MG, Lazarides E. Expression of intermediate filament-associated proteins paranemin and synemin in chicken development. J Cell Biol 97: 1860–1874, 1983. doi: 10.1083/jcb.97.6.1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Prudner BC, Roy PS, Damron DS, Russell MA. α-Synemin localizes to the M-band of the sarcomere through interaction with the M10 region of titin. FEBS Lett 588: 4625–4630, 2014. doi: 10.1016/j.febslet.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Reed PW, Mathews KD, Mills KA, Bloch RJ. The sarcolemma in the Large(myd) mouse. Muscle Nerve 30: 585–595, 2004. doi: 10.1002/mus.20146. [DOI] [PubMed] [Google Scholar]
- 63.Roche JA, Ford-Speelman DL, Ru LW, Densmore AL, Roche R, Reed PW, Bloch RJ. Physiological and histological changes in skeletal muscle following in vivo gene transfer by electroporation. Am J Physiol Cell Physiol 301: C1239–C1250, 2011. doi: 10.1152/ajpcell.00431.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Roche JA, Lovering RM, Roche R, Ru LW, Reed PW, Bloch RJ. Extensive mononuclear infiltration and myogenesis characterize recovery of dysferlin-null skeletal muscle from contraction-induced injuries. Am J Physiol Cell Physiol 298: C298–C312, 2010. doi: 10.1152/ajpcell.00122.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rotty JD, Coulombe PA. A wound-induced keratin inhibits Src activity during keratinocyte migration and tissue repair. J Cell Biol 197: 381–389, 2012. doi: 10.1083/jcb.201107078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Russell M, Lund LM, Haber R, McKeegan K, Cianciola N, Bond M. The intermediate filament protein, synemin, is an AKAP in the heart. Arch Biochem Biophys 456: 204–215, 2006. doi: 10.1016/j.abb.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 67.Sam M, Shah S, Fridén J, Milner DJ, Capetanaki Y, Lieber RL. Desmin knockout muscles generate lower stress and are less vulnerable to injury compared with wild-type muscles. Am J Physiol Cell Physiol 279: C1116–C1122, 2000. doi: 10.1152/ajpcell.2000.279.4.C1116. [DOI] [PubMed] [Google Scholar]
- 68.Sanghvi-Shah R, Weber GF. Intermediate filaments at the junction of mechanotransduction, migration, and development. Front Cell Dev Biol 5: 81, 2017. doi: 10.3389/fcell.2017.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schmid E, Tapscott S, Bennett GS, Croop J, Fellini SA, Holtzer H, Franke WW. Differential location of different types of intermediate-sized filaments in various tissues of the chicken embryo. Differentiation 15: 27–40, 1979. doi: 10.1111/j.1432-0436.1979.tb01031.x. [DOI] [PubMed] [Google Scholar]
- 70.Schweitzer SC, Klymkowsky MW, Bellin RM, Robson RM, Capetanaki Y, Evans RM. Paranemin and the organization of desmin filament networks. J Cell Sci 114: 1079–1089, 2001. [DOI] [PubMed] [Google Scholar]
- 71.Sejersen T, Lendahl U. Transient expression of the intermediate filament nestin during skeletal muscle development. J Cell Sci 106: 1291–1300, 1993. [DOI] [PubMed] [Google Scholar]
- 72.Shah SB, Davis J, Weisleder N, Kostavassili I, McCulloch AD, Ralston E, Capetanaki Y, Lieber RL. Structural and functional roles of desmin in mouse skeletal muscle during passive deformation. Biophys J 86: 2993–3008, 2004. doi: 10.1016/S0006-3495(04)74349-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shah SB, Love JM, O’Neill A, Lovering RM, Bloch RJ. Influences of desmin and keratin 19 on passive biomechanical properties of mouse skeletal muscle. J Biomed Biotechnol 2012: 1–12, 2012. doi: 10.1155/2012/704061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Stone MR, O’Neill A, Catino D, Bloch RJ. Specific interaction of the actin-binding domain of dystrophin with intermediate filaments containing keratin 19. Mol Biol Cell 16: 4280–4293, 2005. doi: 10.1091/mbc.e05-02-0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Stone MR, O’Neill A, Lovering RM, Strong J, Resneck WG, Reed PW, Toivola DM, Ursitti JA, Omary MB, Bloch RJ. Absence of keratin 19 in mice causes skeletal myopathy with mitochondrial and sarcolemmal reorganization. J Cell Sci 120: 3999–4008, 2007. doi: 10.1242/jcs.009241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Street SF. Lateral transmission of tension in frog myofibers: a myofibrillar network and transverse cytoskeletal connections are possible transmitters. J Cell Physiol 114: 346–364, 1983. doi: 10.1002/jcp.1041140314. [DOI] [PubMed] [Google Scholar]
- 77.Toivola DM, Tao GZ, Habtezion A, Liao J, Omary MB. Cellular integrity plus: organelle-related and protein-targeting functions of intermediate filaments. Trends Cell Biol 15: 608–617, 2005. doi: 10.1016/j.tcb.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 78.Tsikitis M, Galata Z, Mavroidis M, Psarras S, Capetanaki Y. Intermediate filaments in cardiomyopathy. Biophys Rev 10: 1007–1031, 2018. doi: 10.1007/s12551-018-0443-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ursitti JA, Lee PC, Resneck WG, McNally MM, Bowman AL, O’Neill A, Stone MR, Bloch RJ. Cloning and characterization of cytokeratins 8 and 19 in adult rat striated muscle. Interaction with the dystrophin glycoprotein complex. J Biol Chem 279: 41830–41838, 2004. doi: 10.1074/jbc.M400128200. [DOI] [PubMed] [Google Scholar]
- 80.Waseem A, Gough AC, Spurr NK, Lane EB. Localization of the gene for human simple epithelial keratin 18 to chromosome 12 using polymerase chain reaction. Genomics 7: 188–194, 1990. doi: 10.1016/0888-7543(90)90540-B. [DOI] [PubMed] [Google Scholar]
- 81.Zhang J, Bang ML, Gokhin DS, Lu Y, Cui L, Li X, Gu Y, Dalton ND, Scimia MC, Peterson KL, Lieber RL, Chen J. Syncoilin is required for generating maximum isometric stress in skeletal muscle but dispensable for muscle cytoarchitecture. Am J Physiol Cell Physiol 294: C1175–C1182, 2008. doi: 10.1152/ajpcell.00049.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]