Keywords: barrier function, chaperone, cystic fibrosis transmembrane conductance regulator
Abstract
Clostridium difficile (CD) is a common pathogen that causes severe gastrointestinal inflammatory diarrhea in patients undergoing antibiotic therapy. Its virulence derives from two toxins, toxin CD, A and B (TcdA and TcdB) (Borriello et al. Rev Infect Dis 12, Suppl 2: S185-191, 1990). Among the prime candidates for CD colonization are patients with cystic fibrosis (CF), who are routinely treated with antibiotics and frequently hospitalized. Indeed, ~50% of patients with CF are colonized with virulent forms of CD but do not exhibit diarrhea (Bauer et al. Clin Microbiol Infect 20: O446–O449, 2014; Binkovitz et al. Am J Roentgenol 172: 517–521, 199; Zemljic et al. Anaerobe 16: 527–532, 2010). We found that TcdB has global effects on colonic cells, including reducing the steady-state levels of sodium-proton exchange regulatory factors, reducing the levels of heat shock protein (Hsp) 27, and increasing the fraction of total Hsp27 bound to the cystic fibrosis transmembrane conductance regulator (CFTR). Also, since some mutations in CFTR seem to be protective, we asked whether CFTR is a target of TcdB. We show here that TcdB increases the maturation of CFTR and transiently increases its function. These combined effects promote increased surface expression of CFTR, resulting in a transient increase in Cl− secretion. This increase is followed by a precipitous decline in both CFTR-dependent Cl− secretion and transepithelial resistance (TER), suggesting a breakdown in the epithelial cells’ tight junctions. We also found that overexpressing Hsp27 reverses some of the deleterious effects of TcdB, in particular preserving TER and therefore likely the maintenance of barrier function. Thus, our data suggest that Hsp27 plays a role in the diarrhea generated by CD infection and is a potential therapeutic target for treating this diarrhea.
NEW & NOTEWORTHY Clostridium difficile (CD) is a common pathogen that causes severe gastrointestinal inflammatory diarrhea in patients undergoing antibiotic therapy. We provide new evidence that heat shock protein (Hsp) 27 is one of the key players in CD pathology and that increasing Hsp27 can prevent the decrease in transepithelial resistance induced by toxin CD B, pointing the way for pharmacologic therapies for patients with chronic CD infection that can increase Hsp27 as a means to mitigate the effects of CD on gastrointestinal pathology.
INTRODUCTION
Clostridium difficile (CD) is a common pathogen that causes severe gastrointestinal inflammatory diarrhea in patients taking antibiotics. It is the leading cause of hospital-acquired diarrhea, resulting in over one billion dollars in additional health care costs (35, 56) and is the leading cause of pseudomembranous colitis (28). The source of its virulence is two toxins, toxin CD (Tcd) A and TcdB (54). Although it was originally thought that TcdA was most potent, it is clear that both contribute to morbidity (47). CD infection usually begins with diarrhea, followed by colitis and pseudomembrane formation. The pseudomembrane, comprised of a combination of necrotic and inflammatory cells, forms yellow plaques on the surface of the colonic epithelium (43a). The pathological effects of CD are thought to begin with CD toxin-induced acute damage to the epithelial barrier in the gut and translocation of bacteria from the gut lumen to the deeper gut layers, thereby inducing an inflammatory response (1).
Mutations in the cystic fibrosis (CF) gene disrupt cystic fibrosis transmembrane regulator (CFTR)’s chloride channel function and cause CF, a disease that in its most advanced forms is associated with dry airways, chronic bacterial infections and bronchiectasis, intestinal obstruction, infertility, failure to properly digest food, and premature mortality (51). Patients with CF are prime candidates for CD colonization because they are routinely treated with antibiotics and are frequently hospitalized. Thus, it is not surprising that patients with CF routinely test positive for CD much more frequently than the population without CF (6, 7, 9, 21, 22, 39, 41, 45). Interestingly, a high percentage of patients with CF are colonized not only with nontoxigenic forms of CD but also with virulent forms, but they do not exhibit the inflammatory diarrhea that would be expected to occur because of a loss of barrier function. Patients with CF may have colitis (41), but the colitis appears to be mutation specific in that it appears to be particularly severe in patients bearing the N1303K mutation (22). Although CD generally causes colonic inflammation and tissue damage (27), the reason why most patients with CF do not experience these clinical signs is still not understood.
One potential reason for the lack of CD-induced pathology in patients with CF is that somehow an absence of CFTR function may be protective. A second possible reason is that a network of proteins involved in dealing with mutant CFTR also happens to protect against CD toxicity. Relevant to this second explanation are the studies of Balch and colleagues (4) and Roth et al. (52), who proposed that mutant forms of CFTR, such as F508-del, alter protein pathways involved in protein processing out of the endoplasmic reticulum (ER). They showed that long-term expression of misfolded proteins such as F508-del causes sustained activation of the heat shock response pathway (52). One of proteins involved in the heat shock response is heat shock protein (Hsp) 27. Hsp27 (HspB1), a member of the small heat shock protein family, is thought to guard against protein aggregation during stress (31). Hsp27 levels are upregulated in certain cancers (59) to protect rapidly dividing cells from protein aggregation and apoptosis (59). Hsp27 is also well known to bind to actin and play a role in actin filament dynamics (20), with high levels being protective against stress fiber formation (18). We have previously shown that Hsp27 is upregulated in cells expressing F508-del and downregulated in cells bearing the N1303K mutation (34, 48). Given that the sensitivity of patients with CF to CD seems to correlate with Hsp27 levels, we have now explored the roles of CFTR and Hsp27 in CD toxicity.
MATERIALS AND METHODS
Cell culture and treatments.
The Caco2 cell line, originally derived from a human adenocarcinoma (American Type Cell Culture Collection HTB37), was grown in MEM containing 20% fetal bovine serum, 50 U/mL penicillin, and 50 μg/mL streptomycin, pH 7.4, in 5% CO2-air at 37°C. TcdB was obtained from List Biological Laboratories (cat. no. 155A) and applied to cells for 30 min to 2 h at 0.1 μg/mL to assess its effect on CFTR. Cells were either harvested after 30 min of treatment for further applications or evaluated by surface protein biotinylation of CFTR.
Transfection.
Overexpression of Hsp27 in Caco2 cells was performed using transient transfection method with HspB1 human untagged clone (Origene, cat. no. SC319285) and Lipofectamine 2000 (Invitrogen) according to the manufacturer’s protocol. Briefly, transfection was performed at ~80% of cell confluence. The Hsp27 overexpression vector and Lipofectamine mix was prepared for one group of cells, and the empty plasmid and Lipofectamine mix was prepared for a blank plasmid control group. The prepared plasmid DNA and liposome complexes were added to the cells that were incubated at 37°C in 5% CO2. After transfection for 48 h, Western blots were executed to determine the transfection efficiency.
Surface protein biotinylation of CFTR.
Plasma membrane proteins of Caco2 cells were labeled using EZ-LinkTM sulfo-NHS-SS-biotin (Thermo Scientific) according to techniques we have reported previously (14, 48, 53). The total cellular protein was determined using the Protein Assay Dye Reagent (Bio-Rad). Surface proteins were isolated using NeutrAvidin Plus UltraLink Resin (Thermo Scientific). Samples mixed with beads were centrifuged, the supernatant was discarded, and the beads were washed five times with lysis buffer. The bound proteins were eluted with 2× Laemmli sample buffer with 5% β-mercaptoethanol, then subjected to SDS-PAGE and immunoblotting as described below.
Immunoblotting.
Caco2 cells were solubilized in lysis buffer containing a protease inhibitor cocktail (Thermo Scientific, cat. no. 78429) and further processed using 10% SDS-PAGE followed by immunoblotting, then enhanced chemiluminescence (SuperSignal West Dura Extended Duration Substrate, Thermo Scientific, cat. no. 34075). We used the following antibodies: monoclonal anti-human CFTR (596; 1:1,000) antibody (provided by Dr. J. Riordan, Department of Biochemistry and Biophysics and Cystic Fibrosis Center of the University of North Carolina), and ezrin (the loading control) monoclonal antibody [1:10,000, Santa Cruz Biotechnology (SCB), cat. no. Sc-58758]. Sodium-proton exchange regulatory factor (NHERF) 1 and chaperones were detected with anti-NHERF1 (1:1,000, SCB, cat. no. Sc-51684); anti-NHERF2 (1:1,000; SCB, cat. no. Sc-365388); anti-Hsp27 (1:1,000, SCB, cat. no. Sc-101700), and GAPDH, (US Biological, cat. no. G8140–11B). Chemiluminescent signals were measured by a FujiFilm LAS-4000.
Immunoprecipitation.
Protein lysates (2,000 μg) were mixed with 80 μL of protein A/G-agarose beads (Santa Cruz Biotechnology) and 5 μg of anti-CFTR antibody (M3A7, Millipore) for 4 h at 4°C; the A/G beads were then washed 4 times with lysis buffer containing a protease inhibitor cocktail. The protein samples were used for immunoblotting as described above.
Short-circuit currents and transepithelial resistance in Caco2 cells.
To measure the CFTR-dependent short-circuit currents (Iscs) and transepithelial electrical resistance (TER), Caco2 cells were seeded onto 12-mm-diameter Costar Snapwell cell culture inserts (Corning Costar, Acton, MA; cat. no. 3801) and assayed when polarized monolayers had been established. The inserts were placed into Ussing chambers, maintained at 37°C, and bubbled with room air. Caco2 cells were exposed to toxin by adding TcdB to the apical culture medium for 30 min to 3 h at 0.1 μg/mL before any experiments were carried out. Iscs was measured by voltage-clamping the transepithelial voltage across the monolayers to 0 mV with a multichannel voltage-current clamp amplifier (model VCC MC6, Physiologic Instruments). TER was measured by using periodic 5-mV bipolar voltage pulses, recording the change in Iscs, and applying Ohm’s law. The apical bath solution was 120 mM Na gluconate, 2 mM CaCl2, 5 mM KCl, 2 mM MgCl2, 10 mM HEPES, and 10 mM d-glucose; the basolateral solution contained 120 mM NaCl, 2 mM CaCl2, 5 mM KCl, 2 mM MgCl2, 10 mM HEPES, and 10 mM d-glucose (adjusted to pH 7.3 with NaOH). Amiloride (100 μM) was added to the apical solution to inhibit the epithelial Na+ channels. The adenylate cyclase activator forskolin (10 μM) and the tyrosine kinase inhibitor genistein (30 μM) were used to phosphorylate CFTR (via PKA) and to potentiate its activity, respectively (44, 60). After stimulation, the CFTR inhibitor CFTRinh172 (10 μM) (55) was added to measure the CFTR-mediated chloride transport. An Acquire and Analyze Data Acquisition System (Physiologic Instruments, version 2.3) was used for data acquisition and analysis. Data are reported as the CFTRinh172-inhibited short-circuit current (ΔIsc), determined by subtracting the current measured after CFTRinh172 treatment from that measured after the addition of forskolin and genistein (34).
Propagation of human enteroid cultures and monolayer formation.
Human colon enteroids from isolated intestinal crypts were obtained from Dr. Mark Donowitz at Johns Hopkins University, Division of Gastroenterology, and Conte NIH/NIDDK Digestive Diseases Basic and Translational Research Core Center and were maintained as cysts embedded in Matrigel (Corning) in 24-well plates. Enteroids were cultured in Wnt3A containing nondifferentiation media at 37°C in 5% CO2 atmosphere. Once multiple cultures had been generated, enteroid monolayers were formed as previously described (42). Briefly, Transwell inserts (polyester membrane with 0.4-μm pores; Costar, cat. no. 3801) were coated with 10 μg/cm2 human collagen IV solution (Sigma-Aldrich, cat. no. C5533) and incubated at 37°C for 4 h. Enteroids were harvested, triturated, and added to the top of Transwell inserts after being resuspended in expansion medium. Each Transwell insert received ~50–100 enteroid fragments and was maintained with 400 μL expansion medium on top and 1.5 mL expansion medium on bottom. Enteroid monolayers were cultured in a 5% CO2 atmosphere at 37°C. Expansion medium was supplemented with Y-27632 and CHIR99021 during the first 2 days after seeding. Formation of enteroid monolayers was monitored by TER measurements using an EVOM2, epithelial volt/ohm meter and the STX2 electrode (WPI Instruments, Sarasota, FL). After they had reached >800 Ω·cm2, monolayers were treated with TcdB for 30 min to 2 h at 0.1 μg/mL in the apical solution only.
Statistical assays.
Analysis of variance with multiple-comparison Tukey analysis or unpaired Student’s t tests were used to evaluate statistical significance (assuming Gaussian distribution) with Prism 7.0 GraphPad Software. All data are present as means ± SE, and values are considered significant at P < 0.05.
Data sharing statement.
Original data will be presented upon request from the corresponding author.
RESULTS
CD TcdB increases CFTR protein and function.
To determine whether CFTR is affected by CD, we treated Caco2 cells with TcdB. Figure 1 shows that a 30-min exposure to TcdB increased both the total and surface expression of CFTR in Caco2 cells. The parallel increase in CFTR in both the total lysate and surface fractions suggests that the increase in surface expression of CFTR can be accounted for simply by increases in the overall expression of CFTR protein.
Fig. 1.
Effect of toxin Clostridium difficile B (Tcd B) on cystic fibrosis transmembrane regulator (CFTR) expression. Caco2 cells were grown to confluence in 10-cm culture dishes and treated with the 0.1 μg/mL of Tcd B for 30 min. A: representative immunoblot of total and surface CFTR expression from five experiments. B: CFTR (C-band) expression in total lysates of cells treated with Tcd B and control (Cntrl) cells. C: CFTR expression (C-band) on the surface of Caco2 cells treated with Tcd B. Values are presented as means ± SE from five independent experiments. *P < 0.05; **P < 0.005. Data were analyzed via Student’s t test. Data are normalized to the loading control, ezrin. Conclusion: Tcd B treatment significantly increases CFTR expression in Caco2 cells in total lysates and at the surface.
To take these results one step further, we grew cells on permeable supports and measured the short-circuit current (Fig. 2, A and B). To estimate the CFTR-driven currents, we stimulated the currents with forskolin and potentiated them with genistein, then inhibited the CFTR-generated currents by using the CFTR inhibitor, CFTRinh172 (see Ref. 11). This protocol allowed us to evaluate the CFTR-generated currents influenced by TcdB. As published previously (64), CFTR-generated currents are readily detectable in Caco2 cells. TcdB clearly caused the CFTR-dependent Cl− current to increase significantly within the first 30 min, with a sharp decline occurring thereafter.
Fig. 2.
Effect of toxin Clostridium difficile B (Tcd B) on cystic fibrosis transmembrane regulator (CFTR) function. Experiments illustrating the effect of Tcd B on the Cl− current across the apical membrane in Caco2 cells. Tcd B was added to both the apical and basolateral solutions in the case of the treated group (x-axis is Tcd B treatment time in h). Data reported are the change in short-circuit current (Isc) following treatment with CFTR inhibitor 172 (A), transepithelial resistance (TER) normalized to control (B), and representative current tracings (actual Isc) (C). Summary data are expressed as the CFTR inh172-sensitive Isc, calculated by subtracting the Isc after CFTR inh172 treatment from the peak forskolin/genistein-stimulated Isc . Amiloride (100 M) was present in the apical solution during the whole experiment to avoid interference by epithelial Na+ channel-mediated Na currents. Data were analyzed by ANOVA followed by Tukey’s multiple comparisons. *P < 0.05; **P < 0.01; ***P < 0.005 (n = 7 for each condition, compared with the control condition). Tcd B was applied for 30 min to 3 h at 0.1 μg/mL in the apical side only. Conclusion: a significant increase in chloride ion channel function occurs in Caco2 cells treated with Tcd B within the first 30 min. Note the rather large drop in TER beginning at 1 h after treatment. C represents the time when Tcd B was added to the solution. ns, not significant.
Measurement of TER detects the leakiness (lower resistance) or tightness (higher resistance) of the junction between epithelial cells (17). We found that TcdB caused a reduction in TER beginning at 1 h posttreatment (Fig. 2C). The time course of the changes in TER was similar to that observed for the changes in Isc. TcdB is known to disrupt the barrier function of intestinal epithelial tissues and the organization of proteins known to be associated with tight junction function, such as Zonula occludens (ZO)-1, ZO-2, and occludin (26, 37, 43). Thus, the decrease in TER was mostly likely caused by a disruption of the tight junction that manifested itself as a decrease in TER (62).
Shown in Fig. 3, A and B, are the steady-state levels of CFTR measured during the same time frame depicted in Fig. 2. We detected only one primary mature band of CFTR in Caco2 cells, consistent with the expression of endogenous wild-type CFTR. Please note that the steady-state levels of CFTR increased within the first 30 min, as already indicated by the data shown in Fig. 1. This increase was sustained throughout the measurement period. Therefore, the reduction in CFTR currents observed in Fig. 2 was not the result of changes in the steady-state level of CFTR.
Fig. 3.
Immunoblot analysis of cystic fibrosis transmembrane regulator (CFTR), CFTR-associated, and chaperone proteins (total lysate). Protein samples were used for immunoblotting, and the membranes were incubated with the primary antibodies indicated in the graphs. A: representative immunoblots showing expression of CFTR and the associated proteins sodium-proton exchange regulatory factor (NHERF) 1, NHERF2, and heat shock protein (HSP) 27 in total lysates from 5 experiments. B–E: data were analyzed by ANOVA followed by a trends test for comparisons of the means. Data are presented as means ± SE from five independent experiments. *P < 0.05; ***P < 0.001; ****P < 0.0001. Data are normalized to GAPDH, the loading control. Conclusion: toxin Clostridium difficile B (Tcd B) treatment increases CFTR expression after 30 min of treatment and, at the same time, alters the steady-state levels of all the proteins studied, indicating that Tcd B has widespread effects on proteins associated with trafficking and processing CFTR.
Steady-state levels of key proteins are altered by TcdB.
To get some insight into how TcdB increases the surface expression of CFTR, we determined the effect of TcdB on the steady-state levels of the sodium-proton exchange regulatory factors NHERF1 and NHERF2, which are known to play a role in the trafficking of CFTR to the plasma membrane (25). Interestingly, TcdB caused a small but significant drop in the steady-state level of NHER1 (Fig. 3C). There was also a dramatic decrease in the levels of NHERF2 (Fig. 3D). These factors stabilize CFTR at the plasma membrane (25), so it is curious that their steady-state levels, particularly that of NHERF2, would fall and that the reduction would be sustained over the course of the experiment. Nevertheless, a reduction in the steady-state levels of both NHERF1 and NHERF2 appears to have contributed to the sharp decrease in CFTR-dependent Isc that followed 1–4 h of treatment with TcdB.
To further characterize the increase in mature CFTR, we evaluated the steady-state levels of the chaperone protein Hsp27. We chose Hsp27 (34) because it is involved in both the degradation of CFTR (63) and the polymerization of filamentous (F)-actin (18). We found that the steady-state level of Hsp27 decreased abruptly after TcdB treatment (Fig. 3E). It is clear that TcdB’s effect on Hsp27 may represent one mechanism, presumably involving altering F-actin polymerization (20), that C. difficile uses to reduce the barrier function of the gastrointestinal (GI) epithelium.
Binding of key proteins to CFTR is altered by TcdB.
As a next step, we assessed the binding of NHERF1, NHERF2, and Hsp27 to CFTR. Our results indicated that, despite a decrease in the steady-state levels of NHERF1 in the cytosol, its binding to CFTR did not change following exposure to TcdB (Fig. 4). In contrast, the binding of NHERF2 to CFTR decreased. The binding of CFTR to Hsp27, like that of NHERF1, did not change after TcdB treatment. Given that the steady-state levels of Hsp27 in the total lysate fell after treatment (Fig. 3E), the lack of change in the amount of Hsp27 protein in the pulldown experiments suggests that TcdB increases the proportion of total cellular Hsp27 that is bound to CFTR.
Fig. 4.
Immunoblot analysis of cystic fibrosis transmembrane regulator (CFTR) binding to recovered proteins after pulldown with CFTR. CFTR was immunoprecipitated with M3A7 (anti-CFTR antibody) and incubated with A/G beads overnight at 4°C. Immunoblotting was performed, with samples incubated with the primary antibodies shown in the graphs. A: representative immunoblots of CFTR-bound CFTR, sodium-proton exchange regulatory factor (NHERF) 1, NHERF2, and heat shock protein (HSP) 27 from 5 experiments. B–E: statistical analysis was performed as in Fig. 3. Data are presented as means ± SE from five to six independent experiments. **P < 0.01. Data are normalized to CFTR in the immunoprecipitated fraction. Samples are from the same experiments shown in Fig. 3. Conclusion: toxin Clostridium difficile B (Tcd B) is involved in altering the binding of proteins involved in the trafficking and processing of CFTR. Note that in many instances the fold change in binding differs from the fold change in the total lysate shown in Fig. 3. ns, not significant.
Overexpression of Hsp27 alters the response to TcdB.
In view of the decrease in the steady-state level of Hsp27 that we observed after exposure to TcdB, we asked whether some of the effects we had noted could be attributed to a decrease in Hsp27 or were independent effects of TcdB. To distinguish between these alternatives, we transfected additional Hsp27 into the Caco2 cells, treated the cells with TcdB, and then measured the short-circuit current (Fig. 5, A and B). A comparison of Figs. 2 and 5 shows that the transfection of Hsp27 increased the magnitude of the Isc in the absence of TcdB. When Hsp27 was overexpressed, TcdB treatment reduced the CFTR-dependent short-circuit current, removing the transient increase in current noted in Fig. 2. Importantly, however, when Hsp27 levels were raised, the transepithelial resistance did not change significantly over the measurement period following TcdB treatment. This result is in contrast to the sharp drop in TER that we noted in Fig. 2, in which TcdB was applied to the untransfected epithelium. This preservation of TER of the epithelial monolayer when Hsp27 levels increased indicates that the additional Hsp27 protected the barrier function from the destructive effects of TcdB on the epithelial cells.
Fig. 5.
Effect of toxin Clostridium difficile B (Tcd B) on cystic fibrosis transmembrane regulator (CFTR) function following heat shock protein (Hsp) 27 overexpression. Legend as in Fig. 2. Conclusion: when Hsp27 levels are increased, a significant decrease in chloride ion channel function occurs in Caco2 cells treated with Tcd B within the first 30 min. Note that the transepithelial electrical resistance (TER) does not change after Tcd B treatment. TER is normalized to cells untreated with ToxB but transfected with Hsp27. Statistics as described in Fig. 3; ***P < 0.001; n = 5 experiments. Isc, short-circuit current; NHREF, sodium-proton exchange regulatory factor; ns, not significant.
Increasing Hsp27 affects the binding of key proteins to CFTR.
Next, we again measured the steady-state levels of CFTR, NHERF1, NHERF2, and Hsp27. Figure 6 shows that when Hsp27 was overexpressed (Fig. 6, A and E), TcdB no longer had any effect on the steady-state levels of CFTR, NHERF1, or NHERF2, suggesting that the overexpression preserved the levels of CFTR, NHERF1, and NHERF2 from changes induced by TcdB. Finally, we measured the effect of overexpressing Hsp27 on the binding of these proteins to CFTR (Fig. 7). Consistent with the reduction in Isc, overexpression of Hsp27 reduced the binding of CFTR to both NHERF1 and NHERF2. Surprisingly, even though Hsp27 was overexpressed, its binding to CFTR actually decreased (Fig. 7E). Note that the levels of CFTR measured in the immunoprecipitation experiment were not affected by TcdB.
Fig. 6.
Immunoblot analysis of cystic fibrosis transmembrane regulator (CFTR), CFTR-associated, and chaperone proteins after heat shock protein (HSP) 27 overexpression (total lysate). Legend as in Fig. 4. A: representative immunoblots showing endogenous expression of CFTR and associated proteins sodium-proton exchange regulatory factor (NHERF) 1 and NHERF2 (top inset). Hsp27 overexpression is verified in the bottom inset. B–E: statistics as described in Fig. 3; *P < 0.05; n = 5 experiments. Conclusion: CFTR and NHERF1 steady-state protein levels do not change after toxin Clostridium difficile B (TcdB) treatment when Hsp27 is overexpressed. The data suggest that overexpression of Hsp27 protects the cells from TcdB treatment-induced changes in these proteins. ns, not significant.
Fig. 7.
Immunoblot analysis of cystic fibrosis transmembrane regulator (CFTR) binding to recovered proteins after pulldown with CFTR in heat shock protein (HSP) 27-overexpressing cells. Legend as in Fig. 4. A: representative immunoblots of CFTR-bound CFTR, sodium-proton exchange regulatory factor (NHERF) 1, NHERF2, and HSP27 from 5 to 6 experiments. B–E: statistics as described in Fig. 3; n = 5 experiments. Conclusion: toxin Clostridium difficile B is involved in altering the binding of proteins involved in the trafficking and processing of CFTR. *P < 0.05; **P < 0.01; ****P < 0.0001. ns, not significant.
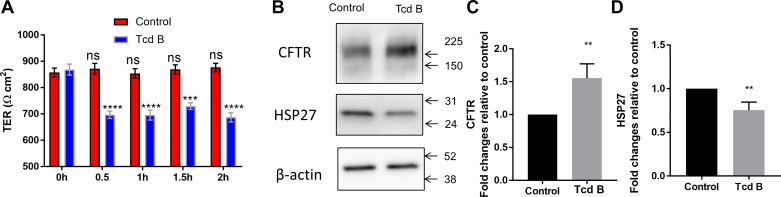
TcdB reduces barrier function and Hsp27 in human enteroids.
We realize that up to this point, we have been using Caco2 cells in our experiments, which may or may not recapitulate the human condition. Thus, to further validate our results, we obtained normal human enteroid cells from the Johns Hopkins Conte Digestive Diseases Basic and Translational Research Core Center and grew them on permeable supports as we did for the Caco2 cells. Figure 8 shows an experiment in which enteroids were grown on permeable supports and exposed to TcdB. In this experiment, we measured TER using an epithelial volt/ohm meter (EVOM2). We used this instrument so that we could measure TER repeatedly on the same cells. For some experiments, we measured the resistance for 30 min and subsequently performed immunoblotting experiments on the same cells. Cells treated with TcdB were compared with nontreated controls. TcdB caused a drop in TER and Hsp27 while increasing the expression of CFTR, as we had already seen in Caco2 cells.
Fig. 8.
Transepithelial electrical resistance, cystic fibrosis transmembrane regulator (CFTR), and heat shock protein (HSP) 27 expression in enteroid monolayers treated with toxin Clostridium difficile B (Tcd B). Human colon enteroid (obtained from isolated intestinal crypts) monolayers were grown on 0.4 μm Transwells (Costar no. 3801). Transepithelial electrical resistance (TER) was monitored in all wells regularly. After they had reached >800 Ω·cm2, monolayers were treated with Tcd B. A: TER was measured in all wells at 30-min intervals until 2 h. Data are compared with the 0 h, no Tcd treatment control group. B: representative immunoblot of CFTR and HSP27 expression after 30-min treatment with Tcd B. C and D: summary data of CFTR (C-band) and HSP27 expression in cells treated with Tcd B and control untreated cells. Values are presented as means ± SE from five to six independent experiments. One-way ANOVA with multiple comparisons; **P < 0.01; ***P < 0.001; ****P < 0.0001. Conclusion: similar results were obtained with human enteroids as observed in the Caco2 cells. ns, not significant.
DISCUSSION
Role of Hsp27.
Li and colleagues (33) studied the host-pathogen response in CD infection in Caco2 cells using a systems biology approach. Although several pathways are affected in Caco2 cells, they found that Hsp90B1 and HspA5 were key targets of CD infection in Caco2 cells. Hsp90B1 is a paralog of Hsp90, located in the ER (16). HspA5, also referred to as Hsp70 binding immunoglobulin protein, is an ER-resident protein as well (57). These investigators concluded that CD reduces the ability of Caco2 cells to respond to unfolded proteins, leading to ER stress. Consistent with this notion is our finding that TcdB reduced the protein levels of Hsp27, another factor involved in the handling of misfolded proteins (40). Given that Hsp27 plays a role in the processing of many proteins (24), it is clear that TcdB has global effects on the cellular machinery, most likely affecting the maturation of many proteins.
Hsp27 is known bind to actin and increase the stability of F-actin (18). Actin is present in cells in two forms, globular actin and F-actin (18). The dynamic interchange between these two forms is critical for the proper functioning of cellular cytoskeleton (18). A reduction in Hsp27 has been shown to have a deleterious effect on cells, particularly in regard to disrupting actin dynamics (18). We propose that CD targets Hsp27 as one mechanism to disrupt cellular function. Thus, our observation that TcdB reduced the electrical resistance of Caco2 cells is consistent with a disruption in barrier function. This drop-in resistance increases the permeability of the epithelium and allows CD toxins to damage the cells underneath, leading to colitis (36). What is novel that we show here is that increasing Hsp27 protects the epithelial cells against a change in electrical resistance induced by TcdB, suggesting that increased steady-state levels of Hsp27 are protective.
Barrier function.
CD is well known to disrupt the barrier function of epithelial cells (26). TcdA and TcdB are, respectively, 308-kDa and 270-kDa glucosyltransferases that inactivate small GTPases such as Rho, Rac, and cell division control protein 42 (12). Small GTPases are monomeric G proteins that share common functional features (16). They bind GTP, allowing them to interact with downstream effectors; hydrolysis of GTP to GDP inactivates them. Many bacterial toxins are known to target the small GTPases (2, 5). CD toxins enter cells via endocytosis and inactivate members of the Rho and Ras small GTPase families by glycosylating a threonine residue critical for their switching function and rendering them permanently inactive (12). Inhibition of the Rho GTPases causes cytoskeletal disruption and ultimately disrupts the epithelial barrier function, resulting in inflammation (46). Thus, the current thinking is that the disruption of barrier function by CD occurs via dysfunction of small GTPases (30). Here, we provide data showing that Hsp27 also plays an important role in maintaining electrical resistance and, therefore, barrier function.
Role of CFTR.
As mentioned above, in patients with CF who appear to be colonized by CD but who do not have diarrhea, a critical question is why the tissues in the GI tract are not damaged by CD, and, therefore, there is no diarrhea associated with the loss of barrier function. We hypothesize that certain mutants of CFTR are protective against diarrhea, not only because of the lack of CFTR chloride channel function but also because they might protect against the breakdown of barrier function. We have previously made the intriguing observation that some CFTR mutations, such as S1235R, a variant of CFTR with nearly normal function, increase the steady-state level of Hsp27, whereas N1303K, which causes severe CF, reduces it (48). Patients who bear the N1303K mutation are, interestingly, severely affected by CD infection (50). We therefore speculate that reducing Hsp27, both as a consequence of the N1303K mutation’s influence and TcdB exposure, can result in severe colitis, as noted in these patients. On the other hand, we have shown that cells bearing the F508-del mutation have increased levels of Hsp27 (34), which, according to our data here, are protective against CD toxicity.
We show here that TcdB increases the total amount of both mature CFTR protein and its surface expression and transiently increases CFTR-dependent Cl− transport, perhaps consistent with a role for CFTR in the early stages of the diarrhea associated with CD infection (61). To gain insight into how TcdB increases the steady-state levels of mature CFTR, we asked whether TcdB affected the ability of Hsp27 to bind to CFTR. Our data show that TcdB reduces the steady-state levels of Hsp27, but the total fraction of cellular Hsp27 bound to CFTR is actually increased by TcdB. When we overexpressed Hsp27, the total cellular fraction of CFTR bound to Hsp27 fell, and TcdB had no effect on the total amount of mature CFTR. Wild-type CFTR is known to be processed inefficiently, with ~50% being incompletely processed and ultimately being degraded in the proteasomes (49). It is well known that Hsp27 plays a role in stabilizing misfolded proteins (40). Thus, an increase in the cellular fraction of Hsp27 bound to CFTR would be consistent with a rise in the total amount of mature CFTR induced by TcdB, perhaps through protection of the CFTR from degradation.
Role of NHERF.
A number of proteins are involved in trafficking CFTR from the Golgi to the plasma membrane and its stabilization there, including NHERF1 and NHERF2 (25). NHERF1 forms a multimolecular complex with CFTR at the plasma membrane (8, 13, 32, 38, 58) and regulates CFTR’s surface expression and CFTR-dependent Cl− transport (23). NHERF2 is important for the overall function of CFTR at the plasma membrane (25). There is little information regarding how either NHERF molecule is regulated by TcdB. Here, we show that TcdB reduces the steady-state levels of NHERF1 and particularly those of NHERF2, reducing their ability to form molecular complexes at the plasma membrane. Although the amount of binding of NHERF1 to CFTR remains intact when cells are treated with TcdB, the amount of binding to NHERF2 to CFTR falls dramatically. Overexpressing Hsp27 protects the cells against changes in the steady-state levels of NHERF1 and NHERF2 induced by TcdB, reducing their binding to CFTR. The latter is also consistent with the reduced CFTR-dependent reduction of Isc by destabilizing CFTR at the plasma membrane.
Conclusion.
We show here that TcdB decreases the steady-state levels of Hsp27 and reduces the transepithelial resistance in both Caco2 and human enteroid cells. We suggest that Hsp27 is one of the key players in CD pathology. We also show that increasing Hsp27 can prevent the decrease in transepithelial resistance induced by TcdB, pointing the way for pharmacologic therapies that can increase Hsp27 as means to mitigate the effects of CD on GI pathology.
Regarding the question of why CD toxicity seems to be milder in patients with CF, we show here that TcdB increases mature CFTR and its surface expression and transiently increases CFTR-dependent transport function. However, this increase in function is short-lived. The level of expression of CFTR remains high following TcdB exposure, whereas the CFTR-driven currents rise transiently and then fall precipitously. This pattern is consistent with the fall in NHERF1 and NHERF2 over time following TcdB exposure and is consistent with CFTR trafficking away from the plasma membrane (15), thereby reducing the CFTR-generated Cl− currents. Moreover, given that CFTR function is severely compromised in CF disease-causing mutations, we hypothesize that CFTR function is not a factor in the resistance to CD toxicity that is evident in some patients with CF. More likely, cells bearing some CFTR mutants, such as F508-del, have an altered proteostatic network of proteins, as suggested by Balch and colleagues (3); these alterations could include elevated Hsp27, which serves to protect them by preserving the barrier function of the GI epithelium.
GRANTS
This work was funded by the US Cystic Fibrosis Foundation and National Institutes of Health DK-072084.
DISCLOSURES
L. Cebotaru has a license agreement with RA Capital, and W. B. Guggino has a consulting agreement with Vertex Corp and Sarepta Therapeutics. Neither of these pertain in any way to this work. None of the other authors has any conflicts of interest, financial or otherwise, to disclose.
AUTHOR CONTRIBUTIONS
L.C. conceived and designed research; M.K.Y. and L.C. performed experiments; L.C. analyzed data; L.C. and W.B.G. interpreted results of experiments; M.K.Y., W.B.G., and L.C. prepared figures; W.B.G. and L.C. drafted manuscript; L.C. and W.B.G. edited and revised manuscript; M.K.Y., W.B.G., and L.C. approved final version of manuscript.
REFERENCES
- 1.Abt MC, McKenney PT, Pamer EG. Clostridium difficile colitis: pathogenesis and host defence. Nat Rev Microbiol 14: 609–620, 2016. doi: 10.1038/nrmicro.2016.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aktories K. Bacterial toxins that target Rho proteins. J Clin Invest 99: 827–829, 1997. doi: 10.1172/JCI119245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balch WE, Morimoto RI, Dillin A, Kelly JW. Adapting proteostasis for disease intervention. Science 319: 916–919, 2008. doi: 10.1126/science.1141448. [DOI] [PubMed] [Google Scholar]
- 4.Balch WE, Roth DM, Hutt DM. Emergent properties of proteostasis in managing cystic fibrosis. Cold Spring Harb Perspect Biol 3: a004499, 2011. doi: 10.1101/cshperspect.a004499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barbieri JT, Riese MJ, Aktories K. Bacterial toxins that modify the actin cytoskeleton. Annu Rev Cell Dev Biol 18: 315–344, 2002. doi: 10.1146/annurev.cellbio.18.012502.134748. [DOI] [PubMed] [Google Scholar]
- 6.Barker HC, Haworth CS, Williams D, Roberts P, Bilton D. Clostridium difficile pancolitis in adults with cystic fibrosis. J Cyst Fibros 7: 444–447, 2008. doi: 10.1016/j.jcf.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 7.Bauer MP, Farid A, Bakker M, Hoek RA, Kuijper EJ, van Dissel JT. Patients with cystic fibrosis have a high carriage rate of non-toxigenic Clostridium difficile. Clin Microbiol Infect 20: O446–O449, 2014. doi: 10.1111/1469-0691.12439. [DOI] [PubMed] [Google Scholar]
- 8.Benharouga M, Sharma M, So J, Haardt M, Drzymala L, Popov M, Schwapach B, Grinstein S, Du K, Lukacs GL. The role of the C terminus and Na+/H+ exchanger regulatory factor in the functional expression of cystic fibrosis transmembrane conductance regulator in nonpolarized cells and epithelia. J Biol Chem 278: 22079–22089, 2003. doi: 10.1074/jbc.M301030200. [DOI] [PubMed] [Google Scholar]
- 9.Binkovitz LA, Allen E, Bloom D, Long F, Hammond S, Buonomo C, Donnelly LF. Atypical presentation of Clostridium difficile colitis in patients with cystic fibrosis. AJR Am J Roentgenol 172: 517–521, 1999. doi: 10.2214/ajr.172.2.9930816. [DOI] [PubMed] [Google Scholar]
- 10.Borriello SP, Davies HA, Kamiya S, Reed PJ, Seddon S. Virulence factors of Clostridium difficile. Rev Infect Dis 12, Suppl 2: S185–S191, 1990. doi: 10.1093/clinids/12.Supplement_2.S185. [DOI] [PubMed] [Google Scholar]
- 11.Cebotaru L, Woodward O, Cebotaru V, Guggino WB. Transcomplementation by a truncation mutant of cystic fibrosis transmembrane conductance regulator (CFTR) enhances ΔF508 processing through a biomolecular interaction. J Biol Chem 288: 10505–10512, 2013. doi: 10.1074/jbc.M112.420489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen S, Sun C, Wang H, Wang J. The role of Rho GTPases in toxicity of Clostridium difficile toxins. Toxins (Basel) 7: 5254–5267, 2015. doi: 10.3390/toxins7124874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheng J, Moyer BD, Milewski M, Loffing J, Ikeda M, Mickle JE, Cutting GR, Li M, Stanton BA, Guggino WB. A Golgi-associated PDZ domain protein modulates cystic fibrosis transmembrane regulator plasma membrane expression. J Biol Chem 277: 3520–3529, 2002. doi: 10.1074/jbc.M110177200. [DOI] [PubMed] [Google Scholar]
- 14.Cheng J, Wang H, Guggino WB. Modulation of mature cystic fibrosis transmembrane regulator protein by the PDZ domain protein CAL. J Biol Chem 279: 1892–1898, 2004. doi: 10.1074/jbc.M308640200. [DOI] [PubMed] [Google Scholar]
- 15.Cheng J, Wang H, Guggino WB. Regulation of cystic fibrosis transmembrane regulator trafficking and protein expression by a Rho family small GTPase TC10. J Biol Chem 280: 3731–3739, 2005. doi: 10.1074/jbc.M410026200. [DOI] [PubMed] [Google Scholar]
- 16.Choi S. (Editor) Encyclopedia of Signaling Molecules. New York: Springer, 2012. [Google Scholar]
- 17.Diamond JM. Twenty-first Bowditch lecture. The epithelial junction: bridge, gate, and fence. Physiologist 20: 10–18, 1977. [PubMed] [Google Scholar]
- 18.Doshi BM, Hightower LE, Lee J. HSPB1, actin filament dynamics, and aging cells. Ann NY Acad Sci 1197: 76–84, 2010. doi: 10.1111/j.1749-6632.2010.05191.x. [DOI] [PubMed] [Google Scholar]
- 20.Doshi BM, Hightower LE, Lee J. The role of Hsp27 and actin in the regulation of movement in human cancer cells responding to heat shock. Cell Stress Chaperones 14: 445–457, 2009. doi: 10.1007/s12192-008-0098-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dunwoody R, Steel A, Landy J, Simmonds N. Clostridium difficile and cystic fibrosis: management strategies and the role of faecal transplantation. Paediatr Respir Rev 26: 16–18, 2018. doi: 10.1016/j.prrv.2017.03.003. [DOI] [PubMed] [Google Scholar]
- 22.Egressy K, Jansen M, Meyer KC. Recurrent Clostridium difficile colitis in cystic fibrosis: an emerging problem. J Cyst Fibros 12: 92–96, 2013. doi: 10.1016/j.jcf.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 23.Favia M, Guerra L, Fanelli T, Cardone RA, Monterisi S, Di Sole F, Castellani S, Chen M, Seidler U, Reshkin SJ, Conese M, Casavola V. Na+/H+ exchanger regulatory factor 1 overexpression-dependent increase of cytoskeleton organization is fundamental in the rescue of F508del cystic fibrosis transmembrane conductance regulator in human airway CFBE41o- cells. Mol Biol Cell 21: 73–86, 2010. doi: 10.1091/mbc.e09-03-0185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gregersen N, Bross P, Vang S, Christensen JH. Protein misfolding and human disease. Annu Rev Genomics Hum Genet 7: 103–124, 2006. doi: 10.1146/annurev.genom.7.080505.115737. [DOI] [PubMed] [Google Scholar]
- 25.Guggino WB, Stanton BA. New insights into cystic fibrosis: molecular switches that regulate CFTR. Nat Rev Mol Cell Biol 7: 426–436, 2006. doi: 10.1038/nrm1949. [DOI] [PubMed] [Google Scholar]
- 26.Hecht G, Koutsouris A, Pothoulakis C, LaMont JT, Madara JL. Clostridium difficile toxin B disrupts the barrier function of T84 monolayers. Gastroenterology 102: 416–423, 1992. doi: 10.1016/0016-5085(92)90085-D. [DOI] [PubMed] [Google Scholar]
- 27.Hirota SA, Iablokov V, Tulk SE, Schenck LP, Becker H, Nguyen J, Al Bashir S, Dingle TC, Laing A, Liu J, Li Y, Bolstad J, Mulvey GL, Armstrong GD, MacNaughton WK, Muruve DA, MacDonald JA, Beck PL. Intrarectal instillation of Clostridium difficile toxin A triggers colonic inflammation and tissue damage: development of a novel and efficient mouse model of Clostridium difficile toxin exposure. Infect Immun 80: 4474–4484, 2012. doi: 10.1128/IAI.00933-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hookman P, Barkin JS. Clostridium difficile associated infection, diarrhea and colitis. World J Gastroenterol 15: 1554–1580, 2009. doi: 10.3748/wjg.15.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kanzaki M, Watson RT, Hou JC, Stamnes M, Saltiel AR, Pessin JE. Small GTP-binding protein TC10 differentially regulates two distinct populations of filamentous actin in 3T3L1 adipocytes. Mol Biol Cell 13: 2334–2346, 2002. doi: 10.1091/mbc.01-10-0490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim YE, Hipp MS, Bracher A, Hayer-Hartl M, Hartl FU. Molecular chaperone functions in protein folding and proteostasis. Annu Rev Biochem 82: 323–355, 2013. doi: 10.1146/annurev-biochem-060208-092442. [DOI] [PubMed] [Google Scholar]
- 32.Kwon SH, Pollard H, Guggino WB. Knockdown of NHERF1 enhances degradation of temperature rescued DeltaF508 CFTR from the cell surface of human airway cells. Cell Physiol Biochem 20: 763–772, 2007. doi: 10.1159/000110436. [DOI] [PubMed] [Google Scholar]
- 33.Li C-W, Su M-H, Chen B-S. Investigation of the cross-talk mechanism in Caco-2 cells during Clostridium difficile infection through genetic-and-epigenetic interspecies networks: big data mining and genome-wide identification. Front Immunol 8: 901, 2017. doi: 10.3389/fimmu.2017.00901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lopes-Pacheco M, Boinot C, Sabirzhanova I, Morales MM, Guggino WB, Cebotaru L. Combination of correctors rescue ΔF508-CFTR by reducing its association with Hsp40 and Hsp27. J Biol Chem 290: 25636–25645, 2015. doi: 10.1074/jbc.M115.671925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mergenhagen KA, Wojciechowski AL, Paladino JA. A review of the economics of treating Clostridium difficile infection. Pharmacoeconomics 32: 639–650, 2014. doi: 10.1007/s40273-014-0161-y. [DOI] [PubMed] [Google Scholar]
- 36.Mitty RD, LaMont JT. Clostridium difficile diarrhea: pathogenesis, epidemiology, and treatment. Gastroenterologist 2: 61–69, 1994. [PubMed] [Google Scholar]
- 37.Moore R, Pothoulakis C, LaMont JT, Carlson S, Madara JL. C. difficile toxin A increases intestinal permeability and induces Cl- secretion. Am J Physiol Gastrointest Liver Physiol 259: G165–G172, 1990. doi: 10.1152/ajpgi.1990.259.2.G165. [DOI] [PubMed] [Google Scholar]
- 38.Moyer BD, Duhaime M, Shaw C, Denton J, Reynolds D, Karlson KH, Pfeiffer J, Wang S, Mickle JE, Milewski M, Cutting GR, Guggino WB, Li M, Stanton BA. The PDZ-interacting domain of cystic fibrosis transmembrane conductance regulator is required for functional expression in the apical plasma membrane. J Biol Chem 275: 27069–27074, 2000. doi: 10.1074/jbc.M004951200. [DOI] [PubMed] [Google Scholar]
- 39.Mulrennan S, Tai A, Eng L, Putsathit P, Collins D, Riley T. Clostridium difficile infection in cystic fibrosis patients (Abstract). Pediatr Pulmonol 53: 393, 2018. [Google Scholar]
- 40.Mymrikov EV, Seit-Nebi AS, Gusev NB. Large potentials of small heat shock proteins. Physiol Rev 91: 1123–1159, 2011. doi: 10.1152/physrev.00023.2010. [DOI] [PubMed] [Google Scholar]
- 41.Nagakumar P. Pseudomembranous colitis in cystic fibrosis. Paediatr Respir Rev 14, Suppl 1: 26–27, 2013. doi: 10.1016/j.prrv.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 42.Noel G, Baetz NW, Staab JF, Donowitz M, Kovbasnjuk O, Pasetti MF, Zachos NC. A primary human macrophage-enteroid co-culture model to investigate mucosal gut physiology and host-pathogen interactions. Sci Rep 7: 45270, 2017. [Erratum in Sci Rep 7: 46790, 2017]. doi: 10.1038/srep45270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nusrat A, von Eichel-Streiber C, Turner JR, Verkade P, Madara JL, Parkos CA. Clostridium difficile toxins disrupt epithelial barrier function by altering membrane microdomain localization of tight junction proteins. Infect Immun 69: 1329–1336, 2001. doi: 10.1128/IAI.69.3.1329-1336.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43a.Oldfield EC IV, Oldfield EC III, Johnson DA. Clinical update for the diagnosis and treatment of Clostridium difficile infection. World J Gastrointest Pharmacol Ther 5: 1–26, 2014. doi: 10.4292/wjgpt.v5.i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ostedgaard LS, Baldursson O, Welsh MJ. Regulation of the cystic fibrosis transmembrane conductance regulator Cl- channel by its R domain. J Biol Chem 276: 7689–7692, 2001. doi: 10.1074/jbc.R100001200. [DOI] [PubMed] [Google Scholar]
- 45.Peach SL, Borriello SP, Gaya H, Barclay FE, Welch AR. Asymptomatic carriage of Clostridium difficile in patients with cystic fibrosis. J Clin Pathol 39: 1013–1018, 1986. doi: 10.1136/jcp.39.9.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pothoulakis C. Effects of Clostridium difficile toxins on epithelial cell barrier. Ann N Y Acad Sci 915: 347–356, 2000. doi: 10.1111/j.1749-6632.2000.tb05263.x. [DOI] [PubMed] [Google Scholar]
- 47.Pruitt RN, Lacy DB. Toward a structural understanding of Clostridium difficile toxins A and B. Front Cell Infect Microbiol 2: 28, 2012. doi: 10.3389/fcimb.2012.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rapino D, Sabirzhanova I, Lopes-Pacheco M, Grover R, Guggino WB, Cebotaru L. Rescue of NBD2 mutants N1303K and S1235R of CFTR by small-molecule correctors and transcomplementation. PLoS One 10: e0119796, 2015. doi: 10.1371/journal.pone.0119796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Riordan JR. Assembly of functional CFTR chloride channels. Annu Rev Physiol 67: 701–718, 2005. doi: 10.1146/annurev.physiol.67.032003.154107. [DOI] [PubMed] [Google Scholar]
- 50.Rivlin J, Lerner A, Augarten A, Wilschanski M, Kerem E, Ephros MA. Severe Clostridium difficile-associated colitis in young patients with cystic fibrosis. J Pediatr 132: 177–179, 1998. doi: 10.1016/S0022-3476(98)70511-6. [DOI] [PubMed] [Google Scholar]
- 51.Rosenstein BJ, Cutting GR; Cystic Fibrosis Foundation Consensus Panel . The diagnosis of cystic fibrosis: a consensus statement. J Pediatr 132: 589–595, 1998. doi: 10.1016/S0022-3476(98)70344-0. [DOI] [PubMed] [Google Scholar]
- 52.Roth DM, Hutt DM, Tong J, Bouchecareilh M, Wang N, Seeley T, Dekkers JF, Beekman JM, Garza D, Drew L, Masliah E, Morimoto RI, Balch WE. Modulation of the maladaptive stress response to manage diseases of protein folding. PLoS Biol 12: e1001998, 2014. doi: 10.1371/journal.pbio.1001998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sabirzhanova I, Lopes Pacheco M, Rapino D, Grover R, Handa JT, Guggino WB, Cebotaru L. Rescuing trafficking mutants of the ATP-binding cassette protein, ABCA4, with small molecule correctors as a treatment for Stargardt eye disease. J Biol Chem 290: 19743–19755, 2015. doi: 10.1074/jbc.M115.647685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shen A. Clostridium difficile toxins: mediators of inflammation. J Innate Immun 4: 149–158, 2012. doi: 10.1159/000332946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Verkman AS, Lukacs GL, Galietta LJ. CFTR chloride channel drug discovery--inhibitors as antidiarrheals and activators for therapy of cystic fibrosis. Curr Pharm Des 12: 2235–2247, 2006. doi: 10.2174/138161206777585148. [DOI] [PubMed] [Google Scholar]
- 56.Voth DE, Ballard JD. Clostridium difficile toxins: mechanism of action and role in disease. Clin Microbiol Rev 18: 247–263, 2005. doi: 10.1128/CMR.18.2.247-263.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang J, Lee J, Liem D, Ping P. HSPA5 gene encoding Hsp70 chaperone BiP in the endoplasmic reticulum. Gene 618: 14–23, 2017. doi: 10.1016/j.gene.2017.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang S, Raab RW, Schatz PJ, Guggino WB, Li M. Peptide binding consensus of the NHE-RF-PDZ1 domain matches the C-terminal sequence of cystic fibrosis transmembrane conductance regulator (CFTR). FEBS Lett 427: 103–108, 1998. doi: 10.1016/S0014-5793(98)00402-5. [DOI] [PubMed] [Google Scholar]
- 59.Wang X, Chen M, Zhou J, Zhang X. HSP27, 70 and 90, anti-apoptotic proteins, in clinical cancer therapy (Review) Int J Oncol 45: 18–30, 2014. doi: 10.3892/ijo.2014.2399. [DOI] [PubMed] [Google Scholar]
- 60.Weinreich F, Wood PG, Riordan JR, Nagel G. Direct action of genistein on CFTR. Pflugers Arch 434: 484–491, 1997. doi: 10.1007/s004240050424. [DOI] [PubMed] [Google Scholar]
- 61.Welkon CJ, Long SS, Thompson CM Jr, Gilligan PH. Clostridium difficile in patients with cystic fibrosis. Am J Dis Child 139: 805–808, 1985. doi: 10.1001/archpedi.1985.02140100067032. [DOI] [PubMed] [Google Scholar]
- 62.Zemljic M, Rupnik M, Scarpa M, Anderluh G, Palù G, Castagliuolo I. Repetitive domain of Clostridium difficile toxin B exhibits cytotoxic effects on human intestinal epithelial cells and decreases epithelial barrier function. Anaerobe 16: 527–532, 2010. doi: 10.1016/j.anaerobe.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 63.Zhang H, Peters KW, Sun F, Marino CR, Lang J, Burgoyne RD, Frizzell RA. Cysteine string protein interacts with and modulates the maturation of the cystic fibrosis transmembrane conductance regulator. J Biol Chem 277: 28948–28958, 2002. doi: 10.1074/jbc.M111706200. [DOI] [PubMed] [Google Scholar]
- 64.Zhu JX, Zhang GH, Yang N, Rowlands DK, Wong HY, Tsang LL, Chung YW, Chan HC. Activation of apical CFTR and basolateral Ca(2+)-activated K+ channels by tetramethylpyrazine in Caco-2 cell line. Eur J Pharmacol 510: 187–195, 2005. doi: 10.1016/j.ejphar.2005.01.026. [DOI] [PubMed] [Google Scholar]