Abstract
Reactive oxygen species (ROS) are important signaling molecules mediating the exercise-induced adaptations in skeletal muscle. Acute exercise also drives the expression of genes involved in reesterification and glyceroneogenesis in white adipose tissue (WAT), but whether ROS play any role in this effect has not been explored. We speculated that exercise-induced ROS would regulate acute exercise-induced responses in WAT. To address this question, we utilized various models to alter redox signaling in WAT. We examined basal and exercise-induced gene expression in a genetically modified mouse model of reduced mitochondrial ROS emission [mitochondrial catalase overexpression (MCAT)]. Additionally, H2O2, various antioxidants, and the β3-adrenergic receptor agonist CL316243 were used to assess gene expression in white adipose tissue culture. MCAT mice have reduced ROS emission from WAT, enlarged WAT depots and adipocytes, and greater pyruvate dehydrogenase kinase-4 (Pdk4) gene expression. In WAT culture, H2O2 reduced glyceroneogenic gene expression. In wild-type mice, acute exercise induced dramatic but transient increases in Pdk4 and phosphoenolpyruvate carboxykinase (Pck1) mRNA in both subcutaneous inguinal WAT and epididymal WAT depots, which was almost completely absent in MCAT mice. Furthermore, the induction of Pdk4 and Pck1 in WAT culture by CL316243 was markedly reduced in the presence of antioxidants N-acetyl-cysteine or vitamin E. Genetic and nutritional approaches that attenuate redox signaling prevent exercise- and β-agonist-induced gene expression within WAT. Combined, these data suggest that ROS represent important mediators of gene expression within WAT.
Keywords: adipose tissue, exercise, glyceroneogenesis, reactive oxygen species, reesterification
INTRODUCTION
Exercise represents a substantial cellular stress in skeletal muscle, increasing ATP requirements by ~100-fold (18). Increased cellular energy demands stimulate mitochondrial activity to maintain ATP levels, but a natural consequence of oxidative phosphorylation is the production of reactive oxygen species (ROS; 9). Classically, the concomitant increase in cellular ADP during exercise was believed to minimize mitochondrial ROS emission (1, 31), and as a result, extramitochondrial ROS sources were primarily believed to contribute to the well-established exercise-induced redox stress (12, 15, 52). However, this notion is starting to be challenged, as we have recently shown that exercise causes a reduction in mitochondrial ADP sensitivity (36), which increases mitochondrial ROS emission in the presence of ADP (19). When exercise-induced ROS production is reduced, we observed attenuated exercise-induced gene expression of peroxisome proliferator-activated receptor-γ coactivator 1-α (Ppargc1a) and pyruvate dehydrogenase kinase-4 (Pdk4) in skeletal muscle (36). Moreover, exercise/muscle contraction induces redox changes in the ryanodine receptor, increasing intracellular calcium concentrations in association with the induction of Ppargc1a, responses that were ablated by the antioxidant N-acetyl-cysteine (NAC; 44). In further support of the notion that ROS is a key signaling molecule regulating exercise-induced gene expression, various antioxidants completely prevent the electrostimulated induction of Ppargc1a, uncoupling protein-3 (Ucp3), and hexokinase 2 (Hk2) in rat skeletal muscle cells (55), whereas antioxidant supplementation (vitamins E and/or C) blocks the beneficial adaptations to exercise in humans [e.g., insulin sensitivity and mitochondrial biogenesis (13, 48)]. Together, these data highlight that ROS are important for exercise-induced adaptations in skeletal muscle.
Beyond skeletal muscle, exercise also causes numerous beneficial metabolic adaptations in white adipose tissue (WAT) such as inducing markers of mitochondrial biogenesis, increasing adipocyte insulin sensitivity, reducing inflammation, and altering the secretion of adipokines (10, 43, 56, 63). We have also shown that acute exercise increases the expression of key genes involved in fatty acid reesterification in WAT (66). During exercise there is a large increase in the rate of lipolysis with a linear and proportionate increase in reesterification (5, 59, 62) that depends on the provision of glycerol-3-phosphate (G3P) to serve as the backbone for triglycerides. Glyceroneogenesis is the primary source of G3P in WAT (27, 40) and is regulated, at least in part, by PDK4 and phosphoenolpyruvate carboxykinase (PEPCK; 6, 11). PDK4 inhibits the pyruvate dehydrogenase complex, thereby shuttling pyruvate away from oxidation and toward G3P (6). PEPCK catalyzes the conversion of oxaloacetate to phosphoenolpyruvate, which eventually becomes G3P, and in this way, PDK4 and PEPCK regulate glyceroneogenesis and reesterification in WAT (6, 11, 59). Despite the importance of glyceroneogenesis for WAT and, ultimately, whole body metabolic homeostasis (6, 11, 59), the molecular signals regulating PDK4 and PEPCK remain incompletely understood.
Whereas redox signaling is implicated in exercise-induced skeletal muscle gene expression, far less is known regarding the possibility that redox signaling is important for exercise-induced adaptations in WAT (25). Much of what is known about oxidative stress in WAT comes from models of obesity and hyperglycemia, both of which increase ROS and oxidative stress in WAT (12, 29). Mice with reduced mitochondrial ROS are protected from high fat diet-induced insulin resistance in WAT (41). Moreover, oxidative stress was associated with reduced WAT expression of peroxisome proliferator-activated receptor-γ (Pparg, also known as Pparg2; 12, 29), a transcription factor that regulates transcription of PDK4 and PEPCK (58, 66), whereas hydrogen peroxide (H2O2) dose-dependently reduced Pparg expression in 3T3-L1 adipocytes (12). Given that ROS regulate exercise-induced gene expression in skeletal muscle (36) and the ability of ROS to directly alter gene expression in WAT (12), we investigated the possibility that exercise-induced ROS may play a role in regulating the expression of glyceroneogenic enzymes in WAT. With this in mind, we used transgenic mice that overexpress human catalase in mitochondria (MCAT mice) to explore WAT metabolism in vivo. We also tested the effects of various antioxidants on adrenergic-stimulated gene expression in WAT culture, using the potent and specific β3-adrenergic receptor agonist CL316243 (CL). We chose to use CL316243 as it is a potent stimulator of lipolysis and has previously been used to examine the regulation of gene expression in adipocytes (37–39). We hypothesized that reducing ROS would attenuate the induction of glyceroneogenic enzymes in WAT.
METHODS
Ethics.
All experiments were approved by the Animal Care Committee at the University of Guelph and were in compliance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Mitochondrial-targeted catalase transgenic mice.
We have previously demonstrated that MCAT mice have reduced ROS production from skeletal muscle (2) and retain insulin sensitivity in WAT after high fat diet-induced obesity (41). Thus, we used these mice to test whether manipulating ROS emission impacts glyceroneogenic gene expression in WAT. Male C57BL/6J [wild-type (WT)] and MCAT mice on the same C57BL/6J background were purchased from the Jackson Laboratory (Bar Harbor, ME). Animals were single housed on a 12:12-h light-dark cycle at room temperature with unrestricted access to a standard chow diet (no. 7004; Teklad) and water. Fifteen-week-old male mice were familiarized to motorized rodent treadmills (Exer-3R treadmill; Columbus Instruments) for 10 min at 15 m/min and a grade of 5% over a 4-day period, as previously described (2). Seventy-two hours after the final acclimation session, mice were randomly selected to remain sedentary or exercise for 90 min (15 m/min and a grade of 5%). Exercised mice were either euthanized immediately postexercise or allowed to recover in their cage for 3 h with free access to food and water. Mice were anesthetized with isoflurane-oxygen (2:98%), and tissues were quickly removed, weighed, snap-frozen in liquid nitrogen, and stored at −80°C until later analysis. Subcutaneous inguinal WAT (iWAT) and epididymal WAT (eWAT) were also fixed in 10% buffered formalin for 24 h, transferred to 70% ethanol, stained with hematoxylin and eosin, and magnified at ×40. Images were captured (Olympus FSX 100 light microscope), and adipocyte cell size was quantified using ImageJ software (60).
Determination of mitochondrial ROS emission.
To measure mitochondrial ROS emission, the rate of H2O2 release from epididymal WAT was determined by Amplex Red fluorescence quantification (Invitrogen) at 37°C as previously reported (2). WAT was added to a constantly stirring cuvette containing 10 mM Amplex Red reagent, 40 U/mL superoxide dismutase (SOD), 1 U/mL horseradish peroxidase, 10 μg/mL digitonin, and 7 μg/mL oligomycin (41). SOD was used to ensure the conversion of superoxide radicals to H2O2. Mitochondrial H2O2 emission was initiated by the addition of either 20 mM succinate, 5 mM pyruvate plus 2 mM malate, or 50 μM palmitoyl-CoA plus 5 mM l-carnitine. Using this basic methodology, in the absence of digitonin and oligomycin, we also assessed the ability of 10 μM CL to stimulate eWAT H2O2 emission, presumably through the liberation of free fatty acids. We further interrogated the possibility of CL stimulating mitochondrial H2O2 emission by examining the ability of a mitochondrial uncoupler [2,4-dinitrophenol (DNP)] to mitigate the CL-mediated drive on ROS. In all experiments, raw fluorescence was calibrated to a standard curve using known concentrations of H2O2. H2O2 emission data were normalized to the mass of WAT following each experiment.
Adipose tissue organ culture.
eWAT or iWAT was collected from ~15-wk-old male chow-fed C57BL/6J mice, quickly weighed, rinsed in warm PBS, placed into 10 mL of M199 (cat. no. 7653; MilliporeSigma) supplemented with 1% antibiotic-antimycotic (cat. no. 30-004-CI; Corning), 50 μU/mL insulin, and 2.5 nM dexamethasone (cat. no. D1159; MilliporeSigma). Approximately 300 mg of tissue were minced in media and kept in an incubator at 37°C to equilibrate for 24 h (57, 66). After 24 h, media were replaced with fresh M199 supplemented with 3% fatty acid-free bovine serum albumin (cat. no. 152401; MPBio). These media were further supplemented with either NAC (10 mM, cat. no. A7250; MilliporeSigma; 12), a reduced thiol donor that supports glutathione resynthesis (42), or α-tocopherol (vitamin E, 500 μM, cat. no. 258024; MilliporeSigma; 4), a hydroperoxyl radical scavenger, for 4 h. Half of the tissue cultures treated with antioxidants were also exposed to CL316243 (CL; 10 μM, cat. no. C5976; MilliporeSigma), a potent β3-adrenergic receptor agonist (37). To assess lipolytic signaling activity [e.g., phosphorylation of adipose triglyceride lipase (ATGL), hormone-sensitive lipase (HSL), and protein kinase A (PKA) substrates], we repeated the NAC and CL experiment with a 30-min incubation (66). Additionally, to further assess the direct effects of ROS, eWAT was cultured with H2O2 for 2 h (500 μM; 12).
Biochemical liver triglyceride quantification.
Liver triglyceride content was biochemically analyzed using a methanol-chloroform protocol (45, 60, 61). Snap-frozen liver (~30 mg) was homogenized in 1 mL of 1:2 methanol-chloroform and gently agitated overnight at 4°C. One milliliter of 4 mM MgCl was added the following day and centrifuged for 1 h at 1,000 g at 4°C. The organic phase was extracted, evaporated overnight, and reconstituted in a 3:2 butanol-Triton X-114 mix. Triacylglycerol content was measured with a commercially available kit (cat. no. F6428; MilliporeSigma).
Western blotting.
Tissue was homogenized in a 4X cocktail of cell lysis buffer (cat. no. FNN0021; Thermo Fisher Scientific) supplemented with phenylmethylsulfonyl fluoride and a protease inhibitor cocktail. Protein content was determined using a bicinchoninic acid assay. Proteins were separated on acrylamide gels and wet transferred onto nitrocellulose membranes. Membranes were incubated overnight with antibodies diluted (1:1,000) in Tris-buffered saline with Tween (TBST)-5% bovine serum albumin at 4°C. Afterward, membranes were briefly washed in TBST and incubated for 1 h at room temperature with horseradish peroxidase-conjugated secondary antibodies (cat. no. 7074; Cell Signaling Technologies; 1:1,000), and signals were detected using chemiluminescence. Primary antibodies against phospho (p)-ATGLSer406 (cat. no. 2138), total ATGL (cat. no. 2439), p-HSLSer563 (cat. no. 4139), total HSL (cat. no. 4107), p-PKA substrates (cat. no. 9624), and phospho-pyruvate dehydrogenase subunit E1α (Ser293) (p-PDHSer293; cat. no. 31866) were from Cell Signaling Technology. An antibody against total pyruvate dehydrogenase subunit E1α was from Abcam (cat. no. 110330). An antibody against PEPCK was from Cayman Chemical (cat. no. 10004943). An antibody against 4-hydroxynonenal (4-HNE) was from R&D Systems (cat. no. MAB3249). An antibody against vinculin was from MilliporeSigma (cat. no. 5386). Phosphorylated proteins were expressed relative to a within-gel loading control, either Ponceau stain or vinculin, before being expressed relative to total protein content, which was also expressed relative to a within-gel loading control (43, 60, 61). Total proteins were expressed relative to a within-gel loading control.
Real-time PCR.
RNA was extracted using TRIzol and Qiagen RNeasy Mini Kits (cat. no. 74106; Qiagen), and genomic DNA was removed using DNase-free treatment (cat. no. AM1906; Thermo Fisher Scientific). cDNA was synthesized using SuperScript II (cat. no. 4368814; Thermo Fisher Scientific), and real-time PCR was run with SYBR Green Supermix (cat. no. 1725271; Bio-Rad) using PCR primers on a Bio-Rad CFX Connect system. All genes (Table 1) are expressed relative to either peptidylprolyl isomerase B (Ppib) or β-actin (Actb) using the 2−ΔΔCt method (where Ct is threshold cycle; 30); housekeeping genes were stable for each experiment. Mouse catalase sequences were as follows (47): forward, AGCGACCAGATGAAGCAGTG; and reverse, TCCGCTCTCTGTCAAAGTGTG. Human catalase sequences were as follows (49): forward, GCCTGGGACCCAATTATCTT; and reverse, GAATCTCCGCACTTCTCCCAG. Both of these genes were normalized to 18S (34): forward, GTTGGTTTTCGGAACTGAGGC; and reverse, GTCGGCATCGTTTATGGTCG.
Table 1.
RT-PCR primer sequences
| Target Gene (Reference) | Forward Sequence | Reverse Sequence |
|---|---|---|
| Acc1 (28) | TGAATCTCACGCGCCTACTATG | ATGACCCTGTTGCCTCCAAAC |
| Actb (67) | GACCCAGATCATGTTTGAGA | GAGCATAGCCCTCGTAGAT |
| Atgl (32) | AATCTCTACCGCCTCTCGAA | GCCCTGTTTGCACATCTCTC |
| Dgat1 (8) | GGAATATCCCCGTGCACAA | CATTTGCTGCTGCCATGTC |
| Dgat2 (8) | CCGCAAAGGCTTTGTGAA | GGAATAAGTGGGAACCAGATCAG |
| Fasn (28) | GGTGTGGTGGGTTTGGTGAATTGT | TTGCTGAGGTTGGACAGCAGGATA |
| Gclm (54) | CTTCGCCTCCGATTGAAGATG | AAAGGCAGTCAAATCTGGTGG |
| Hsl (32) | GGCTTACTGGGCACAGATACCT | CTGAAGGCTCTGAGTTGCTCAA |
| Il6 (46) | TAGTCCTTCCTACCCCAATTTCC | TTGGTCCTTAGCCACTCCTTC |
| Nos2 (65) | CCAAGCCCTCACCTACTTCC | CTCTGAGGGCTGACACAAGG |
| Mcp1 (65) | GGCTGGAGAGCTACAAGAGG | GGTCAGCACAGACCTCTCTC |
| Nqo1 (54) | AGGATGGGAGGTACTCGAATC | TGCTAGAGATGACTCGGAAGG |
| Nrf2 (54) | CTGAACTCCTGGACGGGACTA | CGGTGGGTCTCCGTAAATGG |
| Pck1 (14) | CGGAAGAGGACTTTGAGAAAGCATTC | GCGAGTCTGTCAGTTCAATACCAATC |
| Pdk4 (22) | GGAAGTATCGACCCAAACTGTGA | GGTCGCAGAGCATCTTTGC |
| Ppargc1a (3) | CCCTGCCATTGTTAAGACC | TGCTGCTGTTCCTGTTTTC |
| Pparg2 (51) | TGTTATGGGTGAAACTCTGGG | AGAGCTGATTCCGAAGTTGG |
| Ppib (23) | GGAGATGGCACAGGAGGAA | GCCCGTAGTGCTTCAGCTT |
Acc1, acetyl-CoA carboxylase 1; Actb, β-actin; Atgl, adipose triglyceride lipase; Dgat1 and Dgat2, diglyceride acyltransferase 1 and 2, respectively; Fasn, fatty acid synthase; Gclm, glutamate-cysteine ligase, modifier subunit; Hsl, hormone-sensitive lipase; Il6, interleukin-6; Mcp1, monocyte chemoattractant protein-1; Nos2, inducible nitric oxide synthase; Nqo1, NADPH dehydrogenase quinone 1; Nrf2, nuclear factor (erythroid-derived 2)-like 2; Pck1, phosphoenolpyruvate carboxykinase; Pdk4, pyruvate dehydrogenase kinase-4; Pparg2, peroxisome proliferator-activated receptor-γ; Ppargc1a, peroxisome proliferator-activated receptor-γ coactivator 1-α; Ppib, peptidylprolyl isomerase B.
Statistical analysis.
Statistical tests were completed using GraphPad version 8.0. Normality was assessed by a Shapiro–Wilks test, and if failed, (P < 0.05) data were log10 transformed. Data were then compared by unpaired two-tailed t test (e.g., tissue weights, H2O2 incubation, sedentary MCAT gene expression, and protein content) or two-way ANOVA (e.g., adipose tissue organ culture gene expression, MCAT exercise gene expression, and protein content). For in vivo data from MCAT mice, data are first presented comparing the sedentary groups (unpaired t test) to determine whether the overexpression of catalase altered basal gene expression or protein content. Then, when comparing the effects of exercise, data were normalized to the sedentary group of each genotype (2-way ANOVA); note that sedentary mice are the same for both analyses. Post hoc tests with Tukey’s correction were completed when a significant interaction was identified. All data are presented as box-and-whisker plots, with the line representing the median, the box representing interquartile range, and whiskers indicating minimum to maximum values, unless otherwise stated. Significance level was set at P < 0.05.
RESULTS
H2O2 emission is reduced in eWAT of MCAT mice.
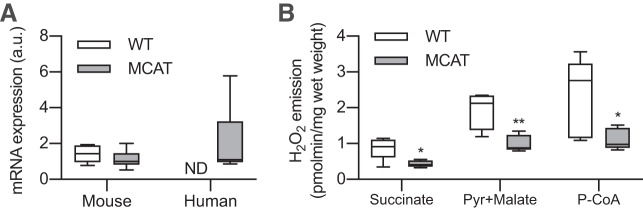
The murine form of mitochondrial catalase was similarly expressed in WT and MCAT mice, but the human catalase gene was present in only MCAT WAT (Fig. 1A). We then confirmed that this overexpression of catalase significantly reduced mitochondrial ROS emission in eWAT of MCAT mice compared with WT (Fig. 1B). Thus, MCAT mice appear to be an appropriate model to test the effects of mitochondrial ROS emission on WAT metabolism.
Fig. 1.
White adipose tissue (WAT) of mitochondrial-targeted catalase transgenic (MCAT) mice overexpresses the human catalase gene and has reduced H2O2 emission. Epididymal WAT (eWAT) was collected from 15-wk-old male wild-type (WT) and MCAT mice to determine expression of catalase via PCR and H2O2 emission via Amplex Red fluorescence quantification. A: mouse and human catalase gene expression in eWAT of WT and MCAT mice (n = 6 mice per group; unpaired Student’s t test; 1 experiment); a.u., arbitrary units; ND, not detected. B: H2O2 emission in eWAT of WT and MCAT mice (n = 5 per group; unpaired Student’s t test); P-CoA, palmitoyl-CoA; Pyr, pyruvate. All data are presented as box-and-whisker plots, with the line representing the median, the box representing interquartile range, and whiskers indicating minimum to maximum values. Data are from a single experiment. *P < 0.05; **P < 0.01 significantly different from WT.
MCAT mice have larger WAT depots and adipocytes.
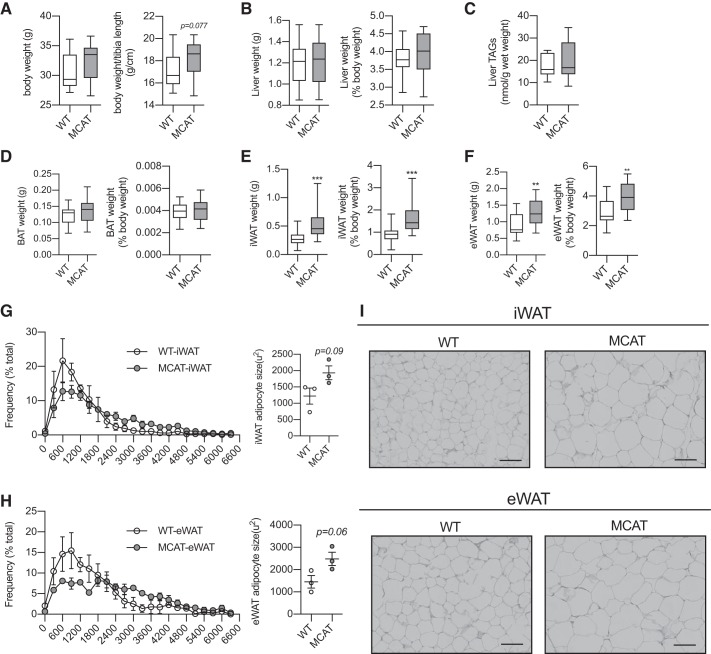
Earlier work has shown that H2O2 dose-dependently reduces the expression of Pparg in adipocytes, which is prevented by cotreatment with NAC (12). Given that Pparg regulates Pdk4 expression, lipogenic/adipogenic gene expression, and WAT expansion (58, 66), we first wanted to explore the effects of lifelong reductions in mitochondrial ROS emission on WAT morphology. MCAT mice had similar body weight to their WT counterparts, although there was a trend for increased body mass when expressed relative to tibia length (Fig. 2A); although not measured here, we and others have previously shown that there is no difference in cardiac muscle in lean mice or lean body mass between WT and MCAT mice on a high-fat diet (2, 50). Liver weights, either absolute or relative to body mass, and liver triglyceride contents were similar between genotypes (Fig. 2, B and C). Brown adipose tissue weights were also similar (Fig. 2D). However, MCAT mice had significantly larger iWAT and eWAT depots, either expressed as absolute mass or relative to body weight (Fig. 2, E and F). In line with this, MCAT mice also tended to have larger adipocytes in both iWAT and eWAT, evident by the diminished peak in smaller-adipocyte frequency (~600–1,200 μm2), the greater distribution of larger adipocytes (3,000–4,000 μm2), and the increased average adipocyte size (Fig. 2, G–I). Overall, these data suggest that reduced mitochondrial ROS emission specifically affects WAT morphology in mice fed a low-fat diet.
Fig. 2.
Mitochondrial-targeted catalase transgenic (MCAT) mice have larger white adipose tissue (WAT) depots and adipocytes. Tissues were weighed and collected from 15-wk-old male chow-fed MCAT and wild-type (WT) mice; weights are from sedentary and exercised mice. Adipocyte size was determined histologically. A: absolute body weight and body weight relative to tibia length (n = 19–21 mice per group; unpaired Student’s t test). B: absolute and relative liver weight (n = 19–21 per group; unpaired Student’s t test). C: biochemical quantification of total liver triacylglycerol (TAG) content from sedentary mice (n = 9 per group; unpaired Student’s t test). D: absolute and relative brown adipose tissue (BAT) weight (n = 19–21 per group; unpaired Student’s t test). E: absolute and relative subcutaneous inguinal white adipose tissue (iWAT) weight (n = 19–21 per group; unpaired Student’s t test). F: absolute and relative epididymal white adipose tissue (eWAT) weight (n = 19–21 per group; unpaired Student’s t test). G: frequency distribution of adipocytes from iWAT (left) and average adipocyte size (right; µm2; n = 3 per group; no statistical test performed and unpaired Student’s t test, respectively). H: frequency distribution of adipocytes from eWAT (left) and average adipocyte size (right; µm2; n = 3 per group; no statistical test performed and unpaired Student’s t test, respectively). I: representative histological images from iWAT and eWAT. Note, scale bar represents 71 μm. A–F are presented as box-and-whisker plots, with the line representing the median, the box representing interquartile range, and whiskers indicating minimum to maximum values. Data are from a single experiment. G and H are presented as means ± SE. **P < 0.01; ***P < 0.001 significantly different from WT.
Sedentary MCAT mice have greater Pdk4 gene expression in WAT.
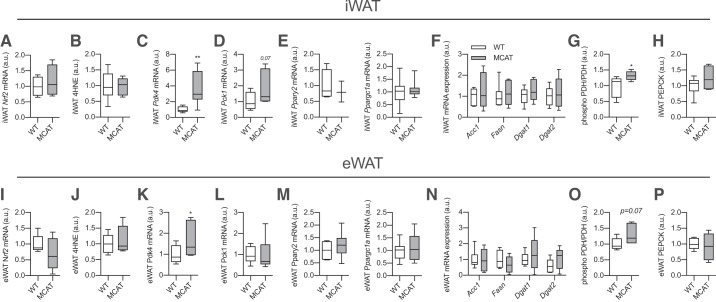
Given the alterations in WAT morphology, we next assessed whether this was associated with alterations in the expression of genes that encode proteins involved in fatty acid reesterification and lipogenesis. Perhaps surprisingly, there was no difference in markers of oxidative stress, including mRNA expression of nuclear factor (erythroid-derived 2)-like 2 (Nrf2) mRNA, a master regulator of oxidative stress response (54; Fig. 3A), or lipid peroxidation based on 4-HNE content (Fig. 3B), although this may reflect a lack of sensitivity for these measures as we observed a clear reduction in mitochondrial ROS emission in WAT from MCAT mice (see Fig. 1). Nevertheless, Pdk4 gene expression was markedly elevated in iWAT of MCAT mice (Fig. 3C). Phosphoenolpyruvate carboxykinase (Pck1) expression was increased ~50% in MCAT mice, but this was not statistically significant (P = 0.07, Fig. 3D). There were no differences in Pparg, its coactivator Ppargc1a, which also regulates Pdk4 expression (24), or any lipogenic genes between genotypes in iWAT (Fig. 3, E and F). In line with greater Pdk4 gene expression, the phosphorylation of PDH was slightly, but significantly, greater in iWAT of MCAT mice, indicative of greater PDK4 activity (Fig. 3G). PEPCK content was similar between genotypes (Fig. 3H). The same results, namely, increased Pdk4 expression and PDH phosphorylation, were also found in eWAT (Fig. 3, I–P). These data show that reduced ROS emission is associated with increased Pdk4 gene expression in WAT, which may contribute to greater reesterification rates and increased WAT mass/size observed in MCAT mice (6, 11). To further support this, we examined the ability of H2O2 to decrease Pdk4 and Pck1 gene expression in a controlled WAT culture model.
Fig. 3.
Sedentary mitochondrial-targeted catalase transgenic (MCAT) mice have increased pyruvate dehydrogenase kinase-4 (Pdk4) gene expression in white adipose tissue (WAT). Gene expression and protein content were assessed in subcutaneous inguinal WAT (iWAT) and epididymal WAT (eWAT) from 15-wk-old male sedentary MCAT and wild-type (WT) mice via RT-PCR and Western blot analysis, respectively. Note that sedentary blots were quantified from the same Western blots as in Fig. 5 and are expressed relative to sedentary WT mice. A: nuclear factor (erythroid-derived 2)-like 2 (Nrf2) mRNA expression in iWAT of sedentary WT and MCAT mice (n = 7–8 mice per group; relative to WT; unpaired Student’s t test). B: 4-hydroxynonenal (4-HNE) content in iWAT (n = 6 per group; unpaired Student’s t test). C–E: Pdk4, phosphoenolpyruvate carboxykinase (Pck1), peroxisome proliferator-activated receptor-γ (Pparg2), and peroxisome proliferator-activated receptor-γ coactivator 1-α (Ppargc1a) gene expression in iWAT from sedentary WT and MCAT mice (n = 7–8 per group; unpaired Student’s t test). F: lipogenic gene expression in iWAT (n = 6–8 per group; unpaired Student’s t test). G and H: phospho-pyruvate dehydrogenase subunit E1α (PDH) and phosphoenolpyruvate carboxykinase (PEPCK) protein content in iWAT (n = 8 per group; relative to WT; unpaired Student’s t test). I: Nrf2 mRNA expression in eWAT of sedentary WT and MCAT mice (n = 7–8 per group; relative to WT; unpaired Student’s t test). J: 4-HNE content in eWAT (n = 6 per group; unpaired Student’s t test). K–M: Pdk4, Pck1, Pparg2, and Ppargc1a gene expression in eWAT from sedentary WT and MCAT mice (n = 7–8 per group; unpaired Student’s t test). N: lipogenic gene expression in eWAT (n = 6–8 per group; unpaired Student’s t test). O and P: phospho-PDH and PEPCK protein content in eWAT (n = 6 per group; unpaired Student’s t test). Here, a.u., arbitrary units; Acc1, acetyl-CoA carboxylase 1; Dgat1 and Dgat2, diglyceride acyltransferase 1 and 2, respectively; Fasn, fatty acid synthase. All data are presented as box-and-whisker plots, with the line representing the median, the box representing interquartile range, and whiskers indicating minimum to maximum values. Data are from a single experiment. *P < 0.05; **P < 0.01 significantly different from WT.
Hydrogen peroxide directly reduces glyceroneogenic gene expression in WAT.
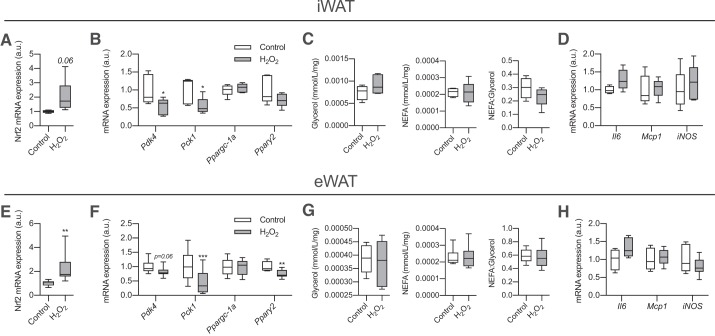
We showed that reduced mitochondrial H2O2 emission was associated with increased Pdk4 expression in WAT from sedentary mice (see Fig. 3, C and K). To test whether H2O2 could have a direct effect on WAT gene expression, we treated iWAT and eWAT with H2O2 for 2 h. Nrf2 expression was increased in H2O2-treated WAT, providing evidence that treatment led to oxidative stress (Fig. 4, A and E). After only 2 h of treatment, far shorter than previously shown [24 h (12)], the mRNA expression of Pdk4 and Pck1 was reduced in both depots (Fig. 4, B and F), whereas Pparg2 expression was reduced only in eWAT. However, at this time point we did not observe any difference in accumulation of glycerol or nonesterified fatty acids (NEFAs) in the media or the NEFA-to-glycerol ratio (Fig. 4, C and G). Importantly, altered gene expression was not associated with markers of inflammation (Fig. 4, D and H), as with longer H2O2 exposure (12), supporting that this is likely a direct effect of ROS. Although culturing adipose tissue increased markers of apoptosis compared with adipose harvested directly from mice, there was no effect of our treatments on indexes of apoptosis (Supplemental Fig. S1; see https://doi.org/10.6084/m9.figshare.10002770.v1). These data show that H2O2 can rapidly reduce mRNA expression of glyceroneogenic genes in WAT.
Fig. 4.
H2O2 rapidly inhibits white adipose tissue (WAT) glyceroneogenic gene expression. Subcutaneous inguinal WAT (iWAT) and epididymal WAT (eWAT) were collected from 15-wk-old male chow-fed C57BL/6J mice and incubated overnight to equilibrate. Tissues were then treated with H2O2 (500 µM) for 2 h to assess the effects of oxidative stress on gene expression. A: nuclear factor (erythroid-derived 2)-like 2 (Nrf2) gene expression in iWAT (n = 8 mice per group; relative to Control; unpaired Student’s t test). B: gene expression in iWAT (n = 8–11 per group; unpaired Student’s t test). C: media glycerol and nonesterified fatty acid (NEFA) concentrations (mmol·mL−1·mg−1 iWAT) and NEFA-to-glycerol ratio at the end of 2-h incubation (n = 8 per group; unpaired Student’s t test). D: inflammatory gene expression in iWAT (n = 8 per group; unpaired Student’s t test). E: Nrf2 gene expression in eWAT (n = 8 per group; relative to Control; unpaired Student’s t test). F: gene expression in eWAT (n = 8–11 per group; unpaired Student’s t test). G: media glycerol and NEFA concentrations (mmol·mL−1·mg−1 eWAT) and NEFA-to-glycerol ratio at the end of 2-h incubation (n = 8 per group; unpaired Student’s t test). H: inflammatory gene expression in eWAT (n = 8 per group; unpaired Student’s t test). Here, a.u., arbitrary units; Il6, interleukin-6; iNOS, inducible nitric oxide synthase (Nos2); Mcp1, monocyte chemoattractant protein-1; Pck1, phosphoenolpyruvate carboxykinase; Pdk4, pyruvate dehydrogenase kinase-4; Pparg2, peroxisome proliferator-activated receptor-γ; Ppargc1a, peroxisome proliferator-activated receptor-γ coactivator 1-α. All data are presented as box-and-whisker plots, with the line representing the median, the box representing interquartile range, and whiskers indicating minimum to maximum values. Data are from a single experiment. *P < 0.05; **P < 0.01; ***P < 0.001 significantly different from Control.
ROS regulates exercise-induced WAT Pdk4 and Pck1 gene expression.
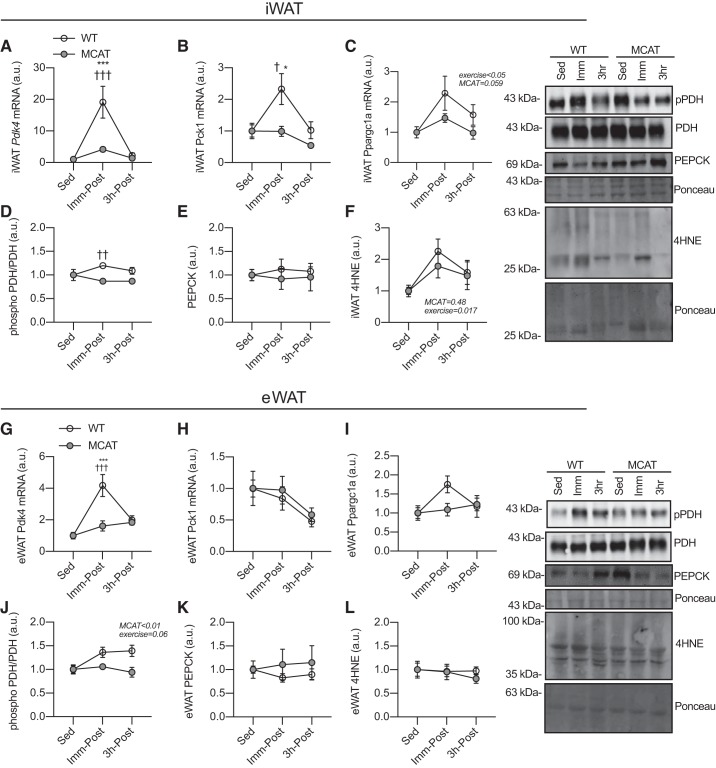
Pdk4 gene expression is increased by exercise in WAT (66), and ROS are required for exercise-induced Pdk4 expression in skeletal muscle (36). Thus, we next assessed whether decreased mitochondrial ROS production could affect exercise-induced WAT gene expression. Exercise robustly increased Pdk4 gene expression in iWAT of WT mice, but this was nearly absent in MCAT mice (Fig. 5A); because sedentary Pdk4 expression was significantly different (Fig. 3), we chose to express these data relative to the sedentary mice of each genotype and for consistency expressed other genes the same way. That ROS appear to be necessary for gene expression in response to exercise but are inhibitory under basal conditions suggests there may be an interaction between ROS and exercise signaling pathways regulating gene expression (see discussion).
Fig. 5.
Exercise-induced pyruvate dehydrogenase kinase-4 (Pdk4) gene expression is absent in white adipose tissue (WAT) of mitochondrial-targeted catalase transgenic (MCAT) mice. Fifteen-week-old male mice either remained sedentary or exercised (90 min, 15 m/min, 5% grade), and WAT was collected either immediately postexercise (Imm-Post) or following a 3-h recovery (3h-Post). Because gene expression and phosphorylation were significantly different for Pdk4 and pyruvate dehydrogenase subunit E1α (PDH), respectively (see Fig. 3), for consistency we expressed all these data relative to sedentary mice of their own genotype. A: Pdk4 gene expression in subcutaneous inguinal WAT (iWAT; n = 7–8 mice per group; 2-way ANOVA). B: phosphoenolpyruvate carboxykinase (Pck1) gene expression in iWAT (n = 7–8 per group; 2-way ANOVA). C: peroxisome proliferator-activated receptor-γ coactivator 1-α (Ppargc1a) gene expression in iWAT (n = 7–8 per group; 2-way ANOVA). D: PDH phosphorylation in iWAT (n = 7–8 per group; 2-way ANOVA). E: phosphoenolpyruvate carboxykinase (PEPCK) content in iWAT (n = 7–8 per group; 2-way ANOVA). F: 4-hydroxynonenal (4-HNE) content in iWAT (n = 6 per group; 2-way ANOVA). G: Pdk4 gene expression in epididymal WAT (eWAT; n = 7–8 per group; 2-way ANOVA). H: Pck1 gene expression in eWAT (n = 7–8 per group; 2-way ANOVA). I: Ppargc1a gene expression in eWAT (n = 7–8 per group; 2-way ANOVA). J: PDH phosphorylation in eWAT (n = 6 per group; 2-way ANOVA). K: PEPCK content in eWAT (n = 6 per group; 2-way ANOVA). L: 4-HNE content in eWAT (n = 6 per group; 2-way ANOVA). Here, pPDH, phospho-PDH. All data are presented as means ± SE. Data are from a single experiment. When significant, main effects are presented with their corresponding P value on each graph. Significant interactions and post hoc tests: *P < 0.05; ***P < 0.001 significantly different from sedentary (Sed) within genotype; †P < 0.05; ††P < 0.01; †††P < 0.001 significantly different from wild-type (WT) group of same time point.
Exercise also drove Pck1 expression in iWAT of WT mice, which was blunted in MCAT mice (Fig. 5B). Ppargc1a expression, a regulator of mitochondrial biogenesis and glyceroneogenic genes (24), was increased by exercise, but to a lesser degree in MCAT mice (P = 0.059; Fig. 5C). There was only a very subtle increase in PDH phosphorylation immediately postexercise in iWAT of WT mice, but not in MCAT mice (Fig. 5D). PEPCK was stable in both genotypes (Fig. 5E). We have shown that the same exercise protocol used in the present report increased markers of oxidative stress (4-HNE) in skeletal muscle (36), and interestingly, exercise led to increased oxidative stress in iWAT regardless of genotype (Fig. 5F) as shown by increases in 4-HNE.
Similar to iWAT, exercise increased Pdk4 gene expression, and this was completely absent in eWAT of MCAT mice (Fig. 5G). Pck1 remained largely unchanged in eWAT, regardless of genotype (Fig. 5H). The induction of Ppargc1a in WT eWAT immediately postexercise was modest (~1.5-fold) and appeared greater in WT than MCAT mice, but this was not significant. However, exercise-induced PDH phosphorylation (P = 0.06) was reduced in MCAT mice (Fig. 5J). PEPCK protein content was unaffected by genotype or exercise (Fig. 5K). Contrary to iWAT, we observed no evidence for exercise-induced lipid peroxidation in eWAT (Fig. 5L). These data show that mitochondrial ROS play an important role in regulating exercise-induced WAT gene expression, particularly in iWAT.
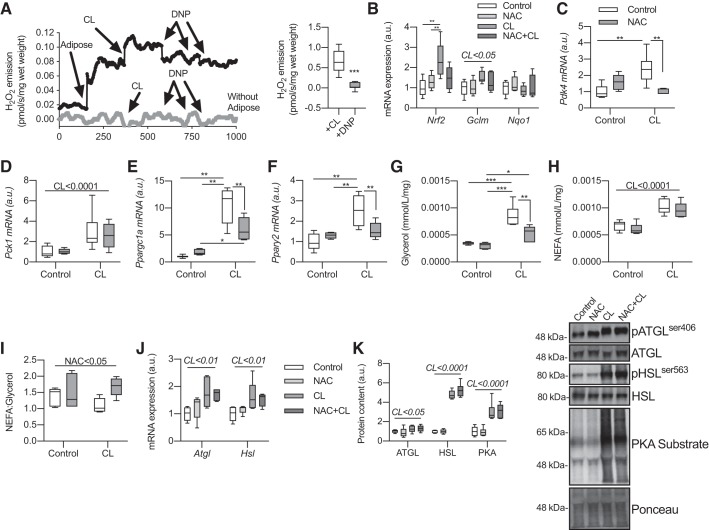
NAC blunts CL-induced increases in gene expression in WAT.
To try and determine whether ROS directly regulate adrenergic-induced glyceroneogenic gene expression, we incubated WAT with the antioxidant NAC in the presence of the β3-adrenergic agonist CL316243 (57, 66). CL is commonly used to activate lipolysis in WAT (37–39). Importantly, CL drove ROS emission from eWAT, which was almost completely inhibited by DNP (Fig. 6A), suggesting that mitochondria are the predominant ROS source, at least in this model. In line with the increase in ROS, CL increased Nrf2 expression, which was blunted by NAC, and there was a slight increase in glutamate-cysteine ligase, modifier subunit (Gclm), supporting that adrenergic activation increases markers of oxidative stress in WAT (Fig. 6B; 54). Not surprisingly, CL caused a robust increase in Pdk4 expression, but this was completely blocked by NAC (Fig. 6C). Pck1 expression was increased in response to CL but was unaffected by cotreatment with NAC (Fig. 6D). Nevertheless, CL-induced Ppargc1a and Pparg2, both reputed transcriptional regulators of β-adrenergic-induced Pdk4 gene expression (66), were almost completely blocked by NAC (Fig. 6, E and F). Interestingly, CL-induced glycerol release was reduced in the presence of NAC (Fig. 6G), whereas NAC had no effect on NEFA release (Fig. 6H). Thus, the NEFA-to-glycerol ratio, a proxy for fatty acid reesterification (35, 59), was increased by NAC (Fig. 7I), suggesting reductions in fatty acid reesterification. NAC had no effect on CL-induced lipolytic (Atgl and Hsl) gene expression (Fig. 6J) or CL-induced activation of ATGL, HSL, or PKA (Fig. 6K), showing that the reduced glycerol release and blunted gene expression observed in the presence of NAC are not secondary to reduced lipolysis. These data show that ROS regulate CL-induced gene expression and markers of reesterification in WAT ex vivo.
Fig. 6.
The antioxidant N-acetyl-cysteine (NAC) blunts CL316243 (CL)-induced gene expression in white adipose tissue (WAT). Epididymal WAT (eWAT) was collected from 15-wk-old male chow-fed C57BL/6J mice and incubated overnight to equilibrate. Tissues were then incubated with NAC (10 mM) and CL (10 µM) for 4 h to assess the effects of oxidative stress on CL-induced gene expression. A, left: timeline of H2O2 emission in the presence (black line) and absence (gray line, negative control) of eWAT. Right: quantified values for CL-mediated stimulation of H2O2 emission in the presence and absence of 2,4-dinitrophenol (DNP; n = 11 mice per group; change from baseline with adipose tissue; unpaired Student’s t test). B: gene expression of markers of oxidative stress (n = 5–6 per group; relative to Control; 2-way ANOVA). C: pyruvate dehydrogenase kinase-4 (Pdk4) gene expression (n = 5–6 per group; 2-way ANOVA). D: phosphoenolpyruvate carboxykinase (Pck1) gene expression (n = 5–6 per group; 2-way ANOVA). E: peroxisome proliferator-activated receptor-γ coactivator 1-α (Ppargc1a) gene expression (n = 5–6 per group; 2-way ANOVA). F: peroxisome proliferator-activated receptor-γ (Pparg2) gene expression (n = 5–6 per group; 2-way ANOVA). G: media glycerol concentrations (mmol·mL−1·mg−1 eWAT) at the end of 4-h incubation (n = 5–6 per group; 2-way ANOVA). H: media nonesterified fatty acid (NEFA) concentrations (mmol·mL−1·mg−1 eWAT) at the end of 4-h incubation (n = 5–6 per group; 2-way ANOVA). I: ratio of NEFA to glycerol (NEFA/glycerol) in media (n = 5–6 per group; 2-way ANOVA). J: lipolytic gene expression in eWAT (n = 5–6 per group; 2-way ANOVA). K: lipolytic protein activation in eWAT after 30-min incubation (n = 6 per group; 2-way ANOVA). Here, a.u., arbitrary units; Atgl, adipose triglyceride lipase; Gclm, glutamate-cysteine ligase, modifier subunit; Hsl, hormone-sensitive lipase; Nqo1, NADPH dehydrogenase quinone 1; Nrf2, nuclear factor (erythroid-derived 2)-like 2; pATGL, phospho-ATGL; pHSL, phospho-HSL. All data are presented as box-and-whisker plots, with the line representing the median, the box representing interquartile range, and whiskers indicating minimum to maximum values. Data are from a single experiment. A horizontal line atop all groups represents a main effect with the factor and corresponding P value given above the line. Significant interactions and post hoc tests are indicated by lines connecting groups: *P < 0.05; **P < 0.01; ***P < 0.001.
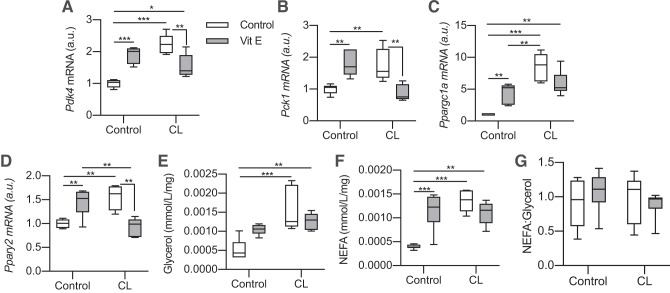
Fig. 7.
Vitamin E blunts CL316243 (CL)-induced gene expression in white adipose tissue (WAT). Epididymal WAT (eWAT) was collected from 15-wk-old male chow-fed C57BL/6J mice and incubated overnight to equilibrate. Tissues were then incubated with vitamin E (α-tocopherol, 500 µM) and CL (10 µM) for 4 h to assess the effects of oxidative stress on CL-induced gene expression. A: pyruvate dehydrogenase kinase-4 (Pdk4) gene expression (n = 5–6 mice per group; relative to Control-Control; 2-way ANOVA). B: phosphoenolpyruvate carboxykinase (Pck1) gene expression (n = 5–6 per group; 2-way ANOVA). C: peroxisome proliferator-activated receptor-γ coactivator 1-α (Ppargc1a) gene expression (n = 5–6 per group; 2-way ANOVA). D: peroxisome proliferator-activated receptor-γ (Pparg2) gene expression (n = 5–6 per group; 2-way ANOVA). E: media glycerol concentrations (mmol·mL−1·mg−1 eWAT) at the end of 4-h incubation (n = 6 per group; 2-way ANOVA). F: media nonesterified fatty acid (NEFA) concentrations (mmol·mL−1·mg−1 eWAT) at the end of 4-h incubation (n = 6 per group; 2-way ANOVA). G: ratio of NEFA to glycerol in media (n = 6 per group; 2-way ANOVA). Here, a.u., arbitrary units; Vit E, vitamin E. All data are presented as box-and-whisker plots, with the line representing the median, the box representing interquartile range, and whiskers indicating minimum to maximum values. Data are from a single experiment. A horizontal line atop all groups represents a main effect with the factor and corresponding P value given above the line. Significant interactions and post hoc tests are indicated by lines connecting groups: *P < 0.05; **P < 0.01; ***P < 0.001.
Vitamin E increases glyceroneogenic gene expression but blunts CL-induced increases in WAT.
To confirm our findings with NAC, we repeated the previous experiment using the fat-soluble ROS scavenging antioxidant α-tocopherol (vitamin E). Interestingly, incubating eWAT with α-tocopherol for 4 h increased Pdk4, Pck1, Ppargc1a, and Pparg expression (Fig. 7, A–D), somewhat reflecting our in vivo sedentary data in MCAT mice. At the same time, α-tocopherol completely blocked CL-induced increases in Pdk4, Pck1, and Pparg while blunting CL-induced Ppargc1a expression (P = 0.07). Perhaps surprisingly, α-tocopherol also increased NEFA accumulation in the media (Fig. 7F) but had no effect on CL-induced NEFA and glycerol release. Considering that vitamin E increased markers of lipolysis and glyceroneogenic gene expression on its own, it is possible that the high concentration used here (500 μM) could have some secondary effects, but these results corroborate that antioxidants alter CL-induced gene expression.
DISCUSSION
Exercise-induced ROS act as signaling molecules in skeletal muscle, mediating the acute and chronic responses to exercise (2, 15, 17, 36). It is now clear that exercise has beneficial effects on WAT (43) that may represent an important factor in the overall systemic metabolic benefits of exercise (56). The present study provides evidence that ROS regulate exercise- and CL-induced glyceroneogenic gene expression in WAT. Overexpression of the antioxidant catalase in mitochondria was associated with larger fat depots and adipocytes, greater Pdk4 gene expression, and a near-complete impairment of exercise-induced Pdk4 gene expression in WAT. Moreover, H2O2 rapidly reduced glyceroneogenic gene expression, whereas antioxidants blunted CL-induced gene expression ex vivo. Taken together, these data strongly support that ROS can regulate the expression of genes involved in fatty acid reesterification in WAT.
We observed that exercise-induced gene expression in WAT was greatly reduced in MCAT mice, similar to what has been observed for skeletal muscle gene expression (2, 15, 17, 36) and whole body adaptations to exercise (13, 48). There are various sources of exercise-induced ROS in skeletal muscle, including mitochondria, NADPH, and xanthine oxidase (15–17, 36). The contribution of these various exercise-induced ROS sources has not been explored in WAT, but our data suggest that there are also multiple sources of exercise-induced ROS in WAT. Moreover, mitochondria-specific overexpression of catalase almost completely prevented exercise-induced gene expression. However, at least in iWAT, we detected similar exercise-induced lipid peroxidation between genotypes, indicative of alternative exercise-induced ROS sources. We cannot rule out the possibility that the lifelong overexpression of mitochondrial catalase produced a compensation in other ROS-producing sources, but since total ROS emission is greatly reduced in MCAT mice, this seems unlikely (41, 50, 53).
Interestingly, in 3T3-L1 adipocytes, specifically inhibiting NADPH oxidase or using the general antioxidant NAC markedly reduced ROS production, whereas targeting xanthine oxidase or mitochondrial complexes failed to suppress ROS production (12), suggesting that NADPH might represent an important ROS source in adipocytes. However, we found that both NAC and α-tocopherol similarly blunted CL-induced gene expression in cultured WAT, DNP almost completely reduced CL-induced ROS emission ex vivo, and mitochondria-specific catalase overexpression blunted exercise-induced gene expression in vivo, suggesting that various ROS sources contribute to regulating gene expression in WAT. It is possible that adrenergic signaling, as during exercise and CL treatment, drives ROS production from alternative sources in WAT compared with unstimulated WAT (12) and that ROS predominantly arise from unique sources between skeletal muscle and WAT, all of which merit future investigation. Regardless of the specific source, our data clearly show that ROS are essential regulators of adrenergically stimulated gene expression in WAT.
In this report, we found that reduced ROS was associated with increased Pdk4 expression in vivo (e.g., sedentary MCAT mice) and ex vivo (e.g., vitamin E incubation); high ROS concentrations reduced Pdk4 (e.g., H2O2 incubation); and ROS were required for Pdk4 induction in the presence of adrenergic stimulation (e.g., exercise- and CL-induced). In other words, depending on the situation, ROS can be either inhibitory or facilitative in regard to WAT gene expression. This phenomenon has been previously described for adipogenesis, where sufficient ROS are required for, but excessive ROS impair, WAT function and adipogenesis (7). Indeed, ROS production is a normal and essential part of adipocyte differentiation (26), but excessive oxidative stress is linked to obesity-associated WAT dysfunction (12). Moreover, low ROS levels can activate insulin signaling, whereas high concentrations are well known to inhibit insulin signaling (21, 41). Along these lines, our data suggest that there may be a “Goldilocks zone” for ROS-regulated WAT gene expression. Another possibility is that the subcellular location (i.e., mitochondrial vs. cytosolic) of ROS production produces distinct cellular effects. Contrary to our mitochondria-specific in vivo model, chronic oral treatment with the nonspecific antioxidant NAC led to smaller WAT depots in chow-fed mice and reduced Pparg expression in high fat diet-fed mice (33). Moreover, oral supplementation with the general antioxidant vitamin C prevented endurance training-induced mitochondrial biogenesis in skeletal muscle (13), whereas specifically targeting xanthine oxidase did not (64). Finally, recently it emerged that cytosolic NADPH oxidase 2 regulates muscle glucose uptake during exercise in skeletal muscle (16). It will be interesting for future work to characterize subcellular ROS production in WAT.
White adipose tissue is closely linked to overall systemic glucose and lipid metabolism (68). The antidiabetic drugs thiazolidinediones (TZDs) work by increasing fatty acid reesterification (27), whereas overexpression of WAT PEPCK leads to increased reesterification and obesity but prevents insulin resistance (11). Inhibiting or knocking down PDK4 impairs these effects of TZDs, demonstrating the important role of PDK4 in fatty acid cycling (6, 59). We previously showed that high fat diet-induced obesity led to greater mitochondrial ROS emission and insulin resistance in WAT and that MCAT mice were protected from these effects (41). Similarly, genetic ablation of peroxiredoxin 3, a mitochondrial-specific thiol peroxidase, also led to glucose intolerance and insulin resistance in mice (20), whereas oral NAC treatment improved insulin and glucose tolerance in high fat diet-fed mice (33). In the present report, we found that lean chow-fed MCAT mice have larger WAT depots and adipocytes with concomitantly elevated Pdk4 expression in WAT. Since H2O2 reduces glyceroneogenic gene expression in cultured WAT, it is appealing to speculate that the reduced ROS production in MCAT mice leads to increased PDK4 activity and reesterification, which contributes to conserved insulin sensitivity in obesity.
A role for ROS in regulating the beneficial adaptations to exercise is well established in skeletal muscle (2, 15, 17, 36). Here we elucidate a previously unappreciated role for ROS in regulating exercise- and CL-induced gene expression in WAT.
GRANTS
This work was funded, in part, through a Natural Sciences and Engineering Research Council of Canada (NSERC) grant to D. C. Wright and G. P. Holloway. D. C. Wright is a Tier II Canada Research Chair in Lipids, Metabolism, and Health. L. K. Townsend was supported by an Ontario Graduate Scholarship, a Dairy Farmers of Canada Doctoral Research Assistantship, and an NSERC doctoral scholarship. A. J. Weber held an NSERC Undergraduate Student Research Award.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
L.K.T., P.-A.B., G.P.H., and D.C.W. conceived and designed research; L.K.T., A.J.W., P.-A.B., and G.P.H. performed experiments; L.K.T., A.J.W., and P.-A.B. analyzed data; L.K.T., G.P.H., and D.C.W. interpreted results of experiments; L.K.T. prepared figures; L.K.T. drafted manuscript; L.K.T., A.J.W., P.-A.B., G.P.H., and D.C.W. edited and revised manuscript; L.K.T., A.J.W., P.-A.B., G.P.H., and D.C.W. approved final version of manuscript.
REFERENCES
- 1.Anderson EJ, Neufer PD. Type II skeletal myofibers possess unique properties that potentiate mitochondrial H2O2 generation. Am J Physiol Cell Physiol 290: C844–C851, 2006. doi: 10.1152/ajpcell.00402.2005. [DOI] [PubMed] [Google Scholar]
- 2.Barbeau PA, Miotto PM, Holloway GP. Mitochondrial-derived reactive oxygen species influence ADP sensitivity, but not CPT-I substrate sensitivity. Biochem J 475: 2997–3008, 2018. doi: 10.1042/BCJ20180419. [DOI] [PubMed] [Google Scholar]
- 3.Boström P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, Rasbach KA, Boström EA, Choi JH, Long JZ, Kajimura S, Zingaretti MC, Vind BF, Tu H, Cinti S, Højlund K, Gygi SP, Spiegelman BM. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 481: 463–468, 2012. doi: 10.1038/nature10777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brookheart RT, Michel CI, Listenberger LL, Ory DS, Schaffer JE. The non-coding RNA gadd7 is a regulator of lipid-induced oxidative and endoplasmic reticulum stress. J Biol Chem 284: 7446–7454, 2009. doi: 10.1074/jbc.M806209200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brooks B, Arch JR, Newsholme EA. Effects of hormones on the rate of the triacylglycerol/fatty acid substrate cycle in adipocytes and epididymal fat pads. FEBS Lett 146: 327–330, 1982. doi: 10.1016/0014-5793(82)80945-9. [DOI] [PubMed] [Google Scholar]
- 6.Cadoudal T, Distel E, Durant S, Fouque F, Blouin JM, Collinet M, Bortoli S, Forest C, Benelli C. Pyruvate dehydrogenase kinase 4: regulation by thiazolidinediones and implication in glyceroneogenesis in adipose tissue. Diabetes 57: 2272–2279, 2008. doi: 10.2337/db08-0477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Castro JP, Grune T, Speckmann B. The two faces of reactive oxygen species (ROS) in adipocyte function and dysfunction. Biol Chem 397: 709–724, 2016. doi: 10.1515/hsz-2015-0305. [DOI] [PubMed] [Google Scholar]
- 8.Chitraju C, Mejhert N, Haas JT, Diaz-Ramirez LG, Grueter CA, Imbriglio JE, Pinto S, Koliwad SK, Walther TC, Farese RV Jr. Triglyceride synthesis by DGAT1 protects adipocytes from lipid-induced ER stress during lipolysis. Cell Metab 26: 407–418.e3, 2017. doi: 10.1016/j.cmet.2017.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dlasková A, Clarke KJ, Porter RK. The role of UCP 1 in production of reactive oxygen species by mitochondria isolated from brown adipose tissue. Biochim Biophys Acta 1797: 1470–1476, 2010. doi: 10.1016/j.bbabio.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 10.Dyck DJ. Adipokines as regulators of muscle metabolism and insulin sensitivity. Appl Physiol Nutr Metab 34: 396–402, 2009. doi: 10.1139/H09-037. [DOI] [PubMed] [Google Scholar]
- 11.Franckhauser S, Muñoz S, Pujol A, Casellas A, Riu E, Otaegui P, Su B, Bosch F. Increased fatty acid re-esterification by PEPCK overexpression in adipose tissue leads to obesity without insulin resistance. Diabetes 51: 624–630, 2002. doi: 10.2337/diabetes.51.3.624. [DOI] [PubMed] [Google Scholar]
- 12.Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, Nakayama O, Makishima M, Matsuda M, Shimomura I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest 114: 1752–1761, 2004. doi: 10.1172/JCI21625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gomez-Cabrera MC, Domenech E, Romagnoli M, Arduini A, Borras C, Pallardo FV, Sastre J, Viña J. Oral administration of vitamin C decreases muscle mitochondrial biogenesis and hampers training-induced adaptations in endurance performance. Am J Clin Nutr 87: 142–149, 2008. doi: 10.1093/ajcn/87.1.142. [DOI] [PubMed] [Google Scholar]
- 14.Gray LR, Sultana MR, Rauckhorst AJ, Oonthonpan L, Tompkins SC, Sharma A, Fu X, Miao R, Pewa AD, Brown KS, Lane EE, Dohlman A, Zepeda-Orozco D, Xie J, Rutter J, Norris AW, Cox JE, Burgess SC, Potthoff MJ, Taylor EB. Hepatic mitochondrial pyruvate carrier 1 is required for efficient regulation of gluconeogenesis and whole-body glucose homeostasis. Cell Metab 22: 669–681, 2015. doi: 10.1016/j.cmet.2015.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Henríquez-Olguín C, Díaz-Vegas A, Utreras-Mendoza Y, Campos C, Arias-Calderón M, Llanos P, Contreras-Ferrat A, Espinosa A, Altamirano F, Jaimovich E, Valladares DM. NOX2 inhibition impairs early muscle gene expression induced by a single exercise bout. Front Physiol 7: 282, 2016. doi: 10.3389/fphys.2016.00282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Henríquez-Olguin C, Knudsen JR, Raun SH, Li Z, Dalbram E, Treebak JT, Sylow L, Holmdahl R, Richter EA, Jaimovich E, Jensen TE. Cytosolic ROS production by NADPH oxidase 2 regulates muscle glucose uptake during exercise. Nat Commun 10: 4623, 2019. doi: 10.1038/s41467-019-12523-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Henríquez-Olguín C, Renani LB, Arab-Ceschia L, Raun SH, Bhatia A, Li Z, Knudsen JR, Holmdahl R, Jensen TE. Adaptations to high-intensity interval training in skeletal muscle require NADPH oxidase 2. Redox Biol 24: 101188, 2019. doi: 10.1016/j.redox.2019.101188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hochachka PW, McClelland GB. Cellular metabolic homeostasis during large-scale change in ATP turnover rates in muscles. J Exp Biol 200: 381–386, 1997. [DOI] [PubMed] [Google Scholar]
- 19.Holloway GP, Holwerda AM, Miotto PM, Dirks ML, Verdijk LB, van Loon LJ. Age-associated impairments in mitochondrial ADP sensitivity contribute to redox stress in senescent human skeletal muscle. Cell Reports 22: 2837–2848, 2018. doi: 10.1016/j.celrep.2018.02.069. [DOI] [PubMed] [Google Scholar]
- 20.Huh JY, Kim Y, Jeong J, Park J, Kim I, Huh KH, Kim YS, Woo HA, Rhee SG, Lee KJ, Ha H. Peroxiredoxin 3 is a key molecule regulating adipocyte oxidative stress, mitochondrial biogenesis, and adipokine expression. Antioxid Redox Signal 16: 229–243, 2012. doi: 10.1089/ars.2010.3766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iwakami S, Misu H, Takeda T, Sugimori M, Matsugo S, Kaneko S, Takamura T. Concentration-dependent dual effects of hydrogen peroxide on insulin signal transduction in H4IIEC hepatocytes. PLoS One 6: e27401, 2011. doi: 10.1371/journal.pone.0027401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jørgensen SB, Wojtaszewski JF, Viollet B, Andreelli F, Birk JB, Hellsten Y, Schjerling P, Vaulont S, Neufer PD, Richter EA, Pilegaard H. Effects of α-AMPK knockout on exercise-induced gene activation in mouse skeletal muscle. FASEB J 19: 1146–1148, 2005. doi: 10.1096/fj.04-3144fje. [DOI] [PubMed] [Google Scholar]
- 23.Kazak L, Chouchani ET, Lu GZ, Jedrychowski MP, Bare CJ, Mina AI, Kumari M, Zhang S, Vuckovic I, Laznik-Bogoslavski D, Dzeja P, Banks AS, Rosen ED, Spiegelman BM. Genetic depletion of adipocyte creatine metabolism inhibits diet-induced thermogenesis and drives obesity. Cell Metab 26: 660–671.e3, 2017. [Erratum in Cell Metab 26: 693, 2017.] doi: 10.1016/j.cmet.2017.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kleiner S, Mepani RJ, Laznik D, Ye L, Jurczak MJ, Jornayvaz FR, Estall JL, Chatterjee Bhowmick D, Shulman GI, Spiegelman BM. Development of insulin resistance in mice lacking PGC-1α in adipose tissues. Proc Natl Acad Sci USA 109: 9635–9640, 2012. doi: 10.1073/pnas.1207287109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kusminski CM, Scherer PE. Mitochondrial dysfunction in white adipose tissue. Trends Endocrinol Metab 23: 435–443, 2012. doi: 10.1016/j.tem.2012.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee H, Lee YJ, Choi H, Ko EH, Kim JW. Reactive oxygen species facilitate adipocyte differentiation by accelerating mitotic clonal expansion. J Biol Chem 284: 10601–10609, 2009. doi: 10.1074/jbc.M808742200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leroyer SN, Tordjman J, Chauvet G, Quette J, Chapron C, Forest C, Antoine B. Rosiglitazone controls fatty acid cycling in human adipose tissue by means of glyceroneogenesis and glycerol phosphorylation. J Biol Chem 281: 13141–13149, 2006. doi: 10.1074/jbc.M512943200. [DOI] [PubMed] [Google Scholar]
- 28.Libby AE, Bales ES, Monks J, Orlicky DJ, McManaman JL. Perilipin-2 deletion promotes carbohydrate-mediated browning of white adipose tissue at ambient temperature. J Lipid Res 59: 1482–1500, 2018. doi: 10.1194/jlr.M086249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin Y, Berg AH, Iyengar P, Lam TK, Giacca A, Combs TP, Rajala MW, Du X, Rollman B, Li W, Hawkins M, Barzilai N, Rhodes CJ, Fantus IG, Brownlee M, Scherer PE. The hyperglycemia-induced inflammatory response in adipocytes: the role of reactive oxygen species. J Biol Chem 280: 4617–4626, 2005. doi: 10.1074/jbc.M411863200. [DOI] [PubMed] [Google Scholar]
- 30.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25: 402–408, 2001. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 31.Ludzki A, Paglialunga S, Smith BK, Herbst EA, Allison MK, Heigenhauser GJ, Neufer PD, Holloway GP. Rapid repression of ADP transport by palmitoyl-CoA is attenuated by exercise training in humans: a potential mechanism to decrease oxidative stress and improve skeletal muscle insulin signaling. Diabetes 64: 2769–2779, 2015. doi: 10.2337/db14-1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luijten IH, Brooks K, Boulet N, Shabalina IG, Jaiprakash A, Carlsson B, Fischer AW, Cannon B, Nedergaard J. Glucocorticoid-induced obesity develops independently of UCP1. Cell Reports 27: 1686–1698.e5, 2019. doi: 10.1016/j.celrep.2019.04.041. [DOI] [PubMed] [Google Scholar]
- 33.Ma Y, Gao M, Liu D. N-acetylcysteine protects mice from high fat diet-induced metabolic disorders. Pharm Res 33: 2033–2042, 2016. doi: 10.1007/s11095-016-1941-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Matravadia S, Martino VB, Sinclair D, Mutch DM, Holloway GP. Exercise training increases the expression and nuclear localization of mRNA destabilizing proteins in skeletal muscle. Am J Physiol Regul Integr Comp Physiol 305: R822–R831, 2013. doi: 10.1152/ajpregu.00590.2012. [DOI] [PubMed] [Google Scholar]
- 35.Mennes E, Dungan CM, Frendo-Cumbo S, Williamson DL, Wright DC. Aging-associated reductions in lipolytic and mitochondrial proteins in mouse adipose tissue are not rescued by metformin treatment. J Gerontol A Biol Sci Med Sci 69: 1060–1068, 2014. doi: 10.1093/gerona/glt156. [DOI] [PubMed] [Google Scholar]
- 36.Miotto PM, Holloway GP. Exercise-induced reductions in mitochondrial ADP sensitivity contribute to the induction of gene expression and mitochondrial biogenesis through enhanced mitochondrial H2O2 emission. Mitochondrion 46: 116–122, 2019. doi: 10.1016/j.mito.2018.03.003. [DOI] [PubMed] [Google Scholar]
- 37.Mottillo EP, Balasubramanian P, Lee YH, Weng C, Kershaw EE, Granneman JG. Coupling of lipolysis and de novo lipogenesis in brown, beige, and white adipose tissues during chronic β3-adrenergic receptor activation. J Lipid Res 55: 2276–2286, 2014. doi: 10.1194/jlr.M050005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mottillo EP, Bloch AE, Leff T, Granneman JG. Lipolytic products activate peroxisome proliferator-activated receptor (PPAR) α and δ in brown adipocytes to match fatty acid oxidation with supply. J Biol Chem 287: 25038–25048, 2012. doi: 10.1074/jbc.M112.374041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mottillo EP, Granneman JG. Intracellular fatty acids suppress β-adrenergic induction of PKA-targeted gene expression in white adipocytes. Am J Physiol Endocrinol Metab 301: E122–E131, 2011. doi: 10.1152/ajpendo.00039.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nye CK, Hanson RW, Kalhan SC. Glyceroneogenesis is the dominant pathway for triglyceride glycerol synthesis in vivo in the rat. J Biol Chem 283: 27565–27574, 2008. doi: 10.1074/jbc.M804393200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Paglialunga S, Ludzki A, Root-McCaig J, Holloway GP. In adipose tissue, increased mitochondrial emission of reactive oxygen species is important for short-term high-fat diet-induced insulin resistance in mice. Diabetologia 58: 1071–1080, 2015. doi: 10.1007/s00125-015-3531-x. [DOI] [PubMed] [Google Scholar]
- 42.Pazdro R, Burgess JR. Differential effects of α-tocopherol and N-acetyl-cysteine on advanced glycation end product-induced oxidative damage and neurite degeneration in SH-SY5Y cells. Biochim Biophys Acta 1822: 550–556, 2012. doi: 10.1016/j.bbadis.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 43.Peppler WT, Townsend LK, Knuth CM, Foster MT, Wright DC. Subcutaneous inguinal white adipose tissue is responsive to, but dispensable for, the metabolic health benefits of exercise. Am J Physiol Endocrinol Metab 314: E66–E77, 2018. doi: 10.1152/ajpendo.00226.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Place N, Ivarsson N, Venckunas T, Neyroud D, Brazaitis M, Cheng AJ, Ochala J, Kamandulis S, Girard S, Volungevičius G, Paužas H, Mekideche A, Kayser B, Martinez-Redondo V, Ruas JL, Bruton J, Truffert A, Lanner JT, Skurvydas A, Westerblad H. Ryanodine receptor fragmentation and sarcoplasmic reticulum Ca2+ leak after one session of high-intensity interval exercise. Proc Natl Acad Sci USA 112: 15492–15497, 2015. doi: 10.1073/pnas.1507176112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rector RS, Thyfault JP, Laye MJ, Morris RT, Borengasser SJ, Uptergrove GM, Chakravarthy MV, Booth FW, Ibdah JA. Cessation of daily exercise dramatically alters precursors of hepatic steatosis in Otsuka Long-Evans Tokushima Fatty (OLETF) rats. J Physiol 586: 4241–4249, 2008. doi: 10.1113/jphysiol.2008.156745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reilly SM, Chiang SH, Decker SJ, Chang L, Uhm M, Larsen MJ, Rubin JR, Mowers J, White NM, Hochberg I, Downes M, Yu RT, Liddle C, Evans RM, Oh D, Li P, Olefsky JM, Saltiel AR. An inhibitor of the protein kinases TBK1 and IKK-ɛ improves obesity-related metabolic dysfunctions in mice. Nat Med 19: 313–321, 2013. doi: 10.1038/nm.3082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rindler PM, Plafker SM, Szweda LI, Kinter M. High dietary fat selectively increases catalase expression within cardiac mitochondria. J Biol Chem 288: 1979–1990, 2013. doi: 10.1074/jbc.M112.412890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ristow M, Zarse K, Oberbach A, Klöting N, Birringer M, Kiehntopf M, Stumvoll M, Kahn CR, Blüher M. Antioxidants prevent health-promoting effects of physical exercise in humans. Proc Natl Acad Sci USA 106: 8665–8670, 2009. doi: 10.1073/pnas.0903485106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rodiño-Janeiro BK, Salgado-Somoza A, Teijeira-Fernández E, González-Juanatey JR, Alvarez E, Eiras S. Receptor for advanced glycation end-products expression in subcutaneous adipose tissue is related to coronary artery disease. Eur J Endocrinol 164: 529–537, 2011. doi: 10.1530/EJE-10-0904. [DOI] [PubMed] [Google Scholar]
- 50.Ryan TE, Schmidt CA, Green TD, Spangenburg EE, Neufer PD, McClung JM. Targeted expression of catalase to mitochondria protects against ischemic myopathy in high-fat diet-fed mice. Diabetes 65: 2553–2568, 2016. doi: 10.2337/db16-0387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sakaguchi M, Fujisaka S, Cai W, Winnay JN, Konishi M, O’Neill BT, Li M, García-Martín R, Takahashi H, Hu J, Kulkarni RN, Kahn CR. Adipocyte dynamics and reversible metabolic syndrome in mice with an inducible adipocyte-specific deletion of the insulin receptor. Cell Metab 25: 448–462, 2017. doi: 10.1016/j.cmet.2016.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sakellariou GK, Vasilaki A, Palomero J, Kayani A, Zibrik L, McArdle A, Jackson MJ. Studies of mitochondrial and nonmitochondrial sources implicate nicotinamide adenine dinucleotide phosphate oxidase(s) in the increased skeletal muscle superoxide generation that occurs during contractile activity. Antioxid Redox Signal 18: 603–621, 2013. doi: 10.1089/ars.2012.4623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schriner SE, Linford NJ, Martin GM, Treuting P, Ogburn CE, Emond M, Coskun PE, Ladiges W, Wolf N, Van Remmen H, Wallace DC, Rabinovitch PS. Extension of murine life span by overexpression of catalase targeted to mitochondria. Science 308: 1909–1911, 2005. doi: 10.1126/science.1106653. [DOI] [PubMed] [Google Scholar]
- 54.Shanmugam G, Challa AK, Litovsky SH, Devarajan A, Wang D, Jones DP, Darley-Usmar VM, Rajasekaran NS. Enhanced Keap1-Nrf2 signaling protects the myocardium from isoproterenol-induced pathological remodeling in mice. Redox Biol 27: 101212, 2019. doi: 10.1016/j.redox.2019.101212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Silveira LR, Pilegaard H, Kusuhara K, Curi R, Hellsten Y. The contraction induced increase in gene expression of peroxisome proliferator-activated receptor (PPAR)-γ coactivator 1α (PGC-1α), mitochondrial uncoupling protein 3 (UCP3) and hexokinase II (HKII) in primary rat skeletal muscle cells is dependent on reactive oxygen species. Biochim Biophys Acta 1763: 969–976, 2006. doi: 10.1016/j.bbamcr.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 56.Stanford KI, Middelbeek RJ, Townsend KL, Lee MY, Takahashi H, So K, Hitchcox KM, Markan KR, Hellbach K, Hirshman MF, Tseng YH, Goodyear LJ. A novel role for subcutaneous adipose tissue in exercise-induced improvements in glucose homeostasis. Diabetes 64: 2002–2014, 2015. doi: 10.2337/db14-0704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sutherland LN, Bomhof MR, Capozzi LC, Basaraba SA, Wright DC. Exercise and adrenaline increase PGC-1α mRNA expression in rat adipose tissue. J Physiol 587: 1607–1617, 2009. doi: 10.1113/jphysiol.2008.165464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tontonoz P, Hu E, Spiegelman BM. Regulation of adipocyte gene expression and differentiation by peroxisome proliferator activated receptor γ. Curr Opin Genet Dev 5: 571–576, 1995. doi: 10.1016/0959-437X(95)80025-5. [DOI] [PubMed] [Google Scholar]
- 59.Townsend LK, Knuth CM, Wright DC. Cycling our way to fit fat. Physiol Rep 5: e13247, 2017. doi: 10.14814/phy2.13247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Townsend LK, Medak KD, Knuth CM, Peppler WT, Charron MJ, Wright DC. Loss of glucagon signaling alters white adipose tissue browning. FASEB J 33: 4824–4835, 2019. doi: 10.1096/fj.201802048RR. [DOI] [PubMed] [Google Scholar]
- 61.Townsend LK, Medak KD, Peppler WT, Meers GM, Rector RS, LeBlanc PJ, Wright DC. High-saturated-fat diet-induced obesity causes hepatic interleukin-6 resistance via endoplasmic reticulum stress. J Lipid Res 60: 1236–1249, 2019. doi: 10.1194/jlr.M092510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Townsend LK, Wright DC. Looking on the “brite” side exercise-induced browning of white adipose tissue. Pflügers Arch 471: 455–465, 2019. doi: 10.1007/s00424-018-2177-1. [DOI] [PubMed] [Google Scholar]
- 63.Trevellin E, Scorzeto M, Olivieri M, Granzotto M, Valerio A, Tedesco L, Fabris R, Serra R, Quarta M, Reggiani C, Nisoli E, Vettor R. Exercise training induces mitochondrial biogenesis and glucose uptake in subcutaneous adipose tissue through eNOS-dependent mechanisms. Diabetes 63: 2800–2811, 2014. doi: 10.2337/db13-1234. [DOI] [PubMed] [Google Scholar]
- 64.Wadley GD, Nicolas MA, Hiam DS, McConell GK. Xanthine oxidase inhibition attenuates skeletal muscle signaling following acute exercise but does not impair mitochondrial adaptations to endurance training. Am J Physiol Endocrinol Metab 304: E853–E862, 2013. doi: 10.1152/ajpendo.00568.2012. [DOI] [PubMed] [Google Scholar]
- 65.Wainright KS, Fleming NJ, Rowles JL, Welly RJ, Zidon TM, Park YM, Gaines TL, Scroggins RJ, Anderson-Baucum EK, Hasty AH, Vieira-Potter VJ, Padilla J. Retention of sedentary obese visceral white adipose tissue phenotype with intermittent physical activity despite reduced adiposity. Am J Physiol Regul Integr Comp Physiol 309: R594–R602, 2015. doi: 10.1152/ajpregu.00042.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wan Z, Thrush AB, Legare M, Frier BC, Sutherland LN, Williams DB, Wright DC. Epinephrine-mediated regulation of PDK4 mRNA in rat adipose tissue. Am J Physiol Cell Physiol 299: C1162–C1170, 2010. doi: 10.1152/ajpcell.00188.2010. [DOI] [PubMed] [Google Scholar]
- 67.Wang QA, Tao C, Jiang L, Shao M, Ye R, Zhu Y, Gordillo R, Ali A, Lian Y, Holland WL, Gupta RK, Scherer PE. Distinct regulatory mechanisms governing embryonic versus adult adipocyte maturation. Nat Cell Biol 17: 1099–1111, 2015. doi: 10.1038/ncb3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest 115: 1111–1119, 2005. doi: 10.1172/JCI25102. [DOI] [PMC free article] [PubMed] [Google Scholar]