Abstract
Cl− is the major extracellular (Cl−out) and intracellular (Cl−in) anion whose concentration is actively regulated by multiple transporters. These transporters generate Cl− gradients across the plasma membrane and between the cytoplasm and intracellular organelles. [Cl−]in changes rapidly in response to cell stimulation and influences many physiological functions, as well as cellular and systemic homeostasis. However, less appreciated is the signaling function of Cl−. Cl− interacts with multiple proteins to directly modify their activity. This review highlights the signaling function of Cl− and argues that Cl− is a bona fide signaling ion, a function deserving extensive exploration.
Keywords: chloride, signaling
INTRODUCTION
As the principal anion in all vertebrate cells, Cl− is mainly studied as a homeostatic, rather than as a signaling ion. Plasma and extracellular Cl− concentration ([Cl−]out) maintains body fluid ionic content, volume homeostasis, and blood pressure. [Cl−]out is, therefore, maintained fairly constant, except for disease state associated with metabolic acidosis and alkalosis (26). However, luminal [Cl−]out can vary considerably during epithelial Cl− secretion and absorption. Nevertheless, the activity of several basolateral and luminal proteins and physiological functions are influenced by changes in [Cl−]out. Plasma Cl− homeostasis is maintained mainly by the kidney (26) and the intestine (47) in close association with Na+, K+, and absorption and secretion (23).
Unlike [Cl−]out, intracellular Cl− concentration ([Cl−]in) varies considerably between cell types and in response to cell stimulation. Resting [Cl−]in in epithelial cells is as high as 60 mM (30, 44, 61, 121), while in neurons and skeletal muscle, it ranges from 10 mM to 30 mM (33, 114). In most cells the major Cl− influx mechanism is the Na+/K+/2 Cl− cotransporter NKCC1 with contribution of the Cl−/ exchanger AE2 when pHin is above 7.2, while Cl− efflux is largely mediated by the K+/Cl− cotransporters KCC (50). These transporters keep [Cl−]in above electrochemical potential in the face of several types of Cl− channels that are expressed in many cells that are likely minimally active in the basal state. [Cl−]in changes in response to many stimuli that involves activation of Cl− channels that drives [Cl−]in toward its electrochemical potential. Prominent Cl− channels are anoctamin 1 (ANO1, TMEM16A), the Ca2+-activated Cl− channel and cystic fibrosis transmembrane conductance regulator (CFTR), a cAMP/PKA activated Cl− channel (57). For example, in the pancreatic and salivary gland acinar cells activation of Gq-coupled receptors evokes Ca2+ oscillations that results in oscillatory activation of ANO1 (36, 73), which reduces [Cl−]in due to luminal Cl− secretion (56, 86). CFTR is the major Cl− channel in secretory gland ducts that are activated in response to stimulation of Gs-coupled receptor and increased cellular cAMP to initial luminal Cl− absorption and secretion (56). Duct [Cl−]in is reduced from ~40 mM to 4 mM by basolateral membrane Cl− extrusion, to allow the high concentration (up to 140 mM) in secreted fluids (44, 56, 115). On the other hand, [Cl−]in increases during regulatory volume increase (30, 56) and in lectin-stimulated T cells (55), largely by activation of NKCC1 (50). The inwardly directed Cl− gradient is coupled to the that of Na+, K+, and . The Na+ gradient mediates most Cl− influx to keep [Cl−]in above electrochemical equilibrium. K+ mediates most Cl− efflux, while the coupling of Cl− to transport has a major role in determining cytoplasmic pH (pHin).
Although the homeostatic functions of [Cl−]in are extensively studied, signaling by [Cl−]in and its regulatory functions are poorly understood and not completely appreciated. [Cl−]in has all the features of a signaling ion. The concentration of a signaling ion should be actively regulated. There should be gradients of the ion across cellular membranes, the gradients should be rapidly dissipated in response to cell stimulation, and the change in the signaling ion concentration should be translated to an altered cell response. [Cl−]in is regulated by secondary active transporters that generate Cl− gradients across the plasma membrane (56) and intracellular organelles (47). Cell stimulation changes [Cl−]in in multiple cell types rapidly and substantially. For example, carbachol stimulation of salivary gland cells reduces [Cl−]in from 60 to 70 to 30 to 35 mM within a few seconds (30), cAMP stimulation reduces [Cl−]in from 75 to 30 mM in MDCK cells (112), and by 10 mM in collecting duct cells (3). Additionally, lectin stimulation of T cells causes persistent [Cl−]in oscillations (55). Oscillations in [Cl−]in are likely a result of apical receptor-stimulated Ca2+ oscillations that activate the Ca2+-activated Cl− channels in acinar cells (73). Changes in [Cl−]in affect cellular processes that function in time scales of milliseconds, such as the membrane potential (9, 39) and protein folding (18, 76), to hours and days, such as cell growth and differentiation (13, 53). In this review, we will discuss what we know about Cl− regulation, what we do not know, and what needs to be elucidated as we seek to understand better signaling by Cl−. We will discuss first the current evidence for signaling and regulatory functions by [Cl−]out, which are only suggestive at this stage. We will then discuss the much better-established evidence for signaling by [Cl−]in. We will attempt to show that the known regulatory functions of [Cl−]in and dynamic changes in [Cl−]in in response to cell stimulation justify considering [Cl−]in as a prominent regulator of cell function and as a bona fide signaling ion that senses and transmits signals.
REGULATION BY [Cl−]out
[Cl−]out Regulates the Function of Signaling Proteins
There is no clear evidence for signaling by [Cl−]out, although [Cl−]out regulates the function of several proteins, including signaling proteins. Changes in [Cl−]out are observed in select environments, such as the luminal space in epithelia and the synaptic cleft (56, 101). In this manner, changes in [Cl−]out indirectly affect cell signaling to modulate cell function. In addition, [Cl−]out is a cofactor in several enzymes. An extensively studied enzyme regulated by [Cl−]out is the ANG I-converting enzyme. [Cl−]out is an essential cofactor for the testis ANG I-converting enzyme (tACE) (62), and somatic ANG-I (sACE) (60). sACE is found on the surface of vascular and renal endothelial cells, proximal tubules, and podocytes. It is also found on the luminal membrane of other epithelial cells (107) and in macrophages (93). sACE is a zinc metallopeptidase that catalyzes the hydrolysis of carboxy-terminal dipeptides of several biologically active peptides (60). The best understood role of sACE is in the renin-angiotensin system. sACE cleaves ANG I to the vasoconstricting ANG II (95). Additionally, sACE has a significant role in innate and adaptive immunity (92), and tACE has a critical role in fertilization (35). The ACE ectodomain has two subdomains with Cl− binding pockets. The N subdomain binds one Cl− ion, and the C subdomain binds two Cl− ions (62). Affinity of the sACE to Cl− is ~30 mM at pH 7.5 and as high as 100 mM at pH 8.6 (24). It is not obvious how sACE senses and responds to Cl−, since plasma [Cl−]out is ~100 mM. On the other hand, [Cl−]out can be as low as 10–20 mM in the epithelial luminal space (56) and seminal fluid (48) that may affect Cl− binding and sensing by the ACEs. Finally, sACE is also present intracellularly, mainly in the nucleus and mitochondria, where it regulates the renin-angiotensin system (1). Cytoplasmic Cl− can be as low as 4 mM (see channels and transporters regulated by [cl−]in), well within the affinity of the ACEs for Cl− (60).
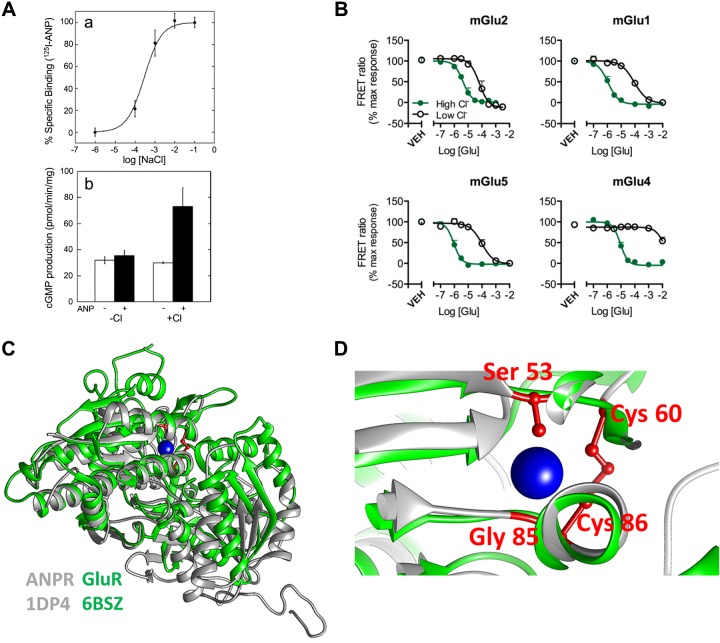
Several guanylyl cyclases and G protein-coupled receptors (GPCRs) sense [Cl−]out, and their signal transduction is controlled or modulated by [Cl−]out. The natriuretic vasodilatory hormone atrial natriuretic peptide (ANP) is secreted by the heart in response to volume expansion to stimulate NaCl and fluid excretion (100). ANP binds to and activates the A-type natriuretic peptide receptor and signals by increasing cellular cGMP (82). The binding of ANP to the ANPR and the stimulation of cGMP is strictly dependent on Cl− with an apparent affinity of ~1 mM (Fig. 1A). The ANP Cl− binding site (Fig. 1, C and D) is next to the ANP binding site in the extracellular domain of the ANPR (106), and it seems to affect the extracellular domain dimerization, which is required for signaling by ANP (65). The very high affinity of the ANPR to [Cl−]out raises the question of at what situation [Cl−]out becomes physiologically relevant. A good example is in the kidney, where the ANPR is expressed at the luminal membrane of intercalated cells (38). Volume depletion and metabolic alkalosis activate the renin-angiotensin system to reduce luminal [Cl−]out to below 10 mM (31).
Fig. 1.
Signaling by [Cl−]out: effect of [Cl−]out on ligand-receptor interaction. A: effect of [Cl−]out on atrial natriuretic peptide (ANP) binding was measured in Chinese hamster ovary (CHO) cells transfected with the full-length ANP receptors. Isolated membranes were incubated in media containing the indicated Cl− concentrations and 125I−-ANP to measure ANP binding to the full-length ANP receptor (a). Isolated membranes from CHO cells transfected with the full-length ANP receptors were used to measure stimulation of guanylyl cyclase activity by ANP in the presence and absence of 100 mM NaCl (b). Results are taken from Ref. 65. B: group II and III mGlu receptors (mGluRs) were cotransfected with a chimeric Gi/Gq protein. The receptors were labeled with blue and green fluorescent tags for measurement of FRET. Labeled cells incubated in solutions containing [Cl−]out of 154.6 mM (green) to 2 mM (black) and FRET between Group II and III mGluRs in response to glutamate stimulation was measured. Shown are the changes in the indicated mGluRs conformation in response to receptor stimulation. Results are taken from Ref. 102. C and D: crystal structures of the rat ANP receptor (ANPR: gray; PDB_ID: 1DP4) and human mGlu8 receptor (GluR: green; PDB_ID: 6BSZ) (C) were obtained from the protein data bank website. The models were aligned and visualized using UCSF Chimera 1.11 (77). In D, a closeup of the ANPR chloride binding site residues are shown in red and the chloride ion in blue, with the mGlu8 Cl− binding site in green.
Notably, the extracellular ligand-binding domain of the metabotropic glutamate receptors (mGluR) fold is similar to the fold of the ANPR extracellular domain [(52, 106) and Fig. 1, C and D)]. The Cl− binding site of the ANPR is highly conserved in the various mGluRs with a nearly identical fold (65), and [Cl−]out regulates signaling by the mGluRs (101, 102). The use of FRET between mGluRs extracellular domains and the measurement of intracellular Ca2+ ([Ca2+]i) has revealed that [Cl−]out markedly increases the apparent affinity of most mGluRs to their agonist (Fig. 1B). The apparent affinity of mGluRs for [Cl−]out is between 60 and 80 mM, within the range of [Cl−]out in synaptic clefts (102). [Cl−]out interaction with mGluRs shows high cooperativity with a Hill coefficient as high as 6, suggesting a response to an exceptionally narrow change in [Cl−]out (101, 102). The inotropic kainate receptors also bind Cl− to stabilize their extracellular ligand binding sites, but this receptor Cl− binding site structure is different than that of the mGluRs. Nevertheless, this is another example of the regulation of a GPCR by [Cl−]out. [Cl−]out regulates kainate receptor desensitization (79, 80).
COUPLED TRANSPORTERS REGULATED BY [Cl−]out
The Cl−/ exchanger AE1 (also called band 3) functions as a dimer and has major roles in erythrocytes and in the kidney. Mutations in the SLC4A1 (encoding AE1) cause renal tubular acidosis and hereditary spherocytosis (2). AE1 is inhibited by stilbene derivatives, such as DBDS (4,4′-dibenzamido-2,2′-stilbenedisulfonate) and DIDS (4,4′-diisothiocyanato-2,2′- stilbenedisulfonate). A critical residue for transport by AE1 is glutamate 681 (E681). Chemical modification of E681 exposes a cryptic [Cl−]out regulatory site in AE1 that binds Cl− with an apparent affinity of 7–10 mM to modulate the conformation of the AE1 dimer (87). These findings were subsequently confirmed by measurements of Cl− and fluxes in erythrocytes (46).
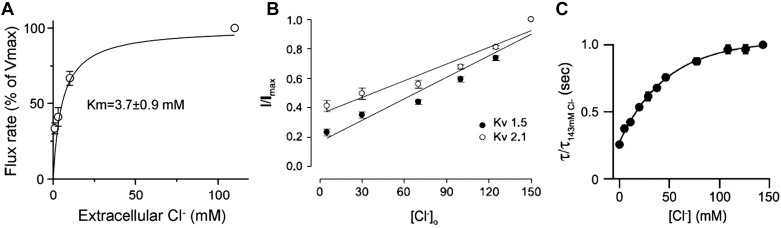
A second family of anion transporters is the SLC26 transporter family, in which several members function as electroneutral and electrogenic Cl−/ exchangers, and several function as Cl− channels (56, 67). Among them are SLC26A2, which functions as a /Cl−/OH− exchanger (66) and has multiple roles in chondrocytes (72), and SLC26A1, which functions as a Cl− and oxalate/Cl− exchanger (110). Measurement of /2OH− exchange by SLC26A2 reveals that Cl−out was required to activate SLC26A2 with apparent affinity of ~4 mM (Fig. 2A). The site displays poor selectivity for Cl− with Cl−, Br−, I−, , and SCN− having similar potency in the activation of SLC26A2 (66). and oxalate transport by SLC26A1 is activated by [Cl−]out with an EC50 of ~13 mM, with [Cl−]out modifying the inhibition of SLC26A1 by acidic pHout (110). The relatively high affinity for regulation of SLC26A2 by [Cl−]out suggests that it functions in low [Cl−]out environment, such as the luminal space of epithelia. There is no structural information on the SLC26 transporters extracellular and cytoplasmic domains except for the STAS domain (110), and therefore, there is no structural information on the potential SLC26 transporters [Cl−]out binding site.
Fig. 2.
Signaling by [Cl−]out: effect of [Cl−]out on ion transporters. A: Xenopus oocytes expressing SLC26A2 and loaded with in Cl−-free solution. The oocytes were then incubated in media containing the indicated Cl− to measure /OH−out exchange. The relative rate of /OH−out exchange is plotted as a function of [Cl−]out. Results are taken from Ref. 66. B: Chinese hamster ovary (CHO) cells transfected with the indicated K+ channel isoforms were used to measure the whole cell K+ current with pipette solution containing 150 mM Cl− and bath solutions containing between 5 and 150 mM Cl−. The figure shows the effect of [Cl−]out on the magnitude of Kv1.5 and Kv2.1 current. Results are taken from Ref. 59. C: ASIC1a channel current rapidly desensitizes when active by external H+. Time constants of desensitization of ASIC1a channel currents are shown when recorded in solutions of varying [Cl−]out, which were normalized to the time constant of desensitization of the current recorded in 143 mM [Cl−]out. Results are taken from Ref. 54.
CHANNELS REGULATED BY [Cl−]out
Several anion and cation channels are regulated by [Cl−]out, which affects channel conductance and/or selectivity. The ionotropic P2X7 receptor (P2X7R) functions as a Cl−-permeable, nonselective cation channel (58). The P2X7R channel pore expands upon continued stimulation to accommodate large molecules the size of ATP and NMDG+ (64). [Cl−]out is required for cation current by P2X7R. Moreover, although [Cl−]out regulates only P2X7R outward current of large molecules, P2X7R pore expansion is also regulated by [Cl−]out (58).
[Cl−]out activates the Cl− channel cystic fibrosis transmembrane conductance regulator (CFTR). Increasing [Cl−]out increases CFTR current by 50–70% with a complex [Cl−]out dependence (109), suggesting complicated modes of [Cl−]out interaction with CFTR. Increasing [Ca2+]i appears to increase the apparent affinity for [Cl−]out without affecting maximal current density. Single-channel analysis showed that [Cl−]out increases CFTR open probability without affecting channel conductance (109). Reduction in [Cl−]out has been reported to increase CFTR Cl−/ permeability in a time-dependent manner (89), but this may relate to the effect of [Cl−]in on CFTR (see Ref. 71 and below). More recent analysis has shown that the effect of [Cl−]out requires Arg-899 in the fourth extracellular loop of CFTR and may affect interaction of ATP with the nucleotide binding domain 1 of CFTR (11). This would argue for the presence of a specific extracellular Cl− binding site in CFTR, although such a site has not been observed in the structure of CFTR (120). Regulation of CFTR by [Cl−]out is significant for epithelial fluid and secretion during which duct luminal Cl− is reduced from about 120 to below 20 mM (56). At 20 mM, [Cl−]out CFTR activity should be substantially reduced.
The 4-Ap-sensitive outward rectifying K+ channel (Kv) is one of the two major K+ channels in the outer hair cell (OHC) and has several kinetic components when activated by a depolarizing pulse (59). The channel is activated by [Cl−]out with a shallow linear dependence on [Cl−]out between 5 and 150 mM (Fig. 2B), with [Cl−]out differentially affecting each of its kinetic components (59). Topical expression of various outward rectifying channels has shown that Kv2.1 and Kv1.5 are activated by [Cl−]out with the properties of Kv2.1 most resembling the K+ channel of OHC and indicating that Kv2.1 accounts for the [Cl−]out sensitivity of OHC (59). The identity and nature of the site mediating the effect of [Cl−]out in Kv channels is unknown.
The acid-sensing ion channels (ASICs) and the epithelial Na+ channel ENaC comprise a group of homologous channels regulated by [Cl−]out. The crystal structure of ASIC1a reveals Cl− binding sites reside at the subunit-subunit interface at low pH. These sites are coordinated by Arg and Glu residues on the thumb domain and by a Lys residue on the palm domain of a neighboring subunit (45). The residues between the thumb and palm domain involved in Cl− bindings are conserved among all ASICs and in ENaC channels. Cl− interacts with these sites to regulate proton-dependent gating, desensitization kinetics, and the tachyphylaxis of ASIC1a with an apparent affinity of ~50 mM [Fig. 2C and (54)]. [Cl−]out inhibits ENaC, in part, by enhancing Na+-self inhibition of the channel (16). Interestingly, the effects of the three [Cl−]out binding sites in ENaC on channel activity are not equivalent and are reduced or eliminated at alkaline pH (16, 17). Indeed, the structure of ASIC shows destabilization of the Cl− binding sites between the thumb and palm domain at high pH, but also reveals Cl− interaction at the mouth of the extracellular fenestrations, just above the transmembrane domain (117). Hence, Cl− binding may control access of the conducted Na+ to the pore.
The effects of [Cl−]out discussed above demonstrate the breath and range of allosteric regulation by [Cl−]out. These regulatory functions require interaction of [Cl−]out with sites of vastly different affinity, some with apparent affinity in the low mM range and others with an affinity close to physiological concentration of 100 mM. At present, there is limited structural information on the nature of the extracellular Cl− binding sites, and therefore, no consensus motifs or even essential residues have emerged to mediate Cl− binding to extracellular sites to allow searching for similar sites in other proteins. Moreover, the physiological significance of regulation by [Cl−]out is rarely demonstrated, even when mutations affecting [Cl−]out sensing are known. Hopefully the availability of a knockout/knockin by CRISPR/Cas will change this to increase understanding and appreciation of the sensing and regulation by [Cl−]out.
SIGNALING BY [Cl−]in
The evidence for signaling by [Cl−]in is quite strong. [Cl−]in rapidly changes in response to cell stimulation to modulate the activity of various cellular proteins, including transcription factors, ion transporters and channels, and protein kinases. Regulation of proteins and physiological functions by [Cl−]in has been described for many years but was not, and for the most part, is not viewed as a signaling by [Cl−]in but rather as a form of regulation. The recognition of [Cl−]in as a signaling ion is in its infancy, but should gain acceptance once signaling by [Cl−]in to various pathways is understood to the extent of [Cl−]in signaling in the WNK [With No lysine (K)] kinases pathway, which was established with the seminal discovery of a Cl− binding site in WNK1 (78). This discovery, more than any other, is drawing attention to [Cl−]in as an important signaling ion, especially in the transport field. We will discuss below regulation by [Cl−]in from genes to proteins to physiological processes, emphasizing the effects of [Cl−]in binding and that are independent of effect of [Cl−]in on the transport of other ions. We close with the most complete studies of the role of [Cl−]in in the regulation of protein kinases, including the WNK pathway, which has been established from the identification of the WNK Cl− binding site to its role in mouse physiological.
GENES REGULATED BY [Cl−]in
Cells respond to changes in [Cl−]in by affecting expression of various Cl− transporters (49, 69, 83). This likely involves sensing of [Cl−]in changes by transcription factors that regulate expression of multiple Cl− transporter genes. A well-demonstrated example is the change in GABA receptor subunits during neuronal maturations in vitro (96) and in vivo (97). A change in GABA receptor subunit mRNA expression during neuronal maturation causes a change in GABAergic responses (25). Embryonic and young neurons express high levels of NKCC1 mRNA and NKCC1 protein, which maintains high [Cl−]in. Neuronal maturation involves upregulation of KCC2 mRNA and KCC2 protein and reduction in [Cl−]in. Reduced [Cl−]in signals a change in the relative expression of α3-1 and δ-containing GABAA receptor subunits to tune the strength of GABAergic responses (96). Although strongly suggestive of gene regulation by [Cl−]in, it should be noted that [Cl−]in regulates the concentrations of other ions like Ca2+, Na+, K+, and H+, as well as cell volume; the contribution of these factors to gene regulation in this case needs a more careful evaluation, to separate them from direct gene regulation by [Cl−]in.
Limited evidence for sensing of and direct regulation of genes by [Cl−]in is available. The gram-positive, halophilic bacterium Halobacillus halophilus lives in an environment of changing salt and is dependent on Cl− for growth. In this bacterium, Cl− directly induces expression of the fliC gene that determines synthesis of the major subunit of the flagellum FliC, and of genes that control five stress-related proteins (85). In ciliated human nasal epithelial cells, [Cl−]in regulates ciliary beating frequency and amplitude (43), although the protein affected by Cl−in that transmit the change in [Cl−]in to the cilia is not known at present. [Cl−]in directly regulates the activity of the epithelial Na+ channel ENaC (see below), but also has a long-term effect by regulating the expression of αENaC subunit mRNA and protein levels (63). Evidence for gene regulation by [Cl−]in was reported in a mammalian bronchial cell line in association with changes in reduced activity of CFTR that resulted in increased [Cl−]in (105). Cl−in appeared to differentially regulate several genes, with inhibition of the RPS27 gene expression at [Cl−]in between 25 and 75 mM, and reverse the inhibition as [Cl−]in was increased between 75 and 125 mM. By contrast, the GLRX5 gene, which encodes glutaredoxin-related protein 5, was activated by Cl−in with an EC50 of 35 mM (104). These [Cl−]in concentrations are well within the physiological changes in [Cl−]in observed in many cell types (56). The nature of the Cl−in interacting protein(s) and the way Cl−in binding is translated to gene regulation are unknown.
CHANNELS AND TRANSPORTERS REGULATED BY [Cl−]in
Several studies have reported direct regulation of ENaC by [Cl−]in. Early work with isolated salivary glands intralobular duct cells has shown that [Cl−]in inhibits ENaC current with half maximal inhibition at 50 mM. The Cl− site mediating this inhibition displays poor anion selectivity with similar inhibition observed with Cl−, Br−, and (20). A subsequent study used excised inside-out patches to show a direct effect of [Cl−]in on ENaC current, again with poor anionic selectivity (6). However, in both studies only high anion concentrations were used, and potential differences in potency among the anions in inhibition of ENaC were not determined. Single channel analysis revealed that [Cl−]in reduced both ENaC open probability and channel conductance with most of the effects observed at [Cl−]in between 4 and 60 mM (34). Importantly, truncation analysis localized the effect of [Cl−]in on ENaC to the cytoplasmic COOH terminus of the α and β ENaC subunits (6).
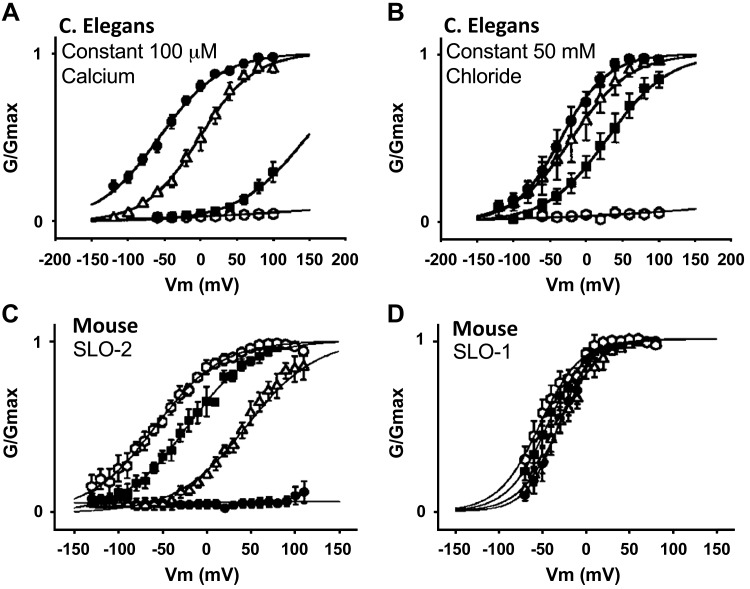
Another group of channels regulated by [Cl−]in are K+ channels. The K+ current in astrocytes mediated by Kv channels is activated when [Cl−]in is increased from 40 to 80 mM, which affects channel activation kinetics (8). Similar regulation by [Cl−]in was observed with the rapidly inactivating K+ channel isoforms Kv1.4 and Kv4.2 (7). The mechanism by which [Cl−]in affects Kv channels function is not known. However, regulation of Kv1.4 and Kv4.2 by [Cl−]in could be observed only after disruption of the cytoskeleton, suggesting that a cytoplasmic channel domain that is shielded by the cytoskeleton senses and mediates channel gating by [Cl−]in (7). The Na+- and Ca2+-activated SLO channels display a unique form of regulation by [Cl−]in. Regulation by [Cl−]in was discovered when the properties of the Caenorhabditis elegans SLO-2 channel were examined. SLO-2, but not SLO-1 and SLO-3, is regulated by [Cl−]in and regulation by [Cl−]in and Ca2+ is synergistic (118). This is illustrated in Fig. 3, showing that [Cl−]in left-shifts the voltage dependence of SLO-2, that [Cl−]in-dependent activation is modulated by [Ca2+]i and that Ca2+-dependent activation is modulated by [Cl−]in. The mammalian SLO channels Slick and SLAK (KCNT1 and KCNT2) are also regulated by Na+ and [Cl−]in with half maximal activation at [Cl−]in of 35 mM (10, 75), which is of physiological relevance.
Fig. 3.
Signaling by [Cl−]in: effect of [Cl−]in on Caenorhabditis elegans and mouse K+ channel activity. [Cl−]in modulates C. elegans SLO-2 K+ channel properties. A: shows C. elegans SLO-2 channel conductance measured at constant 100 µM Ca2+ and at [Cl−]in of 0 (○) 10 (■), 50 (Δ), and 100 mM (●). B shows C. elegans SLO-2 channel conductance measured at constant Cl− of 50 mM and at [Ca2+]i of 0 (○), 25 (■), 100 (Δ), and 250 μM (●). C and D show that mouse SLO-1 is insensitive to [Cl−]in compared with SLO-2. In C and D, channel conductance was measured at constant [Ca2+]i of 250 µM and at [Cl−]in of 0 (●), 10 (Δ), 50 (■), and 100 mM (○). Results are taken from Ref. 118.
Cl− has been well described as a cosubstrate for several neurotransmitter transporters, and evidence for regulation of the transporters by [Cl−]in is also available (28). More recently, a whole endosomal recording of glutamate-mediated current by the vesicular glutamate transporters revealed allosteric activation of glutamate transport by [Cl−]in that took place mostly between 10 and 50 mM [Cl−]in. An arginine in transmembrane domain 4 was required for both the transport and the allosteric regulation by [Cl−]in (14).
A well-understood example of [Cl−]in-mediated regulation of a transporter is the Na+- cotransporter family, in particular, the ubiquitous NBCe1-B isoform (90) that has a prominent role in epithelial secretion (56). NBCe1-B (94, 116), as well as several other transporters (37, 51, 74, 116), are regulated by the scaffolding protein IP3 receptor binding protein released with IP3 (IRBIT). IRBIT binds to and inhibits the IP3 receptors (5), and once released from the IP3Rs by elevation of cytoplasmic IP3, it mediates the synergistic activation of the transporters by Ca2+ and cAMP signaling pathways (74). The basal activity of NBCe1-B is determined by its NH2 terminus autoinhibitory domain (AID) and is minimally inhibited by [Cl−]in. IRBIT interacts with the AID to markedly activate NBCe1-B and exposes a cryptic Cl−in sensing site with an apparent affinity of ~10 mM that potently inhibits the NBCe1-B activity. Inhibition of the NBCe1-B by [Cl−]in occurs with exquisite selectivity and is not affected by Br−, I−, , or SCN− (90).
Although the structure and nature of the NBCe1-B [Cl−]in sensing site is not known, it is mediated by two conserved GXXXP motifs. The first motif is 32GXXXP36 in the NBCe1-B AID, and the second motif is 194GXXXP198. GXXXP motifs function as Cl− sensing sites (22) and participate in Cl− sensing by SLC26A2 (66), NBCe1-B (SLC4A4), and NBCe2-C (SLC4A5) (90). [Cl−]in sensing by the NBCe1-B GXXXP motifs is regulated by protein kinases and phosphatases acting on specific serine residues (Fig. 4). The 32GXXXP36 motif is regulated by Ser-65, which is phosphorylated by the SPAK kinase and dephosphorylated by the phosphatase PP1. The 194GXXXP198 motif is regulated by Ser-12, which is phosphorylated by CaMKII kinase and dephosphorylated by the phosphatase calcineurin [see Fig. 4 and (103)]. IRBIT recruits the kinases and phosphatases to NBCe1-B to determine their phosphorylation state. When Ser-12 and Ser-65 are phosphorylated, the [Cl−]in sensing sites are cryptic and their dephosphorylation uncovers the [Cl−]in sensing sites to allow regulation of NBCe1-B by [Cl−]in (103).
Fig. 4.
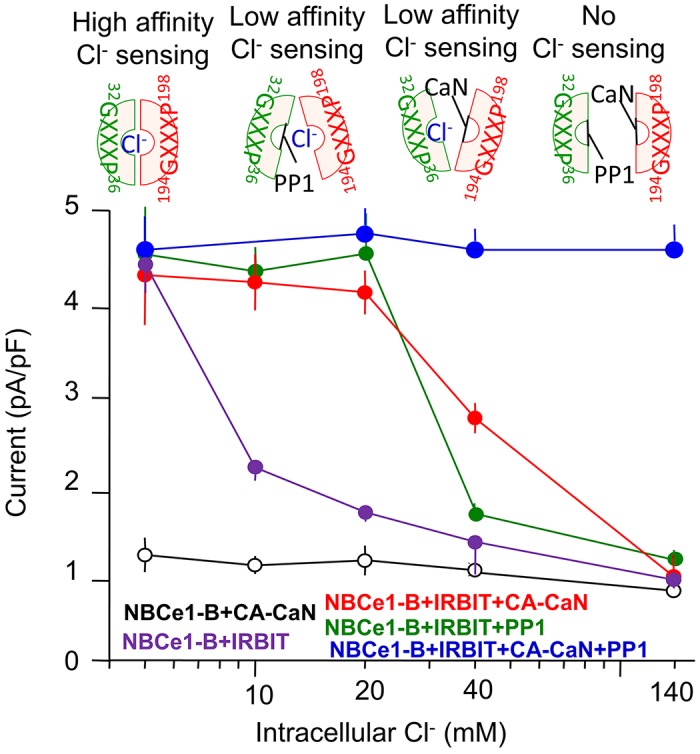
Signaling by [Cl−]in: regulation of the Na+/ cotransporter NBCe1-B by [Cl−]in is modulated by kinases and phosphatases. Na+- cotransport by NBCe1-B was measured as the outward current triggered by the addition of to human embryonic kidney (HEK) cells transfected with NBCe1-B and IRBIT (binding protein released with IP3). IRBIT activates NBCe1-B and exposes its cryptic [Cl−]in sensitive sites 32GXXXP36 and 194GXXXP198 (103). Current was initiated by addition of to the bath and [Cl−]in was set by changes of Cl− concentration in the patch pipette solution. NBCe1-B current due to Na+-2 cotransport in the absence of IRBIT (○) shows low sensitivity to [Cl−]in. IRBIT activates NBCe1-B and exposes regulatory [Cl−]in sites (●). When protein phosphatase 1 (PP1) is activated, regulation of NBCe1-B by [Cl−]in sensing through the 32GXXXP36 site (●) is eliminated, while active constitutively active calcineurin (CA-CaN) eliminates [Cl−]in sensing by the 194GXXXP198 site (●). Activation of both PP1 and CA-CaN eliminates inhibition of NBCe1-B by [Cl-]in (●). Results are taken from Ref. 103.
REGULATION OF PROTEIN KINASES BY [Cl−]in
One of the best understood form of regulation by [Cl−]in is that of various protein kinases. An indirect evidence for activation of Jun kinase 2 (JNK2) by [Cl−]in is suggested by the finding that reduction in [Cl−]in prevents JNK2-dependent induction of the Na+/K+ pump γ subunit in response to hypotonicity (12). In addition, upregulation of the cyclin-dependent kinase inhibitor p21, which is mediated by p38 and JNK kinases, requires an increase in [Cl−]in (68). Regulation of JNK by [Cl−]in may be cell specific since in macrophages low [Cl−]in activates the p38 and the JNK kinases (111). [Cl−]in strongly inhibits the activity of purified apoptosis signal-regulating kinase 1 (ASK1) and mitogen-activated kinase 6 (MEK6) (78), but the physiological function of this inhibition is not clear at this time. Increasing [Cl−]in between 50 and 70 mM, prominently phosphorylates and activates purified glucocorticoid-inducible protein kinase 1 (SGK1) in airway epithelial cells, to mediate the response to LPS-induced reduction in cellular cAMP (119). Although it is not known how [Cl−]in regulates SGK1 and whether the regulation is by direct effect of [Cl−]in on the kinase, the physiological significance of the regulation is evident from the finding that it occurs at the physiological range [Cl−]in and that SGK1 is markedly activated in patients with bronchiectasis, a chronic airway inflammatory disease (119).
One conclusive piece of evidence for the regulation of protein kinases by [Cl−]in with profound physiological consequences is demonstrated with the WNK kinases (41, 91). The WNK kinases were discovered as members of the MAP kinase superfamily (113), with the WNK subfamily consisting of four members with conserved kinase domain and variable NH2 and COOH termini (41). Interest in the WNKs increased greatly with the discovery that mutations in WNK1 and WNK4 cause hypertension in humans (108). The primary action of the WNKs is determining ion transporters surface expression (91) by regulating their endocytosis (32, 42). The WNK kinases can act by directly phosphorylating several transporters, or indirectly by phosphorylating the oxidative stress-responsive kinase 1 and/or the STE20/SPS1-related proline/alanine-rich kinase (SPAK) (19), which phosphorylate the transporters to control their plasma membrane level (40).
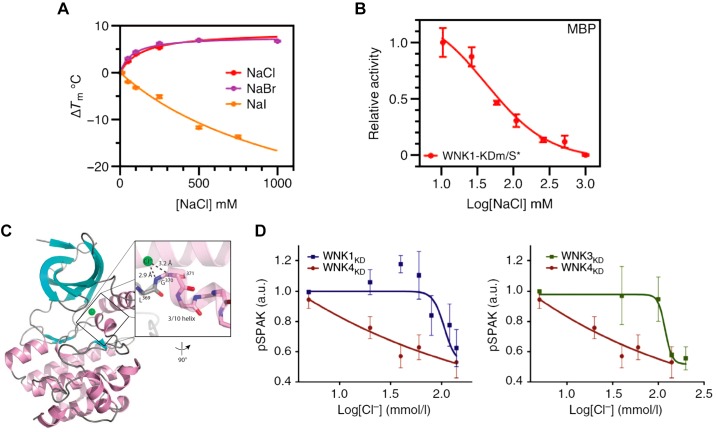
Regulation of the WNK/SPAK pathway by [Cl−]in was established long before direct regulation of the kinases by [Cl−]in and identification of their Cl− binding site was established. For instance, the Na+/K+/2Cl− cotransporter NKCC1 is phosphorylated and activated by SPAK only at low [Cl−]in (21), suggesting that SPAK is inhibited by [Cl−]in. Similar findings were reported for the NaCl cotransporter NCC (70). The phosphorylation of SPAK by WNK3 to regulate NKCC2 function requires low [Cl−]in, suggesting that WNK3 is inhibited by [Cl−]in (81). Phosphorylation of CFTR by WNK1 changes its selectivity from a Cl−-selective to a Cl− and conducting channel, a function observed only at low [Cl−]in (71). Interest in the regulation of the WNKs by [Cl−]in has dramatically changed with the discovery that autophosphorylation, stability (Fig. 5A), and activity (Fig. 5B) of WNK1 are regulated by Cl−, and with the identification of the Cl− binding site in the kinase domain (Fig. 5C) (78). This discovery initiated a number of studies demonstrating that WNK3 and WNK4 are also inhibited by [Cl−]in, with WNK4 showing the highest sensitivity to [Cl−]in [(98) and Fig. 5D)], highlighting the importance of [Cl−]in in the regulatory function of the WNKs (91).
Fig. 5.
Signaling by [Cl−]in: effect of [Cl−]in on the activity of the WNK [With No lysine (K)] kinases. A: effect of Cl−, Br−, and I− concentration on WNK1 kinase stability evaluated from measurement of a change in melting temperature (ΔTm). B: WNK1 kinase activity as a function of Cl− concentration. C: cartoon of WNK1 halide binding site. α helices are pink, β sheets are cyan, and loops are gray. The Cl− position is shown in green. Inset: 3/10 helix and hydrogen bonding distances are shown. Results in A–C are taken from Ref. 78. D: effects of Cl− concentration on in WNK kinase activity was measured using the WNKs kinase domain (WNKxKD) and STE20/SPS1-related proline/alanine-rich kinase (SPAK) as a substrate. The WNK4KD activity is more sensitive to inhibition by Cl− than WNK1KD WNK3KD. Results are taken from Ref. 98.
The physiological in vivo significance was recently established in a notable study, in which a Cl−-insensitive mutant WNK4 replaced the native WNK4 in mice (15). In mammals, a change in systemic K+ is translated to an increase in [Cl−]in, which, in turn, results in inhibition of the WNK4 activity. The altered WNK4 activity changes regulation of KCC and perhaps NKCC2 and, consequently, K+ excretion, resulting in hyperkalemic hypertension (91, 99). Recapitulation in mice expressing the Cl−-insensitive WNK4, the human hypertensive and hyperkalemic disease pseudohypoaldosteronism type II, and the observation that the WNK4 mutant mice lost the renal response to maneuvers that change Cl− homeostasis (15) conclusively establish WNK4 as a physiological [Cl−]in sensor and [Cl−]in as a signaling ion.
CONCLUSIONS AND CHALLENGES
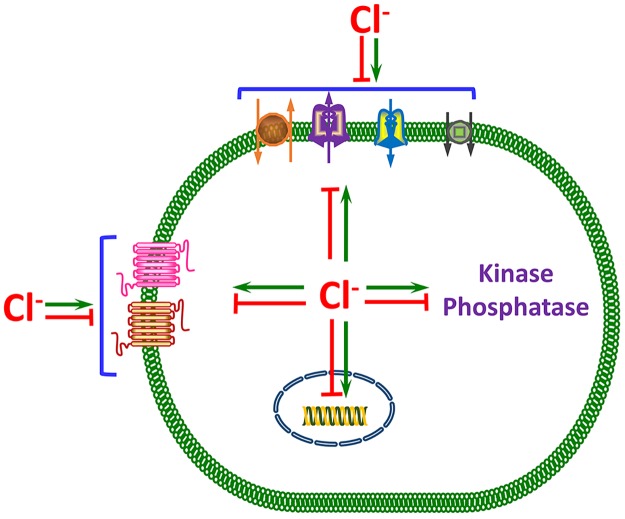
The evidence discussed in the examples above and summarized in Fig. 6 provides a compelling case for Cl− as a signaling ion used by cells to regulate key physiologic functions. Cl− gradients are generated across the plasma and intracellular organelles membranes; the Cl− gradients are dissipated rapidly by cell stimulation to change [Cl−]in and even generate [Cl−]in oscillations; and changes in cellular and systemic Cl− are sensed by a variety of transcription factors to regulate many genes, protein kinases, and ion transporters, to alter cellular activity. These properties fulfill all of the key criteria for an ion to be a bona fide signaling ion. Increasing recognition of Cl− as a signaling ion and its physiological consequences, and not only as a transported ion that affects the concentration of other ions and cell homeostasis through changes in cellular ionic contents poses a major challenge.
Fig. 6.
A model summarizing the signaling functions of Cl−. Intracellular Cl− concentration tightly controls by Cl− influx and efflux transporters that keep [Cl−]in above electrochemical potential. Cell stimulation often rapidly reduces [Cl−]in or causes [Cl−]in oscillations. The changes in [Cl−]in are translated to cellular responses by direct binding of Cl− to a variety of proteins and changes in their activity. The best understood form of signaling by [Cl−]in is regulation of protein kinases and phosphatases. However, regulation of several transporters by binding of Cl− to them, including transporters that do not transport Cl−, is well documented. By regulation of K+ channels, [Cl−]in indirectly affects the membrane potential, in addition to its direct effect on the membrane potential as a conductive ion. [Cl−]in also has long-term effects by binding and activating transcription factors to regulate gene expression. Although [Cl−]in binding to the various proteins and regulation of their activity are well documented, the [Cl−]in binding sites are not always known, and the physiological roles of signaling by [Cl−]in in animal studies is mostly not documented. The exception is the role of [Cl−]in in the WNK/SPAK pathway. Regulation of enzymes, ion channels and transporters, and signaling by G protein-coupled receptors by [Cl−]out is also well documented. However, this is the least understood form of signaling by Cl−, and its physiological role is rarely documented or understood.
A significant difficulty in studying the signaling function of [Cl−]in is separating between the physiological and regulatory function of [Cl−]in. This is exacerbated by the general lack of structural information on the regulatory Cl− binding sites. Because the affinity for Cl− is at the mM range and the presence of [Cl−]in binding sites in cytoplasmic domains that tend to be poorly ordered, the Cl− electron density may not be resolved very well in structural studies. Yet, the case of the WNK kinases illustrates how valuable such information is. With knowledge of the WNK1 Cl− binding site, it became possible to examine the importance of regulation of WNK1, WNK3, and WNK4 by Cl− in epithelial fluid and electrolyte homeostasis, both in model expression system and in vivo. Availability of the structure of the increasing number of regulatory Cl− binding sites should allow better understanding of signaling by Cl− and its physiological relevance.
Second messengers like Ca2+ and cAMP act in cellular microdomains that are generated by compartmentalization of signaling protein complexes in discreet cellular compartments (29, 88). This is also the case for [Cl−]in. Specific Cl− transporters localize to specific membranes, such as the luminal and basolateral membranes of epithelia, and membrane subdomains, such as lipid rafts. In addition, diverse Cl− channels and transporters are present in different organelles. In principal, such compartmentalization allows generation of [Cl−]in signals in specific cellular compartments to exert a selective, rather than a global, effect. Detection and demonstration of localized effects of Ca2+ and cAMP are greatly aided by the availability of genetically targeted protein sensors (4). Genetically encoded fluorescent protein sensors are now available for Cl− (84), and their targeting to various cellular compartments should generate important information on local [Cl−]in signals and their function.
GRANTS
This work was funded by Intramural NIH Grant DE000735-9 from the National Institute of Dental and Craniofacial Research.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
E.O. prepared figures; B.P.L., L.V., and S.M. drafted manuscript; B.P.L., L.V., E.O., and S.M. edited and revised manuscript; B.P.L., L.V., E.O., and S.M. approved final version of manuscript.
REFERENCES
- 1.Abadir PM, Walston JD, Carey RM. Subcellular characteristics of functional intracellular renin-angiotensin systems. Peptides 38: 437–445, 2012. doi: 10.1016/j.peptides.2012.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abbas YM, Toye AM, Rubinstein JL, Reithmeier RAF. Band 3 function and dysfunction in a structural context. Curr Opin Hematol 25: 163–170, 2018. doi: 10.1097/MOH.0000000000000418. [DOI] [PubMed] [Google Scholar]
- 3.Adam G, Ousingsawat J, Schreiber R, Kunzelmann K. Increase in intracellular Cl− concentration by cAMP- and Ca2+-dependent stimulation of M1 collecting duct cells. Pflugers Arch 449: 470–478, 2005. doi: 10.1007/s00424-004-1356-4. [DOI] [PubMed] [Google Scholar]
- 4.Agetsuma M, Matsuda T, Nagai T. Methods for monitoring signaling molecules in cellular compartments. Cell Calcium 64: 12–19, 2017. doi: 10.1016/j.ceca.2016.12.002. [DOI] [PubMed] [Google Scholar]
- 5.Ando H, Mizutani A, Kiefer H, Tsuzurugi D, Michikawa T, Mikoshiba K. IRBIT suppresses IP3 receptor activity by competing with IP3 for the common binding site on the IP3 receptor. Mol Cell 22: 795–806, 2006. doi: 10.1016/j.molcel.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 6.Bachhuber T, König J, Voelcker T, Mürle B, Schreiber R, Kunzelmann K. Cl− interference with the epithelial Na+ channel ENaC. J Biol Chem 280: 31587–31594, 2005. doi: 10.1074/jbc.M504347200. [DOI] [PubMed] [Google Scholar]
- 7.Bekar LK, Loewen ME, Forsyth GW, Walz W. Chloride concentration affects Kv channel voltage-gating kinetics: importance of experimental anion concentrations. Brain Res Bull 67: 142–146, 2005. doi: 10.1016/j.brainresbull.2005.06.023. [DOI] [PubMed] [Google Scholar]
- 8.Bekar LK, Walz W. Intracellular chloride modulates A-type potassium currents in astrocytes. Glia 39: 207–216, 2002. doi: 10.1002/glia.10096. [DOI] [PubMed] [Google Scholar]
- 9.Berg J, Yang H, Jan LY. Ca2+-activated Cl− channels at a glance. J Cell Sci 125: 1367–1371, 2012. doi: 10.1242/jcs.093260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bhattacharjee A, Joiner WJ, Wu M, Yang Y, Sigworth FJ, Kaczmarek LK. Slick (Slo2.1), a rapidly-gating sodium-activated potassium channel inhibited by ATP. J Neurosci 23: 11681–11691, 2003. doi: 10.1523/JNEUROSCI.23-37-11681.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Broadbent SD, Ramjeesingh M, Bear CE, Argent BE, Linsdell P, Gray MA. The cystic fibrosis transmembrane conductance regulator is an extracellular chloride sensor. Pflugers Arch 467: 1783–1794, 2015. doi: 10.1007/s00424-014-1618-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Capasso JM, Rivard CJ, Enomoto LM, Berl T. Chloride, not sodium, stimulates expression of the gamma subunit of Na/K-ATPase and activates JNK in response to hypertonicity in mouse IMCD3 cells. Proc Natl Acad Sci USA 100: 6428–6433, 2003. doi: 10.1073/pnas.1130871100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cellot G, Cherubini E. GABAergic signaling as therapeutic target for autism spectrum disorders. Front Pediatr 2: 70, 2014. doi: 10.3389/fped.2014.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang R, Eriksen J, Edwards RH. The dual role of chloride in synaptic vesicle glutamate transport. eLife 7: e34896, 2018. doi: 10.7554/eLife.34896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen JC, Lo YF, Lin YW, Lin SH, Huang CL, Cheng CJ. WNK4 kinase is a physiological intracellular chloride sensor. Proc Natl Acad Sci USA 116: 4502–4507, 2019. doi: 10.1073/pnas.1817220116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Collier DM, Snyder PM. Extracellular chloride regulates the epithelial sodium channel. J Biol Chem 284: 29320–29325, 2009. doi: 10.1074/jbc.M109.046771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Collier DM, Snyder PM. Identification of epithelial Na+ channel (ENaC) intersubunit Cl− inhibitory residues suggests a trimeric alpha gamma β channel architecture. J Biol Chem 286: 6027–6032, 2011. doi: 10.1074/jbc.M110.198127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Collins KD. Ions from the Hofmeister series and osmolytes: effects on proteins in solution and in the crystallization process. Methods 34: 300–311, 2004. doi: 10.1016/j.ymeth.2004.03.021. [DOI] [PubMed] [Google Scholar]
- 19.Delpire E, Gagnon KB. SPAK and OSR1: STE20 kinases involved in the regulation of ion homoeostasis and volume control in mammalian cells. Biochem J 409: 321–331, 2008. doi: 10.1042/BJ20071324. [DOI] [PubMed] [Google Scholar]
- 20.Dinudom A, Young JA, Cook DI. Na+ and Cl− conductances are controlled by cytosolic Cl− concentration in the intralobular duct cells of mouse mandibular glands. J Membr Biol 135: 289–295, 1993. doi: 10.1007/BF00211100. [DOI] [PubMed] [Google Scholar]
- 21.Dowd BF, Forbush B. PASK (proline-alanine-rich STE20-related kinase), a regulatory kinase of the Na-K-Cl cotransporter (NKCC1). J Biol Chem 278: 27347–27353, 2003. doi: 10.1074/jbc.M301899200. [DOI] [PubMed] [Google Scholar]
- 22.Dutzler R, Campbell EB, Cadene M, Chait BT, MacKinnon R. X-ray structure of a ClC chloride channel at 3.0 A reveals the molecular basis of anion selectivity. Nature 415: 287–294, 2002. doi: 10.1038/415287a. [DOI] [PubMed] [Google Scholar]
- 23.Edwards A, Crambert G. Versatility of NaCl transport mechanisms in the cortical collecting duct. Am J Physiol Renal Physiol 313: F1254–F1263, 2017. doi: 10.1152/ajprenal.00369.2017. [DOI] [PubMed] [Google Scholar]
- 24.Ehlers MR, Kirsch RE. Catalysis of angiotensin I hydrolysis by human angiotensin-converting enzyme: effect of chloride and pH. Biochemistry 27: 5538–5544, 1988. doi: 10.1021/bi00415a023. [DOI] [PubMed] [Google Scholar]
- 25.Ehrlich DE, Ryan SJ, Hazra R, Guo JD, Rainnie DG. Postnatal maturation of GABAergic transmission in the rat basolateral amygdala. J Neurophysiol 110: 926–941, 2013. doi: 10.1152/jn.01105.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eladari D, Chambrey R, Picard N, Hadchouel J. Electroneutral absorption of NaCl by the aldosterone-sensitive distal nephron: implication for normal electrolytes homeostasis and blood pressure regulation. Cell Mol Life Sci 71: 2879–2895, 2014. doi: 10.1007/s00018-014-1585-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eriksen J, Chang R, McGregor M, Silm K, Suzuki T, Edwards RH. Protons regulate vesicular glutamate transporters through an allosteric mechanism. Neuron 90: 768–780, 2016. doi: 10.1016/j.neuron.2016.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Filadi R, Pozzan T. Generation and functions of second messengers microdomains. Cell Calcium 58: 405–414, 2015. doi: 10.1016/j.ceca.2015.03.007. [DOI] [PubMed] [Google Scholar]
- 30.Foskett JK. [Ca2+]i modulation of Cl− content controls cell volume in single salivary acinar cells during fluid secretion. Am J Physiol Cell Physiol 259: C998–C1004, 1990. doi: 10.1152/ajpcell.1990.259.6.C998. [DOI] [PubMed] [Google Scholar]
- 31.Galla JH. Metabolic alkalosis. J Am Soc Nephrol 11: 369–375, 2000. [DOI] [PubMed] [Google Scholar]
- 32.Gallolu Kankanamalage S, Lee AY, Wichaidit C, Lorente-Rodriguez A, Shah AM, Stippec S, Whitehurst AW, Cobb MH. WNK1 is an unexpected autophagy inhibitor. Autophagy 13: 969–970, 2017. doi: 10.1080/15548627.2017.1286431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Glykys J, Dzhala V, Egawa K, Balena T, Saponjian Y, Kuchibhotla KV, Bacskai BJ, Kahle KT, Zeuthen T, Staley KJ. Local impermeant anions establish the neuronal chloride concentration. Science 343: 670–675, 2014. doi: 10.1126/science.1245423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gu Y. Effect of [Cl−]i on ENaC activity from mouse cortical collecting duct cells. J Cell Physiol 216: 453–457, 2008. doi: 10.1002/jcp.21413. [DOI] [PubMed] [Google Scholar]
- 35.Hagaman JR, Moyer JS, Bachman ES, Sibony M, Magyar PL, Welch JE, Smithies O, Krege JH, O’Brien DA. Angiotensin-converting enzyme and male fertility. Proc Natl Acad Sci USA 95: 2552–2557, 1998. doi: 10.1073/pnas.95.5.2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Han Y, Shewan AM, Thorn P. transport through anoctamin/transmembrane protein ANO1/TMEM16A in pancreatic acinar cells regulates luminal pH. J Biol Chem 291: 20345–20352, 2016. doi: 10.1074/jbc.M116.750224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.He P, Zhao L, No YR, Karvar S, Yun CC. The NHERF1 PDZ1 domain and IRBIT interact and mediate the activation of Na+/H+ exchanger 3 by ANG II. Am J Physiol Renal Physiol 311: F343–F351, 2016. doi: 10.1152/ajprenal.00247.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hirsch JR, Kruhøffer M, Adermann K, Heitland A, Maronde E, Meyer M, Forssmann WG, Herter P, Plenz G, Schlatter E. Cellular localization, membrane distribution, and possible function of guanylyl cyclases A and 1 in collecting ducts of rat. Cardiovasc Res 51: 553–561, 2001. doi: 10.1016/S0008-6363(00)00297-2. [DOI] [PubMed] [Google Scholar]
- 39.Hodgkin AL, Horowicz P. The influence of potassium and chloride ions on the membrane potential of single muscle fibres. J Physiol 148: 127–160, 1959. doi: 10.1113/jphysiol.1959.sp006278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hong JH, Park S, Shcheynikov N, Muallem S. Mechanism and synergism in epithelial fluid and electrolyte secretion. Pflugers Arch 466: 1487–1499, 2014. doi: 10.1007/s00424-013-1390-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang CL, Cha SK, Wang HR, Xie J, Cobb MH. WNKs: protein kinases with a unique kinase domain. Exp Mol Med 39: 565–573, 2007. doi: 10.1038/emm.2007.62. [DOI] [PubMed] [Google Scholar]
- 42.Huang CL, Yang SS, Lin SH. Mechanism of regulation of renal ion transport by WNK kinases. Curr Opin Nephrol Hypertens 17: 519–525, 2008. doi: 10.1097/MNH.0b013e32830dd580. [DOI] [PubMed] [Google Scholar]
- 43.Inui TA, Murakami K, Yasuda M, Hirano S, Ikeuchi Y, Kogiso H, Hosogi S, Inui T, Marunaka Y, Nakahari T. Ciliary beating amplitude controlled by intracellular Cl− and a high rate of CO2 production in ciliated human nasal epithelial cells. Pflugers Arch 471: 1127–1142, 2019. doi: 10.1007/s00424-019-02280-5. [DOI] [PubMed] [Google Scholar]
- 44.Ishiguro H, Naruse S, Kitagawa M, Mabuchi T, Kondo T, Hayakawa T, Case RM, Steward MC. Chloride transport in microperfused interlobular ducts isolated from guinea-pig pancreas. J Physiol 539: 175–189, 2002. doi: 10.1113/jphysiol.2001.012490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jasti J, Furukawa H, Gonzales EB, Gouaux E. Structure of acid-sensing ion channel 1 at 1.9 A resolution and low pH. Nature 449: 316–323, 2007. doi: 10.1038/nature06163. [DOI] [PubMed] [Google Scholar]
- 46.Jennings ML. Evidence for a second binding/transport site for chloride in erythrocyte anion transporter AE1 modified at glutamate 681. Biophys J 88: 2681–2691, 2005. doi: 10.1529/biophysj.104.056812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jentsch TJ, Pusch M. CLC chloride channels and transporters: structure, function, physiology, and disease. Physiol Rev 98: 1493–1590, 2018. doi: 10.1152/physrev.00047.2017. [DOI] [PubMed] [Google Scholar]
- 48.Jeyendran RS, Van der Ven HH, Rosecrans R, Perez-Pelaez M, al-Hasani S, Zaneveld LJ. Chemical constituents of human seminal plasma: relationship to fertility. Andrologia 21: 423–428, 1989. doi: 10.1111/j.1439-0272.1989.tb02437.x. [DOI] [PubMed] [Google Scholar]
- 49.Kahle KT, Delpire E. Kinase-KCC2 coupling: Cl− rheostasis, disease susceptibility, therapeutic target. J Neurophysiol 115: 8–18, 2016. doi: 10.1152/jn.00865.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kahle KT, Khanna AR, Alper SL, Adragna NC, Lauf PK, Sun D, Delpire E. K-Cl cotransporters, cell volume homeostasis, and neurological disease. Trends Mol Med 21: 513–523, 2015. doi: 10.1016/j.molmed.2015.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Khamaysi A, Anbtawee-Jomaa S, Fremder M, Eini-Rider H, Shimshilashvili L, Aharon S, Aizenshtein E, Shlomi T, Noguchi A, Springer D, Moe OW, Shcheynikov N, Muallem S, Ohana E. Systemic succinate homeostasis and local succinate signaling affect blood pressure and modify risks for calcium oxalate lithogenesis. J Am Soc Nephrol 30: ASN.2018030277, 2019. doi: 10.1681/ASN.2018030277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kunishima N, Shimada Y, Tsuji Y, Sato T, Yamamoto M, Kumasaka T, Nakanishi S, Jingami H, Morikawa K. Structural basis of glutamate recognition by a dimeric metabotropic glutamate receptor. Nature 407: 971–977, 2000. doi: 10.1038/35039564. [DOI] [PubMed] [Google Scholar]
- 53.Kunzelmann K, Ousingsawat J, Benedetto R, Cabrita I, Schreiber R. Contribution of anoctamins to cell survival and cell death. Cancers (Basel) 11: 382, 2019. doi: 10.3390/cancers11030382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kusama N, Harding AM, Benson CJ. Extracellular chloride modulates the desensitization kinetics of acid-sensing ion channel 1a (ASIC1a). J Biol Chem 285: 17425–17431, 2010. doi: 10.1074/jbc.M109.091561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lai ZF, Chen YZ, Nishi K. Modulation of intracellular Cl− homeostasis by lectin-stimulation in Jurkat T lymphocytes. Eur J Pharmacol 482: 1–8, 2003. doi: 10.1016/S0014-2999(03)02076-4. [DOI] [PubMed] [Google Scholar]
- 56.Lee MG, Ohana E, Park HW, Yang D, Muallem S. Molecular mechanism of pancreatic and salivary gland fluid and secretion. Physiol Rev 92: 39–74, 2012. doi: 10.1152/physrev.00011.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li H, Salomon JJ, Sheppard DN, Mall MA, Galietta LJ. Bypassing CFTR dysfunction in cystic fibrosis with alternative pathways for anion transport. Curr Opin Pharmacol 34: 91–97, 2017. doi: 10.1016/j.coph.2017.10.002. [DOI] [PubMed] [Google Scholar]
- 58.Li Q, Luo X, Muallem S. Regulation of the P2X7 receptor permeability to large molecules by extracellular Cl− and Na+. J Biol Chem 280: 26922–26927, 2005. doi: 10.1074/jbc.M504966200. [DOI] [PubMed] [Google Scholar]
- 59.Li X, Surguchev A, Bian S, Navaratnam D, Santos-Sacchi J. Extracellular chloride regulation of Kv2.1, contributor to the major outward Kv current in mammalian outer hair cells. Am J Physiol Cell Physiol 302: C296–C306, 2012. doi: 10.1152/ajpcell.00177.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Masuyer G, Yates CJ, Sturrock ED, Acharya KR. Angiotensin-I converting enzyme (ACE): structure, biological roles, and molecular basis for chloride ion dependence. Biol Chem 395: 1135–1149, 2014. doi: 10.1515/hsz-2014-0157. [DOI] [PubMed] [Google Scholar]
- 61.Miyazaki H, Shiozaki A, Niisato N, Marunaka Y. Physiological significance of hypotonicity-induced regulatory volume decrease: reduction in intracellular Cl− concentration acting as an intracellular signaling. Am J Physiol Renal Physiol 292: F1411–F1417, 2007. doi: 10.1152/ajprenal.00244.2006. [DOI] [PubMed] [Google Scholar]
- 62.Natesh R, Schwager SL, Sturrock ED, Acharya KR. Crystal structure of the human angiotensin-converting enzyme-lisinopril complex. Nature 421: 551–554, 2003. doi: 10.1038/nature01370. [DOI] [PubMed] [Google Scholar]
- 63.Niisato N, Eaton DC, Marunaka Y. Involvement of cytosolic Cl− in osmoregulation of α-ENaC gene expression. Am J Physiol Renal Physiol 287: F932–F939, 2004. doi: 10.1152/ajprenal.00131.2004. [DOI] [PubMed] [Google Scholar]
- 64.North RA. Molecular physiology of P2X receptors. Physiol Rev 82: 1013–1067, 2002. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- 65.Ogawa H, Qiu Y, Philo JS, Arakawa T, Ogata CM, Misono KS. Reversibly bound chloride in the atrial natriuretic peptide receptor hormone-binding domain: possible allosteric regulation and a conserved structural motif for the chloride-binding site. Protein Sci 19: 544–557, 2010. doi: 10.1002/pro.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ohana E, Shcheynikov N, Park M, Muallem S. Solute carrier family 26 member a2 (Slc26a2) protein functions as an electroneutral SOFormula/OH−/Cl− exchanger regulated by extracellular Cl−. J Biol Chem 287: 5122–5132, 2012. doi: 10.1074/jbc.M111.297192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ohana E, Yang D, Shcheynikov N, Muallem S. Diverse transport modes by the solute carrier 26 family of anion transporters. J Physiol 587: 2179–2185, 2009. doi: 10.1113/jphysiol.2008.164863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ohsawa R, Miyazaki H, Niisato N, Shiozaki A, Iwasaki Y, Otsuji E, Marunaka Y. Intracellular chloride regulates cell proliferation through the activation of stress-activated protein kinases in MKN28 human gastric cancer cells. J Cell Physiol 223: 764–770, 2010. doi: 10.1002/jcp.22088. [DOI] [PubMed] [Google Scholar]
- 69.Okabe A, Yokokura M, Toyoda H, Shimizu-Okabe C, Ohno K, Sato K, Fukuda A. Changes in chloride homeostasis-regulating gene expressions in the rat hippocampus following amygdala kindling. Brain Res 990: 221–226, 2003. doi: 10.1016/S0006-8993(03)03528-5. [DOI] [PubMed] [Google Scholar]
- 70.Pacheco-Alvarez D, Cristóbal PS, Meade P, Moreno E, Vazquez N, Muñoz E, Díaz A, Juárez ME, Giménez I, Gamba G. The Na+:Cl− cotransporter is activated and phosphorylated at the amino-terminal domain upon intracellular chloride depletion. J Biol Chem 281: 28755–28763, 2006. doi: 10.1074/jbc.M603773200. [DOI] [PubMed] [Google Scholar]
- 71.Park HW, Nam JH, Kim JY, Namkung W, Yoon JS, Lee JS, Kim KS, Venglovecz V, Gray MA, Kim KH, Lee MG. Dynamic regulation of CFTR bicarbonate permeability by [Cl−]i and its role in pancreatic bicarbonate secretion. Gastroenterology 139: 620–631, 2010. doi: 10.1053/j.gastro.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 72.Park M, Ohana E, Choi SY, Lee MS, Park JH, Muallem S. Multiple roles of the /Cl−/OH− exchanger protein Slc26a2 in chondrocyte functions. J Biol Chem 289: 1993–2001, 2014. doi: 10.1074/jbc.M113.503466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Park MK, Lomax RB, Tepikin AV, Petersen OH. Local uncaging of caged Ca2+ reveals distribution of Ca2+-activated Cl− channels in pancreatic acinar cells. Proc Natl Acad Sci USA 98: 10948–10953, 2001. doi: 10.1073/pnas.181353798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Park S, Shcheynikov N, Hong JH, Zheng C, Suh SH, Kawaai K, Ando H, Mizutani A, Abe T, Kiyonari H, Seki G, Yule D, Mikoshiba K, Muallem S. Irbit mediates synergy between Ca2+ and cAMP signaling pathways during epithelial transport in mice. Gastroenterology 145: 232–241, 2013. doi: 10.1053/j.gastro.2013.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Paulais M, Lachheb S, Teulon J. A Na+- and Cl− -activated K+ channel in the thick ascending limb of mouse kidney. J Gen Physiol 127: 205–215, 2006. doi: 10.1085/jgp.200509360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pedchenko V, Bauer R, Pokidysheva EN, Al-Shaer A, Forde NR, Fidler AL, Hudson BG, Boudko SP. A chloride ring is an ancient evolutionary innovation mediating the assembly of the collagen IV scaffold of basement membranes. J Biol Chem 294: 7968–7981, 2019. doi: 10.1074/jbc.RA119.007426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE. UCSF Chimera—a visualization system for exploratory research and analysis. J Comput Chem 25: 1605–1612, 2004. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- 78.Piala AT, Moon TM, Akella R, He H, Cobb MH, Goldsmith EJ. Chloride sensing by WNK1 involves inhibition of autophosphorylation. Sci Signal 7: ra41, 2014. doi: 10.1126/scisignal.2005050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Plested AJ. Kainate receptor modulation by sodium and chloride. Adv Exp Med Biol 717: 93–113, 2011. doi: 10.1007/978-1-4419-9557-5_9. [DOI] [PubMed] [Google Scholar]
- 80.Plested AJ, Mayer ML. Structure and mechanism of kainate receptor modulation by anions. Neuron 53: 829–841, 2007. doi: 10.1016/j.neuron.2007.02.025. [DOI] [PubMed] [Google Scholar]
- 81.Ponce-Coria J, San-Cristobal P, Kahle KT, Vazquez N, Pacheco-Alvarez D, de Los Heros P, Juárez P, Muñoz E, Michel G, Bobadilla NA, Gimenez I, Lifton RP, Hebert SC, Gamba G. Regulation of NKCC2 by a chloride-sensing mechanism involving the WNK3 and SPAK kinases. Proc Natl Acad Sci USA 105: 8458–8463, 2008. doi: 10.1073/pnas.0802966105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Potter LR, Yoder AR, Flora DR, Antos LK, Dickey DM. Natriuretic peptides: their structures, receptors, physiologic functions and therapeutic applications. Handb Exp Pharmacol 191: 341–366, 2009. doi: 10.1007/978-3-540-68964-5_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rahmati N, Hoebeek FE, Peter S, De Zeeuw CI. Chloride homeostasis in neurons with special emphasis on the olivocerebellar system: differential roles for transporters and channels. Front Cell Neurosci 12: 101, 2018. doi: 10.3389/fncel.2018.00101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Raimondo JV, Joyce B, Kay L, Schlagheck T, Newey SE, Srinivas S, Akerman CJ. A genetically-encoded chloride and pH sensor for dissociating ion dynamics in the nervous system. Front Cell Neurosci 7: 202, 2013. doi: 10.3389/fncel.2013.00202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Roessler M, Müller V. Chloride, a new environmental signal molecule involved in gene regulation in a moderately halophilic bacterium, Halobacillus halophilus. J Bacteriol 184: 6207–6215, 2002. doi: 10.1128/JB.184.22.6207-6215.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Romanenko VG, Catalán MA, Brown DA, Putzier I, Hartzell HC, Marmorstein AD, Gonzalez-Begne M, Rock JR, Harfe BD, Melvin JE. Tmem16A encodes the Ca2+-activated Cl− channel in mouse submandibular salivary gland acinar cells. J Biol Chem 285: 12990–13001, 2010. doi: 10.1074/jbc.M109.068544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Salhany JM, Sloan RL, Cordes KS. The carboxyl side chain of glutamate 681 interacts with a chloride binding modifier site that allosterically modulates the dimeric conformational state of band 3 (AE1). Implications for the mechanism of anion/proton cotransport. Biochemistry 42: 1589–1602, 2003. doi: 10.1021/bi0205294. [DOI] [PubMed] [Google Scholar]
- 88.Semyanov A. Spatiotemporal pattern of calcium activity in astrocytic network. Cell Calcium 78: 15–25, 2019. doi: 10.1016/j.ceca.2018.12.007. [DOI] [PubMed] [Google Scholar]
- 89.Shcheynikov N, Kim KH, Kim KM, Dorwart MR, Ko SB, Goto H, Naruse S, Thomas PJ, Muallem S. Dynamic control of cystic fibrosis transmembrane conductance regulator Cl−/ selectivity by external Cl−. J Biol Chem 279: 21857–21865, 2004. doi: 10.1074/jbc.M313323200. [DOI] [PubMed] [Google Scholar]
- 90.Shcheynikov N, Son A, Hong JH, Yamazaki O, Ohana E, Kurtz I, Shin DM, Muallem S. Intracellular Cl− as a signaling ion that potently regulates Na+/- transporters. Proc Natl Acad Sci USA 112: E329–E337, 2015. doi: 10.1073/pnas.1415673112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Shekarabi M, Zhang J, Khanna AR, Ellison DH, Delpire E, Kahle KT. WNK kinase signaling in ion homeostasis and human disease. Cell Metab 25: 285–299, 2017. doi: 10.1016/j.cmet.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 92.Shen XZ, Billet S, Lin C, Okwan-Duodu D, Chen X, Lukacher AE, Bernstein KE. The carboxypeptidase ACE shapes the MHC class I peptide repertoire. Nat Immunol 12: 1078–1085, 2011. doi: 10.1038/ni.2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Shen XZ, Li P, Weiss D, Fuchs S, Xiao HD, Adams JA, Williams IR, Capecchi MR, Taylor WR, Bernstein KE. Mice with enhanced macrophage angiotensin-converting enzyme are resistant to melanoma. Am J Pathol 170: 2122–2134, 2007. doi: 10.2353/ajpath.2007.061205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Shirakabe K, Priori G, Yamada H, Ando H, Horita S, Fujita T, Fujimoto I, Mizutani A, Seki G, Mikoshiba K. IRBIT, an inositol 1,4,5-trisphosphate receptor-binding protein, specifically binds to and activates pancreas-type Na+/- cotransporter 1 (pNBC1). Proc Natl Acad Sci USA 103: 9542–9547, 2006. doi: 10.1073/pnas.0602250103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Skeggs LT Jr, Kahn JR, Shumway NP. The preparation and function of the hypertension-converting enzyme. J Exp Med 103: 295–299, 1956. doi: 10.1084/jem.103.3.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Succol F, Fiumelli H, Benfenati F, Cancedda L, Barberis A. Intracellular chloride concentration influences the GABAA receptor subunit composition. Nat Commun 3: 738, 2012. doi: 10.1038/ncomms1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sulis Sato S, Artoni P, Landi S, Cozzolino O, Parra R, Pracucci E, Trovato F, Szczurkowska J, Luin S, Arosio D, Beltram F, Cancedda L, Kaila K, Ratto GM. Simultaneous two-photon imaging of intracellular chloride concentration and pH in mouse pyramidal neurons in vivo. Proc Natl Acad Sci USA 114: E8770–E8779, 2017. doi: 10.1073/pnas.1702861114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Terker AS, Zhang C, Erspamer KJ, Gamba G, Yang CL, Ellison DH. Unique chloride-sensing properties of WNK4 permit the distal nephron to modulate potassium homeostasis. Kidney Int 89: 127–134, 2016. doi: 10.1038/ki.2015.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Terker AS, Zhang C, McCormick JA, Lazelle RA, Zhang C, Meermeier NP, Siler DA, Park HJ, Fu Y, Cohen DM, Weinstein AM, Wang WH, Yang CL, Ellison DH. Potassium modulates electrolyte balance and blood pressure through effects on distal cell voltage and chloride. Cell Metab 21: 39–50, 2015. doi: 10.1016/j.cmet.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Theilig F, Wu Q. ANP-induced signaling cascade and its implications in renal pathophysiology. Am J Physiol Renal Physiol 308: F1047–F1055, 2015. doi: 10.1152/ajprenal.00164.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tora AS, Rovira X, Cao AM, Cabayé A, Olofsson L, Malhaire F, Scholler P, Baik H, Van Eeckhaut A, Smolders I, Rondard P, Margeat E, Acher F, Pin JP, Goudet C. Chloride ions stabilize the glutamate-induced active state of the metabotropic glutamate receptor 3. Neuropharmacology 140: 275–286, 2018. doi: 10.1016/j.neuropharm.2018.08.011. [DOI] [PubMed] [Google Scholar]
- 102.Tora AS, Rovira X, Dione I, Bertrand HO, Brabet I, De Koninck Y, Doyon N, Pin JP, Acher F, Goudet C. Allosteric modulation of metabotropic glutamate receptors by chloride ions. FASEB J 29: 4174–4188, 2015. doi: 10.1096/fj.14-269746. [DOI] [PubMed] [Google Scholar]
- 103.Vachel L, Shcheynikov N, Yamazaki O, Fremder M, Ohana E, Son A, Shin DM, Yamazaki-Nakazawa A, Yang CR, Knepper MA, Muallem S. Modulation of Cl− signaling and ion transport by recruitment of kinases and phosphatases mediated by the regulatory protein IRBIT. Sci Signal 11: eaat5018, 2018. doi: 10.1126/scisignal.aat5018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Valdivieso AG, Clauzure M, Massip-Copiz M, Santa-Coloma TA. The chloride anion acts as a second messenger in mammalian cells: modifying the expression of specific genes. Cell Physiol Biochem 38: 49–64, 2016. doi: 10.1159/000438608. [DOI] [PubMed] [Google Scholar]
- 105.Valdivieso AG, Mori C, Clauzure M, Massip-Copiz M, Santa-Coloma TA. CFTR modulates RPS27 gene expression using chloride anion as signaling effector. Arch Biochem Biophys 633: 103–109, 2017. doi: 10.1016/j.abb.2017.09.014. [DOI] [PubMed] [Google Scholar]
- 106.van den Akker F, Zhang X, Miyagi M, Huo X, Misono KS, Yee VC. Structure of the dimerized hormone-binding domain of a guanylyl-cyclase-coupled receptor. Nature 406: 101–104, 2000. doi: 10.1038/35017602. [DOI] [PubMed] [Google Scholar]
- 107.Vuille-dit-Bille RN, Camargo SM, Emmenegger L, Sasse T, Kummer E, Jando J, Hamie QM, Meier CF, Hunziker S, Forras-Kaufmann Z, Kuyumcu S, Fox M, Schwizer W, Fried M, Lindenmeyer M, Gotze O, and Verrey F. Human intestine luminal ACE2 and amino acid transporter expression increased by ACE-inhibitors. Amino Acids 47: 693–705, 2015. doi: 10.1007/s00726-014-1889-6. [DOI] [PubMed] [Google Scholar]
- 108.Wilson FH, Disse-Nicodème S, Choate KA, Ishikawa K, Nelson-Williams C, Desitter I, Gunel M, Milford DV, Lipkin GW, Achard JM, Feely MP, Dussol B, Berland Y, Unwin RJ, Mayan H, Simon DB, Farfel Z, Jeunemaitre X, Lifton RP. Human hypertension caused by mutations in WNK kinases. Science 293: 1107–1112, 2001. doi: 10.1126/science.1062844. [DOI] [PubMed] [Google Scholar]
- 109.Wright AM, Gong X, Verdon B, Linsdell P, Mehta A, Riordan JR, Argent BE, Gray MA. Novel regulation of cystic fibrosis transmembrane conductance regulator (CFTR) channel gating by external chloride. J Biol Chem 279: 41658–41663, 2004. doi: 10.1074/jbc.M405517200. [DOI] [PubMed] [Google Scholar]
- 110.Wu M, Heneghan JF, Vandorpe DH, Escobar LI, Wu BL, Alper SL. Extracellular Cl− regulates human /anion exchanger SLC26A1 by altering pH sensitivity of anion transport. Pflügers Arch 468: 1311–1332, 2016. doi: 10.1007/s00424-016-1823-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wu QQ, Liu XY, Xiong LX, Shang JY, Mai XY, Pang RP, Su YX, Yu BX, Yuan JN, Yang C, Wang YL, Zhou P, Lv XF, Liu J, Zhou JG, Liang SJ. Reduction of intracellular chloride concentration promotes foam cell formation. Circ J 80: 1024–1033, 2016. doi: 10.1253/circj.CJ-15-1209. [DOI] [PubMed] [Google Scholar]
- 112.Xie Y, Schafer JA. Inhibition of ENaC by intracellular Cl− in an MDCK clone with high ENaC expression. Am J Physiol Renal Physiol 287: F722–F731, 2004. doi: 10.1152/ajprenal.00135.2004. [DOI] [PubMed] [Google Scholar]
- 113.Xu B, English JM, Wilsbacher JL, Stippec S, Goldsmith EJ, Cobb MH. WNK1, a novel mammalian serine/threonine protein kinase lacking the catalytic lysine in subdomain II. J Biol Chem 275: 16795–16801, 2000. doi: 10.1074/jbc.275.22.16795. [DOI] [PubMed] [Google Scholar]
- 114.Yamada J, Okabe A, Toyoda H, Kilb W, Luhmann HJ, Fukuda A. Cl− uptake promoting depolarizing GABA actions in immature rat neocortical neurones is mediated by NKCC1. J Physiol 557: 829–841, 2004. doi: 10.1113/jphysiol.2004.062471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yamaguchi M, Steward MC, Smallbone K, Sohma Y, Yamamoto A, Ko SB, Kondo T, Ishiguro H. Bicarbonate-rich fluid secretion predicted by a computational model of guinea-pig pancreatic duct epithelium. J Physiol 595: 1947–1972, 2017. doi: 10.1113/JP273306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yang D, Li Q, So I, Huang CL, Ando H, Mizutani A, Seki G, Mikoshiba K, Thomas PJ, Muallem S. IRBIT governs epithelial secretion in mice by antagonizing the WNK/SPAK kinase pathway. J Clin Invest 121: 956–965, 2011. doi: 10.1172/JCI43475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Yoder N, Gouaux E. Divalent cation and chloride ion sites of chicken acid sensing ion channel 1a elucidated by X-ray crystallography. PLoS One 13: e0202134, 2018. [Erratum in PLoS One 13: e0209147, 2018.] doi: 10.1371/journal.pone.0202134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yuan A, Dourado M, Butler A, Walton N, Wei A, Salkoff L. SLO-2, a K+ channel with an unusual Cl− dependence. Nat Neurosci 3: 771–779, 2000. doi: 10.1038/77670. [DOI] [PubMed] [Google Scholar]
- 119.Zhang YL, Chen PX, Guan WJ, Guo HM, Qiu ZE, Xu JW, Luo YL, Lan CF, Xu JB, Hao Y, Tan YX, Ye KN, Lun ZR, Zhao L, Zhu YX, Huang J, Ko WH, Zhong WD, Zhou WL, Zhong NS. Increased intracellular Cl− concentration promotes ongoing inflammation in airway epithelium. Mucosal Immunol 11: 1149–1157, 2018. doi: 10.1038/s41385-018-0013-8. [DOI] [PubMed] [Google Scholar]
- 120.Zhang Z, Liu F, Chen J. Conformational changes of CFTR upon phosphorylation and ATP binding. Cell 170: 483–491.e8, 2017. doi: 10.1016/j.cell.2017.06.041. [DOI] [PubMed] [Google Scholar]
- 121.Zhao H, Muallem S. Na+, K+, and Cl− transport in resting pancreatic acinar cells. J Gen Physiol 106: 1225–1242, 1995. doi: 10.1085/jgp.106.6.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]