Abstract
Objective:
Limited English proficiency can be a barrier to asthma care and is associated with poor outcomes. This study examines whether pediatric patients in Ohio with limited English proficiency experience lower asthma care quality or higher morbidity.
Methods:
We used electronic health records for asthma patients aged 2–17 years from a regional, urban, children’s hospital in Ohio during 2011–2015. Community-level demographics were included from U.S. Census data. By using chi-square and t-tests, patients with limited English proficiency and bilingual English-speaking patients were compared with English-only patients. Five asthma outcomes—two quality and three morbidity measures—were modeled using generalized estimating equations.
Results:
The study included 15352 (84%) English-only patients, 1744 (10%) patients with limited English proficiency, and 1147 (6%) bilingual patients. Pulmonary function testing (quality measure) and multiple exacerbation visits (morbidity measure) did not differ by language group. Compared with English-only patients, bilingual patients had higher odds of ever having an exacerbation visit (morbidity measure) (adjusted odds ratio [aOR], 1.4; 95% confidence interval [CI], 1.2–1.6) but lower odds of admission to intensive care (morbidity measure) (aOR, 0.3; 95% CI, 0.2–0.7), while patients with limited English proficiency did not differ on either factor. Recommended follow-up after exacerbation (quality measure) was higher for limited English proficiency (aOR, 1.8; 95% CI, 1.4–2.3) and bilingual (aOR, 1.6; 95% CI, 1.3–2.1), compared with English-only patients.
Conclusions:
In this urban, pediatric population with reliable interpreter services, limited English proficiency was not associated with worse asthma care quality or morbidity.
Keywords: Asthma, pediatrics, language, limited English proficiency, English, Spanish, Somali, quality improvement, electronic health record, disparities
Introduction
One in 12 children in the United States has asthma (1). A growing number of Ohioans speak a non-English language, including more than 14% of households in Columbus (2). Socioeconomic context plays an important role in asthma prevalence and care quality with higher prevalence and morbidity observed among lower income populations (3). Within this context, limited English proficiency presents a unique challenge for asthma patients to access care and adhere to treatment (4). Understanding the impact of English language proficiency on asthma care and treatment is complex because of the relationship of language with cultural factors, health literacy, and socioeconomic status (5,6). Studies have reported that limited English proficiency is a barrier to asthma knowledge and treatment, but these studies have been limited to small, cross-sectional surveys that rely on self-reported asthma and focus on a single language (7–10).
Our aim was to identify asthma disparities associated with language so that quality improvement initiatives could be developed. We hypothesized that lower English proficiency would negatively impact asthma care quality and morbidity.
Methods
We reviewed electronic health records from Hospital System A in Columbus, Ohio, during 2011–2015. Hospital System A is the largest pediatric hospital system in central Ohio with multiple locations including 11 primary care clinics, seven urgent care clinics, and a level 1 pediatric emergency department. Epic (Epic Systems Corporation, Verona, WI) is the single electronic health record system used across all of Hospital System As locations.
Inclusion and exclusion criteria
Patients were included if on December 31 of any year (2011–2015) they met the following: 1) age 2–17 years, 2) hospital system encounter (acute care or routine visit) with an asthma diagnosis within the previous 24 months, and 3) primary care visit in the past 13 months. Patients with missing language or a history of cystic fibrosis, tracheomalacia, tracheostomy, bronchopulmonary dysplasia, or vocal cord paralysis were excluded. Asthma was defined by the International Classification of Diseases, Ninth Edition (ICD-9) (493) and Tenth Edition (ICD-10) (J45) codes as the primary diagnosis.
Language
Language and use of an interpreter are documented in two sections of the electronic health record, and collection methodology is uniform across all hospital system locations. When a patient’s health record is created, preferred language and need for an interpreter are self-reported by the patient and documented by hospital staff in the demographics section. In addition, patients are asked during each visit whether they would like an interpreter and the language requested. The interpreter can be provided for either the patient or the family or guardian and is not distinguished in the record. Documentation of request for interpreter by hospital and clinic staff is used for interpreter scheduling, which improves the reliability of documentation.
Patients who requested an interpreter during any hospital system encounter were considered to have limited English proficiency. Patients who reported a non-English language and never requested an interpreter were considered bilingual. We created a three-category language variable: patients who reported speaking only English, bilingual patients, and patients with limited English proficiency.
Demographic covariates
Tobacco exposure was extracted from a note template with an open-ended question about any tobacco smoke exposure. Any first-, second-, or third-hand reported exposure was included as tobacco exposure. Residential ZIP code-level household income, education, and unemployment were derived from U.S. Census data (12–14).
Asthma care measures
Because the study was conducted for quality improvement purposes, we evaluated two measures of asthma care quality – use of pulmonary function tests (PFTs) during the most recent two years and 30-day outpatient follow-up after exacerbation. Standardized documentation of other quality improvement measures, such as asthma action plans or asthma control test scores, was not implemented until after the start of the study period, and thus were not considered as potential outcome measures. PFT analyses were limited to patients aged 5 years and older. PFTs were determined from billing codes within the most recent two years of study eligibility. Age and time ranges were selected based on the National Asthma Education and Prevention Program Coordinating Committee recommendations, which specify that spirometry be performed a minimum of once every two years over the patient’s lifetime, regardless of asthma severity (11). Follow-up within 30 days was determined by a primary care or pulmonology outpatient encounter within 30 days after the most recent exacerbation.
We also evaluated three morbidity measures because of clinical relevance – asthma exacerbation visit (ever/never), admission to the pediatric intensive care unit (PICU), and multiple exacerbation visits. An asthma exacerbation visit was defined as any urgent care encounter, emergency department encounter, or inpatient admission during the study period for which asthma was the primary diagnosis. Asthma exacerbations treated in a primary care clinic setting were not included because the reason for an asthma visit (well-controlled asthma, uncontrolled asthma, or asthma exacerbation) was not reliably documented in billing codes. Patients who were ever seen for an asthma exacerbation visit during the study period were compared to patients who were never seen for an asthma exacerbation visit. For patients with multiple exacerbation encounters, PICU admission was determined from the most recent exacerbation. We added a third clinical outcome measure, multiple exacerbation visits, as a post hoc analysis. Multiple exacerbation visits were determined based on one calendar year of data using the year of most recent exacerbation. Patients with a single visit in one year were compared with patients who were seen for more than one exacerbation visit.
Lastly, we examined exacerbation treatment location (urgent care, emergency department, and inpatient admission) as a proxy for severity. For patients who were treated at multiple locations in a single encounter, the final destination was used. For example, a patient who arrived at an urgent care, was transferred to the emergency department, and was admitted to a hospital inpatient service was considered an inpatient. Because the relationship between language and 30-day follow-up might vary according to exacerbation severity (effect modification), we examined the 30-day follow-up variable stratified by exacerbation treatment location. We also compared exacerbation treatment location by language group.
Analysis
Statistical analyses were performed using SAS v9.4 (SAS Institute Inc., Cary, NC). Chi-square, Fisher’s exact, and Student’s t-tests were used for significance testing. All covariates except tobacco exposure were missing for < 1% of patients, so patients missing these variables were excluded in multivariable models. Missing tobacco exposure was imputed to 9237 patients. Given the univariate missing data pattern, we applied logistic regression to impute missing tobacco information by using the following variables: language group, age, and race and ethnicity. A total of 20 imputed data sets were created and pooled for analysis. Imputed prevalence of tobacco exposure did not significantly differ from observed prevalence by age, sex, or race and ethnicity.
We used generalized estimating equations with a logit link and an exchangeable correlation structure to adjust for individual and ZIP code-level covariates. Potential confounders were selected a priori on the basis of published literature (3). Age, race, ethnicity, and insurer were individual-level variables included in the adjusted models. Education, household income, and unemployment were ZIP code-level variables included in the adjusted models. Tobacco exposure was considered a priori as a potential confounder for only the morbidity outcomes – asthma exacerbation visit, multiple exacerbation visits, and PICU admission – because we did not expect tobacco to be causally associated with the quality measures. PICU outcome had too few observations to accommodate the full models, so logistic regression without the ZIP code-level variables was used.
We conducted a sensitivity analysis to estimate potential bias of language misclassification on the 30-day follow-up outcome variable. We determined the minimum percent misclassification needed to cause a type II error (failure to detect a disparity by language) for three scenarios. First, patients with limited English proficiency could be misclassified as bilingual patients if they did not request an interpreter. Second, bilingual patients could be misclassified as English-only if they did not report a non-English language. Third, patients with limited English proficiency could be misclassified as English-only if they did not request an interpreter and did not report a non-English language. We also assumed 100% differential misclassification by follow-up (i.e., every misclassified patient failed to follow-up within 30 days).
Ethics and data security
This study was exempted from Hospital System As Institutional Review Board. A non-research determination was obtained through the Centers for Disease Control and Prevention (HSR 2016–00176). To maintain data security and confidentiality, no personal identifying information was used for analysis.
Results
Overall, 18 329 patients met the inclusion criteria. One patient was missing language and 85 had excluding conditions, leaving 18 243 patients for analysis. Of these, 1744 (10%) patients with limited English proficiency and 1147 (6%) bilingual patients were identified. Aside from English, 56 languages were reported (Table 1). For the 1744 patients with limited English proficiency the most common languages were Spanish (936, 54%) and Somali (523, 30%). The most common languages for the 1147 bilingual patients were Somali (502, 44%) and Spanish (282, 25%).
Table 1.
Languages spoken by pediatric asthma patients at Hospital System A, Ohio, 2011–2015.
| No interpreter used (16499) |
Interpreter used (1744) |
||||
|---|---|---|---|---|---|
| Language | N | % | Language | N | % |
| English | 15 352 | 93% | Spanish | 936 | 54% |
| Somali | 502 | 3.0% | Somali | 523 | 30% |
| Spanish | 282 | 1.7% | Arabic | 52 | 3.0% |
| Arabic | 57 | 0.3% | Otherb | 233 | 13% |
| Othera | 306 | 1.9% | |||
Includes 47 additional languages, each with fewer than 50 patients
Includes 31 additional languages, each with fewer than 50 patients
Compared with English-only patients, patients with limited English proficiency were younger with a higher proportion of Hispanic ethnicity and Asian race and a lower proportion of non-Hispanic white and non- Hispanic black patients (Table 2). Bilingual patients were also younger with a higher proportion of Hispanic and Asian but a similar proportion of non-Hispanic black patients. The proportion of Medicaid-insured patients was similar across language groups (83%–85%), but English-only patients had a higher proportion of private or military insurance (8%), compared with bilingual patients (5%) or patients with limited English proficiency (2%). Among the 9006 patients with non-missing data, tobacco exposure was significantly higher among English-only patients (48% versus 9% for bilingual patients and patients with limited English proficiency, p < 0.001). A higher proportion of English-only patients lived in ZIP codes with the lowest income, lowest education, and highest unemployment quartiles (28%, 28%, and 26%, respectively), compared with bilingual patients (16%, 18%, and 16%, respectively) and patients with limited English proficiency (13%, 19%, and 13%, respectively).
Table 2.
Demographics of pediatric asthma patients at Hospital System A by language group, Ohio, 2011–2015.
| Individual-level | Total |
English-only |
Bilingual |
Limited English proficiency |
p value | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | N | % | |||
| Total | 18 243 | 100% | 15 352 | 84% | 1147 | 6% | 1744 | 10% | ||
| Age | ||||||||||
| Under 5 | 6660 | 37% | 5313 | 35% | 531 | 46% | 816 | 47% | <0.001 | |
| 5–<12 | 7719 | 42% | 6491 | 42% | 478 | 42% | 750 | 43% | ||
| 12–<18 | 3864 | 21% | 3548 | 23% | 138 | 12% | 178 | 10% | ||
| Sex | ||||||||||
| Female | 7722 | 42% | 6465 | 42% | 495 | 43% | 762 | 44% | 0.38 | |
| Male | 10 521 | 58% | 8887 | 58% | 652 | 57% | 982 | 56% | ||
| Race and ethnicity | ||||||||||
| Hispanic | 1565 | 9% | 349 | 2% | 281 | 24% | 935 | 54% | <0.001 | |
| Black, non-Hispanic | 11 767 | 65% | 10 436 | 68% | 715 | 62% | 616 | 35% | ||
| White, non-Hispanic | 3141 | 17% | 3042 | 20% | 38 | 3% | 61 | 3% | ||
| Asian, non-Hispanic | 213 | 1% | 74 | 0.5% | 59 | 5% | 80 | 5% | ||
| Other | 1469 | 8% | 1377 | 9% | 46 | 4% | 46 | 3% | ||
| Missing | 88 | 0.5% | 74 | 0.5% | 8 | 0.7% | 6 | 0.3% | ||
| Insurer | ||||||||||
| Medicaid | 15 114 | 83% | 12 666 | 83% | 961 | 84% | 1487 | 85% | <0.001 | |
| Private/Military | 1327 | 7% | 1229 | 8% | 56 | 5% | 42 | 2% | ||
| Self-Pay | 1695 | 9% | 1363 | 9% | 124 | 11% | 208 | 12% | ||
| Other | 37 | 0.2% | 31 | 0.2% | 3 | 0.3% | 3 | 0.2% | ||
| Missing | 70 | 0.4% | 63 | 0.4% | 3 | 0.3% | 4 | 0.2% | ||
| Tobacco exposure | ||||||||||
| Never | 5271 | 29% | 3984 | 26% | 504 | 44% | 783 | 45% | <0.001 | |
| Ever | 3735 | 20% | 3607 | 23% | 49 | 4% | 79 | 5% | ||
| Missing | 9237 | 51% | 7761 | 51% | 594 | 52% | 882 | 51% | ||
| Total |
English-only |
Bilingual |
Limited English proficiency |
|||||||
| Community-level | N | % | N | % | N | % | N | % | p value | |
| Median household income of residential ZIP code | ||||||||||
| $10 362–$33 473 | 4718 | 26% | 4305 | 28% | 180 | 16% | 233 13% | <0.001 | ||
| $33 474–$38 004 | 5278 | 29% | 4323 | 28% | 403 | 35% | 552 32% | |||
| $38 005–$43 810 | 4033 | 22% | 3375 | 22% | 170 | 15% | 488 28% | |||
| $43 811–$120 770 | 4056 | 22% | 3210 | 21% | 385 | 34% | 461 26% | |||
| Missing | 158 | 0.9% | 139 | 0.9% | 9 | 0.8% | 10 0.6% | |||
| Percent of adults over 25 years with at least a bachelor’s deg ree by residential ZIP code | ||||||||||
| 0–9.4% | 4794 | 26% | 4243 | 28% | 212 | 18% | 339 19% | <0.001 | ||
| 9.5%–18.0% | 4546 | 25% | 3847 | 25% | 283 | 25% | 416 24% | |||
| 18.1%–27.0% | 4792 | 26% | 3848 | 25% | 317 | 28% | 627 36% | |||
| ≥27.1% | 3956 | 22% | 3277 | 21% | 326 | 28% | 353 20% | |||
| Missing | 155 | 0.8% | 137 | 0.9% | 9 | 0.8% | 9 0.5% | |||
| Unemployment of adults over 16 years | ||||||||||
| 0–5.9% | 5421 | 30% | 4351 | 28% | 456 | 40% | 614 35% | <0.001 | ||
| 6.0%–9.3% | 4028 | 22% | 2978 | 19% | 357 | 31% | 693 40% | |||
| 9.4%–2.0% | 4316 | 24% | 3966 | 26% | 146 | 13% | 204 12% | |||
| ≥12.1% | 4323 | 24% | 3920 | 26% | 179 | 16% | 224 13% | |||
| Missing | 155 | 0.8% | 137 | 0.9% | 9 | 0.8% | 9 0.5% | |||
In bivariate analyses, bilingual patients and patients with limited English proficiency had higher unadjusted odds of PFTs than English-only patients (Table 3). Follow-up at 30 days was higher for both bilingual patients (OR, 1.8; 95% CI, 1.4–2.2) and patients with limited English proficiency (OR, 2.2; 95% CI, 1.9–2.7) compared with English-only patients. The unadjusted odds of an asthma exacerbation visit was higher for bilingual patients (odds ratio [OR], 1.6; 95% confidence interval [CI], 1.4–1.8) and patients with limited English proficiency (OR, 1.2; 95% CI, 1.1–1.4) compared with English-only patients. Among patients with an asthma exacerbation visit, there were no differences in the unadjusted odds of having multiple exacerbation visits. PICU admission among patients with an asthma exacerbation visit was lower for bilingual patients compared with English-only patients (OR, 0.3; 95% CI, 0.2–0.7) but not for patients with limited English proficiency.
Table 3.
Univariate and multivariable analyses of asthma outcomes among pediatric asthma patients from Hospital System A, Ohio, 2011–2015.
| No | % | Yes | % | Unadjusted odds | 95% CI | Adjusted odds | 95% CI | |
|---|---|---|---|---|---|---|---|---|
| PFT (N = 11583)a | ||||||||
| English-only | 9242 | (92) | 797 | (8) | 1.0 | [Reference] | 1.0 | [Reference] |
| Bilingual | 546 | (89) | 70 | (11) | 1.5 | (1.1,1.9)b | 1.3 | (1.0,1.6) |
| LEP | 835 | (90) | 93 | (10) | 1.3 | (1.0,1.6)b | 0.9 | (0.7,1.1) |
| 30-day follow-up (N = 5348)a | ||||||||
| English-only | 2064 | (48) | 2271 | (52) | 1.0 | [Reference] | 1.0 | [Reference] |
| Bilingual | 152 | (35) | 288 | (65) | 1.8 | (1.4,2.2)b | 1.6 | (1.3,2.1)b |
| LEP | 167 | (29) | 406 | (71) | 2.2 | (1.9,2.7)b | 1.8 | (1.4,2.3)b |
| Exacerbation (N = 18 243)c | ||||||||
| English-only | 11 017 | (72) | 4335 | (28) | 1.0 | [Reference] | 1.0 | [Reference] |
| Bilingual | 707 | (62) | 440 | (38) | 1.6 | (1.4,1.8)b | 1.4 | (1.2,1.6)b |
| LEP | 1171 | (67) | 573 | (33) | 1.2 | (1.1,1.4)b | 1.1 | (1.0,1.3) |
| Multiple visits (N = 5348)c | ||||||||
| English-only | 3299 | (77) | 995 | (23) | 1.0 | [Reference] | 1.0 | [Reference] |
| Bilingual | 344 | (79) | 91 | (21) | 0.9 | (0.7,1.1) | 0.9 | (0.7,1.2) |
| LEP | 447 | (79) | 121 | (21) | 0.9 | (0.7,1.1) | 1.0 | (0.7,1.2) |
| PICU admission (N = 5348)d | ||||||||
| English-only | 4098 | (95) | 237 | (5) | 1.0 | [Reference] | 1.0 | [Reference] |
| Bilingual | 432 | (98) | 8 | (2) | 0.3 | (0.2,0.7)b | 0.3 | (0.2,0.7)b |
| LEP | 546 | (95) | 27 | (5) | 0.9 | (0.6,1.3) | 0.8 | (0.5,1.4) |
CI = confidence interval; LEP = limited English proficiency; PFT = pulmonary function test; PICU = pediatric intensive care unit
adjusted for age, race/ethnicity, insurer, income (ZIP-code level), education (ZIP-code level), and unemployment (ZIP-code level)
significant at 0.05 level
adjusted for age, race/ethnicity, insurer, income (ZIP-code level), education (ZIP-code level), unemployment (ZIP-code level), and tobacco exposure
adjusted for age, race/ethnicity, insurer, and tobacco exposure
After multivariable adjustment, no differences were observed in the odds of PFT use. Both bilingual patients and patients with limited English proficiency had higher adjusted odds of 30-day follow-up after exacerbation, compared with English-only patients (aOR, 1.6; 95% CI, 1.3–2.1 and aOR, 1.8; 95% CI, 1.4–2.3, respectively). Bilingual patients had higher odds of an asthma exacerbation visit (adjusted odds ratio [aOR], 1.4; 95% CI, 1.2–1.6) and decreased odds of PICU admission (aOR, 0.3; 95% CI, 0.2–0.7) than English-only patients. All remaining comparisons were non-significant. When we stratified probability of 30-day follow-up by exacerbation treatment location (Table 4), we found that follow-up was consistently higher for bilingual patients and patients with limited English proficiency compared with English-only patients.
Table 4.
30-day follow-up by exacerbation treatment location among 5348 pediatric patients who experienced an asthma exacerbation, Hospital System A, 2011–2015.
| English-only |
Bilingual |
LEP |
p value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Follow-up visits | Patients | % | Follow-up visits | Patients | % | Follow-up visits | Patients | % | ||
| Urgent care | 950 | 2015 | 47% | 166 | 284 | 58% | 194 | 292 | 66% | <0.001 |
| Emergency department | 854 | 1680 | 51% | 94 | 122 | 77% | 145 | 205 | 71% | <0.001 |
| Inpatient including PICU | 467 | 640 | 73% | 28 | 34 | 82% | 67 | 76 | 88% | 0.009 |
| Overall | 2271 | 4335 | 52% | 288 | 440 | 65% | 406 | 573 | 71% | |
LEP = limited English proficiency; PICU = Pediatric intensive care unit
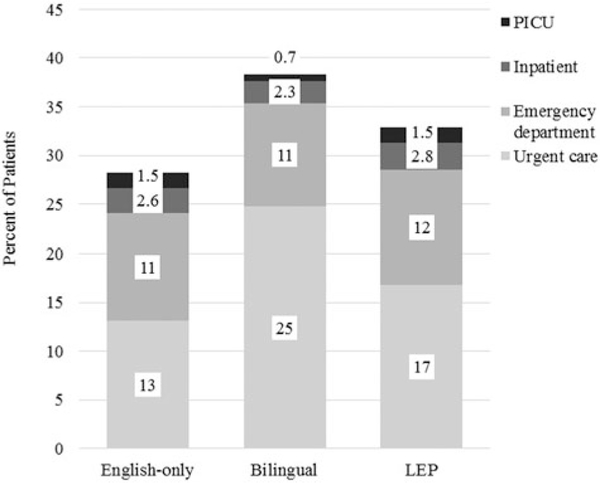
Lastly, we looked at the exacerbation treatment location for all asthma patients as an estimate of asthma exacerbation severity (Figure 1). Among all asthma patients, bilingual patients and patients with limited English proficiency were more likely to be treated at an urgent care facility (25% and 17%, respectively), compared with English-only patients (13%, p < 0.001). Emergency department use (11%–12%) and inpatient admissions (2.3%–2.8%) were similar among the three groups. Bilingual patients were less likely to be admitted to PICU (0.7%), compared with English-only patients (1.5%, p=0.02).
Figure 1.
Distribution of asthma exacerbation encounters among 18243 pediatric asthma patients from Hospital System A, by encounter location and patient language status, Ohio, 2011–2015. Note. Abbreviations: LEP = limited English proficiency; PICU = pediatric intensive care unit. Bars do not equal 100% because not all patients had an exacerbation. Exacerbation location is determined based on the final destination. The following comparisons were significant by using chi-square: English-only versus bilingual: Urgent care p < 0.001, PICU p = 0.02 English-only versus LEP: Urgent care p < 0.001.
Discussion
We analyzed over 18 000 central Ohio pediatric asthma patient records to compare asthma care quality and morbidity by English language proficiency. This is the largest study evaluating the relationship of spoken language and asthma in a hospital system-based population. Contrary to our initial hypothesis, patients with limited English proficiency did not have worse markers of asthma care quality (PFT use or 30-day follow-up) or morbidity (asthma exacerbation visits, multiple exacerbations, or PICU admissions) compared with English-only patients. In fact, follow-up was higher for both bilingual patients and patients with limited English proficiency compared to English-only patients, even after accounting for sociodemographic factors.
These findings differ from previous studies that evaluated slightly different outcomes. A survey of 107 caregivers found that those with limited English proficiency had lower rates of asthma action plan use, even after adjustment for demographic characteristics (15). A larger, multistate telephone survey of 1517 children with parent-reported diagnoses of asthma found that families of Spanish-speaking children were less often instructed how to recognize early exacerbation signs, what to do during an asthma attack, and how to change the living environment, compared with non-Latino white children (8). A cross-sectional, clinic-based survey of405 pediatric asthma patients found lower rates of peak flow monitoring and written action plans among Spanish-speaking Latino patients, compared with English-speaking Latino patients (7).
These differences might have occurred because prior studies evaluated different asthma measures, such as asthma action plans, altering the living environment, or peak flow monitoring, which are thought to modify morbidity. We were unable to evaluate these measures because of inconsistent use or documentation in the electronic health record across the study period. Instead, our study evaluated morbidity outcomes directly and found only one difference. We observed that bilingual patients were more likely to ever have an asthma exacerbation visit compared with English-only patients. This could reflect higher intrinsic disease burden in the bilingual population; however, we suspect it is more likely that bilingual patients seek care for less severe exacerbations or earlier in an exacerbation because of increased access to or higher utilization of care. This explanation is supported by the lower PICU admissions, higher use of urgent care, and higher 30-day follow-up in the bilingual group compared to the English-only group. As in our study, Chan et al. also found no difference in self-reported acute care visits in the past six months among Spanish-speaking Latino patients, compared with English-speaking white patients (7).
One potential limitation to our findings is the low socioeconomic status of the English-only comparison population. We lacked individual-level data for socioeconomic factors, such as household income, unemployment, and education, which might confound the observed relationships between English language proficiency and asthma care quality or morbidity. We used ZIP code-level data as a proxy for socioeconomic factors. Adjustment for ZIP code-level covariates might have also partially adjusted for clustering by clinic site that we were otherwise unable to adjust for. However, residual confounding by socioeconomic factors could mask language barrier effects. Expanding the interpretation of our findings to other populations should be done with caution.
Lastly, the greater asthma care quality or lack of differences we found might be related, in part, to the comprehensive provision of interpreter services in our population. Studies in multiple fields have shown that use of interpreter services can improve health care delivery (16–18) although randomized controlled trials in this field are lacking. The hospital system for our population offers qualified interpreters to all patients for both in-patient and out-patient visits. A staff of 25–30 full-time interpreters provides face-to-face interpretation for over 80 different languages, and third-party phone and video interpreter services are available for additional languages. During orientation, new nursing staff receive training on effective use of interpreters, and additional training for other health care providers is available as needed. Determining how the provision of interpreter services is related to quality of asthma care delivery in this population would require further study.
This study has several limitations. Tobacco exposure was self-reported or reported by proxy (parent or guardian), binary, and often missing. We used multiple imputation to account for missing data. Secondly, no validated measure for defining asthma exacerbation from electronic health records exists, but other published measures are similar to ours (19,20). An expert work-group definition proposed by Fuhlbrigge et al. includes the use of systemic corticosteroids; however, we were unable to reliably determine systemic corticosteroid use during an exacerbation visit, a known limitation of the proposed definition (21). Similarly, no validated measure for English proficiency exists. As in prior published studies, this study relies on self-identified language and self-reported request for interpreter. Using a sensitivity analysis, we found that an estimated 24% misclassification of language status would need to exist before a type II error occurred (Supplemental Table 1). This degree of misclassification is felt to be unlikely. Although Hospital System A serves as the only pediatric emergency department and only inpatient pediatric facility for the region, it is possible that patients could be seen for asthma exacerbations at outside urgent care or emergency facilities; however, we would not expect this to differ by language status. Lastly, although ICD billing codes could determine whether asthma was the primary diagnosis during the follow-up visit, these codes do not distinguish whether patients were seen for follow-up of resolved symptoms or worsening asthma symptoms.
Conclusions
We describe the association between English language proficiency and asthma care quality and morbidity using a large, hospital system-based cohort. In this urban population of pediatric asthma patients where comprehensive interpreter services are provided consistently, we did not find evidence that asthma care (as reflected by PFT utilization and 30-day post-exacerbation follow-up) or morbidity (as reflected by exacerbation visits and PICU admissions) was compromised by limited English proficiency. Other groups working with pediatric asthma populations may consider directly examining the role of interpreter services on reducing language barriers.
Supplementary Material
Acknowledgments
The findings and conclusions in this report are those of the author(s) and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Footnotes
Declaration of interest
The authors have no conflicts of interest to declare.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website. Supplemental Table. Sensitivity analysis of misclassification by language status for pediatric asthma patients with an asthma exacerbation visit, Hospital System A, 2011–2015
References
- 1.Bloom B, Simpson JL. Tables of Summary Health Statistics for U.S. Children: 2015 National Health Interview Survey. National Center for Health Statistics. 2016. Available from: http://www.cdc.gov/nchs/nhis/SHS/tables.htm.
- 2.United States Census Bureau/American FactFinder. S1601: Language spoken at home. 2011–2015 American Communities Survey 5-year Estimates. Available from: https://factfinder.census.gov. Published 2015.
- 3.Williams DR, Sternthal M, Wright RJ. Social determinants: taking the social context of asthma seriously. Pediatrics. 2009;123(3):S174–S184. doi: 10.1542/peds.2008-2233H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lake Snell Perry & Associates. Physician perspectives on communication barriers: insights from focus groups with physicians who treat non-English proficient and limited English proficient patients. Washington, DC: Robert Wood Johnson Foundation; 2004. [Google Scholar]
- 5.Poureslami I, Rootman I, Doyle-Waters MM, Nimmon L, FitzGerald JM. Health literacy, language, and ethnicity-related factors in newcomer asthma patients to canada: a qualitative study. J Immigr Minor Heal. 2011;13(2):315–322. doi: 10.1007/s10903-010-9405-x. [DOI] [PubMed] [Google Scholar]
- 6.Sudore RL, Landefeld CS, Pérez-Stable EJ, Bibbins-Domingo K, Williams BA, Schillinger D. Unraveling the relationship between literacy, language proficiency, and patient-physician communication. Patient Educ Couns. 2009;75(3):398–402. doi: 10.1016/j.pec.2009.02.019. PMID: 19442478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chan KS, Keeler E, Schonlau M, Rosen M, Mangione-Smith R. How do ethnicity and primary language spoken at home affect management practices and outcomes in children and adolescents with asthma? Arch Pediatr Adolesc Med. 2005;159(3):283–289. doi: 10.1001/archpedi.159.3.283. PMID:15753274. [DOI] [PubMed] [Google Scholar]
- 8.Inkelas M, Garro N, McQuaid EL, Ortega AN. Race/ethnicity, language, and asthma care: findings from a 4-state survey. Ann Allergy Asthma Immunol. 2008;100(2):120–127. doi: 10.1016/S1081-1206(10)60420-6. PMID: 18320913. [DOI] [PubMed] [Google Scholar]
- 9.Mosnaim GS, Sadowski LS, Durazo-Arvizu RA, et al. Parental language and asthma among urban Hispanic children. J Allergy Clin Immunol. 2007;120(5):1160–1165. doi: 10.1016/j.jaci.2007.08.040. PMID: 17983874. [DOI] [PubMed] [Google Scholar]
- 10.Wisnivesky J, Kattan M, Evans D, et al. Assessing the relationship between language proficiency and asthma morbidity among innercity asthmatics. Med Care. 2009;47(2):243–249. doi: 10.1097/MLR.0b013e3181847606. PMID: 19169126. [DOI] [PubMed] [Google Scholar]
- 11.National Asthma Education and Prevention Program. Expert panel report 3 (EPR-3): guidelines for the diagnosis and management of asthma–summary report 2007. J Allergy Clin Immunol. 2007;120:94–138. doi: 10.1016/j.jaci.2007.09.029. [DOI] [PubMed] [Google Scholar]
- 12.United States Census Bureau / American FactFinder. S2301: Employment status. 2011–2015 American Communities Survey 5-year Estimates. Available from: https://factfinder.census.gov. Published 2015.
- 13.United States Census Bureau / American FactFinder. S1501: Educational attainment. 2011–2015 American Communities Survey 5-year Estimates. Available from: https://factfinder.census.gov. Published 2015.
- 14.United States Census Bureau / American FactFinder. S1903: Median income in the past 12 months (in 2015 inflation-adjusted dollars). 2011–2015 American Communities Survey 5-year Estimates. Available from: https://factfinder.census.gov. Published 2015. [Google Scholar]
- 15.Riera A, Navas-Nazario A, Shabanova V, Vaca FE. The impact of limited English proficiency on asthma action plan use. J Asthma. 2014;51(2):178–184. doi: 10.3109/02770903.2013.858266. PMID: 24147607. [DOI] [PubMed] [Google Scholar]
- 16.Jacobs EA, Lauderdale DS, Meltzer D, Shorey JM, Levinson W, Thisted RA. Impact of interpreter services on delivery of health care to limited-English-proficient patients. J Gen Intern Med. 2001;16(7):468–474. doi: 10.1046/j.1525-1497.2001.016007468.x. PMID: 11520385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Juckett G, Unger K. Appropriate use of medical interpreters. Am Fam Physician. 2014;90(7):476–480. PMID: 25369625. [PubMed] [Google Scholar]
- 18.Flores G The impact of medical interpreter services on the quality of health care: a systematic review. Med Care Res Rev. 2005;62(3):255–299. doi: 10.1177/1077558705275416. PMID: 15894705. [DOI] [PubMed] [Google Scholar]
- 19.Li P, To T, Guttmann A. Follow-up care after an emergency department visit for asthma and subsequent healthcare utilization in a universal-access healthcare system. J Pediatr. 2012;161(2):208–213.e1. doi: 10.1016/j.jpeds.2012.02.038. PMID: 22484353. [DOI] [PubMed] [Google Scholar]
- 20.Smiley M, Sicignano N, Rush T, Lee R, Allen E. Outcomes of follow-up care after an emergency department visit among pediatric asthmatics in the military health system. J Asthma. 2016;53(8): 816–824. doi: 10.3109/02770903.2016.1170141. PMID: 27115719. [DOI] [PubMed] [Google Scholar]
- 21.Fuhlbrigge A, Peden D, Apter AJ, et al. Asthma outcomes: exacerbations. J Allergy Clin Immunol. 2012; 129(3): S34–S48. doi: 10.1016/j.jaci.2011.12.983. PMID: 22386508. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.