Abstract
Pulmonary fibrosis involves the formation of inappropriate scar tissue in the lungs, but what drives fibrosis is unclear. Sialidases (also called neuraminidases) cleave terminal sialic acids from glycoconjugates. In humans and mice, pulmonary fibrosis is associated with desialylation of glycoconjugates and upregulation of sialidases. Of the four mammalian sialidases, we previously detected only NEU3 in the bronchoalveolar lavage fluid from mice with bleomycin-induced pulmonary fibrosis. In this report, we show that NEU3 upregulates extracellular accumulation of the profibrotic cytokines IL-6 and IL-1β, and IL-6 upregulates NEU3 in human peripheral blood mononuclear cells, suggesting that NEU3 may be part of a positive feedback loop potentiating fibrosis. To further elucidate the role of NEU3 in fibrosis, we used bleomycin to induce lung fibrosis in wild-type C57BL/6 and Neu3−/− mice. At 21 days after bleomycin, compared with male and female C57BL/6 mice, male and female Neu3−/− mice had significantly less inflammation, less upregulation of other sialidases and the profibrotic cytokine active transforming growth factor β1, and less fibrosis in the lungs. Our results suggest that NEU3 participates in fibrosis and that NEU3 could be a target to develop treatments for fibrosis.
Keywords: bleomycin mouse model, idiopathic pulmonary fibrosis, sialidase NEU3, therapeutics
INTRODUCTION
Fibrosis involves the inappropriate formation of scar tissue in an internal organ, and 45% of deaths in the United States are associated with fibrosis (39, 74, 84). Pulmonary fibrosis is the generic term for a broad category of lung diseases that includes idiopathic pulmonary fibrosis (IPF). IPF is a chronic and fatal disease characterized by fibrosis of the lungs with a median survival of 3–5 yr after initial diagnosis (39, 62). IPF affects ~3 million people worldwide, with an incidence of 1 in 400 in the elderly (39, 62).
Sialic acid is a monosaccharide frequently located at the terminal positions of glycoconjugates (67, 78). Sialidases, which are also called neuraminidases, remove the terminal sialic acid from these glycoconjugates (41, 61). There are four known mammalian sialidases, NEU1, NEU2, NEU3, and NEU4, and these have different subcellular localizations and substrate specificities (43). Sialidase activity was detected in the bronchoalveolar lavage (BAL) fluid from 8 of 9 patients with pulmonary fibrosis, but no sialidase activity was detected in the BAL fluid from healthy controls (34). There is upregulation of the sialidase NEU1 in pulmonary fibrosis (37), and in addition to upregulation of NEU1, we detected desialylation of glycoconjugates and upregulation of NEU2 and NEU3 in fibrotic lesions in human and mouse lungs (29). NEU3, but not NEU1, NEU2, or NEU4, was detected in the BAL fluid from mice with bleomycin-induced pulmonary fibrosis; no detectable sialidases were in the BAL fluid from control mice (29). When added to human immune cells, NEU2 and NEU3 upregulated the fibrosis-associated cytokine transforming growth factor (TGF)-β1, and conversely, TGF-β1 added to human lung fibroblasts, lung epithelial cells, and peripheral blood mononuclear cells (PBMCs) upregulated NEU3 expression (29). Serum amyloid P (SAP) is a serum protein that inhibits fibrosis in humans, mice, and rats (55), while fibrocytes are monocyte-derived cells that participate in fibrosis (6, 7, 56). NEU2, NEU3, and NEU4 counteract SAP function and potentiate fibrocyte differentiation in the presence or absence of SAP (29). The small molecules DANA (2,3‐dehydro‐2‐deoxy‐N‐acetylneuraminic acid) and oseltamivir are sialidase inhibitors. We found that DANA and oseltamivir reduce bleomycin-induced pulmonary fibrosis in mice, even when treatment was initiated at day 10 after bleomycin (29). These results suggested that sialidases are involved in pulmonary fibrosis.
Many of the effects of sialidases in fibrosis, such as the sialidase activity in pulmonary fibrosis patient BAL fluid (34), appear to be in the extracellular environment. NEU1, NEU2, and NEU4 are localized inside cells (43), whereas NEU3 is localized on the extracellular side of the plasma membrane (35, 43, 80). NEU3 may thus be a key sialidase involved in fibrosis. NEU3 is upregulated in colon (28), renal (76), ovarian (50), and prostate cancers (31). Overexpression of NEU3 in human colon cancer cells promotes cell proliferation and adhesion, suggesting that the high levels of NEU3 observed in some tumors may potentiate the tumor (30). To elucidate the function of NEU3, NEU3 deficient (Neu3−/−) mice were generated in a C57BL/6 background by disrupting exon 3 of Neu3 (85). Compared with control C57BL/6 mice, Neu3−/− mice have normal lifespans, appearance, fertility, ganglioside composition of tissues, and histology of a variety of tissues, and Neu3−/− mice generated in a Balb/c background are also similar to parental mice (85). In support of the role of NEU3 in cancer, Neu3−/− mice have a reduced incidence of colitis-associated colon cancer (85).
To elucidate the role of NEU3 in pulmonary fibrosis, in this report, we used bleomycin to induce pulmonary fibrosis in wild-type and Neu3−/− mice and find that Neu3−/− mice have strongly attenuated inflammation and fibrosis in response to bleomycin, suggesting that NEU3 plays a major role in bleomycin-induced pulmonary fibrosis in mice.
MATERIALS AND METHODS
Mouse strains.
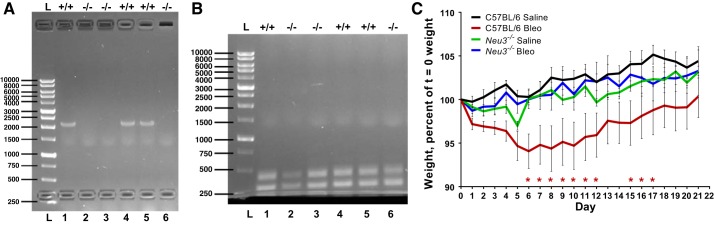
A breeding colony of C57BL/6 background Neu3−/− mice strain B6.129-Neu3tm1Yamk (85), originally from Kazunori Yamaguchi from Miyagi Cancer Center Research Institute, Natori, Japan, was established by Dr. Jamey Marth at the University of California, Santa Barbara, and some of the mice from this colony were sent to Texas A&M University. Sex- and age-matched C57BL/6 wild-type mice were from Jackson Laboratories (Bar Harbor, ME). Tailsnip DNA was collected as described previously (69), and PCR was used to check the Neu3 gene disruption and the presence of the Neu1, Neu2, and Neu4 genes using the primers described in Supplemental Table S1 (all Supplemental Data are available at https://doi.org/10.6084/m9.figshare.9736256). As a control, the presence of the β-actin gene in the tailsnip DNA samples was checked by PCR using the primers described in Supplemental Table S1. Wild-type C57BL/6 mice showed a Neu3 PCR product that was absent in Neu3−/− mice, and actin controls showed that template DNA was present in all samples tested (Fig. 1, A and B). All experiments with the animals were carried out in accordance with National Institutes of Health guidelines. All experimental protocols were approved by the Texas A&M University Institutional Animal Care and Use Committee.
Fig. 1.
Neu3−/− mice are resistant to a decline in body weight after bleomycin treatment. A: tailsnip DNA was prepared from wild-type C57BL/6 (+/+) and Neu3−/− (−/−) mice, and PCR was used to check for the presence of a 2.1 kBP piece of the Neu3 gene. Molecular mass markers in base pair (BP) are at left. B: PCR was used to check for the presence of a 310 BP fragment of the β-actin gene in the DNA samples. C: percent change in body weight after saline or bleomycin (Bleo) treatment at day 0. Values are means ± SE, n = 6 except for bleomycin-treated C57BL/6, where n = 9. *P < 0.05 comparing bleomycin-treated Neu3−/− mice to bleomycin-treated C57BL/6 mice (2-way ANOVA, Bonferroni’s test).
Mouse model of pulmonary fibrosis.
Mice were used in three separate groups to achieve three male and three female Neu3−/− mice treated with saline, three male and three female Neu3−/− mice treated with bleomycin, three male and three female C57BL/6 mice treated with saline, and five male and four female C57BL/6 mice treated with bleomycin. An additional female C57BL/6 mouse treated with bleomycin developed signs of distress on day 10 and was immediately euthanized and not included in the study; an autopsy showed that this mouse had a blocked intestine. All of the mice were 7–9 wk old except for two 18-wk-old females in the Neu3−/− bleomycin group and one 18-wk-old female in the Neu3−/− saline group. In all of the assays, the 18-wk-old females did not show any obvious differences compared with the other Neu3−/− females. All other mice survived until euthanization at day 21 and were used in the study. The mice were sedated for 60 s with 4% isoflurane in oxygen and then treated with an oropharyngeal aspiration of 50 μL of 3 U/kg bleomycin (Calbiochem, Billerica, MA) in 0.9% saline or saline alone as previously described (10, 12, 57, 60). Mice were euthanized 21 days after bleomycin aspiration, and bronchoalveolar lavage fluid (BAL) and blood were collected. The liver, heart, kidneys, and spleen were removed and weighed. After BAL collection, lungs were inflated at equal pressures with, and embedded in, OCT compound (VWR, Radnor, PA), frozen, and stored at −80°C. The cells collected from BAL were counted, and cytospins were prepared as previously described (10, 12, 57, 60). Blood was clarified by centrifugation at 10,000 g for 5 min to obtain serum, which was stored at −80°C.
Histology.
Lung cryosections (10-μm) on glass slides and BAL cytospins were air dried for 48 h before use. Lung cryosections or BAL cytospins were fixed in fresh acetone for 20 min at room temperature and then rehydrated in water for 5 min and then PBS for 5 min. Slides were blocked with PBS containing 2% BSA (PBSB) for 30 min and further processed as described previously (10, 29). Slides with cryosections or BAL cytospins were incubated with 5 μg/mL anti-CD11b (clone M1/70 BioLegend, San Diego, CA) to detect blood and inflammatory macrophages, anti-CD11c (clone N418, BioLegend) to detect resident alveolar macrophages and dendritic cells, anti-CD45 (clone 30-F11, BioLegend) to detect all leukocytes, and anti-Ly6G (1A8, BioLegend) or anti-Ly6C (HK1.4, BioLegend) to detect macrophage subsets and neutrophils, which have been reported to be upregulated in the bleomycin-induced pulmonary fibrosis mouse model (10, 29, 54, 57, 59). Slides with BAL cytospins were also incubated with 5 ug/mL anti-CD3 (clone 17A2, no. 100202, BioLegend) to detect T cells or 5 μg/mL anti-CD19 (90176T, Cell Signaling Technology, Danvers, MA) to detect B cells. Some lung section slides were stained with 1 μg/mL rabbit polyclonal anti-NEU1 (TA335236, Origene, Rockville, MD), anti-NEU2, (TA324482, Origene,), anti-NEU4 (AP52856PU-N, Acris/Origene), or anti-TGF-β1 (AB-100-NA, R&D systems, Minneapolis, MN) in PBSB or 0.5 μg/mL anti-NEU3 (TA590228, Origene) in PBSB/500 mM NaCl–0.1% NP-40 alternative (EMD Millipore, Billerica, MA) for 60 min. Isotype-matched mouse irrelevant antibodies (BioLegend) were used as controls. After washing three times with PBS for 10 min each, the slides were incubated with 1:500 biotinylated donkey-anti-rabbit (cat. no. 711-066-152, Jackson ImmunoResearch Laboratories, West Grove, PA) or biotinylated donkey-anti-goat (cat. no. 713-066-147, Jackson) secondary antibodies and staining was then done as previously described (54, 59). Lung sections were stained with hematoxylin and eosin or Sirius red to detect collagen, as previously described (58). Light microscopy images were taken using a ×4 lens on a Microphot-FX microscope (Nikon, Melville, NY) or ×10 and ×40 lenses on a DM6B microscope (Leica, Buffalo Grove, IL). Polarized light images were also taken on the DM6B microscope. Images of a 1 mm calibration slide (cat. no. MA663, Swift Microscope World, Carlsbad, CA) were used for size bars. Fields were chosen randomly and counted blindly. Image quantification was done as previously described (29). Images were converted to RGB stacks using ImageJ (NIH, Bethesda, MD). The green channel (which shows the red staining) was used to adjust the intensity threshold level. The threshold level was kept the same for analyzing a set of images. The total area of the image and the area stained as a percentage of the total area of the image were then determined using ImageJ.
Immunofluorescence staining.
Glass slides with 10-μm lung cryosections were fixed in acetone for 20 min at room temperature and then rehydrated in water for 5 min and then PBS for 5 min. Slides were blocked with PBSB for 30 min. After blocking, the slides were incubated with 1 μg/mL rabbit anti-NEU3 (21630002, Novus Biologicals LLC, Centennial, CO), 5 μg/mL rat anti-mouse CD11b (BioLegend), rat anti-mouse CD11c (BioLegend), rat anti-mouse CD31 (BioLegend), rat anti-mouse Ly6C (BioLegend), rat anti-α-smooth muscle actin (αSMA; NB300-978, Novus Biologicals LLC) or rat anti-epithelial cell adhesion molecule (EpCAM; BioLegend) in PBSB containing 500 mM NaCl at 4°C overnight. After washing three times with PBS for 10 min, slides were incubated with 2 μg/mL donkey-anti-rat Rhodamine-Red-X (cat. no. 712-296-153, Jackson) or donkey-anti-rabbit Alexa Fluor 488 (cat. no. 711-546-152, Jackson) in PBSB for 30 min at room temperature. After washing three times with PBS, slides were mounted with DAPI containing mounting media (H-1500, Vector Laboratories, Burlingame, CA). The slides were kept in the dark at 4°C for 1 h to harden the mounting media. Images were captured with a ×20 lens on a Nikon ECLIPSE Ti2 microscope. The number of cells stained for NEU3 (green) but not the other cell marker (red), the number stained for both NEU3 and the other cell marker, and the number stained for the other cell marker but not NEU3 were counted in three randomly chosen fields of view. The percentage of cells of a specific type (stained red) that costained for NEU3 was calculated.
Differential cell staining.
BAL cytospins were fixed and treated with Wright–Giemsa stain (cat. no. 08711, Polysciences, Inc., Warrington, PA) following the manufacturer’s instructions. Two hundred cells per mouse were examined and scored for cell type following the manufacturer’s instructions.
Alveolar wall area fraction.
Because alveolar wall thickness increases because of fibrosis or interstitial edema (25), the alveolar wall area fraction was determined from the hematoxylin and eosin-stained lung slides. The quantification of alveolar wall area fraction was performed as described previously (23, 25), in which five randomly selected 1.19 × 0.92 mm fields without blood vessels or airways were imaged using a 4× objective on a Microphot-FX microscope. ImageJ was used to measure the area of all stained tissue as a percentage of the total image area. The alveolar wall area fraction value for 5 fields was averaged for each mouse in the indicated groups.
Analysis of 13-plex mouse inflammation panel.
BAL and serum samples collected at day 21 post-bleomycin aspiration were processed following the manufacturer’s protocol (cat. no. 740150, LEGENDplex Mouse Inflammation Panel (13-plex), Biolegend) for simultaneous quantification of 13 mouse cytokines using an Accuri C6 (BD Bioscience, Franklin Lakes, NJ) flow cytometer.
Western blots.
For Western blots, 20 μL of BAL fluid was mixed with 4 μL 5× Laemmli sample buffer and heated to 95°C for 5 min. Western blotting was done following References 29 and 53 with the exceptions that 4–20% Tris–glycine Mini-Protean TGX gels were used (cat. no. 456-1096, Bio-Rad, Hercules, CA). Equal volumes (1 μL) of BAL fluid were loaded on gels. As a positive control, 50–140 ng of recombinant human NEU1 (TP300386, Origene), NEU2 (TP319858, Origene), NEU3 (TP316537, Origene), NEU4 (TP303948, Origene), or active TGF-β1 (100-21-10UG, PeproTech, Rocky Hill, NJ) was loaded on gels. As a positive control, 0.1–1.0 μg of human serum albumin (A1653-1G, Sigma-Aldrich) was loaded on other gels. For Western blot staining, blocking was in PBS–2% BSA/5% nonfat milk. Blots were incubated with anti-NEU 1, 2, and 4 antibodies (Origene) at 1:1,000 in PBSB for 60 min at room temperature as described previously (29). For anti-NEU3, incubations were at 1:5,000 in PBS/2% BSA/0.1% NP-40 alternative/0.01% SDS for 60 min at room temperature as described previously (29). 1:5,000 peroxidase-conjugated donkey-anti-rabbit (cat. no. 711-036-152, Jackson) was used as secondary antibody. All washes were in PBS/0.1% (vol/vol) Tween 20 (PBST) (Fisher, Fair Lawn, NJ). For Western blot detection of active TGF-β1, nonreducing/no SDS conditions to prepare samples and electrophoresis were used (3). For Western blot staining of albumin, blocking was in 5% nonfat milk in immunoblot buffer (25 mM Tris, 0.15 M NaCl, and 0.1% Triton X-100). In immunoblot buffer were 1:50,000 anti-albumin antibody (AF3329, R&D Systems) incubations. Western blots stained for albumin were washed in Tris-buffered saline (TBS)–0.1% (vol/vol) Tween 20 (TBST). Peroxidase-conjugated donkey anti-rabbit (Jackson) and biotin-conjugated donkey anti-goat (Jackson) were used as secondary antibodies. SuperSignal West Pico Chemiluminescence Substrate (Thermo Scientific, Rockford, IL) was used following the manufacturer’s protocol to visualize the peroxidase using a ChemiDoc XRS+ System (Bio-Rad). Some blots were incubated with 0.1% Ponceau stain (Matheson, Norwood, OH) in 5% acetic acid for 30 min and washed two times with PBST for 10 min each.
Cell isolation and culture.
Human peripheral blood was collected from healthy volunteers who gave written consent and with specific approval from the Texas A&M University human subjects review board. All the methods were performed in accordance with the relevant guidelines and regulations. Peripheral blood mononuclear cells (PBMCs) were isolated and collected from blood using Ficoll-Paque density gradient centrifugation (GE Healthcare, Cincinnati, OH) following the manufacturer’s protocol. PBMCs were cultured at 104 cells/well in a total volume of 200 μL/well in 96-well flat bottom tissue culture plates (VWR) with RS [RPMI-1640 (VWR) supplemented with 10 mM HEPES (VWR), 1× nonessential amino acids (VWR), 1 mM sodium pyruvate (VWR), 2 mM glutamine (VWR), 100 U/mL penicillin, 100 μg/mL streptomycin (VWR), and 1× ITS-3 (Sigma-Aldrich, St. Louis, MO)]. When the cells were plated, recombinant human NEU3 (TP316537, Origene), was added to a final concentration of 0–500 ng/mL. The NEU3 was diluted in RS and added to cells to make the total volume of 200 μL in a well. The cells were then incubated at 37°C with 5% CO2. The culture media supernatants were collected after 48 h and assayed using interleukin-1β (IL-1β), IL-4, IL-6, IL-10, IL-12, IL-13, or IFN-γ ELISA kits (Biolegend) following the manufacturer’s protocols, reading absorbance with a SynergyMX plate reader (BioTek, Winooski, VT).
Flow cytometry analysis of sialidases expression in human PBMCs.
Human PBMCs were cultured as above with 8.3 × 105 cells/well with 2 mL/well in 6-well tissue culture plates (VWR) in the presence or absence of recombinant human IL-6 (Biolegend) and IL-1β. After 72 h, the medium was carefully removed and the cells were washed with 1 mL of sterile sterile phosphate-buffered saline (PBS) at room temperature. The cells were detached with 500 μL of Accutase cell detachment solution (VWR) per well for 6 min at 37°C. One milliliter of RS was added per well. After pipetting the cell solutions four times, the cells were placed in sterile 1.7 mL microtube tubes (Genesee Scientific, San Diego, CA) and cells were collected by centrifugation at 500 × g for 10 min at 4°C. The pelleted cells were washed twice by resuspension with 1 mL of ice-cold PBS and centrifugation. The cells were resuspended in 200 μL of ice-cold 2% (wt/vol) paraformaldehyde (EMS, Hatfield, PA), in PBS for 10 min on ice for fixation. One milliliter of ice-cold PBS was added and cells were collected by centrifugation. The cells were resuspended in 200 μL of ice-cold PBSB for 10 min on ice for blocking. One milliliter of ice-cold PBS was added and cells were collected by centrifugation. The pellet was resuspended in 200 μL of ice-cold 0.1% (wt/vol) Triton X-100 (Alfa Aesar, Ward Hill, MA) in PBS and cell membranes were lysed for 10 min on ice. One ml of ice-cold PBS was added and cells were collected by centrifugation. Cells were resuspended in 500 μL of PBSB, and 125 μL was then collected by centrifugation for a staining reaction.
The pelleted cells for a staining reaction were resuspended in 100 μL of 1 μg/mL rabbit polyclonal anti-NEU1 (Origene), anti-NEU2, (Origene), anti-NEU4 (Acris/Origene), irrelevant rabbit polyclonal antibody (AB-105-C, R&D Systems, Minneapolis, MN), or no antibody in 2% (wt/vol) PBSB, or 1 μg/mL anti-NEU3 (Origene) in 2% (wt/vol) PBSB with 0.1% (vol/vol) NP-40 alternative (EMD Millipore, Billerica, MA). Cells were incubated with antibodies for 60 min on ice. Five hundred microliters of ice-cold PBS was added, and cells were collected by centrifugation. Cells were washed twice by resuspension in 1 mL of ice-cold PBS followed by centrifugation. The cells were then incubated with 100 μL of 1:1,000 goat anti-rabbit Alexa Fluor 647 (Life Technologies, Carlsbad, CA), in PBSB for 30 min on ice. The cells were then washed twice as described above. The cells were then resuspended in 100 μL of PBSB and kept on ice, and the fluorescence of cells was analyzed on an Accuri C6 flow cytometer (BD Bioscience), using forward and side-scatter to identify monocytes and lymphocytes, as described previously (10).
Lung tissue lysate preparation.
Approximately 10–12 mg of mouse lung tissue frozen in OCT was cut off, thawed, washed twice in 0.5 mL PBS by centrifugation at 2,000 × g for 5 min in a preweighed Eppendorf tube and resuspension. After the last centrifugation, the supernatant was carefully removed, the tissue was weighed, and the tube with tissue was frozen in liquid nitrogen. Five hundred microliters of Pierce RIPA buffer (Thermo Scientific, Rockford, IL) with 1× protease and phosphatase inhibitor cocktail (Cell Signaling Technology, Danvers, MA) was added per 5 mg of tissue, and tissue was crushed with a pestle. The mixture was then incubated on a rotator for 2 h at 4°C. After centrifugation at 18,000 × g for 20 min at 4°C, the supernatant was collected as lung tissue lysate. Total protein was measured by optical density of 280/260 with a SynergyMX plate reader, (BioTek, Winooski, VT) using RIPA buffer with protease and phosphatase inhibitor as a blank.
NEU3 ELISA.
ELISA assays for NEU3 from BAL fluid were performed as described previously (29), with the exception that BAL fluid was diluted to 10 μg of protein in 100 μL of PBS. Serial dilutions of human recombinant NEU3 (Origene) in PBS were also incubated and used as standard curves.
Hydroxyproline assay.
Approximately half lobes of lungs frozen in OCT were cut off, thawed, and washed three times with PBS to remove OCT in preweighed Eppendorf tubes as described above. After the last centrifugation, the tubes were kept inverted for 5 min to allow PBS to blot onto blotting paper, and the tissue was then weighed. Tissues were then processed using a hydroxyproline quantification kit (MAK008-1KT, Sigma-Aldrich, St. Louis, MO) following the manufacturer’s directions.
Statistical analysis.
Data were analyzed by t test or ANOVA using Prism 7 (Graphpad, La Jolla, CA). Significance was defined as P < 0.05.
RESULTS
Neu3−/− mice do not lose weight after bleomycin treatment.
We previously observed that fibrotic lesions from human and mouse lungs have elevated levels of NEU3 and that in the bleomycin mouse model of pulmonary fibrosis, sialidase inhibitors attenuate fibrosis (29). To determine if loss of NEU3 affects bleomycin-induced pulmonary inflammation and fibrosis, wild-type C57BL/6 and Neu3−/− mice (85) were treated with an oropharyngeal aspiration of saline or bleomycin. The Neu3−/− mice had a disruption of the Neu3−/− gene (Fig. 1, A and B) and undisrupted fragments of the Neu1, Neu2, and Neu4 genes (Supplemental Fig. S1, A–C). All of the mice survived the saline or bleomycin treatment and were included in the study, except for one female C57BL/6 mouse, which developed a blocked intestine on day 10 after bleomycin aspiration and was euthanized on day 10; this mouse was not included in the study. As previously observed (20, 86), compared with saline-treated C57BL/6 mice (control), bleomycin-treated C57BL/6 mice had lower body weights from days 6 to 12 after bleomycin (Fig. 1C). The weight gain of saline or bleomycin-treated Neu3−/− mice was not significantly different from controls (Fig. 1C). Although there are differential effects of bleomycin in male and female mice (19, 79), similar trends were observed for male and female mice (Supplemental Fig. S1, D and E). Bleomycin treatment and/or loss of NEU3 did not significantly affect liver, heart, kidney, or spleen weights (as a percent of total body weight) for combined male and female mice (Supplemental Fig. S1F), although Neu3−/− female mice had slightly increased liver weights after bleomycin (Supplemental Fig. S1G). These data suggest that loss of NEU3 attenuates bleomycin-induced total body weight loss in mice.
Neu3−/− mice have an attenuated protein increase in the BAL at day 21 after bleomycin treatment.
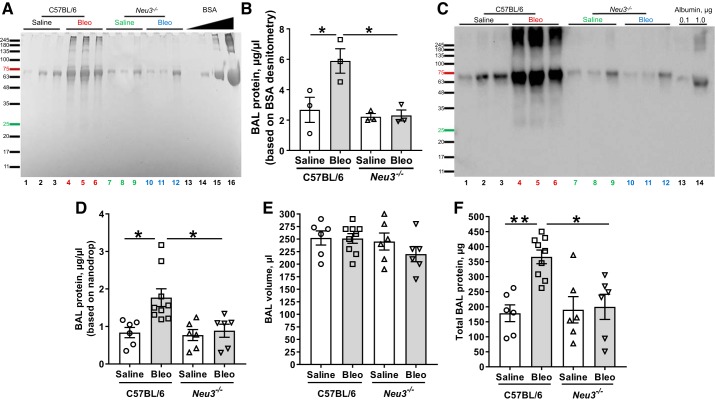
Bleomycin treatment of mouse lungs causes protein levels in the BAL fluid to increase (33, 51, 57). To determine if loss of NEU3 affects this, we measured protein in the BAL. As previously observed (29, 57), at day 21, bleomycin caused an upregulation of protein levels in BAL fluid in C57BL/6 mice as determined by scanning protein gels or by nanodrop assays (Fig. 2, A, B, D, and F). Saline-treated Neu3−/− mice had comparable levels of protein in BAL to those of C57BL/6 mice, and bleomycin did not significantly affect the protein levels in Neu3−/− mouse BAL (Fig. 2, A and B). As previously observed (51), the bleomycin-induced protein in BAL appeared to be mainly albumin, which was confirmed by an immunoblot with anti-albumin antibodies (Fig. 2C). Similar results were observed for female and male mice (Supplemental Fig. S2, A–F), although as previously observed (79), C57BL/6 female mice had an attenuated response to bleomycin compared with C57BL/6 male mice. Although bleomycin seemed to increase BAL protein levels in female Neu3−/− mice, the effect was not statistically significant. Together these data suggest that loss of NEU3 attenuates bleomycin-induced upregulation of protein in BAL.
Fig. 2.
Neu3−/− mice do not have elevated bronchoalveolar lavage (BAL) protein following bleomycin aspiration. BAL was collected at day 21 following saline or bleomycin (Bleo) treatment. A: BAL from 3 randomly selected mice per group were analyzed by PAGE on a 4–12% reducing gel and stained with Coomassie. Molecular mass markers in kilodaltons are at left. Bovine serum albumin at 0.01, 0.1, 1.0, and 10.0 μg was loaded at right. From left, lane nos. 1–12, the sex of the mice is M, F, F, M, M, F, M, F, M, M, M, F, with M, male and F, female. B: quantification of protein in lane nos. 1–12, using BSA band densities as standards. Values are means ± SE, n = 3. *P < 0.05 (1-way ANOVA, Bonferroni’s test). C: BAL from the same mice were analyzed by PAGE on a reducing gel and stained with anti-albumin antibodies. Molecular mass markers in kDa are at left. Human albumin at 0.1 and 1.0 μg are in lanes 13 and 14. D: BAL protein was measured using nanodrop (spectrophotometry). Values are means ± SE, n = 6 except for bleomycin-treated C57BL/6, where n = 9. *P < 0.05 (t test). E: BAL volumes. Values are means ± SE, n = 6 except for bleomycin-treated C57BL/6, where n = 9. F: total BAL protein. BAL protein concentration was assessed by nanodrop spectrophotometry for each mouse and multiplied by the BAL volume from that mouse to obtain total BAL protein. Values are means ± SE, n = 6 except for bleomycin-treated C57BL/6, where n = 9. *P < 0.05; **P < 0.01 (1-way ANOVA, Bonferroni’s test).
Neu3−/− mice have an attenuated inflammatory cell increase at day 21 after bleomycin treatment.
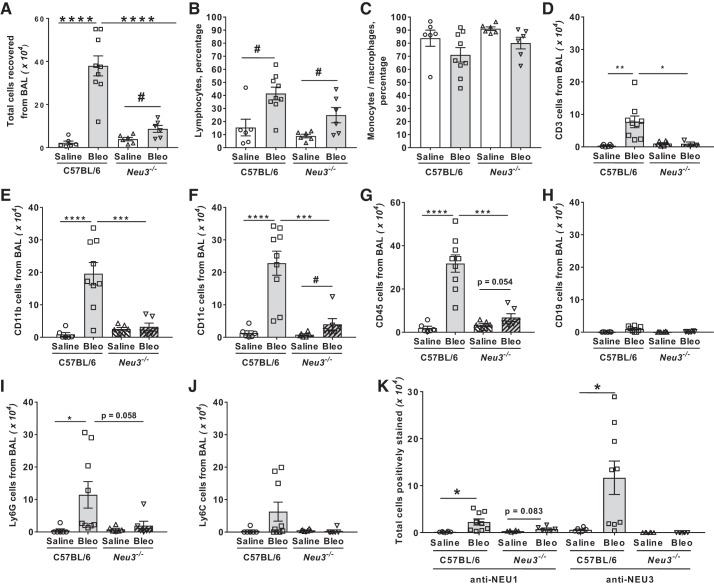
Bleomycin upregulates inflammatory cell counts in mouse lung BAL (10, 48, 57, 60). Compared with saline-treated C57BL/6 mice, saline-treated Neu3−/− mice had similar BAL cell numbers (Fig. 3A). Compared with saline-treated Neu3−/− mice, bleomycin-treated Neu3−/− mice had upregulated BAL cell counts, but these were significantly less compared with bleomycin-treated C57BL/6 mice (Fig. 3A). Similar effects were seen with male and female mice (Supplemental Figs. S3, A and B). Wright–Giemsa staining indicated that bleomycin treatment increased the lymphocyte percentage in both C57BL/6 and Neu3−/− BALs (Fig. 3B) but did not significantly affect monocyte/macrophage percentages compared with saline treatment (Fig. 3C). In C57BL/6 BAL, as previously observed (10, 57, 60), bleomycin increased the numbers of CD3-positive lymphocytes (Fig. 3D), CD11b-positive inflammatory macrophages (Fig. 3E), CD11c-positive resident alveolar macrophages (Fig. 3F), CD45-positive leukocytes (Fig. 3G), and Ly6G-positive cells in the BAL (Fig. 3I). Significant differences were not observed for CD19-positive lymphocytes (Fig. 3H) and Ly6C-positive cells in the BAL (Fig. 3J). Compared with saline-treated C57BL/6 mice, saline-treated Neu3−/− mice had similar BAL cell numbers for the above cell types, and in Neu3−/− mice, bleomycin upregulated CD11c positive cells in the BAL (Fig. 3F). Similar effects were seen in male and female mice with the exception that bleomycin caused an increase in CD45-positive cells in the BAL in Neu3−/− female mice but not Neu3−/− male mice (Supplemental Fig. S3, C and D). Bleomycin-treated C57BL/6 mice had increased numbers of NEU1 and NEU3-expressing cells in BAL compared with saline-treated C57BL/6 mice (Fig. 3K). We did not observe any significant difference because of loss of NEU3 or presence of bleomycin in the percent of the above cell types in the BAL (Supplemental Fig. 3E). Together, these data suggest that loss of NEU3 attenuates the bleomycin-induced increase of inflammatory cell counts in the BAL of Neu3−/− mice.
Fig. 3.
Neu3−/− mice have fewer bronchoalveolar lavage (BAL) cells after bleomycin treatment. A: the total number of cells collected from the BAL at day 21. Values are means ± SE, n = 6 mice per group except for bleomycin-treated C57BL/6, where n = 9 mice per group. ****P < 0.0001 (1-way ANOVA, Bonferroni’s test), #P < 0.05 (t test). B and C: cytospins of BAL at day 21 were stained with Wright-Giemsa stain and the percentage of lymphocytes (B) and monocytes/macrophages (C) was determined by examining 200 cells per mouse BAL sample. Values are means ± SE, n = 6 except for bleomycin-treated C57BL/6, where n = 9. #P < 0.05 (t test). D–K: cytospins of BAL at day 21 were stained for the markers CD3 (D), CD11b (E), CD11c (F), CD45 (G), CD19 (H), Ly6G (I), Ly6C (J), and NEU1 and NEU3 (K). The percentage of cells stained was determined in 5 randomly chosen fields of 100–150 cells, and the percentage was multiplied by the total number of BAL cells for that mouse to obtain the total number of BAL cells staining for the marker. Values are means ± SE, n = 6 except for bleomycin-treated C57BL/6, where n = 9. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001 (1-way ANOVA, Bonferroni’s test); #P < 0.05 (t test).
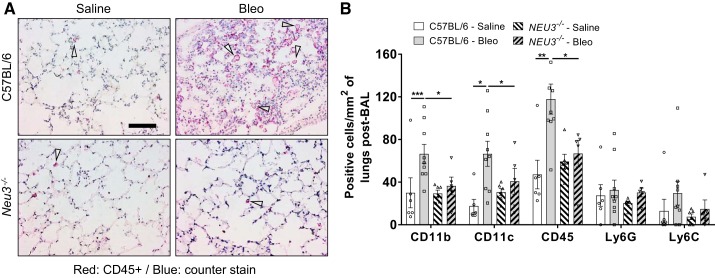
Following BAL, lungs were sectioned and stained to detect cells that were not removed by BAL. As previously observed (10, 57, 60), in C57BL/6 mice, bleomycin increased the numbers of CD11b+, CD11c+, and CD45+ cells remaining in the lungs after BAL (Fig. 4, A and B, Supplemental Fig. S4, A–C). Compared with saline-treated C57BL/6 mice, saline-treated Neu3−/− mice had similar numbers for the above cell types, and in Neu3−/− mice, bleomycin did not significantly affect counts for the above cell types (Fig. 4, A and B). As previously observed (25, 26), bleomycin did not affect counts of Ly6G+ or Ly6C+ cells in C57BL/6 lungs after BAL at day 21, and loss of NEU3 did not affect Ly6G+ or Ly6C+ counts (Fig. 4B, Supplemental Fig. S4, A–C). Female mice tended to have lower densities of the above cell types in the lungs (Supplemental Figs. S4, A and B). The positively stained cells were primarily clustered in the fibrotic regions of bleomycin-treated C57BL/6 lungs (Supplemental Fig. S4D). Together, these data indicate that Neu3−/− mice show an attenuated inflammatory response at day 21 after bleomycin aspiration.
Fig. 4.
Immune cells remaining in the lungs after bronchoalveolar lavage (BAL). A: cryosections of day 21 lungs were stained with antibodies against CD45. Red is positive staining; blue is counterstain. Bar is 100 μm. Arrows indicate representative positive staining. Image is representative of n = 6 mice per group, except for bleomycin-treated C57BL/6, where n = 9. Bleo, bleomycin. B: cryosections of mouse lungs were stained for the indicated markers, and cells in 5 randomly chosen 45-μm diameter fields of view were counted, and the number was then calculated to number per mm2. Values are means ± SE, n = 6 except for bleomycin-treated C57BL/6, where n = 9. *P < 0.05; **P < 0.01; ***P < 0.001 (1-way ANOVA, Bonferroni’s test).
NEU3 upregulates the profibrotic cytokines IL-6 and IL-1β, and IL-6 upregulates NEU3 expression in human PBMCs.
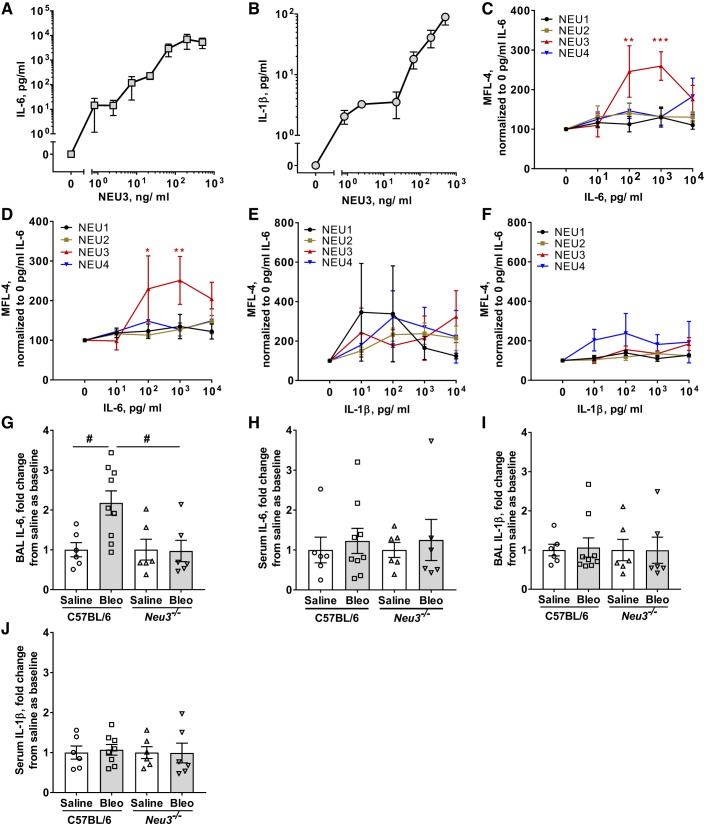
We previously observed significant upregulation of extracellular and intracellular active TGF-β1 from human peripheral blood mononuclear cells (PBMCs) when cultured with human recombinant NEU3 (29). To further investigate the role of NEU3 in upregulation of fibrotic cytokines, we cultured human PBMCs with recombinant human NEU3 for 48 h and then assayed the media supernatant by ELISA for the cytokines IL-1β, IL-4, IL-6, IL-10, IL-12, IL-13, and IFN-γ. We observed significant upregulation of extracellular accumulation of IL-6 and IL-1β with increasing concentrations of recombinant human NEU3 (Fig. 5, A and B). We did not observe any significant effect of NEU3 on extracellular accumulation of IL-10, IL-13, and IFN-γ (Supplemental Figs. S5, A–C). The levels of extracellular IL-4 and IL-12 were below the level of detection.
Fig. 5.
NEU3 upregulates IL-6 and IL-1β, IL-6 upregulates NEU3, and Neu3−/− mice have less bronchoalveolar lavage (BAL) IL-6 after bleomycin treatment. A and B: human peripheral blood mononuclear cells (PBMCs) were cultured with recombinant human NEU3 and culture supernatants were assayed by ELISA for IL-6 (A) and IL-1β (B). Values are means ± SE, n = 3. C and D: human monocytes (C) and human lymphocytes (D) were cultured in human recombinant IL-6 and cells were assayed for NEU 1–4 expression by flow cytometry. Values are means ± SE, n = 3. *P < 0.05; **P < 0.01; ***P < 0.001 (2-way ANOVA, Bonferroni’s test). E and F: human monocytes (E) and human lymphocytes (F) were cultured in human recombinant IL-1β and cells were assayed for NEU1–4 expression by flow cytometry. MFL, median fluorescence intensity. Values are means ± SE, n = 3. G and H: BAL (G) and serum (H) from the indicated mouse groups were collected at day 21 and assayed by ELISA for IL-6. Values are means ± SE, n = 6 except for bleomycin-treated C57BL/6, where n = 9. #P < 0.05 (t test). Bleo, bleomycin. I and J: BAL (I) and serum (J) from the indicated mouse groups were collected at day 21 and assayed by ELISA for IL-1β. Values are means ± SE, n = 6 except for bleomycin-treated C57BL/6, where n = 9. For G and I, IL-6 and IL-1β were measured as μg per mg of total BAL protein, and values were normalized to the C57BL/6 saline mean values (4.6 ± 0.8 μg IL-6/mg BAL protein and 1.8 ± 0.3 μg IL-1β/mg BAL protein). For H and J, values were measured as pg/ml of serum and were similarly normalized to the C57BL/6 saline mean values (8.1 ± 2.5 pg IL-6/mL serum and 2.8 ± 0.5 pg IL-1β/mL serum).
IL-6 upregulates NEU3 expression in carcinomas (42, 71). To determine the effects of IL-6 and IL-1β on NEU3 expression by human PBMCs, PBMCs were cultured in the presence or absence of recombinant human IL-6 and IL-1β. One hundred and 1,000 pg/mL of IL-6 significantly increased NEU3 expression in monocytes (Fig. 5C) and lymphocytes (Fig. 5D). IL-1β did not significantly affect expression of NEU 1–4 in monocytes (Fig. 5E) or lymphocytes (Fig. 5F). Together, these results suggest that NEU3 can upregulate profibrotic cytokines, such as TGF-β1 (29), IL-6, and IL-1β. Conversely, the ability of TGF-β1 (29) and IL-6 to upregulate NEU3 suggests that under some conditions, a positive feedback loop involving NEU3 and profibrotic cytokines might form.
Neu3−/− mice have an attenuated IL-6 increase in the BAL at day 21 after bleomycin treatment.
To further investigate the role of NEU3 in bleomycin-induced pulmonary fibrosis, we tested if Neu3−/− mice have altered levels of fibrosis-associated cytokines in the BAL and serum. In C57BL/6 mice, bleomycin increased levels of the cytokine IL-6 in the BAL but not the serum (Fig. 5, G and H). Saline-treated C57BL/6 and Neu3−/− mice had comparable levels of IL-6 in the BAL and serum, but bleomycin did not increase IL-6 in Neu3−/− BAL (Fig. 5, G and H). No significant changes were observed in the levels of BAL and serum IL-1β in saline and bleomycin-treated C57BL/6 and Neu3−/− mice (Fig. 5, I and J). As observed previously (32, 46, 72), for C57BL/6 at day 21, bleomycin did not significantly affect levels of IL-1α, IL-10, IL-12p70, IL-17A, IL-23, IL-27, monocyte chemoattractant protein 1 (MCP-1), TNF-α, granulocyte-macrophage colony-stimulating factor (GM-CSF), IFN-β, and IFN-γ in BAL or serum (Supplemental Fig. S5, G and H). With or without bleomycin, the loss of NEU3 did not affect the levels of these cytokines in BAL or serum (Supplemental Fig. S5, G and H). These data suggest that NEU3 is required for a bleomycin-induced increase in BAL IL-6 at day 21 in mice.
Neu3−/− mice show an attenuated upregulation of other sialidases at day 21 after bleomycin treatment.
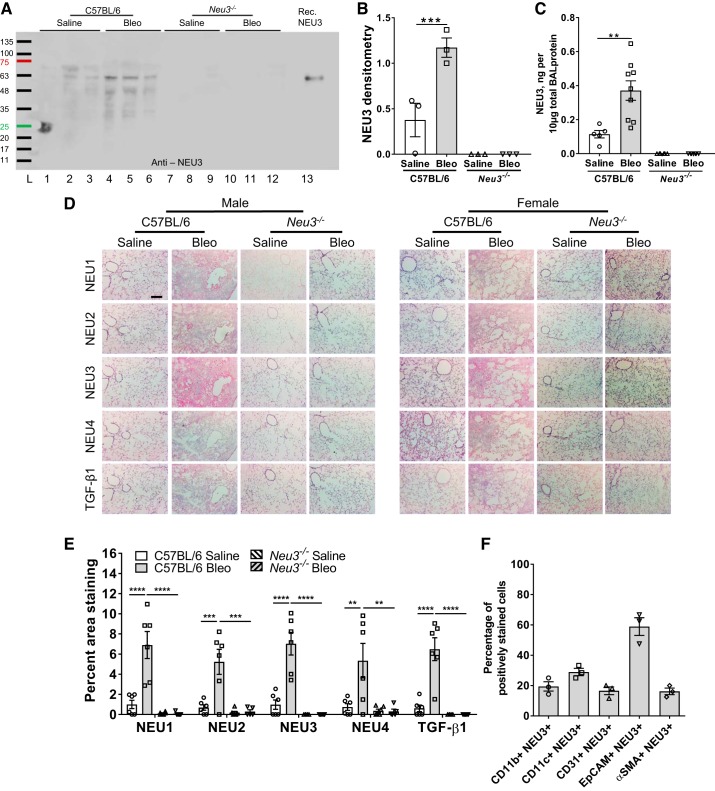
As observed previously (29), compared with the BAL from saline-treated C57BL/6 mice, upregulated levels of NEU3 were detected in the BAL from bleomycin-treated C57BL/6 mice (Fig. 6, A–C). No NEU3 was detected in saline or bleomycin-treated Neu3−/− mouse BAL when analyzed by Western blot staining or ELISA for NEU3 (Fig. 6, A–C). Mice and humans have three other sialidases (NEU1, NEU2, and NEU4) in addition to NEU3 (43). We previously observed that anti-NEU1, -NEU2, -NEU3, and -NEU4 antibodies are specific for the different sialidases (29). As observed previously (29), the sialidases NEU1, NEU2, and NEU4 were not detected in the BAL from saline or bleomycin-treated C57BL/6 mice (Supplemental Fig. S6, A–C), and these sialidases were also not detected in the BAL from saline-treated or bleomycin-treated Neu3−/− mice, even though some protein was present in these samples, as determined by Ponceau-stained blots (Supplemental Fig. S6, A–D).
Fig. 6.
Neu3−/− mice have fewer sialidases and transforming growth factor (TGF)-β1 in the lungs after bleomycin (Bleo) treatment. A: bronchoalveolar lavage (BAL) was collected at day 21 and BAL from 3 randomly chosen mice per group was analyzed by staining a Western blot with anti-NEU3 antibodies. From left, in lane no. 1–12, the sex of mice is M, F, F, M, M, F, M, F, M, M, M, F, with M, male and F, female. Recombinant human NEU3 (50 ng) was loaded in lane no. 13. Molecular mass markers in kDa are at left. B: densitometry of ~51 kDa NEU3 band, arbitrary units. ***P < 0.001 (1-way ANOVA, Bonferroni’s test). C: BAL from the indicated groups was assayed by direct ELISA for NEU3 protein. Values are means ± SE, n = 6 except for bleomycin-treated C57BL/6, where n = 9. D: cryosections of male and female mouse lungs at day 21 were stained with antibodies against NEU1, NEU2, NEU3, NEU4, and active TGF-β1. Bar is 200 μm. Images are representative of six mice per group. E: for each mouse, for each stain, 3 randomly chosen fields of view of the size shown in D were imaged, the percent area of the field of view showing staining was measured and the average was calculated. Values are means ± SE, n = 6 averages. **P < 0.01; ***P < 0.001; ****P < 0.0001 (1-way ANOVA, Bonferroni’s test). F: cryosections of bleomycin-treated C57BL/6 mice were costained with antibodies against the indicated cell markers and NEU3. The percentage of cells stained for the indicated marker that also stained for NEU3 was calculated. Values are means ± SE, n = 3.
Following BAL, lungs were sectioned and stained. As previously observed for C57BL/6 mice (15, 29, 37, 83), bleomycin increased staining for NEU1, NEU2, NEU3, and TGF-β1 in the lungs (Fig. 6, D and E). As we observed for fibrotic human lungs, we also observed that bleomycin increased staining for NEU4 in the C57BL/6 lungs (Fig. 6, D and E); this was due to bleomycin increasing staining for NEU4 in female but not male lungs (Supplemental Fig. S6, E and F). Furthermore, as previously observed for C57BL/6 mice (40), females have smaller alveoli than males, and this appears to also be the case for Neu3−/− mice. (Fig. 6D).
Compared with saline-treated C57BL/6 mice, saline-treated Neu3−/− mice had similar amounts of staining for NEU1, NEU2, NEU3, NEU4, and TGF-β1 in the lungs, and in Neu3−/− mice, bleomycin did not significantly affect staining for the above markers (Fig. 6, D and E). The positive staining for NEU3 was primarily observed in the fibrotic regions of bleomycin-treated C57BL/6 lungs (Supplemental Fig. S7A). The lung sections from bleomycin-treated C57BL/6 mice were costained with antibodies against different cellular markers and antibodies against NEU3. In agreement with previous reports (29, 36), where NEU3 is primarily expressed by epithelial cells, we observed NEU3 expressed in CD11b-positive monocyte-derived macrophages, CD11c-positive alveolar macrophages, CD31-positive cells, EpCAM-positive epithelial cells, and αSMA-positive fibroblasts, with a high percentage of EpCAM-positive epithelial cells expressing NEU3 (Fig. 6F and Supplemental Fig. S7B).
Western blots of lung tissue lysates were stained for NEU1, NEU2, NEU3, NEU4, and TGF-β1 (Supplemental Fig. S8, A–E). The relative protein levels were normalized to total protein from Coomassie gels (Supplemental Fig. S8F). Compared with saline-treated C57BL/6 lysates, bleomycin-treated C57BL/6 lysates had upregulated levels of NEU1 (Supplemental Fig. S8, A and G), NEU2 (Supplemental Fig. S8, B and H), NEU3 (Supplemental Fig. S8, C and I), NEU4 (Supplemental Fig. S8, D and J), and active TGF-β1 (Supplemental Fig. S8, E and K). Compared with saline-treated C57BL/6 mice, saline-treated Neu3−/− mice had normal levels of NEU2 and active TGF-β1 and less NEU1, NEU3, and NEU4 in lung lysates (Supplemental Fig. S8, A–K). Bleomycin did not upregulate NEU2, NEU3, or active TGF-β1 but did upregulate NEU1 and NEU4 in Neu3−/− lysates, albeit to a lesser level than in C57BL/6 lysates (Supplemental Fig. S8, A–K). Together, these results suggest that NEU3 protein is not detected in Neu3−/− mouse lungs and that loss of NEU3 reduces bleomycin-induced increases in the levels of NEU1, NEU2, NEU4, and active TGF-β1 in the lungs.
Neu3−/− mice have decreased fibrosis at day 21 after bleomycin treatment.
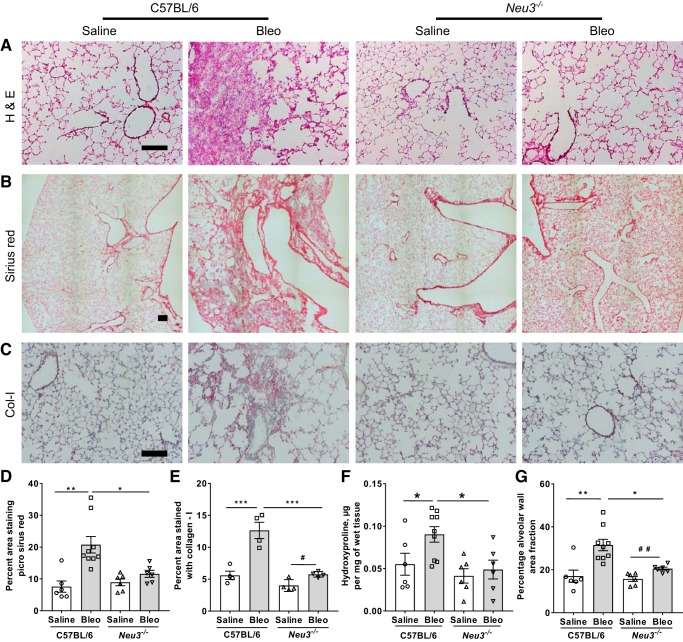
To determine if loss of NEU3 affects the fibrotic response to bleomycin, lung sections were stained with hematoxylin and eosin to detect tissue, antibodies to detect collagen-I, and picrosirius red to detect total collagen (which was also imaged using polarized light microscopy for qualitative analysis), and hydroxyproline levels were measured in tissue hydrolysates. As previously observed for C57BL/6 mice (1, 23, 47, 81), bleomycin caused fibrosis in the mouse lungs (Fig. 7, A–F). Bleomycin did not significantly affect Sirius red staining for collagen or hydroxyproline levels but increased collagen-I staining and increased alveolar wall area fraction in Neu3−/− mouse lungs, but the increase was less than in bleomycin-treated C57BL/6 mice (Fig. 7, D–F; Supplemental Fig. S9A). Similar results were observed for male and female mice (Supplemental Fig. S9, B–G) with the exception that bleomycin-treated Neu3−/− female mice had significantly upregulated Sirius red staining compared with saline-treated Neu3−/− female mice, whereas this was not observed for male mice (Supplemental Fig. S9, B and C). Conversely, bleomycin-treated Neu3−/− male mice had significantly upregulated alveolar wall area fraction compared with saline-treated Neu3−/− male mice, whereas this effect did not reach significance for female mice (Supplemental Fig. S9, F and G). Polarized light microscopy of lung sections stained with Sirius red showed qualitative upregulation of collagen in bleomycin-treated C57BL/6 mice compared with saline-treated C57BL/6 mice or saline and bleomycin-treated Neu3−/− mice (Supplemental Fig. S9H). These results suggest that loss of NEU3 significantly decreases the fibrotic response to bleomycin in mouse lungs.
Fig. 7.
Neu3−/− mice have less fibrosis in the lungs after bleomycin treatment. A–C: day 21 lung cryosections were stained with H&E (A), picrosirius red to show collagen content (B), and antibodies against collagen-I (Col-I) (C). Bars are 200 μm. For A and B, images are representative of n = 6 mice per group except for bleomycin-treated C57BL/6 (Bleo), where n = 9. For C, images are representative of 4 randomly chosen (2 male and 2 female) mice per group. For picrosirius red quantification (D) and collagen-I quantification (E), 5 randomly chosen fields of view of the sizes shown in B and C were imaged for each mouse, and the average percent area of the field of view showing staining for each mouse was measured. F: estimation of hydroxyproline from saline or bleomycin-treated C57BL/6 wild-type and Neu3−/− mice lungs. G: percentage of alveolar wall area fraction was determined by the ratio of area of alveolar walls to the total image area. For D, F, and G, values are means ± SE, n = 6 except for bleomycin-treated C57BL/6, where n = 9, and for E, values are means ± SE, n = 4. *P < 0.05; **P < 0.01; ***P < 0.001 (1-way ANOVA, Bonferroni’s test); #P < 0.05; ##P < 0.01 (t test).
DISCUSSION
In this report, we observed that loss of the sialidase NEU3 in male and female mice attenuated bleomycin-induced weight loss, upregulation of protein levels in the BAL, inflammation, TGF-β1 upregulation, and fibrosis, and that the loss of NEU3 did not appear to be compensated by the upregulation of other sialidases. These results suggest that NEU3 may have a major role in bleomycin-induced pulmonary fibrosis in mice.
We observed attenuated total inflammatory cell counts but no significant differences in the percentages of the different cell types in the BAL of bleomycin-treated Neu3−/− compared with bleomycin-treated C57BL/6 mice, suggesting that attenuation of bleomycin-induced pulmonary fibrosis in the Neu3−/− mice is not dependent on the ratios of the different cell types in the BAL.
Changes in cytokine levels are associated with, and appear to drive, fibrosis (73). We observed that NEU3 upregulates extracellular accumulation of IL-6 by human PBMCs, which is in agreement with previous reports where the addition of NEU3 to cells upregulates IL-6 (42, 71). IL-6 potentiates fibrosis (16) and is actively involved in epithelial-to-mesenchymal transition, which is associated with fibrosis (27, 70). We observed bleomycin-induced upregulation of IL-6 in the BAL fluid of C57BL/6 mice but not Neu3−/− mice, suggesting that NEU3 may be an important driver of the upregulation of IL-6 levels in fibrosis. Conversely, we observed upregulation of NEU3 in human PBMCs by IL-6, which is in agreement with a previous report where IL-6 increases NEU3 expression in renal cell carcinoma cells (76). Together, these results suggest that NEU3-mediated upregulation of IL-6 and IL-6-mediated upregulation of NEU3 might form a NEU3-IL-6-NEU3-positive feedback loop that potentiates fibrosis.
IL-1β is also associated with inflammatory and fibrotic responses (5). In the presence of serum, IL-1β upregulates NEU1 but not NEU3 in human neutrophils (82) and in monocytes/macrophages in a positive feedback loop (68). In the absence of serum, we observed that NEU3 upregulates IL-1β, but IL-1β does not upregulate NEU3 in human PBMCs. Together, these results suggest that as with IL6, NEU3 may form a positive feedback loop with IL-1β and that something in serum is necessary for IL-1β upregulation of NEU3.
We previously reported upregulation of NEU1, NEU2, and NEU3 but not NEU4 in fibrotic lesions of lung tissue from male mice at day 21 after bleomycin aspiration (29). A recent report found that NEU1 is downregulated in IPF, Neu1−/− fibroblasts have characteristics of myofibroblasts, and Neu1−/− fibroblasts release the profibrotic cytokine TGF-β1, suggesting that NEU1 does not potentiate fibrosis (77). Here, we observed upregulation of NEU4 in lung tissue from C57BL/6 female mice but not male mice at day 21 after bleomycin. Bleomycin has different effects on male versus female mice (19, 79), and the upregulation of NEU4 appears to be an additional sex-linked differential effect of bleomycin in mice. The lack of NEU4 upregulation in fibrosis in male mice suggests that NEU4 upregulation is not a key driver of fibrosis. The observation that mice lacking NEU3 do not upregulate NEU2, and the above observations about NEU1 and NEU4, suggest that NEU2 and/or NEU3 may be responsible for the extensive desialylation that occurs in the lungs in response to bleomycin (29).
The Ensembl database ENSG00000162139 for human (87) shows that for NEU3 there are seven different protein coding splice variants. Similarly, for mouse, the National Center for Biotechnology Information gene ID 50877 shows at least three NEU3 protein coding transcripts. In agreement with the concept of different NEU3 splice variant transcripts, at least five variants of NEU3 transcripts have been observed in zebrafish (38). Transcriptomic data show that there are at least nine different splice variants for NEU3 from human IPF patient lungs (49). There are multiple NEU3 proteins in human cell lines (65). Other workers also found multiple NEU3 variant proteins in human cells (11, 36, 37), and, looking at 10 different human cell lines, we also observed multiple variants, with different variants expressed by different cell types (29). In the same publication, we observed that TGF-β1 causes upregulation of NEU3 in PBMCs, airway epithelial cells, A549 cells, and lung fibroblasts. We observed NEU3 expressed in multiple cell types in fibrotic mouse lungs. Together, this suggests the possibility that in bleomycin-induced fibrosis, multiple cell types express multiple NEU3 variants, leading to the multiple NEU3 bands we observed in Fig. 6A and Supplemental Fig. S8C. In Fig. 6A and Supplemental Fig. S8C, we observed that several of these bands were upregulated by bleomycin treatment. None of these bands were observed in the BAL or lung tissue from saline-treated or bleomycin-treated NEU3 knockout mice, indicating that the multiple NEU3 bands observed in the WT mice are bona fide NEU3 proteins.
There are upregulated levels of the mRNA encoding NEU1 in the lung fibroblasts from some pulmonary fibrosis patients (37). Single-cell transcriptomic analysis also showed upregulated levels of NEU1 transcripts in lung cells from pulmonary fibrosis patients (63). A different study, however, found no significant increases in the mRNAs encoding NEU1–4 in IPF patients compared with healthy individuals (49). However, we consistently found elevated levels of NEU3 protein in the fibrotic lesions in human and mouse lungs (29). We also found that the profibrotic cytokine TGF-β1 (15, 83) causes a variety of cell types to upregulate NEU3 (29). Since TGF-β1 regulates the levels of some proteins by a post-transcriptional mechanism (9), we previously hypothesized that the increased levels of NEU3 observed in pulmonary fibrosis may be due to a post-transcriptional mechanism (29). The observation that the antibody we used for the previous work does not detect proteins in mice lacking NEU3 reinforces the validity of this assay and, in combination with the NEU3 transcriptomic analysis, suggests that in fibrosis there may be post-transcriptional regulation of NEU3 levels.
Active TGF-β1 is a key driver of fibrosis (15, 83). We previously discussed a possible positive feedback loop involving NEU3-TGF-β1-NEU3 in the progression of pulmonary fibrosis (29). In this report, we observed upregulated levels of active TGF-β1 in lung tissues of bleomycin-treated C57BL/6 mice but not in the lung tissues of bleomycin-treated Neu3−/− mice. TGF-β1 is sequestered in an inactive state by latency-associated peptide (LAP) in a complex called latent TGF-β1 protein (LTGF-β1) (22). LTGF-β1 has three glycosylated asparagine residues at position 82, 136, and 176, all in the LAP sequence (22, 75). Bacterial and viral sialidases can desialylate LAP, causing a conformational change that releases active TGF-β1, and this is thought to explain the elevated TGF-β1 levels observed during some infections (4, 8, 21, 44, 66). In bleomycin-treated Neu3−/− mice, we did not observe upregulated levels of active TGF-β1 (~25 kDa). On Western blots of saline-treated or bleomycin-treated Neu3−/− mouse lung tissue electrophoresed on nonreducing/no SDS gels [which preserve the LAP/TGF-β1 complex (44, 45)], we observed anti-TGF-β1 antibodies staining a ~60 kDa band, which is the previously reported size of the LAP/TGF-β1 complex (Supplemental Fig. S8E). These results suggest that NEU3 may be involved in releasing active TGF-β1 from LTGF-β1 in bleomycin-induced pulmonary fibrosis.
The loss of NEU3 attenuates bleomycin-induced increases in protein and albumin in BAL fluid, which may well be due to vascular leakage. IL-6 causes vascular leakage (2, 13, 14). The loss of NEU3 also attenuates bleomycin-induced inflammation, and IL-6 can cause inflammation (18, 52, 64). NEU3 upregulates IL-6, and loss of NEU3 inhibits bleomycin-induced IL6 upregulation in the BAL. Thus, one possible explanation for the reduced inflammation in, and reduced levels of protein and albumin in the BAL of, bleomycin-treated Neu3−/− cells is that in a wild-type lung, the damage caused by bleomycin causes an upregulation of NEU3, the upregulated NEU3 upregulates IL-6, and the IL-6 induces inflammation and vascular leakage. In the absence of NEU3, the upregulation of IL-6, inflammation, and leakage then do not occur.
We previously observed that TGF-β1 upregulates NEU3 and that NEU3 upregulates TGF-β1, which suggested that a NEU3-TGF-β1-NEU3 positive feedback loop potentiates fibrosis (29). In this report, we observed that IL-6 upregulates NEU3 and that NEU3 upregulates IL-6 and IL-1β. These results suggest that NEU3 participates in at least two positive feedback loops, one with TGF-β1 driving fibrosis and the other with IL-6 driving vascular leakage, inflammation, and fibrosis. Thus, the extent to which NEU3-dependent bleomycin-induced vascular leakage and inflammation contribute to NEU3-dependent bleomycin-induced fibrosis is unclear.
Given that we previously observed increased levels of NEU3 in the fibrotic lesions in the lungs of patients with IPF and that the sialidase inhibitors DANA and oseltamivir attenuate bleomycin-induced pulmonary fibrosis in mice, our results suggest that NEU3 may be a potential target for the development of therapeutics for pulmonary fibrosis.
GRANTS
This work was supported by NIH Grant HL-132919.
DISCLOSURES
T. R. Karhadkar and R. H. Gomer are coinventors on patent applications for sialidase inhibitors used as antifibrotics. W. Chen does not have any conflicts of interest, financial or otherwise, to disclose.
AUTHOR CONTRIBUTIONS
T.R.K. and R.H.G. conceived and designed research; T.R.K. and W.C. performed experiments; T.R.K. and R.H.G. analyzed data; T.R.K. and R.H.G. interpreted results of experiments; T.R.K. prepared figures; T.R.K. and R.H.G. drafted manuscript; T.R.K. and R.H.G. edited and revised manuscript; T.R.K. and R.H.G. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Jamey Marth and Douglas Heithoff for sending mice, Darrell Pilling for assistance with BAL sample collection, and the Laboratory Animal Resources and Research staff at Texas A&M for animal care. We deeply thank Dr. Takeo Miyagi and Dr. Kazunori Yamaguchi for the original generation of the Neu3−/− mice.
REFERENCES
- 1.Adamson IY, Bowden DH. The pathogenesis of bleomycin-induced pulmonary fibrosis in mice. Am J Pathol 77: 185–197, 1974. [PMC free article] [PubMed] [Google Scholar]
- 2.Alsaffar H, Martino N, Garrett JP, Adam AP. Interleukin-6 promotes a sustained loss of endothelial barrier function via Janus kinase-mediated STAT3 phosphorylation and de novo protein synthesis. Am J Physiol Cell Physiol 314: C589–C602, 2018. doi: 10.1152/ajpcell.00235.2017. [DOI] [PubMed] [Google Scholar]
- 3.Arndt C, Koristka S, Bartsch H, Bachmann M. Native polyacrylamide gels. Methods Mol Biol 869: 49–53, 2012. doi: 10.1007/978-1-61779-821-4_5. [DOI] [PubMed] [Google Scholar]
- 4.Biernacka A, Dobaczewski M, Frangogiannis NG. TGF-β signaling in fibrosis. Growth Factors 29: 196–202, 2011. doi: 10.3109/08977194.2011.595714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borthwick LA. The IL-1 cytokine family and its role in inflammation and fibrosis in the lung. Semin Immunopathol 38: 517–534, 2016. doi: 10.1007/s00281-016-0559-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bucala R. Review series–inflammation & fibrosis. fibrocytes and fibrosis. QJM 105: 505–508, 2012. doi: 10.1093/qjmed/hcs068. [DOI] [PubMed] [Google Scholar]
- 7.Bucala R, Spiegel LA, Chesney J, Hogan M, Cerami A. Circulating fibrocytes define a new leukocyte subpopulation that mediates tissue repair. Mol Med 1: 71–81, 1994. doi: 10.1007/BF03403533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carlson CM, Turpin EA, Moser LA, O’Brien KB, Cline TD, Jones JC, Tumpey TM, Katz JM, Kelley LA, Gauldie J, Schultz-Cherry S. Transforming growth factor-β: activation by neuraminidase and role in highly pathogenic H5N1 influenza pathogenesis. PLoS Pathog 6: e1001136, 2010. doi: 10.1371/journal.ppat.1001136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chaudhury A, Hussey GS, Ray PS, Jin G, Fox PL, Howe PH. TGF-beta-mediated phosphorylation of hnRNP E1 induces EMT via transcript-selective translational induction of Dab2 and ILEI. Nat Cell Biol 12: 286–293, 2010. doi: 10.1038/ncb2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cox N, Pilling D, Gomer RH. DC-SIGN activation mediates the differential effects of SAP and CRP on the innate immune system and inhibits fibrosis in mice. Proc Natl Acad Sci USA 112: 8385–8390, 2015. doi: 10.1073/pnas.1500956112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cross AS, Hyun SW, Miranda-Ribera A, Feng C, Liu A, Nguyen C, Zhang L, Luzina IG, Atamas SP, Twaddell WS, Guang W, Lillehoj EP, Puché AC, Huang W, Wang LX, Passaniti A, Goldblum SE. NEU1 and NEU3 sialidase activity expressed in human lung microvascular endothelia: NEU1 restrains endothelial cell migration, whereas NEU3 does not. J Biol Chem 287: 15966–15980, 2012. doi: 10.1074/jbc.M112.346817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Daubeuf F, Frossard N. Performing bronchoalveolar lavage in the mouse. Curr Protoc Mouse Biol 2: 167–175, 2012. doi: 10.1002/9780470942390.mo110201. [DOI] [PubMed] [Google Scholar]
- 13.Desai TR, Leeper NJ, Hynes KL, Gewertz BL. Interleukin-6 causes endothelial barrier dysfunction via the protein kinase C pathway. J Surg Res 104: 118–123, 2002. doi: 10.1006/jsre.2002.6415. [DOI] [PubMed] [Google Scholar]
- 14.Didion SP. Cellular and oxidative mechanisms associated with interleukin-6 signaling in the vasculature. Int J Mol Sci 18: 2563, 2017. doi: 10.3390/ijms18122563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fernandez IE, Eickelberg O. The impact of TGF-β on lung fibrosis: from targeting to biomarkers. Proc Am Thorac Soc 9: 111–116, 2012. doi: 10.1513/pats.201203-023AW. [DOI] [PubMed] [Google Scholar]
- 16.Fielding CA, Jones GW, McLoughlin RM, McLeod L, Hammond VJ, Uceda J, Williams AS, Lambie M, Foster TL, Liao CT, Rice CM, Greenhill CJ, Colmont CS, Hams E, Coles B, Kift-Morgan A, Newton Z, Craig KJ, Williams JD, Williams GT, Davies SJ, Humphreys IR, O’Donnell VB, Taylor PR, Jenkins BJ, Topley N, Jones SA. Interleukin-6 signaling drives fibrosis in unresolved inflammation. Immunity 40: 40–50, 2014. doi: 10.1016/j.immuni.2013.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gabay C. Interleukin-6 and chronic inflammation. Arthritis Res Ther 8, Suppl 2: S3, 2006. doi: 10.1186/ar1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gharaee-Kermani M, Hatano K, Nozaki Y, Phan SH. Gender-based differences in bleomycin-induced pulmonary fibrosis. Am J Pathol 166: 1593–1606, 2005. doi: 10.1016/S0002-9440(10)62470-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gilhodes J-C, Julé Y, Kreuz S, Stierstorfer B, Stiller D, Wollin L. Quantification of pulmonary fibrosis in a bleomycin mouse model using automated histological image analysis. PLoS One 12: e0170561, 2017. doi: 10.1371/journal.pone.0170561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gratz N, Loh LN, Mann B, Gao G, Carter R, Rosch J, Tuomanen EI. Pneumococcal neuraminidase activates TGF-β signalling. Microbiology 163: 1198–1207, 2017. doi: 10.1099/mic.0.000511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hinck AP, Mueller TD, Springer TA. Structural biology and evolution of the TGF-β family. Cold Spring Harb Perspect Biol 8: a022103, 2016. doi: 10.1101/cshperspect.a022103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Izbicki G, Or R, Christensen TG, Segel MJ, Fine A, Goldstein RH, Breuer R. Bleomycin-induced lung fibrosis in IL-4-overexpressing and knockout mice. Am J Physiol Lung Cell Mol Physiol 283: L1110–L1116, 2002. doi: 10.1152/ajplung.00107.2002. [DOI] [PubMed] [Google Scholar]
- 25.Izbicki G, Segel MJ, Christensen TG, Conner MW, Breuer R. Time course of bleomycin-induced lung fibrosis. Int J Exp Pathol 83: 111–119, 2002. doi: 10.1046/j.1365-2613.2002.00220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ji WJ, Ma YQ, Zhou X, Zhang YD, Lu RY, Guo ZZ, Sun HY, Hu DC, Yang GH, Li YM, Wei LQ. Spironolactone attenuates bleomycin-induced pulmonary injury partially via modulating mononuclear phagocyte phenotype switching in circulating and alveolar compartments. PLoS One 8: e81090, 2013. doi: 10.1371/journal.pone.0081090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jiang GX, Cao LP, Kang PC, Zhong XY, Lin TY, Cui YF. Interleukin-6 induces epithelial-mesenchymal transition in human intrahepatic biliary epithelial cells. Mol Med Rep 13: 1563–1569, 2016. doi: 10.3892/mmr.2015.4706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kakugawa Y, Wada T, Yamaguchi K, Yamanami H, Ouchi K, Sato I, Miyagi T. Up-regulation of plasma membrane-associated ganglioside sialidase (Neu3) in human colon cancer and its involvement in apoptosis suppression. Proc Natl Acad Sci USA 99: 10718–10723, 2002. doi: 10.1073/pnas.152597199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karhadkar TR, Pilling D, Cox N, Gomer RH. Sialidase inhibitors attenuate pulmonary fibrosis in a mouse model. Sci Rep 7: 15069, 2017. doi: 10.1038/s41598-017-15198-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kato K, Shiga K, Yamaguchi K, Hata K, Kobayashi T, Miyazaki K, Saijo S, Miyagi T. Plasma-membrane-associated sialidase (NEU3) differentially regulates integrin-mediated cell proliferation through laminin- and fibronectin-derived signalling. Biochem J 394: 647–656, 2006. doi: 10.1042/BJ20050737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kawamura S, Sato I, Wada T, Yamaguchi K, Li Y, Li D, Zhao X, Ueno S, Aoki H, Tochigi T, Kuwahara M, Kitamura T, Takahashi K, Moriya S, Miyagi T. Plasma membrane-associated sialidase (NEU3) regulates progression of prostate cancer to androgen-independent growth through modulation of androgen receptor signaling. Cell Death Differ 19: 170–179, 2012. doi: 10.1038/cdd.2011.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim MS, Kim SH, Jeon D, Kim HY, Lee K. Changes in expression of cytokines in polyhexamethylene guanidine-induced lung fibrosis in mice: comparison of bleomycin-induced lung fibrosis. Toxicology 393: 185–192, 2018. doi: 10.1016/j.tox.2017.11.017. [DOI] [PubMed] [Google Scholar]
- 33.Kulkarni AA, Thatcher TH, Hsiao H-M, Olsen KC, Kottmann RM, Morrissette J, Wright TW, Phipps RP, Sime PJ. The triterpenoid CDDO-Me inhibits bleomycin-induced lung inflammation and fibrosis. PLoS One 8: e63798, 2013. doi: 10.1371/journal.pone.0063798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lambré CR, Pilatte Y, Le Maho S, Greffard A, De Crémoux H, Bignon J. Sialidase activity and antibodies to sialidase-treated autologous erythrocytes in bronchoalveolar lavages from patients with idiopathic pulmonary fibrosis or sarcoidosis. Clin Exp Immunol 73: 230–235, 1988. [PMC free article] [PubMed] [Google Scholar]
- 35.Li SC, Li YT, Moriya S, Miyagi T. Degradation of G(M1) and G(M2) by mammalian sialidases. Biochem J 360: 233–237, 2001. doi: 10.1042/bj3600233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lillehoj EP, Hyun SW, Feng C, Zhang L, Liu A, Guang W, Nguyen C, Sun W, Luzina IG, Webb TJ, Atamas SP, Passaniti A, Twaddell WS, Puché AC, Wang LX, Cross AS, Goldblum SE. Human airway epithelia express catalytically active NEU3 sialidase. Am J Physiol Lung Cell Mol Physiol 306: L876–L886, 2014. doi: 10.1152/ajplung.00322.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Luzina IG, Lockatell V, Hyun SW, Kopach P, Kang PH, Noor Z, Liu A, Lillehoj EP, Lee C, Miranda-Ribera A, Todd NW, Goldblum SE, Atamas SP. Elevated expression of NEU1 sialidase in idiopathic pulmonary fibrosis provokes pulmonary collagen deposition, lymphocytosis, and fibrosis. Am J Physiol Lung Cell Mol Physiol 310: L940–L954, 2016. doi: 10.1152/ajplung.00346.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Manzoni M, Colombi P, Papini N, Rubaga L, Tiso N, Preti A, Venerando B, Tettamanti G, Bresciani R, Argenton F, Borsani G, Monti E. Molecular cloning and biochemical characterization of sialidases from zebrafish (Danio rerio). Biochem J 408: 395–406, 2007. doi: 10.1042/BJ20070627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Martinez FJ, Collard HR, Pardo A, Raghu G, Richeldi L, Selman M, Swigris JJ, Taniguchi H, Wells AU. Idiopathic pulmonary fibrosis. Nat Rev Dis Primers 3: 17074, 2017. doi: 10.1038/nrdp.2017.74. [DOI] [PubMed] [Google Scholar]
- 40.Massaro GD, Mortola JP, Massaro D. Sexual dimorphism in the architecture of the lung’s gas-exchange region. Proc Natl Acad Sci USA 92: 1105–1107, 1995. doi: 10.1073/pnas.92.4.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miyagi T, Takahashi K, Hata K, Shiozaki K, Yamaguchi K. Sialidase significance for cancer progression. Glycoconj J 29: 567–577, 2012. doi: 10.1007/s10719-012-9394-1. [DOI] [PubMed] [Google Scholar]
- 42.Miyagi T, Wada T, Yamaguchi K. Roles of plasma membrane-associated sialidase NEU3 in human cancers [BBA]. Biochim Biophys Acta 1780: 532–537, 2008. doi: 10.1016/j.bbagen.2007.09.016. [DOI] [PubMed] [Google Scholar]
- 43.Miyagi T, Yamaguchi K. Mammalian sialidases: physiological and pathological roles in cellular functions. Glycobiology 22: 880–896, 2012. doi: 10.1093/glycob/cws057. [DOI] [PubMed] [Google Scholar]
- 44.Miyazono K, Heldin CH. Role for carbohydrate structures in TGF-beta 1 latency. Nature 338: 158–160, 1989. doi: 10.1038/338158a0. [DOI] [PubMed] [Google Scholar]
- 45.Miyazono K, Olofsson A, Colosetti P, Heldin CH. A role of the latent TGF-beta 1-binding protein in the assembly and secretion of TGF-beta 1. EMBO J 10: 1091–1101, 1991. doi: 10.1002/j.1460-2075.1991.tb08049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Monnier J, Zabel BA. Anti-asialo GM1 NK cell depleting antibody does not alter the development of bleomycin induced pulmonary fibrosis. PLoS One 9: e99350, 2014. doi: 10.1371/journal.pone.0099350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moore BB, Lawson WE, Oury TD, Sisson TH, Raghavendran K, Hogaboam CM. Animal models of fibrotic lung disease. Am J Respir Cell Mol Biol 49: 167–179, 2013. doi: 10.1165/rcmb.2013-0094TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Murray LA, Rosada R, Moreira AP, Joshi A, Kramer MS, Hesson DP, Argentieri RL, Mathai S, Gulati M, Herzog EL, Hogaboam CM. Serum amyloid P therapeutically attenuates murine bleomycin-induced pulmonary fibrosis via its effects on macrophages. PLoS One 5: e9683, 2010. doi: 10.1371/journal.pone.0009683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nance T, Smith KS, Anaya V, Richardson R, Ho L, Pala M, Mostafavi S, Battle A, Feghali-Bostwick C, Rosen G, Montgomery SB. Transcriptome analysis reveals differential splicing events in IPF lung tissue. PLoS One 9: e92111, 2014. [Erratum in PLoS ONE 9: e97550, 2014]. doi: 10.1371/journal.pone.0092111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nomura H, Tamada Y, Miyagi T, Suzuki A, Taira M, Suzuki N, Susumu N, Irimura T, Aoki D. Expression of NEU3 (plasma membrane-associated sialidase) in clear cell adenocarcinoma of the ovary: its relationship with T factor of pTNM classification. Oncol Res 16: 289–297, 2006. doi: 10.3727/000000006783981035. [DOI] [PubMed] [Google Scholar]
- 51.Parker JC, Townsley MI. Evaluation of lung injury in rats and mice. Am J Physiol Lung Cell Mol Physiol 286: L231–L246, 2004. doi: 10.1152/ajplung.00049.2003. [DOI] [PubMed] [Google Scholar]
- 52.Pedroza M, Schneider DJ, Karmouty-Quintana H, Coote J, Shaw S, Corrigan R, Molina JG, Alcorn JL, Galas D, Gelinas R, Blackburn MR. Interleukin-6 contributes to inflammation and remodeling in a model of adenosine mediated lung injury. PLoS One 6: e22667, 2011. doi: 10.1371/journal.pone.0022667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pilling D, Buckley CD, Salmon M, Gomer RH. Inhibition of fibrocyte differentiation by serum amyloid P. J Immunol 171: 5537–5546, 2003. doi: 10.4049/jimmunol.171.10.5537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pilling D, Fan T, Huang D, Kaul B, Gomer RH. Identification of markers that distinguish monocyte-derived fibrocytes from monocytes, macrophages, and fibroblasts. PLoS One 4: e7475, 2009. doi: 10.1371/journal.pone.0007475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pilling D, Gomer RH. The development of serum amyloid P as a possible therapeutic. Front Immunol 9: 2328, 2018. doi: 10.3389/fimmu.2018.02328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pilling D, Gomer RH. Differentiation of circulating monocytes into fibroblast-like cells. Methods Mol Biol 904: 191–206, 2012. doi: 10.1007/978-1-61779-943-3_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pilling D, Gomer RH. Persistent lung inflammation and fibrosis in serum amyloid P component (APCs−/−) knockout mice. PLoS One 9: e93730, 2014. doi: 10.1371/journal.pone.0093730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pilling D, Roife D, Wang M, Ronkainen SD, Crawford JR, Travis EL, Gomer RH. Reduction of bleomycin-induced pulmonary fibrosis by serum amyloid P. J Immunol 179: 4035–4044, 2007. doi: 10.4049/jimmunol.179.6.4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pilling D, Vakil V, Gomer RH. Improved serum-free culture conditions for the differentiation of human and murine fibrocytes. J Immunol Methods 351: 62–70, 2009. doi: 10.1016/j.jim.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pilling D, Zheng Z, Vakil V, Gomer RH. Fibroblasts secrete Slit2 to inhibit fibrocyte differentiation and fibrosis. Proc Natl Acad Sci USA 111: 18291–18296, 2014. doi: 10.1073/pnas.1417426112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pshezhetsky AV, Ashmarina LI. Desialylation of surface receptors as a new dimension in cell signaling. Biochemistry (Mosc) 78: 736–745, 2013. doi: 10.1134/S0006297913070067. [DOI] [PubMed] [Google Scholar]
- 62.Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, Colby TV, Cordier JF, Flaherty KR, Lasky JA, Lynch DA, Ryu JH, Swigris JJ, Wells AU, Ancochea J, Bouros D, Carvalho C, Costabel U, Ebina M, Hansell DM, Johkoh T, Kim DS, King TE Jr, Kondoh Y, Myers J, Müller NL, Nicholson AG, Richeldi L, Selman M, Dudden RF, Griss BS, Protzko SL, Schünemann HJ; ATS/ERS/JRS/ALAT Committee on Idiopathic Pulmonary Fibrosis . An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med 183: 788–824, 2011. doi: 10.1164/rccm.2009-040GL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Reyfman PA, Walter JM, Joshi N, Anekalla KR, McQuattie-Pimentel AC, Chiu S, Fernandez R, Akbarpour M, Chen CI, Ren Z, Verma R, Abdala-Valencia H, Nam K, Chi M, Han S, Gonzalez-Gonzalez FJ, Soberanes S, Watanabe S, Williams KJN, Flozak AS, Nicholson TT, Morgan VK, Winter DR, Hinchcliff M, Hrusch CL, Guzy RD, Bonham CA, Sperling AI, Bag R, Hamanaka RB, Mutlu GM, Yeldandi AV, Marshall SA, Shilatifard A, Amaral LAN, Perlman H, Sznajder JI, Argento AC, Gillespie CT, Dematte J, Jain M, Singer BD, Ridge KM, Lam AP, Bharat A, Bhorade SM, Gottardi CJ, Budinger GRS, Misharin AV. Single-cell transcriptomic analysis of human lung provides insights into the pathobiology of pulmonary fibrosis. Am J Respir Crit Care Med, 199: 1517–1536, 2019. doi: 10.1164/rccm.201712-2410OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rincon M. Interleukin-6: from an inflammatory marker to a target for inflammatory diseases. Trends Immunol 33: 571–577, 2012. doi: 10.1016/j.it.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 65.Rodriguez-Walker M, Daniotti JL. Human sialidase Neu3 is S-acylated and behaves like an integral membrane protein. Sci Rep 7: 4167, 2017. doi: 10.1038/s41598-017-04488-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schultz-Cherry S, Hinshaw VS. Influenza virus neuraminidase activates latent transforming growth factor beta. J Virol 70: 8624–8629, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schwab I, Nimmerjahn F. Intravenous immunoglobulin therapy: how does IgG modulate the immune system? Nat Rev Immunol 13: 176–189, 2013. doi: 10.1038/nri3401. [DOI] [PubMed] [Google Scholar]
- 68.Sieve I, Ricke-Hoch M, Kasten M, Battmer K, Stapel B, Falk CS, Leisegang MS, Haverich A, Scherr M, Hilfiker-Kleiner D. A positive feedback loop between IL-1β, LPS and NEU1 may promote atherosclerosis by enhancing a pro-inflammatory state in monocytes and macrophages. Vascul Pharmacol 103-105: 16–28, 2018. doi: 10.1016/j.vph.2018.01.005. [DOI] [PubMed] [Google Scholar]
- 69.Strauss WM. Preparation of genomic DNA from mammalian tissue. Curr Protoc Mol Biol 42: 2.2.1–2.2.3, 2001. doi: 10.1002/0471142727.mb0202s42. [DOI] [PubMed] [Google Scholar]
- 70.Sullivan NJ, Sasser AK, Axel AE, Vesuna F, Raman V, Ramirez N, Oberyszyn TM, Hall BM. Interleukin-6 induces an epithelial-mesenchymal transition phenotype in human breast cancer cells. Oncogene 28: 2940–2947, 2009. doi: 10.1038/onc.2009.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sundararaj K, Rodgers JI, Marimuthu S, Siskind LJ, Bruner E, Nowling TK. Neuraminidase activity mediates IL-6 production by activated lupus-prone mesangial cells. Am J Physiol Renal Physiol 314: F630–F642, 2018. doi: 10.1152/ajprenal.00421.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Taooka Y, Maeda A, Hiyama K, Ishioka S, Yamakido M. Effects of neutrophil elastase inhibitor on bleomycin-induced pulmonary fibrosis in mice. Am J Respir Crit Care Med 156: 260–265, 1997. doi: 10.1164/ajrccm.156.1.9612077. [DOI] [PubMed] [Google Scholar]
- 73.Thannickal VJ, Toews GB, White ES, Lynch JP III, Martinez FJ. Mechanisms of pulmonary fibrosis. Annu Rev Med 55: 395–417, 2004. doi: 10.1146/annurev.med.55.091902.103810. [DOI] [PubMed] [Google Scholar]
- 74.Thannickal VJ, Zhou Y, Gaggar A, Duncan SR. Fibrosis: ultimate and proximate causes. J Clin Invest 124: 4673–4677, 2014. doi: 10.1172/JCI74368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Travis MA, Sheppard D. TGF-β activation and function in immunity. Annu Rev Immunol 32: 51–82, 2014. doi: 10.1146/annurev-immunol-032713-120257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ueno S, Saito S, Wada T, Yamaguchi K, Satoh M, Arai Y, Miyagi T. Plasma membrane-associated sialidase is up-regulated in renal cell carcinoma and promotes interleukin-6-induced apoptosis suppression and cell motility. J Biol Chem 281: 7756–7764, 2006. doi: 10.1074/jbc.M509668200. [DOI] [PubMed] [Google Scholar]
- 77.van de Vlekkert D, Demmers J, Nguyen XX, Campos Y, Machado E, Annunziata I, Hu H, Gomero E, Qiu X, Bongiovanni A, Feghali-Bostwick CA, d’Azzo A. Excessive exosome release is the pathogenic pathway linking a lysosomal deficiency to generalized fibrosis. Sci Adv 5: eaav3270, 2019. doi: 10.1126/sciadv.aav3270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Varki A, Gagneux P. Multifarious roles of sialic acids in immunity. Ann N Y Acad Sci 1253: 16–36, 2012. doi: 10.1111/j.1749-6632.2012.06517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Voltz JW, Card JW, Carey MA, Degraff LM, Ferguson CD, Flake GP, Bonner JC, Korach KS, Zeldin DC. Male sex hormones exacerbate lung function impairment after bleomycin-induced pulmonary fibrosis. Am J Respir Cell Mol Biol 39: 45–52, 2008. doi: 10.1165/rcmb.2007-0340OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wada T, Yoshikawa Y, Tokuyama S, Kuwabara M, Akita H, Miyagi T. Cloning, expression, and chromosomal mapping of a human ganglioside sialidase. Biochem Biophys Res Commun 261: 21–27, 1999. doi: 10.1006/bbrc.1999.0973. [DOI] [PubMed] [Google Scholar]
- 81.Walters DM, Kleeberger SR. Mouse models of bleomycin-induced pulmonary fibrosis. Curr Protoc Pharmacol 40: 5.46.1–5.46.17, 2008. doi: 10.1002/0471141755.ph0546s40. [DOI] [PubMed] [Google Scholar]
- 82.Wang P, Zhang J, Bian H, Wu P, Kuvelkar R, Kung TT, Crawley Y, Egan RW, Billah MM. Induction of lysosomal and plasma membrane-bound sialidases in human T-cells via T-cell receptor. Biochem J 380: 425–433, 2004. doi: 10.1042/bj20031896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wolters PJ, Collard HR, Jones KD. Pathogenesis of idiopathic pulmonary fibrosis. Annu Rev Pathol 9: 157–179, 2014. doi: 10.1146/annurev-pathol-012513-104706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wynn TA, Ramalingam TR. Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat Med 18: 1028–1040, 2012. doi: 10.1038/nm.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yamaguchi K, Shiozaki K, Moriya S, Koseki K, Wada T, Tateno H, Sato I, Asano M, Iwakura Y, Miyagi T. Reduced susceptibility to colitis-associated colon carcinogenesis in mice lacking plasma membrane-associated sialidase. PLoS One 7: e41132, 2012. doi: 10.1371/journal.pone.0041132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yamauchi K, Kasuya Y, Kuroda F, Tanaka K, Tsuyusaki J, Ishizaki S, Matsunaga H, Iwamura C, Nakayama T, Tatsumi K. Attenuation of lung inflammation and fibrosis in CD69-deficient mice after intratracheal bleomycin. Respir Res 12: 131, 2011. doi: 10.1186/1465-9921-12-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zerbino DR, Achuthan P, Akanni W, Amode MR, Barrell D, Bhai J, Billis K, Cummins C, Gall A, Girón CG, Gil L, Gordon L, Haggerty L, Haskell E, Hourlier T, Izuogu OG, Janacek SH, Juettemann T, To JK, Laird MR, Lavidas I, Liu Z, Loveland JE, Maurel T, McLaren W, Moore B, Mudge J, Murphy DN, Newman V, Nuhn M, Ogeh D, Ong CK, Parker A, Patricio M, Riat HS, Schuilenburg H, Sheppard D, Sparrow H, Taylor K, Thormann A, Vullo A, Walts B, Zadissa A, Frankish A, Hunt SE, Kostadima M, Langridge N, Martin FJ, Muffato M, Perry E, Ruffier M, Staines DM, Trevanion SJ, Aken BL, Cunningham F, Yates A, Flicek P. Ensembl 2018. Nucleic Acids Res 46: D754–D761, 2018. doi: 10.1093/nar/gkx1098. [DOI] [PMC free article] [PubMed] [Google Scholar]