Abstract
Disturbances in mitochondrial structure and function in lung epithelial cells have been implicated in the pathogenesis of various lung diseases, including chronic obstructive pulmonary disease (COPD). Such disturbances affect not only cellular energy metabolism but also alter a range of indispensable cellular homeostatic functions in which mitochondria are known to be involved. These range from cellular differentiation, cell death pathways, and cellular remodeling to physical barrier function and innate immunity, all of which are known to be impacted by exposure to cigarette smoke and have been linked to COPD pathogenesis. Next to their well-established role as the first physical frontline against external insults, lung epithelial cells are immunologically active. Malfunctioning epithelial cells with defective mitochondria are unable to maintain homeostasis and respond adequately to further stress or injury, which may ultimately shape the phenotype of lung diseases. In this review, we provide a comprehensive overview of the impact of cigarette smoke on the development of mitochondrial dysfunction in the lung epithelium and highlight the consequences for cell function, innate immune responses, epithelial remodeling, and epithelial barrier function in COPD. We also discuss the applicability and potential therapeutic value of recently proposed strategies for the restoration of mitochondrial function in the treatment of COPD.
Keywords: COPD, cigarette smoke, lung epithelial cells, mitochondrial dysfunction
INTRODUCTION
Chronic obstructive pulmonary disease (COPD) is a chronic lung disease caused mainly by cigarette smoking and characterized by chronic inflammation, airway obstruction, and emphysema, culminating in airway obstruction and a progressive decline in lung function (50). Despite the fact that smoking is the most important risk factor for the development of COPD, 10–30% of COPD patients have never smoked (112), indicating that environmental factors distinct from cigarette smoke (CS) contribute to COPD, including occupational exposures and indoor and outdoor air pollution (6, 71). Furthermore, only 20% of smokers develop COPD, which indicates that, in addition to environmental factors, genetic factors may also be involved (112). A wide variety of cells have been implicated in the complex pathogenesis of COPD. In particular, epithelial cells are considered a central player (47, 60). Lung epithelial cells, extending from the upper airways to the terminal alveoli, act as the first line of protection against pathogens and noxious particles in the inhaled air (123). Chronic exposure of lung epithelial cells to these toxicants, in particular CS, triggers oxidative stress, inflammation (116), cell death, cellular remodeling, cellular senescence, and cellular dysfunction (84), all of which are key processes involved in the pathogenesis of COPD. Emerging evidence indicates a regulatory role for mitochondria in these cellular processes alongside with their traditional role as the cell’s powerhouse (24).
Disturbances in mitochondrial morphology and function have been reported in airway epithelial cells of both non-COPD smokers as well as COPD patients, with more pronounced changes in COPD (40, 49). Impaired mitochondrial function in in these cell types may have severe consequences for cell fate and functionality, particularly in cells with high energy demands, including ciliated cells of the airway epithelium and type II alveolar epithelial cells (AECII) that need substantial levels of ATP for ciliary beating and surfactant production, respectively (24). In addition to energy depletion, mitochondrial dysfunction leads to excessive production of mitochondrial reactive oxygen species (mtROS), stress, and cell death, which ultimately can contribute to tissue injury. In this review, we provide a comprehensive overview of the potential links between CS exposure, mitochondrial dysfunction, and cardinal cellular responses involved in the pathophysiology of COPD, including loss of epithelial barrier integrity, innate immunity, disturbed redox balance, autophagy, and cellular senescence.
MITOCHONDRIA IN LUNG EPITHELIAL CELLS DURING HOMEOSTASIS
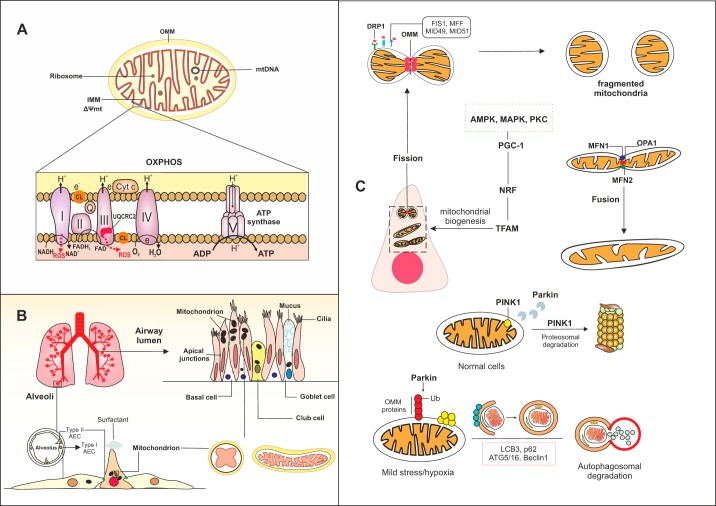
Mitochondria are double-membrane organelles with prokaryotic ancestors that are thought to have been incorporated into the eukaryotic cell cytoplasm during evolution (136). As a result, mitochondria have preserved their own specific structures, such as CpG-rich DNA, membrane lipids (in particular cardiolipin), N-formylated peptides, and bioenergetic pathways, while functioning as a part of an integrated intracellular system predominantly regulated by genomic DNA. Mitochondrial DNA (mtDNA) encodes 37 genes, most of which encode for redox active proteins located in the inner mitochondrial membrane (IMM) that are involved in oxidative phosphorylation (OXPHOS), a process involving the transfer of electrons through a chain of enzymatic complexes (I–IV) leading to the production of ATP (Fig. 1A) (24). Generation of ATP by the mitochondrion provides the energy required for a wide variety of cellular processes. Interestingly, different subtypes of airway epithelial cells, including ciliated cells, secretory goblet and club cells, and progenitor basal cells, as well as type I alveolar (AECI) and AECII, contain a distinct number of mitochondria with different intracellular organization to optimally facilitate energy demand and cellular function. For instance, in ciliated airway epithelial cells, mitochondria with highly folded cristae and a dense matrix are concentrated mostly apically (125), whereas in airway goblet cells and AECII mitochondria reside around the nucleus (17, 18). In basal and club cells of the airway epithelium, mitochondria are found scattered throughout the cytoplasm (Fig. 1B) (92, 97). This cell-specific distribution of mitochondria affects ATP and metabolite levels that are subsequently used for specific functions of these different cell types (ciliary beating or mucus secretion in differentiated airway epithelial cells and surfactant production in AECII). In addition, different epithelial cell subsets vary in their sensitivity to mitochondrial damage. For example, mitochondria in club cells are more susceptible to metabolic changes compared with other cell types within the airway epithelium (63), which is not surprising given recent evidence from single-cell transcriptomics demonstrating that human club cells are metabolically active and contain a large number of functional mitochondria (137), rendering them highly sensitive to oxidative damage.
Fig. 1.
Schematic of mitochondrial function and localization in various lung epithelial cells during homeostasis. A: within the mitochondria, the double membrane with a folded inner membrane (IMM) forms cristae. The mitochondrial matrix contains circular mitochondrial DNA (mtDNA). Oxidative phosphorylation (OXPHOS), which provides the ATP required for cellular functions, occurs in this hyper-folded IMM. During OXPHOS, a proton gradient is established over the IMM through the flow of electrons through chains of enzymatic complexes (I–V), which leads to the production of reactive oxygen species (ROS), H2O, and as ATP. Cytochrome c and coenzyme Q facilitate the transfer of electrons during OXPHOS. Complexes I and III are the major sites of ROS production in the electron transport chain (ETC). Ubiquinol-cytochrome c reductase core II (UQCRC2) is a damage-sensitive protein that contributes to ROS generation in the complex III of ETC. Oxidation of cardiolipin (CL), the mitochondrial-specific lipid in the IMM, can induce oxidative damage. B: in the upper respiratory tract, the airway epithelium constitutes the first physical defensive barrier against inhaled pathogens and toxic gases and particles, whereas the type I and type II alveolar epithelial cells (AEC) that cover the alveolar surface facilitate gas exchange. C: the mitochondrial network is highly dynamic in lung epithelial cells. Various processes contribute to generation and degradation of mitochondria in the cells. These processes include fusion, fission, mitochondrial-specific autophagy (mitophagy), and mitochondrial biogenesis. During fission, recognition of activated dynamin-related protein 1 (DRP1) by surface receptors (FIS1, MFF, MID49, and MID51) leads to fragmentation of the mitochondrial outer membrane (OMM) and formation of new mitochondria. Mitochondria can form an elongated shape by fusion, in which mitochondrial membranes tether through fusion proteins in the IMM such as optic atrophy 1 (OPA1) and outer membranes mitofusin 1 (MFN1) and MFN2. Mitophagy is regulated by several proteins, including PTEN-induced kinase 1 (PINK1) and Parkin. In homeostatic conditions, translocation of PINK1 to the IMM triggers recruitment of E3 ligase Parkin, which leads to proteasomal degradation of PINK1. Upon mild stress mitochondria can undergo complete degradation by mitophagy, in which accumulation of PINK-1 in the OMM and subsequent ubiquitination of several proteins at the OMM by Parkin lead to engulfment of the damaged mitochondrion by autophagy compartments. Autophagy proteins LCB3, p62, ATG5/16, and beclin 1 facilitate the generation of phagophore and autophagosomes. Formation of new mitochondria can be triggered by activation of peroxisome proliferator-activated receptor-γ coactivator1α (PGC-1α) and downstream transcription factors, nuclear respiratory factors 1 and 2 (NRF1/2), and mitochondrial transcription factor A (TFAM). Activation of several proteins, including AMP-activated protein kinase (AMPK), mitogen-activated protein kinase (MAPK), and protein kinase C (PKC), may activate the PGC-1α signaling pathway and in turn lead to synthesis of mitochondria.
Disturbances in the production of reactive oxygen species (ROS) by the mitochondrion contribute to cellular injury. Under normal physiological conditions, ROS released from mitochondria act as a second messenger to maintain cellular homeostasis. Several mitochondrial proteins, in particular those in the electron transport chain (ETC), contribute to ROS formation, including complex I and III of the ETC, ubiquinol-cytochrome c reductase core II (UQCRC2) (4), mitochondrial uncoupling protein 2 (UCP2) (73), and the adaptor protein P66shc (54). When generated in excessive amounts by damaged mitochondria, ROS contribute to cellular injury, further propagating cellular stress response or programmed cell death pathways (16). Besides being major endogenous producers of ROS, mitochondria are also the main targets of ROS, resulting in oxidative damage to mitochondrial proteins, mtDNA damage, and mutations with excessive electron leakage, inflicting further oxidative stress in a vicious cycle.
The mitochondrial network is highly dynamic, allowing adaptation to changes in homeostatic conditions and cellular responses to damage. Processes involved in remodeling of the mitochondrial network include fusion and fission events, e.g., to exchange mtDNA during repair processes, the creation of new mitochondria (biogenesis), or the removal of damaged mitochondria by mitochondrial-specific autophagy (mitophagy) (Fig. 1C). The biogenesis of mitochondria is regulated largely by peroxisome proliferator-activated receptor-γ coactivator-1 molecules (PGC-1α and PGC-1β), with mitochondrial transcription factor A (TFAM) and nuclear respiratory factors 1 and 2 (NRF1/2) acting as key downstream regulators (87). Activation of several kinases can contribute to mitochondrial biogenesis via regulation of PGC-1α. Among these, protein kinase C, mitogen-activated protein kinases (MAPK), and AMP-activated protein kinase (AMPK), an upstream regulator of mechanistic target of rapamycin (mTOR) (46), have been implicated in CS-induced epithelial cell dysfunction (7). Although increased mitochondrial biogenesis is expected to be beneficial for cellular functions, increased ROS production as a result of an increase in the number or activity of mitochondria may render the cells more vulnerable to deleterious processes such as irreversible growth arrest or senescence (41), especially when accompanied by an imbalance between oxidants and antioxidant capacity, as observed in COPD (72).
Mitochondrial fusion and fission events are regulated primarily by specialized proteins located on the outer and inner mitochondrial membrane. These proteins belong to the guanosine triphosphatase (GTPases) family of dynamin proteins, which are regulated chiefly by the proteolytic machinery (77). During fission, the outer mitochondrial membrane (OMM) is segregated by dynamin-related protein 1 (DRP1) upon recognition by the OMM receptors fission protein 1 (FIS1), mitochondrial fission factor (MFF), mitochondrial dynamic protein 49 (MID49), and MID51, resulting in a fragmented mitochondrial network (78). On the other hand, during fusion, the OMM proteins mitofusin 1 (MFN1) and MFN2, as well as the IMM protein optic atrophy 1 (OPA1), tether mitochondrial membranes, encouraging fusion, allowing neighboring mitochondria to exchange genetic material as well as promoting OXPHOS capacity (77). Silencing of fusion factors OPA1 and MFN leads to mitochondrial fragmentation and subsequent irreversible cell growth arrest in airway epithelial cells, whereas inhibition of FIS1 and DRP1 does not impact cell growth (40), suggesting an essential role of especially mitochondrial fusion in the regulation of cell growth during homeostasis.
Under conditions of mitochondrial injury, damaged or dysfunctional mitochondria are removed by mitophagy, the selective removal of mitochondria by autophagy. PTEN-induced kinase 1 (PINK1), a master regulator of mitophagy (41), undergoes proteasomal degradation in healthy cells via recruitment of E3 ligase PARK2-encoded Parkin upon translocation to the IMM (130). Under stressful conditions (e.g., hypoxia, oxidative stress) this proteasomal degradation is impaired, leading to accumulation of PINK1 on the OMM and subsequent recruitment of Parkin. Parkin then ubiquitinates several proteins in the OMM, acting as a tagging signal for the engulfment of stressed mitochondria into a double-membrane autophagosome. Autophagosomal degradation of mitochondria is mediated by several proteins, including autophagy-related genes (ATGs), beclin-1, microtubule-associated protein 1 light chain 3 (LCB3), and p62 (99). Additionally, PINK1-independent dephosphorylation of the OMM receptor FUN14 domain containing 1 (FUNDC1) and PINK1-dependent phosphorylation of BLC2-interacting protein 3 (BNIP3), BNIP3-like (BNP3L) proteins, optineurin (OPTN), and nuclear dot protein 52 (NDP52) may act as adaptors for the formation of the autophagosome around mitochondria, facilitating mitophagy (129).
MITOCHONDRIAL ABNORMALITIES IN LUNG EPITHELIAL CELLS EXPOSED TO CIGARETTE SMOKE
Several studies have provided evidence for mitochondrial abnormalities in lung epithelial cells of subjects with COPD or emphysema (79). These studies reported loss of cristae, abnormally branched, swollen, and fragmented organelles in primary airway epithelial cells isolated from COPD patients (40, 49), and mitochondrial swelling in primary AECII from patients with emphysema (62). In support of a causative role of CS exposure in mediating these morphological abnormalities, long-term exposure of human airway epithelial cells to CS extract (CSE) recapitulates observations in COPD subjects, with CSE inducing similar aberrations in mitochondrial morphology (24, 40, 49). Given that the above studies utilized airway epithelial cells (from both COPD and non-COPD subjects) in submerged cultures instead of cells differentiated in an air-liquid interface, the main cell type investigated in these studies is most likely a basal-like undifferentiated cell. Further studies are needed to assess whether exposure of differentiated cultures of human airway epithelial cells containing ciliated cells, goblet, and club cells to CS in vitro results in similar changes in mitochondrial morphology.
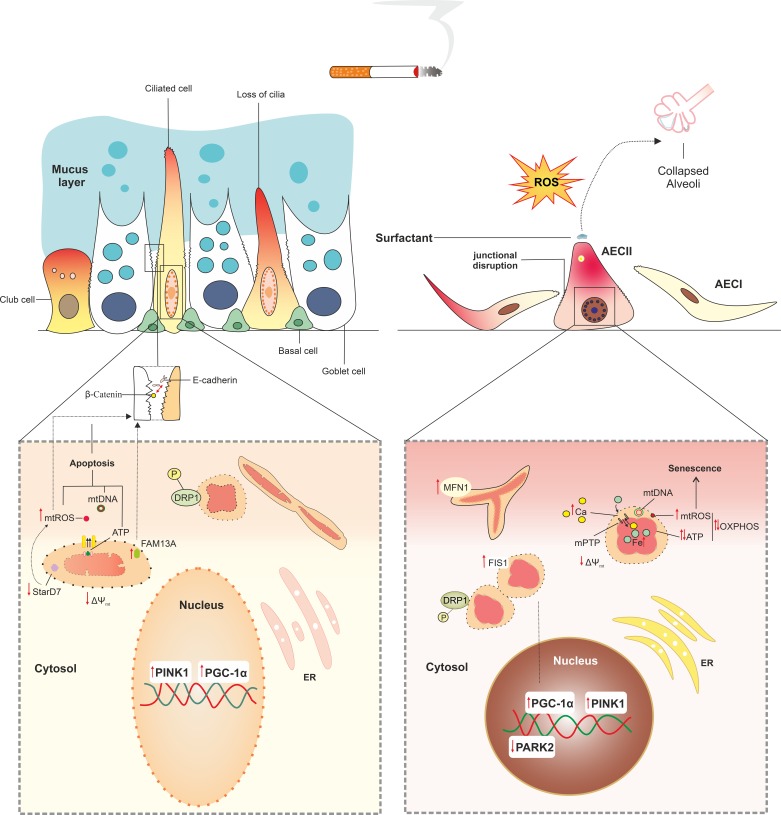
In line with the above-mentioned in vitro studies using human cells, 3 months in vivo exposure of mice to CS also resulted in mitochondrial damage in both the airway and alveolar compartment (79). Suggestive of a potential link between these morphological changes and impairments in mitochondrial function as well as aberrations in cellular functionality in response to CS exposure, abnormalities in mitochondrial morphology were associated with a lower mtDNA content, decreased oxygen consumption rates, reduced ATP levels, decreased activity of the ETC, loss of mitochondrial membrane potential, increased levels of mtROS, loss of ciliary function, and decreased production of surfactant (Fig. 2) (1, 24, 40, 49, 62, 74, 79, 114, 116). Decreases in cellular energy production in response to airway epithelial exposure to CS in these studies likely originated from impairments in the ETC, as exposure of airway epithelial cells to CS has been shown to result in decreased activity levels of ETC complexes I–III (114, 116) as well as increased autophagosomal breakdown of ETC complexes III and V (79). Taken together, these studies convincingly demonstrate that CS exposure results in aberrations in mitochondrial morphology and function in lung epithelial cells, which is in line with observations in primary lung epithelial cells from COPD patients.
Fig. 2.
Cigarette smoke (CS) induces abnormalities in mitochondrial morphology and function in lung epithelial cells. CS exposure of lung epithelial cells results in swollen and branched mitochondria with a condensed matrix. These morphological features are accompanied by functional alterations in mitochondria, including altered oxidative phosphorylation (OXPHOS) with higher reactive oxygen species (ROS) production and lower ATP generation, depolarization of mitochondrial membrane, and impaired mitophagy, all of which cause subsequent changes at the cellular level. These changes include loss of ciliary function, increase in mucus production in the airway epithelium, and impaired production of surfactant in type II alveolar epithelial cells (AECII), leading to alveolar collapse. CS increases the permeability of the mitochondrial membranes and opens ion channels such as mitochondrial permeability transition pore (mPTP) in the inner membrane, leading to overload of iron in mitochondria and cytoplasmic accumulation of mitochondrial danger-associated molecular patterns (mtDAMPs), including Ca2+, ATP, mitochondrial ROS (mtROS), mtDNA, cardiolipin, and N-formylated peptides, further inducing oxidative damage and cell death. CS-induced mitochondrial dysfunction may contribute to a leaky manifestation of the airway epithelium by increased mtROS and subsequent weakening cellular junctions. Furthermore, increase in FAM13A has been associated with disrupted airway epithelial barrier by increasing mtROS. Excessive mtROS has also consequences for cell fate and growth, inducing permanent damage to mtDNA, leading to increased mitophagy and cell apoptosis, and irreversible arrest in cell growth. Short-term CS exposure may enhance mitochondrial biogenesis via increase in PPAR-γ coactivator1α (PGC-1α) transcript levels, whereas longer exposure times suppress this process. CS induces an imbalance in fusion/fission process by more trends to fission, leading to fragmentation of mitochondria in lung epithelial cells. CS enhances clearance of fragmented mitochondria by mitophagy through increase in PTEN-induced kinase 1 (PINK1) mRNA and protein levels in both airway epithelial cells and AECII.
Interestingly, in apparent contrast to these studies, Ballweg et al. (11) reported hyperfusion of mitochondria and increased metabolic activity in primary mouse AECII in response to low and non-toxic doses of CSE, suggestive of mitochondrial adaptation. More specifically, these authors observed that exposure of these cells to 25% CSE resulted in hyperfused mitochondria as early as 6 h and persisting to 48 h postexposure, whereas increased cellular metabolic activity was observed with concentrations of CSE of ≤10–25%, with the effects being most pronounced 24–48 h postexposure. In line with this, 8 wk of exposure of mice to CS increased expression of genes encoding ETC complexes II, III, IV, and V as well as activity levels of ETC complexes II, IV, and V and upregulated genes involved in metabolism, mitochondrial transport, and dynamics in lung tissue (2). The increased expression of subunits of ETC complexes II, III, and VF1α was also reported in immortalized human bronchial BEAS2-B epithelial cells exposed to 10% CSE for 6 mo, whereas complex VF1α was higher in COPD-derived airway epithelial cells compared with control-derived cells, and may be illustrative of a compensatory mechanism (49). These apparently conflicting results in the effect of CSE on mitochondrial function are most likely attributed to the different concentrations of CSE used and possibly relate to an initial adaptation response by mitochondria (mitohormesis) at lower concentrations, whereas higher concentrations or prolonged exposure may result in mitochondrial dysfunction (19).
In addition to deteriorative effects of CS on mitochondrial morphology and function in lung epithelial cells, studies have also reported similar detrimental effects of CS in other cells of the airways, including human airway smooth muscle cells and lung fibroblasts, indicating that detrimental effects of CS components on mitochondrial function likely extend beyond the epithelium (5, 8). Collectively, these studies indicate that CS alters mitochondrial structure and function in various cells of the airway walls and lung parenchyma.
CHANGES IN MITOCHONDRIAL BIOGENESIS AND MITOPHAGY IN LUNG EPITHELIAL CELLS IN RESPONSE TO CIGARETTE SMOKE
Given that mitochondrial function and mitochondrial content (i.e., mtDNA copy number) in airway epithelial cells are significantly impacted by exposure to CS, it is not surprising that the pathways controlling mitochondrial biogenesis and mitophagy are also affected in these cell types in response to CS and in the airways of COPD patients. Indeed, Li et al. (65) demonstrated that PGC-1α protein levels were higher in peripheral lung tissue from mild COPD patients compared with controls, with levels progressively decreasing with increasing disease severity to levels comparable with the controls. In addition, Hoffmann et al. (49) reported significantly higher transcript levels of PGC-1α in primary airway epithelial cells from patients with advanced COPD compared with those from control subjects. Consistent with these observations, short-term exposure (24 h) of human bronchial epithelial cells (16HBE) to CSE increased PGC-1α transcript levels (Fig. 2) (118). Because PGC-1α is also known to control the expression of genes involved in mitochondrial fusion, the observation that hyperfusion of mitochondria in primary mouse AECII exposed to CSE was associated with increased expression of the mitochondrial fusion protein MFN-1 is also in line with this (11). In this context, increased levels of PGC-1α may reflect an adaptive cellular mechanism in response to reductions in mitochondrial content and function, whereas decreased levels in more advanced disease stages or in response to longer exposure or a higher dose of CS may reflect an inability to compensate for these changes in a chronic setting. In line with this notion, in rats exposed to CS for 30 days, pulmonary PGC-1α protein levels were significantly reduced (122). Besides smoke exposure, rats were also challenged twice (on days 1 and 14) with 200 µg of LPS instilled intratracheally. Because intratracheal LPS instillation has been shown to suppress PGC-1α expression in AECII (27), it cannot be excluded that reduced levels of PGC-1α in this model may be the result of LPS instillation instead of smoke exposure.
With regard to mitophagy, Mizumura et al. (79) showed that PINK1 protein levels were significantly higher in airway epithelial cells of COPD patients compared with controls. In line with this, Hoffmann et al. (49) showed increased levels of PINK1 mRNA in primary airway epithelial cells of COPD patients compared with controls. In the study from Mizumura et al. (79), higher PINK1 protein levels in COPD lung tissue were associated with increased abundance of the activated form of the fission protein DRP1. This likely resulted from exposure of lung epithelial cells to CS components, as in the same study CSE exposure of BEAS-2B cells resulted in significant mtROS-mediated increases in both PINK1, and phosphorylated DRP1 protein abundance and smoke exposure (3 wk) of mice similarly increased the abundance of phosphorylated DRP1 protein in the lung (79). In line with this, protein levels of phosphorylated DRP1 have been shown to be increased in AECII of smokers compared with nonsmokers (62). These changes were associated with CS-induced activation of mitophagy, as evidenced by enhanced mitochondrial clearance, and were directly linked to CS-induced mtROS production, as the mitochondrial-targeted antioxidant molecule Mitoquinone prevented these changes (79). Other studies confirmed this by demonstrating increases in PINK1 protein levels, increased abundance of activated fission proteins, and decreased mRNA levels of fusion genes in airway epithelial cells in response to CS in vitro (49, 106) and in vivo (5). In addition to increased mRNA and protein abundance of PINK1, decreased protein levels of PARK2 have also been reported in COPD lung tissue (55). In another study, PARK2-deficient mice demonstrated enhanced airway wall thickening with aggravated emphysematous changes following CS exposure in comparison with wild-type mice. AECII of CS-exposed PARK2-knockout animals also displayed an accumulation of damaged mitochondria, increased oxidative modifications and accelerated cellular senescence compared with wild-type animals (Fig. 2) (9). These results collectively suggest that disturbances in the PINK/PARK2 pathway may play a pivotal role in (CS-induced) COPD pathogenesis by regulating mitophagy. The lower levels of PARK2 observed in COPD (55) could lead to the accumulation of damaged mitochondria, and PARK2 induction may mitigate the progression of COPD. Nevertheless, whether mitophagy in the context of COPD and smoking is mainly protective or contributes to epithelial injury remains controversial. This controversy is illustrated, for example, by the observations that blocking mitophagy prevented CS-induced airspace enlargement and inhibition of ciliary function in mice (79), whereas activation of mitophagy prevented smoke-induced cellular senescence of cultured small airway epithelial cells (5). Further studies assessing the cell-specific function of mitophagy and mitochondrial fission/fusion in the pathogenesis and progression of COPD will help shed light on these outstanding questions.
CONTRIBUTION OF CIGARETTE SMOKE-INDUCED MITOCHONDRIAL DYSFUNCTION TO CELLULAR SENESCENCE IN LUNG EPITHELIAL CELLS
Cellular senescence has been implicated in the complex pathogenesis of COPD (13), and recent studies have suggested that mitochondrial dysfunction may act as a driver of cellular senescence in both human and murine lung cells. Several studies have shown that CS exposure leads to the activation of senescence in structural cells of the airways (40, 55, 94, 119, 128).
Interestingly, in vivo overexpression of sirtuin1 (SIRT1), an activator of mitochondrial biogenesis, protected against CS-induced senescence of airway epithelial cells in mice via SIRT1-mediated deacetylation of stress response transcription factor forkhead box O3 (FOXO3) (127). FOXO3 activation has previously been shown to result in enhancement of mitochondrial mass, increased ATP production, and clearance of defective mitochondria, likely via transcription of key anti-oxidant enzymes, thereby reducing ROS production and oxidative damage to the mitochondria and limiting cellular senescence (39, 96). However, SIRT1 also has other intracellular targets independent of the pathways regulating mitochondrial biogenesis, and whether or not the induction of senescence involved mitochondrial dysfunction was not assessed in the above-mentioned study (127). Recent studies provided further evidence directly linking impaired mitochondrial quality control processes and mitochondrial dysfunction to cellular senescence in structural cells of the airways. More specifically, several studies suggest a causal involvement of the PINK1/PARK2 pathway in CS-induced senescence in airway epithelial cells (5, 9, 55). For example, CSE-induced mitochondrial damage in primary human airway epithelial cells was associated with increased ROS production and cellular senescence, and knockdown of PINK1 and PARK2 aggravated both mtROS production as well as senescence (55). More recently, airway epithelial cells from PARK2-deficient mice displayed a greater accumulation of damaged mitochondria and accelerated senescence in response to CS exposure compared with wild-type animals. In the same study, these authors also showed that in vitro overexpression of PARK2 in airway epithelial cells was sufficient to attenuate smoke-induced mtROS production and cellular senescence (9). The link between the PINK1/PARK2 pathway and CS-induced senescence was further confirmed by another study demonstrating that impaired mitophagy leads to CS-induced senescence in primary human airway epithelial cells (5). The direct link between mitochondria and cellular senescence in the airways is further illustrated by a recent study showing that the pathways involved in mitochondrial biogenesis can contribute to cellular senescence, as activation of the mTORC1/PGC1 axis promoted mitochondrial biogenesis and enhanced OXPHOS as well as mtROS production and induced cellular senescence in rat AECII (111). Although this provided more evidence for the link between mitochondrial function and cellular senescence, understanding the complex link between mitochondrial dysfunction and cellular senescence requires further mechanistic studies. Collectively, these studies suggest that mitochondrial function, and alterations in homeostatic response to CS exposure, may contribute to cellular senescence in lung epithelial cells and as such can contribute to COPD pathogenesis.
LINKS BETWEEN MITOCHONDRIAL FUNCTION AND LUNG EPITHELIAL INTERCELLULAR JUNCTIONS
In the airways, epithelial cell contacts such as tight junctions and adherens junctions, as well as the trapping function of the mucociliary system, constitute a robust physical barrier against pathogens and inhaled toxicants such as CS (3). Interestingly, mitochondrial function has been shown to modulate epithelial integrity in various organs (52, 100, 113, 120, 126). More specifically, ATP starvation as well as high levels of mtROS and mitochondrial calcium overload disrupt tight junctions in airway and renal epithelial cells (20, 102, 113). Moreover, increased mtROS disrupts airway epithelial barrier integrity in response to the viral mimic poly (I:C) as well as rhinovirus (113), and loss of steroidogenic acute regulatory protein D7 (STARD7), a lipid transfer protein, disrupts airway epithelial tight and adherens junctions by enhancing mtROS and subsequent mtDNA damage in vitro and in vivo (126) (Fig. 2). This may contribute to COPD pathogenesis, since the lipid metabolism is dysregulated in airway epithelium of COPD subjects (115). Although CS was shown to disrupt apical and basal junctions between airway epithelial cells, to our knowledge there is no direct evidence for a causal link between smoking, mitochondrial dysfunction, and disruption of the lung epithelial barrier. However, studies in the airway and intestinal epithelium highlight the concept that mitochondrial dysfunction may contribute to the breakdown of the epithelial barrier in a mtROS-dependent mechanism (53, 102, 120).
An excessive oxidative burden is considered one of the underlying mechanisms of epithelial barrier disruption in COPD (3, 44), and mitochondrial dysfunction induces oxidative stress by overproduction of mtROS, which increases permeability of the epithelial barrier (120). Indeed, the CS-induced increase in mtROS has been shown to dissociate tight junctions through activation of epidermal growth factor receptor (EGFR) and extracellular signal-regulated kinase (ERK) signaling (3). Thus, mitochondrial dysfunction may lead to increased permeability of the lung epithelial barrier. In support of this, the barrier-disruptive effect of CS was reversible by mitochondrial-targeted antioxidant therapy (126). In addition, mtDNA damage, ATP depletion, and oxidative stress enhance apoptosis (68), which may eventually lead to epithelial barrier disruption (Fig. 2). For example, the CS-induced increase in FAM13A, a reported COPD susceptibility gene (82), causes a shift in mitochondrial metabolic activity in AECII and increases mtROS production from the fatty acid β-oxidation by interaction with SIRT1 in damaged mitochondria (43, 81). This in turn may lead to mtROS-mediated epithelial barrier impairment and apoptosis (Fig. 2). Therefore, regulation of FAM13A may have implications for mitochondrial-mediated epithelial barrier impairment in COPD in part by the ability of FAM13A to regulate mitochondrial fatty acid oxidation and thus mtROS levels in lung epithelial cells.
Furthermore, mitochondrial regulation of the epithelial barrier is thought to be important for polarization and differentiation of airway epithelial cells (98) and, for example, could impact mucociliary function. Indeed, it was shown that CS-induced increases in mtROS mediate autophagy-dependent overexpression of mucin 5AC (MUC5AC) in an immortalized bronchial epithelial cell line (CRL-2741) (134). In addition, mtROS promote mitophagy and mitochondrial fission by upregulation of PINK1 and phosphorylation of DRP1 (79). Inhibition of mitochondrial fission and mitophagy by a pharmacological inhibitor and genetic deletion of PINK1, respectively, was shown to protect against CS-induced reduction in mucociliary clearance in the airways of mice (79), suggesting a contribution of mitochondria to mucociliary function.
Together, the aforementioned studies suggest that mitochondrial dysfunction may induce oxidative damage that may in turn disrupt cell-cell contacts in lung epithelial cells. Epithelial damage not only may lead to increased susceptibility to environmental factors such as smoke and pathogens but is thought to contribute to proinflammatory and remodeling processes as well (3).
MITOCHONDRIAL DAMPs: POTENT INDUCERS OF LUNG INFLAMMATION IN COPD
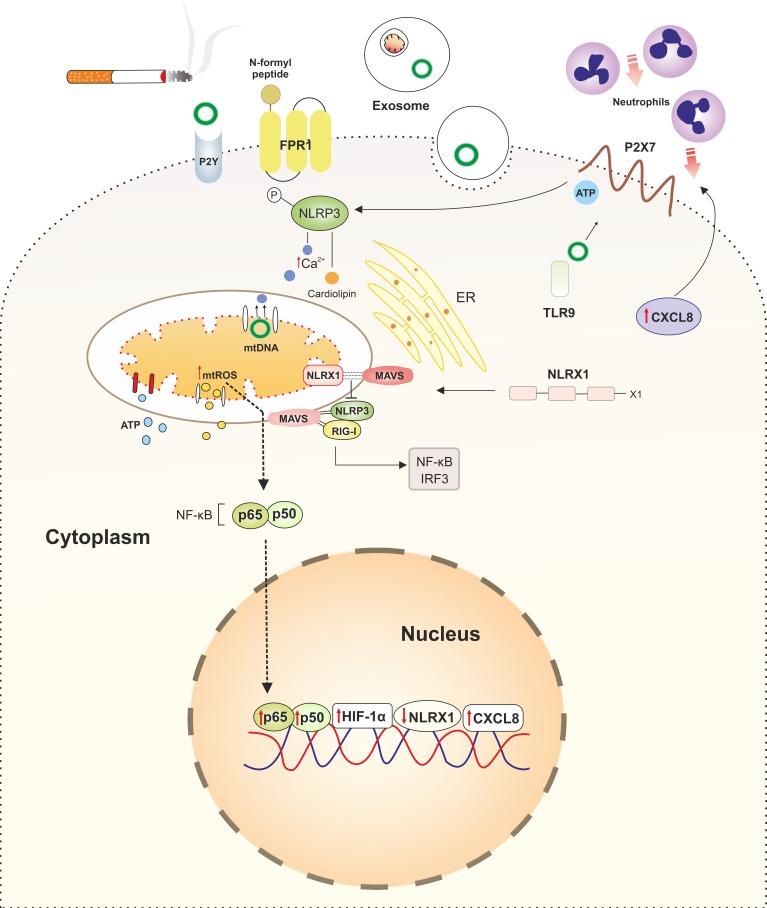
In addition to the activation of proinflammatory responses through ROS production, the accumulation of damaged mitochondria may contribute to lung inflammation by the release of so-called damage-associated molecular patterns (DAMPs). Besides infectious agents originating from “strangers” that carry pathogen-associated molecular patterns (PAMPs), endogenous danger signals originating from damaged or necrotic cells (“dangers”; DAMPs) are able to induce innate immune signaling pathways (76). A DAMP is defined as a molecule that has no proinflammatory function under physiological conditions but after being released during cellular damage or immunogenic cell death elicits a proinflammatory response by activating pattern recognition receptors (PRRs) (89). A wide variety of mitochondrial-derived molecules, which at normal physiological concentrations act as second messengers, can also behave as mitochondrial damage-associated molecular patterns (mtDAMPs) when produced in surplus or in an alternative cellular compartment (38). Upon cellular damage or necrotic cell death, released mtDAMPs are highly abundant and are potent activators of proinflammatory responses, which is partially due to their high level of homology to PAMPs. This may be explained by the endosymbiotic theory (95), as described earlier. Homology is evident by the high number of unmethylated CpG repeats in mtDNA compared with genomic DNA and the fact that peptides encoded by mtDNA are N-formylated, which is a characteristic of bacterial proteins (42). Such formyl peptides act as DAMPs by activating the formyl peptide 1 receptor (FPR1) (57). In mice, genetic ablation of the FPR1 gene, encoding the receptor for N-formyl peptides, resulted in a significant protection against CS-induced emphysema and airway inflammation (21). In line with this, treatment of wild-type mice with the FPR1 antagonist cyclosporin H also provided protection against CS-induced acute airway inflammation (21). Although it is technically challenging to measure extracellular levels of N-formyl peptides, various studies suggest that N-formyl peptides are important in the pathophysiology of COPD (10, 21, 75). The best-studied mtDAMP is mtDNA, which can activate Toll-like receptor 9 (TLR9) as well as multiprotein complexes that mediate inflammatory responses termed inflammasomes, including the nuclear oligomerization domain (NOD), leucine-rich repeats, and pyrin domain-containing protein 3 (NLRP3) inflammasome (89). MtDNA can be passively released upon immunogenic cell death and actively secreted via exosomes or from neutrophils as neutrophil extracellular traps (NETs) (57). Next to mtDNA and N-formyl peptides, other mitochondrial molecules can act as DAMPs, including ATP, TFAM, cardiolipin, carbamoyl phosphate synthetase, and cytochrome c (38). MtDAMPs are involved in the pathophysiology of various diseases, including sepsis, trauma, and autoimmune diseases (28). However, their role in COPD is less well characterized. Few studies have assessed the extracellular role of mtDAMPs. Nonetheless, it was shown that exposure of airway epithelial cell lines to CSE induces necroptotic and necrotic cell death, followed by the release of mtDNA alongside other non-mitochondrial-derived DAMPs (91). Furthermore, it was shown that 37 wk of CS exposure significantly increased serum mtDNA levels in vivo (131). Additionally, primary airway epithelial cells isolated from either healthy controls or COPD patients exposed to CSE released significant levels of mtDNA (45, 90). Stimulation of airway epithelial cells from either healthy controls or COPD patients with mtDAMPs induced a strong CXCL8 response (90). Acute exposure to CS in BALB/c mice resulted in significant mtDNA release in their bronchoalveolar lavage (BAL) fluid compared with air-exposed mice, leading to higher levels of the CXCL8 analog KC and neutrophilic infiltration (90). Next to mtDNA, other mtDAMPs have been studied in the context of COPD. ATP, which can activate the proinflammatory purinergic P2Y and P2X receptors, is produced by the mitochondrion and can be released both actively and passively from multiple subcellular compartments, including mitochondria, endoplasmic reticulum, and the cytoplasm (Fig. 3) (12). Extracellular ATP levels are increased in COPD patients compared with non-COPD smokers and showed a positive correlation with disease severity (33, 69). Together, mtDAMPs may invoke and perpetuate the inflammatory reaction as well as induce lung tissue damage, e.g., by attraction of neutrophils and subsequent release of neutrophil elastase during COPD.
Fig. 3.
Links between cigarette smoke (CS)-induced mitochondrial dysfunction and altered innate immune responses in chronic obstructive pulmonary disease (COPD). Damaged mitochondria as a result of CS exposure release their contents into to the cytoplasm, acting as damage-associated molecular patterns (DAMPs) and subsequently activating the innate immune system. Cytoplasmic levels of mitochondrial danger-associated molecular patterns (mtDAMPs), including mitochondrial reactive oxygen species (mtROS), mtDNA, ATP, cardiolipin, and Ca2+, are increased in lung epithelial cells in COPD. Increased levels of cytoplasmic mtDNA, mtROS, Ca2+, and cardiolipin activate innate immune responses by stimulation of intracellular pattern recognition receptors (PRRs), in particular the NLRP3 inflammasome. mtDNA acts as a ligand for Toll-like receptor 9 (TLR9), further activating NLRP3. mtDNA can be transferred to the neighboring cells via exosomes. N-formyl peptides encoded by mtDNA are released upon cellular damage, acting as DAMPs by activating formyl peptide receptor 1 (FPR1), which induces attraction of neutrophils to the site of damage. Extracellular ATP also activates NLRP3 via purinergic receptors P2X7 and P2Y2R. Activated NLRP3 translocates to mitochondria, which subsequently induces more damage by enhancing mtROS production. This increase in mtROS levels directly induces proinflammatory responses by increasing nuclear translocation of p65 and activating hypoxia-inducible factor-1α (HIF-1α) transcription factor. Furthermore, mtDAMPs elicit proinflammatory responses by inducing strong CXCL8 responses in airway epithelial cells, recruiting neutrophils to the site of damage. Another PRR, NLRX1, is also localized into mitochondria and interacts with mitochondrial antiviral signaling (MAVS), exerting anti-inflammatory responses by precluding interaction of NLRX1 with NLRP3 and retinoic acid-inducible gene-I (RIG-I) and subsequent activation of NF-κB and interferon regulatory transcription factor 3 (IRF3). CS reduces the expression of NLRX1 in the lung at both gene and posttranscriptional levels, perpetuating inflammation.
INTERPLAY BETWEEN MITOCHONDRIAL FUNCTION AND INNATE IMMUNE RESPONSES IN LUNG EPITHELIUM
Besides providing a physical barrier against environmental insults, the lung epithelium is important for the production of antimicrobial peptides, antioxidants, and proinflammatory mediators, which are able to attract and activate cells of both the innate and adaptive immune systems (47). Lung epithelial cells express a wide array of proinflammatory PRRs, including TLRs, NOD (nucleotide-binding oligomerization domain)-like receptors (NLRs), RIG (retinoic acid-inducible gene)-I-like receptors (RLRs), and the receptor for advanced glycation end products (RAGE), which can be activated by PAMPs and DAMPs, including mtDAMPs. Next to mtDAMPs, it has been shown that mtROS are able to induce a proinflammatory response by directly activating the NF-κB pathway and hypoxia-inducible factor-1α (HIF-1α) (12). mtROS also regulate inflammation through activation of inflammasomes, and selected NLR family members oligomerize to form inflammasomes. The best-studied NLR inflammasome is NLRP3, which has a strong link to mitochondrial dysfunction. NLRP3 can be activated by various mtDAMPs either indirectly by extracellular ATP, which activates the P2X7 receptor, or directly by mtDNA and cardiolipin (56, 103). Furthermore, activated NLRP3 inflammasomes colocalize with mitochondria, which may act as a scaffold for NLRP3 assembly and activation (32, 135). Activation of NLRP3 inflammasomes has been reported in lung epithelium of COPD patients undergoing an exacerbation (35). Several studies have shown that the NLRP3 inflammasome anchors at the mitochondria via mitochondrial antiviral signaling protein (MAVS), whereas cardiolipin and the antiapoptotic c-FLIP protein have also been suggested as mitochondrial anchors (12, 34, 110, 124). Activation of NLRP3 induces mitochondrial damage and the subsequent release of mtROS and vice versa (83, 135). In line with this, dysfunctional mitophagy augments the activation of NLRP3 (70). The correlation between mitochondrial dysfunction and NLRP3 activation may be caused by high cytosolic calcium levels, which are needed for NLRP3 activation and which may induce damage to the mitochondria (12). Another NLR family member, NLRX1, also colocalizes with mitochondria by binding to MAVS (80). Therefore, NLRX1 acts as an anti-inflammatory decoy receptor, limiting the binding and activation of NLRP3 and RIG-I, which subsequently results in decreased NF-κB and interferon regulatory factor 3 (IRF3) activation (124). However, recently, it was shown that NLRX1 may also have proinflammatory functions under specific conditions e.g., viral infections (36), suggesting that NLRX1 may have pro- as well as anti-inflammatory function and is thus important for the modulation of inflammatory responses. NLRX1 expression is lower in CS-exposed mice as well as in severe stages of COPD compared with healthy controls at both gene and protein levels (58). Interestingly, it was shown that loss of NLRX1 levels induces mtROS-mediated oxidative stress in ischemia-reperfusion injury model in renal epithelial cells by interacting with UQCRC2 (109), thus, acting as a mediator between innate immune compartments and mitochondrial ETC. Collectively, dysfunctional mitochondria as a result of CS exposure can potentially affect innate immune responses by the release of both mtDAMPs and mtROS and by anchoring and modulating the proinflammatory inflammasomes in lung epithelial cells, as depicted in Fig. 3.
Whereas these observations clearly link dysfunctional mitochondria to inflammation, mitochondrial dysfunction may also contribute to increased susceptibility to infection by changing the energy balance in affected cells as well as through direct involvement of mitochondria in antimicrobial activity of cells. Mitochondria have been shown to contribute to antibacterial activity through various mechanisms, including the aforementioned regulation of innate immune responses but also through direction of mitochondria toward the phagolysosome and subsequent delivery of mtROS, which in concert with phagosomal NADPH-oxidase-derived ROS contribute to bacterial killing within the phagolysosome (reviewed in Ref. 86). Such studies help to explain the observed link between mitochondrial dysfunction and decreased antibacterial activity of macrophages in COPD (14, 15). In addition, mitochondria contribute to antiviral responses in cells by facilitating RLR signaling, including that of the aforementioned mitochondria-associated MAVS, thus regulating the production of antiviral type I and type III interferons and proinflammatory cytokines following viral infection (reviewed in Refs. 59 and 88). This was first shown in studies demonstrating that mitochondrial fragmentation and loss of mitochondrial membrane potential, as observed in cells exposed to CS (40, 49), result in a marked impairment of MAVS-mediated antiviral immunity (22, 61). Viruses exploit this mechanism by inducing mitochondrial dysfunction (e.g., by increasing mitophagy), thus escaping efficient antiviral host defense. These observations may help to explain how exposure of cultured airway epithelial cells to CS increases infection by, e.g., rhinoviruses (30, 93), as well as the observed antiviral immunity in, e.g., COPD patients with frequent exacerbations (105). Collectively, these studies show that mitochondrial dysfunction as observed in smokers with and without COPD may contribute to their increased susceptibility to respiratory infections. Conversely, these findings suggest that such infections may contribute to the observed mitochondrial dysfunction.
RESTORATION OF MITOCHONDRIAL FUNCTION IN COPD: FROM BASICS TO CLINIC
Emerging mitochondrial-specific therapeutics may assist in alleviating COPD symptoms or even improve pathophysiological features of the disease. To restore the impaired function of lung epithelial cells in COPD, strategies have been developed to specifically target mitochondria in these particular cells. These strategies are aimed at amelioration of impaired mitochondrial bioenergetics and biogenesis, improved regulation of mitophagy, and inhibition of excessive mtROS generation, as summarized in Table 1.
Table 1.
Potential therapeutic targets for regulation of mitochondrial-mediated cellular dysfunction in cigarette smoke-exposed lung epithelial cells
| Therapeutic Strategies | Drug/Inhibitor/Activator | Lung Epithelial Cells | Therapeutic Effects | Potential Adverse Effects | Ref. No. |
|---|---|---|---|---|---|
| Mitochondrial transfer | Stem cells (iPSC-MSCs) | HBECs HBECs in vitro and in vivo |
∙ Inhibited CSE-induced apoptosis and cellular damage | Not reported | 66, 67 |
| ∙ Restored ATP levels and improved lung repair in CS-induced cellular damage | |||||
| Mitophagy inhibitors | Mdivi1 | HBECs Murine BECs in vitro and in vivo |
∙ Reduced CSE-induced decrease in Δψmt | Not reported | 79, 106, 133 |
| ∙ Prevented apoptosis emphysema and MCC dysfunction | |||||
| Quercetogetin | HBECs | ∙ Inhibited CSE-induced mitophagy and apoptosis | |||
| Mitochondrial ROS scavengers | MitoTEMPO | HBECs HBECs and mouse airways HBECs |
∙ Inhibited PINK1 stabilization ∙ Reduced DRP-1 phosphorylation |
∙ Global reduction of ROS in immune cells ∙ Enhanced proliferation of malignant cells |
5, 40, 79, 106, 134 |
| ∙ Inhibited CSE-induced autophagy and muc5AC mRNA and protein expression | |||||
| ∙ Inhibited CSE-induced mitochondrial fragmentation ∙ Suppressed CSE-induced cellular senescence | |||||
| Iron chelators | Deferiprone | In vivo | ∙ Improved MCC ∙ Reduced lung inflammation |
∙ Nephrotoxicity ∙ Low bioavailibilty ∙ Exacerbating preexisting anemia |
25 |
| Mitochondrial biogenesis stimulators | AMPK activators | HBECs and mouse airways | ∙ Decreased cellular senescence ∙ Lowered inflammatory responses ∙ Reduced emphysema |
Not reported | 23, 127 |
| SIRT1 overexpression/activation | Mouse airways | ∙ Attenuated CS-induced cellular senescence |
AMPK, AMP-activated protein kinase; CSE, cigarette smoke extract; DRP1, dynamin-related protein 1; HBEC, human bronchial epithelial cells; iPSC, induced pluripotent stem cells; MCC, mucociliary clearance; MSC, mesenchymal stem/stromal cells; PINK1, PTEN-induced kinase 1; SIRT1, sirtuin1; Δψmt: mitochondrial membrane potential.
Another interesting approach involves the transfer of mitochondria from mesenchymal stromal cells to damaged lung epithelial cells. This approach ameliorates the imbalance in oxidative respiration, improves energy levels, and enhances mitochondrial biogenesis in damaged epithelial cells (85). This transfer can be mediated by various cellular dynamic compartments, including gap junctions, microvesicles, and nanotube structures (85), and has proven to be efficient in mediating protection against LPS-induced acute lung injury as well as in smoke-exposed airway epithelial cells (67). Interestingly, exosomes in airway cells were shown to carry mtDNA to neighboring cells (51), making these vesicles potential factors for reversing CS-induced mtDNA damage. Replacement of CS-induced damaged mitochondria by healthy mitochondria through nanotube structures from human pluripotent stem cells accelerates lung repair upon damage in vivo (67). Additionally, coculture of CS-stimulated airway epithelial cells with mesenchymal stromal cells (MSCs) reduces apoptosis by interfering with mitochondrial cytochrome c release (66). This type of intervention not only reduces cell death but may also be able to rescue mtDNA damage in lung epithelial cells, as transfer of healthy mitochondria from wild-type MSCs was shown to recover oxidative respiration in mtDNA-damaged AECII (108).
Correction of the imbalance in oxidant/antioxidant by mitochondrial-targeted antioxidants that specifically target mtROS or pathways involved in generation of mtROS may also have potential as a therapeutic strategy for the restoration of mitochondrial function in COPD. Indeed, it has been shown that mtROS scavengers counteract CS-induced cellular dysfunction in airway epithelial cells in vitro (5, 40). Furthermore, studies demonstrated that mitochondrial-targeted antioxidants such as MitoTEMPO, Quercetogetin and Resveratrol reduce mucus overproduction, cellular senescence, and apoptosis in CS-exposed airway epithelial cells (79, 106, 107, 134). Despite all advances in preclinical development of this strategy, serious complications may limit the clinical applications of mtROS scavengers in COPD patients. MtDNA damage induced by short-lived molecules such as ROS is thought to be irreversible, particularly upon persistent oxidative damage, when repair mechanisms of mtDNA also become impaired (117). Because ROS levels are essential for the adequate activation of immune compartments in COPD (15), systemic inhibition of mtROS may interfere with innate immune cell function. Furthermore, mitochondrial antioxidants not only protect healthy cells but may also promote the survival and migration of cells undergoing malignant changes (64, 101).
Although it is not completely clear whether mitophagy is protective in COPD, regulation of mitophagy by inhibition of mitochondrial degradation via blocking fission or enhancing OXPHOS capacity by activation of fusion factors, particularly in the early stages of the disease, may improve mitochondrial function and subsequently restore cellular functionality in lung epithelial cells. Indeed, inhibition of fission improves mitochondrial respiration as well as mtROS production. Mdivi-1, a well-known fission inhibitor that has been broadly used in experimental studies, was shown to inhibit fission-related protein DRP1 and reverse CS-induced decrease in mitochondrial integrity in airway epithelial cells (79). Interestingly, Mdivi-1 was also able to inhibit cathepsin-E/parkin-mediated apoptosis and emphysema as well as mucociliary dysfunction in lung epithelial cells of CS-exposed mice (79, 133). On the other hand, diminished mitophagy acts as an upstream regulator of cellular senescence in response to CSE through increased oxidative burden, as demonstrated both in vitro and in vivo (9, 55). Thus, enhancement of mitophagy may be able to inhibit cellular senescence induced by CS. Other therapeutics that were reported to promote mitochondrial turnover include mitochondrial-targeted antioxidants, SIRT1 activators, transcription factor nuclear factor E2-related factor 2 (Nrf2) inducers, mTORC1/2 inhibitors, and iron chelators (37, 48). Together, strategies based on recovery of factors involved in mitophagy, fission, and fusion in lung epithelial cells may be effective in alleviation of COPD progression.
Iron also contributes to the regulation of the redox balance in lung epithelial cells, and its metabolism has been shown to be disturbed in COPD (26), which may have implications for mitochondrial function (132). Smokers exhibit higher levels of iron in the lung, and CS-induced increase in cellular load of iron was shown to induce mitochondrial dysfunction in lung epithelial cells (132). Cloonan et al. (25) showed that iron binding protein 2 (IRP-2), which is a susceptibility gene for COPD (29), regulates iron uptake to the mitochondria, leading to increased cytochrome c oxidase (COX) in complex IV of the ETC and subsequent mitochondrial dysfunction. This may lead to an imbalance in the oxidant/antioxidant responses in lung epithelial cells as one of the main features of COPD. Thus, iron chelators (deferiprone and other siderophores) that specifically reduce the uptake of iron could restore mitochondrial function as well as mucociliary clearance in COPD (25). However, because many COPD patients suffer from anemia in late stages (104), systemic administration of iron chelators may worsen the condition. Alternatively, delivery of aerosolized forms of these compounds directly to the lung using nebulizers may circumvent the disadvantage of systemic therapy, providing beneficial effects.
Other strategies that could potentially be used to counteract mitochondrial dysfunction in COPD include reducing excessive mtROS production by blocking calcium and iron flux channels in mitochondrial membrane and stimulating mitochondrial biogenesis by AMPK and SIRT1 activators, which was already reported to reverse CS-induced cellular senescence in airway epithelial cells (23, 127).
CONCLUSIONS AND FUTURE DIRECTION
The contribution of mitochondrial dysfunction to the pathophysiology of lung diseases is an emerging area of research with many unknown aspects. More specifically, in COPD patients, there is evidence for abnormalities at the level of the mitochondrion in various cell types of the airway and alveolar epithelium. CS likely contributes to these mitochondrial changes observed in lung epithelial cells by interfering with OXPHOS, mitophagy, and mitochondrial membrane integrity and mediating mtDNA damage. COPD patients may be more susceptible to the development of severe smoking-induced mitochondrial abnormalities than smokers with normal lung function. These abnormalities may eventually lead to alterations in epithelial cell growth, innate immunity, and barrier function. Although several studies have highlighted the potential contribution of CS to progression of mitochondrial dysfunction in COPD, there are still many unanswered questions. For instance, there are no studies comparing the effects of CS on mitochondrial function in different lineages of differentiated lung epithelium. It is important in this regard to carefully consider cell-specific abnormalities in the mitochondria of various lung epithelial cells during COPD. Single-cell sequencing may help uncover the susceptibility of distinct lineages of lung epithelial cells to develop mitochondrial abnormalities in COPD. Furthermore, it is not clear whether current COPD therapeutics such as corticosteroids, inhaled bronchodilators (β2-agonists, anticholinergics and theophyllin), and antibiotics alter mitochondrial function in lung epithelial cells. From a therapeutic perspective, mitochondria targeting strategies such as increasing ETC function, antioxidant therapy, and inducing mitochondrial biogenesis have displayed beneficial effects in several clinical trials in aging-related diseases (31). Future experimental developments in therapeutic approaches based on similar strategies as well as other strategies aiming to promote mtDNA repair in COPD will stimulate progress of these strategies to the clinical phase.
GRANTS
M. Aghapour receives a fellowship from the Kommission zur Förderung des wissenschaftlichen Nachwuchses from the Medical Faculty, Otto-von-Guericke University, Magdeburg, and is supported by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) no. 361210922/GRK2408. A. H. V. Remels is funded by a grant from the Dutch National Institute of Public Health and the Environment. S. M. Cloonan is supported by National Institutes of Health National Heart, Lung, and Blood Institute Grant R00-HL125899. D. Bruder is supported by DFG nos. 361210922/GRK2408 and BR 2221/4-1.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.A. prepared figures; M.A., A.H.V.R., and S.D.P. drafted manuscript; A.H.V.R., S.D.P., D.B., P.S.H., S.M.C., and I.H.H. edited and revised manuscript; M.A., A.H.V.R., S.D.P., D.B., P.S.H., S.M.C., and I.H.H. approved final version of manuscript.
REFERENCES
- 1.Agarwal AR, Yin F, Cadenas E. Short-term cigarette smoke exposure leads to metabolic alterations in lung alveolar cells. Am J Respir Cell Mol Biol 51: 284–293, 2014. doi: 10.1165/rcmb.2013-0523OC. [DOI] [PubMed] [Google Scholar]
- 2.Agarwal AR, Zhao L, Sancheti H, Sundar IK, Rahman I, Cadenas E. Short-term cigarette smoke exposure induces reversible changes in energy metabolism and cellular redox status independent of inflammatory responses in mouse lungs. Am J Physiol Lung Cell Mol Physiol 303: L889–L898, 2012. doi: 10.1152/ajplung.00219.2012. [DOI] [PubMed] [Google Scholar]
- 3.Aghapour M, Raee P, Moghaddam SJ, Hiemstra PS, Heijink IH. Airway epithelial barrier dysfunction in chronic obstructive pulmonary disease: role of cigarette smoke exposure. Am J Respir Cell Mol Biol 58: 157–169, 2018. doi: 10.1165/rcmb.2017-0200TR. [DOI] [PubMed] [Google Scholar]
- 4.Aguilera-Aguirre L, Bacsi A, Saavedra-Molina A, Kurosky A, Sur S, Boldogh I. Mitochondrial dysfunction increases allergic airway inflammation. J Immunol 183: 5379–5387, 2009. doi: 10.4049/jimmunol.0900228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahmad T, Sundar IK, Lerner CA, Gerloff J, Tormos AM, Yao H, Rahman I. Impaired mitophagy leads to cigarette smoke stress-induced cellular senescence: implications for chronic obstructive pulmonary disease. FASEB J 29: 2912–2929, 2015. doi: 10.1096/fj.14-268276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andersen ZJ, Hvidberg M, Jensen SS, Ketzel M, Loft S, Sørensen M, Tjønneland A, Overvad K, Raaschou-Nielsen O. Chronic obstructive pulmonary disease and long-term exposure to traffic-related air pollution: a cohort study. Am J Respir Crit Care Med 183: 455–461, 2011. doi: 10.1164/rccm.201006-0937OC. [DOI] [PubMed] [Google Scholar]
- 7.Antico Arciuch VG, Elguero ME, Poderoso JJ, Carreras MC. Mitochondrial regulation of cell cycle and proliferation. Antioxid Redox Signal 16: 1150–1180, 2012. doi: 10.1089/ars.2011.4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aravamudan B, Kiel A, Freeman M, Delmotte P, Thompson M, Vassallo R, Sieck GC, Pabelick CM, Prakash YS. Cigarette smoke-induced mitochondrial fragmentation and dysfunction in human airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 306: L840–L854, 2014. doi: 10.1152/ajplung.00155.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Araya J, Tsubouchi K, Sato N, Ito S, Minagawa S, Hara H, Hosaka Y, Ichikawa A, Saito N, Kadota T, Yoshida M, Fujita Y, Utsumi H, Kobayashi K, Yanagisawa H, Hashimoto M, Wakui H, Ishikawa T, Numata T, Kaneko Y, Asano H, Yamashita M, Odaka M, Morikawa T, Nishimura SL, Nakayama K, Kuwano K. PRKN-regulated mitophagy and cellular senescence during COPD pathogenesis. Autophagy 15: 510–526, 2019. doi: 10.1080/15548627.2018.1532259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Atzori L, Lucattelli M, Scotton CJ, Laurent GJ, Bartalesi B, De Cunto G, Lunghi B, Chambers RC, Lungarella G. Absence of proteinase-activated receptor-1 signaling in mice confers protection from fMLP-induced goblet cell metaplasia. Am J Respir Cell Mol Biol 41: 680–687, 2009. doi: 10.1165/rcmb.2007-0386OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ballweg K, Mutze K, Königshoff M, Eickelberg O, Meiners S. Cigarette smoke extract affects mitochondrial function in alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol 307: L895–L907, 2014. doi: 10.1152/ajplung.00180.2014. [DOI] [PubMed] [Google Scholar]
- 12.Banoth B, Cassel SL. Mitochondria in innate immune signaling. Transl Res 202: 52–68, 2018. doi: 10.1016/j.trsl.2018.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barnes PJ, Baker J, Donnelly LE. Cellular senescence as a mechanism and target in chronic lung diseases. Am J Respir Crit Care Med 200: 556–564, 2019. doi: 10.1164/rccm.201810-1975TR. [DOI] [PubMed] [Google Scholar]
- 14.Belchamber KBR, Singh R, Batista CM, Whyte MK, Dockrell DH, Kilty I, Robinson MJ, Wedzicha JA, Barnes PJ, Donnelly LE; COPD-MAP consortium . Defective bacterial phagocytosis is associated with dysfunctional mitochondria in COPD macrophages. Eur Respir J 54: 1802244, 2019. doi: 10.1183/13993003.02244-2018. [DOI] [PubMed] [Google Scholar]
- 15.Bewley MA, Preston JA, Mohasin M, Marriott HM, Budd RC, Swales J, Collini P, Greaves DR, Craig RW, Brightling CE, Donnelly LE, Barnes PJ, Singh D, Shapiro SD, Whyte MKB, Dockrell DH. Impaired mitochondrial microbicidal responses in chronic obstructive pulmonary disease macrophages. Am J Respir Crit Care Med 196: 845–855, 2017. doi: 10.1164/rccm.201608-1714OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bolisetty S, Jaimes EA. Mitochondria and reactive oxygen species: physiology and pathophysiology. Int J Mol Sci 14: 6306–6344, 2013. doi: 10.3390/ijms14036306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bueno M, Lai YC, Romero Y, Brands J, St Croix CM, Kamga C, Corey C, Herazo-Maya JD, Sembrat J, Lee JS, Duncan SR, Rojas M, Shiva S, Chu CT, Mora AL. PINK1 deficiency impairs mitochondrial homeostasis and promotes lung fibrosis. J Clin Invest 125: 521–538, 2015. doi: 10.1172/JCI74942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bustamante-Marin XM, Ostrowski LE. Cilia and mucociliary clearance. Cold Spring Harb Perspect Biol 9: a028241, 2017. doi: 10.1101/cshperspect.a028241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Calabrese EJ. Hormesis: from marginalization to mainstream: a case for hormesis as the default dose-response model in risk assessment. Toxicol Appl Pharmacol 197: 125–136, 2004. doi: 10.1016/j.taap.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 20.Canfield PE, Geerdes AM, Molitoris BA. Effect of reversible ATP depletion on tight-junction integrity in LLC-PK1 cells. Am J Physiol 261: F1038–F1045, 1991. doi: 10.1152/ajprenal.1991.261.6.F1038. [DOI] [PubMed] [Google Scholar]
- 21.Cardini S, Dalli J, Fineschi S, Perretti M, Lungarella G, Lucattelli M. Genetic ablation of the fpr1 gene confers protection from smoking-induced lung emphysema in mice. Am J Respir Cell Mol Biol 47: 332–339, 2012. doi: 10.1165/rcmb.2012-0036OC. [DOI] [PubMed] [Google Scholar]
- 22.Castanier C, Garcin D, Vazquez A, Arnoult D. Mitochondrial dynamics regulate the RIG-I-like receptor antiviral pathway. EMBO Rep 11: 133–138, 2010. doi: 10.1038/embor.2009.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheng XY, Li YY, Huang C, Li J, Yao HW. AMP-activated protein kinase reduces inflammatory responses and cellular senescence in pulmonary emphysema. Oncotarget 8: 22513–22523, 2017. doi: 10.18632/oncotarget.15116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cloonan SM, Choi AM. Mitochondria in lung disease. J Clin Invest 126: 809–820, 2016. doi: 10.1172/JCI81113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cloonan SM, Glass K, Laucho-Contreras ME, Bhashyam AR, Cervo M, Pabón MA, Konrad C, Polverino F, Siempos II, Perez E, Mizumura K, Ghosh MC, Parameswaran H, Williams NC, Rooney KT, Chen ZH, Goldklang MP, Yuan GC, Moore SC, Demeo DL, Rouault TA, D’Armiento JM, Schon EA, Manfredi G, Quackenbush J, Mahmood A, Silverman EK, Owen CA, Choi AM. Mitochondrial iron chelation ameliorates cigarette smoke-induced bronchitis and emphysema in mice. Nat Med 22: 163–174, 2016. doi: 10.1038/nm.4021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cloonan SM, Mumby S, Adcock IM, Choi AMK, Chung KF, Quinlan GJ. The “iron”-y of iron overload and iron deficiency in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 196: 1103–1112, 2017. doi: 10.1164/rccm.201702-0311PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cui H, Xie N, Banerjee S, Ge J, Guo S, Liu G. Impairment of fatty acid oxidation in alveolar epithelial cells mediates acute lung injury. Am J Respir Cell Mol Biol 60: 167–178, 2019. doi: 10.1165/rcmb.2018-0152OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dela Cruz CS, Kang MJ. Mitochondrial dysfunction and damage associated molecular patterns (DAMPs) in chronic inflammatory diseases. Mitochondrion 41: 37–44, 2018. doi: 10.1016/j.mito.2017.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.DeMeo DL, Mariani T, Bhattacharya S, Srisuma S, Lange C, Litonjua A, Bueno R, Pillai SG, Lomas DA, Sparrow D, Shapiro SD, Criner GJ, Kim HP, Chen Z, Choi AM, Reilly J, Silverman EK. Integration of genomic and genetic approaches implicates IREB2 as a COPD susceptibility gene. Am J Hum Genet 85: 493–502, 2009. doi: 10.1016/j.ajhg.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eddleston J, Lee RU, Doerner AM, Herschbach J, Zuraw BL. Cigarette smoke decreases innate responses of epithelial cells to rhinovirus infection. Am J Respir Cell Mol Biol 44: 118–126, 2011. doi: 10.1165/rcmb.2009-0266OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.El-Hattab AW, Zarante AM, Almannai M, Scaglia F. Therapies for mitochondrial diseases and current clinical trials. Mol Genet Metab 122: 1–9, 2017. doi: 10.1016/j.ymgme.2017.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Elliott EI, Miller AN, Banoth B, Iyer SS, Stotland A, Weiss JP, Gottlieb RA, Sutterwala FS, Cassel SL. Cutting edge: mitochondrial assembly of the NLRP3 inflammasome complex is initiated at priming. J Immunol 200: 3047–3052, 2018. doi: 10.4049/jimmunol.1701723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eltom S, Belvisi MG, Stevenson CS, Maher SA, Dubuis E, Fitzgerald KA, Birrell MA. Role of the inflammasome-caspase1/11-IL-1/18 axis in cigarette smoke driven airway inflammation: an insight into the pathogenesis of COPD. PLoS One 9: e112829, 2014. doi: 10.1371/journal.pone.0112829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Faiz A, Heijink IH, Vermeulen CJ, Guryev V, van den Berge M, Nawijn MC, Pouwels SD. Cigarette smoke exposure decreases CFLAR expression in the bronchial epithelium, augmenting susceptibility for lung epithelial cell death and DAMP release. Sci Rep 8: 12426, 2018. doi: 10.1038/s41598-018-30602-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Faner R, Sobradillo P, Noguera A, Gomez C, Cruz T, López-Giraldo A, Ballester E, Soler N, Arostegui JI, Pelegrín P, Rodriguez-Roisin R, Yagüe J, Cosio BG, Juan M, Agustí A. The inflammasome pathway in stable COPD and acute exacerbations. ERJ Open Res 2: 00002-2016, 2016. doi: 10.1183/23120541.00002-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Feng H, Lenarcic EM, Yamane D, Wauthier E, Mo J, Guo H, McGivern DR, González-López O, Misumi I, Reid LM, Whitmire JK, Ting JP, Duncan JA, Moorman NJ, Lemon SM. NLRX1 promotes immediate IRF1-directed antiviral responses by limiting dsRNA-activated translational inhibition mediated by PKR. Nat Immunol 18: 1299–1309, 2017. doi: 10.1038/ni.3853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Georgakopoulos ND, Wells G, Campanella M. The pharmacological regulation of cellular mitophagy. Nat Chem Biol 13: 136–146, 2017. doi: 10.1038/nchembio.2287. [DOI] [PubMed] [Google Scholar]
- 38.Grazioli S, Pugin J. Mitochondrial damage-associated molecular patterns: from inflammatory signaling to human diseases. Front Immunol 9: 832, 2018. doi: 10.3389/fimmu.2018.00832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hagenbuchner J, Ausserlechner MJ. Mitochondria and FOXO3: breath or die. Front Physiol 4: 147, 2013. doi: 10.3389/fphys.2013.00147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hara H, Araya J, Ito S, Kobayashi K, Takasaka N, Yoshii Y, Wakui H, Kojima J, Shimizu K, Numata T, Kawaishi M, Kamiya N, Odaka M, Morikawa T, Kaneko Y, Nakayama K, Kuwano K. Mitochondrial fragmentation in cigarette smoke-induced bronchial epithelial cell senescence. Am J Physiol Lung Cell Mol Physiol 305: L737–L746, 2013. doi: 10.1152/ajplung.00146.2013. [DOI] [PubMed] [Google Scholar]
- 41.Hara H, Kuwano K, Araya J. Mitochondrial quality control in COPD and IPF. Cells 7: 86, 2018. doi: 10.3390/cells7080086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hauser CJ, Otterbein LE. Danger signals from mitochondrial DAMPS in trauma and post-injury sepsis. Eur J Trauma Emerg Surg 44: 317–324, 2018. doi: 10.1007/s00068-018-0963-2. [DOI] [PubMed] [Google Scholar]
- 43.Hawkins GA, Mora AL. FAM13A, a fatty acid oxidation switch in mitochondria. Friend or foe in chronic obstructive pulmonary disease pathogenesis? Am J Respir Cell Mol Biol 56: 689–691, 2017. doi: 10.1165/rcmb.2017-0080ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Heijink I, van Oosterhout A, Kliphuis N, Jonker M, Hoffmann R, Telenga E, Klooster K, Slebos DJ, ten Hacken N, Postma D, van den Berge M. Oxidant-induced corticosteroid unresponsiveness in human bronchial epithelial cells. Thorax 69: 5–13, 2014. doi: 10.1136/thoraxjnl-2013-203520. [DOI] [PubMed] [Google Scholar]
- 45.Heijink IH, Pouwels SD, Leijendekker C, de Bruin HG, Zijlstra GJ, van der Vaart H, ten Hacken NH, van Oosterhout AJ, Nawijn MC, van der Toorn M. Cigarette smoke-induced damage-associated molecular pattern release from necrotic neutrophils triggers proinflammatory mediator release. Am J Respir Cell Mol Biol 52: 554–562, 2015. doi: 10.1165/rcmb.2013-0505OC. [DOI] [PubMed] [Google Scholar]
- 46.Herzig S, Shaw RJ. AMPK: guardian of metabolism and mitochondrial homeostasis. Nat Rev Mol Cell Biol 19: 121–135, 2018. doi: 10.1038/nrm.2017.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hiemstra PS, McCray PB Jr, Bals R. The innate immune function of airway epithelial cells in inflammatory lung disease. Eur Respir J 45: 1150–1162, 2015. doi: 10.1183/09031936.00141514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Higgins GC, Coughlan MT. Mitochondrial dysfunction and mitophagy: the beginning and end to diabetic nephropathy? Br J Pharmacol 171: 1917–1942, 2014. doi: 10.1111/bph.12503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hoffmann RF, Zarrintan S, Brandenburg SM, Kol A, de Bruin HG, Jafari S, Dijk F, Kalicharan D, Kelders M, Gosker HR, Ten Hacken NH, van der Want JJ, van Oosterhout AJ, Heijink IH. Prolonged cigarette smoke exposure alters mitochondrial structure and function in airway epithelial cells. Respir Res 14: 97, 2013. doi: 10.1186/1465-9921-14-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hogg JC. Pathophysiology of airflow limitation in chronic obstructive pulmonary disease. Lancet 364: 709–721, 2004. doi: 10.1016/S0140-6736(04)16900-6. [DOI] [PubMed] [Google Scholar]
- 51.Hough KP, Trevor JL, Strenkowski JG, Wang Y, Chacko BK, Tousif S, Chanda D, Steele C, Antony VB, Dokland T, Ouyang X, Zhang J, Duncan SR, Thannickal VJ, Darley-Usmar VM, Deshane JS. Exosomal transfer of mitochondria from airway myeloid-derived regulatory cells to T cells. Redox Biol 18: 54–64, 2018. doi: 10.1016/j.redox.2018.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hu Q, Ren H, Ren J, Liu Q, Wu J, Wu X, Li G, Wang G, Gu G, Guo K, Hong Z, Liu S, Li J. Released mitochondrial DNA following intestinal ischemia reperfusion induces the inflammatory response and gut barrier dysfunction. Sci Rep 8: 7350, 2018. doi: 10.1038/s41598-018-25387-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hu Q, Ren J, Li G, Wu J, Wu X, Wang G, Gu G, Ren H, Hong Z, Li J. The mitochondrially targeted antioxidant MitoQ protects the intestinal barrier by ameliorating mitochondrial DNA damage via the Nrf2/ARE signaling pathway. Cell Death Dis 9: 403, 2018. doi: 10.1038/s41419-018-0436-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hüttemann M, Pecina P, Rainbolt M, Sanderson TH, Kagan VE, Samavati L, Doan JW, Lee I. The multiple functions of cytochrome c and their regulation in life and death decisions of the mammalian cell: From respiration to apoptosis. Mitochondrion 11: 369–381, 2011. doi: 10.1016/j.mito.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ito S, Araya J, Kurita Y, Kobayashi K, Takasaka N, Yoshida M, Hara H, Minagawa S, Wakui H, Fujii S, Kojima J, Shimizu K, Numata T, Kawaishi M, Odaka M, Morikawa T, Harada T, Nishimura SL, Kaneko Y, Nakayama K, Kuwano K. PARK2-mediated mitophagy is involved in regulation of HBEC senescence in COPD pathogenesis. Autophagy 11: 547–559, 2015. doi: 10.1080/15548627.2015.1017190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Iyer SS, He Q, Janczy JR, Elliott EI, Zhong Z, Olivier AK, Sadler JJ, Knepper-Adrian V, Han R, Qiao L, Eisenbarth SC, Nauseef WM, Cassel SL, Sutterwala FS. Mitochondrial cardiolipin is required for Nlrp3 inflammasome activation. Immunity 39: 311–323, 2013. doi: 10.1016/j.immuni.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kaczmarek E, Hauser CJ, Kwon WY, Riça I, Chen L, Sandler N, Otterbein LE, Campbell Y, Cook CH, Yaffe MB, Marusich MF, Itagaki K. A subset of five human mitochondrial formyl peptides mimics bacterial peptides and functionally deactivates human neutrophils. J Trauma Acute Care Surg 85: 936–943, 2018. doi: 10.1097/TA.0000000000001971. [DOI] [PubMed] [Google Scholar]
- 58.Kang M-J, Yoon CM, Kim BH, Lee C-M, Zhou Y, Sauler M, Homer R, Dhamija A, Boffa D, West AP, Shadel GS, Ting JP, Tedrow JR, Kaminski N, Kim WJ, Lee CG, Oh Y-M, Elias JA. Suppression of NLRX1 in chronic obstructive pulmonary disease. J Clin Invest 125: 2458–2462, 2015. doi: 10.1172/JCI71747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim SJ, Ahn DG, Syed GH, Siddiqui A. The essential role of mitochondrial dynamics in antiviral immunity. Mitochondrion 41: 21–27, 2018. doi: 10.1016/j.mito.2017.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.King PT. Inflammation in chronic obstructive pulmonary disease and its role in cardiovascular disease and lung cancer. Clin Transl Med 4: 68, 2015. doi: 10.1186/s40169-015-0068-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Koshiba T, Yasukawa K, Yanagi Y, Kawabata S. Mitochondrial membrane potential is required for MAVS-mediated antiviral signaling. Sci Signal 4: ra7, 2011. doi: 10.1126/scisignal.2001147. [DOI] [PubMed] [Google Scholar]
- 62.Kosmider B, Lin CR, Karim L, Tomar D, Vlasenko L, Marchetti N, Bolla S, Madesh M, Criner GJ, Bahmed K. Mitochondrial dysfunction in human primary alveolar type II cells in emphysema. EBioMedicine 46: 305–316, 2019. doi: 10.1016/j.ebiom.2019.07.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kuroda A, Hegab AE, Jingtao G, Yamashita S, Hizawa N, Sakamoto T, Yamada H, Suzuki S, Ishii M, Namkoong H, Asakura T, Ozaki M, Yasuda H, Hamamoto J, Kagawa S, Soejima K, Betsuyaku T. Effects of the common polymorphism in the human aldehyde dehydrogenase 2 (ALDH2) gene on the lung. Respir Res 18: 69, 2017. doi: 10.1186/s12931-017-0554-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Le Gal K, Ibrahim MX, Wiel C, Sayin VI, Akula MK, Karlsson C, Dalin MG, Akyürek LM, Lindahl P, Nilsson J, Bergo MO. Antioxidants can increase melanoma metastasis in mice. Sci Transl Med 7: 308re8, 2015. doi: 10.1126/scitranslmed.aad3740. [DOI] [PubMed] [Google Scholar]
- 65.Li J, Dai A, Hu R, Zhu L, Tan S. Positive correlation between PPARγ/PGC-1α and gamma-GCS in lungs of rats and patients with chronic obstructive pulmonary disease. Acta Biochim Biophys Sin (Shanghai) 42: 603–614, 2010. doi: 10.1093/abbs/gmq071. [DOI] [PubMed] [Google Scholar]
- 66.Li X, Zhang Y, Liang Y, Cui Y, Yeung SC, Ip MS, Tse HF, Lian Q, Mak JC. iPSC-derived mesenchymal stem cells exert SCF-dependent recovery of cigarette smoke-induced apoptosis/proliferation imbalance in airway cells. J Cell Mol Med 21: 265–277, 2017. doi: 10.1111/jcmm.12962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li X, Zhang Y, Yeung SC, Liang Y, Liang X, Ding Y, Ip MS, Tse HF, Mak JC, Lian Q. Mitochondrial transfer of induced pluripotent stem cell-derived mesenchymal stem cells to airway epithelial cells attenuates cigarette smoke-induced damage. Am J Respir Cell Mol Biol 51: 455–465, 2014. doi: 10.1165/rcmb.2013-0529OC. [DOI] [PubMed] [Google Scholar]
- 68.Liu X, Chen Z. The pathophysiological role of mitochondrial oxidative stress in lung diseases. J Transl Med 15: 207, 2017. doi: 10.1186/s12967-017-1306-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lommatzsch M, Cicko S, Müller T, Lucattelli M, Bratke K, Stoll P, Grimm M, Dürk T, Zissel G, Ferrari D, Di Virgilio F, Sorichter S, Lungarella G, Virchow JC, Idzko M. Extracellular adenosine triphosphate and chronic obstructive pulmonary disease. Am J Respir Crit Care Med 181: 928–934, 2010. doi: 10.1164/rccm.200910-1506OC. [DOI] [PubMed] [Google Scholar]
- 70.Lupfer C, Thomas PG, Anand PK, Vogel P, Milasta S, Martinez J, Huang G, Green M, Kundu M, Chi H, Xavier RJ, Green DR, Lamkanfi M, Dinarello CA, Doherty PC, Kanneganti TD. Receptor interacting protein kinase 2-mediated mitophagy regulates inflammasome activation during virus infection. Nat Immunol 14: 480–488, 2013. doi: 10.1038/ni.2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lytras T, Kogevinas M, Kromhout H, Carsin AE, Antó JM, Bentouhami H, Weyler J, Heinrich J, Nowak D, Urrutia I, Martinez-Moratalla J, Gullón JA, Pereira-Vega A, Raherison-Semjen C, Pin I, Demoly P, Leynaert B, Villani S, Gislason T, Svanes C, Holm M, Forsberg B, Norbäck D, Mehta AJ, Probst-Hensch N, Benke G, Jogi R, Torén K, Sigsgaard T, Schlünssen V, Olivieri M, Blanc PD, Vermeulen R, Garcia-Aymerich J, Jarvis D, Zock JP. Occupational exposures and 20-year incidence of COPD: the European Community Respiratory Health Survey. Thorax 73: 1008–1015, 2018. doi: 10.1136/thoraxjnl-2017-211158. [DOI] [PubMed] [Google Scholar]
- 72.MacNee W. Pulmonary and systemic oxidant/antioxidant imbalance in chronic obstructive pulmonary disease. Proc Am Thorac Soc 2: 50–60, 2005. doi: 10.1513/pats.200411-056SF. [DOI] [PubMed] [Google Scholar]
- 73.Mailloux RJ, Harper ME. Uncoupling proteins and the control of mitochondrial reactive oxygen species production. Free Radic Biol Med 51: 1106–1115, 2011. doi: 10.1016/j.freeradbiomed.2011.06.022. [DOI] [PubMed] [Google Scholar]
- 74.Malinska D, Szymański J, Patalas-Krawczyk P, Michalska B, Wojtala A, Prill M, Partyka M, Drabik K, Walczak J, Sewer A, Johne S, Luettich K, Peitsch MC, Hoeng J, Duszyński J, Szczepanowska J, van der Toorn M, Wieckowski MR. Assessment of mitochondrial function following short- and long-term exposure of human bronchial epithelial cells to total particulate matter from a candidate modified-risk tobacco product and reference cigarettes. Food Chem Toxicol 115: 1–12, 2018. doi: 10.1016/j.fct.2018.02.013. [DOI] [PubMed] [Google Scholar]
- 75.Matheson M, Rynell AC, McClean M, Berend N. Cigarette smoking increases neutrophil formyl methionyl leucyl phenylalanine receptor numbers. Chest 123: 1642–1646, 2003. doi: 10.1378/chest.123.5.1642. [DOI] [PubMed] [Google Scholar]
- 76.Matzinger P. Tolerance, danger, and the extended family. Annu Rev Immunol 12: 991–1045, 1994. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- 77.Mishra P, Chan DC. Metabolic regulation of mitochondrial dynamics. J Cell Biol 212: 379–387, 2016. doi: 10.1083/jcb.201511036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mishra P, Chan DC. Mitochondrial dynamics and inheritance during cell division, development and disease. Nat Rev Mol Cell Biol 15: 634–646, 2014. doi: 10.1038/nrm3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mizumura K, Cloonan SM, Nakahira K, Bhashyam AR, Cervo M, Kitada T, Glass K, Owen CA, Mahmood A, Washko GR, Hashimoto S, Ryter SW, Choi AMK. Mitophagy-dependent necroptosis contributes to the pathogenesis of COPD. J Clin Invest 124: 3987–4003, 2014. doi: 10.1172/JCI74985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Moore CB, Bergstralh DT, Duncan JA, Lei Y, Morrison TE, Zimmermann AG, Accavitti-Loper MA, Madden VJ, Sun L, Ye Z, Lich JD, Heise MT, Chen Z, Ting JP. NLRX1 is a regulator of mitochondrial antiviral immunity. Nature 451: 573–577, 2008. doi: 10.1038/nature06501. [DOI] [PubMed] [Google Scholar]
- 81.Mora AL, Bueno M, Rojas M. Mitochondria in the spotlight of aging and idiopathic pulmonary fibrosis. J Clin Invest 127: 405–414, 2017. doi: 10.1172/JCI87440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Morrow JD, Zhou X, Lao T, Jiang Z, DeMeo DL, Cho MH, Qiu W, Cloonan S, Pinto-Plata V, Celli B, Marchetti N, Criner GJ, Bueno R, Washko GR, Glass K, Quackenbush J, Choi AM, Silverman EK, Hersh CP. Functional interactors of three genome-wide association study genes are differentially expressed in severe chronic obstructive pulmonary disease lung tissue. Sci Rep 7: 44232, 2017. doi: 10.1038/srep44232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Murakami T, Ockinger J, Yu J, Byles V, McColl A, Hofer AM, Horng T. Critical role for calcium mobilization in activation of the NLRP3 inflammasome. Proc Natl Acad Sci USA 109: 11282–11287, 2012. doi: 10.1073/pnas.1117765109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nyunoya T, Mebratu Y, Contreras A, Delgado M, Chand HS, Tesfaigzi Y. Molecular processes that drive cigarette smoke-induced epithelial cell fate of the lung. Am J Respir Cell Mol Biol 50: 471–482, 2014. doi: 10.1165/rcmb.2013-0348TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Paliwal S, Chaudhuri R, Agrawal A, Mohanty S. Regenerative abilities of mesenchymal stem cells through mitochondrial transfer. J Biomed Sci 25: 31, 2018. doi: 10.1186/s12929-018-0429-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pinegin B, Vorobjeva N, Pashenkov M, Chernyak B. The role of mitochondrial ROS in antibacterial immunity. J Cell Physiol 233: 3745–3754, 2018. doi: 10.1002/jcp.26117. [DOI] [PubMed] [Google Scholar]
- 87.Ploumi C, Daskalaki I, Tavernarakis N. Mitochondrial biogenesis and clearance: a balancing act. FEBS J 284: 183–195, 2017. doi: 10.1111/febs.13820. [DOI] [PubMed] [Google Scholar]
- 88.Pourcelot M, Arnoult D. Mitochondrial dynamics and the innate antiviral immune response. FEBS J 281: 3791–3802, 2014. doi: 10.1111/febs.12940. [DOI] [PubMed] [Google Scholar]
- 89.Pouwels SD, Heijink IH, ten Hacken NH, Vandenabeele P, Krysko DV, Nawijn MC, van Oosterhout AJ. DAMPs activating innate and adaptive immune responses in COPD. Mucosal Immunol 7: 215–226, 2014. doi: 10.1038/mi.2013.77. [DOI] [PubMed] [Google Scholar]
- 90.Pouwels SD, Hesse L, Faiz A, Lubbers J, Bodha PK, Ten Hacken NH, van Oosterhout AJ, Nawijn MC, Heijink IH. Susceptibility for cigarette smoke-induced DAMP release and DAMP-induced inflammation in COPD. Am J Physiol Lung Cell Mol Physiol 311: L881–L892, 2016. doi: 10.1152/ajplung.00135.2016. [DOI] [PubMed] [Google Scholar]
- 91.Pouwels SD, Zijlstra GJ, van der Toorn M, Hesse L, Gras R, Ten Hacken NH, Krysko DV, Vandenabeele P, de Vries M, van Oosterhout AJ, Heijink IH, Nawijn MC. Cigarette smoke-induced necroptosis and DAMP release trigger neutrophilic airway inflammation in mice. Am J Physiol Lung Cell Mol Physiol 310: L377–L386, 2016. doi: 10.1152/ajplung.00174.2015. [DOI] [PubMed] [Google Scholar]
- 92.De Proost I, Pintelon I, Brouns I, Kroese ABA, Riccardi D, Kemp PJ, Timmermans J-P, Adriaensen D. Functional live cell imaging of the pulmonary neuroepithelial body microenvironment. Am J Respir Cell Mol Biol 39: 180–189, 2008. doi: 10.1165/rcmb.2008-0011OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Proud D, Hudy MH, Wiehler S, Zaheer RS, Amin MA, Pelikan JB, Tacon CE, Tonsaker TO, Walker BL, Kooi C, Traves SL, Leigh R. Cigarette smoke modulates expression of human rhinovirus-induced airway epithelial host defense genes. PLoS One 7: e40762, 2012. doi: 10.1371/journal.pone.0040762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rashid K, Sundar IK, Gerloff J, Li D, Rahman I. Lung cellular senescence is independent of aging in a mouse model of COPD/emphysema. Sci Rep 8: 9023, 2018. doi: 10.1038/s41598-018-27209-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Reijnders L. The origin of mitochondria. J Mol Evol 5: 167–176, 1975. doi: 10.1007/BF01741239. [DOI] [PubMed] [Google Scholar]
- 96.Rimmele P, d’Escamard V, Fatih K, Zhang C, Sadek HA, Ghaffari S. Metabolic cross talk between Foxo3 and mTOR is essential for hematopoietic stem cell function. Blood 120: 856, 2012. doi: 10.1182/blood.V120.21.856.856. [DOI] [Google Scholar]
- 97.Rokicki W, Rokicki M, Wojtacha J, Dżeljijli A. The role and importance of club cells (Clara cells) in the pathogenesis of some respiratory diseases. Kardiochir Torakochirurgia Pol 13: 26–30, 2016. doi: 10.5114/kitp.2016.58961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rowart P, Wu J, Caplan MJ, Jouret F. Implications of AMPK in the formation of epithelial tight junctions. Int J Mol Sci 19: 2040, 2018. doi: 10.3390/ijms19072040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ryter SW, Choi AM. Autophagy in the lung. Proc Am Thorac Soc 7: 13–21, 2010. doi: 10.1513/pats.200909-101JS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Saxena A, Lopes F, Poon KKH, McKay DM. Absence of the NOD2 protein renders epithelia more susceptible to barrier dysfunction due to mitochondrial dysfunction. Am J Physiol Gastrointest Liver Physiol 313: G26–G38, 2017. doi: 10.1152/ajpgi.00070.2017. [DOI] [PubMed] [Google Scholar]
- 101.Sayin VI, Ibrahim MX, Larsson E, Nilsson JA, Lindahl P, Bergo MO. Antioxidants accelerate lung cancer progression in mice. Sci Transl Med 6: 221ra15, 2014. doi: 10.1126/scitranslmed.3007653. [DOI] [PubMed] [Google Scholar]
- 102.Sebag SC, Koval OM, Paschke JD, Winters CJ, Comellas AP, Grumbach IM. Inhibition of the mitochondrial calcium uniporter prevents IL-13 and allergen-mediated airway epithelial apoptosis and loss of barrier function. Exp Cell Res 362: 400–411, 2018. doi: 10.1016/j.yexcr.2017.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Shimada K, Crother TR, Karlin J, Dagvadorj J, Chiba N, Chen S, Ramanujan VK, Wolf AJ, Vergnes L, Ojcius DM, Rentsendorj A, Vargas M, Guerrero C, Wang Y, Fitzgerald KA, Underhill DM, Town T, Arditi M. Oxidized mitochondrial DNA activates the NLRP3 inflammasome during apoptosis. Immunity 36: 401–414, 2012. doi: 10.1016/j.immuni.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Silverberg DS, Mor R, Weu MT, Schwartz D, Schwartz IF, Chernin G. Anemia and iron deficiency in COPD patients: prevalence and the effects of correction of the anemia with erythropoiesis stimulating agents and intravenous iron. BMC Pulm Med 14: 24, 2014. doi: 10.1186/1471-2466-14-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Singanayagam A, Loo SL, Calderazzo MA, Finney LJ, Trujillo Torralbo MB, Bakhsoliani E, Girkin J, Veerati PC, Pathinayake PS, Nichol KS, Reid AT, Footitt J, Johnston SL, Bartlett NW, Mallia P. Anti-viral immunity is impaired in COPD patients with frequent exacerbations. Am J Physiol Lung Cell Mol Physiol ajplung.00253.2019, 2019. doi: 10.1152/ajplung.00253.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Son ES, Kim SH, Ryter SW, Yeo EJ, Kyung SY, Kim YJ, Jeong SH, Lee CS, Park JW. Quercetogetin protects against cigarette smoke extract-induced apoptosis in epithelial cells by inhibiting mitophagy. Toxicol In Vitro 48: 170–178, 2018. doi: 10.1016/j.tiv.2018.01.011. [DOI] [PubMed] [Google Scholar]
- 107.Song C, Luo B, Gong L. Resveratrol reduces the apoptosis induced by cigarette smoke extract by upregulating MFN2. PLoS One 12: e0175009, 2017. [Erratum in PLoS One 14: e0213877, 2019.] doi: 10.1371/journal.pone.0175009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Spees JL, Olson SD, Whitney MJ, Prockop DJ. Mitochondrial transfer between cells can rescue aerobic respiration. Proc Natl Acad Sci USA 103: 1283–1288, 2006. doi: 10.1073/pnas.0510511103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Stokman G, Kors L, Bakker PJ, Rampanelli E, Claessen N, Teske GJD, Butter L, van Andel H, van den Bergh Weerman MA, Larsen PWB, Dessing MC, Zuurbier CJ, Girardin SE, Florquin S, Leemans JC. NLRX1 dampens oxidative stress and apoptosis in tissue injury via control of mitochondrial activity. J Exp Med 214: 2405–2420, 2017. doi: 10.1084/jem.20161031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Subramanian N, Natarajan K, Clatworthy MR, Wang Z, Germain RN. The adaptor MAVS promotes NLRP3 mitochondrial localization and inflammasome activation. Cell 153: 348–361, 2013. doi: 10.1016/j.cell.2013.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Summer R, Shaghaghi H, Schriner D, Roque W, Sales D, Cuevas-Mora K, Desai V, Bhushan A, Ramirez MI, Romero F. Activation of the mTORC1/PGC-1 axis promotes mitochondrial biogenesis and induces cellular senescence in the lung epithelium. Am J Physiol Lung Cell Mol Physiol 316: L1049–L1060, 2019. doi: 10.1152/ajplung.00244.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Terzikhan N, Verhamme KM, Hofman A, Stricker BH, Brusselle GG, Lahousse L. Prevalence and incidence of COPD in smokers and non-smokers: the Rotterdam Study. Eur J Epidemiol 31: 785–792, 2016. doi: 10.1007/s10654-016-0132-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Unger BL, Ganesan S, Comstock AT, Faris AN, Hershenson MB, Sajjan US. Nod-like receptor X-1 is required for rhinovirus-induced barrier dysfunction in airway epithelial cells. J Virol 88: 3705–3718, 2014. doi: 10.1128/JVI.03039-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Valdivieso ÁG, Dugour AV, Sotomayor V, Clauzure M, Figueroa JM, Santa-Coloma TA. N-acetyl cysteine reverts the proinflammatory state induced by cigarette smoke extract in lung Calu-3 cells. Redox Biol 16: 294–302, 2018. doi: 10.1016/j.redox.2018.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.van der Does AM, Heijink M, Mayboroda OA, Persson LJ, Aanerud M, Bakke P, Eagan TM, Hiemstra PS, Giera M. Dynamic differences in dietary polyunsaturated fatty acid metabolism in sputum of COPD patients and controls. Biochim Biophys Acta Mol Cell Biol Lipids 1864: 224–233, 2019. doi: 10.1016/j.bbalip.2018.11.012. [DOI] [PubMed] [Google Scholar]
- 116.van der Toorn M, Rezayat D, Kauffman HF, Bakker SJ, Gans RO, Koëter GH, Choi AM, van Oosterhout AJ, Slebos DJ. Lipid-soluble components in cigarette smoke induce mitochondrial production of reactive oxygen species in lung epithelial cells. Am J Physiol Lung Cell Mol Physiol 297: L109–L114, 2009. doi: 10.1152/ajplung.90461.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Van Houten B, Hunter SE, Meyer JN. Mitochondrial DNA damage induced autophagy, cell death, and disease. Front Biosci 21: 42–54, 2016. doi: 10.2741/4375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Vanella L, Li Volti G, Distefano A, Raffaele M, Zingales V, Avola R, Tibullo D, Barbagallo I. A new antioxidant formulation reduces the apoptotic and damaging effect of cigarette smoke extract on human bronchial epithelial cells. Eur Rev Med Pharmacol Sci 21: 5478–5484, 2017. doi: 10.26355/eurrev_201712_13938. [DOI] [PubMed] [Google Scholar]
- 119.Vij N, Chandramani-Shivalingappa P, Van Westphal C, Hole R, Bodas M. Cigarette smoke-induced autophagy impairment accelerates lung aging, COPD-emphysema exacerbations and pathogenesis. Am J Physiol Cell Physiol 314: C73–C87, 2018. doi: 10.1152/ajpcell.00110.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wang A, Keita AV, Phan V, McKay CM, Schoultz I, Lee J, Murphy MP, Fernando M, Ronaghan N, Balce D, Yates R, Dicay M, Beck PL, MacNaughton WK, Söderholm JD, McKay DM. Targeting mitochondria-derived reactive oxygen species to reduce epithelial barrier dysfunction and colitis. Am J Pathol 184: 2516–2527, 2014. doi: 10.1016/j.ajpath.2014.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wang XL, Li T, Li JH, Miao SY, Xiao XZ. The effects of resveratrol on inflammation and oxidative stress in a rat model of chronic obstructive pulmonary disease. Molecules 22: 1529, 2017. doi: 10.3390/molecules22091529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Whitsett JA, Alenghat T. Respiratory epithelial cells orchestrate pulmonary innate immunity. Nat Immunol 16: 27–35, 2015. doi: 10.1038/ni.3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wu YH, Kuo WC, Wu YJ, Yang KT, Chen ST, Jiang ST, Gordy C, He YW, Lai MZ. Participation of c-FLIP in NLRP3 and AIM2 inflammasome activation. Cell Death Differ 21: 451–461, 2014. doi: 10.1038/cdd.2013.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Xu W, Janocha AJ, Leahy RA, Klatte R, Dudzinski D, Mavrakis LA, Comhair SA, Lauer ME, Cotton CU, Erzurum SC. A novel method for pulmonary research: assessment of bioenergetic function at the air-liquid interface. Redox Biol 2: 513–519, 2014. doi: 10.1016/j.redox.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Yang L, Na CL, Luo S, Wu D, Hogan S, Huang T, Weaver TE. The phosphatidylcholine transfer protein Stard7 is required for mitochondrial and epithelial cell homeostasis. Sci Rep 7: 46416, 2017. doi: 10.1038/srep46416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Yao H, Chung S, Hwang JW, Rajendrasozhan S, Sundar IK, Dean DA, McBurney MW, Guarente L, Gu W, Rönty M, Kinnula VL, Rahman I. SIRT1 protects against emphysema via FOXO3-mediated reduction of premature senescence in mice. J Clin Invest 122: 2032–2045, 2012. doi: 10.1172/JCI60132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Yao H, Sundar IK, Gorbunova V, Rahman I. P21-PARP-1 pathway is involved in cigarette smoke-induced lung DNA damage and cellular senescence. PLoS One 8: e80007, 2013. doi: 10.1371/journal.pone.0080007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Yoo SM, Jung YK. A molecular approach to mitophagy and mitochondrial dynamics. Mol Cells 41: 18–26, 2018. doi: 10.14348/molcells.2018.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Youle RJ, Narendra DP. Mechanisms of mitophagy. Nat Rev Mol Cell Biol 12: 9–14, 2011. doi: 10.1038/nrm3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zhang J, Wang J, Wang X, Liu Z, Ren J, Sun T. Early surgery increases mitochondrial DNA release and lung injury in a model of elderly hip fracture and chronic obstructive pulmonary disease. Exp Ther Med 14: 4541–4546, 2017. doi: 10.3892/etm.2017.5044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zhang WZ, Butler JJ, Cloonan SM. Smoking-induced iron dysregulation in the lung. Free Radic Biol Med 133: 238–247, 2019. doi: 10.1016/j.freeradbiomed.2018.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zhang X, Shan P, Homer R, Zhang Y, Petrache I, Mannam P, Lee PJ. Cathepsin E promotes pulmonary emphysema via mitochondrial fission. Am J Pathol 184: 2730–2741, 2014. doi: 10.1016/j.ajpath.2014.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zhou JS, Zhao Y, Zhou HB, Wang Y, Wu YF, Li ZY, Xuan NX, Zhang C, Hua W, Ying SM, Li W, Shen HH, Chen ZH. Autophagy plays an essential role in cigarette smoke-induced expression of MUC5AC in airway epithelium. Am J Physiol Lung Cell Mol Physiol 310: L1042–L1052, 2016. doi: 10.1152/ajplung.00418.2015. [DOI] [PubMed] [Google Scholar]
- 135.Zhou R, Yazdi AS, Menu P, Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature 469: 221–225, 2011. [Erratum in Nature 475: 122, 2011.] doi: 10.1038/nature09663. [DOI] [PubMed] [Google Scholar]
- 136.Zimorski V, Ku C, Martin WF, Gould SB. Endosymbiotic theory for organelle origins. Curr Opin Microbiol 22: 38–48, 2014. doi: 10.1016/j.mib.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 137.Zuo WL, Shenoy SA, Li S, O’Beirne SL, Strulovici-Barel Y, Leopold PL, Wang G, Staudt MR, Walters MS, Mason C, Kaner RJ, Mezey JG, Crystal RG. Ontogeny and biology of human small airway epithelial club cells. Am J Respir Crit Care Med 198: 1375–1388, 2018. doi: 10.1164/rccm.201710-2107OC. [DOI] [PMC free article] [PubMed] [Google Scholar]