Keywords: amino acid, cecum, colon, LC-MS, metabolome, NMR, transporter, Ussing chamber
Abstract
The capacity of the colon to absorb microbially produced amino acids (AAs) and the underlying mechanisms of AA transport are incompletely defined. We measured the profile of 16 fecal AAs along the rat ceco-colonic axis and compared unidirectional absorptive AA fluxes across mucosal tissues isolated from the rat jejunum, cecum, and proximal colon using an Ussing chamber approach, in conjunction with 1H-NMR and ultra-performance liquid chromatography-mass spectrometry chemical analyses. Passage of stool from cecum to midcolon was associated with segment-specific changes in fecal AA composition and a decrease in total AA content. Simultaneous measurement of up to 16 AA fluxes under native luminal conditions, with correction for endogenous AA release, demonstrated absorptive transfer of AAs across the cecum and proximal colon at rates comparable (30–80%) to those across the jejunum, with significant Na+-dependent and H+-stimulated components. Expression profiling of 30 major AA transporter genes by quantitative PCR revealed comparatively high levels of transcripts for 20 AA transporters in the cecum and/or colon, with the levels of 12 exceeding those in the small intestine. Our results suggest a more detailed model of major apical and basolateral AA transporters in rat colonocytes and provide evidence for a previously unappreciated transfer of AAs across the colonic epithelium that could link the prodigious metabolic capacities of the luminal microbiota, the colonocytes, and the body tissues.
NEW & NOTEWORTHY This study provides evidence for a previously unappreciated transfer of microbially generated amino acids across the colonic epithelium under physiological conditions that could link the prodigious metabolic capacities of the luminal microbiota, the colonocytes, and the body tissues. The segment-specific expression of at least 20 amino acid transporter genes along the colon provides a detailed mechanistic basis for uniport, heteroexchange, Na+-cotransport, and H+-cotransport components of colonic amino acid absorption.
INTRODUCTION
Amino acids (AA) are essential precursors for the synthesis of all proteins and numerous substances with enormous biological importance. Of the 20 standard proteinogenic L-amino acids, 9 cannot be synthesized within the body and must be obtained from the diet or from intestinal microbes. The nutritional needs for AAs are mainly met by the assimilation of dietary proteins along the small intestine. Proteolysis in the stomach and proximal intestine releases free AAs and oligopeptides, which are then vigorously absorbed by villus enterocytes of the duodenum, jejunum, and ileum.
Intestinal absorption of AAs is known to involve multiple interdependent epithelial transport systems with broad specificities for neutral, cationic, anionic, or β-AAs and differing co-substrate (Na+, H+, Cl−) dependencies. The transfer of AAs across the enterocyte involves an assemblage of transporters in the apical membrane operating in series with different transporters in the basolateral membrane (7, 8, 48). All known intestinal AA transporters are members of the solute carrier (SLC) superfamily, with multiple constituents of the SLC1, -6, -7, -16, -36, -38, and -43 families (8). Many function as obligate AA exchangers or cotransporters driven by Na+, H+, or Cl− electrochemical gradients (23). In addition to AA transporters, intestinal epithelial cells are equipped with an apical H+-peptide cotransporter (PepT-1, SLC15A1) that is responsible for a substantial fraction of dietary protein assimilation (46). The integrated operation of multiple transporters with overlapping substrate preferences allows for gradient coupling and efficient scavenging of diverse AAs from the intestinal lumen. For example, the accumulation of a given AA within the enterocyte by a concentrative (ion-driven) cotransporter can secondarily drive the uptake of other AAs via an adjacent exchange transporter. Studies of AA transport and SLC gene transcripts in the small intestine have firmly established that different ensembles of AA transporters are expressed in proximal (jejunum) and distal (ileum) segments (8). By contrast, the stratification of AA transporters along the large intestine, and their physiological functions, remains incompletely defined.
The large intestine, particularly the cecum and proximal colon, functions as an elaborate bioreactor that cultivates trillions of beneficial bacteria (36). This microbial ecosystem works in a mutualistic relationship with the colonic epithelium to harvest energy, metabolize xenobiotics, exclude pathogenic invaders, and synthesize essential nutrients, including vitamins and AAs (9). Although most of the ingested proteins, desquamated enterocyte proteins, and secreted mucin glycoproteins are efficiently assimilated as oligopeptides and free AAs along the small intestine, significant amounts of distally released endogenous proteins can enter the large intestine (47), where they are extensively catabolized by the resident microbes (13, 20, 26) into various nitrogenous metabolites, including free AAs (4, 14). The reservoir of fecal AAs is further enriched by de novo synthesis of certain AAs by the colonic microbiota (14, 19, 31, 55).
The prodigious capacity of the colonic microbiota to both liberate and synthesize free AAs raises the possibility that the added AAs, if absorbed across the colonic epithelium, could contribute to whole body AA and protein homeostasis (4, 55). Although it has been proposed that vigorous microbial deamination and decarboxylation reactions might outcompete AA transporters in colonocytes for available AA substrates (4, 27), many AAs are in fact present in colonic fecal fluid at concentrations well above the affinities of several major AA transporters, and even higher concentrations may be available to AA transporters in the microbially restricted juxtamucosal microenvironment underlying the adherent mucus layer.
Although early animal studies concluded that AAs are not, under normal circumstances, absorbed in nutritionally relevant quantities by the colon (19, 47, 55), other observations have suggested that the colonic epithelium may, nevertheless, engage in rapid bidirectional exchange of AAs between the luminal microbiota, the colonocyte, and the circulating blood. First, there is firm evidence that epithelial cells in the colon, like those in the small intestine, express a variety of AA transporters at comparably high levels. For example, Ugawa et al. (54) demonstrated that surface epithelial cells of the mouse colon strongly express an apical membrane protein, designated mCATB0,+ (now recognized as SLC6A14) that transports neutral and cationic AAs with high affinity in a Na+-dependent manner. Second, studies with colonic mucosa isolated from a variety of mammalian species have demonstrated that some AAs are rapidly accumulated within colonocytes, at least in part through concentrative uptake across the basolateral membrane (43). Third, recent studies comparing the concentrations of small organic substances in colonic stool fluid, colonic mucosal tissues, and portal blood plasma isolated from paired germ-free and conventional mice have suggested that at least 11 microbially generated AAs (Arg, Gly, His, Ile, Leu, Lys, Phe, Pro, Ser, Thr, Val) may be added to the circulating blood in significant quantities via colonic absorption, although direct evidence for epithelial AA absorption was not presented (30).
The goal of this study was to provide new information on microbially generated AAs that may be afforded access to the blood circulation by absorptive transport processes in the colon. We show that substantial quantities of several AAs are lost from fecal fluid on transit from the cecum to the middle colon, presumably through microbial metabolism and colonic absorption. To evaluate the potential contribution of epithelial absorption, we compared luminal-to-serosal fluxes of AAs across mucosal tissues isolated from the rat jejunum, cecum, and proximal colon using an Ussing chamber approach in conjunction with 1H-NMR and ultra-performance liquid chromatography-mass spectrometry (UPLC-MS) chemical analysis, correcting for the release of endogenous AAs by the mucosal tissue. Finally, to explore the molecular basis for these transport activities, we mapped the relative expression of transcripts encoding 30 prospective AA transporters along the small intestine and colon by quantitative PCR (qPCR).
METHODS
Tissue isolation and fecal fluid collection.
Nine-week-old female Sprague-Dawley rats (220–260 g) were obtained from Taconic Biosciences and housed at 23 ± 1°C on a 12:12-h light-dark cycle and allowed free access to water and a standard rodent diet. All animal protocols were approved by the University of California Riverside Institutional Animal Care and Use Committee under conditions consistent with Animals in Research: Reporting In Vivo Experiments (ARRIVE) guidelines. Rats were euthanized by gradual displacement of air with CO2. The intestine was removed through an abdominal incision and pinned onto a cold silicone tray. Samples of stool were removed from the cecum, proximal colon (0–20% colon length), and middle colon (20–60% colon length) (53) through incisions along the mesenteric border and sealed in preweighed microfuge tubes on ice. Microbes and solids were removed by four cycles of centrifugation (15,500 g, 7 min), followed by filtration (0.45 μm); the clarified bacteria-free supernatant (native fecal fluid) was stored at −80°C until analysis by 1H-NMR.
Ussing chamber methods.
Absorptive AA flux (nmol·cm−2·h−1), transmucosal potential difference (VT), transmucosal electrical resistance (TER), and short-circuit current (Isc) across isolated rat intestinal mucosae were measured using an Ussing chamber technique (10) with minor modifications. Tissues were rinsed in ice-cold Parson’s solution composed of the following (in mM): 110 NaCl, 25 NaHCO3, 4 KCl, 2 Na2HPO4, 1.25 CaCl2, 1 MgSO4, and 12 deuterated d-glucose-1,2,3,4,5,6,6-d7 (Sigma-Aldrich), saturated with 5% CO2-95% O2 and adjusted to 305 mosmol/kgH2O by the addition of NaCl or water. To suppress prostanoid influences, indomethacin (1 μM) was included in all solutions, added from a 64 mM stock solution prepared in deuterated DMSO (Cambridge Isotope Laboratories, Tewksbury, MA). Use of deuterated forms of glucose and DMSO circumvented NMR resonance overlap with measured metabolites. Segments of jejunum (15–20 cm distal to pylorus), ileum (2–7 cm proximal to cecum), cecum, proximal colon (0–20% colon length), and middle colon (20–60% colon length) were investigated (53). Each segment was opened along the mesenteric border, rinsed thoroughly, and pinned mucosal side down onto an ice-cold silicone tray. To preserve viability, reduce intrinsic neural influences, and minimize subepithelial barriers to diffusion, the wall of intestine was stripped of its outer serosal and muscle layers by dissection under a stereomicroscope to obtain a conventional mucosa-submucosa preparation (10). Tissues were mounted on pins across an oblong aperture (5 × 22 mm) with an exposed tissue area of 1.0 cm2 (Physiologic Instruments P2252) and incubated in a series of Ussing chambers (Physiologic Instruments EM-CSYS-2). Chambers were maintained at 37°C by heated water jackets and continuously mixed by gas lift (5% CO2-95% O2) with 2 mL of solution in each compartment. Fluxes were initiated by concurrently replacing the luminal “donor” solution with a test solution and the serosal “receiver” solution with fresh Parson’s solution. Where indicated, the luminal solution contained different sets of 9–16 AAs (isosmotically replacing NaCl or KCl).
VT was measured through 170 mM KCl agar bridges connected to a pair of calomel electrodes and monitored with a voltage-clamp amplifier (Physiological Instruments VCC-MC2). The mucosa was maintained in an “open circuit” mode, except during periodic (every 5 min) measurements of TER to monitor tissue integrity: VT was clamped briefly (15 s) at 0 mV by applying a current (Isc) through a pair of Ag/AgCl electrodes kept in contact with the luminal and serosal solutions via 170 mM KCl agar bridges. TER (in Ω·cm2) was calculated using Ohm’s law after recording the change in Isc evoked by a 2-mV pulse.
Glucose was excluded from all luminal solutions and isosmotically replaced with NaCl or KCl. Glucose in the serosal Parson’s solution, in contrast, was required to maintain tissue viability. In all experiments, both sides of the tissue were initially incubated with Parson’s solution for 30 min to establish a basal steady-state condition. Tissues were then incubated for 20 min on the luminal side with prewarmed glucose-free Parson’s solution containing various mixtures of AAs at either 0.5 mM or at their native luminal concentrations (see Table 2). Solutions were adjusted to 305 osmol/kgH2O by varying luminal [KCl] or serosal [NaCl] (where brackets denote concentration). To assess the Na+ dependence of AA absorptive fluxes, experiments were repeated using a Na+-free luminal AA solution, with Na+ replaced on an equimolar basis by K+; in some of these experiments, serosal-to-mucosal leakage of Na+ was monitored with a Na+-sensitive electrode (MI-420, Microelectrodes, Bedford, NH) placed in the luminal chamber. In experiments evaluating the effects of native luminal pH (6.2) on absorptive AA fluxes (see Fig. 7), luminal was replaced with either 15 mM Na-HEPES (pH 7.4) or 15 mM Na-PIPES (pH 6.2), and the chambers were equilibrated with 100% O2 (luminal compartment) and 95% O2-5% CO2 (serosal compartment).
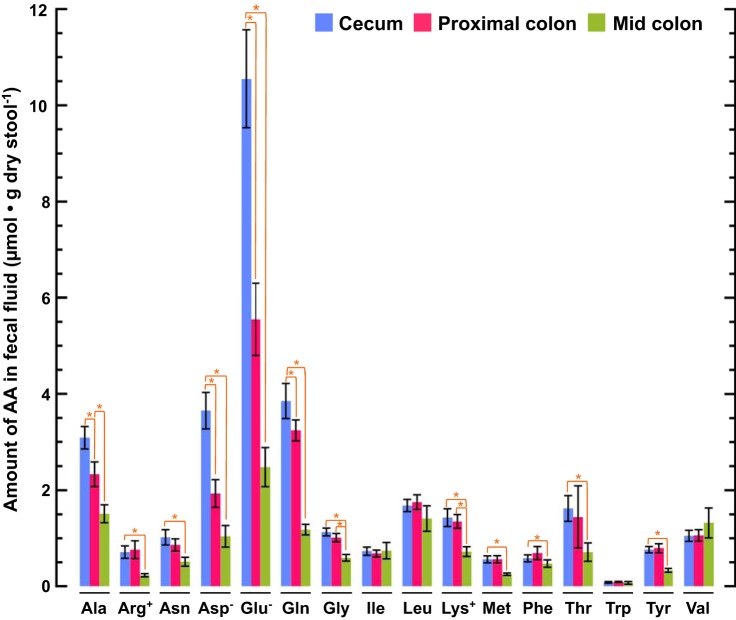
Table 2.
Concentrations of amino acids in rat cecal stool fluid
| Amino Acid | Cecum | Proximal Colon | Middle Colon |
|---|---|---|---|
| ALA | 0.686 ± 0.051 | 0.423 ± 0.047 | 0.700 ± 0.087 |
| ARG | 0.158 ± 0.027 | 0.139 ± 0.035 | 0.109 ± 0.016 |
| ASN | 0.227 ± 0.035 | 0.156 ± 0.023 | 0.235 ± 0.043 |
| ASP | 0.811 ± 0.084 | 0.350 ± 0.053 | 0.484 ± 0.104 |
| GLU | 2.345 ± 0.227 | 1.009 ± 0.136 | 1.155 ± 0.189 |
| GLN | 0.855 ± 0.081 | 0.589 ± 0.040 | 0.550 ± 0.051 |
| GLY | 0.251 ± 0.018 | 0.184 ± 0.016 | 0.275 ± 0.031 |
| ILE | 0.162 ± 0.019 | 0.124 ± 0.013 | 0.346 ± 0.078 |
| LEU | 0.373 ± 0.028 | 0.319 ± 0.028 | 0.657 ± 0.124 |
| LYS | 0.318 ± 0.041 | 0.245 ± 0.026 | 0.333 ± 0.046 |
| MET | 0.124 ± 0.016 | 0.102 ± 0.014 | 0.116 ± 0.012 |
| PHE | 0.129 ± 0.016 | 0.125 ± 0.025 | 0.218 ± 0.035 |
| THR | 0.359 ± 0.059 | 0.261 ± 0.118 | 0.329 ± 0.089 |
| TRP | 0.018 ± 0.003 | 0.016 ± 0.002 | 0.033 ± 0.012 |
| TYR | 0.168 ± 0.014 | 0.144 ± 0.017 | 0.153 ± 0.018 |
| VAL | 0.234 ± 0.026 | 0.192 ± 0.022 | 0.613 ± 0.145 |
Values are means ± SE in mM. Stool samples were isolated from the cecum, proximal colon, and middle colon of 8 rats, cleared of microbes and particulates by centrifugation and filtration, and analyzed by 1H-NMR.
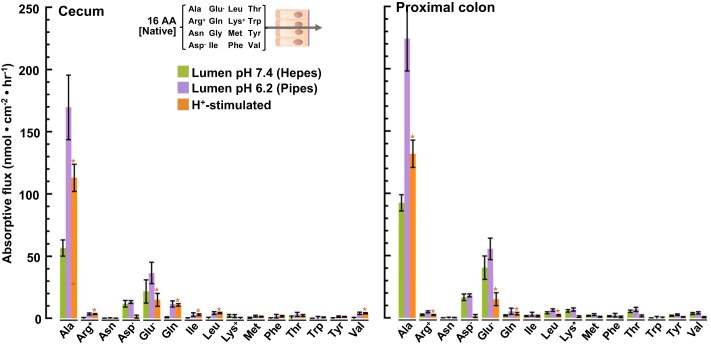
Fig. 7.
Absorptive fluxes of 15 amino acids (AAs) (native concentrations): luminal H+ stimulation. Chambered mucosae isolated from cecum (left) and proximal colon (right) were incubated with luminal Parson’s solution (buffered to pH 7.4 with HEPES) containing 15 AAs at their native cecal concentrations. Samples of the serosal bath were collected after 45 min and analyzed by UPLC-MS (green bars). Replicate experiments were carried out in an otherwise identical luminal solution buffered to pH 6.2 with PIPES (lavender bars). The component stimulated by luminal H+ is indicated by the orange bars. Values are means ± SE obtained with tissues isolated from the rat cecum (n = 8) and proximal colon (n = 7). *P < 0.05.
Unidirectional luminal-to serosal (absorptive) fluxes were measured using a total sample-and-replace method: after various times, luminal and serosal solutions were harvested and replaced concurrently with 2 mL of fresh prewarmed solution. Samples (2 mL) were snap frozen and stored at −80°C until chemical analysis. At the conclusion of each flux assay, the tissue was incubated in bilateral Parson’s solution for 10 min to restore a baseline steady-state condition. Forskolin (10 µM) was then added (from a 1000-fold stock in deuterated DMSO) to the serosal bath to evoke electrogenic Cl− secretion, and the peak secretory Isc response was recorded after 10 min. Tissues exhibiting signs of functional impairment, i.e., low TER (≤30 Ω·cm2) or a weak secretory Isc response (≤40 μA/cm2), were excluded from analyses.
1H-NMR analysis.
The concentrations of free L-AA in fecal fluid samples or Ussing chamber solutions were analyzed by 1H-NMR using a Bruker Avance III NMR spectrometer (Bruker, Billerica, MA), as described previously (17). A 65-μL aliquot of deuterated 50 mM sodium phosphate buffer in D2O (pD 7.4) containing 0.35 or 0.45 mM 3-(trimethylsilyl)-propane-1-sulfonic acid (DSS-d6, Cambridge Isotope Laboratories, Tewksbury, MA) as a chemical shift and quantitation reference, and 0.2 mM ethylenediaminetetraacetic acid-d16 (EDTA-d16, Sigma-Aldrich) was added to a 5-mm NMR tube (Wilmad 535-pp or equivalent) containing 585 μL of Ussing chamber sample. The DSS concentration was determined in a separate NMR experiment relative to the concentration of the primary standard potassium hydrogen phthalate (minimum 99.95% purity; Sigma-Aldrich). AA concentrations were measured relative to the integrated intensity of the resonance of the internal standard DSS. The concentration of the AA of interest was calculated from the ratio of the predetermined DSS concentration and its corresponding T1-corrected and normalized peak area, along with the dilution factor (D) applied when deuterated buffer is added to the sample before NMR analysis.
UPLC-MS analysis.
Where indicated, free L-AA concentrations were analyzed by the University of California Riverside Metabolomics Core Facility using UPLC-MS, with results comparable to 1H-NMR. Calibration curves were generated for each set of unknown samples by UPLC-MS analysis of serially diluted luminal AA solution containing 16 AAs at 0.5 mM. Clarified Ussing chamber samples (300 µL) were mixed with an internal standard (Metabolomics AA Mix Standard, Cambridge Isotope Laboratories) and 900 μL of acetonitrile. After centrifugation (15 min, 16,000 g, 4°C), the supernatant was transferred to 2 mL autosampler vials and analyzed by a UPLC (ACQUITY UPLC I-Class, Waters Corporation) and MS (Xevo TQ-XS, Waters Corporation) tandem system. Peak areas were normalized to their respective internal standards, and calibration curves were used to determine the concentration of each AA in the sample. To estimate the concentration of alanine, a minor component of the alanine peak obscured by an overlapping peak of unknown identity was excluded; thus values for alanine may slightly underestimate the true concentration of this AA.
RNA extraction and real-time PCR.
Intestinal segments, including jejunum (15–20 cm distal to pylorus), ileum (2–7 cm proximal to cecum), cecum, proximal colon (0–20% colon length), and middle colon (20–60% colon length), were isolated from euthanized rats, pinned on an ice-cold silicone tray, and opened along the mesenteric border. Debris and mucus adhering to the luminal surface were removed by gentle rinsing and blotting. The mucosal layer was scrapped off with a glass slide and collected into microfuge tubes on ice. The tissue was rinsed twice with ice-cold Parson’s solution by sequential centrifugation, snap frozen, and then homogenized in TRIzol reagent. Total RNA was isolated by TRIzol extraction, and dry RNA pellets were resolubilized in RNase-free water. Purity was assessed from the A260/A280 ratio (between 1.9 and 2.1) with a NanoDrop ND-1000 spectrophotometer (Thermo Scientific). First-strand cDNA was then synthesized from 1 mg of total RNA with a SuperScript III reverse transcriptase kit (Invitrogen, CA). cDNA synthesis and pre-denaturation were performed at 50°C for 1 h, followed by 75°C for 10 min. Primers were selected based on published reports and their corresponding rat sequences, as listed in Table 1. The reaction mixture (25 μL) contained 2 μL of cDNA template, 0.5 μL of 10 μM primer, 12.5 μL of 2 × iTaq Universal SYBR Green Supermix (Bio-Rad), and 9.5 μL of RNase-free water. Up to 42 amplification cycles were performed with the following parameters: predenature at 95°C for 1 min, denature with reaction cycle at 95°C for 15 s, renaturation and elongation at 60°C for 30 s. Fluorescence signal was detected at the end of each cycle. The standard curve of each sample was obtained, and the cycle threshold value was calculated by CFX384 Touch Real-Time PCR Detection System (Bio-Rad). mRNA levels were normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH).
Table 1.
Primers used for real-time PCR of rat amino acid transporter genes
| Gene | Protein Alias | Direction | Sequence | Reference No. |
|---|---|---|---|---|
| GAPDH | Forward | GGCAAGTTCAACGGCACAGT | 50 | |
| Reverse | TGGTGAAGACGCCAGTAGACTC | |||
| SLC1A1 | EAAT3 | Forward | TGAGCATCAAGCCTGGTGTCACT | 34 |
| Reverse | GGCTTCACTTCTTCACGCTTGGT | |||
| SLC1A2 | EAAT2 | Forward | TGTCTATGCCGCACACAACT | 21 |
| Reverse | TCCTCAACACTGCAGTCAGC | |||
| SLC1A3 | EAAT1 | Forward | GAGATTGGTAGCGGTGATAATGTG | 60 |
| Reverse | CACCCATATCTTCCATCTCAACAA | |||
| SLC1A4 | ASCT1 | Forward | ACGCGGGACAGATTTTCAC | 58 |
| Reverse | ACACCCGCTGCTCCAAC | |||
| SLC1A5 | ASCT2 or ATB0 | Forward | GGAGAAATGGACTGGGTGTG | 59 |
| Reverse | CCAGCAAGAAGGCTCTGAAT | |||
| SLC3A1 | rBAT | Forward | GGGTGTTGATGGGTTTAGTTTC | 61 |
| Reverse | GTGGTGAAGTCGTGGTACAG | |||
| SLC3A2 | 4F2hc or CD98hc | Forward | CAGGGTAGGAGCCTCCAACC | 37 |
| Reverse | TGCCCTCCTCACGGCTT | |||
| SLC6A6 | TAUT | Forward | CGCTCCGCGTGAGAATCAAA | 39 |
| Reverse | CCGTTCATGAGGGTTGCTCT | |||
| SLC6A9 | GLYT1 | Forward | CCAGCTTCTCCTTGGTTGTCAT | 16 |
| Reverse | AGCATCATCTGAATGTCCTGGAA | |||
| SLC6A14 | ATB0,+ | Forward | ATTGGGATAAAGTGACGC | 12 |
| Reverse | AGAAGAAGGCAAAGTGCTAAG | |||
| SLC6A15 | B0AT2 | Forward | ATAGCCTGTACTTATCTTTGG | 40 |
| Reverse | AAACCCTCGATCACATCTGG | |||
| SLC6A18 | XTRP2 | Forward | AGCACGGGGAAGGTGATCTA | Designed |
| Reverse | GTTAGGCCCTCTGTTGCTCC | |||
| SLC6A19 | B0AT1 | Forward | AACCAGAACCAGACAGGCTAT | 38 |
| Reverse | AGAACACTCCAGGCACAT | |||
| SLC6A20 | SIT1 | Forward | GTCATCAACAGCTCCACCTC | 52 |
| Reverse | ATGGCCGCTGTATTTCCAAG | |||
| SLC7A1 | CAT-1 | Forward | CGTCCCTCCTCATTTGCT | 44 |
| Reverse | CTTCCCAGCCCCTGTACT | |||
| SLC7A5 | LAT1 | Forward | TGGCCGTGAAAGAAACCT | 1 |
| Reverse | CAGTCCCCAAAGTCAGAAAGA | |||
| SLC7A6 | y+LAT2 | Forward | GGCTGTGACATTTGCTGACC | Designed |
| Reverse | GACAGAAGGTTCGGGAGGTG | |||
| SLC7A7 | y+LAT1 | Forward | CTCGGAACTTGCTTTTGAGG | 22 |
| Reverse | CCGAAACAACACGTAGCAAA | |||
| SLC7A8 | LAT2 | Forward | CTCCACTGGAAAAAAGGTAGCA | 57 |
| Reverse | TGGTGAATGAAGCCACATCTG | |||
| SLC7A9 | b0,+AT | Forward | TGGGAATATCTCTACTGCGTGTTG | 51 |
| Reverse | TTACTCTGGGTCCTTCTCTGGTG | |||
| SLC7A10 | ASC1 | Forward | GTGGGGTCGGCATCATCATT | Designed |
| Reverse | GCGTGTCATGGACTCTGTGAA | |||
| SLC16A10 | TAT1 | Forward | TGCAGCTGTAGGATTCGTTG | 22 |
| Reverse | GCAGGCAAATACGACTCCAT | |||
| SLC36A1 | PAT1 | Forward | TGGTTGTACCAGTCGGTGAA | 6 |
| Reverse | GGCCAGAACACATGTCACAC | |||
| SLC36A2 | PAT2 | Forward | CACAAGAACGAAACGGTAG | 49 |
| Reverse | GCCAACAGGGAGAAGATG | |||
| SLC38A1 | SNAT1 | Forward | TCAGCCTGGTACGTCGATGG | 42 |
| Reverse | CCAGGTTCTTCAAGAGACACAG | |||
| SLC38A2 | SNAT2 | Forward | AGAGCAATTCCAGTATTAGC | 42 |
| Reverse | TTAATCTGAGCAATGCGATTGTG | |||
| SLC38A3 | SNAT3 | Forward | CGGTTCCGATCGTTCTGTTC | 56 |
| Reverse | GAACTCCTGGTTCTGAAACAGCAT | |||
| SLC38A5 | SNAT5 | Forward | GCCCATTTACACGGAACTTTG | 56 |
| Reverse | GGACATGTTGGCCACAGCTT | |||
| SLC43A1 | LAT3 | Forward | CCTGGTAGGCAGTGCCTGCT | Designed |
| Reverse | CCCATTCAAGGACAGTGCCA | |||
| SLC43A2 | LAT4 | Forward | CCTAGGTGCTCACCATCTTCC | Designed |
| Reverse | AAGGACAACCCACACCTCTG |
Statistical analysis.
Data are shown as means ± SE from three to nine replicate experiments (as specified in Fig. 1–8 legends) using different rats. Real-time PCR data represent the mean ± SE for triplicate measurements on each tissue, with sets of tissues isolated from three rats. IBM SPSS Statistics 20.0 was used for data analysis. Statistical differences were analyzed by one-way ANOVA, and the least significant difference test was performed to evaluate the significant differences of means at P < 0.05 level.
Fig. 1.
Loss of amino acids (AAs) from stool in transit. Stool samples were isolated from the rat cecum, proximal colon, and middle colon. AA content was calculated as the product of regional AA concentration (Table 1) and the corresponding regional stool water content (53). Values are means ± SE; n = 8 rats. *P < 0.05.
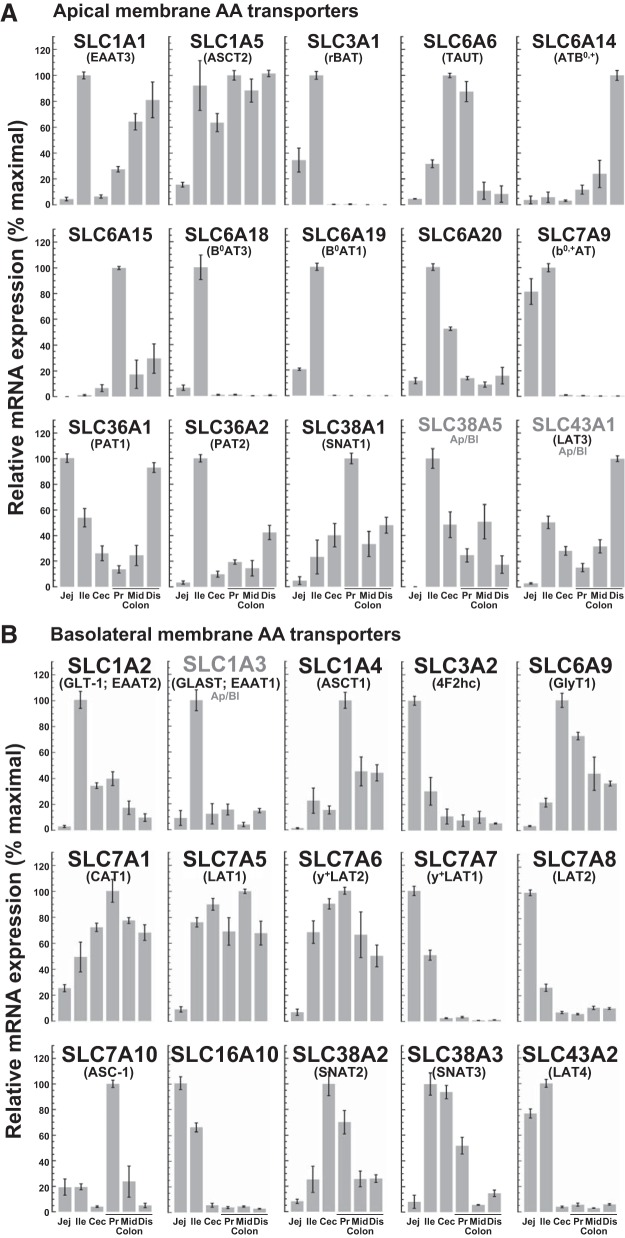
Fig. 8.
Expression of major apical membrane (A) and basolateral membrane (B) AA transporters along the rat intestine estimated by quantitative PCR. Transcript abundance, normalized to GAPDH, is plotted as a percentage of maximal levels detected in mucosal tissue isolated from (bars left to right): jejunum, ileum, cecum, proximal colon, middle colon, and distal colon of 3 rats. The distribution of SLC43A1 between the apical and basolateral membrane domains of intestinal epithelial cells is uncertain. Values are means ± SE; n = 3.
RESULTS
AA concentrations in fecal fluid.
The concentrations of 16 L-AAs in fecal fluid isolated from the cecum, proximal colon, and middle colon of eight rats was measured by 1H-NMR (Table 2). The other four proteinogenic AAs (His, Pro, Ser, Cys) could not be analyzed directly due to resonance overlap with other chemical signatures in the fecal metabolome. Analysis of samples from the distal colon (60–100% colon length) was precluded by the low fluid content of stool in this segment. Despite noticeable interindividual differences in regional stool volumes and possible variations in motility-dependent stool transit, the AA composition of stool within a given intestinal segment was found to be remarkably consistent between rats. The AA composition of stool from different intestinal segments, however, differed markedly.
To exclude the effect of water absorption, regional AA concentrations (mmol/L) were multiplied by the corresponding regional stool water content (µL/mg dry stool) (53) to yield AA content (µmol/mg dry stool−1). Fig. 1 shows that substantial quantities of AAs are lost from the stool as it passes from the cecum to the proximal colon, and then on to the midcolon; the aggregate content (in µmol/mg dry stool, means ± SE) of the 16 measured AAs decreased from 32.5 ± 1.26 (cecum) to 24.1 ± 1.16 (proximal colon) to 13.6 ± 5.2 (middle colon). Three AA (Asp, Glu, Gln) were lost in excess proportion to water, resulting in a marked decrease in their concentrations (Table 2). Eight others (Ala, Arg, Asn, Gly, Lys, Met, Thr, Tyr) were lost in proportion to water without a significant change in their concentrations. Four others, including the three essential branched-chain AAs (Ile, Leu, Val), along with Trp, were neither lost nor gained appreciably, causing their concentrations to increase as water was absorbed in transit. The data indicate that the passage of stool from the cecum to the proximal colon to the middle colon is associated with an altered balance between local AA generation (microbial degradation of endogenous proteins and de novo synthesis) and local AA removal (microbial metabolism and epithelial absorption).
Time course of AA absorptive flux.
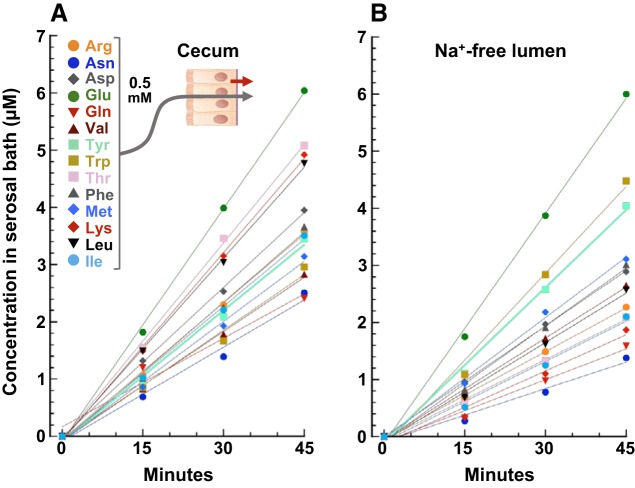
To evaluate the distal intestine’s capacity to absorb AAs, mucosal tissues were mounted in Ussing chambers containing Parson’s solution. The luminal (donor) bath was isosmotically supplemented with a fixed concentration (0.5 mM) of 14 AAs whose 1H-NMR signatures do not overlap. After a 20-min preincubation period to establish a steady state, the accumulation of AAs in the serosal (receiver) bath was measured over three successive 15-min intervals using a total sample-and-replace method. These time course experiments indicated that the serosal accumulation of all 14 AAs remains proportional to time over the 45-min period with an intercept near zero (Fig. 2A). Longer time course assays established that flux remains linear for up to 90 min, affirming that the tissue remains viable and that retrograde (serosal-to-luminal) fluxes of absorbed AAs are negligible over this interval. Accordingly, absorptive flux was calculated from linear regression of consecutive timed data points or from single 45-min data points (expressed in the conventional notation of nmol·cm−2·h−1). Flux experiments were repeated with a luminal solution lacking Na+ (isosmotically replaced with K+, the predominant cation of fecal fluid). In pilot experiments, a Na+-sensitive electrode placed in the luminal chamber indicated that leakage of serosal Na+ across the tissue increased luminal [Na+] from 0 to ~7 mM after 45 min, a level that could potentially support Na+-dependent AA absorption. Time course data (Fig. 2B) revealed the slow leak of sodium into the luminal chamber was not sufficient to support an additional Na+-dependent component of absorptive AA flux.
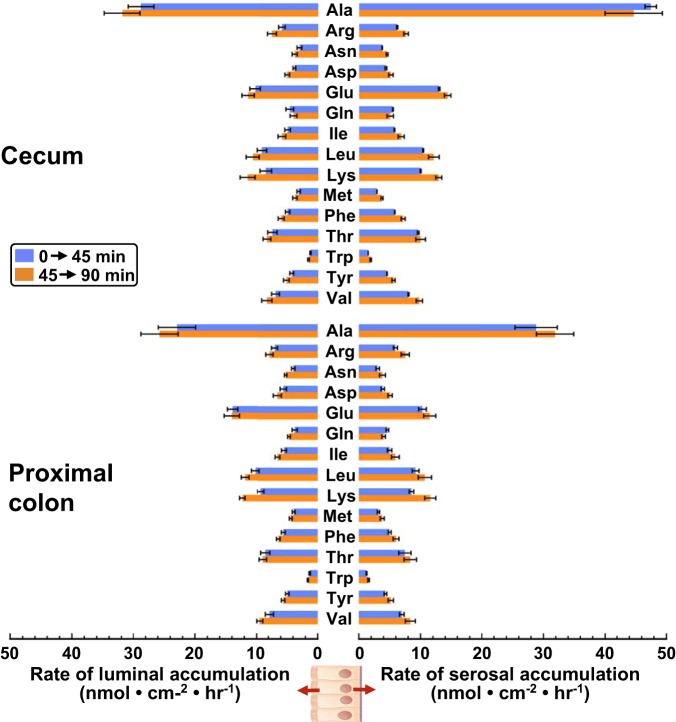
Fig. 2.
Time course of serosal amino acid (AA) accumulation by rat cecum. A: cecum was incubated in an Ussing chamber with luminal Parson’s solution containing 14 AAs (as designated in the figure key), all at 0.5 mM, and serosal Parson’s solution. Every 15 min, the entire serosal bath was collected and replaced. Samples were analyzed by ultra-performance liquid chromatography-mass spectrometry. B: replicate experiment with an otherwise identical Na+-free (K+ replacement) luminal solution. Each plot is representative of several time course experiments.
Tonic release of endogenous AAs.
Not all of the serosal accumulation of AAs could be attributed to absorptive fluxes. We have noted previously (17) that, when the rat cecum is rinsed thoroughly and incubated in the complete absence of extracellular AAs (bilateral Parson’s solution), several AAs are released into the serosal bath at steady rates. Our present study confirmed this finding and revealed that AAs are also released into the luminal bath (Fig. 3). Individual AAs were released both luminally and serosally at similar rates, with an average ratio of 1.13 ± 0.088. The ensemble of AA released from tissues bathed in the complete absence of extracellular AAs included those that can be synthesized by animal cells de novo (Arg, Asn, Asp, Glu, Gln, Tyr) and those that cannot (“essential” AAs: Ile, Lys, Leu, Met, Phe, Thr, Trp, Val). The rate of AA release did not diminish over two successive 45-min intervals, even after multiple rinses, discounting the possibility that the process reflects washout of AAs from the mucosal interstitium or efflux from nonepithelial cells in the lamina propria or submucosa. The release of endogenous Ala from the cecum and proximal colon to the serosal solution was especially rapid (Fig. 3). Because endogenous AA release contributed significantly to the total serosal accumulation of AAs, in all subsequent experiments this component was subtracted from the rates of serosal AA accumulation to estimate the true transmucosal “absorptive flux.”
Fig. 3.
Release of endogenous amino acids (AAs). Ussing chambered rat cecum (top, n = 5) and proximal colon (bottom, n = 6) were preincubated for 20 min in bilateral Parson’s solution lacking AAs and then rinsed thoroughly with fresh solution twice. Samples of the luminal bath and serosal bath were collected over two successive 45-min intervals (0–45 min: blue bars; 45–90 min: orange bars) and analyzed by ultra-performance liquid chromatography-mass spectrometry. Values are presented as rate of AA release (means ± SE) into the luminal bath (left) or into the serosal bath (right).
Absorptive flux of luminal AAs at 0.5 mM.
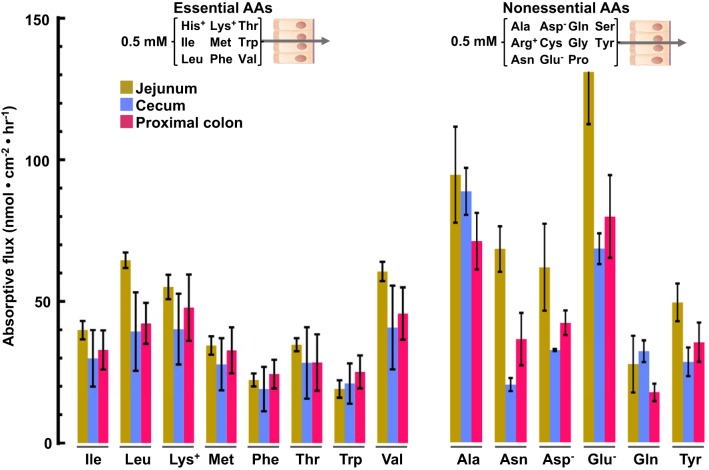
The most detailed information on intestinal AA transport has come from studies of the small intestine, particularly the jejunum. Accordingly, it was instructive to compare, under identical luminal and serosal conditions, absorptive fluxes of AAs by the colon and the jejunum. Initial experiments were carried out with an ensemble of 9 essential AAs (Fig. 4, left) or 11 nonessential AAs (Fig. 4, right) in the luminal bath, each at a fixed concentration of 0.5 mM. Eight of the 9 essential AAs, and 6 of the 11 nonessential AAs in the serosal solution were analyzed. Mucosal tissues isolated from the jejunum, cecum, and proximal colon were found to absorb AAs at roughly comparable rates, with slightly larger fluxes across the jejunum. The flux of lysine (in nmol·cm−2·h−1) recorded in our experiments (55 ± 9 with 0.5 mM lysine in the luminal bath) is comparable to that reported previously in rat jejunum (90 ± 20 with 1 mM lysine in the luminal bath (32, 33) and in human jejunum (115 ± 19 with 3 mM lysine in the luminal bath (15), supporting the validity of our multiplex flux assay. Similar experiments were carried out with an expanded set of 16 AAs in the luminal compartment, each at a fixed concentration of 0.5 mM (Fig. 5). The presence of additional AAs in the lumen markedly reduced the absorptive fluxes of all measured AAs across the cecum and proximal colon (compare Figs. 4 and 5), presumably reflecting additional cross-competition between multiple apical transporters with overlapping specificity for AAs.
Fig. 4.
Absorptive fluxes of amino acids (AAs) (0.5 mM) by jejunum, cecum, and proximal colon. Chambered mucosae isolated from the jejunum (brown bars), cecum (blue bars), and proximal colon (red bars) were preincubated for 20 min with luminal Parson’s solution containing 11 nonessential AAs (as designated on left) or 9 essential AAs (as designated on right). After rinsing the chambers with fresh solution, samples of the serosal bath were collected after 45 min and analyzed for the indicated AAs by 1H-NMR. Absorptive flux is defined as the rate of serosal AA accumulation minus basal serosal AA release. Basal AA release from the jejunum was not measured directly and was assumed to equal that from the cecum. Values are means ± SE; n = 3–5.
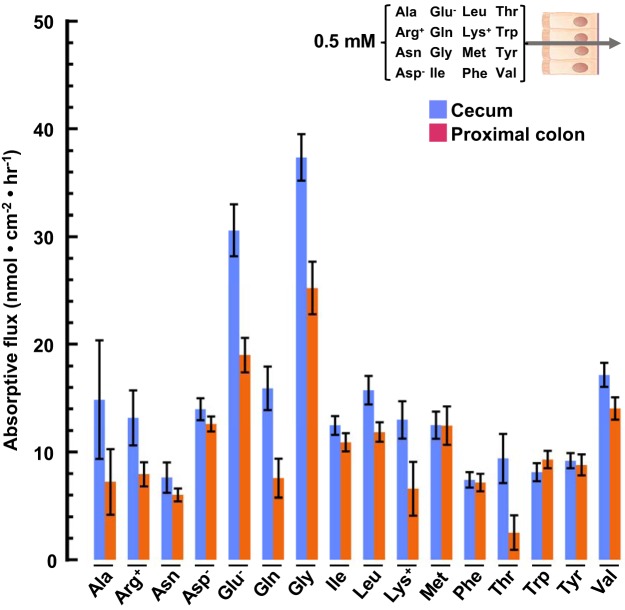
Fig. 5.
Absorptive fluxes of amino acids (AAs) (0.5 mM) by cecum and proximal colon. Chambered mucosal tissues were incubated with luminal Parson’s solution containing the 16 AA designated in the inset, each at 0.5 mM. Samples of the serosal bath were collected after 45 min and analyzed by 1H-NMR. Values are means ± SE. Cecum: blue bars, n = 8; proximal colon: orange bars, n = 6.
Absorptive flux of luminal AAs at native concentrations.
To better approximate the absorptive profile of microbially generated AAs in vivo, flux measurements were repeated with the luminal bath containing 16 AAs (Fig. 6) at concentrations matching those measured in fecal fluid isolated from the rat cecum (Table 2). The presence of a physiological ensemble of AAs at their native concentrations resulted in a distinctly different pattern of AA fluxes across the cecum and proximal colon (compare Fig. 5 with Fig. 6, control), again reflecting the competitive and cooperative nature of intestinal AA transport. This observation underscores the importance, seldom considered in transport studies, of measuring aggregate transport activity simultaneously under native conditions. Under these conditions, Glu, among the most abundant AAs in cecal stool fluid, was also the most rapidly absorbed by both the cecum and the proximal colon (Fig. 6, control).
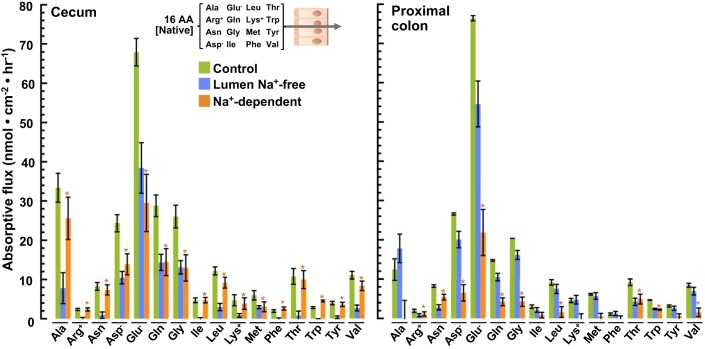
Fig. 6.
Absorptive fluxes of amino acids (AAs) (native concentrations): luminal Na+ dependence. Chambered mucosae isolated from the cecum (left) and proximal colon (right) were preincubated with luminal Parson’s solution containing 16 AAs at native concentrations (measured in fecal fluid isolated from cecum and listed in Table 1) for 20 min. After being rinsed in fresh solutions, samples of the serosal bath were collected after 45 min and analyzed by 1H-NMR (green bars). Replicate experiments were carried out in an otherwise identical luminal solution lacking Na+ (K+ replacement) (blue bars). Differences between these absorptive fluxes represent the component that requires luminal Na+ (orange bars). Values are means ± SE obtained with tissues isolated from the rat cecum (n = 6 with Na+ and 7 without) and proximal colon (n = 7 with Na+ and 7 without). *P < 0.05.
Luminal Na+ dependence of absorptive AA flux.
Because several intestinally expressed AA transporters are known to obligately cotransport Na+, we evaluated the dependence of colonic AA absorption on luminal Na+. Flux experiments were repeated with a luminal solution lacking Na+ (isosmotically replaced with K+, the predominant cation of fecal fluid). Significant components of Na+-dependent absorption were observed for all 16 measured AAs in the cecum, where the native luminal [Na+] is ~58 mM (53) (Fig. 6, left). In the proximal colon, where the native luminal [Na+] is ~45 mM (53), significant components of Na+-dependent absorption were observed for all of the measured AAs, except Ala, Ile, Lys, Met, Phe, and Tyr (Fig. 6, right).
Luminal H+ dependence of absorptive AA flux.
In vivo measurements of intraluminal pH using a miniature electrode (53) have established that that mucosal surface of the proximal colon is markedly more acidic (pH 6.20) than in the cecum (pH 7.45). Because intestinal AA transport is often energetically coupled to the transmembrane H+ gradient, we measured the effect of this pH difference on the absorptive fluxes of 15 AAs at their native luminal concentrations, shown in Fig. 7. Reducing luminal pH from 7.4 to 6.20 significantly stimulated the absorptive fluxes of Ala, Arg, Glu, Gln, Ile, Leu, and Val in the cecum (Fig. 7, left) and of Ala, Arg, Glu, Gln, and Leu in the proximal colon (Fig. 7, right). In contrast, luminal acidification had no detectable effect on the absorptive fluxes of Asp, Lys, Met, Phe, Thr, and Tyr in either the cecum or proximal colon.
Profile of intestinal AA transporter gene expression.
The mechanisms that move AAs across the apical and basolateral membranes of colonic epithelial cells remain largely undefined. Of the 52 known families of solute carrier (SLC) transporters, seven (SLC1, -6, -7, -16, -36, -38, -43) include members that are known to transport AAs across the plasma membrane of vertebrate animal cells (8). To map the expression of major AA transporter genes along the intestinal tract, mucosal tissues were isolated from rat jejunum, ileum, cecum, proximal colon, middle colon, and distal colon, and the relative abundance of transcripts was measured by qPCR using the primer pairs listed in Table 2 and GAPDH as an internal control. The intestinal profiles of transcripts for 15 putative apical membrane transporters are shown in Fig. 8A and for 15 putative basolateral membrane transporters in Fig. 8B.
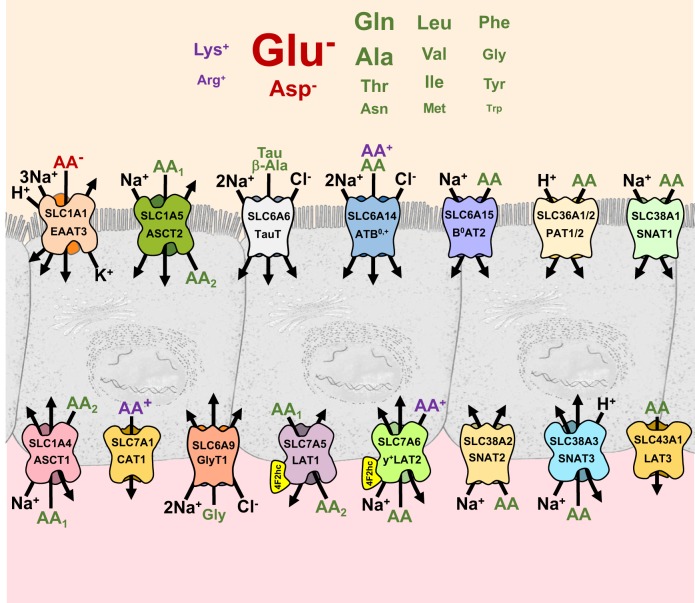
Of these 30 AA transporter genes, 20 were expressed at comparatively high levels in the cecum and/or colon. Among these 20 genes, 5 encoding apically disposed (SLC6A6, -6A14, -6A15, -38A1, and -43A1) transporters and 5 encoding basolaterally disposed (SLC1A4, -6A9, -7A1, -7A10, and -38A2) transporters were expressed either exclusively or more prominently in the cecum/colon than in the small intestine. Our aggregate findings suggest a rudimentary model of the major apical and basolateral AA transporters and their reported operational modes in rat colonocytes (Fig. 9).
Fig. 9.
Proposed model of amino acids (AA) transport in the rat colon based on observed absorptive AA transport properties (Figs. 5–7), expression of transporter genes (Fig. 8), and putative functional modes of transport (8). The sizes of luminal AA labels are roughly proportional to their measured concentrations in rat cecal stool fluid (Table 2).
DISCUSSION
The purpose of this study was to assess whether microbially generated AAs in the fecal fluid are afforded access to the blood circulation by absorptive transport processes in the colon. Our results provide evidence for a previously unappreciated transfer of AAs across the colonic epithelium that could link the prodigious metabolic capacities of the luminal microbiota, the colonocytes, and the body tissues.
Vigorous microbial degradation of endogenous proteins (sloughed epithelial cells, mucus glycoproteins, dead bacteria) and de novo biosynthesis give rise to a diverse mixture of free AAs within the fecal fluid, with concentrations ranging from 0.02 mM (Trp) to 2.34 mM (Glu). Our results indicate that, as stool passes from the cecum to the proximal colon to the middle colon, the AA composition of the fecal fluid changes in a consistent manner, with a stepwise decrease in total AA content. The loss of AAs was attributed to a selective removal of Ala, Arg, Asn, Asp, Gln, Glu, Gly, Lys, Met, Phe, Thr, and Tyr. The regional loss of each AA must reflect a localized predominance of its removal (microbial AA degradation plus colonic epithelial absorption) over its addition (de novo biosynthesis plus endogenous protein degradation plus colonic epithelial release). With regard to microbial degradation, the preferred AA substrates for bacteria in the colon include Arg, Gly, Ile, Leu, Lys, and Val (13, 25), perhaps accounting for the relative scarcity of these AAs in fecal fluid (Table 2; Fig. 1). Our results suggest that two additional processes, epithelial release and absorption, may contribute importantly to fecal AA balance.
Confirming previous observations with pig jejunum (2) and rat cecum (17), we found that the cecum and proximal colon, when incubated in the complete absence of extracellular AAs, steadily release a diverse ensemble of AAs into the serosal solution. As in the pig jejunum (2), the release of Ala from the cecum and proximal colon was especially rapid. Our analysis indicated that AA release into the serosal solution is accompanied by nearly equivalent release into the lumen. The AAs discharged from the tissues do not appear to originate from bacteria adhering to the luminal surface: 1) before and during our experiments, the tissues are thoroughly rinsed free of visible stool and mucus; 2) the continuous presence of dissolved oxygen in our experiments would be expected to incapacitate the majority of colonic bacteria, which are obligate anaerobes; and 3) the release of endogenous AAs from the rat cecum is not affected by pretreating the tissues with a cocktail of broad-coverage antibiotics (17), suggesting that adherent facultative anaerobes and aerotolerant bacteria are not major sources of AAs in our experiments. Furthermore, the endogenous AAs do not appear to arise through degradation of mucins secreted into the lumen by goblet cells (2): 1) in our experiments, the major stimuli of mucin secretion (prostaglandins and muscarinic agonists) were inhibited with indomethacin or absent; 2) mucus glycoproteins are relatively resistant to mammalian proteases compared with bacterial proteases (25), and there is no known capacity of the colonic brush border for contact proteolysis; and 3) luminally released and digested mucus could account for the observed release of AAs into the luminal bath, but not into the serosal bath. We conclude that a significant component of serosal AA accumulation represents release from a relatively stable pool of AAs within the epithelial cell component of the mucosa, possibly derived from ongoing synthesis/transformation of intracellular AAs or catabolism of cytosolic protein stores. The preferential release of Ala from colonic epithelial cells may account for the observed enrichment of Ala in the inferior mesenteric venous plasma exiting the human colon (35), supporting the hypothesis that the colon, like the liver and skeletal muscle, adds appreciable quantities of Ala to the systemic circulation. These measurements of arteriovenous differences also suggested smaller net releases of Gly, Lys, and taurine by the human colon, although these differences did not reach statistical significance, perhaps related to a small data set (35). Our result suggest that colonic epithelial cells also add AAs, especially Ala, to the fecal fluid.
Our current understanding of intestinal AA transport has been derived almost entirely from studies of the small intestine, particularly the jejunum, which is the major site of dietary AA assimilation. Studies employing a variety of preparations (intestinal loops, perfused segments, isolated mucosa, cultured enterocytes) have established that the absorption of dietary AAs involves an ensemble of interdependent epithelial transport systems with broad specificities for neutral, cationic, anionic, or β-AAs (8). In the mammalian small intestine, at least six transport systems contribute importantly to AA absorption across the apical membrane of villus enterocytes (8, 48). These include the 2Na+-AA cotransporter SLC6A19 (B0AT1) for neutral AAs, the heteromeric AA+/AA exchanger SLC3A1/SLC7A9 (rBAT/b0,+AT), which trades cationic AAs for neutral AAs, and SLC36A1 (PAT1) and SLC6A20 (SIT1), which absorb Pro. Anionic AAs are absorbed by SLC1A1 (EAAT3), which exchanges a complex of 3Na+-1H+-1AA− for 1K+. In the small intestine, much if not most dietary protein is absorbed, not in the form of free AAs, but rather as di- and tripeptides by the proton-oligopeptide cotransporter PepT1 (SLC15A1) (46).
Because these AA transporters carry different AAs and co-substrate ions (Na+, H+, Cl−), the absorptive flux of each AA is expected to depend on the following: 1) the assemblage of accumulative and exchange transporters in the apical and basolateral membranes of regional epithelial cells; 2) the intrinsic affinities of apical transporters for AAs and their co-substrate ions; 3) the regional availability of luminal substrates (AA, Na+, H+, Cl−), and 4) the regulated activity of the rate-limiting transport process. How these factors differ between each functional segment of the intestine remains incompletely defined.
Our results raise interest in the distal intestine as a potentially important site of AA transfer between the colonic microbiota, colonocyte, and portal blood. Measurements of RNA transcripts by qPCR indicate that 10 AA transporter genes (SLC1A4, -6A6, -6A9, -6A14, -6A15, -7A1, -7A10, -38A1,-38A2, and -43A1) are expressed more prominently in the large intestine (cecum/colon) than in the small intestine. The concentrations of AAs within fecal fluid are well within the known affinities of these AA transporters.
When exposed to a mixture of AAs at their native fecal concentrations, the cecum and proximal colon transfer AAs in the absorptive direction at rates nearly on par (30–80%) with those in the jejunum. It should be noted, however, that direct comparison of flux rates in different intestinal segments is problematic, since native luminal AA and co-ion concentrations probably differ markedly along the intestinal axis and at the absorptive surface as a consequence of brush-border peptidase activity.
Our results demonstrate that AA transport activities in the colon, as in the small intestine, exhibit cross-competition between other luminal AAs and include appreciable sodium-dependent and proton-stimulated components. In the cecum, replacing luminal Na+ with K+ reduces the absorptive fluxes of all 16 measured AAs, including anionic AAs (Asp, Glu), cationic AAs (Arg, Lys), and neutral AAs (Ala, Asn, Gln, Gly, Ile, Leu, Met, Phe, Thr, Trp, Tyr, and Val), consistent with the prominent expression of several Na+-AA cotransport proteins (SLC1A5, -6A6, -6A20, and -38A1) in the cecum. Although generally smaller, significant Na+-dependent components of Arg, Asn, Asp, Glu, Gln, Gly, Leu, Thr, Trp, and Val absorption were also observed in the proximal colon, where the same ensemble of Na+-AA cotransport proteins is expressed, albeit in different proportions. In addition, the rat colon, particularly the proximal segment, appears to uniquely express SLC6A15 (B0AT2), which cotransports Na+ with branched-chain AAs (Leu, Ile, Val), Pro, and Met with high affinity. Interestingly, several Na-AA cotransporters (SLC1A1, -1A5, -6A14, -6A15, and -38A1) are expressed in the middle and distal rat colon, even though the concentration of sodium within the stool and along the mucosal surface in these distal segments falls to relatively low levels (~15 mM) (53) and may limit AA scavenging.
In the small intestine, assimilation of AAs by SLC1A1 (EAAT3) and SLC36A1 (PAT1) and oligopeptides via PepT1 (SLC15A1) is energetically dependent on the maintenance of an acidic microenvironment along the mucosal surface through the secretion of protons by Na/H exchanger-3 (SLC9A3) (8, 24). In the rat proximal colon, an equally acidic (pH 6.2) mucosal surface, maintained by Na/H exchanger-3 activity and microbial fermentation, drives short-chain fatty acid absorption (SCFA) via nonionic diffusion of H-SCFA (53). Our results suggest that the proximal colon may also utilize this proton gradient to drive AA absorption; this segment exhibited significant components of H+-stimulated Glu and Ala absorption and expression of accumulative H+-AA cotransporters, such as SLC1A1 and SLC36A1/2. Although the stratum of fluid overlaying the epithelium in the rat proximal colon is exceptionally acidic (pH 6.2), in the cecum it is normally alkaline (pH 7.50) due to vigorous secretion via SCFA/HCO3 exchange and SLC26A3-mediated Cl/HCO3 exchange in this segment (53). Despite the apparent absence of a proton motive force along its surface in vivo, the cecum exhibited significant components of H+-stimulated AA absorption in our experiments.
A common design strategy for AA transport in epithelial cells of the gut (7, 8), placenta (11), and kidney (29) is functional cooperation between concentrative and dissipative transport systems by means of AA gradient coupling. In the colonocyte, outward AA gradients created by concentrative AA transporters in the apical membrane (SLC1A1, -1A5, -6A6, -6A14, -6A15, and -38A1) and basolateral membrane (SLC1A4, -6A9, -7A6, -38A2, and -38A3) could be harnessed by AA exchangers (SLC1A5, -1A4, -7A5, and -7A6) to the uptake of other extracellular AAs. For instance, the accumulation of Ala within the colonocyte, driven by the combined electrochemical gradients of H+ (SLC36A1/2) and Na+ (SLC38A1, -38A2, -1A4, -1A5, and -6A14), could promote the apical uptake of SLC1A5 substrates (Asn, Cys, Gln, Ser, Thr) via heteroexchange. This manner of functional coupling may contribute to the H+-stimulated components of Ala and Gln absorption, and Na+-stimulated components of Asn, Gln, and Thr absorption, observed in our experiments.
A limitation of our experimental approach is the inability to measure net AA transfer, the difference between unidirectional absorptive and secretory fluxes, under physiologically relevant conditions, i.e., with AAs simultaneously present in the luminal and serosal chambers at their native concentrations. While the capacity for net absorptive transfer of AAs against lumen-to-blood concentration gradients is well established in the small intestine (15, 33), it remains less clear in the large intestine (55). A net transfer of AAs from the luminal to serosal side of the colon against a concentration gradient could be demonstrated in some mammalian species, e.g., dog (41) and newborn pig (45), but not in others (5, 18). Nevertheless, the steady-state mucosal-to-serosal fluxes measured under native luminal conditions in our experiments suggest some degree of net absorptive transfer or rapid bidirectional heteroexchange of AAs across the colonic epithelium, consistent with the observed expression of functionally cooperative AA cotransporters and exchangers.
Another limitation concerns the interpretation of qPCR data. Measurements of relative mRNA expression in mucosal tissues may not proportionately reflect the functional activities of the encoded AA transporters in colonocytes. Differences in mRNA translation, delivery of the protein to its functional residence in the apical or basolateral membranes, or biochemical regulation could significantly impact overall transport activity. Nevertheless, our data provide a conceptual framework for potential mechanisms of AA transport in the colon.
Interpretation of intestinal AA transport data may also be complicated by “first-pass” metabolism of AAs during intracellular transit. In the small intestine, AAs serve as major energy fuels, and many (e.g., Asp, Gln, Glu, Pro), but not others (e.g., His, Lys, Met, Phe, Thr, Trp), may be extensively metabolized after entering the enterocyte from either the lumen or the blood. Indeed, animal studies have suggested that the small intestinal mucosa captures or transforms up to one-half of all dietary AAs presented to it (3, 4). In addition, rapid AA exchange between enterocytes and the portal blood, facilitated by numerous basolateral membrane AA transport systems, may allow for interorgan AA flow and metabolic collaboration (28). Thus intestinal epithelial cells appear to modulate not only the quantity and type of dietary AAs delivered to the portal circulation, but also the AA composition of the systemic blood. The corresponding capacity of colonocytes for first-pass AA metabolism remains uncertain. Although peptides were not measured in our experiments, a portion of the AAs entering the portal blood in vivo may be derived from the absorption and cytosolic degradation of fecal oligopeptides by colonocytes.
In summary, measurements of unidirectional transport and AA transporter gene expression in the rat cecum and proximal colon suggest that microbially generated AAs traffic between the fecal fluid and the portal blood at significant rates. Net transport or heteroexchange of AAs across the colonic epithelium could contribute, together with microbial metabolism, to the selective loss of AAs from fecal fluid during stool transit. The segment-specific expression of at least 20 AA transporters along the ceco-colonic axis provides a mechanistic basis for uniport, Na+-cotransport, and H+-cotransport components of AA absorptive flux.
GRANTS
This work was supported by National Institutes of Health Diabetes, Digestive, and Kidney Diseases Grant R21DK110516 (to C. Lytle).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
C.L. conceived and designed research; Y.C., M.M.D., A.G., and S.E.C. performed experiments; Y.C., M.M.D., A.G., S.E.C., and C.K.L. analyzed data; C.L. interpreted results of experiments; C.L. prepared figures; Y.C. drafted manuscript; C.L. edited and revised manuscript; C.K.L. and C.L. approved final version of manuscript.
ACKNOWLEDGMENTS
We are grateful to Alyssa Vollaro and Dr. Jay Kirk (University of California, Riverside, Metabolomics Core Facility) for conducting ultra-performance liquid chromatography-mass spectrometry analysis.
REFERENCES
- 1.Alemán G, López A, Ordaz G, Torres N, Tovar AR. Changes in messenger RNA abundance of amino acid transporters in rat mammary gland during pregnancy, lactation, and weaning. Metabolism 58: 594–601, 2009. doi: 10.1016/j.metabol.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 2.Awati A, Rutherfurd SM, Plugge W, Reynolds GW, Marrant H, Kies AK, Moughan PJ. Ussing chamber results for amino acid absorption of protein hydrolysates in porcine jejunum must be corrected for endogenous protein. J Sci Food Agric 89: 1857–1861, 2009. doi: 10.1002/jsfa.3662. [DOI] [Google Scholar]
- 3.Baracos VE. Animal models of amino acid metabolism: a focus on the intestine. J Nutr 134, Suppl: 1656S–1659S, 2004. doi: 10.1093/jn/134.6.1656S. [DOI] [PubMed] [Google Scholar]
- 4.Bergen WG, Wu G. Intestinal nitrogen recycling and utilization in health and disease. J Nutr 139: 821–825, 2009. doi: 10.3945/jn.109.104497. [DOI] [PubMed] [Google Scholar]
- 5.Binder HJ. Amino acid absorption in the mammalian colon. Biochim Biophys Acta 219: 503–506, 1970. doi: 10.1016/0005-2736(70)90233-6. [DOI] [PubMed] [Google Scholar]
- 6.Broberg M, Holm R, Tønsberg H, Frølund S, Ewon KB, Nielsen A, Brodin B, Jensen A, Kall MA, Christensen KV, Nielsen CU. Function and expression of the proton-coupled amino acid transporter PAT1 along the rat gastrointestinal tract: implications for intestinal absorption of gaboxadol. Br J Pharmacol 167: 654–665, 2012. doi: 10.1111/j.1476-5381.2012.02030.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bröer S. Amino acid transport across mammalian intestinal and renal epithelia. Physiol Rev 88: 249–286, 2008. doi: 10.1152/physrev.00018.2006. [DOI] [PubMed] [Google Scholar]
- 8.Bröer S, Fairweather SJ. Amino acid transport across the mammalian intestine. Compr Physiol 9: 343–373, 2018. doi: 10.1002/cphy.c170041. [DOI] [PubMed] [Google Scholar]
- 9.Cani PD. Microbiota and metabolites in metabolic diseases. Nat Rev Endocrinol 15: 69–70, 2019. doi: 10.1038/s41574-018-0143-9. [DOI] [PubMed] [Google Scholar]
- 10.Clarke LL. A guide to Ussing chamber studies of mouse intestine. Am J Physiol Gastrointest Liver Physiol 296: G1151–G1166, 2009. doi: 10.1152/ajpgi.90649.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cleal JK, Lofthouse EM, Sengers BG, Lewis RM. A systems perspective on placental amino acid transport. J Physiol 596: 5511–5522, 2018. doi: 10.1113/JP274883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.D’Argenio G, Calvani M, Casamassimi A, Petillo O, Margarucci S, Rienzo M, Peluso I, Calvani R, Ciccodicola A, Caporaso N, Peluso G. Experimental colitis: decreased Octn2 and Atb0+ expression in rat colonocytes induces carnitine depletion that is reversible by carnitine-loaded liposomes. FASEB J 20: 2544–2546, 2006. doi: 10.1096/fj.06-5950fje. [DOI] [PubMed] [Google Scholar]
- 13.Dai ZL, Wu G, Zhu WY. Amino acid metabolism in intestinal bacteria: links between gut ecology and host health. Front Biosci 16: 1768–1786, 2011. doi: 10.2741/3820. [DOI] [PubMed] [Google Scholar]
- 14.Davila AM, Blachier F, Gotteland M, Andriamihaja M, Benetti PH, Sanz Y, Tomé D. Intestinal luminal nitrogen metabolism: role of the gut microbiota and consequences for the host. Pharmacol Res 68: 95–107, 2013. doi: 10.1016/j.phrs.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 15.Desjeux JF, Rajantie J, Simell O, Dumontier AM, Perheentupa J. Lysine fluxes across the jejunal epithelium in lysinuric protein intolerance. J Clin Invest 65: 1382–1387, 1980. doi: 10.1172/JCI109802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dijk F, Kamphuis W. An immunocytochemical study on specific amacrine cell subpopulations in the rat retina after ischemia. Brain Res 1026: 205–217, 2004. doi: 10.1016/j.brainres.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 17.Dinges MM, Lytle C, Larive CK. 1H NMR-based identification of intestinally absorbed metabolites by Ussing chamber analysis of the rat cecum. Anal Chem 90: 4196–4202, 2018. doi: 10.1021/acs.analchem.8b00393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Evered DF, Nunn PB. Uptake of amino acids by mucosa of rat colon in vitro. Eur J Biochem 4: 301–304, 1968. doi: 10.1111/j.1432-1033.1968.tb00208.x. [DOI] [PubMed] [Google Scholar]
- 19.Fuller M. Determination of protein and amino acid digestibility in foods including implications of gut microbial amino acid synthesis. Br J Nutr 108, Suppl 2: S238–S246, 2012. doi: 10.1017/S0007114512002279. [DOI] [PubMed] [Google Scholar]
- 20.Gill SR, Pop M, Deboy RT, Eckburg PB, Turnbaugh PJ, Samuel BS, Gordon JI, Relman DA, Fraser-Liggett CM, Nelson KE. Metagenomic analysis of the human distal gut microbiome. Science 312: 1355–1359, 2006. doi: 10.1126/science.1124234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goursaud S, Maloteaux JM, Hermans E. Distinct expression and regulation of the glutamate transporter isoforms GLT-1a and GLT-1b in cultured astrocytes from a rat model of amyotrophic lateral sclerosis (hSOD1G93A). Neurochem Int 55: 28–34, 2009. doi: 10.1016/j.neuint.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 22.Holland AM, Hyatt HW, Smuder AJ, Sollanek KJ, Morton AB, Roberts MD, Kavazis AN. Influence of endurance exercise training on antioxidant enzymes, tight junction proteins, and inflammatory markers in the rat ileum. BMC Res Notes 8: 514, 2015. doi: 10.1186/s13104-015-1500-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kandasamy P, Gyimesi G, Kanai Y, Hediger MA. Amino acid transporters revisited: New views in health and disease. Trends Biochem Sci 43: 752–789, 2018. doi: 10.1016/j.tibs.2018.05.003. [DOI] [PubMed] [Google Scholar]
- 24.Kennedy DJ, Leibach FH, Ganapathy V, Thwaites DT. Optimal absorptive transport of the dipeptide glycylsarcosine is dependent on functional Na+/H+ exchange activity. Pflugers Arch 445: 139–146, 2002. doi: 10.1007/s00424-002-0910-1. [DOI] [PubMed] [Google Scholar]
- 25.Macfarlane GT, Allison C, Gibson SA, Cummings JH. Contribution of the microflora to proteolysis in the human large intestine. J Appl Bacteriol 64: 37–46, 1988. doi: 10.1111/j.1365-2672.1988.tb02427.x. [DOI] [PubMed] [Google Scholar]
- 26.Macfarlane GT, Macfarlane S. Bacteria, colonic fermentation, and gastrointestinal health. J AOAC Int 95: 50–60, 2012. doi: 10.5740/jaoacint.SGE_Macfarlane. [DOI] [PubMed] [Google Scholar]
- 27.Macfarlane GT, Macfarlane S. Human colonic microbiota: ecology, physiology and metabolic potential of intestinal bacteria. Scand J Gastroenterol Suppl 32, Suppl 222: 3–9, 1997. doi: 10.1080/00365521.1997.11720708. [DOI] [PubMed] [Google Scholar]
- 28.Mailliard ME, Stevens BR, Mann GE. Amino acid transport by small intestinal, hepatic, and pancreatic epithelia. Gastroenterology 108: 888–910, 1995. doi: 10.1016/0016-5085(95)90466-2. [DOI] [PubMed] [Google Scholar]
- 29.Makrides V, Camargo SM, Verrey F. Transport of amino acids in the kidney. Compr Physiol 4: 367–403, 2014. doi: 10.1002/cphy.c130028. [DOI] [PubMed] [Google Scholar]
- 30.Matsumoto M, Ooga T, Kibe R, Aiba Y, Koga Y, Benno Y. Colonic absorption of low-molecular-weight metabolites influenced by the intestinal microbiome: a pilot study. PLoS One 12: e0169207, 2017. doi: 10.1371/journal.pone.0169207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Metges CC. Contribution of microbial amino acids to amino acid homeostasis of the host. J Nutr 130: 1857S–1864S, 2000. doi: 10.1093/jn/130.7.1857S. [DOI] [PubMed] [Google Scholar]
- 32.Munck BG, Rasmussen SN. Characteristics of rat jejunal transport of tryptophan. Biochim Biophys Acta 389: 261–280, 1975. doi: 10.1016/0005-2736(75)90320-X. [DOI] [PubMed] [Google Scholar]
- 33.Munck BG, Rasmussen SN. Lysine transport across rat jejunum: distribution between the transcellular and the paracellular routes. J Physiol 291: 291–303, 1979. doi: 10.1113/jphysiol.1979.sp012813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nakamura E, Hasumura M, San Gabriel A, Uneyama H, Torii K. New frontiers in gut nutrient sensor research: luminal glutamate-sensing cells in rat gastric mucosa. J Pharmacol Sci 112: 13–18, 2010. doi: 10.1254/jphs.09R16FM. [DOI] [PubMed] [Google Scholar]
- 35.Neis EP, Sabrkhany S, Hundscheid I, Schellekens D, Lenaerts K, Olde Damink SW, Blaak EE, Dejong CH, Rensen SS. Human splanchnic amino-acid metabolism. Amino Acids 49: 161–172, 2017. [Erratum in Amino Acids 49: 1145, 2017.] doi: 10.1007/s00726-016-2344-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nicholson JK, Holmes E, Kinross J, Burcelin R, Gibson G, Jia W, Pettersson S. Host-gut microbiota metabolic interactions. Science 336: 1262–1267, 2012. doi: 10.1126/science.1223813. [DOI] [PubMed] [Google Scholar]
- 37.Nishiya T, Mori K, Hattori C, Kai K, Kataoka H, Masubuchi N, Jindo T, Manabe S. The crucial protective role of glutathione against tienilic acid hepatotoxicity in rats. Toxicol Appl Pharmacol 232: 280–291, 2008. doi: 10.1016/j.taap.2008.06.024. [DOI] [PubMed] [Google Scholar]
- 38.Pinho MJ, Serrão MP, José PA, Soares-da-Silva P. Organ specific underexpression renal of Na+-dependent B0AT1 in the SHR correlates positively with overexpression of NHE3 and salt intake. Mol Cell Biochem 306: 9–18, 2007. doi: 10.1007/s11010-007-9548-9. [DOI] [PubMed] [Google Scholar]
- 39.Rasmussen RN, Lagunas C, Plum J, Holm R, Nielsen CU. Interaction of GABA-mimetics with the taurine transporter (TauT, Slc6a6) in hyperosmotic treated Caco-2, LLC-PK1 and rat renal SKPT cells. Eur J Pharm Sci 82: 138–146, 2016. doi: 10.1016/j.ejps.2015.11.020. [DOI] [PubMed] [Google Scholar]
- 40.Rho SW, Choi GS, Ko EJ, Kim SK, Lee YS, Lee HJ, Hong MC, Shin MK, Min BI, Kee HJ, Lee CK, Bae HS. Molecular changes in remote tissues induced by electro-acupuncture stimulation at acupoint ST36. Mol Cells 25: 178–183, 2008. [PubMed] [Google Scholar]
- 41.Robinson JW, Luisier AL, Mirkovitch V. Transport of amino-acids and sugars by the dog colonic mucosa. Pflugers Arch 345: 317–326, 1973. doi: 10.1007/BF00585850. [DOI] [PubMed] [Google Scholar]
- 42.Sathishkumar K, Elkins R, Chinnathambi V, Gao H, Hankins GD, Yallampalli C. Prenatal testosterone-induced fetal growth restriction is associated with down-regulation of rat placental amino acid transport. Reprod Biol Endocrinol 9: 110, 2011. doi: 10.1186/1477-7827-9-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Scharrer E, Amann B. Concentrative amino acid uptake at the serosal side of colon mucosa. Pflugers Arch 376: 245–249, 1978. doi: 10.1007/BF00584958. [DOI] [PubMed] [Google Scholar]
- 44.Scumpia PO, Sarcia PJ, DeMarco VG, Stevens BR, Skimming JW. Hypothermia attenuates iNOS, CAT-1, CAT-2, and nitric oxide expression in lungs of endotoxemic rats. Am J Physiol Lung Cell Mol Physiol 283: L1231–L1238, 2002. doi: 10.1152/ajplung.00102.2002. [DOI] [PubMed] [Google Scholar]
- 45.Smith MW, James PS. Amino acid transport by the helicoidal colon of the new-born pig. Biochim Biophys Acta 419: 391–394, 1976. doi: 10.1016/0005-2736(76)90366-7. [DOI] [PubMed] [Google Scholar]
- 46.Spanier B, Rohm F. Proton coupled oligopeptide transporter 1 (PepT1) function, regulation, and influence on the intestinal homeostasis. Compr Physiol 8: 843–869, 2018. doi: 10.1002/cphy.c170038. [DOI] [PubMed] [Google Scholar]
- 47.Starck CS, Wolfe RR, Moughan PJ. Endogenous amino acid losses from the gastrointestinal tract of the adult human-A quantitative model. J Nutr 148: 1871–1881, 2018. doi: 10.1093/jn/nxy162. [DOI] [PubMed] [Google Scholar]
- 48.Stevens BR. Amino acid transport by epithelial membranes. : Epithelial Transport Physiology, edited by Gerencser GA. New York: Humana, 2010, p. 355–378. [Google Scholar]
- 49.Sundberg BE, Wååg E, Jacobsson JA, Stephansson O, Rumaks J, Svirskis S, Alsiö J, Roman E, Ebendal T, Klusa V, Fredriksson R. The evolutionary history and tissue mapping of amino acid transporters belonging to solute carrier families SLC32, SLC36, and SLC38. J Mol Neurosci 35: 179–193, 2008. doi: 10.1007/s12031-008-9046-x. [DOI] [PubMed] [Google Scholar]
- 50.Suzuki H, Ito Y, Shinohara M, Yamashita S, Ichinose S, Kishida A, Oyaizu T, Kayama T, Nakamichi R, Koda N, Yagishita K, Lotz MK, Okawa A, Asahara H. Gene targeting of the transcription factor Mohawk in rats causes heterotopic ossification of Achilles tendon via failed tenogenesis. Proc Natl Acad Sci USA 113: 7840–7845, 2016. doi: 10.1073/pnas.1522054113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Suzuki K, Kawakami F, Sasaki H, Maruyama H, Ohtsuki K. Biochemical characterization of tau protein and its associated syndapin 1 and protein kinase Cε for their functional regulation in rat brain. Biochim Biophys Acta 1790: 188–197, 2009. doi: 10.1016/j.bbagen.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 52.Takanaga H, Mackenzie B, Suzuki Y, Hediger MA. Identification of mammalian proline transporter SIT1 (SLC6A20) with characteristics of classical system imino. J Biol Chem 280: 8974–8984, 2005. doi: 10.1074/jbc.M413027200. [DOI] [PubMed] [Google Scholar]
- 53.Talbot C, Lytle C. Segregation of Na/H exchanger-3 and Cl/HCO3 exchanger SLC26A3 (DRA) in rodent cecum and colon. Am J Physiol Gastrointest Liver Physiol 299: G358–G367, 2010. doi: 10.1152/ajpgi.00151.2010. [DOI] [PubMed] [Google Scholar]
- 54.Ugawa S, Sunouchi Y, Ueda T, Takahashi E, Saishin Y, Shimada S. Characterization of a mouse colonic system B(0+) amino acid transporter related to amino acid absorption in colon. Am J Physiol Gastrointest Liver Physiol 281: G365–G370, 2001. doi: 10.1152/ajpgi.2001.281.2.G365. [DOI] [PubMed] [Google Scholar]
- 55.van der Wielen N, Moughan PJ, Mensink M. Amino acid absorption in the large intestine of humans and porcine models. J Nutr 147: 1493–1498, 2017. doi: 10.3945/jn.117.248187. [DOI] [PubMed] [Google Scholar]
- 56.Wang W, Li Y, Zhang W, Zhang F, Li J. Changes of plasma glutamine concentration and hepatocyte membrane system N transporters expression in early endotoxemia. J Surg Res 166: 290–297, 2011. doi: 10.1016/j.jss.2009.08.027. [DOI] [PubMed] [Google Scholar]
- 57.Wu Y, Yin Q, Lin S, Huang X, Xia Q, Chen Z, Zhang X, Yang D. Increased SLC7A8 expression mediates L-DOPA uptake by renal tubular epithelial cells. Mol Med Rep 16: 887–893, 2017. doi: 10.3892/mmr.2017.6620. [DOI] [PubMed] [Google Scholar]
- 58.Yamagata K, Yamamoto M, Kawakami K, Ohara H, Nabika T. Arginine vasopressin regulated ASCT1 expression in astrocytes from stroke-prone spontaneously hypertensive rats and congenic SHRpch1_18 rats. Neuroscience 267: 277–285, 2014. doi: 10.1016/j.neuroscience.2014.02.039. [DOI] [PubMed] [Google Scholar]
- 59.Yan Q, Tong H, Tang S, Tan Z, Han X, Zhou C. l-Theanine administration modulates the absorption of dietary nutrients and expression of transporters and receptors in the intestinal mucosa of rats. BioMed Res Int 2017: 9747256, 2017. doi: 10.1155/2017/9747256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yeh TH, Hwang HM, Chen JJ, Wu T, Li AH, Wang HL. Glutamate transporter function of rat hippocampal astrocytes is impaired following the global ischemia. Neurobiol Dis 18: 476–483, 2005. doi: 10.1016/j.nbd.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 61.Zhang S, Amstein T, Shen J, Brush FR, Gershenfeld HK. Molecular correlates of emotional learning using genetically selected rat lines. Genes Brain Behav 4: 99–109, 2005. doi: 10.1111/j.1601-183X.2004.00099.x. [DOI] [PubMed] [Google Scholar]