Abstract
Endocrine-disrupting chemicals interact with transcription factors essential for adipocyte differentiation. Exposure to endocrine-disrupting chemicals corresponds with elevated risks of obesity, but the effects of these compounds on human cells remain largely undefined. Widespread use of bisphenol AF (BPAF) as a bisphenol A (BPA) alternative in the plastics industry presents unknown health risks. To this end, we discovered that BPAF interferes with the metabolic function of mature human adipocytes. Although 4-day exposures to BPAF accelerated adipocyte differentiation, we observed no effect on mature fat cell marker genes. Additional gene and protein expression analysis showed that BPAF treatment during human adipocyte differentiation failed to suppress the proinflammatory transcription factor STAT1. Microscopy and respirometry experiments demonstrated that BPAF impaired mitochondrial function and structure. To test the hypothesis that BPAF fosters vulnerabilities to STAT1 activation, we treated mature adipocytes previously exposed to BPAF with interferon-γ (IFNγ). BPAF increased IFNγ activation of STAT1 and exposed mitochondrial vulnerabilities that disrupt adipocyte lipid and carbohydrate metabolism. Collectively, our data establish that BPAF activates inflammatory signaling pathways that degrade metabolic activity in human adipocytes. These findings suggest how the BPA alternative BPAF contributes to metabolic changes that correspond with obesity.
Keywords: adipocyte, inflammation, metabolism, PPARγ, transcription
INTRODUCTION
Approximately 40% of adults in the United States are classified as obese (24). This number primarily results from increased caloric intake and reduced energy expenditure. However, the rate of obesity in the United States cannot be totally explained by lifestyle choices and, in recent decades, may be correlated with persistent environmental exposures to synthetic chemicals that possess endocrine-disrupting capabilities (67). Although controversial, exposure to endocrine-disrupting chemicals (EDCs) can be associated with obesity. The widespread presence of EDCs in the food and water supply coupled with the obesity epidemic in developed countries warrants mechanistic understanding and risk assessment in human biological models (18).
White adipose tissue (WAT) is an organ that regulates energy balance by storing and mobilizing lipids. Subcutaneous (peripheral) WAT constitutes most of the body’s fat mass, and its expansion allows appropriate storage of lipids. An inability to expand the number of subcutaneous fat cells correlates with metabolic dysfunction and ectopic lipid deposition in intra-abdominal organs (17). Many studies demonstrate that subcutaneous WAT and adipocytes in vitro show a capability to acquire hallmark features of brown adipose tissue (BAT), including expression of uncoupling protein 1 (UCP1), increased mitochondrial biogenesis, enhanced energy expenditure, and dissipation of chemical energy as heat (14, 49, 51, 58, 63, 70). Although white adipocytes exhibit less oxidative capacity than brown adipocytes (5, 20, 72), energy expenditure in subcutaneous fat cells might critically regulate metabolic homeostasis because of the sheer amount of peripheral WAT as a percentage of body mass.
In humans and mice, persistent WAT inflammation has been implicated in the metabolic consequences of obesity, including insulin resistance. Obesity-linked inflammation critically restricts sequestration of lipids in subcutaneous WAT leading to accumulation of intra-abdominal ectopic fat deposition, insulin resistance, and type 2 diabetes mellitus. Supporting these observations, proinflammatory mediators secreted by immune cells, such as interferon-γ (IFNγ) and tumor necrosis factor-α (TNFα), correlate with insulin resistance, as well as failure of integral lipid metabolism (15, 29, 30, 57) and mitochondrial functions (31, 34, 45). There is physiological evidence that EDCs interfere with normal weight control mechanisms, particularly those that impact the function of WAT. Accordingly, the obesogen hypothesis proposes that EDCs may contribute to obesity and its comorbidities by impacting WAT expandability (28). EDCs may also elevate intrinsic inflammatory responses that compromise white adipocyte function and, ultimately, adaptation to metabolic demands.
Here, we show that the EDC bisphenol AF (BPAF; CAS no. 6807176) sensitizes human adipocytes to the effects of the obesity-linked cytokine IFNγ. Furthermore, BPAF treatment does not allow full suppression of STAT1, which communicates IFNγ signals to inflammatory gene transcription. The National Institute of Environmental Health Sciences nominated BPAF for comprehensive characterization on the basis of the lack of adequate toxicology data. BPAF, like the ubiquitous plasticizer bisphenol A (BPA), persists in the environment as a component of polymer mixtures used for high-temperature composites, electronic materials, and gas-permeable membranes. The constant efforts to replace BPA with similar derivatives raise the possibility for future exposures that may influence and accelerate the obesity crisis in the United States. Despite the potential impact of BPAF on disease risk, there is a gap in our knowledge of BPAF activity in human models. This study identifies BPAF as a critical effector of inflammation in human fat cells and metabolic responses associated with obesity.
MATERIALS AND METHODS
Cell culture and differentiation.
Cryopreserved, subcutaneous primary human preadipocytes from normal female donors with an average body mass index of 27.51 were provided by Zen-Bio Inc. Cells were received at passage 2, and experiments were performed before passage 10. Experiments were performed using pooled human preadipocytes from five individual female donors (lot SL0065). Human preadipocytes were maintained at 5% CO2 and 37°C in DMEM/F-12 (GIBCO) with 10% fetal bovine serum (FBS; Gemini Bio-Products), 100 U/mL penicillin, and 100 µg/mL streptomycin (growth media). Medium was replaced during routine maintenance every 2 days. Confluent cells were differentiated using growth media supplemented with 100 nM human insulin, 0.250 mM 3-isobutyl-1-methylxanthine (IBMX), 500 nM dexamethasone, and 3 µM rosiglitazone (BRL-49653; Cayman Chemical Company). BPAF (Sigma Chemical Company) or ethanol (EtOH, control) was added to media throughout differentiation. Cells were maintained in these media for 4 days, after which the media were changed to growth media supplemented with 100 nM human insulin, 500 nM dexamethasone, and 3 µM rosiglitazone. Cells were maintained in this manner with media changed every 2 days until their use for end point experiments. Experiments performed in Fig. 1 used adipocytes treated with differentiation media for 4 days. All other end point experiments were profiled for functional readouts at 14 days. IBMX, insulin, and dexamethasone were purchased from Sigma Chemical Company (St. Louis, MO). Recombinant human interferon-γ was purchased from R&D Systems. HepG2 cells were maintained in Eagle’s minimum essential medium (American Type Culture Collection) containing 10% FBS, 100 U/mL penicillin, and 100 µg/mL streptomycin. In experiments related to Fig. 5, mature human adipocytes were treated with recombinant human 100 ng/mL IFNγ (R&D Systems) or 0.0001% BSA (vehicle control) for up to 24 h.
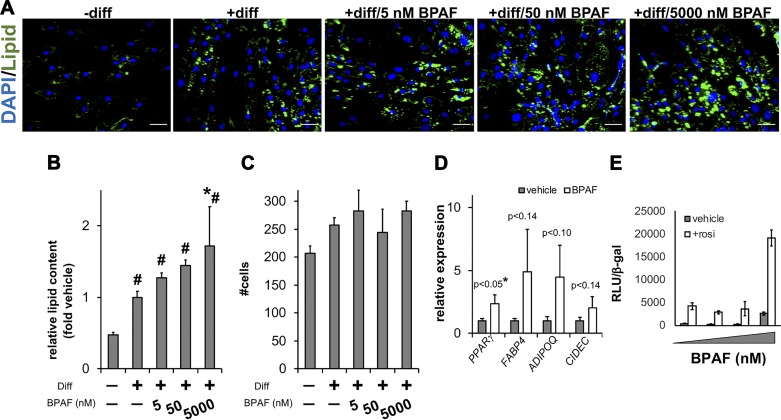
Fig. 1.
Impacts of bisphenol AF (BPAF) on lipid accumulation in human adipocytes. A: human preadipocytes were induced to differentiate for 96 h in the presence or absence of BPAF concentrations up to 5 μM. Cells were then stained with DAPI (nucleus) and LipidTOX (lipid). Representative images are shown. Scale bars are 50 μm. B: lipid accumulation was quantified by image analysis. C: nuclei were counted to analyze toxicity (n ≥ 8 wells; means ± SE; *P < 0.05 relative to vehicle; #P < 0.05 relative to no differentiation). D: in parallel, relative mRNA levels were determined for canonical adipocyte marker genes (n = 6; means ± SE). E: to further validate effects on peroxisome proliferator-activated receptor-γ (PPARγ)-mediated transcription, HepG2 cells were treated with increasing doses of BPAF ± 1 µM rosiglitazone (rosi). Transcriptional activity was determined by measuring PPAR responsive element (PPRE)-luciferase normalized to β-galactosidase (β-gal; n = 4; means ± SE). ADIPOQ, adiponectin, C1Q and collagen domain containing; CIDEC, cell death-inducing DFFA-like effector c; diff, differentiation; FABP4, fatty acid-binding protein 4; RLU, relative light units.
Fig. 5.
The interferon-γ response in human adipocytes is primed by bisphenol AF (BPAF) treatment. A: immunoblots of total cell lysates from human preadipocytes differentiated 14 days ± 5 µM BPAF followed by treatment with vehicle (0.0001% BSA) or recombinant human IFNγ (100 ng/mL) for 10 min (short = 3–5-s film exposure; long = 60-s film exposure). B–D: quantitative PCR analysis of STAT1 (B), interferon regulatory factor 1 (IRF1; C), and uncoupling protein 1 (UCP1; D; n ≥ 3 experiments; means ± SE) expression in preadipocytes differentiated 14 days ± 5 μM BPAF treated with vehicle or 100 ng/mL IFNγ for 24 h. *P < 0.05 relative to vehicle; #P < 0.05 relative to IFNγ. E: HepG2 cells were transfected with a UCP1 −5.5-kb-luciferase reporter (UCP1-luc) and vectors expressing peroxisome proliferator-activated receptor-γ (PPARγ) and STAT1. Forty-eight hours after transfection, HepG2 cells were exposed to 1 μM rosiglitazone and BPAF concentrations up to 5 μM ± 100 ng/mL IFNγ. Transcriptional activity was determined by measuring UCP1-luc normalized to β-galactosidase (β-gal; n = 6; means ± SE, *P < 0.05 relative to vehicle; #P < 0.05 relative to IFNγ). F and G: oxygen consumption rate (OCR; F) and immunoblots of total cell lysates (G) in preadipocytes differentiated 14 days ± 5 µM BPAF treated with vehicle or 100 ng/mL IFNγ for 24 h. OCR was measured over time with the addition of oligomycin (α), carbonyl cyanide p-trifluoromethoxyphenylhydrazone (FCCP; β), and antimycin A/rotenone (γ). For Seahorse assays, statistical significance indicates changes in maximal respiration relative to vehicle controls (*P < 0.05, compared with the black curve in F). Cyt C, cytochrome c; Hsp60 and Hsp90, 60- and 90-kDa heat shock proteins, respectively; rel, relative; RLU, relative light units.
Plate processing for high-throughput microscopy.
For fluorescence microscopy performed on 384-well plates (Greiner), the following protocol was performed on the BioMek NX (Beckman Coulter). Aspirations and plate washes were performed with an ELx405 (BioTek). All plate washes were performed with Tris-buffered saline (TBS). Following differentiation, media were aspirated, and 4% formaldehyde (Electron Microscopy Sciences) in PBS was immediately added for 30 min at room temperature. Plates were then quenched with 100 mM ammonium chloride. The quench was followed by permeabilization with 0.1% Triton X-100 in TBS for 10 min. Then, cells were incubated with CellMask Blue (1 μg/mL, Life Technologies, a general protein dye), LipidTOX Green (1:1,000, Life Technologies, a nonpolar lipid-binding dye), and DAPI (10 μg/mL, a DNA-specific dye) in PBS for 45 min at room temperature. Dyes were aspirated, and PBS-0.01% NaN3 was added. Finally, plates were sealed and imaged immediately.
High-content analysis.
Cells were imaged using the Cell Laboratory IC-100 Image Cytometer (Beckman Coulter) equipped with a Nikon S Fluor ×20/0.75-numerical aperture objective. The imaging camera (Hamamatsu) captured 8-bit images at 2 × 2 binning (672 × 512 pixels, 0.684 × 0.684 μm2/pixel). Sixteen images were captured per well. Images were analyzed using custom algorithms developed with the Pipeline Pilot (v7.5) software platform (Accelrys). After background subtraction, nuclear and cell masks are generated using a combination of nonlinear least squares and watershed-from-markers image manipulations of the DAPI images. Cell populations were filtered to discard events with cell aggregates, mitotic cells, apoptotic cells, cellular debris, or poor segmentation. All events with whole cell masks bordering the edge of the image were additionally eliminated from analysis. Postanalysis measurements were exported to spreadsheet software (Microsoft Excel) for further analysis.
Plasmids and luciferase expression experiments.
We used Lipofectamine 2000 (Invitrogen) to express transcription factor cDNAs and peroxisome proliferator-activated receptor (PPAR) responsive element (PPRE)-luciferase or a −5.5-kb UCP1-NanoLuc-green fluorescent protein (GFP) fusion (see below) in HepG2 cells cultured in 24-well plates. 3XPPRE-luciferase (37) was provided by David D. Moore (Baylor College of Medicine). Fred Schaufele provided the PPARγ2 cDNA (25, 56), and the STAT1 construct was acquired from Addgene (George Stark laboratory, Addgene no. 71454). To detect effects of BPAF on PPRE-luciferase, cells were transfected with Lipofectamine 2000, 500 ng of reporter constructs, 500 ng of PPARγ2 cDNA, and 200 ng of cytomegalovirus (CMV)-β-galactosidase. After 48-h transfection, cells were treated with increasing doses of BPAF (up to 5 μM) with vehicle (0.1% DMSO) or 1 μM rosiglitazone in growth media for an additional 48 h. To measure effects of BPAF on −5.5-kb UCP1-NanoLuc-GFP reporter activity, cells were transfected with Lipofectamine 2000, 500 ng of reporter construct (−5.5-kb UCP1-NanoLuc-GFP), 500 ng of PPARγ2 cDNA, 500 ng of STAT1 cDNA, and 200 ng of CMV-β-galactosidase. After 48-h transfection, cells were treated with 1 μM rosiglitazone, 100 ng/mL human IFNγ (R&D Systems) or 0.0001% BSA (vehicle control), and increasing doses of BPAF (up to 5 μM) for an additional 48 h. In all experiments, reporter gene activity was detected using the Promega Luciferase Assay Kit. Relative luminescence units were normalized to β-galactosidase activity (Promega).
For this study, we generated a −5.5-kb UCP1-NanoLuc-GFP fusion to measure in vitro activity of the UCP1 enhancer region. The −5.5-kb UCP1-TurboGFP was a gift from Irfan Lodhi (Addgene plasmid no. 104585). To construct the −5.5-kb UCP1 NanoLuc-GFP reporter, −5.5-kb UCP1-TurboGFP was digested by XhoI/PmeI, and the 10,704-bp fragment was gel purified. NanoLuc and TurboGFP were PCR amplified using the following oligonucleotides and then cloned into the XhoI/PmeI sites using an In-Fusion Cloning kit (Takara): NanoLuc-forward, CGCCACCATGCTCGA GCCACCATGGTCT; NanoLuc-P2A-reverse, AGGTCCAGGGTTCTCCTCC; TurboGFP-forward, GAGAACCCTGGACCTATGGAGAGCGACGAGAGCG; and TurboGFP-reverse, CGCGGCCGGCCGTTTAAAC. pCDH-EF1-NLuc-P2A-copGFP-T2A-Puro was the template for the NanoLuc-GFP fusion (Kazuhiro Oka, Addgene no. 73022). The nucleotide sequence was verified by DNA sequence analysis. This reporter was generated by the Gene Vector Core at the Baylor College of Medicine.
RNA extraction and quantitative PCR analysis.
Total RNA was extracted from cells using the Direct-Zol kit (Zymo Research). cDNA was synthesized using SuperScript ViLO Master Mix (Invitrogen). To measure relative mRNA expression, quantitative PCR (qPCR) was performed with TaqMan reagents using a QuantStudio 3 PCR system (Applied Biosystems). The invariant control was TATA box-binding protein (TBP). TaqMan and Roche Universal ProbeLibrary Gene Expression Assays (Table 1) were used as previously described (33, 34).
Table 1.
Roche Universal ProbeLibrary primer sequences and TaqMan assays used in quantitative RT-PCR for markers of adipocyte differentiation, function, inflammation, and thermogenesis in human fat cells
| Gene | Species | Accession No. | L Primer | R Primer | UPL No. |
|---|---|---|---|---|---|
| ADIPOQ | Human | NM_004797.2 | GGTGAGAAGGGTGAGAAAGGA | TTTCACCGATGTCTCCCTTAG | 85 |
| ISG15 | Human | NM_005101.3 | GAGGCAGCGAACTCATCTTT | AGCATCTTCACCGTCAGGTC | 76 |
| LEP | Human | NM_000230.2 | TTGTCACCAGGATCAATGACA | GTCCAAACCGGTGACTTTCT | 25 |
| AGT | Human | NM_000029.3 | ACCTACGTCCACTTCCAAGG | GTTGTCCACCCAGAACTCCT | 7 |
| PRDM16 | Human | NM_022114.2 | TACACTGTGCAGGCAGGCTA | GTGTGGAGAGGAGTGTCTTCG | 56 |
| TBP | Human | NM_003194.4 | CCCATGACTCCCATGACC | TTTACAACCAAGATTCACTGTGG | 51 |
| Gene | Species | TaqMan Assay | |||
|---|---|---|---|---|---|
| PPARG | Human | Hs01115513_m1 | |||
| FABP4 | Human | Hs01086177_m1 | |||
| PPARGC1A | Human | Hs01016719_m1 | |||
| CIDEC | Human | Hs01032998_m1 | |||
| GLUT4 | Human | Hs00168966_m1 | |||
| UCP1 | Human | Hs00222453_m1 | |||
| CIDEA | Human | Hs00154455_m1 | |||
| TNF | Human | Hs00174128_m1 | |||
| STAT1 | Human | Hs01013996_m1 |
ADIPOQ, adiponectin, C1Q and collagen domain containing; AGT, angiotensinogen; CIDEA and CIDEC, cell death-inducing DFFA-like effector a and c, respectively; FABP4, fatty acid-binding protein 4; GLUT4, solute carrier family 2 member 4; ISG15, ISG15 ubiquitin-like modifier; L, left; LEP, leptin; PPARG, peroxisome proliferator-activated receptor-γ; PPARGC1A, PPARγ coactivator 1α; PRDM16, PR/SET domain 16; R, right; TBP, TATA box-binding protein; UCP1, uncoupling protein 1; UPL, Universal ProbeLibrary.
Fluorescence microscopy.
Mitochondria were labeled using MitoTracker CMX-ROS (Life Technologies). Live cells were pulsed with 500 nM MitoTracker for 15 min. Mitochondrial labeling was followed by cell fixation in 4% paraformaldehyde. Ammonium chloride was used to quench autofluorescence derived from residual paraformaldehyde. Fixed adipocytes were permeabilized with 2% BSA in PBS-0.01% saponin for 30 min at room temperature followed by three PBS washes. Cells were incubated with LipidTOX Green (Invitrogen) and DAPI in PBS for 45 min at room temperature. Dyes were then aspirated, and PBS-0.01% azide was added before mounting coverslips onto glass slides. Imaging was performed with the DeltaVision Core Image Restoration Microscope (Applied Precision).
Cellular respiration.
Respiration was determined with human adipocytes using a Seahorse XF24 analyzer (Agilent Technologies). Preadipocytes were allowed to grow to confluence before differentiation. After treatments, media were replaced with 37°C unbuffered DMEM containing 4.5 g/L glucose, sodium pyruvate (1 mmol/L), and l-glutamine (2 mmol/L). Measurement was at 37°C using 2-2-2 intervals. Basal respiration was defined before sequential addition of oligomycin, FCCP (maximal respiration), rotenone, and antimycin A.
Antibodies.
The following antibodies were used for immunoblot analysis: phospho-STAT1 Y701 (Cell Signaling Technology no. 9167); STAT1 (GeneTex no. GTX-113030); adiponectin, C1Q and collagen domain containing (ADIPOQ; GeneTex no. GTX-112777); PPARγ (Cell Signaling Technology no. 2435); cytochrome c (GeneTex no. GTX-108585); 60-kDa heat shock protein (HSP60; GeneTex no. GTX-110089); and β-actin (Sigma no. A-5441).
Immunoblot analysis.
Cells were collected by scraping and lysed in radioimmunoprecipitation assay buffer supplemented with the appropriate protease and phosphatase inhibitors. Immunoblot analysis was performed with whole cell lysates run on 4–12% Bis-Tris NuPage (Millipore) gels and transferred onto Immobilon-P Transfer Membranes (Millipore) followed by antibody incubation. Immunoreactive bands were visualized by chemiluminescence.
Chromatin immunoprecipitation.
Chromatin immunoprecipitation (ChIP) was performed using the ChIP-IT High Sensitivity kit (Active Motif) per the manufacturer’s instructions. Chromatin fragmentation was performed by sonication using a Bioruptor (Diagenode). Sheared protein-DNA complexes were immunoprecipitated with anti-PPARγ (Santa Cruz Biotechnology no. H-100) or rabbit IgG control (Santa Cruz Biotechnology). For ChIP-qPCR, enrichment was measured using SYBR Green (Applied Biosystems). Primer sequences can be found in Table 2.
Table 2.
Primers used in ChIP assays for PPARγ binding near the UCP1 and ADIPOQ coding regions
| Gene | L Primer | R Primer |
|---|---|---|
| UCP1 enhancer | CACAAAGAAGAAGCAGAGAGGT | TTGCTGCCACTCCTTTGCTA |
| ADIPOQ downstream | GTGTCTCTGGGTACTTGAGATTG | TGCAGGGTGTGCTTTGTTA |
| Cyclin D1 intron 4 | ACAGCCAGAAGCTCCAAAAA | TGCCACACACCAGTGACTTT |
A commonly used cyclin D1 intronic region was used as negative control. ADIPOQ, adiponectin, C1Q and collagen domain containing; ChIP, chromatin immunoprecipitation; L, left; PPARγ, peroxisome proliferator-activated receptor-γ; R, right; UCP1, uncoupling protein 1.
Statistical analyses.
Data presented were acquired from a minimum of three independent experiments performed on multiple days, unless otherwise indicated. Statistical significance was assessed by unpaired Student’s t test.
RESULTS
BPAF increases lipid accumulation during human adipocyte differentiation.
The effects of BPAF on adipocyte differentiation and broad metabolic end points remain undefined. To this end, we used quantitative microscopy (25, 26) to identify how varying BPAF concentrations impact lipid accumulation in human adipocytes. Human preadipocytes differentiated for 4 days in the presence of 5 µM BPAF grossly showed elevated lipid accumulation (Fig. 1A). We next quantified changes in lipid content using automated cell and nucleus identification. As indicated in Fig. 1B, only 5 µM BPAF increased lipid content significantly (1.7-fold) relative to adipocytes treated with standard adipocyte differentiation cocktail. Total nuclei counts indicated no signs of cytotoxicity at the concentrations of BPAF used (Fig. 1C). In follow-up studies, we found that PPARγ and adipocyte-specific gene expression trended higher during continuous 5 µM BPAF exposure (Fig. 1D). We also tested the capability of BPAF to activate PPARγ using a transient transfection receptor activation assay. Rosiglitazone produced a 10-fold induction over DMSO, whereas 5 µM BPAF also increased reporter activity (Fig. 1E). The combination of 5 µM BPAF and 1 µM rosiglitazone significantly enhanced luciferase induction over single-compound treatments. These data indicate that BPAF displays some PPARγ agonist activities, particularly during early stages of human adipocyte differentiation.
Continuous BPAF exposure during human adipocyte differentiation does not affect PPARγ binding to DNA.
PPARγ transactivation of insulin sensitivity genes is necessary for adipocyte differentiation (69). Higher activation of PPARγ reporter gene activity led us to hypothesize that BPAF impacted PPARγ binding to regulatory regions required for transcriptional induction of adipocyte marker genes. We investigated whether the increased transcriptional activity of PPARγ corresponded with increased occupancy of PPARγ on enhancer regions upstream of UCP1 (6, 33, 35) and downstream of ADIPOQ (34, 44) in human adipocytes treated with 5 µM BPAF. ChIP-qPCR revealed that PPARγ occupancy on the ADIPOQ (Fig. 2A) and UCP1 (Fig. 2B) enhancers was not changed following BPAF treatment during human adipocyte differentiation. As a control, DNA regions deficient in transcription factor binding sites (22) were not occupied by PPARγ under any treatment conditions (Fig. 2C). Together, our findings demonstrate that BPAF does not alter DNA occupancy of PPARγ despite in vitro data demonstrating that BPAF displays agonist activities in human adipocytes.
Fig. 2.
Bisphenol AF (BPAF) does not alter occupancy of peroxisome proliferator-activated receptor-γ (PPARγ) near DNA sequences that regulate transcription of adipocyte marker genes. A and B: after 14-day treatment, PPARγ chromatin immunoprecipitation was performed in human adipocytes. A and B: quantitative PCR was used to analyze genomic occupancy using primers flanking PPARγ binding sites downstream of adiponectin, C1Q and collagen domain containing (ADIPOQ_dwnstrm; A) and within the uncoupling protein 1 enhancer (UCP1_enh; B). C: an intronic region of cyclin D1 (CCND1_intr4) served as a negative control (n = 4 independent experiments). Here, veh, vehicle.
Human adipocytes treated with BPAF exhibit impaired mitochondrial performance.
Mitochondrial biogenesis accompanies adipocyte differentiation to meet the higher demands for fatty acid metabolic processes (70). To determine the effect of BPAF on cellular mitochondrial content, we differentiated human adipocytes in the presence of EtOH (−BPAF) or 5 µM BPAF (+BPAF) for 14 days followed by end point mitochondria labeling with MitoTracker. Lipids were stained with LipidTOX. Adipocytes differentiated in the presence of BPAF showed less intense MitoTracker staining and fewer puncta compared with differentiation controls (Fig. 3A). Consistent with reduced mitochondrial biogenesis, 5 μM BPAF treatment reduced maximal respiratory capacity in adipocytes as measured by oxygen consumption in the presence of the protonophoric uncoupler FCCP (Fig. 3, B and C). In sum, chronic BPAF treatment degrades mitochondrial capacity and reduces cellular respiratory capacity.
Fig. 3.
Bisphenol AF (BPAF) reduces the metabolic performance of human adipocytes. A: mitochondria (MitoTracker), nuclei (DAPI), and lipids (LipidTOX) were labeled in human adipocytes differentiated ± 5 µM BPAF. Scale bars are 20 μm. B: respiration [as oxygen consumption rate (OCR)] was measured in human adipocytes differentiated 14 days in the presence or absence of BPAF concentrations up to 5 μM BPAF. OCR was measured over time with the addition of oligomycin (α), carbonyl cyanide p-trifluoromethoxyphenylhydrazone (FCCP; β), and antimycin A/rotenone (γ). C: maximal respiration capacity was calculated from OCR measurements performed after FCCP addition. Here, n ≥ 4 independent experiments per group. EtOH, ethanol. *P < 0.05 relative to treatment.
BPAF does not suppress inflammatory genes during human adipocyte differentiation.
The observation that BPAF activated PPARγ activity in the early phases of human adipocyte differentiation (Fig. 1) suggested that BPAF increased mature adipocyte formation. To test this hypothesis, we analyzed the expression of adipocyte marker genes that represent insulin sensitivity and metabolic activities in mature fat cells. After differentiation for 14 days, we observed substantial increases in adipocyte marker genes (Fig. 4A). Consistent with the induction of thermogenic and fatty acid metabolism genes during human adipocyte differentiation (1, 5, 19), PPARγ coactivator 1α (PGC1A), cell death-inducing DFFA-like effector a (CIDEA), and UCP1 gene levels were also significantly increased during differentiation (Fig. 4B). Although thermogenic genes increased during human adipocyte differentiation, expression of the brown fat determination marker PR/SET domain 16 (PRDM16; 58) did not change. The presence of BPAF during differentiation did not affect the induction or regulation of these eight marker genes. However, we noticed that BPAF did not allow suppression of the type 1 inflammatory genes STAT1, ISG15 ubiquitin-like modifier (ISG15), and interferon regulatory factor 1 (IRF1) during human adipocyte differentiation (Fig. 4C). We also profiled other inflammatory cytokines and the adipokine leptin (Fig. 4D). Whereas the inflammatory marker angiotensinogen (AGT) was not altered by BPAF during adipocyte differentiation, TNF was only partly suppressed. Although differentiation robustly induced the mRNA encoding the adipokine leptin (LEP), BPAF exposure altered expression. mRNA changes in adipocyte marker proteins and STAT1 were confirmed by immunoblot analysis (Fig. 4E). BPAF treatment did not impact protein expression of adipocyte and mitochondrial markers known to increase during differentiation including fatty acid-binding protein 4 (FABP4), ADIPOQ, PPARγ, and cytochrome c (Cyt C). Adipocyte differentiation (+d) caused a 91% reduction in STAT1 protein levels (−d/−BPAF vs. +d/+BPAF). However, STAT1 showed threefold higher levels in adipocytes differentiated with BPAF (+d/+BPAF) relative to controls (+d/−BPAF). Therefore, our data indicate that BPAF does not largely impact the acquisition of mature adipocyte identity but fails to silence inflammatory genes during differentiation.
Fig. 4.
Inflammatory genes and proteins are not fully suppressed by bisphenol AF (BPAF) during human adipocyte differentiation. A–D: relative mRNA levels were determined for canonical white adipocyte marker genes (A), mitochondrial function genes (B), STAT1 target genes (C), and cytokine genes (D; n ≥ 6 experiments; means ± SE) in human preadipocytes treated ± differentiation (Diff) ± 5 µM BPAF for 14 days. Gene expression was normalized to TATA box-binding protein (TBP) and presented as relative mRNA. *P < 0.05 relative to vehicle; #P < 0.05 relative to no differentiation. E: immunoblot analysis of selected adipocyte metabolic markers and STAT1 in human preadipocytes treated ± differentiation ± 5 µM BPAF for 14 days. Quantification of STAT1 protein levels is expressed as relative change (Δ) vs. no-differentiation (−d) controls. ADIPOQ, adiponectin, C1Q and collagen domain containing; AGT, angiotensinogen; CIDEA, cell death-inducing DFFA-like effector a; Cyt C, cytochrome c; FABP4, fatty acid-binding protein 4; GLUT4, solute carrier family 2 member 4; Hsp60 and Hsp90, 60- and 90-kDa heat shock proteins, respectively; IRF1, interferon regulatory factor 1; ISG15, ISG15 ubiquitin-like modifier; LEP, leptin; PGC1A, peroxisome proliferator-activated receptor-γ coactivator 1α; PPARG, peroxisome proliferator-activated receptor-γ; PRDM16, PR/SET domain 16; rel, relative; UCP1, uncoupling protein 1.
Interferon-γ response in human adipocytes is primed by BPAF treatment.
WAT insulin resistance corresponds with local inflammation (52). STAT1 provides the primary transcriptional response to the type 1 inflammatory cytokine IFNγ. The sustained expression of inflammatory genes during BPAF treatment provided evidence that BPAF contributes to differentiation of an inflammatory adipocyte. We subsequently asked whether BPAF also increases IFNγ sensitivity. To answer this question, we exposed mature adipocytes to IFNγ for short (Fig. 5A) and long intervals (Fig. 5, B–G). In mature adipocytes, IFNγ treatment for 10 min strongly activated STAT1. Adipocytes differentiated in the presence of BPAF showed stronger IFNγ stimulation of STAT1 levels (Fig. 5A). In cells treated continuously with BPAF, STAT1 activation for 24 h corresponded with greater transcription of STAT1 target genes, including STAT1 (Fig. 5B) itself and IRF1 (Fig. 5C). STAT1 activation by IFNγ in BPAF-treated adipocytes also showed more robust suppression of UCP1 indicative of impacted mitochondrial function (Fig. 5D). To further define the transcriptional regulation of UCP1 in this setting, we investigated how BPAF and IFNγ affected the activity of a conserved enhancer that permits PPARγ regulation of the UCP1 gene (12, 41). For this experiment, we compared the abilities of PPARγ and STAT1 to regulate a UCP1-NanoLuc-GFP reporter construct (Fig. 5E). After cotransfection of PPARγ and STAT1, all cells received 1 μM rosiglitazone to potently activate reporter activity. Only 5 μM BPAF showed significant inhibition of luciferase activity. Consistent with BPAF causing increased sensitivity to IFNγ, UCP1-NanoLuc levels showed additive repression under IFNγ and two BPAF concentrations (0.05 and 5 µM). Seahorse assays demonstrated that metabolic activity of adipocytes differentiated with BPAF exhibited lower maximal oxygen consumption rates that resembled the response to IFNγ (Fig. 5F). Consistent with the metabolic effects, we observed that adipocytes that received BPAF showed greater sensitivity to the mitochondrial effects of longer-term IFNγ exposure (Fig. 5G). Mitochondrial proteins that reflect respiratory capacity, including HSP60 and Cyt C, were depleted by IFNγ, especially in adipocytes that received BPAF. These observations suggested that human adipocytes exposed to BPAF harbor intrinsic activation of type 1 inflammatory signaling that contributes to metabolic disruption and blunted mitochondrial gene regulation.
DISCUSSION
In this study, we demonstrated that the EDC BPAF impacts human subcutaneous adipocyte function. Gene expression and metabolic profiles argue that BPAF regulates the activity of PPARγ and, ultimately, impairs the function of mature subcutaneous fat cells. Although many EDCs demonstrate varying and debated effects on adipocyte differentiation (9, 16, 55), BPAF accelerates expression of some early adipocyte marker genes. In mature fat cells, BPAF primarily influences metabolic behaviors and intrinsic receptivity to obesity-related inflammatory signals. These findings portray that persistent BPAF exposure targets metabolic functions of subcutaneous adipocytes and outline a mechanism that links a poorly characterized BPA alternative to risks for the development of obesity and metabolic disorders.
Countless studies document that BPA and its analogs bind a number of nuclear receptors to alter gene regulation in developmental, endocrine, and metabolic contexts (67). Estrogenic activities of BPAF are well established (4, 38, 39), but much less is known about activities on other nuclear receptors. Bulkier halogenation of BPA biases binding toward PPARγ and weakens transactivation of estrogen receptors (53). In our study, we demonstrated that BPAF transactivated PPARγ-dependent reporter gene activity in HepG2 cells and modestly stimulated PPARγ gene transcription during early phases of human adipocyte differentiation. Although our heterologous luciferase reporter assays contrast with other studies (60), molecular dynamic studies predict that BPAF binds to the PPARγ ligand-binding domain and bridges interactions with PPARγ residues in the helix H3, helix H5, and β-sheet structural regions (73). PPARγ full agonists, such as rosiglitazone, attain receptor activation by directly interacting with and stabilizing helix H12, whereas compounds that do not directly contact H12 behave as weak or partial agonists by stabilizing H3 and the β-sheet regions (10). Our functional assays and structural studies support this model of partial PPARγ agonism by BPAF. These molecular dynamics studies indicate that PPARγ activity assays performed with synthetic reporter fusions (60) may not fully uncover how BPAF impacts transcription. Although BPAF exerts agonist and antagonist activities toward other nuclear receptors (32, 60), the effects on lipid synthesis and mitochondrial function in adipocytes implicate that BPAF regulates PPARγ activity.
PPARγ is essential for adipocyte differentiation and regulates the entire cassette of genes that specify mature fat cells (69). Microarray and RNA-sequencing analyses (23, 26, 59) indicate that transcription of mature adipocyte genes coincides with repression of STAT1 and STAT1 target genes. As such, part of the antidiabetic functions of PPARγ agonists requires suppression of transactivators downstream of IFNγ or other cytokines (61). We previously demonstrated that STAT1 interferes with PPARγ activity, which explains how IFNγ restricts insulin sensitivity and lipid metabolism in adipocytes (34). Here we show that BPAF fails to suppress STAT1 in mature human adipocytes, which may allow STAT1 to impede PPARγ activity in the genome. Although BPAF performs PPARγ partial agonist functions, BPAF cultivates an intracellular signaling environment that is silent until triggers for inflammation are sensed. Our data suggest that BPAF leverages STAT1 function to amplify inflammatory responses and disrupt mitochondrial function. Other EDCs including BPA (3, 9, 13, 16, 65, 66), BPA analogs (7, 8), and tributyltin (59) likely also leverage proinflammatory pathways to induce mitochondrial dysfunction in adipocytes. A prominent effect of chronic interferon activation in adipocytes is reduced mitochondrial function and morphology (31, 34). Although the mechanisms by which IFNγ activation reduces mitochondrial function remain unclear, one possibility is that IFNγ activation of STAT1 represses transcription of UCP1 and other important mitochondrial genes. Along these lines, it will now be important to determine whether EDCs amplify effects of other obesity-related cytokines, such as TNFα, that likely exploit STAT1 or other inflammatory transcription factors to transduce signals that disrupt mitochondrial gene transcription in adipose tissues.
Efforts to synthesize alternative plasticizers include combinations of halide substitutions to the propane bridge of BPA. The potential toxicity of BPAF raises concern in part because -CF3 groups are more electronegative and potentially reactive than the -CH3 group of BPA (43). Broadly, BPAF damages DNA (46) and disrupts nuclear morphology. These impacts generate reproductive toxicities and sublethal effects in model organisms (40, 62, 64, 71). We did not detect any toxic effects of BPAF on human adipocytes. However, BPAF diminished mitochondrial activity and volume coupled with reduced respiratory capacity. In the respiration assays, the effects of the mitochondrial electron transport chain inhibitor FCCP on oxygen consumption rate allow estimation of maximal respiratory capacity and indirect assessment of the ability of cells to respond to increased energy demands (11). Adipocytes differentiated with BPAF show restricted respiratory capacity, which suggests that these cells cannot adequately metabolize sugars, fats, and amino acids when exposed to EDC stresses. Ultimately, these structural and metabolic defects rendered adipocytes vulnerable to the metabolic effects of IFNγ, including disruption of metabolic flexibility and depletion of critical mitochondrial proteins. Like BPA, BPAF presumably partitions into adipocyte mitochondrial membranes that contain interior hydrophobic proteins (36, 48, 50). Oxidative stresses likely follow to damage nucleic acids, lipids, and proteins, contributing to the onset of metabolic dysfunction in fat cells (21, 27) and mitochondrial impairments observed in WAT of obese individuals (5, 15).
In 2008, the National Toxicology Program noted concern regarding potential human exposures to BPAF (47). BPAF accumulates in human adipose tissues and, consequently, may preferentially target and alter the endocrine function of adipocytes. It is important to emphasize the ongoing fallacy in assuming that increased adipocyte differentiation drives obesity and its comorbidities (54). Obesity imparts complex effects on adipogenesis and fat cell hypertrophy that represent the whole body protective response to overnutrition. Consistent with these notions, reduced adipocyte differentiation correlates with insulin resistance and other comorbidities of obesity. Our observations point to the concept that BPAF and other endocrine disruptors (16, 59) predispose adipocytes to inflammatory signals that impinge upon the endocrine and metabolic functions of WAT.
To our knowledge, we are the first to show that BPAF degrades the function of human adipocytes. Surprisingly, despite the fact that BPAF and BPA share similar structures, BPAF shows reduced clearance rates in mice, rats, and humans (68) and exerts 1,000-fold greater toxicity in aquatic organisms (2). It is difficult to determine, on the basis of in vitro exposures, whether the potency of BPAF influences physiology sufficiently to affect the metabolic profile of obesity. Estimated dietary intake of BPAF is ~0.5 ng/kg body wt or ~1 order of magnitude lower than that of BPA (42). Nonetheless, the increased environmental accumulation of BPA alternatives motivates a need to understand how BPAF exerts effects on endocrine tissues. The wide-ranging potency of BPAF toward other metabolic transcription factors (60) coupled with the proinflammatory effects in human adipocytes should motivate future toxicology studies that investigate the influences of BPAF on energy balance and physiology.
GRANTS
This work was funded by NIH Grants K01-DK-096093, R03-DK-105006, and R01-DK-114356 and American Diabetes Association Grant 1-18-IBS-105 (S. M. Hartig).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
N.C., P.M., and S.M.H. conceived and designed research; N.C., P.M., and S.M.H. performed experiments; N.C., P.M., and S.M.H. analyzed data; N.C., P.M., A.R.C., and S.M.H. interpreted results of experiments; S.M.H. prepared figures; S.M.H. drafted manuscript; N.C., P.M., A.R.C., and S.M.H. edited and revised manuscript; N.C., P.M., A.R.C., and S.M.H. approved final version of manuscript.
REFERENCES
- 1.Adams M, Montague CT, Prins JB, Holder JC, Smith SA, Sanders L, Digby JE, Sewter CP, Lazar MA, Chatterjee VK, O’Rahilly S. Activators of peroxisome proliferator-activated receptor gamma have depot-specific effects on human preadipocyte differentiation. J Clin Invest 100: 3149–3153, 1997. doi: 10.1172/JCI119870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arancio AL, Cole KD, Dominguez AR, Cohenour ER, Kadie J, Maloney WC, Cilliers C, Schuh SM. Bisphenol A, Bisphenol AF, di-n-butyl phthalate, and 17β-estradiol have shared and unique dose-dependent effects on early embryo cleavage divisions and development in Xenopus laevis. Reprod Toxicol 84: 65–74, 2019. doi: 10.1016/j.reprotox.2018.12.005. [DOI] [PubMed] [Google Scholar]
- 3.Ariemma F, D’Esposito V, Liguoro D, Oriente F, Cabaro S, Liotti A, Cimmino I, Longo M, Beguinot F, Formisano P, Valentino R. Low dose Bisphenol A impairs adipogenesis and generates dysfunctional 3T3-L1 adipocytes. PLoS One 11: e0150762, 2016. doi: 10.1371/journal.pone.0150762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bermudez DS, Gray LE Jr, Wilson VS. Modeling the interaction of binary and ternary mixtures of estradiol with bisphenol A and bisphenol AF in an in vitro estrogen-mediated transcriptional activation assay (T47D-KBluc). Toxicol Sci 116: 477–487, 2010. doi: 10.1093/toxsci/kfq156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bogacka I, Ukropcova B, McNeil M, Gimble JM, Smith SR. Structural and functional consequences of mitochondrial biogenesis in human adipocytes in vitro. J Clin Endocrinol Metab 90: 6650–6656, 2005. doi: 10.1210/jc.2005-1024. [DOI] [PubMed] [Google Scholar]
- 6.Bordicchia M, Liu D, Amri EZ, Ailhaud G, Dessì-Fulgheri P, Zhang C, Takahashi N, Sarzani R, Collins S. Cardiac natriuretic peptides act via p38 MAPK to induce the brown fat thermogenic program in mouse and human adipocytes. J Clin Invest 122: 1022–1036, 2012. doi: 10.1172/JCI59701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boucher JG, Ahmed S, Atlas E. Bisphenol S induces adipogenesis in primary human preadipocytes from female donors. Endocrinology 157: 1397–1407, 2016. doi: 10.1210/en.2015-1872. [DOI] [PubMed] [Google Scholar]
- 8.Boucher JG, Gagné R, Rowan-Carroll A, Boudreau A, Yauk CL, Atlas E. Bisphenol A and Bisphenol S induce distinct transcriptional profiles in differentiating human primary preadipocytes. PLoS One 11: e0163318, 2016. doi: 10.1371/journal.pone.0163318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boucher JG, Husain M, Rowan-Carroll A, Williams A, Yauk CL, Atlas E. Identification of mechanisms of action of bisphenol a-induced human preadipocyte differentiation by transcriptional profiling. Obesity (Silver Spring) 22: 2333–2343, 2014. doi: 10.1002/oby.20848. [DOI] [PubMed] [Google Scholar]
- 10.Bruning JB, Chalmers MJ, Prasad S, Busby SA, Kamenecka TM, He Y, Nettles KW, Griffin PR. Partial agonists activate PPARγ using a helix 12 independent mechanism. Structure 15: 1258–1271, 2007. doi: 10.1016/j.str.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 11.Bugge A, Dib L, Collins S. Measuring respiratory activity of adipocytes and adipose tissues in real time. Methods Enzymol 538: 233–247, 2014. doi: 10.1016/B978-0-12-800280-3.00013-X. [DOI] [PubMed] [Google Scholar]
- 12.Cao W, Daniel KW, Robidoux J, Puigserver P, Medvedev AV, Bai X, Floering LM, Spiegelman BM, Collins S. p38 mitogen-activated protein kinase is the central regulator of cyclic AMP-dependent transcription of the brown fat uncoupling protein 1 gene. Mol Cell Biol 24: 3057–3067, 2004. doi: 10.1128/MCB.24.7.3057-3067.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cimmino I, Oriente F, D’Esposito V, Liguoro D, Liguoro P, Ambrosio MR, Cabaro S, D’Andrea F, Beguinot F, Formisano P, Valentino R. Low dose Bisphenol A regulates inflammatory cytokines through GPR30 in mammary adipose cells. J Mol Endocrinol 63: 273–283, 2019. doi: 10.1530/JME-18-0265. [DOI] [PubMed] [Google Scholar]
- 14.Cohen P, Levy JD, Zhang Y, Frontini A, Kolodin DP, Svensson KJ, Lo JC, Zeng X, Ye L, Khandekar MJ, Wu J, Gunawardana SC, Banks AS, Camporez JP, Jurczak MJ, Kajimura S, Piston DW, Mathis D, Cinti S, Shulman GI, Seale P, Spiegelman BM. Ablation of PRDM16 and beige adipose causes metabolic dysfunction and a subcutaneous to visceral fat switch. Cell 156: 304–316, 2014. doi: 10.1016/j.cell.2013.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dahlman I, Forsgren M, Sjögren A, Nordström EA, Kaaman M, Näslund E, Attersand A, Arner P. Downregulation of electron transport chain genes in visceral adipose tissue in type 2 diabetes independent of obesity and possibly involving tumor necrosis factor-α. Diabetes 55: 1792–1799, 2006. doi: 10.2337/db05-1421. [DOI] [PubMed] [Google Scholar]
- 16.De Filippis E, Li T, Rosen ED. Exposure of adipocytes to bisphenol-A in vitro interferes with insulin action without enhancing adipogenesis. PLoS One 13: e0201122, 2018. doi: 10.1371/journal.pone.0201122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Després JP. Body fat distribution and risk of cardiovascular disease: an update. Circulation 126: 1301–1313, 2012. doi: 10.1161/CIRCULATIONAHA.111.067264. [DOI] [PubMed] [Google Scholar]
- 18.Diamanti-Kandarakis E, Bourguignon JP, Giudice LC, Hauser R, Prins GS, Soto AM, Zoeller RT, Gore AC. Endocrine-disrupting chemicals: an Endocrine Society scientific statement. Endocr Rev 30: 293–342, 2009. doi: 10.1210/er.2009-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Digby JE, Montague CT, Sewter CP, Sanders L, Wilkison WO, O’Rahilly S, Prins JB. Thiazolidinedione exposure increases the expression of uncoupling protein 1 in cultured human preadipocytes. Diabetes 47: 138–141, 1998. doi: 10.2337/diab.47.1.138. [DOI] [PubMed] [Google Scholar]
- 20.Frayn KN, Langin D, Karpe F. Fatty acid-induced mitochondrial uncoupling in adipocytes is not a promising target for treatment of insulin resistance unless adipocyte oxidative capacity is increased. Diabetologia 51: 394–397, 2008. doi: 10.1007/s00125-007-0901-z. [DOI] [PubMed] [Google Scholar]
- 21.Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, Nakayama O, Makishima M, Matsuda M, Shimomura I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest 114: 1752–1761, 2004. doi: 10.1172/JCI21625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gates LA, Gu G, Chen Y, Rohira AD, Lei JT, Hamilton RA, Yu Y, Lonard DM, Wang J, Wang SP, Edwards DG, Lavere PF, Shao J, Yi P, Jain A, Jung SY, Malovannaya A, Li S, Shao J, Roeder RG, Ellis MJ, Qin J, Fuqua SA, O’Malley BW, Foulds CE. Proteomic profiling identifies key coactivators utilized by mutant ERα proteins as potential new therapeutic targets. Oncogene 37: 4581–4598, 2018. doi: 10.1038/s41388-018-0284-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gerhold DL, Liu F, Jiang G, Li Z, Xu J, Lu M, Sachs JR, Bagchi A, Fridman A, Holder DJ, Doebber TW, Berger J, Elbrecht A, Moller DE, Zhang BB. Gene expression profile of adipocyte differentiation and its regulation by peroxisome proliferator-activated receptor-γ agonists. Endocrinology 143: 2106–2118, 2002. doi: 10.1210/endo.143.6.8842. [DOI] [PubMed] [Google Scholar]
- 24.Hales CM, Fryar CD, Carroll MD, Freedman DS, Aoki Y, Ogden CL. Differences in obesity prevalence by demographic characteristics and urbanization level among adults in the United States, 2013–2016. JAMA 319: 2419–2429, 2018. doi: 10.1001/jama.2018.7270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hartig SM, He B, Long W, Buehrer BM, Mancini MA. Homeostatic levels of SRC-2 and SRC-3 promote early human adipogenesis. J Cell Biol 192: 55–67, 2011. doi: 10.1083/jcb.201004026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hartig SM, He B, Newberg JY, Ochsner SA, Loose DS, Lanz RB, McKenna NJ, Buehrer BM, McGuire SE, Marcelli M, Mancini MA. Feed-forward inhibition of androgen receptor activity by glucocorticoid action in human adipocytes. Chem Biol 19: 1126–1141, 2012. doi: 10.1016/j.chembiol.2012.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hauck AK, Huang Y, Hertzel AV, Bernlohr DA. Adipose oxidative stress and protein carbonylation. J Biol Chem 294: 1083–1088, 2019. doi: 10.1074/jbc.R118.003214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heindel JJ, Blumberg B. Environmental obesogens: mechanisms and controversies. Annu Rev Pharmacol Toxicol 59: 89–106, 2019. doi: 10.1146/annurev-pharmtox-010818-021304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hotamisligil GS, Arner P, Caro JF, Atkinson RL, Spiegelman BM. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J Clin Invest 95: 2409–2415, 1995. doi: 10.1172/JCI117936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kintscher U, Hartge M, Hess K, Foryst-Ludwig A, Clemenz M, Wabitsch M, Fischer-Posovszky P, Barth TF, Dragun D, Skurk T, Hauner H, Blüher M, Unger T, Wolf AM, Knippschild U, Hombach V, Marx N. T-lymphocyte infiltration in visceral adipose tissue: a primary event in adipose tissue inflammation and the development of obesity-mediated insulin resistance. Arterioscler Thromb Vasc Biol 28: 1304–1310, 2008. doi: 10.1161/ATVBAHA.108.165100. [DOI] [PubMed] [Google Scholar]
- 31.Kissig M, Ishibashi J, Harms MJ, Lim HW, Stine RR, Won KJ, Seale P. PRDM16 represses the type I interferon response in adipocytes to promote mitochondrial and thermogenic programing. EMBO J 36: 1528–1542, 2017. doi: 10.15252/embj.201695588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kitamura S, Suzuki T, Sanoh S, Kohta R, Jinno N, Sugihara K, Yoshihara S, Fujimoto N, Watanabe H, Ohta S. Comparative study of the endocrine-disrupting activity of bisphenol A and 19 related compounds. Toxicol Sci 84: 249–259, 2005. doi: 10.1093/toxsci/kfi074. [DOI] [PubMed] [Google Scholar]
- 33.Koh EH, Chen Y, Bader DA, Hamilton MP, He B, York B, Kajimura S, McGuire SE, Hartig SM. Mitochondrial activity in human white adipocytes is regulated by the ubiquitin carrier protein 9/microRNA-30a axis. J Biol Chem 291: 24747–24755, 2016. doi: 10.1074/jbc.M116.749408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koh EH, Chernis N, Saha PK, Xiao L, Bader DA, Zhu B, Rajapakshe K, Hamilton MP, Liu X, Perera D, Chen X, York B, Trauner M, Coarfa C, Bajaj M, Moore DD, Deng T, McGuire SE, Hartig SM. miR-30a remodels subcutaneous adipose tissue inflammation to improve insulin sensitivity in obesity. Diabetes 67: 2541–2553, 2018. doi: 10.2337/db17-1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kozak UC, Kopecky J, Teisinger J, Enerbäck S, Boyer B, Kozak LP. An upstream enhancer regulating brown-fat-specific expression of the mitochondrial uncoupling protein gene. Mol Cell Biol 14: 59–67, 1994. doi: 10.1128/MCB.14.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Law P, Campbell SD, Lepock JR, Kruuv J. Effects of butylated hydroxytoluene on membrane lipid fluidity and freeze-thaw survival in mammalian cells. Cryobiology 23: 317–322, 1986. doi: 10.1016/0011-2240(86)90037-4. [DOI] [PubMed] [Google Scholar]
- 37.Lee JM, Wagner M, Xiao R, Kim KH, Feng D, Lazar MA, Moore DD. Nutrient-sensing nuclear receptors coordinate autophagy. Nature 516: 112–115, 2014. doi: 10.1038/nature13961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li Y, Burns KA, Arao Y, Luh CJ, Korach KS. Differential estrogenic actions of endocrine-disrupting chemicals bisphenol A, bisphenol AF, and zearalenone through estrogen receptor α and β in vitro. Environ Health Perspect 120: 1029–1035, 2012. doi: 10.1289/ehp.1104689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li Y, Perera L, Coons LA, Burns KA, Tyler Ramsey J, Pelch KE, Houtman R, van Beuningen R, Teng CT, Korach KS. Differential in vitro biological action, coregulator interactions, and molecular dynamic analysis of Bisphenol A (BPA), BPAF, and BPS ligand-ERα complexes. Environ Health Perspect 126: 017012, 2018. doi: 10.1289/EHP2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liang S, Yin L, Shengyang Yu K, Hofmann MC, Yu X. High-content analysis provides mechanistic insights into the testicular toxicity of bisphenol A and selected analogues in mouse spermatogonial cells. Toxicol Sci 155: 43–60, 2017. doi: 10.1093/toxsci/kfw178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lodhi IJ, Dean JM, He A, Park H, Tan M, Feng C, Song H, Hsu FF, Semenkovich CF. PexRAP inhibits PRDM16-mediated thermogenic gene expression. Cell Reports 20: 2766–2774, 2017. doi: 10.1016/j.celrep.2017.08.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lorber M, Schecter A, Paepke O, Shropshire W, Christensen K, Birnbaum L. Exposure assessment of adult intake of bisphenol A (BPA) with emphasis on canned food dietary exposures. Environ Int 77: 55–62, 2015. doi: 10.1016/j.envint.2015.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Matsushima A, Liu X, Okada H, Shimohigashi M, Shimohigashi Y. Bisphenol AF is a full agonist for the estrogen receptor ERα but a highly specific antagonist for ERβ. Environ Health Perspect 118: 1267–1272, 2010. doi: 10.1289/ehp.0901819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mikkelsen TS, Xu Z, Zhang X, Wang L, Gimble JM, Lander ES, Rosen ED. Comparative epigenomic analysis of murine and human adipogenesis. Cell 143: 156–169, 2010. doi: 10.1016/j.cell.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moisan A, Lee YK, Zhang JD, Hudak CS, Meyer CA, Prummer M, Zoffmann S, Truong HH, Ebeling M, Kiialainen A, Gérard R, Xia F, Schinzel RT, Amrein KE, Cowan CA. White-to-brown metabolic conversion of human adipocytes by JAK inhibition. Nat Cell Biol 17: 57–67, 2015. doi: 10.1038/ncb3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mokra K, Woźniak K, Bukowska B, Sicińska P, Michałowicz J. Low-concentration exposure to BPA, BPF and BPAF induces oxidative DNA bases lesions in human peripheral blood mononuclear cells. Chemosphere 201: 119–126, 2018. doi: 10.1016/j.chemosphere.2018.02.166. [DOI] [PubMed] [Google Scholar]
- 47.National Toxicology Program Chemical Information Profile for Bisphenol AF [CAS No. 1478-61-1]: Supporting Nomination for Toxicological Evaluation by the National Toxicology Program. Research Triangle Park, NC: U.S. Department of Health and Human Services, 2008. [Google Scholar]
- 48.Nunez AA, Kannan K, Giesy JP, Fang J, Clemens LG. Effects of Bisphenol A on energy balance and accumulation in brown adipose tissue in rats. Chemosphere 42: 917–922, 2001. doi: 10.1016/S0045-6535(00)00196-X. [DOI] [PubMed] [Google Scholar]
- 49.Ohno H, Shinoda K, Spiegelman BM, Kajimura S. PPARγ agonists induce a white-to-brown fat conversion through stabilization of PRDM16 protein. Cell Metab 15: 395–404, 2012. doi: 10.1016/j.cmet.2012.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ooe H, Taira T, Iguchi-Ariga SM, Ariga H. Induction of reactive oxygen species by bisphenol A and abrogation of bisphenol A-induced cell injury by DJ-1. Toxicol Sci 88: 114–126, 2005. doi: 10.1093/toxsci/kfi278. [DOI] [PubMed] [Google Scholar]
- 51.Petrovic N, Walden TB, Shabalina IG, Timmons JA, Cannon B, Nedergaard J. Chronic peroxisome proliferator-activated receptor γ (PPARγ) activation of epididymally derived white adipocyte cultures reveals a population of thermogenically competent, UCP1-containing adipocytes molecularly distinct from classic brown adipocytes. J Biol Chem 285: 7153–7164, 2010. doi: 10.1074/jbc.M109.053942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Reilly SM, Saltiel AR. Adapting to obesity with adipose tissue inflammation. Nat Rev Endocrinol 13: 633–643, 2017. doi: 10.1038/nrendo.2017.90. [DOI] [PubMed] [Google Scholar]
- 53.Riu A, Grimaldi M, le Maire A, Bey G, Phillips K, Boulahtouf A, Perdu E, Zalko D, Bourguet W, Balaguer P. Peroxisome proliferator-activated receptor γ is a target for halogenated analogs of bisphenol A. Environ Health Perspect 119: 1227–1232, 2011. doi: 10.1289/ehp.1003328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rosen ED, Spiegelman BM. What we talk about when we talk about fat. Cell 156: 20–44, 2014. doi: 10.1016/j.cell.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sargis RM, Johnson DN, Choudhury RA, Brady MJ. Environmental endocrine disruptors promote adipogenesis in the 3T3-L1 cell line through glucocorticoid receptor activation. Obesity (Silver Spring) 18: 1283–1288, 2010. doi: 10.1038/oby.2009.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schaufele F, Carbonell X, Guerbadot M, Borngraeber S, Chapman MS, Ma AA, Miner JN, Diamond MI. The structural basis of androgen receptor activation: intramolecular and intermolecular amino-carboxy interactions. Proc Natl Acad Sci USA 102: 9802–9807, 2005. doi: 10.1073/pnas.0408819102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schmidt FM, Weschenfelder J, Sander C, Minkwitz J, Thormann J, Chittka T, Mergl R, Kirkby KC, Faßhauer M, Stumvoll M, Holdt LM, Teupser D, Hegerl U, Himmerich H. Inflammatory cytokines in general and central obesity and modulating effects of physical activity. PLoS One 10: e0121971, 2015. doi: 10.1371/journal.pone.0121971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Seale P, Conroe HM, Estall J, Kajimura S, Frontini A, Ishibashi J, Cohen P, Cinti S, Spiegelman BM. Prdm16 determines the thermogenic program of subcutaneous white adipose tissue in mice. J Clin Invest 121: 96–105, 2011. doi: 10.1172/JCI44271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shoucri BM, Hung VT, Chamorro-García R, Shioda T, Blumberg B. Retinoid X receptor activation during adipogenesis of female mesenchymal stem cells programs a dysfunctional adipocyte. Endocrinology 159: 2863–2883, 2018. doi: 10.1210/en.2018-00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Skledar DG, Carino A, Trontelj J, Troberg J, Distrutti E, Marchianò S, Tomašič T, Zega A, Finel M, Fiorucci S, Mašič LP. Endocrine activities and adipogenic effects of bisphenol AF and its main metabolite. Chemosphere 215: 870–880, 2019. doi: 10.1016/j.chemosphere.2018.10.129. [DOI] [PubMed] [Google Scholar]
- 61.Soccio RE, Li Z, Chen ER, Foong YH, Benson KK, Dispirito JR, Mullican SE, Emmett MJ, Briggs ER, Peed LC, Dzeng RK, Medina CJ, Jolivert JF, Kissig M, Rajapurkar SR, Damle M, Lim HW, Won KJ, Seale P, Steger DJ, Lazar MA. Targeting PPARγ in the epigenome rescues genetic metabolic defects in mice. J Clin Invest 127: 1451–1462, 2017. doi: 10.1172/JCI91211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Song M, Liang D, Liang Y, Chen M, Wang F, Wang H, Jiang G. Assessing developmental toxicity and estrogenic activity of halogenated bisphenol A on zebrafish (Danio rerio). Chemosphere 112: 275–281, 2014. doi: 10.1016/j.chemosphere.2014.04.084. [DOI] [PubMed] [Google Scholar]
- 63.Tiraby C, Tavernier G, Lefort C, Larrouy D, Bouillaud F, Ricquier D, Langin D. Acquirement of brown fat cell features by human white adipocytes. J Biol Chem 278: 33370–33376, 2003. doi: 10.1074/jbc.M305235200. [DOI] [PubMed] [Google Scholar]
- 64.Tišler T, Krel A, Gerželj U, Erjavec B, Dolenc MS, Pintar A. Hazard identification and risk characterization of bisphenols A, F and AF to aquatic organisms. Environ Pollut 212: 472–479, 2016. doi: 10.1016/j.envpol.2016.02.045. [DOI] [PubMed] [Google Scholar]
- 65.Tsou TC, Yeh SC, Hsu JW, Tsai FY. Estrogenic chemicals at body burden levels attenuate energy metabolism in 3T3-L1 adipocytes. J Appl Toxicol 37: 1537–1546, 2017. doi: 10.1002/jat.3508. [DOI] [PubMed] [Google Scholar]
- 66.Valentino R, D’Esposito V, Passaretti F, Liotti A, Cabaro S, Longo M, Perruolo G, Oriente F, Beguinot F, Formisano P. Bisphenol-A impairs insulin action and up-regulates inflammatory pathways in human subcutaneous adipocytes and 3T3-L1 cells. PLoS One 8: e82099, 2013. doi: 10.1371/journal.pone.0082099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Veiga-Lopez A, Pu Y, Gingrich J, Padmanabhan V. Obesogenic endocrine disrupting chemicals: identifying knowledge gaps. Trends Endocrinol Metab 29: 607–625, 2018. doi: 10.1016/j.tem.2018.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Waidyanatha S, Mathews JM, Patel PR, Black SR, Snyder RW, Fennell TR. Disposition of bisphenol AF, a bisphenol A analogue, in hepatocytes in vitro and in male and female Harlan Sprague-Dawley rats and B6C3F1/N mice following oral and intravenous administration. Xenobiotica 45: 811–819, 2015. doi: 10.3109/00498254.2015.1021732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang F, Mullican SE, DiSpirito JR, Peed LC, Lazar MA. Lipoatrophy and severe metabolic disturbance in mice with fat-specific deletion of PPARγ. Proc Natl Acad Sci USA 110: 18656–18661, 2013. doi: 10.1073/pnas.1314863110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wilson-Fritch L, Burkart A, Bell G, Mendelson K, Leszyk J, Nicoloro S, Czech M, Corvera S. Mitochondrial biogenesis and remodeling during adipogenesis and in response to the insulin sensitizer rosiglitazone. Mol Cell Biol 23: 1085–1094, 2003. doi: 10.1128/MCB.23.3.1085-1094.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yang X, Liu Y, Li J, Chen M, Peng D, Liang Y, Song M, Zhang J, Jiang G. Exposure to Bisphenol AF disrupts sex hormone levels and vitellogenin expression in zebrafish. Environ Toxicol 31: 285–294, 2016. doi: 10.1002/tox.22043. [DOI] [PubMed] [Google Scholar]
- 72.Yehuda-Shnaidman E, Buehrer B, Pi J, Kumar N, Collins S. Acute stimulation of white adipocyte respiration by PKA-induced lipolysis. Diabetes 59: 2474–2483, 2010. [Erratum in Diabetes 60: 691, 2011]. doi: 10.2337/db10-0245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhuang S, Zhang C, Liu W. Atomic insights into distinct hormonal activities of Bisphenol A analogues toward PPARγ and ERα receptors. Chem Res Toxicol 27: 1769–1779, 2014. doi: 10.1021/tx500232b. [DOI] [PubMed] [Google Scholar]