Abstract
Fluorescence recovery after photobleaching (FRAP) has been useful in delineating cardiac myofilament biology, and innovations in fluorophore chemistry have expanded the array of microscopic assays used. However, one assumption in FRAP is the irreversible photobleaching of fluorescent proteins after laser excitation. Here we demonstrate reversible photobleaching regarding the photoconvertible fluorescent protein mEos3.2. We used CRISPR/Cas9 genome editing in human induced pluripotent stem cells (hiPSCs) to knock-in mEos3.2 into the COOH terminus of titin to visualize sarcomeric titin incorporation and turnover. Upon cardiac induction, the titin-mEos3.2 fusion protein is expressed and integrated in the sarcomeres of hiPSC-derived cardiomyocytes (CMs). STORM imaging shows M-band clustered regions of bound titin-mEos3.2 with few soluble titin-mEos3.2 molecules. FRAP revealed a baseline titin-mEos3.2 fluorescence recovery of 68% and half-life of ~1.2 h, suggesting a rapid exchange of sarcomeric titin with soluble titin. However, paraformaldehyde-fixed and permeabilized titin-mEos3.2 hiPSC-CMs surprisingly revealed a 55% fluorescence recovery. Whole cell FRAP analysis in paraformaldehyde-fixed, cycloheximide-treated, and untreated titin-mEos3.2 hiPSC-CMs displayed no significant differences in fluorescence recovery. FRAP in fixed HEK 293T expressing cytosolic mEos3.2 demonstrates a 58% fluorescence recovery. These data suggest that titin-mEos3.2 is subject to reversible photobleaching following FRAP. Using a mouse titin-eGFP model, we demonstrate that no reversible photobleaching occurs. Our results reveal that reversible photobleaching accounts for the majority of titin recovery in the titin-mEos3.2 hiPSC-CM model and should warrant as a caution in the extrapolation of reliable FRAP data from specific fluorescent proteins in long-term cell imaging.
Keywords: FRAP, hiPSC-CM, mEos3.2, sarcomere, titin
INTRODUCTION
During the average human life span, the heart beats over 2.5 billion times (22). This remarkable feat is accomplished by terminally differentiated mechanically contracting cardiac myocytes. In turn, cardiomyocyte contraction at the subcellular level is orchestrated by a highly complex and integrated protein system known as the sarcomere, where force is generated by bidirectional sliding of thin actin filaments past the thick myosin filaments. To maintain functional sarcomeres, it is assumed that precise turnover of proteins is required that balances protein synthesis and incorporation into the sarcomere with removal and degradation of worn out or damaged proteins. With the use of radioisotopes, the protein half-lives have been estimated for myosin heavy chain (5.4 days), actin (10 days), troponin T (3.5 days), troponin I (3.2 days), and troponin C (5.3 days) in the adult rat heart (10, 25). These long protein half-lives supported the early notion that the sarcomere was a static structure with proteins turning over at their respective rates.
Fluorescence recovery after photobleaching (FRAP) is a commonly used microscopy technique to quantify fluorescently tagged protein dynamics in living cells (33). Specifically, in the heart, FRAP has allowed investigators to examine the dynamic state of the sarcomere in real-time. In neonatal ventricular myocytes, α-actin and β-actin have exchange half-lives on the order of minutes (37) whereas β-myosin heavy chain has an exchange half-life of 32 min in adult rat cardiomyocytes (43). Cardiac troponin T and C have exchange half-lives on the order of minutes in mouse embryonic cardiomyocytes (41). Collectively, these observations suggest that sarcomere protein components may in fact recycle among myofibrils either through a pool of sarcomeric (bound) and nonsarcomeric (unbound) molecules. This is particularly germane to titin, an enormous (up to 4 mDa) structural component of the thick filament spanning the half-sarcomere.
Titin serves as a molecular scaffold for sarcomere assembly, maintenance, and function (23, 39). With its NH2-terminal domain anchored at the sarcomeric Z line and its COOH-terminal domain at the M band, titin also functions to center the thick filament between the thick filaments and to stabilize relative positions of the two filaments to prevent overstretch. In addition, titin possesses a bidirectional spring domain responsible for most of the sarcomere’s elastic recoil (14, 16, 24). Given titin’s vital importance in sarcomere structure and function, surprisingly little is known about the dynamics of titin turnover in a continuously contracting cardiomyocyte.
With multiple isoforms ranging in molecular weights of 2.97–3.7 mDa, modeling titin dynamics has been problematic. To study sarcomere turnover in situ, da Silva Lopes et al. (8) created a titin-eGFP knock-in mouse model to isolate neonatal mouse cardiomyocytes and study titin-eGFP mobility using FRAP. They reported that the exchange half-life for fluorescent titin recovery in the sarcomere was 2.1 h, which was unexpected given that the molecular protein half-life of titin is 72 h by pulse-chase experiments (19). They concluded that the sarcomere is a dynamic rather than a static structure with rapid exchange between a pool of sarcomeric (bound) and nonsarcomeric (unbound) titin molecules.
The reprogramming of adult somatic cells into induced pluripotent stem cells has revolutionized biomedical research (38). With standard protocols available coupled to emerging technologies (i.e., (Matrigel Mattress, microcontact printing), human induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CMs) are increasingly being valued as a useful model and surrogate for human CM studies (3, 11, 34). Complemented with the versatility of CRISPR/Cas9 gene editing, one can generate genetically modified hiPSC-CMs for research (32). To more closely examine the dynamics of sarcomeric titin turnover, in situ, we used the CRISPR/Cas9 genome editing technology to generate the first-of-its-kind titin fused photoconvertible fluorescent protein to directly track titin incorporation and turnover in sarcomeres of live, beating hiPSC-CMs. We used our model to test the hypothesis that titin is a dynamic and mobile protein in hiPSC-CMs.
MATERIALS AND METHODS
Institutional Review Board Statement.
A human iPSC line was generated from a healthy control subject using an episomal approach and validated, as we have previously described (7). All protocols were approved by the Vanderbilt University Institutional Review Board, and written informed consent was provided.
hiPSC reprograming.
Dermal fibroblasts were isolated from a 2-mm dermal punch biopsy from a 25-yr-old healthy male volunteer with no history of heart disease. Fibroblasts from the second passage were reprogrammed into hiPSCs using electroporation delivery (Neon) of nonintegrating episomal vectors (Epi5 Episomal iPSC Reprogramming Kit; ThermoFisher). After episomal delivery, fibroblasts were plated on a standard cell culture grade 10-cm plate. iPSC-like colonies were manually moved to hES-grade Matrigel (Corning)-coated 24-well plates between days 14 and 30 postelectroporation. Individual colonies were expanded for validation and line maintenance.
hiPSC validation: stem cell markers.
hiPSCs were validated by the presence of stem cell-associated markers via immunostain for OCT4 (no. 2750; Cell Signaling), SSEA4 (MC-813-70; Developmental Studies Hybridoma Bank), SSEA3 (MAB4303; Millipore), TRA-1-60 (MAB4360; Millipore). Secondary antibodies, Alexa Fluor 488-labeled goat anti-mouse IgG (A-11029; ThermoFisher Scientific), Alexa Fluor 488-labeled goat anti-rat IgG (A-11006; ThermoFisher), and Alexa Fluor 568-labeled goat anti-rabbit IgG (A-11036; ThermoFisher), were used to visualize specimens. The presence of stem cell-associated alkaline phosphatase was detected by commercially available colorimetric assay (00-0055; Stemgent).
hiPSC validation: pluripotency.
hiPSCs were grown in suspension on low adherence plates in DMEM/F-12 w/GlutamaX (10565; Invitrogen) supplemented with 20% knockout serum replacement (10828-028; Invitrogen), 1% nonessential amino acids (M7145; Sigma), and 1% penicillin-streptomycin (15140122; MediaTech). On day 5 of differentiation, individual embryoid bodies were replated on growth factor-reduced Matrigel (Corning) for 14 days in media listed above. The presence of cells in the endoderm, mesoderm, and ectoderm lineages was visualized by immunofluorescent detection of proteins associated with each of the three germ layers: GATA4 (ab84593; Abcam) for endoderm, α-smooth muscle actin (ab7817; Abcam) for mesoderm, and βIII-tubulin (MAB1637f; Millipore) for ectoderm. Secondary antibodies, Alexa Fluor 488-labeled goat anti-mouse IgG (A-11029; ThermoFisher Scientific), and Alexa 568-labeled goat anti-rabbit IgG (A-11036; ThermoFisher), were used to visualize specimens. An Olympus IX81 inverted fluorescent microscope was used to capture images.
hiPSC validation: chromosomal assessment.
Twenty hiPSCs were screened for large chromosomal abnormalities by commercial karyotype analysis (Genetics Associates, Nashville, TN).
hiPSC maintenance.
hiPSCs were maintained as described in Feaster et al. (11). Briefly, hiPSCs were on cultured on growth factor-reduced Matrigel (Corning)-coated plates (1:200 dilution, DMEM/F-12) in mTeSR1 medium (StemCell Technologies). Cells were passaged every 4 days using 0.5 mM EDTA (Life Technologies) in D-PBS without CaCl2 or MgCl2 (Life Technologies). The 10-μM Rho kinase inhibitor Y-27632 (CalBiochem) was added for the first 24 h after passaging. Cells were maintained at 37°C, with 5% CO2 and 5% O2.
Guide RNA design.
The human titin exon 363 sequence was uploaded into the CRISPR Design Tool (https://zlab.bio/guide-design-resources) for guide (g)RNA generation. Guide sequence 5′-TCTGTACGTCCATGATGATC-3′ was cloned into pSpCas9(BB)-2A-Puro (PX459) (gift from Feng Zhang, Addgene plasmid no. 4813). Sanger sequencing confirmed correct gRNA insertion.
T7 endonuclease assay.
HEK 293 cells were transiently transfected via lipofectamine delivery with 1 μg of PX459 plasmid encoding the titin gRNA and Cas9 for 36 h. HEK 293 cells were then subjected to antibiotic selection (1 μg/mL ampicillin) for 48 h. HEK 293 cells were then harvested for DNA extraction using QuickExtract solution (QE0905T; Epicenter). Indels were detected using GeneArt Genomic Cleavage Detection Kit (A24372; Invitrogen) per manufacturer’s protocol. A 423-bp loci where the double-stranded breaks occurred was PCR amplified using left primer sequence 5′-AGCAGGCATAAGAGGTGAGC-3′ and right primer sequence 5′-CAGTGGCAGAGTCAGATCCA-3′. DNA was run on 2% agarose gel and was visualized by ethidium bromide staining. Percent cleavage [(sum of cleaved band intensities/total band intensities) × 100] was quantified by band densitometry.
CRISPR/Cas9 gene editing.
hiPSCs were dissociated with electroporated with 10 μg of gRNA and 500 ng of titin mEos3.2 homology-directed repair template. Cells were plated on 1:200 Matrigel-coated plates with in mTesSR1 with 10 μM Y-27632 for 24 h. Media were changed after 24 h to remove Y-27632. At 36 h postelectroporation, cells were subjected to puromycin selection at 500 ng/mL for 48 h. Individual colonies were picked and expanded for screening. The hiPSC individual colonies were screened for mEos3.2 insertion by PCR using primers flanking the outside of the repair template (right primer sequence 5′-CAGCGTAAAATGAGCGAACA-3′ and left primer sequence 5′-TGCAAGGAAGCTTCTCGTCT-3′). mEos3.2 was confirmed via Sanger sequencing.
hiPSC cardiac differentiation and culture.
Cardiac induction was achieved by small molecule delivery as previously described (11). Briefly, at day 0 hiPSCs were switched from mTeSR1 medium to RPMI 1640 (11875; Life Technologies) supplemented with B27 minus insulin (A1895601; Life Technologies) and 6 μM CHIR99021 (Selleck Chemicals) for 2 days. At day 2, media were changed to RPMI 1640 with 2% B27 minus insulin. At day 3, media wre changed to RPMI 1640 with B27 minus insulin and 5 μM IWR-1 (Sigma) for 2 days. At day 5, medium was changed to RPMI 1640 with 2% B27 minus insulin for 2 days and changed every other day until day 10. At day 10, media were changed to RPMI 1640, no glucose (11879; Life Technologies) for 2 days and changed every other day until day 16. At day 16, medium was changed to RPMI 1640 with 2% B27 complete (17504044; Life Technologies) with 1% penicillin-streptomycin (15140122; Gibco) for 2 days and changed every other day until day 30.
hiPSC-CM dissociation.
Day-30 hiPSC-CMs were washed with PBS without CaCl2 and MgCl2 and dissociated with TrypLE Express (12604013; ThermoFisher Scientific) for 15 min at 37°C. Single hiPSC-CMs were replated on Growth Factor Reduced Matrigel (Corning) (1:200 dilution in DMEM/F-12)-coated MatTek) dishes (P35G-1.5-14-C; MatTek) with phenol free RPMI 1640 with 2% B27 complete and 1% penicillin-streptomycin.
Video-based edge detection.
Day-30 control or titin-mEos3.2 hiPSC-CMs were dissociated as described above and replated on the Matrigel Mattress for 5 days as in Feaster et al. (11). Cellular shortening was assessed by video edge-based detection as in Feaster et al. (11).
Drug treatments.
Day-30 titin-mEos3.2 hiPSC-CMs were treated with 0.1% DMSO (Sigma) and 10 μg/mL cycloheximide (CHX; Sigma), 1 h before and throughout image acquisition.
Protein synthesis assay.
Global protein synthesis was detected via the Protein Synthesis Assay kit (Cayman Chemicals) per manufacturer’s protocol. Briefly, day 30 hiPSC-CMs were plated on a 1:200 Matrigel-coated 96-well plate. Cells were pretreated with vehicle or CHX (10 μg/mL) for 30 min in culture. Cells were then treated with a metabolic probe O-propargyl-puromycin (OPP), OPP + CHX, or no OPP for 1 h in culture. Cells were then fixed and treated with 5-FAM-Azide for OPP probe detection. Fluorescence was detected (excitation/emission: 485/535 nm) via Promega Glomax reader.
HEK 293T cell transfection.
HEK 293T cells were maintained in DMEM/F-12 (11330-032; ThermoFisher) and 10% FBS (16140-071; ThermoFisher) on MatTek dishes (P35G-0-14-C; MatTek) coated with 0.1% gelatin. Cells were transfected 2 μg pBABE or 2 μg mEos3.2-C1 GFP using Lipofectamine 2000 (11668-027; ThermoFisher) per manufacturer’s instruction. Addgene plasmid no. 54550 was a gift from Michael Davidson. pBABE GFP was a gift from Young-Jae Nam.
Mouse neonatal cardiomyocyte isolation.
All experiments using animal models were approved by Institutional Animal Care and Use Committee at Vanderbilt University Medical Center. Mice expressing titin-eGFP were a gift from Michael Gotthardt. Neonatal mouse cardiomyocytes expressing titin-eGFP were isolated, plated, and cultured as previously described in da Silva Lopes et al. (8). Briefly, the titin-eGFP mouse model harbors an eGFP reporter replacing the stop codon at titin’s M-band exon 6. Six-week-old mice homozygous titin-eGFP mice were bred. The mouse background is 129/S6. Eight neonatal mouse pups were harvested at postnatal day 2 using the Cellutron NEOMYT KIT (NC9073658; ThermoFisher) per manufacturer’s instruction. Neonatal pups were placed on a 10-cm plastic petri dish on ice to be anesthetized via hypothermia followed by decapitation using a sharp scissor. The pups were euthanized in accordance with IACUC.SOP.AWEL.12-Euthanasia, Anesthesia, and Analgesia of Rodent Fetuses and Neonates. Neonatal mouse titin-eGFP cardiomyocytes were fixed and permeabilized as described below. FRAP experiments were carried out as described below using RPMI 1640 without phenol red with 2% B27 complete (17504044; Life Technologies) with 1% penicillin-streptomycin (15140122; Gibco).
hiPSC-CM immunocytochemistry.
Immunostaining of hiPSC-CMs was performed as previously described (11, 46). Briefly, hiPSC-CMs were fixed in 4% paraformaldehyde/PBS for 15 min at room temperature followed by permeabilization with 0.2% Triton X-100 (Sigma) for 15 min. Specimens were blocked in PBS with 1% BSA for 1 h at room temperature. Specimens were incubated with primary antibody myomesin (B4; University of Iowa Hybridoma Bank) or α-actinin (ab137346; Abcam) in PBS with 1% BSA at 1:100 dilution overnight at 4°C. Specimens were washed in PBS three times at 5-min intervals. Specimens were then incubated with PBS with 1% PSA containing fluorophore-conjugated secondary antibodies, Alexa 568-labeled goat anti-rabbit IgG (A-11036; ThermoFisher), and Alexa Fluor 568 goat anti-mouse IgG (A-11031; ThermoFisher), specific to the primary antibody IgG isotype (1:1,000 dilution) for 1 h in the dark at room temperature. Specimens were washed in PBS for 15 min three times before mounting with VectaShield (Vector Laboratories). Specimens were visualized using a Zeiss laser scanning LSM 710 META Inverted confocal microscope described below.
Titin-mEos3.2 hiPSC-CM, titin-eGFP NCM, and HEK 293T cell fixation and permeabilization.
Cells were fixed in 4% paraformaldehyde/PBS for 15 min at room temperature followed by permeabilization with 0.2% Triton X-100 (Sigma) for 15 min. Cells washed in PBS three times for 5 min. FRAP experiments were carried out as described below using RPMI 1640 without phenol red with 2% B27 complete (17504044; Life Technologies) with 1% penicillin-streptomycin (15140122; Gibco).
Fluorescence recovery after photobleaching.
FRAP experiments were conducted by using a Zeiss laser scanning LSM 710 META Inverted confocal microscope. Titin-mEos3.2 hiPSC-CMs on MatTek dishes were kept at 37°C and 5% CO2 using the ZEISS live cell system. For FRAP, a ×63/1.40 Plan-APOCHROMAT OIL objective was used with a pinhole set at 1.4 μm. Images were taken before and after photobleaching in the 488-nm channel at 4% laser power. Spectral detection was used to collect emission between 493 and 630 nm. A region of interest (ROI) of 2 sarcomeres was bleached by 100% laser power in the 488-nm channel for 20 iterations. FRAP images were acquired every 30 min for 9 h. Pixel intensities of the ROI, background, and whole cell were acquired using Zen software. Background was subtracted from ROI and whole cell measurements. The ROI was normalized to whole cell pixel intensities. Data were fitted into a one-phase association equation to calculate titin-mEos3.2 exchange half-life. Mobile fractions were calculated by (end recovered fluorescence intensity − postbleach fluorescence intensity/(prebleach fluorescence intensity − postbleach fluorescence intensity) × 100.
For whole cell FRAP, images were taken before and after photobleaching in the 488-nm channel at 4% laser power. A region encompassing the whole cell was bleached by 100% laser power in the 488-nm channel for 200 iterations. FRAP images were acquired every 30 min for 9 h. Pixel intensities of the ROI (2 sarcomeres) and background were acquired. Background was subtracted from ROI. Images were normalized to prebleach intensities. Data were fitted into a one-phase association. Mobile fractions were calculated as described above.
For FRAP in fixed and permeabilized HEK 293 cells expressing mEos3.2 or neonatal mouse titin-eGFP cardiomyocytes, images were taken before and after photobleaching in the 488-nm channel at 4% laser power. An ROI was bleached by 100% laser power in the 488-nm channel for 20 iterations. FRAP images were acquired every 30 min for 9 h. Background was subtracted from ROI and whole cell measurements. The ROI was normalized to whole cell pixel intensities. Data was fitted into a one-phase association. Mobile fractions were calculated as described above.
STORM imaging.
For stochastic optical reconstruction microscopy (STORM) imaging, titin-mEos3.2 hiPSC-CMs were fixed on MatTek dishes (no. 1.5) and immunostained for myomesin [detection with Alexa 647-conjugated goat anti-mouse IgG secondary antibody (A32728; ThermoFisher)]. Labeled cells were dried and imaged with fresh oxygen-scavenging buffer (10 mmol/L cysteamine, 0.5 mg/mL glucose oxidase, 40 μg/mL catalase, and 10% glucose in 50 mmol/L Tris with 10 mmol/L NaCl) as previously reported in Fu et al. (12). In brief, all STORM imaging was performed on a Nikon Eclipse Ti microscope with a ×100 1.49 NA TIRF objective and NIS Elements software. Images were acquired using lasers (488 and 647) from a self-contained four-line laser module with acousto-optic tunable filters and captured by a high-speed iXon DU897 Ultra EMCCD camera. With the use of the STORM module, 20,000 frames were acquired from each channel to generate both two-dimensional whole cell STORM images and three-dimensional projections of ROIs (2 sarcomeres) of titin-mEos3.2 and myomesin images at nanoscale resolution.
Statistical analysis.
All data displayed as means ± SE. Differences between two groups were assessed by a two-tailed Student’s t test. Differences between more than two groups were assessed by a one-way ANOVA with a Dunnett’s multiple comparison post hoc test. P < 0.05 was considered statistically significant. Statistical analysis was performed using GraphPad Prism 6.
RESULTS
Generation and validation of titin-mEos3.2 hiPSC model.
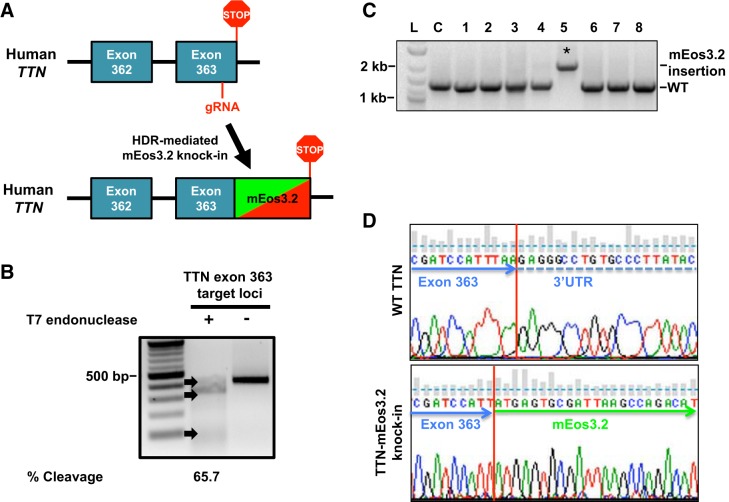
Briefly, dermal fibroblasts were isolated from a healthy male volunteer with no history of heart disease and reprogrammed into hiPSCs. Isolated control hiPSC colonies displayed stem cell morphology via the presence of pluripotent markers Oct4, SSEA4, SSEA3, and Tra-1–60 as well as exhibited alkaline phosphatase activity with no changes in genomic integrity (see Supplemental Fig. S1, A–C; All Supplemental materials is available at https://doi.org/10.6084/m9.figshare.9946871). Pluripotency was validated via an in vitro differentiation assay of hiPSC (Supplemental Fig. S1D). We next used CRISPR/Cas9 gene editing in hiPSC to replace the stop codon in titin’s terminal exon 363 with an in-frame knock-in of the photoconvertible fluorescent protein mEos3.2 (45) (Fig. 1A). We designed a gRNA to target titin COOH-terminal exon 363 for cleavage since NH2-terminal tagging of titin with GFP was shown to result in sarcomere disassembly (40). T7 endonuclease assay demonstrated gRNA cleavage activity (65.7%) in HEK 293 cells (Fig. 1B) Next, we electroporated hiPSCs with the titin-targeting gRNA and the mEos3.2 homology-directed repair template. Successful and correct genomic mEos3.2 integration was confirmed by screening single hiPSC colonies by PCR using primers that flanked outside the repair template (Fig. 1C). Sequence integration of mEos3.2 was confirmed by Sanger sequencing (Fig. 1D). The newly generated titin-mEos3.2 hiPSC-retained stem cell markers (Supplemental Fig. S2).
Fig. 1.
Generation of titin-mEos3.2 knock-in model in human induced pluripotent stem cells (hiPSCs). A: titin-mEos3.2 knock-in strategy to replace the stop codon in titin’s terminal exon 363 with an in frame mEos3.2 knock-in into titin’s terminal exon 363. A guide (g)RNA targeting titin exon 363 for cleavage was coelectroporated with a homology directed repair template (mEos3.2, with 600-bp titin flanking homology arms) in hiPSCs. B: gel image of T7 endonuclease assay demonstrates titin-specific gRNA targets exon 363 for cleavage in HEK 293 cells. Briefly, HEK 293 were transiently transfected with 1 μg of PX 459 plasmid encoding a titin-specific gRNA and Cas9 for 36 h. HEK 293 cells were then subjected to antibiotic selection (500 ng/mL, puromycin) for 48 h. HEK 293 cells were then harvested for DNA extraction. A 421-bp loci where the double-stranded breaks occurred was PCR amplified. The PCR product was denatured and reannealed before being subjected to T7 endonuclease. Lane 1: 100-bp ladder. Lane 2: treated with T7 endonuclease. Lane 3: not treated. DNA was visualized by ethidium bromide (EtBr) staining and %cleavage {[sum of cleaved band intensities/(total band intensities)] × 100} was quantified by band densitometry. C: gel image of mEos3.2 sequence integration in hiPSC. hiPSC were electroporated with 10 μg of gRNA and 500 ng of titin-mEos3.2 HDR template. Individual colonies hiPSC were screened for mEos3.2 insertion by PCR using primers flanking outside of the repair template. PCR products were ran on a 1% agarose gel and stained with EtBr. Lane L: 100-bp ladder. Lane C: control. Lanes 1–8: individual hiPSC clones. *mEos3.2 sequence integration. D: chromatogram of genomic sequences from hiPSC with and without mEos3.2 sequence integration. WT, wild type.
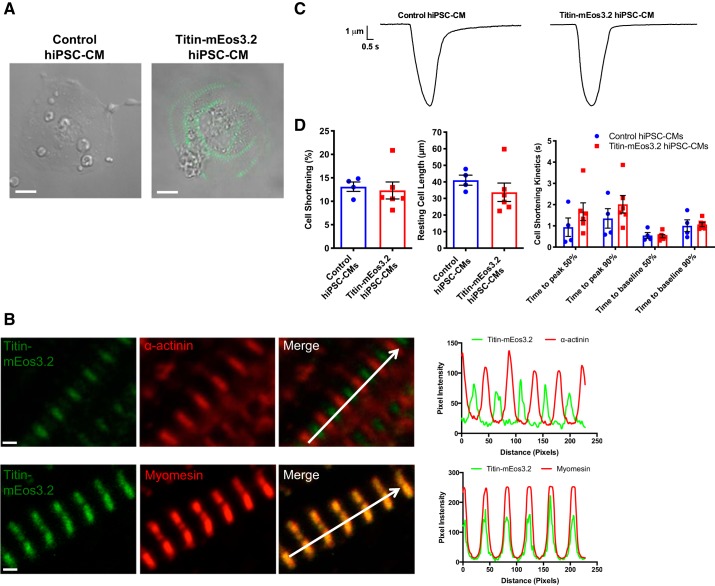
Cardiac differentiation of the titin-mEos3.2 hiPSC resulted in the expression of striated titin-mEos3.2 protein at the sarcomere, which was not detectable in the unedited control hiPSC-CMs by confocal microscopy (Fig. 2A). Our titin-mEos3.2 model labels all main titin isoforms with the exception of the novex isoforms (2, 5). Correct sarcomeric localization was confirmed by coimmunostaining for α-actinin (Z disk) and myomesin (M band) (Fig. 2B and Supplemental Fig. S3). The alternating signal peaks of α-actinin and colocalization peaks of myomesin confirmed proper titin-mEos3.2 integration at the sarcomere. We next determined whether the mEos3.2 tag affected cardiomyocyte function by assessing cell-shortening mechanics. We found no change in resting cell length and no differences in the contraction and relaxation kinetics in titin-mEos3.2 hiPSC-CMs when compared with controls (Fig. 2, C and D). Our cellular shortening percent is comparable to a previously reported ~9% cell shortening in control hiPSC-CMs (11). Our data support the claim that the mEos3.2 tag does not disrupt titin localization and sarcomere function.
Fig. 2.
Titin-mEos3.2 human induced pluripotent stem cell-cardiomyocytes (hiPSCs-CMs) express endogenous striated titin-mEos3.2 and are functional. A: live cell bright-field image overlaid with green fluorescence image of day 30 unedited control and titin-mEos3.2 hiPSC-CM. Scale bar = 10 μm. B: day-30 titin-mEos3.2 hiPSC-CMs were fixed and immunostained for Z-disk protein, α-actinin (red, top), or M-band protein, myomesin (red, bottom). Scale bar = 1 μm. Top: alternating bands of titin-mEos3.2 (green) with α-actinin (red). Bottom: colocalziation of myomesin (red) and titin-mEos3.2 (green). Right: corresponding pixel intensity along the white arrow in the merged images. Scale bar = 1 μm. C: representative contraction trace of control hiPSC-CMs and titin-mEos3.2 (unpaced). D: contractility evaluation of cell shortening (left) resting cell length (middle) and cell shortening kinetics in control (n = 6) and titin-mEos3.2 (n = 4) hiPSC-CMs.
Titin-mEos3.2 mobility in live hiPSC-CMs.
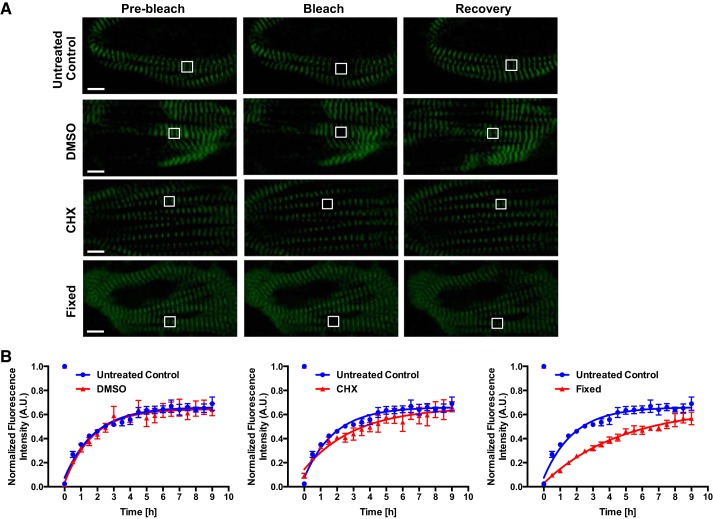
We assessed the baseline kinetics of endogenous titin-mEos3.2 exchange using day 30 hiPSC-CMs. As a metric for quality control, all cells imaged were spontaneously beating unless stated otherwise. We used FRAP to quantify titin-mEos3.2 mobility and exchange. At baseline, we found titin-mEos3.2’s fluorescence recovery reached a steady state within 9 h after photoablation. We found the titin-mEos3.2 fluorescence recovery was 68.1 ± 5.7% with a half-life of ~1.2 h (Fig. 3, A and B, and Table 1) in untreated control titin-mEos3.2 hiPSC-CMs. These results are consistent with those of da Silva Lopes et al. (8).
Fig. 3.
Titin protein dynamics in live and fixed human induced pluripotent stem cell-cardiomyocytes (hiPSCs-CMs). A: representative fluorescence recovery after photobleaching (FRAP) images (prebleach, bleach, recovery) of live and fixed day-30 hiPSC-CMs. Live hiPSC-CMs were treated with 10 μg/mL cycloheximide (CHX), 0.1% DMSO or untreated throughout image acquisition. Fixed hiPSC-CMs were treated with 4% PFA for 15 min and permeabilized with 0.5% Triton X-100 in PBS for 15 min before FRAP. A region of interest (ROI; white outlined box) of 2 sarcomere m-lines were bleached. Scale bar = 5 μm. B: quantification of FRAP images. FRAP images were acquired every 30 min for 9 h. Pixel intensities of the ROI, background, and whole cell were acquired. Background was subtracted from ROI and whole cell measurements. The ROI was normalized to whole cell pixel intensities. Data were fitted into a one-phase association equation to calculate titin-mEos3.2 exchange half-life; n = 4 cells for control and DMSO groups; n = 3 cells for CHX and fixed groups. All data are displayed as means ± SE. AU, arbitrary units.
Table 1.
Mobile fractions and exchange half-lives of titin-mEos3.2 hiPSC-CMs
| Treatment | Mobile Fraction % | t1/2, h |
|---|---|---|
| Untreated control (n = 4) | 68.1 ± 5.7 | 1.2 ± 0.19 |
| DMSO (n = 4) | 63.4 ± 5.0 | 1.3 ± 0.20 |
| Cycloheximide (n = 3) | 62.8 ± 5.2 | 2.1 ± 0.02* |
| Fixed (n = 3) | 55.5 ± 5.3 | 3.3 ± 0.35* |
Data are presented as means ± SE. hiPSCs-CMs, human induced pluripotent stem cell-cardiomyocytes.
P < 0.05 vs. control, groups compared by ANOVA.
To determine whether the rapid sarcomeric titin-mEos3.2 recovery was dependent on de novo synthesis, we treated hiPSC-CMs with the CHX, translational inhibitor, for 1 h before and during FRAP. We found no significant difference in titin-mEos3.2 fluorescence recovery (68.1 ± 5.7% untreated control vs. 62.8 ± 5.2% CHX) and a significant increase in the fluorescence recovery half-life from 1.2 h (Untreated Control) to 2.1 h (CHX) (Fig. 3, A and B, and Table 1). Since CHX was dissolved in DMSO, we treated titin-mEos3.2 hiPSC-CMs with 0.1% DMSO to account for potential solvent toxicity and found no difference in titin fluorescence recovery and half-life (Fig. 3, A and B, and Table 1). As a metric for CHX functionality, we assayed hiPSC-CMs for global protein synthesis and found that our 10 μg/mL CHX treatment reduced global protein translation indicating that CHX treated titin-mEos3.2 recovery may be due to preexisting titin molecules (Supplemental Fig S4). Our initial results agree with those of da Silva Lopes et al. (8) suggesting that sarcomeric titin molecules are freely 1) recycled between sarcomeres and/or 2) exchanged between a precursor pool.
Reversible photobleaching occurs in fixed titin-mEos3.2 hiPSC-CMs.
To determine if titin-mEos3.2 recovery was due to de facto titin movement, we fixed titin-mEos3.2 hiPSC-CMs in 4% PFA for 15 min and permeabilized their membranes before performing FRAP. Surprisingly, fixed titin-mEos3.2 hiPSC-CMs had a fluorescence recovery of 55.5 ± 5.3% and a ~3.1 h half-life (Fig. 3, A and B). We found substantial recovery in fixed cells suggesting that the rate and mobile recovery of fixed titin-mEos3.2 were not due to physical movement of titin molecules per se but to an intrinsic GFP phenomena known as reversible photobleaching (Fig. 3, A and B) (26).
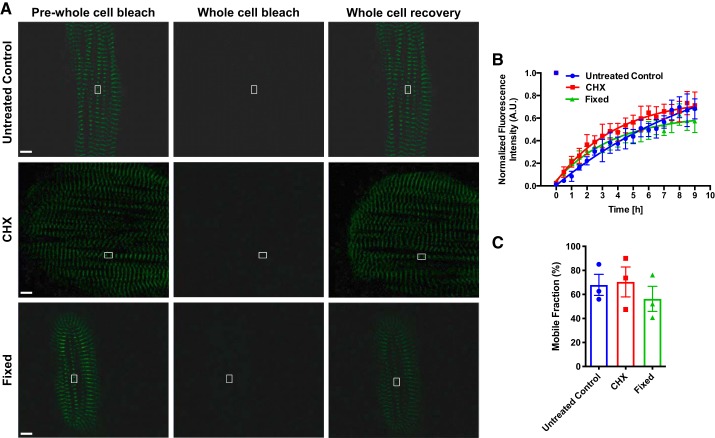
Our initial FRAP assays only quantified the recovery area of two sarcomeres in the presence of preexisting fluorescent titin-mEos3.2 molecules, therefore our observable recovery in fixed cells may in fact be due to, but unlikely, poor sample fixation. However, we postulated that this was not the case; therefore, we employed a secondary confirmatory method where we bleached the entire titin-mEos3.2 cell to quench all fluorescence so that any observable recovery would not be due to poorly fixed preexisting molecules. We first performed whole cell FRAP in untreated control cells and observed a 67.9 ± 8.0 mobile fluorescence recovery (Fig. 4, A–C). We then performed whole cell FRAP in CHX and fixed titin-mEos3.2 cells and saw no differences in fluorescence recovery compared with control (Fig. 4, A–C). CHX treatment was included to ensure that any recovery observed must occur via reversible photobleaching and not de novo synthesis.
Fig. 4.
Whole cell titin protein dynamics in live and fixed human induced pluripotent stem cell-cardiomyocytes (hiPSCs-CMs). A: representative fluorescence recovery after photobleaching (FRAP) images (prewhole cell postwhole cell bleach and whole cell recovery) of day 30 hiPSC-CMs. Scale bar = 5 μm. B: quantification of FRAP images. FRAP images were acquired every 30 min for 9 h. Pixel intensities of the region of interest (ROI) and background were acquired. Background was subtracted from ROI. Images were normalized to prebleach intensities. Data were fitted into a one-phase association equation to extrapolate a synthesis rate (n = 3 cells for each group). C: titin-mEos3.2 mobile fractions of untreated control, CHX treated, and fixed hiPSC-CMs. AU, arbitrary units.
Cytosolic mEos3.2 display reversible photobleaching properties in non-hiPSC-CMs.
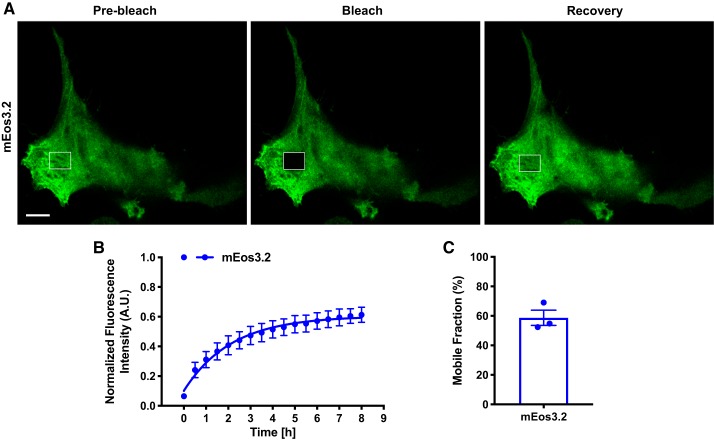
To ensure that the fusion of mEos3.2 to titin did not augment mEos3.2 function, we performed FRAP in fixed HEK 293T cells expressing free soluble mEos3.2 protein. FRAP analysis confirmed reversible photobleaching (58 ± 5.81% fluorescence recovery), thus demonstrating that the mEos3.2 tag itself was undergoing this phenomenon and not limited to cell type (Fig. 5, A–C).
Fig. 5.
Cytosolic mEo3.2 displays reversible photobleaching in HEK 293T cells. A: representative fluorescence recovery after photobleaching (FRAP) images (prebleach, bleach, and recovery) of HEK 293T cells expressing mEos3.2. Scale bar = 10 μm. B: quantification of FRAP images. FRAP images were acquired every 30 min for 9 h. Pixel intensities of the region of interest (ROI), cell, and background were acquired. Background was subtracted from ROI and whole cell. Images were normalized to prebleach intensities. Data were fitted into a one-phase association equation to extrapolate a reversible photoswitching rate (n = 3 cells). C: mobile fractions of fixed mEos3.2-expressing HEK 293T cells. AU, arbitrary units.
Titin-eGFP expressing cardiomyocytes does not undergo reversible photobleaching.
Given our findings regarding mEos3.2, we used a previously published mouse model expressing a titin-eGFP fusion protein (8). FRAP analysis in titin-eGFP neonatal mouse cardiomyocytes demonstrates a 2.6% fluorescence recovery suggesting that eGFP does not undergo extensive reversible photobleaching (Supplemental Fig. S5).
DISCUSSION
Our findings strongly suggest that the mEos3.2 protein undergoes reversible photobleaching. One presumption in FRAP is the irreversible quenching of GFP from a bright state to a dark state upon photoablation (33); however, accumulating evidence has demonstrated that reversible photobleaching can impact FRAP studies leading to an artifactual overestimation of protein mobility by as much as 9, 15, and 60% under differential experimental conditions (9, 26, 36).
Although the titin-mEos3.2 hiPSC-CMs were spontaneously beating, they remained stable throughout the FRAP assays. Along with the precision of the photobleaching laser, we were able to bleach two sarcomeres without any issue. Our 9 h two sarcomere FRAP time-lapse acquisition showed that fixed titin-mEos3.2 hiPSC-CMs recovered to the degree of untreated titin-mEos3.2 hiPSC-CMs and accounted for at least 81% of the observed mobile fraction. Although the fixed titin-mEos3.2 rate was significantly slower, we postulate that the rate represents mEos3.2 achieving a reversible fluorescence equilibrium. These initial observations were performed in fixed cells where the ROI was two sarcomere M lines. Poor sample fixation could have accounted for the fluorescence recovery as preexisting soluble titin molecules could have diffused to the ROI. However, we propose that poor sample fixation could not have accounted for the majority of titin-mEos3.2 fluorescence recovery. Our whole cell FRAP experiments in untreated control, CHX-treated, and fixed titin-mEos3.2 cells demonstrated no changes in the mobile fraction measured suggesting that reversible photobleaching accounted for the recovery in fluorescence. Our data demonstrates that mEos3.2 reversibility is intrinsically associated with mEos3.2 molecule itself and not its fusion to titin as fixed HEK 293 cells expressing mEos3.2 protein can undergo fluorescence recovery.
Both titin-mEos3.2 hiPSC-CM and mouse titin-eGFP models have a genetically encoded in frame fluorescent protein at terminal exon of the titin gene. The main difference between the fluorescent proteins is that mEos3.2 photoconverts from green (maximum excitation: 507 nm; maximum emission: 516 nm) to red (maximum excitation: 572 nm; maximum emission: 580 nm) after ultraviolet irradiation, whereas eGFP only emits green light (maximum excitation: 489 nm and maximum emission: 509 nm) (6, 45). The mEos3.2 photoconvertible protein is derived from the EosFP that was isolated from the stony coral Lobophyllia hemprichii with a chromophore consisting of a key His residue of the His62, Tyr63, and Gly64 peptide sequence that is required for photoconversion (42, 45). eGFP is derived from GFP that was isolated from the jellyfish Aequorea Victoria, and its chromophore region consists of Thr65, Tyr66, and Gly67 sequences (1, 44).
With respect to the mouse titin-eGFP studies, it is important to state that our mEos3.2 findings in no way alters the conclusions that have been reported by da Silva Lopes et al. (8). To determine if reversible photobleaching accounted for previously reported 56% titin-eGFP mobile fraction in neonatal cardiomyocytes, we fixed and performed FRAP using the titin-eGFP mouse model (8). Our data regarding a key control experiment in fixed titin-eGFP mouse neonatal cardiomyocytes demonstrated a ~2.5% reversed photobleached mobile fraction. The fixed titin-eGFP mobile fraction is significantly less than the reported 56% mobile fraction suggesting the eGFP reversible photobleaching does not significantly account for measured recovery (8). Exactly how reversible photobleaching occurs among different fluorophores is not entirely known; however, our data regarding eGFP undergoing reversible photobleaching agree with Sinnecker et al. (36) reporting of eGFP’s reversible photobleaching property. Our results may have significant impact on other investigator’s work where long-term FRAP imaging using photoconvertible fluorescent proteins was used (28).
At the sarcomere, the giant protein titin binds to over 25 identified different proteins each with different affinities (24). For proper incorporation and removal, titin not only has to overcome these interactions but also the interactions that the binding proteins have with other sarcomere proteins. For example, within the M band, the titin, obscurin, obscurin-like-1, and myomesin are all cross linked to form ternary stable network. Both the titin-obscurin and obscurin-like-1 interactions require at least 30 pN for disruption, whereas obsurcin-1-myomesin complex can withstand forces up to ~135 pN thus making the M band stable (30, 31). Regarding the A band, titin is tightly associated with myosin heavy chain and regarded as a molecular ruler for thick filament assembly due to the coincidental ~43-nm spacing of myosin molecules to the spacing of titin’s 11-domain FnIII/Ig super repeat along the A band (13, 18). With respect to the Z disk, the multiple titin/alpha actinin interactions contribute to long-term stability (15). Therefore, all of these described interactions contribute to the notion that titin movement needs to overcome multiple strong interactions at the sarcomere to move fluidly. Given our titin-mEos3.2 hiPSC-CM model’s caveat, much of this dynamic process remains to be explored.
Despite our initial studies, the titin-mEos3.2 model still has potential uses that extend beyond that of sarcomere biology; the hiPSC titin-mEos3.2 cell line can be used as a robust high throughput in vitro platform for preclinical discovery and/or screening of drugs that affect the contractile properties of the heart. Recently, a hiPSC-CM titin Z-disk-tagged eGFP reporter line was constructed to investigate the contractile properties of live cardiomyocytes (35).
Given the earlier observations of titin exchange by da Silva Lopes et al. (8) in neonatal mouse cardiomyocytes, we sought to determine if these observations were recapitulated in human cardiomyocytes by using hiPSC-CMs as a model system as well as a surrogate for human cardiac cellular biology. However, there are several limitations in this model system that need to be acknowledged such as cell heterogeneity, titin isoform expression, and hiPSC-CM maturation. Although hiPSC-CMs are a heterogenous population, we employ small molecule differentiation and chemical selection protocol to mitigate cell heterogeneity by producing primarily ventricular-like cardiomyocytes at day 30 (3). Titin isoform expression varies throughout development ultimately resulting in a higher N2B/N2BA (~60/40%) ratio which is seen in healthy adult human left ventricular myocardium (21, 24, 29). In comparison, Hinson et al. (17) reported that day-30 to -40 hiPSC-CMs express N2B, N2BA, and fetal N2BA titin isoforms with the fetal being the predominate isoform expressed. da Silva Lopes and colleagues (8, 21) primary used neonatal and embryonic mouse cardiomyocytes for their titin-eGFP FRAP studies, which express a higher fetal N2BA isoform. Taken together, both titin-eGFP and titin-mEos3.2 models express a higher fetal N2BA titin isoform ratio thus having the same limitation of not expressing a higher N2B to N2BA ratio as in adult human nonfailing heart samples (27). Our FRAP studies were performed at the single cell level. To date, it is unknown what the individual titin protein isoform expression levels and individual titin isoform half-lives are at the single cell level. To circumvent the influence isoform discrepancies, we tagged the COOH-terminal end with mEos3.2 to label all titin isoforms. Maturation of hiPSC-CMs into a more adult-like ventricular phenotype is an active area of investigation. We have previously demonstrated that culturing hiPSC-CMs on undiluted Matrigel enhances morphological and contractile properties of single hiPSC-CMs (11). However, time-lapse microscopy of hiPSC-CMs cultured on the Matrigel Mattress demonstrated enhanced cell mobility (4, 11) thus making FRAP studies problematic. According to the manufacturer, Corning Matrigel Matrix does fluoresce. Taken together, mobile hiPSC-CMs coupled with an autofluorescence substrate generate a limitation in using titin-mEos3.2 hiPSC-CMs plated on the Matrigel Mattress for live cell imaging; therefore, we used a 1:200 dilution of Matrigel, which creates hiPSC-CMs with an immature cellular architectural phenotype. It is unknown what the titin exchange rate is in isolated adult mouse cardiomyocytes. da Silva et al. (8) reported that they could not quantity titin exchange in isolated adult cardiomyocytes due to cellular viability; therefore, they used primary neonatal mouse and embryonic cardiomyocytes as their model system, which also have an immature cellular phenotype. Although hiPSC-CM, neonatal mouse cardiomyocyte, and mouse embryonic cardiomyocyte model systems display immature phenotypes, they are invaluable as a research tool for in vitro studies.
Long-term imaging of single cell dynamics requires effort in the maintenance of culture conditions, minimization of phototoxicity, and maximization of data acquisition (20). Our results heed caution in the extrapolation of reliable FRAP data in long-term cell imaging using fluorophores that have not been validated for reversible photobleaching properties.
GRANTS
This study was funded in part by National Institutes of Health (NIH) Grant R01-HL-095813 and Vanderbilt University Stahlman (to C. C. Lim); NIH Grants 5R01-HL-104040, 5R01-HL-095813, R01-HL-135129-A1, and P50-GM-115305 (to C. C. Hong); NIH Grant R01-HL-133286 (to T. Hong); and Graduate Research Assistant Supplement R01-HL-095813-S1 and Center for Advancing Translational Sciences Award UL1TR000445 (to A. G. Cadar). Live cell imaging was performed in part through the use of the Vanderbilt University School of Medicine Cell Imaging Shared Resource (supported by NIH Grants CA-68485, DK-20593, DK-58404, DK-59637, EY-08126, and 1S10-RR-027396-01).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.G.C., T.K.F., C.C.L., and C.C.H. conceived and designed research; A.G.C., T.K.F., K.R.B., L.W., T.H., J.A.B., Z.Z., and Y.-J.N. performed experiments; A.G.C., L.W., T.H., Z.Z., Y.W.C., B.C.K., C.C.L., and C.C.H. analyzed data; A.G.C., K.R.B., L.W., T.H., M.G., B.C.K., D.M.R., C.C.L., and C.C.H. interpreted results of experiments; A.G.C., L.W., and T.H. prepared figures; A.G.C., C.C.L., and C.C.H. drafted manuscript; A.G.C., T.H., Z.Z., Y.W.C., Y.-J.N., M.G., C.C.L., and C.C.H. edited and revised manuscript; A.G.C., T.K.F., K.R.B., L.W., T.H., J.A.B., Z.Z., Y.W.C., Y.-J.N., M.G., B.C.K., D.M.R., C.C.L., and C.C.H. approved final version of manuscript.
ACKNOWLEDGMENTS
Correspondence may also be addressed to: C. Lim, Clinical and Integrative Cardiovascular Sciences, Center for Scientific Review, 6701 Rockledge Dr., Rm 4128, MSC 7814, Bethesda, MD 20892 (20817 for courier mail). (e-mail: chee.lim@nih.gov).
REFERENCES
- 1.Arpino JAJ, Rizkallah PJ, Jones DD. Crystal structure of enhanced green fluorescent protein to 1.35 Å resolution reveals alternative conformations for Glu222. PLoS One 7: e47132, 2012. doi: 10.1371/journal.pone.0047132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bang ML, Centner T, Fornoff F, Geach AJ, Gotthardt M, McNabb M, Witt CC, Labeit D, Gregorio CC, Granzier H, Labeit S. The complete gene sequence of titin, expression of an unusual approximately 700-kDa titin isoform, and its interaction with obscurin identify a novel Z-line to I-band linking system. Circ Res 89: 1065–1072, 2001. doi: 10.1161/hh2301.100981. [DOI] [PubMed] [Google Scholar]
- 3.Burridge PW, Matsa E, Shukla P, Lin ZC, Churko JM, Ebert AD, Lan F, Diecke S, Huber B, Mordwinkin NM, Plews JR, Abilez OJ, Cui B, Gold JD, Wu JC. Chemically defined generation of human cardiomyocytes. Nat Methods 11: 855–860, 2014. doi: 10.1038/nmeth.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cadar AG, Feaster TK, Durbin MD, Hong CC. Production of single contracting human induced pluripotent stem cell-derived cardiomyocytes: Matrigel mattress technique. Curr Protoc Stem Cell Biol 42: 1, 2017. doi: 10.1002/cpsc.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cazorla O, Freiburg A, Helmes M, Centner T, McNabb M, Wu Y, Trombitás K, Labeit S, Granzier H. Differential expression of cardiac titin isoforms and modulation of cellular stiffness. Circ Res 86: 59–67, 2000. doi: 10.1161/01.RES.86.1.59. [DOI] [PubMed] [Google Scholar]
- 6.Chudakov DM, Matz MV, Lukyanov S, Lukyanov KA. Fluorescent proteins and their applications in imaging living cells and tissues. Physiol Rev 90: 1103–1163, 2010. doi: 10.1152/physrev.00038.2009. [DOI] [PubMed] [Google Scholar]
- 7.Chun YW, Balikov DA, Feaster TK, Williams CH, Sheng CC, Lee JB, Boire TC, Neely MD, Bellan LM, Ess KC, Bowman AB, Sung HJ, Hong CC. Combinatorial polymer matrices enhance in vitro maturation of human induced pluripotent stem cell-derived cardiomyocytes. Biomaterials 67: 52–64, 2015. doi: 10.1016/j.biomaterials.2015.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.da Silva Lopes K, Pietas A, Radke MH, Gotthardt M. Titin visualization in real time reveals an unexpected level of mobility within and between sarcomeres. J Cell Biol 193: 785–798, 2011. doi: 10.1083/jcb.201010099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dayel MJ, Hom EF, Verkman AS. Diffusion of green fluorescent protein in the aqueous-phase lumen of endoplasmic reticulum. Biophys J 76: 2843–2851, 1999. doi: 10.1016/S0006-3495(99)77438-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Everett AW, Prior G, Zak R. Equilibration of leucine between the plasma compartment and leucyl-tRNA in the heart, and turnover of cardiac myosin heavy chain. Biochem J 194: 365–368, 1981. doi: 10.1042/bj1940365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feaster TK, Cadar AG, Wang L, Williams CH, Chun YW, Hempel JE, Bloodworth N, Merryman WD, Lim CC, Wu JC, Knollmann BC, Hong CC. Matrigel mattress: a method for the generation of single contracting human-induced pluripotent stem cell-derived cardiomyocytes. Circ Res 117: 995–1000, 2015. doi: 10.1161/CIRCRESAHA.115.307580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fu Y, Shaw SA, Naami R, Vuong CL, Basheer WA, Guo X, Hong T. Isoproterenol promotes rapid ryanodine receptor movement to bridging integrator 1 (BIN1)-organized dyads. Circulation 133: 388–397, 2016. doi: 10.1161/CIRCULATIONAHA.115.018535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fürst DO, Nave R, Osborn M, Weber K. Repetitive titin epitopes with a 42 nm spacing coincide in relative position with known A band striations also identified by major myosin-associated proteins. An immunoelectron-microscopical study on myofibrils. J Cell Sci 94: 119–125, 1989. [DOI] [PubMed] [Google Scholar]
- 14.Granzier HL, Labeit S. The giant protein titin: a major player in myocardial mechanics, signaling, and disease. Circ Res 94: 284–295, 2004. doi: 10.1161/01.RES.0000117769.88862.F8. [DOI] [PubMed] [Google Scholar]
- 15.Grison M, Merkel U, Kostan J, Djinović-Carugo K, Rief M. α-Actinin/titin interaction: A dynamic and mechanically stable cluster of bonds in the muscle Z-disk. Proc Natl Acad Sci USA 114: 1015–1020, 2017. doi: 10.1073/pnas.1612681114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Helmes M, Trombitás K, Granzier H. Titin develops restoring force in rat cardiac myocytes. Circ Res 79: 619–626, 1996. doi: 10.1161/01.RES.79.3.619. [DOI] [PubMed] [Google Scholar]
- 17.Hinson JT, Chopra A, Nafissi N, Polacheck WJ, Benson CC, Swist S, Gorham J, Yang L, Schafer S, Sheng CC, Haghighi A, Homsy J, Hubner N, Church G, Cook SA, Linke WA, Chen CS, Seidman JG, Seidman CE. Titin mutations in iPS cells define sarcomere insufficiency as a cause of dilated cardiomyopathy. Science 349: 982–986, 2015. doi: 10.1126/science.aaa5458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Isaacs WB, Kim IS, Struve A, Fulton AB. Association of titin and myosin heavy chain in developing skeletal muscle. Proc Natl Acad Sci USA 89: 7496–7500, 1992. doi: 10.1073/pnas.89.16.7496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Isaacs WB, Kim IS, Struve A, Fulton AB. Biosynthesis of titin in cultured skeletal muscle cells. J Cell Biol 109: 2189–2195, 1989. doi: 10.1083/jcb.109.5.2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jones RB, Mueller S, O’Connor R, Rimpel K, Sloan DD, Karel D, Wong HC, Jeng EK, Thomas AS, Whitney JB, Lim SY, Kovacs C, Benko E, Karandish S, Huang SH, Buzon MJ, Lichterfeld M, Irrinki A, Murry JP, Tsai A, Yu H, Geleziunas R, Trocha A, Ostrowski MA, Irvine DJ, Walker BD. A subset of latency-reversing agents expose HIV-infected resting CD4+ T-cells to recognition by cytotoxic T-lymphocytes. PLoS Pathog 12: e1005545, 2016. doi: 10.1371/journal.ppat.1005545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lahmers S, Wu Y, Call DR, Labeit S, Granzier H. Developmental control of titin isoform expression and passive stiffness in fetal and neonatal myocardium. Circ Res 94: 505–513, 2004. doi: 10.1161/01.RES.0000115522.52554.86. [DOI] [PubMed] [Google Scholar]
- 22.Levine HJ. Rest heart rate and life expectancy. J Am Coll Cardiol 30: 1104–1106, 1997. doi: 10.1016/s0735-1097(97)00246-5. [DOI] [PubMed] [Google Scholar]
- 23.LeWinter MM, Granzier H. Cardiac titin: a multifunctional giant. Circulation 121: 2137–2145, 2010. doi: 10.1161/CIRCULATIONAHA.109.860171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Linke WA, Hamdani N. Gigantic business: titin properties and function through thick and thin. Circ Res 114: 1052–1068, 2014. doi: 10.1161/CIRCRESAHA.114.301286. [DOI] [PubMed] [Google Scholar]
- 25.Martin AF. Turnover of cardiac troponin subunits. Kinetic evidence for a precursor pool of troponin-I. J Biol Chem 256: 964–968, 1981. [PubMed] [Google Scholar]
- 26.Mueller F, Morisaki T, Mazza D, McNally JG. Minimizing the impact of photoswitching of fluorescent proteins on FRAP analysis. Biophys J 102: 1656–1665, 2012. doi: 10.1016/j.bpj.2012.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neagoe C, Kulke M, del Monte F, Gwathmey JK, de Tombe PP, Hajjar RJ, Linke WA. Titin isoform switch in ischemic human heart disease. Circulation 106: 1333–1341, 2002. doi: 10.1161/01.CIR.0000029803.93022.93. [DOI] [PubMed] [Google Scholar]
- 28.Ojima K, Ichimura E, Yasukawa Y, Wakamatsu J, Nishimura T. Dynamics of myosin replacement in skeletal muscle cells. Am J Physiol Cell Physiol 309: C669–C679, 2015. doi: 10.1152/ajpcell.00170.2015. [DOI] [PubMed] [Google Scholar]
- 29.Opitz CA, Leake MC, Makarenko I, Benes V, Linke WA. Developmentally regulated switching of titin size alters myofibrillar stiffness in the perinatal heart. Circ Res 94: 967–975, 2004. doi: 10.1161/01.RES.0000124301.48193.E1. [DOI] [PubMed] [Google Scholar]
- 30.Pernigo S, Fukuzawa A, Beedle AEM, Holt M, Round A, Pandini A, Garcia-Manyes S, Gautel M, Steiner RA. Binding of myomesin to obscurin-like-1 at the muscle M-band provides a strategy for isoform-specific mechanical protection. Structure 25: 107–120, 2017. doi: 10.1016/j.str.2016.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pernigo S, Fukuzawa A, Bertz M, Holt M, Rief M, Steiner RA, Gautel M. Structural insight into M-band assembly and mechanics from the titin-obscurin-like-1 complex. Proc Natl Acad Sci USA 107: 2908–2913, 2010. doi: 10.1073/pnas.0913736107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ran FA, Hsu PD, Wright J, Agarwala V, Scott DA, Zhang F. Genome engineering using the CRISPR-Cas9 system. Nat Protoc 8: 2281–2308, 2013. doi: 10.1038/nprot.2013.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reits EA, Neefjes JJ. From fixed to FRAP: measuring protein mobility and activity in living cells. Nat Cell Biol 3: E145–E147, 2001. doi: 10.1038/35078615. [DOI] [PubMed] [Google Scholar]
- 34.Ribeiro AJ, Ang YS, Fu JD, Rivas RN, Mohamed TM, Higgs GC, Srivastava D, Pruitt BL. Contractility of single cardiomyocytes differentiated from pluripotent stem cells depends on physiological shape and substrate stiffness. Proc Natl Acad Sci USA 112: 12705–12710, 2015. doi: 10.1073/pnas.1508073112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sharma A, Toepfer CN, Schmid M, Garfinkel AC, Seidman CE. Differentiation and contractile analysis of GFP-Sarcomere reporter hiPSC-cardiomyocytes. Curr Protoc Hum Genet 96: 21.12.1–21.12, 2018. doi: 10.1002/cphg.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sinnecker D, Voigt P, Hellwig N, Schaefer M. Reversible photobleaching of enhanced green fluorescent proteins. Biochemistry 44: 7085–7094, 2005. doi: 10.1021/bi047881x. [DOI] [PubMed] [Google Scholar]
- 37.Skwarek-Maruszewska A, Hotulainen P, Mattila PK, Lappalainen P. Contractility-dependent actin dynamics in cardiomyocyte sarcomeres. J Cell Sci 122: 2119–2126, 2009. doi: 10.1242/jcs.046805. [DOI] [PubMed] [Google Scholar]
- 38.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126: 663–676, 2006. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 39.Tskhovrebova L, Trinick J. Titin: properties and family relationships. Nat Rev Mol Cell Biol 4: 679–689, 2003. doi: 10.1038/nrm1198. [DOI] [PubMed] [Google Scholar]
- 40.Turnacioglu KK, Mittal B, Dabiri GA, Sanger JM, Sanger JW. An N-terminal fragment of titin coupled to green fluorescent protein localizes to the Z-bands in living muscle cells: overexpression leads to myofibril disassembly. Mol Biol Cell 8: 705–717, 1997. doi: 10.1091/mbc.8.4.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang J, Fan Y, Dube DK, Sanger JM, Sanger JW. Jasplakinolide reduces actin and tropomyosin dynamics during myofibrillogenesis. Cytoskeleton (Hoboken) 71: 513–529, 2014. doi: 10.1002/cm.21189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wiedenmann J, Ivanchenko S, Oswald F, Schmitt F, Röcker C, Salih A, Spindler K-D, Nienhaus GU. EosFP, a fluorescent marker protein with UV-inducible green-to-red fluorescence conversion. Proc Natl Acad Sci USA 101: 15905–15910, 2004. doi: 10.1073/pnas.0403668101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wolny M, Colegrave M, Colman L, White E, Knight PJ, Peckham M. Cardiomyopathy mutations in the tail of β-cardiac myosin modify the coiled-coil structure and affect integration into thick filaments in muscle sarcomeres in adult cardiomyocytes. J Biol Chem 288: 31952–31962, 2013. doi: 10.1074/jbc.M113.513291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang G, Gurtu V, Kain SR. An enhanced green fluorescent protein allows sensitive detection of gene transfer in mammalian cells. Biochem Biophys Res Commun 227: 707–711, 1996. doi: 10.1006/bbrc.1996.1573. [DOI] [PubMed] [Google Scholar]
- 45.Zhang M, Chang H, Zhang Y, Yu J, Wu L, Ji W, Chen J, Liu B, Lu J, Liu Y, Zhang J, Xu P, Xu T. Rational design of true monomeric and bright photoactivatable fluorescent proteins. Nat Methods 9: 727–729, 2012. doi: 10.1038/nmeth.2021. [DOI] [PubMed] [Google Scholar]
- 46.Zhong L, Chiusa M, Cadar AG, Lin A, Samaras S, Davidson JM, Lim CC. Targeted inhibition of ANKRD1 disrupts sarcomeric ERK-GATA4 signal transduction and abrogates phenylephrine-induced cardiomyocyte hypertrophy. Cardiovasc Res 106: 261–271, 2015. doi: 10.1093/cvr/cvv108. [DOI] [PMC free article] [PubMed] [Google Scholar]