Abstract
Commissural systems are essential components of motor circuits that coordinate left–right activity of the skeletomuscular system. Commissural systems are found at many levels of the neuraxis including the cortex, brainstem, and spinal cord. In this review we will discuss aspects of the mammalian spinal commissural system. We will focus on commissural interneurons, which project from one side of the cord to the other and form axonal terminations that are confined to the cord itself. Commissural interneurons form heterogeneous populations and influence a variety of spinal circuits. They can be defined according to a variety of criteria including, location in the spinal gray matter, axonal projections and targets, neurotransmitter phenotype, activation properties, and embryological origin. At present, we do not have a comprehensive classification of these cells, but it is clear that cells located within different areas of the gray matter have characteristic properties and make particular contributions to motor circuits. The contribution of commissural interneurons to locomotor function and posture is well established and briefly discussed. However, their role in other goal-orientated behaviors such as grasping, reaching, and bimanual tasks is less clear. This is partly because we only have limited information about the organization and functional properties of commissural interneurons in the cervical spinal cord of primates, including humans. In this review we shall discuss these various issues. First, we will consider the properties of commissural interneurons and subsequently examine what is known about their functions. We then discuss how they may contribute to restoration of function following spinal injury and stroke.
Keywords: bilateral, commissural, movement
INTRODUCTION
The most basic definition of a spinal commissural interneuron (CIN) is a cell with an axonal projection that crosses the midline and terminates within the confines of the contralateral spinal cord. Although all CINs share the common feature of contralateral axonal projections, they consist of a heterogeneous population with a variety of properties and it is not easy to produce a comprehensive classification for them. Initially in this review, we will discuss their importance in bilateral control systems and examine their properties before discussing the roles of CINs in the maintenance of posture and locomotion. We will then examine their potential importance for more sophisticated voluntary tasks such as reaching, grasping, and bimanual coordination. We shall focus on the properties of mammalian CINs and their role on control of the limbs. For information on CINs in nonmammalian species, the reader is referred to the following reviews: Berg et al. (2018); Berkowitz et al. (2010); Goulding (2009); Grillner and Jessell (2009).
WHY ARE SPINAL COMMISSURAL CELLS IMPORTANT?
One of the principal characteristics of animals is the ability to locomote. As almost all vertebrates are bilaterally symmetrical, this imposes a fundamental constraint on how locomotory movements can be executed via the skeletomotor system. Although the locomotor apparatus can vary substantially, from body to limbs to wings, ultimately what one side of the body does must be coordinated with what the other side is doing.
The bilateral axis of symmetry is also mirrored in the organization of brain systems responsible for control of movement. For bilateral control of locomotion to occur successfully, there needs to be some communication between motor controllers on either side of the body. There are many possible, and nonmutually exclusive, schemata that mediate this.
In all cases, however, there needs to be a transfer of information from one side of the brain to the other, and this necessitates that neurons send axons across the midline, through commissures found in all levels of the neuraxis. The nature of the information crossing the midline will depend on the details of the system mediating bilateral coordination; it could include information on the command signals aimed at driving muscles on one side, or it might consist of sensory feedback of the contralateral movement (or a combination of both). The importance of commissural systems for motor control is supported by at least two lines of evidence:
First, during development there are many molecular mechanisms controlling axonal crossing of the midline, and the chemical cocktails that attract axons to the midline and ensure that they stay on the contralateral side (see review by Kaprielian et al. 2001). This gatekeeping mechanism is conserved in mammalian species separated by millions of years of evolution. Contralaterally projecting axons are attracted to the central nervous system (CNS) midline by diffusible factors including netrins, identified in vertebrates, and subsequently recognized to be homologous with Unc-6 protein in the nematode Caenorhabditis elegans (Kennedy et al. 1994). Netrins were successively identified in Drosophila, where they are also expressed at the midline and assist with commissural axon attraction (Mitchell et al. 1996).
Second, most areas that have a role in sensorimotor control of movement are interlinked through commissures. In the mammalian CNS, the corpus callosum forms a very large bundle of primarily unmyelinated fibers interconnecting precentral and postcentral sensorimotor areas (Karol and Pandya 1971), as well as mediating bilateral projections from the cortex to the basal ganglia (Wilson 1986). Subcortically, structures such as the superior colliculus also show commissural interconnectivity (Edwards 1977; Tardif and Clarke 2002) as do some of the output structures of the cerebellum, including but not limited to, the fastigial (Batton et al. 1977) and vestibular nuclei (Carleton and Carpenter 1983; Shinoda et al. 1986).
At the lowest level of the neuraxis is the spinal commissural system. This system consists of a heterogeneous mix of neurons, which project from one side of the spinal cord to the other and have a critical role in coordinating circuits that control posture and locomotion. The earliest anatomical descriptions of spinal commissural cells were obtained from the Golgi studies of Ramón y Cajal (1909) and similar cells were later observed by Scheibel and Scheibel (Scheibel and Scheibel 1968). Classical physiological studies of crossed-extensor reflexes in spinal animals (Sherrington 1910) and observations of Sherrington, Graham Brown, and others, showing that alternate stepping can occur in cats with complete spinal transections, provided further evidence of the existence and importance of spinal commissural systems (Brown 1911; Sherrington 1910).
In this review we will focus on the mammalian spinal commissural system. Much of our knowledge comes from studies of the cat, rat, and mouse, with a focus on the hindlimb and locomotion. There is limited knowledge of the neuroanatomy and the functional organization of commissural systems in humans and other primates, especially with respect to their contribution to circuits that control the upper limb.
PROPERTIES AND CLASSIFICATIONS OF SPINAL COMMISSURAL INTERNEURONS
CINs can be classified according to a variety of criteria including, their location in the spinal gray matter, axonal projections and targets, neurotransmitter phenotypes, activation properties, and embryological origin, but complete classifications derived from several criteria only exist for a limited number of cells such as those of cat midlumbar segments (Bannatyne et al. 2003, 2006, 2009; Jankowska et al. 2005b, 2009) and the cells of origin of the large cholinergic C boutons that directly contact motoneurons (Zagoraiou et al. 2009). The challenge we face is how to integrate these various criteria to create meaningful classifications of CINs that will provide further insight into their functions within spinal circuits. A further complication, which was discussed by Jankowska in her 2008 review (Jankowska 2008), is that the boundaries between the various classes of CIN are not sharp and functional properties of CINs may change depending on the context that they are operating within.
PROJECTIONS OF CINS
Spinal CINs can have segmental, intersegmental, and long propriospinal projections. Segmental cells are defined as those cells with axons that are confined to the contralateral segment, whereas intersegmental cells project over several segments and long propriospinal cells project to and from the lumbar and cervical enlargements (Molenaar and Kuypers 1978). A problem with this approach is that the majority of CINs have both segmental and intersegmental projections, so a simpler classification would be to consider cells with short axons and long propriospinal neurons (LPNs) as two basic types. Short axon CINs project contralaterally at the same segmental level as their cell bodies via the ventral commissure to run in the contralateral, the ventromedial, or the contralateral lateral funiculi. The axons of these cells can ascend, descend, or bifurcate to project both rostrally and caudally (Bannatyne et al. 2009; Eide et al. 1999; Jankowska et al. 2009; Matsuyama et al. 2006; Petkó and Antal 2012; Petkó et al. 2004; Stokke et al. 2002) and may travel through two to five segments (Matsuyama et al. 2006). Anterogradely labeled CIN axons with cell bodies in contralateral lamina VIII of cat midlumbar segments give off from 6 to 32 axon collaterals, which form complex ramifications within the contralateral gray matter. Cells with axons in the ventral funiculus tend to form terminations in laminae VII and VIII whereas those in the lateral funiculus tend to terminate in lamina IX (Matsuyama et al. 2006). In adult rat, lumbar cord, cells located in laminae III–IV project contralaterally via the ventral commissure to form arbors in the corresponding region of the contralateral gray matter (Petkó and Antal 2000, 2012; Petkó et al. 2004). The contralateral terminations of CINs can be very complex but there is evidence of a relationship between the configuration of the axonal arbor and other properties of the cell such as the types of input it receives and the neurotransmitter it uses (Bannatyne et al. 2003, 2006, 2009; Jankowska et al. 2009) (see studies of cins in cat midlumbar segments: inputs, axonal terminations, neurotransmitters, and targets for details). Other types of spinal commissural cells that project to the brain such as those belonging to the spinothalamic tract or the ventral spinocerebellar tract (VSCT) would not normally be classified as CINs. However, it should be noted that VSCT cells form axonal terminations in the contralateral spinal gray matter (Bras et al. 1988) and therefore, in addition to their central actions, they act as CINs and influence local contralateral spinal circuits.
LOCATIONS OF CINS WITHIN SPINAL GRAY MATTER
Lumbar CINs with Short Axons
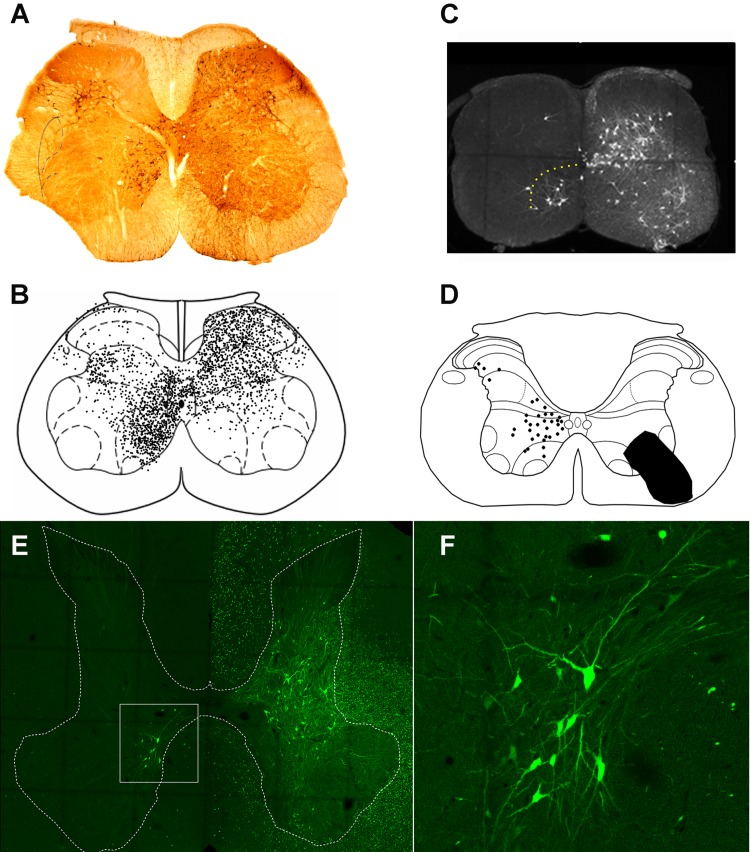
Studies using tracing methods have revealed much about the locations of CIN cell bodies within the spinal gray matter. These studies have focused principally on cells within the lumbar enlargement of adult cats, rats, and mice (Birinyi et al. 2003; Eide et al. 1999; Harrison et al. 1986; Hoover and Durkovic 1992; Liu et al. 2010; Puskár and Antal 1997; Stokke et al. 2002). In the lumbar cord, a pattern of distribution is emerging; the vast majority of cells is located within lamina VIII, but additional groups are located in the medial intermediate gray matter, lamina X, and the lateral deep dorsal horn (laminae IV–VI; Liu et al. 2010; Puskár and Antal 1997; Stokke et al. 2002) (Fig. 1, A and B). Occasional cells in the intermediate gray matter were found to be double labeled in a study where fluorescent spheres were injected into both sides of the cat lumbar cord (Hoover and Durkovic 1992) and thus had bilateral projections. Trans-synaptic viral tracing methods confirm that many CINs form synaptic contacts with motoneurons (Coulon et al. 2011; Stepien et al. 2010; Fig. 1C).
Fig. 1.
Locations of commissural interneuron cell bodies in the lumbar and cervical spinal cord. Commissural interneuron (CIN) cell bodies are shown on the left side of transverse sections and all injections were made on the right side. A: CINs labeled with the b subunit of cholera toxin (CTb) in L5 of the adult rat spinal cord. CTb was injected into the contralateral L3 intermediate gray matter. B: distribution of cells labeled as in A made from eight sections from L5. Adapted from Liu et al. (2010) with permission from Elsevier. Note the clusters of cells in lamina VIII, the medial intermediate gray matter and the lateral deep dorsal horn. C: transverse section of a C4 lumbar segment from a young mouse. Last-order premotor interneurons are labeled transsynaptically with a rabies virus that was injected into the right tibialis anterior muscle. Note the concentration of CINs in lamina VIII (demarcated by yellow dots; A. Bannatyne, B. S. Bhumbra, A. J. Todd, D. J. Maxwell, M. Beato, unpublished observations). D: plot of distribution of Fluorogold-labeled CINs in a single C4 section of an adult rat. Adapted from Mitchell et al. (2016). The black area on the right represents the injection site. E: cells in segment C4 labeled with a retrograde virus [Hiret-GFP (Kinoshita et al. 2012)] that was injected unilaterally into C7–C8 segment of an adult macaque. The area demarcated by the box is shown at higher magnification in F; note the labeled CINs in lamina VIII (A. Bannatyne, E. Bagdatlioglu, D. J. Maxwell, D. S. Soteropoulos, unpublished observations).
Cervical CINs with Short Axons
Less information is available about the organization of CINs in cervical segments when compared with lumbar segments; however, the pattern of cell body locations is similar to the lumbar cord as the majority of cells are found in lamina VIII and additional cells are present in medial lamina VII and laterally in the deep dorsal horn (Alstermark and Kümmel 1990; Bolton et al. 1991; Mitchell et al. 2016; Sugiuchi et al. 1992) (Fig. 1D). Recently viral tracing techniques have revealed a similar distribution in the nonhuman primate (Fig. 1, E and F). In addition to their roles in limb movement and posture, some cervical CINs are components of respiratory circuits (Lipski and Duffin 1986; Zholudeva et al. 2018).
Thoracic CINs with Short Axons
There are a small number of studies showing the existence of CINs at thoracic levels (Kasumacic et al. 2010, 2015; Kirkwood et al. 1988; Saywell et al. 2011; Schmid et al. 1993). Some of these cells are concerned with postural adjustments as they have input from vestibular systems and activate axial motoneurons (Kasumacic et al. 2010) or they are involved in respiratory function (Saywell et al. 2011). The latter cells are located mainly within the ventral horn.
Long Propriospinal Cells
A number of studies have revealed the existence of LPNs that project to the contralateral gray matter in lumbar and cervical enlargements of rats, mice, cats and monkeys (Brockett et al. 2013; Dutton et al. 2006; English et al. 1985; Flynn et al. 2017; Matsushita and Ikeda 1973; Miller et al. 1998; Molenaar and Kuypers 1978; Ni et al. 2014; Reed et al. 2006; Villanueva et al. 1991). These studies are generally in agreement that commissural LPNs are located principally within lamina VII and VIII.
NEUROTRANSMITTERS
CINs with Short Axons
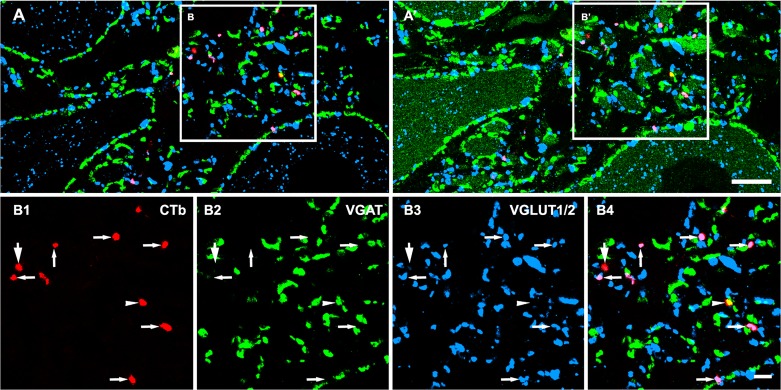
Electrophysiological studies have shown that CINs with short axons have excitatory and inhibitory effects on contralateral motoneurons (Butt and Kiehn 2003; Jankowska et al. 2003, 2005c, 2006; Quinlan and Kiehn 2007) and pharmacological studies indicate that excitatory effects are mediated via glutamate whereas the inhibitory effects are mediated via glycine or combined actions of glycine and GABA (Cowley and Schmidt 1995; Quinlan and Kiehn 2007). Anatomical studies using immunocytochemistry, in situ hybridization, or genetically modified mice show that terminals of CINs contain glutamate, GABA, glycine, or a combination of GABA and glycine (Bannatyne et al. 2003, 2006, 2009; Jankowska et al. 2009; Liu et al. 2010; Petkó and Antal 2012; Restrepo et al. 2009; Wéber et al. 2007). In a study using a combination of immunocytochemistry and anterograde labeling of CIN axons in the neonatal rat, Wéber et al. (2007) concluded that the majority (58%) of CIN axon terminals were inhibitory; ~76% of labeled inhibitory terminals were immunoreactive for the glycine transporter 2, 53% displayed immunoreactivity for glutamate decarboxylase, and 30% were likely to contain a mixture of GABA and glycine. Approximately 27% of the total labeled terminals were immunoreactive for the vesicular glutamate transporter 2. Similar proportions were reported by Restrepo and colleagues for the neonatal mouse (Restrepo et al. 2009). In a study of the adult rat lumbar cord, Liu et al. (2010) examined immunochemical properties of labeled CIN axon terminals associated with motoneurons within the lateral motor nucleus (Fig. 2). Injections of the b subunit of cholera toxin (CTb) were made in L1 or L3. They made a comparison between segmental and intersegmental projections. In the L5 contralateral lateral motor nucleus most (75%) axon terminals labeled from L1 or L3 were excitatory and expressed the vesicular glutamate transporter 2. The remaining 25% were in inhibitory and mainly contained a mixture of GABA and glycine. However, in segmental CIN projections a different picture emerged; 19% of axon terminals were found to be purely glycinergic whereas 17% contained both inhibitory transmitters. This suggests that there are differences in the transmitter phenotypes between CINs with axons that project over a few segments than those which have short segmental axons. In adult rat, Petkó et al. (2004) found that the majority (75%) of axons originating from dorsal horn CINs were inhibitory and most of these contained a mixture of GABA and glycine. Studies in the cat (Bannatyne et al. 2003, 2006, 2009; Jankowska et al. 2009) of identified CINs have shown that they use either glutamate or glycine as a neurotransmitter and no examples of GABAergic cells or cells that contained a mixture of GABA and glycine were found (see below, studies of cins in cat midlumbar segments: inputs, axonal terminations, neurotransmitters, and targets). There can be a variety of reasons for the apparent differences between the findings of these various studies. Obviously, species and developmental stage are important factors but there could be other reasons for discrepancies in the findings; for example, all the glycinergic cells examined in the cat belong to specific functionally identified populations of CINs whereas the tracing studies examined mixed populations of CINs.
Fig. 2.
Neurotransmitters associated with commissural interneuron (CIN) axon terminals in rat lumbar motor nuclei. A and A′ show general overviews of CIN terminals and their neurotransmitter content before (A) and after (A′) a sequential reaction with an antibody against choline acetyltransferase to identify motoneurons (additional green structures in A′). Details of the areas demarcated by the boxes are shown in B1–B4. B1–B4: single optical sections illustrating the neurotransmitter content of terminals that were labeled with the b subunit of cholera toxin (CTb; shown in red). Immunoreactivity for the vesicular GABA transporter (VGAT), which labels GABAergic and glycinergic terminals) is shown in green, and immunoreactivity for vesicular glutamate transporters (VGLUT1/2) is blue. B4 shows a merged image confirming the immunocytochemical characteristics of the terminals examined. Arrows in B1–B4 indicate CTb-labeled excitatory terminals that were positive for VGLUT but negative for VGAT. Arrowheads in B1–B4 indicate CTb-labeled inhibitory terminals that were positive for VGAT but negative for VGLUT1/2. The larger arrows in B1–B4 indicate a CTb-labeled terminal without immunolabeling for either VGAT or VGLUT. Reprinted from Liu et al. (2010) with permission from Elsevier. Scale bars: A = 10 µm; B1–B4 = 5 µm.
Propriospinal Cells
Brockett et al. (2013) have described a population of ascending LPNs that project from the lumbar cord to the caudal cervical cord in the adult rat. Although these projections are predominantly ipsilateral there is also a contralateral component. Many axon terminals form contacts with motoneurons of the contralateral lateral motor nucleus; ~60% of these axon terminals are glutamatergic and the remaining 40% consist of populations that contain GABA, glycine, or a mixture of the two inhibitory transmitters. In a population of descending LPNs Flynn et al. (2017) reported that in the adult mouse, relatively few contralaterally projecting cells contained inhibitory transmitters (12–14%).
Cholinergic Cells
There is a population of partition cells in laminae VII and X that are cholinergic (Liu et al. 2010; Stepien et al. 2010; Zagoraiou et al. 2009). A subpopulation of these cells has bilateral projections and some are the source of the large cholinergic C boutons found on motoneurons (Zagoraiou et al. 2009).
INPUTS TO CINS
CINs have complex patterns of input and can be activated monosynaptically by primary afferent axons and/or descending systems (see Table 1). Much of our knowledge of activation patterns comes from electrophysiological studies of cat midlumbar CINs which are discussed in detail further below.
Table 1.
Identified commissural interneurons in cat midlumbar segments
| Monosynaptic Activation | Location | Projection | Action | Contralateral Terminations | References |
|---|---|---|---|---|---|
| RF | VIII | contralateral | excitatory | VII, VIII, IX, MN | Bannatyne et al. (2003) |
| RF | VIII | contralateral | inhibitory | VII, VIII, MN | Bannatyne et al. (2003) |
| GpII | VIII | contralateral | excitatory | VIII, IX | Jankowska et al. (2009) |
| GpII | VIII | contralateral | Inhibitory | VI–IX, MN | Jankowska et al. (2009) |
| GpII | VIII | bilateral | excitatory | VI–VIII, MN | Jankowska et al. (2009) |
| GpI&II | VI–VII | contralateral | excitatory | VII, IX, MN | Bannatyne et al. (2009); Jankowska et al. (2009) |
| GPI&II | VI–VII | bilateral | excitatory | VII, VIII | Bannatyne et al. (2009); Jankowska et al. (2009) |
| GpII&Cut | IV–V | bilateral | inhibitory | VII–IX, MN | Bannatyne et al. (2006) |
Cut, cutaneous afferent; GpII, group II muscle afferent; GpI, group I muscle afferent; MN, contacts on contralateral motoneurons; RF, reticular formation. Roman numerals refer to laminae of Rexed.
The same sources of inputs can also evoke di- and polysynaptic excitatory postsynaptic potentials (EPSPs) and inhibitory postsynaptic potentials (IPSPs) in CINs via other spinal interneurons. In addition, they may change input patterns depending on the context in which they are operating; input properties can be modified by descending monoaminergic systems (Hammar et al. 2004; Jankowska 2001) or presynaptic inhibition (Edgley et al. 2003; Jankowska et al. 2002a, 2002b, 2003). This adds further complications when attempting to classify CINs according to patterns of activation.
Anatomical Studies on Descending Inputs
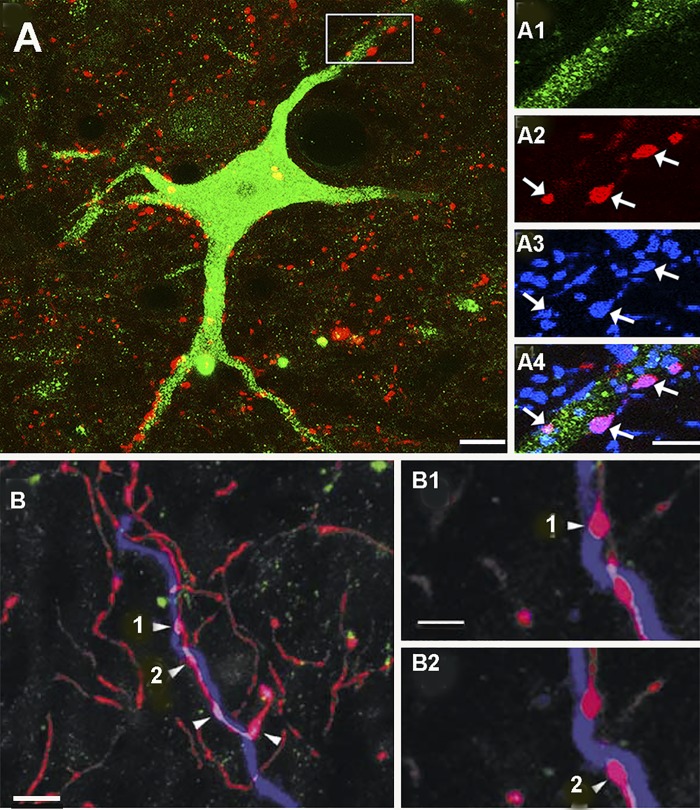
In a study of the rat cervical spinal cord, Mitchell et al. (2016) examined CINs with short axons and LPNs with crossed axons to determine whether they have direct contacts from corticospinal and reticulospinal fibers (RF). Both types of cell had very few contacts from the corticospinal tract (CST) axons but significant contacts from RF fibers were found (Fig. 3A). The majority (80%) of RF axons formed boutonlike contacts that were immunolabeled for the vesicular glutamate transporter 2 but a minority contained the vesicular GABA transporter. However, information on the anatomical relationships between other descending systems such as the vestibulospinal tract (VST) with CINs is lacking.
Fig. 3.
Contacts on commissural interneuron (CINs) from descending systems. A: commissural interneuron in lamina VIII (green) of the rat cervical spinal cord. Red structures are terminals of reticulospinal (RS) neurons. The area within the box is shown at a larger magnification in the series of images on the right. A1 shows the dendrite of the cell. A2 shows RF terminals. A3 shows immunoreactivity for the vesicular glutamate transporter 2. A4 is a merged image showing that several glutamatergic RF terminals contact the dendrite (arrows). Adapted from Mitchell et al. (2016). Scale bars A = 20 µm; A1–A4 = 5 µm. B: a dendrite of a lamina VIII commissural cell injected with Neurobiotin in the cat lumbar cord (blue). Red structures are serotonin-immunoreactive terminals and green terminals are dopamine-β hydroxylase immunoreactive; a marker for noradrenergic terminals. Several serotonin terminals make contacts with the dendrite (B1 and B2). From Hammar et al. (2004). Scale bars: B = 5 µm; B1 and B2 = 2.5 µm.
Monoamines
Hindlimb movements can be induced in spinal cats following intravenous injections of l-DOPA or drugs acting at monoamine receptors (Jankowska et al. 1967). In such preparations, the gait can be changed from an alternating movement to a near-synchronous galloping-like gait. (Grillner and Zangger 1979). Thus, monoamines can influence commissural systems which, in turn, influence half-center pattern generators to produce different patterns of gait. Monoamines are known to have profound effects on CINs and can significantly change their activation properties (Aggelopoulos et al. 1996; Dougherty et al. 2005; Hammar et al. 2004). There is evidence that the inhibitory effects of group II activated CINs on motoneurons are modulated by serotonin (Aggelopoulos et al. 1996). Serotonin and noradrenaline have differential effects on lamina VIII CINs activated from the reticular formation (RF) or by group II afferents (Hammar et al. 2004). RF activation is enhanced by both serotonin and noradrenaline whereas group II activation is facilitated by serotonin but is depressed by noradrenaline. Studies of labeled lamina VIII CINs show that both subtypes of cell are associated with dense innervation from serotonin-immunoreactive axon terminals but receive smaller numbers of noradrenergic terminals (Fig. 3B; Hammar et al. 2004). Excitatory transmission to dorsal horn group II-activated cells is depressed by agonists operating at 5-HT1&7 and 5-HT3 receptors (Dougherty et al. 2005). The action via 5-HT3 receptors is probably presynaptic as immunoreactivity for 5-HT3 receptors is present on axon terminals that surround a subgroup of group II CINs.
Interneurons
Little is understood about the relationships between CINs and other interneurons. However, it is very likely that some of the ipsilateral disynaptic and polysynaptic effects from group II afferents observed in interneurons in laminae VII and VIII are mediated via dorsal horn group II interneurons with ipsilateral projections (Jankowska et al. 2002b, 2005b). Excitatory group II interneurons have limited axonal projections that are mainly confined to the dorsal horn and intermediate gray matter and therefore may contact group I/II activated CINs in this region (Bannatyne et al. 2006). In addition to ipsilateral effects, there is evidence that contralateral CINs target ipsilateral CINs in lamina VIII (Birinyi et al. 2003; Jankowska et al. 2005a, 2005b; Matsuyama et al. 2006).
CONTRALATERAL TARGETS OF CINS
Many CINs form synaptic contacts with both contralateral motoneurons and premotor interneurons but some may operate exclusively via contralateral interneurons. Details of some of the target cells of CINs are discussed in the next two sections on cat midlumbar CINs and the embryological origin of CINs.
STUDIES OF CINS IN CAT MIDLUMBAR SEGMENTS: INPUTS, AXONAL TERMINATIONS, NEUROTRANSMITTERS, AND TARGETS
The work of Jankowska and her colleagues on the cat lumbar cord has enabled us to begin to construct a broad-based classification of commissural cells based on location, input, neurotransmitters, projections, and target cells (Bannatyne et al. 2003, 2006, 2009; Jankowska et al. 2005b, 2009). These studies employed combinations of electrophysiology, intracellular staining, immunocytochemistry (to identify neurotransmitters), and identification of target cells (Fig. 4).
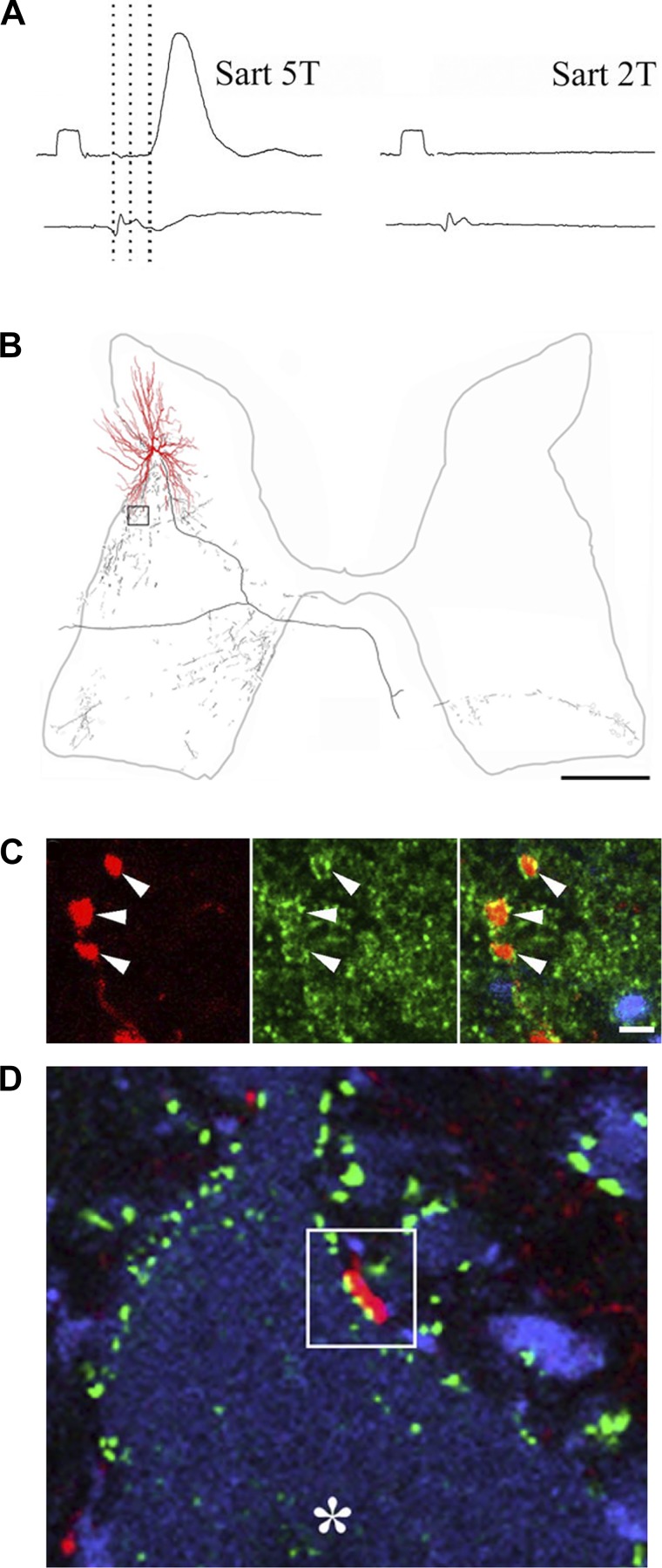
Fig. 4.
Properties dorsal horn commissural interneurons activated by group II muscle afferents. A shows an intracellular recording from a cell (top traces). An excitatory postsynaptic potential (EPSP) was evoked by stimulation the sartorius nerve at five times the threshold for the most sensitive fibers but no EPSP could be evoked at twice the threshold. The latency (3rd dotted line) is consistent with monosynaptic activation from group II fibers. Bottom traces show the cord dorsum potentials. The calibration pulse = 0.5 mV and 2 ms. B is a reconstruction of a group II activated cell that was intracellularly labeled with Neurobiotin. Dendrites are shown in red and the axonal arbor in black. Note the extent of the axonal projections within the ipsilateral gray matter and the contralateral ventral horn. C shows images of labeled terminals from the cell (red), immunoreactivity for the glycine transporter T2 (green), and a merged image. The terminals are associates with immunoreactivity and hence are glycinergic. D shows a terminal (red), immunoreactivity for gephyrin (green), and immunoreactivity for choline acetyltransferase (blue). The terminal shown in the box opposes gephyrin puncta, which are associated with the postsynaptic densities of inhibitory synapses and a motoneuron (*) in the contralateral motor nucleus. Adapted from Bannatyne et al. (2006); © 2006 Society for Neuroscience.
Lamina VIII CINs
Intracellular electrophysiological studies of cat lamina VIII CINs have revealed much about their input properties (Bannatyne et al. 2003, 2006, 2009; Jankowska et al. 2005a, 2005b, 2005c, 2009; Krutki et al. 2003). Basically, two classes of cell have been identified. The first class of cell is monosynaptically activated by group II muscle afferents but is not directly activated from the reticular formation, whereas the second class of cell is monosynaptically activated from the reticular formation but is not directly activated by group II muscle afferents.
Group II activated cells can also be excited disynaptically from ipsilateral and contralateral group II afferents and reticulospinal axons. Di- and polysynaptic IPSPs are evoked from contralateral group II afferents, ipsilateral group I fibers, and reticulospinal axons (Cavallari et al. 1987; Edgley and Jankowska 1987b; Jankowska et al. 2005b, 2009). These cells are located within lamina VIII or at the border between lamina VII and VIII and are a mixed population of glycinergic or glutamatergic neurons. Most of them project contralaterally although very occasional examples of bilaterally projecting cells have been described (Jankowska et al. 2009). Group II activated cells project to lamina VIII and motor nuclei. They have predominant inhibitory actions on contralateral motoneurons (Arya et al. 1991) and also influence activity of contralateral group II activated premotor interneurons (Bajwa et al. 1992).
The second class of lamina VIII cell is activated monosynaptically by both reticulospinal (RF) and vestibulospinal systems. These cells may also be excited disynaptically by ipsi- and contralateral group II afferents, ipsilateral group I afferents, and vestibulospinal fibers. IPSPs are evoked di- and polysynaptically from ipsi- and contralateral group II fibers along with reticulospinal and vestibulospinal systems and some ipsilateral group I fibers. Activation of these cells from the reticular formation evokes disynaptic EPSPs and IPSPs in contralateral motoneurons supplying hindlimb muscles (Edgley et al. 2003; Jankowska et al. 2003; Krutki et al. 2003) and also modifies activity of group I and II activated premotor interneurons within the intermediate gray matter (Cabaj et al. 2006). These cells can be activated indirectly from the ipsilateral and contralateral pyramidal tracts via the reticulospinal tract (Edgley et al. 2004; Jankowska et al. 2005a; see Fig. 5) and are rhythmically active during fictive locomotion (Matsuyama et al. 2004). They can further be subdivided into excitatory (glutamatergic) or inhibitory (glycinergic) cells but there was no evidence that GABA was present in any of their terminals. Their axons project to the gastrocnemius-soleus motor nucleus in L7 but they also have collateral terminations in motor nuclei of the 4th and 5th lumbar segments. They ramify within contralateral laminae VII and VIII but do not form ipsilateral collaterals (see also Matsuyama et al. 2004).
Fig. 5.
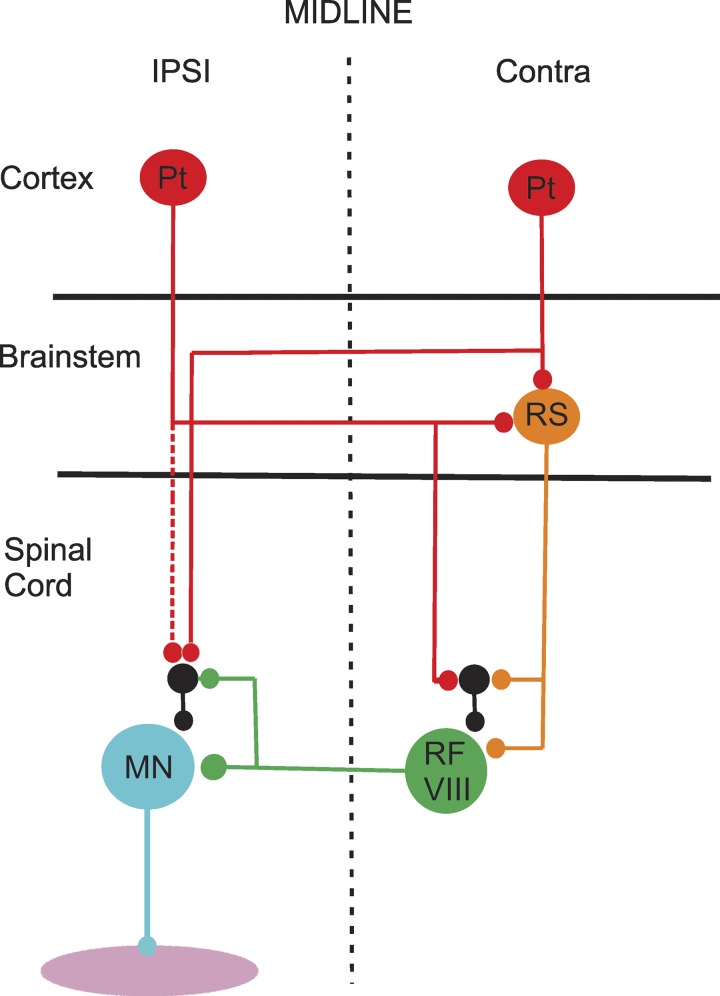
Organization of corticospinal and reticulospinal inputs to lamina VIII commissural interneurons (CINs). The diagram shows how both ipsilateral (IPSI) and contralateral (Contra) pyramidal tract (Pt) cells can indirectly influence the activity of lamina VIII commissural interneurons (RF VIII) via the reticulospinal (RS) tract. The commissural cells project directly to motoneurons (MN), which can also be indirectly influenced from Pt cells and RF CINs. Black cells represent interneurons with ipsilateral projections to motoneurons and CINs. Based on data from Stecina et al. (2008a).
Cells in the Intermediate Gray Matter
Jankowska and Edgley (2010) recently argued that interneurons in the intermediate gray matter that are coexcited by group I and II afferents should be classified as belong to the same population and not as separate populations according to the dominant types of peripheral afferent activation patterns. This would include excitatory CINs in lamina VI–VII that are monosynaptically coexcited by these classes of afferent fiber (Bannatyne et al. 2006). In addition, they have various forms of input from descending systems from the cortex, red nucleus, and reticular formation (Davies and Edgley 1994; Harrison and Jankowska 1985a, 1985b; Jankowska et al. 2006; Stecina et al. 2008a, 2008b). Commissural neurons belonging to this group are exclusively excitatory and project contralaterally or bilaterally. Cells with bilateral projections tended to form “mirrorlike” ramifications within the intermediate gray matter and rarely make direct contacts with motoneurons whereas those with contralateral projections form terminations within the ventral horn and motor nuclei (Bannatyne et al. 2009). Bilateral cells have a principal axon that runs both rostrally and caudally in the contralateral ventromedial white matter. The ipsilateral collaterals are highly localized and are restricted to the same segment as the cell body whereas contralateral collaterals project more extensively. Contralateral cells have their principal axon within the contralateral ventromedial white matter and may bifurcate or ascend through this funiculus.
CINs in the Dorsal Horn
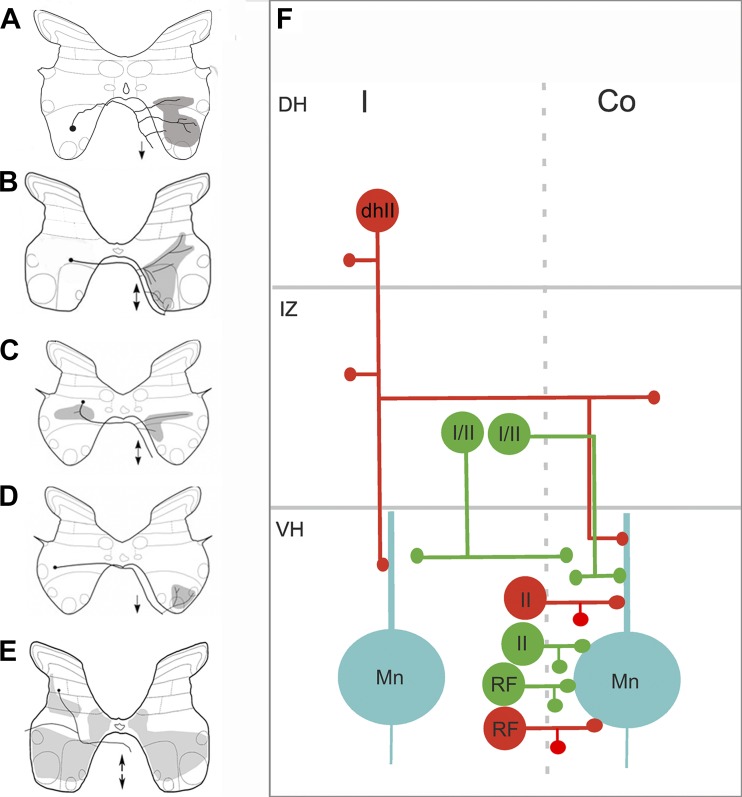
Inhibitory cells in the dorsal horn with bilateral projections are excited monosynaptically by group II muscle and cutaneous afferents (Bannatyne et al. 2006; Edgley and Jankowska 1987a; Edgley and Jankowska 1987b; see also Fig. 4). These cells may also be activated via the corticospinal tract (Davies and Edgley 1994). This population of CINs has cell bodies within the lateral deep dorsal horn (laminae IV–VI) (Bannatyne et al. 2006; Bras et al. 1989; Edgley et al. 2003; Jankowska et al. 2002a; Maxwell et al. 1997) and are exclusively glycinergic. They form extensive bilateral projections within the ipsilateral (IV–IX) and contralateral (VII–IX) gray matter (Bannatyne et al. 2006) and form synaptic contacts with ipsilateral and contralateral motoneurons. They often have axons that bifurcate within the ipsilateral gray matter and form two branches: one that runs in the contralateral ventromedial white matter and one that projects via the ipsilateral ventrolateral white matter. Both ipsi- and contralateral branches frequently formed rostral and caudal projections within these funiculi. They are likely candidates for the mediation of crossed inhibitory reflexes evoked in motoneurons by group II afferents (Aggelopoulos et al. 1996). Projections of the various types of cat midlumbar CINs are shown in Fig. 6.
Fig. 6.
Projections of identified commissural interneurons (CINs) in cat midlumbar segments. A–E: cartoons illustrating typical projections of cells [adapted from Bannatyne et al. 2003, 2006 (© 2006 Society for Neuroscience); 2009; Jankowska et al. 2009]. The black dot represents the location of the cell body; black lines show axonal projections, and shaded gray areas show areas of the gray matter innervated by axon terminals. Double-headed arrows in B, C, and E indicate axons that bifurcate and ascend and descend in the contralateral ventral funiculus. Single-headed arrows in A and D indicate descending axons. A: lamina VIII cell monosynaptically activated by reticulospinal fibers. B: a group II-activated lamina VIII cell. C: a group I/II cell in the intermediate gray matter with bilateral projections. D: a group I/II cell with a contralateral projection. E: a dorsal horn group II activated cell with extensive ipsilateral and contralateral projections. F: a schematic diagram showing these projections. Inhibitory cells are in red and excitatory cells are in green. Co, contralateral; DH, dorsal horn; dhII, dorsal horn group II activated cell; I, ipsilateral; I/II, cells in the intermediate gray activated by group I and II afferent fibers; II, lamina VIII group II activated cell; IZ, intermediate zone; Mn, motoneuron; RF, lamina VIII cell activated by reticulospinal axons; VH, ventral horn.
EMBRYOLOGICAL DERIVATION OF CINS
Netrins are essential chemotropic guidance factors that bind to the DCC (Deleted in Colorectal Cancer) netrin receptor and enable axons to cross the midline of the spinal cord during development (Kennedy et al. 1994). Netrin-1 knockout mice do not form contralateral projections (Serafini et al. 1996) and mice lacking the DCC receptor have disrupted locomotor patterns (Rabe Bernhardt et al. 2012). Thus all CINs, by definition, express the DCC receptor. Classes of CINs can be distinguished according to combinations of transcription factors and there is a relationship between these combinations and neurotransmitter phenotypes and other features such as projection patterns and target cells (see Fig. 5). CINs are derived from both dorsal and ventral progenitor cells (Chédotal 2014; Lu et al. 2015; Vallstedt and Kullander 2013). Cells with axons restricted to the spinal cord are derived from dI5, dI6, V0D, V0V, V0C, and V3 cells (see Fig. 7) whereas those originating from dI1C and dI2 with contralateral axons project to the brain (Table 2).
Fig. 7.
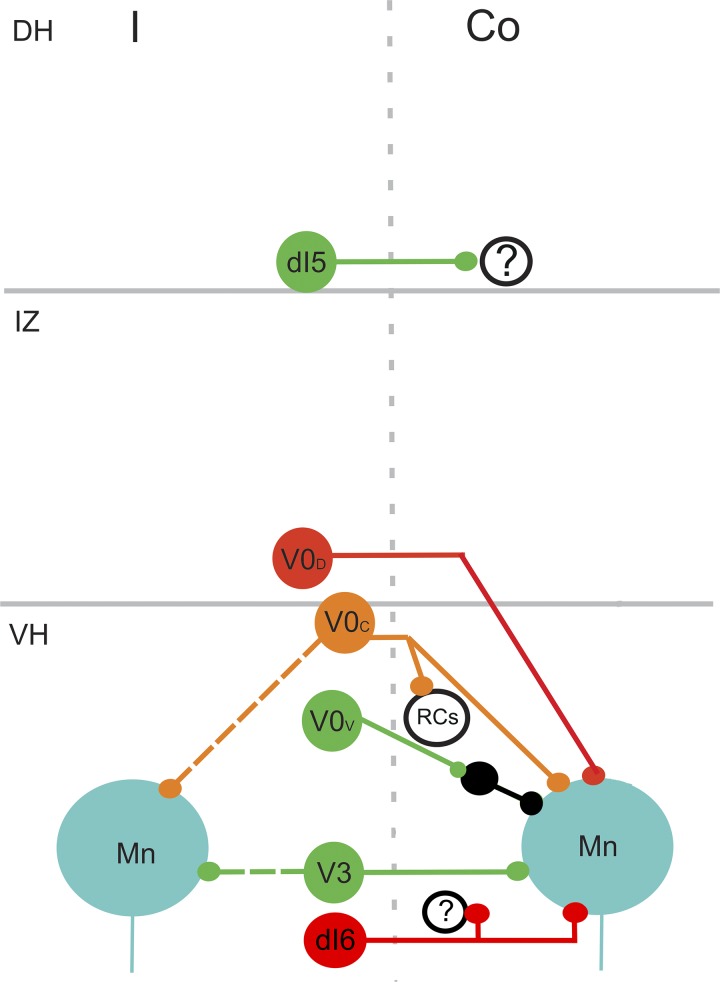
Organization and projections of various classes of commissural interneuron (CIN) according to embryological origin. Glutamatergic dI5 cells settle in the deep dorsal horn (DH) and project to the contralateral dorsal horn. Their target neurons are unknown. V0D cells are inhibitory. They settle within the intermediate gray matter (IZ) or ventral horn (VH) and project to motoneurons (Mn). V0C are cholinergic and found in lamina X and VII close to the midline. Some of them project bilaterally to contralateral and ipsilateral motoneurons and may have additional projections to Renshaw cells (RCs). V0V cells are excitatory and project to motoneurons and Ia inhibitory interneurons (Ia INs). V3 cells are excitatory and have bilateral projections to ipsilateral and contralateral motoneurons. Inhibitory dI6 cells are present in laminae VII and VIII. They project to contralateral motoneurons and additional ventral horn cells. Green, excitatory cells; red, inhibitory cells; orange, cholinergic cells. Co, contralateral; I, ipsilateral. Based on data from Alaynick et al. (2011); Briscoe et al. (1999); Kiehn (2016); Vallstedt and Kullander (2013); Zagoraiou et al. (2009).
Table 2.
Embryological origin of commissural interneurons
| Cell Type | Location | Projection | Transmitter | Transcription Factor | Contralateral Terminations | Action | References |
|---|---|---|---|---|---|---|---|
| dI5 | VI | contralateral | Glutamate | Lmx1b | dorsal horn | ? | Lu et al. (2015); Vallstedt and Kullander (2013) |
| dI6 | VII–VIII | contralateral | GABA/glycine | Dmrt3 | VII–IX, MN | Control of gait | Andersson et al. (2012); Dyck et al. (2012) |
| V0D | VII–VIII | contralateral | GABA/glycine | Dbx1 | VII–IX, MN | Coordination of left–right alternation at low speed | Talpalar et al. (2013) |
| V0V | VII–VIII | contralateral | Glutamate | Dbx1 | VII–VIII | Coordination of left–right alternation at high speed | Talpalar et al. (2013) |
| V0C | VII, X | contralateral/bilateral | Acetylcholine | Dbx1 | IX, VII, MN | Modulation of motoneuron firing frequency | Stepien et al. (2010); Zagoraiou et al. (2009) |
| V3 | VII | contralateral/bilateral | Glutamate | Nkx2.2 | VII–IX, MN | Control of rhythmicity | Briscoe et al. 1999); Zhang et al. (2008) |
Roman numerals refer to laminae of Rexed. MN, contacts on contralateral motoneurons; ?, unknown action.
Of the dorsal groups, dI5 CINs settle deep in the dorsal horn and express lbx1 and Lmx1b transcription factors (Chédotal 2014; Vallstedt and Kullander 2013). They appear to be exclusively glutamatergic (Lu et al. 2015), but at present their function is unknown. The dI6 group express the Dmrt3 transcription factor, which is essential for normal development of the motor network (Andersson et al. 2012; Dyck et al. 2012; Vallstedt and Kullander 2013). Mutations of Dmrt3 produce gait changes in horses: for example, the tölt gait associated with the Icelandic horse, which is a regular ambling gait (Andersson et al. 2012; Vallstedt and Kullander 2013). dI6 cells settle in laminae VII–VIII and are inhibitory (GABA/glycinergic). Their axons ascend and descend for a few segments contralaterally and there is evidence that they contact motoneurons and other ventral horn cells.
Of the ventral groups, V0 cells all express the Dbx1 homeodomain protein (Kiehn 2016; Lanuza et al. 2004; Pierani et al. 2001). Three subtypes of V0 cells have contralateral projections: V0D, V0V, and V0C. V0D form an inhibitory (GABA/glycinergic) group and settle in laminae VII and VIII. They form direct contacts with motoneurons and have an important function in left–right coordination at low speeds (Kiehn 2016; Talpalar et al. 2013). V0V cells are excitatory (glutamatergic) and settle in laminae VII and VIII. Their axons ascend and descend for a few segments contralaterally and make contacts inhibitory premotor interneurons (Kiehn 2016). V0V knockout mice exhibit a hopping gait and these cells are essential for normal left–right alternating locomotor coordination at high speeds (Talpalar et al. 2013). The V0C subgroup of V0 cells settles in medial lamina VII and lamina X. These cells express Pitx2 and are cholinergic (Stepien et al. 2010; Zagoraiou et al. 2009). They form contralateral and bilateral projections. They are the source of the C boutons on motoneurons and modulate motoneuron firing frequency. They have also been shown to form contacts with Renshaw cells (Zagoraiou et al. 2009). The final group of ventral cells that project contralaterally is the V3 group. These cells arise from Nkx2.2-positive p3 progenitors and are mainly excitatory (glutamatergic) (Briscoe et al. 1999). They settle within lamina VIII and some of them project bilaterally. They contact motoneurons and other ventral horn cells and are rhythmically active during fictive locomotion (Briscoe et al. 1999; Zhang et al. 2008).
How Do Studies of Embryological Origin of CINs Relate to the Findings in Adult Cats?
Although it is likely that properties of spinal commissural systems are conserved throughout mammalian species, it is difficult to reconcile some of the differences in findings from studies that use transcription factors with those for the adult cat. For example, the V3 group of cells, which settle in lamina VIII, have very different properties than the lamina VIII cells identified in the cat studies (see diagrams in Figs. 6 and 7). First, they belong to a group of cells that is predominantly excitatory, and, second, they often have bilateral projections. Cat lamina VIII cells are a mixed population of excitatory and inhibitory cells and rarely form bilateral projections. However, like the V3 group (Zhang et al. 2008), they are rhythmically active during fictive locomotion (Matsuyama et al. 2004). Differences may be due to the developmental stages of the animals studied or differences in species. Some of cells, like the V0C cholinergic cells (Kjaerulff and Kiehn 1996), may correspond to types that have not been studied in cats, but the precise reasons for these differences is, at present, not clear.
SPINAL COMMISSURAL SYSTEMS AND MOVEMENT — LOCOMOTION
There are at least three criteria that need to be fulfilled before the commissural system can be ascribed a functional role in locomotion: 1) There should be evidence for a close connectivity with the spinal sensorimotor apparatus, 2) the activity of CINs must be related to the motor pattern, and 3) disruption of CINs should have an impact on motor output.
-
1)
In the previous sections we have already discussed the close connectivity of CINs with both motoneurons as well as with sensory and/or descending inputs (see Table 1). This means that CINs can directly relay sensory feedback or descending commands directly onto the motoneurons they innervate in the contralateral spinal cord.
-
2)
Evidence regarding correlated activity with the locomotor pattern also exists, although mostly in reduced preparations. CINs have been shown to be phase locked to the locomotor cycle in the cat (Matsuyama et al. 2004), the rodent (Butt et al. 2002; Raastad and Kiehn 2000; Zhong et al. 2012), and other vertebrates (Roberts and Sillar 1990; Soffe et al. 1984). Many CINs showed activity that was time locked to various aspects of the locomotor rhythm, although interestingly in some cases this activity was phase locked to contralateral rather than ipsilateral muscle activity patterns. In the cat, evidence for commissural interactions between spinal circuits across the midline exists mostly through the modulation of crossed spinal reflexes during locomotion that survive spinal transection (Forssberg et al. 1977, 1980; Frigon 2017; Frigon and Rossignol 2008; Grillner and Rossignol 1978; Rossignol and Gauthier 1980). Crossed spinal reflexes can also be elicited in humans in the lower limb (Stevenson et al. 2013, 2015b) and can be modulated during walking (Gervasio et al. 2013; Stevenson et al. 2015a). There is also some evidence that they are under cortical influence (Mrachacz-Kersting et al. 2018).
-
3)
The effects of CIN disruption on locomotion have been supported by two main methodologies. The first is disruption of commissural fibers that connect the two halves of the spinal cord. In the isolated neonatal rat spinal cord preparation, midsagittal lesions uncouple left and right central pattern generators, especially if the ventral commissure is disrupted (Cowley and Schmidt 1997; Cowley et al. 2009; Kjaerulff and Kiehn 1996; Kudo and Yamada 1987). In cats, isolation of the lumbar cord (through a combined lateral spinal hemisection and ensuing caudal midline transection) revealed that, although the affected hindlimb was able to recover the ability to locomote, this was uncoupled from the locomotor rhythm of the other limbs (Kato 1990). Similarly, in reduced preparations of nonmammalian vertebrate species such as the lamprey, a midline section of the spinal cord abolishes the bilaterally coherent locomotor rhythm (Buchanan and McPherson 1995).
The second approach is through a specific genetic lesion of commissural neurons. Mouse mutants were created with a genetic ablation of a specific subgroup of spinal interneurons (V0V; section embryological derivation of cins), which includes CINs (Bellardita and Kiehn 2015; Talpalar et al. 2013). The mutant mice were still able to locomote but were unable to unlock the actions of limbs on either side of the body, such that the animals were able to move only with a “hopping” pattern rather than the usual alternation of flexors and extensors on either side.
SPINAL COMMISSURAL SYSTEMS AND MOVEMENT: POSTURE
Postural adjustments are essential during locomotion and it has been known since the days of Sherrington (Sherrington 1910) that spinal animals can stand and display locomotor-like activity. Studies by Rossingnol (Barbeau and Rossignol 1987; Forssberg et al. 1980; Rossignol et al. 2008) and others show that chronically spinalized cats on treadmills can adapt to perturbations of the stance and swing phases of locomotion and thus there are intrinsic mechanisms within the cord that facilitate these adjustments. CINs with group II input will provide the contralateral motor circuitry with information about muscle length on the ipsilateral side and therefore are good candidates for the cells that mediate these postural changes (Jankowska and Edgley 1993). Furthermore, lamina VIII cells with monosynaptic input from the RF and vestibulospinal tract are obvious candidates for the integration of postural and locomotor control in intact animals. CINs in thoracic segments of neonatal mice have input from the lateral vestibular tract and influence the activity of axial motoneurons and are therefore likely to be primarily involved in postural adjustments of the axial musculature (Kasumacic et al. 2010). Thus, integrated mechanisms exist within the spinal cord to coordinate these activities and it is highly probable that the same CINs make contributions to both locomotor and postural motor output patterns.
SPINAL COMMISSURAL SYSTEMS AND MOVEMENT: OBJECT-ORIENTED ACTIONS
In quadrupeds, the forelimbs have a dual role to play. During locomotion, their motor output is comparable to that of the hindlimbs and indeed the locomotor rhythm is coordinated across all four limbs. In many quadrupeds, the forelimbs have an additional role which typically involves manipulative actions targeted toward a given object, typically food. The “secondary” role of obtaining and manipulating food before it is consumed is particularly prominent in many primates, while in humans this has become the primary function of the upper limbs.
As discussed in the previous sections, the role of the CINs for locomotion and posture is well supported. Their role in more object-oriented movements though is far less clear, but fulfilment of the same three criteria discussed in the previous section would provide strong evidence for a role also in manipulative actions. Before considering whether CINS play a role in movements beyond locomotion, it would be first worth to address the role of the spinal cord in general for such actions. It is becoming increasingly evident that spinal circuits can have very specific roles during voluntary movements with the forelimbs that extend beyond fast reflexes, locomotion, and posture. Reaching and grasping have been the most studied movements as they form a big part of our everyday movement repertoire.
In mice, recent work has genetically characterized groups of neurons that are likely to be involved in reaching and grasping. A specific group of spinal cord interneurons (dI3 cells) seem to facilitate integration of cutaneous feedback with the control of grasping actions. A genetic ablation of dI3 cells causes specific deficit in grasping in mice but other movements are more or less unaffected (Bui et al. 2013). A different group of cervical spinal interneurons has also been shown by the Jessel group (Azim et al. 2014) to be important for reaching in mice. Genetic ablation or optogenetic activation of cervical V2a interneurons can disrupt reaching but leave other forelimb actions relatively unaffected. These cells were located in the C3–C4 cervical segments and likely form part of the C3–C4 propriospinal system, a well-established circuit within the upper cervical spinal cord that specifically contributes, in conjunction with supraspinal areas, to reaching movements (Alstermark et al. 2007). This system has been studied extensively in the cat, has been shown to be under descending control from both corticospinal and reticulospinal systems (Alstermark et al. 1984; Illert et al. 1975, 1977, 1981; Illert and Tanaka 1978), and also receives oligosynaptic afferent inputs from the upper limbs (Alstermark et al. 1984). Such a system also exists in the primate cervical cord, although its function seems to be more related to grasping rather than reaching (Kinoshita et al. 2012). Following genetic inactivation, monkeys showed deficits in being able to coordinate the fingers of the hand for the purpose of grasping a food reward, without any obvious deficits in reaching. The C3–C4 propriospinal system has also been studied noninvasively in humans (Pierrot-Deseilligny and Burke 2012), where there are similar indications for a role in reaching to grasp movements (Giboin et al. 2012; Iglesias et al. 2007).
Cervical spinal circuits are likely to have an involvement in activities beyond reaching and grasping. During precision grasping the activity of premotor interneurons in the monkey spinal cord has been shown to be closely related to the activity of muscles involved in grasping both in terms of movement onset as well as force production (Takei and Seki 2013). The same cells were found to have diverse connectivity to intrinsic hand muscles involved in grasping (Takei and Seki 2010). Spinal circuits can contribute to more isolated finger movements; during slow finger movements, primate spinal interneurons may also be involved in higher order functions such as tremor cancellation (Williams et al. 2010), which would improve the accuracy of such manipulative actions. Beyond the execution aspects of movements, spinal interneurons can show set related activity during the delay period tasks, and such activity is not simply a mirror of the activity during the upcoming movement (Prut and Fetz 1999). This preparatory activation is well established for higher motor centers such as motor cortex and premotor areas and is taken to represent various aspects of movement planning. Studies in humans have also found evidence for a contribution of spinal circuits to precision grip (Bunday et al. 2014). Although descending pathways are critical for voluntary and nonlocomotive movements with the upper limb, particularly in the primate, multiple lines of evidence show that spinal circuity is also engaged during such actions.
Commissural interneurons in the cervical spinal cord
Information on commissural systems in the cervical cord is limited but we would predict that they would mirror, at least in part, the lumbar CIN organization for limb movements. This would be most obvious for quadrupeds, but there is even evidence that coordinated motor activity of the arms occurs during bipedal locomotion in humans. It has been known for some time that swaying of the arms is phase locked to the rhythm of the legs and that this is not a passive process (Dietz 2002). This might represent an echo of quadrupedal locomotion in the upper limb, and, if so, CINs would almost certainly play a role in this process. To consider the role of cervical CINs beyond locomotion and posture, we need to apply the criteria mentioned earlier, namely we need to show a close association of CINs with spinal motor activity during voluntary arm movements and an impact on motor function following CINs inactivation. Evidence of direct inputs from, or modulation through, a prominent descending pathway (such as the CST or RST) would also be supportive. However, there is very little direct evidence for the involvement of commissural circuits in the cervical cord, for any type of movement in any mammalian species.
In the cat, work has been carried out in lamina VIII CINs from the upper cervical cord (segments rostral to C4) looking at their potential contribution to vestibulocolic reflexes with neck muscles (Bolton et al. 1991, 1993; Endo et al. 1994). They were shown to receive vestibular information (most likely through the VST) and interestingly some of them were shown to project at least as far down as C5 on the contralateral side, which would contain motoneurons and spinal circuits controlling the proximal forelimb. More recently, we have looked at the descending inputs from the CST and RST systems in the lower cervical cord in the rat (Mitchell et al. 2016) and found that, as in the lumbar cord in the cat, RST inputs were much more prominent than CST.
While there is some anatomical (Molenaar and Kuypers 1978; Rathelot et al. 2017) evidence for the presence of CINs in the lower cervical cord in the primate, the evidence for a role in reaching and grasping is far less clear and indirect. In humans, nonnoxious sensory stimulation in one upper limb rarely elicits responses in muscles in the contralateral arm, unless trains of stimuli are used (Schrafl-Altermatt and Dietz 2016; Thomas et al. 2018; Zehr et al. 2001), which makes localization to the spinal cord less certain. Even so, such crossed responses are modulated with locomotor like or bimanual movements of the upper limb (Dietz and Schrafl-Altermatt 2016; Thomas et al. 2018; Zehr and Kido 2001). Earlier experiments in humans showed that reciprocal inhibition between extensor and flexor muscles in the forearm could be modulated, with a very short delay, by stimulation of sensory afferents in the contralateral wrist (Delwaide and Pepin 1991) or by activity in the contralateral hand (Delwaide et al. 1988); this would likely be mediated through commissural interneurons located in the cervical spinal cord.
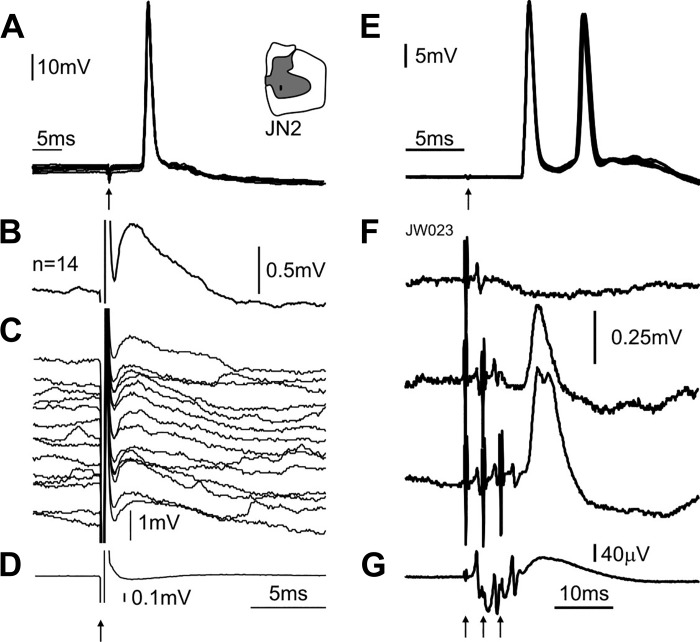
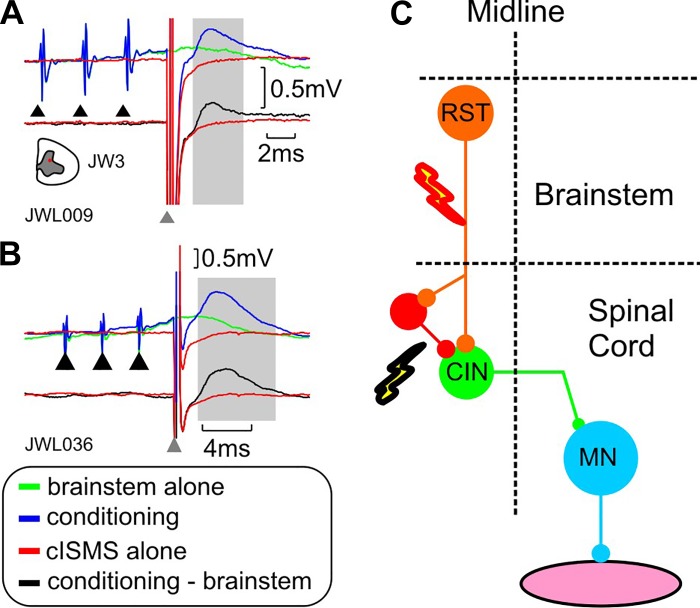
Further evidence for commissural inputs to upper limb motoneurons comes from experiments in the anesthetized primate (Soteropoulos et al. 2013). Weak microstimulation of one side of the lower cervical cord was shown to evoke monosynaptic responses in distal motoneurons on the contralateral side (Fig. 8, A–D). Indeed, in that study, most (>70%) of tested MNs received direct and oligosynaptic inputs from the contralateral spinal cord. In the same experiments, some motoneurons also responded (indirectly) to nonnoxious stimulation of sensory afferents on the contralateral side (Fig. 8, E and F), showing that sensory afferents from one upper limb can impact on motoneurons on the other side (Soteropoulos et al. 2013). The evidence for commissural inputs in distal motoneurons is not inconsistent with a role during locomotion, particularly in a primarily arboreal species such as the macaque monkey.
Fig. 8.
Crossed responses in motoneurons supplying intrinsic hand muscle. A: antidromic activation from ulnar nerve at the wrist. Inset on the right shows location of intraspinal electrode eliciting the crossed response. B: mean intracellular response to contralateral intraspinal microstimulation (100-μA stimulus current). C: single-sweep intracellular potentials that contributed to the average in B. D: averaged cord dorsum potential response recorded simultaneously as B. Arrows indicate time of stimulation. E: same as A, but for a different motoneuron from an intrinsic hand muscle. F: mean intracellular responses to stimulation of the contralateral median nerve at the arm (3× motor threshold). The top trace shows the lack of response to a single shock the middle trace shows the EPSP response to a double shock to the contralateral nerve and the lowest trace shows the response to a triple shock to the contralateral nerve. G: averaged cord dorsum potential response recorded simultaneously as F for triple shock stimulation of the contralateral median nerve. Modified from Soteropoulos et al. (2013).
There is also indirect evidence for descending inputs to the lower cervical CINs in the primate (as shown in the rat). Activation of brainstem descending pathways (mediated through stimulation of the medial longitudinal fasciculus; MLF) can in some cases potentiate crossed responses in cervical MNs (Fig. 9), which suggests that in the primate, as in the rat and cat, the CINs receive inputs from this descending pathway. The RF is associated with posture and locomotion, but recent work has highlighted its role in voluntary reaching and manipulative movements (Buford and Davidson 2004; Soteropoulos et al. 2012) and this might be an avenue for modulation of cervical CINs during such actions.
Fig. 9.
Interaction of brainstem descending pathways with commissural circuits in the primate. A: intracellular records of responses of a motoneuron from the lower cervical cord in a primate. The green trace shows the mean response to stimulation of the brainstem descending pathways alone (train of 3 shocks), the red trace shows the response to stimulation of the contralateral spinal cord (cISMS) while the blue trace shows the response to combined brainstem and cISMS stimulation. The black trace underneath shows the net response to the cISMS after brainstem responses have been subtracted. Note the presence of the excitatory postsynaptic potential (EPSP) to cISMS that was absent to cISMS stimulation alone (red). The black triangles indicate the time of brainstem stimulation and the gray arrow shows the time of spinal stimulation. B: same as A but for a different motoneuron (MN). C: schematic showing the stimulated pathways and circuits that could be mediating the conditioned response. CIN, commissural interneuron; RST, reticulospinal tract. Modified from Soteropoulos et al. (2013).
Previous sections have highlighted the importance of descending pathways on CIN activation, based mostly on cat and rodent experiments. The termination pattern of some descending pathways, such as the vestibulospinal and reticulospinal (RF), is generally well conserved across mammalian species to primates and humans, but for other pathways this is less robust. This is especially true for the corticospinal pathway. Its laminar termination pattern shows major differences between rodents and primates (Armand 1982; Kuypers 1981), with a shift toward an increasingly ventral termination pattern from rodents to cats to primates. In humans and some nonhuman primates, the CST can contact motoneurons directly (Bernhard and Bohm 1954), and these direct connections are thought to underlie the high levels of manual dexterity in these species (Lawrence and Hopkins 1976; Lemon 2019). In adult rodents (Yang and Lemon 2003) and cats (Illert et al. 1976) there is no evidence for such direct connections from cortex to motoneurons. Another example is the rubrospinal pathway. Although a critical motor pathway for reaching and grasping in rodents (Küchler et al. 2002; Morris et al. 2015; Whishaw et al. 1998) and cats (Batson and Amassian 1986; Horn et al. 2002), its relative prominence is decreased in primates (Padel et al. 1981) and particularly in humans (Nathan and Smith 1982). The importance of the CST system in the primate CIN system remains unresolved.
Commissural lesions in the cervical cord.
To the best of our knowledge there are no animal studies that investigated the impact of cervical commissural lesions on movements, other than for locomotion. In humans, however, spinal commissurotomy (sometimes referred to as midline myelotomy) is a procedure that was first established in the earlier part of the 20th century (Putnam 1934), primarily with the aim of offering some relief in patients with intractable pain (most often due to cancer). The rationale was that lesioning of the commissural fibers in the midline would disrupt the transmission of nociceptive signals to the cortex (through the thalamus). The location and extent of the lesions varies substantially from patient to patient and the approach used has evolved over the years (see (Konrad 2014; Tasker and North 1997). It is most often applied in the lower thoracic and lumbar segments to avoid respiratory complications, but some higher cervical myeolotomies are also occasionally carried out. As descending pathways should remain unaffected following the procedure, severe motor deficits are not often reported in the literature. Although we might expect to see some deficits in bimanual actions, these were unlikely to be have been explicitly tested in this patient cohort.
Thus, there is some support for commissural interactions in the upper limb and some tentative evidence that they could play a role during movements other than locomotion, but currently there is no direct evidence to define these contributions.
IMPACT FOR RECOVERY OF FUNCTION AFTER MOTOR DAMAGE
Beyond normal motor function, however, the CIN system has the potential to play an important role following damage to motor systems. An obvious possibility is as a component offering a reroute passage for descending commands (Jankowska et al. 2005a; Jankowska and Edgley 2006; Stecina and Jankowska 2007), in which case the CINS would be an essential component for positive rehabilitation (Cowley et al. 2015). However, CINS could also have a less benevolent role following motor damage. The commissural system might be under tight regularity control from descending systems, as is the case for the C3–C4 propriospinal system mentioned earlier (Alstermark et al. 1984, 1999; Illert and Tanaka 1978). If, following motor damage, this control is much reduced, then CINs might mediate aberrant interactions between the two sides, which could impede rather than support recovery and useful functioning of the hands. Some recent work in stroke patients has shown that crossed reflexes in the lower limb mediated through commissural systems are affected following stroke (Stubbs et al. 2012).
These two roles are not mutually exclusive. Some recent work on the reticulospinal system following CST damage offers a good example of this “Jekyll and Hyde” scenario: following a unilateral CST transection in the primate (Zaaimi et al. 2012), denervated motoneurons in the upper limb showed a strengthening of inputs from the RST and very likely contributed to the impressive recovery the animals were able to exhibit. Animals were still able to carry out everyday tasks such as locomotion, climbing, and power grasping, consistent with previous reports (Lawrence and Kuypers 1968). However, the increased RST influence was uneven across muscle groups; forearm and intrinsic hand muscles received much stronger inputs from the RST than was the case for forearm extensor muscles. Hence the RST system might mediate some functional recovery following stroke, but it might also be the reason for the poor recovery in extensor muscles seen in stroke patients (Baker et al. 2015).
Injuries of most relevance to the commissural system are stroke and spinal cord injury (SCI). Although the etiology and neurological signs are different, a common symptom in both is a deficit in motor control of the upper limbs, with residual muscle weakness, particularly for distal muscles. This is likely to arise, at least in part, because of the disruption of CST input to spinal circuits and MNs. Although motor deficits are most often lateralized, there is evidence for aberrant bilateral interactions. In stroke patients, although one hemisphere is typically undamaged, it has been known for some time that even the “unaffected” upper limb shows some manual deficits (Desrosiers et al. 1996; Jones et al. 1989), with some of these deficits being amplified during bimanual actions (e.g., Lewis and Byblow 2004). This is most often attributed to an imbalanced interaction between lesioned and unlesioned cortical hemispheres and affected corticospinal tracts, e.g., Buetefisch 2015; Schrafl-Altermatt and Dietz 2016), but bilateral spinal contributions are less often considered. Similarly, in SCI patients, the performance of the “least affected” arm is hindered during bimanual actions with the more affected arm (Calabro and Perez 2016), but the focus is most often on cortical contributions (Scharfenberger et al. 2018). Whether the aberrant interactions during bimanual movements is related to the fact that interlimb reflexes become much easier to evoke after SCI (Calancie et al. 1996) is not clear and the actual impact of CINs after motor damage remains to be elucidated.
FINAL THOUGHTS
In this review, we have tried to cover what is known about the organization of commissural systems in the mammalian spinal cord. Commissural circuits are formed from groups of interneurons with diverse properties and there is diversity even within the groups that we can define. They should be thought of as multifunctional cells that form critical components of spinal circuitry, pivotal for the bilateral organization of locomotion and posture. However, their possible roles in goal-orientated movements remain an open question. Some outstanding questions about the properties and functions of CINs are outlined below:
-
1)
How do we assemble the fragmentary information available to produce a functionally meaningful classification of CINs?
-
2)
As the spinal commissural system is phylogenetically old, how well it is conserved across species, particularly with respect to primates?
-
3)
What role does the cervical commissural system, if any, play during object-oriented actions such as reaching and grasping? Is this role specific, or is it a function of the same CINs that contribute to locomotion and posture?
-
4)
What is the relationship between the cervical CIN system and the corticospinal tract, particularly in the primate, given the importance of the CST for manipulative movements? Does the CST provide direct inputs to CINs in primates?
-
5)
Following stroke or spinal cord, injury recovery is dictated by the surviving circuitry. What, if any, is the contribution of CINs to the recovery process?
GRANTS
D. J. Maxwell and D. S. Soteropoulos are supported by a joint Biotechnology and Biological Sciences Research Council project grant (BB/P019897/1 and BB/P019757/1, respectively).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
D.J.M. and D.S.S. prepared figures; D.J.M. and D.S.S. drafted manuscript; D.J.M. and D.S.S. edited and revised manuscript; D.J.M. and D.S.S. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Elzbieta Jankowska, Roger Lemon, and Karen Fisher for constructive feedback. We also thank Anne Bannatyne and Emine Bagdatlioglu for helpful discussions during the writing of this article.
REFERENCES
- Aggelopoulos NC, Burton MJ, Clarke RW, Edgley SA. Characterization of a descending system that enables crossed group II inhibitory reflex pathways in the cat spinal cord. J Neurosci 16: 723–729, 1996. doi: 10.1523/JNEUROSCI.16-02-00723.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alaynick WA, Jessell TM, Pfaff SL. SnapShot: spinal cord development. Cell 146: 178–178.e1, 2011. doi: 10.1016/j.cell.2011.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alstermark B, Isa T, Ohki Y, Saito Y. Disynaptic pyramidal excitation in forelimb motoneurons mediated via C3–C4 propriospinal neurons in the Macaca fuscata. J Neurophysiol 82: 3580–3585, 1999. doi: 10.1152/jn.1999.82.6.3580. [DOI] [PubMed] [Google Scholar]
- Alstermark B, Isa T, Pettersson LG, Sasaki S. The C3-C4 propriospinal system in the cat and monkey: a spinal pre-motoneuronal centre for voluntary motor control. Acta Physiol (Oxf) 189: 123–140, 2007. doi: 10.1111/j.1748-1716.2006.01655.x. [DOI] [PubMed] [Google Scholar]
- Alstermark B, Kümmel H. Transneuronal transport of wheat germ agglutinin conjugated horseradish peroxidase into last order spinal interneurones projecting to acromio- and spinodeltoideus motoneurones in the cat. 1. Location of labelled interneurones and influence of synaptic activity on the transneuronal transport. Exp Brain Res 80: 83–95, 1990. doi: 10.1007/BF00228850. [DOI] [PubMed] [Google Scholar]
- Alstermark B, Lundberg A, Sasaki S. Integration in descending motor pathways controlling the forelimb in the cat. 11. Inhibitory pathways from higher motor centres and forelimb afferents to C3-C4 propriospinal neurones. Exp Brain Res 56: 293–307, 1984. doi: 10.1007/BF00236285. [DOI] [PubMed] [Google Scholar]
- Andersson LS, Larhammar M, Memic F, Wootz H, Schwochow D, Rubin C-J, Patra K, Arnason T, Wellbring L, Hjälm G, Imsland F, Petersen JL, McCue ME, Mickelson JR, Cothran G, Ahituv N, Roepstorff L, Mikko S, Vallstedt A, Lindgren G, Andersson L, Kullander K. Mutations in DMRT3 affect locomotion in horses and spinal circuit function in mice. Nature 488: 642–646, 2012. doi: 10.1038/nature11399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armand J. The origin, course and terminations of corticospinal fibers in various mammals. Prog Brain Res 57: 329–360, 1982. doi: 10.1016/S0079-6123(08)64136-9. [DOI] [PubMed] [Google Scholar]
- Arya T, Bajwa S, Edgley SA. Crossed reflex actions from group II muscle afferents in the lumbar spinal cord of the anaesthetized cat. J Physiol 444: 117–131, 1991. doi: 10.1113/jphysiol.1991.sp018869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azim E, Jiang J, Alstermark B, Jessell TM. Skilled reaching relies on a V2a propriospinal internal copy circuit. Nature 508: 357–363, 2014. doi: 10.1038/nature13021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajwa S, Edgley SA, Harrison PJ. Crossed actions on group II-activated interneurones in the midlumbar segments of the cat spinal cord. J Physiol 445: 205–217, 1992. doi: 10.1113/jphysiol.1992.sp019297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker SN, Zaaimi B, Fisher KM, Edgley SA, Soteropoulos DS. Pathways mediating functional recovery. Prog Brain Res 218: 389–412, 2015. doi: 10.1016/bs.pbr.2014.12.010. [DOI] [PubMed] [Google Scholar]
- Bannatyne BA, Edgley SA, Hammar I, Jankowska E, Maxwell DJ. Networks of inhibitory and excitatory commissural interneurons mediating crossed reticulospinal actions. Eur J Neurosci 18: 2273–2284, 2003. doi: 10.1046/j.1460-9568.2003.02973.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannatyne BA, Edgley SA, Hammar I, Jankowska E, Maxwell DJ. Differential projections of excitatory and inhibitory dorsal horn interneurons relaying information from group II muscle afferents in the cat spinal cord. J Neurosci 26: 2871–2880, 2006. doi: 10.1523/JNEUROSCI.5172-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannatyne BA, Liu TT, Hammar I, Stecina K, Jankowska E, Maxwell DJ. Excitatory and inhibitory intermediate zone interneurons in pathways from feline group I and II afferents: differences in axonal projections and input. J Physiol 587: 379–399, 2009. doi: 10.1113/jphysiol.2008.159129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbeau H, Rossignol S. Recovery of locomotion after chronic spinalization in the adult cat. Brain Res 412: 84–95, 1987. doi: 10.1016/0006-8993(87)91442-9. [DOI] [PubMed] [Google Scholar]
- Batson DE, Amassian VE. A dynamic role of rubral neurons in contact placing by the adult cat. J Neurophysiol 56: 835–856, 1986. doi: 10.1152/jn.1986.56.3.835. [DOI] [PubMed] [Google Scholar]
- Batton RR III, Jayaraman A, Ruggiero D, Carpenter MB. Fastigial efferent projections in the monkey: an autoradiographic study. J Comp Neurol 174: 281–305, 1977. doi: 10.1002/cne.901740206. [DOI] [PubMed] [Google Scholar]
- Bellardita C, Kiehn O. Phenotypic characterization of speed-associated gait changes in mice reveals modular organization of locomotor networks. Curr Biol 25: 1426–1436, 2015. doi: 10.1016/j.cub.2015.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg EM, Björnfors ER, Pallucchi I, Picton LD, El Manira A. Principles governing locomotion in vertebrates: lessons from zebrafish. Front Neural Circuits 12: 73, 2018. doi: 10.3389/fncir.2018.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkowitz A, Roberts A, Soffe SR. Roles for multifunctional and specialized spinal interneurons during motor pattern generation in tadpoles, zebrafish larvae, and turtles. Front Behav Neurosci 4: 36, 2010. doi: 10.3389/fnbeh.2010.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhard CG, Bohm E. Cortical representation and functional significance of the corticomotoneuronal system. AMA Arch Neurol Psychiatry 72: 473–502, 1954. doi: 10.1001/archneurpsyc.1954.02330040075006. [DOI] [PubMed] [Google Scholar]
- Birinyi A, Viszokay K, Wéber I, Kiehn O, Antal M. Synaptic targets of commissural interneurons in the lumbar spinal cord of neonatal rats. J Comp Neurol 461: 429–440, 2003. doi: 10.1002/cne.10696. [DOI] [PubMed] [Google Scholar]
- Bolton PS, Goto T, Wilson VJ. Commissural neurons in the cat upper cervical spinal cord. Neuroreport 2: 743–746, 1991. doi: 10.1097/00001756-199112000-00003. [DOI] [PubMed] [Google Scholar]
- Bolton PS, Goto T, Wilson VJ. Horizontal canal input to upper cervical commissural neurons. Exp Brain Res 92: 549–552, 1993. doi: 10.1007/BF00229046. [DOI] [PubMed] [Google Scholar]
- Bras H, Cavallari P, Jankowska E. Demonstration of initial axon collaterals of cells of origin of the ventral spinocerebellar tract in the cat. J Comp Neurol 273: 584–592, 1988. doi: 10.1002/cne.902730412. [DOI] [PubMed] [Google Scholar]
- Bras H, Cavallari P, Jankowska E, Kubin L. Morphology of midlumbar interneurones relaying information from group II muscle afferents in the cat spinal cord. J Comp Neurol 290: 1–15, 1989. doi: 10.1002/cne.902900102. [DOI] [PubMed] [Google Scholar]
- Briscoe J, Sussel L, Serup P, Hartigan-O’Connor D, Jessell TM, Rubenstein JLR, Ericson J. Homeobox gene Nkx2.2 and specification of neuronal identity by graded Sonic hedgehog signalling. Nature 398: 622–627, 1999. doi: 10.1038/19315. [DOI] [PubMed] [Google Scholar]
- Brockett EG, Seenan PG, Bannatyne BA, Maxwell DJ. Ascending and descending propriospinal pathways between lumbar and cervical segments in the rat: evidence for a substantial ascending excitatory pathway. Neuroscience 240: 83–97, 2013. doi: 10.1016/j.neuroscience.2013.02.039. [DOI] [PubMed] [Google Scholar]
- Brown TG. The intrinsic factors in the act of progression in the mammal. Proc R Soc Biol Sci 84: 308–319, 1911. [Google Scholar]
- Buchanan JT, McPherson DR. The neuronal network for locomotion in the lamprey spinal cord: evidence for the involvement of commissural interneurons. J Physiol Paris 89: 221–233, 1995. doi: 10.1016/0928-4257(96)83638-2. [DOI] [PubMed] [Google Scholar]
- Buetefisch CM. Role of the contralesional hemisphere in post-stroke recovery of upper extremity motor function. Front Neurol 6: 214, 2015. doi: 10.3389/fneur.2015.00214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buford JA, Davidson AG. Movement-related and preparatory activity in the reticulospinal system of the monkey. Exp Brain Res 159: 284–300, 2004. doi: 10.1007/s00221-004-1956-4. [DOI] [PubMed] [Google Scholar]
- Bui TV, Akay T, Loubani O, Hnasko TS, Jessell TM, Brownstone RM. Circuits for grasping: spinal dI3 interneurons mediate cutaneous control of motor behavior. Neuron 78: 191–204, 2013. doi: 10.1016/j.neuron.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunday KL, Tazoe T, Rothwell JC, Perez MA. Subcortical control of precision grip after human spinal cord injury. J Neurosci 34: 7341–7350, 2014. doi: 10.1523/JNEUROSCI.0390-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butt SJ, Harris-Warrick RM, Kiehn O. Firing properties of identified interneuron populations in the mammalian hindlimb central pattern generator. J Neurosci 22: 9961–9971, 2002. doi: 10.1523/JNEUROSCI.22-22-09961.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butt SJ, Kiehn O. Functional identification of interneurons responsible for left-right coordination of hindlimbs in mammals. Neuron 38: 953–963, 2003. doi: 10.1016/S0896-6273(03)00353-2. [DOI] [PubMed] [Google Scholar]
- Cabaj A, Stecina K, Jankowska E. Same spinal interneurons mediate reflex actions of group Ib and group II afferents and crossed reticulospinal actions. J Neurophysiol 95: 3911–3922, 2006. doi: 10.1152/jn.01262.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabro FJ, Perez MA. Bilateral reach-to-grasp movement asymmetries after human spinal cord injury. J Neurophysiol 115: 157–167, 2016. doi: 10.1152/jn.00692.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calancie B, Lutton S, Broton JG. Central nervous system plasticity after spinal cord injury in man: interlimb reflexes and the influence of cutaneous stimulation. Electroencephalogr Clin Neurophysiol 101: 304–315, 1996. doi: 10.1016/0924-980X(96)95194-2. [DOI] [PubMed] [Google Scholar]
- Carleton SC, Carpenter MB. Afferent and efferent connections of the medial, inferior and lateral vestibular nuclei in the cat and monkey. Brain Res 278: 29–51, 1983. doi: 10.1016/0006-8993(83)90223-8. [DOI] [PubMed] [Google Scholar]
- Cavallari P, Edgley SA, Jankowska E. Post-synaptic actions of midlumbar interneurones on motoneurones of hind-limb muscles in the cat. J Physiol 389: 675–699, 1987. doi: 10.1113/jphysiol.1987.sp016677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chédotal A. Development and plasticity of commissural circuits: from locomotion to brain repair. Trends Neurosci 37: 551–562, 2014. doi: 10.1016/j.tins.2014.08.009. [DOI] [PubMed] [Google Scholar]
- Coulon P, Bras H, Vinay L. Characterization of last-order premotor interneurons by transneuronal tracing with rabies virus in the neonatal mouse spinal cord. J Comp Neurol 519: 3470–3487, 2011. doi: 10.1002/cne.22717. [DOI] [PubMed] [Google Scholar]
- Cowley KC, MacNeil BJ, Chopek JW, Sutherland S, Schmidt BJ. Neurochemical excitation of thoracic propriospinal neurons improves hindlimb stepping in adult rats with spinal cord lesions. Exp Neurol 264: 174–187, 2015. doi: 10.1016/j.expneurol.2014.12.006. [DOI] [PubMed] [Google Scholar]
- Cowley KC, Schmidt BJ. Effects of inhibitory amino acid antagonists on reciprocal inhibitory interactions during rhythmic motor activity in the in vitro neonatal rat spinal cord. J Neurophysiol 74: 1109–1117, 1995. doi: 10.1152/jn.1995.74.3.1109. [DOI] [PubMed] [Google Scholar]
- Cowley KC, Schmidt BJ. Regional distribution of the locomotor pattern-generating network in the neonatal rat spinal cord. J Neurophysiol 77: 247–259, 1997. doi: 10.1152/jn.1997.77.1.247. [DOI] [PubMed] [Google Scholar]
- Cowley KC, Zaporozhets E, Joundi RA, Schmidt BJ. Contribution of commissural projections to bulbospinal activation of locomotion in the in vitro neonatal rat spinal cord. J Neurophysiol 101: 1171–1178, 2009. doi: 10.1152/jn.91212.2008. [DOI] [PubMed] [Google Scholar]
- Davies HE, Edgley SA. Inputs to group II-activated midlumbar interneurones from descending motor pathways in the cat. J Physiol 479: 463–473, 1994. doi: 10.1113/jphysiol.1994.sp020310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delwaide PJ, Pepin JL. The influence of contralateral primary afferents on Ia inhibitory interneurones in humans. J Physiol 439: 161–179, 1991. doi: 10.1113/jphysiol.1991.sp018662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delwaide PJ, Sabatino M, Pepin JL, La Grutta V. Reinforcement of reciprocal inhibition by contralateral movements in man. Exp Neurol 99: 10–16, 1988. doi: 10.1016/0014-4886(88)90122-7. [DOI] [PubMed] [Google Scholar]
- Desrosiers J, Bourbonnais D, Bravo G, Roy PM, Guay M. Performance of the ‘unaffected’ upper extremity of elderly stroke patients. Stroke 27: 1564–1570, 1996. doi: 10.1161/01.STR.27.9.1564. [DOI] [PubMed] [Google Scholar]
- Dietz V. Do human bipeds use quadrupedal coordination? Trends Neurosci 25: 462–467, 2002. doi: 10.1016/S0166-2236(02)02229-4. [DOI] [PubMed] [Google Scholar]
- Dietz V, Schrafl-Altermatt M. Control of functional movements in healthy and post-stroke subjects: Role of neural interlimb coupling. Clin Neurophysiol 127: 2286–2293, 2016. doi: 10.1016/j.clinph.2016.02.014. [DOI] [PubMed] [Google Scholar]
- Dougherty KJ, Bannatyne BA, Jankowska E, Krutki P, Maxwell DJ. Membrane receptors involved in modulation of responses of spinal dorsal horn interneurons evoked by feline group II muscle afferents. J Neurosci 25: 584–593, 2005. doi: 10.1523/JNEUROSCI.3797-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutton RC, Carstens MI, Antognini JF, Carstens E. Long ascending propriospinal projections from lumbosacral to upper cervical spinal cord in the rat. Brain Res 1119: 76–85, 2006. doi: 10.1016/j.brainres.2006.08.063. [DOI] [PubMed] [Google Scholar]
- Dyck J, Lanuza GM, Gosgnach S. Functional characterization of dI6 interneurons in the neonatal mouse spinal cord. J Neurophysiol 107: 3256–3266, 2012. doi: 10.1152/jn.01132.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgley SA, Jankowska E. Field potentials generated by group II muscle afferents in the middle lumbar segments of the cat spinal cord. J Physiol 385: 393–413, 1987a. doi: 10.1113/jphysiol.1987.sp016498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgley SA, Jankowska E. An interneuronal relay for group I and II muscle afferents in the midlumbar segments of the cat spinal cord. J Physiol 389: 647–674, 1987b. doi: 10.1113/jphysiol.1987.sp016676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgley SA, Jankowska E, Hammar I. Ipsilateral actions of feline corticospinal tract neurons on limb motoneurons. J Neurosci 24: 7804–7813, 2004. doi: 10.1523/JNEUROSCI.1941-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgley SA, Jankowska E, Krutki P, Hammar I. Both dorsal horn and lamina VIII interneurones contribute to crossed reflexes from feline group II muscle afferents. J Physiol 552: 961–974, 2003. doi: 10.1113/jphysiol.2003.048009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards SB. The commissural projection of the superior colliculus in the cat. J Comp Neurol 173: 23–40, 1977. doi: 10.1002/cne.901730103. [DOI] [PubMed] [Google Scholar]
- Eide AL, Glover J, Kjaerulff O, Kiehn O. Characterization of commissural interneurons in the lumbar region of the neonatal rat spinal cord. J Comp Neurol 403: 332–345, 1999. doi:. [DOI] [PubMed] [Google Scholar]
- Endo K, Kasper J, Wilson VJ, Yates BJ. Response of commissural and other upper cervical ventral horn neurons to vestibular stimuli in vertical planes. J Neurophysiol 71: 11–16, 1994. doi: 10.1152/jn.1994.71.1.11. [DOI] [PubMed] [Google Scholar]
- English AW, Tigges J, Lennard PR. Anatomical organization of long ascending propriospinal neurons in the cat spinal cord. J Comp Neurol 240: 349–358, 1985. doi: 10.1002/cne.902400403. [DOI] [PubMed] [Google Scholar]
- Flynn JR, Conn VL, Boyle KA, Hughes DI, Watanabe M, Velasquez T, Goulding MD, Callister RJ, Graham BA. Anatomical and molecular properties of long descending propriospinal neurons in mice. Front Neuroanat 11: 5, 2017. doi: 10.3389/fnana.2017.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forssberg H, Grillner S, Halbertsma J, Rossignol S. The locomotion of the low spinal cat. II. Interlimb coordination. Acta Physiol Scand 108: 283–295, 1980. doi: 10.1111/j.1748-1716.1980.tb06534.x. [DOI] [PubMed] [Google Scholar]
- Forssberg H, Grillner S, Rossignol S. Phasic gain control of reflexes from the dorsum of the paw during spinal locomotion. Brain Res 132: 121–139, 1977. doi: 10.1016/0006-8993(77)90710-7. [DOI] [PubMed] [Google Scholar]
- Frigon A. The neural control of interlimb coordination during mammalian locomotion. J Neurophysiol 117: 2224–2241, 2017. doi: 10.1152/jn.00978.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frigon A, Rossignol S. Short-latency crossed inhibitory responses in extensor muscles during locomotion in the cat. J Neurophysiol 99: 989–998, 2008. doi: 10.1152/jn.01274.2007. [DOI] [PubMed] [Google Scholar]
- Gervasio S, Farina D, Sinkjær T, Mrachacz-Kersting N. Crossed reflex reversal during human locomotion. J Neurophysiol 109: 2335–2344, 2013. doi: 10.1152/jn.01086.2012. [DOI] [PubMed] [Google Scholar]
- Giboin LS, Lackmy-Vallée A, Burke D, Marchand-Pauvert V. Enhanced propriospinal excitation from hand muscles to wrist flexors during reach-to-grasp in humans. J Neurophysiol 107: 532–543, 2012. doi: 10.1152/jn.00774.2011. [DOI] [PubMed] [Google Scholar]
- Goulding M. Circuits controlling vertebrate locomotion: moving in a new direction. Nat Rev Neurosci 10: 507–518, 2009. doi: 10.1038/nrn2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillner S, Jessell TM. Measured motion: searching for simplicity in spinal locomotor networks. Curr Opin Neurobiol 19: 572–586, 2009. doi: 10.1016/j.conb.2009.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillner S, Rossignol S. Contralateral reflex reversal controlled by limb position in the acute spinal cat injected with clonidine i.v. Brain Res 144: 411–414, 1978. doi: 10.1016/0006-8993(78)90169-5. [DOI] [PubMed] [Google Scholar]
- Grillner S, Zangger P. On the central generation of locomotion in the low spinal cat. Exp Brain Res 34: 241–261, 1979. doi: 10.1007/BF00235671. [DOI] [PubMed] [Google Scholar]
- Hammar I, Bannatyne BA, Maxwell DJ, Edgley SA, Jankowska E. The actions of monoamines and distribution of noradrenergic and serotoninergic contacts on different subpopulations of commissural interneurons in the cat spinal cord. Eur J Neurosci 19: 1305–1316, 2004. doi: 10.1111/j.1460-9568.2004.03239.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison PJ, Jankowska E. Organization of input to the interneurones mediating group I non-reciprocal inhibition of motoneurones in the cat. J Physiol 361: 403–418, 1985a. doi: 10.1113/jphysiol.1985.sp015652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison PJ, Jankowska E. Sources of input to interneurones mediating group I non-reciprocal inhibition of motoneurones in the cat. J Physiol 361: 379–401, 1985b. doi: 10.1113/jphysiol.1985.sp015651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison PJ, Jankowska E, Zytnicki D. Lamina VIII interneurones interposed in crossed reflex pathways in the cat. J Physiol 371: 147–166, 1986. doi: 10.1113/jphysiol.1986.sp015965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover JE, Durkovic RG. Retrograde labeling of lumbosacral interneurons following injections of red and green fluorescent microspheres into hindlimb motor nuclei of the cat. Somatosens Mot Res 9: 211–226, 1992. doi: 10.3109/08990229209144772. [DOI] [PubMed] [Google Scholar]
- Horn KM, Pong M, Batni SR, Levy SM, Gibson AR. Functional specialization within the cat red nucleus. J Neurophysiol 87: 469–477, 2002. doi: 10.1152/jn.00949.2000. [DOI] [PubMed] [Google Scholar]
- Iglesias C, Marchand-Pauvert V, Lourenco G, Burke D, Pierrot-Deseilligny E. Task-related changes in propriospinal excitation from hand muscles to human flexor carpi radialis motoneurones. J Physiol 582: 1361–1379, 2007. doi: 10.1113/jphysiol.2007.133199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illert M, Jankowska E, Lundberg A, Odutola A. Integration in descending motor pathways controlling the forelimb in the cat. 7. Effects from the reticular formation on C3-C4 propriospinal neurones. Exp Brain Res 42: 269–281, 1981. [DOI] [PubMed] [Google Scholar]
- Illert M, Lundberg A, Padel Y, Tanaka R. Convergence on propriospinal neurones which may mediate disynaptic corticospinal excitation to forelimb motoneurones in the cat. Brain Res 93: 530–534, 1975. doi: 10.1016/0006-8993(75)90194-8. [DOI] [PubMed] [Google Scholar]
- Illert M, Lundberg A, Tanaka R. Integration in descending motor pathways controlling the forelimb in the cat. 1. Pyramidal effects on motoneurones. Exp Brain Res 26: 509–519, 1976. doi: 10.1007/BF00238824. [DOI] [PubMed] [Google Scholar]
- Illert M, Lundberg A, Tanaka R. Integration in descending motor pathways controlling the forelimb in the cat. 3. Convergence on propriospinal neurones transmitting disynaptic excitation from the corticospinal tract and other descending tracts. Exp Brain Res 29: 323–346, 1977. doi: 10.1007/bf00236174. [DOI] [PubMed] [Google Scholar]
- Illert M, Tanaka R. Integration in descending motor pathways controlling the forelimb in the cat. 4. Corticospinal inhibition of forelimb motoneurones mediated by short propriospinal neurones. Exp Brain Res 31: 131–141, 1978. doi: 10.1007/BF00235810. [DOI] [PubMed] [Google Scholar]
- Jankowska E. Spinal interneuronal systems: identification, multifunctional character and reconfigurations in mammals. J Physiol 533: 31–40, 2001. doi: 10.1111/j.1469-7793.2001.0031b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E. Spinal interneuronal networks in the cat: elementary components. Brain Res Brain Res Rev 57: 46–55, 2008. doi: 10.1016/j.brainresrev.2007.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E, Bannatyne BA, Stecina K, Hammar I, Cabaj A, Maxwell DJ. Commissural interneurons with input from group I and II muscle afferents in feline lumbar segments: neurotransmitters, projections and target cells. J Physiol 587: 401–418, 2009. doi: 10.1113/jphysiol.2008.159236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E, Cabaj A, Pettersson LG. How to enhance ipsilateral actions of pyramidal tract neurons. J Neurosci 25: 7401–7405, 2005a. doi: 10.1523/JNEUROSCI.1838-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E, Edgley S. Interactions between pathways controlling posture and gait at the level of spinal interneurones in the cat. Prog Brain Res 97: 161–171, 1993. doi: 10.1016/S0079-6123(08)62274-8. [DOI] [PubMed] [Google Scholar]
- Jankowska E, Edgley SA. How can corticospinal tract neurons contribute to ipsilateral movements? A question with implications for recovery of motor functions. Neuroscientist 12: 67–79, 2006. doi: 10.1177/1073858405283392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E, Edgley SA. Functional subdivision of feline spinal interneurons in reflex pathways from group Ib and II muscle afferents; an update. Eur J Neurosci 32: 881–893, 2010. doi: 10.1111/j.1460-9568.2010.07354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E, Edgley SA, Krutki P, Hammar I. Functional differentiation and organization of feline midlumbar commissural interneurones. J Physiol 565: 645–658, 2005b. doi: 10.1113/jphysiol.2005.083014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E, Hammar I, Slawinska U, Maleszak K, Edgley SA. Neuronal basis of crossed actions from the reticular formation on feline hindlimb motoneurons. J Neurosci 23: 1867–1878, 2003. doi: 10.1523/JNEUROSCI.23-05-01867.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E, Jukes MGM, Lund S, Lundberg A. The effect of DOPA on the spinal cord. 6. Half-centre organization of interneurones transmitting effects from the flexor reflex afferents. Acta Physiol Scand 70: 389–402, 1967. doi: 10.1111/j.1748-1716.1967.tb03637.x. [DOI] [PubMed] [Google Scholar]
- Jankowska E, Krutki P, Matsuyama K. Relative contribution of Ia inhibitory interneurones to inhibition of feline contralateral motoneurones evoked via commissural interneurones. J Physiol 568: 617–628, 2005c. doi: 10.1113/jphysiol.2005.088351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E, Slawinska U, Hammar I. Differential presynaptic inhibition of actions of group II afferents in di- and polysynaptic pathways to feline motoneurones. J Physiol 542: 287–299, 2002a. doi: 10.1113/jphysiol.2001.014068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E, Slawinska U, Hammar I. On organization of a neuronal network in pathways from group II muscle afferents in feline lumbar spinal segments. J Physiol 542: 301–314, 2002b. doi: 10.1113/jphysiol.2001.014076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E, Stecina K, Cabaj A, Pettersson LG, Edgley SA. Neuronal relays in double crossed pathways between feline motor cortex and ipsilateral hindlimb motoneurones. J Physiol 575: 527–541, 2006. doi: 10.1113/jphysiol.2006.112425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones RD, Donaldson IM, Parkin PJ. Impairment and recovery of ipsilateral sensory-motor function following unilateral cerebral infarction. Brain 112: 113–132, 1989. doi: 10.1093/brain/112.1.113. [DOI] [PubMed] [Google Scholar]
- Kaprielian Z, Runko E, Imondi R. Axon guidance at the midline choice point. Dev Dyn 221: 154–181, 2001. doi: 10.1002/dvdy.1143. [DOI] [PubMed] [Google Scholar]
- Karol EA, Pandya DN. The distribution of the corpus callosum in the Rhesus monkey. Brain 94: 471–486, 1971. doi: 10.1093/brain/94.3.471. [DOI] [PubMed] [Google Scholar]
- Kasumacic N, Glover JC, Perreault MC. Segmental patterns of vestibular-mediated synaptic inputs to axial and limb motoneurons in the neonatal mouse assessed by optical recording. J Physiol 588: 4905–4925, 2010. doi: 10.1113/jphysiol.2010.195644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasumacic N, Lambert FM, Coulon P, Bras H, Vinay L, Perreault MC, Glover JC. Segmental organization of vestibulospinal inputs to spinal interneurons mediating crossed activation of thoracolumbar motoneurons in the neonatal mouse. J Neurosci 35: 8158–8169, 2015. doi: 10.1523/JNEUROSCI.5188-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato M. Chronically isolated lumbar half spinal cord generates locomotor activities in the ipsilateral hindlimb of the cat. Neurosci Res 9: 22–34, 1990. doi: 10.1016/0168-0102(90)90042-D. [DOI] [PubMed] [Google Scholar]
- Kennedy TE, Serafini T, de la Torre JR, Tessier-Lavigne M. Netrins are diffusible chemotropic factors for commissural axons in the embryonic spinal cord. Cell 78: 425–435, 1994. doi: 10.1016/0092-8674(94)90421-9. [DOI] [PubMed] [Google Scholar]
- Kiehn O. Decoding the organization of spinal circuits that control locomotion. Nat Rev Neurosci 17: 224–238, 2016. doi: 10.1038/nrn.2016.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita M, Matsui R, Kato S, Hasegawa T, Kasahara H, Isa K, Watakabe A, Yamamori T, Nishimura Y, Alstermark B, Watanabe D, Kobayashi K, Isa T. Genetic dissection of the circuit for hand dexterity in primates. Nature 487: 235–238, 2012. doi: 10.1038/nature11206. [DOI] [PubMed] [Google Scholar]
- Kirkwood PA, Munson JB, Sears TA, Westgaard RH. Respiratory interneurones in the thoracic spinal cord of the cat. J Physiol 395: 161–192, 1988. doi: 10.1113/jphysiol.1988.sp016913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjaerulff O, Kiehn O. Distribution of networks generating and coordinating locomotor activity in the neonatal rat spinal cord in vitro: a lesion study. J Neurosci 16: 5777–5794, 1996. doi: 10.1523/JNEUROSCI.16-18-05777.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konrad P. Dorsal root entry zone lesion, midline myelotomy and anterolateral cordotomy. Neurosurg Clin N Am 25: 699–722, 2014. doi: 10.1016/j.nec.2014.07.010. [DOI] [PubMed] [Google Scholar]
- Krutki P, Jankowska E, Edgley SA. Are crossed actions of reticulospinal and vestibulospinal neurons on feline motoneurons mediated by the same or separate commissural neurons? J Neurosci 23: 8041–8050, 2003. doi: 10.1523/JNEUROSCI.23-22-08041.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Küchler M, Fouad K, Weinmann O, Schwab ME, Raineteau O. Red nucleus projections to distinct motor neuron pools in the rat spinal cord. J Comp Neurol 448: 349–359, 2002. doi: 10.1002/cne.10259. [DOI] [PubMed] [Google Scholar]
- Kudo N, Yamada T. N-methyl-d,l-aspartate-induced locomotor activity in a spinal cord-hindlimb muscles preparation of the newborn rat studied in vitro. Neurosci Lett 75: 43–48, 1987. doi: 10.1016/0304-3940(87)90072-3. [DOI] [PubMed] [Google Scholar]
- Kuypers H. Anatomy of the descending pathways. In: Handbook of Physiology, The Nervous System, Motor Control, edited by Brookhart JM, Mountcastle VB. Bethesda, MD: American Physiological Society, 1981, p. 597–666. [Google Scholar]
- Lanuza GM, Gosgnach S, Pierani A, Jessell TM, Goulding M. Genetic identification of spinal interneurons that coordinate left-right locomotor activity necessary for walking movements. Neuron 42: 375–386, 2004. doi: 10.1016/S0896-6273(04)00249-1. [DOI] [PubMed] [Google Scholar]
- Lawrence DG, Hopkins DA. The development of motor control in the rhesus monkey: evidence concerning the role of corticomotoneuronal connections. Brain 99: 235–254, 1976. doi: 10.1093/brain/99.2.235. [DOI] [PubMed] [Google Scholar]
- Lawrence DG, Kuypers HG. The functional organization of the motor system in the monkey. I. The effects of bilateral pyramidal lesions. Brain 91: 1–14, 1968. doi: 10.1093/brain/91.1.1. [DOI] [PubMed] [Google Scholar]
- Lemon R. Recent advances in our understanding of the primate corticospinal system. F1000 Res 8: 274, 2019. doi: 10.12688/f1000research.17445.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis GN, Byblow WD. Bimanual coordination dynamics in poststroke hemiparetics. J Mot Behav 36: 174–188, 2004. doi: 10.3200/JMBR.36.2.174-188. [DOI] [PubMed] [Google Scholar]
- Lipski J, Duffin J. An electrophysiological investigation of propriospinal inspiratory neurons in the upper cervical cord of the cat. Exp Brain Res 61: 625–637, 1986. doi: 10.1007/BF00237589. [DOI] [PubMed] [Google Scholar]
- Liu TT, Bannatyne BA, Maxwell DJ. Organization and neurochemical properties of intersegmental interneurons in the lumbar enlargement of the adult rat. Neuroscience 171: 461–484, 2010. doi: 10.1016/j.neuroscience.2010.09.012. [DOI] [PubMed] [Google Scholar]
- Lu DC, Niu T, Alaynick WA. Molecular and cellular development of spinal cord locomotor circuitry. Front Mol Neurosci 8: 25, 2015. doi: 10.3389/fnmol.2015.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushita M, Ikeda M. Propriospinal fiber connections of the cervical motor nuclei in the cat: a light and electron microscope study. J Comp Neurol 150: 1–31, 1973. doi: 10.1002/cne.901500102. [DOI] [PubMed] [Google Scholar]
- Matsuyama K, Kobayashi S, Aoki M. Projection patterns of lamina VIII commissural neurons in the lumbar spinal cord of the adult cat: an anterograde neural tracing study. Neuroscience 140: 203–218, 2006. doi: 10.1016/j.neuroscience.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Matsuyama K, Nakajima K, Mori F, Aoki M, Mori S. Lumbar commissural interneurons with reticulospinal inputs in the cat: morphology and discharge patterns during fictive locomotion. J Comp Neurol 474: 546–561, 2004. doi: 10.1002/cne.20131. [DOI] [PubMed] [Google Scholar]
- Maxwell DJ, Kerr R, Jankowska E, Riddell JS. Synaptic connections of dorsal horn group II spinal interneurons: synapses formed with the interneurons and by their axon collaterals. J Comp Neurol 380: 51–69, 1997. doi:. [DOI] [PubMed] [Google Scholar]
- Miller KE, Douglas VD, Richards AB, Chandler MJ, Foreman RD. Propriospinal neurons in the C1-C2 spinal segments project to the L5-S1 segments of the rat spinal cord. Brain Res Bull 47: 43–47, 1998. doi: 10.1016/S0361-9230(98)00065-3. [DOI] [PubMed] [Google Scholar]
- Mitchell EJ, McCallum S, Dewar D, Maxwell DJ. Corticospinal and reticulospinal contacts on cervical commissural and long descending propriospinal neurons in the adult rat spinal cord; evidence for powerful reticulospinal connections. PLoS One 11: e0152094, 2016. [Erratum in PLoS One 11: e0155664, 2016.] doi: 10.1371/journal.pone.0152094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell KJ, Doyle JL, Serafini T, Kennedy TE, Tessier-Lavigne M, Goodman CS, Dickson BJ. Genetic analysis of Netrin genes in Drosophila: netrins guide CNS commissural axons and peripheral motor axons. Neuron 17: 203–215, 1996. doi: 10.1016/S0896-6273(00)80153-1. [DOI] [PubMed] [Google Scholar]
- Molenaar I, Kuypers HG. Cells of origin of propriospinal fibers and of fibers ascending to supraspinal levels. A HRP study in cat and rhesus monkey. Brain Res 152: 429–450, 1978. doi: 10.1016/0006-8993(78)91102-2. [DOI] [PubMed] [Google Scholar]
- Morris R, Vallester KK, Newton SS, Kearsley AP, Whishaw IQ. The differential contributions of the parvocellular and the magnocellular subdivisions of the red nucleus to skilled reaching in the rat. Neuroscience 295: 48–57, 2015. doi: 10.1016/j.neuroscience.2015.03.027. [DOI] [PubMed] [Google Scholar]
- Mrachacz-Kersting N, Gervasio S, Marchand-Pauvert V. Evidence for a supraspinal contribution to the human crossed reflex response during human walking. Front Hum Neurosci 12: 260, 2018. doi: 10.3389/fnhum.2018.00260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan PW, Smith MC. The rubrospinal and central tegmental tracts in man. Brain 105: 223–269, 1982. doi: 10.1093/brain/105.2.223. [DOI] [PubMed] [Google Scholar]
- Ni Y, Nawabi H, Liu X, Yang L, Miyamichi K, Tedeschi A, Xu B, Wall NR, Callaway EM, He Z. Characterization of long descending premotor propriospinal neurons in the spinal cord. J Neurosci 34: 9404–9417, 2014. doi: 10.1523/JNEUROSCI.1771-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padel Y, Angaut P, Massion J, Sedan R. Comparative study of the posterior red nucleus in baboons and gibbons. J Comp Neurol 202: 421–438, 1981. doi: 10.1002/cne.902020311. [DOI] [PubMed] [Google Scholar]
- Petkó M, Antal M. Propriospinal afferent and efferent connections of the lateral and medial areas of the dorsal horn (laminae I–IV) in the rat lumbar spinal cord. J Comp Neurol 422: 312–325, 2000. doi:. [DOI] [PubMed] [Google Scholar]
- Petkó M, Antal M. Propriospinal pathways in the dorsal horn (laminae I–IV) of the rat lumbar spinal cord. Brain Res Bull 89: 41–49, 2012. doi: 10.1016/j.brainresbull.2012.06.005. [DOI] [PubMed] [Google Scholar]
- Petkó M, Veress G, Vereb G, Storm-Mathisen J, Antal M. Commissural propriospinal connections between the lateral aspects of laminae III–IV in the lumbar spinal cord of rats. J Comp Neurol 480: 364–377, 2004. doi: 10.1002/cne.20356. [DOI] [PubMed] [Google Scholar]
- Pierani A, Moran-Rivard L, Sunshine MJ, Littman DR, Goulding M, Jessell TM. Control of interneuron fate in the developing spinal cord by the progenitor homeodomain protein Dbx1. Neuron 29: 367–384, 2001. doi: 10.1016/S0896-6273(01)00212-4. [DOI] [PubMed] [Google Scholar]
- Pierrot-Deseilligny E, Burke DC. The Circuitry of the Human Spinal Cord: Spinal and Corticospinal Mechanisms of Movement. Cambridge, UK: Cambridge University Press, 2012, p. xxiii. [Google Scholar]
- Prut Y, Fetz EE. Primate spinal interneurons show pre-movement instructed delay activity. Nature 401: 590–594, 1999. doi: 10.1038/44145. [DOI] [PubMed] [Google Scholar]
- Puskár Z, Antal M. Localization of last-order premotor interneurons in the lumbar spinal cord of rats. J Comp Neurol 389: 377–389, 1997. doi:. [DOI] [PubMed] [Google Scholar]
- Putnam TJ. Myelotomy of the commissure: a new method of treatment for pain in the upper extremities. Arch Neurol Psychiatry 32: 1189–1193, 1934. doi: 10.1001/archneurpsyc.1934.02250120066006. [DOI] [Google Scholar]
- Quinlan KA, Kiehn O. Segmental, synaptic actions of commissural interneurons in the mouse spinal cord. J Neurosci 27: 6521–6530, 2007. doi: 10.1523/JNEUROSCI.1618-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raastad M, Kiehn O. Spike coding during locomotor network activity in ventrally located neurons in the isolated spinal cord from neonatal rat. J Neurophysiol 83: 2825–2834, 2000. doi: 10.1152/jn.2000.83.5.2825. [DOI] [PubMed] [Google Scholar]
- Rabe Bernhardt N, Memic F, Gezelius H, Thiebes AL, Vallstedt A, Kullander K. DCC mediated axon guidance of spinal interneurons is essential for normal locomotor central pattern generator function. Dev Biol 366: 279–289, 2012. doi: 10.1016/j.ydbio.2012.03.017. [DOI] [PubMed] [Google Scholar]
- Ramón y Cajal S. Histologie du systeme nerveux de l’homme et des vertébrés. Madrid, Spain: Instituto Cajal, 1909. [Google Scholar]
- Rathelot JA, Dum RP, Strick PL. Posterior parietal cortex contains a command apparatus for hand movements. Proc Natl Acad Sci USA 114: 4255–4260, 2017. doi: 10.1073/pnas.1608132114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed WR, Shum-Siu A, Onifer SM, Magnuson DS. Inter-enlargement pathways in the ventrolateral funiculus of the adult rat spinal cord. Neuroscience 142: 1195–1207, 2006. doi: 10.1016/j.neuroscience.2006.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Restrepo CE, Lundfald L, Szabó G, Erdélyi F, Zeilhofer HU, Glover JC, Kiehn O. Transmitter-phenotypes of commissural interneurons in the lumbar spinal cord of newborn mice. J Comp Neurol 517: 177–192, 2009. doi: 10.1002/cne.22144. [DOI] [PubMed] [Google Scholar]
- Roberts A, Sillar KT. Characterization and function of spinal excitatory interneurons with commissural projections in Xenopus laevis embryos. Eur J Neurosci 2: 1051–1062, 1990. doi: 10.1111/j.1460-9568.1990.tb00017.x. [DOI] [PubMed] [Google Scholar]
- Rossignol S, Barrière G, Frigon A, Barthélemy D, Bouyer L, Provencher J, Leblond H, Bernard G. Plasticity of locomotor sensorimotor interactions after peripheral and/or spinal lesions. Brain Res Brain Res Rev 57: 228–240, 2008. doi: 10.1016/j.brainresrev.2007.06.019. [DOI] [PubMed] [Google Scholar]
- Rossignol S, Gauthier L. An analysis of mechanisms controlling the reversal of crossed spinal reflexes. Brain Res 182: 31–45, 1980. doi: 10.1016/0006-8993(80)90828-8. [DOI] [PubMed] [Google Scholar]
- Saywell SA, Ford TW, Meehan CF, Todd AJ, Kirkwood PA. Electrophysiological and morphological characterization of propriospinal interneurons in the thoracic spinal cord. J Neurophysiol 105: 806–826, 2011. doi: 10.1152/jn.00738.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfenberger TM, Schrafl-Altermatt M, Dietz V. Cooperative hand movements in tetraplegic spinal cord injury patients: preserved neural coupling. Clin Neurophysiol 129: 2059–2064, 2018. doi: 10.1016/j.clinph.2018.06.028. [DOI] [PubMed] [Google Scholar]
- Scheibel ME, Scheibel AB. Terminal axonal patterns in cat spinal cord. II. The dorsal horn. Brain Res 9: 32–58, 1968. doi: 10.1016/0006-8993(68)90256-4. [DOI] [PubMed] [Google Scholar]
- Schmid K, Kirkwood PA, Munson JB, Shen E, Sears TA. Contralateral projections of thoracic respiratory interneurones in the cat. J Physiol 461: 647–665, 1993. doi: 10.1113/jphysiol.1993.sp019534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrafl-Altermatt M, Dietz V. Cooperative hand movements in post-stroke subjects: Neural reorganization. Clin Neurophysiol 127: 748–754, 2016. doi: 10.1016/j.clinph.2015.07.004. [DOI] [PubMed] [Google Scholar]
- Serafini T, Colamarino SA, Leonardo ED, Wang H, Beddington R, Skarnes WC, Tessier-Lavigne M. Netrin-1 is required for commissural axon guidance in the developing vertebrate nervous system. Cell 87: 1001–1014, 1996. doi: 10.1016/S0092-8674(00)81795-X. [DOI] [PubMed] [Google Scholar]
- Sherrington CS. Flexion-reflex of the limb, crossed extension-reflex, and reflex stepping and standing. J Physiol 40: 28–121, 1910. doi: 10.1113/jphysiol.1910.sp001362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinoda Y, Ohgaki T, Futami T. The morphology of single lateral vestibulospinal tract axons in the lower cervical spinal cord of the cat. J Comp Neurol 249: 226–241, 1986. doi: 10.1002/cne.902490208. [DOI] [PubMed] [Google Scholar]
- Soffe SR, Clarke JD, Roberts A. Activity of commissural interneurons in spinal cord of Xenopus embryos. J Neurophysiol 51: 1257–1267, 1984. doi: 10.1152/jn.1984.51.6.1257. [DOI] [PubMed] [Google Scholar]
- Soteropoulos DS, Edgley SA, Baker SN. Spinal commissural connections to motoneurons controlling the primate hand and wrist. J Neurosci 33: 9614–9625, 2013. doi: 10.1523/JNEUROSCI.0269-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soteropoulos DS, Williams ER, Baker SN. Cells in the monkey ponto-medullary reticular formation modulate their activity with slow finger movements. J Physiol 590: 4011–4027, 2012. doi: 10.1113/jphysiol.2011.225169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stecina K, Jankowska E. Uncrossed actions of feline corticospinal tract neurones on hindlimb motoneurones evoked via ipsilaterally descending pathways. J Physiol 580: 119–132, 2007. doi: 10.1113/jphysiol.2006.122721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stecina K, Jankowska E, Cabaj A, Pettersson LG, Bannatyne BA, Maxwell DJ. Premotor interneurones contributing to actions of feline pyramidal tract neurones on ipsilateral hindlimb motoneurones. J Physiol 586: 557–574, 2008a. doi: 10.1113/jphysiol.2007.145466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stecina K, Slawinska U, Jankowska E. Ipsilateral actions from the feline red nucleus on hindlimb motoneurones. J Physiol 586: 5865–5884, 2008b. doi: 10.1113/jphysiol.2008.163998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepien AE, Tripodi M, Arber S. Monosynaptic rabies virus reveals premotor network organization and synaptic specificity of cholinergic partition cells. Neuron 68: 456–472, 2010. doi: 10.1016/j.neuron.2010.10.019. [DOI] [PubMed] [Google Scholar]
- Stevenson AJ, Geertsen SS, Andersen JB, Sinkjær T, Nielsen JB, Mrachacz-Kersting N. Interlimb communication to the knee flexors during walking in humans. J Physiol 591: 4921–4935, 2013. doi: 10.1113/jphysiol.2013.257949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson AJ, Geertsen SS, Sinkjær T, Nielsen JB, Mrachacz-Kersting N. Interlimb communication following unexpected changes in treadmill velocity during human walking. J Neurophysiol 113: 3151–3158, 2015a. doi: 10.1152/jn.00794.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson AJ, Kamavuako EN, Geertsen SS, Farina D, Mrachacz-Kersting N. Short-latency crossed responses in the human biceps femoris muscle. J Physiol 593: 3657–3671, 2015b. doi: 10.1113/JP270422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokke MF, Nissen UV, Glover JC, Kiehn O. Projection patterns of commissural interneurons in the lumbar spinal cord of the neonatal rat. J Comp Neurol 446: 349–359, 2002. doi: 10.1002/cne.10211. [DOI] [PubMed] [Google Scholar]
- Stubbs PW, Nielsen JF, Sinkjær T, Mrachacz-Kersting N. Short-latency crossed spinal responses are impaired differently in sub-acute and chronic stroke patients. Clin Neurophysiol 123: 541–549, 2012. doi: 10.1016/j.clinph.2011.07.033. [DOI] [PubMed] [Google Scholar]
- Sugiuchi Y, Kakei S, Shinoda Y. Spinal commissural neurons mediating vestibular input to neck motoneurons in the cat upper cervical spinal cord. Neurosci Lett 145: 221–224, 1992. doi: 10.1016/0304-3940(92)90027-5. [DOI] [PubMed] [Google Scholar]
- Takei T, Seki K. Spinal interneurons facilitate coactivation of hand muscles during a precision grip task in monkeys. J Neurosci 30: 17041–17050, 2010. doi: 10.1523/JNEUROSCI.4297-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takei T, Seki K. Spinal premotor interneurons mediate dynamic and static motor commands for precision grip in monkeys. J Neurosci 33: 8850–8860, 2013. doi: 10.1523/JNEUROSCI.4032-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talpalar AE, Bouvier J, Borgius L, Fortin G, Pierani A, Kiehn O. Dual-mode operation of neuronal networks involved in left-right alternation. Nature 500: 85–88, 2013. doi: 10.1038/nature12286. [DOI] [PubMed] [Google Scholar]
- Tardif E, Clarke S. Commissural connections of human superior colliculus. Neuroscience 111: 363–372, 2002. doi: 10.1016/S0306-4522(01)00600-5. [DOI] [PubMed] [Google Scholar]
- Tasker RR, North R. Cordotomy and myelotomy. In: Neurosurgical Management of Pain, edited by North RB, Levy RM. New York: Springer, 1997, p. 191–220. [Google Scholar]
- Thomas FA, Dietz V, Schrafl-Altermatt M. Automatic gain control of neural coupling during cooperative hand movements. Sci Rep 8: 5959, 2018. doi: 10.1038/s41598-018-24498-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallstedt A, Kullander K. Dorsally derived spinal interneurons in locomotor circuits. Ann N Y Acad Sci 1279: 32–42, 2013. doi: 10.1111/j.1749-6632.2012.06801.x. [DOI] [PubMed] [Google Scholar]
- Villanueva L, de Pommery J, Menétrey D, Le Bars D. Spinal afferent projections to subnucleus reticularis dorsalis in the rat. Neurosci Lett 134: 98–102, 1991. doi: 10.1016/0304-3940(91)90517-W. [DOI] [PubMed] [Google Scholar]
- Wéber I, Veress G, Szucs P, Antal M, Birinyi A. Neurotransmitter systems of commissural interneurons in the lumbar spinal cord of neonatal rats. Brain Res 1178: 65–72, 2007. doi: 10.1016/j.brainres.2007.06.109. [DOI] [PubMed] [Google Scholar]
- Whishaw IQ, Gorny B, Sarna J. Paw and limb use in skilled and spontaneous reaching after pyramidal tract, red nucleus and combined lesions in the rat: behavioral and anatomical dissociations. Behav Brain Res 93: 167–183, 1998. doi: 10.1016/S0166-4328(97)00152-6. [DOI] [PubMed] [Google Scholar]
- Williams ER, Soteropoulos DS, Baker SN. Spinal interneuron circuits reduce approximately 10-Hz movement discontinuities by phase cancellation. Proc Natl Acad Sci USA 107: 11098–11103, 2010. doi: 10.1073/pnas.0913373107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson CJ. Postsynaptic potentials evoked in spiny neostriatal projection neurons by stimulation of ipsilateral and contralateral neocortex. Brain Res 367: 201–213, 1986. doi: 10.1016/0006-8993(86)91593-3. [DOI] [PubMed] [Google Scholar]
- Yang HW, Lemon RN. An electron microscopic examination of the corticospinal projection to the cervical spinal cord in the rat: lack of evidence for cortico-motoneuronal synapses. Exp Brain Res 149: 458–469, 2003. doi: 10.1007/s00221-003-1393-9. [DOI] [PubMed] [Google Scholar]
- Zaaimi B, Edgley SA, Soteropoulos DS, Baker SN. Changes in descending motor pathway connectivity after corticospinal tract lesion in macaque monkey. Brain 135: 2277–2289, 2012. doi: 10.1093/brain/aws115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagoraiou L, Akay T, Martin JF, Brownstone RM, Jessell TM, Miles GB. A cluster of cholinergic premotor interneurons modulates mouse locomotor activity. Neuron 64: 645–662, 2009. doi: 10.1016/j.neuron.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zehr EP, Collins DF, Chua R. Human interlimb reflexes evoked by electrical stimulation of cutaneous nerves innervating the hand and foot. Exp Brain Res 140: 495–504, 2001. doi: 10.1007/s002210100857. [DOI] [PubMed] [Google Scholar]
- Zehr EP, Kido A. Neural control of rhythmic, cyclical human arm movement: task dependency, nerve specificity and phase modulation of cutaneous reflexes. J Physiol 537: 1033–1045, 2001. doi: 10.1113/jphysiol.2001.012878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Narayan S, Geiman E, Lanuza GM, Velasquez T, Shanks B, Akay T, Dyck J, Pearson K, Gosgnach S, Fan CM, Goulding M. V3 spinal neurons establish a robust and balanced locomotor rhythm during walking. Neuron 60: 84–96, 2008. doi: 10.1016/j.neuron.2008.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zholudeva LV, Qiang L, Marchenko V, Dougherty KJ, Sakiyama-Elbert SE, Lane MA. The neuroplastic and therapeutic potential of spinal interneurons in the injured spinal cord. Trends Neurosci 41: 625–639, 2018. doi: 10.1016/j.tins.2018.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong G, Shevtsova NA, Rybak IA, Harris-Warrick RM. Neuronal activity in the isolated mouse spinal cord during spontaneous deletions in fictive locomotion: insights into locomotor central pattern generator organization. J Physiol 590: 4735–4759, 2012. doi: 10.1113/jphysiol.2012.240895. [DOI] [PMC free article] [PubMed] [Google Scholar]