Abstract
The most common cause of acute lung injury is ischemia-reperfusion injury (IRI), during which mitochondrial damage occurs. We have previously demonstrated that mitochondrial transplantation is an efficacious therapy to replace or augment mitochondria damaged by IRI, allowing for enhanced muscle viability and function in cardiac tissue. Here, we investigate the efficacy of mitochondrial transplantation in a murine lung IRI model using male C57BL/6J mice. Transient ischemia was induced by applying a microvascular clamp on the left hilum for 2 h. Upon reperfusion mice received either vehicle or vehicle-containing mitochondria either by vascular delivery (Mito V) through the pulmonary artery or by aerosol delivery (Mito Neb) via the trachea (nebulization). Sham control mice underwent thoracotomy without hilar clamping and were ventilated for 2 h before returning to the cage. After 24 h recovery, lung mechanics were assessed and lungs were collected for analysis. Our results demonstrated that at 24 h of reperfusion, dynamic compliance and inspiratory capacity were significantly increased and resistance, tissue damping, elastance, and peak inspiratory pressure (Mito V only) were significantly decreased (P < 0.05) in Mito groups as compared with their respective vehicle groups. Neutrophil infiltration, interstitial edema, and apoptosis were significantly decreased (P < 0.05) in Mito groups as compared with vehicles. No significant differences in cytokines and chemokines between groups were shown. All lung mechanics results in Mito groups except peak inspiratory pressure in Mito Neb showed no significant differences (P > 0.05) as compared with Sham. These results conclude that mitochondrial transplantation by vascular delivery or nebulization improves lung mechanics and decreases lung tissue injury.
Keywords: acute lung injury, ischemia-reperfusion injury, mitochondrial transplantation
INTRODUCTION
Acute lung injury (ALI) complicates a wide variety of medical and surgical conditions and reflects the pulmonary response to lung insult or precipitating factors (4, 16, 29). One of the most common insults leading to ALI is ischemia-reperfusion injury (IRI). IRI can occur with extracorporeal gas exchange, cardiopulmonary bypass, and lung ventilation and has been shown to have profound effects on lung function and cellular viability and to significantly contribute to increased morbidity and mortality in both pediatric and adult patients (2, 4, 9, 16, 29, 36). The incidence of ALI in adult patients undergoing cardiac surgery has been estimated to 2% (38). In pediatric patients, ALI is present in 8–10% of patients requiring mechanical ventilation. In both pediatric and adult patients, ALI has an estimated mortality of 20–75% (2, 9, 12, 24, 31, 36, 42).
Previous studies have demonstrated that mitochondria suffer damage during ischemia-reperfusion and that mitochondrial damage plays a significant role in ALI (1, 7, 8, 28, 32). These data suggest that targeting the mitochondria could provide a promising strategy for the management of lung IRI.
Recently, we have pioneered a novel therapy for cardiac IRI based on mitochondrial transplantation (5, 6, 11, 17, 19–22, 25, 26, 30). This approach uses replacement of native mitochondria with viable, respiration-competent mitochondria isolated from nonischemic autologous tissue to overcome the many deleterious effects of IRI on native mitochondria. Autologous mitochondrial transplantation has been demonstrated to provide salvage of tissue affected by IRI (19, 21, 25). Healthy mitochondria can be harvested from nonischemic skeletal muscle in the patient’s own body and processed within <30 min in the surgical suite (6, 17, 25, 30). The isolated mitochondria are then transplanted into the tissue, where they restore normal cellular energetics (17, 25, 30). In this study, we investigate the safety and efficacy of syngeneic mitochondrial transplantation by vascular delivery through the pulmonary artery and nebulization via the trachea in a murine model of lung IRI.
METHODS
Animals
Male C57BL/6J mice (8–10 wk, n = 36; Jackson Laboratory, Bar Harbor, ME) were used. All experiments were approved by the Institutional Animal Care and Use Committee at Boston Children’s Hospital and conformed to the National Institutes of Health guidelines on animal care and use.
Mitochondrial Isolation
Gastrocnemius muscle from C57BL/6J male mice (n = 6) was dissected and used immediately to isolate syngeneic mitochondria. Mitochondria were isolated and the number and viability of the isolated mitochondria was determined as previously described (22, 25, 26). The isolated mitochondria were suspended in either 0.3 mL buffer (250 mmol/L sucrose, 20 mmol/L K+-HEPES buffer, pH 7.2, 0.5 mmol/L K+-EGTA, pH 8.0) for vascular delivery group or in 90 μL buffer for nebulization delivery group and used immediately for mitochondrial transplantation.
Murine Lung IRI Model
The experimental protocol is shown in Fig. 1. Surgery was performed according to the procedure previously described with modifications (18). In brief, C57BL/6J male mice (n = 30) were anesthetized with intraperitoneal injection of sodium pentobarbital (100 mg/kg) and maintained during surgery on 0.5% inhaled isoflurane. Oral endotracheal intubation using 20 G plastic catheter was performed and mice were mechanically ventilated with tidal volume 10 mL/kg and respiratory rate 130–140 breaths/min. Left thoracotomy was performed in third intercostal space and the left pulmonary hilum was exposed. Transient ischemia of the left lung was induced by occluding the left hilum at the end of expiration for 2 h using a microvascular clamp (Roboz Surgical Instrument, Gaithersburg, MD) and tidal volume was reduced to 7.5 mL/kg. The surgical incision was covered by gauze soaked in heparin solution containing (60 IU heparin/mL). At the end of ischemia period, the clip was removed. The animals were randomized into two experimental groups: vascular delivery (V) or nebulization (Neb) group.
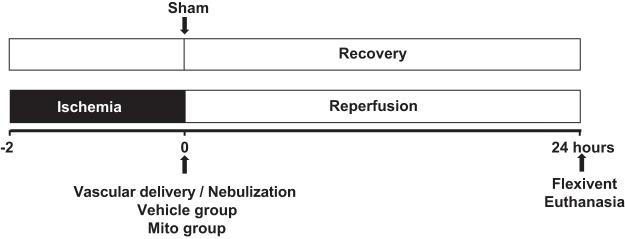
Fig. 1.
Experimental protocol. C57BL/6J male mice (8–10 wk, n = 36) were used for all experimental groups. Mice underwent left thoracotomy and the left pulmonary hilum was occluded at the end of expiration for 2 h using a microvascular clamp to create ischemia. At the end of the ischemia period, the clip was removed to allow for reperfusion, and the animals were randomized to receive either Vehicle or Vehicle containing mitochondria delivered either by vascular delivery (Mito V) via the pulmonary artery or by aerosol delivery (Mito Neb) via the trachea via nebulization. In the vascular delivery group at the beginning of reperfusion, either 1 × 108 mitochondria in 0.3 mL respiration buffer (Mito V, n = 6) or 0.3 mL respiration buffer (Vehicle V, n = 7) was injected as a bolus antegrade via the left pulmonary artery using a tuberculin syringe with a 40 G needle. In the nebulization group at the beginning of reperfusion, either 3 × 108 mitochondria in 90 μL respiration buffer (Mito Neb, n = 6) or 90 μL respiration buffer (Vehicle Neb, n = 6) were delivered to the whole lung over 40 s using the Aeroneb ultrasonic nebulizer following delivery protocol: 10 s nebulization followed by 1 min of regular mechanical ventilation, repeated four times. The mice were allowed to recover and returned to their cage for 24 h. Sham control mice (Sham, n = 5) underwent thoracotomy without hilar clamping and were mechanically ventilated for 2 h before returning to the cage. Following 24 h recovery, lung mechanics were assessed using FlexiVent system, bronchoalveolar lavage was obtained and lungs were collected for analysis.
Delivery of Mitochondria
Vascular delivery.
Vascular delivery of mitochondria to the left lung was achieved by injection of mitochondria in buffer directly into the left pulmonary artery at the beginning of reperfusion. In brief, vehicle alone (0.3 mL buffer, Vehicle V, n = 7) or vehicle-containing mitochondria (1 × 108 mitochondria suspended in 0.3 mL buffer, Mito V, n = 6) was injected as a bolus antegrade via the left pulmonary artery using a tuberculin syringe with a 40-gauge needle. The mice were allowed to recover for 24 h.
Nebulization.
Aerosol delivery of mitochondria was achieved by nebulization using the FlexiVent nebulizer system (FlexiVent FX2, SCIREQ, Montreal, Quebec, Canada). In brief, the FlexiVent was equipped with the Aeroneb ultrasonic nebulizer and Y-tubing to deliver the aerosol containing vehicle alone (Vehicle Neb) or buffer containing mitochondria (Mito Neb). The nebulization mouse default template was selected from the operating software and the FlexiVent system was calibrated according to the manufacturer’s directions. At the beginning of reperfusion, the oral endotracheal 20-gauge plastic catheter was connected to the FlexiVent system and mechanical ventilation was initiated at 130–140 breaths/min and 10 mL/kg tidal volume. The Aeroneb ultrasonic nebulizer was primed with vehicle alone (90 μL buffer, Vehicle Neb, n = 6) or with mitochondria in vehicle (3 × 108 in 90 μL buffer, Mito Neb, n = 6). Vehicle or mitochondria were delivered over 40 s using the following protocol: 10 s nebulization followed by 1 min of regular mechanical ventilation, repeated 4 times. The mice were allowed to recover for 24 h. Sham control mice (Sham, n = 5) underwent thoracotomy without hilar clamping and were mechanically ventilated for 2 h before returning to the cage.
Mitochondrial Biodistribution
Male Wistar rats (200–250 g, n = 8, Charles River Laboratories, Worcester, MA) were used for visualization of mitochondrial uptake in the lung. Male donor Wistar rats (n = 2) were used for syngeneic mitochondria isolation and 18F-labeled rhodamine 6G mitochondrial labeling as previously described (3, 19, 25, 26). Wistar rats were anesthetized and maintained on 2–3% inhaled isoflurane. In rats, receiving mitochondria (n = 3) via the pulmonary artery, a sternotomy was performed and the pulmonary trunk was exposed. 18F-labeled rhodamine 6G-labeled mitochondria (1 × 109 in 0.5 mL buffer) were injected to the lungs as a bolus antegrade via injection to the pulmonary trunk using a tuberculin syringe with a 30-gauge needle.
A separate group of Wistar rats (n = 3), received 18F-labeled rhodamine 6G-labeled mitochondria (1 × 109 in 0.3 mL buffer) as an aerosol to the lungs via the trachea via nebulization. Nebulization was performed as described above with the following modification. The endotracheal tube was connected to the FlexiVent system and mechanical ventilation was initiated at 90 breaths/min and 10 mL/kg tidal volume. The Aeroneb ultrasonic nebulizer was primed with 18F-labeled rhodamine 6G mitochondria (1 × 109 in 0.3 mL buffer) and the mitochondria were delivered using the following protocol: 10 s nebulization followed by 1 min of regular mechanical ventilation, repeated 6 times.
Ten minutes after delivery of mitochondria, the animals were euthanized in a CO2 chamber and imaged by positron emission tomography (PET) and microcomputed tomography using an Albira Si SPECT/CT/PET System (Bruker Corporation, Billerica, MA) (35).
Mitochondrial Tissue Uptake
In a separate set of C57BL/6J male mice (n = 6), mitochondria were isolated from the culture of human cardiac fibroblasts as previously described and delivered to mouse lung via pulmonary artery and via trachea via nebulization to assess mitochondrial tissue uptake in IRI model described above (5). In brief, mice were anesthetized with intraperitoneal injection of sodium pentobarbital (100 mg/kg) and maintained during surgery on 0.5% inhaled isoflurane and were mechanically ventilated with tidal volume 10 mL/kg and respiratory rate 130–140 breaths/min. Left thoracotomy was performed in third intercostal space and the left pulmonary hilum was exposed. Transient ischemia of the left lung was induced by occluding the left hilum at the end of expiration for 2 h using a microvascular clamp (Roboz Surgical Instrument) and tidal volume was reduced to 7.5 mL/kg. At the beginning of reperfusion, human mitochondria were delivered either by vascular delivery or by delivery via nebulization. In the mice receiving human mitochondria via vascular delivery, a vehicle-containing human mitochondria (1 × 108 mitochondria suspended in 0.3 mL buffer, n = 3) was injected as a bolus antegrade via the left pulmonary artery using a tuberculin syringe with a 40-gauge needle. In mice receiving human mitochondria via nebulization, mice were ventilated with FlexiVent equipped with the Aeroneb ultrasonic nebulizer. The Aeroneb ultrasonic nebulizer was primed with human mitochondria in vehicle (3 × 108 in 90 μL buffer, n = 3). Human mitochondria were delivered over 40 s using the following protocol: 10 s nebulization followed by 1 min of regular mechanical ventilation, repeated 4 times. After delivery of human mitochondria, animals were allowed to recover for 30 min, and left lung tissue was then harvested for further histological analysis.
The use of human mitochondria allowed for differentiation between endogenous mouse mitochondria and transplanted human mitochondria based on immune reactivity to a monoclonal anti-human mitochondrial antibody (biotin-MTCO2, Abcam, Cambridge, MA) (6, 19, 21). Mitochondria were detected using the anti-human mitochondria antibody and visualized using Vectastain (Vector Laboratories, Burlingame, CA) and AEC+ substrate chromogen (Dako, Agilent, Santa Clara, CA) as previously described (5).
Lung Mechanics
After 24 h recovery, mice were anesthetized with an intraperitoneal injection of sodium pentobarbital (70 mg/kg) and maintained on 0.5% inhaled isoflurane. Tracheostomy and endotracheal intubation using plastic 18-gauge catheter were performed and mice were mechanically ventilated with tidal volume 10 mL/kg and respiratory rate 130–140 breaths/min. A midline laparotomy and sternotomy were performed to expose the lung and right hilum was clamped to assess the mechanics of the left injured lung. Tidal volume was reduced to 5 mL/kg and mice were then connected to the specialized rodent ventilator (FlexiVent, SCIREQ, Montreal, Quebec, Canada) to assess dynamic compliance of respiratory system (dynamic compliance), resistance of respiratory system (resistance), tissue damping (as an indicator of peripheral tissue resistance), elastance of respiratory system (elastance), peak inspiratory pressure, and inspiratory capacity of the left lung.
Bronchoalveolar Lavage Analysis
After the assessment of lung mechanics, the left lung underwent bronchoalveolar lavage (BAL) using 1 mL saline solution. The BAL fluid was immediately centrifuged at 4°C at 500 g for 5 min, and the supernatant was collected and stored at −80°C until further analysis to determine the expression levels of cytokine and chemokine by Eve Technology (Calgary, AB, Canada) using the Millipore (Milliplex) mouse cytokine/chemokine panel (MilliporeSigma, Burlington, MA) (27). The Millipore (Milliplex) multiplex assay is a derivative of an ELISA using beads for binding the capture antibody. In a multiplex assay, microspheres of designated colors are coated with specific antibodies and read by flow cytometry. The following biomarkers were tested: Eotaxin, granulocyte colony-stimulating factor (G-CSF), granulocyte-macrophage colony-stimulating factor (GM-GSF), IFNγ, IL-1α, IL-1β, IL-2, IL-3, IL-4, IL-5, IL-6, IL-7, IL-9, IL-10, IL-12 (p40), IL-12 (p70), IL-13, IL-15, IL-17A, IP-10, keratinocyte chemoattractant (KC), leukemia inhibitory factor (LIF), lipopolysachharide-induced CXC chemokine (LIX), monocyte chemoattractant protein 1 (MCP-1), macrophage colony-stimulating factor (M-CSF), monokine induced by γ-interferon (MIG), macrophage inflammatory proteins MIP-1α, MIP-1β, MIP-2, regulated on activation, normal T expressed and secreted cytokines (RANTES), TNFα, VEGF. All samples were run in triplicate.
Tissue Analysis
After 24 h of recovery, the left lung was collected and each lung was dissected into three equal transverse sections. All analyses were performed by a blinded examiner.
The upper section of the left lung was used to assess tissue edema by calculating wet-to-dry weight ratio. Immediately after the collection, the lung was weighed (wet weight), put in an oven at 70°C for 72 h, then weighed again (dry weight), and wet-to-dry weight ratio was calculated (39).
The middle section of the left lung was immediately fixed in 10% formalin. The tissue was paraffin embedded and sectioned at 5-μm thickness. Serial sections were used for hematoxylin and eosin (H&E) staining, myeloperoxidase (MPO) staining, and TUNEL assay. Lung sections were evaluated for severity of lung injury by H&E staining as previously described using a pedigreed grading system (10). Five random (×15) visual fields per section were analyzed. Lung injury was graded using the following: grade 1 represented normal pulmonary histology; grade 2 indicated mild neutrophil leukocyte infiltrations and mild to moderate interstitial congestion; grade 3 represented moderate neutrophil leukocyte infiltration, perivascular edema formation and partial destruction of pulmonary architecture and grade 4 included dense neutrophil leukocyte infiltration, abscess formation and complete destruction of pulmonary architecture. MPO staining was performed as previously described and sections were evaluated for neutrophil infiltration (22). The number of neutrophils was determined in five random (×15) visual fields per section. TUNEL assay was performed as previously described (33). The number of TUNEL-positive nuclei was expressed as a percentage of all cells counted in 16 random (×10) visual fields per section.
The basal part of the left lung was used as previously described for transmission electron microscopy (TEM) to analyze structural damage in the lung (33).
Statistical Analysis
All data were collected by observers blinded to the treatments. All data are expressed as means ± SE. Statistical analysis was performed by nonparametric Mann-Whitney U test for all data except BAL analysis results, where between-group comparisons were evaluated with one-way ANOVA. Statistical significance was defined by an exact two-tailed P < 0.05.
RESULTS
Mitochondrial Biodistribution
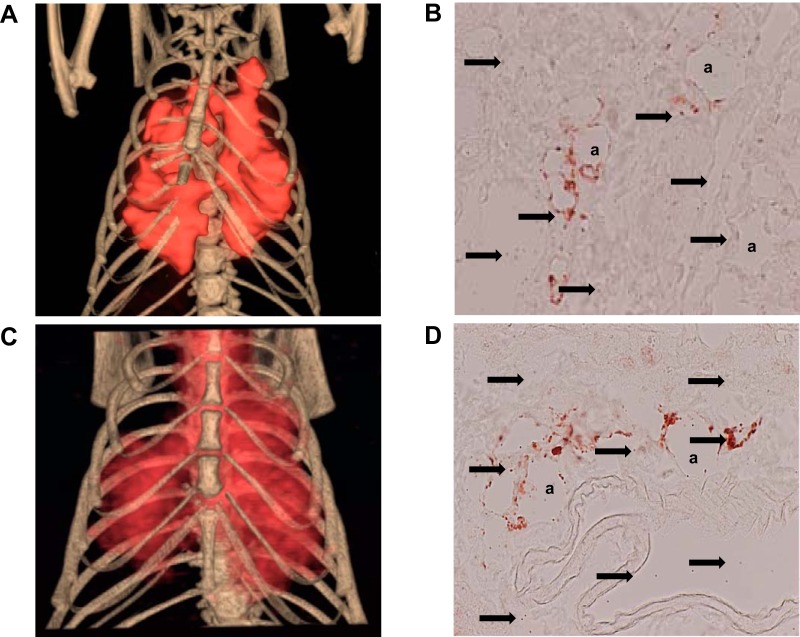
18F-Labeled rhodamine 6G radiolabeled mitochondria delivered either via vascular delivery (Fig. 2A) or via nebulization (Fig. 2C) were taken up diffusely by the lungs. 18F-Rhodamine 6G radiolabeled mitochondria were not detected in any other region of the body.
Fig. 2.
Mitochondrial biodistribution and tissue uptake. PET-microcomputed tomography imaging of 18F-labeled rhodamine 6G-labeled mitochondria (1 × 109, red) delivered to the lungs of Wistar male rats (n = 6; A and C). A: in rats receiving mitochondria via the pulmonary artery (n = 3), 18F-labeled rhodamine 6G mitochondria (1 × 109 mitochondria in 0.5 mL buffer) were injected to the lungs as a bolus antegrade via the pulmonary trunk using a tuberculin syringe with a 30-gauge needle. C: in rats receiving mitochondria via nebulization (n = 3), 18F-labeled rhodamine 6G labeled mitochondria (1 × 109 in 0.3 mL buffer) were delivered as an aerosol to the lungs via the trachea via nebulization using the Aeroneb ultrasonic nebulizer connected to the FlexVent system. Wistar rats were allowed to recover for 10 min after mitochondrial delivery and were then euthanized in a CO2 chamber before imaging. Labeled mitochondria (red signal on the imaging) were detected only in the lungs; radiolabeled mitochondria were not detectable in any other organ or region of the body (A and C). Representative immunohistochemical images (×100) of human mitochondria delivered to the lungs of C57BL/6J male mice at the beginning of reperfusion following 2 h of ischemia (n = 6; B and D). B: in mice receiving human mitochondria via the pulmonary artery (n = 3), human mitochondria (1 × 108 mitochondria in 0.3 mL buffer) were injected to the lung as a bolus antegrade via the left pulmonary artery using a tuberculin syringe with a 40-gauge needle. D: in mice receiving human mitochondria via nebulization (n = 3), human mitochondria (3 × 108 in 0.3 mL buffer) were delivered as an aerosol to the lungs via the trachea via nebulization using the Aeroneb ultrasonic nebulizer connected to the FlexVent system. C57BL/6J mice were allowed to recover for 30 min after mitochondrial delivery. Left lung tissue was then harvested for further immnohistological staining. Human mitochondria were detected using monoclonal anti-human mitochondrial antibody (biotin-MTCO2, Abcam, Cambridge, MA) and visualized using Vectastain (Vector Laboratories, Burlingame, CA) and AEC+ substrate chromogen (Dako, Agilent, Santa Clara, CA). Human mitochondria indicated by black arrows were globally distributed throughout the lung and detected within and around lung alveoli (a) and connective tissue (B and D). μCT, microcomputed tomography.
Mitochondrial Tissue Uptake
Exogenous mitochondria were isolated from human cardiac fibroblasts and delivered either via vascular delivery (Fig. 2B) or via nebulization (Fig. 2D). Analysis of mitochondrial uptake showed the majority of mitochondria based on signal intensity were globally distributed throughout the lung in Mito V and in Mito Neb (Fig. 2, B and D). Immunohistochemical analysis showed that the transplanted mitochondria were detected in alveoli and in connective tissue in both Mito V and Mito Neb (Fig. 2, B and D).
Lung Mechanics
After 2 h of ischemia and 24 h of reperfusion, lung mechanics were evaluated in mice receiving vehicle only and in mice receiving vehicle-containing mitochondria by both vascular delivery and nebulization.
Vascular Delivery
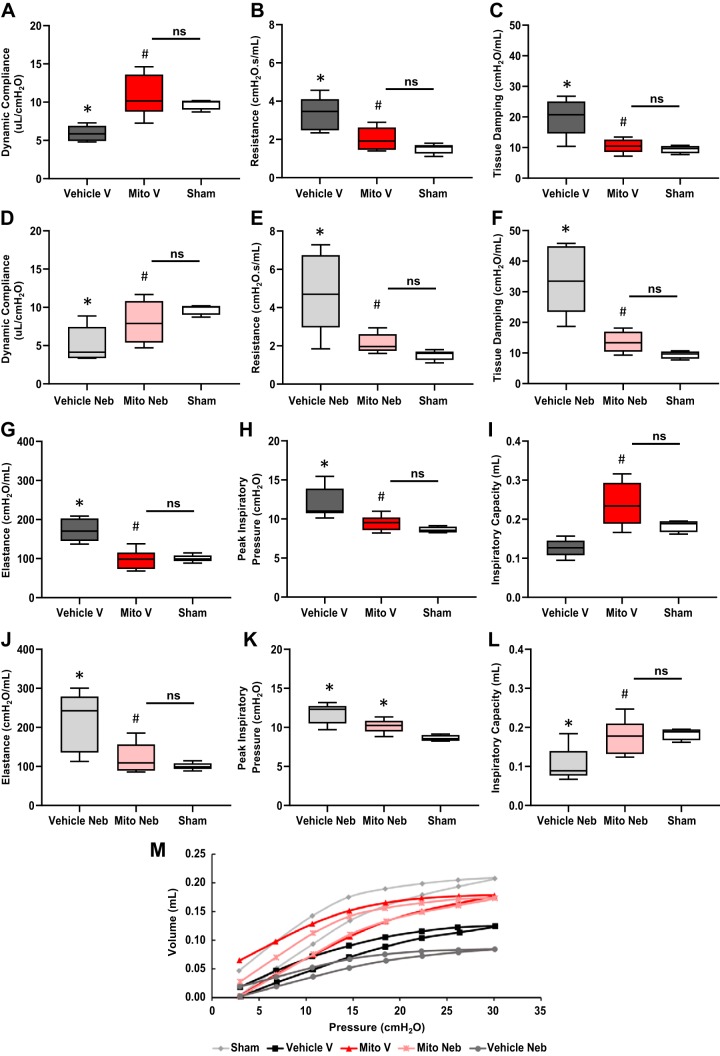
After 2 h of ischemia and 24 h of reperfusion, dynamic compliance was significantly decreased to 5.91 ± 0.46 μL/cmH2O, resistance was significantly increased to 3.33 ± 0.41 cmH2O, tissue damping was significantly increased to 20.2 ± 2.7 cmH2O/mL, elastance was significantly increased to 173.31 ± 13.40 cmH2O/mL and peak inspiratory pressure was significantly increased to 11.96 ± 0.74 cmH2O in Vehicle V lungs as compared with Sham lungs (9.75 ± 0.35 μL/cmH2O, 1.51 ± 0.12 cmH2O, 9.46 ± 0.63 cmH2O/mL, 100.41 ± 4.18 cmH2O/mL, and 8.62 ± 0.20 cmH2O for dynamic compliance, resistance, tissue damping, elastance, and peak inspiratory pressure, respectively; P < 0.05; Fig. 3, A–C, G, H). There was no significant difference (P > 0.05) in Vehicle V inspiratory capacity 0.13 ± 0.01 mL as compared with Sham 0.18 ± 0.01 mL (Fig. 3I).
Fig. 3.
Lung mechanics analysis using FlexiVent. After 2 h of ischemia and 24 h of reperfusion, improved lung mechanics were observed in mice receiving mitochondria via vascular delivery (Mito V) as compared with mice receiving vehicle only (Vehicle V). A–C and G–I: in Mito V lungs, dynamic compliance (A) was significantly increased to 10.79 ± 1.11 μL/cmH2O; resistance (B) was significantly decreased to 2.02 ± 0.28 cmH2O; tissue damping (C) was significantly decreased to 10.49 ± 0.91 cmH2O/min; elastance (G) was significantly decreased to 97.65 ± 10.28 cmH2O/mL; peak inspiratory pressure (H) was significantly decreased to 9.48 ± 0.40 cmH2O; and inspiratory capacity (I) was significantly increased to 0.24 ± 0.02 as compared with Vehicle V lungs (5.91 ± 0.46 μL/cmH2O, 3.33 ± 0.41 cmH2O, 20.2 ± 2.7 cmH2O/mL, 173.31 ± 13.40 cmH2O/mL, 11.96 ± 0.74 cmH2O. and 0.13 ± 0.01 mL, respectively, for Vehicle V; P < 0.05). There were no significant differences in any lung mechanics parameters observed between Mito V and Sham lungs (A–C, G–I: 9.75 ± 0.35 μL/cmH2O, 1.51 ± 0.12 cmH2O, 9.46 ± 0.63 cmH2O/mL, 100.41 ± 4.18 cmH2O/mL, 8.62 ± 0.20 cmH2O, and 0.18 ± 0.01 mL, respectively, for Sham; P > 0.05). Following 2 h of ischemia and 24 h of reperfusion, improved lung mechanics were observed in mice receiving mitochondria via nebulization (Mito Neb) as compared with mice receiving vehicle only (Vehicle Neb). D–F, J, and L: in Mito Neb lungs, dynamic compliance (D) was significantly increased to 7.88 ± 1.05 μL/cmH2O; resistance (E) was significantly decreased to 2.13 ± 0.23 cmH2O; tissue damping (F) was significantly decreased to 13.63 ± 1.55 cmH2O/mL; elastance (J) was significantly decreased to 120.06 ± 17.91 cmH2O/mL; and inspiratory capacity (L) was significantly increased to 0.18 ± 0.02 mL as compared with Vehicle Neb lungs (D–F, J, L: 5.13 ± 0.93 μL/cmH2O, 4.74 ± 0.83 cmH2O, 34.02 ± 5.04 cmH2O/mL, 218.93 ± 30.64 cmH2O/mL, and 0.10 ± 0.02 mL, respectively, for Vehicle Neb; P < 0.05). K: there was no significant difference observed in peak inspiratory pressure in Mito Neb 10.18 ± 0.40 cmH2O as compared with Vehicle (11.82 ± 0.54 cmH2O; P > 0.05). There were no significant differences in dynamic compliance, resistance, tissue damping, elastance, and inspiratory capacity observed between Mito Neb and Sham lungs (D–F, J, L). However, Mito Neb peak inspiratory pressure was significantly increased as compared with Sham (K; P < 0.05). M: representative pressure-volume loops demonstrate Vehicle V and Vehicle Neb had a lower lung volume capacity despite higher pressures as compared with Mito V, Mito Neb, and Sham groups. These pressure-volumes loops show narrower hysteresis and describe lower compliance in both Vehicle V and Vehicle Neb when compared with Mito V, Mito Neb, and Sham groups. All values are means ± SE *P < 0.05 vs. Sham; #P < 0.05 vs. Vehicle; n = 7 for Vehicle V, n = 6 for Mito V, n = 6 for Vehicle Neb, n = 6 for Mito Neb, and n = 5 for Sham group.
In Mito V lungs dynamic compliance was significantly increased to 10.79 ± 1.11 μL/cmH2O, resistance was significantly decreased to 2.02 ± 0.28 cmH2O, tissue damping was significantly decreased to 10.49 ± 0.91 cmH2O/min, elastance was significantly decreased to 97.65 ± 10.28 cmH2O/mL, peak inspiratory pressure was significantly decreased to 9.48 ± 0.40 cmH2O and inspiratory capacity was significantly increased to 0.24 ± 0.02 mL following 2 h of ischemia and 24 h of reperfusion as compared with Vehicle V lungs (P < 0.05; Fig. 3, A–C, G–I).
There were no significant differences in any lung mechanics parameters observed between Sham control and Mito V (P > 0.05; Fig. 3, A–C, G–I).
Nebulization
After 2 h of ischemia and 24 h of reperfusion, dynamic compliance was significantly decreased to 5.13 ± 0.93 μL/cmH2O, resistance was significantly increased to 4.74 ± 0.83, cmH2O tissue damping was significantly increased to 34.02 ± 5.04 cmH2O/mL, elastance was significantly increased to 218.93 ± 30.64 cmH2O/mL, peak inspiratory pressure was significantly increased to 11.82 ± 0.54 cmH2O and inspiratory capacity was significantly decreased to 0.10 ± 0.02 mL in Vehicle Neb lungs as compared with Sham lungs (P < 0.05; Fig. 3, D–F, J–L).
In Mito Neb lungs dynamic compliance was significantly increased to 7.88 ± 1.05 μL/cmH2O, resistance was significantly decreased to 2.13 ± 0.23 cmH2O, tissue damping was significantly decreased to 13.63 ± 1.55 cmH2O/mL, elastance was significantly decreased to 120.06 ± 17.91 cmH2O/mL and inspiratory capacity was significantly increased to 0.18 ± 0.02 mL following 2 h of ischemia and 24 h of reperfusion as compared with Vehicle Neb lungs (P < 0.05; Fig. 3, D–F, J, L). There was no significant difference observed in peak inspiratory pressure in Mito Neb 10.18 ± 0.40 cmH2O as compared with Vehicle Neb (P > 0.05; Fig. 3K).
There were no significant differences in dynamic compliance, resistance, tissue damping, elastance, and inspiratory capacity observed between Mito Neb and Sham lungs (P > 0.05; Fig. 3, D–F, J, L). However, Mito Neb peak inspiratory pressure was significantly increased as compared with Sham (P < 0.05; Fig. 3K).
Pressure Volume
Representative pressure-volume loops demonstrated that Vehicle V and Vehicle Neb had a lower lung volume capacity and higher pressures as compared with Mito V, Mito Neb, and Sham groups (Fig. 3M). These pressure-volume loops show narrower hysteresis and describe lower compliance in both Vehicle V and Vehicle Neb when compared with Mito V, Mito Neb, and Sham lungs.
Lung Tissue Injury
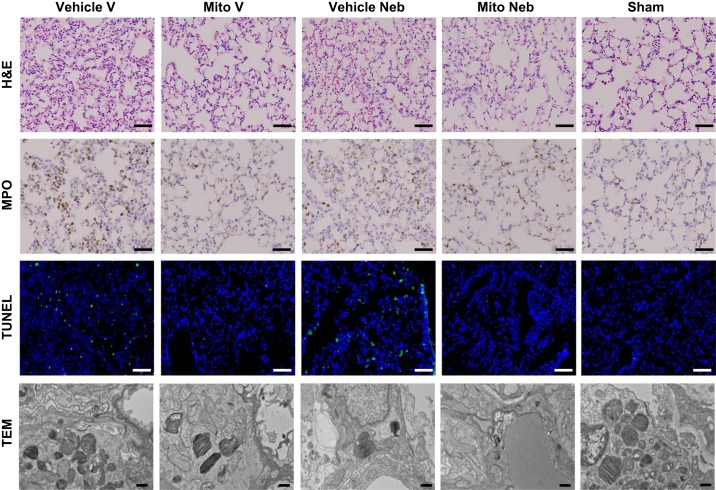
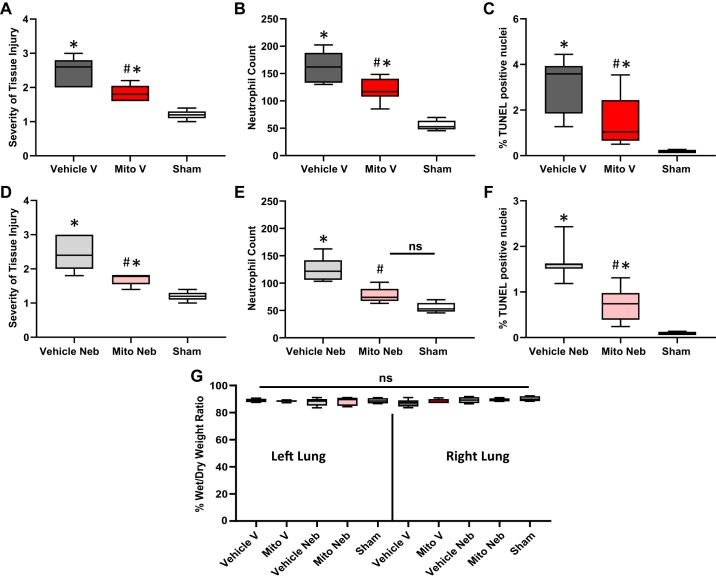
H&E analysis showed significantly decreased inflammatory cells infiltration and interstitial congestion with no signs of destruction of lung architecture in Mito V and Mito Neb lungs as compared with Vehicle V and Vehicle Neb, respectively (grade 1.8 ± 0.1, grade 2.5 ± 0.1; respectively for vascular delivery group, grade 1.7 ± 0.1, grade 2.5 ± 0.1; respectively for nebulization group, P < 0.05, both; Figs. 4, and 5, A and D). Significantly impaired preservation of the lung tissue was observed in all groups as compared with Sham lungs (grade 1.3 ± 0.1, P < 0.05).
Fig. 4.
Lung tissue injury at 24 h of reperfusion following 2 h of ischemia. Representative hematoxylin and eosin (H&E), myeloperoxidase staining (MPO), TUNEL, and transmission electron microscope (TEM) images of lung tissue sections are shown. Improved preservation of lung tissue was observed in lungs in mice receiving mitochondria via vascular delivery (Mito V) and via nebulization (Mito Neb) as compared with mice receiving vehicle only (Vehicle V, Vehicle Neb). Mito V and Mito Neb lungs demonstrated decreased inflammatory cells infiltration and interstitial congestion with decreased destruction of lung architecture as shown on H&E and MPO tissue sections. Scale bars: 40 μm. Representative TUNEL images show decreased count of apoptosis-positive nuclei (green) in Mito V and Mito Neb groups as compared with Vehicle V and Vehicle Neb groups. Blue stain represents DAPI-stained nuclei. Scale bars: 20 μm. Representative TEM images from the inferior left lobe showed preserved endothelia and no difference in alveolar space, indicating no base membrane damage in Vehicle V, Vehicle Neb, Mito V, Mito Neb, and Sham lungs. Scale bars: 500 μm.
Fig. 5.
Lung tissue injury at 24 h of reperfusion. After 2 h of ischemia and 24 h of reperfusion, H&E stained sections (×15) were evaluated for severity of lung injury using the previously described grading system; the worse the tissue injury, the higher the grade (grades 1–5). A and D: severity of tissue injury analysis shows significantly decreased inflammatory cell infiltration and interstitial congestion with no signs of destruction of lung architecture in Mito V and Mito Neb lungs as compared with Vehicle V and Vehicle Neb, respectively (grade 1.8 ± 0.1, grade 2.5 ± 0.1; respectively for vascular delivery group; grade 1.7 ± 0.1, grade 2.5 ± 0.1; respectively for nebulization group, P < 0.05, both). Significantly decreased preservation of the lung tissue was observed in all groups as compared with Sham lungs (grade 1.3 ± 0.1, P < 0.05). Neutrophil infiltration was determined in myeloperoxidase-stained sections (×15). B and E: significantly decreased neutrophil count was observed in Mito V and Mito Neb groups as compared with Vehicle V and Vehicle Neb, respectively (120.2 ± 5.5, 159.9 ± 6.5; respectively, for vascular delivery group; 77.8 ± 3.8, 125.7 ± 4.9; respectively, for nebulization group, P < 0.05, both). The neutrophil count was significantly increased in Mito V, Vehicle V, and Vehicle Neb lungs as compared with Sham lungs (55.3 ± 4.1, P < 0.05). There was no significant difference observed in the number of neutrophils in Sham group as compared with Mito Neb (P > 0.05). TUNEL-positive nuclei per 100 nuclei was determined in 16 random visual fields per section (×10). C and F: significantly decreased count of apoptosis-positive nuclei observed in Mito V and Mito Neb groups as compared with Vehicle V and Vehicle Neb, respectively (1.48 ± 0.47% vs. 3.13 ± 0.41%; respectively, for vascular delivery group; 1.41 ± 0.28% vs. 3.28 ± 0.29%; respectively, for nebulization group, P < 0.05, both). The count of apoptosis-positive nuclei was significantly increased in all groups as compared with Sham lung (0.18 ± 0.03%; P < 0.05). G: following 24 h of reperfusion, no significant difference was found in wet-to-dry weight percentages between Vehicle V left and right lung, Mito V left and right lung, Vehicle Neb left and right lung and Mito Neb left and right lung, and Sham left and right lung. All values are means ± SE. *P < 0.05 vs. Sham; #P <0.05 vs. Vehicle; n = 7 for Vehicle V, n = 6 for Mito V, n = 6 forVehicle Neb, n = 6 for Mito Neb and n = 5 for Sham group.
A significantly decreased neutrophil count was observed in Mito V and Mito Neb groups as compared with Vehicle V and Vehicle Neb, respectively (120.2 ± 5.5, 159.9 ± 6.5; respectively, for vascular delivery group; 77.8 ± 3.8, 125.7 ± 4.9; respectively, for nebulization group, P < 0.05, both, Figs. 4 and 5, B and E). The neutrophil count was significantly increased Mito V, Vehicle V, and Vehicle Neb lungs as compared with Sham lungs (55.3 ± 4.1, P < 0.05). There was no significant difference observed in number of neutrophils in Sham group as compared with Mito Neb (P > 0.05).
TUNEL analysis showed significantly decreased count of apoptosis-positive nuclei observed in Mito V and Mito Neb groups as compared with Vehicle V and Vehicle Neb, respectively (1.48 ± 0.47% vs. 3.13 ± 0.41%; respectively, for vascular delivery group; 1.41 ± 0.28% vs. 3.28 ± 0.29%; respectively, for nebulization group, P < 0.05; Figs. 4 and 5, C and F). The count of apoptosis-positive nuclei was significantly increased in all groups as compared with Sham lung (0.18 ± 0.03%, P < 0.05).
After 24 h of reperfusion, TEM from the inferior left lobe showed preserved endothelia and no difference in alveolar space indicating no base membrane damage in Vehicle V, Vehicle Neb, Mito V, Mito Neb, and Sham lungs (Fig. 4).
No significant difference was found in wet-to-dry weight percentages between Vehicle V left (88.9 ± 0.5%) and right lung (87.1 ± 1.1%), Mito V left (88.5 ± 0.3%) and right lung (88.3 ± 0.6%), Vehicle Neb left (87.5 ± 1.1%) and right lung (89.2 ± 1.1%), Mito Neb left (88.5 ± 1.2%) and right lung (89.3 ± 0.6%), and Sham left (88.7 ± 1.0%) and right lung (90.1 ± 1.0%; P > 0.05 each; Fig. 5G).
Bronchoalveolar Lavage Cytokines
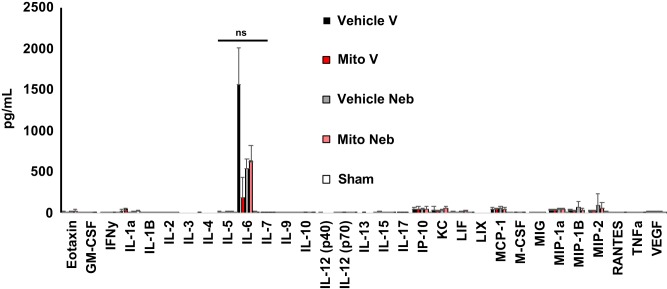
At 24 h of reperfusion, there were no significant differences in cytokines and chemokines between groups (Fig. 6).
Fig. 6.
Bronchoalveolar lavage cytokine analysis. The expression levels of cytokines and chemokines of the left lung demonstrated no significant differences in cytokines and chemokines between groups. All values are expressed as means ± SE; n = 4 for all groups.
DISCUSSION
Previous studies have demonstrated that mitochondria suffer damage during ischemia-reperfusion and that mitochondrial damage plays a significant role in ALI (1, 7, 8, 28, 32). In this study, we have used syngeneic mitochondrial transplantation to overcome these deleterious effects. Our studies demonstrate that mitochondria delivered by either direct vascular perfusion to the lungs via the pulmonary artery or by aerosol delivery via trachea via nebulization improves lung mechanics and enhances tissue recovery following 2 h of ischemia and 24 h of reperfusion in a murine model.
Sommer et al. (32) have used rat lung model of IRI similar to the mouse model used in this study, in which left pulmonary hilum was clamped and then reperfused to investigated mitochondrial damage. Sommer et al. (32) have shown significant mitochondrial dysfunction presented by decay in respiratory complexes I-V, II-V, and III-V and significant decrease in mitochondrial viability expressed by the presence of Ca2+-induced mitochondrial swelling.
The correlation between lung IRI and mitochondrial damage has been previously shown (1, 7, 8, 28, 32). The mechanism of mitochondrial transplantation in lung tissue recovery and lung mechanics enhancement remains unclear and is beyond the scope of this study. However, based on our previous experiments conducted in heart IRI models, we can speculate that the transplanted fully viable and functional mitochondria would support the mitochondria damaged during IRI. Previously in our heart IRI studies, we have shown that transplanted mitochondria increase tissue ATP content, high energy synthesis, replace damaged mitochondrial DNA, and enhance proteomic pathways for the mitochondrion and the generation of precursor metabolites for energy and cellular respiration (5, 6, 19, 27). We hypothesize that similar mechanisms are undergoing in the lung, and further experiments should be warranted to enlighten these processes.
In our previous studies, we have already demonstrated the viability and functionality of mitochondria isolated from skeletal muscle, similarly used in this study (19, 21, 26). Mitochondrial viability of isolated mitochondria has been previously shown using MitoTracker fluorescent dye that stains respiration-competent mitochondria depending upon preserved membrane potential (19, 21). Mitochondrial function of isolated mitochondria has been previously shown using Clark-type electrodes that assess mitochondrial complexes of electron transport chin (26). Furthermore, isolated mitochondria continue to produce ATP as demonstrated in our previous studies using ATP luminescence assay (19, 21, 26).
To demonstrate distribution of mitochondria delivered by pulmonary artery (vascular delivery) and by aerosol delivery via the trachea via nebulization, we have performed PET imaging studies using 18F-labeled rhodamine 6G radiolabeled mitochondria (5). Our results show that mitochondria delivered specifically to the lungs via the pulmonary artery or aerosol delivery through the trachea are rapidly taken up by the lungs and distributed throughout. In Mito V, the mitochondria were also observed in the pulmonary artery; in Mito Neb, the mitochondria were also found along trachea and bronchial tree. These studies replicate our previous studies demonstrating the specific uptake of mitochondria in the end organ. In preliminary experiments, we have found that systemic injection results in localized uptake in the vein and does not circulate significantly beyond the site of injection.
To demonstrate uptake of the transplanted mitochondria into lung tissue, we have used mitochondria isolated from human adult cardiac fibroblasts. The use of human mitochondria in a murine model allows for the identification of the transplanted mitochondria based on immuno-reactivity to a human-specific mitochondrial antibody (19). The transplanted mitochondria were found throughout the lung tissue, demonstrating that mitochondria delivered by either infusion into pulmonary artery or by aerosol delivery via the trachea via nebulization were effectively taken up in the lung. This is in agreement with our previous studies in the heart in which we have demonstrated that the mitochondria are rapidly taken up in heart tissue through actin-dependent endocytosis (6, 23). We did not replicate those mechanistic studies in the current study; however, our results are also in agreement with those of others that show uptake of exogenous mitochondria in the lung (40, 41).
Others have already shown that lung ischemia followed by reperfusion causes significant lung injury (13–15, 18, 34, 37). Lung IRI was previously demonstrated by BAL and tissue analysis. Others have shown increased level of total protein, MPO, and macrophages count in BAL, which corelated with immunohistochemistry findings of an increased number of neutrophils, macrophages, and apoptosis level in the lung tissue (13–15, 18, 34, 37). In our study, severity of IRI was demonstrated by tissue injury, showing increased number of neutrophils and apoptosis levels, which is in agreement with other researchers’ findings (14, 15). Of note, although other researchers have provided detailed analysis of lung tissue damage, lung mechanics analysis was either lacking or was measured in a nonphysiological, isolated ex vivo system. This paper provides for the first time in vivo evidence of lung IRI by measuring lung mechanics using FlexiVent analysis.
Other researchers have investigated ischemia time of 60 to 90 min followed by 1 to 4 h of reperfusion (13–15, 18, 34, 37). In this paper, we have researched 2 h ischemia followed by 24 h of reperfusion to determine the efficacy of mitochondrial transplantation for lung IRI. This duration was based on preliminary experiments that demonstrated that shorter ischemia-reperfusion times resulted in insufficient lung IRI and did not allow for lung mechanics evaluation and comparative analysis between our study groups, despite the use of FlexiVent analysis for in vivo lung mechanics measurements.
In the nebulization group, a specific protocol for aerosol delivery was used in five consecutively repeating cycles. This protocol was selected based on manufacturer recommendation to maximize the effective delivery of mitochondria.
Our results show that following 2 h of ischemia and 24 h of reperfusion, both vascular delivery of mitochondria (Mito V) via the pulmonary artery and aerosol delivery of mitochondria (Mito Neb) via the trachea via nebulization provide for significant post-ischemic lung mechanics improvement as compared with lungs receiving vehicle alone. Almost all lung mechanics indices for Mito V and Mito Neb were not significantly different from that in Sham lungs (Fig. 3). The only exception was peak inspiratory pressure in Mito Neb, which was not significantly increased as compared with Sham lungs but was significantly different compared with Vehicle Neb (P < 0.05; Fig. 3K).
The observed differences in peak inspiratory pressure in Mito V and Mito Neb lungs compared with their respective Vehicle V and Vehicle Neb and Sham lungs are not due to changes in cellular viability. Our results demonstrate that there is significant improvement in cell viability after 24 h of reperfusion in both Mito V and Mito Neb when compared with their Vehicle groups. Thus, the reasons for this difference remain to be elucidated.
In conclusion, we have demonstrated the efficacy and safety of mitochondrial transplantation in a murine model of lung IRI. After 24 h of reperfusion following ischemic injury, mitochondrial transplantation improves lung mechanics and decreases lung tissue injury. These results suggest that this therapy can be used to protect lung tissue from IRI, thus reducing morbidity and mortality in several clinical scenarios such as lung transplantation, cardiopulmonary resuscitation, pulmonary embolism, sepsis and hypotensive events, and procedures during cardiopulmonary bypass or mechanical circulatory support.
Limitations
This study is limited in that we have used only a single mitochondria concentration and recovery was limited to 24 h. In future studies, we will examine the effects of varied mitochondrial concentrations with extended recovery time. An additional limitation is that BAL was collected for cytokine analysis at 24 h of reperfusion. Cytokine release peaks at the early reperfusion; therefore, cytokine upregulation in our study may be decreased. This study is also limited in that the same animal group was used for BAL collection and wet-to-dry weight analysis, contributing to potential inaccuracy in the collected data.
GRANTS
This work was supported by Boston Children’s Hospital Anesthesia Foundation, Ryan Family Endowment, the Cardiac Conduction Fund, NIH National Heart, Lung, and Blood Institute Grants 5 T32 HL 007734 and 5 R01 HL108107, the Richard A. and Susan F. Smith President’s Innovation Award, Michael B. Klein and Family, The Sidman Family Foundation, The Michael B. Rukin Charitable Foundation, The Kenneth C. Griffin Charitable Research Fund, and The Boston Investment Council.
DISCLOSURES
J. D. McCully, D. B. Cowan, and P. J. del Nido have patents pending for the isolation and usage of mitochondria. None of the other authors has any conflicts of interest, financial or otherwise, to disclose.
AUTHOR CONTRIBUTIONS
K.M., A.O., K.L., A.B.P., D.B.C., G.A.V., P.J.d.N., and J.D.M. conceived and designed research; K.M., A.O., K.L., G.R.-B., J.K.T., R.Y., E.R.S., J.A.I., A.B.P., D.B.C., and J.D.M. performed experiments; K.M., A.O., G.R.-B., J.K.T., R.Y., A.G., I.P.D., D.B., B.S., K.I., A.B.P., D.B.C., and J.D.M. analyzed data; K.M., A.O., G.A.V., and J.D.M. interpreted results of experiments; K.M., A.O., and J.D.M. prepared figures; K.M., A.O., and J.D.M. drafted manuscript; K.M., A.O., G.A.V., and J.D.M. edited and revised manuscript; K.M., A.O., K.L., G.R.-B., J.K.T., R.Y., A.G., I.P.D., D.B., B.S., E.R.S., J.A.I., K.I., A.B.P., D.B.C., G.A.V., P.J.d.N., and J.D.M. approved final version of manuscript.
REFERENCES
- 1.Aggarwal S, Mannam P, Zhang J. Differential regulation of autophagy and mitophagy in pulmonary diseases. Am J Physiol Lung Cell Mol Physiol 311: L433–L452, 2016. doi: 10.1152/ajplung.00128.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asimakopoulos G, Smith PL, Ratnatunga CP, Taylor KM. Lung injury and acute respiratory distress syndrome after cardiopulmonary bypass. Ann Thorac Surg 68: 1107–1115, 1999. doi: 10.1016/S0003-4975(99)00781-X. [DOI] [PubMed] [Google Scholar]
- 3.Bartholomä MD, Zhang S, Akurathi V, Pacak CA, Dunning P, Fahey FH, Cowan DB, Treves ST, Packard AB. 18F-labeled rhodamines as potential myocardial perfusion agents: comparison of pharmacokinetic properties of several rhodamines. Nucl Med Biol 42: 796–803, 2015. doi: 10.1016/j.nucmedbio.2015.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, Lamy M, Legall JR, Morris A, Spragg R; Consensus Committee The American-European Consensus Conference on ARDS . The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med 149: 818–824, 1994. doi: 10.1164/ajrccm.149.3.7509706. [DOI] [PubMed] [Google Scholar]
- 5.Cowan DB, Yao R, Akurathi V, Snay ER, Thedsanamoorthy JK, Zurakowski D, Ericsson M, Friehs I, Wu Y, Levitsky S, Del Nido PJ, Packard AB, McCully JD. Intracoronary delivery of mitochondria to the ischemic heart for cardioprotection. PLoS One 11: e0160889, 2016. doi: 10.1371/journal.pone.0160889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cowan DB, Yao R, Thedsanamoorthy JK, Zurakowski D, Del Nido PJ, McCully JD. Transit and integration of extracellular mitochondria in human heart cells. Sci Rep 7: 17450, 2017. doi: 10.1038/s41598-017-17813-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.den Hengst WA, Gielis JF, Lin JY, Van Schil PE, De Windt LJ, Moens AL. Lung ischemia-reperfusion injury: a molecular and clinical view on a complex pathophysiological process. Am J Physiol Heart Circ Physiol 299: H1283–H1299, 2010. doi: 10.1152/ajpheart.00251.2010. [DOI] [PubMed] [Google Scholar]
- 8.Dobson AW, Grishko V, LeDoux SP, Kelley MR, Wilson GL, Gillespie MN. Enhanced mtDNA repair capacity protects pulmonary artery endothelial cells from oxidant-mediated death. Am J Physiol Lung Cell Mol Physiol 283: L205–L210, 2002. doi: 10.1152/ajplung.00443.2001. [DOI] [PubMed] [Google Scholar]
- 9.Edens JW, Chung KK, Pamplin JC, Allan PF, Jones JA, King BT, Cancio LC, Renz EM, Wolf SE, Wade CE, Holcomb JB, Blackbourne LH. Predictors of early acute lung injury at a combat support hospital: a prospective observational study. J Trauma 69, Suppl 1: S81–S86, 2010. doi: 10.1097/TA.0b013e3181e44a32. [DOI] [PubMed] [Google Scholar]
- 10.Ehrentraut H, Clambey ET, McNamee EN, Brodsky KS, Ehrentraut SF, Poth JM, Riegel AK, Westrich JA, Colgan SP, Eltzschig HK. CD73+ regulatory T cells contribute to adenosine-mediated resolution of acute lung injury. FASEB J 27: 2207–2219, 2013. doi: 10.1096/fj.12-225201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Emani SM, Piekarski BL, Harrild D, Del Nido PJ, McCully JD. Autologous mitochondrial transplantation for dysfunction after ischemia-reperfusion injury. J Thorac Cardiovasc Surg 154: 286–289, 2017. doi: 10.1016/j.jtcvs.2017.02.018. [DOI] [PubMed] [Google Scholar]
- 12.Erickson S, Schibler A, Numa A, Nuthall G, Yung M, Pascoe E, Wilkins B; Paediatric Study Group; Australian and New Zealand intensive care society . Acute lung injury in pediatric intensive care in Australia and New Zealand—A prospective, multicenter, observational study. Pediatr Crit Care Med 8: 317–323, 2007. doi: 10.1097/01.PCC.0000269408.64179.FF. [DOI] [PubMed] [Google Scholar]
- 13.Fei L, Jifeng F, Tiantian W, Yi H, Linghui P. Glycyrrhizin ameliorate ischemia reperfusion lung injury through downregulate TLR2 signaling cascade in alveolar macrophages. Front Pharmacol 8: 389, 2017. doi: 10.3389/fphar.2017.00389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gielis JF, Jungraithmayr W, Boulet GA, Bogers JP, Weder W, Cos P, Van Schil PEY. A murine model of lung ischemia and reperfusion injury: tricks of the trade. J Surg Res 194: 659–666, 2015. doi: 10.1016/j.jss.2014.12.008. [DOI] [PubMed] [Google Scholar]
- 15.Huerter ME, Sharma AK, Zhao Y, Charles EJ, Kron IL, Laubach VE. Attenuation of pulmonary ischemia-reperfusion injury by adenosine A2B receptor antagonism. Ann Thorac Surg 102: 385–393, 2016. doi: 10.1016/j.athoracsur.2016.02.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huffmyer JL, Groves DS. Pulmonary complications of cardiopulmonary bypass. Best Pract Res Clin Anaesthesiol 29: 163–175, 2015. doi: 10.1016/j.bpa.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaza AK, Wamala I, Friehs I, Kuebler JD, Rathod RH, Berra I, Ericsson M, Yao R, Thedsanamoorthy JK, Zurakowski D, Levitsky S, Del Nido PJ, Cowan DB, McCully JD. Myocardial rescue with autologous mitochondrial transplantation in a porcine model of ischemia/reperfusion. J Thorac Cardiovasc Surg 153: 934–943, 2017. doi: 10.1016/j.jtcvs.2016.10.077. [DOI] [PubMed] [Google Scholar]
- 18.LaPar DJ, Hajzus VA, Zhao Y, Lau CL, French BA, Kron IL, Sharma AK, Laubach VE. Acute hyperglycemic exacerbation of lung ischemia-reperfusion injury is mediated by receptor for advanced glycation end-products signaling. Am J Respir Cell Mol Biol 46: 299–305, 2012. doi: 10.1165/rcmb.2011-0247OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Masuzawa A, Black KM, Pacak CA, Ericsson M, Barnett RJ, Drumm C, Seth P, Bloch DB, Levitsky S, Cowan DB, McCully JD. Transplantation of autologously derived mitochondria protects the heart from ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol 304: H966–H982, 2013. doi: 10.1152/ajpheart.00883.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McCully JD, Cowan DB, Emani SM, Del Nido PJ. Mitochondrial transplantation: from animal models to clinical use in humans. Mitochondrion 34: 127–134, 2017. doi: 10.1016/j.mito.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 21.McCully JD, Cowan DB, Pacak CA, Toumpoulis IK, Dayalan H, Levitsky S. Injection of isolated mitochondria during early reperfusion for cardioprotection. Am J Physiol Heart Circ Physiol 296: H94–H105, 2009. doi: 10.1152/ajpheart.00567.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moskowitzova K, Shin B, Liu K, Ramirez-Barbieri G, Guariento A, Blitzer D, Thedsanamoorthy JK, Yao R, Snay ER, Inkster JAH, Orfany A, Zurakowski D, Cowan DB, Packard AB, Visner GA, Del Nido PJ, McCully JD. Mitochondrial transplantation prolongs cold ischemia time in murine heart transplantation. J Heart Lung Transplant 38: 92–99, 2019. doi: 10.1016/j.healun.2018.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pacak CA, Preble JM, Kondo H, Seibel P, Levitsky S, Del Nido PJ, Cowan DB, McCully JD. Actin-dependent mitochondrial internalization in cardiomyocytes: evidence for rescue of mitochondrial function. Biol Open 4: 622–626, 2015. doi: 10.1242/bio.201511478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pan X, Lu J, Cheng W, Yang Y, Zhu J, Jin M. Independent factors related to preoperative acute lung injury in 130 adults undergoing Stanford type-A acute aortic dissection surgery: a single-center cross-sectional clinical study. J Thorac Dis 10: 4413–4423, 2018. doi: 10.21037/jtd.2018.06.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Preble JMKH, Levitsky S, McCully JD. Quality control parameters for mitochondria transplant in cardiac tissue. JSM Biochem Mol Biol 2: 1008, 2014. [Google Scholar]
- 26.Preble JM, Pacak CA, Kondo H, MacKay AA, Cowan DB, McCully JD. Rapid isolation and purification of mitochondria for transplantation by tissue dissociation and differential filtration. J Vis Exp 6: e51682, 2014. doi: 10.3791/51682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ramirez-Barbieri G, Moskowitzova K, Shin B, Blitzer D, Orfany A, Guariento A, Iken K, Friehs I, Zurakowski D, Del Nido PJ, McCully JD. Alloreactivity and allorecognition of syngeneic and allogeneic mitochondria. Mitochondrion 46: 103–115, 2019. doi: 10.1016/j.mito.2018.03.002. [DOI] [PubMed] [Google Scholar]
- 28.Schumacker PT, Gillespie MN, Nakahira K, Choi AM, Crouser ED, Piantadosi CA, Bhattacharya J. Mitochondria in lung biology and pathology: more than just a powerhouse. Am J Physiol Lung Cell Mol Physiol 306: L962–L974, 2014. doi: 10.1152/ajplung.00073.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shah CV, Localio AR, Lanken PN, Kahn JM, Bellamy S, Gallop R, Finkel B, Gracias VH, Fuchs BD, Christie JD. The impact of development of acute lung injury on hospital mortality in critically ill trauma patients. Crit Care Med 36: 2309–2315, 2008. doi: 10.1097/CCM.0b013e318180dc74. [DOI] [PubMed] [Google Scholar]
- 30.Shin B, Cowan DB, Emani SM, Del Nido PJ, McCully JD. Mitochondrial transplantation in myocardial ischemia and reperfusion injury. Adv Exp Med Biol 982: 595–619, 2017. doi: 10.1007/978-3-319-55330-6_31. [DOI] [PubMed] [Google Scholar]
- 31.Silachev DN, Plotnikov EY, Pevzner IB, Zorova LD, Babenko VA, Zorov SD, Popkov VA, Jankauskas SS, Zinchenko VP, Sukhikh GT, Zorov DB. The mitochondrion as a key regulator of ischaemic tolerance and injury. Heart Lung Circ 23: 897–904, 2014. doi: 10.1016/j.hlc.2014.05.022. [DOI] [PubMed] [Google Scholar]
- 32.Sommer SP, Sommer S, Sinha B, Wiedemann J, Otto C, Aleksic I, Schimmer C, Leyh RG. Ischemia-reperfusion injury-induced pulmonary mitochondrial damage. J Heart Lung Transplant 30: 811–818, 2011. doi: 10.1016/j.healun.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 33.Stadler B, Phillips J, Toyoda Y, Federman M, Levitsky S, McCully JD. Adenosine-enhanced ischemic preconditioning modulates necrosis and apoptosis: effects of stunning and ischemia-reperfusion. Ann Thorac Surg 72: 555–563, 2001. doi: 10.1016/S0003-4975(01)02665-0. [DOI] [PubMed] [Google Scholar]
- 34.Stone ML, Sharma AK, Zhao Y, Charles EJ, Huerter ME, Johnston WF, Kron IL, Lynch KR, Laubach VE. Sphingosine-1-phosphate receptor 1 agonism attenuates lung ischemia-reperfusion injury. Am J Physiol Lung Cell Mol Physiol 308: L1245–L1252, 2015. doi: 10.1152/ajplung.00302.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tansey EE, Kwaku KF, Hammer PE, Cowan DB, Federman M, Levitsky S, McCully JD. Reduction and redistribution of gap and adherens junction proteins after ischemia and reperfusion. Ann Thorac Surg 82: 1472–1479, 2006. doi: 10.1016/j.athoracsur.2006.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Van Putte BP, Kesecioglu J, Hendriks JM, Persy VP, van Marck E, Van Schil PE, De Broe ME. Cellular infiltrates and injury evaluation in a rat model of warm pulmonary ischemia-reperfusion. Crit Care 9: R1–R8, 2005. doi: 10.1186/cc2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang ML, Wei CH, Wang WD, Wang JS, Zhang J, Wang JJ. Melatonin attenuates lung ischaemia-reperfusion injury via inhibition of oxidative stress and inflammation. Interact Cardiovasc Thorac Surg 26: 761–767, 2018. doi: 10.1093/icvts/ivx440. [DOI] [PubMed] [Google Scholar]
- 38.Weissman C. Pulmonary complications after cardiac surgery. Semin Cardiothorac Vasc Anesth 8: 185–211, 2004. doi: 10.1177/108925320400800303. [DOI] [PubMed] [Google Scholar]
- 39.Zhang XY, Chen C, Bai YP, Ma G, Zhang YB, Liu B. RNase attenuates acute lung injury induced by ischemia-reperfusion in mice. Int Immunopharmacol 40: 288–293, 2016. doi: 10.1016/j.intimp.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 40.Zhou J, Zhang J, Lu Y, Huang S, Xiao R, Zeng X, Zhang X, Li J, Wang T, Li T, Zhu L, Hu Q, Wang T, Li T.. Mitochondrial transplantation attenuates hypoxic pulmonary vasoconstriction. Oncotarget 7: 31284–31298, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhu L, Zhang J, Zhou J, Lu Y, Huang S, Xiao R, Yu X, Zeng X, Liu B, Liu F, Sun M, Dai M, Hao Q, Li J, Wang T, Li T, Hu Q. Mitochondrial transplantation attenuates hypoxic pulmonary hypertension. Oncotarget 7: 48925–48940, 2016. doi: 10.18632/oncotarget.10596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zimmerman JJ, Akhtar SR, Caldwell E, Rubenfeld GD. Incidence and outcomes of pediatric acute lung injury. Pediatrics 124: 87–95, 2009. doi: 10.1542/peds.2007-2462. [DOI] [PubMed] [Google Scholar]