Abstract
Purpose
Identification of patient characteristics that are associated with behavioral weight loss success among bariatric surgery candidates could inform selection of optimal bariatric surgery candidates. We examined the associations between psychosocial characteristics and weight loss in a group of Veterans with severe obesity who participated in a behavioral weight loss intervention.
Methods
The MAINTAIN trial involved a 16-week weight loss program followed by randomization among participants losing at least 4 kg to a maintenance intervention or usual care. This secondary analysis was performed on Veterans who participated in the 16-week weight loss program and met NIH criteria for bariatric surgery (body mass index [BMI] 35.0–39.9 with at least 1 obesity-related comorbidity or BMI≥40). Unadjusted and adjusted associations between baseline patient characteristics and weight loss during the 16-week induction phase were evaluated with linear regression. Missing weight measurements were multiply imputed, and results combined across ten imputations.
Results
Among the 206 patients who met inclusion criteria, mean initial BMI was 40.8 kg/m2 (SD 6.0), and mean age was 59.2 years (SD 9.4). Approximately 20% of participants were female, 51.5% were Black, and 44.7% were White. Estimated mean 16-week weight loss was 5.16 kg (SD 4.31). In adjusted analyses, greater social support and older age were associated with greater weight loss (p< 0.05). None of the nine psychosocial characteristics we examined were associated with greater weight loss.
Conclusions
Understanding and strengthening the level of social support for bariatric surgery candidates may be important given that it appears to be strongly correlated with behavioral weight loss success.
Level of evidence
Level II, Evidence obtained from well-designed controlled trials without randomization.
Keywords: clinical trials, weight loss, severe obesity, bariatric surgery
INTRODUCTION
Bariatric surgery is an effective treatment for severe obesity (body mass index [BMI] ≥ 35 kg/m2). On average, patients maintain over 60% excess body weight loss at least five years after bariatric surgery and have high rates of resolution of obesity-related comorbidities including diabetes and hypertension [1]. Findings from randomized controlled trials suggest that, compared to medical therapy, quality of life improves significantly after bariatric surgery and that this effect is durable [2]. Population-level observational studies have found that patients who underwent bariatric surgery lived longer compared to patients with severe obesity who did not undergo bariatric surgery [3]. Weight loss among patients who underwent bariatric surgery was durable for at least a decade [3].
Despite favorable outcomes on average, not all bariatric surgery patients experience sustained weight loss and comorbidity resolution. Cooper and colleagues reported that more than one-third of patients who underwent a laparoscopic Roux-en-Y gastric bypass (RYGB) regained at least 25% of their maximum weight loss at a mean follow-up interval of 7 years [4]. Golomb and colleagues found that 50% of patients who underwent laparoscopic sleeve gastrectomy - the most commonly performed bariatric operation in the U.S. - experienced diabetes remission after one year. However, only 20% were still in remission after 5 years [5]. The authors posited that weight regain was a likely a significant factor in reduced diabetes remission rates over time. Thus, identifying which bariatric surgery patients will achieve significant and sustained weight loss is key. Some patient characteristics that have been associated with greater weight loss success following bariatric surgery are lower initial BMI, younger age, lack of diabetes, and no preoperative weight gain [6].
Multiple studies have found that patients who experience greater weight loss during the pre-operative period are more likely to have better bariatric surgery outcomes, including lower complication rates and greater weight loss [7,8]. However, the patient characteristics that are associated with a higher likelihood of behavioral weight management success prior to undergoing bariatric surgery are unknown. The objective of this study was to identify patient characteristics that were associated with improved weight loss during a weight loss intervention that was administered to a group of Veterans with severe obesity who met NIH criteria for bariatric surgery. Veterans were selected for this study given that it was part of a larger, Veterans Health Administration (VA) funded study of obese Veterans participating in a weight loss maintenance intervention [9]. In this study, we investigated patient demographic characteristics, weight loss behaviors, and psychosocial factors that might be associated with weight loss.
METHODS
Setting
Participants were enrolled from three primary clinics associated with the Durham Veterans Affairs Medical Center (VAMC) in North Carolina, USA. The study protocol was approved by the Durham VAMC Institutional Review Board (IRB) and Research and Development Committee and the Duke University Medical Center IRB.
Design
Data were obtained during the weight loss initiation phase of the MAINTAIN study, which involved a 16-week weight loss program focusing on calorie and fat restriction for all participants who met initial eligibility criteria. Participants who lost at least 4 kg were then randomized to either a maintenance intervention (42 weeks followed by 14 weeks of no intervention) or usual care (56 weeks).[10] This report includes results from the 16-week weight loss induction phase among the subset of participants who met National Institutes of Health (NIH) eligibility criteria for bariatric surgery: BMI 35.0–39.9 with at least 1 obesity-related comorbidity or BMI ≥ 40.
Screening and Recruitment
The study was conducted in six cohorts. Each cohort was recruited over a 6 to 8-week period. Patients called study staff in response to a mailed recruitment letter or flyer posted at the medical center. Providers could also refer patients by placing a consult in the electronic medical record (EMR). Eligibility was determined through a combination of EMR review, telephone screening, and in-person screening [10]. Inclusion criteria were: body mass index (BMI) ≥ 30 kg/m2; cared for by a VA primary care provider; age 18–75; desire to lose weight and agreement to attend study visits; access to a telephone; and access to reliable transportation. Exclusion criteria were unstable health defined by one of the following: kidney or liver disease, type I diabetes, uncontrolled hypertension (average systolic blood pressure > 160 during the previous year and during the most recent clinic visit), uncontrolled hyperglycemia (hemoglobin A1c > 12 during the previous six months), cancer not in remission, organ transplant recipient, or a heart condition. Additional exclusion criteria included current enrollment in another behavioral weight management program; psychiatric illness; pregnancy or plans to become pregnant; breastfeeding; previous bariatric surgery; current use of weight loss medication or appetite suppressants; weight loss of at least 10 pounds in the previous 3 months; pacemaker (due to use of bioeletric impedance scale); and inability to stand for measurements. Written informed consent was obtained at the in-person screening appointment. Eligible patients chose one of six meeting times for the group-based weight loss program.
Procedures and Measures
For ease of describing the study time points, we refer to in-person screening as week −17, the first group weight loss session as −16, and the time of randomization as week 0. Participants were not compensated for the week −17 visit but received $20 for the week 0 visit. From weeks −16 to −2, participants attended biweekly group sessions delivered by a registered dietitian that addressed dietary education and behavioral weight loss strategies (e.g., goal setting, mindful eating). The weight loss intervention was an abbreviated version of a protocol that our team has evaluated previously.
Dependent Variable
Weight obtained at week −16 served as the study entry weight, while the weight obtained at week 0 served as the final weight for the induction phase. Weight was assessed on a calibrated digital scale in light clothing and with shoes removed.
Independent variables
The following variables were obtained at the week −17 screening visit:
Demographic characteristics
Self-reported age, sex, race and whether the participant had engaged in a previous weight loss attempt were assessed. Educational level and employment status were also obtained.
Clinical characteristics
Height was assessed with a stadiometer for calculation of BMI. The presence of six obesity-related comorbidities in the past one year, as indicated by ICD-9 codes recorded in the EMR, was identified via the VA Informatics and Computing Infrastructure (VINCI) [11]. These included hypertension, type 2 diabetes mellitus, gastroesophageal reflux disease, obstructive sleep apnea, coronary artery disease and dyslipidemia. The patient’s smoking history was also assessed.
Psychosocial characteristics
The following self-report measures correspond to our conceptual model regarding behavior initiation and maintenance [12]. The conceptual model proposes that initial behavior change (vs. maintenance of behavior) is influenced by a focus on anticipated positive outcomes of weight loss (i.e., favorable expectations), self-efficacy for taking action towards diet and physical activity change (i.e., action self-efficacy).
Favorable expectations about future weight loss were assessed in the domains of enjoyment of food, health, physical attractiveness, fit of clothes, physical fitness, ability to complete tasks requiring physical exertion, social life, and positive feedback about weight loss [13]. The measure used for favorable expectations in this study has previously demonstrated a coefficient alpha of 0.78 and scores have been associated with weight loss [14].
Self-efficacy to initiate dietary and physical activity changes (action self-efficacy) was assessed with items developed for this study following the methods of Schwarzer [15]. The 11 dietary self-efficacy items began with the stem, “I am sure I can start a low-fat diet even if…” and included endings such as “my weight doesn’t improve immediately.” The nine physical activity self-efficacy items began with the stem, “I am sure I can start getting regular physical activity” and included endings such as “I have to start all over again several times until I succeed.” This method has produced good coefficient alphas in previous research [15].
The 15-item Treatment Self-Regulation for Diet questionnaire assessed the extent to which motivation for dieting was autonomous (6 items), controlled (6 items), or lacking (amotivation; 3 items) [16]. The 15-item Treatment Self-Regulation for Exercise questionnaire similarly assessed source of motivation for physical activity. In the current study, the amotivation subscale from each measure was unreliable (alpha = 0.43 for the dietary measure and alpha = 0.32 for the exercise measure) and thus excluded from analyses. The autonomous motivation subscale has been associated with weight loss in past research [16].
Behavioral intentions for diet and physical activity were assessed separately with five semantic differential items ranging from 1 to 7 (unlikely to likely; impossible to possible; definitely would not to definitely would; no chance to certain; and probably not to probably) following the methods of Ajzen [17]. Previous measures developed with this method have produced coefficient alphas of 0.79 for physical activity and 0.56 for fruit and vegetable intake [15].
Participants also indicated whether they had a social support person (“Do you have a friend, spouse, partner, acquaintance, coworker or other person whom you confide in regularly?”).
Analyses
Means and standard deviations were calculated for continuous variables, and frequencies (N, %) were calculated for categorical variables. Weight loss was calculated as weight at week −16 minus weight at week 0; positive values correspond to weight loss. Unadjusted (bivariate) relationships between weight loss and clinical, demographic, and psychosocial variables were characterized with linear regression. Variables with p < 0.10 in unadjusted analyses were entered simultaneously into a linear regression model to estimate adjusted relationships. Maximum likelihood estimation via the EM algorithm was used to calculate weight loss mean and standard deviation [18]. Additionally, missing weight measurements at week 0 were multiply imputed under a multivariate normal model via the MCMC option in PROC MI (SAS 9.4, Cary, NC). All independent variables in the unadjusted analyses were included in the imputation model; additionally, all observed weights measured bi-weekly at weeks −16 through week 0 were included in the imputation model. Ten imputed datasets were created, and all unadjusted and adjusted analyses were run on each of the imputations. Model estimates and standard errors were combined across the imputations via PROC MIANALYZE. P values <0.05 were considered to be statistically significant.
RESULTS
Participants
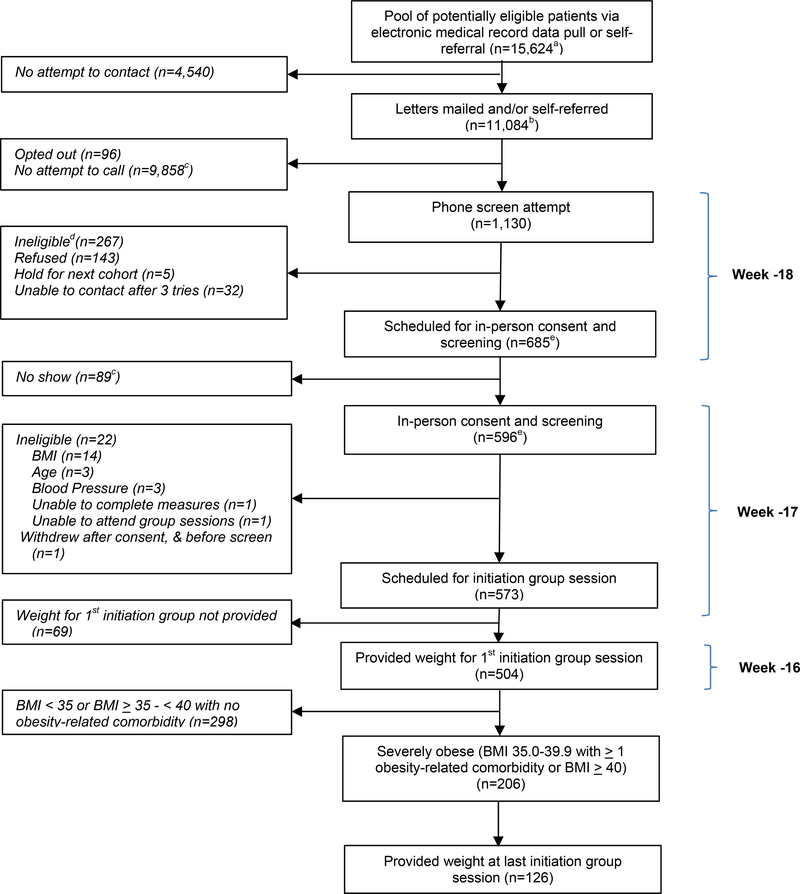
As reported elsewhere [9], our research team attempted to screen 1,130 patients, of whom 267 were ineligible, 143 declined to participate, 32 were unable to be contacted after three attempts, and five asked to be held for a future cohort but never enrolled. In-person screening appointments were scheduled for 685 patients. Of those, 504 initiated participation in our weight management program, of which 206 met NIH BMI and comorbidity criteria for bariatric surgery and were included in current analyses.
Patient characteristics
Table 1 displays descriptive statistics for the observed data of the 206 participants who met NIH criteria for bariatric surgery and initiated treatment. The mean age was 59.2 (SD 9.4). Females comprised 18.4% of the study population; 51.5% were Black, and 44.7% were White. Retired, employed, and “other/disabled” participants comprised 43.7%, 31.6%, and 24.8% of the cohort, respectively. The mean week −16 BMI and weight of the group were 40.8 kg/m2 (SD 6.0) and 123.2 kg (SD 21.6), respectively. Nearly 90% had attempted weight loss previously. At least one obesity-related comorbidity was present for 94% of the cohort. The most common obesity-related comorbidity was hypertension (69%), followed by diabetes (43%) and dyslipidemia (43%). The majority of participants reported having a support person (88%).
Table 1.
Characteristics of participants who met NIH criteria for bariatric surgery
| Characteristic | Overall (n=206) |
|---|---|
| Age, M (SD) | 59.2 (9.4) |
| Female, N (%) | 38 (18.4) |
| Race/ethnicity, N (%) | |
| Black | 106 (51.5) |
| White | 92 (44.7) |
| Multiracial/Other | 6 (2.9) |
| High school graduate, N (%) | 201 (97.6) |
| Employment status, N (%) | |
| Retired | 90 (43.7) |
| Employed | 65 (31.6) |
| Other/disabled | 51 (24.8) |
| Body mass index, kg/M2, M (SD) | 40.6 (5.7) |
| Weight, kg, M (SD) | 122.7 (21.0) |
| Obesity class | |
| Class II obesity (BMI 35.0–39.9) | 110 (53.4) |
| Class III obesity (BMI>=40) | 96 (46.6) |
| Comorbid condition | |
| Hypertension | 142 (68.9) |
| Type II diabetes mellitus | 88 (42.7) |
| Dyslipidemia | 88 (42.7) |
| Obstructive sleep apnea | 72 (35.0) |
| Coronary artery disease | 39 (18.9) |
| Gastroesophageal reflux disease | 31 (15.0) |
| >1 obesity-related comorbid condition | 193 (93.7) |
| Current tobacco user, N (%) | 18 (8.7) |
| Attempted weight loss previously, N (%) | 180 (87.4) |
| Identify a support person, N (%) | 182 (88.3) |
Psychosocial characteristics of the participants at initiation of the weight loss program are shown in Table 2. Among the six constructs scored on a 1–7 scale, participants scored highest in autonomous motivation for physical activity (6.5; SD 0.9), autonomous motivation for eating healthy (6.5; SD 0.7), and intentions to change their diet (6.3; SD 0.8) and engage in physical activity (6.1; SD 1.3).
Table 2.
Psychosocial measures upon initiation of the weight loss program (n=206a)
| Measure | Possible range | Mean (SD) | Cronbach’s alpha |
|---|---|---|---|
| Favorable expectations about weight lossb | −4 – +4 | 2.5 (1.0) | 0.89 |
| Self-efficacy to initiate diet | 0–3 | 2.1 (0.4) | 0.89 |
| Self-efficacy to initiate physical activity | 0–3 | 2.1 (0.5) | 0.93 |
| Autonomous motivation for physical activity | 1–7 | 6.5 (0.9) | 0.91 |
| Autonomous motivation for eating healthy | 1–7 | 6.5 (0.7) | 0.86 |
| Intentions to change diet | 1–7 | 6.3 (0.8) | 0.95 |
| Intentions to engage in physical activity | 1–7 | 6.1 (1.3) | 0.98 |
| Controlled motivation for eating healthy | 1–7 | 3.7 (1.6) | 0.86 |
| Controlled motivation for physical activity | 1–7 | 3.7 (1.7) | 0.88 |
None of the measures contained missing data for the 206 eligible patients.
Negative numbers indicate unfavorable expectations (e.g., −4=health will worsen a great deal); positive numbers indicate favorable expectations (e.g., +4=health will improve a great deal).
Weight loss
Follow-up weights at week 0 were obtained for 61.2% (n=126) of the cohort. Estimated mean weight loss during the initiation phase of the intervention was 5.16 kg (SD 4.31). In unadjusted analyses, six of the fifteen characteristics were associated with greater weight loss at the p ≤ 0.1 level of significance: older age, White vs. non-White race, current tobacco use, and presence of a support person (Table 3). Female gender and at least one previous weight loss attempt were inversely associated with greater weight loss. Each of these characteristics was associated with a differential mean weight loss of at least 1.06 kg; for example, patients who had a social support person lost an estimated mean 2.31 kg (95% CI: 0.30, 4.32) more than patients without social support. In the adjusted model, the associations remained significant for age (estimated mean weight loss of 0.84 kg [95% CI: 0.02, 1.67] greater for every 10 year increase in age) and presence of a social support person (estimated mean weight loss of 2.17 kg [95% CI 0.20, 4.14] more than patients without a social support person).
Table 3.
Linear regression analysis between the association of demographic, clinical, and psychosocial factors and greater weight loss (n=206)
| Factor | Unadjusted | Adjusted | ||
|---|---|---|---|---|
| Parameter Estimate (95% CI) | p-value | Parameter Estimate (95% CI) | p-value | |
| Age (10-unit increase) | 1.06 (0.27, 1.85) | 0.009 | 0.84 (0.02, 1.67) | 0.046 |
| White | 1.54 (0.15, 2.92) | 0.03 | 0.76 (−0.74, 2.26) | 0.32 |
| Female | −2.40 (−4.11, −0.68) | 0.006 | −1.34 (−3.06, 0.38) | 0.13 |
| Current tobacco user | 2.00 (−0.32, 4.33) | 0.09 | 1.74 (−0.53, 4.02) | 0.13 |
| Had ≥ 1 previous weight loss attempt | −2.26 (−4.52, 0.01) | 0.051 | −2.08 (−4.34, 0.19) | 0.072 |
| Presence of social support person | 2.31 (0.30, 4.32) | 0.02 | 2.17 (0.20, 4.14) | 0.031 |
| Favorable expectations about weight loss | −0.53 (−1.22, 0.16) | 0.13 | ||
| Self-efficacy to initiate diet | −0.84 (−2.50, 0.81) | 0.32 | ||
| Intentions to change diet | −0.26 (−1.13, 0.62) | 0.57 | ||
| Autonomous motivation for eating healthy | −0.03 (−1.00, 0.93) | 0.95 | ||
| Controlled motivation for eating healthy | 0.21 (−0.22, 0.64) | 0.35 | ||
| Self-efficacy to initiate physical activity | −0.05 (−1.38, 1.27) | 0.94 | ||
| Intentions to engage in physical activity | −0.32 (−0.86, 0.23) | 0.25 | ||
| Autonomous motivation for physical activity | −0.24 (−1.04, 0.55) | 0.55 | ||
| Controlled motivation for physical activity | 0.15 (−0.29, 0.59) | 0.50 | ||
95% CI indicates 95% confidence interval; all parameter estimates were combined across ten imputed datasets via PROC MIANALYZE. Positive regression coefficients indicate weight (kg) loss, whereas negative coefficients indicate weight gain. For example, in adjusted analyses, holding all else equal, patients with social support lost approximately 2.17 kilograms (95% CI: 0.20, 4.14) more than patients without social support. The multiple linear regression (adjusted) model included only those characteristics significant at the α < 0.10 level of significance in unadjusted analyses.
DISCUSSION
Identifying which patients are most likely to maintain significant and sustained weight loss and comorbidity resolution following bariatric surgery is critical. Our findings suggest that severely obese patients who have greater social support and are older are more likely to achieve success with behavioral weight management. We did not identify any psychosocial factors that were associated with improved weight loss. Given that behavioral weight loss success is associated with improved bariatric surgery outcomes, these characteristics may be important to consider when evaluating bariatric surgery patients preoperatively.
The strongest predictor of weight loss success among this group of U.S. Veterans with severe obesity was having a “friend, spouse, partner, acquaintance, coworker or other person whom you confide in regularly.” Other investigators have also found that stronger social support was important for achieving success with behavioral weight management. In a meta-analysis of weight loss studies, Lemstra and colleagues reported that interventions offering social support for obese patients had higher rates of adherence to weight loss interventions compared to those without social support [19]. Social support included teaching in group vs. individual sessions, peer coaches, and “buddy programs.” Participants in studies that included social support achieved intervention adherence rates of 73.4% vs. 57.1% in studies where no social support was offered.
Stronger social support has also been found to be associated with greater weight loss after bariatric surgery. In a systematic review that included 10 studies that reported on social support and weight loss outcomes, Livhits and colleagues found a positive association between post-operative social support groups and weight loss [20]. For this reason, support groups are strongly supported by the American Society for Metabolic and Bariatric Surgery (ASMBS) for bariatric surgery patients [21].
A correlation between improved social support and better outcomes has been described in other surgical specialties. Many of these studies use marital status as a proxy for social support. Patients who are married are more likely to have a higher quality-of-life after undergoing anti-reflux surgery for treatment of gastroesophageal reflux disease (GERD)[22]. Married patients undergoing cardiac surgery have been found to have lower mortality rates and increased functional ability after surgery [23–25]. Having a spouse has been associated with improved survival for various types of cancers [26–28]. The authors from one study posited that married individuals may have pursued treatment earlier due to earlier diagnosis [27].
The finding from our study and others that social support is associated with improved outcomes adds to the concept that relationships between people are highly influential in healthcare and in surgery. However, having someone available to confide in, as our social support question asked, is likely only a proxy for the overall strength and richness of a person’s social network. In their seminal publication on how obesity spreads, Christakis and colleagues found that obesity spread through social ties over a 30-year period [29]. Very little is known about how relationships may influence an individual’s likelihood to lose weight, either with or without bariatric surgery. Almost nothing is known about how these relationships change over time and how those changes influence an individual’s likelihood of achieving weight loss success. This is an area where research is needed for the future.
None of the psychosocial characteristics we examined in our linear regression analyses was associated with increased weight loss. Patients’ expectations about their weight loss, their beliefs in their ability to start and maintain a healthier diet, and their intentions to begin and sustain increased physical activity had no measurable impact on the amount of weight patients lost. These findings contrast with analyses from this same trial including the entire pool of participants (i.e., BMI ≥30kg/m2), which found that poor motivation for physical activity was associated with decreased weight loss [30]. Other studies have found that certain psychosocial characteristics, including higher self-efficacy to initiate exercise [31], emotional eating [32] and lack of autonomous regulation [33], are associated with less weight loss. Our findings suggest that despite having positive intentions and self-belief in their ability to make behavior change, patients with severe obesity may encounter other barriers to weight loss that patients with class I obesity do not experience. This is an important area for future investigation.
One of these potential barriers is that, compared to patients with class I obesity, severely obese patients are much more likely to have comorbidities that limit their ability to exercise vigorously. Nearly 94% of our cohort had at least one obesity-related comorbidity, including lifestyle impairing health conditions such as obstructive sleep apnea and coronary artery disease. In contrast, only 76% of patients with class I obesity in our larger trial had at least one obesity-related comorbidity at the time of study initiation. A focus group study of primary care providers found that limited physical mobility for severely obese patients was a major challenge to implementing obesity treatment plans [34].
The association between older age and increased weight loss was clinically small, but statistically significant in the adjusted analyses. For every increase in age by 10 years, Veterans lost an additional 0.8 kg. One reason for this relationship may have been that older patients were more likely to have the capacity to adhere to the components in our intervention, which included multiple meetings during the week in the afternoon. Other investigators have also reported that older age is associated with higher adherence to behavioral weight management interventions [35–37].
Our study has several limitations. First, it was performed at a single VAMC and may not generalize to other VA and non-VA settings. Second, although the Veterans met NIH BMI criteria, some would not have met other program-specific criteria for bariatric surgery. For example, many bariatric surgery programs exclude active smokers from bariatric surgery consideration. In our study, 8.7% of patients were actively smoking. Third, the mean age in our cohort was 59 years old, which is more than a decade older than a typical bariatric surgery cohort [38]. Thus, our findings may not generalize to a cohort of patients considering bariatric surgery. However, a systematic review published in 2015 found that bariatric surgery outcomes in patients over 60 were similar to younger patients [39]. Further, Larjani and colleagues reported in a Canadian study that older patients were more likely to return consistently for scheduled follow-up after bariatric surgery [40]. The elements that underlie stronger social support are still unknown and may involve an individual’s overall social network rather than one relationship. Finally, we did not explicitly assess the barriers to behavior change which are known to be important determinants of weight loss success [41].
In conclusion, stronger social support and older age were associated with improved weight loss in our 16-week intervention on a group of U.S. Veterans with severe obesity. Psychosocial parameters, in contrast, were not associated with the amount of weight loss. Improving social support systems and understanding their social networks should continue to be critical evaluation and treatment components for bariatric surgery patients. When evaluating older patients who are considering bariatric surgery, their increased likelihood to adhere to the treatment plan postoperatively may also be an important consideration.
Figure 1. Patient Flow for Participants with Severe Obesity.
a Exclusion criteria determined by data pull included most recent serum creatinine >2.0 mg/dL in men or >1.7 mg/dL in women; liver disease; type 1 diabetes; most recent hemoglobin A1c, in past six months ≥12%; average systolic blood pressure over the past year of ≥ 160 mmHg and most recent BP ≥ 160 mmHg; history of weight loss surgery, dementia, severe psychiatric illness, or substance abuse
b N=10,807 were mailed letters; n=38 were mailed letters as well as being self-referred; n=239 were self-referred with no letter sent
c Obtained by subtraction
d Potential reasons for ineligibility assessed by telephone included: body mass index (BMI) <30 kg/m2 based on self-reported weight and height (reduced threshold to allow for error in reporting); self-reported age < 18 or > 75; weight loss ≥10 pounds in the previous 3 months; current enrollment in a lifestyle program; history of weight loss surgery; current use of weight loss medication or appetite suppressant; pregnancy or plan to become pregnant in upcoming 6 months, breastfeeding, or lack of birth control if premenopausal (female); organ transplant recipient; type 1 diabetes; heart disease with new treatment in past 3 months; liver disease; cancer not in remission; pacemaker or defibrillator due to use of bioelectronic impedance scale; major depression or emotional problems that would prevent following a diet closely or interacting with others in a group environment; illicit drug use or alcohol problems in the past year; inability to stand for study measurements; desire to lose weight; agreement to attend study visits; access to telephone; reliable transportation
e N=2 of the n=267 ineligibles at phone screen (1 due to BMI, and 1 due to age) are included in both the “Scheduled for in-person consent and screening” and “In-person consent and screening” boxes. One was ineligible at phone screen due to BMI < 30 kg/m2, but then was erroneously re-screened in-person and excluded at that point for the same reason. The second was listed as excluded due to age > 75 at both phone and in-person screen. Both exclusions were erroneous as the patient was 75 at both time points; however, the patient was not included in study after the in-person screen.
ACKNOWLEDGMENTS
The authors thank Jahdai Dawes, Marsha Turner, and Terry Ervin, RN for assistance with recruitment and conducting measurements; Elizabeth Strawbridge, MPH, RD, and Leslie Gaillard, RD, for delivery of the weight loss intervention; and Lesa Powell and Aviel Alkon for programming and database development. Finally, the authors thank all study participants.
FUNDING
This study was funded by a grant awarded to Drs. Voils and Yancy by the Department of Veterans Affairs (DVA) Health Services Research & Development Service (IIR 11–040). Effort on this study and manuscript were also made possible by a VA Career Development Award to Dr. Funk (CDA 15–060), an NIH career development award to Dr. McVay (K23 HL127334), and a Research Career Scientist award to Dr. Voils (RCS 14–443). This work was supported by the Center of Innovation for Health Services Research in Primary Care (CIN 13–410) at the Durham VA Medical Center. The views represented in this article represent those of the authors and not those of the DVA or the US Government.
Footnotes
CONFLICT OF INTEREST
Effort on this study and manuscript was made possible by a VA Career Development Award to Dr. Funk, an NIH career development award to Dr. McVay, and a Research Career Scientist award to Dr. Voils. This work was also supported by the Center of Innovation for Health Services Research in Primary Care at the Durham VA Medical Center. The views represented in this article represent those of the authors and not those of the DVA or the US Government. The authors declare no conflicts of interest related to these funding sources.
Trial registration: ClinicalTrials.gov http://clinicaltrials.gov/show/NCT01357551
REFERENCES
- 1.Chang SH, Stoll CR, Song J, Varela JE, Eagon CJ, Colditz GA. The Effectiveness and Risks of Bariatric Surgery: An Updated Systematic Review and Meta-analysis, 2003–2012. JAMA Surg 2013;149:275–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schauer PR, Bhatt DL, Kirwan JP, Wolski K, Brethauer SA, Navaneethan SD, et al. Bariatric Surgery versus Intensive Medical Therapy for Diabetes - 3-Year Outcomes. N Engl J Med 2014;370:2002–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arterburn DE, Olsen MK, Smith VA, Livingston EH, Van Scoyoc L, Yancy WS Jr., et al. Association between bariatric surgery and long-term survival. JAMA 2015;313:62–70. [DOI] [PubMed] [Google Scholar]
- 4.Cooper TC, Simmons EB, Webb K, Burns JL, Kushner RF. Trends in Weight Regain Following Roux-en-Y Gastric Bypass (RYGB) Bariatric Surgery. Obes Surg 2015;25:1474–81. [DOI] [PubMed] [Google Scholar]
- 5.Golomb I, Ben David M, Glass A, Kolitz T, Keidar A. Long-term Metabolic Effects of Laparoscopic Sleeve Gastrectomy. JAMA Surg 2015;150:1051–7. [DOI] [PubMed] [Google Scholar]
- 6.Al-Khyatt W, Ryall R, Leeder P, Ahmed J, Awad S. Predictors of Inadequate Weight Loss After Laparoscopic Gastric Bypass for Morbid Obesity. Obes Surg 2017;27:1446–52. [DOI] [PubMed] [Google Scholar]
- 7.Sethi M, Beitner M, Magrath M, Schwack B, Kurian M, Fielding G, et al. Previous weight loss as a predictor of weight loss outcomes after laparoscopic adjustable gastric banding. Surg Endosc 2016;30:1771–7. [DOI] [PubMed] [Google Scholar]
- 8.Gerber P, Anderin C, Thorell A. Weight loss prior to bariatric surgery: an updated review of the literature. Scand J Surg 2015;104:33–9. [DOI] [PubMed] [Google Scholar]
- 9.Voils CI, Olsen MK, Gierisch JM, McVay MA, Grubber JM, Gaillard L, et al. Maintenance of Weight Loss After Initiation of Nutrition Training: A Randomized Trial. Ann Intern Med 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Voils CI, Gierisch JM, Olsen MK, Maciejewski ML, Grubber J, McVay MA, et al. Study design and protocol for a theory-based behavioral intervention focusing on maintenance of weight loss: the Maintenance After Initiation of Nutrition TrAINing (MAINTAIN) study. Contemp Clin Trials 2014;39:95–105. [DOI] [PubMed] [Google Scholar]
- 11.VA Informatics and Computer Infrastructure (VINCI), VA HSR HIR 08–204, U.S. Department of Veterans Affairs. 2008. (Accessed October 12, 2016, 2016, at https://vaww.VINCI.med.va.gov.)
- 12.Voils CI, Gierisch JM, Yancy WS Jr, Sandelowski M, Smith R, Bolton J, et al. Differentiating behavior initiation and maintenance: Theoretical framework and proof of concept. Health Education & Behavior 2014;41:325–36. [DOI] [PubMed] [Google Scholar]
- 13.Baldwin AS, Rothman AJ, Jeffery RW. Satisfaction with weight loss: Examining the longitudinal covariation between people’s weight-loss-related outcomes and experiences and their satisfaction. Annals of Behavioral Medicine 2009;38:213–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Finch EA, Linde JA, Jeffery RW, Rothman AJ, King CM, Levy RL. The effects of outcome expectations and satisfaction on weight loss and maintenance: correlational and experimental analyses--a randomized trial. Health Psychol 2005;24:608–16. [DOI] [PubMed] [Google Scholar]
- 15.Schwarzer R, Schuz B, Ziegelmann JP, Lippke S, Luszczynska A, Scholz U. Adoption and maintenance of four health behaviors: theory-guided longitudinal studies on dental flossing, seat belt use, dietary behavior, and physical activity. Ann Behav Med 2007;33:156–66. [DOI] [PubMed] [Google Scholar]
- 16.Williams GC, Grow VM, Freedman ZR, Ryan RM, Deci EL. Motivational predictors of weight loss and weight-loss maintenance. J Pers Soc Psychol 1996;70:115–26. [DOI] [PubMed] [Google Scholar]
- 17.Ajzen I, Fishbien M. Understanding attitudes and predicting social behavior. Englewood Cliffs, NJ: Prentice-Hall; 1980. [Google Scholar]
- 18.Allison PD. Missing data: Sage University Paper Series on quantitative applications in the social sciences. Thousand Oaks, CA2001. [Google Scholar]
- 19.Lemstra M, Bird Y, Nwankwo C, Rogers M, Moraros J. Weight loss intervention adherence and factors promoting adherence: a meta-analysis. Patient Prefer Adherence 2016;10:1547–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Livhits M, Mercado C, Yermilov I, Parikh JA, Dutson E, Mehran A, et al. Is social support associated with greater weight loss after bariatric surgery?: a systematic review. Obes Rev 2011;12:142–8. [DOI] [PubMed] [Google Scholar]
- 21.ASMBS Bariatric Surgery Support Group Facilitator Manual. (Accessed March 6, 2017, at https://asmbs.org/resources/asmbs-bariatric-surgery-support-group-facilitator-manual.)
- 22.Statz AK, A. MS, Jolles SA, Greenberg JA, Lidor AO, A.L. S, et al. Psychosocial factors are associated with quality of life after laparoscopic anti-reflux surgery Surg Endosc 2017. [DOI] [PubMed] [Google Scholar]
- 23.Idler EL, Boulifard DA, Contrada RJ. Mending broken hearts: marriage and survival following cardiac surgery. J Health Soc Behav 2012;53:33–49. [DOI] [PubMed] [Google Scholar]
- 24.King KB, Reis HT. Marriage and long-term survival after coronary artery bypass grafting. Health Psychol 2012;31:55–62. [DOI] [PubMed] [Google Scholar]
- 25.Neuman MD, Werner RM. Marital Status and Postoperative Functional Recovery. JAMA Surg 2016;151:194–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Greenleaf EK, Cooper AB, Hollenbeak CS. Marital Status and Survival in Patients with Carcinoid Tumors. Health Serv Insights 2016;9:3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang L, Wilson SE, Stewart DB, Hollenbeak CS. Marital status and colon cancer outcomes in US Surveillance, Epidemiology and End Results registries: does marriage affect cancer survival by gender and stage? Cancer Epidemiol 2011;35:417–22. [DOI] [PubMed] [Google Scholar]
- 28.Eskander MF, Schapira EF, Bliss LA, Burish NM, Tadikonda A, Ng SC, et al. Keeping it in the family: the impact of marital status and next of kin on cancer treatment and survival. Am J Surg 2016;212:691–9. [DOI] [PubMed] [Google Scholar]
- 29.Christakis NA, Fowler JH. The spread of obesity in a large social network over 32 years. N Engl J Med 2007;357:370–9. [DOI] [PubMed] [Google Scholar]
- 30.Voils CI, Grubber JM, McVay MA, Olsen MK, Bolton J, Gierisch JM, et al. Recruitment and Retention for a Weight Loss Maintenance Trial Involving Weight Loss Prior to Randomization. Obes Sci Pract 2016;2:355–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Byrne S, Barry D, Petry NM. Predictors of weight loss success. Exercise vs. dietary self-efficacy and treatment attendance. Appetite 2012;58:695–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Annesi JJ, Mareno N, McEwen K. Psychosocial predictors of emotional eating and their weight-loss treatment-induced changes in women with obesity. Eat Weight Disord 2016;21:289–95. [DOI] [PubMed] [Google Scholar]
- 33.Teixeira PJ, Silva MN, Mata J, Palmeira AL, Markland D. Motivation, self-determination, and long-term weight control. Int J Behav Nutr Phys Act 2012;9:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Funk LM, Jolles SA, Greenberg CC, Schwarze ML, Safdar N, M.A. M, et al. Primary care physician decision making regarding severe obesity treatment and bariatric surgery: A qualitative study. Surg Obes Relat Dis 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Austin JL, Smith JE, Gianini L, Campos-Melady M. Attitudinal familism predicts weight management adherence in Mexican-American women. J Behav Med 2013;36:259–69. [DOI] [PubMed] [Google Scholar]
- 36.Colley RC, Hills AP, O’Moore-Sullivan TM, Hickman IJ, Prins JB, Byrne NM. Variability in adherence to an unsupervised exercise prescription in obese women. Int J Obes (Lond) 2008;32:837–44. [DOI] [PubMed] [Google Scholar]
- 37.Steinberg DM, Levine EL, Lane I, Askew S, Foley PB, Puleo E, et al. Adherence to self-monitoring via interactive voice response technology in an eHealth intervention targeting weight gain prevention among Black women: randomized controlled trial. J Med Internet Res 2014;16:e114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abraham A, Ikramuddin S, Jahansouz C, Arafat F, Hevelone N, Leslie D. Trends in Bariatric Surgery: Procedure Selection, Revisional Surgeries, and Readmissions. Obes Surg 2016;26:1371–7. [DOI] [PubMed] [Google Scholar]
- 39.Giordano S, Victorzon M. Bariatric surgery in elderly patients: a systematic review. Clin Interv Aging 2015;10:1627–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Larjani S, Spivak I, Hao Guo M, Aliarzadeh B, Wang W, Robinson S, et al. Preoperative predictors of adherence to multidisciplinary follow-up care postbariatric surgery. Surg Obes Relat Dis 2016;12:350–6. [DOI] [PubMed] [Google Scholar]
- 41.Burgess E, Hassmen P, Pumpa KL. Determinants of adherence to lifestyle intervention in adults with obesity: a systematic review. Clin Obes 2017;7:123–35. [DOI] [PubMed] [Google Scholar]