Abstract
The type 5 metabotropic glutamate receptor (mGluR5) represents a novel therapeutic target for schizophrenia and other disorders. Schizophrenia is associated with progressive abnormalities in cortical oscillatory processes including reduced spindles (8–15 Hz) during sleep and increased delta (0.5–4 Hz)- and gamma-band activity (30–80 Hz) during wakefulness. mGluR5 knockout (KO) mice demonstrate many schizophrenia-like behaviors, including abnormal sleep. To examine the effects of mGluR5 on the maintenance of the neocortical circuitry responsible for such neural oscillations, we analyzed sleep/wake electroencephalographic (EEG) activity of mGluR5 KO mice at baseline, after 6 h of sleep deprivation, and during a visual method of cortical entrainment (visual steady state response). We hypothesized mGluR5-KO mice would exhibit translationally relevant abnormalities in sleep and neural oscillations that mimic schizophrenia. Power spectral and spindle density analyses were performed across 24-h EEG recordings in mGluR5-KO mice and wild-type (WT) controls. Novel findings in mGluR5 KO mice include deficits in sleep spindle density, wake alpha power, and 40-Hz visual task-evoked gamma power and phase locking. Sigma power (10–15 Hz), an approximation of spindle activity, was also reduced during non-rapid eye movement sleep transitions. Our observations on abnormal sleep/wake are generally in agreement with previous reports, although we did not replicate changes in rapid eye movement sleep. The timing of these phenotypes may suggest an impaired circadian process in mGluR5 KO mice. In conclusion, EEG phenotypes in mGluR5 KO mice resemble deficits observed in patients with schizophrenia. These findings implicate mGluR5-mediated pathways in several translationally relevant phenotypes associated with schizophrenia, and suggest that agents targeting this receptor may have harmful consequences on sleep health and daily patterns of EEG power.
NEW & NOTEWORTHY Metabotropic glutamate receptor type 5 (mGluR5) knockout (KO) mice show several translationally relevant abnormalities in neural oscillatory activity associated with schizophrenia. These include deficits in sleep spindle density, sigma and alpha power, and 40-Hz task-evoked gamma power. The timing of these phenotypes suggests an impaired circadian process in these mice. Previously reported rapid eye movement sleep deficits in this model were not observed. These findings suggest mGluR5-enhancing drugs may improve sleep stability and sleep spindle density, which could impact memory and cognition.
Keywords: metabotropic glutamate receptor 5, N-methyl-d-aspartate receptors, polysomnography, sigma, visual evoked potentials
INTRODUCTION
Schizophrenia is a chronic and multidimensional neuropsychiatric disorder, with devastating consequences for patient outcome. The complex nature of schizophrenia, which involves a combination of genetic, developmental, and environmental factors, has made identification of the exact etiology of this disorder difficult and impaired the development of novel therapeutic approaches. This fact has been highlighted by recent large-scale genetic studies, which have implicated numerous candidate genes for schizophrenia, as well as other neuropsychiatric conditions (Schizophrenia Working Group of the Psychiatric Genomics Consortium 2014). Such findings underscore the complex polygenetic origin of schizophrenia and suggest that candidate gene assessment is required to determine functional significance, especially where such candidates are linked to mechanisms that are believed to be related to disease pathophysiology (Matosin et al. 2018; Wilkening et al. 2009). Abnormalities in glutamate transmission have been strongly implicated in the development of schizophrenia-related symptoms, specifically, impaired cognitive performance (McNally and McCarley 2016). Thus the characterization of mechanisms and/or therapeutic pathways that enhance glutamatergic receptor activity have been of great interest.
Recently, the type 5 metabotropic glutamate receptor (mGluR5) has emerged as a potential therapeutic target for schizophrenia and other psychiatric disorders. This is specifically due to its role in modulating N-methyl-d-aspartate receptor (NMDAR) function, linking this receptor to critical cellular processes disrupted in schizophrenia. The gene that codes for mGluR5, Grm5, was identified as a top candidate gene for schizophrenia in a convergent functional genomic analysis study that combined findings of the International Schizophrenia Consortium genome-wide association studies (ISC GWAS) with animal modeling evidence and associated human studies (Ayalew et al. 2012). mGluR5 may contribute to abnormalities in neural excitation, putatively underpinning the manifestation of cognitive impairment and psychotic-like behavior in schizophrenia and related disorders (de Bartolomeis and Szumlinski 2012). Recently, several Grm5 variants have been associated with cognitive impairment and hippocampal volume reduction (Matosin et al. 2018). Furthermore, disruption of mGluR5-mediated signal transduction pathways is associated with schizophrenia (Wang and Zhuo 2012). Despite this, the exact mechanism for how mGluR5 is involved in specific psychiatric disorders remains unclear. Thus it is important to assess mGluR5 dysfunction’s role in schizophrenia pathophysiology and the development of schizophrenia-related symptom classes.
Although not included in the diagnostic criteria for schizophrenia, sleep disturbances are consistently reported in patients (Kaskie et al. 2017; Palmese et al. 2011). Impaired sleep represents the most commonly reported symptom during the prodromal phase of the illness and has been suggested to be a predictive factor in determining who will develop psychosis among clinically high-risk subjects (Ruhrmann et al. 2010). Several prior studies suggest that mGluR5 disruption can lead to sleep/wake abnormalities (Ahnaou et al. 2015a, 2015b; Holst et al. 2017). Glutamate release shows rhythmic fluctuations across sleep/wake states with peak levels reached during wake and rapid eye movement (REM) sleep (Dash et al. 2009). mGluR5, in conjunction with associated protein Homer, is required for maintenance of behavioral state and NMDAR expression patterns (Ango et al. 2002; Diering et al. 2017). This is supported by more recent findings showing an increase in mGluR5 expression following sleep deprivation (Holst et al. 2017). Additionally, mGluR5 activation is necessary for developmental alterations in NMDAR subunit expression (Matta et al. 2011), suggesting a fundamental role for mGluR5 in developmental aspects of schizophrenia.
Abnormal corticothalamic network dynamics and circadian patterns have been reported in a number of neurological and psychiatric disorders including schizophrenia (Andreasen et al. 1994; Avram et al. 2018; Llinás et al. 2005; Martin et al. 2005). Most commonly associated with increased delta (0.5–4 Hz) and reduced alpha activity (8–13 Hz) during wakefulness (Boutros et al. 2008; Newson and Thiagarajan 2019), patients with schizophrenia also consistently show a reduction in sleep spindles, which are 10- to 15-Hz oscillations that occur during non-rapid eye movement (NREM) sleep (Manoach et al. 2016). These spindle abnormalities are implicated in impaired sleep-dependent memory consolidation (Goder et al. 2015; Wamsley et al. 2012) and may represent an endophenotype for schizophrenia that contributes to cognitive symptoms (Bódizs et al. 2005; Manoach et al. 2016). mGluR5 dynamically regulates the thalamic reticular nucleus (TRN), which is a brain region necessary for sleep spindle generation (Sun et al. 2016), and mGluR5 knockout (KO) mice exhibit memory impairment (Lu et al. 1997). Together, this suggests a potential role of mGluR5 in spindle generation.
In this study we utilize the mGluR5 KO mouse model to examine the role of mGluR5 disruption on disease-relevant phenotypes, with a specific focus on sleep spindle generation and abnormalities in gamma-band activity, which potentially represent endophenotypes for cognitive dysfunction in schizophrenia. We find evidence of sleep spindle, EEG power, and task-evoked gamma deficits in mGluR5 KO mice that mimic schizophrenia. The existence of these deficits suggests a mechanistic link of these translationally relevant phenotypes to mGluR5, informing future therapeutic options targeting this receptor.
MATERIALS AND METHODS
Animals
Both wild-type (WT) and mGluR5 KO (−/−) C57BL6J mice (B6.129-Grm5tm1Rod/J) were bred in-house, originally obtained from Jackson Laboratories. Mice were housed at room temperature with a 12:12-h light-dark cycle [7:00 AM lights on = Zeitgeber time (ZT) 0] with food and water provided ad libitum. All experiments conformed to US Veterans Affairs (VA), Harvard University, and US National Institutes of Health guidelines. VA Boston Healthcare System’s Institutional Animal Care and Use Committee prospectively granted approval for the experiments in this study. Mice used for sleep recordings were 4+ mo old with the following sex distribution: WT, n = 5 male, 3 female; KO, n = 8 male, 4 female.
Stereotaxic Surgery
Adult mice were deeply anesthetized with isoflurane (5% induction, 1–2% maintenance) and placed in a stereotaxic device with body temperature maintained by a chemical heating pad. Epidural EEG screw electrodes were implanted bilaterally into the skull above frontal cortices [from bregma: anteroposterior (AP) +1.9 mm, mediolateral (ML) ±1 mm] with a reference screw above the parietal cortex (from bregma: AP −3.4 mm, M/L ±1.85 mm) and a ground above the cerebellum (from lambda: AP −1 mm, ML +0 mm). Two electromyography (EMG) wires were placed in the nuchal muscle to record movement. EEG/EMG signals were acquired via three-channel amplifier (Pinnacle Technology), sampled at 1 kHz, and low-pass filtered at 200 Hz.
Sleep/Wake Behavior
Animals were habituated for ~48 h and then recorded for 24 h. A subgroup of animals (8 WT, 10 KO) were recorded again for a sleep deprivation experiment. This second 48-h recording consisted of a new 24-h baseline, 6 h of sleep deprivation from ZT 0–6 (7:00 AM–1:00 PM), and 18 h of recovery from ZT 6–24 (1:00 PM–7:00 AM). Sleep deprivation was achieved using a horizontal bar-style automated sleep deprivation system (Pinnacle Technology; bar speed 7, rotation direction changed every 10–30 s). EEG data were recorded and then manually sleep scored in 10-s epochs using Sirenia software (Pinnacle Technology). Sleep spindles were detected using an automated MATLAB-based algorithm developed in-house (Uygun et al. 2019). Briefly, the raw EEG is bandpass filtered from 10 to 15 Hz, a Butterworth filter is applied, the root mean square (RMS) of the filtered EEG data is taken (750-ms window), and RMS data are cubed. Putative spindles must pass a lower (1.2 times mean cubed RMS) and upper (3.5 times mean cubed RMS) threshold and last between 0.5 and 10 s with an interspindle interval of 0.5 s. Custom MATLAB scripts were additionally employed to examine power spectral density. A multitaper spectral analysis (Chronux toolbox, http://chronux.org/; 2 tapers, bandwidth 3) was performed on each 10-s scored epoch (Mitra and Bokil 2007). Within each behavioral state (wake, NREM, REM), each epoch was normalized to the sum power of all epochs (0–100 Hz) in the baseline day hours 0–24.
Visual Steady-State Response
To examine task-evoked gamma power, we utilized a visual analog to the acoustic steady-state response (ASSR). A 2-s train of light flashes (LED array, 550 lux) is presented above the open home cage of a freely moving EEG-tethered mouse in complete darkness to entrain the cortex at a range of frequencies (10–60 Hz in 10-Hz steps). The test phase for each frequency consisted of 75 repetitions of 4-s trials: 1 s of prestimulus, 2 s of flashing light stimulus, 1 s of poststimulus. Visually evoked EEG data are recorded with WinWCP (Strathclyde University). The fold change in power is measured by taking the average evoked power (stimulus frequency ± 3 Hz) during the stimulus (2.05–2.6 s) divided by the average power during baseline (0.25–0.8 s). Phase-locking value is a measure of phase synchronization for each stimulation frequency during the period of stimulation. We calculated the phase-locking value of the wavelet-transformed 40-Hz stimulation data (37–43 Hz) during the stimulus time period (2.05–2.6 s). Visual steady-state response (VSSR) measurements were taken after all sleep recordings had concluded, and some animals were no longer available for recording. Sixty percent of the animals in this study were from the sleep recording data, and the remaining 40% were age-matched littermates and controls with the following sex distribution: WT, n = 7 male, 2 female; KO, n = 3 male, 3 female.
Statistics
Statistical analysis was performed using two-way repeated-measures analysis of variance (2-way RM ANOVA, where between measure is strain, and within measure is either time or vigilance state). Significant interactions were followed up with a Holm–Sidak multiple comparisons test. In instances with missing data points, RM ANOVA was not appropriate and the Holm–Sidak method was used alone. To minimize outliers, each hour with ≤120 s of NREM sleep was removed from the spindle analysis. All spindle data were analyzed in 3-h bins to minimize missing data from hours without NREM sleep. One WT animal was awake for 7 consecutive hours and was removed from the entire data set. All data are means ± SE.
RESULTS
mGluR5 KO Mice Exhibit Decreased Wake and Increased NREM Sleep
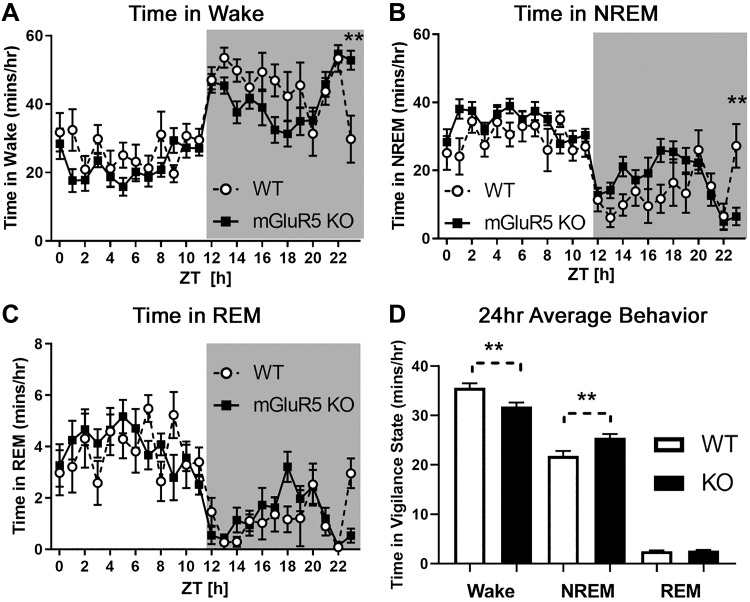
mGluR5 KO mice exhibited significantly decreased time in wake and increased time in NREM sleep across 24 h compared with WT controls [Fig. 1; 2-way RM ANOVAs; wake: main effect of strain, F(1,18) = 9.638, P < 0.01, WT = 35.61 ± 2.30 min/h, KO = 31.82 ± 2.39 min/h; NREM: main effect of strain, F(1,18) = 9.178, P < 0.01, WT = 21.81 ± 1.98 min/h, KO = 25.51 ± 2.09 min/h]. Significant interactions (P < 0.05) between strain and time were also present in all three vigilance states, and a Holm–Sidak follow-up test revealed mGluR5 KO mice spend more time in wake and less time in NREM sleep at ZT 23–24 [P < 0.01; wake interaction, F(23,414) = 2.197; NREM interaction, F(23,414) = 2.286; REM interaction, F(23,414) = 1.620]. No significant differences between strains were observed during REM sleep.
Fig. 1.
Metabotropic glutamate receptor type 5 (mGluR5) knockout (KO) mice (n = 12) had decreased time in wake (A) and increased time in non-rapid eye movement (NREM) sleep (B) across 24 h compared with wild-type (WT; n = 8) controls (D; 2-way RM ANOVA; main effects, P < 0.01). No differences in rapid eye movement (REM) sleep were observed (C). In contrast to the 24-h average trends represented in D, KO mice had more wake and less NREM activity during Zeitgeber time (ZT) 23–24 (A and B). In A and B: **Significant Holm–Sidak post hoc test (P < 0.01) following a 2-way RM ANOVA significant interaction (P < 0.01). In D: **Significant main effect of strain (P < 0.01, 2-way RM ANOVA). EEG data from mice were sleep scored using 10-s epochs. Values are means (error bars are SE). Shaded area represents the dark cycle/lights-off period (ZT 12–24, or 7:00 PM–7:00 AM).
Recovery from Sleep Deprivation Is Unchanged in mGluR5 KO Mice
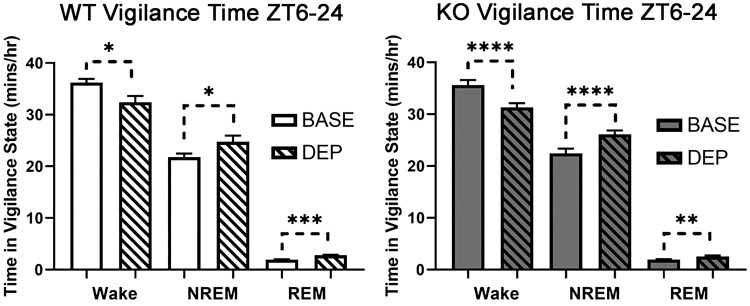
The effects of 6 h of sleep deprivation (ZT 0–6) were additionally examined in a subset of mice (n = 8 WT, 10 KO). The 6-h sleep deprivation led WT and KO mice to experience decreased wake and increased NREM and REM activity from ZT 6–24 relative to their own baseline activity 24 h prior (Fig. 2; main effects of sleep deprivation, P < 0.05). In Supplemental Fig. S1 (see https://doi.org/10.6084/m9.figshare.9337397.v1), we have also analyzed these data in smaller time segments (ZT 6–12 and ZT 12–24) to display the time course of the homeostatic sleep response in WT and KO animals. Specifically, the rebound sleep response occurs during ZT 12–24 in WT mice, whereas it occurs during ZT 6–12 and ZT 12–24 in KO mice. Increases in wake theta during sleep deprivation can represent homeostatic sleep pressure and predict a subsequent increase in NREM delta activity (Vyazovskiy and Tobler 2005). In Supplemental Fig. S2 (see https://doi.org/10.6084/m9.figshare.9337415.v1), we compared wake theta (7–9 Hz) and NREM delta (0.5–4 Hz) power during ZT 0–24 of baseline and sleep deprivation days to confirm the efficacy of the sleep deprivation procedure. The wake theta (7–9 Hz) power showed an initial increase, perhaps due to higher activity/locomotion that decreases as the mice slow down toward the end of deprivation. The NREM delta power is highest in the first 2 h of recovery sleep as was expected. In sum, these analyses suggest that the sleep deprivation procedure caused a homeostatic sleep response in both WT and KO animals.
Fig. 2.
Sleep deprivation from Zeitgeber time (ZT) 0–6 decreased wake and increased non-rapid eye movement (NREM) and rapid eye movement (REM) sleep in both wild-type (WT; n = 8; left) and metabotropic glutamate receptor type 5 knockout (KO; n = 10; right) animals. This baseline recording was taken 24 h before the automated sleep deprivation. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001, significant main effects of sleep deprivation for WT and KO (2-way RM ANOVA). BASE, baseline; DEP, sleep deprivation.
mGluR5 KO Mice Display More Fragmented Sleep and Wake in the Dark (Active) Period
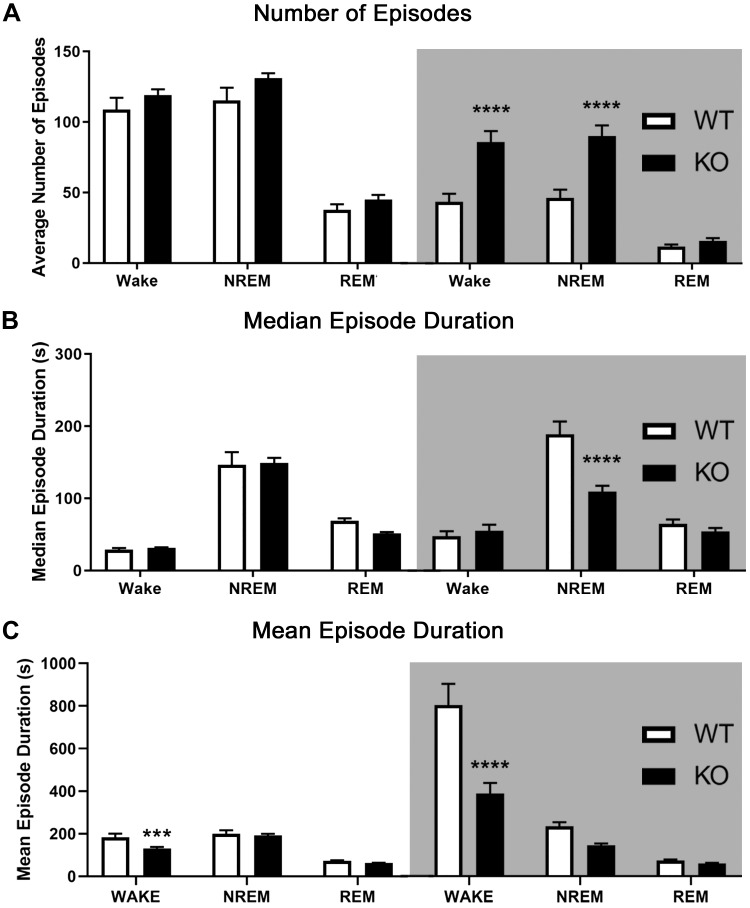
Bout analysis of sleep/wake records was performed for both the light (ZT 0–12) and dark periods (ZT 12–24) separately. All significant interactions are between the strain (WT/KO) and vigilance state (wake/NREM/REM) within the time period specified. Across baseline and after sleep deprivation, mGluR5 KO mice showed a significant increase in the number of wake and NREM sleep episodes compared with WT in the dark cycle [Fig. 3A; baseline: interaction, F(2,36) = 13.82, P < 0.0001, Holm–Sidak P < 0.001; sleep deprivation (not shown): interaction, F(2,32) = 10.96, P < 0.001, Holm–Sidak P < 0.01]. Next, the median duration of episodes was calculated for each mouse and then averaged within each strain. Analysis of median episode duration revealed shorter NREM episodes in mGluR5 KO mice than in WT during the dark cycle [Fig. 3B; baseline: interaction, F(2,36) = 13.67, P < 0.0001, Holm–Sidak P < 0.0001; sleep deprivation (not shown): interaction, F(2,32) = 16.21, P < 0.0001, Holm–Sidak P < 0.0001]. The mean episode duration of wake events was significantly decreased in mGluR5 KO mice for both light and dark cycles [Fig. 3C; baseline: light cycle interaction, F(2,36) = 4.299, dark cycle interaction, F(2,36) = 14.14, P < 0.05 for each, Holm–Sidak P < 0.001 for each; sleep deprivation (not shown): dark cycle interaction, F(2,32) = 4.687, P < 0.05, Holm–Sidak P < 0.001]. Following sleep deprivation, the light cycle (ZT 6–12) had no significant differences in mean wake duration (not shown). Note that in the sleep deprivation data, the hours of sleep deprivation (ZT 0–6) were excluded from light cycle analysis. Further evidence of enhanced fragmentation in KO animals (histograms of episode duration) is presented in Supplemental Fig. S3 (see https://doi.org/10.6084/m9.figshare.9337424.v1). Specifically, KO mice have a smaller proportion of extremely long-duration NREM episodes (>600 s), a larger proportion of short (<90 s)- and medium-length (240–480 s) NREM and REM episodes, and an increased number of wake, NREM, and REM episodes, which suggests KO mice have enhanced sleep fragmentation.
Fig. 3.
Metabotropic glutamate receptor type 5 (mGluR5) knockout (KO) mice displayed more fragmented sleep and wake activity in the dark (active) period. Light [Zeitgeber time (ZT) 0–12, or 7:00 AM–7:00 PM) and dark periods (ZT 12–24, or 7:00 PM–7:00 AM; shaded areas) were analyzed separately. mGluR5 KO mice (n = 12) showed a greatly increased number of non-rapid eye movement (NREM) sleep and wake episodes compared with wild-type (WT) controls (n = 8) in the dark period (A). This pattern persisted after 6 h of sleep deprivation (not shown). Next, the median duration of episodes was calculated for each mouse and then averaged within each strain. Analysis of median episode duration revealed shorter NREM episodes in mGluR5 KO mice than in WT during the dark cycle in baseline (B) and post-sleep-deprived conditions (not shown). Mean episode duration analysis revealed significantly shorter wake episodes in KO mice than in WT across both light and dark cycles at baseline (C) and across the dark cycle after sleep deprivation (not shown). ***P < 0.001, ****0.0001, significant difference in the Holm–Sidak post hoc test following a significant interaction (P < 0.05) in a 2-way RM ANOVA. REM, rapid eye movement.
mGluR5 KO Mice Exhibit Decreased Delta, Theta, and Alpha Power
We analyzed the power spectral density of multiple frequency bands across the 24-h sleep/wake cycle in WT and KO mice. We focused the following analysis on bands clinically observed to be abnormal in schizophrenia, including resting state (wake) delta, alpha, and gamma power. Analysis of a wider frequency band (1–50 Hz) is presented in Supplemental Fig. S4 (see https://doi.org/10.6084/m9.figshare.9337427.v1), whereas additional power spectral density differences are summarized in Supplemental Table S1 (https://doi.org/10.6084/m9.figshare.9890576.v1).
Delta (0.5–4 Hz).
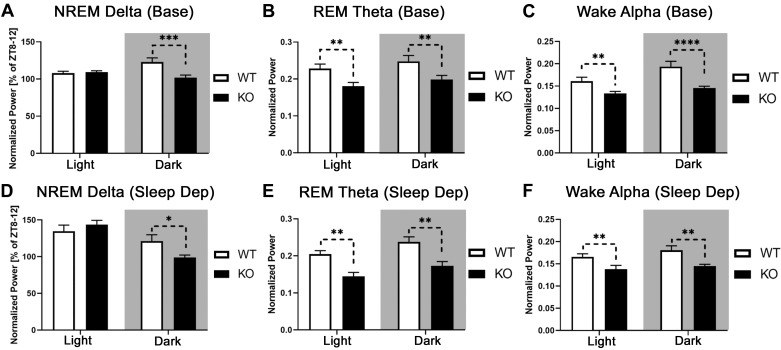
Wake delta power is enhanced in patients with schizophrenia (Boutros et al. 2008; Newson and Thiagarajan 2019) but was unchanged in mGluR5 KO mice. In two mGluR5 KO mouse sleep studies, NREM delta was normalized to ZT 8–12, a time period when delta power is lowest (Ahnaou et al. 2015b; Holst et al. 2017). When normalized with this method, mGluR5 KO mice had lower NREM delta power than WT mice during the dark cycle, during both baseline and the recovery period following 6 h of sleep deprivation [Fig. 4, A and D; 2-way RM ANOVAs; strain × light cycle interactions, P < 0.01, Holm–Sidak P < 0.05; baseline: F(1,18) = 14.45; sleep deprivation: F(1,16) = 18.95].
Fig. 4.
Metabotropic glutamate receptor type 5 (mGluR5) knockout (KO) mice demonstrated decreased delta (0.5–4 Hz), theta (7–9 Hz), and alpha power (8–13 Hz). Normalized non-rapid eye movement (NREM) delta power was analyzed as a percentage of individual mean delta power from the last 4 h of the light period [Zeitgeber time (ZT) 8–12, as seen in Ahnaou et al. (2015b) and Holst et al. (2017)]. This represents a block of time when delta power is normally lowest. For other frequency bands, each epoch was normalized to the sum power (0–100 Hz) of all epochs within a behavioral state across the baseline day ZT 0–24. mGluR5 KO mice (n = 12) had lower normalized NREM delta power than wild-type (WT) controls (n = 8) during the dark cycle (A). A similar relationship was observed in mice after sleep deprivation (D; WT, n = 8; KO, n = 10). Rapid eye movement (REM) theta power was decreased across light and dark cycles in mGluR5 KO mice at baseline (B) and after sleep deprivation (E). Compared with WT animals, mGluR5 KO mice demonstrated a decrease in wake alpha power across light and dark cycles during baseline (C) and after sleep deprivation (F). In A, C, and D: *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001, significant Holm–Sidak following a strain × light cycle interaction in a 2-way RM ANOVA. In B, E, and F: **P < 0.01, significant main effect of strain in a 2-way RM ANOVA. In the recovery sleep condition (D–F), the hours of active sleep deprivation (ZT 0–6) have been omitted from the light cycle. Sleep Dep, sleep deprivation.
Theta (7–9 Hz).
REM theta was decreased in KO mice compared with WT controls during the light and dark cycles across baseline and recovery sleep conditions [Fig. 4, B and E; 2-way RM ANOVAs; main effects of strain, P < 0.01; baseline ZT 0–24, F(1,18) = 9.041; sleep deprivation ZT 6–24, F(1,16) = 15.45]. Wake theta was also reduced in KO mice during the first 6 h of recovery sleep (Supplemental Table S1; see https://doi.org/10.6084/m9.figshare.9890576.v1).
Alpha (8–13 Hz).
A decrease in resting-state alpha-band power (8–13 Hz) is a common observation in patients with schizophrenia (Boutros et al. 2008; Howells et al. 2018; Merrin and Floyd 1996; Newson and Thiagarajan 2019). Compared with WT animals, mGluR5 KO mice demonstrate a decrease in normalized wake alpha power over light and dark cycles during baseline and recovery sleep [2-way RM ANOVAs; baseline (Fig. 4C): interaction, F(1,18) = 8.863, P < 0.01, with Holm–Sidak light and dark, P < 0.01; sleep deprivation (Fig. 4F): main effect of strain, F(1,16) = 10.24, P < 0.01].
Elevated Baseline Gamma Is Present During Sleep in mGluR5 KO mice
Gamma (30–80 Hz).
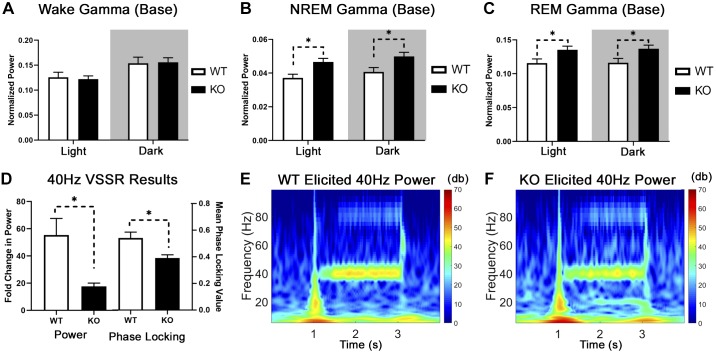
Increased resting-state gamma power occurs in animal models of NMDA receptor dysfunction and a subset of patients in the early stages of psychosis (Grent-’t-Jong et al. 2018; Reilly et al. 2018; White and Siegel 2016), yet we observed no differences in resting (wake) gamma activity in our KO mice (Fig. 5A). However, KO mice have higher baseline gamma power than WT during NREM (Fig. 5B) and REM sleep (Fig. 5C) during both the light and dark cycles [2-way RM ANOVAs; main effect of strain, P < 0.05; NREM, F(1,18) = 7.473; REM, F(1,18) = 6.224]. After sleep deprivation, KO mice continue to have higher gamma power than WT during the light and dark cycle, but only during REM sleep [2-way RM ANOVA; main effect of strain, F(1,16) = 4.646, P < 0.05; WT mean = 0.1275, KO mean = 0.1457 (data not shown)].
Fig. 5.
Elevated baseline gamma and reduced 40-Hz task-evoked gamma are present in metabotropic glutamate receptor type 5 (mGluR5) knockout (KO) mice. Baseline gamma activity was unchanged in our KO animals during normal waking conditions (A) but enhanced during non-rapid eye movement (NREM; B) and rapid eye movement (REM: C) sleep compared with wild-type (WT) mice (n = 8 WT, n = 12 KO; 2-way RM ANOVA, main effect of strain, *P < 0.05). We utilized the visual steady-state response (VSSR) to measure task-evoked gamma power in 9 WT (7 males, 2 females) and 6 KO animals (3 males, 3 females). This is a visual analog to the auditory steady-state response (ASSR), where a 2-s train of light flashes was presented to a mouse in complete darkness at a range of frequencies (10–60 Hz in 10-Hz steps) while frontal cortical EEG response was measured. The evoked response (elicited power of stimulus period/average power of baseline period) resembles the ASSR with a distribution peaking around 30 Hz. KO mice have a smaller fold increase in power (D, left) and reduced phase-locking value (D, right) than WT mice at 40 Hz, which mimics the ASSR in patients with schizophrenia (Welch’s t test, *P < 0.05). Grand-averaged spectrograms of task-evoked power during a 40-Hz light stimulus are shown for WT (E) and mGluR5 KO animals (F). Colors represent the elicited power in decibels (dB) with blues representing lower power and reds representing higher power. Base, baseline.
Reduced Task-evoked Gamma in KO mice: 40-Hz Visual Steady State Response
The acoustic steady-state response (ASSR) produced a limited response from our WT and KO mice, potentially due to hearing impairment. Therefore, we utilized a visual analog to this technique where a 2-s train of light flashes is presented to a mouse in complete darkness at a range of frequencies (10–60 Hz in 10-Hz steps) while frontal cortical EEG response was measured. This produced an evoked response comparable to the ASSR with a peak in evoked power around 30 Hz (Supplemental Fig. S5; see https://doi.org/10.6084/m9.figshare.9337433.v1). The fold change in power (Fig. 5D, left) was measured by taking the average evoked power (stimulus frequency ± 3 Hz) during the stimulus (2.05–2.6 s) divided by the average power during baseline (0.25–0.8 s). We predicted a smaller fold change in evoked power for KO mice at 40-Hz stimulation a priori based on similar deficits in the ASSR for patients with schizophrenia (Kwon et al. 1999; Thuné et al. 2016). Indeed, KO animals have a smaller fold increase in power [17.65 ± 2.37 arbitrary units (a.u.)] than WT (55.39 ± 12.20 a.u.) during the 40-Hz stimulation [unpaired 2-tailed Welch’s unequal variances t test, t(8.598) = 3.036, P = 0.0149]. Grand-averaged spectrograms of a 40-Hz light stimulus are shown for WT (Fig. 5E; n = 9) and mGluR5 KO animals (Fig. 5F, n = 6). The phase-locking value during the 40-Hz stimulus period (Fig. 5D, right) was also significantly reduced in mGluR5 KO animals (0.39 ± 0.02 a.u.) compared with WT [0.54 ± 0.04 a.u.; unpaired 2-tailed Welch’s unequal variances t test, t(11.84) = 3.023, P = 0.0108].
Sleep Spindle Density and NREM Sigma Power Is Decreased During Transition Periods in mGluR5 KO Mice
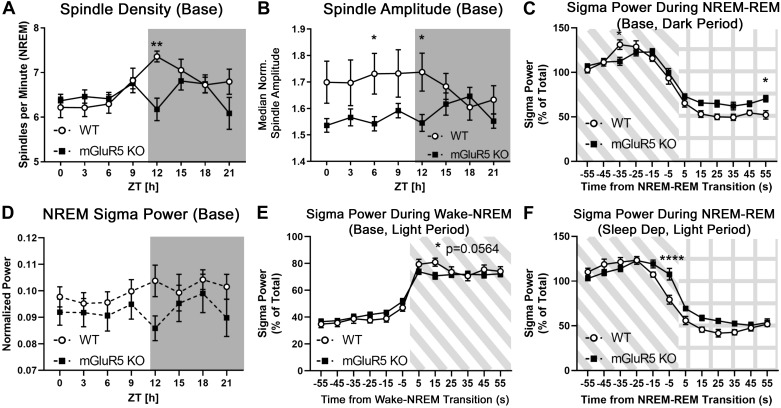
NREM sleep spindle activity was examined using an automated detection algorithm (Uygun et al. 2019). Spindle density (NREM spindles/min), median spindle duration, and median normalized spindle amplitude were analyzed at baseline and during recovery sleep. Spindle abnormalities were observed in KO animals during the transition into the dark cycle. mGluR5 KO animals exhibited significantly lower sleep spindle density than WT controls at ZT 12–15 (Fig. 6A; Holm–Sidak multiple t tests, P < 0.01; WT 7.35 ± 0.12 vs. KO 6.18 ± 0.25 spindles/min at ZT 12–15). During ZT 6–9 and ZT 12–15, median normalized spindle amplitude was lower in KO animals than in WT controls (Fig. 6B; Holm–Sidak multiple t tests, P < 0.05; WT 1.73 ± 0.08 vs. KO 1.54 ± 0.03 a.u. at ZT 6–9; WT 1.74 ± 0.07 vs. KO 1.55 ± 0.03 a.u. at ZT 12–15). These differences disappeared after sleep deprivation (not shown). Spindle duration was unchanged in both conditions.
Fig. 6.
Sleep spindle density and non-rapid eye movement (NREM) sigma power is decreased during transition periods in metabotropic glutamate receptor type 5 (mGluR5) knockout (KO) mice. A custom MATLAB script (Uygun et al. 2019) was used to extract the following NREM sleep spindle data from the EEG trace: spindle density (NREM spindles per minute), median spindle duration, and median normalized spindle amplitude. During baseline activity, mGluR5 KO animals (n = 12) exhibited significantly lower NREM spindle density than wild-type (WT) controls (n = 8) during the first 3 hours of the dark (active) period at Zeitgeber time (ZT) 12–15 (A) and lower spindle amplitude at ZT 6–9 and 12–15 (B). These differences disappeared after sleep deprivation (data not shown). NREM sigma power (10–15 Hz), which is sometimes used to approximate spindle activity, was not significantly different between groups (D). Sigma power was measured during the transitions to and from NREM sleep [C, E, and F; diagonal shaded lines indicate NREM sleep, grid indicates rapid eye movement (REM) sleep]. At baseline, the surge in sigma power is lower in KO than in WT mice 35 s before the NREM-REM transition (C, dark period) and 15 s after the wake-NREM transition (E, light period; Holm–Sidak P = 0.0564). Conversely, after 6 h of sleep deprivation, mGluR5 KO mice (n = 10) had increased sigma power when transitioning from NREM to REM sleep compared with WT controls (n = 8) (F, light period; −5 to +5 s of transition, and main effect P < 0.05). Together, these results suggest abnormal regulation of sigma power during transitions to and from NREM sleep in KO mice. *P < 0.05; **P < 0.01; ****P < 0.0001, significance from multiple t tests with a Holm–Sidak correction alone (A, B, D) or following a significant interaction in a 2-way RM ANOVA (C, E, F). Base, baseline.
An alternative method of approximating sleep spindle activity is measuring NREM sigma power (10–15 Hz) (Campbell and Feinberg 2016). NREM sigma was not significantly different in KO animals despite the observed changes in spindle density, although its pattern of activity (Fig. 6D) appears to imitate sleep spindle density (Fig. 6A). A surge in sigma power immediately preceding the NREM-REM transition is an additional metric of mouse spindle activity (Astori et al. 2011). Sigma power was measured during the transitions from wake to NREM sleep and from NREM to REM sleep (Fig. 6, C–F). At baseline, the surge in sigma power is lower in KO mice than WT 35 s before the NREM-REM transition (Fig. 6C; dark period: interaction P < 0.0001, Holm–Sidak P < 0.05). A similar deficit in NREM sigma power emerged 15 s into the transition from wake to NREM sleep (Fig. 6E; light period: interaction P < 0.001, Holm–Sidak P = 0.0564). Conversely, after sleep deprivation, mGluR5 KO mice had increased sigma power when transitioning from NREM to REM sleep (Fig. 6F;light period: main effect P < 0.05 and interaction P < 0.0001, Holm–Sidak P < 0.0001 at −5 to +5 s of transition). Together, these results suggest abnormal regulation of sigma power during transitions to and from NREM sleep in KO mice.
DISCUSSION
In this study we have examined the consequences of global deletion of mGluR5 on sleep/wake state, homeostatic sleep response, and oscillatory activity relevant to psychiatric disorders including schizophrenia. We have observed that mGluR5 KO mice recapitulate several important phenomena seen in patients with schizophrenia, including a modest deficit in sleep spindles and decreased alpha power (Newson and Thiagarajan 2019; Wamsley et al. 2012). However, unlike schizophrenia, we did not observe enhanced wake delta or wake gamma power at baseline but did see evidence of a reduction in 40-Hz task-evoked gamma and 40-Hz phase locking (visual steady-state response) that mimics auditory and visual tests in patients with schizophrenia (Brenner et al. 2009; Kwon et al. 1999; Thuné et al. 2016).
Sleep Spindles are Reduced in mGluR5 KO mice and Patients with Schizophrenia
Utilizing a recently validated method to identify mouse sleep spindles (Uygun et al. 2019; see Supplemental Fig. S6; https://doi.org/10.6084/m9.figshare.9337436.v1), we extended the scope of previous sleep studies of mGluR5 KO mice to include analysis of sleep spindle activity. For the first 3 h of the dark (active) period, mGluR5 KO mice demonstrated reductions in NREM spindle density that mimic those in patients with schizophrenia (Manoach et al. 2016; Wamsley et al. 2012). Reduced sleep spindle density is a heritable biomarker of schizophrenia that influences memory consolidation and cognition (Manoach et al. 2016). We have additionally observed abnormalities in sigma power, a commonly employed proxy for assessing spindle activity, during transitions to and from NREM sleep in mGluR5 KO mice. This aligns with prior reports of abnormal NREM sleep-state transitions in this model (Ahnaou et al. 2015b). Unit recordings in mice suggest the same TRN neurons that drive spindle activity during sleep are also associated with alpha oscillations during attentive wake (Chen et al. 2016). In addition to the deficit in sleep spindle density, we observed lower wake alpha power in mGluR5 KO mice. Therefore, these deficits may have a common underlying mechanism of TRN neuron dysfunction. Indeed, the absence of mGluR5 may disrupt TRN neuron activity by altering cholinergic regulation of this region (Sun et al. 2016). There may be other compensatory changes in the global KO mice that we cannot account for, given that loss of mGluR5 can prevent thalamocortical-cortical synapse formation (Ballester-Rosado et al. 2016) or result in chronic disruption of intracellular signaling cascades.
Power Spectral Density Changes Above Theta Range Align with Clinical Literature
Changes in resting-state EEG power in patients with schizophrenia can be variable due to the complex nature of the disorder and inconsistencies in collection methods across different studies (Boutros et al. 2008). Increased resting-state gamma power occurs in some animal models of NMDA receptor dysfunction and in a subset of patients in the early stages of psychosis, yet this is an inconsistent finding that may depend on the stage of mental illness, method of analysis, brain region recorded, or presence of a task requiring attention or cognitive load (Grent-’t-Jong et al. 2018; Hirano et al. 2015; Reilly et al. 2018; White and Siegel 2016). We observed no differences in resting (wake) gamma activity in our adult KO mice, which mimics the chronic schizophrenia condition (Di Lorenzo et al. 2015). mGluR5 KO mice represent a chronic constitutive model of NMDA receptor hypofunction, and it is possible that this phenotype may have emerged temporarily at an earlier point in development. Enhanced resting-state delta and theta power are reliable biomarkers in patients with schizophrenia that were completely absent in our mGluR5 KO mice (Boutros et al. 2008; Newson and Thiagarajan 2019). However, we observed a decrease in resting-state alpha-band power (8–13 Hz) that is a common observation in patients with schizophrenia (Boutros et al. 2008; Howells et al. 2018; Merrin and Floyd 1996; Newson and Thiagarajan 2019). Rats administered negative allosteric modulators of mGluR5 show decreased theta and alpha power (Ahnaou et al. 2015a; Harvey et al. 2013), which aligns with our findings in mGluR5 KO mice. Optogenetic inhibition of REM theta power impairs sleep-dependent memory consolidation in mice (Boyce et al. 2016). Therefore, our observation of decreased REM theta power (7–9 Hz) complements reports of sleep-dependent memory impairment in mGluR5 KO mice (Lu et al. 1997).
NREM delta power (0.5–4 Hz) represents a homeostatic sleep drive that builds during periods of extended wake and is dispelled during sleep, reaching a minimum in the last 4 h of the light phase (ZT 8–12) (Borbély et al. 2016; Holst et al. 2017). Our mGluR5 KO mice showed an attenuation in NREM delta power normalized to ZT 8–12 of baseline. This finding is consistent with results of prior reports, from studies using a similar methodological approach (Ahnaou et al. 2015b; Holst et al. 2017). However, we note that if these mice demonstrate abnormalities in circadian or daily EEG patterns, then this method of normalization may be inappropriate due to potentially altered delta power around the light-dark transitions (ZT 0 and ZT 12). Nevertheless, 24-h normalized delta power during ZT 8–12 was consistently low and overlapped between WT and KO mice, suggesting this baseline period was appropriate.
Deficit in 40-Hz Cortical Entrainment Mimics Schizophrenia
Abnormal steady-state responses within gamma frequencies may indicate dysregulation of the excitatory/inhibitory balance between pyramidal neurons and fast-spiking inhibitory interneurons, NMDA receptor dysfunction, or underlying structural changes in sensory pathways (Brenner et al. 2009). The visual steady-state response (VSSR) is a paradigm that entrains the cortex using a sequence of light flashes at specific frequencies and provides a means to assess rhythmic entrainment of neural activity. Our mGluR5 KO mice had impairments in 40-Hz stimulus-evoked gamma power and phase locking in the VSSR, which generally mimic the results of auditory and visual tests in patients with schizophrenia (Brenner et al. 2009; Kwon et al. 1999; Thuné et al. 2016). Similar visual tests in patients with schizophrenia found stimulus-evoked power deficits at low frequencies that reached into the gamma range (Krishnan et al. 2005; Rice et al. 1989) and phase-locking deficits during 40-Hz light stimulation (Riečanský et al. 2010). Barnes et al. (2015) reported increases in both baseline and evoked gamma-band power (40–54 Hz) in mice with mGluR5 genetically ablated from parvalbumin-containing neurons during postnatal development. mGluR5 is expressed in multiple cell types in rodents and primates, including parvalbumin-containing inhibitory interneurons and pyramidal neurons (López-Bendito et al. 2002; Muly et al. 2003). Therefore, it appears mGluR5 is necessary for normal entrainment of 40-Hz gamma-band oscillations, and the direction of the effect may depend on the specificity of its absence (constitutive vs. parvalbumin-specific) or the developmental timing of the knockout (prenatal vs. postnatal), which could lead to differing compensatory responses.
mGluR5 KO Mice Exhibit Abnormal Sleep Profile That Does Not Closely Mimic Schizophrenia
Although sleep disorders are extremely common in patients with schizophrenia, the specific sleep abnormality varies greatly and can include insomnia, circadian rhythm disorder, restless leg syndrome, obstructive sleep apnea, or other disturbances (Annamalai et al. 2015; Kang et al. 2007; Kaskie et al. 2017; Palmese et al. 2011; Wulff et al. 2012). Our data, supported by Holst et al. (2017), demonstrate that mGluR5 KO mice exhibit an increase in NREM sleep time and decreased time awake compared with controls. Increased NREM sleep time is also observed in Sprague-Dawley rats following allosteric blockade of mGluR5 (Ahnaou et al. 2015a). These phenotypes do not reflect those of unmedicated schizophrenia patients, many of whom suffer from insomnia (Kaskie et al. 2017; Palmese et al. 2011). However, sleep-onset insomnia can counterintuitively increase total sleep time in some patients with schizophrenia compared with healthy controls (Poulin et al. 2010). KO mice had inconsistent sleep with more fragmented NREM sleep and wake patterns, which has been observed in patients with schizophrenia but is not particularly common (Chiu et al. 2016; Wulff et al. 2012). Two independent groups observed significant decreases in REM sleep of mGluR5 KO mice at baseline and after sleep deprivation that were not reflected in our study (Ahnaou et al. 2015b; Holst et al. 2017). Our use of 10-s epochs (and episodes of 20+ s) may have affected REM sleep comparisons, given that this behavior occurs in shorter bursts than during wake or NREM sleep. In sum, the sleep phenotype of mGluR5 KO mice may mimic abnormalities observed in a small subset of patients with schizophrenia but are not reflective of disease-related sleep abnormalities overall.
Sleep Deprivation Affects WT and KO Sleep Similarly
Sleep deprivation (ZT 0–6) decreased wake and increased NREM and REM activity in all animals. These results are largely in agreement with Ahnaou et al. (2015b), except they observed no enhancement in REM sleep for mGluR5 KO mice in the dark period following sleep deprivation. Nevertheless, the REM sleep rebound we observed in KO animals (43% increase in dark period) appears markedly smaller than the REM sleep rebound in WT animals (100% increase in dark period; Supplemental Fig. S1, see https://doi.org/10.6084/m9.figshare.9337397.v1), which is consistent with their findings. Our sleep data are less consistent with those of Holst et al. (2017), who found excessive wake and minimal sleep in mGluR5 KO mice 8–20 h after sleep deprivation. The inconsistencies described above may be due to differences in the sleep deprivation methods used. Our study and that of Ahnaou et al. (2015b) used the forced locomotion method of sleep deprivation, whereas Holst et al. (2017) used gentle handling. Both WT and KO animals showed an enhancement in wake theta power during sleep deprivation and an increase in NREM delta power during recovery sleep. This suggests that sleep deprivation did enhance homeostatic sleep pressure in both strains.
mGluR5 Contributes to Circadian or Daily Rhythms in Sleep, Spindles, and EEG Power
Circadian rhythm disorder is one of the most common sleep disorders present in patients with schizophrenia (Kaskie et al. 2017; Poulin et al. 2010; Wulff et al. 2012). KO animals have dark period-related changes in sleep spindle density and sleep fragmentation, as well as reduced daily fluctuation across certain frequency bands at baseline. Specifically, the change in 12-h EEG power from the light to the dark period is greater in WT than KO animals at delta and alpha frequencies (NREM delta: WT +13%, KO −7%; wake alpha: WT+19%, KO +8%). These spindle, fragmentation, and EEG phenotypes may be influenced by an abnormal circadian pattern in mGluR5 KO mice. Indeed, human and rodent mGluR5 receptor availability fluctuates across 24 h in some (DeLorenzo et al. 2017; Elmenhorst et al. 2016) but not all (Ahnaou et al. 2015a) reports. This receptor also has an integral role in synaptic homeostasis, which is a circadian process by which synapses may be weakened during sleep (de Vivo et al. 2017). Prolonged wakefulness increases adenosine concentrations, which recruits Homer1a to the postsynaptic density of excitatory neurons, which disrupts the second messaging systems of mGluR5 during sleep (Diering et al. 2017). If this pathway was contributing to the KO-induced phenotypes, the largest differences should occur during the dark (active) period when adenosine concentrations are highest in rodents (McKenna et al. 2003). Indeed, this aligns with our observed data. This evidence suggests the mGluR5 KO mouse is missing the circadian or daily variation of a key glutamatergic receptor, which leads to abnormalities in EEG power, sleep, and spindles during the dark period.
Limitations
We note that the WT and mGluR5 KO mice were not littermates. The KO mice (Grm5tm1Rod) were a cryopreserved transgenic line reconstituted into the same C57BL/6J background strain as WT mice at the same facility (Jackson Laboratories). These congenic KO animals have been backcrossed for at least six generations into the C57BL/6J strain, suggesting their genetic differences from the background strain should be limited to the mGluR5 encoding gene Grm5. Our mGluR5 KO mouse line resulted from a mix of homozygous and heterozygous breeding; they were bred in-house with Jackson Laboratories’ C57BL/6J mice one to three generations before this study. Therefore, we used C57BL/6J mice (nonlittermate) from Jackson Laboratories as our WT control. Our WT sleep time and NREM delta power were comparable to those reported in previous literature in this strain (C57BL/6J) at baseline and after sleep deprivation (Franken et al. 1999). Although we observed no sleep deprivation-induced changes in vigilance time during the first 6 h of recovery sleep (ZT 6–12) in WT mice, our data did replicate an enhancement in NREM minutes per hour and an impairment in wake minutes per hour during the dark period following recovery sleep (ZT 12–24; Supplemental Fig. S1, see https://doi.org/10.6084/m9.figshare.9337397.v1) (Franken et al. 1999).
Conclusion
In summary, mGluR5 KO mice demonstrate several phenotypes that mimic symptoms associated with schizophrenia, including the novel observations of reduced sleep spindle density, reduced alpha power, and impaired 40-Hz visual task-evoked gamma and phase locking. These findings suggest that mGluR5-enhancing drugs may alleviate sleep spindle deficits, task-evoked gamma deficits, and specific sleep disturbances in addition to rescuing more prominent symptoms of schizophrenia. Understanding the consequences of mGluR5 modulation on sleep health is significant, because the wake-promoting properties of mGluR5-enhancing drugs could exacerbate the insomnia associated with a subset of patients with schizophrenia (Ahnaou et al. 2015a; Kaskie et al. 2017; Loomis et al. 2015; Palmese et al. 2011) . However, eszopiclone or certain atypical antipsychotics may be able to counteract this effect (Tek et al. 2014; Wiegand et al. 2008). In contrast, blocking mGluR5 is a prospective therapeutic approach in other mental illnesses and disorders, including autism, obsessive compulsive disorder, Alzheimer’s disease, and Fragile X syndrome (Harvey et al. 2013; Matosin and Siegel 2016; Sokol et al. 2011). Our data suggest mGluR5 antagonists may impair sleep spindle density, enhance sleep fragmentation and time spent in NREM sleep, and disrupt NREM sleep-state transitions. Future work should test the consequences of mGluR5-targeting compounds on sleep spindles and sleep health in diverse animal models across multiple treatment lengths and developmental periods. Aging patient populations are especially at risk because they may already be experiencing circadian abnormalities, fragmented sleep, and decreases in sleep spindles (Pace-Schott and Spencer 2011). In sum, our findings support the mGluR5 receptor as a target for novel therapeutics for schizophrenia and other neuropsychiatric conditions. However, careful consideration may be needed regarding the timing and regional targeting of experimental mGluR5-modulating drugs, so their desired effects are reached without disrupting sleep or sleep spindle activity.
GRANTS
This study was supported by US Department of Veterans Affairs Career Development Award BX002130 (to J. M. McNally), Merit Awards I01 BX004500 (to J. M. McNally), I01 BX001356 (to R. E Brown), I01 BX002774 (to R. E. Strecker), and I01 BX001404 (to R. Basheer), and National Institutes of Health Grants R01 MH39683 (to R. E. Brown), T32 MH016259 (to D. D. Aguilar and M. E. Shenton), and P01 HL095491 (to R. E. Strecker).
DISCLAIMERS
The contents of this work do not represent the views of the US Department of Veterans Affairs or the United States Government.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
R.E.S., R.B., and J.M.M. conceived and designed research; D.D.A. performed experiments; D.D.A. analyzed data; D.D.A. and J.M.M. interpreted results of experiments; D.D.A. prepared figures; D.D.A. drafted manuscript; D.D.A., R.E.S., R.B., and J.M.M. edited and revised manuscript; D.D.A., R.E.S., R.B., and J.M.M. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Ritchie E. Brown for helpful discussion, Yunren Bolortuya for assistance with EEG implantation and sleep scoring, and MacKenzie Gamble for assistance with data analysis.
REFERENCES
- Ahnaou A, Langlois X, Steckler T, Bartolome-Nebreda JM, Drinkenburg WH. Negative versus positive allosteric modulation of metabotropic glutamate receptors (mGluR5): indices for potential pro-cognitive drug properties based on EEG network oscillations and sleep-wake organization in rats. Psychopharmacology (Berl) 232: 1107–1122, 2015a. doi: 10.1007/s00213-014-3746-4. [DOI] [PubMed] [Google Scholar]
- Ahnaou A, Raeymaekers L, Steckler T, Drinkenbrug WH. Relevance of the metabotropic glutamate receptor (mGluR5) in the regulation of NREM-REM sleep cycle and homeostasis: evidence from mGluR5 (−/−) mice. Behav Brain Res 282: 218–226, 2015b. doi: 10.1016/j.bbr.2015.01.009. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, Arndt S, Swayze V 2nd, Cizadlo T, Flaum M, O’Leary D, Ehrhardt JC, Yuh WT. Thalamic abnormalities in schizophrenia visualized through magnetic resonance image averaging. Science 266: 294–298, 1994. doi: 10.1126/science.7939669. [DOI] [PubMed] [Google Scholar]
- Ango F, Robbe D, Tu JC, Xiao B, Worley PF, Pin JP, Bockaert J, Fagni L. Homer-dependent cell surface expression of metabotropic glutamate receptor type 5 in neurons. Mol Cell Neurosci 20: 323–329, 2002. doi: 10.1006/mcne.2002.1100. [DOI] [PubMed] [Google Scholar]
- Annamalai A, Palmese LB, Chwastiak LA, Srihari VH, Tek C. High rates of obstructive sleep apnea symptoms among patients with schizophrenia. Psychosomatics 56: 59–66, 2015. doi: 10.1016/j.psym.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astori S, Wimmer RD, Prosser HM, Corti C, Corsi M, Liaudet N, Volterra A, Franken P, Adelman JP, Lüthi A. The CaV3.3 calcium channel is the major sleep spindle pacemaker in thalamus. Proc Natl Acad Sci USA 108: 13823–13828, 2011. doi: 10.1073/pnas.1105115108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avram M, Brandl F, Bäuml J, Sorg C. Cortico-thalamic hypo- and hyperconnectivity extend consistently to basal ganglia in schizophrenia. Neuropsychopharmacology 43: 2239–2248, 2018. doi: 10.1038/s41386-018-0059-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayalew M, Le-Niculescu H, Levey DF, Jain N, Changala B, Patel SD, Winiger E, Breier A, Shekhar A, Amdur R, Koller D, Nurnberger JI, Corvin A, Geyer M, Tsuang MT, Salomon D, Schork NJ, Fanous AH, O’Donovan MC, Niculescu AB. Convergent functional genomics of schizophrenia: from comprehensive understanding to genetic risk prediction. Mol Psychiatry 17: 887–905, 2012. doi: 10.1038/mp.2012.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballester-Rosado CJ, Sun H, Huang JY, Lu HC. mGluR5 exerts cell-autonomous influences on the functional and anatomical development of layer IV cortical neurons in the mouse primary somatosensory cortex. J Neurosci 36: 8802–8814, 2016. doi: 10.1523/JNEUROSCI.1224-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes SA, Pinto-Duarte A, Kappe A, Zembrzycki A, Metzler A, Mukamel EA, Lucero J, Wang X, Sejnowski TJ, Markou A, Behrens MM. Disruption of mGluR5 in parvalbumin-positive interneurons induces core features of neurodevelopmental disorders. Mol Psychiatry 20: 1161–1172, 2015. doi: 10.1038/mp.2015.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bódizs R, Kis T, Lázár AS, Havrán L, Rigó P, Clemens Z, Halász P. Prediction of general mental ability based on neural oscillation measures of sleep. J Sleep Res 14: 285–292, 2005. doi: 10.1111/j.1365-2869.2005.00472.x. [DOI] [PubMed] [Google Scholar]
- Borbély AA, Daan S, Wirz-Justice A, Deboer T. The two-process model of sleep regulation: a reappraisal. J Sleep Res 25: 131–143, 2016. doi: 10.1111/jsr.12371. [DOI] [PubMed] [Google Scholar]
- Boutros NN, Arfken C, Galderisi S, Warrick J, Pratt G, Iacono W. The status of spectral EEG abnormality as a diagnostic test for schizophrenia. Schizophr Res 99: 225–237, 2008. doi: 10.1016/j.schres.2007.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyce R, Glasgow SD, Williams S, Adamantidis A. Causal evidence for the role of REM sleep theta rhythm in contextual memory consolidation. Science 352: 812–816, 2016. doi: 10.1126/science.aad5252. [DOI] [PubMed] [Google Scholar]
- Brenner CA, Krishnan GP, Vohs JL, Ahn WY, Hetrick WP, Morzorati SL, O’Donnell BF. Steady state responses: electrophysiological assessment of sensory function in schizophrenia. Schizophr Bull 35: 1065–1077, 2009. doi: 10.1093/schbul/sbp091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell IG, Feinberg I. Maturational patterns of sigma frequency power across childhood and adolescence: a longitudinal study. Sleep (Basel) 39: 193–201, 2016. doi: 10.5665/sleep.5346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Wimmer RD, Wilson MA, Halassa MM. Thalamic circuit mechanisms link sensory processing in sleep and attention. Front Neural Circuits 9: 83, 2016. doi: 10.3389/fncir.2015.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu VW, Ree M, Janca A, Waters F. Sleep in schizophrenia: exploring subjective experiences of sleep problems, and implications for treatment. Psychiatr Q 87: 633–648, 2016. doi: 10.1007/s11126-015-9415-x. [DOI] [PubMed] [Google Scholar]
- Dash MB, Douglas CL, Vyazovskiy VV, Cirelli C, Tononi G. Long-term homeostasis of extracellular glutamate in the rat cerebral cortex across sleep and waking states. J Neurosci 29: 620–629, 2009. doi: 10.1523/JNEUROSCI.5486-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bartolomeis A, Szumlinski K. Group 1 metabotropic glutamate receptors and schizophrenia. WIREs Membr Transp Signal 1: 94–103, 2012. doi: 10.1002/wmts.15. [DOI] [Google Scholar]
- de Vivo L, Bellesi M, Marshall W, Bushong EA, Ellisman MH, Tononi G, Cirelli C. Ultrastructural evidence for synaptic scaling across the wake/sleep cycle. Science 355: 507–510, 2017. doi: 10.1126/science.aah5982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLorenzo C, Gallezot JD, Gardus J, Yang J, Planeta B, Nabulsi N, Ogden RT, Labaree DC, Huang YH, Mann JJ, Gasparini F, Lin X, Javitch JA, Parsey RV, Carson RE, Esterlis I. In vivo variation in same-day estimates of metabotropic glutamate receptor subtype 5 binding using [11C]ABP688 and [18F]FPEB. J Cereb Blood Flow Metab 37: 2716–2727, 2017. doi: 10.1177/0271678X16673646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lorenzo G, Daverio A, Ferrentino F, Santarnecchi E, Ciabattini F, Monaco L, Lisi G, Barone Y, Di Lorenzo C, Niolu C, Seri S, Siracusano A. Altered resting-state EEG source functional connectivity in schizophrenia: the effect of illness duration. Front Hum Neurosci 9: 234, 2015. doi: 10.3389/fnhum.2015.00234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diering GH, Nirujogi RS, Roth RH, Worley PF, Pandey A, Huganir RL. Homer1a drives homeostatic scaling-down of excitatory synapses during sleep. Science 355: 511–515, 2017. doi: 10.1126/science.aai8355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmenhorst D, Mertens K, Kroll T, Oskamp A, Ermert J, Elmenhorst EM, Wedekind F, Beer S, Coenen HH, Bauer A. Circadian variation of metabotropic glutamate receptor 5 availability in the rat brain. J Sleep Res 25: 754–761, 2016. doi: 10.1111/jsr.12432. [DOI] [PubMed] [Google Scholar]
- Franken P, Malafosse A, Tafti M. Genetic determinants of sleep regulation in inbred mice. Sleep 22: 155–169, 1999. [PubMed] [Google Scholar]
- Goder R, Graf A, Ballhausen F, Weinhold S, Baier PC, Junghanns K, Prehn-Kristensen A. Impairment of sleep-related memory consolidation in schizophrenia: relevance of sleep spindles? Sleep Med 16: 564–569, 2015. doi: 10.1016/j.sleep.2014.12.022. [DOI] [PubMed] [Google Scholar]
- Grent-’t-Jong T, Gross J, Goense J, Wibral M, Gajwani R, Gumley AI, Lawrie SM, Schwannauer M, Schultze-Lutter F, Navarro Schröder T, Koethe D, Leweke FM, Singer W, Uhlhaas PJ. Resting-state gamma-band power alterations in schizophrenia reveal E/I-balance abnormalities across illness-stages. eLife 7: e37799, 2018. doi: 10.7554/eLife.37799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey BD, Siok CJ, Kiss T, Volfson D, Grimwood S, Shaffer CL, Hajós M. Neurophysiological signals as potential translatable biomarkers for modulation of metabotropic glutamate 5 receptors. Neuropharmacology 75: 19–30, 2013. doi: 10.1016/j.neuropharm.2013.06.020. [DOI] [PubMed] [Google Scholar]
- Hirano Y, Oribe N, Kanba S, Onitsuka T, Nestor PG, Spencer KM. Spontaneous gamma activity in schizophrenia. JAMA Psychiatry 72: 813–821, 2015. doi: 10.1001/jamapsychiatry.2014.2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holst SC, Sousek A, Hefti K, Saberi-Moghadam S, Buck A, Ametamey SM, Scheidegger M, Franken P, Henning A, Seifritz E, Tafti M, Landolt HP. Cerebral mGluR5 availability contributes to elevated sleep need and behavioral adjustment after sleep deprivation. eLife 6: e28751, 2017. doi: 10.7554/eLife.28751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howells FM, Temmingh HS, Hsieh JH, van Dijen AV, Baldwin DS, Stein DJ. Electroencephalographic delta/alpha frequency activity differentiates psychotic disorders: a study of schizophrenia, bipolar disorder and methamphetamine-induced psychotic disorder. Transl Psychiatry 8: 75, 2018. doi: 10.1038/s41398-018-0105-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang SG, Lee HJ, Jung SW, Cho SN, Han C, Kim YK, Kim SH, Lee MS, Joe SH, Jung IK, Kim L. Characteristics and clinical correlates of restless legs syndrome in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry 31: 1078–1083, 2007. doi: 10.1016/j.pnpbp.2007.03.011. [DOI] [PubMed] [Google Scholar]
- Kaskie RE, Graziano B, Ferrarelli F. Schizophrenia and sleep disorders: links, risks, and management challenges. Nat Sci Sleep 9: 227–239, 2017. doi: 10.2147/NSS.S121076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan GP, Vohs JL, Hetrick WP, Carroll CA, Shekhar A, Bockbrader MA, O’Donnell BF. Steady state visual evoked potential abnormalities in schizophrenia. Clin Neurophysiol 116: 614–624, 2005. doi: 10.1016/j.clinph.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Kwon JS, O’Donnell BF, Wallenstein GV, Greene RW, Hirayasu Y, Nestor PG, Hasselmo ME, Potts GF, Shenton ME, McCarley RW. Gamma frequency-range abnormalities to auditory stimulation in schizophrenia. Arch Gen Psychiatry 56: 1001–1005, 1999. doi: 10.1001/archpsyc.56.11.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R, Urbano FJ, Leznik E, Ramírez RR, van Marle HJ. Rhythmic and dysrhythmic thalamocortical dynamics: GABA systems and the edge effect. Trends Neurosci 28: 325–333, 2005. doi: 10.1016/j.tins.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Loomis S, McCarthy A, Baxter C, Kellett DO, Edgar DM, Tricklebank M, Gilmour G. Distinct pro-vigilant profile induced in rats by the mGluR5 potentiator LSN2814617. Psychopharmacology (Berl) 232: 3977–3989, 2015. doi: 10.1007/s00213-015-3936-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Bendito G, Shigemoto R, Fairén A, Luján R. Differential distribution of group I metabotropic glutamate receptors during rat cortical development. Cereb Cortex 12: 625–638, 2002. doi: 10.1093/cercor/12.6.625. [DOI] [PubMed] [Google Scholar]
- Lu YM, Jia Z, Janus C, Henderson JT, Gerlai R, Wojtowicz JM, Roder JC. Mice lacking metabotropic glutamate receptor 5 show impaired learning and reduced CA1 long-term potentiation (LTP) but normal CA3 LTP. J Neurosci 17: 5196–5205, 1997. doi: 10.1523/JNEUROSCI.17-13-05196.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoach DS, Pan JQ, Purcell SM, Stickgold R. Reduced sleep spindles in schizophrenia: a treatable endophenotype that links risk genes to impaired cognition? Biol Psychiatry 80: 599–608, 2016. doi: 10.1016/j.biopsych.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin JL, Jeste DV, Ancoli-Israel S. Older schizophrenia patients have more disrupted sleep and circadian rhythms than age-matched comparison subjects. J Psychiatr Res 39: 251–259, 2005. doi: 10.1016/j.jpsychires.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Matosin N, Newell KA, Quidé Y, Andrews JL, Teroganova N, Green MJ, Fernandez F. Effects of common GRM5 genetic variants on cognition, hippocampal volume and mGluR5 protein levels in schizophrenia. Brain Imaging Behav 12: 509–517, 2018. doi: 10.1007/s11682-017-9712-0. [DOI] [PubMed] [Google Scholar]
- Matosin N, Siegel SJ. Metabotropic glutamate receptor 5 as a point of convergence for models of obsessive-compulsive disorder and autism spectrum disorder. Biol Psychiatry 80: 504–506, 2016. doi: 10.1016/j.biopsych.2016.08.005. [DOI] [PubMed] [Google Scholar]
- Matta JA, Ashby MC, Sanz-Clemente A, Roche KW, Isaac JT. mGluR5 and NMDA receptors drive the experience- and activity-dependent NMDA receptor NR2B to NR2A subunit switch. Neuron 70: 339–351, 2011. doi: 10.1016/j.neuron.2011.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna JT, Dauphin LJ, Mulkern KJ, Stronge AM, McCarley RW, Strecker RE. Nocturnal elevation of extracellular adenosine in the rat basal forebrain. Sleep Res Online 5: 155–160, 2003. [Google Scholar]
- McNally JM, McCarley RW. Gamma band oscillations: a key to understanding schizophrenia symptoms and neural circuit abnormalities. Curr Opin Psychiatry 29: 202–210, 2016. doi: 10.1097/YCO.0000000000000244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrin EL, Floyd TC. Negative symptoms and EEG alpha in schizophrenia: a replication. Schizophr Res 19: 151–161, 1996. doi: 10.1016/0920-9964(96)88522-7. [DOI] [PubMed] [Google Scholar]
- Mitra P, Bokil H. Observed Brain Dynamics. New York: Oxford University Press, 2007. [Google Scholar]
- Muly EC, Maddox M, Smith Y. Distribution of mGluR1alpha and mGluR5 immunolabeling in primate prefrontal cortex. J Comp Neurol 467: 521–535, 2003. doi: 10.1002/cne.10937. [DOI] [PubMed] [Google Scholar]
- Newson JJ, Thiagarajan TC. EEG frequency bands in psychiatric disorders: a review of resting state studies. Front Hum Neurosci 12: 521, 2019. doi: 10.3389/fnhum.2018.00521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace-Schott EF, Spencer RM. Age-related changes in the cognitive function of sleep. Prog Brain Res 191: 75–89, 2011. doi: 10.1016/B978-0-444-53752-2.00012-6. [DOI] [PubMed] [Google Scholar]
- Palmese LB, DeGeorge PC, Ratliff JC, Srihari VH, Wexler BE, Krystal AD, Tek C. Insomnia is frequent in schizophrenia and associated with night eating and obesity. Schizophr Res 133: 238–243, 2011. doi: 10.1016/j.schres.2011.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulin J, Chouinard S, Pampoulova T, Lecomte Y, Stip E, Godbout R. Sleep habits in middle-aged, non-hospitalized men and women with schizophrenia: a comparison with healthy controls. Psychiatry Res 179: 274–278, 2010. doi: 10.1016/j.psychres.2009.08.009. [DOI] [PubMed] [Google Scholar]
- Reilly TJ, Nottage JF, Studerus E, Rutigliano G, Micheli AI, Fusar-Poli P, McGuire P. Gamma band oscillations in the early phase of psychosis: a systematic review. Neurosci Biobehav Rev 90: 381–399, 2018. doi: 10.1016/j.neubiorev.2018.04.006. [DOI] [PubMed] [Google Scholar]
- Rice DM, Potkin SG, Jin Y, Isenhart R, Heh CW, Sramek J, Costa J, Sandman CA. EEG alpha photic driving abnormalities in chronic schizophrenia. Psychiatry Res 30: 313–324, 1989. doi: 10.1016/0165-1781(89)90022-X. [DOI] [PubMed] [Google Scholar]
- Riečanský I, Kašpárek T, Rehulová J, Katina S, Přikryl R. Aberrant EEG responses to gamma-frequency visual stimulation in schizophrenia. Schizophr Res 124: 101–109, 2010. doi: 10.1016/j.schres.2010.06.022. [DOI] [PubMed] [Google Scholar]
- Ruhrmann S, Schultze-Lutter F, Salokangas RK, Heinimaa M, Linszen D, Dingemans P, Birchwood M, Patterson P, Juckel G, Heinz A, Morrison A, Lewis S, von Reventlow HG, Klosterkötter J. Prediction of psychosis in adolescents and young adults at high risk: results from the prospective European prediction of psychosis study. Arch Gen Psychiatry 67: 241–251, 2010. doi: 10.1001/archgenpsychiatry.2009.206. [DOI] [PubMed] [Google Scholar]
- Schizophrenia Working Group of the Psychiatric Genomics Consortium Biological insights from 108 schizophrenia-associated genetic loci. Nature 511: 421–427, 2014. doi: 10.1038/nature13595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokol DK, Maloney B, Long JM, Ray B, Lahiri DK. Autism, Alzheimer disease, and fragile X: APP, FMRP, and mGluR5 are molecular links. Neurology 76: 1344–1352, 2011. doi: 10.1212/WNL.0b013e3182166dc7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun YG, Rupprecht V, Zhou L, Dasgupta R, Seibt F, Beierlein M. mGluR1 and mGluR5 synergistically control cholinergic synaptic transmission in the thalamic reticular nucleus. J Neurosci 36: 7886–7896, 2016. doi: 10.1523/JNEUROSCI.0409-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tek C, Palmese LB, Krystal AD, Srihari VH, DeGeorge PC, Reutenauer EL, Guloksuz S. The impact of eszopiclone on sleep and cognition in patients with schizophrenia and insomnia: a double-blind, randomized, placebo-controlled trial. Schizophr Res 160: 180–185, 2014. doi: 10.1016/j.schres.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thuné H, Recasens M, Uhlhaas PJ. The 40-Hz auditory steady-state response in patients with schizophrenia: a meta-analysis. JAMA Psychiatry 73: 1145–1153, 2016. doi: 10.1001/jamapsychiatry.2016.2619. [DOI] [PubMed] [Google Scholar]
- Uygun DS, Katsuki F, Bolortuya Y, Aguilar DD, McKenna JT, Thankachan S, McCarley RW, Basheer R, Brown RE, Strecker RE, McNally JM. Validation of an automated sleep spindle detection method for mouse EEG. Sleep 42: 2019. doi: 10.1093/sleep/zsy218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyazovskiy VV, Tobler I. Theta activity in the waking EEG is a marker of sleep propensity in the rat. Brain Res 1050: 64–71, 2005. doi: 10.1016/j.brainres.2005.05.022. [DOI] [PubMed] [Google Scholar]
- Wamsley EJ, Tucker MA, Shinn AK, Ono KE, McKinley SK, Ely AV, Goff DC, Stickgold R, Manoach DS. Reduced sleep spindles and spindle coherence in schizophrenia: mechanisms of impaired memory consolidation? Biol Psychiatry 71: 154–161, 2012. doi: 10.1016/j.biopsych.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Zhuo M. Group I metabotropic glutamate receptor-mediated gene transcription and implications for synaptic plasticity and diseases. Front Pharmacol 3: 189, 2012. doi: 10.3389/fphar.2012.00189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White RS, Siegel SJ. Cellular and circuit models of increased resting-state network gamma activity in schizophrenia. Neuroscience 321: 66–76, 2016. doi: 10.1016/j.neuroscience.2015.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiegand MH, Landry F, Brückner T, Pohl C, Veselý Z, Jahn T. Quetiapine in primary insomnia: a pilot study. Psychopharmacology (Berl) 196: 337–338, 2008. doi: 10.1007/s00213-007-0968-8. [DOI] [PubMed] [Google Scholar]
- Wilkening S, Chen B, Bermejo JL, Canzian F. Is there still a need for candidate gene approaches in the era of genome-wide association studies? Genomics 93: 415–419, 2009. doi: 10.1016/j.ygeno.2008.12.011. [DOI] [PubMed] [Google Scholar]
- Wulff K, Dijk DJ, Middleton B, Foster RG, Joyce EM. Sleep and circadian rhythm disruption in schizophrenia. Br J Psychiatry 200: 308–316, 2012. doi: 10.1192/bjp.bp.111.096321. [DOI] [PMC free article] [PubMed] [Google Scholar]