INTRODUCTION
Status epilepticus is a condition resulting either from the failure of the mechanisms responsible for seizure termination or from the initiation of mechanisms, which lead to abnormally prolonged seizures (after time point t1). The time t1 is 5 minutes for generalized tonic-clonic seizure, 10 minutes for focal seizure.[1] This state identifies a state of neuronal excitation characterized by an excess production of glutamate that activates the N-methyl-D-Aspartate (NMDA) receptor and promotes entry of calcium. Excess glutamate leads to the sensitization and internalization of gamma aminobutyric acid receptor A (GABAA), increases the expression of proconvulsive neuropeptide and maintains the living circle of self-sustained epilepsies.[2] Progress in magnetic resonance imaging (MRI) offers new opportunities to identify early neuronal lesions and identify areas of epileptic seizures.
POST ICTAL CHANGES IN MRI
A large range of ictal and early postictal changes have been described on MRI. These changes can be confined to the area of epileptic activity or remote from this region (Table 1). Knowledge of these anomalies may be important to not be confused with other focal pathology such as brain tumor, stroke or encephalitis. Local MRI findings include restricted diffusion in diffusion weighted images (DWIs), hyperintensity T2 better seen in fluid-attenuated inversion recovery (FLAIR) images, swelling of the focal structure, cerebral hyperperfusion in magnetic resonance perfusion, and increased vascularity in MR angiography.[2] These techniques make it possible to highlight focal lesions and remote lesions responsible for these epileptic seizures. These MRI images are often reversible but can also become permanent and irreversible in severe and prolonged epilepsy (Figure 1).[2] The physiopathologic basis of remote periictal findings is not well understood and several hypotheses remain. Ipsilateral diencephalic and contralateral lateral cerebellar lesions appear after an abnormality in these structures triggered by epileptic activity. Transient splenium lesions may also reflect abnormal activity of the white substance during epileptic activity. The mechanism by which the signal T2 increases and the diffusion decreases is not yet known.[3] Huang et al. studied 15 patients with status epilepticus by comparing imaging lesions with electroencephalogram (EEG) lesions. In their series, the topography of periodic discharge is comparable to MRI images.[2] These MRI abnormalities consist of decreased diffusion in DWI, decreased apparent diffusion coefficient (ADC), and increased signal in T2 usually accompanied by focal cerebral edema and increased vascularization. The cytotoxic edema in T2 signal can be seen in different kinds of central nervous injury: including ischemia, trauma, metabolic insult and status epilepticus.[4] These lesions are usually reversible but can lead to permanent lesions such as cerebral atrophy, laminar necrosis or medial temporal sclerosis.[1] DWI combined with ADC is a very sensitive tool for detecting seizures-related focal. The diffusion is diminished because of the cytotoxic edema, potentialized by the sodium and calcium channels leading to an influx of water and ions.[5] It has been experimentally shown in rats that a decrease in extracellular space associated with an increase in extracellular tortuosity is correlated with a decrease in ADC.[6,7] These lesions are more frequently reversible in epilepsy than other etiologies such as migraine or thrombolysis due to stroke.[8] If a status epilepticus is present, we have a depletion of ATP, energy reserve, pump function and augmentation of permeability resulting in increase of extracellular potassium and accumulation of intracellular calcium together with cellular swelling. The intracellular calcium activate Ca2+ dependent enzyme such as protease or phospholipidase, which can lead to cellular damage. The change of ADC reflects this cellular damage.[9]
Table 1.
Classification of periictal imaging abnormalities
| Local | Remote |
|---|---|
| Mass effect | Posterior leukoencephalopathy |
| Hippocampal swelling | Uni/bilateral diencephalic lesions |
| Focal cortical lesion | Splenium abnormalities |
| Migratory lesion | Cerebellar diaschisis |
| Blood brain barrier breakdown | |
| Increased vessel caliber flow |
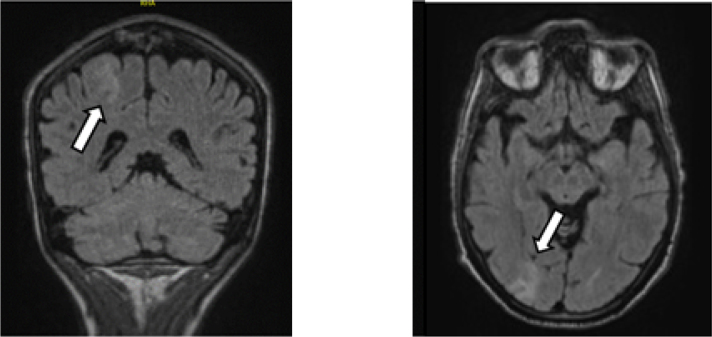
Figure 1.
Two examples of epilepsy focus. Restriction of diffusion at the fronto-parietal and right occipital cortical level with anomaly of signal in the form of cortico-subcortical hypersignals on a FLAIR sequence.
Table 2.
Physiopathological mechanism of cerebral ischemia versus epilepsy
| Cerebral ischemia | Epilepsy |
|---|---|
| ↓ cerebral blood flow | ↑ cerebral blood flow |
| ↓ metabolism glucose | ↑ metabolism glucose |
| ↓ pump activity | ↑oxygen metabolism |
| ↑ permeability and cellular swelling | If blood flow insufficient, anaerobic metabolisms and lactate production. |
| Cell necrosis |
DIFFERENTIAL WITH ACUTE CEREBRAL ISCHEMIA
Local MRI findings can be very similar to those seen in early stroke. Lesions may appear hyperintense on T2 and FLAIR with increased DWI and decreased ADC values. However, the physiopathological mechanisms are completely different. The locations involved in periictal changes include cerebral cortex, sometimes associated with hippocampal and pulvinar lesions,[10] with no arterial distribution. Besides the absence of a typical vascular territory, the perfusion parameters are another important imaging clue for the diagnosis of epilepsy. Perfusion-weighted MRI may reveal hyperperfusion in the epileptogenic area during the acute phase, which is uncharacteristic of stroke.[11] Time-of-flight MR angiography (TOF-MRI) may demonstrate prominent arteries facing the area on the seizure focus. Gyral enhancement occurs earlier than expected for an acute ischemic stroke.[12]
CONCLUSION
EEG is the formal prove of a synchrone abnormal electric activity of a group of neurons. However, seizures and status epilepticus are sometimes diagnosed just on the basis of the clinic because EEG is not available. In these cases, MRI can help the clinicians to put a diagnosis of status epilepticus when there is strong suspicion based on medical examination.
Footnotes
Conflict of Interests
The authors declare to have no competing interests.
REFERENCES
- 1.Trinka E, Kälviäinen R. 25 years of advances in the definition, classification and treatment of status epilepticus. Seizure. 2017;44:65–73. doi: 10.1016/j.seizure.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 2.Huang YC, Weng HH, Tsai YT, Huang YC, Hsiao MC, Wu CY. et al. Periictal magnetic resonance imaging in status epilepticus. Epilepsy Res. 2009;86:72–81. doi: 10.1016/j.eplepsyres.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 3.Cole AJ. Status epilepticus and periictal imaging. Epilepsia. 2004;45(Suppl 4):72–7. doi: 10.1111/j.0013-9580.2004.04014.x. [DOI] [PubMed] [Google Scholar]
- 4.Chan S, Chin SS, Kartha K, Nordli DR, Goodman RR, Pedley TA. Reversible signal abnormalities in the hippocampus and neocortex after prolonged seizures. AJNR Am J Neuroradiol. 1996;17:1725–31. [PMC free article] [PubMed] [Google Scholar]
- 5.Wang Y, Majors A, Najm I, Xue M, Comair Y, Modic M. Postictal alteration of sodium content and apparent diffusion coefficient in epileptic rat brain induced by kainic acid. Epilepsia. 1996;37:1000–6. doi: 10.1111/j.1528-1157.1996.tb00539.x. [DOI] [PubMed] [Google Scholar]
- 6.Dijkhuizen RM, de Graaf RA, Tulleken KA, Nicolay K. Changes in the diffusion of water and intracellular metabolites after excitotoxic injury and global ischemia in neonatal rat brain. J Cereb Blood Flow Metab. 1999;19:341–9. doi: 10.1097/00004647-199903000-00012. [DOI] [PubMed] [Google Scholar]
- 7.van der Toorn A, Syková E, Dijkhuizen RM, Vorísek I, Vargová L, Skobisová E. Dynamic changes in water ADC, energy metabolism, extracellular space volume, and tortuosity in neonatal rat brain during global ischemia. Magn Reson Med. 1996;36:52–60. doi: 10.1002/mrm.1910360110. [DOI] [PubMed] [Google Scholar]
- 8.Grant PE1, He J, Halpern EF, Wu O, Schaefer PW, Schwamm LH. Frequency and clinical context of decreased apparent diffusion coefficient reversal in the human brain. Radiology. 2001;221:43–50. doi: 10.1148/radiol.2211001523. [DOI] [PubMed] [Google Scholar]
- 9.Kim J, Chung JI, Yoon PH, Kim DI, Chung TS, Kim EJ. Transient MR signal changes in patients with generalized tonicoclonic seizure or status epilepticus: periictal diffusion-weighted imaging. AJNR Am J Neuroradiol. 2001;22:1149–60. [PMC free article] [PubMed] [Google Scholar]
- 10.Nakae Y, Kudo Y, Yamamoto R, Dobashi Y, Kawabata Y, Ikeda S. et al. Relationship between cortex and pulvinar abnormalities on diffusion-weighted imaging in status epilepticus. J Neurol. 2016;263:127–32. doi: 10.1007/s00415-015-7948-4. [DOI] [PubMed] [Google Scholar]
- 11.Szabo K, Poepel A, Pohlmann-Eden B, Hirsch J, Back T, Sedlaczek O. et al. Diffusion weighted and perfusion MRI demonstrates parenchymal changes in complex partial status epilepticus. Brain. 2005;128:1369–76. doi: 10.1093/brain/awh454. [DOI] [PubMed] [Google Scholar]
- 12.Doherty CP, Cole AJ, Grant PE, Fischman A, Dooling E, Hoch DB. et al. Multimodal longitudinal imaging of focal status epilepticus. Can J Neurol Sci. 2004;31:276–81. doi: 10.1017/s031716710005397x. [DOI] [PubMed] [Google Scholar]