Abstract
We evaluated the mechanisms underlying protease-activated receptor 1 (PAR1)-mediated activation of nodose C-fibers in mouse lungs. The PAR1-induced action potential discharge at the terminals was strongly inhibited in phospholipase C-β3 (PLCβ3)-deficient animals. At the level of the cell soma, PAR1 activation led to an increase in cytosolic calcium that was largely inhibited by transient receptor potential (TRP) A1 antagonism. Patch-clamp recordings, however, revealed that neither TRPA1 nor TRPV1 or any other ruthenium red-sensitive ion channels are required for the PAR1-mediated inward current or membrane depolarization in isolated nodose neurons. Consistent with these findings, PAR1-mediated action potential discharge in mouse lung nodose C-fiber terminals was unaltered in Trpa1/Trpv1 double-knockout animals and Trpc3/Trpc6 double-knockout animals. The activation of the C-fibers was also not inhibited by ruthenium red at concentrations that blocked TRPV1- and TRPA1-dependent responses. The biophysical data show that PAR1/Gq-mediated activation of nodose C-fibers may involve multiple ion channels downstream from PLCβ3 activation. TRPA1 is an ion channel that participates in PAR1/Gq-mediated elevation in intracellular calcium. There is little evidence, however, that TRPA1, TRPV1, TRPC3, TRPC6, or other ruthenium red-sensitive TRP channels are required for PAR1/Gq-PLCβ3-mediated membrane depolarization and action potential discharge in bronchopulmonary nodose C-fibers in the mouse.
Keywords: airway C-fiber, GPCR, mouse nodose, protease-activated receptor 1, TRP channel
INTRODUCTION
The majority of the vagal afferent nerves innervating the respiratory tract are unmyelinated C-fibers that detect various types of noxious stimuli in a graded fashion, i.e., nociceptors. In health, these nerves can serve as a warning, resulting in conscious sensations, such as the urge to cough and dyspnea, or subconscious autonomic reflexes, such as changing bronchial smooth muscle tone and inducing glandular secretions, aimed at ridding the noxious stimulus. These nerves may become dysregulated or overstimulated in inflammatory airway disorders, leading to excessive bronchospasm, secretions, dyspnea, and unproductive coughing (26).
The involvement of C-fibers in the pathophysiology of visceral inflammatory disease in general is explained by the fact that many inflammatory mediators can act on receptors that directly lead to activation (action potential discharge) of C-fiber terminals. The receptors that inflammatory mediators act on to stimulate afferent nerves can be subdivided into two major types: ionotropic receptors and metabotropic receptors [most commonly G protein-coupled receptors (GPCRs)]. The mechanisms by which ionotropic receptor activation leads to a depolarizing generator potential is straightforward: most often the receptor is a ligand-gated nonselective cation channel. The mechanisms underlying GPCR activation-induced terminal membrane depolarization are less obvious. In general terms, some signal transduction process must be set in place that ultimately leads to the opening or closing of an ion channel to cause membrane depolarization (referred to as a generator potential).
Transient receptor potential (TRP) channels, in particular, TRPV1 and TRPA1, have been implicated in the transduction of GPCR activation into membrane depolarizations in nociceptive neurons. Most of the work in this area focuses on behavioral studies of pain, itch, and cough and is often combined with studies at the level of cell bodies [either in heterologous systems or in dissociated dorsal root ganglion (DRG) neurons] (6, 8, 10, 13, 25, 29, 32, 39, 41), in which the microenvironment of the organelles relevant to signal transduction may be substantively different from that at the nerve terminals (16, 17). Here, we address the role of TRP channels in GPCR-dependent activation of vagal C-fibers at the level of cell bodies isolated from the vagal sensory ganglion, as well as the C-fiber terminals in mouse lungs.
The cell bodies of vagal sensory nerves innervating mouse lungs are situated in the nodose and jugular ganglia (28). In this study, our aim was to focus solely on nodose C-fibers. In the respiratory tract and esophagus, nodose C-fibers are phenotypically distinct from jugular C-fibers and DRG-derived C-fibers with respect to embryonic origins, growth factor regulation, neuropeptide content, and chemical activation profiles (19, 23, 28, 37). It would therefore not be surprising if GPCR signaling mechanisms also differ between nodose and jugular C-fibers. In mouse lungs, it can be difficult to ascertain whether the vagal afferent C-fiber under study is derived from nodose or jugular ganglion neurons. Therefore, to focus specifically on GPCR-mediated activation of nodose C-fibers, we have elected to mainly investigate the effect of TFLLR (Thr-Phe-Leu-Leu-Arg-NH2), a selective agonist for protease-activated receptor 1 (PAR1) (12). PAR1 is a GPCR that is expressed by mouse nodose C-fiber neurons but not jugular ganglion neurons. Moreover, PAR1 activation causes a consistent and reproducible discharge of action potentials in mouse lung nodose C-fiber terminals without activating jugular C-fibers (21, 40).
METHODS
Ethical approval.
The experiments were approved by The Johns Hopkins Animal Care and Use Committee and conformed to the committee’s principles and regulations, as described in Ref. 9.
Animals.
C57BL/6J male wild-type and various genetically modified mice (22–27 g) were used in this study. The Trpa1−/− and Trpv1−/− mice were obtained from Jackson’s Laboratory. The Trpa1−/−/Trpv1−/− double-knockout animals were obtained by mating these homozygous mice in Dr. Xingzhong Dong’s laboratory at Johns Hopkins School of Medicine. The Trpc 3−/−/Trpc6−/− double-knockout mice were obtained from the laboratory of Dr. D. Kass (Johns Hopkins School of Medicine) but were originally developed in the laboratory of Dr. L. Birnbaumer (National Institutes of Environmental Health). The Plcb3−/− mice were developed in the laboratory of Dr. M. Simon and provided by Dr. K.-W. Yau (Johns Hopkins School of Medicine). Pirt-GCaMP3 heterozygote mice were generously provided by Dr. Xinzhong Dong (Johns Hopkins University).
Cell labeling and cell isolation.
In some studies, the bronchopulmonary afferent neurons were retrogradely labeled using Dil [1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate; DiC18 (3); Molecular Probes/Invitrogen, Eugene, OR] as previously described (27). Briefly, mice were anesthetized with a mixture of ketamine (2 mg ip) and xylazine (0.2 mg ip) and were orotracheally intubated. Dil solution (0.1%, 50 μL) was instilled into the tracheal lumen 7–10 days before experiments. The distribution of dye in the lungs but not in esophagus was verified at the time the animals were euthanized for cell isolation.
Mice were killed by CO2 inhalation and subsequent exsanguinations. Both sides of jugular/nodose ganglia were dissected and cleared of adhering connective tissues. The lower two-thirds of ganglion [most likely nodose component (28)] were cut out for subsequent enzymatic digestion using type 1A collagenase (2 mg/mL) and dispase II (2 mg/mL), as previously described (21). The isolated nodose neurons were kept at 37°C in L-15 medium containing 10% fetal bovine serum for use within 24 h. The interactions between Gq/GPCR signaling and TRPV1 are observed at room temperature (18), and, therefore, we elected to carry out our studies on dissociated neurons at 22°C–25°C to improve the stability of the responses.
Intracellular Ca2+ measurements.
Intracellular Ca2+ concentrations ([Ca2+]i) were measured in nodose neurons isolated from Pirt-GCaMP3 heterozygote mice, in which the genetically encoded Ca2+ indicator GCaMP3 is expressed under the Pirt promoter, as we have previously described (40). GCaMP3 was excited at 488 nm, and the emission of its green fluorescence at 525 nm was captured by a Nikon DSLR camera (30–60 frames/s) under the control of imaging software NIS-Elements AR. The intensity of whole cell fluorescence for each cell under study was plotted against time, using the same software. The changes in [Ca2+]i were expressed as the ratio of fluorescence change over the resting fluorescence (ΔF/F0). Bath solution contained 136 mM NaCl, 5.4 mM KCl, 1 mM MgCl2, 1.5 mM CaCl2, 10 mM HEPES, and 10 mM glucose with pH adjusted to 7.35 with NaOH. The fluorescent signal was recorded at rest for at least 30 s before the PAR1 agonist was applied via bath perfusion, and in some experiments, together with TRPV1 and/or TRPA1 blockers following 15-min pretreatment with the TRP channel blockers. The resting fluorescent signal (F0) is usually very stable. If the increase in the fluorescence intensity following application of the agonist was greater than or equal to three times the noise level of the resting fluorescence, we considered it to be a real Ca2+ response. Accordingly, the neuron was considered responsive. Capsaicin (1 μM) was applied at the end of each experiment to evaluate the expression of TRPV1 in neurons.
Patch-clamp recordings.
Amphotericin B-perforated whole cell patch-clamp technique was employed to record the membrane potential and current under current-clamp mode and voltage-clamp mode, respectively, using an Axopatch 200B amplifier interfaced with Axon Digidata 1550A and driven by pCLAMP 10 software (Molecular Devices, Sunnyvale, CA). Bath solution was the same as for Ca2+ measurement. Pipette solution contained 30 or 20 mM KCl, 115 or 125 mM K-gluconate, and 10 mM HEPES with pH adjusted to 7.2 with KOH. Freshly prepared amphotericin B was added to the pipette solution (300 μg/mL) before experiments. The junction potential (−13.8 or −14.7 mV, estimated using Clampex calculator) was corrected. Membrane currents were recorded at −70 mV, sampled at 2 kHz, and filtered at 1 kHz. In some experiments, repetitive ramp voltage commands from −100 to −50 mV (0.2 mV/ms) were applied at 0.5 or 1 Hz from a holding potential of −70 mV. In these experiments, membrane currents were sampled at 10 kHz and filtered at 2–5 kHz. The cell capacitance and series resistance (80%) were compensated. Data were analyzed using Clampfit 10 and SigmaPlot software.
Extracellular recordings.
Extracellular recordings of action potentials in nodose lung C-fibers were carried out as previously described, using a mouse ex vivo lung-vagus nerve preparation (15). The lungs, along with the vagus nerve and ganglia, were dissected and pinned in a chamber and superfused and tracheally perfused with Krebs bicarbonate buffer solutions (37°C). The recording electrode was positioned in the vagal nodose/jugular complex and micromanipulated until a receptive field in the lungs was found. After at least 60 min of equilibration, the receptive field of a single C-fiber was searched for and identified with a small concentric electrode. The conduction velocity was then determined. In mouse lungs, the P2X3 selective agonist α,β-methylene ATP (α,β-mATP) consistently activates nodose C-fibers but not jugular C-fibers, so we focused only on α,β-mATP-sensitive C-fibers. The antagonists were both superfused over the lungs and perfused via the trachea for at least 15 min before the application of a C-fiber stimulant. The C-fiber stimulants (TFLLR, bradykinin, or α,β-mATP) were applied by 1 mL infusion via the trachea. The recorded action potentials were amplified (Microelectrode AC Amplifier 1800; A-M Systems, Everett, WA), filtered (0.3 kHz low cutoff and 1 kHz high cutoff), and monitored on an oscilloscope (TDS340; Tektronix, Beaverton, OR) and a chart recorder (TA240; Gould, Valley View, OH). The scaled output from the amplifier was captured and analyzed by a Macintosh computer using NerveOfIt software (Phocis, Baltimore, MD). On the occasion when background activity unrelated to the nerve ending under study was present, NerveOfIt was used to define only those peak shapes that were in precise accordance with the action potential evoked by punctate stimulation of the receptive field. In this way, “fit spikes” were culled from any background activity. The action potential discharge was quantified offline as peak frequency (peak number of action potentials in any 1-s bin) and total number of action potentials (the number of action potentials assessed until the discharge returned to baseline levels, generally <90 s).
Chemicals and drugs.
L-threonyl-L-phenylalanyl-L-leucyl-L-leucyl- L-argininamide trifluoroacetate salt, Thr-Phe-Leu-Leu-Arg-NH2 trifluoroacetate salt (TFLLR), AMG9810, and ruthenium red stocks were prepared in Millipore water. The stock solution of HC 030031 (HC) was prepared in DMSO. Capsaicin stock was prepared in ethanol. All drug stocks were at concentrations at least 1,000 times those used in experiments.
Statistical analysis.
All statistical analyses were performed using SigmaPlot software. Pooled data are expressed as mean ± SE. The significance of differences between two means was evaluated by Student’s t test or rank sum test. When two means were derived from data before and after a drug treatment, paired Student’s t test or Wilcoxon signed-rank test was used; otherwise, Student’s t test or Mann–Whitney rank sum test for unpaired values was used. The significance of differences between multiple means was evaluated by one-way ANOVA with Holm–Sidak test as a post hoc analysis for multiple pairwise comparisons.
RESULTS
PAR1-mediated nodose C-fiber activation requires phospholipase C-β3.
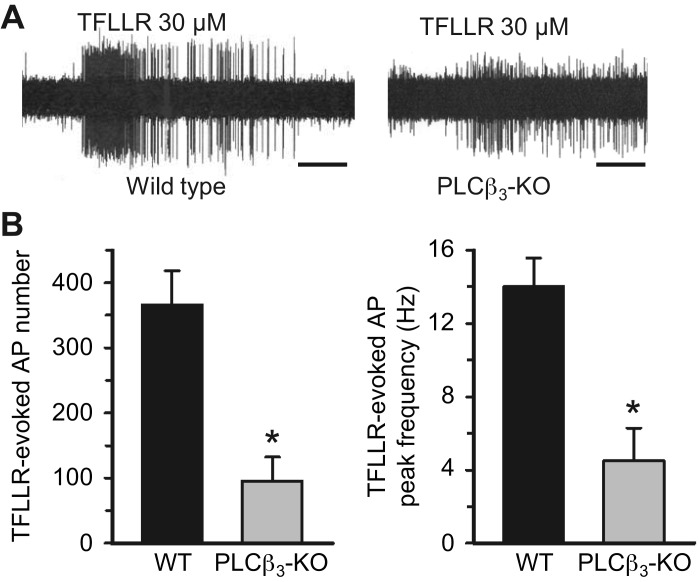
We have previously published that the PAR1 receptor is expressed in airway-specific nodose C-fiber neurons but not jugular C-fiber neurons, that the PAR1-selective agonist TFLLR strongly activates nodose C-fibers but not jugular C-fibers in mouse lungs, and that this activation is absent in PAR1 knockout animals (21, 40). Therefore, TFLLR is an ideally suited GPCR agonist for specifically evaluating nodose C-fiber activation. PAR1 signaling often involves Gq-coupled phospholipase C (PLC) (34, 38). We evaluated the expression of PLC mRNAs in our previously obtained data set from an RNAseq analysis specifically of nodose C-fiber neurons. Since it is the β isoform of PLC that is often linked to GPCRs, we determined which among the four PLCβs was most expressed in the nodose neurons. The count per million for PLCβ1, PLCβ2, PLCβ3, and PLCβ4 was found to be 10, 0, 218, and 93, respectively, indicating that PLCβ3 is the dominant, but not exclusive, PLCβ isozyme expressed (see supplement in Ref. 40). We therefore compared TFLLR activation of nodose C-fibers in mouse lungs ex vivo in wild-type versus PLCβ3-knockout animals. Consistent with the gene expression analysis, we found that TFLLR-induced action potential discharge largely, but not exclusively, depends on PLCβ3 (Fig. 1). The action potential discharge to α,β-mATP, which stimulates nodose C-fibers via ionotropic P2X2,3 receptors (28), was not significantly different between wild-type and Plcb3−/− mice (total number of action potentials was 134 ± 20, range 17–460, n = 24 vs. 80 ± 15, range 40–170, n = 8, P = 0.15).
Fig. 1.
Protease-activated receptor 1 (PAR1)-induced activation of mouse airway nodose C-fibers is mainly mediated by phospholipase C β3 (PLCβ3). A: representative recordings of TFLLR-evoked action potential (AP) discharges in the bronchopulmonary C-fibers from wild-type (WT) and PLCβ3-knockout (KO) mouse lungs. Note that PAR1 activation elicited 103 APs (peak frequency at 22 Hz) and 32 APs (peak frequency at 3 Hz) in the WT and PLCβ3-deficient C-fibers, respectively. The horizontal bars represent 10 s. Note that some spikes recorded in the PLCβ3-KO lungs are background activities. B: pooled data showing the mean numbers and peak frequency of TFLLR-evoked AP discharges from WT (n = 24) and PLCβ3-KO (n = 8) C-fibers. *P < 0.01 (Mann–Whitney rank sum test).
TRP channels and PAR1-induced activation at nodose cell bodies.
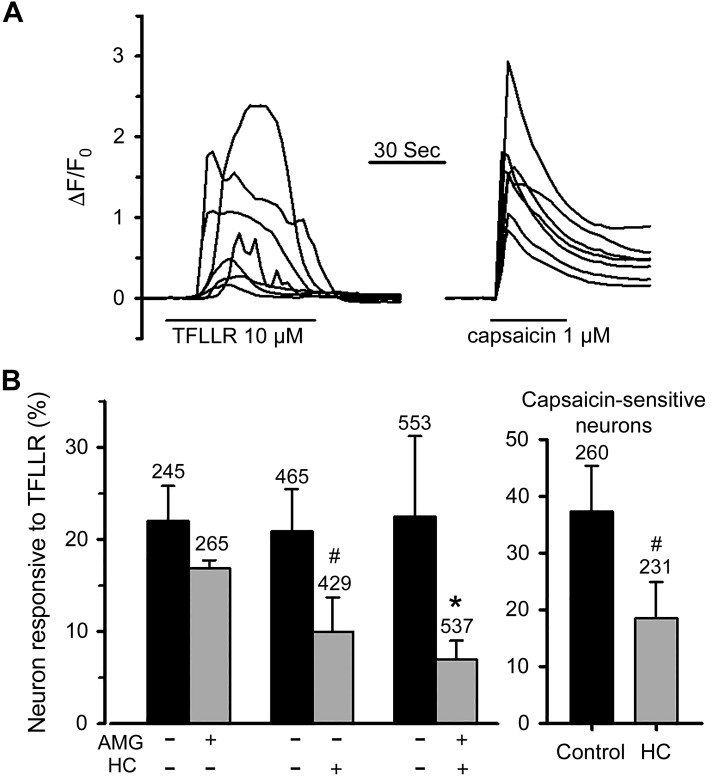
GPCR-Gq/PLC-dependent signaling is often associated with the activation of TRP channels. Among the many TRP channels, selective stimulation of TRPA1 or TRPV1 mimics the effect of TFLLR in evoking strong action potential discharge in nodose C-fibers. As TRP channels carry calcium, a convenient way to evaluate TRPA1 and TRPV1 activation is to measure the changes in intracellular calcium concentrations. We therefore examined the PAR1 agonist-induced increases in free intracellular calcium and its dependence on TRPV1 and/or TRPA1 channels in isolated nodose neurons of mice expressing the genetically encoded Ca2+ indicator GCaMP3. Bath application of TFLLR (10 μM) elicited an increase in [Ca2+]i in 23% of cells (249/1,084 cells). The majority of TFLLR-sensitive cells (227/249 cells) also responded to capsaicin (1 μM) with robust Ca2+ transients (Fig. 2A). AMG9810 is a selective TRPV1 antagonist (7). Treatment of cells with AMG9810 (3 μM, incubation for 15 min and then presence in the bath throughout the experiments) significantly and strongly reduced the number of cells that responded to capsaicin (9% vs. 57% in control, P < 0.001) but did not affect the response to PAR1 activation. The percentage of TFLLR-responsive cells was, however, significantly reduced by TRPA1 antagonist HC 030031 [30 μM, a concentration that blocks TRPA1 in nodose neurons (5, 35)]. Combined use of HC 030031 and AMG9810 also reduced the number of cells responsive to TFLLR compared with control (Fig. 2B), but the effect was not significantly different from that produced by HC 030031 alone (one-way ANOVA analysis). We have previously reported that TRPA1-expressing neurons in mouse nodose ganglion also express TRPV1 and all C-fibers activated by a TRPA1 agonist were also activated by capsaicin (27). Some TFLLR-responsive neurons were capsaicin insensitive and thus also likely to be insensitive to TRPA1 stimulation. We therefore carried out an analysis of the effect of HC 030031 specifically on capsaicin-sensitive (and presumed TRPA1-expressing) neurons. Again, HC 030031 significantly inhibited the TFLLR response in these C-fiber neurons (Fig. 2B, right). These results suggest that the PAR1 activation-induced Ca2+ response involves the opening of TRPA1 but not TRPV1.
Fig. 2.
Effects of transient receptor potential (TRP) A1 and TRPV1 inhibitors on the intracellular Ca2+ response to protease-activated receptor 1 (PAR1) activation in nodose neurons isolated from Pirt-GCaMP3 heterozygote mice. A: representative recordings of Ca2+ signals, expressed as ΔF/F0, in response to PAR1 agonist TFLLR and to capsaicin obtained from the same group of neurons. Capsaicin was applied 10 min after washout of TFLLR. B: bar graphs showing percentage of nodose neurons (left) or capsaicin-sensitive nodose neurons (right) responding to PAR1 activation in the presence or absence of different drugs as indicated. Numbers on the top of bars indicate the number of neurons studied in each group. Data from different treatment groups and their respective controls were obtained from neurons isolated from the same animals (n = 4–5). *P < 0.05 and #P < 0.01 compared with the respective control assessed by paired t test. One-way ANOVA analysis of pooled control, AMG9810 (AMG), HC 030031 (HC), and HC + AMG treatment groups revealed that the percentage of TFLLR-responsive neurons was significantly reduced by treatments with HC or AMG + HC compared with control, but the result was not different between the two treatment groups. F0, resting fluorescence level before agonist application used to normalize Ca2+ response in each neuron; ΔF, changes in the fluorescent intensity with respect to F0.
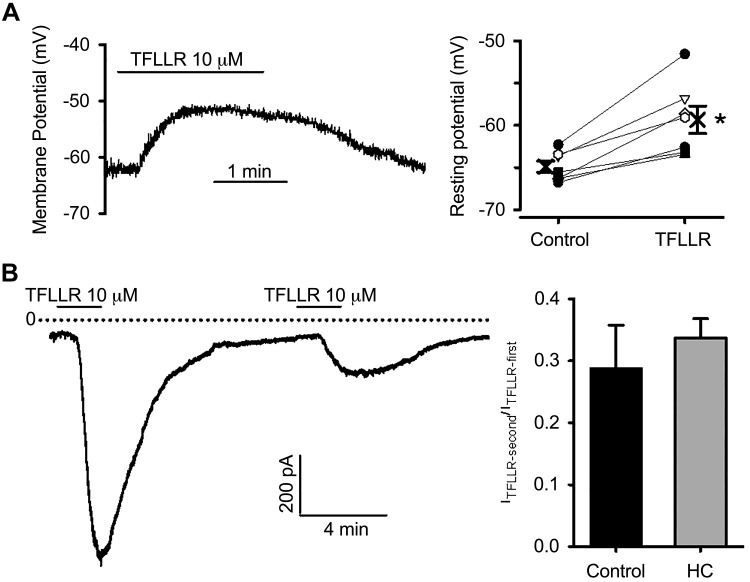
We next employed the perforated patch-clamp technique to address the hypothesis that TFLLR-induced depolarizing current is dependent on TRP channels. Bath application of TFLLR evoked a membrane depolarization (Fig. 3A) in 7 of 8 cells; −64.9 ± 0.7 to −59.3 ± 1.6 mV (P < 0.01). Consistent with this observation, TFLLR elicited an inward current (ITFLLR) at a holding potential of −70 mV in 78% of neurons (82/105 cells) with an average current density of −3.92 ± 0.49 pA/pF. We also examined the effects of TFLLR on the membrane currents in a subset of retrogradely labeled bronchopulmonary afferent neurons and found a similar percentage of cells responding to PAR1 activation (6/7 cells) with a similar current density (−4.55 ± 1.06 pA/pF).
Fig. 3.
Protease-activated receptor 1 (PAR1) activation evokes membrane depolarization and inward currents. A: typical recordings (left) and individual and pooled data (right) showing PAR1 agonist-evoked membrane depolarization in nodose neurons. Each symbol and the connecting line represent results obtained from individual neurons, and the bold cross indicates the mean from 7 neurons. *P = 0.003 (paired t test). B: TRPA1 is not required for PAR1 activation-evoked inward currents. Example of inward currents evoked by repetitive TFLLR applications at −70 mV (left). Averaged fraction of current remaining during second agonist application (right) in the absence (control) and presence of TRPA1 blocker HC 030031 (HC), between two TFLLR applications (8–10 min).
Bath perfusion of TFLLR for 2 min significantly desensitized the receptor, as evidenced by a marked reduction in current amplitude upon a subsequent TFLLR application (Fig. 3B), which complicates the pharmacological studies of ITFLLR in the same cells. Nevertheless, we evaluated the effects of TRPA1 inhibition by HC 030031 on ITFLLR by comparing the ratio of the second ITFLLR over the first ITFLLR obtained in the absence or presence of the TRPA1 inhibitor between two applications of the PAR1 agonist (8–10 min). HC 030031 at 30 μM did not affect the amplitude of current evoked by second PAR1 activation compared with control conditions (Fig. 3B). To further evaluate whether TRP channels are involved in PAR1 agonist-evoked activation of nodose neurons, we applied 30 μM ruthenium red 2–3 min before and during the application of TFLLR to the isolated neurons. At a concentration (10 μM) that blocks TRPV1, TRPA1, and most other TRP channels (and many other cation channels), ruthenium red did not inhibit PAR1 activation-evoked inward currents in a group of 5 neurons (ITFLLR −3.43 ± 1.93 pA/pF). Comparing the ITFLLR amplitude for control-first response (−5.21 ± 1.02 pA/pF), HC-first response (−3.81 ± 1.35 pA/pF), and in the presence of ruthenium red with one-way ANOVA analysis showed no statistical difference (P = 0.33). Additional evidence against a critical role of TRPV1 in TFLLR-induced responses was obtained by evaluating the effect of TFLLR on capsaicin-insensitive neurons. We noted that, in a group of 12 TFLLR-responsive neurons that were also tested for capsaicin sensitivity, the ITFLLR in 6 of 12 capsaicin-insensitive neurons (−3.89 ± 1.77 pA/pF) was comparable with that recorded in 6 of 12 capsaicin-sensitive neurons (−5.17 ± 1.66 pA/pF, P = 0.6). The same results were obtained in labeled bronchopulmonary afferent neurons where TFLLR evoked ITFLLR in three capsaicin-insensitive neurons.
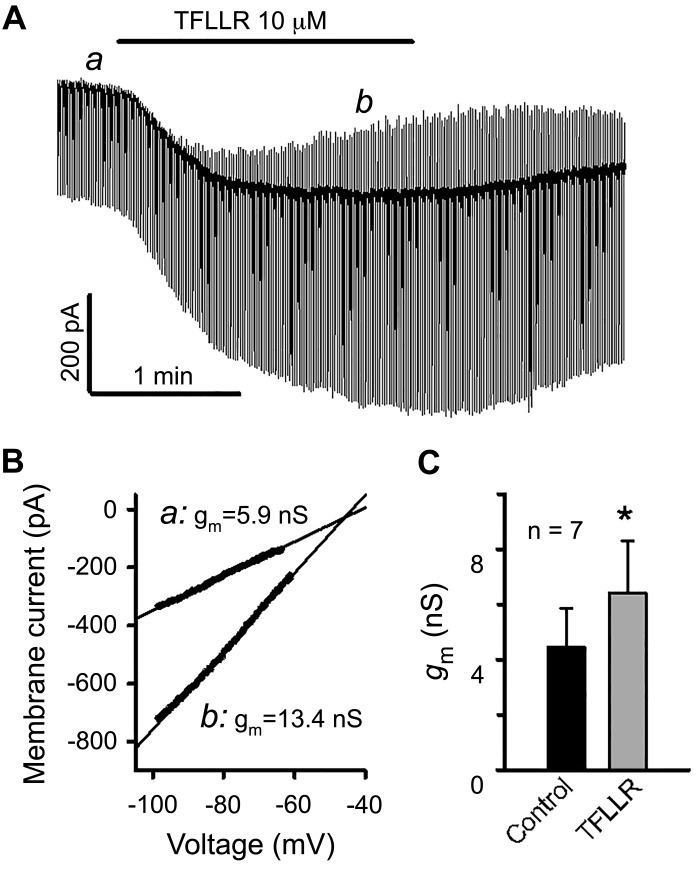
We measured the effects of TFLLR on membrane conductance (gm) to gain insight into the general ionic mechanisms underlying PAR1 stimulation-induced inward currents, i.e., whether it was associated with the opening of depolarizing channels and/or closing of hyperpolarizing channels. Repetitive voltage ramp commends from −100 to −50 mV (at 0.5 or 1 Hz) were applied before and during bath perfusion of TFLLR, and gm was estimated from the slope of the linear portion of ramp current-voltage plots determined by linear curve fitting (Fig. 4). TFLLR caused a substantial increase in gm measured at the peak of the agonist-induced inward currents in five of seven neurons, indicating that TFLLR stimulation is predominantly associated with the opening of ion channels at voltages around the resting potential. In the other two neurons, a transiently reduced or unchanged gm was observed at the onset and/or during a portion of increasing phase of TFLLR-induced inward currents, indicating that PAR1 activation also led to channel closing. In these neurons, gm at the peak of inward currents was unchanged compared with that of baseline, suggesting that PAR1-induced reduction in outward conductance (i.e., closing of channels conducting outward currents) effectively counteracted the increase in inward conductance (i.e., opening of channels conducting inward currents). The averaged data from the seven neurons indicate that the PAR1 agonist increased the membrane conductance at the peak of inward currents (6.4 ± 1.9 vs. 4.4 ± 1.4 nS, P = 0.016, Fig. 4C), despite a highly variable basal gm (ranging from 0.4 to 11.2 nS). We also observed that the TFLLR-evoked inward current reverses at a wide range of voltages (−46 to 1 mV, averaged −18 ± 8 mV), further indicating that conductance changes as a consequence of PAR1 activation are unlikely to be explained by a single type of ion channel.
Fig. 4.
Effect of protease-activated receptor 1 (PAR1) activation on membrane current and membrane conductance. A: continuous display of membrane current elicited by repetitive ramp voltage commands (from −100 to −50 mV for 300 ms, 1 Hz) from a holding potential of −70 mV before and after bath application of TFLLR. B: current-voltage plots for the linear portion of voltage ramp-elicited currents taken at indicated time points (a and b in A). Solid lines represent the fits of currents to a linear function. The slope of the curves gave an estimate of membrane conductance (gm). In this neuron, PAR1 activation increased gm, and the TFLLR-induced current reversed at −45.8 mV. C: averaged gm increase caused by PAR-1 activation. *P < 0.05 (paired t test).
TRP channels and PAR1-induced activation at nodose C-fiber terminals in the lungs.
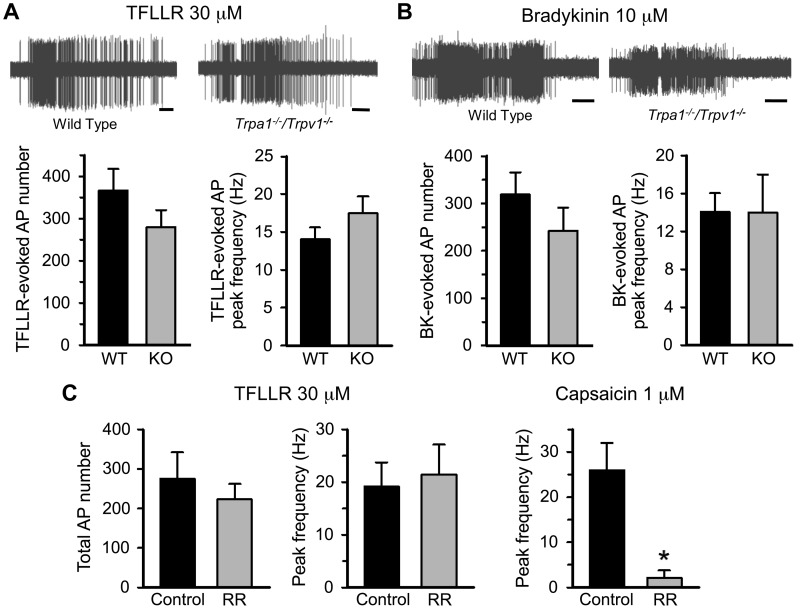
The studies at the cells’ somas indicate that, although TRPA1 plays a role in TFLLR-induced increases in intracellular calcium in many mouse nodose neurons, neither TRPA1 nor TRPV1 is necessary for the inward depolarizing electrical currents. Consistent with our previous studies, applying TFFLR to the lungs resulted in strong and consistent activation (action potential discharge) in nodose C-fiber terminals. We compared the effect of TFLLR on mouse lung nodose C-fibers in wild-type versus Trpa1/Trpv1 double-knockout mice. We found that the action potential number and peak discharge frequency were comparable between the two groups (Fig. 5A). This was also the case with bradykinin, a stimulus that depends on the bradykinin B2 receptors (Fig. 5B). Studies have shown that TRPC channels, notably TRPC3 and TRPC6, can be influenced by signaling events downstream of GPCR activation (11), and GPCR-mediated activation of a subset of DRG neurons was found to be dependent on TRPC3 (36). We therefore investigated the effect of TFLLR on nodose C-fibers in the lungs of Trpc3/Trpc6 double-knockout mice. Again, the PAR1-mediated action potential discharge was not different from that observed in C-fibers of wild-type mouse lungs (total action potential number: 366 ± 52 for wild-type vs. 421 ± 148 for Trpc3/Trpc6 knockout mice).
Fig. 5.
Protease-activated receptor 1 (PAR1) activation-induced action potential discharges in bronchopulmonary C-fibers is not affected by genetic or pharmacological inhibition of transient receptor potential (TRP) channels. A: representative recordings (top) and averaged total numbers and peak frequency (bottom) of TFLLR-evoked action potential (AP) firings in bronchopulmonary C-fibers from wild-type (WT, n = 24) and Trpa1−/−/Trpv1−/− double-knockout (KO, n = 14) mice. B: representative recordings (top) and averaged total AP number and peak frequency (bottom) of bradykinin (BK)-evoked AP discharges in WT (n = 16) and Trpa1−/−/Trpv1−/− double-KO (n = 8) mice. Horizontal calibration bars under the recordings in A and B represent 10 s. C: averaged total AP number and peak frequency of TFLLR-evoked AP discharges (left) and mean peak frequency of capsaicin-induced AP firings (right) in C-fibers under control conditions (n = 6 for both agonists) and after treatment with 30 μM ruthenium red (RR, n = 6 for both agonists). *P < 0.001 (unpaired t test).
To further evaluate the role of TRP channels at the nerve terminals, we evaluated the effect of ruthenium red. In a paired analysis, the response to TFLLR was the same before and after treatment with ruthenium red (30 μM). Ruthenium red did, however, block the response of nodose C-fibers to capsaicin (Fig. 5C), and we have previously published that, in the same experimental setting, this concentration of ruthenium red blocks the nodose C-fiber response to the TRPA1 stimulant cinnamaldehyde (27).
DISCUSSION
The results provide evidence that action potential discharge of pulmonary nodose C-fibers in response to PAR1 receptor activation depends largely on PLC and, more specifically, the PLCβ3 isozyme. Selectively blocking PLCβ3 in the airways with an inhaled PLCβ3 antagonist may be a strategy that would inhibit the activation of nodose C-fibers by multiple inflammatory mediators that are agonists for Gq-coupled GPCRs. The downstream ion channels involved with the PAR1/PLCβ3-dependent membrane depolarization in the nodose C-fibers are likely to be manifold and as of yet to be determined, but the data provide strong evidence against an important role for TRPV1, TRPA1, TRPC3, TRPC6, or any other ruthenium red-sensitive ion channel.
It is not surprising that PLCβ is involved in the signaling mechanisms between PAR1 activation and action potential discharge, as PAR1 is often linked to Gq, and Gq is linked to PLCβ (34, 38). That specifically β3 isoform is involved is consistent with data from our previous RNAseq analysis of capsaicin-sensitive (C-fiber) nodose neurons, in which the β3 isozyme was the dominant PLC expressed (40).
Several studies have shown that TRPV1 may be a receptor-mediated ion channel underlying GPCR-Gq receptor activation of nociceptors (39). One mechanism by which this occurs is through the phospholipase C-mediated cleavage of the inositol moiety of phosphatidylinositol 4,5-bisphosphate (4,5P2) that then disinhibits the channel (6). The observation that the action potential discharge at nodose C-fiber terminals in response to TFLLR or bradykinin in mouse lungs is the same in wild-type and TRPA1/TRPV1 double-knockout animals indicates that these channels are not essential for GPCR-PLC-dependent activation. The genetic deletion studies also rule out a critical role of TRPC3 or TRPC6. The results with genetic approaches leave open the possibility that TRP channels are involved but that there is a redundancy among different channel subtypes. Alternatively, the gene deletion may have led to some compensatory changes. These explanations are unlikely to explain our findings, as the response to TFLLR was also unaffected by ruthenium red at concentrations that block both TRPV1-dependent activation of the nodose C-fibers by capsaicin (present study) and the TRPA1-dependent activation by cinnamaldehyde (27).
These results are different from findings in jugular C-fibers innervating guinea pig trachea, in which blocking TRPV1 partially inhibits bradykinin-induced action potential discharge (4). Activation of jugular C-fibers can lead to cough in conscious guinea pigs (3), and TRPV1 and TRPA1 inhibitors inhibit bradykinin-induced cough (1, 8). The discrepancy with the findings here could be explained by a species difference (mouse vs. guinea pig) or, perhaps more likely, by the fact that in the present study we purposely focused only on nodose C-fibers. As mentioned earlier, jugular vagal C-fibers are neural crest-derived neurons with properties similar to DRG-derived C-fibers but quite distinct from placode-derived nodose C-fibers (26). It is worth mentioning in this context that bradykinin-induced activation of nodose C-fibers terminating in the guinea pigs’ esophagi are unaffected by TRPA1 blockade (42).
The patch-clamp data indicate that the electrical consequences of PAR1 activation are probably because of a combination of increased inward excitatory ionic currents and reduced outward (most likely potassium) currents. Consistent with the findings at the nerve terminals, there is little evidence that TRPA1, TRPV1, or any ruthenium red-sensitive cation channel plays a key role in PAR-1-mediated inward current or membrane depolarization. This conclusion is supported by the pharmacological data and also by the findings that the electrophysiological consequence of TFLLR stimulation were similar in capsaicin-sensitive and capsaicin-insensitive neurons. Here it is worth noting that in the mouse vagal sensory ganglia, we and others have noted a concordance in the expression of TRPV1 and TRPA1 mRNA, i.e., TRPA1 is expressed in TRPV1-expressing neurons and is seldom expressed in TRPV1-negative neurons, and thus the capsaicin-insensitive neurons are also likely to lack TRPA1 responsivity (20, 27, 40). The lack of involvement of TRPV1/TRPA1 in the PAR1-mediated action potential discharge from nodose C-fibers is consistent with the finding that activation of mouse lung C-fibers via the sphingosine 1-phosphate GPCR S1PR3 is not inhibited by ruthenium red (30). These data may help to explain why recent clinical trials with effective TRPV1 blockers were found to be disappointing in the treatment of chronic cough (2).
The nature of the non-TRP channel-dependent TFLLR-evoked depolarization in mouse nodose neurons has not been defined. Based on the variability in the reversal potentials of TFLLR-induced currents, it is likely that more than one ionic mechanism underlies the net PAR-1-induced membrane depolarization. Previous studies have reported that the inward ionic current caused by bradykinin in guinea pig nodose neurons is secondary to combined effects of Ca2+-activated chloride conductance and inhibition of a potassium current (29). This was also the case in patch-clamped airway-specific jugular neurons of guinea pigs (22), but the nature of the Cl− channels involved was not determined. Potassium channel subtypes in nodose nerves innervating the airways that can be inhibited by PAR-1 or other GPCRs require further investigation. KCNQ/KV7 channels mediate the muscarinic receptor-inhibited current (M-current). The M-current is a noninactivating potassium current that is activated at or near resting membrane potentials. It is well recognized that the GPCR agonists can inhibit this current, leading to membrane depolarization (24, 25). We have recently noted that nodose C-fiber neurons express KCNQ/KV7 channels (mainly KCNQ3 and KCNQ2) and that virtually every neuron dissociated from the mouse nodose ganglion has an M-current. Complete inhibition of this current, however, only caused a very modest membrane depolarization and is unlikely to explain PAR-1-induced action potential discharge at the nerve terminals (33).
It was interesting to note that, at the level of the nodose cell bodies, TRPA1 appears to play a major role in the PAR1-mediated rise of intracellular calcium without having a substantive role in causing membrane depolarization. This finding raises a caveat about inferring knowledge regarding electrophysiological activities of C-fibers based solely on increases in intracellular-free calcium concentrations at the cell bodies. Similar discrepancies have been made in the somatosensory system, where TRPV1 was found to have no effect on bradykinin-induced action potential discharge in cutaneous C-fibers, yet played a substantive role in the bradykinin-induced elevation of intracellular calcium at the cell bodies (14). Genetic deletion of both TRPA1 and TRPV1 or pharmacological inhibition of these channels has also shown little effect on GPCR (MrgprA1/MrgprC11)-induced action potential discharge of cutaneous C-fibers in mouse skin (31) yet may play a major role in regulating the elevation in intracellular calcium when studied at the level of the cell bodies isolated from the DRG (41). Although we found that TRPA1 regulation of the intracellular calcium response was uncoupled from the electrical excitability of the nerve, it still may have physiological relevance. For example, regulation of increases in intracellular calcium at the peripheral terminals via TRPA1 may be involved with neurokinin release from the C-fiber terminals in the lungs and the consequent neurogenic inflammation.
GRANTS
This work was supported in part by National Institutes of Health National Heart, Lung, and Blood Institute Grant R01 HL137807 and a Blaustein Pain Research Fund Award from Johns Hopkins University.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
H.S. and B.J.U. conceived and designed research; H.S. and S.M. performed experiments; H.S., S.M., and B.J.U. analyzed data; H.S. and B.J.U. interpreted results of experiments; H.S. and S.M. prepared figures; H.S. and B.J.U. drafted manuscript; H.S. and B.J.U. edited and revised manuscript; H.S., S.M., and B.J.U. approved final version of manuscript.
REFERENCES
- 1.Al-Shamlan F, El-Hashim AZ. Bradykinin sensitizes the cough reflex via a B2 receptor dependent activation of TRPV1 and TRPA1 channels through metabolites of cyclooxygenase and 12-lipoxygenase. Respir Res 20: 110, 2019. doi: 10.1186/s12931-019-1060-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Belvisi MG, Birrell MA, Wortley MA, Maher SA, Satia I, Badri H, Holt K, Round P, McGarvey L, Ford J, Smith JA. XEN-D0501, a novel transient receptor potential vanilloid 1 antagonist, does not reduce cough in patients with refractory cough. Am J Respir Crit Care Med 196: 1255–1263, 2017. doi: 10.1164/rccm.201704-0769OC. [DOI] [PubMed] [Google Scholar]
- 3.Canning BJ, Mazzone SB, Meeker SN, Mori N, Reynolds SM, Undem BJ. Identification of the tracheal and laryngeal afferent neurones mediating cough in anaesthetized guinea-pigs. J Physiol 557: 543–558, 2004. doi: 10.1113/jphysiol.2003.057885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carr MJ, Kollarik M, Meeker SN, Undem BJ. A role for TRPV1 in bradykinin-induced excitation of vagal airway afferent nerve terminals. J Pharmacol Exp Ther 304: 1275–1279, 2003. doi: 10.1124/jpet.102.043422. [DOI] [PubMed] [Google Scholar]
- 5.Choi MJ, Jin Z, Park YS, Rhee YK, Jin YH. Transient receptor potential (TRP) A1 activated currents in TRPV1 and cholecystokinin-sensitive cranial visceral afferent neurons. Brain Res 1383: 36–42, 2011. doi: 10.1016/j.brainres.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 6.Chuang HH, Prescott ED, Kong H, Shields S, Jordt SE, Basbaum AI, Chao MV, Julius D. Bradykinin and nerve growth factor release the capsaicin receptor from PtdIns(4,5)P2-mediated inhibition. Nature 411: 957–962, 2001. doi: 10.1038/35082088. [DOI] [PubMed] [Google Scholar]
- 7.Gavva NR, Tamir R, Qu Y, Klionsky L, Zhang TJ, Immke D, Wang J, Zhu D, Vanderah TW, Porreca F, Doherty EM, Norman MH, Wild KD, Bannon AW, Louis JC, Treanor JJ. AMG 9810 [(E)-3-(4-t-butylphenyl)-N-(2,3-dihydrobenzo[b][1,4] dioxin-6-yl)acrylamide], a novel vanilloid receptor 1 (TRPV1) antagonist with antihyperalgesic properties. J Pharmacol Exp Ther 313: 474–484, 2005. doi: 10.1124/jpet.104.079855. [DOI] [PubMed] [Google Scholar]
- 8.Grace M, Birrell MA, Dubuis E, Maher SA, Belvisi MG. Transient receptor potential channels mediate the tussive response to prostaglandin E2 and bradykinin. Thorax 67: 891–900, 2012. doi: 10.1136/thoraxjnl-2011-201443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grundy D. Principles and standards for reporting animal experiments in The Journal of Physiology and Experimental Physiology. J Physiol 593: 2547–2549, 2015. doi: 10.1113/JP270818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hill RZ, Morita T, Brem RB, Bautista DM. S1PR3 mediates itch and pain via distinct TRP channel-dependent pathways. J Neurosci 38: 7833–7843, 2018. doi: 10.1523/JNEUROSCI.1266-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hofmann T, Obukhov AG, Schaefer M, Harteneck C, Gudermann T, Schultz G. Direct activation of human TRPC6 and TRPC3 channels by diacylglycerol. Nature 397: 259–263, 1999. doi: 10.1038/16711. [DOI] [PubMed] [Google Scholar]
- 12.Hollenberg MD, Saifeddine M, al-Ani B, Kawabata A. Proteinase-activated receptors: structural requirements for activity, receptor cross-reactivity, and receptor selectivity of receptor-activating peptides. Can J Physiol Pharmacol 75: 832–841, 1997. doi: 10.1139/y97-110. [DOI] [PubMed] [Google Scholar]
- 13.Jin X, Shah S, Liu Y, Zhang H, Lees M, Fu Z, Lippiat JD, Beech DJ, Sivaprasadarao A, Baldwin SA, Zhang H, Gamper N. Activation of the Cl− channel ANO1 by localized calcium signals in nociceptive sensory neurons requires coupling with the IP3 receptor. Sci Signal 6: ra73, 2013. doi: 10.1126/scisignal.2004184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Katanosaka K, Banik RK, Giron R, Higashi T, Tominaga M, Mizumura K. Contribution of TRPV1 to the bradykinin-evoked nociceptive behavior and excitation of cutaneous sensory neurons. Neurosci Res 62: 168–175, 2008. doi: 10.1016/j.neures.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 15.Kollarik M, Dinh QT, Fischer A, Undem BJ. Capsaicin-sensitive and -insensitive vagal bronchopulmonary C-fibres in the mouse. J Physiol 551: 869–879, 2003. doi: 10.1113/jphysiol.2003.042028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kruger L, Kavookjian AM, Kumazawa T, Light AR, Mizumura K. Nociceptor structural specialization in canine and rodent testicular “free” nerve endings. J Comp Neurol 463: 197–211, 2003. doi: 10.1002/cne.10754. [DOI] [PubMed] [Google Scholar]
- 17.Kruger L, Light AR, Schweizer FE. Axonal terminals of sensory neurons and their morphological diversity. J Neurocytol 32: 205–216, 2003. doi: 10.1023/B:NEUR.0000010080.62031.f0. [DOI] [PubMed] [Google Scholar]
- 18.Kumar R, Hazan A, Geron M, Steinberg R, Livni L, Matzner H, Priel A. Activation of transient receptor potential vanilloid 1 by lipoxygenase metabolites depends on PKC phosphorylation. FASEB J 31: 1238–1247, 2017. doi: 10.1096/fj.201601132R. [DOI] [PubMed] [Google Scholar]
- 19.Kummer W, Fischer A, Kurkowski R, Heym C. The sensory and sympathetic innervation of guinea-pig lung and trachea as studied by retrograde neuronal tracing and double-labelling immunohistochemistry. Neuroscience 49: 715–737, 1992. doi: 10.1016/0306-4522(92)90239-X. [DOI] [PubMed] [Google Scholar]
- 20.Kupari J, Häring M, Agirre E, Castelo-Branco G, Ernfors P. An atlas of vagal sensory neurons and their molecular specialization. Cell Reports 27: 2508–2523.e4, 2019. doi: 10.1016/j.celrep.2019.04.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kwong K, Nassenstein C, de Garavilla L, Meeker S, Undem BJ. Thrombin and trypsin directly activate vagal C-fibres in mouse lung via protease-activated receptor-1. J Physiol 588: 1171–1177, 2010. doi: 10.1113/jphysiol.2009.181669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee MG, Macglashan DW Jr, Undem BJ. Role of chloride channels in bradykinin-induced guinea pig airway vagal C-fibre activation. J Physiol 566: 205–212, 2005. doi: 10.1113/jphysiol.2005.087577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lieu T, Kollarik M, Myers AC, Undem BJ. Neurotrophin and GDNF family ligand receptor expression in vagal sensory nerve subtypes innervating the adult guinea pig respiratory tract. Am J Physiol Lung Cell Mol Physiol 300: L790–L798, 2011. doi: 10.1152/ajplung.00449.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Linley JE, Rose K, Patil M, Robertson B, Akopian AN, Gamper N. Inhibition of M current in sensory neurons by exogenous proteases: a signaling pathway mediating inflammatory nociception. J Neurosci 28: 11240–11249, 2008. doi: 10.1523/JNEUROSCI.2297-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu B, Linley JE, Du X, Zhang X, Ooi L, Zhang H, Gamper N. The acute nociceptive signals induced by bradykinin in rat sensory neurons are mediated by inhibition of M-type K+ channels and activation of Ca2+-activated Cl− channels. J Clin Invest 120: 1240–1252, 2010. doi: 10.1172/JCI41084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mazzone SB, Undem BJ. Vagal afferent innervation of the airways in health and disease. Physiol Rev 96: 975–1024, 2016. doi: 10.1152/physrev.00039.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nassenstein C, Kwong K, Taylor-Clark T, Kollarik M, Macglashan DM, Braun A, Undem BJ. Expression and function of the ion channel TRPA1 in vagal afferent nerves innervating mouse lungs. J Physiol 586: 1595–1604, 2008. doi: 10.1113/jphysiol.2007.148379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nassenstein C, Taylor-Clark TE, Myers AC, Ru F, Nandigama R, Bettner W, Undem BJ. Phenotypic distinctions between neural crest and placodal derived vagal C-fibres in mouse lungs. J Physiol 588: 4769–4783, 2010. doi: 10.1113/jphysiol.2010.195339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oh EJ, Weinreich D. Bradykinin decreases K+ and increases Cl− conductances in vagal afferent neurones of the guinea pig. J Physiol 558: 513–526, 2004. doi: 10.1113/jphysiol.2004.066381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patil MJ, Meeker S, Bautista D, Dong X, Undem BJ. Sphingosine-1-phosphate activates mouse vagal airway afferent C-fibres via S1PR3 receptors. J Physiol 597: 2007–2019, 2019. doi: 10.1113/JP277521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ru F, Sun H, Jurcakova D, Herbstsomer RA, Meixong J, Dong X, Undem BJ. Mechanisms of pruritogen-induced activation of itch nerves in isolated mouse skin. J Physiol 595: 3651–3666, 2017. doi: 10.1113/JP273795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shin J, Cho H, Hwang SW, Jung J, Shin CY, Lee SY, Kim SH, Lee MG, Choi YH, Kim J, Haber NA, Reichling DB, Khasar S, Levine JD, Oh U. Bradykinin-12-lipoxygenase-VR1 signaling pathway for inflammatory hyperalgesia. Proc Natl Acad Sci USA 99: 10150–10155, 2002. doi: 10.1073/pnas.152002699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sun H, Lin AH, Ru F, Patil MJ, Meeker S, Lee LY, Undem BJ. KCNQ/M-channels regulate mouse vagal bronchopulmonary C-fiber excitability and cough sensitivity. JCI Insight 4: e124467, 2019. doi: 10.1172/jci.insight.124467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Swift S, Leger AJ, Talavera J, Zhang L, Bohm A, Kuliopulos A. Role of the PAR1 receptor 8th helix in signaling: the 7-8-1 receptor activation mechanism. J Biol Chem 281: 4109–4116, 2006. doi: 10.1074/jbc.M509525200. [DOI] [PubMed] [Google Scholar]
- 35.Taylor-Clark TE, Undem BJ, Macglashan DW Jr, Ghatta S, Carr MJ, McAlexander MA. Prostaglandin-induced activation of nociceptive neurons via direct interaction with transient receptor potential A1 (TRPA1). Mol Pharmacol 73: 274–281, 2008. doi: 10.1124/mol.107.040832. [DOI] [PubMed] [Google Scholar]
- 36.Than JY, Li L, Hasan R, Zhang X. Excitation and modulation of TRPA1, TRPV1, and TRPM8 channel-expressing sensory neurons by the pruritogen chloroquine. J Biol Chem 288: 12818–12827, 2013. doi: 10.1074/jbc.M113.450072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Undem BJ, Chuaychoo B, Lee MG, Weinreich D, Myers AC, Kollarik M. Subtypes of vagal afferent C-fibres in guinea-pig lungs. J Physiol 556: 905–917, 2004. doi: 10.1113/jphysiol.2003.060079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vaidyula VR, Rao AK. Role of Gαq and phospholipase C-β2 in human platelets activation by thrombin receptors PAR1 and PAR4: studies in human platelets deficient in Galphaq and phospholipase C-β2. Br J Haematol 121: 491–496, 2003. doi: 10.1046/j.1365-2141.2003.04296.x. [DOI] [PubMed] [Google Scholar]
- 39.Veldhuis NA, Poole DP, Grace M, McIntyre P, Bunnett NW. The G protein-coupled receptor-transient receptor potential channel axis: molecular insights for targeting disorders of sensation and inflammation. Pharmacol Rev 67: 36–73, 2015. doi: 10.1124/pr.114.009555. [DOI] [PubMed] [Google Scholar]
- 40.Wang J, Kollarik M, Ru F, Sun H, McNeil B, Dong X, Stephens G, Korolevich S, Brohawn P, Kolbeck R, Undem B. Distinct and common expression of receptors for inflammatory mediators in vagal nodose versus jugular capsaicin-sensitive/TRPV1-positive neurons detected by low input RNA sequencing. PLoS One 12: e0185985, 2017. doi: 10.1371/journal.pone.0185985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wilson SR, Gerhold KA, Bifolck-Fisher A, Liu Q, Patel KN, Dong X, Bautista DM. TRPA1 is required for histamine-independent, Mas-related G protein-coupled receptor-mediated itch. Nat Neurosci 14: 595–602, 2011. doi: 10.1038/nn.2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yu S, Ouyang A. TRPA1 in bradykinin-induced mechanical hypersensitivity of vagal C fibers in guinea pig esophagus. Am J Physiol Gastrointest Liver Physiol 296: G255–G265, 2009. doi: 10.1152/ajpgi.90530.2008. [DOI] [PubMed] [Google Scholar]