Abstract
Liquiritin apioside (LA), a main flavonoid component of licorice, reportedly suppresses cough responses to inhalation of aerosolized capsaicin [CAP; a stimulant to transient receptor potential vanilloid 1 (TRPV1)] in conscious guinea pigs via acting on peripheral nerves. However, the evidence of LA having a direct effect on airway sensory fibers is lacking. Considering the important role laryngeal chemoreceptors and mechanoreceptors play in triggering apnea and cough, we studied whether LA suppressed the apneic responses to stimulation of these receptors via directly acting on the superior laryngeal nerve (SLN). Intralaryngeal delivery of chemical [CAP, HCl, and distilled water (DW)] and mechanical [an air-pulse (AP)] stimulations was applied in anesthetized rat pups to evoke the apnea. These stimuli were repeated after intralaryngeal LA treatment or peri-SLN LA treatment to determine the direct effect of LA on the SLN. Our results showed that all stimuli triggered an immediate apnea. Intralaryngeal LA treatment significantly attenuated the apneic response to chemical but not mechanical stimulations. The same attenuation was observed after peri-SLN LA treatment. Owing that TRPV1 receptors of laryngeal C fibers are responsible for the CAP-triggered apneas, the LA impact on the activity of laryngeal C neurons retrogradely traced by DiI was subsequently studied using a patch-clamp approach. LA pretreatment significantly altered the electrophysiological kinetics of CAP-induced currents in laryngeal C neurons by reducing their amplitudes, increasing the rise times, and prolonging the decay times. In conclusion, our results, for the first time, reveal that LA suppresses the laryngeal chemoreceptor-mediated apnea by directly acting on the SLN (TRPV1 receptors of laryngeal C fibers).
Keywords: chemoreceptor, mechanoreceptor, superior laryngeal nerve, TRPV1
INTRODUCTION
Licorice, derived from the roots of Glycyrrhiza inflata, has been used as an herbal medicine for more than two millennia in China (82). It has particularly been employed as a cough suppressant in clinical settings (5, 63). Recent studies show that systemic administration of liquiritin apioside (LA), a main flavonoid component of licorice, greatly blunts cough responses to inhalation of aerosolized capsaicin (CAP) (34, 35), citric acid (51, 61), and ammonia (38, 75) in conscious guinea pigs and mice. This antitussive effect of LA is, at least in part, achieved by acting on peripheral nerves because the effect is significantly reduced by systemic administration of glibenclamide (34, 38), an ATP-sensitive K+ channel antagonist that is poorly permeable to the blood-brain barrier (39). It is well-known that multiple peripheral afferent nerves are responsible for cough, such as sensory afferents from the airways (especially the larynx, tracheobronchial tree, and bronchopulmonary C fibers), heart, and splanchnic bed (for review, see Ref. 29). To date, it remains unexplored which types of peripheral sensory nerves are involved in the antitussive effect of LA.
The superior laryngeal nerve (SLN) that conveys most of the laryngeal sensory afferent fibers innervating the larynx plays a crucial role in cardiorespiratory modulation. The SLN is composed of both unmyelinated C fibers and myelinated Aδ-fibers (13, 30, 78), but the myelinated component as compared with unmyelinated C fibers is much lower in neonate animals (13). Its cell bodies are located in nodose/jugular ganglia (30, 83) and central afferents terminate onto the second-order neurons within the ipsilateral nucleus tractus solitarius, and these neurons further project to pontomedullary respiratory-related nuclei (58). For example, the projections from the nucleus tractus solitarius to the nucleus ambiguous contain the preganglionic cardiac vagal cell bodies and the recurrent laryngeal nerve motoneurons. Stimulations applied onto the laryngeal mucosa reflexly trigger an apnea (bradycardia and hypertension) in the immature newborn, such as the rat pup and the preterm lamb or human, and evoke cough, swallowing, and brief laryngeal closure in a mature mammal (19, 21, 40, 57, 58, 64, 65, 71–73). In general, there are two types of laryngeal reflexes according to the different receptor activated. One is laryngeal chemoreflex (LCR), such as the responses to CAP, acid, and distilled water (DW) (9, 16, 19, 40, 50, 64, 73), and another is laryngeal mechanoreflex (LMR) evoked by air pulse/pressure (AP), air puff, and mucosal vibration (6, 10, 17, 49). In addition, cold and L‐menthol air flow or CO2 can also induce the larynx-mediated reflex (58). Usually, intralaryngeal administration of CAP selectively activates the local transient receptor potential vanilloid 1 (TRPV1) of laryngeal C fibers (12, 22, 46, 50), while acid [hydrochloric acid solution (HCl)], DW, and AP activate acid-sensing ion channels, osmotic receptors, and mechanoreceptors of Aδ-fibers, respectively (1, 4, 11, 37, 67). The globular corpuscles of the fine myelinated afferent fiber (Aδ-fiber) endings in the laryngeal mucosa reportedly operate as slowly adapting mechanoreceptors and the conical corpuscles function as rapidly adapting mechanoreceptors, and both fibers are sensitive to changes in intralaryngeal pressure (1, 62, 69). The bilateral section of the SLN abolishes both LCR and LMR (9, 19). Postoperative sore throat and cough in the clinical setting, the undesirable side effects of endotracheal intubation, are significantly diminished by medicated lozenges of licorice (26) or licorice gargles before the endotracheal intubation (2). These results imply an ability of licorice to depress the laryngeal disturbance-induced nociceptive and tussive sensations that are mainly mediated by TRPV1. However, the effects of LA (an important component of licorice) on the LCR and LMR and the involvement of the SLN (particularly TRPV1 of laryngeal C fibers) in the LA effect have not been investigated.
In the present experiments, we examined the effects of intralaryngeal perfusion of LA and perineural treatment of the SLN with LA (peri-SLN LA treatment) on LCR and LMR in anesthetized rat pups. The LA impact on the CAP-induced currents in laryngeal sensory C neurons was subsequently studied using a patch-clamp approach. Our results show that LA significantly suppresses LCR but not LMR by directly affecting the SLN and especially the TRPV1 receptors of laryngeal C neurons.
MATERIALS AND METHODS
Pathogen-free pregnant Sprague-Dawley rats (250–350 g) were purchased from Charles River Laboratories (Wilmington, MA). They were housed in the animal facility at Lovelace Respiratory Research Institute in filter top cages and provided with water and food ad libitum. The room was constantly ventilated, and the temperature was kept at 23°C. The animals were quarantined for 1 wk before the experiments. The experimental protocols were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee of the Lovelace Respiratory Research Institute, which is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International.
Use of Rat Pups
Rat pups born by spontaneous vaginal delivery were housed with their mother and siblings (24–25°C, and 12:12-h light-dark cycle). In all experiments, no more than three male pups from each litter with a similar overall litter size were used in each study to minimize the possible effect of genetic difference between litters on the results. Similar to previous studies (18, 23), male pups at postnatal day 14–18 (P14–18) (n = 68) were chosen in this study due to the higher sensitivity of LCR in males than females at this period (8). All studies were performed between 9:00 AM and 4:00 PM to avoid any influence from the circadian rhythm (66).
Experimental Protocols
Study series I was designed to confirm the effects of laryngeal LA pretreatment on the LCR evoked by intralaryngeal perfusion of CAP, HCl, and DW and on the LMR induced by AP. To this end, intralaryngeal perfusion of 50 µl CAP (23) (10 µg/mL, n = 9, group 1), 100 µl HCl (77) (pH at 2.0, n = 10, group 2), and 100 µl DW (18) (n = 10, group 3) for 10 s was performed during inspiration (79). The temperature of all solutions was ~35°C before the injection. The perfusion rate was 5 µl/s for CAP and 10 µl/s for HCl and DW (23). The vehicle of CAP (1% Tween 80, 1% ethanol, and 98% saline) and vehicle of HCl and DW (saline) were also tested. These stimulations were applied immediately after loading 50 µl LA (10 µg/µl) or its vehicle (saline) for 10 min in the larynx. To examine the effect of laryngeal LA pretreatment on LMR, an AP generated from a ventilator (detailed below) for 3 s with peak pressure of ~30 mmHg was applied after the same laryngeal LA treatment (n = 8, group 4). The order of laryngeal LA and saline pretreatments was random with an interval of up to 3 h.
Study series II aimed to determine whether LA exerts its effect directly via acting on the SLN. Owing to the lack of LA effect on the apneic response to the AP, we just focused on the LCR. The SLN was bilaterally isolated in anesthetized and spontaneously breathing rat pups. The same chemical stimuli (CAP, n = 6; HCl, n = 7; and DW, n = 8) mentioned above were applied after peri-SLN pretreatment with LA (10 µg/µl) and saline for 20 min [similar to the previous report (50)] and the order of the LA and saline treatments was random too.
Study series III was carried out to verify the influences of LA on the electrophysiological properties of CAP-induced currents in laryngeal C neurons because TRPV1 receptors of laryngeal C fibers are responsible for intralaryngeal CAP perfusion-triggered apneas (12, 22, 46, 50). Pups at P5–6 were pretreated with the retrograde tracer 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiI) injected into the SLN (n = 10/per group). Nine to twelve days later, the nodose/jugular ganglia were collected for primary culture of the neurons. The neurons were cultured overnight and pretreated with LA (0.1 mM, overnight) or its vehicle. The LA concentration and treatment time were chosen because lower concentrations and LA pretreatment for several hours could not constantly and strikingly affect CAP currents in our pilot studies (data not shown). Laryngeal C neurons labeled by DiI with a cell size <25 µm were identified as laryngeal C neurons (23) and chosen for whole cell patch clamp. The whole cell patch-clamp technique was utilized, and the CAP-induced currents, current amplitudes, rising and decay times were analyzed and compared before and after LA bath application.
Animal Preparations and Experimental Stimulations
Preparation for anesthetized and spontaneously breathing pups.
The pups were anesthetized (urethane, 1,400 mg/kg ip) to record cardiorespiratory activity. Supplemental doses of anesthetics (urethane, 120–240 mg/kg iv) were provided as needed to suppress corneal and withdrawal reflexes. The trachea was opened with a transverse incision to expose the lumen but leave the posterior tracheal wall intact. Both the rostral and caudal segments of the trachea were cannulated separately. The caudal tracheal opening was cannulated and connected to a pneumotachograph (Frank’s Manufacturing Co., Albuquerque, NM) for recording airflow (84), while rostral tracheal opening was also cannulated as detailed below. The left femoral artery was cannulated for monitoring arterial blood pressure (ABP). The core temperature of the animal was monitored with a rectal probe and maintained at ~36°C with a heating pad and radiant heat. The animal was exposed to 30% O2 throughout the experiment to prevent hypoxia.
The chemical stimuli applied in the anesthetized and spontaneously breathing pups.
Briefly (23, 45), a laryngeal catheter (PE-190) with a premade window (~2.5 mm long × 1.2 mm wide) was inserted cranially from the rostral tracheal opening and extended out of the mouth bypassing the pharynx and mouth. The window was positioned just caudal to the vocal cord where most of the laryngeal mucosal surface was exposed to the perfused solution. The catheter was then fixed at both ends. The solution in the catheter was perfused from the tracheal end to the mouth end of the catheter at a constant flow rate (5–10 µl/s) (23) by using a programmable single-syringe infusion pump (model SP100i; WPI, Sarasota, FL). Perfusion at this flow rate for 10 s did not induce detectable leaking because the same amount of solution volume was collected from the mouth end of the catheter in our studies. After laryngeal delivery of LA, CAP, HCl, or DW, the catheter was flushed with saline twice.
AP generation.
A rodent ventilator (UGO Basile 7025, Varese, Italy) was employed to generate AP. Its expiratory outlet was connected to the caudal end of the laryngeal catheter through a three-way stopcock opening to room air. During application of an AP, the path of the three-way stopcock was switched (at the end of an expiration) from ventilator-room air to ventilator-catheter for 3 s; the mouth end of the laryngeal catheter was blocked. The air pressure generated from the ventilator produced a transient (~3 s) intralaryngeal pulse with peak value of ~30 mmHg that was measured with a pressure transducer (Statham Instruments Inc., Hato Rey, Puerto Rico) connected to the side branch of laryngeal catheter. The setting of respiratory frequency and tidal volume (VT) was adjusted to generate the AP before the test. This AP peak value was chosen because 10–20 mmHg applied in infants (74) only prolonged expiratory duration (TE) by ~80% and failed to induce an apnea in our pilot study in rat pups.
Peri-SLN LA pretreatment.
Perivagal and peri-SLN treatment with CAP or different antagonists has been previously used in rats and dogs, in which CAP blocks the sensory C-fiber signal conduction and the antagonists block the corresponding agonist effect on the nerve afferent endings (44, 45, 50). Briefly, a segment (~2 mm) of the SLN was bilaterally exposed. A vertical slitting in the diameter line was made in a PE50 catheter with a length equal to 2 mm to make two grooved pieces. The segments of the isolated SLN on both sides were gently positioned into the groove. The edge of the groove was bedded and covered with petroleum jelly so that the SLN segment within the groove was isolated from neighboring tissues. LA or saline (2–3 µl) was carefully injected into the grooves to immerse the SLN segments. The solution in the grooves was removed 20 min later with a subsequent saline washout (twice).
Retrograde labeling of laryngeal C neurons in the nodose/jugular ganglia.
Pups at P5–6 were anesthetized with continuous inhalation of isoflurane (1–2%) administered via a nose cone connected to a vaporizing machine (SurgiVet, Waukesha, WI). A midline incision (1~1.5 cm) was made in the neck to expose both sides of the SLN. As reported previously (32), a beveled glass micropipette needle (10-μm tip diameter) was pulled by a DMZ universal puller (Dagon, Minneapolis, MN), sharpened with a microforge (MF-900; Narishige, Japan), and connected to a Harvard PHD syringe pump (Harvard Apparatus). The micropipette needle was filled with DiI, a retrograde tracer to mark laryngeal neurons within the nodose/jugular ganglia. The tip of the needle was gently inserted into the isolated SLN to microinject 0.5 µl of DiI solution at a rate of 0.1 µl/min. The skin incision was sutured following bilateral microinjections. Nine to twelve days later, the pups were anesthetized again for primary nodose/jugular ganglia neuron culture.
Cultured laryngeal C neurons in the nodose/jugular ganglia.
The primary nodose/jugular ganglia neurons were cultured in the same as the previous report (24). Briefly, after decapitation, the rat head was immediately immersed in ice-cold Dulbecco’s modified Eagle’s medium (DMEM)/F-12 solution. This was followed by quick extraction of the nodose/jugular ganglia under a dissecting microscope. Each ganglion was desheathed, cut into eight pieces, placed in a 0.08% type IV collagenase, and incubated for 60 min in 5% CO2 in air at 37°C. The ganglia suspension was centrifuged (150 g, 5 min), and the supernatant was aspirated. The cell pellet was resuspended in 0.05% trypsin for 1 min and centrifuged (150 g, 5 min); the pellet was then resuspended in a modified DMEM/F-12 solution (supplemented with 10% heat-inactivated fetal bovine serum, 100 U/mL penicillin, 100 μg/mL streptomycin, and 100 μM minimum essential media nonessential amino acids) and gently triturated with a small-bore fire-polished Pasteur pipette. Myelin debris was separated and discarded after centrifugation of the dispersed cell suspension (500 g, 8 min) through a layer of 15% bovine serum albumin. The cell pellet was resuspended in the modified DMEM/F-12 solution, plated onto poly-l-lysine-coated glass coverslips and incubated overnight (5% CO2 in air at 37°C). For LA group, LA (0.1 mM) will be added to medium overnight.
Experimental Recordings
Respiratory parameters including expiratory duration (TE), tidal volume (VT), respiratory frequency (fR), and minute ventilatory volume (VE) were derived and calculated from respiratory airflow signals. TE longer than or equal to threefold of the baseline TE was defined as an apnea (54, 80). Mean arterial blood pressure (MABP) and heart rate (HR) were derived from the ABP signals. Raw data of VT, fR, VE, TE, HR, and MABP were digitized and recorded using a PowerLab/8sp unit (model ML 785; ADInstruments Inc., Colorado Springs, CO) and a computer with LabChart Pro 7 software.
For whole cell patch-clamp recording, similar to the previous report (28), all recordings were made on the neurons cultured on the coverslip. Neurons were superfused (2 mL/min) continuously with standard extracellular solution containing the following chemicals (in mM): 136 NaCl, 5.4 KCl, 1.8 CaCl2, 1 MgCl2, 0.33 NaH2PO4, 10 glucose, and 10 HEPES; pH was adjusted to 7.4 with NaOH, and in the LA group, LA (0.1 mM) was added into the solution. The whole cell patch clamp was performed by using Axopatch 200B, Digidata 1440A, and pClamp 10.5 software (Molecular Devices, Palo Alto, CA). Patch pipettes were pulled from borosilicate glass capillary tubing (G-1.5; Narishige) with a Narishige PC-10 two-stage electrode puller (Narishige International Inc., Amityville, NY). The pipette solution had the following composition (in mM): 92 potassium gluconate, 40 KCl, 8 NaCl, 1 CaCl2, 0.5 MgCl2, 10 EGTA, and 10 HEPES; pH was adjusted to 7.2 with KOH. The pipette resistance was 3–5 MΩ when filled with the above saline. The chemical stimulants (CAP, 1.5 µM) were applied through a glass-pipette (tip diameter: 10 μm) by a pressure-driven microinjection system (Picospritzer II; General Valve Corp., Fairfield, NJ). Laryngeal C neurons were identified by DiI retrograde labeling and the neural size (<25 μm) (80) with fluorescence microscopy (56). Neural signals were filtered at 2 kHz and sampled at 10 kHz. Signal acquisition mode was set as gap-free with the duration of the recording over 30 s. Series resistance (6–18 MΩ) was monitored throughout the recordings, and data were discarded if the resistance changed by >20%. The experiments were performed at room temperature (~22°C).
Chemicals
Capsaicin was purchased from Sigma-Aldrich (St. Louis, MO), and a stock solution of capsaicin (1 mM) was prepared in 1% Tween80, 1% ethanol, and 98% saline. The HCl solution was diluted in saline (pH = 2). LA was purchased from Chengdu Alfa Biotechnology (Chengdu, Sichuan, China) and diluted in saline.
Data Acquisition and Statistical Analysis
All variables are expressed as absolute values with the exception that TE, HR, and MABP responses are also presented as percent change from baseline value. Group data are reported as means ± SE in tables and as means, medians, and interquartile ranges in figures. Paired t test was used to compare the difference in the responses to LA and saline treatments (intralaryngeal or peri-SLN approach), while Student’s t test was used to compare CAP-induced currents with and without LA pretreatment. P < 0.05 was considered significant.
RESULTS
Intralaryngeal Application of CAP, HCl, DW, or AP Induces an Immediate Apnea
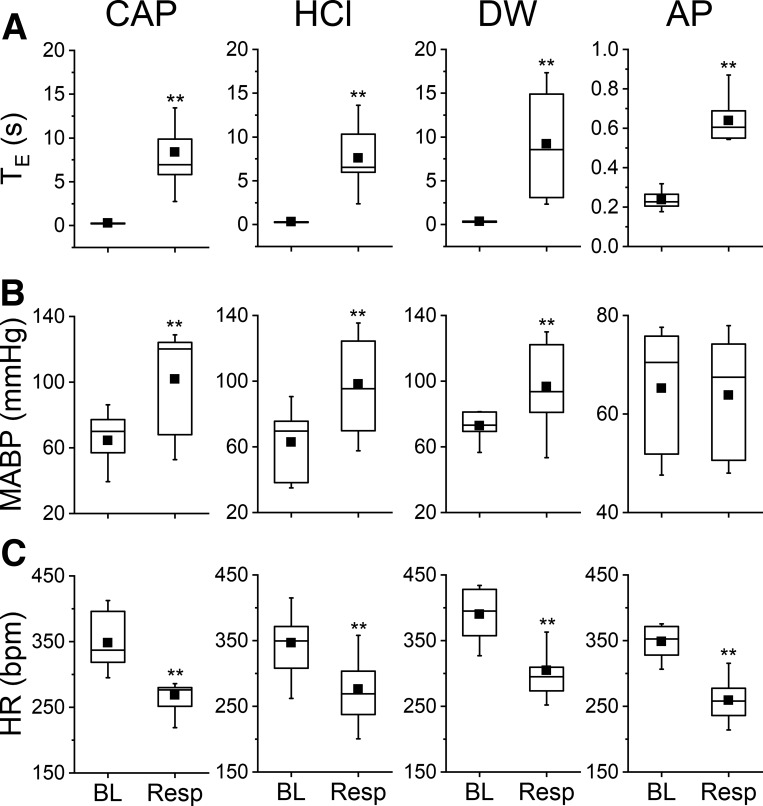
The cardiorespiratory responses to intralaryngeal delivery of CAP, HCl, DW, and AP are depicted in Fig. 1. Intralaryngeal delivery of vehicle failed to cause significant change in baseline cardiorespiratory activity. However, laryngeal delivery of CAP, HCl, or DW triggered an immediate apnea with TE prolonged by 31-, 24-, and 27-fold, respectively. All stimulations also induced a remarkable cardiovascular response characterized by hypertension and bradycardia. The responses of hypertension and bradycardia evoked by CAP were 58 and −23%, by HCl were 56% and −20% and by DW were 33% and −22%. In addition, intralaryngeal delivery of AP evoked an apnea with TE prolonged by threefold and bradycardia (−26%) without an impact on MABP. The evoked LCR and LMR were repeatable with an interval of 30 min or 3 h.
Fig. 1.
The cardiorespiratory responses to intralaryngeal delivery of capsaicin (CAP; 10 μg/mL), HCl (pH 2), distilled water (DW), and air pulse (AP; ~30 mmHg for 3 s). A–C: laryngeal stimulation-evoked changes in expiratory duration (TE; A), mean arterial blood pressure (MABP; B), and heart rate (HR; C); n = 9 for CAP, 10 for HCl, 10 for DW, and 8 for AP. Mean values are presented as square symbols in the boxes. BL, baseline; Resp, response. **P < 0.01 compared with the baseline (vehicle).
Laryngeal LA Pretreatment Fails to Alter Pups’ Baseline Cardiorespiratory Activity
We compared baseline cardiorespiratory activity after laryngeal application of saline and LA in the above 37 pups. As presented in Table 1, laryngeal LA pretreatment failed to significantly alter baseline VT, fR, VE, TE, HR, and MABP in these pups.
Table 1.
Effects of laryngeal LA pretreatment on baseline cardiorespiratory activity
| Variable | Saline | LA |
|---|---|---|
| VT, mL/kg | 8.0 ± 0.4 | 8.1 ± 0.4 |
| fR, breaths/min | 124 ± 5 | 126 ± 5 |
| VE, mL·min−1·kg−1 | 990 ± 60 | 1,008 ± 50 |
| TE, s | 0.31 ± 0.02 | 0.30 ± 0.02 |
| HR, beats/min | 347 ± 14 | 346 ± 13 |
| MABP, mmHg | 69 ± 6 | 66 ± 6 |
Values are means ± SE. LA, liquiritin apioside; TE, expiratory duration; VT, tidal volume; fR, respiratory frequency; VE, minute ventilatory volume; HR, heart rate; MABP, mean arterial blood pressure. The data were obtained from 4 groups of pups in series I: n = 9 for capsaicin, 10 for HCl, 10 for distilled water, and 8 for air pulse.
LA Pretreatment Reduces the CAP-, HCl-, and DW-Evoked Response But Not the AP-Evoked Response
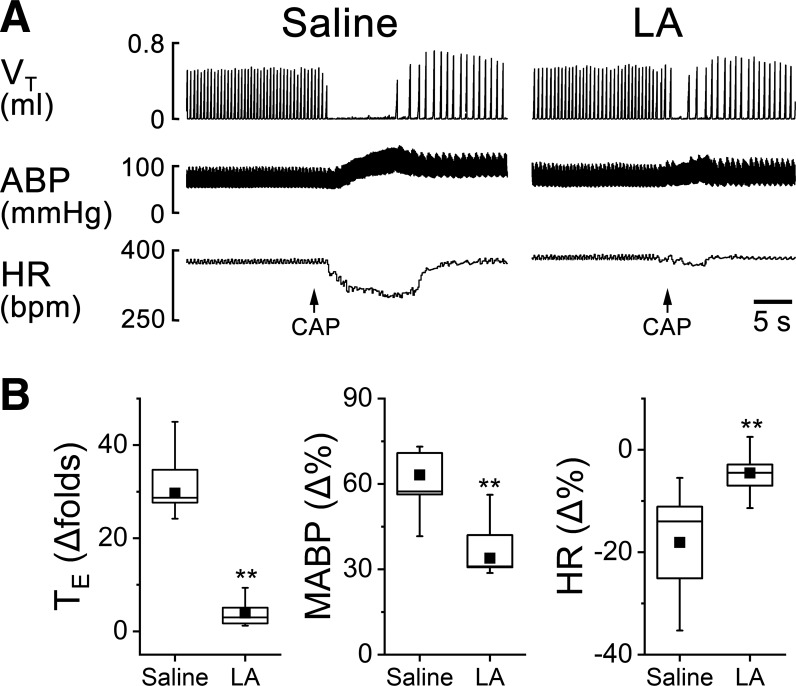
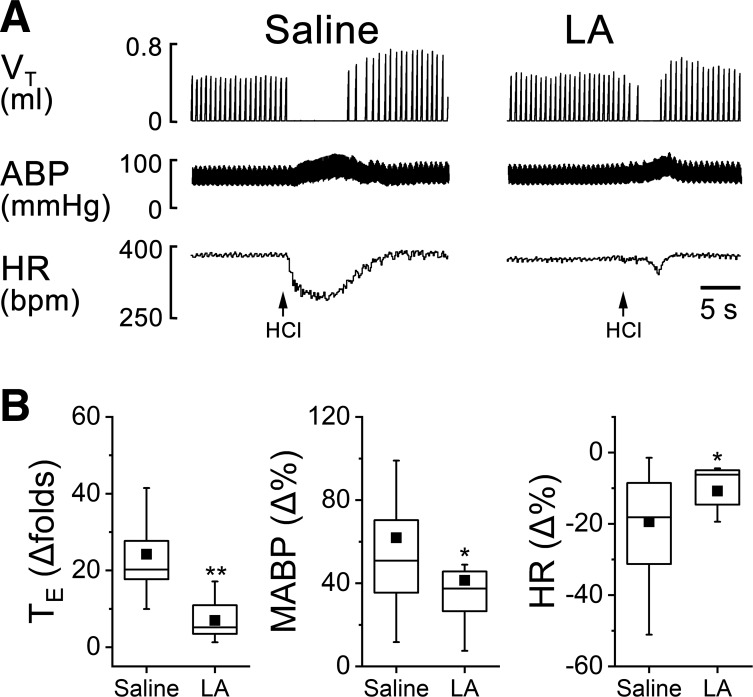
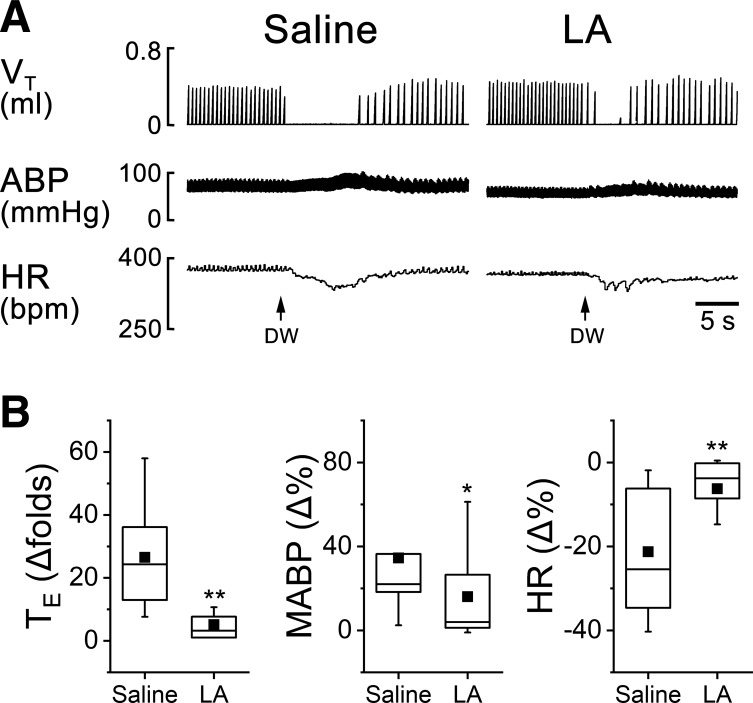
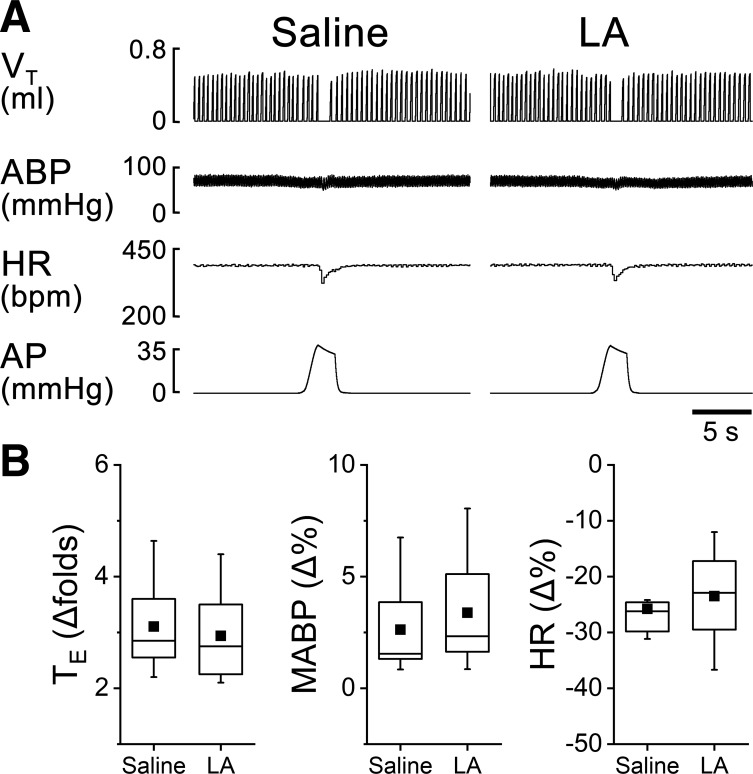
The cardiorespiratory responses to intralaryngeal delivery of CAP, HCl, DW, and AP were measured before and after laryngeal LA pretreatment. The results for LCR could be summarized as follows (Figs. 2, 3, and 4): LA profoundly shortened the apneic responses to CAP, HCl, and DW by 72%, 71%, and 81% and attenuated hypertension and bradycardia responses to CAP, HCl, and DW by 41% and 59%, 33% and 44%, and 54% and 71%, respectively. Interestingly, laryngeal LA pretreatment did not change the cardiorespiratory responses to AP (Fig. 5). The suppressive effect of LA usually disappeared 3 h after the intralaryngeal perfusion.
Fig. 2.
The effects of laryngeal application of liquiritin apioside (LA) on capsaicin (CAP)-mediated cardiorespiratory responses. A: typical recordings of cardiorespiratory responses to intralaryngeal application of CAP after saline and LA pretreatment in an anesthetized and spontaneously breathing pup. Traces from top to bottom are tidal volume (VT), arterial blood pressure (ABP), and heart rate (HR). B: corresponding group data of expiratory duration (TE), mean ABP (MABP), and HR. Responses of apnea, hypertension, and bradycardia to CAP are inhibited by LA. Mean values are presented as square symbols in the boxes; n = 9. **P < 0.01 compared with saline.
Fig. 3.
Cardiorespiratory responses to intralaryngeal application of hydrochloric acid solution before and after laryngeal LA pretreatment. A: typical recordings in an anesthetized and spontaneously breathing pup. Traces from top to bottom are tidal volume (VT), arterial blood pressure (ABP), and heart rate (HR). B: corresponding group data of expiratory duration (TE), mean ABP (MABP), and HR. Responses of apnea, hypertension, and bradycardia to HCl are significantly reduced by intralaryngeal LA pretreatment. Mean values are presented as square symbols in the boxes; n = 10. *P < 0.05 and **P < 0.01 compared with saline.
Fig. 4.
Effects of laryngeal liquiritin apioside (LA) pretreatment on the cardiorespiratory response to distilled water. A: typical recordings in an anesthetized and spontaneously breathing pup. Traces from top to bottom are tidal volume (VT), arterial blood pressure (ABP), and heart rate (HR). B: corresponding group data of expiratory duration (TE), mean ABP (MABP), and HR. Responses of apnea, hypertension, and bradycardia to distilled water are significantly reduced by intralaryngeal LA pretreatment: n = 10. Mean values are presented as square symbols in the boxes. *P < 0.05 and **P < 0.01 compared with saline.
Fig. 5.
Cardiorespiratory responses to an intralaryngeal applied air pulse (AP) before and after laryngeal liquiritin apioside (LA) pretreatment. A: typical recordings in an anesthetized and spontaneously breathing pup. Traces from top to bottom are tidal volume (VT), arterial blood pressure (ABP), and heart rate (HR). B: corresponding group data of expiratory duration (TE), mean ABP (MABP), and HR. Responses of apnea, hypertension, and bradycardia to AP are not significantly changed by intralaryngeal LA pretreatment. Mean values are presented as square symbols in the boxes; n = 8.
Peri-SLN LA Pretreatment Inhibits the LCR
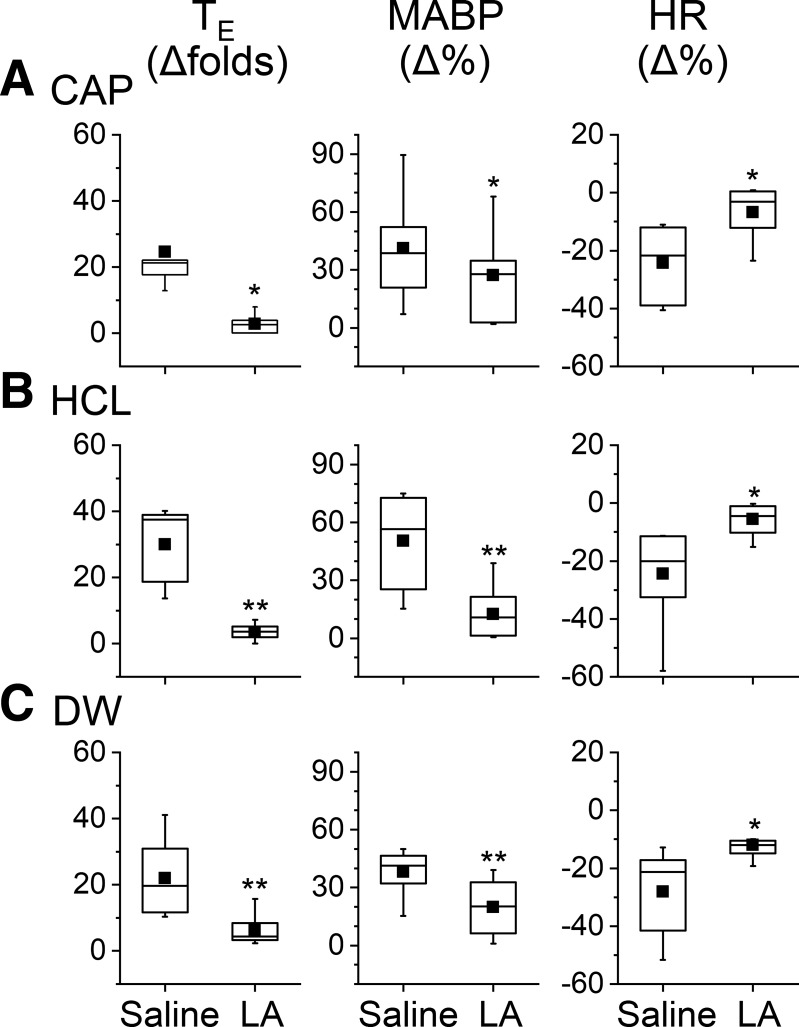
Peri-SLN pretreatment with LA (10 µg/µl, 2–3 µl) was applied to determine the direct effect of LA on the SLN. The same intralaryngeal chemical stimulations (CAP, HCl, and DW) were applied immediately after peri-SLN treatment with LA or saline. Owing to the lack of intralaryngeal LA effect on the LMR (as exhibited in Fig. 5), peri-SLN LA pretreatment was not conducted for the LMR. As shown in Table 2, there was no remarkable difference in baseline cardiorespiratory activity after peri-SLN saline and LA pretreatment. In sharp contrast, peri-SLN LA pretreatment strikingly suppressed the apnea, hypertension, and bradycardia responses to CAP by 88%, 34%, and 72%; HCl by 72%, 48%, and 57%; and DW by 88%, 75%, and 77% (Fig. 6). This is similar to those induced by the laryngeal LA pretreatment (see Figs. 2, 3, and 4). It is worth noting that there was no significant difference in baseline cardiorespiratory activity in the pups with (Table 2) and without (see Table 1) surgical operation for peri-SLN treatment.
Table 2.
Effects of peri-SLN LA treatment of SLN on baseline cardiorespiratory activity
| Variable | Saline | LA |
|---|---|---|
| VT, mL/kg | 8.4 ± 0.4 | 8.3 ± 0.5 |
| fR, breaths/min | 118 ± 5 | 118 ± 5 |
| VE, mL·min−1·kg−1 | 991 ± 68 | 988 ± 68 |
| TE, s | 0.35 ± 0.02 | 0.36 ± 0.02 |
| HR, beats/min | 379 ± 12 | 378 ± 12 |
| MABP, mmHg | 65 ± 2 | 64 ± 2 |
Values are means ± SE; n = 21 (n = 6 for CAP, n = 7 for HCl, and n = 8 for DW). SLN, superior laryngeal nerve; LA, liquiritin apioside; TE, expiratory duration; VT, tidal volume; fR, respiratory frequency; VE, minute ventilatory volume; HR, heart rate; MABP, mean arterial blood pressure.
Fig. 6.
Cardiorespiratory responses to capsaicin (CAP; A), HCl (B), and distilled water (DW; C) before and after perisuperior laryngeal nerve liquiritin apioside (LA) treatment (10 μg/μL, 20 min). This treatment significantly decreases the chemical stimulation-evoked responses of apnea, hypertension, and bradycardia: n = 6, 7, and 8 for CAP, HCl, and DW group, respectively. Mean values are presented as square symbols in the boxes. *P < 0.05 and **P < 0.01 compared with saline.
LA Pretreatment Inhibits CAP-Induced Currents in Laryngeal C Neurons
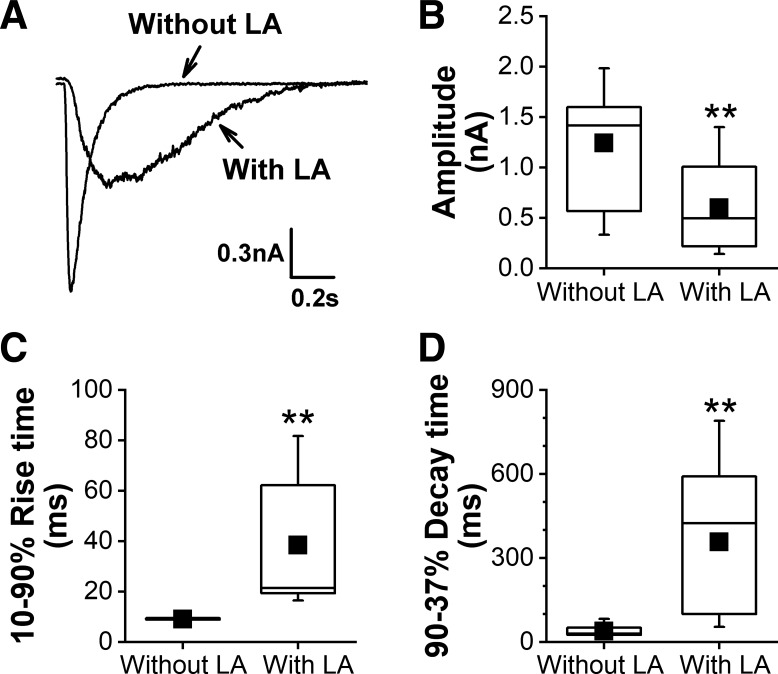
To test if LA would affect CAP-induced current in laryngeal C neurons, the whole cell patch-clamp technique was utilized to record the CAP-induced currents with the current amplitudes and rising and decay times analyzed before and after LA bath application. Although some cells with a larger size (>25 µm) were also labeled with DiI in the cultured neurons derived from the nodose and jugular ganglions, only laryngeal C neurons identified by DiI labeling with a cell size <25 µm were tested in this study. Of the 18 laryngeal C neurons tested, three neurons (16%) were not CAP responsive. LA pretreatment inhibited the CAP-induced currents in laryngeal C neurons by reducing the current amplitudes, increasing the rise times, and prolonging the decay times (Fig. 7).
Fig. 7.
The effect of liquiritin apioside (LA) pretreatment (0.1 mM, overnight) on the capsaicin (CAP; 1.5 μM) triggered CAP-induced current in laryngeal C neurons. A: typical recordings of a CAP-induced current in a laryngeal C neuron without and with LA pretreatment. B–D: LA pretreatment markedly inhibited these neurons’ response to CAP by reducing the current amplitude (B), increasing the rise time (C), and prolonging the decay time (D). Neurons = 15 for either without or with LA pretreatment. Mean values are presented as square symbols in the boxes. **P < 0.01 vs. without LA pretreatment.
DISCUSSION
The followings are the three major findings in this study (1). Laryngeal LA pretreatment profoundly attenuates CAP-, HCl-, and DW-induced LCR without an effect on AP-induced LMR in anesthetized and spontaneously breathing rat pups (2). Peri-SLN LA pretreatment causes similar attenuation of LCR as induced by laryngeal LA pretreatment (3). LA pretreatment markedly inhibits laryngeal C-neuron responses to CAP in vitro by reducing the current amplitude, increasing the rise time, and prolonging the decay time.
Intralaryngeal application of CAP, HCl, DW, or AP provoked an immediate apnea in anesthetized rat pups associated with hypertension and/or bradycardia in this study. These data are consistent with previous reports in anesthetized and unanesthetized mammals, especially rat pups, piglets, puppies, newborn lambs, and premature infants (6, 7, 9, 10, 16, 19, 23, 40, 50, 64, 73, 77). So far as we know, this is the first report showing the apneic responses to intralaryngeal application of HCl and AP in anesthetized rat pups. Intralaryngeal stimulations can also evoke laryngeal closure through activation of the recurrent laryngeal nerve motoneurons (58). In this study, ventilation was recorded from intratracheal cannulation that bypassed the upper airway. Thus the possible presence of the effect of LA on laryngeal closure response to these stimuli cannot be ruled out.
To evaluate the effect of LA applied onto the larynx on the LCR and LMR, both LCR and LMR before and after laryngeal LA pretreatment were compared. We found that this pretreatment suppressed the apneic responses to CAP, HCl, and DW by 72, 71, and 81%, respectively, without effect on AP-induced apnea. Moreover, this pretreatment also attenuated hypertension and bradycardia in response to laryngeal application of CAP, HCl, and DW without effect on AP-induced bradycardia. These data clearly lead to a conclusion that laryngeal LA pretreatment is able to blunt the LCR but not LMR. The reason for the selectivity of LA suppressing LCR rather than LMR is unclear. One possibility for the lack of LA effect on the LMR is that the mechanoreceptors are sensitive to pressure, tension, or stretch, etc., and this mechanosensitivity is not affected by LA. Another one is that the laryngeal mechanoreceptors compared with the intralaryngeal chemoreceptors are located more deeply underneath the epithelial lining of the mucous membrane. Upon activation, airway cells, especially macrophages and epithelial cells, are capable of promoting release of mediators/neuropeptides, such as, PGE2 (3, 33), IL-1β (14, 15), and substance P (52), which are stimulants of sensory fibers (for review, see Ref. 41). One may question whether LA exerts its effect by directly acting on the SLN rather than indirectly by acting on laryngeal cells that release these mediators/neuropeptides to secondarily affect SLN activity. Thus, we compared the LCR before and after peri-SLN LA pretreatment. The peri-SLN or the perivagal pretreatment has been reported in rats and dogs (44, 45, 50). In these studies, the agents applied for the perineural treatment are absorbed into the axon and conveyed to the nerve terminals in the airways/lungs via an axonal transport system of the afferents (44). Interestingly, peri-SLN LA pretreatment utilized in this study suppressed the apnea, hypertension, and bradycardia in response to CAP, HCl, and DW to the degrees similar to those induced by laryngeal LA pretreatment (Figs. 2, 3, and 4 versus Fig. 6). The fact of no difference in baseline cardiorespiratory activity with (Table 2) and without (Table 1) surgical operation for the peri-SLN points to limited damage on the SLN by this surgical approach. It is generally accepted that intralaryngeal application of CAP selectively activates local TRPV1 of C fibers (12, 46, 50), while acid (HCl), DW, and AP predominantly activate acid-sensing ion channels, osmotic receptors, and mechanoreceptors of Aδ-fibers, respectively (1, 4, 11, 37, 67). Therefore, our data suggest that LA has a direct impact on laryngeal C and Aδ-fibers to suppress the LCR. This study did not test the effect of laryngeal LA pretreatment on cough because of the difficulty to provoke cough in anesthetized rat pups.
The impact of LA on the CAP-induced currents in laryngeal sensory C neurons was subsequently investigated by using the patch-clamp approach in this study since laryngeal CAP triggers apneas via acting on TRPV1 receptor of local C fibers (12, 46, 50). We found that the CAP-induced currents in laryngeal sensory C neurons were inhibited by LA pretreatment by reducing the current amplitude, increasing the rise time, and prolonging the decay time (Fig. 7). This finding in vitro, along with above results in vivo, suggest that LA applied on the laryngeal mucosa is able to depress the LCR via directly acting on laryngeal sensory fibers, such as TRPV1 of laryngeal sensory C fibers.
The mechanisms underlying the suppressive effect of LA on LCR are unknown. One possibility is that LA suppresses reactive oxygen species (ROS) and NADPH oxidase and in turn attenuates LCR, especially laryngeal C-fiber-mediated apnea via ROS interaction with TRPV1. Inhalation of aerosolized H2O2 (0.2–0.4%) leads to activation of pulmonary CAP-sensitive fibers attenuated by suppression of ROS in rats via inhibition of TRPV1 (59, 60). Moreover, ROS and NADPH oxidase are responsible for the potentiated LCR induced by intralaryngeal application of CAP, adenosine, and α,β-methylene-ATP after chronic intermittent hypoxia (81). These findings are consistent with the results in vitro that ROS sensitizes TRPV1 in dorsal root ganglia neurons (68). In fact, we recently found that the apneic duration evoked by intralaryngeal perfusion of CAP was significantly shortened by 46% after local perfusion of dimethylthiourea, a ROS inhibitor, in the rat pups (unpublished observation by W. Wei and F. Xu). This finding seems to support the activation of ROS in the SLN and the facilitatory effect of this activation on the laryngeal C-fiber-mediated apnea in the normal and anesthetized rat pups. In fact, previous studies have suggested that licorice extract and LA are able to decrease ROS by lowering myeloperoxidase activity, suppressing the depletion of glutathione and promoting superoxide dismutase activity (2 antioxidants) (25, 35a). These results point to the plausible involvement of LA suppressing ROS and NADPH in attenuation of the LCR, especially laryngeal C-fiber-mediated apnea. In addition, LA may function as a TRPV1 antagonist to account for LA effects on the LCR. These assumptions warrant further proof.
Our novel finding that LA suppresses the LCR via directly acting on laryngeal sensory fibers, particularly TRPV1, has clinical relevance. First, the sensitized and strengthened LCR in premature infants could lead to severe bradycardia and life-threatening apneas (9, 53), which has been linked to the pathogenesis of some SIDS victims (79). The “near-miss” SIDS has been reported in the clinical setting (20); one subject showed a very long apneic response to laryngeal stimulation and died later unexpectedly from SIDS (76). Therefore, LA would appear to be an attractive potential therapeutic candidate for this subgroup of infants. Second, the SLN-mediated apnea in the newborn becomes less pronounced by age (72) and the same SLN activation triggers highly protective reflexes to prevent subglottal aspiration, primarily cough and swallowing in the mature stage (21, 71, 72). Thus the impact of LA on the SLN may be accountable for interpretation of LA antitussive effect. Third, previous research has revealed that licorice plays important roles in antianaphylaxis (48), anti-inflammation (36), neuroprotection (31, 42), and analgesia (55). SLN sensory fibers are involved in local neurogenic anaphylaxis (70), neurogenic inflammation (43, 47), and nociceptive sensation (2, 26). Thus our data may extend LA application in treating these SLN-mediated responses. Other drugs have been previously reported to attenuate the LCR, such as aminophylline, caffeine, antihistamine agents, acetazolamide, β‐adrenergic agonists, topical lidocaine, and pretreatment by antagonists of the calcitonin gene‐related peptide (58). It would be difficult to compare the inhibitory effects of LA and these drugs on the LCR because of the great differences in the laryngeal stimulating intensities, doses of the drugs, and species used in our and these studies. Certainly, further study is necessary to determine whether laryngeal LA treatment is able to 1) alleviate the severe bradycardia and life-threatening apneas in SIDS models, such as those induced by prenatal nicotinic exposure in rat pups (23, 85); 2) suppress the cough responses (especially those mediated by the SLN) in animals; and 3) affect neurogenic anaphylaxis and inflammation as well as nociceptive sensation in the larynx.
GRANTS
This study was supported in part by National Heart, Lung, and Blood Institute Grant HL-119683 (to F. Xu) and National Natural Science Foundation of China Grant NSFC-81573970 (to Y. Jiao).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
W.W., X.G., L.Z., Y.J., and F.X. conceived and designed research; W.W., X.G., and L.Z. performed experiments; W.W., X.G., and J.Z. analyzed data; W.W., X.G., J.Z., Y.J., and F.X. interpreted results of experiments; W.W., X.G., and J.Z. prepared figures; W.W., X.G., and F.X. drafted manuscript; W.W., X.G., J.Z., Y.J., and F.X. edited and revised manuscript; Y.J. and F.X. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors are grateful to Ellen Blake for editing.
REFERENCES
- 1.Adzaku FK. The morphological and functional characteristics of the innervation of the subglottic mucosa of the larynx. Ann R Coll Surg Engl 62: 426–431, 1980. [PMC free article] [PubMed] [Google Scholar]
- 2.Agarwal A, Gupta D, Yadav G, Goyal P, Singh PK, Singh U. An evaluation of the efficacy of licorice gargle for attenuating postoperative sore throat: a prospective, randomized, single-blind study. Anesth Analg 109: 77–81, 2009. doi: 10.1213/ane.0b013e3181a6ad47. [DOI] [PubMed] [Google Scholar]
- 3.Aksoy MO, Li X, Borenstein M, Yi Y, Kelsen SG. Effects of topical corticosteroids on inflammatory mediator-induced eicosanoid release by human airway epithelial cells. J Allergy Clin Immunol 103: 1081–1091, 1999. doi: 10.1016/S0091-6749(99)70183-1. [DOI] [PubMed] [Google Scholar]
- 4.Anderson JW, Sant’Ambrogio FB, Mathew OP, Sant’Ambrogio G. Water-responsive laryngeal receptors in the dog are not specialized endings. Respir Physiol 79: 33–43, 1990. doi: 10.1016/0034-5687(90)90058-7. [DOI] [PubMed] [Google Scholar]
- 5.Asl MN, Hosseinzadeh H. Review of pharmacological effects of Glycyrrhiza sp. and its bioactive compounds. Phytother Res 22: 709–724, 2008. doi: 10.1002/ptr.2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aviv JE, Martin JH, Keen MS, Debell M, Blitzer A. Air pulse quantification of supraglottic and pharyngeal sensation: a new technique. Ann Otol Rhinol Laryngol 102: 777–780, 1993. doi: 10.1177/000348949310201007. [DOI] [PubMed] [Google Scholar]
- 7.Aviv JE, Martin JH, Kim T, Sacco RL, Thomson JE, Diamond B, Close LG. Laryngopharyngeal sensory discrimination testing and the laryngeal adductor reflex. Ann Otol Rhinol Laryngol 108: 725–730, 1999. doi: 10.1177/000348949910800802. [DOI] [PubMed] [Google Scholar]
- 8.Baldy C, Chamberland S, Fournier S, Kinkead R. Sex-specific consequences of neonatal stress on cardio-respiratory inhibition following laryngeal stimulation in rat pups. eNeuro 4: ENEURO.0393-17.2017, 2018. doi: 10.1523/ENEURO.0393-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boggs DF, Bartlett D Jr. Chemical specificity of a laryngeal apneic reflex in puppies. J Appl Physiol 53: 455–462, 1982. doi: 10.1152/jappl.1982.53.2.455. [DOI] [PubMed] [Google Scholar]
- 10.Boushey HA, Richardson PS, Widdicombe JG, Wise JC. The response of laryngeal afferent fibres to mechanical and chemical stimuli. J Physiol 240: 153–175, 1974. doi: 10.1113/jphysiol.1974.sp010605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bradley RM. Sensory receptors of the larynx. Am J Med 108, Suppl 4a: 47–50, 2000. doi: 10.1016/S0002-9343(99)00339-3. [DOI] [PubMed] [Google Scholar]
- 12.Carreau AM, Patural H, Samson N, Doueik AA, Hamon J, Fortier PH, Praud JP. Effects of simulated reflux laryngitis on laryngeal chemoreflexes in newborn lambs. J Appl Physiol (1985) 111: 400–406, 2011. doi: 10.1152/japplphysiol.00105.2011. [DOI] [PubMed] [Google Scholar]
- 13.Chung KF, Sant’Ambrogio G. The fiber composition of the superior laryngeal nerve (Abstract). FASEB J 7: A402, 1993. [Google Scholar]
- 14.Costa JJ, Matossian K, Resnick MB, Beil WJ, Wong DT, Gordon JR, Dvorak AM, Weller PF, Galli SJ. Human eosinophils can express the cytokines tumor necrosis factor-alpha and macrophage inflammatory protein-1 alpha. J Clin Invest 91: 2673–2684, 1993. doi: 10.1172/JCI116506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dantzer R. Cytokine-induced sickness behavior: mechanisms and implications. Ann NY Acad Sci 933: 222–234, 2001. doi: 10.1111/j.1749-6632.2001.tb05827.x. [DOI] [PubMed] [Google Scholar]
- 16.Davies AM, Koenig JS, Thach BT. Upper airway chemoreflex responses to saline and water in preterm infants. J Appl Physiol (1985) 64: 1412–1420, 1988. doi: 10.1152/jappl.1988.64.4.1412. [DOI] [PubMed] [Google Scholar]
- 17.Davis PJ. The sensitivity of laryngeal epithelial receptors to static and dynamic forms of mechanical stimulation. In: Vocal Physiology: Voice Production, edited by Fujimura O. New York: Raven, 1988. [Google Scholar]
- 18.Donnelly WT, Xia L, Bartlett D, Leiter JC. Activation of serotonergic neurons in the medullary caudal raphe shortens the laryngeal chemoreflex in anaesthetized neonatal rats. Exp Physiol 102: 1007–1018, 2017. doi: 10.1113/EP086082. [DOI] [PubMed] [Google Scholar]
- 19.Downing SE, Lee JC. Laryngeal chemosensitivity: a possible mechanism for sudden infant death. Pediatrics 55: 640–649, 1975. [PubMed] [Google Scholar]
- 20.Duffty P, Bryan MH. Home apnea monitoring in ‘near-miss’ sudden infant death syndrome (SIDS) and in siblings of SIDS victims. Pediatrics 70: 69–74, 1982. [PubMed] [Google Scholar]
- 21.Ebihara S, Ebihara T, Kohzuki M. Effect of aging on cough and swallowing reflexes: implications for preventing aspiration pneumonia. Hai 190: 29–33, 2012. doi: 10.1007/s00408-011-9334-z. [DOI] [PubMed] [Google Scholar]
- 22.Forsberg K, Karlsson JA, Theodorsson E, Lundberg JM, Persson CG. Cough and bronchoconstriction mediated by capsaicin-sensitive sensory neurons in the guinea-pig. Pulm Pharmacol 1: 33–39, 1988. doi: 10.1016/0952-0600(88)90008-7. [DOI] [PubMed] [Google Scholar]
- 23.Gao X, Zhao L, Zhuang J, Zang N, Xu F. Prenatal nicotinic exposure prolongs superior laryngeal C-fiber-mediated apnea and bradycardia through enhancing neuronal TRPV1 expression and excitation. FASEB J 31: 4325–4334, 2017. doi: 10.1096/fj.201700163R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gu QD, Moss CR 2nd, Kettelhut KL, Gilbert CA, Hu H. Activation of TRPV4 regulates respiration through indirect activation of bronchopulmonary sensory neurons. Front Physiol 7: 65, 2016. doi: 10.3389/fphys.2016.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guan Y, Li FF, Hong L, Yan XF, Tan GL, He JS, Dong XW, Bao MJ, Xie QM. Protective effects of liquiritin apioside on cigarette smoke-induced lung epithelial cell injury. Fundam Clin Pharmacol 26: 473–483, 2012. doi: 10.1111/j.1472-8206.2011.00956.x. [DOI] [PubMed] [Google Scholar]
- 26.Gupta D, Agrawal S, Sharma JP. Effect of preoperative licorice lozenges on incidence of postextubation cough and sore throat in smokers undergoing general anesthesia and endotracheal intubation. Middle East J Anaesthesiol 22: 173–178, 2013. [PubMed] [Google Scholar]
- 28.Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch 391: 85–100, 1981. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- 29.Hanacek J, Tatar M, Widdicombe J. Regulation of cough by secondary sensory inputs. Respir Physiol Neurobiol 152: 282–297, 2006. doi: 10.1016/j.resp.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 30.Hishida N, Tsubone H, Sugano S. Fiber composition of the superior laryngeal nerve in rats and guinea pigs. J Vet Med Sci 59: 499–501, 1997. doi: 10.1292/jvms.59.499. [DOI] [PubMed] [Google Scholar]
- 31.Hwang IK, Lim SS, Choi KH, Yoo KY, Shin HK, Kim EJ, Yoon-Park JH, Kang TC, Kim YS, Kwon DY, Kim DW, Moon WK, Won MH. Neuroprotective effects of roasted licorice, not raw form, on neuronal injury in gerbil hippocampus after transient forebrain ischemia. Acta Pharmacol Sin 27: 959–965, 2006. doi: 10.1111/j.1745-7254.2006.00346.x. [DOI] [PubMed] [Google Scholar]
- 32.Iyer SM, Montgomery KL, Towne C, Lee SY, Ramakrishnan C, Deisseroth K, Delp SL. Virally mediated optogenetic excitation and inhibition of pain in freely moving nontransgenic mice. Nat Biotechnol 32: 274–278, 2014. doi: 10.1038/nbt.2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jallat-Daloz I, Cognard JL, Badet JM, Regnard J. Neural-epithelial cell interplay: in vitro evidence that vagal mediators increase PGE2 production by human nasal epithelial cells. Allergy Asthma Proc 22: 17–23, 2001. doi: 10.2500/108854101778249267. [DOI] [PubMed] [Google Scholar]
- 34.Kamei J, Nakamura R, Ichiki H, Kubo M. Antitussive principles of Glycyrrhizae radix, a main component of the Kampo preparations Bakumondo-to (Mai-men-dong-tang). Eur J Pharmacol 469: 159–163, 2003. doi: 10.1016/S0014-2999(03)01728-X. [DOI] [PubMed] [Google Scholar]
- 35.Kamei J, Saitoh A, Asano T, Nakamura R, Ichiki H, Iiduka A, Kubo M. Pharmacokinetic and pharmacodynamic profiles of the antitussive principles of Glycyrrhizae radix (licorice), a main component of the Kampo preparation Bakumondo-to (Mai-men-dong-tang). Eur J Pharmacol 507: 163–168, 2005. doi: 10.1016/j.ejphar.2004.11.042. [DOI] [PubMed] [Google Scholar]
- 35a.Khattab HA, Abdel-Dayem UA, Jambi HA, Abba AT, Abdul-Jawad MTA, El-Shitany NAEF. Licorice (Glycyrrhizza glabra) extract prevents production of Th2 cytokines and free radicals induced by ova albumin in mice. Int J Pharmacol 14: 1072–1079, 2018. doi: 10.3923/ijp.2018.1072.1079. [DOI] [Google Scholar]
- 36.Kim JK, Oh SM, Kwon HS, Oh YS, Lim SS, Shin HK. Anti-inflammatory effect of roasted licorice extracts on lipopolysaccharide-induced inflammatory responses in murine macrophages. Biochem Biophys Res Commun 345: 1215–1223, 2006. doi: 10.1016/j.bbrc.2006.05.035. [DOI] [PubMed] [Google Scholar]
- 37.Kollarik M, Undem BJ. Mechanisms of acid-induced activation of airway afferent nerve fibres in guinea-pig. J Physiol 543: 591–600, 2002. doi: 10.1113/jphysiol.2002.022848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kuang Y, Li B, Fan J, Qiao X, Ye M. Antitussive and expectorant activities of licorice and its major compounds. Bioorg Med Chem 26: 278–284, 2018. doi: 10.1016/j.bmc.2017.11.046. [DOI] [PubMed] [Google Scholar]
- 39.Lahmann C, Kramer HB, Ashcroft FM. Systemic administration of glibenclamide fails to achieve therapeutic levels in the brain and cerebrospinal fluid of rodents. PLoS One 10: e0134476, 2015. [Erratum in PLoS One 14: e0215989, 2019.] doi: 10.1371/journal.pone.0134476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee JC, Stoll BJ, Downing SE. Properties of the laryngeal chemoreflex in neonatal piglets. Am J Physiol Regul Integr Comp Physiol 233: R30–R36, 1977. doi: 10.1152/ajpregu.1977.233.1.R30. [DOI] [PubMed] [Google Scholar]
- 41.Lee LY, Pisarri TE. Afferent properties and reflex functions of bronchopulmonary C-fibers. Respir Physiol 125: 47–65, 2001. doi: 10.1016/S0034-5687(00)00204-8. [DOI] [PubMed] [Google Scholar]
- 42.Li H, Ye M, Zhang Y, Huang M, Xu W, Chu K, Chen L, Que J. Blood-brain barrier permeability of Gualou Guizhi granules and neuroprotective effects in ischemia/reperfusion injury. Mol Med Rep 12: 1272–1278, 2015. doi: 10.3892/mmr.2015.3520. [DOI] [PubMed] [Google Scholar]
- 43.Lima-Rodrigues M, Valle-Fernandes A, Lamas N, Cruz A, Baltazar F, Milanezi F, Nunes R, Reis RM, Pedrosa J, Castro AG, Almeida A. A new model of laryngitis: neuropeptide, cyclooxygenase, and cytokine profile. Laryngoscope 118: 78–86, 2008. doi: 10.1097/MLG.0b013e3181492400. [DOI] [PubMed] [Google Scholar]
- 44.Lin YJ, Lin YS, Lai CJ, Yuan ZF, Ruan T, Kou YR. Perivagal antagonist treatment in rats selectively blocks the reflex and afferent responses of vagal lung C fibers to intravenous agonists. J Appl Physiol (1985) 114: 361–370, 2013. doi: 10.1152/japplphysiol.00977.2012. [DOI] [PubMed] [Google Scholar]
- 45.Liu BY, Lin YJ, Lee HF, Ho CY, Ruan T, Kou YR. Menthol suppresses laryngeal C-fiber hypersensitivity to cigarette smoke in a rat model of gastroesophageal reflux disease: the role of TRPM8. J Appl Physiol (1985) 118: 635–645, 2015. doi: 10.1152/japplphysiol.00717.2014. [DOI] [PubMed] [Google Scholar]
- 46.Liu BY, Tsai TL, Ho CY, Lu SH, Lai CJ, Kou YR. Role of TRPA1 and TRPV1 in the ROS-dependent sensory irritation of superior laryngeal capsaicin-sensitive afferents by cigarette smoke in anesthetized rats. Pulm Pharmacol Ther 26: 364–372, 2013. doi: 10.1016/j.pupt.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 47.Lundberg JM, Brodin E, Hua X, Saria A. Vascular permeability changes and smooth muscle contraction in relation to capsaicin-sensitive substance P afferents in the guinea-pig. Acta Physiol Scand 120: 217–227, 1984. doi: 10.1111/j.1748-1716.1984.tb00127.x. [DOI] [PubMed] [Google Scholar]
- 48.Majima T, Yamada T, Tega E, Sakurai H, Saiki I, Tani T. Pharmaceutical evaluation of liquorice before and after roasting in mice. J Pharm Pharmacol 56: 589–595, 2004. doi: 10.1211/0022357023286. [DOI] [PubMed] [Google Scholar]
- 49.Murakami Y, Kirchner JA. Mechanical and physiological properties of reflex laryngeal closure. Ann Otol Rhinol Laryngol 81: 59–71, 1972. doi: 10.1177/000348947208100106. [DOI] [PubMed] [Google Scholar]
- 50.Mutoh T, Kanamaru A, Kojima K, Nishimura R, Sasaki N, Tsubone H. Effects of perineural capsaicin treatment on cardiopulmonary reflexes elicited by laryngeal instillations of capsaicin and distilled water in sevoflurane-anesthetized dogs. J Vet Med Sci 62: 665–668, 2000. doi: 10.1292/jvms.62.665. [DOI] [PubMed] [Google Scholar]
- 51.Nosalova G, Fleskova D, Jurecek L, Sadlonova V, Ray B. Herbal polysaccharides and cough reflex. Respir Physiol Neurobiol 187: 47–51, 2013. doi: 10.1016/j.resp.2013.03.015. [DOI] [PubMed] [Google Scholar]
- 52.O’Connor TM, O’Connell J, O’Brien DI, Goode T, Bredin CP, Shanahan F. The role of substance P in inflammatory disease. J Cell Physiol 201: 167–180, 2004. doi: 10.1002/jcp.20061. [DOI] [PubMed] [Google Scholar]
- 53.Page M, Jeffery H. The role of gastro-oesophageal reflux in the aetiology of SIDS. Early Hum Dev 59: 127–149, 2000. doi: 10.1016/S0378-3782(00)00093-1. [DOI] [PubMed] [Google Scholar]
- 54.Pendlebury JD, Wilson RJ, Bano S, Lumb KJ, Schneider JM, Hasan SU. Respiratory control in neonatal rats exposed to prenatal cigarette smoke. Am J Respir Crit Care Med 177: 1255–1261, 2008. doi: 10.1164/rccm.200711-1739OC. [DOI] [PubMed] [Google Scholar]
- 55.Peng ZC, Lu H, Yi SF. The analgesic effect of processed licorice. Chin J Chin Mater Med 14: 22–23, 1989. [Google Scholar]
- 56.Potenzieri C, Meeker S, Undem BJ. Activation of mouse bronchopulmonary C-fibres by serotonin and allergen-ovalbumin challenge. J Physiol 590: 5449–5459, 2012. doi: 10.1113/jphysiol.2012.237115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Negus V. The Comparative Anatomy and Physiology of the Larynx. New York: Hafner Publishing, 1962. [Google Scholar]
- 58.Reix P, St-Hilaire M, Praud JP. Laryngeal sensitivity in the neonatal period: from bench to bedside. Pediatr Pulmonol 42: 674–682, 2007. doi: 10.1002/ppul.20645. [DOI] [PubMed] [Google Scholar]
- 59.Ruan T, Ho CY, Kou YR. Afferent vagal pathways mediating respiratory reflexes evoked by ROS in the lungs of anesthetized rats. J Appl Physiol (1985) 94: 1987–1998, 2003. doi: 10.1152/japplphysiol.01047.2002. [DOI] [PubMed] [Google Scholar]
- 60.Ruan T, Lin YS, Lin KS, Kou YR. Sensory transduction of pulmonary reactive oxygen species by capsaicin-sensitive vagal lung afferent fibres in rats. J Physiol 565: 563–578, 2005. doi: 10.1113/jphysiol.2005.086181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Saha S, Nosál’ová G, Ghosh D, Flešková D, Capek P, Ray B. Structural features and in vivo antitussive activity of the water extracted polymer from Glycyrrhiza glabra. Int J Biol Macromol 48: 634–638, 2011. doi: 10.1016/j.ijbiomac.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 62.Sampson S, Eyzaguirre C. Some functional characteristics of mechanoreceptors in the larynx of the cat. J Neurophysiol 27: 464–480, 1964. doi: 10.1152/jn.1964.27.3.464. [DOI] [PubMed] [Google Scholar]
- 63.Shergis JL, Wu L, May BH, Zhang AL, Guo X, Lu C, Xue CC. Natural products for chronic cough: text mining the East Asian historical literature for future therapeutics. Chron Respir Dis 12: 204–211, 2015. doi: 10.1177/1479972315583043. [DOI] [PubMed] [Google Scholar]
- 64.St-Hilaire M, Nsegbe E, Gagnon-Gervais K, Samson N, Moreau-Bussière F, Fortier PH, Praud JP. Laryngeal chemoreflexes induced by acid, water, and saline in nonsedated newborn lambs during quiet sleep. J Appl Physiol (1985) 98: 2197–2203, 2005. doi: 10.1152/japplphysiol.01346.2004. [DOI] [PubMed] [Google Scholar]
- 65.St-Hilaire M, Samson N, Nsegbe E, Duvareille C, Moreau-Bussière F, Micheau P, Lebon J, Praud JP. Postnatal maturation of laryngeal chemoreflexes in the preterm lamb. J Appl Physiol (1985) 102: 1429–1438, 2007. doi: 10.1152/japplphysiol.00977.2006. [DOI] [PubMed] [Google Scholar]
- 66.Stephenson R, Liao KS, Hamrahi H, Horner RL. Circadian rhythms and sleep have additive effects on respiration in the rat. J Physiol 536: 225–235, 2001. doi: 10.1111/j.1469-7793.2001.00225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Storey AT, Johnson P. Laryngeal water receptors initiating apnea in the lamb. Exp Neurol 47: 42–55, 1975. doi: 10.1016/0014-4886(75)90235-6. [DOI] [PubMed] [Google Scholar]
- 68.Susankova K, Tousova K, Vyklicky L, Teisinger J, Vlachova V. Reducing and oxidizing agents sensitize heat-activated vanilloid receptor (TRPV1) current. Mol Pharmacol 70: 383–394, 2006. doi: 10.1124/mol.106.023069. [DOI] [PubMed] [Google Scholar]
- 69.Suzuki M, Kirchner JA. Afferent nerve fibers in the external branch of the superior larygneal nerve in the cat. Ann Otol Rhinol Laryngol 77: 1059–1070, 1968. doi: 10.1177/000348946807700606. [DOI] [PubMed] [Google Scholar]
- 70.Szereda-Przestaszewska M. The effect of anaphylactic shock on laryngeal calibre in rabbits. Respir Physiol 25: 1–7, 1975. doi: 10.1016/0034-5687(75)90046-8. [DOI] [PubMed] [Google Scholar]
- 71.Thach BT. Maturation and transformation of reflexes that protect the laryngeal airway from liquid aspiration from fetal to adult life. Am J Med 111, Suppl 8A: 69–77, 2001. doi: 10.1016/S0002-9343(01)00860-9. [DOI] [PubMed] [Google Scholar]
- 72.Thach BT. Maturation of cough and other reflexes that protect the fetal and neonatal airway. Pulm Pharmacol Ther 20: 365–370, 2007. doi: 10.1016/j.pupt.2006.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Thach BT. Reflux associated apnea in infants: evidence for a laryngeal chemoreflex. Am J Med 103: 120S–124S, 1997. doi: 10.1016/S0002-9343(97)00336-7. [DOI] [PubMed] [Google Scholar]
- 74.Thompson DM, Rutter MJ, Willging JP, Rudolph CD, Cotton RT. Altered laryngeal sensation: a potential cause of apnea of infancy. Ann Otol Rhinol Laryngol 114: 258–263, 2005. doi: 10.1177/000348940511400402. [DOI] [PubMed] [Google Scholar]
- 75.Wang M, Zhang M, Tang Q, Li X. Influence of honey-roasting on the main pharmacological activities and the water-soluble active glycosides of licorice. Afr J Tradit Complement Altern Med 9: 189–196, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wennergren G, Hertzberg T, Milerad J, Bjure J, Lagercrantz H. Hypoxia reinforces laryngeal reflex bradycardia in infants. Acta Paediatr Scand 78: 11–17, 1989. doi: 10.1111/j.1651-2227.1989.tb10879.x. [DOI] [PubMed] [Google Scholar]
- 77.Wetmore RF. Effects of acid on the larynx of the maturing rabbit and their possible significance to the sudden infant death syndrome. Laryngoscope 103: 1242–1254, 1993. doi: 10.1288/00005537-199311000-00006. [DOI] [PubMed] [Google Scholar]
- 78.Widdicombe JG, Tatar M. Upper airway reflex control. Ann NY Acad Sci 533: 252–261, 1988. doi: 10.1111/j.1749-6632.1988.tb37254.x. [DOI] [PubMed] [Google Scholar]
- 79.Xia L, Leiter JC, Bartlett D Jr. Laryngeal apnea in rat pups: effects of age and body temperature. J Appl Physiol (1985) 104: 269–274, 2008. doi: 10.1152/japplphysiol.00721.2007. [DOI] [PubMed] [Google Scholar]
- 80.Xu F, Gu QH, Zhou T, Lee LY. Acute hypoxia prolongs the apnea induced by right atrial injection of capsaicin. J Appl Physiol (1985) 94: 1446–1454, 2003. doi: 10.1152/japplphysiol.00767.2002. [DOI] [PubMed] [Google Scholar]
- 81.Yang CH, Zhuang WL, Shen YJ, Lai CJ, Kou YR. NADPH oxidase-derived ROS induced by chronic intermittent hypoxia mediates hypersensitivity of lung vagal c fibers in rats. Front Physiol 7: 166, 2016. doi: 10.3389/fphys.2016.00166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yang R, Wang LQ, Yuan BC, Liu Y. The pharmacological activities of licorice. Planta Med 81: 1654–1669, 2015. doi: 10.1055/s-0035-1557893. [DOI] [PubMed] [Google Scholar]
- 83.Yoshida Y, Tanaka Y, Hirano M, Nakashima T. Sensory innervation of the pharynx and larynx. Am J Med 108, Suppl 4a: 51–61, 2000. doi: 10.1016/S0002-9343(99)00342-3. [DOI] [PubMed] [Google Scholar]
- 84.Zhang Z, Zhuang J, Zhang C, Xu F. Isoflurane depolarizes bronchopulmonary C neurons by inhibiting transient A-type and delayed rectifier potassium channels. Respir Physiol Neurobiol 186: 164–172, 2013. doi: 10.1016/j.resp.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 85.Zhuang J, Zhao L, Zang N, Xu F. Prenatal nicotinic exposure augments cardiorespiratory responses to activation of bronchopulmonary C-fibers. Am J Physiol Lung Cell Mol Physiol 308: L922–L930, 2015. doi: 10.1152/ajplung.00241.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]