Keywords: brain-gut, ENS, microbiome, motility, serotonin
Abstract
Gastrointestinal (GI) comorbidities are common in individuals with mood and behavioral dysfunction. Similarly, patients with GI problems more commonly suffer from co-morbid psychiatric diagnoses. Although the central and enteric nervous systems (CNS and ENS, respectively) have largely been studied separately, there is emerging interest in factors that may contribute to disease states involving both systems. There is strong evidence to suggest that serotonin may be an important contributor to these brain-gut conditions. Serotonin has long been recognized for its critical functions in CNS development and function. The majority of the body’s serotonin, however, is produced in the GI tract, where it plays key roles in ENS development and function. Further understanding of the specific impact that enteric serotonin has on brain-gut disease may lay the foundation for the creation of novel therapeutic targets. This review summarizes the current data focusing on the important roles that serotonin plays in ENS development and motility, with a focus on novel aspects of serotonergic signaling in medical conditions in which CNS and ENS co-morbidities are common, including autism spectrum disorders and depression.
INTRODUCTION
Although serotonin (5-hydroxytryptamine, 5-HT) was first noted in the gastrointestinal (GI) tract in rabbits (28), its later discovery in the central nervous system (CNS) quickly attracted overwhelming attention (129) for its key CNS roles in brain development, sleep, mood, and appetite (13). Approximately 95% of the body’s serotonin, however, is produced in the intestine, where it continues to be increasingly recognized for its paracrine and endocrine actions (127). For instance, enteric 5-HT has been found to modulate enteric nervous system (ENS) development and neurogenesis, motility, secretion, inflammation, sensation, and epithelial development (75, 83, 84). In recent years, there has also been an emerging interest in the role that 5-HT plays in disease conditions that affect the brain and the gut. Some of these disorders are considered to be CNS centric, including autism spectrum disorders (ASD) and depression, both of which are highly co-morbid with GI dysfunction. The precise roles that 5-HT plays in brain-gut conditions, such as these, are unknown.
One way in which 5-HT may impact brain and gut function is based on its ability to affect the “brain-gut axis.” The brain-gut axis is defined as the bidirectional communication between the CNS and ENS. This interaction can result in CNS modulation of GI functions, including GI immune responses, secretion, and motility (88), as well as the transmission of sensations, such as nausea, to the CNS. The interactions between the CNS and ENS occur on neural, immunologic, and hormonal levels (87) and may also be heavily influenced by the intestinal microbiome (89). Dysregulation of the “brain-gut-microbiome” axis has increasingly been implicated in the etiology of several disease processes in which CNS and GI dysfunction often coexist. Alternatively, the overlapping developmental and functional processes that 5-HT affects in the CNS (e.g., mood and cognition) (66) and the intestine (e.g., motility) (75) may also occur simultaneously and manifest as co-occurring brain-gut conditions.
In this review, we will discuss major roles of enteric 5-HT in neurodevelopment and motility. We will then apply those concepts to the current understanding of how 5-HT may modulate co-occurring brain-gut dysfunction that is seen in clinical subsets of individuals with ASD and depression. Finally, we will discuss how the mechanistic findings in these studies could potentially lead to the development of novel therapeutic options for brain-gut disorders.
BACKGROUND
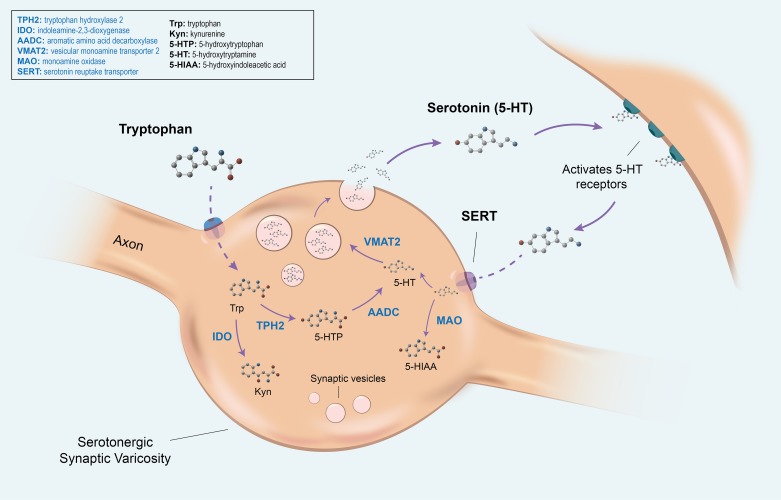
In the intestine, 5-HT is synthesized and released from both the intestinal mucosa and enteric neurons (37). 5-HT release from these distinct locales can have different effects that have important implications on physiology, which will be discussed in this review. The majority of intestinal 5-HT is synthesized in the enterochromaffin (EC) cells that line the GI mucosa. This source of 5-HT has been shown to stimulate peristaltic and secretory reflexes and may also send signals to the CNS by activation of sensory afferent connections, including those that mediate nausea and discomfort (46, 114, 115). 5-HT production in EC cells and enteric neurons is controlled by two different isoforms of the same rate-limiting enzyme: tryptophan hydroxylase (TPH). Synthesis of neuronal 5-HT, in both the CNS and ENS, is regulated by TPH2. Intestinal mucosal 5-HT, however, is produced by TPH1 in EC cells. After being released into the GI lumen or the synaptic cleft, 5-HT is transported intracellularly, primarily by the presynaptic serotonin reuptake transporter (SERT; Slc6a4), where it is inactivated by intracellular monoamine oxidase (MAO). Increased or decreased expression of SERT can thus result in decreased or increased levels, respectively, of the 5-HT available for neurotransmission (Fig. 1).
Fig. 1.
The pathways of tryptophan metabolism. In the serotonergic synaptic varicosity, tryptophan (Trp) enters the cell through an amino acid transporter, where it is primarily converted to 5-hydroxytryptophan (5-HTP) through the actions of the rate-limiting tryptophan hydroxylase 2 (TPH2). It is then converted to serotonin (5-hydroxytryptamine, 5-HT) by aromatic L-amino acid decarboxylase (AADC). Alternatively, tryptophan can enter the kynurenine (Kyn) pathway through metabolism by the ubiquitous indoleamine-2,3-dioxygenase enzyme (IDO) or the hepatic tryptophan-2,3-dioxygenase enzyme (not depicted). The vesicular monoamine transporter 2 (VMAT2) transports monoamines, including 5-HT, from the cellular cytosol into vesicles. Upon receiving a stimulus, 5-HT vesicles fuse with the plasma membrane, releasing 5-HT into the synaptic cleft, where it can activate 5-HT receptors on the target cell. 5-HT is then inactivated by reverse transport back into the presynaptic neuron through the actions of the serotonin reuptake transporter (SERT). There, it can either be recycled into vesicles or converted into the metabolite 5-hydroxyindoleacetic acid (5-HIAA) by the actions of monoamine oxidase (MAO).
In addition to changes in SERT, 5-HT availability can also be affected by alterations in its synthetic pathway. This often occurs at the point where tryptophan is converted into 5-HT or alternatively, degraded to kynurenine (Fig. 1). Once tryptophan is converted to 5-hydroxytryptophan (5-HTP), via TPH, 5-HTP can be converted to 5-HT via aromatic amino acid decarboxylase (AADC). Alternatively, however, tryptophan degradation can occur through the kynurenine pathway (131) via the hepatic enzyme tryptophan-2,3-dioxygenase or the ubiquitous enzyme indoleamine-2,3-dioxygenase (IDO) (34) (Fig. 1). Although beyond the scope of this article, it is important to note that these different pathways, and thus the ratio of 5-HT to kynurenine synthesized, are often regulated by proinflammatory mediators. The products of these pathways (kynurenine and 5-HT) are not only affected by inflammation but may also, consequently, have an impact on inflammatory conditions, such as inflammatory bowel disease (IBD) (105, 109). This is reviewed in Savitz (108) and Waclawikova and El Aidy (133).
5-HT exerts its varying effects, in large part, by specific 5-HT receptor activation. The determination of receptor selectivity can be a daunting task; 7 different classes of the 5-HT receptor (5-HT1 to 5-HT7), with 13 different subtypes, have been identified (20, 38). Each of the receptors exists in slightly different locations in the CNS and peripheral nervous systems and elicits its actions through different second-messenger pathways, including inhibition or activation of adenylate cyclase (5-HT1 and 5-HT4, 5-HT6, 5-HT7, respectively), phospholipase C activation (5-HT2), or as a ligand-gated ion channel (5-HT3) (100). This diversity in receptor subtype explains a critical way by which 5-HT is able to implement so many different actions. Instances where activation of different receptors results in similar physiologic effects may also serve to create redundancy and thus preservation of function in the system (99).
ROLE OF SEROTONIN IN NEUROGENESIS AND GI MOTILITY
Serotonin As a Modulator of Neurodevelopment
5-HT is a critical modulator of brain and intestinal neurodevelopment. During CNS development, 5-HT is necessary for neuronal differentiation and migration, neurite outgrowth, myelination, and synapse formation (35, 55). Similar to what is seen in the CNS, enteric neuronal 5-HT also drives the development of enteric neurons through its actions as a growth factor, both prenatally and throughout life (75, 77). During ENS development, serotonergic neurons are among the first neurons to develop, where they influence neurogenesis and drive the development and survival of later-born enteric neurons, including those expressing calcitonin gene-related peptide (CGRP), dopamine, and γ-aminobutyric acid (GABA) (75, 76). The influence of 5-HT on later-developing neurons has been shown in vitro, in cell cultures of isolated enteric neural crest-derived precursor cells (ENCDCs), where 5-HT has been demonstrated to promote development of dopaminergic neurons (75). It has also been demonstrated in vivo, in mice that lack TPH2 [TPH2 knockout (KO)]; these mice have enteric neuronal hypoplasia (Table 1) that is accompanied by significant decreases in dopaminergic, GABAergic and CGRP-expressing neurons compared with their wild-type (WT) littermates (75). Of note, enteric serotonergic neurons have also been shown to impact their own subsequent development (83). The mechanism by which this occurs in the ENS has not yet been demonstrated. In TPH2KO mouse models, specific region-dependent differences in brain 5-HT innervation and synaptic density are observed, suggesting that CNS 5-HT also plays roles in establishing and maintaining innervation during development (24). Serotonergic neurons in the CNS, however, have been shown to express inhibitory 5-HT1A autoreceptors that regulate levels of serotonergic neurotransmission during development; genetic and pharmacological ablation of 5-HT1A receptors in the CNS results in increased 5-HT neurotransmission, whereas overexpression of 5-HT1A receptors leads to decreased 5-HT neurotransmission (2). It is not yet known whether the 5-HT1A receptor modulates similar processes in the ENS.
Table 1.
Modulation of gastrointestinal 5-HT in murine motility models
| Model | Effect | Findings (Compared With Control) | |
|---|---|---|---|
| Heredia et al. (51) and Li et al. (75) | TPH1KO mice | Lack EC cell-derived 5-HT (intestinal mucosa) | Elongated colon; poorly disseminated CMMCs that moved in retrograde fashion; lack of response to 5-HT3 antagonist, ondansetron (51); no change in in vivo motility (75) |
| Israelyan et al. (61) | TPH2-R439H mice | Reduced neuronal 5-HT (CNS and ENS) | Slower in vivo motility; slower and less frequent CMMC compared with WT; decreased neuronal number in ENS |
| Li et al. (75) | TPH2KO mice | Lack neuronal 5-HT (CNS and ENS) | Slowed in vivo motility; fewer enteric neurons |
| Margolis et al. (83) | SERT Ala56 mice | Increased 5-HT inactivation → decreased 5-HT availability (CNS and ENS) |
Slower and less frequent CMMCs; fewer enteric neurons |
| Margolis et al. (83) | SERT KO mice | Decreased 5-HT inactivation → increased 5-HT availability (CNS and ENS) |
Faster and more frequent CMMCs compared with WT; increased neurogenesis |
| Margolis et al. (83) | Fluoxetine exposed | Inhibition of SERT; increased 5-HT availability | Slowed in vivo motility; faster and more frequent CMMCs compared with WT; increased enteric neurons |
5-HT, serotonin (5-hydroxytryptamine); CNS, central nervous system; CMMC, colonic migrating motor complexes; EC, endothelial cell; ENS, enteric nervous system; KO, knockout; SERT, serotonin reuptake transporter; TPH, tryptophan hydroxylase; WT, wild type.
The notion that 5-HT is important for neurogenesis is further confirmed in mice that lack SERT (SERT KO). In contrast to TPH2KO, SERT KO leads to decreased inactivation of 5-HT and thus a greater availability of 5-HT for neurotransmission. Consequently, SERT KO mice exhibit enteric neuronal hyperplasia (Table 1), whereas mice with a hyperefficient SERT variant, SERT Ala56, have significantly fewer enteric neurons in the small and large intestines, as well as decreases in subsets of late-born enteric neurons (83) (Table 1).
The differences in neuron quantity and proportions demonstrated in the TPH2KO and SERT KO models do not distinguish whether the effects on neurogenesis occur solely during development or whether the abnormalities in 5-HT levels continue to influence neuron development throughout life. CNS and ENS neuronal quantity can be affected by 5-HT during early life but also in adulthood (27). For example, in the CNS, selective serotonin reuptake inhibitors (SSRIs) increase hippocampal neurogenesis in adult mice (116) through activation of glucocorticoid receptors (3). There is also evidence that 5-HT is involved in the transcriptional regulation of cell adhesion molecules that are critical for plasticity in the adult brain (72).
Although the majority of studies that have examined the effects of 5-HT on adult neurogenesis have been done in the CNS, it has recently been shown that 5-HT may also have effects on adult neurogenesis in the intestine. Most recently, it was shown that chronic administration of a slow-release version of the 5-HT precursor, 5-HTP (5-HTP SR), resulted in an increase in enteric neurons in adult mice that expressed a genetic variant of TPH2 (61). 5-HT promotes enteric neurogenesis, at least partially through its actions on 5-HT2B and/or 5-HT4 receptors (30, 77). 5-HT2B and 5-HT4 receptors have both been demonstrated to impact neurogenesis during development (30, 77). In addition to its effects during development, 5-HT4 agonism may also induce neurogenesis postnatally; neuron proliferation and differentiation increase significantly in postnatal gut-derived enteric neural stem/progenitor cells and colon explants cultured with 5-HT4 receptor agonist (RS67506)-loaded liposomal nanoparticles, as well as in vivo (57). Similarly, ENCDC proliferation and differentiation were induced in a murine model of hypoganglionosis after 5-HT4 agonist administration (138). These pro-neurogenic effects of 5-HT4 agonism may, in part, result from its ability to shield neurons against toxic oxidative stress (12). Although these data largely support a role for 5-HT in the promotion of ENS and CNS neurogenesis, the mechanism(s) through which this occurs require further study. In the CNS, the relative density of 5-HT1A autoreceptors has also been suggested to contribute to the physiological diversity of 5-HT neurons and the differences in subsequent neuronal density, although this has not been studied in the ENS (126).
It has recently been suggested, for the first time, that enteric neurogenesis occurs at the same pace or even more rapidly throughout life than has been observed in development. Based on studies involving pulse labeling with thymidine analogs, it had been thought that rapid enteric neurogenesis did not occur after postnatal day 21 and furthermore, that enteric neurons were long lived and underwent limited levels of adult neurogenesis only after exposure to 5-HT or under conditions of gut stress (e.g., inflammation) (5, 6, 61, 77, 81, 83). This paradigm was recently challenged by a study in which the investigators demonstrated that neuronal turnover and neurogenesis continue during adulthood at an astounding rate in healthy WT mice. The investigators showed that 11% of myenteric neurons in the adult murine small intestine were undergoing apoptosis that could be partially prevented with application of a pan-caspase inhibitor (70). To compensate for this high rate of neuronal death, the investigators suggested that proliferation of new neurons was ongoing and demonstrated proof of their hypothesis by the identification of Nestin+ cells as a marker for cells with neurogenic potential. Nestin+ cells were capable of giving rise to neurons in vivo, suggesting that Nestin is a viable marker for progenitors that can replenish dying enteric neurons (70). Further pulse-chase experiments with thymidine analogs revealed an astounding rate of neurogenesis, with ~88% of neurons being born every 2 weeks (70). There is limited data examining whether this effect is mediated via 5-HT. When 5-HTP SR is administered to conventionally raised WT mice, there does not appear to be an increase in the total amount of enteric neurons (61). It therefore does not appear thus far that 5-HT alters this process, at least in terms of neuronal numbers. Studies that evaluate the roles of 5-HT agonists or antagonists in the evaluation of Nestin+ cell neurogenesis and cleaved caspase-3+ cell apoptosis would be necessary to discern further the role of 5-HT in adult neurogenesis. It would be an exciting prospect to be able to use 5-HT mediators to manipulate this dynamic state to treat medical conditions that impact neurogenesis and other aspects of ENS function. Before these questions can be thoroughly investigated, however, the hypothesis that enteric neurogenesis rapidly occurs throughout life must be thoroughly tested and replicated.
Serotonin As a Mediator of GI Motility
A key component of GI motility is the peristaltic reflex, for which its purpose is to propel the movement of GI contents down the intestinal tract in a consecutive manner. The peristaltic reflex consists of coordinated oral contractions and distal relaxations, which occur in response to elevations of intraluminal pressure. These are measured in the colon, in part, by measurement of colonic migrating motor complexes (CMMCs). Genetic mouse models, complemented by ex vivo studies, have helped in beginning to elucidate specific roles and necessity for EC cell-derived and neuronal 5-HT in motility and in the propagation of the peristaltic reflex (75, 115).
In vivo motility in the small intestine, colon, and throughout the GI tract (total GI transit) is significantly slower in TPH2KO mice compared with controls (75) (Table 1), indicating an important role for neuronal 5-HT in GI motility. Although 5-HT neurons are estimated to comprise only ~2% of neurons in the myenteric plexus, motility is likely influenced by the extensive projections that serotonergic neurons have preferentially with other serotonergic neurons but also with major inhibitory neurons (e.g., nitric oxide synthase expressing), as well as the interstitial cells of Cajal (50, 52, 86). SERT hyperefficiency, which like TPH2KO, results in a decreased amount of serotonergic neurotransmission, also leads to slower and less frequent CMMCs (83) (Table 1). In contrast to the CMMCs observed in models of TPH2KO and SERT hyperefficiency, SERT KO leads to faster and more frequent CMMCs (83) (Table 1).
Although there is general agreement in the literature that the release of endogenous 5-HT can occur during peristalsis and CMMCs and furthermore, that 5-HT can evoke peristaltic contractions, there has been a lack of consensus as to whether the presence of mucosal and/or neuronal 5-HT is mandatory for colonic peristalsis and the propagation of CMMCs (117–119). It was suggested in the mid-1950s that endogenous mucosal 5-HT release initiates the peristaltic reflex and is critical for CMMCs (15). These data were further supported by a paper by Heredia et al. (50) that showed abolition of spontaneous CMMCs after enteric mucosal removal. These latter findings, however, were disputed by a series of experiments by Spencer et al. (117), which showed that peristalsis and spontaneous-evoked CMMCs could be reliably recorded despite the removal of the entire mucosa from the isolated guinea pig colon. The authors of this latter study thus concluded that peristalsis and CMMCs can occur without mucosal 5-HT. The necessity for mucosal 5-HT for CMMC propagation was evaluated in a different way by Heredia et al. (51), who examined CMMCs in TPH1KO mice that specifically lack mucosal 5-HT (Table 1). Although in vivo motility parameters are unaffected in mice that lack TPH1 (75), they found that stimuli restricted to the mucosa cannot evoke CMMCs in the TPH1KO colon and also that the motor complexes that triggered the peristaltic waves elicited in these ex vivo TPH1KO colons were poorly disseminated and moved in a largely retrograde fashion (4, 51, 115). Because the CMMCs elicited in TPH1KO mice are not propagated normally, the authors of that paper (51) refer to them as “CMMC-like” and not as true CMMCs. Altogether, both groups have data supporting their complementary points. One difference of opinion here is not whether 5-HT can evoke peristaltic contractions but in the definition of a CMMC. Spencer et al. (119) interpret the findings of the latter Heredia et al. (50) paper to mean that CMMCs are present in the TPH1KO mouse, whereas the authors of that Heredia et al. (51) paper call them not true CMMCs but CMMC-like. It may thus be that mucosal 5-HT is not necessary for propulsion in general but yet needs to be present for fully intact, functional CMMCs.
Two important ways that 5-HT affects motility is by binding to 5-HT3 and/or 5-HT4 receptors (86). 5-HT3 antagonists and 5-HT4 agonists evoke peristaltic reflexes (9, 33, 44, 54). Both receptor subtypes are present on neurons within the myenteric and submucosal plexuses of the ENS, intrinsic and extrinsic sensory neurons, and EC cells. 5-HT4 receptor agonists, however, are thought to enhance naturally occurring reflex activity rather than to generate neurotransmission (53). Further evidence that endogenous 5-HT may be necessary for peristalsis is based on the demonstration that 5-HT3 agonists and 5-HT4 antagonists can substantially inhibit or block colonic peristalsis in the whole ex vivo colon (92, 115). The argument made against this suggests that these same agonists and antagonists can also substantially inhibit colonic peristalsis in colonic preparations from mucosa-free, reserpine-treated animals (118). If endogenous 5-HT is truly depleted in the latter model, then it makes sense that endogenous 5-HT may not be necessary for activation of 5-HT3 and 5-HT4 receptors, thus supporting the arguments of Spencer et al. (117, 118). 5-HT3 and 5-HT4 have, in fact, been shown to be constitutively active in the absence of 5-HT, further supporting this idea (8, 58, 113). The conclusion that 5-HT is not necessary for peristalsis in the reserpine-treated animal model, however, needs to be further explored because constitutive 5-HT synthesis and release still occur in reserpine-treated animals, albeit at low levels. This is because reserpine inhibits the vesicular monoamine transporter (VMAT) but does not inhibit 5-HT biosynthesis or its constitutive release (49, 114); constitutive 5-HT release in reserpine-treated animals has been shown to enhance intestinal motility, indicating that there may still be interactions between 5-HT with 5-HT3 and/or 5-HT receptors (14). Because eradication of 5-HT biosynthesis requires deletion or inhibition of TPH, the studies done by Heredia et al. (51) (above) highlight the requirement of TPH1 for fully intact, functional CMMCs (114). Importantly, this same study also demonstrated a lack of effect of 5-HT3 antagonists on CMMC-like activity in TPH1KO mice, indicating that mucosal 5-HT presence is important for preventing 5-HT3-mediated actions (51).
There thus exists interesting data that support assertions from both groups. There is clear evidence from both, as noted above, supporting the notion that 5-HT can have impact on GI motility and peristaltic reflexes. It may be that the “perfect” CMMC cannot be implemented without endogenous 5-HT, but CMMC-like activity can progress, which along with other intact neural circuitry, may facilitate propulsion. Experiments evaluating the full capabilities of the constitutive activity of 5-HT3 and 5-HT4 receptors must be done in the complete absence of colonic 5-HT to offer some clarity to this aspect of the current controversy. Certainly, the multiplicity and redundancy of enteric 5-HT targets and receptors complicate ascertainment of the precise physiological roles of these interactions.
In addition to the studies that focus solely on enteric 5-HT, further work is also needed to evaluate the role(s) of CNS 5-HT on motility. The current TPH2KO model that has been used to evaluate GI motility lacks neuronal 5-HT in both the CNS and the ENS, so it does not answer this question directly (75). It is conceivable that the CNS abnormalities in the TPH2KO mice could impact gut motility; however, the ex vivo motility findings in these mice, in CMMC recordings, mimic those seen in vivo (slower colonic motility), suggesting that motility in TPH2KO mice is ENS mediated (61, 76).
ROLE OF ENTERIC 5-HT IN DISEASES THAT AFFECT THE BRAIN AND THE INTESTINE
5-HT impacts the development and long-term function of both the ENS and CNS. It is thus likely that mutations that affect 5-HT-mediated development and function will simultaneously impact those aspects of brain and intestinal development and function. In the brain, these functions include mood and behavior and in the intestine, GI motility (35, 75, 121). Genetic polymorphisms in the enzymes involved in neuronal serotonin synthesis and homeostasis have been associated with disease states involving both the CNS and ENS (61, 83, 97, 134). We will focus on 5-HT-related mutations in conditions classically recognized to be CNS centric but that have been commonly associated with GI dysfunction, including those involved in ASDs and depression (86, 94, 139).
ASDs, GI Dysfunction, and 5-HT
GI problems are fourfold more common in children with ASD than in their neurotypical peers (91). The most commonly diagnosed GI problems in ASD are constipation, diarrhea, and gastroesophageal reflux disease (82, 91), although among the three conditions, constipation is the most prevalent GI diagnosis rendered in the majority of studies. Interestingly, GI dysfunction in individuals with ASD has also been associated with other CNS-associated behaviors and mood disorders, including rigid compulsivity, anxiety, and depression (29, 43, 99).
Serotonin could be a nexus between ASD and GI dysfunction. High blood 5-HT levels, or hyperserotonemia, occur in approximately one-third of individuals with ASD. The origin of the hyperserotonemia is not known, but a likely source is the GI tract (127). Because platelets do not produce 5-HT, but they do express SERT, the 5-HT within them is the result of uptake via SERT. The quantity of 5-HT found in platelets is therefore dependent on the quantity available to take up as they circulate peripherally. Because 95% of the body’s 5-HT is produced in the intestine, it is likely that blood 5-HT levels are reflective of what platelets take up as they circulate through the intestine. Hyperserotonemia is thus suggestive of abnormal 5-HT secretion from the GI tract and/or GI dysfunction (62). The postulate that hyperserotonemia is linked to GI dysfunction was supported in a clinical study that showed an association between whole blood serotonin levels and lower GI dysfunction, most commonly constipation, in children with ASD (85). These data suggest that a subset of children with ASD and hyperserotonemia is a subset that may be more likely to suffer from GI problems.
The evaluation of 5-HT-related genetic abnormalities in individuals with ASD has revealed a possible relationship to Slc6a4, the gene encoding SERT. A genome-wide study in individuals with ASD revealed associations with SERT, and linkage studies further implicated the 17q11.2 region (18, 60). Following these studies, rare variants of Slc6a4 were screened for in families demonstrating a linkage to 17q11.2. Five variants were identified to be overexpressed in ASD. All of these variants conferred a gain-of-function mutation with consequent SERT hyperactivity (39, 93). SERT Ala56, a mutation in which glycine is replaced by alanine at the 56th amino acid position, was the most common variant identified and was found to be associated with rigid-compulsive behaviors and sensory aversion (122). In accordance with the other variants identified in this study, the SERT Ala56 mutation results in a hyperfunctional SERT protein that takes up 5-HT with increased efficacy, thus leading to less 5-HT in the synaptic cleft that is available for signaling (132).
To examine the abnormalities in nervous system development and function caused by SERT Ala56, the mutation was transgenically engineered into a mouse model. The SERT Ala56 mice have hyperserotonemia and furthermore, demonstrate behavioral anomalies that mimic those seen in ASD, including repetitive behaviors, deficits in social interaction, and delayed communication (132). The SERT Ala56 mice also have CNS abnormalities that include increased 5-HT clearance and hypersensitivity of the 5-HT1A and 5-HT2A receptors, demonstrated by exaggerated responses to 5-HT1A and 5-HT2A receptor agonists (132). The ENS is also abnormal in the SERT Ala56 mice. The mice exhibit significantly fewer neurons in both the myenteric and submucosal plexuses with a greater loss of the later-developing types of neurons that are known to be 5-HT dependent (83). As a result of an abnormal ENS, the SERT Ala56 mice exhibit slowed motility throughout the entire intestine, including a decrease in frequency and speed of CMMCs that are known to be ENS dependent. Administration of the 5-HT4 agonist prucalopride during development was shown to rescue the defects in enteric neurogenesis and motility in the SERT Ala56 mice (83).
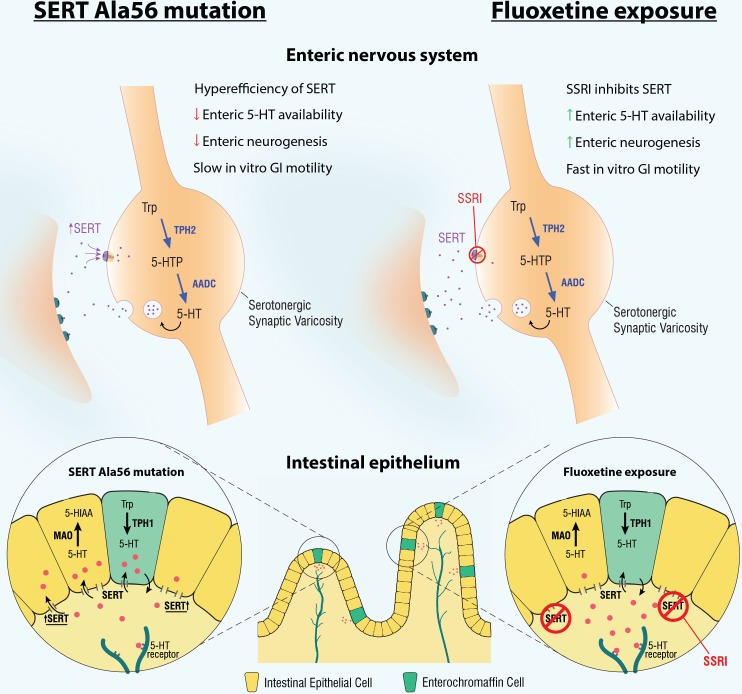
The hyperefficiency of SERT that results from the SERT Ala56 mutation leads to decreased availability of enteric neuronal 5-HT, as well as alterations in the intestinal mucosa. These mice demonstrate a higher EC cell density and a greater quantity of Tph1 transcripts than WT mice (83). These changes in the enteric mucosa are indicative of an increase in mucosal 5-HT production and secretion and may thus account for the hyperserotonemia that is associated with this mutation. One possible reason why the differences in 5-HT levels exist in enteric neurons (less TPH2) compared with the mucosa (more TPH1) is based on the differences in colocalization between TPH and SERT in enteric neurons compared with the mucosa. In neurons, TPH2 and SERT are colocalized within the same cell. A hyperefficient SERT will thus bring more 5-HT back into the neurons and cause negative-feedback inhibition on TPH2, resulting in a decreased quantity of neuronal 5-HT production. In the mucosa, however, TPH1 and SERT are in different cells; TPH1 is found in EC cells, and SERT is in intestinal epithelial cells. Thus when 5-HT is taken up by SERT in intestinal epithelial cells, TPH1 cannot sense this increased uptake because of the lack of colocalization. TPH1 may thus upregulate 5-HT production to compensate for the lower levels of 5-HT sensed in the luminal environment (37, 83) (Fig. 2).
Fig. 2.
Changes in the enteric nervous system (ENS) and intestinal epithelium in mice with the serotonin reuptake transporter (SERT) Ala56 mutation compared with mice exposed developmentally to fluoxetine. SERT Ala56 mice have a hyperfunctional SERT that enhances the rate of serotonin (5-hydroxytryptamine, 5-HT) reuptake and thus inactivation, leading to a decrease in the amount of 5-HT neurotransmission. This results in a hypoplastic ENS, with deficits in total and late-born neurons, slower ex vivo gastrointestinal (GI) motility, and shorter intestinal villus height when compared with wild-type mice. In contrast, fluoxetine exposure during development results in an increase in 5-HT neurotransmission, a hyperplastic ENS, faster ex vivo GI motility, and taller intestinal villi compared with vehicle-exposed mice. Of note, tryptophan hydroxylase 2 (TPH2) is the rate-limiting enzyme responsible for 5-HT synthesis in the ENS, whereas TPH1 is the rate-limiting enzyme responsible for 5-HT synthesis in the intestinal epithelium. 5-HIAA, 5-hydroxyindoleacetic acid; 5-HTP, 5-hydroxytryptophan; AADC, amino acid decarboxylase; MAO, monoamine oxidase; SSRI, serotonin reuptake inhibitor; Trp, tryptophan.
If ENS development and function are inhibited by less neuronal 5-HT availability, it follows that an increase in 5-HT, available for neurotransmission, should result in opposing anomalies in ENS development and function. This concept has been studied by the evaluation of the effects of perinatal SSRI exposure on ENS development and function (Fig. 2). SSRIs block the actions of SERT, leading to an extended period of 5-HT acting on postsynaptic receptors in the synaptic cleft (107). The ENS of mice exposed to the SSRI, fluoxetine, during development is persistently hyperplastic, with greater increases in the same late-born neuronal subsets that are deficient in SERT Ala56 mice. ENS-driven colonic contractions, as measured by CMMC frequency and velocity, are also markedly faster in fluoxetine-exposed mice (Table 1). The ENS was not, however, the sole influence on GI motility in this model. External neuronal influence on gut motility was also demonstrated; fluoxetine-mediated SERT inhibition during development resulted in an enhancement of activity in descending sympathetic activity, which resulted in slowed GI motility in vivo (83) (Table 1), an effect that was reversed after use of a chemical sympathectomy with 6-hydroxydopamine, an agent that denatures input from descending sympathetic neurons. Of note, developmental SSRI exposure also leads to abnormal CNS development (47) and maladaptive behaviors in animal models and humans, including anxiety and depression, that persist into adulthood (90). The data in SERT Ala56 and fluoxetine-exposed mice thus suggest that there may be a critical range for neuronal serotonin homeostasis and that perturbations outside of this range can result in long-lasting deficits in brain and gut behavior and furthermore, that SERT is important in this control.
The demonstration that SSRIs impact nervous system development and function may have important clinical implications. SSRIs are the chief drug class prescribed for depression during pregnancy. Developmental SSRI exposure has been associated significantly with adverse outcomes, including a twofold increased risk of birth defects (135) and a higher risk of preterm delivery (22). Although there is significant clinical data to suggest changes in brain wiring, mood, and behavior in children exposed to SSRIs during development, there has been only one clinical retrospective study examining the effects of SSRIs on GI function. In that study, however, children exposed to SSRIs in utero were 10-fold more likely to require laxatives for constipation (95). Further study is thus warranted to understand the clinical relationship between developmental SSRI exposure and ensuing GI dysfunction.
Depression, GI Dysfunction, and 5-HT: a Potential Connection Through TPH2
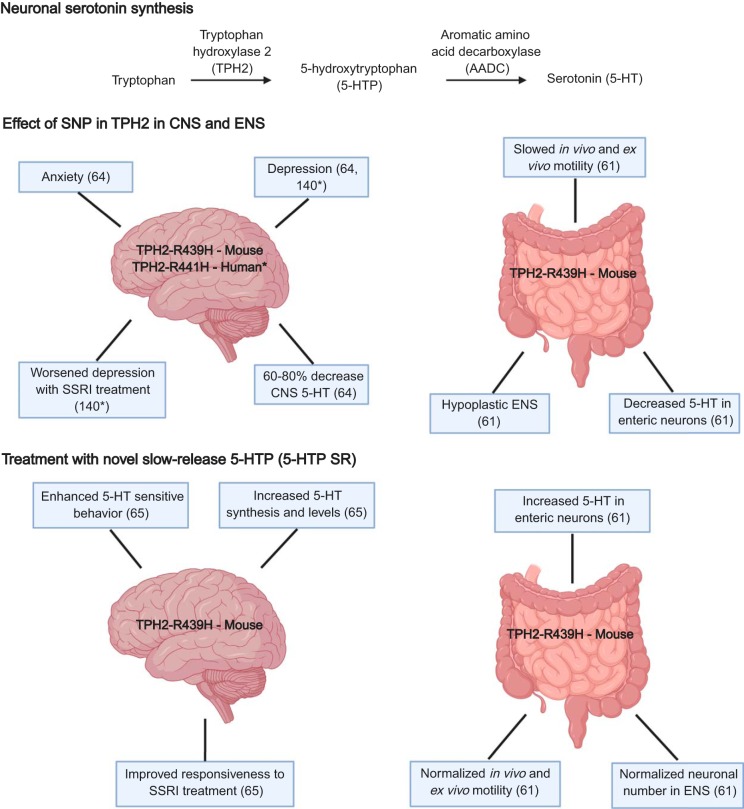
TPH2 is the rate-limiting enzyme that is critical for 5-HT biosynthesis in serotonergic neurons of both the CNS and the ENS. Polymorphisms in TPH2 have been identified in a range of conditions thought to co-occur in both the brain and the intestine. These include depression (128, 134, 141) and anxiety (7, 48), which are often co-morbid with GI disorders, commonly constipation and/or irritable bowel syndrome (IBS) (68). Conversely, constipation and IBS often co-occur with psychiatric conditions, including anxiety and/or depression (56). One such TPH2-related single nucleotide polymorphism (SNP), in which a conserved arginine residue was replaced with histidine (TPH2-R441H), was identified in a cohort of patients with unipolar depression (140) (Fig. 3). The TPH2-R441H polymorphism was shown to confer hypoefficiency to TPH2. Interestingly, individuals in this cohort also expressed a worsened depressive state upon treatment with an SSRI (140).
Fig. 3.
The effects of the single nucleotide polymorphism (SNP) R441H mutation in tryptophan hydroxylase 2 (TPH2), enteric nervous system (ENS), and central nervous system (CNS) development and function in mice. The SNP R441H was identified in a cohort of patients with unipolar depression. This mutation was knocked in to mice with an analogous R439H mutation. These mice exhibit CNS defects, including depression, anxiety, worsened depression with serotonin reuptake inhibitor (SSRI) treatment, and a 60–80% decrease in CNS 5-hydroxytryptamine (5-HT). In the ENS, these mice exhibit slowed in vivo and ex vivo motility, a hypoplastic ENS, and a decreased 5-HT in enteric neurons, as demonstrated by Ca2+ imaging following tryptamine exposure. When a novel slow-release version of 5-hydroxytryptophan (5-HTP SR) is administered in the mouse chow, R439H mice show increased central levels of 5-HT synthesis and improved central responsiveness to SSRI treatment. They also have increased 5-HT in enteric neurons, normalized in vivo and ex vivo motility, and normalized neuronal numbers in the ENS.
A transgenic mouse model incorporating an analogous version of the human SNP (TPH2-R439H) was generated. TPH2-R439H mice exhibit a 60–80% decrease in CNS 5-HT, as well as anxiety- and depressive-like behaviors (64) (Fig. 3). Interestingly, treatment of TPH2-R439H mice with an SSRI further depletes tissue levels of 5-HT (65, 106). Treatment of TPH2-R439H mice with a novel slow-release version of the 5-HT precursor, 5-HTP SR, however, appears to ameliorate these defects (Fig. 3). Treatment with 5-HTP SR in these mice was also shown to maintain therapeutically relevant levels of 5-HT in the CNS (63). This is likely because 5-HTP effectively bypasses TPH2 and thus the genetic abnormality in the TPH2-R439H model. Parallel success was noted in clinical studies; in humans, acute adjunctive 5-HTP was shown to enhance the effects of SSRIs on the neuroendocrine markers of extracellular 5-HT levels (78).
We have recently demonstrated that TPH2-R439H exhibits significant defects in ENS development and ENS-mediated GI functions (61) (Fig. 3). The ENS of TPH2-R439H mice is hypoplastic, with a particular defect in late-born enteric neurons, similar to what is observed in other mouse models where neuronal 5-HT is deficient (e.g., SERT Ala56 and TPH2KO mice) (83). There are also less detectable enteric serotonin-containing neurons in TPH2-R439H mice and less 5-HT present in the serotonergic neurons that do exist, as detected by calcium channel signaling after neuronal tryptamine exposure (61) (Fig. 3); tryptamine has been shown to release endogenous 5-HT from enteric serotonergic neurons (125). When enteric serotonergic neurons take up tryptamine, it displaces 5-HT in intracellular synaptic vesicles, causing 5-HT release and thus eliciting Ca2+ transients. Transients, evoked by an initial application of tryptamine, were significantly greater in WT than in TPH2-R439H animals, suggesting that endogenous stores of releasable 5-HT are significantly lower in enteric neurons of TPH2-R439H mice than in WT animals (61). Consequently, GI motility is markedly altered in TPH2-R439H mice, which demonstrate slowed motility throughout the GI tract, including a decrease in the frequency and velocity of ENS-mediated CMMCs (Table 1). Strikingly, treatment with 5-HTP SR reverses the defects associated with ENS development and GI function in TPH2-R439H mice (Fig. 3). Neuronal numbers in the ENS are normalized in these mice, and this change is accompanied by a normalization of colonic motility to control levels, both in vivo and ex vivo (61). These findings reveal that both CNS and ENS development and function are highly sensitive to levels of neuronal 5-HT and that these neuronally mediated functions may be alterable during adulthood with application of a novel slow-release delivery of 5-HTP. Importantly, treatment with 5-HTP SR also demonstrates the capacity for neurogenesis in the adult ENS, suggesting that ENS plasticity may be able to be modulated through mediation of 5-HT signaling.
5-HT AND THE INTESTINAL MICROBIOME
Although not a major focus of this review, the insights available on the interactions between 5-HT and the enteric microbiome are important to include, because these interactions are increasingly being recognized for their ability to alter host physiology and disease (10, 19, 31, 67, 96, 101, 102, 112). Intestinal microbiota interact with 5-HT in a bidirectional fashion; alterations in the enteric microbiome lead to changes in 5-HT homeostasis, and manipulations in elements of the serotonergic pathway can result in changes in the composition of enteric microbiota communities. The resulting microbial composition could then be a trigger or alternatively, a modifying factor in brain-gut disease.
Intestinal bacteria play a role in intestinal 5-HT production. Germ-free (GF) mice exhibit deficiencies in serum and colonic EC cell 5-HT concentration and TPH1 expression, as well as an increase in SERT expression (137), implying that intestinal epithelial 5-HT production is decreased in GF animals. Furthermore, colonization of GF mice with spore-forming microbes, dominated by the Clostridial species, has been found to restore these concentrations to levels equivalent to those demonstrated in conventionally raised mice (137), indicating that specific bacteria synthesize 5-HT. The gram-negative probiotic, Escherichia coli Nissle 1917, has also been shown to enhance 5-HT biosynthesis and bioavailability in ileal tissue (98).
A greater understanding of how enteric microbes are stimulated to affect 5-HT production may result in novel ways to manipulate enteric 5-HT for therapeutic benefit. One way in which gut microbes may be influenced to increase enteric 5-HT production is through diet; gut microbiota in mice generate short-chain fatty acids (SCFAs) by fermenting dietary saccharides, and this process has been demonstrated to result in an increase in TPH1 expression and levels of 5-HT EC cell synthesis (103). Another way that enteric 5-HT levels may be modulated is through fecal microbiota transplantation (FMT) (69), a therapy that is currently used to treat recurrent Clostridium difficile infection (69) and some cases of IBD (73). FMT is also being evaluated for its possible therapeutic role in several mental health conditions, including anxiety and depression (71). FMT of GF mice with normally colonized stool results in an increase in small intestinal 5-HT concentration, increased TPH1 expression, and faster motility (120, 136). The increase in 5-HT and TPH1 elicited through FMT may be due to microbial production of SCFAs (103). Despite its therapeutic potential, FMT could be disadvantageous because of the administration of highly nonspecific groups of organisms, many of which are unknown and may be harmful. This possibility was actualized recently when two immunocompromised patients who received FMT from stool not screened for drug-resistant bacteria died from infections caused by multi-drug-resistant organisms (42). A more specific therapeutic approach than FMT would thus be preferred. Probiotics may ultimately provide this option. Probiotic strains, such as Lactobacillus acidophilus, Lactobacillus reuteri (17, 104), and Bifidobacterium longum (17), have been shown to increase serum 5-HT (104), as well as intestinal epithelial SERT protein and mRNA levels (16, 17).
Microbial modulation of 5-HT that affects gut neurogenesis and/or motility may require activation of the 5-HT4 receptor. Colonization of GF mice with normal microbiota results in 5-HT release, 5-HT4 receptor activation, and consequent plasticity in the ENS (23). In this study, colonization of GF mice treated with the 5-HT4 agonist sc-53116 resulted in faster intestinal transit, neurogenesis in the myenteric plexus, and an increase in innervation of the colonic mucosa (23).
The mechanisms by which microbiota detect enteric host 5-HT remain largely unanswered, as do the potential consequences of these interactions in disease conditions (103, 137). A recent paper by Fung et al. (32), however, suggests that the spore-forming bacteria, Turicibacter, may have 5-HT “sensors” with some homology to SERT. These bacteria may respond to 5-HT in ways that impact their own colonization, as well as host physiological functions, such as lipid homeostasis (32). Whether other bacteria express similar kinds of machinery and if other sensing mechanisms exist have not yet been evaluated.
Individuals with ASD or depression have been shown, in some studies, to possess an intestinal microbiota that is distinct from control populations (21, 25, 59, 79). The microbiota may play a role in the initiation or perpetuation of either of these disorders; transfer of microbiota from individuals with ASD or depression into GF mice has been shown to replicate these behaviors (74, 110). It is not yet known whether bacteria, such as Turicibacter, or any others for that matter, play a role in these conditions by interacting with the host’s serotonergic system.
FUTURE DIRECTIONS
Over the last decades, it has been increasingly recognized that 5-HT is likely to play important roles in ENS development and long-term functions. The current phase of research that is building on these foundational blocks may well lend insight to novel therapeutics that modulate 5-HT signaling in ways that treat GI motility disorders, as well as other disease states that affect the brain and the intestine simultaneously.
Although not the focus of this review, but important to mention, is the mounting evidence that inflammation also plays a critical role in the pathophysiology of mood disorders and how this connection may link to intestinal inflammatory conditions. Patients with major depression have been shown to possess elevated levels of proinflammatory cytokines, and conversely, many inflammatory conditions, including IBD, have been linked to a higher risk of mood disorders (40, 80, 130) [nicely outlined in Abautret-Daly et al. (1)]. Treatment of psychological symptoms may also improve both the quality-of-life measures as well relapse rates in some individuals with IBD (41, 123). Conversely, management of GI symptoms in these patients may also improve psychological outcomes. In line with these findings, both clinical and preclinical studies have shown that induction of a proinflammatory state in otherwise healthy subjects results in behavioral phenotypes resembling depression (26, 45). Potential mechanisms underlying the inflammatory induction of behavioral changes include effects of cytokines on hypothalamic-pituitary-adrenal axis dysregulation and changes in the modulation of the serotonergic and/or the kynurenine pathways (1). Manipulation of these pathways through 5-HT mediation should thus be considered a goal of future therapies.
The idea that the ENS is continuously regenerating (70) and may be more plastic than previously thought may lead to novel ways to harness this plasticity and modulate it, via 5-HT signaling, to treat ENS-based conditions. Enteric neuropathies and degenerative diseases are often not diagnosed until after birth. It is thus exciting that 5-HT4 agonists may have therapeutic benefits in the postnatal period. Older 5-HT4 agonists, such as cisapride and tegaserod, have been restricted in use, secondary to adverse cardiovascular side effects, based on their off-target effects on human ether à-go-go-related gene (hERG) potassium channels (124). Newer, more highly selective 5-HT4 agonists, however, have not been associated with these side effects (111). Prucalopride, a newer, more targeted 5-HT4 agonist, has been found to increase neurogenesis in vivo, although it has not yet been trialed in neuropathic conditions (36, 83). A slow-release formulation of 5-HTP may also be beneficial in not only treating both depression and constipation (61) but also in mobilizing adult ENS neurogenesis. As shown above, microbial mediators are also currently being evaluated and based on their interactions with 5-HT, may ultimately be used to drive 5-HT-associated brain and gut physiology and function (11, 137). Finally, many other mediators may be involved in the disease conditions discussed in this article. 5-HT is also likely to be involved in multiple brain-gut conditions discussed outside of this review. These data are consistent, however, with the notion that 5-HT modulation will be an important consideration in the future research of brain, gut, and brain-gut diseases.
GRANTS
Support for this work was provided by the National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-093786 (to K. Gross Margolis), National Institute of Neurological Disorders and Stroke Grant NS15547 (to K. Gross Margolis), T35AG044303 (to N. Israelyan), Department of Defense Grant PR160365 (to K. Gross Margolis), and gifts from the Phyllis and Ivan Seidenberg Family Fund for Children’s Digestive Health (to K. Gross Margolis).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.D.C., N.I., and K.G.M. prepared figures; A.D.C., N.I., and K.G.M. drafted manuscript; A.D.C., N.I., and K.G.M. edited and revised manuscript; A.D.C., N.I., and K.G.M. approved final version of manuscript.
REFERENCES
- 1.Abautret-Daly Á, Dempsey E, Parra-Blanco A, Medina C, Harkin A. Gut-brain actions underlying comorbid anxiety and depression associated with inflammatory bowel disease. Acta Neuropsychiatr 30: 275–296, 2018. doi: 10.1017/neu.2017.3. [DOI] [PubMed] [Google Scholar]
- 2.Albert PR. Transcriptional regulation of the 5-HT1A receptor: implications for mental illness. Philos Trans R Soc Lond B Biol Sci 367: 2402–2415, 2012. doi: 10.1098/rstb.2011.0376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anacker C, Zunszain PA, Cattaneo A, Carvalho LA, Garabedian MJ, Thuret S, Price J, Pariante CM. Antidepressants increase human hippocampal neurogenesis by activating the glucocorticoid receptor. Mol Psychiatry 16: 738–750, 2011. doi: 10.1038/mp.2011.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balasuriya GK, Hill-Yardin EL, Gershon MD, Bornstein JC. A sexually dimorphic effect of cholera toxin: rapid changes in colonic motility mediated via a 5-HT3 receptor-dependent pathway in female C57Bl/6 mice. J Physiol 594: 4325–4338, 2016. doi: 10.1113/JP272071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Belkind-Gerson J, Graham HK, Reynolds J, Hotta R, Nagy N, Cheng L, Kamionek M, Shi HN, Aherne CM, Goldstein AM. Colitis promotes neuronal differentiation of Sox2+ and PLP1+ enteric cells. Sci Rep 7: 2525, 2017. doi: 10.1038/s41598-017-02890-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Belkind-Gerson J, Hotta R, Nagy N, Thomas AR, Graham H, Cheng L, Solorzano J, Nguyen D, Kamionek M, Dietrich J, Cherayil BJ, Goldstein AM. Colitis induces enteric neurogenesis through a 5-HT4-dependent mechanism. Inflamm Bowel Dis 21: 870–878, 2015. doi: 10.1097/MIB.0000000000000326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berger SM, Weber T, Perreau-Lenz S, Vogt MA, Gartside SE, Maser-Gluth C, Lanfumey L, Gass P, Spanagel R, Bartsch D. A functional Tph2 C1473G polymorphism causes an anxiety phenotype via compensatory changes in the serotonergic system. Neuropsychopharmacology 37: 1986–1998, 2012. doi: 10.1038/npp.2012.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berthouze M, Ayoub M, Russo O, Rivail L, Sicsic S, Fischmeister R, Berque-Bestel I, Jockers R, Lezoualc’h F. Constitutive dimerization of human serotonin 5-HT4 receptors in living cells. FEBS Lett 579: 2973–2980, 2005. doi: 10.1016/j.febslet.2005.04.040. [DOI] [PubMed] [Google Scholar]
- 9.Bertrand PP, Kunze WA, Furness JB, Bornstein JC. The terminals of myenteric intrinsic primary afferent neurons of the guinea-pig ileum are excited by 5-hydroxytryptamine acting at 5-hydroxytryptamine-3 receptors. Neuroscience 101: 459–469, 2000. doi: 10.1016/S0306-4522(00)00363-8. [DOI] [PubMed] [Google Scholar]
- 10.Bhattarai Y, Schmidt BA, Linden DR, Larson ED, Grover M, Beyder A, Farrugia G, Kashyap PC. Human-derived gut microbiota modulates colonic secretion in mice by regulating 5-HT3 receptor expression via acetate production. Am J Physiol Gastrointest Liver Physiol 313: G80–G87, 2017. doi: 10.1152/ajpgi.00448.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bhattarai Y, Williams BB, Battaglioli EJ, Whitaker WR, Till L, Grover M, Linden DR, Akiba Y, Kandimalla KK, Zachos NC, Kaunitz JD, Sonnenburg JL, Fischbach MA, Farrugia G, Kashyap PC. Gut microbiota-produced tryptamine activates an epithelial G-protein-coupled receptor to increase colonic secretion. Cell Host Microbe 23: 775–785.e5, 2018. doi: 10.1016/j.chom.2018.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bianco F, Bonora E, Natarajan D, Vargiolu M, Thapar N, Torresan F, Giancola F, Boschetti E, Volta U, Bazzoli F, Mazzoni M, Seri M, Clavenzani P, Stanghellini V, Sternini C, De Giorgio R. Prucalopride exerts neuroprotection in human enteric neurons. Am J Physiol Gastrointest Liver Physiol 310: G768–G775, 2016. doi: 10.1152/ajpgi.00036.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brummelte S, Mc Glanaghy E, Bonnin A, Oberlander TF. Developmental changes in serotonin signaling: implications for early brain function, behavior and adaptation. Neuroscience 342: 212–231, 2017. doi: 10.1016/j.neuroscience.2016.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bülbring E, Crema A. The action of 5-hydroxytryptamine, 5-hydroxytryptophan and reserpine on intestinal peristalsis in anaesthetized guinea-pigs. J Physiol 146: 29–53, 1959. doi: 10.1113/jphysiol.1959.sp006176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bülbring E, Lin RC. The effect of intraluminal application of 5-hydroxytryptamine and 5-hydroxytryptophan on peristalsis; the local production of 5-HT and its release in relation to intraluminal pressure and propulsive activity. J Physiol 140: 381–407, 1958. [PMC free article] [PubMed] [Google Scholar]
- 16.Cao YN, Feng LJ, Liu YY, Jiang K, Zhang MJ, Gu YX, Wang BM, Gao J, Wang ZL, Wang YM. Effect of Lactobacillus rhamnosus GG supernatant on serotonin transporter expression in rats with post-infectious irritable bowel syndrome. World J Gastroenterol 24: 338–350, 2018. doi: 10.3748/wjg.v24.i3.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cao YN, Feng LJ, Wang BM, Jiang K, Li S, Xu X, Wang WQ, Zhao JW, Wang YM. Lactobacillus acidophilus and Bifidobacterium longum supernatants upregulate the serotonin transporter expression in intestinal epithelial cells. Saudi J Gastroenterol 24: 59–66, 2018. doi: 10.4103/sjg.SJG_333_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carneiro AM, Cook EH, Murphy DL, Blakely RD. Interactions between integrin alphaIIbbeta3 and the serotonin transporter regulate serotonin transport and platelet aggregation in mice and humans. J Clin Invest 118: 1544–1552, 2008. doi: 10.1172/JCI33374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chang EB, Rao MC. A new role for microbiota? Dulling the thrust of serotonin and 5-HT3 signaling cascade. Am J Physiol Gastrointest Liver Physiol 313: G14–G15, 2017. doi: 10.1152/ajpgi.00166.2017. [DOI] [PubMed] [Google Scholar]
- 20.Coates MD, Tekin I, Vrana KE, Mawe GM. Review article: the many potential roles of intestinal serotonin (5-hydroxytryptamine, 5-HT) signalling in inflammatory bowel disease. Aliment Pharmacol Ther 46: 569–580, 2017. doi: 10.1111/apt.14226. [DOI] [PubMed] [Google Scholar]
- 21.Coretti L, Paparo L, Riccio MP, Amato F, Cuomo M, Natale A, Borrelli L, Corrado G, Comegna M, Buommino E, Castaldo G, Bravaccio C, Chiariotti L, Berni Canani R, Lembo F. Gut microbiota features in young children with autism spectrum disorders. Front Microbiol 9: 3146, 2018. [Erratum in Front Microbiol 10: 920, 2019.]. doi: 10.3389/fmicb.2018.03146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davis RL, Rubanowice D, McPhillips H, Raebel MA, Andrade SE, Smith D, Yood MU, Platt R; HMO Research Network Center for Education, Research in Therapeutics . Risks of congenital malformations and perinatal events among infants exposed to antidepressant medications during pregnancy. Pharmacoepidemiol Drug Saf 16: 1086–1094, 2007. doi: 10.1002/pds.1462. [DOI] [PubMed] [Google Scholar]
- 23.De Vadder F, Grasset E, Mannerås Holm L, Karsenty G, Macpherson AJ, Olofsson LE, Bäckhed F. Gut microbiota regulates maturation of the adult enteric nervous system via enteric serotonin networks. Proc Natl Acad Sci USA 115: 6458–6463, 2018. doi: 10.1073/pnas.1720017115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Vitry F, Hamon M, Catelon J, Dubois M, Thibault J. Serotonin initiates and autoamplifies its own synthesis during mouse central nervous system development. Proc Natl Acad Sci USA 83: 8629–8633, 1986. doi: 10.1073/pnas.83.22.8629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ding HT, Taur Y, Walkup JT. Gut microbiota and sutism: key concepts and findings. J Autism Dev Disord 47: 480–489, 2017. doi: 10.1007/s10803-016-2960-9. [DOI] [PubMed] [Google Scholar]
- 26.Eisenberger NI, Berkman ET, Inagaki TK, Rameson LT, Mashal NM, Irwin MR. Inflammation-induced anhedonia: endotoxin reduces ventral striatum responses to reward. Biol Psychiatry 68: 748–754, 2010. doi: 10.1016/j.biopsych.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eriksson PS, Perfilieva E, Björk-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH. Neurogenesis in the adult human hippocampus. Nat Med 4: 1313–1317, 1998. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- 28.Erspamer V. Pharmacology of enteramine. Arch Exp Pathol Pharmakol 196: 343–365, 1940. doi: 10.1007/BF01861121. [DOI] [Google Scholar]
- 29.Ferguson BJ, Marler S, Altstein LL, Lee EB, Mazurek MO, McLaughlin A, Macklin EA, McDonnell E, Davis DJ, Belenchia AM, Gillespie CH, Peterson CA, Bauman ML, Margolis KG, Veenstra-VanderWeele J, Beversdorf DQ. Associations between cytokines, endocrine stress response, and gastrointestinal symptoms in autism spectrum disorder. Brain Behav Immun 58: 57–62, 2016. doi: 10.1016/j.bbi.2016.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fiorica-Howells E, Maroteaux L, Gershon MD. Serotonin and the 5-HT(2B) receptor in the development of enteric neurons. J Neurosci 20: 294–305, 2000. doi: 10.1523/JNEUROSCI.20-01-00294.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Frank DN, St. Amand AL, Feldman RA, Boedeker EC, Harpaz N, Pace NR. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci USA 104: 13780–13785, 2007. doi: 10.1073/pnas.0706625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fung TC, Vuong HE, Luna CDG, Pronovost GN, Aleksandrova AA, Riley NG, Vavilina A, McGinn J, Rendon T, Forrest LR, Hsiao EY. Intestinal serotonin and fluoxetine exposure modulate bacterial colonization in the gut. Nat Microbiol In press. doi: 10.1038/s41564-019-0540-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Galligan JJ, LePard KJ, Schneider DA, Zhou X. Multiple mechanisms of fast excitatory synaptic transmission in the enteric nervous system. J Auton Nerv Syst 81: 97–103, 2000. doi: 10.1016/S0165-1838(00)00130-2. [DOI] [PubMed] [Google Scholar]
- 34.Gao J, Xu K, Liu H, Liu G, Bai M, Peng C, Li T, Yin Y. Impact of the gut microbiota on intestinal immunity mediated by tryptophan metabolism. Front Cell Infect Microbiol 8: 13, 2018. doi: 10.3389/fcimb.2018.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gaspar P, Cases O, Maroteaux L. The developmental role of serotonin: news from mouse molecular genetics. Nat Rev Neurosci 4: 1002–1012, 2003. doi: 10.1038/nrn1256. [DOI] [PubMed] [Google Scholar]
- 36.Gershon MD. 5-HT4-mediated neuroprotection: a new therapeutic modality on the way? Am J Physiol Gastrointest Liver Physiol 310: G766–G767, 2016. doi: 10.1152/ajpgi.00120.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gershon MD. 5-Hydroxytryptamine (serotonin) in the gastrointestinal tract. Curr Opin Endocrinol Diabetes Obes 20: 14–21, 2013. doi: 10.1097/MED.0b013e32835bc703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gershon MD. Review article: serotonin receptors and transporters—roles in normal and abnormal gastrointestinal motility. Aliment Pharmacol Ther 20, Suppl 7: 3–14, 2004. doi: 10.1111/j.1365-2036.2004.02180.x. [DOI] [PubMed] [Google Scholar]
- 39.Glatt CE, DeYoung JA, Delgado S, Service SK, Giacomini KM, Edwards RH, Risch N, Freimer NB. Screening a large reference sample to identify very low frequency sequence variants: comparisons between two genes. Nat Genet 27: 435–438, 2001. doi: 10.1038/86948. [DOI] [PubMed] [Google Scholar]
- 40.Goldsmith DR, Rapaport MH, Miller BJ. A meta-analysis of blood cytokine network alterations in psychiatric patients: comparisons between schizophrenia, bipolar disorder and depression. Mol Psychiatry 21: 1696–1709, 2016. doi: 10.1038/mp.2016.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Goodhand JR, Greig FI, Koodun Y, McDermott A, Wahed M, Langmead L, Rampton DS. Do antidepressants influence the disease course in inflammatory bowel disease? A retrospective case-matched observational study. Inflamm Bowel Dis 18: 1232–1239, 2012. doi: 10.1002/ibd.21846. [DOI] [PubMed] [Google Scholar]
- 42.Grady D. Fecal transplant is linked to a patient’s death, the F.D.A. warns. New York Times; https://www.nytimes.com/2019/06/13/health/fecal-transplant-fda.html. [September 18, 2019]. [Google Scholar]
- 43.Greenlee JL, Mosley AS, Shui AM, Veenstra-VanderWeele J, Gotham KO. Medical and behavioral correlates of depression history in children and adolescents with autism spectrum disorder. Pediatrics 137, Suppl 2: S105–S114, 2016. doi: 10.1542/peds.2015-2851I. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grider JR, Foxx-Orenstein AE, Jin JG. 5-Hydroxytryptamine4 receptor agonists initiate the peristaltic reflex in human, rat, and guinea pig intestine. Gastroenterology 115: 370–380, 1998. doi: 10.1016/S0016-5085(98)70203-3. [DOI] [PubMed] [Google Scholar]
- 45.Grigoleit JS, Kullmann JS, Wolf OT, Hammes F, Wegner A, Jablonowski S, Engler H, Gizewski E, Oberbeck R, Schedlowski M. Dose-dependent effects of endotoxin on neurobehavioral functions in humans. PLoS One 6: e28330, 2011. doi: 10.1371/journal.pone.0028330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Grundy D, Blackshaw LA, Hillsley K. Role of 5-hydroxytryptamine in gastrointestinal chemosensitivity. Dig Dis Sci 39, Suppl: 44S–47S, 1994. doi: 10.1007/BF02300369. [DOI] [PubMed] [Google Scholar]
- 47.Gur TL, Kim DR, Epperson CN. Central nervous system effects of prenatal selective serotonin reuptake inhibitors: sensing the signal through the noise. Psychopharmacology (Berl) 227: 567–582, 2013. doi: 10.1007/s00213-013-3115-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gutknecht L, Jacob C, Strobel A, Kriegebaum C, Müller J, Zeng Y, Markert C, Escher A, Wendland J, Reif A, Mössner R, Gross C, Brocke B, Lesch KP. Tryptophan hydroxylase-2 gene variation influences personality traits and disorders related to emotional dysregulation. Int J Neuropsychopharmacol 10: 309–320, 2007. doi: 10.1017/S1461145706007437. [DOI] [PubMed] [Google Scholar]
- 49.Henry JP, Sagné C, Botton D, Isambert MF, Gasnier B. Molecular pharmacology of the vesicular monoamine transporter. Adv Pharmacol 42: 236–239, 1998. doi: 10.1016/S1054-3589(08)60736-X. [DOI] [PubMed] [Google Scholar]
- 50.Heredia DJ, Dickson EJ, Bayguinov PO, Hennig GW, Smith TK. Localized release of serotonin (5-hydroxytryptamine) by a fecal pellet regulates migrating motor complexes in murine colon. Gastroenterology 136: 1328–1338, 2009. doi: 10.1053/j.gastro.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Heredia DJ, Gershon MD, Koh SD, Corrigan RD, Okamoto T, Smith TK. Important role of mucosal serotonin in colonic propulsion and peristaltic reflexes: in vitro analyses in mice lacking tryptophan hydroxylase 1. J Physiol 591: 5939–5957, 2013. doi: 10.1113/jphysiol.2013.256230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hirst GD, Edwards FR. Role of interstitial cells of Cajal in the control of gastric motility. J Pharmacol Sci 96: 1–10, 2004. doi: 10.1254/jphs.CRJ04002X. [DOI] [PubMed] [Google Scholar]
- 53.Hoffman JM, McKnight ND, Sharkey KA, Mawe GM. The relationship between inflammation-induced neuronal excitability and disrupted motor activity in the guinea pig distal colon. Neurogastroenterol Motil 23: 673-e279, 2011. doi: 10.1111/j.1365-2982.2011.01702.x. [DOI] [PubMed] [Google Scholar]
- 54.Hoffman JM, Tyler K, MacEachern SJ, Balemba OB, Johnson AC, Brooks EM, Zhao H, Swain GM, Moses PL, Galligan JJ, Sharkey KA, Greenwood-Van Meerveld B, Mawe GM. Activation of colonic mucosal 5-HT(4) receptors accelerates propulsive motility and inhibits visceral hypersensitivity. Gastroenterology 142: 844–854.e4, 2012. doi: 10.1053/j.gastro.2011.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Homberg JR, Kolk SM, Schubert D. Editorial perspective of the research topic “deciphering serotonin’s role in neurodevelopment.” Front Cell Neurosci 7: 212, 2013. doi: 10.3389/fncel.2013.00212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hosseinzadeh ST, Poorsaadati S, Radkani B, Forootan M. Psychological disorders in patients with chronic constipation. Gastroenterol Hepatol Bed Bench 4: 159–163, 2011. [PMC free article] [PubMed] [Google Scholar]
- 57.Hotta R, Cheng L, Graham HK, Nagy N, Belkind-Gerson J, Mattheolabakis G, Amiji MM, Goldstein AM. Delivery of enteric neural progenitors with 5-HT4 agonist-loaded nanoparticles and thermosensitive hydrogel enhances cell proliferation and differentiation following transplantation in vivo. Biomaterials 88: 1–11, 2016. doi: 10.1016/j.biomaterials.2016.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hu XQ, Peoples RW. The 5-HT3B subunit confers spontaneous channel opening and altered ligand properties of the 5-HT3 receptor. J Biol Chem 283: 6826–6831, 2008. doi: 10.1074/jbc.M707571200. [DOI] [PubMed] [Google Scholar]
- 59.Huang TT, Lai JB, Du YL, Xu Y, Ruan LM, Hu SH. Current understanding of gut microbiota in mood disorders: an update of human studies. Front Genet 10: 98, 2019. doi: 10.3389/fgene.2019.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.International Molecular Genetic Study of Autism Consortium (IMGSAC) A genomewide screen for autism: strong evidence for linkage to chromosomes 2q, 7q, and 16p. Am J Hum Genet 69: 570–581, 2001. doi: 10.1086/323264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Israelyan N, Del Colle A, Li Z, Park Y, Xing A, Jacobsen JPR, Luna RA, Jensen DD, Madra M, Saurman V, Rahim R, Latorre R, Law K, Carson W, Bunnett NW, Caron MG, Margolis KG. Effects of serotonin and slow-release 5-hydroxytryptophan on gastrointestinal motility in a mouse model of depression. Gastroenterology 157: 507–521.e4, 2019. doi: 10.1053/j.gastro.2019.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Israelyan N, Margolis KG. Reprint of: serotonin as a link between the gut-brain-microbiome axis in autism spectrum disorders. Pharmacol Res 140: 115–120, 2019. doi: 10.1016/j.phrs.2018.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jacobsen JP, Rudder ML, Roberts W, Royer EL, Robinson TJ, Oh A, Spasojevic I, Sachs BD, Caron MG. SSRI augmentation by 5-hydroxytryptophan slow release: mouse pharmacodynamic proof of concept. Neuropsychopharmacology 41: 2324–2334, 2016. doi: 10.1038/npp.2016.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jacobsen JP, Siesser WB, Sachs BD, Peterson S, Cools MJ, Setola V, Folgering JH, Flik G, Caron MG. Deficient serotonin neurotransmission and depression-like serotonin biomarker alterations in tryptophan hydroxylase 2 (Tph2) loss-of-function mice. Mol Psychiatry 17: 694–704, 2012. doi: 10.1038/mp.2011.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jacobsen JPR, Oh A, Bangle R, Roberts WL, Royer EL, Modesto N, Windermere SA, Yi Z, Vernon R, Cajina M, Urs NM, Snyder JC, Nicholls PJ, Sachs BD, Caron MG. Slow-release delivery enhances the pharmacological properties of oral 5-hydroxytryptophan: mouse proof-of-concept. Neuropsychopharmacology 44: 2082–2090, 2019. doi: 10.1038/s41386-019-0400-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jenkins TA, Nguyen JC, Polglaze KE, Bertrand PP. Influence of tryptophan and serotonin on mood and cognition with a possible role of the gut-brain axis. Nutrients 8: E56, 2016. doi: 10.3390/nu8010056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jostins L, Ripke S, Weersma RK, Duerr RH, McGovern DP, Hui KY, Lee JC, Schumm LP, Sharma Y, Anderson CA, Essers J, Mitrovic M, Ning K, Cleynen I, Theatre E, Spain SL, Raychaudhuri S, Goyette P, Wei Z, Abraham C, Achkar JP, Ahmad T, Amininejad L, Ananthakrishnan AN, Andersen V, Andrews JM, Baidoo L, Balschun T, Bampton PA, Bitton A, Boucher G, Brand S, Büning C, Cohain A, Cichon S, D’Amato M, De Jong D, Devaney KL, Dubinsky M, Edwards C, Ellinghaus D, Ferguson LR, Franchimont D, Fransen K, Gearry R, Georges M, Gieger C, Glas J, Haritunians T, Hart A, Hawkey C, Hedl M, Hu X, Karlsen TH, Kupcinskas L, Kugathasan S, Latiano A, Laukens D, Lawrance IC, Lees CW, Louis E, Mahy G, Mansfield J, Morgan AR, Mowat C, Newman W, Palmieri O, Ponsioen CY, Potocnik U, Prescott NJ, Regueiro M, Rotter JI, Russell RK, Sanderson JD, Sans M, Satsangi J, Schreiber S, Simms LA, Sventoraityte J, Targan SR, Taylor KD, Tremelling M, Verspaget HW, De Vos M, Wijmenga C, Wilson DC, Winkelmann J, Xavier RJ, Zeissig S, Zhang B, Zhang CK, Zhao H, Silverberg MS, Annese V, Hakonarson H, Brant SR, Radford-Smith G, Mathew CG, Rioux JD, Schadt EE, Daly MJ, Franke A, Parkes M, Vermeire S, Barrett JC, Cho JH; International IBD Genetics Consortium (IIBDGC) . Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature 491: 119–124, 2012. doi: 10.1038/nature11582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jun S, Kohen R, Cain KC, Jarrett ME, Heitkemper MM. Associations of tryptophan hydroxylase gene polymorphisms with irritable bowel syndrome. Neurogastroenterol Motil 23: 233–239, 2011. doi: 10.1111/j.1365-2982.2010.01623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kim P, Gadani A, Abdul-Baki H, Mitre R, Mitre M. Fecal microbiota transplantation in recurrent Clostridium difficile infection: A retrospective single-center chart review. JGH Open 3: 4–9, 2019. doi: 10.1002/jgh3.12093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kulkarni S, Micci MA, Leser J, Shin C, Tang SC, Fu YY, Liu L, Li Q, Saha M, Li C, Enikolopov G, Becker L, Rakhilin N, Anderson M, Shen X, Dong X, Butte MJ, Song H, Southard-Smith EM, Kapur RP, Bogunovic M, Pasricha PJ. Adult enteric nervous system in health is maintained by a dynamic balance between neuronal apoptosis and neurogenesis. Proc Natl Acad Sci USA 114: E3709–E3718, 2017. doi: 10.1073/pnas.1619406114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kurokawa S, Kishimoto T, Mizuno S, Masaoka T, Naganuma M, Liang KC, Kitazawa M, Nakashima M, Shindo C, Suda W, Hattori M, Kanai T, Mimura M. The effect of fecal microbiota transplantation on psychiatric symptoms among patients with irritable bowel syndrome, functional diarrhea and functional constipation: An open-label observational study. J Affect Disord 235: 506–512, 2018. doi: 10.1016/j.jad.2018.04.038. [DOI] [PubMed] [Google Scholar]
- 72.Lesch KP, Waider J. Serotonin in the modulation of neural plasticity and networks: implications for neurodevelopmental disorders. Neuron 76: 175–191, 2012. doi: 10.1016/j.neuron.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 73.Levy AN, Allegretti JR. Insights into the role of fecal microbiota transplantation for the treatment of inflammatory bowel disease. Therap Adv Gastroenterol 12: 1756284819836893, 2019. doi: 10.1177/1756284819836893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li N, Wang Q, Wang Y, Sun A, Lin Y, Jin Y, Li X. Fecal microbiota transplantation from chronic unpredictable mild stress mice donors affects anxiety-like and depression-like behavior in recipient mice via the gut microbiota-inflammation-brain axis. Stress 22: 592–602, 2019. doi: 10.1080/10253890.2019.1617267. [DOI] [PubMed] [Google Scholar]
- 75.Li Z, Chalazonitis A, Huang YY, Mann JJ, Margolis KG, Yang QM, Kim DO, Côté F, Mallet J, Gershon MD. Essential roles of enteric neuronal serotonin in gastrointestinal motility and the development/survival of enteric dopaminergic neurons. J Neurosci 31: 8998–9009, 2011. doi: 10.1523/JNEUROSCI.6684-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li ZS, Pham TD, Tamir H, Chen JJ, Gershon MD. Enteric dopaminergic neurons: definition, developmental lineage, and effects of extrinsic denervation. J Neurosci 24: 1330–1339, 2004. doi: 10.1523/JNEUROSCI.3982-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liu MT, Kuan YH, Wang J, Hen R, Gershon MD. 5-HT4 receptor-mediated neuroprotection and neurogenesis in the enteric nervous system of adult mice. J Neurosci 29: 9683–9699, 2009. doi: 10.1523/JNEUROSCI.1145-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lowe SL, Yeo KP, Teng L, Soon DK, Pan A, Wise SD, Peck RW. L-5-Hydroxytryptophan augments the neuroendocrine response to a SSRI. Psychoneuroendocrinology 31: 473–484, 2006. doi: 10.1016/j.psyneuen.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 79.Mangiola F, Ianiro G, Franceschi F, Fagiuoli S, Gasbarrini G, Gasbarrini A. Gut microbiota in autism and mood disorders. World J Gastroenterol 22: 361–368, 2016. doi: 10.3748/wjg.v22.i1.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Margaretten M, Julian L, Katz P, Yelin E. Depression in patients with rheumatoid arthritis: description, causes and mechanisms. Int J Clin Rheumatol 6: 617–623, 2011. doi: 10.2217/ijr.11.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Margolis KG. A role for the serotonin reuptake transporter in the brain and intestinal features of autism spectrum disorders and developmental antidepressant exposure. J Chem Neuroanat 83–84: 36–40, 2017. doi: 10.1016/j.jchemneu.2017.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Margolis KG, Buie TM, Turner JB, Silberman AE, Feldman JF, Murray KF, McSwiggan-Hardin M, Levy J, Bauman ML, Veenstra-VanderWeele J, Whitaker AH, Winter HS. Development of a brief parent-report screen for common gastrointestinal disorders in autism spectrum disorder. J Autism Dev Disord 49: 349–362, 2019. doi: 10.1007/s10803-018-3767-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Margolis KG, Li Z, Stevanovic K, Saurman V, Israelyan N, Anderson GM, Snyder I, Veenstra-VanderWeele J, Blakely RD, Gershon MD. Serotonin transporter variant drives preventable gastrointestinal abnormalities in development and function. J Clin Invest 126: 2221–2235, 2016. doi: 10.1172/JCI84877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Margolis KG, Stevanovic K, Li Z, Yang QM, Oravecz T, Zambrowicz B, Jhaver KG, Diacou A, Gershon MD. Pharmacological reduction of mucosal but not neuronal serotonin opposes inflammation in mouse intestine. Gut 63: 928–937, 2014. doi: 10.1136/gutjnl-2013-304901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Marler S, Ferguson BJ, Lee EB, Peters B, Williams KC, McDonnell E, Macklin EA, Levitt P, Gillespie CH, Anderson GM, Margolis KG, Beversdorf DQ, Veenstra-VanderWeele J. Brief report: whole blood serotonin levels and gastrointestinal symptoms in autism spectrum disorder. J Autism Dev Disord 46: 1124–1130, 2016. doi: 10.1007/s10803-015-2646-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mawe GM, Hoffman JM. Serotonin signalling in the gut—functions, dysfunctions and therapeutic targets. Nat Rev Gastroenterol Hepatol 10: 473–486, 2013. [Erratum in Nat Rev Gastroenterol Hepatol 10: 564, 2013.] doi: 10.1038/nrgastro.2013.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mayer EA. Gut feelings: the emerging biology of gut-brain communication. Nat Rev Neurosci 12: 453–466, 2011. doi: 10.1038/nrn3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mayer EA. The neurobiology of stress and gastrointestinal disease. Gut 47: 861–869, 2000. doi: 10.1136/gut.47.6.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mayer EA, Knight R, Mazmanian SK, Cryan JF, Tillisch K. Gut microbes and the brain: paradigm shift in neuroscience. J Neurosci 34: 15490–15496, 2014. doi: 10.1523/JNEUROSCI.3299-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.McAllister BB, Kiryanova V, Dyck RH. Behavioural outcomes of perinatal maternal fluoxetine treatment. Neuroscience 226: 356–366, 2012. doi: 10.1016/j.neuroscience.2012.09.024. [DOI] [PubMed] [Google Scholar]
- 91.McElhanon BO, McCracken C, Karpen S, Sharp WG. Gastrointestinal symptoms in autism spectrum disorder: a meta-analysis. Pediatrics 133: 872–883, 2014. doi: 10.1542/peds.2013-3995. [DOI] [PubMed] [Google Scholar]
- 92.Monro RL, Bertrand PP, Bornstein JC. ATP and 5-HT are the principal neurotransmitters in the descending excitatory reflex pathway of the guinea-pig ileum. Neurogastroenterol Motil 14: 255–264, 2002. doi: 10.1046/j.1365-2982.2002.00325.x. [DOI] [PubMed] [Google Scholar]
- 93.Muller CL, Anacker AMJ, Veenstra-VanderWeele J. The serotonin system in autism spectrum disorder: From biomarker to animal models. Neuroscience 321: 24–41, 2016. doi: 10.1016/j.neuroscience.2015.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Murphy DL, Moya PR. Human serotonin transporter gene (SLC6A4) variants: their contributions to understanding pharmacogenomic and other functional G×G and G×E differences in health and disease. Curr Opin Pharmacol 11: 3–10, 2011. doi: 10.1016/j.coph.2011.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nijenhuis CM, ter Horst PG, van Rein N, Wilffert B, de Jong-van den Berg LT. Disturbed development of the enteric nervous system after in utero exposure of selective serotonin re-uptake inhibitors and tricyclic antidepressants. Part 2: testing the hypotheses. Br J Clin Pharmacol 73: 126–134, 2012. doi: 10.1111/j.1365-2125.2011.04081.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nitzan O, Elias M, Peretz A, Saliba W. Role of antibiotics for treatment of inflammatory bowel disease. World J Gastroenterol 22: 1078–1087, 2016. doi: 10.3748/wjg.v22.i3.1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nordquist N, Oreland L. Serotonin, genetic variability, behaviour, and psychiatric disorders—a review. Ups J Med Sci 115: 2–10, 2010. doi: 10.3109/03009730903573246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nzakizwanayo J, Dedi C, Standen G, Macfarlane WM, Patel BA, Jones BV. Escherichia coli Nissle 1917 enhances bioavailability of serotonin in gut tissues through modulation of synthesis and clearance. Sci Rep 5: 17324, 2015. doi: 10.1038/srep17324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Peters B, Williams KC, Gorrindo P, Rosenberg D, Lee EB, Levitt P, Veenstra-VanderWeele J. Rigid-compulsive behaviors are associated with mixed bowel symptoms in autism spectrum disorder. J Autism Dev Disord 44: 1425–1432, 2014. doi: 10.1007/s10803-013-2009-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pithadia AB, Jain SM. 5-Hydroxytryptamine receptor subtypes and their modulators with therapeutic potentials. J Clin Med Res 1: 72–80, 2009. doi: 10.4021/jocmr2009.05.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Prado C, Michels M, Avila P, Burger H, Milioli MVM, Dal-Pizzol F. The protective effects of fecal microbiota transplantation in an experimental model of necrotizing enterocolitis. J Pediatr Surg 54: 1578–1583, 2019. doi: 10.1016/j.jpedsurg.2018.10.045. [DOI] [PubMed] [Google Scholar]
- 102.Ramos GP, Papadakis KA. Mechanisms of disease: inflammatory bowel diseases. Mayo Clin Proc 94: 155–165, 2019. doi: 10.1016/j.mayocp.2018.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Reigstad CS, Salmonson CE, Rainey JF III, Szurszewski JH, Linden DR, Sonnenburg JL, Farrugia G, Kashyap PC. Gut microbes promote colonic serotonin production through an effect of short-chain fatty acids on enterochromaffin cells. FASEB J 29: 1395–1403, 2015. doi: 10.1096/fj.14-259598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Riezzo G, Chimienti G, Orlando A, D’Attoma B, Clemente C, Russo F. Effects of long-term administration of Lactobacillus reuteri DSM-17938 on circulating levels of 5-HT and BDNF in adults with functional constipation. Benef Microbes 10: 137–147, 2019. doi: 10.3920/BM2018.0050. [DOI] [PubMed] [Google Scholar]
- 105.Ruddick JP, Evans AK, Nutt DJ, Lightman SL, Rook GA, Lowry CA. Tryptophan metabolism in the central nervous system: medical implications. Expert Rev Mol Med 8: 1–27, 2006. doi: 10.1017/S1462399406000068. [DOI] [PubMed] [Google Scholar]
- 106.Sachs BD, Jacobsen JP, Thomas TL, Siesser WB, Roberts WL, Caron MG. The effects of congenital brain serotonin deficiency on responses to chronic fluoxetine. Transl Psychiatry 3: e291, 2013. doi: 10.1038/tp.2013.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sangkuhl K, Klein TE, Altman RB. Selective serotonin reuptake inhibitors pathway. Pharmacogenet Genomics 19: 907–909, 2009. doi: 10.1097/FPC.0b013e32833132cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Savitz J. The kynurenine pathway: a finger in every pie. Mol Psychiatry In press. doi: 10.1038/s41380-019-0414-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Savitz J, Drevets WC, Wurfel BE, Ford BN, Bellgowan PS, Victor TA, Bodurka J, Teague TK, Dantzer R. Reduction of kynurenic acid to quinolinic acid ratio in both the depressed and remitted phases of major depressive disorder. Brain Behav Immun 46: 55–59, 2015. doi: 10.1016/j.bbi.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sharon G, Cruz NJ, Kang DW, Gandal MJ, Wang B, Kim YM, Zink EM, Casey CP, Taylor BC, Lane CJ, Bramer LM, Isern NG, Hoyt DW, Noecker C, Sweredoski MJ, Moradian A, Borenstein E, Jansson JK, Knight R, Metz TO, Lois C, Geschwind DH, Krajmalnik-Brown R, Mazmanian SK. Human gut microbiota from autism spectrum disorder promote behavioral symptoms in mice. Cell 177: 1600–1618.e17, 2019. doi: 10.1016/j.cell.2019.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Shin A, Camilleri M, Kolar G, Erwin P, West CP, Murad MH. Systematic review with meta-analysis: highly selective 5-HT4 agonists (prucalopride, velusetrag or naronapride) in chronic constipation. Aliment Pharmacol Ther 39: 239–253, 2014. doi: 10.1111/apt.12571. [DOI] [PubMed] [Google Scholar]
- 112.Shin A, Preidis GA, Shulman R, Kashyap PC. The gut microbiome in adult and pediatric functional gastrointestinal disorders. Clin Gastroenterol Hepatol 17: 256–274, 2019. doi: 10.1016/j.cgh.2018.08.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sia TC, Whiting M, Kyloh M, Nicholas SJ, Oliver J, Brookes SJ, Dinning PG, Wattchow DA, Spencer NJ. 5-HT3 and 5-HT4 antagonists inhibit peristaltic contractions in guinea-pig distal colon by mechanisms independent of endogenous 5-HT. Front Neurosci 7: 136, 2013. doi: 10.3389/fnins.2013.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Smith TK, Gershon MD. CrossTalk proposal: 5-HT is necessary for peristalsis. J Physiol 593: 3225–3227, 2015. doi: 10.1113/JP270182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Smith TK, Park KJ, Hennig GW. Colonic migrating motor complexes, high amplitude propagating contractions, neural reflexes and the importance of neuronal and mucosal serotonin. J Neurogastroenterol Motil 20: 423–446, 2014. doi: 10.5056/jnm14092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Song NN, Huang Y, Yu X, Lang B, Ding YQ, Zhang L. Divergent roles of central serotonin in adult hippocampal neurogenesis. Front Cell Neurosci 11: 185, 2017. doi: 10.3389/fncel.2017.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Spencer NJ, Nicholas SJ, Robinson L, Kyloh M, Flack N, Brookes SJ, Zagorodnyuk VP, Keating DJ. Mechanisms underlying distension-evoked peristalsis in guinea pig distal colon: is there a role for enterochromaffin cells? Am J Physiol Gastrointest Liver Physiol 301: G519–G527, 2011. doi: 10.1152/ajpgi.00101.2011. [DOI] [PubMed] [Google Scholar]
- 118.Spencer NJ, Nicholas SJ, Sia TC, Staikopoulos V, Kyloh M, Beckett EA. By what mechanism does ondansetron inhibit colonic migrating motor complexes: does it require endogenous serotonin in the gut wall? Neurogastroenterol Motil 25: 677–685, 2013. doi: 10.1111/nmo.12136. [DOI] [PubMed] [Google Scholar]
- 119.Spencer NJ, Sia TC, Brookes SJ, Costa M, Keating DJ. CrossTalk opposing view: 5-HT is not necessary for peristalsis. J Physiol 593: 3229–3231, 2015. doi: 10.1113/JP270183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sun W, Guo Y, Zhang S, Chen Z, Wu K, Liu Q, Liu K, Wen L, Wei Y, Wang B, Chen D. Fecal microbiota transplantation can alleviate gastrointestinal transit in rats with high-fat diet-induced obesity via regulation of serotonin biosynthesis. BioMed Res Int 2018: 8308671, 2018. doi: 10.1155/2018/8308671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Suri D, Teixeira CM, Cagliostro MK, Mahadevia D, Ansorge MS. Monoamine-sensitive developmental periods impacting adult emotional and cognitive behaviors. Neuropsychopharmacology 40: 88–112, 2015. doi: 10.1038/npp.2014.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sutcliffe JS, Delahanty RJ, Prasad HC, McCauley JL, Han Q, Jiang L, Li C, Folstein SE, Blakely RD. Allelic heterogeneity at the serotonin transporter locus (SLC6A4) confers susceptibility to autism and rigid-compulsive behaviors. Am J Hum Genet 77: 265–279, 2005. doi: 10.1086/432648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Szigethy E, Bujoreanu SI, Youk AO, Weisz J, Benhayon D, Fairclough D, Ducharme P, Gonzalez-Heydrich J, Keljo D, Srinath A, Bousvaros A, Kirshner M, Newara M, Kupfer D, DeMaso DR. Randomized efficacy trial of two psychotherapies for depression in youth with inflammatory bowel disease. J Am Acad Child Adolesc Psychiatry 53: 726–735, 2014. doi: 10.1016/j.jaac.2014.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Tack J, Camilleri M, Chang L, Chey WD, Galligan JJ, Lacy BE, Müller-Lissner S, Quigley EM, Schuurkes J, De Maeyer JH, Stanghellini V. Systematic review: cardiovascular safety profile of 5-HT(4) agonists developed for gastrointestinal disorders. Aliment Pharmacol Ther 35: 745–767, 2012. doi: 10.1111/j.1365-2036.2012.05011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Takaki M, Mawe GM, Barasch JM, Gershon MD, Gershon MD. Physiological responses of guinea-pig myenteric neurons secondary to the release of endogenous serotonin by tryptamine. Neuroscience 16: 223–240, 1985. doi: 10.1016/0306-4522(85)90059-4. [DOI] [PubMed] [Google Scholar]
- 126.Teissier A, Soiza-Reilly M, Gaspar P. Refining the role of 5-HT in postnatal development of brain circuits. Front Cell Neurosci 11: 139, 2017. doi: 10.3389/fncel.2017.00139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Terry N, Margolis KG. Serotonergic mechanisms regulating the GI tract: experimental evidence and therapeutic relevance. Handb Exp Pharmacol 239: 319–342, 2017. doi: 10.1007/164_2016_103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Tsai SJ, Hong CJ, Liou YJ, Yu YW, Chen TJ, Hou SJ, Yen FC. Tryptophan hydroxylase 2 gene is associated with major depression and antidepressant treatment response. Prog Neuropsychopharmacol Biol Psychiatry 33: 637–641, 2009. doi: 10.1016/j.pnpbp.2009.02.020. [DOI] [PubMed] [Google Scholar]
- 129.Twarog BM. Responses of a molluscan smooth muscle to acetylcholine and 5-hydroxytryptamine. J Cell Comp Physiol 44: 141–163, 1954. doi: 10.1002/jcp.1030440112. [DOI] [PubMed] [Google Scholar]
- 130.Tyring S, Gottlieb A, Papp K, Gordon K, Leonardi C, Wang A, Lalla D, Woolley M, Jahreis A, Zitnik R, Cella D, Krishnan R. Etanercept and clinical outcomes, fatigue, and depression in psoriasis: double-blind placebo-controlled randomised phase III trial. Lancet 367: 29–35, 2006. doi: 10.1016/S0140-6736(05)67763-X. [DOI] [PubMed] [Google Scholar]
- 131.Vécsei L, Szalárdy L, Fülöp F, Toldi J. Kynurenines in the CNS: recent advances and new questions. Nat Rev Drug Discov 12: 64–82, 2013. doi: 10.1038/nrd3793. [DOI] [PubMed] [Google Scholar]
- 132.Veenstra-VanderWeele J, Muller CL, Iwamoto H, Sauer JE, Owens WA, Shah CR, Cohen J, Mannangatti P, Jessen T, Thompson BJ, Ye R, Kerr TM, Carneiro AM, Crawley JN, Sanders-Bush E, McMahon DG, Ramamoorthy S, Daws LC, Sutcliffe JS, Blakely RD. Autism gene variant causes hyperserotonemia, serotonin receptor hypersensitivity, social impairment and repetitive behavior. Proc Natl Acad Sci USA 109: 5469–5474, 2012. doi: 10.1073/pnas.1112345109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Waclawiková B, El Aidy S. Role of microbiota and tryptophan metabolites in the remote effect of intestinal inflammation on brain and depression. Pharmaceuticals (Basel) 11: E63, 2018. doi: 10.3390/ph11030063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wigner P, Czarny P, Synowiec E, Bijak M, Białek K, Talarowska M, Galecki P, Szemraj J, Sliwinski T. Association between single nucleotide polymorphisms of TPH1 and TPH2 genes, and depressive disorders. J Cell Mol Med 22: 1778–1791, 2018. doi: 10.1111/jcmm.13459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wogelius P, Nørgaard M, Gislum M, Pedersen L, Munk E, Mortensen PB, Lipworth L, Sørensen HT. Maternal use of selective serotonin reuptake inhibitors and risk of congenital malformations. Epidemiology 17: 701–704, 2006. doi: 10.1097/01.ede.0000239581.76793.ae. [DOI] [PubMed] [Google Scholar]
- 136.Yang M, Fukui H, Eda H, Kitayama Y, Hara K, Kodani M, Tomita T, Oshima T, Watari J, Miwa H. Involvement of gut microbiota in the association between gastrointestinal motility and 5-HT expression/M2 macrophage abundance in the gastrointestinal tract. Mol Med Rep 16: 3482–3488, 2017. doi: 10.3892/mmr.2017.6955. [DOI] [PubMed] [Google Scholar]
- 137.Yano JM, Yu K, Donaldson GP, Shastri GG, Ann P, Ma L, Nagler CR, Ismagilov RF, Mazmanian SK, Hsiao EY. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell 161: 264–276, 2015. [Erratum in Cell 163: 258, 2015.] doi: 10.1016/j.cell.2015.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Yu H, Zheng BJ, Pan WK, Wang HJ, Xie C, Zhao YY, Chen XL, Liu Y, Gao Y. Combination of exogenous cell transplantation and 5-HT4 receptor agonism induce endogenous enteric neural crest-derived cells in a rat hypoganglionosis model. Exp Cell Res 351: 36–42, 2017. doi: 10.1016/j.yexcr.2016.12.022. [DOI] [PubMed] [Google Scholar]
- 139.Zhang X, Beaulieu JM, Gainetdinov RR, Caron MG. Functional polymorphisms of the brain serotonin synthesizing enzyme tryptophan hydroxylase-2. Cell Mol Life Sci 63: 6–11, 2006. doi: 10.1007/s00018-005-5417-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zhang X, Gainetdinov RR, Beaulieu JM, Sotnikova TD, Burch LH, Williams RB, Schwartz DA, Krishnan KR, Caron MG. Loss-of-function mutation in tryptophan hydroxylase-2 identified in unipolar major depression. Neuron 45: 11–16, 2005. doi: 10.1016/j.neuron.2004.12.014. [DOI] [PubMed] [Google Scholar]
- 141.Zill P, Baghai TC, Zwanzger P, Schüle C, Eser D, Rupprecht R, Möller HJ, Bondy B, Ackenheil M. SNP and haplotype analysis of a novel tryptophan hydroxylase isoform (TPH2) gene provide evidence for association with major depression. Mol Psychiatry 9: 1030–1036, 2004. doi: 10.1038/sj.mp.4001525. [DOI] [PubMed] [Google Scholar]