Abstract
Sensorimotor training providing motion-dependent somatosensory feedback to spinal locomotor networks restores treadmill weight-bearing stepping on flat surfaces in spinal cats. In this study, we examined if locomotor ability on flat surfaces transfers to sloped surfaces and the contribution of length-dependent sensory feedback from lateral gastrocnemius (LG) and soleus (Sol) to locomotor recovery after spinal transection and locomotor training. We compared kinematics and muscle activity at different slopes (±10° and ±25°) in spinalized cats (n = 8) trained to walk on a flat treadmill. Half of those animals had their right hindlimb LG/Sol nerve cut and reattached before spinal transection and locomotor training, a procedure called muscle self-reinnervation that leads to elimination of autogenic monosynaptic length feedback in spinally intact animals. All spinal animals trained on a flat surface were able to walk on slopes with minimal differences in walking kinematics and muscle activity between animals with/without LG/Sol self-reinnervation. We found minimal changes in kinematics and muscle activity at lower slopes (±10°), indicating that walking patterns obtained on flat surfaces are robust enough to accommodate low slopes. Contrary to results in spinal intact animals, force responses to muscle stretch largely returned in both SELF-REINNERVATED muscles for the trained spinalized animals. Overall, our results indicate that the locomotor patterns acquired with training on a level surface transfer to walking on low slopes and that spinalization may allow the recovery of autogenic monosynaptic length feedback following muscle self-reinnervation.
NEW & NOTEWORTHY Spinal locomotor networks locomotor trained on a flat surface can adapt the locomotor output to slope walking, up to ±25° of slope, even with total absence of supraspinal CONTROL. Autogenic length feedback (stretch reflex) shows signs of recovery in spinalized animals, contrary to results in spinally intact animals.
Keywords: afferent feedback, locomotion, reinnervation, spinal cord injury
INTRODUCTION
Complete spinal cord injury (SCI) removes supraspinal input to the spinal locomotor network, causing spastic paralysis of the hindlimbs. Sensorimotor training that provides motion-dependent somatosensory feedback to spinal locomotor networks restores treadmill weight-bearing stepping in spinal cats and rats and improves locomotion in people with incomplete spinal cord injury (Edgerton et al. 2008; Hubli and Dietz 2013; Rossignol 2006). The benefits of somatosensory training are thought to arise from functionally appropriate changes in gain of proprioceptive and cutaneous afferent pathways (Côté and Gossard 2004; Côté et al. 2003) and from plastic changes in the spinal interneuronal networks mediated by motion-dependent sensory input (Côté et al. 2011; Edgerton et al. 2001).
In an intact animal, adaptation to slope walking is easily accomplished by modifying the motor output from the supraspinal locomotor centers. A complete spinal cord injury removes the modulation in supraspinal output to the spinal locomotor networks. Although sensorimotor treadmill training restores walking on a flat treadmill in spinalized cats (Bélanger et al. 1988; Lovely et al. 1986), the gait pattern does not seem to adapt to changes in slopes (Bélanger et al. 1998), an observation also reported in incomplete spinal cord injury subjects walking upslope (Leroux et al. 1999). In addition to descending commands to the spinal networks CONTROLling hindlimbs (Gottschall and Nichols 2007; Matsuyama and Drew 2000), local afferent feedback also contributes to hindlimb locomotor kinematics on flat and inclined surfaces (Abelew et al. 2000; Maas et al. 2007; McVea et al. 2005), yet the relative contribution of supraspinal input and afferent feedback on the locomotor adaptation to slope is not fully understood.
Modeling studies using a neuromechanical model of the cat’s hindlimbs and locomotor circuitry have shown that in the absence of modulation in the supraspinal drive or afferent feedback gains, a model with a constant drive and gains adjusted to produce stable locomotion on a level surface was incapable of upslope or downslope walking (±50% or ±27°; Klishko et al. 2015). No feedback gain values could be found that allowed 27° upslope walking, although changes in the supraspinal drive to the locomotor circuitry components allowed the model to walk upslope or downslope. In a spinal cord injury animal model, potential adaptations in the locomotor patterns of spinalized animals in which modulation of the supraspinal drive is no longer possible would have to be dependent on afferent feedback.
Various studies have established the role of sensory feedback in the locomotor recovery obtained with body weight-supported training. Removal of sensory input from muscle spindles (Takeoka and Arber 2019; Takeoka et al. 2014) or foot cutaneous afferents (Bouyer and Rossignol 2003) prevents recovery of proper weight-bearing stepping in spinal mice and cats, respectively. Although the studies of Takeoka and colleagues (Takeoka and Arber 2019; Takeoka et al. 2014) clearly demonstrated the importance of muscle length-dependent sensory input for locomotor recovery by removing muscle spindles in one hindlimb by genetic manipulations in mice, it did not address the contribution of length-dependent sensory inputs from distinct muscle groups in locomotor recovery. It is known that activation of group I and II sensory afferents from ankle flexors and extensors and from hip flexors directly affects phase and rhythm of fictive locomotion (Conway et al. 1987; Perreault et al. 1995; Stecina et al. 2005). It has also been shown that stretch of the triceps surae during walking in the decerebrate cat substantially increases triceps surae electromyographic (EMG) activity (Stein et al. 2000). Finally, removal of stretch reflex from ankle extensors by muscle self-reinnervation (Cope et al. 1994; Lyle et al. 2016) in otherwise intact cats leads to locomotor deficits during the stance phase of downslope walking as opposed to flat or upslope walking (Abelew et al. 2000; Gregor et al. 2018; Maas et al. 2007). This result is consistent with the fact that muscle fascicles stretch to a much larger extent during the stance phase of downslope walking than that of flat or upslope walking (Maas et al. 2009).
Thus proprioceptive feedback of the ankle extensors plays a particular role in regulating the locomotor output as discussed above. In the intact (nonspinalized) cat, complete denervation of the soleus (Sol) and lateral gastrocnemius (LG) results in an increase in activity of the remaining synergists, including the medial gastrocnemius (MG) (Donelan et al. 2009; Maas et al. 2010) and plantaris (Gregor et al. 2018). In a locomotor-trained complete spinal animal, the ankle yield at paw contact increases following LG/Sol nerve section, but returns to functional levels within a few day as synergist muscles’ activity increases, highlighting the adaptability of the isolated spinal locomotor networks (Bouyer et al. 2001; Frigon and Rossignol 2008).
Nerve section leads to a loss of all proprioceptive feedback and motor output from the denervated muscles. A more focused preparation to study the role of length sensory feedback has been muscle self-reinnervation, particularly of the ankle extensors (Abelew et al. 2000; Maas et al. 2007). This technique involves transecting the nerve and immediately repairing it by suturing or gluing the proximal and distal nerve stumps to permit axonal regeneration (English 2005). The motor and sensory axons regenerate and restore functional activity in the muscles they innervate (Gregor et al. 2018; Haftel et al. 2005). However, a consequence of this nerve disruption is the loss of the autogenic stretch reflex in self-reinnervated muscles (Cope et al. 1994; Lyle et al. 2016), which might explain an abnormal increase in ankle joint yield during stance of downslope walking in this preparation (Abelew et al. 2000; Maas et al. 2007; Pantall et al. 2016).
The goals of this study were 1) to evaluate if locomotion at different inclines is possible in a complete spinal cat trained on a flat treadmill and 2) to determine the contribution of stretch feedback from LG and Sol muscles to recovery of locomotion at different slopes following self-reinnervation of those two muscles in the absence of supraspinal influences. To address these goals, we examined the hindlimb joint kinematics, including joint angles, and muscle activity during flat, upslope, and downslope locomotion before and after spinalization in two groups of cats locomotor trained on a flat surface following spinalization, one group with intact and one with self-reinnervated LG and Sol in the right hindlimb. Surprisingly, recordings of the muscle force responses to stretch-hold-release perturbations during a terminal experiment showed signs of stretch reflex recovery in the self-reinnervated muscles in contrast to results in spinally intact animals. This recovery may have contributed to the similar recovery of treadmill weight-bearing stepping in the two groups of spinalized animals with limited adaptation to ±10° slope, which we took as an indicator of the robustness of the spinal motor output to slope. When tested at ±25° slope, the kinematics of gait changed markedly in the one spinal animal with LG/Sol self-reinnervation tested, suggesting that adaptation to locomotion on slope has limits in complete spinal animals.
METHODS
Timeline, Procedures, and Recordings
Experimental timeline.
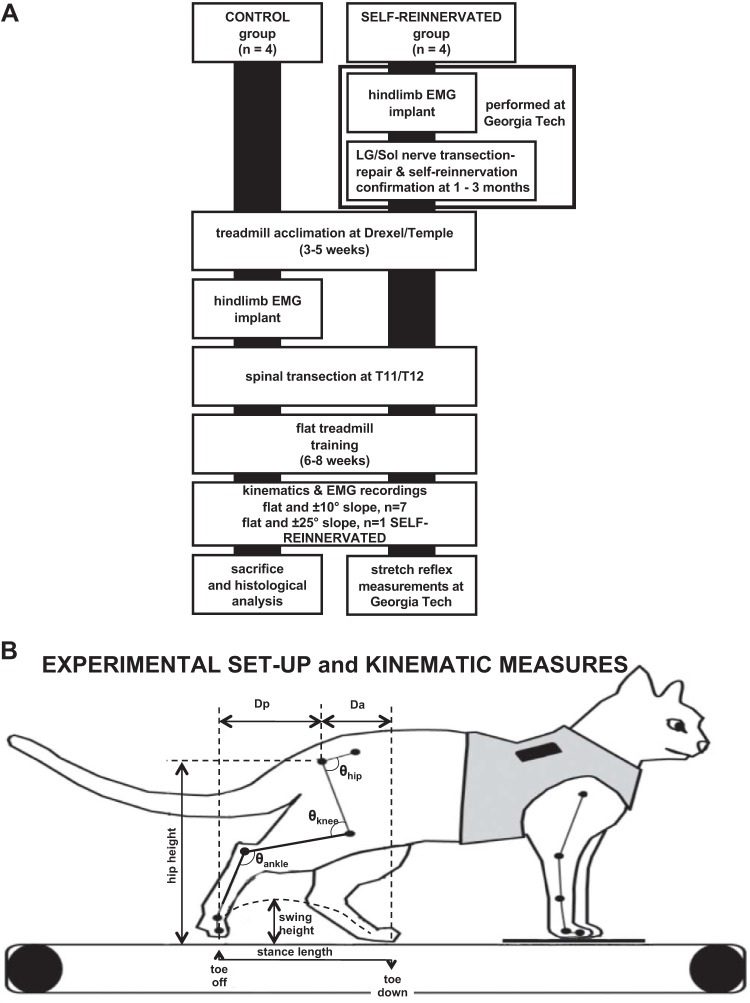
All animal care and procedures were approved by the Institutional Animal Care and Use Committee of Drexel University, Temple University, and Georgia Institute of Technology (Georgia Tech) and were performed according to NIH guidelines. Eight adult domestic shorthair female cats (2.6–4.1 kg) were used in these experiments. Females were used to facilitate manual bladder management. Four of these animals underwent implantation of muscle EMG electrodes, a transection and repair of the nerves to LG and Sol muscles in the right hindlimb, and recordings of muscle activity during locomotion pre-nerve transection/repair surgery, at 1–2 wk postsurgery to confirm muscle denervation, and at 5 wk to 3 mo postsurgery to confirm muscle self-reinnervation. These experiments were performed at Georgia Tech (see Pantall et al. 2016; Prilutsky et al. 2011) before animals were transported to Drexel University (or Temple University, 1 animal: SELF-REINNERVATED subject 4). The other four animals were implanted with EMG electrodes and maintained at Drexel University throughout the study. The experimental timeline for both groups is presented in Fig. 1A.
Fig. 1.
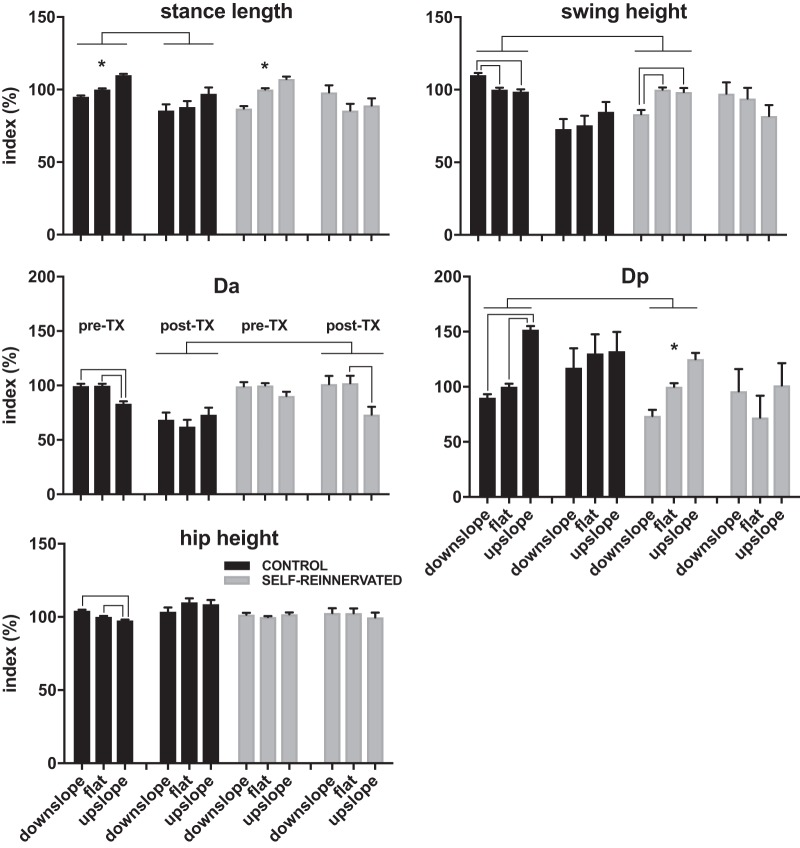
A: experimental timeline for the animals in the study. CONTROL animals were acclimated to walking on a flat treadmill, and baseline kinematics recordings were taken before and after muscle electromyogram (EMG) electrode implantation during walking on level and 10° upslope/downslope treadmill surfaces. Animals received from Georgia Tech (SELF-REINNERVATED) had already undergone EMG electrode implantation and lateral gastrocnemius/soleus (LG/Sol) nerve transection-repair surgery and had been evaluated to confirm muscle self-reinnervation 5 wk to 3 mo after surgery during walking on flat and ±27° (50%) grade as described in previous studies (Gregor et al. 2018; Pantall et al. 2016). Recordings were taken at Georgia Tech before and after EMG implantation and at various time points following LG/Sol nerve transection-repair. After baseline recordings were taken on all animals at Drexel/Temple, a complete spinal transection was performed at T11/T12. Animals were locomotor trained following transection for ~6–8 wk, at which point weight-bearing stepping had returned and performance plateaued. Posttransection recordings were then taken on a flat, 10° upslope, and 10° downslope grade over multiple sessions. One animal with LG/Sol self-reinnervation was also evaluated at ±25° of slope. SELF-REINNERVATED animals were then returned to Georgia Tech for terminal measurements (Lyle et al. 2016) that evaluated the stretch reflex response in the Sol and LG muscles. B: schematic of experimental apparatus showing the marker locations and kinematics measures used to evaluate the changes in locomotion. The animal’s forelimbs were standing on a fixed platform while the hindlimbs walked over the treadmill belt. Kinematics measures extracted included stance length (horizontal distance from toe down to toe off), swing height (maximum height of the metatarsophalangeal marker above the treadmill during swing), hip height (average height of the hip marker over the step), Dp (horizontal displacement of the toe marker relative to the hip at toe off), Da (horizontal displacement of the toe marker relative to the hip at toe down), and hip, knee, and ankle joint angles (θ). EMGs from several right hindlimb muscles were simultaneously acquired with the kinematics data.
The cats without muscle self-reinnervation surgery (CONTROL group) underwent 3–5 wk of daily level treadmill walking sessions, and kinematics of locomotion were recorded once the animals were acclimated to the treadmill. The cats were then implanted with chronic EMG bipolar electrodes and allowed to recover for at least 1 wk. After recovery, the cats received one final week of acclimation training where walking performance was monitored to ensure that the implanted electrodes did not alter gait. Pretransection EMG and kinematic recordings were taken on flat, upslope (10°), and downslope (−10°) treadmill as a baseline for comparison. Cats were then transected at the T11/T12 spinal level and given 1 wk for recovery.
The animals from Georgia Tech (SELF-REINNERVATED group) were acclimated and evaluated on overground walking at Georgia Tech. They were transported to Drexel/Temple University at least 5 wk after the LG/Sol nerve transection-repair surgery when recordings of Sol muscle activity during flat, upslope, and downslope (±27°) walking showed a return of muscle innervation in all animals (Fig. 2), as was the case in our previous studies (Gregor et al. 2018; Pantall et al. 2016). Once at Drexel/Temple University, each cat underwent 1–2 wk of daily level treadmill walking sessions, and pre-spinal transection EMG and kinematic recordings were taken on flat, upslope, and downslope treadmill as a baseline for comparison. Cats were then transected at the T11/T12 spinal level and given 1 wk for recovery.
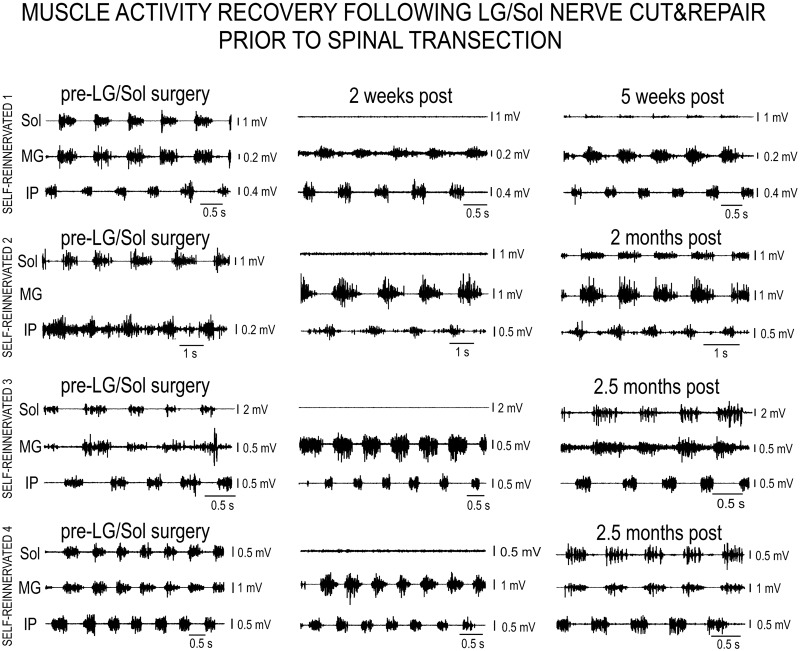
Fig. 2.
Electromyographic (EMG) activity of selected muscles for Self-reinnervated animals (SELF-REINNERVATED 1–4) before lateral gastrocnemius/soleus (pre-LG/Sol) nerve cut and repair (left column), 2 wk post nerve repair (middle column), and 5 wk to 2.5 mo post nerve repair (right column). Motor activity during walking is present in the Sol muscle before nerve cut, is absent at 2 wk post-repair, and returns by ~5 wk, with the activity getting more intense by 2.5 mo. The progression shows the denervation brought on by the nerve cut and subsequent motor reinnervation.
Following recovery from the spinal transection, all cats were treadmill trained (20 min/day, 5 days/wk for 6 wk at 0° slope) using perineal stimulation, as needed, to assist in full weight-bearing stepping (Boyce et al. 2007). After 2–4 wk of training, animals were capable of stepping for periods of 10–20 min without requiring perineal stimulation. Locomotor performance data were recorded for each animal to monitor progress until the animals reached a performance plateau where stance length and swing height (Krupka et al. 2017) were stable from session to session; this generally occurred after 6–8 wk of training. Animals’ locomotor kinematics and muscle activity were then recorded on a flat, upslope (10°), and downslope (−10°) treadmill at 0.4 m/s. In one SELF-REINNERVATED animal, kinematics were also measured at a 25° upslope and downslope grade. To be considered successful stepping, animals had to make 10 consecutive plantar weight-bearing steps at 0.4 m/s. On completion of recordings, animals from Drexel University were euthanized and perfused with 4% paraformaldehyde, and the spinal tissue was retrieved to confirm completeness of the lesions. Animals from Georgia Tech were returned to Georgia Tech, and autogenic stretch reflex responses in the self-reinnervated Sol and LG, as well as in the intact right hindlimb medial gastrocnemius (MG) synergist and intact contralateral Sol and LG muscles, were measured during a terminal experiment using previously described procedures (Lyle et al. 2016).
Nerve transection-repair and EMG electrode implantation procedures.
All EMG electrode implantation and nerve transection-repair survival surgeries at Georgia Tech were performed under general anesthesia and aseptic conditions. Each animal was first anesthetized using ketamine (10 mg/kg sc), atropine (0.05 mg/kg sc), and isoflurane (inhalation, 5%). Subsequently, the animal was intubated and anesthesia was maintained with isoflurane (1–3%). Temperature, heart rate, blood pressure, and respiration were continuously monitored throughout the surgery. At the end of surgery, the following pain medication was administered: fentanyl transdermal patch (2–25 µg/h, 72 h) and/or buprenorphine (0.01 mg/kg sc) or ketoprofen (2 mg/kg sc). The animal recovered from several days (after nerve transection and repair) to 2 wk (after electrode implantation) with the pain medication administered for at least 3 days postoperatively and antibiotics (cefovecin, 8 mg/kg sc; or ceftiofur, 4 mg/kg sc) for 10 days.
EMG activity was recorded unilaterally from 11 hindlimb muscles. These included one-joint muscles: hip flexor, iliopsoas (IP); knee extensor, vastus lateralis (VL); ankle extensor, soleus (Sol); and ankle flexor, tibialis anterior (TA); and two-joint muscles: hip flexor/knee extensor, sartorius anterior (SartA); hip and knee flexor, sartorius medialis (SartM); hip extensor/knee flexor, biceps femoris posterior (BFP); hip and knee extensor, biceps femoris anterior (BFA); knee flexor/ankle extensor, gastrocnemius medialis (MG); ankle flexor/toe extensor, extensor digitorum longus (EDL); and ankle extensor/toe flexor, flexor digitorum longus (FDL). Muscles were implanted with bifilar electrodes constructed of insulated multistranded stainless steel wires (AS 633; Cooner Wire, Chatsworth, CA) (Loeb and Gans 1986; Prilutsky et al. 2011). Proper localization of the electrodes into the muscle of interest was verified with electrical stimulation of the muscle and postmortem dissection. For the CONTROL cats, the remaining length of each wire electrode was passed subcutaneously along the lateral aspect of the hindlimb and back, exiting through the skin above the cervical vertebra. Wires were attached to a connector mounted on a custom jacket (LOMIR biomedical, Notre-Dame-de-L’Île Perrot, Canada) worn by the animal following electrode implantation. For the SELF-REINNERVATED cats, the EMG electrodes were similarly tunneled and connected to a headpiece as described in Prilutsky et al. (2011).
Spinalization procedure.
Animals were spinalized at the T11/T12 vertebral level under aseptic conditions after completion of the pretransection recordings. Anesthesia was induced with ketamine HCl (25 mg/kg im) given in combination with atropine (0.05 mg/kg im) and maintained with isoflurane (1.5–3.0% in oxygen) administered through an endotracheal tube. Buprenorphine (0.01 mg/kg sc) was given before first incision. An incision was made over the T11/T12 vertebrae, and the dorsal vertebral periosteal surface was exposed using blunt dissection. A laminectomy was performed to create an opening at the T11/T12 junction, and the dorsal surface of the dura was opened with microscissors. Xylocaine (1%) was applied to the dorsal surface of the exposed spinal cord and injected into the spinal cord to anesthetize the cord and eliminate injury discharge. The spinal cord was severed completely with microscissors between T11 and T12. The completeness of transection was verified with the aid of an operating microscope. On completion of spinal transection, the dura was sutured closed, and then the muscles in layers and skin. Postoperative spinal care was as described in our previous work (Krupka et al. 2017). A transdermal fentanyl patch (25 µg/h, 72 h) provided analgesic relief for 72 h. Additional pain relief was provided as needed under the direction of veterinary staff; this included extra fentanyl patches or doses of buprenorphine. Ampicillin (15 mg/kg sc, every 12 h) was administered for 10 days after surgery to prevent infections.
EMG recordings.
Electromyograms for hindlimb muscles were recorded using differential alternating current amplifiers (model 1700; A-M Systems, Sequim, WA) and the VICON motion capture data acquisition system (Nexus Motion System version 1.8.2; VICON Denver, CO). EMG signals were analog filtered with a high-pass cutoff of 10 Hz and a low-pass cutoff at 1,000 Hz and amplified at a gain of 1,000 or 10,000 depending on the strength of the EMG signal for each channel. The EMG signals were digitized and sampled at 2,400 Hz.
Kinematic recordings.
Kinematic data were captured at 300 Hz via the VICON motion capture data acquisition system (Nexus Motion System version 1.8.2; VICON, Denver, CO) using three cameras. Forelimb anatomical markers were placed on skin above the humeral head, elbow, lateral malleolus, metacarpophalangeal joint, and the second toe of the forelimb. Hindlimb anatomical markers were placed on the skin above the ischium, femoral head, knee, ankle, metatarsophalangeal joint, and the second toe of the hindlimb (Fig. 1B).
Autogenic stretch reflex responses.
A full description of the procedure used to assess the muscle’s autogenic stretch reflex is given in Lyle et al. (2016). Animals were anesthetized in an induction box with isoflurane (pre-anesthetics were not used because they interfere with the expression of spinal cord activity). All surgical procedures up to and including the precollicular decerebration were performed under anesthesia. Depth of anesthesia was assessed using physiological parameters and lack of a withdrawal reflex. The LG and Sol muscles were dissected in both the right (self-reinnervated) and left (untreated) hindlimbs. The distal tendons of those muscles were then attached to strain-gauge myographs mounted in series with linear actuators. Following a precollicular decerebration where all brain tissue rostral to the transection was removed, anesthesia was gradually withdrawn to avoid sudden increases in blood pressure. Data were collected beginning ~1 h following the removal of anesthesia and when active reflexes were detected. At that point, the muscle’s force responses to ramp-hold-release muscle stretches were measured. The left hindlimb acted as control for the force responses to stretch in muscles with intact sensory innervation, whereas responses in the right hindlimb were used to evaluate the changes in self-reinnervated muscles.
Data Analysis
Data organization.
Analyses were chosen to establish the effects of slope, spinal transection, and self-reinnervation of the LG and Sol muscles on hindlimb kinematics and muscle activity. Data were arranged based on spinal condition (pretransection and posttransection), slope [flat (0°), upslope (10°), and downslope (−10°)], and state of the LG and Sol muscles (CONTROL and SELF-REINNERVATED groups).
Kinematic data processing.
Kinematic data was analyzed off-line and processed using the following techniques implemented in several commercially available numerical packages.
Digital videos of the walking sessions were used to determine the quality of kinematic data and choose acceptable trials for processing. Acceptable trials were selected based on the animal taking 20 consecutive plantar weight-bearing steps on the treadmill with minimal head motion. Motion of hindlimb markers in the sagittal plane was divided into individual strides. A stride was defined as toe off to consecutive toe off and was identified by a semiautomated algorithm implemented into MATLAB (The MathWorks, Natick, MA). All subsequent processing of walking cycle marker data was conducted using custom routines implemented in Igor Pro (Wavemetrics, Lake Oswego, OR). Knee and elbow marker positions were triangulated based on femur and tibia bone lengths for the knee marker and humerus and ulna bone lengths for the elbow marker to correct for skin slippage over those joints (Goslow et al. 1973). Hindlimb gait parameters analyzed included hip height, toe height, timing of toe down and toe off, stance length, and anterior and posterior displacements of the toe for each stride (see Fig. 1B and Ollivier-Lanvin et al. 2015). Hip height was defined as the distance between the hip and the surface of the treadmill belt. Stance length was defined as the distance the foot travels from paw contact to paw off. Toe height was defined as the maximum height the toe travels off the treadmill belt during swing. The anterior displacement, measured from the horizontal position of the hip, is the maximum distance the toe travels forward of the hip. The posterior displacement is the furthest distance the toe travels behind the horizontal hip position.
Performance indexes were calculated by dividing the mean of the kinematic parameter (over 20 steps) by its mean baseline value (typically, the value for walking on a flat surface) for each cat, and multiplying by 100 to give the performance index as a percentage of baseline.
Joint angle data processing.
Average joint angles (over 20 strides) as a function of phase of walking cycle were calculated for each cat walking on flat, upslope, and downslope treadmill pre- or posttransection. The minimum, maximum, and range of each angle joint (hip, knee, ankle) were extracted from the average of that joint. Those values were compared across slopes using the same type of linear mixed model as for the kinematic parameters with the minimum, maximum, and range of each angle joint as the dependent variables. Statistical significance was set at P < 0.05. Bonferroni analysis was used to reduce multiple comparisons type I error for the post hoc comparisons of the estimated marginal means. Comparisons between groups and conditions were conducted using the same type of linear mixed model as for the kinematic parameters with the angle parameters serving as dependent variables.
EMG data processing.
Envelopes of muscle activity were calculated by filtering raw voltage records with a high-pass filter (2-pole Butterworth, 15 Hz), followed by full-wave rectification and low-pass filtering (zero-lag, fourth-order Butterworth, 20-Hz cutoff frequency). A muscle burst was defined as the time between muscle contraction onset and offset. These bursts were identified with a semiautomated algorithm based on the generalized likelihood ratio test. The automatically detected onsets/offsets were visually verified and manually corrected using MATLAB scripts.
Cluster analysis of EMG onset vs. EMG offset was performed. The onset and offset times were normalized to the walking cycle duration based on the mean swing onset (represented by 0), stance onset, and stance offset (represented by 1) times for the slope and condition being analyzed. Each normalized burst was plotted as a function of its onset (x-axis) and offset (y-axis) as in Krouchev et al. (2006). Next, following the procedure in Markin et al. (2012), we calculated the “statistical distance” between the centers of the scatter clouds of points representing each individual muscle burst’s onsets and offsets. The scatterplot of burst onsets and offsets was considered as an edge-weighted graph G with the vertices at the centers of the clouds of muscle burst onsets and offsets, and the edges representing the distances between cloud centers. Muscles were then clustered by establishing the minimal spanning tree of graph G in two steps. Muscles with minimal overlap of their onset/offset times were first divided into clusters using the “graphminsspantree” procedure of MATLAB (The MathWorks, Natick, MA). The resulting clusters that contained more than three muscles were then further examined by using the validity index proposed in Jana and Naik (2009). This index calculates the intra- to intercluster distance ratio as individual muscles are joined and is used to establish the cluster divisions that optimize the index.
EMG burst characteristics (duration, onset, offset) of each stride were also calculated to determine if there were condition- or slope-related changes in muscle activation timings.
Autogenic stretch reflex evaluation.
As further described in Lyle et al. (2016), the baseline force before stretch was subtracted from the raw force profiles before analyses. Stretches that had a sudden or spontaneous force change unrelated to muscle stretch were not further analyzed. To determine whether the force responses of the SELF-REINNERVATED muscles contained a reflexive component, we assessed whether the stiffness of the muscle in response to the full 2-mm ramp stretch (incremental stiffness, i.e., Ke in Fig. 11) was close to the short-range stiffness (Ki), measured over the first 0.2 mm of the ramp (Huyghues-Despointes et al. 2003a). In the absence of the stretch reflex, the incremental stiffness is less than the short-range stiffness due to the yielding, which leads to a ratio of Ke/Ki less than 1 (Huyghues-Despointes et al. 2003a). This yielding occurs in all muscle types but is more pronounced in slow twitch muscles such as the soleus (Malamud et al. 1996).
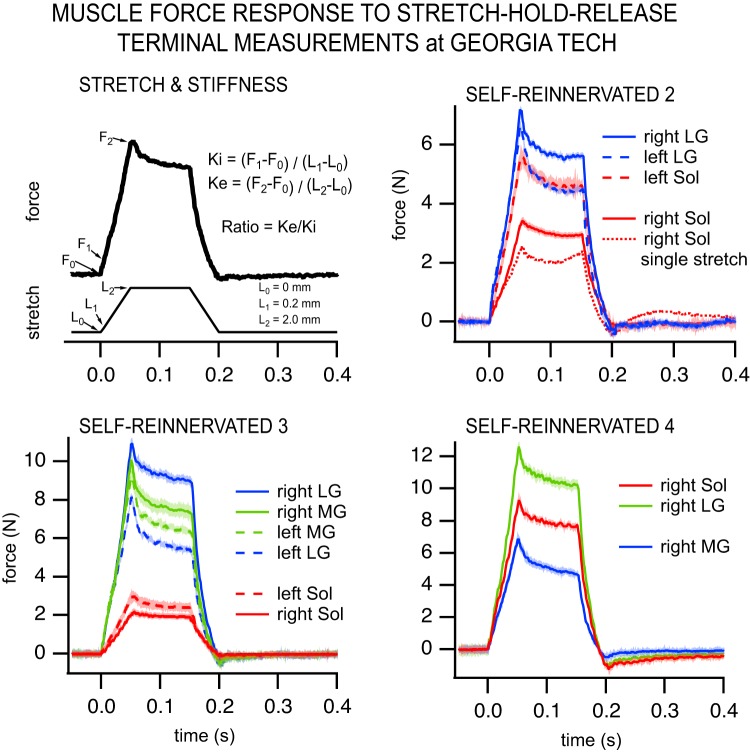
Fig. 11.
Muscle force responses to stretch-hold-release perturbations. Top left: depiction of the stretch applied and stiffness measurements calculation. The stiffness ratio (Ke/Ki) was calculated as incremental stiffness (Ke), measured over the ramp of 2-mm amplitude, divided by the short-range stiffness (Ki), measured over the first 0.2 mm. Color graphs show the muscle force responses to stretch-hold-release measured in a terminal experiment following spinalization and locomotor training for SELF-REINNERVATED 2–4 (not obtained for SELF-REINNERVATED 1). The average (over 20 stretches) muscle force response is displayed for ankle extensors of the right [lateral gastrocnemius/soleus (LG/Sol) nerve cut and repair] and left (intact) hindlimbs. Shading around the response lines indicates ±2 SD. Example of a soleus single-stretch response in SELF-REINNERVATED 2 (top right) clearly demonstrates an increase in force during the hold period that is indicative of a stretch reflex response. Note from Table 3 that most values of Ke/Ki were ≥1.0, and all were >0.7. MG, gastrocnemius medialis.
Statistical analysis.
Kinematic parameter indexes (hip height, toe height, etc.) for all cats were compared across slopes at each condition (pre- and posttransection) for both groups using a linear mixed model (SPSS, IBM, Chicago, IL) with slope as a repeated measure factor. The dependent variables used were hip height, stance length, toe height, anterior displacement of the toe, and the posterior displacement of the toe indexes. Statistical significance was set at P < 0.05. Bonferroni correction was used to reduce multiple comparisons type I error for the post hoc comparisons of the estimated marginal means. Comparisons between groups and conditions were conducted using a linear mixed model with slope and condition as repeated measure factors and group as factor. The performance indexes were the dependent variables. Lack of overlap in the estimated marginal means confidence intervals was used to establish significant difference between groups/conditions.
Joint angle parameters were compared across slopes using a linear mixed model with the minimum, maximum, and range of each angle joint as the dependent variables. Statistical significance was set at P < 0.05. Bonferroni analysis was used to reduce multiple comparisons type I error for the post hoc comparisons of the estimated marginal means. Comparisons between groups and conditions were conducted using the same type of linear mixed model as for the kinematic parameters with the angle parameters serving as dependent variables.
Linear mixed models with the EMG burst characteristics as dependent variables and slope as the factor with slope and stride as repeated variables were used to evaluate the effects of slope on burst characteristics in the pre- or postinjury condition for the animals in each group (CONTROL and SELF-REINNERVATED). Linear mixed models with the burst characteristics as dependent variables and condition (pre- or posttransection) as the factor with slope and stride as repeated variables were used to evaluate the changes in burst characteristics from pre- to postinjury at the different slopes. The estimates of the marginal means were used as a measure of the changes, and post hoc pairwise comparisons were used to evaluate if the changes were significant (at P < 0.05).
The Ke/Ki ratios of the left and right hindlimb muscles were compared with the Ke/Ki ratios for the self-reinnervated Sol and gastrocnemius muscles of published experiments (Huyghues-Despointes et al. 2003a; see their Fig. 6, top, initial force <12 N) using nonparametric one-way ANOVA (Kruskal–Wallis, at P < 0.05; R-Studio version 1.1.423). Pairwise comparisons of the average Ke/Ki between our untreated and self-reinnervated muscles and the self-reinnervated muscles of Huyghues-Despointes et al. (2003a) were done using Dwass–Steel–Critchlow–Fligner pairwise comparisons (at P < 0.05).
RESULTS
Locomotor Characteristics on Flat, Uphill and Downhill Treadmill Walking
CONTROL cats.
Pre-EMG implantation kinematics recordings were taken between 1 and 2 wk before implantation for the animals that did not undergo LG and Sol self-reinnervation. Following implantation of chronic EMG electrodes, pretransection recordings on flat, upslope, and downslope treadmill were taken for CONTROL subjects 2, 3, and 4. CONTROL subject 1 was only evaluated for walking on a flat treadmill pretransection. Following transection, recordings on flat, upslope, and downslope were taken for each subject. Posttransection recording sessions began when stable full weight-bearing stepping was observed in the cat following training, generally between 6 and 8 wk postinjury, at which time performance had plateaued. All cats trained on a flat surface following spinal transection could continuously step on a 10° upslope and 10° downslope surface. Figure 3 shows representative walking strides for flat and sloped surface walking for CONTROL and SELF-REINNERVATED subjects.
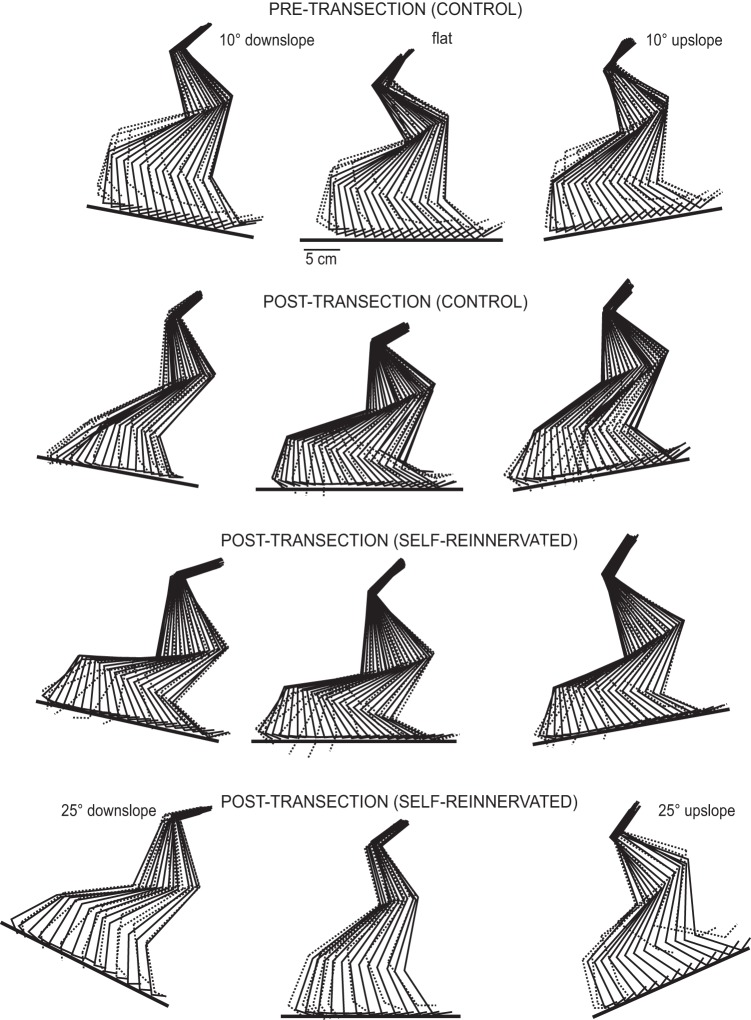
Fig. 3.
Stick figure representations of exemplar steps at the different slopes. The stick figures represent hindlimb segments over the time course of a stride for a walking speed of 0.4 m/s. Stance phase segments are indicated by solid lines, and swing phase segments by dotted lines. First row shows 1 step at 10° downslope, flat, and 10° upslope for a CONTROL cat pretransection. Second row shows the same steps for the same animal posttransection and following locomotor training. Third row shows the same steps for a SELF-REINNERVATED animal posttransection and posttraining. Fourth row shows steps at ±25° of slope and flat for another SELF-REINNERVATED animal posttransection and posttraining. Spinal animals locomotor trained on a flat surface were able to locomote on inclined surfaces, up to ±25° of grade.
SELF-REINNERVATED cats.
Three of the animals that underwent LG/Sol self-reinnervation were tested on flat and 10° upslope and downslope. Pretransection flat, upslope, and downslope treadmill walking recordings were performed on SELF-REINNERVATED subjects 2 and 3; however, only pretransection flat recordings were performed on SELF-REINNERVATED subject 1. Recordings of flat and ±10° slope walking were obtained for all three subjects posttransection. SELF-REINNERVATED subject 4 was evaluated on flat and 25° upslope and downslope before and after spinal transection. Animals with self-reinnervated LG and Sol muscles could continuously step on a ±10° or ±25° (1 animal) sloped surface following sensorimotor training on a flat treadmill (see Fig. 3).
Effects of Slope on Kinematics Variables
Variables analyzed and statistical testing.
Averages (over 20 steps) of hip height, stance length, swing height, anterior displacement of the toe, and posterior displacement of the toe indexes were calculated for each cat. Estimated marginal means and 95% confidence intervals for each normalized variable are reported in Fig. 4 for walking on flat, 10° upslope, and 10° downslope before and after spinal transection for each group.
Fig. 4.
Estimated marginal means ± 95% confidence intervals of the normalized stance length, swing height, anterior displacement (Da), posterior displacement (Dp), and hip height for all animals evaluated on flat and ±10° slope of walking. Full factorial linear mixed models of each of the indexes in the pre- and posttransection conditions with group and slope as factors found significant differences (no overlap in 95% confidence interval of the estimated marginal means) in swing height and Dp between the CONTROL and SELF-REINNERVATED animals pretransection, and in Da between the same two groups posttransection. Overall, the differences between CONTROL and SELF-REINNERVATED animals were small, confirming prior results in spinally intact animals showing that lateral gastrocnemius/soleus self-reinnervation has limited effects on the kinematics of locomotion even at much higher (27°) slopes (Gregor et al. 2018). Slope had a significant effect on stance length, swing height, and Dp in spinally intact animals, but not for the spinalized animals, suggesting that the adaptation to slope may be supraspinal in origin. Significant lowering of the anterior displacement between flat and upslope was the only change with grade found in spinalized animals. *Estimated marginal means do not overlap at any of the grades; lines between groups/slopes indicate no overlap in estimated marginal means between groups/slopes. Number of steps: 20 steps for each condition/slope/subject; number of subjects: 4 CONTROL at all slopes postspinalization and flat prespinalization, 3 CONTROL at ±10° slopes prespinalization, 3 SELF-REINNERVATED at all slopes postspinalization and flat prespinalization, and 2 SELF-REINNERVATED at ±10° slope prespinalization.
Difference in the effects of slope between the two groups (animals with/without LG/Sol self-reinnervation).
Significant differences in pretransection swing height and posterior displacement and posttransection anterior displacement were found between the groups (see Fig. 4). Overall, the differences in the values of the kinematic parameters between CONTROL and SELF-REINNERVATED animals were small.
Effects of slope within each group.
Although the kinematics of walking for each group were similar, the slope of the treadmill surface had a significant effect on a number of stride parameters before transection for both CONTROL and SELF-REINNERVATED animals (see Fig. 4). All five kinematic parameters reported were affected by slope in the CONTROL animals, whereas only swing height was affected in the SELF-REINNERVATED animals. At the slopes used (±10°), it is unlikely that this difference in the number of kinematic variables affected is due to the LG/Sol self-reinnervation, since reports in spinally intact animals following ankle extensor self-reinnervation surgeries show modest differences in gait, and only for downslope walking at a significantly higher slope (27°) (Gregor et al. 2018; Maas et al. 2007; Pantall et al. 2016).
The effect of slope was reduced in spinal animals; a drop in the anterior displacement during upslope walking in animals with LG/Sol self-reinnervation was the only significant adaptation to slope in spinal animals. This supports the hypothesis that adaptation to incline surfaces may be mostly driven by supraspinal control.
Our results at ±25° of slope in a spinalized (i.e., posttransection) animal with LG/Sol self-reinnervation showed significant changes in nearly all stride parameters with slope (linear mixed model, post hoc comparison with Bonferroni correction, P < 0.05; see Table 1 for estimated marginal means). Hip height and swing height indexes were significantly different between slopes (larger in downslope and smaller in upslope), stance length was significantly longer on upslope, and anterior displacement significantly decreased on upslope and downslope, whereas posterior displacement significantly increased. We unfortunately did not obtain the treadmill with ±25° slope capability in time to evaluate other spinalized animals at this level of slope, but results in this one animal show the spinal locomotor center’s ability to adapt to a 25° incline. Because the LG and Sol muscle stretch reflexes largely returned in this animal (SELF-REINNERVATED subject 4; see below), these adaptations appear to be due to loss of the supraspinal command and not lack of LG/Sol ankle extensor stretch reflex. Results in spinally intact animals also suggest that the contributions of the ankle extensors’ length feedback to the changes in kinematics are minimal and limited to an increased ankle yield during stance for 50% (27°) downslope walking (Maas et al. 2007).
Table 1.
Estimated marginal means of kinematic parameters of gait for SELF-REINNERVATED subject 4
| Parameter Indexes |
|||||
|---|---|---|---|---|---|
| Hip Height | Stance Length | Swing Height | Da | Dp | |
| Downslope | 1.19 ± 0.03* | 0.85 ± 0.01a | 0.59 ± 0.05* | 0.68 ± 0.03c | 1.13 ± 0.03e |
| Flat | 1.09 ± 0.03* | 0.86 ± 0.01b | 0.91 ± 0.05* | 0.85 ± 0.03c,d | 0.88 ± 0.03e,f |
| Upslope | 1.00 ± 0.03* | 0.91 ± 0.01a,b | 1.44 ± 0.05* | 0.74 ± 0.03d | 1.18 ± 0.03f |
Values are estimated marginal means ± 95% confidence intervals of the kinematic parameters of gait (performance indexes calculated as a percentage of baseline) for SELF-REINNERVATED subject 4 walking on flat and ±25° slope at 0.4 m/s posttransection. Da, anterior displacement; Dp, posterior displacement.
Significant differences between each row.
Same letters on a row indicate significant differences between the rows.
Effects of Slope on Joint Angles
Effects of slope on joint angle profiles.
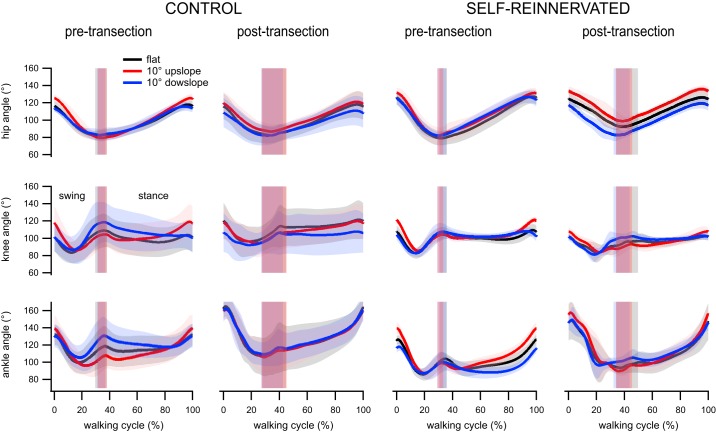
Joint angles were processed and normalized in duration (with 0 or 0% corresponding to swing onset and 1 or 100% corresponding to stance offset). Minimum, maximum, and angle range were averaged for hip, knee, and ankle joints over 20 steps for each cat. Averages of the averaged hip, knee, and ankle angles across cats for each condition and slope combinations for both groups were used to illustrate joint angles over a complete walking cycle (see Fig. 5). The patterns of the joint angles were similar across conditions and slopes for both groups. The hip angle decreased throughout swing and increased throughout stance. The knee flexed in early swing, extended before paw contact, and stayed relatively constant during stance. The ankle also flexed in early swing, extended before paw contact, and briefly flexed after contact (ankle yield at toe-down) before extending throughout the rest of stance. The adaptations in joint angles to a ±10° slope were minimal for both groups, especially after spinalization. The confidence intervals of the joint angles at all three slopes of walking overlapped for the spinalized CONTROL animals, whereas only the hip angle during uphill compared with downhill walking differed during most of the gait cycle for the spinalized SELF-REINNERVATED animals. The lack of significant angle changes during slope walking was similar in the spinally intact condition, where only the ankle angle varied with slope during part of the gait cycle in both groups.
Fig. 5.
Average hip, knee, and ankle angles over the walking cycle from swing onset (0%) to stance offset (100%) for animals with/without lateral gastrocnemius/soleus self-reinnervation walking on flat and ±10° slope pretransection and following spinal transection and locomotor training on a flat treadmill. Lines represent averages and the thicknesses of shading around each line indicates the 95% confidence interval. Vertical shaded bars indicate stance onset ± 95% confidence interval. The joint angles did not vary much with grades in both groups, pre- and postspinalization. The results suggest that the joint angles during flat walking will result in weight-bearing stepping at slopes of ±10°. Number of steps: 20 steps for each condition/slope/subject; number of subjects: 4 CONTROL at all slopes postspinalization and flat prespinalization, 3 CONTROL at ±10° slopes prespinalization, 3 SELF-REINNERVATED at all slopes postspinalization and flat prespinalization, and 2 SELF-REINNERVATED at ±10° slope prespinalization.
Effects of slope on joint angle minima, maxima, and ranges of motion.
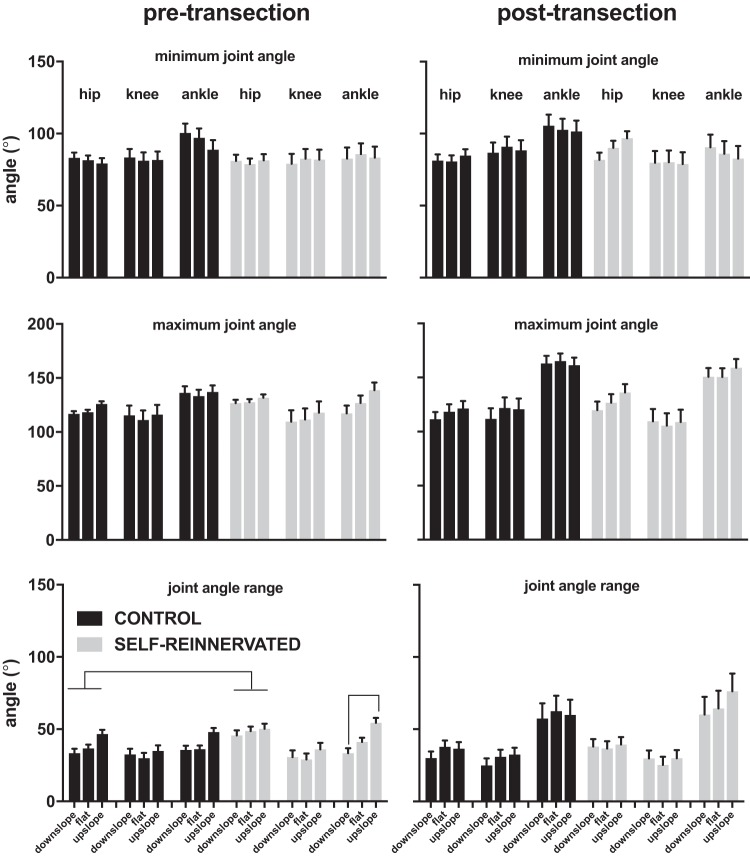
The similarity of the joint angle profiles at the various slopes was confirmed by analysis of the angles’ maxima, minima, and ranges across conditions and slope for both groups (Fig. 6). In terms of differences between groups, only the hip range of motion pretransection was significantly larger in the animals with LG/Sol self-innervation than without it (no overlap in estimated marginal means for that angle parameter pre- and posttransection using a full factorial linear mixed model with group and slope as factors). Differences due to slope were limited to difference in the ankle range of motion between upslope and downslope for animals with LG/Sol self-reinnervation before spinalization (post hoc test at P < 0.05 with Bonferroni correction). The ankle range of motion was reduced for downslope compared with upslope walking, mostly due to a progressive increase in ankle extension from downslope to upslope. We did not observe the significant increase in ankle peak flexion (minimum ankle angle) reported at higher downslope in LG/Sol self-reinnervated cats (Maas et al. 2007). At the downslope used, we saw no significant effect of slope in peak ankle flexion in our SELF-REINNERVATED group. For the one SELF-REINNERVATED animal tested at ±25° of slope posttransection, we obtained no increase in peak ankle flexion for downslope walking but a significant increase for upslope walking [similar increase observed at 27° upslope walking in spinally intact cats in the early period following LG/S self-reinnervation (Maas et al. 2007)]. Most joint angle parameters were affected by slope when a slope of ±25° was used (linear mixed models with stride and slope as repeated factors, see Table 2). This level of slope had a significant effect on the minima (typically decreased from downslope to upslope) and range of motion values of all angle joints (typically increased from downslope to upslope), and on the maxima of the hip and ankle joints (hip maximum increased from downslope to upslope; ankle maximum decreased from downslope to upslope). The only joint angle parameter that was not affected by slope was the knee maximum angle, i.e., extension. In summary, the ±25° slope caused significant adaptations in the spinal gait for the one animal tested.
Fig. 6.
Estimated marginal means ± 95% confidence intervals of the averaged (over 20 steps) hip, knee, and ankle angle minima, maxima, and ranges during walking on flat, 10° upslope, or 10° downslope for all animals evaluated at those grades. Full factorial linear mixed models of each of the angle parameters in the pre- (left column) and posttransection (right column) conditions with group and slope as factors indicated a significant difference between the CONTROL and SELF-REINNERVATED groups for the hip range of motion pretransection. No other significant differences between groups were found. The only effect of slope was on the ankle range of motion, which was larger for upslope walking (compared with downslope) in spinally intact SELF-REINNERVATED animals (no overlap in 95% confidence interval of the estimated marginal means). Overall, the results indicate that the angular parameters were not affected by slope in either condition (intact or spinal) for animals with/without lateral gastrocnemius/soleus self-reinnervation surgery. Lines between groups/slopes indicate no overlap in estimated marginal means between groups/slopes. Number of steps: 20 steps for each condition/slope/subject; number of subjects: 4 CONTROL at all slopes postspinalization and flat prespinalization, 3 CONTROL at ±10° slopes prespinalization, 3 SELF-REINNERVATED at all slopes postspinalization and flat prespinalization, and 2 SELF-REINNERVATED at ±10° slope prespinalization.
Table 2.
Estimated marginal means of minima, maxima, and ranges of joint angles for SELF-REINNERVATED subject 4
| Hip |
Knee |
Ankle |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Max | Min | Range | Max | Min | Range | Max | Min | Range | |
| Downslope | 88 ± 0.6* | 49 ± 0.7* | 38 ± 0.7* | 123 ± 0.9 | 99 ± 0.9* | 24 ± 1.3* | 153 ± 1.5* | 109 ± 1a | 44 ± 1.2* |
| Flat | 82 ± 0.6* | 40 ± 0.7* | 43 ± 0.7* | 122 ± 0.9 | 91 ± 0.9* | 31 ± 1.3* | 145 ± 1.5* | 108 ± 1b | 37 ± 1.2* |
| Upslope | 97 ± 0.6* | 44 ± 0.7* | 54 ± 0.7* | 121 ± 0.9 | 73 ± 0.9* | 48 ± 1.3* | 130 ± 1.5* | 73 ± 1a,b | 56 ± 1.2* |
Values are estimated marginal means ± 95% confidence intervals of the minima, maxima and ranges of joint angles (in degrees) for SELF-REINNERVATED subject 4 walking on flat and ±25° slope at 0.4 m/s post-transection.
Significant differences between each row.
Same letters on a row indicate significant differences between the rows.
EMG Changes
Muscles active in each animal.
All CONTROL animals had functional EMG electrodes in BFA, VL, Sol, MG (active in stance and designated as extensors), TA, and EDL (active in swing and designated as flexors) for the length of the study. The bifunctional BFP only showed a flexor burst and was functional in CONTROL subjects 1, 2, and 3. The SartA was functional in CONTROL subjects 1, 3, and 4. The FDL was functional in CONTROL subjects 2, 3, and 4. The IP was only functional in CONTROL subject 4. EMG offset vs. onset burst times were clustered, and shifts in centroids were analyzed using minimal spanning tree (as described in methods).
EMG signals could not be collected on SELF-REINNERVATED subject 4 that was tested on ±25° slope. All other SELF-REINNERVATED cats (3 animals) had functional EMG electrodes in BFA, Sol, MG, FDL (extensors), and IP (flexor). The bifunctional BFP burst was split into its flexor burst (BFP F), which was obtained only in SELF-REINNERVATED subject 1, and its extensor portion (BFP E), which was obtained only in SELF-REINNERVATED subject 2. SELF-REINNERVATED subjects 1 and 3 had a functioning SartM electrode, and SELF-REINNERVATED subjects 2 and 3 had a functioning TA electrode (flexor). EMG offsets vs. onsets were processed as for the CONTROL cats.
Changes in flexor and extensor activation timing with slope were examined with separate linear mixed models for each condition (pre- and posttransection) in each of the two groups. To determine the effects of spinal transection on flexor and extensor activation timing, post- to pretransection comparisons were made between cluster centers for flat, 10° upslope, and 10° downslope walking. Linear mixed models and post hoc comparisons (least significant difference, LSD) of each muscle’s onset and offset times were used to identify significant shifts in muscle activation timing with slope or spinalization.
Muscle activity clustering.
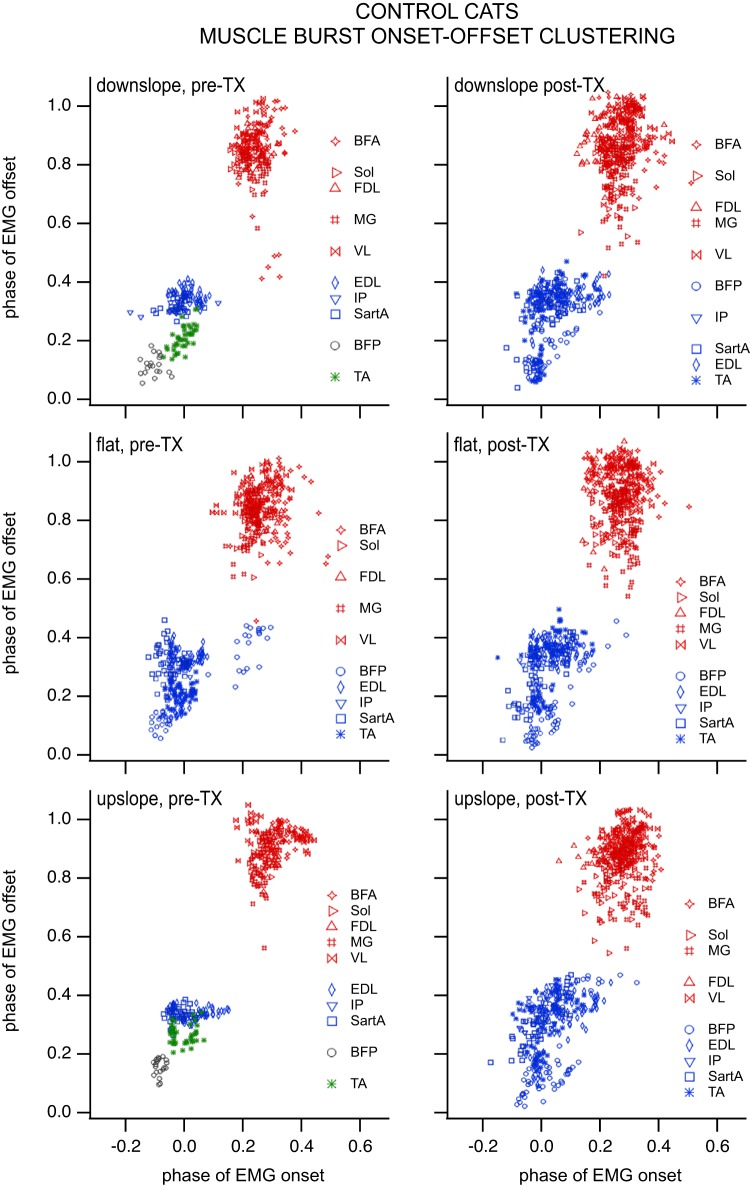
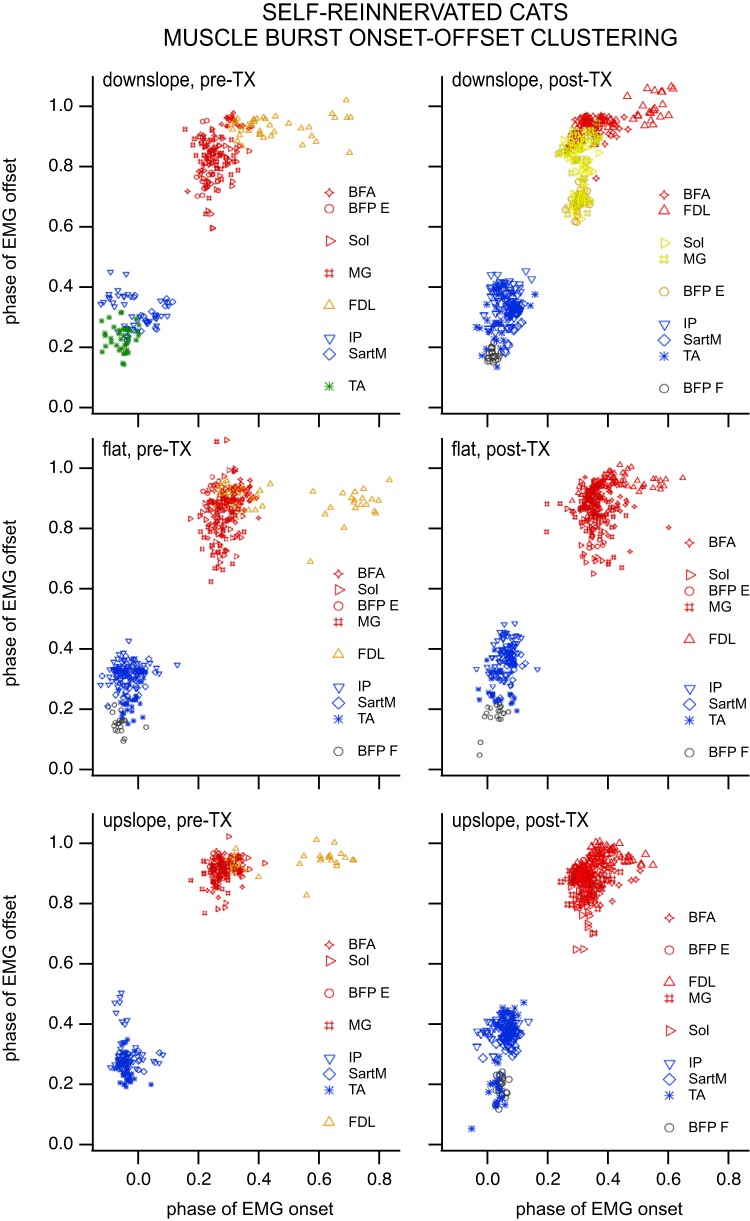
The 10 muscles (BFA, VL, Sol, MG, TA, and EDL for all CONTROL animals; BFP F for CONTROL subjects 1, 2, and 3; SartA for CONTROL subjects 1, 3, and 4; FDL for CONTROL subjects 2, 3, and 4; IP for CONTROL subject 4) implanted in CONTROL cats separated into five clusters on flat, seven clusters on downslope, and four clusters on upslope pretransection (Fig. 7). Posttransection, the muscles separated into two clusters on flat, seven clusters on downslope, and four clusters on upslope (Fig. 7). Membership fell for the most part along the traditional flexor/extensor divisions when the minimal span tree method was used, especially following spinal transection. The validity index produced optimal subclusters within the two major divisions. An exception to this major division into flexors and extensors was the single muscle clusters containing BFP or TA for pretransection slope walking. These single muscle clusters may be indicative of a supraspinal modulation of muscle activity for slope walking. These individual muscle clusters (using the minimal span tree method) were not present when supraspinal input was removed, i.e., posttransection.
Fig. 7.
Clustering of muscle burst onset/offset for CONTROL cats during locomotion on a flat, 10° upslope, and 10° downslope treadmill in the intact (left column) and spinalized (right column) conditions (i.e., pre- and posttransection). Each symbol represents a muscle burst onset/offset combination during a cycle. Different symbol colors indicate distinct clusters of muscle electromyographic (EMG) burst onsets/offsets based on the “graphminsspantree” procedure, whereas blank lines between muscles/symbols of the same colors in the key indicate that the main cluster identified with the graphminsspantree procedure subdivided into smaller clusters using the validity index. Biceps femoris posterior (BFP) and tibialis anterior (TA) formed distinct clusters for upslope and downslope walking pretransection but grouped with the other flexors posttransection. The number of clusters (especially those identified with the graphminsspantree procedure) went down in the spinal condition, suggesting that the spinal control of locomotion may be more primitive and produce coarser flexor and extensor groups. Number of subjects: 4 at all slopes postspinalization and flat prespinalization, 3 at ±10° slopes prespinalization. Number of bursts prespinalization (pre-TX): n = 20 for iliopsoas (IP) at each slope and BFP at ±10° slopes, n = 40 for all other muscles at ±10° slopes, whereas n = 60 of those muscles for flat, except for BFP and flexor digitorum longus (FDL), where n = 40. Number of bursts postspinalization (post-TX): n = 20 for IP, whereas n = 60 for BFP, sartorius medialis (SartA), and FDL at each slope; n = 80 for the remaining muscles at each slope (BFA, biceps femoris anterior; EDL, extensor digitorum longus; MG, gastrocnemius medialis; Sol, soleus; VL, vastus lateralis).
The nine muscles (BFA, Sol, MG, FDL, and IP for all SELF-REINNERVATED animals; BFP F for SELF-REINNERVATED subject 1; BFP E for SELF-REINNERVATED subject 2; SartM for SELF-REINNERVATED subjects 2 and 3; TA for SELF-REINNERVATED subjects 2 and 3) implanted in SELF-REINNERVATED cats separated into four clusters on flat, six clusters on downslope, and five clusters on upslope pretransection. Posttransection, the muscles separated into five clusters on flat, five clusters on downslope, and six clusters on upslope (Fig. 8). Muscle separation was more pronounced in the SELF-REINNERVATED animals, with the minimal span tree method producing distinct subclusters of flexor or extensor muscles pre- and posttransection at the various slopes. Muscles particular to these animals, such as the FDL or the flexor/extensor portions of the BFP, tended to form very tight configurations of burst onsets/offsets separate from the main extensor or flexor group bursts.
Fig. 8.
Clustering of muscle burst onset/offset for SELF-REINNERVATED cats during locomotion on a flat, 10° upslope, and 10° downslope treadmill in the intact (left column) and spinalized (right column) conditions (i.e., pre- and posttransection). Each symbol represents a muscle burst onset/offset combination during a cycle. Different symbol colors indicate distinct clusters of muscle electromyographic (EMG) burst onsets/offsets based on the “graphminsspantree” procedure, whereas blank lines between muscles/symbols of the same colors in the key indicate that the main cluster identified with the graphminsspantree procedure subdivided into smaller clusters using the validity index. A reduction in the number of clusters posttransection was also observed for this group. As in the CONTROL group, the biceps femoris posterior flexor burst (BFP F) and tibialis anterior (TA) tended to form distinct clusters. Interestingly, the soleus (Sol) and gastrocnemius medialis (MG) muscles formed a very distinct cluster (graphminsspantree procedure) for downslope walking posttransection. This phenomenon was not observed in animals with intact lateral gastrocnemius (LG)/Sol nerve, and prior studies have shown that the greatest deficits during slope walking in animals with LG/Sol self-reinnervation occur on downslope. The distinct clustering of both ankle extensors may be related to the loss of heteronymous stretch reflex from the LG and Sol muscles in this group, since homonymous stretch reflex recovered (Fig. 11). Number of subjects: 3 at all slopes postspinalization and flat prespinalization, 2 at ±10° slope prespinalization. Number of bursts prespinalization (pre-TX): n = 20 for BFP at each slope and sartorius medialis (SartM) at ±10° slopes, n = 40 for all other muscles at ±10° slopes, and n = 60 at flat, except for TA, which remains at n = 40 for flat. Number of bursts postspinalization (post-TX): n = 20 for BFP at each slope, n = 20 for SartM and TA, and n = 60 for the remaining muscles at each slope (BFP E, biceps femoris posterior extensor burst; EDL, extensor digitorum longus; FDL, flexor digitorum longus; IP, iliopsoas; VL, vastus lateralis).
As in the CONTROL group, BFP F and TA tended to form distinct clusters. Interestingly, the Sol and MG muscles formed a very distinct cluster (graphminsspantree procedure) for downslope walking posttransection. This phenomenon was not observed in animals with intact LG and Sol muscles.
ANOVA with number of clusters as the dependent variable and slope and group as factors (full factorial) showed a significant effect of slope (but not group) on the number of clusters. Number of clusters increased for downslope walking compared with flat walking (LSD post hoc comparison); no other difference in the number of clusters between slopes was significant.
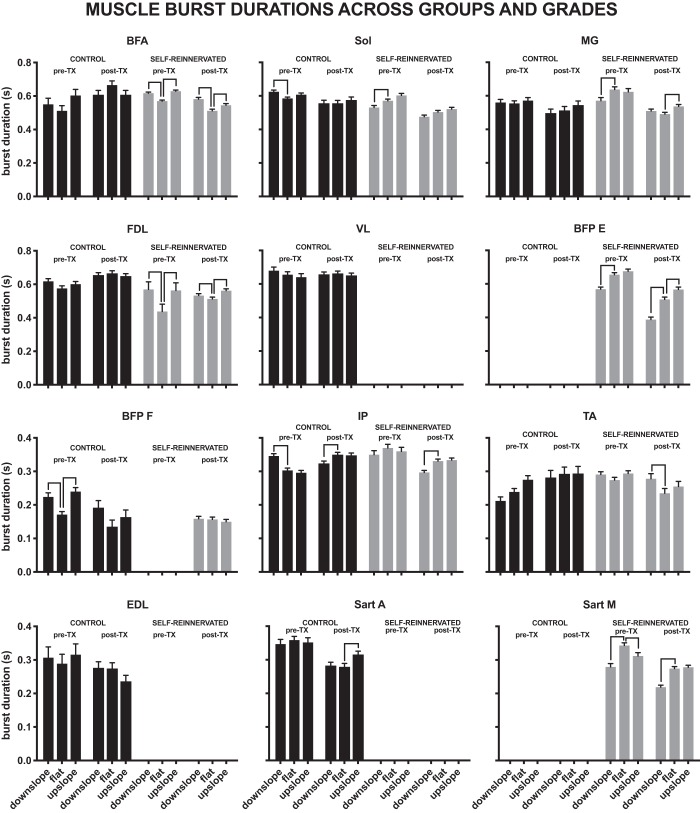
Changes in muscle burst durations with slope.
Figure 9 illustrates the changes in muscle burst durations with slope for the two groups, pre- and posttransection. For the range of slopes studied (±10°), muscle burst durations were not greatly affected by slope. In CONTROL cats, few muscle burst durations were affected by the slope of walking pre- or posttransection. Only the BFP (flexor burst) increased significantly from flat to upslope pretransection, and only the SartA posttransection. For comparisons from flat to downslope, three (BFP, IP, and Sol) muscle burst durations increased pretransection, and the IP burst duration declined posttransection.
Fig. 9.
Individual muscle burst durations for both groups (CONTROL and SELF-REINNERVATED), before (pre-TX) and after spinalization (post-TX), at each slope of walking (flat, 10° upslope, and 10° downslope). Significant differences due to slope are indicated by line segments between the bars representing the burst durations at each slope. Burst durations were not affected by a ±10° change in elevation for most of the muscles studied in animals with either an intact or lateral gastrocnemius/soleus self-reinnervation, before or after spinalization. Bars indicate the estimated marginal means ± 95% confidence interval of burst durations. Number of subjects and bursts for each bar are reported in Figs. 7 and 8.
Changes in muscle burst durations were more prevalent in the SELF-REINNERVATED animals. Before injury, two extensor burst durations (BFA and FDL) increased from flat to upslope, and one flexor burst duration (SartM) decreased. From flat to downslope, two (BFA and FDL) muscles’ burst durations significantly increased, whereas they decreased for four muscles (BFP-E, MG, SartM, Sol). Posttransection, muscle burst durations for the BFA, BFP-E, FDL, and MG increased from flat to upslope walking. From flat to downslope, burst duration increased for three muscles (BFA, FDL, TA) and decreased for three muscles (BFP-E, IP, SartM).
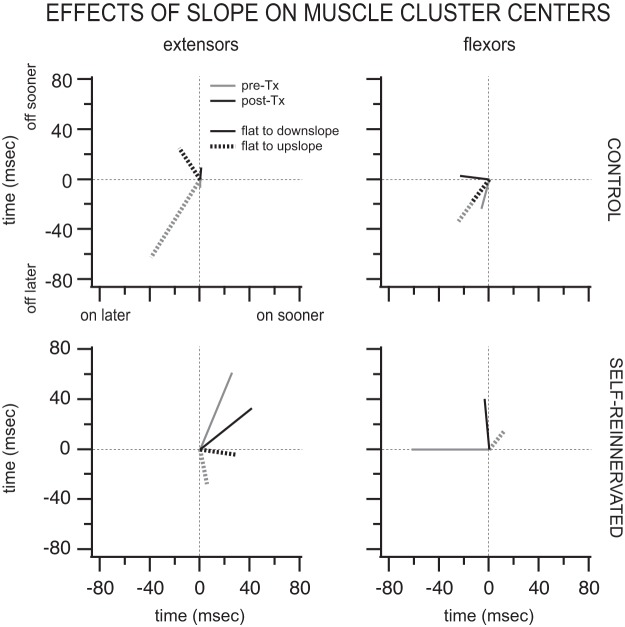
Changes in muscle activation timing with slope.
The effects of slope on the muscle burst onset and offset times are shown in Fig. 10. Displayed are the vector averages of the significant shifts in muscle burst onset/offset between flat and upslope or flat and downslope for the flexors and extensors of the hindlimb. In CONTROL spinally intact animals, the extensors turned on (39 ms) and off (62 ms) later for upslope walking than for flat walking. Following spinalization, the changes in onset/offset were smaller, with the extensors turning on 16 ms later and off 25 ms sooner for upslope walking. For downslope walking, the extensors’ onsets/offsets were largely unaffected (<7 ms) pre- and postspinalization. In the SELF-REINNERVATED animals, the largest changes in extensor activation timing were seen for downslope walking, where the extensors turned on (26 ms prespinalization and 42 ms postspinalization) and off (61 ms prespinalization and 33 ms postspinalization) sooner pre- and postspinalization. The onset/offset of flexor muscles showed limited changes with slope for either groups (CONTROL or SELF-REINNERVATED) or conditions (pre- or posttransection); the two largest changes were in SELF-REINNERVATED animals, where flexors turned off 40 ms later for downslope walking, and in CONTROL, where they turned on (24 ms) and off (33 ms) later for upslope walking. The number of flexor muscles with significant changes in muscle burst onset/offset was also low, one to two in all instances but one. Overall, the shifts in muscle activation timing during slope walking were minimal for the range of slope reported (±10°).
Fig. 10.
Averages of significant shifts in extensor and flexor muscle burst onset/offset cluster centers from flat to upslope (dashed lines) and flat to downslope (solid lines). Graphs show the averages (for the muscles studied) of significant changes in muscle burst onsets and offsets with slope of walking for CONTROL (top) and SELF-REINNERVATED (bottom) animals before (pre-Tx; gray lines) and after spinal transection (post-Tx; black lines). Whereas extensor activation was delayed by walking upslope in CONTROL animals postspinalization, it was advanced in SELF-REINNERVATED animals for both upslope and downslope walking both pre- and postspinalization. Extensors tended to turn off later during upslope walking prespinalization, but turned off earlier postspinalization in CONTROL animals. For SELF-REINNERVATED animals, extensors turned off earlier during downslope walking and later during upslope walking, irrespective of spinalization. Flexors tended to be active earlier for slope walking in both groups, with minimal changes in burst offsets. All changes were relatively small (∼20–60 ms). Number of subjects: 4 CONTROL at all slopes postspinalization and flat prespinalization, 3 CONTROL at ±10° slopes prespinalization, 3 SELF-REINNERVATED at all slopes postspinalization and flat prespinalization, 2 SELF-REINNERVATED at ±10° slope prespinalization. Number of muscles with significant shifts in muscle burst onset/offset cluster centers: in CONTROL animals prespinalization, for flat to upslope, n = 5 extensor and n = 3 flexor muscles, and for flat to downslope, n = 3 extensor and n = 3 flexor muscles; in CONTROL animals postspinalization, for flat to upslope, n = 2 extensor and n = 2 flexor muscles, and for flat to downslope, n = 3 extensor and n = 3 flexor muscles; in SELF-REINNERVATED animals prespinalization, for flat to upslope, n = 4 extensor and n = 2 flexor muscles, and for flat to downslope, n = 4 extensor and n = 1 flexor muscles; and in SELF-REINNERVATED animals postspinalization, for flat to upslope, n = 5 extensor and n = 1 flexor muscles, and for flat to downslope, n = 2 extensor and n = 3 flexor muscles.
Terminal Measurements of the Autogenic Stretch Reflex
The average force responses to stretch of the muscles in all the SELF-REINNERVATED animals are presented in Fig. 11. Animals were at minimum 12 wk postspinalization (range 12–43 wk) at the time of the terminal experiment. Background forces before stretch were <3.5 N in all cases. Not all muscles could be evaluated in all animals due to technical considerations during measurements (spontaneous contractions, presence of locomotor patterns, etc.). In past experiments in spinally intact animals, the stiffness ratio for the areflexive Sol for low-to-moderate background forces (<12 N) was between 0.3 and 0.4, whereas for the whole gastrocnemius muscle it was between 0.4 and 0.5 (Huyghues-Despointes et al. 2003a). In the presence of the stretch reflex, the stiffness ratio for Sol and gastrocnemius muscles for low-to-moderate forces varied from 0.75 to >2 (Huyghues-Despointes et al. 2003a). For most of the cases shown in the present study (Table 3), values of the stiffness ratio for self-reinnervated muscles (right hindlimb) were close to or in excess of 1.0, except for LG in SELF-REINNERVATED subject 2, suggesting the presence of a reflex component in addition to stiffness due to passive elastic structures (Nichols and Houk 1976). Pairwise comparisons showed no significant differences between our self-reinnervated (right hindlimb) and untreated (left hindlimb) muscles (P = 0.272, n = 6 elf-reinnervated muscles and n = 4 untreated muscles), but the average Ke/Ki ratios for both set of muscles were significantly higher than for self-reinnervated soleus and gastrocnemius muscles of spinally intact animals [P = 0.006 and P < 0.001, respectively, n = 20 muscles/initial force; from Huyghues-Despointes et al. (2003a)]. Our results are therefore consistent with the apparent reappearance of the stretch reflex following reinnervation and spinal cord injury.
Table 3.
Short-range and incremental stiffnesses and resulting Ke/Ki ratio for muscles studied
| Ki, N/mm | Ke, N/mm | Ke/Ki | |
|---|---|---|---|
| Self-reinnervated muscles | |||
| SELF-REINNERVATED 2 right LG | 4.7 | 3.6 | 0.77 |
| SELF-REINNERVATED 3 right LG | 5.0 | 5.5 | 1.1 |
| SELF-REINNERVATED 4 right LG | 5.0 | 6.0 | 1.2 |
| SELF-REINNERVATED 2 right Sol | 1.3 | 1.7 | 1.3 |
| SELF-REINNERVATED 3 right Sol | 1.0 | 1.0 | 1.0 |
| SELF-REINNERVATED 4 right Sol | 4.4 | 4.5 | 1.0 |
| Untreated muscles | |||
| SELF-REINNERVATED 2 left LG | 5 | 3.35 | 0.67 |
| SELF-REINNERVATED 3 left LG | 5.0 | 4.0 | 0.8 |
| SELF-REINNERVATED 2 left Sol | 2.8 | 2.8 | 1.0 |
| SELF-REINNERVATED 3 left Sol | 1.5 | 1.5 | 1.0 |
Values are short-range (Ki) and incremental (Ke) stiffnesses for the muscles studied, and the resulting Ke ratio. LG, lateral gastrocnemius; Sol, soleus.
DISCUSSION
The goals of this study were twofold: 1) evaluate if locomotion on slope is possible in the complete spinal cat trained on a flat treadmill, and 2) determine the contribution of LG/Sol stretch feedback to recovery of locomotion on flat and slope surfaces in the absence of supraspinal influences.
We found that complete spinal animals locomotor trained on a flat treadmill can walk on a sloped surface, with or without LG/Sol self-reinnervation. A number of stride kinematic parameters change with slope in spinal animals, and these changes were similar whether the animal had undergone an LG/Sol self-reinnervation or not. Joint angles over the walking cycles had greater variability in spinal animals, but the joint angles profiles were similar across slopes. Finally, muscle burst characteristics also showed minimal changes with slope following spinal transection. These results show a lack of adaptation to low slope (±10°) in spinalized animals, and thus demonstrate the robustness of the locomotor patterns obtained with training on a flat surface to moderate changes in terrain.
Overall, we did not observe significant differences between animals with or without an LG/Sol self-innervation, which could be due to our unexpected finding that the autogenic stretch reflex largely returned in the animals with LG/Sol self-innervation or to the low slopes (±10°) used (see below). One of the few major differences found between the two animal groups was an increase in the number of clusters in the muscle burst onset/offset groupings in SELF-REINNERVATED animals, especially for downhill walking. This finding would be consistent with the greater effects of stretch feedback on muscle activity during the eccentric contractions that the ankle extensor muscles undergo during downslope walking (Gregor et al. 2018; Gregor et al. 2006; Maas et al. 2009; Pantall et al. 2016) and may indicate some deficits in heterogenic muscle feedback that were particularly difficult to test in spinalized animals.
Our results suggest that body weight-supported training on a flat surface would permit stepping on a variety of terrains. They also suggest that the lack of stretch reflex responses in muscles following nerve axotomy and repair may depend on the presence of supraspinal inputs to the lumbar cord.
Adaptations to Slope in Spinally Intact Animals with/without Ankle Extensor Self-Reinnervation
The comparison of upslope and downslope slope walking to flat walking in our intact cats without self-reinnervation of LG and Sol revealed changes in stride kinematic parameters with slope that closely resembled reported data in intact cats (Carlson-Kuhta et al. 1998; Gregor et al. 2006; Smith et al. 1998). Hip height remained relatively unchanged at all slopes as previously reported by Smith et al. and Gregor et al. Posterior displacement increased from downslope to flat to upslope (Carlson-Kuhta et al. 1998; Gregor et al. 2006; Smith et al. 1998), whereas anterior displacement decreased on the upslope but was similar between flat and downslope. As in Gregor et al. (2006), we saw a monotonic increase in stance length from downslope to upslope.
Following spinalization, most of those adaptations disappeared, except for anterior displacement, which significantly decreased on downslope for animals with LG/Sol self-reinnervation. Our hip, knee, and ankle joint angles during slope and flat walking in spinally intact animals resembled published data (Gregor et al. 2006; their Fig. 3) but showed greater overlap in the average angles at all slopes, most likely due to smaller slopes tested. Our hip, knee, and ankle joint angles during level walking post-spinal transection also resembled published joint angles data for locomotor-trained spinalized animals walking on a level treadmill (Lovely et al. 1990; their Fig. 2) and (Bélanger et al. 1988; their Fig. 6). Overall, our results in intact and spinal animals match published reports for the same conditions, suggesting that our methodology and measurement techniques were appropriate to determine the changes in hindlimb kinematics in spinal animals walking on slopes.
We observed major changes in gait kinematics in the one spinal SELF-REINNERVATED animal tested at slopes of ±25°. Those measurements were taken 30 wk postspinalization, suggesting that the LG and Sol stretch reflexes had returned at the time of the measurements (see below). The extent of the kinematic changes seen in that animal versus the changes seen in a spinal intact animal with actually missing LG and Sol stretch reflexes suggests that supraspinal control is the main contributor to adaptations at higher slopes. Nevertheless, the spinal locomotor networks can adapt to significant changes in slope in the absence of supraspinal drive, although the kinematics are significantly changed compared with the changes observed in spinally intact animals.
Muscle Burst Characteristics and Clustering
Onset and offset of muscle bursts during a stride were plotted to reveal groupings in muscle activation timing (Desrochers et al. 2019; Krouchev et al. 2006; Markin et al. 2012). Our muscle groupings during level walking in animals before spinal transection were similar to prior reports in intact animals. SartA and SartM grouped with EDL, IP, and TA during swing, whereas during stance, BFA grouped with SOL, and FDL, MG, and VL formed independent groups (Fig. 6, intact cats). Our clusters were more inclusive than in Krouchev et al. (2006) or Markin et al. (2012), who found that the TA formed its own cluster during the swing phase (see also Desrochers et al. 2019), as did the VL during stance (also the case in our results). Whereas the FDL clustered with the other extensors in Krouchev et al. (2006) and Desrochers et al. (2019), it clustered by itself in our animals.
Comparisons of pre-spinal transection walking on a slope with walking on flat showed limited changes in muscle activation phase or burst durations (Figs. 8 and 9). In addition, few muscles showed a significant shift in activation timing with slope, and those shifts were relatively small and not consistent between the animal groups with and without self-reinnervation of LG and Sol. For upslope walking, Carlson-Kuhta et al. (1998) similarly observed limited changes in muscle activity timing (e.g., prolonged FDL activity in the swing phase for upslope), especially at the lower slope (14°). For downslope walking, Smith et al. (1998) found significant activity in the IP (a hip flexor) and TA (an ankle flexor) during stance that acted to counteract the external moments exerted by the body’s gravity during downslope walking (Gregor et al. 2006). This increase was limited at the lower slope (14°) in the study of Smith et al. and in our study (slope 10°). Ankle extensors have been shown to prolong their activity during 27° upslope walking (Gregor et al. 2018). We saw no such trends in increased ankle extensor burst duration in our animals, which may be due to the much lower slope used. The above-mentioned changes in muscle activity during slope walking resulted in some limited changes in muscle clusters: the single flexor cluster observed in flat walking split into three, forming two new clusters of BFP and TA. Multiple extensor clusters during stance of flat and downslope walking united in a single cluster in upslope walking. Such reorganization of clusters could be caused by changes in supraspinal (Gottschall and Nichols 2007; Matsuyama and Drew 2000) or afferent inputs (Gregor et al. 2006; McVea et al. 2005) to the spinal locomotor networks.
Axotomy and repair of LG/Sol nerve did affect muscle burst clustering within the same slope. In flat walking, most of the hindlimb extensors formed a single cluster, whereas in upslope walking, multiple clusters of extensors were formed, as opposed to cats with intact LG and Sol. The exact reasons for the effect of LG/Sol self-reinnervation on reorganization of muscle clusters is unclear but could be related to the reorganization of spinal circuitry in response to self-reinnervation (Alvarez et al. 2011; Rotterman et al. 2014). It is also unknown if remaining deficits in length feedback (e.g., to heteronymous muscles) remain in our SELF-REINNERVATED animals following spinalization and locomotor training.
Muscle clusters in spinal cats were generally similar to those in spinal cats walking on a treadmill reported in Desrochers et al. (2019), supporting the conclusion of Desrochers et al. (2019) that muscle clusters observed during walking are formed primarily in the spinal cord.
Slope Adaptations in Spinalized Individuals
A similar lack of adaptation in joint angles to slope has been observed in ambulatory incomplete SCI individuals walking uphill at slopes up to 15°. In addition, muscle activity also poorly modulates with slope in the SCI individuals, whereas it shows a clear adaptation based on muscular demands in uninjured individuals (Leroux et al. 1999). The authors also found that SCI subjects used a forward tilt of the trunk and pelvic segments during both uphill and downhill walking and associated this adaptation to a need for greater stability brought on by the lack of leg kinematic adaptations to slope (Leroux et al. 2006). Therefore, although our results indicate that the locomotor pattern obtained with treadmill training after complete SCI may be sufficient to navigate the American Disability Act’s (ADA) requirements for inclined walkways, the results in human partial spinal injury suggest that the lack of adaptation in the kinematics may lead to body posture adaptation particular to the stability demand of bipedal gait.
Recovery of Muscle Force Response to Stretch in Self-Reinnervated Ankle Extensors of Spinal Cats
Our terminal measurements of the force response to stretch in the self-reinnervated muscles showed a return of the muscle’s stretch reflex (i.e., lack of significant yielding to ramp stretches) never observed in spinally intact animals following nerve transection and repair (Cope et al. 1994; Huyghues-Despointes et al. 2003a, 2003b; Lyle et al. 2016; Maas et al. 2007). We unfortunately have no information about the time course of the recovery, or if the return of the response to stretch extends to influence on heteronymous muscles. Although exercise is known to limit the stripping of afferent synaptic inputs to motoneurons that occurs following nerve axotomy (Krakowiak et al. 2015), results in spinally intact cats did not show a return of stretch reflex responses in self-reinnervated muscles even in animals that underwent significant locomotor training [weekly recording sessions, 3–4 days/wk, 1–2 h/day for 3 mo (Gregor et al. 2018) and free to move in their animal facility room otherwise]. It is thus unlikely that the recovery of the stretch reflex in the spinalized animals is exercise driven. A more likely mechanism for the reflex recovery is the removal of descending synaptic inputs following spinalization. A number of studies have shown that supraspinal descending inputs compete with proprioceptive afferents for synaptic contact onto spinal neurons in general, and motoneurons in particular. Increased proprioceptive afferent activity leads to decreased corticospinal projections to interneurons and an increase in afferent terminations to motoneurons, whereas deafferentation leads to increased corticospinal projections to interneurons (Jiang et al. 2016). Unilateral pyramidal tract section does lead to increased VGlut1-positive boutons (indicative of Ia afferent terminals) on contralateral motoneurons (Tan et al. 2012). Genetic manipulations in mice have shown a similar competitive balance between vestibular and proprioceptive inputs to motoneurons (Basaldella et al. 2015). The removal of all descending input in the spinal transection model is likely to have facilitated reattachment of the Ia fibers onto motoneuron soma rather than on the dendritic arbor as occurs in spinally intact rats following muscle reinnervation (Alvarez et al. 2011).
The stretch reflex recovery makes it impossible to establish if the lack of locomotor deficits, particularly an increased ankle flexion during stance [as reported during 27° downslope walking for spinal intact animals with self-reinnervated ankle extensors (Abelew et al. 2000; Maas et al. 2007)], during downslope walking in our animals was due to the lower slope used, the return of length feedback from the self-reinnervated muscles, or compensatory activity in other ankle extensors, notably shown for the MG after a cut to the LG/S nerve (Bouyer et al. 2001). Our previous modeling work suggests that ankle extensor length and velocity feedback have minimal effect on gait. Removal of all length-dependent feedback from the ankle extensors did not substantially affect maintenance of level walking in the neuromechanical model with no adaptation in the supraspinal drive to the central pattern generator (CPG) (Markin et al. 2016). On the other hand, removal of sensory feedback from hip flexor or extensor group Ia and II afferents, ankle extensor Ib afferents, or foot pad cutaneous feedback in the same conditions resulted in the model’s inability to maintain stable level walking (Markin et al. 2016).
Further exploration of the adaptation to slope with the model showed that in the absence of modulation in the supraspinal drive or afferent feedback gains, the model was incapable of upslope or downslope walking (±50% or ±27°) (Klishko et al. 2015). The model was unfortunately not tested at the lower slopes (±10°) used in our study, but the animal tested at ±25° was capable of stable plantar weight-bearing stepping. For the model, no feedback gain values could be found that allowed 27° upslope walking, although changes in the supraspinal drive to the CPG components allowed the model to walk upslope or downslope. The feedback gains during walking were assumed to be constant during locomotion, although they are known to be dependent on the locomotor phase (Lam and Pearson 2002; McCrea et al. 1995; Pearson 2000). Modulation of the feedback gain throughout the phase cycle may provide greater adaptability to the locomotor cycle and be responsible for our spinal animal’s ability to walk at ±25° of slope.
Potential changes in perineal stimulation could also mimic changes in the supraspinal drive and be responsible for the adaptations to increased slope, but we believe this is unlikely to be the mechanism underlying the spinal animal’s ability to walk at ±25° of slope. Our animals were extensively trained to locomote on the treadmill, and perineal stimulation was no longer required to obtain prolonged bouts of locomotion. Reviews of the videos taken during the sessions show that the animals were being held loosely by the base of the tail, which was often horizontal because the animals could bear full weight. No apparent differences could be seen in the way the tail was being held at the different slopes. It is nevertheless possible that the forces applied to the perineum area may have varied between the different slopes and that these variations could simulate variations in the supraspinal drive as perineal, cutaneous, and muscle afferents converge onto interneurons (Angel et al. 1994) that are likely targets of the descending motor commands. It seems unlikely that the potential increase or decrease in perineal input with slope would always have been beneficial to walking under all conditions, and more likely that only minimal changes in perineum feedback occurred at the different slopes. In light of the modeling work of Klishko et al. (2015), this would suggest that the gait cycle-dependent variations in afferent feedback may account for the robustness of gait to changes in slope, a hypothesis that would be worth exploring with the model in the future.
Conclusions
Spinalized animals locomotor trained on a flat treadmill are capable of walking on ±10° slopes. After spinal transection, the spinal locomotor networks made minimal adjustments to muscle activation for low slopes and the joint excursions showed minimal changes. Muscle synergies maintained an extensor and flexor grouping with no major changes in the timing of muscle activity. Whereas minimal changes were observed for ±10° slopes, the kinematic parameters were significantly altered for walking at ±25° in the cat with spinal transection and LG/Sol nerve repair. Spinalization, and possibly training, fosters the return of the muscle force response to stretch (i.e., stretch reflex) following self-reinnervation. The results of this study demonstrate the significant capacity of the spinal locomotor networks and motion-dependent sensory inputs at adapting locomotion to changes in slope in the absence of supraspinal drive.
GRANTS
This research was supported by National Institutes of Health Grants NS074406, NS11605, NS100928, and EB012855
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
D.H., A.K., A.N.K., T.R.N., M.A. Lyle, B.I.P., and M.A. Lemay conceived and designed research; D.H., A.K., A.N.K., T.R.N., M.A. Lyle, B.I.P., and M.A. Lemay performed experiments; D.H., A.K., A.N.K., T.R.N., M.A. Lyle, B.I.P., and M.A. Lemay analyzed data; D.H., A.K., A.N.K., T.R.N., M.A. Lyle, B.I.P., and M.A. Lemay interpreted results of experiments; D.H., A.K., O.H.M., T.R.N., M.A. Lyle, and M.A. Lemay prepared figures; D.H., A.K., and M.A. Lemay drafted manuscript; D.H., A.K., A.N.K., T.R.N., M.A. Lyle, B.I.P., and M.A. Lemay edited and revised manuscript; D.H., A.K., O.H.M., A.N.K., T.R.N., M.A. Lyle, B.I.P., and M.A. Lemay approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Kassi Miller and Jennifer Dashkova for assistance with animal care and training.
Present address of M. A. Lyle: Dept. of Rehabilitation Medicine, Emory University School of Medicine, Atlanta, GA.
REFERENCES
- Abelew TA, Miller MD, Cope TC, Nichols TR. Local loss of proprioception results in disruption of interjoint coordination during locomotion in the cat. J Neurophysiol 84: 2709–2714, 2000. doi: 10.1152/jn.2000.84.5.2709. [DOI] [PubMed] [Google Scholar]
- Alvarez FJ, Titus-Mitchell HE, Bullinger KL, Kraszpulski M, Nardelli P, Cope TC. Permanent central synaptic disconnection of proprioceptors after nerve injury and regeneration. I. Loss of VGLUT1/IA synapses on motoneurons. J Neurophysiol 106: 2450–2470, 2011. doi: 10.1152/jn.01095.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angel MJ, Fyda D, McCrea DA, Shefchyk SJ. Primary afferent depolarization of cat pudendal afferents during micturition and segmental afferent stimulation. J Physiol 479: 451–461, 1994. doi: 10.1113/jphysiol.1994.sp020309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basaldella E, Takeoka A, Sigrist M, Arber S. Multisensory signaling shapes vestibulo-motor circuit specificity. Cell 163: 301–312, 2015. doi: 10.1016/j.cell.2015.09.023. [DOI] [PubMed] [Google Scholar]
- Bélanger M, Drew T, Provencher J, Rossignol S.. Locomotion on an inclined plane before and after spinalization in the same cat (Abstract). 28th Annual Meeting of the Society for Neuroscience Los Angeles, CA, November 7–12, 1998, p. 14.265. [Google Scholar]
- Bélanger M, Drew T, Rossignol S. Spinal locomotion: a comparison of the kinematics and the electromyographic activity in the same animal before and after spinalization. Acta Biol Hung 39: 151–154, 1988. [PubMed] [Google Scholar]
- Bouyer LJ, Rossignol S. Contribution of cutaneous inputs from the hindpaw to the control of locomotion. II. Spinal cats. J Neurophysiol 90: 3640–3653, 2003. doi: 10.1152/jn.00497.2003. [DOI] [PubMed] [Google Scholar]
- Bouyer LJ, Whelan PJ, Pearson KG, Rossignol S. Adaptive locomotor plasticity in chronic spinal cats after ankle extensors neurectomy. J Neurosci 21: 3531–3541, 2001. doi: 10.1523/JNEUROSCI.21-10-03531.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyce VS, Tumolo M, Fischer I, Murray M, Lemay MA. Neurotrophic factors promote and enhance locomotor recovery in untrained spinalized cats. J Neurophysiol 98: 1988–1996, 2007. doi: 10.1152/jn.00391.2007. [DOI] [PubMed] [Google Scholar]
- Carlson-Kuhta P, Trank TV, Smith JL. Forms of forward quadrupedal locomotion. II. A comparison of posture, hindlimb kinematics, and motor patterns for upslope and level walking. J Neurophysiol 79: 1687–1701, 1998. doi: 10.1152/jn.1998.79.4.1687. [DOI] [PubMed] [Google Scholar]
- Conway BA, Hultborn H, Kiehn O. Proprioceptive input resets central locomotor rhythm in the spinal cat. Exp Brain Res 68: 643–656, 1987. doi: 10.1007/BF00249807. [DOI] [PubMed] [Google Scholar]
- Cope TC, Bonasera SJ, Nichols TR. Reinnervated muscles fail to produce stretch reflexes. J Neurophysiol 71: 817–820, 1994. doi: 10.1152/jn.1994.71.2.817. [DOI] [PubMed] [Google Scholar]
- Côté MP, Azzam GA, Lemay MA, Zhukareva V, Houlé JD. Activity-dependent increase in neurotrophic factors is associated with an enhanced modulation of spinal reflexes after spinal cord injury. J Neurotrauma 28: 299–309, 2011. doi: 10.1089/neu.2010.1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Côté MP, Gossard JP. Step training-dependent plasticity in spinal cutaneous pathways. J Neurosci 24: 11317–11327, 2004. doi: 10.1523/JNEUROSCI.1486-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Côté MP, Ménard A, Gossard JP. Spinal cats on the treadmill: changes in load pathways. J Neurosci 23: 2789–2796, 2003. doi: 10.1523/JNEUROSCI.23-07-02789.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desrochers E, Harnie J, Doelman A, Hurteau MF, Frigon A. Spinal control of muscle synergies for adult mammalian locomotion. J Physiol 597: 333–350, 2019. doi: 10.1113/JP277018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donelan JM, McVea DA, Pearson KG. Force regulation of ankle extensor muscle activity in freely walking cats. J Neurophysiol 101: 360–371, 2009. doi: 10.1152/jn.90918.2008. [DOI] [PubMed] [Google Scholar]
- Edgerton VR, Courtine G, Gerasimenko YP, Lavrov I, Ichiyama RM, Fong AJ, Cai LL, Otoshi CK, Tillakaratne NJ, Burdick JW, Roy RR. Training locomotor networks. Brain Res Brain Res Rev 57: 241–254, 2008. doi: 10.1016/j.brainresrev.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgerton VR, de Leon RD, Harkema SJ, Hodgson JA, London N, Reinkensmeyer DJ, Roy RR, Talmadge RJ, Tillakaratne NJ, Timoszyk W, Tobin A. Retraining the injured spinal cord. J Physiol 533: 15–22, 2001. doi: 10.1111/j.1469-7793.2001.0015b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- English AW. Enhancing axon regeneration in peripheral nerves also increases functionally inappropriate reinnervation of targets. J Comp Neurol 490: 427–441, 2005. doi: 10.1002/cne.20678. [DOI] [PubMed] [Google Scholar]
- Frigon A, Rossignol S. Locomotor and reflex adaptation after partial denervation of ankle extensors in chronic spinal cats. J Neurophysiol 100: 1513–1522, 2008. doi: 10.1152/jn.90321.2008. [DOI] [PubMed] [Google Scholar]
- Goslow GE Jr, Reinking RM, Stuart DG. The cat step cycle: hind limb joint angles and muscle lengths during unrestrained locomotion. J Morphol 141: 1–41, 1973. doi: 10.1002/jmor.1051410102. [DOI] [PubMed] [Google Scholar]
- Gottschall JS, Nichols TR. Head pitch affects muscle activity in the decerebrate cat hindlimb during walking. Exp Brain Res 182: 131–135, 2007. doi: 10.1007/s00221-007-1084-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregor RJ, Maas H, Bulgakova MA, Oliver A, English AW, Prilutsky BI. Time course of functional recovery during the first 3 mo after surgical transection and repair of nerves to the feline soleus and lateral gastrocnemius muscles. J Neurophysiol 119: 1166–1185, 2018. doi: 10.1152/jn.00661.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregor RJ, Smith DW, Prilutsky BI. Mechanics of slope walking in the cat: quantification of muscle load, length change, and ankle extensor EMG patterns. J Neurophysiol 95: 1397–1409, 2006. 10.1152/jn.01300.2004. [DOI] [PubMed] [Google Scholar]
- Haftel VK, Bichler EK, Wang QB, Prather JF, Pinter MJ, Cope TC. Central suppression of regenerated proprioceptive afferents. J Neurosci 25: 4733–4742, 2005. doi: 10.1523/JNEUROSCI.4895-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubli M, Dietz V. The physiological basis of neurorehabilitation–locomotor training after spinal cord injury. J Neuroeng Rehabil 10: 5, 2013. doi: 10.1186/1743-0003-10-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huyghues-Despointes CM, Cope TC, Nichols TR. Intrinsic properties and reflex compensation in reinnervated triceps surae muscles of the cat: effect of activation level. J Neurophysiol 90: 1537–1546, 2003a. doi: 10.1152/jn.00718.2002. [DOI] [PubMed] [Google Scholar]
- Huyghues-Despointes CM, Cope TC, Nichols TR. Intrinsic properties and reflex compensation in reinnervated triceps surae muscles of the cat: effect of movement history. J Neurophysiol 90: 1547–1555, 2003b. doi: 10.1152/jn.00719.2002. [DOI] [PubMed] [Google Scholar]
- Jana PK, Naik A. An efficient minimum spanning tree based clustering algorithm. 2009 Proceeding of International Conference on Methods and Models in Computer Science (ICM2CS). 2009, p. 1–5. doi: 10.1109/ICM2CS.2009.5397966. [DOI] [Google Scholar]
- Jiang YQ, Zaaimi B, Martin JH. Competition with primary sensory afferents drives remodeling of corticospinal axons in mature spinal motor circuits. J Neurosci 36: 193–203, 2016. doi: 10.1523/JNEUROSCI.3441-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klishko AN, Markin SN, Shevtsova NA, Lemay MA, Rybak IA, Prilutsky BI. Computer simulations of slope walking in the cat: role of supraspinal input to extensor interneurons. Program No. 799.08 2015 Neuroscience Meeting Planner. Washington, DC: Society for Neuroscience, 2015. [Google Scholar]
- Krakowiak J, Liu C, Papudesu C, Ward PJ, Wilhelm JC, English AW. Neuronal BDNF signaling is necessary for the effects of treadmill exercise on synaptic stripping of axotomized motoneurons. Neural Plast 2015: 392591, 2015. doi: 10.1155/2015/392591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krouchev N, Kalaska JF, Drew T. Sequential activation of muscle synergies during locomotion in the intact cat as revealed by cluster analysis and direct decomposition. J Neurophysiol 96: 1991–2010, 2006. doi: 10.1152/jn.00241.2006. [DOI] [PubMed] [Google Scholar]
- Krupka AJ, Fischer I, Lemay MA. Transplants of neurotrophin-producing autologous fibroblasts promote recovery of treadmill stepping in the acute, sub-chronic, and chronic spinal cat. J Neurotrauma 34: 1858–1872, 2017. doi: 10.1089/neu.2016.4559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam T, Pearson KG. The role of proprioceptive feedback in the regulation and adaptation of locomotor activity. Adv Exp Med Biol 508: 343–355, 2002. doi: 10.1007/978-1-4615-0713-0_40. [DOI] [PubMed] [Google Scholar]
- Leroux A, Fung J, Barbeau H. Adaptation of the walking pattern to uphill walking in normal and spinal-cord injured subjects. Exp Brain Res 126: 359–368, 1999. doi: 10.1007/s002210050743. [DOI] [PubMed] [Google Scholar]
- Leroux A, Fung J, Barbeau H. Postural adaptation to walking on inclined surfaces: II. Strategies following spinal cord injury. Clin Neurophysiol 117: 1273–1282, 2006. doi: 10.1016/j.clinph.2006.02.012. [DOI] [PubMed] [Google Scholar]
- Loeb GE, Gans C. Electromyography for Experimentalists. Chicago, IL: The University of Chicago Press, 1986. [Google Scholar]
- Lovely RG, Gregor RJ, Roy RR, Edgerton VR. Effects of training on the recovery of full-weight-bearing stepping in the adult spinal cat. Exp Neurol 92: 421–435, 1986. doi: 10.1016/0014-4886(86)90094-4. [DOI] [PubMed] [Google Scholar]
- Lovely RG, Gregor RJ, Roy RR, Edgerton VR. Weight-bearing hindlimb stepping in treadmill-exercised adult spinal cats. Brain Res 514: 206–218, 1990. doi: 10.1016/0006-8993(90)91417-F. [DOI] [PubMed] [Google Scholar]
- Lyle MA, Prilutsky BI, Gregor RJ, Abelew TA, Nichols TR. Self-reinnervated muscles lose autogenic length feedback, but intermuscular feedback can recover functional connectivity. J Neurophysiol 116: 1055–1067, 2016. doi: 10.1152/jn.00335.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maas H, Gregor RJ, Hodson-Tole EF, Farrell BJ, English AW, Prilutsky BI. Locomotor changes in length and EMG activity of feline medial gastrocnemius muscle following paralysis of two synergists. Exp Brain Res 203: 681–692, 2010. doi: 10.1007/s00221-010-2279-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maas H, Gregor RJ, Hodson-Tole EF, Farrell BJ, Prilutsky BI. Distinct muscle fascicle length changes in feline medial gastrocnemius and soleus muscles during slope walking. J Appl Physiol (1985) 106: 1169–1180, 2009. doi: 10.1152/japplphysiol.01306.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maas H, Prilutsky BI, Nichols TR, Gregor RJ. The effects of self-reinnervation of cat medial and lateral gastrocnemius muscles on hindlimb kinematics in slope walking. Exp Brain Res 181: 377–393, 2007. doi: 10.1007/s00221-007-0938-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malamud JG, Godt RE, Nichols TR. Relationship between short-range stiffness and yielding in type-identified, chemically skinned muscle fibers from the cat triceps surae muscles. J Neurophysiol 76: 2280–2289, 1996. doi: 10.1152/jn.1996.76.4.2280. [DOI] [PubMed] [Google Scholar]
- Markin SN, Klishko AN, Shevtsova NA, Lemay MA, Prilutsky BI, Rybak IA. A neuromechanical model of spinal control of locomotion. In: Neuromechanical Modeling of Posture and Locomotion, edited by Prilutsky BI, Edwards DH. New York: Springer, 2016, p. 21–65. [Google Scholar]
- Markin SN, Lemay MA, Prilutsky BI, Rybak IA. Motoneuronal and muscle synergies involved in cat hindlimb control during fictive and real locomotion: a comparison study. J Neurophysiol 107: 2057–2071, 2012. doi: 10.1152/jn.00865.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuyama K, Drew T. Vestibulospinal and reticulospinal neuronal activity during locomotion in the intact cat. II. Walking on an inclined plane. J Neurophysiol 84: 2257–2276, 2000. doi: 10.1152/jn.2000.84.5.2257. [DOI] [PubMed] [Google Scholar]
- McCrea DA, Shefchyk SJ, Stephens MJ, Pearson KG. Disynaptic group I excitation of synergist ankle extensor motoneurones during fictive locomotion in the cat. J Physiol 487: 527–539, 1995. doi: 10.1113/jphysiol.1995.sp020897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McVea DA, Donelan JM, Tachibana A, Pearson KG. A role for hip position in initiating the swing-to-stance transition in walking cats. J Neurophysiol 94: 3497–3508, 2005. doi: 10.1152/jn.00511.2005. [DOI] [PubMed] [Google Scholar]
- Nichols TR, Houk JC. Improvement in linearity and regulation of stiffness that results from actions of stretch reflex. J Neurophysiol 39: 119–142, 1976. doi: 10.1152/jn.1976.39.1.119. [DOI] [PubMed] [Google Scholar]
- Ollivier-Lanvin K, Fischer I, Tom V, Houlé JD, Lemay MA. Either brain-derived neurotrophic factor or neurotrophin-3 only neurotrophin-producing grafts promote locomotor recovery in untrained spinalized cats. Neurorehabil Neural Repair 29: 90–100, 2015. doi: 10.1177/1545968314532834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantall A, Hodson-Tole EF, Gregor RJ, Prilutsky BI. Increased intensity and reduced frequency of EMG signals from feline self-reinnervated ankle extensors during walking do not normalize excessive lengthening. J Neurophysiol 115: 2406–2420, 2016. doi: 10.1152/jn.00565.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson KG. Neural adaptation in the generation of rhythmic behavior. Annu Rev Physiol 62: 723–753, 2000. doi: 10.1146/annurev.physiol.62.1.723. [DOI] [PubMed] [Google Scholar]
- Perreault MC, Angel MJ, Guertin P, McCrea DA. Effects of stimulation of hindlimb flexor group II afferents during fictive locomotion in the cat. J Physiol 487: 211–220, 1995. doi: 10.1113/jphysiol.1995.sp020872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prilutsky BI, Maas H, Bulgakova M, Hodson-Tole EF, Gregor RJ. Short-term motor compensations to denervation of feline soleus and lateral gastrocnemius result in preservation of ankle mechanical output during locomotion. Cells Tissues Organs 193: 310–324, 2011. doi: 10.1159/000323678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossignol S. Plasticity of connections underlying locomotor recovery after central and/or peripheral lesions in the adult mammals. Philos Trans R Soc Lond B Biol Sci 361: 1647–1671, 2006. doi: 10.1098/rstb.2006.1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotterman TM, Nardelli P, Cope TC, Alvarez FJ. Normal distribution of VGLUT1 synapses on spinal motoneuron dendrites and their reorganization after nerve injury. J Neurosci 34: 3475–3492, 2014. doi: 10.1523/JNEUROSCI.4768-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JL, Carlson-Kuhta P, Trank TV. Forms of forward quadrupedal locomotion. III. A comparison of posture, hindlimb kinematics, and motor patterns for downslope and level walking. J Neurophysiol 79: 1702–1716, 1998. doi: 10.1152/jn.1998.79.4.1702. [DOI] [PubMed] [Google Scholar]
- Stecina K, Quevedo J, McCrea DA. Parallel reflex pathways from flexor muscle afferents evoking resetting and flexion enhancement during fictive locomotion and scratch in the cat. J Physiol 569: 275–290, 2005. doi: 10.1113/jphysiol.2005.095505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein RB, Misiaszek JE, Pearson KG. Functional role of muscle reflexes for force generation in the decerebrate walking cat. J Physiol 525: 781–791, 2000. doi: 10.1111/j.1469-7793.2000.00781.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeoka A, Arber S. Functional local proprioceptive feedback circuits initiate and maintain locomotor recovery after spinal cord injury. Cell Rep 27: 71–85.e3, 2019. doi: 10.1016/j.celrep.2019.03.010. [DOI] [PubMed] [Google Scholar]
- Takeoka A, Vollenweider I, Courtine G, Arber S. Muscle spindle feedback directs locomotor recovery and circuit reorganization after spinal cord injury. Cell 159: 1626–1639, 2014. doi: 10.1016/j.cell.2014.11.019. [DOI] [PubMed] [Google Scholar]
- Tan AM, Chakrabarty S, Kimura H, Martin JH. Selective corticospinal tract injury in the rat induces primary afferent fiber sprouting in the spinal cord and hyperreflexia. J Neurosci 32: 12896–12908, 2012. doi: 10.1523/JNEUROSCI.6451-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]