Keywords: immunology, inflammatory bowel diseases, interferon γ-antisense 1, lncRNA, rs7134599
Abstract
The inflammatory bowel diseases (IBD) are a complex set of chronic gastrointestinal inflammatory conditions arising from the interplay of genetic and environmental factors. This study focuses on noncoding RNA transcripts as potential mediators of IBD pathophysiology. One particular gene, interferon γ-antisense 1 (IFNG-AS1), has been consistently observed to be elevated in the intestinal mucosa of patients with actively inflamed IBD versus healthy controls. This study builds on these observations, demonstrating that the second splice variant is specifically altered, and this alteration even stratifies within inflamed patients. With the use of a CRISPR-based overexpression system, IFNG-AS1 was selectively overexpressed directly from its genomic loci in T cells. An unbiased mRNA array on these cells identified a large increase in many inflammatory cytokines and a decrease in anti-inflammatory cytokines after IFNG-AS1 overexpression. Media from T cells overexpressing IFNG-AS1 elicited an inflammatory signaling cascade in primary human peripheral blood mononuclear cells, suggesting the potential functional importance of IFNG-AS1 in IBD pathophysiology. The significance of these results is amplified by studies suggesting that a single-nucleotide polymorphism in IFNG-AS1, rs7134599, was associated with both subtypes of patients with IBD independently of race.
NEW & NOTEWORTHY Long noncoding RNAs are an emerging field of inflammatory bowel disease (IBD) research. This study mechanistically analyzes the role of a commonly upregulated gene in IBD and shows IFNG-AS1 as a mediator of an inflammatory signaling cascade.
INTRODUCTION
The inflammatory bowel diseases (IBD) are characterized by chronic inflammation in the gastrointestinal tract and are typically subdivided into Crohn's disease, which can cause transmural inflammation throughout the entire GI tract, and ulcerative colitis (UC), which only affects the mucosal layer of the colon. Although modern treatments, such as anti-TNFs, have improved the lives of many patients, ~20–30% of patients do not respond, whereas 30–40% lose response to these agents within 1 yr (7). Novel therapeutic approaches are needed to aid these patients. Along these lines, one approach to identify these novel therapeutic targets is to perform profiling studies comparing biological differences, such as genotypes and/or intestinal microbiome composition, between patients with IBD and controls. Although exome sequencing studies have identified many of the present drug targets, most of the genome is transcribed into non-protein-coding genes known as noncoding RNAs (15). One subset of these genes is long noncoding RNAs (lncRNAs), which are >200 base pairs and do not contain an open reading frame (14). lncRNAs can act enzymatically, regulate protein function, inhibit miRNAs, and have also been shown to regulate the transcription of many genes (14).
Three studies have used unbiased methods to profile lncRNAs in patients with IBD compared with healthy controls (9, 11, 23). Of the few genes that overlapped between these studies, interferon-γ-antisense 1 (IFNG-AS1), was seen to be elevated in patients with UC vs. healthy controls. Two other aspects of IFNG-AS1 biology make it an especially interesting IBD target: GWAS studies on non-Hispanic whites identified an association between IFNG-AS1 and IBD (18), and IFNG-AS1 genomically resides next to the potent inflammatory cytokines interferon-γ (IFNG) and IL22. In the colon, IFNG-AS1 is expressed by CD4 and CD8 T cells, B cells, and NK cells (4, 11, 19, 21). IFNG-AS1 has also been shown to regulate IFNG transcription in mouse and human immune cells (3, 4, 11, 19, 21). One proposed mechanism for this regulation is that IFNG-AS1 binds to the MLL/SET1 histone methylation complex, and gene activating methylation of histones surrounding IFNG occurs after IFNG-AS1 overexpression (4).
In this study, we will investigate further into each aspect of IFNG-AS1 biology in immune cell function and in IBD samples. First, we aim to identify specific splice variants in IFNG-AS1 correlating with differing degrees of endoscopic inflammation. Second, we aim to evaluate the association between the IFNG-AS1 risk allele and IBD in patients of primarily Hispanic origin. Third, to broaden the understanding of which genes other than IFNG are regulated by IFNG-AS1, we employed a CRISPR-based technique to overexpress IFNG-AS1 in T cells. Resulting microarray results identified IFNG to be the most altered cytokine by IFNG-AS1 overexpression. However, there were a few other cytokines that demonstrated major differences between the two groups that could be important for understanding how IFNG-AS1 participates in the pathogenesis of IBD.
MATERIALS AND METHODS
Mild vs. severe array.
Colonic UC tissues used for the mild (n = 7) and severe (n = 4) inflammation based on pathology reports analysis were obtained from the UCLA Center for Inflammatory Bowel Diseases under IRB approved protocol no. 18-000209. Disease severity was determined by both endoscopic and histologic data with endoscopic disease severity greater than or equal to Mayo score of 2, defined as moderate to severe inflammation. Histological data were also used to corroborate the inflammatory burden. A miRNeasy mini RNA purification kit was used to isolate RNA from biopsies (Qiagen). Biopsies were homogenized in 500 μL of lysis buffer, and the manufacturer’s instructions were followed. One-hundred nanograms of total RNA were used for NanoString analysis (22). Each target gene of interest was detected using a pair of reporter and capture probes carrying 35- to 50-base target-specific sequences. Hybridization between target RNA and reporter-capture probe pairs was performed at 65°C for 20 h. Posthybridization processing was carried out on a fully automated nCounter Prep station liquid-handling robot. Excess probes were removed, and the probe/target complexes were aligned and immobilized in the nCounter cartridge, which were then placed in a digital analyzer for image acquisition and data processing per the manufacturer’s protocol. The expression level of a gene was measured by counting the number of times the specific barcode for that gene was detected, and the barcode counts were then tabulated. The raw digital count of expression was exported from nSolver v3.0 software for downstream analysis. Background levels were calculated and subtracted from the samples, which were then normalized against the positive control and housekeeping gene probes.
Cell culture and cell line production.
Jurkat cells (ATCC, TIB-152) were cultured at 37°C with 5% CO2 in DMEM (Corning) supplemented with 10% fetal bovine serum (Sigma-Aldrich). To stimulate Jurkat cells, 0.4 mg/mL PMA and 5 mM ionomycin (I0634, Sigma-Aldrich) were both used at 1:5,000 for 18 h. Controls contained the vehicle, DMSO, at a concentration of 1:2,500. For inhibitory IFNG antibody treatment, 1 μg/mL of antibody was used (MAB285, R&D Systems). To produce overexpressing cells, we used a modified version of the SAM system from the Gersbach laboratory (12). To make lentiviruses, either the dCas9-VP64 vector (50918, Addgene) or gRNA vector (VB180119-1195qxv, VectorBuilder) were transfected into HEK293T cells (CRL-1573, ATCC) with appropriate additional packaging vectors (16). A p24 ELISA (PerkinElmer) was used to normalize viral particles per cell between the two groups. Jurkat cells were first transduced with the dCas9 vector, selected with Puromycin (Sigma-Aldrich), and then three clonal dCas9 cell lines were created. dCas9-expressing Jurkat cells were then transduced with gRNA-containing lentiviruses and then selected with Hygromycin B (Corning) to produce the control gRNA or IFNG-AS1 gRNA-overexpressing cell lines. Peripheral blood mononuclear cells (PBMCs) from anonymous donors were obtained from the UCLA virology core, IRB no. 11-000443. PBMCs were cultured in RPMI with 10% FBS.
Microarray analysis and RT-qPCR.
An Aurum RNA purification kit was used to lyse cells, DNAase samples, and purify RNA (Bio-Rad). The RNA samples were sent to Arraystar for microarray analysis. RNA from each sample was linearly amplified and labeled with Cy3-UTP. An RNeasy mini kit was used to purify labeled RNA (Qiagen). The concentration and specific activity of the labeled RNAs were 1 pmol Cy3 per μg labeled RNA. One microgram of each labeled RNA was fragmented and incubated with the microarray slides for 17 h at 65°C in an Agilent Hybridization Oven. The hybridized arrays were washed, fixed and scanned using the Agilent DNA Microarray Scanner. Agilent Feature Extraction software (version 11.0.1.1) was used to analyze the acquired array images. Quantile normalization and subsequent data processing were performed using the GeneSpring GX v12.1 software (Agilent Technologies). To validate microarray results by RT-PCR and iScript cDNA synthesis kit, iTaq Universal SYBR green reagent and CFX384 connect PCR machine were used (Bio-Rad). The comparative Ct method was used to calculate fold change relative to the housekeeping gene, HPRT1 (17). The following primers were used: IFNG-AS1 transcript 1 F 5′-GATGACAGATATTTGGTCAAAC-3′, R 5′-CTCCTTCTGGGCTCTCATAGC-3′; IFNG-AS1 transcript 2 F 5′-GGTACATGTGGCTAGAAGCCC-3′, R 5′-GTTCTTAGGCCTCGGTTGCT-3′; IFNG-AS1 transcript 3 F 5′-GTGCTCTCTGATGGTGGGAAR-3′, R 5′-TGATTCAGGGCACAACCCA-3′; IL2 F 5′-ATGCTGATGAGACAGCAACCA-3′, R 5′-GAGCCCCTAGGGCTTACAAAA-3′; IL3 F 5′-AACGTCCAGCTCGTTCTCTG-3′, R 5′-GAGAACACAACCGCACAAGG-3′; IFNG F 5′-GAGTGTGGAGACCATCAAGGA-3′, R 5′-GTATTGCTTTGCGTTGGACA-3′; HPRT1 F 5′-GACCAGTCAACAGGGGACAT-3′, R 5′-GCTTGCGACCTTGACCATCT-3′. Gene Set Enrichment Analysis (GSEA) was performed using the Java GSEA Desktop Application (v2.2.4) on all annotated mRNAs (n = 21,918) using default parameters against the Biocarta “inflammation pathway” and the Gene Ontology (GO) “cellular response to interferon gamma” gene sets obtained from Molecular Signatures Database (v6.0) (1, 20).
SNP analysis.
Genomic DNA from control (n = 198) or IBD (n = 200) patients was obtained from the Los Angeles County Hospital under Ling Shao's University of Southern California IRB no. HS-09-00543. A Quick Extract Solution was used to prepared buccal swab DNA (Epicenter). To genotype the samples, a predesigned TaqMan-based SNP assay, C____348319_10, was performed according the manufacturer’s instructions (ThermoFisher).
Protein assays.
The xMax Bio-Plex 3D system and the Bio-Plex Human Cytokine 27-Plex Group I Standards were used to assess cytokine levels (Bio-Rad). The capture sandwich immunoassay format was designed on magnetic beads, according to the manufacturer's instructions. The capture antibody-coupled beads were first incubated with antigen standards, samples, or controls, followed by incubation with biotinylated detection antibodies and reporter streptavidin-phycoerythrin conjugate. The cell supernatants were diluted to 1:4 in medium with 0.5% of FBS. The Bio-Plex Manager 6.0 software was used to calculate the concentrations. To detect IL2 protein by ELISA, a Quantikine assay was performed on 100 μL media from gRNA-expressing cells (R&D Systems).
PBMC treatment and flow cytometry.
Media was collected from cells and centrifuged at 4,000 revolutions per minute for 10 min. Media was then dialyzed in 4-kDa MWCO cassettes twice 1:10,000 overnight. PBMCs from anonymous donors were obtained from the UCLA virology core, IRB 11-000443. PBMCs were treated with 10 μL of media for 48 h before immunolabeling the cells. Flow cytometry was performed on a BD LSR11 cytometer at the UCLA Jonsson Comprehensive Cancer Center and Center for AIDS Research Flow Cytometry Core Facility. Immunophenotyping by flow cytometry was performed on 1E5 cells by prestaining with a viability dye (Invitrogen LIVE/DEAD aqua no. L34957) followed by a five-color study panel: CD3-Pacific Blue (BD PharMingen no. 558117), CD4-FITC (BD no. 340133), CD8-APC (BD no. 340584), CD25 Alexa-Fluor 700 (Biolegend no. 302622), and CD69-PE (BD-PharMingen no. 555531), for 30 min in the dark at room temperature. After two washes in PBS containing 1% BSA, cells were resuspended in 2% paraformaldehyde for flow cytometric analysis. Compensation was set individually for each fluorophore in the panel using BD-positive and -negative compensation beads (BD Biosciences no. 552843). Compensation staining for the Aqua fluorescent dye was performed separately using the Arc Amine Reactive Compensation Bead Kit (Invitrogen no. A10346). Flow cytometric analysis was performed using FlowJo (Treestar). Lymphocytes were gated initially on FSC vs. SSC, followed by live CD3-positive events. CD4-positive cells were gated to reveal the percentage of CD25 and CD69 expression.
RESULTS
The second splice variant of IFNG-AS1 positively correlates with UC severity.
It has been shown in multiple studies that IFNG-AS1 is elevated in patients with UC compared with healthy controls or those with inactive UC. However, IFNG-AS1 is thought to have three splice variants, and it is unknown which splice variant is associated with disease. To address this question, probes specific to each of the three variants were hybridized to RNA samples obtained from endoscopies of patients with UC with mild and severe colitis (Fig. 1, A and B). It was determined that the second transcript, exons 1–2-3–5 spliced together, dominates the colonic expression of IFNG-AS1 and that variant is the only one significantly elevated in patients with severe colitis compared with patients with mild colitis (Fig. 1C). We next used PCR to verify which transcripts were expressed in colonic biopsies and cells from either immune or epithelial origin. Similar to the results from the disease severity array, only the second transcript was able to be detected in the colon by PCR (Fig. 1D). All three primer sets were able to readily detect IFNG-AS1 in human PBMCs (Fig. 1D). We were not able to detect IFNG-AS1 in epithelial cell lines or in purified primary epithelial cells (data not shown). Similar to PBMCs and colon, Jurkat T cells also expressed the second transcript of IFNG-AS1 (Fig. 1D). Therefore, to better understand the role for IFNG-AS1 in IBD, we chose to overexpress IFNG-AS1 in Jurkat cells. To accomplish this, gRNAs against the IFNG-AS1 transcriptional start site were used to target a transcriptional activating complex to the promoter of IFNG-AS1 (Fig. 1A). The control for this experiment contained all of the same components except that the gRNA sequence displayed little similarity to any known genomic sequence. Using this system, we were able to obtain a stable overexpression of IFNG-AS1 in T cells (Fig. 1D). We next used this new cell line to probe the effects of IFNG-AS1 on T-cell functions.
Fig. 1.
The second splice variant of IFNG-AS1 positively correlates with ulcerative colitis (UC) severity. A: graphic explaining the design for transcript-specific primers and CRISPR-based overexpression overlaid with the gene structure of IFNG-AS1. B: endoscopic and histological findings were used to define mildly inflamed and severely inflamed patients with UC. C: probes specific to each splice variant were used to measure IFNG-AS1 levels in mildly (n = 7) and severely inflamed (n = 4) patients with UC. Values are means ± SD, *P < 0.05. D: RT-PCR was used to amplify the 3 splice variants in peripheral blood mononuclear cells (PBMCs), Jurkat T-cell line, and colonic biopsies. E: RT-PCR was used to measure the level of IFNG-AS1 in control gRNA or IFNG-AS1 gRNA Jurkat cells. Representative of 3 independent experiments. Values are means ± SD, ***P < 0.001.
Overexpression of IFNG-AS1 alters cytokine expression.
The main function that has been ascribed to IFNG-AS1 is its positive regulation of IFNG transcription after immune cell stimulation (3, 4, 11, 19, 21). However, an unbiased approach to identifying which genes are regulated by IFNG-AS1 has not yet been performed. To stimulate the gRNA-expressing cells, both PMA, a broad PKC activator that acts similar to CD28 engagement, and ionomycin, a calcium channel activator that mimics T-cell receptor engagement, were used. A microarray was then performed on both unstimulated and stimulated cells. When we compared stimulated control and IFNG-AS1-overexpressing cells, many genes were altered between the two groups (Fig. 2A). With the use of the GSEA-recommended threshold of FDR <0.25, both the Biocarta “inflammation pathway” (FDR = 0.11) and the GO “cellular response to interferon gamma” (FDR = 0.20) were significantly different between IFNG-AS1 vs. control PMA-stimulated cells. Expression of genes in the Biocarta “inflammation pathway” gene set were the most strongly enriched (Fig. 2B). Although IFNG was one of the most significantly altered genes by IFNG-AS1, increased expression of other inflammatory cytokines, such as IL2, IL3, and IL1A, was also evident (Fig. 2B). In addition to inflammatory cytokine genes, many anti-inflammatory genes, such as IL10 and IL13, were downregulated by IFNG-AS1 overexpression. We next validated our microarray results by RT-PCR and confirmed that IL2, IL3, and IFNG mRNA levels were elevated in stimulated IFNG-AS1-overexpressing cells (Fig. 2, C–E).
Fig. 2.
Many cytokines are altered by overexpression of IFNG-AS1. A: volcano plot plotting the significance vs. fold change for genes that were considered altered by IFNG-AS1 overexpression after stimulation. An FDR of 0.05 equals 1 on the y-axis. B: relative expression of Biocarta “inflammation pathway” genes in stimulated IFNG-AS1 vs. control gRNA-expressing Jurkat cells. Red, pink, light blue, and blue indicate high, moderate, low, and lowest expression levels of indicated genes. C–E: RT-PCR on an independent cohort was used to validate microarray results. n = 3 samples per group. Values are means ± SD, *P < 0.05. ***P < 0.001.
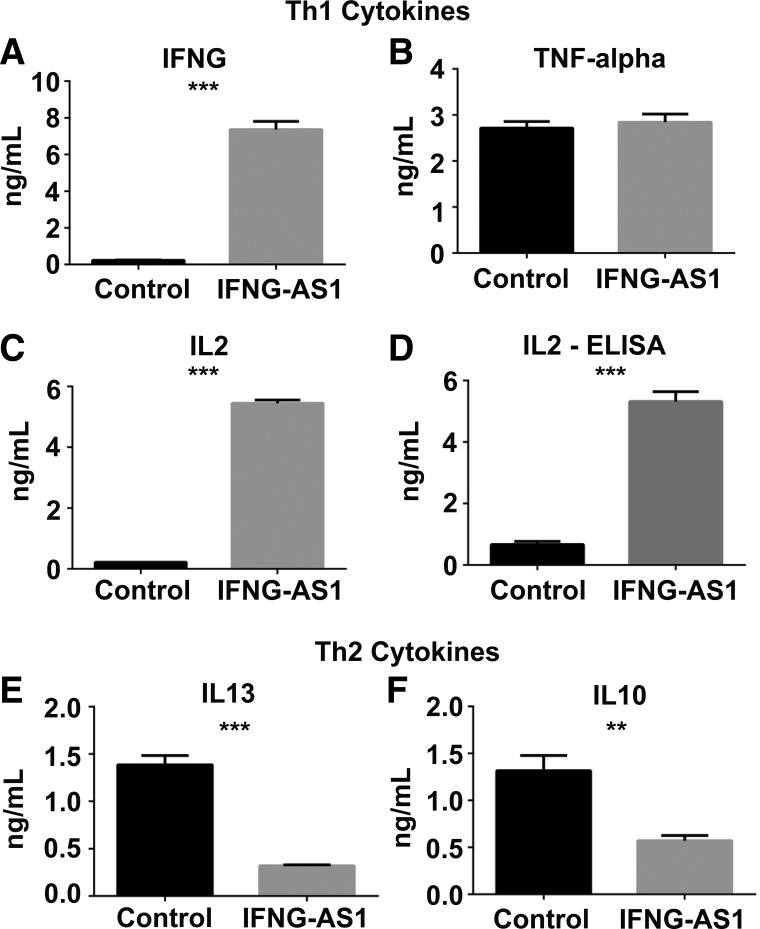
IFNG-AS1 has differential effects on Th1 vs. Th2 cytokine production.
To test whether overexpression of IFNG-AS1 caused a switch from anti-inflammatory cytokine production to inflammatory cytokine production at the protein level, an array was next performed on the media of gRNA-expressing cells. We measured five cytokines that were altered in the microarray and observed that four were also significantly altered in the media of IFNG-AS1-overexpressing cells compared with controls. The Th1 inflammatory cytokines IFNG and IL2 were both highly upregulated in the media of IFNG-AS1-overexpressing cells (Fig. 3, A and C). Conversely, the Th2 anti-inflammatory cytokines IL10 and IL13 were both highly downregulated in these cells (Fig. 3, E and F). Interestingly, although TNF-α mRNA levels were high in overexpressing cells, this did not correlate with protein levels (Fig. 3B). Lastly, as an alternative method for protein detection, an IL2 ELISA was used on supernatants from overexpressing or control cells. Similar to the protein array data, our ELISA results confirmed that IL2 levels were increased 10-fold in IFNG-AS1-overexpressing cells compared with controls (Fig. 3D).
Fig. 3.
IFNG-AS1 has differential effects on Th1 vs. Th2 cytokine production. A–F: after the stimulation of control or IFNG-AS1-overexpressing Jurkat cells, a protein array (A, B, C, E, and F) or an IL2-specific ELISA was performed on the media (D). 3 independent samples were used per group. Values are means ± SD, **P < 0.01, ***P < 0.001.
IFNG-AS1 regulates IL2.
To test whether IFNG-AS1 regulation of IL2 is independent or dependent on IFNG, cells were treated with an excess of inhibitory IFNG antibody before stimulation. Treatment with the inhibitory IFNG antibody did not alter the enhanced IFNG levels in IFNG-AS1-overexpressing cells compared with controls, nor did the inhibitory antibody reduce IFNG expression in either control or overexpressing cells (Fig. 4A). Although, in IFNG-AS1-overexpressing cells, inhibitory IFNG antibody treatment caused IL2 levels to decrease compared with controls, there was still a significantly elevated amount of IL2 in IFNG-AS1-overexpressing cells. These results suggest that IFNG-AS1 could regulate the production of IL2 independent of IFNG.
Fig. 4.
IFNG-AS1 regulates IL2. A and B: before stimulation, both control and IFNG-AS1-overexpressing cells were treated with an inhibitory IFNG antibody. After stimulation, RT-PCR was performed against IFNG and IL2. Values are means ± SD, 3 samples per group, 2 independent experiments, *P < 0.05, **P < 0.01, ***P < 0.001.
T cells overexpressing IFNG-AS1 act to hyperstimulate primary immune cells.
We next tested whether the media from IFNG-AS1-overexpressing cells would act to hyperstimulate naive immune cells compared with media from control cells. To accomplish this, PBMCs were treated with media from control gRNA cells or IFNG-AS1-overexpressing cells (Fig. 5A). After 2 days of treatment, cells were immunolabeled and fixed, and flow cytometry was performed. When analyzing the percentage of CD3+, CD4+ T cells that were also positive for the early activation marker CD69, there were nearly triple the number of cells when treated with the IFNG-AS1 overexpression media compared with controls (Fig. 5B) (2). These results suggest that the inflammatory cytokines caused by IFNG-AS1 overexpression have functionally significant effects on the surrounding tissue.
Fig. 5.
T cells overexpressing IFNG-AS1 act to hyperstimulate primary immune cells. A: graphic explaining the experimental design. After stimulation of control or IFNG-AS1-overexpressing cells, the resulting media was used to treat peripheral blood mononuclear cells (PBMCs). B: after immunolabel of media-treated PBMCs, flow cytometry was performed against the early activation marker CD69. The graph is the composite of 3 independent experiments. Values are means ± SD, ***P < 0.001.
The risk allele in IFNG-AS1 is present in Hispanics.
Given the negative effects of elevated IFNG-AS1 on proinflammatory cytokines and the association of an SNP in IFNG-AS1 in patients with UC of non-Hispanic white background, we next sought to determine whether the risk allele in IFNG-AS1 could associate with patients with IBD from other races, broadening the implications of IFNG-AS1 in IBD. We were able to use a PCR-based genotyping technique to determine the genotypes of ~200 controls and patients with IBD of primarily Hispanic origin (Fig. 6, A and B). A χ2 analysis comparing AA versus AG/GG resulted in a significant association between being homozygous for the risk allele and IBD (Fig. 6C).
Fig. 6.
The risk allele in IFNG-AS1 is present in Hispanics. A: patient characteristics of the samples used from the USC IBD biobank. B: after the performance of PCR-based genotyping of the IFNG-AS1 SNP, rs7134599, the numbers of AA, AG, and GG samples were quantified from 197 controls and 200 patients with inflammatory bowel disease (IBD). C: χ2 analysis of genotyping results. UC, ulcerative colitis, CD, Crohn’s disease.
DISCUSSION
Most of the research into causes and treatments for IBD has focused on protein-coding genes. However, most of the genome is transcribed into non-protein-coding genes. This study focused on the lncRNA, IFNG-AS1, as two out of the three profiling studies on lncRNAs on patients with UC identified IFNG-AS1 to be elevated in patients with active UC compared with healthy controls and patients with noninflamed UC (9, 11, 23). Our results add to these findings, as we identified that the IFNG-AS1 transcript containing exons 1, 2, 3, and 5 spliced together is specifically elevated in severely inflamed vs. mildly inflamed patients with UC. These findings, coupled with previously published studies, suggest that patients with UC do not have a constitutive upregulation of IFNG-AS1 but instead demonstrate conditional upregulation depending on the inflammatory state. It is possible that in patients with IBD there is a dysregulation in the elevation of IFNG-AS1 in response to inflammation. However, it is still unclear whether elevation of IFNG-AS1 alone can cause inflammation, whether it is a byproduct of inflammation, or whether it is a mixture between the two.
Besides being elevated in patients with inflamed UC, IFNG-AS1 also contains an IBD risk allele within an intron. This SNP, rs7134599, was found to be associated with patients with UC of European descent (18). We were able to find an association between rs7134599 and IBD patients of primarily Hispanic origin. Taken together, it appears that rs7134599 is penetrant in disease regardless of racial or IBD subtype. It is still unclear how rs7134599 could be genetically predisposing people to IBD. Two of the most likely scenarios are that the SNP could regulate the splicing of IFNG-AS1 or even contribute to the elevated IFNG-AS1 levels seen in patients with IBD.
Most studies have focused on solely IFNG when studying the possible functions for IFNG-AS1. The CRISPR-based overexpression and microarray approach presented within this study was able to determine a pathway that best defined the function of IFNG-AS1. Not surprisingly, bioinformatic analysis of the microarray data suggested that inflammatory pathways were the most altered pathways by IFNG-AS1. In reviewing the literature, one study measured the effects from overexpression of IFNG-AS1 in murine CD8 T cells and identified Ifng, Il2, and Mip1a as the three most upregulated genes (4). In our study, we took advantage of a more targeted approach and also identified IFNG and IL2 as two of the top genes altered by IFNG-AS1. Additionally, our study found IL2 production to be downstream of IFNG-AS1, which appeared independent of IFNG. That independence from IFNG signaling may mean that a histone methylation event might occur at multiple spots on the genome in reaction to elevated IFNG-AS1 levels, not just at the IFNG genomic loci. One drawback of the CRISPR-based system to overexpress IFNG-AS1 is its inability to specifically express specific splice variants. As such, we cannot directly attribute the alteration of cytokines (Figs. 3–5) to the second splice variant. However, any method to overexpress IFNG-AS1 has its drawbacks. Specifically, cDNA-based lentiviral- or plasmid-mediated methods would not recapitulate the genomic context because of random integration or lack of integration at all.
Although we observed many inflammatory cytokines to be increased, we also observed decreases in many anti-inflammatory cytokines. To regulate IFNG levels, IFNG-AS1 has been shown to bind and positively regulate the histone methyltransferase complex MLL/SET1 (4). The MLL/SET1 complex has, not only the ability to turn genes on via methylation of histone H3 at lysine 4, but also the ability to turn genes off (13). Therefore, it is possible that the effect of Th1 (IFNG, IL2) cytokine induction and Th2 cytokine (IL10, IL13) reduction induced by IFNG-AS1 occurs via a MLL/SET1 mechanism. The mechanism behind cytokine switching could also involve T-bet, as T-bet regulates both T-cell differentiation and IFNG-AS1 expression (6).
Our results on the lncRNA IFNG-AS1 may pose an exciting opportunity for IBD research. New biomarkers and new therapeutic targets can be gleaned from the myriad of differentially expressed lncRNAs (5, 8, 10). However, mechanistic insights are still required for these lncRNAs to allow us to fully realize their potential. This study helps to elucidate the mechanism by which the lncRNA, IFNG-AS1, could function to elicit a colitis phenotype. By regulating a broad proinflammatory cascade, IFNG-AS1 may be an attractive target for disruption in patients with colitis. Future research is needed to determine the best approaches to downregulate this lncRNA in patients. Additionally, its association with the SNP rs7134599 poses an interesting observation, not only in European Caucasian cohorts, but also in previously undescribed Hispanic populations, further broadening the importance of this genomic locus.
GRANTS
C. Pothoulakis is supported by NIDDK Grants RO1-DK-60729, RO1 DK110003-01, U01 AI124290, and P30-DK-41301. D. Padua is supported by a Crohn’s and Colitis Foundation Career Development Award, CURE: Digestive Diseases Research Center (DDRC) Grant DK-41301 and UCLA Clinical and Translational Science Institute (CTSI) Grant UL1-TR-0001881. The Center for AIDS Research (CFAR) UCLA Virology Core/UCLA AIDS Institute was funded by CFAR Grant 5P30-AI-028697. The UCLA flow cytometry core is supported by National Institutes of Health awards P30 CA016042 and 5P30 AI028697 and by the JCCC, the UCLA AIDS Institute, the David Geffen School of Medicine at UCLA, the UCLA Chancellor's Office, and the UCLA Vice Chancellor's Office of Research. L. Shao is supported by a K-08 award from the NIDDK (DK100462). The UCLA IMT core is supported by CURE/P30 DK041301.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
C.R.R., J.N.B., J.S.S., N.A., C.P., and D.M.P. conceived and designed research; C.R.R., L.S., J.E., L.R., A.P., J.S.S., N.A., M.C., O.A., A.G., and D.M.P. performed experiments; C.R.R., J.E., L.R., A.P., E.J.V., and D.M.P. analyzed data; C.R.R., J.E., L.R., A.P., and D.M.P. interpreted results of experiments; C.R.R. prepared figures; C.R.R. drafted manuscript; C.R.R., J.S.S., C.P., and D.M.P. edited and revised manuscript; C.R.R., L.S., J.E., L.R., A.P., E.J.V., J.N.B., J.S.S., N.A., M.C., O.A., A.G., C.P., and D.M.P. approved final version of manuscript.
ACKNOWLEDGMENTS
We acknowledge Jenn Fulcher for help with the experimental design.
REFERENCES
- 1.Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT, Harris MA, Hill DP, Issel-Tarver L, Kasarskis A, Lewis S, Matese JC, Richardson JE, Ringwald M, Rubin GM, Sherlock G; The Gene Ontology Consortium . Gene ontology: tool for the unification of biology. Nat Genet 25: 25–29, 2000. doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cibrián D, Sánchez-Madrid F. CD69: from activation marker to metabolic gatekeeper. Eur J Immunol 47: 946–953, 2017. doi: 10.1002/eji.201646837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Collier SP, Henderson MA, Tossberg JT, Aune TM. Regulation of the Th1 genomic locus from Ifng through Tmevpg1 by T-bet. J Immunol 193: 3959–3965, 2014. doi: 10.4049/jimmunol.1401099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gomez JA, Wapinski OL, Yang YW, Bureau JF, Gopinath S, Monack DM, Chang HY, Brahic M, Kirkegaard K. The NeST long ncRNA controls microbial susceptibility and epigenetic activation of the interferon-γ locus. Cell 152: 743–754, 2013. doi: 10.1016/j.cell.2013.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Indrieri A, Grimaldi C, Zucchelli S, Tammaro R, Gustincich S, Franco B. Synthetic long noncoding RNAs [SINEUPs] rescue defective gene expression in vivo. Sci Rep 6: 27315, 2016. doi: 10.1038/srep27315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kanhere A, Hertweck A, Bhatia U, Gökmen MR, Perucha E, Jackson I, Lord GM, Jenner RG. T-bet and GATA3 orchestrate Th1 and Th2 differentiation through lineage-specific targeting of distal regulatory elements. Nat Commun 3: 1268, 2012. doi: 10.1038/ncomms2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Katsanos KH, Papamichael K, Feuerstein JD, Christodoulou DK, Cheifetz AS. Biological therapies in inflammatory bowel disease: beyond anti-TNF therapies. Clin Immunol, 206: 9–14, 2019. doi: 10.1016/j.clim.2018.03.004. [DOI] [PubMed] [Google Scholar]
- 8.Meng L, Ward AJ, Chun S, Bennett CF, Beaudet AL, Rigo F. Towards a therapy for Angelman syndrome by targeting a long noncoding RNA. Nature 518: 409–412, 2015. doi: 10.1038/nature13975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mirza AH, Berthelsen CH, Seemann SE, Pan X, Frederiksen KS, Vilien M, Gorodkin J, Pociot F. Transcriptomic landscape of lncRNAs in inflammatory bowel disease. Genome Med 7: 39, 2015. doi: 10.1186/s13073-015-0162-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Özeş AR, Wang Y, Zong X, Fang F, Pilrose J, Nephew KP. Therapeutic targeting using tumor specific peptides inhibits long noncoding RNA HOTAIR activity in ovarian and breast cancer. Sci Rep 7: 894, 2017. doi: 10.1038/s41598-017-00966-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Padua D, Mahurkar-Joshi S, Law IK, Polytarchou C, Vu JP, Pisegna JR, Shih D, Iliopoulos D, Pothoulakis C. A long noncoding RNA signature for ulcerative colitis identifies IFNG-AS1 as an enhancer of inflammation. Am J Physiol Gastrointest Liver Physiol 311: G446–G457, 2016. doi: 10.1152/ajpgi.00212.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perez-Pinera P, Kocak DD, Vockley CM, Adler AF, Kabadi AM, Polstein LR, Thakore PI, Glass KA, Ousterout DG, Leong KW, Guilak F, Crawford GE, Reddy TE, Gersbach CA. RNA-guided gene activation by CRISPR-Cas9-based transcription factors. Nat Methods 10: 973–976, 2013. doi: 10.1038/nmeth.2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Popovic R, Zeleznik-Le NJ. MLL: how complex does it get? J Cell Biochem 95: 234–242, 2005. doi: 10.1002/jcb.20430. [DOI] [PubMed] [Google Scholar]
- 14.Quinn JJ, Chang HY. Unique features of long noncoding RNA biogenesis and function. Nat Rev Genet 17: 47–62, 2016. doi: 10.1038/nrg.2015.10. [DOI] [PubMed] [Google Scholar]
- 15.Rankin CR, Theodorou E, Man Law IK, Rowe L, Kokkotou E, Pekow J, Wang J, Martín MG, Pothoulakis C, Padua D. Identification of novel mRNAs and lncRNAs associated with mouse experimental colitis and human inflammatory bowel disease. Am J Physiol Gastrointest Liver Physiol 315: G722–G733, 2018. doi: 10.1152/ajpgi.00077.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rankin CR, Treger J, Faure-Kumar E, Benhammou J, Anisman-Posner D, Bollinger AE, Pothoulakis C, Padua DM. Overexpressing long noncoding RNAs using gene-activating CRISPR. J Vis Exp (145): 2019. doi: 10.3791/59233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc 3: 1101–1108, 2008. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 18.Silverberg MS, Cho JH, Rioux JD, McGovern DP, Wu J, Annese V, Achkar JP, Goyette P, Scott R, Xu W, Barmada MM, Klei L, Daly MJ, Abraham C, Bayless TM, Bossa F, Griffiths AM, Ippoliti AF, Lahaie RG, Latiano A, Paré P, Proctor DD, Regueiro MD, Steinhart AH, Targan SR, Schumm LP, Kistner EO, Lee AT, Gregersen PK, Rotter JI, Brant SR, Taylor KD, Roeder K, Duerr RH. Ulcerative colitis-risk loci on chromosomes 1p36 and 12q15 found by genome-wide association study. Nat Genet 41: 216–220, 2009. [Erratum in: Nat Genet 41: 762, 2009.] doi: 10.1038/ng.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stein N, Berhani O, Schmiedel D, Duev-Cohen A, Seidel E, Kol I, Tsukerman P, Hecht M, Reches A, Gamliel M, Obeidat A, Charpak-Amikam Y, Yamin R, Mandelboim O. IFNG-AS1 enhances interferon gamma production in human natural killer cells. iScience 11: 466–473, 2019. doi: 10.1016/j.isci.2018.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, Mesirov JP. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA 102: 15545–15550, 2005. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vigneau S, Rohrlich PS, Brahic M, Bureau JF. Tmevpg1, a candidate gene for the control of Theiler’s virus persistence, could be implicated in the regulation of gamma interferon. J Virol 77: 5632–5638, 2003. doi: 10.1128/JVI.77.10.5632-5638.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Warren S. Simultaneous, multiplexed detection of RNA and protein on the NanoString® nCounter® platform. Methods Mol Biol 1783: 105–120, 2018. doi: 10.1007/978-1-4939-7834-2_5. [DOI] [PubMed] [Google Scholar]
- 23.Wu F, Huang Y, Dong F, Kwon JH. Ulcerative colitis-associated long noncoding RNA, BC012900, regulates intestinal epithelial cell apoptosis. Inflamm Bowel Dis 22: 782–795, 2016. doi: 10.1097/MIB.0000000000000691. [DOI] [PubMed] [Google Scholar]