Keywords: Botox, crural diaphragm paralysis, esophagogastric junction, esophageal motor disorders, high-resolution manometry, lower esophageal sphincter
Abstract
Background: Endoscopic intrasphincteric injection of Botox (ISIB) is used routinely for the treatment of achalasia esophagus and other spastic motor disorders. Studies show that the ISIB reduces the smooth muscle lower esophageal sphincter (LES) pressure. The esophageal hiatus, formed by the right crus of diaphragm, surrounds the cranial half of the LES and works like an external LES. We studied the effects of ISIB on the LES and hiatal contraction and gastroesophageal reflux (GER). Fourteen patients treated with ISIB were studied. Esophageal manometry-impedance recordings were performed before and after the ISIB. Hiatal contraction was assessed during tidal inspiration, forced inspiration, Müller’s maneuver, and straight leg raise. In 6 subjects, the manometry were repeated 6–12 mo after the ISIB. The esophagogastric junction (EGJ) pressure was measured at end expiration (LES pressure) and at the peak of maneuvers (hiatal contraction). Transdiaphragmatic pressure (pdi; force of diaphragmatic contraction) was measured at the peak of forced inspiration. GER was measured from the impedance recordings. The EGJ pressure at end expiration (LES pressure) decreased significantly after the Botox injection. The peak EGJ pressure at tidal inspiration, forced inspiration, Müller’s maneuver, and straight leg raise was also dramatically reduced by the ISIB. There was no effect of Botox on the pdi during forced inspiration. Seven of 10 subjects demonstrated GER during maneuvers following the ISIB. Six to 12 mo after ISIB, the LES and hiatal contraction pressure returned to the pre-ISIB levels. ISIB, in addition to decreasing LES pressure, paralyzes the esophageal hiatus (crural diaphragm) and induces GER.
NEW & NOTEWORTHY The sphincter mechanism at the lower end of the esophagus comprises smooth muscle lower esophageal sphincter (LES) and skeletal muscle crural diaphragm (hiatus). Current thinking is that the endoscopic intrasphincteric injection of Botox (ISIB), used routinely for the treatment of achalasia esophagus, reduces LES pressure. Our study shows that ISIB, even though injected into the LES, diffuses into the hiatus and causes its paralysis. These findings emphasize the importance of esophageal hiatus as an important component of the antireflux barrier and that the ISIB is refluxogenic.
INTRODUCTION
Botulinum toxin is a neurotoxic protein produced by the clostridium botulinum and related species of bacteria. It is the most lethal toxin known, with an estimated human median lethal dose of 1.3–2.1 ng/kg intravenously or intramuscularly and 10–13 ng/kg when inhaled. It is composed of a heavy protein chain bound to a light chain by the disulfide bond. Botox specifically enters the cholinergic nerve endings through a membrane receptor. The light chain is liberated in cytosol, which hydrolyzes the synaptosomal nerve-associated protein 25, a part of the soluble N-ethylmaleimide-sensitive fusion protein attachment protein receptor (SNARE) complex. The latter is involved in docking and fusing the vesicle membrane to plasma membrane (fusion machine), which allows exocytosis of the neurotransmitters. Botulinum toxin prevents the release of acetylcholine from the axonal endings at the neuromuscular junction and causes flaccid paralysis of the skeletal muscles (3, 5). Alan Scott (20) studied the effect of Botox on strabismus. Botox is now widely used in the treatment of torticollis, laryngeal dystonia, tremors, hemifacial spasm, and myoclonus (all skeletal muscle spastic disorders). Pasricha et al. (17) studied the use of Botox for the treatment of achalasia esophagus, a spastic condition of the smooth muscle lower esophageal sphincter (LES) in 1995. Since then, a large number of studies have shown the efficacy of Botox in the treatment of achalasia and other spastic esophageal motor disorders (9, 10, 19, 21).
For the treatment of achalasia esophagus, Botox is injected endoscopically into the LES. Since LES is not recognizable on endoscopy, the squamocolumnar junction (Z-line) is used as a surrogate of the LES, and the Botox injections are generally made 1–2 cm above the Z-line. There is a close anatomical relationship between the LES and esophageal hiatus (12, 16). The latter is formed by the right crus of diaphragm, which surrounds the cranial/proximal half of the LES. Studies show that the hiatal contraction exerts a sphincter-like action on the esophagus (external LES). Intraluminal pressure measured at the esophagogastric junction (EGJ) is due to the contraction of smooth muscle LES and/or skeletal muscles crural diaphragm. Generally, the EGJ pressure at end expiration is due to the LES (1, 12), and the increase in pressure with inspiration is due to crural diaphragm/hiatal contraction. The increase in EGJ pressure with inspiration is directly related to the depth of inspiration or the force of diaphragmatic contraction (15). The hiatal contraction also plays a major role in the increase in end-expiratory EGJ pressure during abdominal compression, straight leg raise, and all those maneuvers that increase intra-abdominal pressure (13).
Studies show that the intrasphincteric injection of Botox (ISIB) into the LES causes reduction in LES pressure (17, 18). To the best of our knowledge, whether Botox affects the esophageal hiatus/crural diaphragm contraction has never been studied. Also, it is also not clear whether ISIB injection increases the propensity for gastroesophageal reflux (GER). The goals of our studies were to determine 1) the effects of endoscopic ISIB on the LES and hiatal contraction using high-resolution manometry (HRM) and 2) if there is an increase in the GER during physical maneuvers that increase gastroesophageal pressure gradient.
METHODS
Studies were conducted in 14 patients (age range: 40–74 yr; 6 male) who had undergone ISIB for the treatment of achalasia esophagus and other spastic esophageal motor disorders {i.e., diffuse esophageal spasm, nutcracker esophagus, and outflow obstruction [defined as per Chicago Classification (4)]} (Table 1). All patients had bothersome dysphagia and chest pain as their major symptoms. Patients had undergone evaluation with high-resolution esophageal impedance manometry (HRMZ) as a part of the clinical workup before the ISIB. Botox A, 100 units, in the powder form was mixed with 5 mL of saline just before the injection. Five injections of 20 units (1 mL) each were delivered 1–2 cm above the Z-line all around the circumference of the esophagus. Subjects were invited to participate in the research study after they had the ISIB. The study required symptom evaluation using the standardized questionnaires (reflux and dysphagia questionnaires) and an esophageal manometry 2–4 wk after the ISIB. In 6 subjects, symptom evaluation and HRMZ recordings were repeated 6–12 mo after the ISIB. The University of California San Diego approved the study protocol, and each patient signed informed consent before their participation in the study.
Table 1.
Subject demographics, manometry diagnosis (based on Chicago Classification), and symptom
| Heartburn |
||||||
|---|---|---|---|---|---|---|
| Patient No. | Age, yr | Sex | Diagnosis | Pre-Botox | Post-Botox 2–4 wk |
Post-Botox 6–12 mo |
| 1 | 74 | M | Achalasia 2 | N | N | N |
| 2 | 51 | F | Achalasia 3 | N | Y | Y |
| 3 | 59 | F | Achalasia 3 | N | N | N |
| 4 | 40 | M | Nutcracker | N | Y | Y |
| 5 | 60 | F | Nutcracker | N | Y | |
| 6 | 57 | F | Nutcracker | Y | Y | |
| 7 | 35 | F | Nutcracker | N | Y | |
| 8 | 68 | M | DES | N | Y | N |
| 9 | 74 | M | DES | N | Y | N |
| 10 | 48 | F | DES | Y | Y | |
| 11 | 65 | M | DES | N | Y | |
| 12 | 43 | F | DES | Y | Y | |
| 13 | 68 | M | Jackhammer | Y | Y | |
| 14 | 58 | F | Outflow obstruction | Y | Y | |
DES, diffuse esophageal spasm; F, female; M, male.
The protocol for the clinical manometry test at the University of California San Diego includes 10–12 swallows of 0.5 N saline and 3–5 deep breaths. The research manometry study included placement of the HRMZ catheter after local anesthesia of nasal mucosa and throat with viscus lidocaine and spray. The catheter was positioned via nose into the esophagus and stomach. After baseline accommodation period of 10 min, subjects were asked to swallow 10 times, 30 s apart. Each swallow was 5 mL of 0.5 N saline. Subjects then performed maximal deep breaths, Müller’s maneuver, sniffs, and straight leg raises 3–4 times each. Müller’s maneuver was performed by asking the subjects to inspire against the closed mouth (palm of the hand placed over the mouth). Straight leg raise was performed by asking subjects to lift both legs at the same time with straight knees while continuing to breathe normally.
Data analysis.
The EGJ pressure was determined from the HRMZ recordings at the following time points: 1) end expiration, 2) the peak of tidal inspiration, 3) the peak of forced inspiration, 4) the peak pressure during Müller’s maneuver, and 5) during SLR. For the end expiration EGJ pressure, five 2-min periods were randomly selected in the recordings (total of 10 min data). The E-Sleeve function in the HRM analysis software was used to determine the mean end-expiration pressure. To calculate the transdiaphragmatic pressure (pdi), a time point in the recording at the peak of inspiration was chosen, and using the smart mouse function, esophageal and gastric pressure were measured at the peak of inspiration. The difference between the gastric (positive number) and esophageal pressure (negative number) at the peak of inspiration represents the pdi. Mean of 3–4 observations was made in each subject. The Z (impedance) line tracing of the HRMZ recording was used to determine the GER events. A drop in impedance value of ≥100 Ω moving in a retrograde fashion was the criterion for the detection of a GER event during each maneuver.
Statistical analysis.
Data are expressed as median and interquartile range, unless otherwise stated. Normality was assessed through the Shapiro-Wilk test. Paired t test and Wilcoxon test for normal or non-normal samples were used for comparisons. A general linear model with repeated measures for comparison between the 3 time points (i.e., pre-Botox, post-Botox; 2–4 wk and 6–12 mo) was used. The Bonferroni correction was used when appropriate. Differences with a two-tailed value of P < 0.05 were considered statistically significant.
RESULTS
Effect of Botox on EGJ pressure.
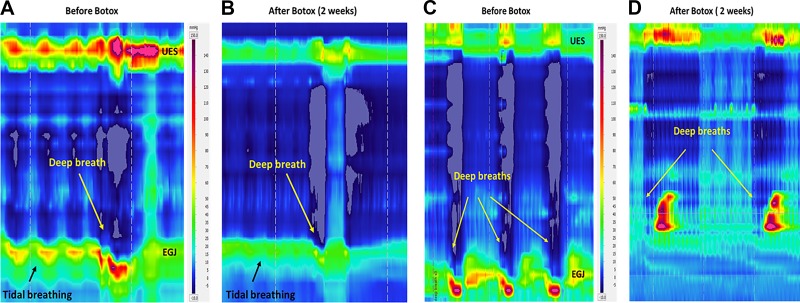
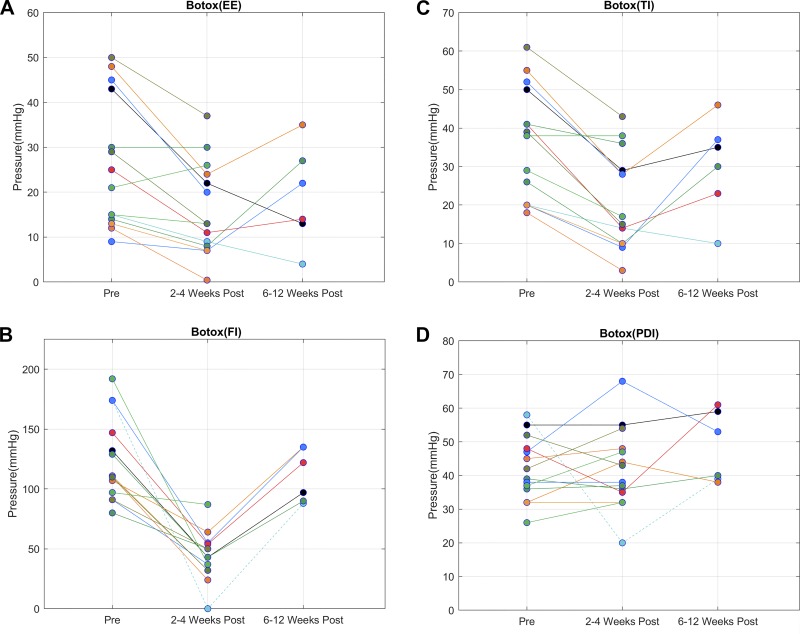
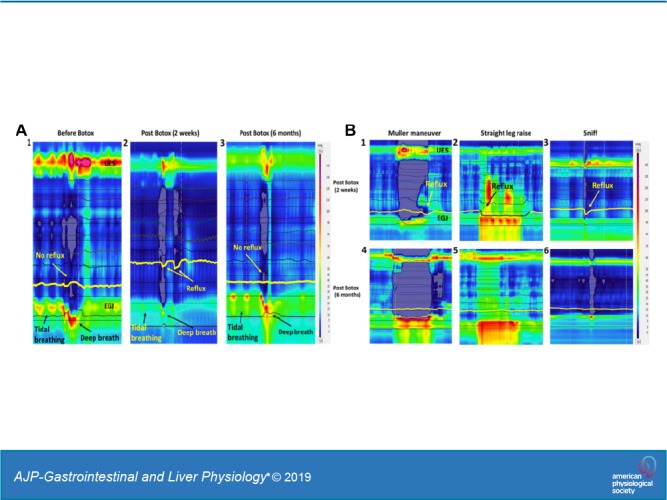
The EGJ pressure increases with each tidal inspiration and decreases with expiration (Fig. 1A). End-expiratory EGJ pressure is generally considered to be due to the tonic LES contraction (1). On the other hand, the increase in EGJ pressure with tidal inspiration is generally due to the effect of crural diaphragm/hiatal contraction. Forced inspiration (deep breath) results in a large increase in EGJ pressure, ranging from 100 to 150 mmHg. The HRMZ study conducted 2–4 wk after the ISIB revealed significant reduction in the end-expiratory EGJ pressure (LES pressure) (Fig. 2A) from 23 (29) mmHg to 13 (16) mmHg, P < 0.01. There was also a dramatic reduction in the increase in EGJ pressure associated with tidal inspiration [39 (30) mmHg to 16 (19) mmHg, P < 0.01] (Fig. 2C) and forced inspiration [111 (50) mmHg to 43 (22) mmHg, P < 0.01] (Fig. 2B). In most of the subjects, there was 70% or more reduction in the EGJ pressure with forced inspiration. In one subject, there was complete absence of the increase in EGJ pressure with forced inspiration (Fig. 1D). In fact, the EGJ pressure showed reduction, resulting in near equalization of the stomach and esophageal pressures (Figs. 1D and 2D). The pdi during forced inspiration [41 (12) mmHg], a measure of the force of diaphragmatic contraction, was not altered [40.5 (13) mmHg] by the Botox injection, P = 0.66 (Fig. 2). In one subject in whom there was complete absence of hiatal contraction with forced inspiration, there was marked reduction of the pdi, from 60 to 20 mmHg. The EGJ descent associated with forced inspiration (seen on the HRM recording) was not affected by the Botox injection [pre-Botox: 30 (10) mm and post-Botox (2–4 wk): 20 (10) mm, P > 0.05].
Fig. 1.
Esophagogastric junction (EGJ) pressure during tidal inspiration and deep breaths before Botox injection (A) and at 2–4 wk of Botox injection (B). Note marked reduction in the EGJ pressure with tidal inspiration and deep breath. Complete paralysis of the hiatal contraction following the Botox injection (D); note negative esophageal pressure and positive stomach pressure before Botox injection (C). After Botox injection, there is a complete absence of hiatal contraction in this patient. UES, upper esophageal sphincter.
Fig. 2.
Line graphs showing comparison of end expiration (EE) (A), tidal inspiration (TI) (C), forced inspiration (FI) (B) and transdiaphragmatic pressure (pdi) (D) before and after the Botox injection at 2–4 wk and at 6–12 mo. Note reduction in the esophagogastric junction (EGJ) pressures at end expiration (P < 0.01), tidal inspiration (P < 0.01), and deep breath at 2–4 wk (P < 0.01) and return of pressures at 6–12 mo after the Botox injection. The pdi pressures were not affected by the Botox injection (P = 0.66) except in one patient who had complete paralysis of hiatal contraction by Botox at 2 wk.
GER during maneuvers that increase gastroesophageal pressure gradient.
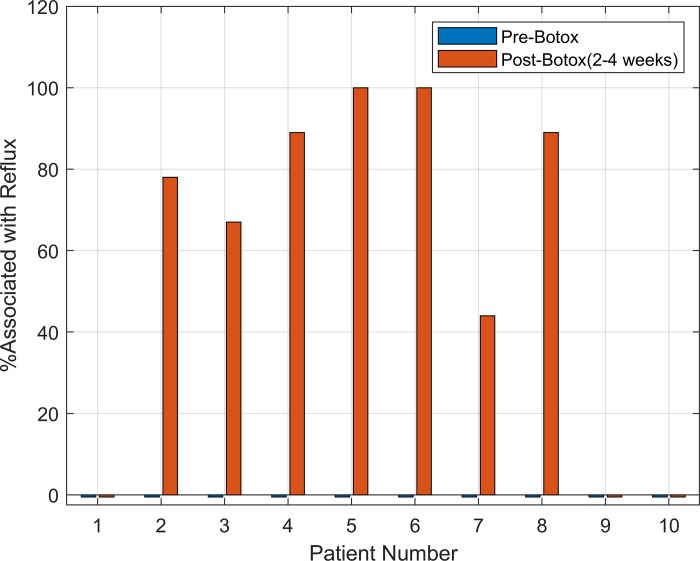
None of the forced inspirations were associated with GER events before the Botox injection in any of the studied subjects. On the other hand, 7 of the 10 subjects in whom the impedance recordings were available before and after the Botox injection showed evidence of GER after the ISIB (Fig. 3). In four subjects, malfunction of the impedance electrodes did not allow reflux measurements. Some subjects complained of regurgitation and gurgling sounds in chest with deep inspiration; they were aware of the movement of stomach contents into the esophagus and chest. Similar to forced inspiration, Müller’s maneuver and straight leg raise were also associated with GER after the ISIB.
Fig. 3.
x-Axis shows each individual subject, and y-axis is the percent of maneuvers associated with gastroesophageal reflux events in each subject (deep breaths, leg raise, Müller’s maneuver) following the Botox injection.
Effects of Botox on EGJ pressure, 6–12 mo after Botox injection.
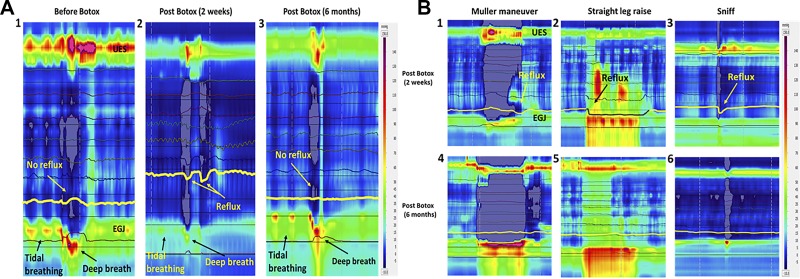
In 6 subjects, HRMZ recordings were obtained 6–12 mo after the ISIB. These data show that the end-expiratory EGJ pressure as well as tidal and forced inspiration-related increase in the EGJ pressure returned back to the pre-Botox injection levels (Fig. 4, A and B). Similarly, the increase in the EGJ pressure associated with straight leg raise, Müller’s maneuver, and sniffs were higher at the 6–8 mo study as compared with the 2–4 wk study. Gastroesophageal reflux during deep breaths, SLR, and Müller’s maneuver that was frequently observed at 2–4 wk post-ISIB was not observed at the 6–12 mo post-ISIB.
Fig. 4.
A: esophagogastric junction (EGJ) pressure before Botox (1), at 2 wk (2), and at 6 mo (3) after Botox injection. Note reduction in the EGJ pressure and reflux with deep inspiration at 2 wk and return of EGJ pressure at 6 mo. B: EGJ pressure during Müller’s maneuver, straight leg raise, and sniffing, 2 wk (1–3) and 6 mo (4–6) after the Botox injection. Note return of EGJ pressure and absence of reflux during the maneuvers at 6 mo. UES, upper esophageal sphincter.
Symptoms post-Botox injection.
Nine of the 14 subjects did not complain of GER symptoms before the Botox injection. Seven of these 9 subjects developed GER symptoms at the 2–4 wk evaluation. They described regurgitation with deep breaths, bending over, and other physical activities. Some patients complained of new heartburn symptom; others who had heartburn and reflux before the ISIB complained that their symptoms increased after the ISIB. Of the 6 patients who were studied at both 2–4 wk and 6–12 mo after the ISIB, 3 patients developed reflux symptoms at 2–4 wk after the ISIB, which disappeared at 6–12 mo post-ISIB. The other 2 patients did not complain of reflux symptoms at any time during the study. One patient who had no reflux before the ISIB developed reflux at 2 wk, which was present even at the 8-mo study (Table 1).
DISCUSSION
In summary, our data show that the ISIB, besides decreasing the end-expiratory EGJ pressure, which is generally considered as the LES pressure, has significant influence on the crural diaphragm/hiatal contraction. The ISIB induces partial or complete paralysis of the esophageal hiatus. Symptoms of GER were experienced by several patients for several weeks after the Botox injection. The physiological maneuvers, such as deep inspiration and Müller’s maneuver, that are normally associated with strong hiatal contraction and no GER were associated with GER following ISIB. The end-expiratory EGJ pressure and hiatal contraction pressure returned back to normal (pre-Botox) level 6–12 mo after Botox injection, at which point physiological maneuvers did not induce GER.
The sphincter mechanism at the junction of the esophagus and stomach (EGJ) is quite complex. Although commonly referred to as just LES, the intraluminal pressure at the EGJ is related to the intrinsic or smooth muscle LES and extrinsic or skeletal muscle crural diaphragm. Generally, the EGJ pressure at end expiration is considered to be due to the smooth muscle LES, and with each inspiration (tidal or forced inspiration), the increase in EGJ pressure is due to the contraction of crural diaphragm/esophageal hiatus (1). The latter can also contribute to the end-expiratory EGJ pressure during maneuvers such as abdominal compression and straight leg raise (13). Coughing, sneezing, and Valsalva maneuvers are also associated with strong contraction of the crural diaphragm (12). All of the above maneuvers are associated with an increase in the gastroesophageal pressure gradient and thus a propensity for GER. The large increase in EGJ pressure provided by the hiatal contraction during these physiological maneuvers strengthens the antireflux barrier and prevents GER. Since ISIB causes partial or complete paralysis of the crural diaphragm, it is not surprising that there is high incidence of GER during all those maneuvers that are associated with an increase in the gastroesophageal pressure gradient. At the end of 6–12 mo and with the return of LES and hiatal contraction functions, these maneuvers were not associated with GER, proving that the ISIB is refluxogenic, at least temporarily.
One has to ensure similar depth of inspiration or force of contraction in the pre and post-Botox studies to prove that the difference in the EGJ pressures is not related to the differences in the force of diaphragmatic contraction. Transdiaphragmatic pressure (pdi) is a measure of the force of diaphragmatic contraction. Overall, we found no difference in the pdi values before and after Botox injection. In one subject, we observed almost complete paralysis of the hiatus and almost zero EGJ pressure following the ISIB; the same subjects also showed marked reduction in the pdi value with forced inspiration from the pre-ISIB value. However, pdi can only be considered to be representative of the diaphragmatic force in a setting in which there is no movement of gastric contents into the esophagus, in other words, an intact EGJ barrier function. Furthermore, we also observed that the LES descent during inspiration, which is related to the contraction of diaphragm, was not different before and after the ISIB.
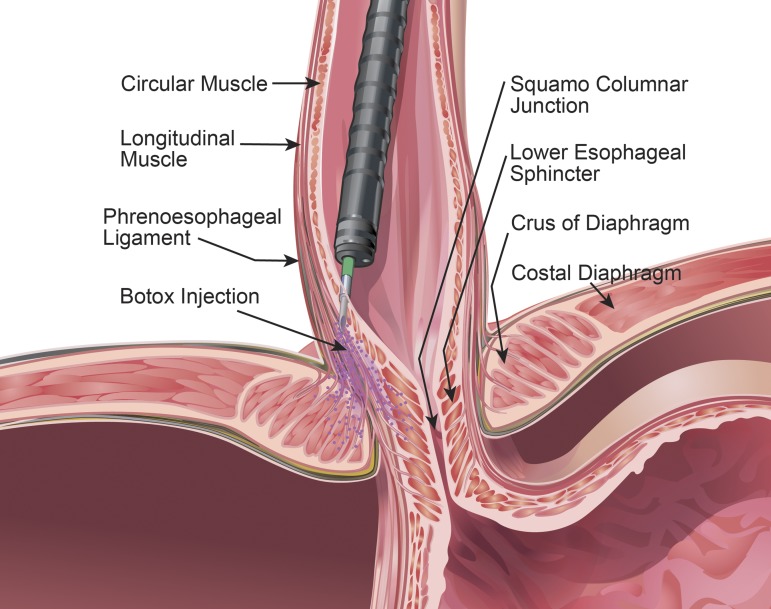
There are three possibilities as to why injection of Botox into the LES may affect hiatal contraction. The needle used for the endoscopic injection protrudes 5 mm beyond the tip of the catheter. It can penetrate 5 mm deeper from the surface, which is also approximately the thickness of the tissue (mucosa + submucosa + muscularis propria) at the level of the LES (based on the measurements with ultrasound imaging) (8, 14). Since the hiatus surrounds the proximal half of the LES (16), it is possible that the needle traverses through the LES into the hiatus. Second, Botox powder is water soluble and may easily diffuse from the LES tissue into the surrounding hiatus. Third, phrenesophageal ligaments originate from the superior and inferior surfaces of the diaphragm and penetrate into the muscularis propria of the esophagus (6). It is possible that Botox, even though injected into the muscularis propria, diffuses along the phrenesophageal ligament into the hiatus to cause its partial/complete paralysis (Fig. 5). Diffusion of Botox into the muscles adjacent to the primary target of injection is well known, and therefore, our results should not be surprising (22).
Fig. 5.
Schematic of the intrasphincteric injection of Botox into the distal esophagus and diffusion of Botox into the lower esophageal sphincter and hiatus.
We did not perform prolonged esophageal pH studies to document GER disease in our patients, which may be considered as a limitation of our study. However, several patients reported reflux of gastric contents into the chest with deep breathing and straining following the ISIB, and impedance monitoring showed clear evidence of GER with physical maneuvers. None of the subjects reported difficulty breathing following Botox injection when questioned, which raises an important physiological question: is crural diaphragm a part of the respiratory muscle whose major function is ventilation of the lungs? The costal part of the diaphragm originates from the thoracic ribs and the crural diaphragm originates from the lumbar vertebrae, the two important components of the respiratory diaphragm. These two parts of the diaphragm have different embryological origins (7). The costal diaphragm develops from the third, fourth, and fifth cervical myotomes. On the other hand, the crural diaphragm develops in the mesentery of the esophagus. Under normal conditions, the two parts of the diaphragm contract in unison; the crural diaphragm may contract slightly ahead of the costal diaphragm. Experimental studies in animals show that electrical stimulation of the costal and crural diaphragm individually increases the pdi, and therefore, both of these muscles are important in the ventilatory function of the diaphragm (2). Crural diaphragm contraction clearly increases the EGJ pressure, and therefore, it must have two functions: ventilatory and sphincter. One may wonder why injection of Botox into the LES affects only the sphincter function of diaphragm and not its ventilatory function. Right and left crus muscles originate from the lumbar vertebra, and they are inserted into the central tendon of the diaphragm. The esophageal hiatus formed by the right crus of diaphragm divides into two distinct bundles to surround the esophagus to form the esophageal hiatus (23). We found that the muscle fibers of the two bundles of the right crus cross each other in a “scissor-like fashion” and encircle the esophagus to form the esophageal hiatus, a unique myoarchitecture that is responsible its “sphincter-like” action. We suspect it is only the hiatus and not the entire right and left crus muscles (pillars) that are affected by ISIB. The latter explains why ISIB selectively impairs the lower esophageal sphincter and not the ventilatory function of crural diaphragm.
In summary, our study shows that the ISIB, in addition to the LES, has a major effect on the crural diaphragm, the two major important components of the EGJ. The ISIB is “refluxogenic.” Several studies (10, 21), but not all (11), show the beneficial effects of Botox in the treatment of dysphagia and esophageal pain in spastic motor disorders. Reduction in the LES pressure by Botox provides a good rationale for the beneficial effects of Botox for the relief of dysphagia. However, the mechanism by which Botox may reduce esophageal pain and the precise site of Botox injection (LES or the body of esophagus) is debatable. If GER caused esophageal pain in our subjects, one would expect worsening of pain following the ISIB, especially if the pain was related to the GER, which is not the case. We suggest that the esophageal pain may be related to muscle spasm rather than GER in these patients. We did not study changes in dysphagia and chest pain scores with ISIB systematically using standardized questionnaires, which could be considered as a limitation of our study; however, the main goal of our study was to determine the effects of ISIB on the LES and hiatal contraction.
GRANTS
This work was supported by National Institutes of Health Grant DK-060733.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
D.K. and R.K.M. conceived and designed research; D.K. and R.K.M. performed experiments; D.K., A.Z., and R.K.M. analyzed data; D.K., A.Z., and R.K.M. interpreted results of experiments; D.K. and A.Z. prepared figures; R.K.M. drafted manuscript; D.K., A.Z., and R.K.M. edited and revised manuscript; D.K., A.Z., and R.K.M. approved final version of manuscript.
REFERENCES
- 1.Boyle JT, Altschuler SM, Nixon TE, Tuchman DN, Pack AI, Cohen S. Role of the diaphragm in the genesis of lower esophageal sphincter pressure in the cat. Gastroenterology 88: 723–730, 1985. doi: 10.1016/0016-5085(85)90143-X. [DOI] [PubMed] [Google Scholar]
- 2.De Troyer A, Sampson M, Sigrist S, Macklem PT. The diaphragm: two muscles. Science 213: 237–238, 1981. doi: 10.1126/science.7244632. [DOI] [PubMed] [Google Scholar]
- 3.Frevert J. Pharmaceutical, biological, and clinical properties of botulinum neurotoxin type A products. Drugs R D 15: 1–9, 2015. doi: 10.1007/s40268-014-0077-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kahrilas PJ, Bredenoord AJ, Fox M, Gyawali CP, Roman S, Smout AJ, Pandolfino JE; International High Resolution Manometry Working Group . The Chicago Classification of esophageal motility disorders, v3.0. Neurogastroenterol Motil 27: 160–174, 2015. doi: 10.1111/nmo.12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kumar R, Dhaliwal HP, Kukreja RV, Singh BR. The botulinum toxin as a therapeutic agent: molecular structure and mechanism of action in motor and sensory systems. Semin Neurol 36: 10–19, 2016. doi: 10.1055/s-0035-1571215. [DOI] [PubMed] [Google Scholar]
- 6.Kwok H, Marriz Y, Al-Ali S, Windsor JA. Phrenoesophageal ligament re-visited. Clin Anat 12: 164–170, 1999. doi:. [DOI] [PubMed] [Google Scholar]
- 7.Langman J. Medical Embryology: Human Developement-Normal and Abnormal. Philadelphia, PA: William & Wilkins, 1975. [Google Scholar]
- 8.Liu J, Parashar VK, Mittal RK. Asymmetry of lower esophageal sphincter pressure: is it related to the muscle thickness or its shape? Am J Physiol Gastrointest Liver Physiol 272: G1509–G1517, 1997. doi: 10.1152/ajpgi.1997.272.6.G1509. [DOI] [PubMed] [Google Scholar]
- 9.Miller LS, Parkman HP, Schiano TD, Cassidy MJ, Ter RB, Dabezies MA, Cohen S, Fisher RS. Treatment of symptomatic nonachalasia esophageal motor disorders with botulinum toxin injection at the lower esophageal sphincter. Dig Dis Sci 41: 2025–2031, 1996. doi: 10.1007/BF02093606. [DOI] [PubMed] [Google Scholar]
- 10.Miller LS, Pullela SV, Parkman HP, Schiano TD, Cassidy MJ, Cohen S, Fisher RS. Treatment of chest pain in patients with noncardiac, nonreflux, nonachalasia spastic esophageal motor disorders using botulinum toxin injection into the gastroesophageal junction. Am J Gastroenterol 97: 1640–1646, 2002. doi: 10.1111/j.1572-0241.2002.05821.x. [DOI] [PubMed] [Google Scholar]
- 11.Mion F, Marjoux S, Subtil F, Pioche M, Rivory J, Roman S, Zerbib F. Botulinum toxin for the treatment of hypercontractile esophagus: results of a double-blind randomized sham-controlled study. Neurogastroenterol Motil 31: e13587, 2019. doi: 10.1111/nmo.13587. [DOI] [PubMed] [Google Scholar]
- 12.Mittal RK, Balaban DH. The esophagogastric junction. N Engl J Med 336: 924–932, 1997. doi: 10.1056/NEJM199703273361306. [DOI] [PubMed] [Google Scholar]
- 13.Mittal RK, Fisher M, McCallum RW, Rochester DF, Dent J, Sluss J. Human lower esophageal sphincter pressure response to increased intra-abdominal pressure. Am J Physiol Gastrointest Liver Physiol 258: G624–G630, 1990. doi: 10.1152/ajpgi.1990.258.4.G624. [DOI] [PubMed] [Google Scholar]
- 14.Mittal RK, Liu J, Puckett JL, Bhalla V, Bhargava V, Tipnis N, Kassab G. Sensory and motor function of the esophagus: lessons from ultrasound imaging. Gastroenterology 128: 487–497, 2005. doi: 10.1053/j.gastro.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 15.Mittal RK, Rochester DF, McCallum RW. Electrical and mechanical activity in the human lower esophageal sphincter during diaphragmatic contraction. J Clin Invest 81: 1182–1189, 1988. doi: 10.1172/JCI113433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mittal RK, Zifan A, Kumar D, Ledgerwood-Lee M, Ruppert E, Ghahremani G. Functional morphology of the lower esophageal sphincter and crural diaphragm determined by three-dimensional high-resolution esophago-gastric junction pressure profile and CT imaging. Am J Physiol Gastrointest Liver Physiol 313: G212–G219, 2017. doi: 10.1152/ajpgi.00130.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pasricha PJ, Ravich WJ, Hendrix TR, Sostre S, Jones B, Kalloo AN. Intrasphincteric botulinum toxin for the treatment of achalasia. N Engl J Med 332: 774–778, 1995. doi: 10.1056/NEJM199503233321203. [DOI] [PubMed] [Google Scholar]
- 18.Pasricha PJ, Ravich WJ, Kalloo AN. Effects of intrasphincteric botulinum toxin on the lower esophageal sphincter in piglets. Gastroenterology 105: 1045–1049, 1993. doi: 10.1016/0016-5085(93)90947-B. [DOI] [PubMed] [Google Scholar]
- 19.Pehlivanov N, Pasricha PJ. Achalasia: Botox, dilatation or laparoscopic surgery in 2006. Neurogastroenterol Motil 18: 799–804, 2006. doi: 10.1111/j.1365-2982.2006.00802.x. [DOI] [PubMed] [Google Scholar]
- 20.Scott AB. Botulinum toxin injection into extraocular muscles as an alternative to strabismus surgery. Ophthalmology 87: 1044–1049, 1980. doi: 10.1016/S0161-6420(80)35127-0. [DOI] [PubMed] [Google Scholar]
- 21.Vanuytsel T, Bisschops R, Farre R, Pauwels A, Holvoet L, Arts J, Caenepeel P, De Wulf D, Mimidis K, Rommel N, Tack J. Botulinum toxin reduces Dysphagia in patients with nonachalasia primary esophageal motility disorders. Clin Gastroenterol Hepatol 11: 1115–1121.e2, 2013. doi: 10.1016/j.cgh.2013.03.021. [DOI] [PubMed] [Google Scholar]
- 22.Venkatesan NN, Johns MM, Hapner ER, DelGaudio JM. Abductor paralysis after Botox injection for adductor spasmodic dysphonia. Laryngoscope 120: 1177–1180, 2010. doi: 10.1002/lary.20855. [DOI] [PubMed] [Google Scholar]
- 23.Zifan A, Kumar D, Cheng LK, Mittal RK. Three-dimensional myoarchitecture of the lower esophageal sphincter and esophageal hiatus using optical sectioning microscopy. Sci Rep 7: 13188, 2017. doi: 10.1038/s41598-017-13342-y. [DOI] [PMC free article] [PubMed] [Google Scholar]