Abstract
The prefrontal cortex has been implicated in various cognitive processes, including working memory, executive control, decision making, and relational learning. One core computational requirement underlying all these processes is the integration of information across time. When rodents and rabbits associate two temporally discontiguous stimuli, some neurons in the medial prefrontal cortex (mPFC) change firing rates in response to the preceding stimulus and sustain the firing rate during the subsequent temporal interval. These firing patterns are thought to serve as a mechanism to buffer the previously presented stimuli and signal the upcoming stimuli; however, how these critical properties are distributed across different neuron types remains unknown. We investigated the firing selectivity of regular-firing, burst-firing, and fast-spiking neurons in the prelimbic region of the mPFC while rats associated two neutral conditioned stimuli (CS) with one aversive stimulus (US). Analyses of firing patterns of individual neurons and neuron ensembles revealed that regular-firing neurons maintained rich information about CS identity and CS-US contingency during intervals separating the CS and US. Moreover, they further strengthened the latter selectivity with repeated conditioning sessions over a month. The selectivity of burst-firing neurons for both stimulus features was weaker than that of regular-firing neurons, indicating the difference in task engagement between two subpopulations of putative excitatory neurons. In contrast, putative inhibitory, fast-spiking neurons showed a stronger selectivity for CS identity than for CS-US contingency, suggesting their potential role in sensory discrimination. These results reveal a fine-scaled functional organization in the prefrontal network supporting the formation of temporal stimulus associations.
NEW & NOTEWORTHY To associate stimuli that occurred separately in time, the brain needs to bridge the temporal gap by maintaining what was presented and predicting what would follow. We show that in rat medial prefrontal cortex, the former function is associated with a subpopulation of putative inhibitory neurons, whereas the latter is supported by a subpopulation of putative excitatory neurons. Our results reveal a distinct contribution of these microcircuit components to neural representations of temporal stimulus associations.
Keywords: associative memory, excitatory neurons, interneurons, unit recording, working memory
INTRODUCTION
Many cognitive processes, such as episodic memory and decision-making, rely on the ability to form associations between two events that occur separately in time. The formation of these temporal associations depends on neural representations of at least three types of information (Honig and Thompson 1982; Zentall 2010). First, the brain must continue representing events after they have passed (trace holding). Second, expectation signals need to be formed regarding which event would follow the first event (temporal expectation). Third, the brain needs to represent the exact timing at which the subsequent event would take place (explicit timing).
Previous works showed that all of these information types are represented in neuron firing responses in brain regions necessary for temporal stimulus associations, including the prefrontal cortex, hippocampus, and striatum (Gu et al. 2015; Pilkiw and Takehara-Nishiuchi 2018). For example, when rodents and rabbits associate a neutral conditioned stimulus (CS) with an innately salient, unconditioned stimulus (US) across a short temporal interval, some neurons in the medial prefrontal cortex (mPFC) change firing rates in response to the CS and sustain the firing rate during the subsequent temporal gap (Baeg et al. 2001; Gilmartin and McEchron 2005; Hattori et al. 2014; Morrissey et al. 2017; Siegel et al. 2012; Siegel and Mauk 2013; Takehara-Nishiuchi and McNaughton 2008). Moreover, by training rats to associate two different CS with the same US in a manner dependent on temporal context, we found that some neurons in rat mPFC sustained stimulus-evoked firing responses only to one of two CS, whereas others changed the magnitude of firing responses to the same CS depending on its predictability of the US (Morrissey et al. 2017). Furthermore, over a month of daily conditioning, the selectivity of ensemble firings for CS-US contingency was strengthened while the selectivity for CS identity was weakened. These findings indicate that the mPFC network performs parallel computations supporting trace holding and temporal expectation; however, how these critical functional properties are organized within the mPFC microcircuits remains unknown.
The PFC contains excitatory projection neurons and inhibitory interneurons, each of which is further categorized into many subpopulations with distinct electrophysiological, morphological, and neurochemical properties (Connors and Gutnick 1990; Dembrow and Johnston 2014; Kepecs and Fishell 2014; Molnár and Cheung 2006; Spruston 2008). Among them, two types of excitatory neurons and one type of inhibitory neuron exhibit distinct spike waveforms and temporal firing patterns, which makes it possible to isolate them from extracellularly recorded spike data (Baeg et al. 2001; Insel and Barnes 2015; Jung et al. 1998; Rao et al. 1999; Wilson et al. 1994). With the same criteria used in Insel and Barnes (2015), the present study extracted regular-firing, burst-firing, and fast-spiking neurons from the data set previously collected from the prelimbic region of the mPFC while rats associated two CS with the same US over a temporal gap (Morrissey et al. 2017). Analyses on firing patterns of individual neurons and neuron ensembles reveal the major differences in the firing selectivity and its experience-dependent changes between three neuron types, indicating their distinct contributions to neural processes supporting the formation and expression of temporal stimulus associations.
MATERIALS AND METHODS
Subjects, Surgery, Data Acquisition
The present study analyzed the spiking activity of multiple single neurons recorded in our previous study (Morrissey et al. 2017). These data were collected from four male Long-Evans rats (Charles River Laboratories, St. Constant, QC, Canada) between 16 and 25 wk old at the time of surgery. A protocol that was originally used to obtain these data was approved by the Animal Care and Use Committees at the University of Toronto (AUP no. 20011400). All surgeries were performed in a stereotaxic frame under isoflurane anesthesia, with recording started ~1 mo after the surgery. Recordings were performed using a chronically implanted array of 14 microdrives, each of which guided a tetrode to the prelimbic region of the mPFC (Fig. 1A; Kloosterman et al. 2009; Wilson and McNaughton 1993). Signals from each tetrode were recorded as differential signals between the tetrode and a reference tetrode placed in the secondary motor cortex, with a stainless steel screw implanted above the cerebellum used for grounding. Also, electromyogram (EMG) was recorded from the left upper eyelid through a pair of chronically implanted stainless steel wires. Neural and EMG signals were recorded, amplified, and filtered (neural: between 600 and 6,000 Hz; EMG: 300–3,000 Hz) with the Cheetah data acquisition system (Digital Lynx and Cheetah software; Neuralynx, Bozeman, MT). A threshold voltage was set at 40–50 µV, and if the voltage on any channel of a tetrode exceeded this threshold, signals were sampled from all four channels of the tetrode for 1 ms at 32 kHz. EMG activity was continuously sampled at 6,108 Hz. Putative single neurons were isolated offline using a specialized software package (MClust; https://redishlab.neuroscience.umn.edu/MClust/MClust.html) in MATLAB (The MathWorks, Natick, MA). Both automatic and manual spike-sorting procedures were used to assign each action potential to one of the neurons recorded simultaneously on one tetrode. The result was a collection of timestamps associated with each action potential from a given neuron. Only neurons with <1% of interspike intervals distribution falling within a 2-ms refractory period were used in the final analysis. If a neuron did not show more than 1,500 spikes during the entire recording session (i.e., one with basal firing rates less than ~0.2 Hz), it was removed from further analyses due to the insufficient number of spikes for the subsequent analysis. To collect neural activity from comparable recording locations across successive stages of learning, we minimized the movement of tetrodes across days. By comparing spike waveforms of units recorded on each tetrode across consecutive days, we found that on most tetrodes, different units were recorded across days, with the exception of some rare cases. Based on these qualitative observations, the across-day overlap in recorded units was estimated as less than ~10% (for detail, see Morrissey et al. 2017). Each of these units was treated as a separate sample.
Fig. 1.
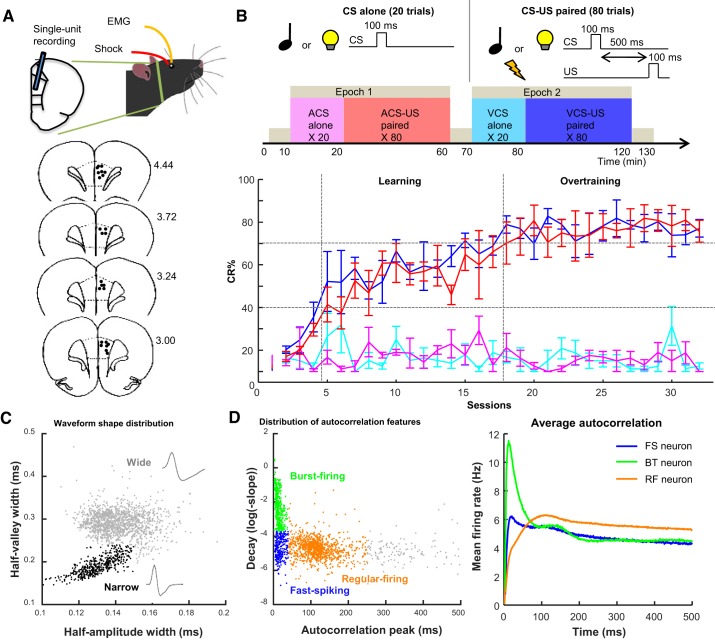
Behavior and neuron classification. A: rats were implanted with 4 wires into the eyelid and an array of 14 tetrodes in the prelimbic region of the medial prefrontal cortex (mPFC). In schematic diagrams of coronal sections (bottom), black dots indicate the final location of tetrode tips; numbers at right show the anterior-posterior coordinates from bregma. B: rats received 2 epochs of trace eyeblink conditioning, during which either a tone (auditory conditioned stimulus, ACS) or light (visual conditioned stimulus, VCS) was presented alone (CS alone; 20 trials) or preceding mild eyelid shock (US) by 500 ms (CS-US; 80 trials). The rate of expression of the conditioned response (CR%; n = 4 rats; means ± SE) was increased over days for both ACS-US (red) and VCS-US (blue) trials, but not during the respective CS-alone trials (magenta, turquoise). Based on CR% in CS-US trials, sessions were divided into learning and overtraining phases. C: distribution of half-amplitude (depolarization) width and half-valley (afterhyperpolarization) width of spike waveforms of all neurons. Neurons with shorter half-valley width (narrow waveform; black) were classified as putative inhibitory neurons. The remaining neurons with wide waveforms (gray) were classified as putative excitatory projection neurons. D: peak latency and downward slope of autocorrelograms were calculated in each neuron. The distribution of these features (left) allowed for dividing neurons into 3 groups: fast-spiking (FS; early peaks and slow decay; blue), burst-firing (BT; early peaks and rapid decay; green) and regular-firing (RF; later peaks and slower decay; orange) types. The averaged autocorrelograms of all neurons in each type (right) confirmed their difference in firing characteristics.
Behavior
All four rats underwent two epochs of the trace eyeblink conditioning paradigm daily, in which the conditioned stimulus (CS) was paired with the unconditioned stimulus (US) that was presented 500 ms after CS offset (Takehara-Nishiuchi 2018; Woodruff-Pak and Disterhoft 2008). In both epochs, rats were placed in the same conditioning chamber and received the presentation of either 100-ms pure tone (85 dB, 2.5 kHz) or 100-ms LED light (blinking at 50 Hz) as the CS. The different CS was used during the first and second epoch, with the CS order and schedule pseudorandomized across days and rats. The US was a 100-ms mild electrical shock near the eyelid (100-Hz square pulse, 0.3–2.0 mA), and the intensity was carefully monitored via a webcam and adjusted to ensure a proper eyeblink/head-turn response. Each epoch included a block of 20 presentations of the CS alone, followed by another block of 80 trials in which the CS was paired with the US (Fig. 1B). An interval between CS presentations ranged from 20 to 40 s, with an average of 30 s. This design generated four CS presentation types for comparison: presentations of the auditory CS alone (ACS alone), pairings of the auditory CS and US (ACS-US paired), presentations of the visual CS alone (VCS alone), and pairings of the VCS and US (VCS-US paired). Before and after each epoch, the rat was placed in a comfortable rest box separated from the conditioning chamber for 10 min. Each daily session lasted for ~130 min.
The conditioned response (CR) was defined as a fivefold increase in eyelid EMG amplitude during a 200-ms time window before US onset from baseline (see Morrissey et al. 2012, 2017 for detail). The proportion of trials with CR expression (CR%) was used to monitor the progress of associative learning. The conditioning sessions were divided into two successive stages of learning based on CR% during CS-US paired trials: a learning phase (sessions during which CR% was above 30% and progressed to an asymptotic level of learning) and an overtraining phase (remaining sessions after the CR% reached asymptote).
Neuron Type Classification
All isolated units were categorized into eight types based on the waveform shape and firing patterns, as described in Insel and Barnes (2015). The waveform shape was used to divide neurons into putative interneuron/inhibitory neurons and projection/excitatory neurons. Neurons having half-amplitude width < 0.15 ms and half-valley width < 0.24 ms were classified as interneuron/inhibitory neurons. The rest of the neurons were classified as projection/excitatory neurons. Neurons were then further divided into four types based on two features of autocorrelograms of their spike activity (1-ms bin). Fast-spiking neurons were defined as those with the peak latency of their autocorrelogram < 40 ms and log of decay slope smaller than −3.7. Neurons having the peak latency < 40 ms and log of decay slope larger than −3.7 were classified as burst-firing neurons. Regular-firing neurons were defined as those with the peak latency > 40 ms and log of the decay slope < 250. Neurons whose features did not meet these criteria were left as “ungrouped.” These parameters were chosen based on the visual inspection of the present data (Fig. 1, C and D) and consistent with those used in the other data set collected from rat rostral anterior cingulate and prelimbic cortex during a decision-making task (Insel and Barnes 2015).
Stimulus Selectivity of Firing Responses of Individual Neurons
To test whether a neuron significantly changed firing rates after CS presentations, in each neuron the difference between baseline (a 600-ms period before CS onset) and CS-evoked (a 600-ms period from CS onset to US onset) firing rate was calculated in each trial and then averaged across all trials. Random permutation tests (1,000 repetitions) were performed by reassigning the labels (i.e., baseline or CS evoked) to the firing rate in randomly chosen trials and calculating the firing rate change in the same manner as that with the real labels (Supplemental Fig. S1A; see https://doi.org/10.6084/m9.figshare.11348024). This generated the probability distribution of CS-evoked firing rate change at the chance level. If the actual firing rate difference of a neuron fell in the top or bottom 2.5% of this distribution, the neuron was considered as having a significant firing rate increase or decrease, respectively.
The differential index (DI) was used to quantify the degree of differentiation of binned firing rates between two CS presentation types:
where the FR1 and FR2 are averaged firing rates across trials in two CS presentation types. To quantify the selectivity for CS-US contingency, two DIs were calculated, one with firing rates during auditory CS alone and auditory CS-US paired trials, and the other with firing rates during visual CS alone and visual CS-US paired trials. Among the two DIs, the higher DI was chosen. We used this approach because ~20% of neurons exhibited firing differentiation between CS alone and CS-US paired trials in one of two CS types (Morrissey et al. 2017). To quantify the selectivity for CS identity, DI was calculated with firing rates during auditory CS-US paired trials and visual CS-US paired trials. To test whether the selectivity of each neuron for these features was statistically significant, random permutation tests (1,000 iterations) were performed by shuffling assigned labels of trial types to determine the probability distribution of DIs at the chance level (Supplemental Fig. S1B; see https://doi.org/10.6084/m9.figshare.11348024). Because the DI value ranges from 0 to 1, the actual DI fell in the top 5% of this distribution and was identified as significant. The difference between the actual DI and top 5% value of the chance distribution was calculated as the corrected DI.
Stimulus Selectivity of Ensemble Firing Patterns
Principal component analysis.
To examine the similarity between firing rates of a population of neurons across four CS presentation types (auditory CS-US paired, visual CS-US paired, auditory CS alone, or visual CS alone), we constructed a population firing rate matrix (neuron × trial), which contained the firing rate of all neurons in each type during a 500-ms period from CS offset and US onset. For each neuron, the firing rate in each trial was converted to a z score with the mean and SD of the firing rate across all trials. A two-dimensional projection of the firing rate matrix was obtained using principal component analysis for data visualization purposes in a low-dimensional space (see Fig. 3B).
Fig. 3.
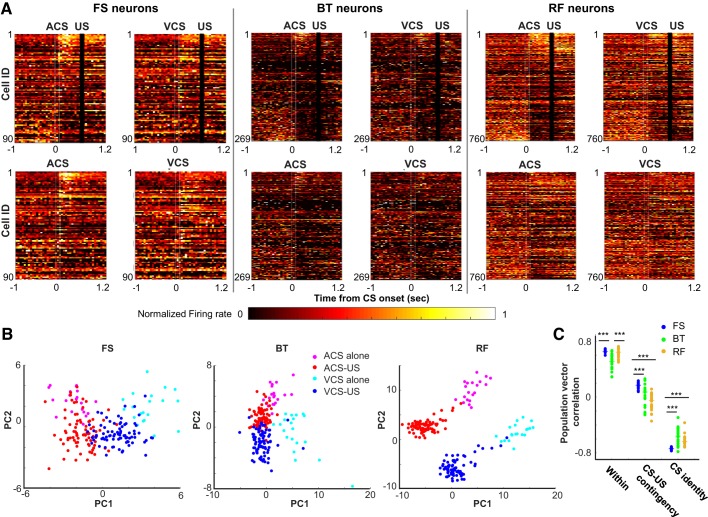
Differentiation of ensemble firing patterns across 4 types of conditioned stimulus (CS) presentations. A: neuron × time matrices of normalized firing rate (maximum = 1) for fast-spiking neurons with narrow waveforms (FS neurons; n = 90), burst-firing neurons with wide waveforms (BT neurons; n = 269), and regular-firing neurons with wide waveforms (RF neurons; n = 760). Neurons were sorted based on the auditory CS-evoked firing rate in the auditory CS-unconditioned stimulus (US) paired trials from the largest increase (top) to the largest decrease (bottom). White lines indicate CS onset and offset, and black bars mask US artifacts. B: first and second principal components (PC1 and PC2) of ensemble firing patterns during CS-US intervals in each of 3 neuron types. C: correlation coefficient of CS-evoked ensemble firing patterns between odd- and even-numbered auditory CS-US paired trials (Within), between auditory CS-US paired and auditory CS-alone trials (CS-US contingency), and between auditory and visual CS-US paired trials (CS identity). Each data point indicates the correlation coefficient in each run with a different set of 80 subsampled neurons, and horizontal bars indicate the median of 20 runs. ***P < 0.001, Kruskal–Wallis test and post hoc Dunn’s test. ACS, auditory CS; VCS, visual CS.
Population vector analysis.
The above-mentioned population firing rate matrix was constructed with 80 neurons subsampled from all neurons in each neuron type. A population vector (PV) was constructed by averaging the z-scored firing rates across all trials in each CS presentation type. Pearson correlation coefficient was calculated between PVs in the auditory CS alone and auditory CS-US paired type (CS-US contingency) as well as between PVs in the auditory CS-US paired and visual CS-US paired type (CS modality). To compare the degree of trial-by-trial variation in firing responses, we also calculated the highest possible correlation value in each neuron type by correlating PVs in odd- and even-numbered auditory CS-US paired trials (Within). The procedure was repeated 20 times with a different set of 80 subsampled neurons.
SVM decoding analysis.
As in our previous study (Morrissey et al. 2017), we quantified the selectivity of ensemble firing patterns by using decoding accuracy and errors of support vector machine (SVM) classifiers. SVM classifiers produce a model from training data attributes and then predict the target values using only the test data attributes. In our case, the attributes were the normalized binned firing rates of neurons, and the target values were the CS presentation type during which they were recorded. All algorithms were run in MATLAB using the freely accessible LIBSVM library (Chang and Lin 2011). This library implements the one-against-one approach for multiclass classifications. Specifically, binary classifications were conducted with all possible pairs of the target values. Each binary classification assigned a target value to each attribute. In the end, each attribute was classified into the target value to which it was assigned most frequently across all the binary classifications. A firing rate matrix was constructed by concatenating the binned firing rates of a set of N neurons during 20 trials randomly drawn, without replacement, from each of four CS presentation types (total 80 trials; Supplemental Fig. S2; see https://doi.org/10.6084/m9.figshare.11348021). In each neuron, the firing rate in each trial was divided by the maximum firing rate of the neuron across the 80 trials. The classifiers were trained with the radial basis function (RBF) kernels. Two parameters in the RBF kernel, cost (C) and γ, were first identified by performing a grid search in which fivefold cross-validation accuracy was compared across multiple SVM runs with a different combination of C (11 values) and γ (10 values). Because the degree of overfitting should be negatively correlated with the accuracy, we selected the set of C and γ that resulted in the highest accuracy. These parameters were then used for the training of SVM classifiers with half of the trials (10 trials from each CS presentation type, 40 trials in total). The remaining half were used for testing. The training and testing were repeated 200 times, with the use each time of a different set of 40 trials for training and another set of 40 trials for testing. Decoding accuracy was defined as the proportion of test trials correctly classified. Incorrectly classified trials were divided into three error types, errors in discriminating CS alone from CS-US paired trials (CS-US contingency), auditory CS trials from visual CS trials (CS identity), or both (mixed).
To examine how decoding accuracy changes depending on a number of neurons used to construct input vectors (set size), decoding accuracy was calculated with various set sizes and fitted to the following custom function using Curve Fitting Toolbox in MATLAB:
where x is the set size and y is decoding accuracy. To examine how decoding accuracy would be improved with a larger number of neurons than that available in our data set, the range of b was set to [0, inf] and the range of c was set to [−inf, 100]. In this way, the horizontal asymptote, defined by c, indicates the highest possible decoding accuracy, whereas a and b indicate the rate of improvement. The goodness of fit was measured by R2. The least absolute residuals procedure was used to minimize the effect of outliers.
Statistical Analyses
All statistical tests were two sided. The corrected DI, population vector correlation, SVM decoding accuracy, and error rate were compared across three neuron types with nonparametric statistics including Wilcoxon rank-sum tests and Kruskal–Wallis tests, followed by post hoc Dunn’s test where appropriate.
RESULTS
Behavior
As we reported previously (Morrissey et al. 2017), all four rats gradually increased the proportion of trials in which they expressed anticipatory blinking responses (CR%) during two CS presentation types in which the CS was paired with the US (CS-US paired; Fig. 1B). CR% was comparable between two CS types, suggesting that the rats formed two associations to the same degree. Moreover, CR% stayed low during the other CS presentation types in which the CS was presented alone (CS alone), suggesting that the rats learned the changes in CS-US associative strength depending on the temporal context. Based on CR% in each rat, the conditioning sessions were divided into two successive stages of learning: learning and overtraining phases (see materials and methods for more detail).
Neuron Types
During the conditioning sessions, a total of 1,701 neurons were recorded from the prelimbic region of the mPFC (see Table 1). Among them, 444 neurons (26.1%) exhibited narrow spike waveforms, whereas the remaining 1,257 neurons (73.9%) showed wide spike waveforms (Fig. 1C). In parallel, the examination of two features of their autocorrelograms (Fig. 1D) revealed that about half of these neurons showed regular-firing patterns (55.6%, n = 945). Smaller subsets showed burst-firing (24.0%, n = 408) and fast-spiking patterns (14.0%, n = 238). In subsequent analyses, we focused on three of these neuron types: 1) fast-spiking neurons with narrow waveforms (FS neurons, putative inhibitory neurons), 2) burst-firing neurons with wide waveforms (BT neurons, putative excitatory neurons), and 3) regular-firing neurons with wide waveforms (RF neurons, putative excitatory neurons).
Table 1.
Numbers and proportions of recorded neurons across different classes
| Learning Phase |
Overtraining Phase |
||||
|---|---|---|---|---|---|
| Wide waveform | Narrow waveform | Wide waveform | Narrow waveform | Total | |
| Fast spiking | 79 (9.4%) | 41 (4.9%) | 69 (8.0%) | 49 (5.7%) | 238 (14.0%) |
| Burst firing | 134 (15.9%) | 64 (7.6%) | 135 (15.7%) | 75 (8.7%) | 408 (24.0%) |
| Regular firing | 376 (44.6%) | 79 (9.3%) | 384 (44.7%) | 106 (12.3%) | 945 (55.6%) |
| Ungrouped | 48 (5.7%) | 22 (2.6%) | 32 (3.7%) | 8 (0.9%) | 110 (6.4%) |
| Total | 637 (75.5%) | 206 (24.4%) | 620 (72.2%) | 238 (27.7%) | 1,701 |
Data are from a total of 1,701 neurons recorded from 4 rats across 2 successive stages of learning, the learning (n = 843 neurons) and overtraining (n = 858 neurons) phases. Neurons were categorized into 8 types based on the shape of their waveforms and temporal firing patterns. Data in bold type indicate neurons used for subsequent analyses, and those included fast-spiking neurons with narrow waveform, burst-firing neurons with wide waveform, and regular-firing neurons with wide waveform.
Stimulus-Evoked Firing Responses of Individual Neurons
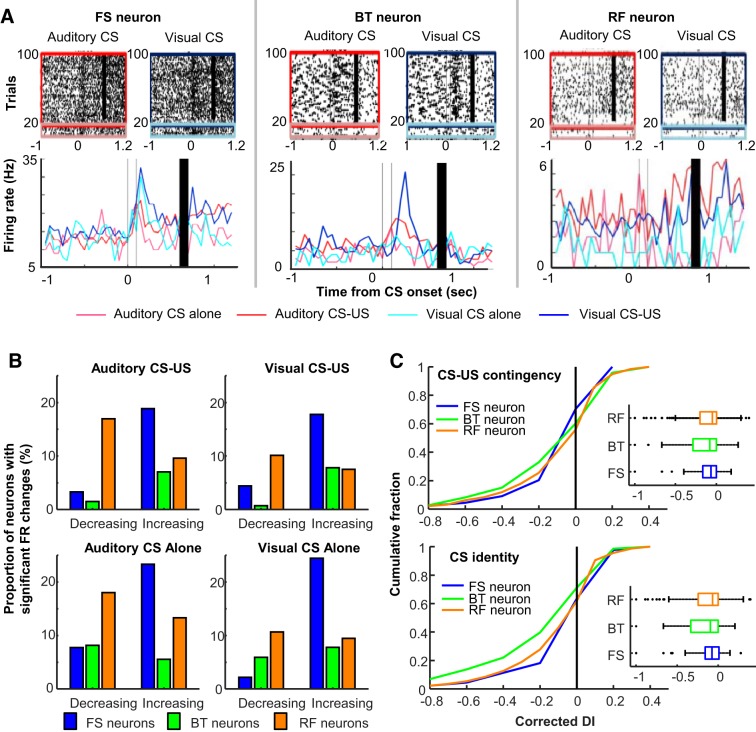
During blocks of CS alone and CS-US paired trials in two epochs, a sizable proportion of neurons in each neuron type markedly changed their firing rates during and after CS presentations. Also, some neurons showed different CS-evoked firing responses across four types of CS presentations (Fig. 2A). The degree of firing rate changes reached statistical significance (random permutation test, α = 0.05) in ~10–30% of neurons in each neuron type (Fig. 2B). The proportion of these “CS-responding” neurons was lower in BT neurons (~7%) than in FS (~25%) and RF neurons (~25%). Also, the proportion of RF neurons with decreased firing rates was greater than that with increased firing rates. In contrast, in most FS and BT neurons, the degree of firing rate decrease did not reach statistical significance, leading to a greater proportion of neurons with increased firing rates than those with decreased firing rates.
Fig. 2.
Stimulus-evoked firing responses of individual neurons. A: representative raster plots and peristimulus time histograms (1-ms bins, smoothed with a 50-ms Hanning window) for a fast-spiking neuron with narrow waveforms (FS neuron), a burst-firing neuron with wide waveforms (BT neurons), and a regular-firing neuron with wide waveforms (RF neuron). Line color indicates the firing response during each of 4 conditioned stimulus (CS) presentation types [presentations of the auditory CS alone, magenta; pairings of the auditory CS and unconditioned stimulus (US), red; presentations of the visual CS alone, turquois; pairings of the visual CS and US, blue]. B: proportion of neurons with significant firing rate (FR) changes during and after CS presentations in 4 CS presentation types. C: cumulative density plots (left) and box plots (right) comparing the corrected differential index (DI; positive values indicate significant firing differentiation, random permutation test, P < 0.05) for CS-US contingency (top) and CS identity (bottom). Box plots show median, first and third quartiles, and minimum and maximum values within 1.5 times the interquartile range from each quantile.
By calculating the differential index (DI) with these CS-evoked firing patterns, we then examined their selectivity for two stimulus features: the identity of the CS (i.e., which CS was presented) and the CS-US contingency (i.e., whether the US followed the CS). Raw DIs were corrected with the top 5% of the chance distribution in each neuron. Therefore, the positive DI values indicate that the firing differentiation between the two CS presentation types was statistically significant. Across three neuron types, ~40% of CS-responding neurons significantly differentiated their CS-evoked firing rates between the CS presented with the US and the CS presented alone (Fig. 2C, top). In parallel, ~35% of CS-responding neurons differentiated firing responses between the auditory and visual CS (Fig. 2C, bottom). In both cases, three neuron types included a comparable proportion of neurons with the significant firing differentiation (Kruskal–Wallis test, CS-US contingency, P = 0.065; CS identity, P = 0.999).
Stimulus Selectivity of Ensemble Firing Patterns
Next, we examined the degree to which neuron ensembles of each neuron type differentiated CS-evoked firing patterns across four CS presentation types (auditory CS-US paired, visual CS-US paired, auditory CS alone, or visual CS alone). Consistent with the distribution of DI values (Fig. 2C), in all three neuron types, some neurons differentiated their firing rates between CS-alone trials and CS-US trials whereas others differentiated firing rates between visual and auditory CS trials (Fig. 3A). A small proportion of neurons (particularly in BT neurons) exhibited action potentials in some CS presentation types whereas they were completely silent in other CS presentation types. A principal component analysis of CS-evoked ensemble firing patterns showed that in all three neuron types, the top two principal components were sensitive to CS-US contingency (i.e., CS alone or CS-US paired trials) and CS identity (i.e., auditory or visual CS trials; Fig. 3B). RF neuron ensembles appeared to show greater differentiation of CS-evoked firing patterns across four CS presentation types than the other neuron types.
To quantify the visual impression, we then conducted a population vector (PV) analysis that calculated correlation coefficients of CS-evoked ensemble firing patterns between the CS presentation types. To match the number of neurons used to construct PVs, 80 neurons were randomly sampled from all neurons in each neuron type, because this is the largest set size allowing for reasonable subsampling from 90 FS neurons. The similarity of ensemble firing patterns between auditory CS-US and auditory CS-alone trials was higher in FS neurons than BT and RF neurons (Fig. 3C; n = 20 runs; CS-US contingency; Kruskal–Wallis test, P < 0.001; post hoc Dunn’s test, RF vs. FS, P < 0.001; RF vs. BT, P = 0.092; FS vs. BT, P < 0.001). In parallel, the similarity of ensemble firing patterns between auditory CS-US and visual CS-US trials was lower in FS neurons than in BT and RF neurons (CS identity; Kruskal–Wallis test, P < 0.001; post hoc Dunn’s test, FS vs. BT, P < 0.001; FS vs. RF, P < 0.001), whereas BT and RF neurons showed a comparable level of the similarity (P = 0.776). The differences in the stimulus selectivity between FS and RF neurons were not due to the differences in their trial-by-trial firing variations, because the similarity of odd- and even-numbered trials of the auditory CS-US paired type was comparable between FS and RF neurons (Within; Kruskal-Wallis test, P < 0.001; post hoc Dunn’s test, P = 0.744). In contrast, the similarity was significantly lower in BT neurons than in the other neuron types (P values < 0.001).
Ensemble Decoding Accuracy and Errors
As another measure of ensemble selectivity, we applied SVM classifiers to CS-evoked ensemble firing patterns, as in our previous work (Morrissey et al. 2017). In the original study, SVM classifiers were applied to input vectors consisting of firing patterns of 200 subsampled neurons. After neuron classification, we no longer had 200 neurons in some of the neuron types. To optimize decoding accuracy with a smaller number of neurons, we first conducted preliminary analyses by testing several different ways to construct input vectors. When the input vector was constructed from binned firing rates covering the first 100–500 ms of the CS-US intervals, decoding accuracy was improved with the larger bin size (Fig. 4A; Wilcoxon rank-sum test against accuracy with 100-ms bins, all except with 300-ms bins, P values < 0.05/5). In all neuron types, however, decoding accuracy appeared to reach asymptote with the bin size of 250 ms or larger.
Fig. 4.
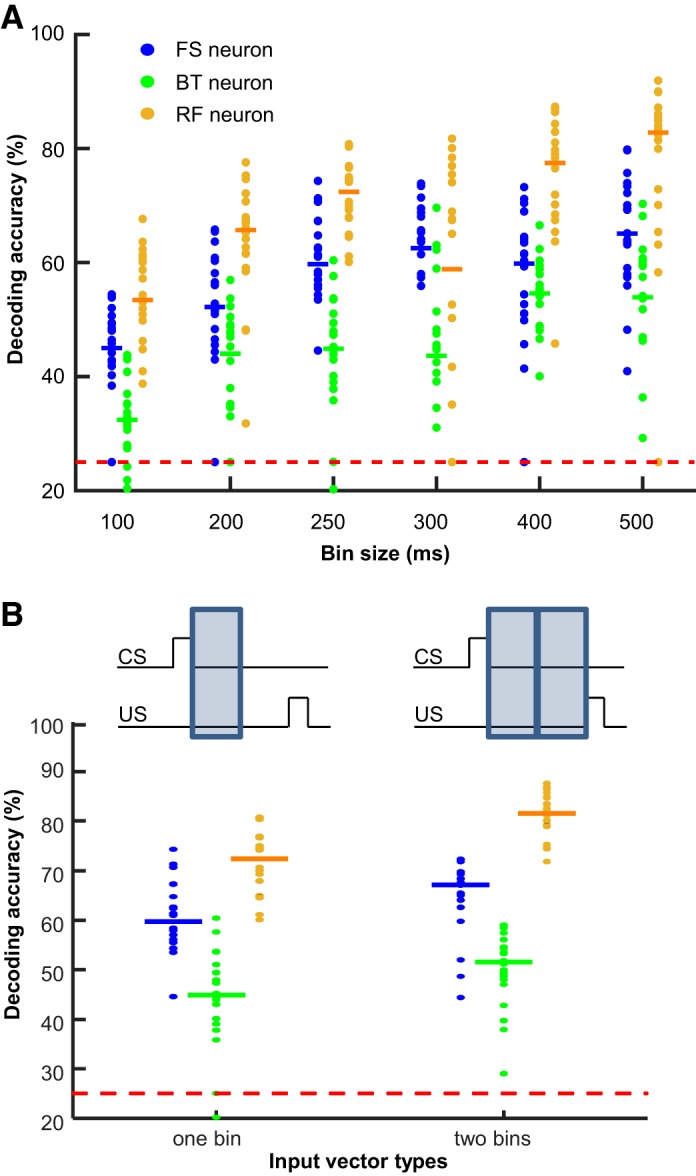
Optimization of input firing vectors for ensemble decoding. A: input vectors for support vector machine (SVM) decoding analysis were constructed from firing rates during a variable size of a time bin within the 500-ms interval between the conditioned stimulus (CS) and the unconditioned stimulus (US). In all 3 neuron types, decoding accuracy was improved with the larger bin size. BT, burst-firing neuron; FS, fast-spiking neuron; RF, regular-firing neuron. B: comparison of decoding accuracy with firing rates during the first 250-ms bin (left) vs. with those during both the first and last bins (right). Each data point indicates decoding accuracy of each SVM run with a different set of 80 subsampled neurons, and horizontal bars indicate the median of 20 runs. Red dashed line represents 25% chance level.
In our previous analysis of the same data set, we were able to decode whether an ensemble firing pattern was from the first or second half of the CS-US interval (Pilkiw and Takehara-Nishiuchi 2018), suggesting the change in ensemble firing patterns between the early and late phases of CS-US intervals. By incorporating this firing property into input vectors, we were able to further improve decoding accuracy: decoding accuracy was higher when firing rates during both the first and second 250-ms bins were used as inputs rather than those during the first 250-ms bin alone (Fig. 4B; Wilcoxon rank-sum test, FS, P = 0.039; BT, P = 0.036; RF, P < 0.001). Based on these findings, we conducted the main analysis by using two sets of 250-ms binned firing rates that covered the entire CS-US interval.
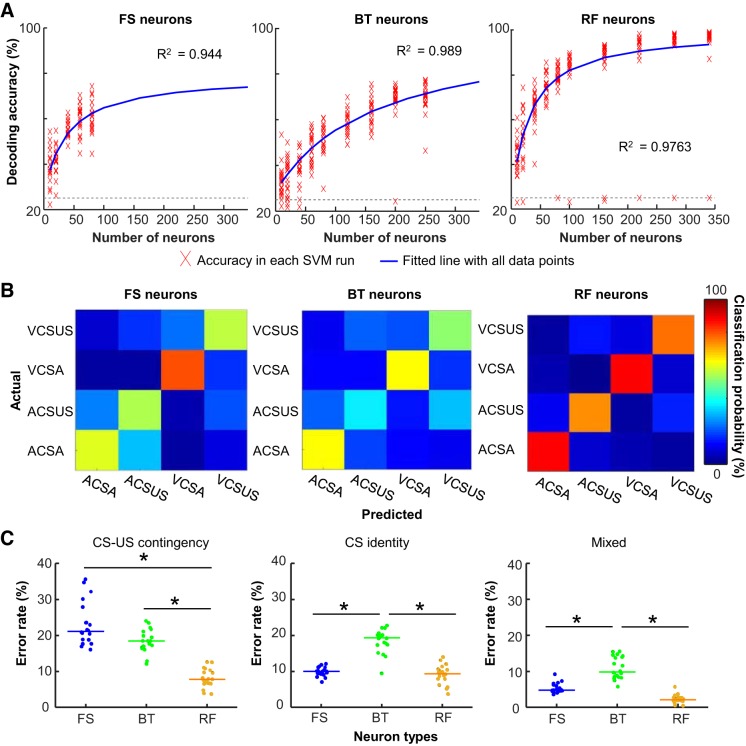
We also examined the change in decoding accuracy as a function of the number of neurons included in input vectors (set size). In each neuron type, we fit a custom function to a series of decoding accuracy calculated with 10–350 subsampled neurons (see materials and methods for detail). In all three neuron types, decoding accuracy was dramatically improved as the set size became larger (Fig. 5A). Compared with RF neurons, FS neurons showed a similar rate of the improvement (parameters a and b in Table 2), but they showed lower theoretical maximum accuracy (parameter c in Table 2). BT neurons, on the other hand, showed a slower rate of improvement than the other neuron types, but decoding accuracy was expected to reach 100% with a large number of BT neurons.
Fig. 5.
Differential selectivity of ensemble firing patterns for relational and sensory stimulus features. A: decoding analyses were repeated with input vectors containing a varying number of subsampled neurons in each type. Red crosses indicate decoding accuracy in each support vector machine (SVM) run. Resultant decoding accuracy was fitted to a custom function (blue line). Gray dashed line represents 25% chance level. BT, burst-firing neurons; FS, fast-spiking neurons; RF, regular-firing neurons. B: representative confusion matrices from an SVM run with 80 subsampled neurons showing the proportion of trials in each condition (rows) that were classified as 1 of 4 conditioned stimulus (CS) presentation types (columns). Hotter color along diagonal illustrates the high accuracy of decoding. ACSA, auditory CS presented alone; ACS-US, auditory CS paired with unconditioned stimulus (US); VCSA, visual CS presented alone; VCSUS, visual CS paired with US. C: proportion of trials classified as those with incorrect CS-US contingency (left), incorrect CS identity (middle), or both (mixed; right). Each data point represents the proportion of errors in an SVM run with 80 subsampled neurons, and horizontal bars show the median. *P < 0.001, Kruskal–Wallis test, P < 0.05, post hoc Dunn’s test.
Table 2.
Parameters used for curve fitting to changes in decoding accuracy with an increasing number of neurons
| Curve Fit Parameters |
||||
|---|---|---|---|---|
| R2 | a | b | c | |
| FS neurons | 0.944 | −1,963 | 36.92 | 79.16 |
| BT neurons | 0.989 | −11,460 | 159 | 100 |
| RF neurons | 0.976 | −1,998 | 23.52 | 100 |
Values for R2 indicate goodness of fit for fast-spiking neurons with narrow waveform (FS neurons), burst-firing neurons with wide waveform (BT neurons), and regular-firing neurons with wide waveform (RF neurons). Parameters a and b indicate the rate of improvement in decoding accuracy with an increasing number of neurons included in input firing vectors. Parameter c indicates the theoretical maximum of decoding accuracy with an infinite number of neurons.
In the set size of 80 neurons (the largest set size allowing for reasonable subsampling from 90 FS neurons), the decoding accuracy was the highest in RF neurons (Kruskal–Wallis test, P < 0.001; post hoc Dunn’s test, RF vs. FS, P < 0.001; RF vs. BT, P < 0.001) and the lowest in BT neurons (FS vs. BT, P = 0.027). Examination of the specific classification errors (confusion matrices; Fig. 5B) demonstrated major differences in ensemble selectivity between the three neurons types. With FS neurons, the majority of inaccurate classifications resulted from errors in discriminating CS-US paired trials from CS-alone trials, whereas errors in discriminating auditory CS trials from visual CS trials were rare, suggesting that FS neuron ensembles were more selective for CS identity than CS-US contingency. With BT neurons, decoding accuracy was higher for CS-alone trials than CS-US paired trials. In the latter trials, inaccurate classifications were mostly due to errors in discriminating auditory CS-US trials from visual CS-US trials, suggesting the stronger selectivity for CS-US contingency than CS identity. With RF neurons, classifiers were able to decode CS-alone trials almost perfectly. Most incorrect classifications resulted from errors in discriminating auditory CS-US paired from visual CS-US paired trials, suggesting the stronger selectivity for CS-US contingency than CS identity.
Among three neuron types, the rate of errors in differentiating CS-alone trials and CS-US trials was the lowest in RF neurons (Fig. 5C; Kruskal–Wallis test, P < 0.001; post hoc Dunn’s test, P values < 0.001), and the rates in FS and BT neurons were comparable to one another (P = 0.267). In contrast, RF and FS neurons showed a similar rate of errors in differentiating auditory CS-US and visual CS-US trials (Kruskal–Wallis test, P < 0.001; post-hoc Dunn’s test, P = 0.770), which was significantly lower than that of BT neurons (P values < 0.001). The combined error in CS identity and CS-US contingency was lowest in RF neurons, higher in FS neurons, and highest in BT neurons (Kruskal–Wallis test, P < 0.001; post hoc Dunn’s test, FS vs. BT, P = 0.011; FS vs. RF, P < 0.001; BT vs. RF, P < 0.001).
Influence of Repeated Experiences on Ensemble Selectivity
By applying SVM analyses to all recorded neurons, we previously reported that throughout learning and subsequent overtraining, prefrontal neuron ensembles improved their selectivity for CS-US contingency while they concomitantly reduced the selectivity for CS identity (Morrissey et al. 2017). To address which neuron types contribute to the experience-dependent change in ensemble selectivity, we separately ran SVM decoding analyses with ensemble firing patterns during two successive stages of learning: learning and overtraining phases (Fig. 1B). The smallest sample size was 41 FS neurons recorded during the learning phase. Therefore, the set size of 40 neurons was used for the subsequent analyses.
With FS neurons, decoding accuracy during the overtraining phase was lower than that during the learning phase (Fig. 6; Wilcoxon rank-sum test, P = 0.001), although the rate of any error type was not significantly increased (Wilcoxon rank-sum test, CS-US contingency, P = 0.323; CS modality, P = 0.091; mixed, P = 0.123). Similarly, decoding accuracy with BT neurons was also decreased with repeated conditioning sessions (P < 0.001), which was due to the increased errors on CS identity and mixed errors on both CS identity and CS-US contingency (P values < 0.001). In contrast, decoding accuracy with RF neurons was improved with repeated conditioning sessions (P = 0.030), which resulted from the significant decrease in errors on CS-US contingency (P < 0.001) as well as the mixed errors (P = 0.008). In parallel, the rate of errors on CS identity was increased (P = 0.004).
Fig. 6.
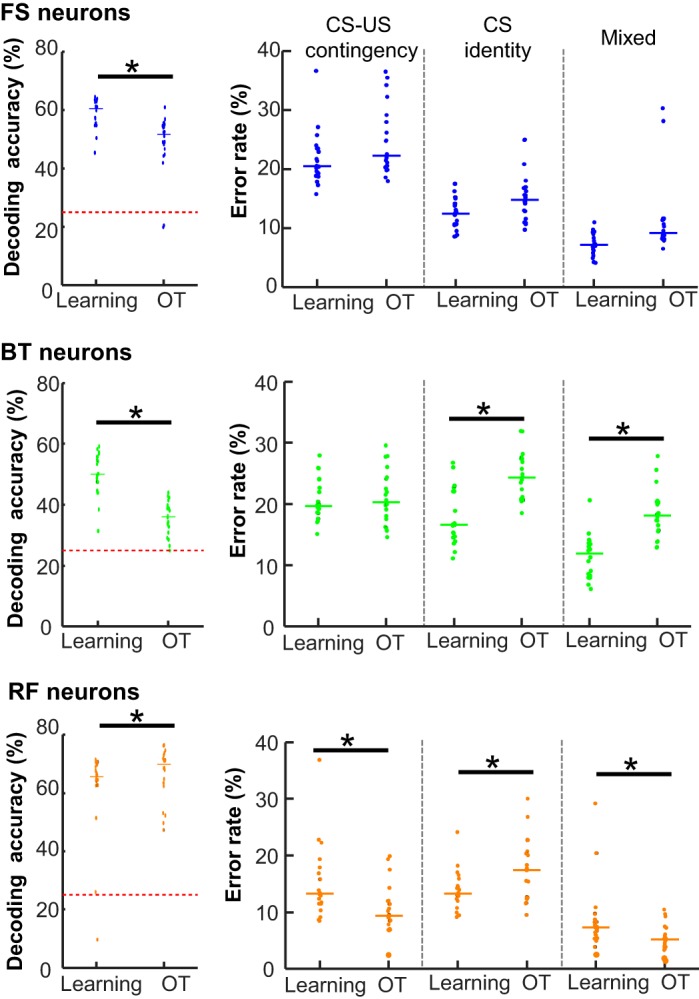
Experience-dependent changes in ensemble selectivity. Decoding accuracy (left) and error rates (right) of 20 support vector machine (SVM) runs with 40 subsampled neurons during learning and overtraining (OT) phases in fast-spiking (FS), burst-firing (BT), and regular-firing (RF) neurons. Each data point represents the data in an SVM run, and horizontal bars show the median. *P < 0.05, Wilcoxon rank-sum test. CS, conditioned stimulus; US, unconditioned stimulus.
DISCUSSION
Considerable evidence from electrophysiological (Baeg et al. 2001; Gilmartin and McEchron 2005; Hattori et al. 2014; Morrissey et al. 2017; Siegel et al. 2012; Siegel and Mauk 2013; Takehara-Nishiuchi and McNaughton 2008) and behavioral studies (Gilmartin et al. 2013; Jarovi et al. 2018; Volle et al. 2016) suggests that prefrontal firing activity during intervals between temporally discontiguous stimuli is essential for the encoding and expression of temporal stimulus associations. What remains unknown, however, is how different types of neurons within the prefrontal network are activated by the stimulus pairings and how their activity patterns change with repeated experiences. By isolating one putative inhibitory and two putative excitatory neuron types from a data set of the extracellularly recorded spiking activity (Morrissey et al. 2017), we found major differences in stimulus selectivity among the three neuron types, indicating their differential contribution to the encoding and expression of temporal stimulus associations. Specifically, with two parallel analyses on ensemble selectivity, we found that putative excitatory, regular-firing neurons showed a stronger selectivity for stimulus contingency than putative inhibitory, fast-spiking neurons. Fast-spiking neurons, on the other hand, showed the strong selectivity for CS identity and maintained the selectivity across repeated experiences over a month.
Functional Dissociation Between Two Subpopulations of Excitatory Neurons
Two subpopulations of putative excitatory neurons, RF and BT neurons, contained a comparable proportion of neurons that significantly changed firing rates during and after CS presentations (Fig. 2B). Consistent with previous studies (Baeg et al. 2001; Hattori et al. 2014; Takehara-Nishiuchi and McNaughton 2008), some of these neurons increased firing rates whereas others decreased firing rates. The increased firing rates during the CS-US interval are an essential neural process for associating the CS and US because optogenetic inhibition of neural firings during the intervals impairs the formation of CS-US association (Gilmartin et al. 2013), whereas their chemogenetic enhancement facilitates the association formation (Jarovi et al. 2018; Volle et al. 2016). In contrast, it remains unclear how the decreased firing rates of excitatory neurons are related to association formation. A potential function would be to enhance the differentiation of ensemble activity before and after CS presentations, thereby increasing the contrast of neural representations between foreground events and background context.
In parallel, detailed examinations of ensemble similarity and decoding accuracy revealed that RF neurons showed a stronger selectivity for CS identity and CS-US contingency than BT neurons (Fig. 3C and Fig. 5, B and C). Moreover, over a month of repeated conditioning sessions, only RF neurons improved the selectivity for CS-US contingency, whereas both neuron types weakened the selectivity for CS identity (Fig. 6). These findings expanded our previous findings of time-dependent changes in the ensemble selectivity of unclassified neurons (Morrissey et al. 2017; Takehara-Nishiuchi and McNaughton 2008) by revealing that the improved selectivity for the relational stimulus feature is uniquely attributable to RF neural ensembles, whereas both RF and BT neurons contribute to the weakened selectivity for the physical stimulus feature. Of note, the improved selectivity for the relational feature occurs as the mPFC becomes necessary for memory expression (Oswald et al. 2010; Simon et al. 2005; Takehara-Nishiuchi et al. 2006; Takehara et al. 2003). Also, this circuit property is a prerequisite for the proposed role of the mPFC as a hub of the neocortical network supporting long-lasting memories (Frankland and Bontempi 2005; Insel and Takehara-Nishiuchi 2013; Wiltgen et al. 2004). The present finding, therefore, adds to the literature by identifying RF neurons as the circuit component subserving the proposed role.
Relation to Anatomical Characteristics of Subpopulations of Excitatory Neurons
By combining in vitro intracellular recordings with morphological characterizations, Yang et al. (1996) identified four subpopulations of pyramidal neurons in deep layers of rat prelimbic region. Among them, one subpopulation exhibits single spikes at a regular pace and has a rounded pyramidal soma and dendrites of simple branching. These morphological features match those of intratelencephalic neurons that project to the rhinal cortex, amygdala, striatum, and neocortex (Dembrow et al. 2010; Dembrow and Johnston 2014; Hirai et al. 2012; Molnár and Cheung 2006). The other subpopulation, on the other hand, exhibits burst firings and maintains a thick apical dendritic truck that is profusely bifurcated (Yang et al. 1996). These features are consistent with the morphology of pyramidal tract neurons that project to subcortical structures, such as pons, brain stem, and thalamus (Dembrow et al. 2010; Dembrow and Johnston 2014; Molnár and Cheung 2006). These findings raise the possibility that RF and BT neurons might include these cortically and subcortically projecting neurons, respectively. If so, the present findings allude to a potential functional division between cortically and subcortically projecting neurons: the former may carry rich information about the paired stimuli, including behaviorally relevant, relational features (i.e., CS-US contingency) as well as incidental, sensory features (i.e., CS identity), whereas the latter may carry simple information, mostly focusing on the relational features. Of note, the ratio of regular-firing and burst-firing neurons in the present data was the inverse of that in the in vitro study (Yang et al. 1996). This may be due to differences between the two preparations, particularly the recording sites: we recorded from both superficial and deep layers, whereas Yang et al. (1996) characterized neurons in deep layers in which subcortically projecting neurons are preferentially located (Dembrow and Johnston 2014). Nonetheless, future studies combining the single-unit recording with the characterization of morphology and projection targets are necessary to investigate the potential functional division among subpopulations of excitatory neurons depending on the projection targets.
Role of Fast-Spiking Neurons in Sensory Discrimination
Among diverse types of GABAergic, interneurons in the cortex, those expressing parvalbumin are unique in that they exhibit high-frequency firings without adaptation (Connors and Gutnick 1990; Kawaguchi and Kubota 1997). This feature, along with their narrow spike waveforms, makes it possible to identify these neurons as FS neurons in extracellularly recorded spike data in vivo (Baeg et al. 2001; Insel and Barnes 2015; Jung et al. 1998; Rao et al. 1999; Wilson et al. 1994). In our data set, ~30% of FS neurons significantly changed firing rates during and after CS presentations, and the majority sharply increased firing rates at the onset of the CS (Fig. 3A). Also, FS neuron ensembles showed the strong selectivity for the identity of the CS (i.e., auditory or visual stimulus) regardless of its associative strength with the US (Figs. 3C and 5C), and they were the only subpopulation that maintained the comparable level of the selectivity for CS identity over a month (Fig. 6). These findings suggest a potential contribution of prefrontal parvalbumin-positive interneurons to sensory discrimination. This role was overlooked in previous studies using differential associative learning paradigms (Courtin et al. 2014) or go/no-go tasks (Kamigaki and Dan 2017; Pinto and Dan 2015), in which stimulus contingency covaries with stimulus identity. Each of the parvalbumin-positive interneurons may receive a specific modality of sensory inputs and form feedforward inhibitory connections onto nearby excitatory neurons receiving different modalities of sensory inputs. Such a microcircuit organization enables parvalbumin-positive interneurons to shape the selectivity of pyramidal neurons for specific sensory modality, as shown in the posterior parietal cortex (Song et al. 2017).
In conclusion, we showed the major differences in the stimulus selectivity and its experience-dependent changes among three subpopulations of prefrontal neurons. A subpopulation of putative excitatory neurons maintained rich information about the sensory and relational stimulus features and further strengthened the latter selectivity with repeated experiences. In contrast, the other subpopulation of putative excitatory neurons encoded simpler information focusing on the relational stimulus features, which might play a role in driving the expression of learned behavior. Putative inhibitory neurons prioritized the sensory feature over the relational feature, suggesting their potential role in sensory discrimination. These data expand the growing literature on functional division among different cell types and provide several testable hypotheses on distinct contributions of specific components of prefrontal microcircuits to the encoding and expression of temporal stimulus associations.
GRANTS
This work was supported by National Sciences and Engineering Research Council Discovery Grant, Ontario Research Fund Early Researcher Awards, and CFI Leaders Opportunity Fund (to K. Takehara-Nishiuchi).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.D.M. and K.T.-N. conceived and designed research; M.D.M. performed experiments; B.X. analyzed data; K.T.-N. interpreted results of experiments; B.X. prepared figures; B.X. and K.T.-N. drafted manuscript; K.T.-N. edited and revised manuscript; B.X., M.D.M., and K.T.-N. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Nathan Insel for generously providing the codes for neuron classification.
REFERENCES
- Baeg EH, Kim YB, Jang J, Kim HT, Mook-Jung I, Jung MW. Fast spiking and regular spiking neural correlates of fear conditioning in the medial prefrontal cortex of the rat. Cereb Cortex 11: 441–451, 2001. doi: 10.1093/cercor/11.5.441. [DOI] [PubMed] [Google Scholar]
- Chang CC, Lin CJ. LIBSVM: a library for support vector machines. ACM Trans Intell Syst Technol 2: 27, 2011. doi: 10.1145/1961189.1961199. [DOI] [Google Scholar]
- Connors BW, Gutnick MJ. Intrinsic firing patterns of diverse neocortical neurons. Trends Neurosci 13: 99–104, 1990. doi: 10.1016/0166-2236(90)90185-D. [DOI] [PubMed] [Google Scholar]
- Courtin J, Chaudun F, Rozeske RR, Karalis N, Gonzalez-Campo C, Wurtz H, Abdi A, Baufreton J, Bienvenu TC, Herry C. Prefrontal parvalbumin interneurons shape neuronal activity to drive fear expression. Nature 505: 92–96, 2014. doi: 10.1038/nature12755. [DOI] [PubMed] [Google Scholar]
- Dembrow N, Johnston D. Subcircuit-specific neuromodulation in the prefrontal cortex. Front Neural Circuits 8: 54, 2014. doi: 10.3389/fncir.2014.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dembrow NC, Chitwood RA, Johnston D. Projection-specific neuromodulation of medial prefrontal cortex neurons. J Neurosci 30: 16922–16937, 2010. doi: 10.1523/JNEUROSCI.3644-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankland PW, Bontempi B. The organization of recent and remote memories. Nat Rev Neurosci 6: 119–130, 2005. doi: 10.1038/nrn1607. [DOI] [PubMed] [Google Scholar]
- Gilmartin MR, McEchron MD. Single neurons in the medial prefrontal cortex of the rat exhibit tonic and phasic coding during trace fear conditioning. Behav Neurosci 119: 1496–1510, 2005. doi: 10.1037/0735-7044.119.6.1496. [DOI] [PubMed] [Google Scholar]
- Gilmartin MR, Miyawaki H, Helmstetter FJ, Diba K. Prefrontal activity links nonoverlapping events in memory. J Neurosci 33: 10910–10914, 2013. doi: 10.1523/JNEUROSCI.0144-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu BM, van Rijn H, Meck WH. Oscillatory multiplexing of neural population codes for interval timing and working memory. Neurosci Biobehav Rev 48: 160–185, 2015. doi: 10.1016/j.neubiorev.2014.10.008. [DOI] [PubMed] [Google Scholar]
- Hattori S, Yoon T, Disterhoft JF, Weiss C. Functional reorganization of a prefrontal cortical network mediating consolidation of trace eyeblink conditioning. J Neurosci 34: 1432–1445, 2014. doi: 10.1523/JNEUROSCI.4428-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai Y, Morishima M, Karube F, Kawaguchi Y. Specialized cortical subnetworks differentially connect frontal cortex to parahippocampal areas. J Neurosci 32: 1898–1913, 2012. doi: 10.1523/JNEUROSCI.2810-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honig W, Thompson RK. Retrospective and prospective processing in animal working memory. In: The Psychology of Learning and Motivation: Advances in Research and Theory, edited by Bower GH. New York: Academic, 1982, vol. 16, p. 239–283. [Google Scholar]
- Insel N, Barnes CA. Differential activation of fast-spiking and regular-firing neuron populations during movement and reward in the dorsal medial frontal cortex. Cereb Cortex 25: 2631–2647, 2015. doi: 10.1093/cercor/bhu062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel N, Takehara-Nishiuchi K. The cortical structure of consolidated memory: a hypothesis on the role of the cingulate-entorhinal cortical connection. Neurobiol Learn Mem 106: 343–350, 2013. doi: 10.1016/j.nlm.2013.07.019. [DOI] [PubMed] [Google Scholar]
- Jarovi J, Volle J, Yu X, Guan L, Takehara-Nishiuchi K. Prefrontal theta oscillations promote selective encoding of behaviorally relevant events. eNeuro 5: ENEURO.0407-18.2018, 2018. doi: 10.1523/ENEURO.0407-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung MW, Qin Y, McNaughton BL, Barnes CA. Firing characteristics of deep layer neurons in prefrontal cortex in rats performing spatial working memory tasks. Cereb Cortex 8: 437–450, 1998. doi: 10.1093/cercor/8.5.437. [DOI] [PubMed] [Google Scholar]
- Kamigaki T, Dan Y. Delay activity of specific prefrontal interneuron subtypes modulates memory-guided behavior. Nat Neurosci 20: 854–863, 2017. doi: 10.1038/nn.4554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y, Kubota Y. GABAergic cell subtypes and their synaptic connections in rat frontal cortex. Cereb Cortex 7: 476–486, 1997. doi: 10.1093/cercor/7.6.476. [DOI] [PubMed] [Google Scholar]
- Kepecs A, Fishell G. Interneuron cell types are fit to function. Nature 505: 318–326, 2014. doi: 10.1038/nature12983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloosterman F, Davidson TJ, Gomperts SN, Layton SP, Hale G, Nguyen DP, Wilson MA. Micro-drive array for chronic in vivo recording: drive fabrication. J Vis Exp 1094, 2009. doi: 10.3791/1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molnár Z, Cheung AF. Towards the classification of subpopulations of layer V pyramidal projection neurons. Neurosci Res 55: 105–115, 2006. doi: 10.1016/j.neures.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Morrissey MD, Insel N, Takehara-Nishiuchi K. Generalizable knowledge outweighs incidental details in prefrontal ensemble code over time. eLife 6: e22177, 2017. doi: 10.7554/eLife.22177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrissey MD, Maal-Bared G, Brady S, Takehara-Nishiuchi K. Functional dissociation within the entorhinal cortex for memory retrieval of an association between temporally discontiguous stimuli. J Neurosci 32: 5356–5361, 2012. doi: 10.1523/JNEUROSCI.5227-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oswald BB, Maddox SA, Tisdale N, Powell DA. Encoding and retrieval are differentially processed by the anterior cingulate and prelimbic cortices: a study based on trace eyeblink conditioning in the rabbit. Neurobiol Learn Mem 93: 37–45, 2010. doi: 10.1016/j.nlm.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilkiw M, Takehara-Nishiuchi K. Neural representations of time-linked memory. Neurobiol Learn Mem 153: 57–70, 2018. doi: 10.1016/j.nlm.2018.03.024. [DOI] [PubMed] [Google Scholar]
- Pinto L, Dan Y. Cell-type-specific activity in prefrontal cortex during goal-directed behavior. Neuron 87: 437–450, 2015. doi: 10.1016/j.neuron.2015.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao SG, Williams GV, Goldman-Rakic PS. Isodirectional tuning of adjacent interneurons and pyramidal cells during working memory: evidence for microcolumnar organization in PFC. J Neurophysiol 81: 1903–1916, 1999. doi: 10.1152/jn.1999.81.4.1903. [DOI] [PubMed] [Google Scholar]
- Siegel JJ, Kalmbach B, Chitwood RA, Mauk MD. Persistent activity in a cortical-to-subcortical circuit: bridging the temporal gap in trace eyelid conditioning. J Neurophysiol 107: 50–64, 2012. doi: 10.1152/jn.00689.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel JJ, Mauk MD. Persistent activity in prefrontal cortex during trace eyelid conditioning: dissociating responses that reflect cerebellar output from those that do not. J Neurosci 33: 15272–15284, 2013. doi: 10.1523/JNEUROSCI.1238-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon B, Knuckley B, Churchwell J, Powell DA. Post-training lesions of the medial prefrontal cortex interfere with subsequent performance of trace eyeblink conditioning. J Neurosci 25: 10740–10746, 2005. doi: 10.1523/JNEUROSCI.3003-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song YH, Kim JH, Jeong HW, Choi I, Jeong D, Kim K, Lee SH. A neural circuit for auditory dominance over visual perception. Neuron 93: 940–954.e6, 2017. [Erratum in Neuron 93: 1236–1237, 2017.] doi: 10.1016/j.neuron.2017.01.006. [DOI] [PubMed] [Google Scholar]
- Spruston N. Pyramidal neurons: dendritic structure and synaptic integration. Nat Rev Neurosci 9: 206–221, 2008. doi: 10.1038/nrn2286. [DOI] [PubMed] [Google Scholar]
- Takehara K, Kawahara S, Kirino Y. Time-dependent reorganization of the brain components underlying memory retention in trace eyeblink conditioning. J Neurosci 23: 9897–9905, 2003. doi: 10.1523/JNEUROSCI.23-30-09897.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takehara-Nishiuchi K. The anatomy and physiology of eyeblink classical conditioning. Curr Top Behav Neurosci 37: 297–323, 2018. doi: 10.1007/7854_2016_455. [DOI] [PubMed] [Google Scholar]
- Takehara-Nishiuchi K, McNaughton BL. Spontaneous changes of neocortical code for associative memory during consolidation. Science 322: 960–963, 2008. doi: 10.1126/science.1161299. [DOI] [PubMed] [Google Scholar]
- Takehara-Nishiuchi K, Nakao K, Kawahara S, Matsuki N, Kirino Y. Systems consolidation requires postlearning activation of NMDA receptors in the medial prefrontal cortex in trace eyeblink conditioning. J Neurosci 26: 5049–5058, 2006. doi: 10.1523/JNEUROSCI.4381-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volle J, Yu X, Sun H, Tanninen SE, Insel N, Takehara-Nishiuchi K. Enhancing prefrontal neuron activity enables associative learning of temporally disparate events. Cell Reports 15: 2400–2410, 2016. doi: 10.1016/j.celrep.2016.05.021. [DOI] [PubMed] [Google Scholar]
- Wilson FA, O’Scalaidhe SP, Goldman-Rakic PS. Functional synergism between putative gamma-aminobutyrate-containing neurons and pyramidal neurons in prefrontal cortex. Proc Natl Acad Sci USA 91: 4009–4013, 1994. doi: 10.1073/pnas.91.9.4009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson MA, McNaughton BL. Dynamics of the hippocampal ensemble code for space. Science 261: 1055–1058, 1993. doi: 10.1126/science.8351520. [DOI] [PubMed] [Google Scholar]
- Wiltgen BJ, Brown RA, Talton LE, Silva AJ. New circuits for old memories: the role of the neocortex in consolidation. Neuron 44: 101–108, 2004. doi: 10.1016/j.neuron.2004.09.015. [DOI] [PubMed] [Google Scholar]
- Woodruff-Pak DS, Disterhoft JF. Where is the trace in trace conditioning? Trends Neurosci 31: 105–112, 2008. doi: 10.1016/j.tins.2007.11.006. [DOI] [PubMed] [Google Scholar]
- Yang CR, Seamans JK, Gorelova N. Electrophysiological and morphological properties of layers V-VI principal pyramidal cells in rat prefrontal cortex in vitro. J Neurosci 16: 1904–1921, 1996. doi: 10.1523/JNEUROSCI.16-05-01904.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zentall TR. Coding of stimuli by animals: retrospection, prospection, episodic memory and future planning. Learn Motiv 41: 225–240, 2010. doi: 10.1016/j.lmot.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]