Abstract
We investigated the relationship between self-reported adherence to pancreatic enzyme replacement therapy (PERT), nutritional status, and all-cause hospitalization in cystic fibrosis (CF) patients with a record of PERT use. Association of self-reported annual PERT use rate (adherence) with annual hospital admission rate (HAR) and annual total hospital nights (THNs) were analyzed for 5301 children (2000–2012) and 13,989 adults (2000–2013) from the CF Foundation Patient Registry. Multivariate linear regression was used to determine the association of HAR and THN with mean annual PERT use rate, cumulative PERT use rate, mean body mass index (BMI) (adult) or BMI percentile (pediatric), age, and sex. The median annual PERT use rate was 87% in children and 80% in adults. Statistically, higher annual PERT use, longer cumulative PERT, and higher BMI percentile (children) or BMI (adults) were significantly (p < 0.0001) associated with lower annual HAR and fewer annual THN in children and adults. Female sex was associated with higher annual HAR and more annual THN in children and adults (p < 0.05). Results indicate self-reported adherence to PERT, increased BMI, and male sex were associated with fewer hospital admissions and annual hospital nights in CF patients.
Keywords: Exocrine pancreatic insufficiency, pancreatic enzyme replacement therapy, treatment adherence, hospitalization, pediatric, adult
Introduction
Cystic fibrosis (CF) is a genetic disease with clinical manifestations that result from involvement of multiple organ systems, most notably the lungs and gastrointestinal tract, which can lead to respiratory failure and exocrine pancreatic insufficiency (EPI), respectively.1 EPI is present in 85% to 90% of patients with CF2; the lack of pancreatic enzymes in these patients leads to maldigestion and malabsorption3,4 contributing to poor nutritional status.5 In children with CF, reduced lung function is associated with frequent hospitalization and poor nutritional status,6,7 whereas nutritional status is positively associated with lung function and survival in pediatric patients and young adults with CF.7,8
Complicated and time-consuming treatment regimens, which may result in low adherence to medications, are a challenge in the management of patients with CF.9 Low adherence to pulmonary medications in patients with CF is associated with more frequent pulmonary exacerbations10 and more CF-related hospitalizations and higher health-care costs,11 all of which may result in reduced health care-related quality of life.12 In addition to pulmonary medications, more than 80% of patients with CF younger than 40 years are prescribed pancreatic enzyme replacement therapy (PERT) to treat EPI.13 Low adherence to PERT is a common cause of ongoing malnutrition despite prescriptions of appropriate doses in patients with CF.14 Malnutrition in CF may require surgical placement of a gastrostomy tube in order to provide enteral nutrition therapy15,16 or for the short-term placement of a peripherally inserted central catheter for total parenteral nutrition.16 The high cost and associated morbidities of these treatments are well documented.15 Managing complications of malabsorption or malnutrition in patients with CF often requires hospitalization.
The relationship between reported adherence to PERT and health-care utilization in children and adults with CF is not well-studied. Because of the importance of medication adherence to clinical outcomes, nutritional status, hospitalization, and quality of life, our objective was to investigate the relationship of PERT use rates and annual all-cause hospital admission rates (HARs), annual total hospital nights (THNs), and body mass index (BMI) in children and adults with EPI due to CF. We also evaluated the influence of nutritional status on hospitalizations in patients with CF. We used data from the CF Foundation (CFF) Patient Registry, which was established in 1966 and contained information on 28,676 patients with CF as of 2014.13 The registry is used to track trends in patient care and outcomes, facilitate the design of clinical trials, conduct epidemiologic research, and execute post-marketing studies.
Methods
Data source
We performed a retrospective analysis of the CFF Patient Registry using data from 2000 to 2012 for pediatric patients and from 2000 to 2013 for adults.17,18 To be included in the analysis, pediatric patients had to have been born in or after 2000, have been diagnosed with CF between 2000 and 2007, and have received PERT during at least their final year of enrollment in the CFF Patient Registry. Adult patients were ≥20 years of age and had to have at least one dose of PERT reported during at least their final year of enrollment in the CFF Patient Registry. Pediatric and adult patients were excluded from analysis if their only registry record was in the year of their death; patients with anomalous data (i.e. THN >365 days in a year or impossible timing of diagnosis or referral (diagnosis of CF in a year before birth year, review before diagnosis, review before birth)) were also excluded. By design, in order to focus specifically upon the separate pediatric and adult populations, no analyses were conducted in adolescent patients 14 to 19 years of age. Pancreatic status (sufficient vs. insufficient) is not captured in the registry.
Assessments
The self-reported annual PERT use rate (adherence) was defined as the percentage of CF center outpatient visits per year at which PERT use was recorded as being used for each patient. Annual HAR was the number of inpatient care episodes reported per year in the CF registry for each patient. If there was no hospitalization record for a particular review year, the assumption was that the days of hospitalization for that year were zero. The annual THN was calculated as the sum of the total hospital nights reported for each patient encounter in a given year for each patient.
Statistical analysis
The data were evaluated using two general approaches: cumulative longitudinal patient-level data and cross-sectional data at discrete time points. A cumulative longitudinal analysis was used to evaluate and summarize patient level data over the entire analysis period. A cross-sectional analysis was used to evaluate pediatric patient-level data at discrete time points, specifically age in years. The mean annual PERT use rate, cumulative PERT use rate, annual HAR, and annual THN were calculated for each patient. Multivariate linear regression, correlation analysis, and graphical methods were used to determine the association of annual HAR and THN with the mean annual PERT use rate, cumulative PERT use rate, mean BMI (in adults) or BMI percentile (BMI%, which is more appropriate for children19), age, and sex.
Results
Patients and PERT use
After excluding patients with anomalous data, 5301 children with CF met the inclusion criteria and were included in the analysis (Figure 1). For adults, 16,994 adults were identified after excluding patients with anomalous data, as above, and those who died during their final year of enrollment in the CFF Patient Registry (Figure 1). Of these adults, 13,989 used at least 1 dose of PERT in their final year of enrollment in the CFF Patient Registry and were included in the analysis. The mean follow-up was 6.9 years for children and 5.3 years for adults (Table 1). Averaged over all review years, the median annual PERT use was 87% (range, 5–100%; Table 1) in children and 80% (range, 0.02–100%) in adults.
Figure 1.
Disposition of CFF registry patients with cystic fibrosis. CFF: Cystic Fibrosis Foundation..
Table 1.
Characteristics of the analysis populations.
| Characteristic | Pediatric patients (N = 5301) | Adult patients (N = 13,989) |
|---|---|---|
| Male, n (%) | 2610 (49.2) | 7667 (54.8) |
| Follow-up, years | ||
| Mean ± SD | 6.9 ± 1.6 | 5.3 ± 2.9 |
| Median (range) | 8 (1–8) | 5 (1–9) |
| Age at CF diagnosis, years | ||
| Mean ± SD | 1.6 ± 1.2 | 5.6 ± 9.6 |
| Median (range) | 1 (1–8) | 2 (1–82) |
| Over all review years | ||
| BMI,a kg/m2 | ||
| Mean ± SD | — | 21.8 ± 3.5 |
| Median (range) | — | 21.3 (12.4–44.8) |
| BMI%b | ||
| Mean ± SD | 52.3 ± 21.7 | |
| Median (range) | 52.7 (0–100) | |
| Annual PERT use rate, % | ||
| Mean ± SD | 83 ± 15 | 77 ± 20 |
| Median (range) | 87 (5–100) | 80 (0.02–100) |
| Cumulative PERT use, yearsc | ||
| Mean ± SD | 5.7 ± 1.6 | 4.0 ± 2.4 |
| Median (range) | 5.9 (0.05–8) | 3.8 (0.02–9.0) |
| Cumulative number of years of PERT used | ||
| Mean ± SD | 6.8 ± 1.7 | 5.2 ± 2.9 |
| Median (range) | 8 (1–8) | 5.0 (1.0–9.0) |
BMI: body mass index; BMI%: BMI percentile; CF: cystic fibrosis; PERT: pancreatic enzyme replacement therapy.
a N = 13,739 (adult patients).
b N = 5299 (pediatric patients).
c Reflects PERT use prorated within each year of follow-up to account for years with partial use (e.g. due to treatment interruptions or missing data).
d Reflects overall duration of PERT use regardless of partial use during follow-up years. Excludes follow-up years before a patient turned 21 years of age.
Hospitalization data
Cumulative longitudinal analysis
Cumulative longitudinal analysis indicated that the median annual HAR was 0.3 admissions per patient (range, 0–9.2; Table 2) for all children and 0.78 admissions per patient (range, 0–13.5) for all adults. The median annual THN was 1.5 nights per patient (range, 0–203 nights; Table 2) for children and 3.78 nights per patient (range, 0–245.5 nights) for adults. For children, the three most common reasons for hospital admission were pulmonary exacerbation (69%), gastrointestinal complication (9%), and pulmonary complication (3%). Approximately 80% of hospital admissions for adult patients were because of pulmonary exacerbation, followed by gastrointestinal complication and pulmonary complication.
Table 2.
HAR and total hospital nights per year.
| Pediatric patients (N = 5301) | Adult patients (N = 13,989) | |||
|---|---|---|---|---|
| Mean ± SD | Median (range) | Mean ± SD | Median (range) | |
| Annual HAR per patient | 0.5 ± 0.7 | 0.3 (0–9.2) | 1.2 ± 1.4 | 0.78 (0–13.5) |
| Annual THN per patient | 4.4 ± 8.8 | 1.5 (0–203) | 11.3 ± 19.2 | 3.78 (0–245.5) |
HAR: hospital admission rate; THN: total hospital nights.
Multivariate linear regression analysis
Multivariate analysis of patient-level data indicated that higher annual PERT use rate and longer cumulative PERT use rate were significantly (p < 0.0001) associated with fewer annual hospitalizations and hospital nights in children and adults (Table 3). Controlling for the other covariates (including cumulative PERT use, mean BMI% or BMI, and sex), each 10% absolute increase in mean annual PERT use rate in children was associated with a decrease of 0.11 admissions in the mean annual HAR and a decrease of 1.0 nights in the mean annual THN. In children, for each 1-year increase in the cumulative use of PERT, mean annual HAR decreased by 0.02 admissions and mean annual THN decreased by 0.56 nights. In adults, each 10% increase in the mean annual PERT use rate was associated with a decrease of 0.18 admissions in the mean annual HAR and a decrease of 2.4 nights in the mean annual THN. For each 1-year increase in the cumulative use of PERT in adults, there was a decrease in the mean annual HAR of 0.08 admissions and 1.2 fewer nights in mean annual THN.
Table 3.
Multivariate linear regression analysis of associations between HAR and total hospital nights per year with PERT use, BMI, and sex.a
| Factor | Pediatric patients Regression coefficient ± SE |
Adult patients Regression coefficient ± SE |
||
|---|---|---|---|---|
| HAR | THN | HAR | THN | |
| Mean annual PERT use rateb | −0.11 ± 0.007e | −1.00 ± 0.09e | −0.18 ± 0.005e | −2.37 ± 0.08e |
| Cumulative use of PERTc | −0.02 ± 0.01e | −0.56 ± 0.08e | −0.08 ± 0.005e | −1.18 ± 0.07e |
| Mean BMId | −0.03 ± 0.004e | −0.45 ± 0.05e | −0.09 ± 0.003e | −1.12 ± 0.04e |
| Female versus male sex | 0.04 ± 0.02f | 0.47 ± 0.23f | 0.13 ± 0.02e | 0.74 ± 0.30f |
BMI: body mass index; BMI%: BMI percentile; HAR: hospital admission rate; PERT: pancreatic enzyme replacement therapy; THN: total hospital nights.
a Because cumulative PERT use has a linear relationship with age, this model does not include age.
b A single unit change was defined as 10%.
c A single unit change was defined as 1 year.
d Mean BMI% was used for pediatric patients. A single unit change was defined as 10 percentile for pediatric patients or 1 kg/m2 for adult patients.
e p < 0.0001.
f p < 0.05.
Higher BMI% (children) or BMI (adults) was significantly (p < 0.0001) associated with fewer annual hospitalizations and hospital nights in children and adults (Table 3). Controlling for the other covariates, an increase of 10% in mean BMI% in children was associated with a reduction of 0.03 admissions in mean annual HAR and 0.45 nights in the mean annual THN. An increase of 1 kg/m2 in mean BMI in adults was associated with a reduction of 0.09 admissions in annual HAR and 1.1 nights in the mean annual THN. Female sex was significantly associated with more frequent and longer annual hospitalizations in children and adults. Compared with boys, girls had an increase in the mean annual HAR of 0.04 admissions and 0.47 more nights in mean annual THN. Compared with men, women had an increase in the mean annual HAR of 0.13 admissions and 0.74 more nights in mean annual THN.
Cross-sectional analysis by age
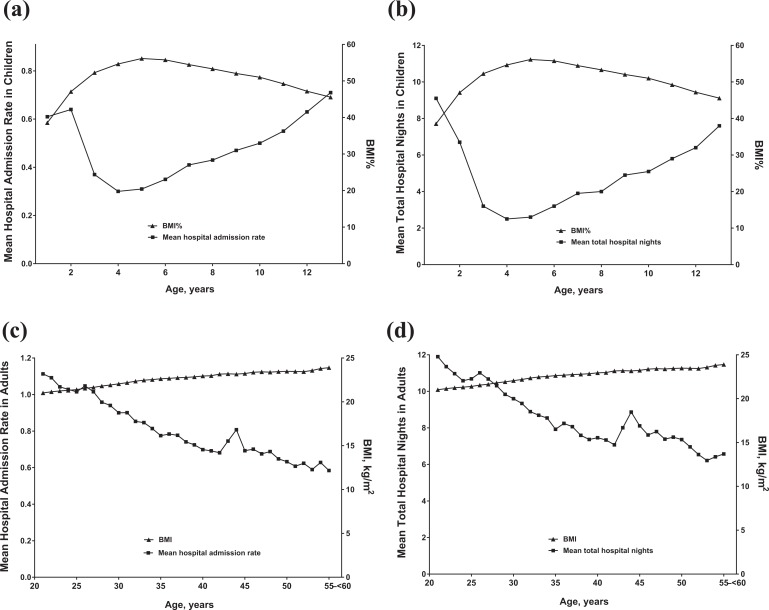
In children, cross-sectional analysis by age revealed that BMI% was greatest at 5 years of age; the lowest HAR and THN occurred at 4 years of age (Figure 2(a) and (b)). In adults, BMI increased steadily with age, whereas mean HAR and mean annual THN decreased with age; however, there was a peak between ages 40 and 45 years in HAR and annual THN (Figure 2(c) and (d)). Boys and girls had differential annual HAR and THN starting at 8 and 9 years of age, respectively (p < 0.05; Figure 3), controlling for PERT use rates (mean, 83% in boys and girls; p = 0.7 for comparison).
Figure 2.
HAR (a) or total hospital nights (b) per year versus BMI percentile in pediatric patients by age or HAR (c) or total hospital nights (d) per year versus BMI in adult patients. BMI: body mass index; BMI%: BMI percentile; HAR: hospital admission rate.
Figure 3.
HAR per year (a) and total hospital nights per year (b) in boys and girls by age. *p < 0.05, boys versus girls. HAR: hospital admission rate.
Discussion
The results of this analysis of 19,290 children and adults with CF from the CFF Patient Registry indicate that among patients using PERT, self-reported adherence to PERT was associated with a reduction in annual hospital admissions and number of annual hospital nights. In addition, increasing BMI or BMI% was associated with a reduction in hospitalization and number of hospital nights. Notably, women and girls had higher annual HARs and more hospital nights than did men and boys; this sex-related difference was evident by approximately 8 to 9 years of age.
Treatment of patients with CF is complex; it involves numerous medications and is time-consuming for patients.20 Because of these and other factors,9 overall treatment adherence to CF medications in general is poor; adherence to PERT has been reported to range from 27% to 49%.21,22 Poor treatment adherence to CF medications is associated with increased pulmonary exacerbations, hospitalizations, and health-care costs.10,11 The cost of hospitalizations as a proportion of total CF-related health-care spending varies widely (e.g. ranging from 12% to 77%) according to country and patient characteristics.23,24 In the United States, an analysis of the 2007 Nationwide Inpatient Sample database estimated that total hospital charges for adults (18–44 years) with CF averaged US$ 53,095 to US$ 59,627, depending on the type of hospital.25 Thus, reducing CF-related hospitalizations might be expected to reduce overall CF-related health-care costs.
In patients with CF, good nutritional status is associated with improved lung function,7,26 survival,8 and increased health-related quality of life.27 In this retrospective analysis, increased BMI or BMI%, which was used as a surrogate for nutritional status, was associated with a reduction in hospitalization admissions and number of hospital nights. In children, there was an inverse curvilinear relationship between BMI% and hospitalization. In adults, BMI continued to increase linearly over time, possibly as the result of the long-term survival of those patients who had better nutritional status; a potential contributing factor that was not measured is that these patients may have had milder genotypes and subsequently less pancreatic insufficiency. These results are consistent with a number of studies showing that nutritional status, especially in early childhood, has a major impact on pulmonary function and mortality. For example, in children with CF, better nutritional indices at 3 years of age were associated with better lung function at 6 years of age compared with poorer nutritional indices.28 In an analysis of the 2007 European Cystic Fibrosis Society Patient Registry database, BMI was positively associated with lung function in both children and adults; patients with a low BMI had a sixfold greater risk, compared with patients with normal BMI, of having a forced expiratory volume in 1 second measurement that was <40% of the predicted value.29 Finally, in children with CF, weight-for-age percentile >50% at 4 years of age was associated with better lung function, fewer CF-related complications, fewer annual hospital nights, and lower mortality at 18 years of age than children with a weight-for-age percentile <50% at 4 years of age.8
In our analysis, girls had a higher annual hospitalization rate and more total hospital nights per year than boys, starting at 8 and 9 years of age, respectively. Moreover, adult female patients had a slightly but significantly increased annual hospitalizations and more hospital nights per year compared with adult male patients. These results are consistent with an analysis using the Canadian CF Patient Registry that showed greater rates of hospitalization in female patients than in male patients aged 7 to 19 years and ≥20 years.30 The reasons for these sex-related differences are unclear. Previous studies have reported that compared with boys with CF, adolescent girls with CF have a lower adherence to some aspects of CF management, including coughing as an airway clearance technique, consumption of high-calorie, high-fat foods, and taking medications.31,32 Differences in caloric intake may be related to perceived body image and self-esteem, with female patients with CF preferring a lower BMI than male patients.33,34 Girls and women also reported more CF-related emotional and physical strains than boys and men.32,33
This study has several limitations. It was an observational retrospective study of open-label treatment, without randomization or stratification, and was thus subject to various kinds of bias and confounding factors. Although occasional outliers from the typical demographic profile of patients with CF were observed (e.g. age of 82 years or BMI of 44.8 kg/m2), the large analysis sample sizes prevented skewing of the overall results. The need for exclusion of anomalous data such as more total annual hospital nights than days in a year highlights the fact that data from registries, though invaluable, require curation to identify potential reporting errors. In this study, use of PERT was interpreted as indicating pancreatic insufficiency. Because the CFF registry does not capture pancreas status, it is possible some patients may have received PERT despite having sufficient pancreatic function due to more mild genotypes. In addition, self-reported PERT use rates (extrapolated from the percentage of CF center outpatient visits per year with PERT use recorded for each patient) may not accurately reflect actual daily PERT utilization and may be subject to data entry error or center-to-center variations in data collection and reporting. Adherence may have been influenced by the number of health-care provider visits, whereby more visits equal more data points, as well as by the “white coat phenomenon” of increased adherence as visits approach or with more frequent HCP visits. Moreover, among patients using PERT, higher self-reported PERT adherence may have been associated with better adherence to other medications and lifestyle modifications/management techniques, which could have contributed to reduced hospitalization. Of note, adherence to pulmonary medications was not measured in this analysis. Therefore, it could not be determined what impact adherence to pulmonary medications may have had on hospitalizations. By intention, no analyses were conducted for adolescent patients aged 14 to 19 years (n = 12,074). However, a recent study reported that adherence rates to pulmonary medications in CF were greatest in patients aged 6 to 10 years (59%) and decreased to approximately 40% to 50% in older children and adults.11 Thus, in patients with CF, if PERT adherence is similar to adherence to pulmonary medications, we would not expect the results in patients aged 14 to 19 years to be substantially different from those presented for the age-groups in this report. Finally, this analysis identified both PERT and BMI or BMI% as statistically significant factors in reduced hospitalizations. PERT is prescribed specifically to address malnutrition caused by pancreatic insufficiency and to improve nutritional status (which would be reflected in BMI/BMI%); therefore, confirmatory analysis of the relationship between self-reported PERT adherence and BMI/BMI% could provide valuable information regarding the overall effect of PERT as a contributing factor in reduced hospitalizations related to improved nutritional status.
The results of this analysis among patients using PERT show the importance of adherence to PERT and of nutritional status in reducing the risk of hospitalization in children and adults with CF. Although there are certainly other contributing factors, self-reported adherence to PERT and increased BMI were associated with a reduction in hospitalization rate and annual number of hospital nights, thus reinforcing the close association between nutritional status and CF-related outcomes that needs to be communicated to patients with CF. Strategies to improve PERT adherence may provide benefits related to hospitalization in patients with CF.
Acknowledgments
The authors would like to thank the CFF for the use of CFF Patient Registry data to conduct this study. Additionally, the authors would like to thank the patients, care providers, and clinic coordinators at CF Centers throughout the United States for their contributions to the CFF Patient Registry.
Footnotes
Author contributions: The authors and AbbVie scientists designed the study and analyzed and interpreted the data. All authors contributed to the development of the content; all authors and AbbVie reviewed and approved the manuscript; and the authors maintained control over the final content. Medical writing support was provided by Richard M Edwards, PhD, and Michael J Theisen, PhD, of Complete Publication Solutions, LLC, North Wales, PA, USA.
Declaration of conflicting interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Bruce Trapnell has no financial conflicts to disclose. Su Chen, Amit Bodhani, and Mudra Kapoor are employees of AbbVie and may own AbbVie stocks or options. Mark Haupt and Rupal Khurmi were employees of AbbVie at the time of the analysis and manuscript preparation and may own AbbVie stocks or options.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by AbbVie.
References
- 1. Cutting GR. Cystic fibrosis genetics: from molecular understanding to clinical application. Nat Rev Genet 2015; 16(1): 45–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ooi CY, Durie PR. Cystic fibrosis transmembrane conductance regulator (CFTR) gene mutations in pancreatitis. J Cyst Fibros 2012; 11(5): 355–362. [DOI] [PubMed] [Google Scholar]
- 3. Li L, Somerset S. Digestive system dysfunction in cystic fibrosis: challenges for nutrition therapy. Dig Liver Dis 2014; 46(10): 865–874. [DOI] [PubMed] [Google Scholar]
- 4. Dominguez-Munoz JE. Pancreatic exocrine insufficiency: diagnosis and treatment. J Gastroenterol Hepatol 2011; 26(suppl 2): 12–16. [DOI] [PubMed] [Google Scholar]
- 5. Kalnins D, Wilschanski M. Maintenance of nutritional status in patients with cystic fibrosis: new and emerging therapies. Drug Des Devel Ther 2012; 6: 151–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Com G, Carroll JL, Castro MM, et al. Predictors and outcome of low initial forced expiratory volume in 1 second measurement in children with cystic fibrosis. J Pediatr 2014; 164(4): 832–838. [DOI] [PubMed] [Google Scholar]
- 7. Steinkamp G, Wiedemann B. Relationship between nutritional status and lung function in cystic fibrosis: cross sectional and longitudinal analyses from the German CF quality assurance (CFQA) project. Thorax 2002; 57(7): 596–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yen EH, Quinton H, Borowitz D. Better nutritional status in early childhood is associated with improved clinical outcomes and survival in patients with cystic fibrosis. J Pediatr 2013; 162(3): 530–535. [DOI] [PubMed] [Google Scholar]
- 9. Jennings MT, Riekert KA, Boyle MP. Update on key emerging challenges in cystic fibrosis. Med Princ Pract 2014; 23(5): 393–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Eakin MN, Bilderback A, Boyle MP, et al. Longitudinal association between medication adherence and lung health in people with cystic fibrosis. J Cyst Fibros 2011; 10(4): 258–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Quittner AL, Zhang J, Marynchenko M, et al. Pulmonary medication adherence and health-care use in cystic fibrosis. Chest 2014; 146(1): 142–151. [DOI] [PubMed] [Google Scholar]
- 12. Bradley JM, Blume SW, Balp MM, et al. Quality of life and healthcare utilisation in cystic fibrosis: a multicentre study. Eur Respir J 2013; 41(3): 571–577. [DOI] [PubMed] [Google Scholar]
- 13. Cystic Fibrosis Foundation Patient Registry. 2014. Annual Data Report, https://www.cff.org/2014-Annual-Data-Report.pdf (accessed 19 August 2016).
- 14. Littlewood JM, Wolfe SP, Conway SP. Diagnosis and treatment of intestinal malabsorption in cystic fibrosis. Pediatr Pulmonol 2006; 41(1): 35–49. [DOI] [PubMed] [Google Scholar]
- 15. Schwarzenberg SJ, Hempstead SE, McDonald CM, et al. Enteral tube feeding for individuals with cystic fibrosis: cystic fibrosis foundation evidence-informed guidelines. J Cyst Fibros 2016; 15(6): 724–735. [DOI] [PubMed] [Google Scholar]
- 16. Turck D, Braegger CP, Colombo C, et al. ESPEN-ESPGHAN-ECFS guidelines on nutrition care for infants, children, and adults with cystic fibrosis. Clin Nutr 2016; 35(3): 557–577. [DOI] [PubMed] [Google Scholar]
- 17. Trapnell B, Jerkins T, Haupt M, et al. Relationship between pancreatic enzyme replacement therapy and healthcare use in children with cystic fibrosis. Presented at the North American Cystic Fibrosis Conference, 9–11 October 2014, Atlanta, GA. [Google Scholar]
- 18. Trapnell B, Jerkins T, Chen S, et al. Adherence with pancreatic enzyme replacement therapy is associated with reduced hospitalization in adults with cystic fibrosis. Presented at the North American Cystic Fibrosis Conference, 8–10 October 2015, Phoenix, AZ. [Google Scholar]
- 19. Ogden CL, Flegal KM. Changes in terminology for childhood overweight and obesity. Natl Health Stat Report 2010; 25: 1–5. [PubMed] [Google Scholar]
- 20. Sawicki GS, Sellers DE, Robinson WM. High treatment burden in adults with cystic fibrosis: challenges to disease self-management. J Cyst Fibros 2009; 8(2): 91–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Barker DH, Quittner AL. Parental depression and pancreatic enzymes adherence in children with cystic fibrosis. Pediatrics 2016; 137(2): 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Modi AC, Lim CS, Yu N, et al. A multi-method assessment of treatment adherence for children with cystic fibrosis. J Cyst Fibros 2006; 5(3): 177–185. [DOI] [PubMed] [Google Scholar]
- 23. Colombo C, Dacco V, Alicandro G, et al. Cost of cystic fibrosis: analysis of treatment costs in a specialized center in northern Italy. Adv Ther 2013; 30(2): 165–175. [DOI] [PubMed] [Google Scholar]
- 24. van Gool K, Norman R, Delatycki MB, et al. Understanding the costs of care for cystic fibrosis: an analysis by age and health state. Value Health 2013; 16(2): 345–355. [DOI] [PubMed] [Google Scholar]
- 25. Kopp BT, Wang W, Chisolm DJ, et al. Inpatient healthcare trends among adult cystic fibrosis patients in the US. Pediatr Pulmonol 2012; 47(3): 245–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Peterson ML, Jacobs DR, Jr, Milla CE. Longitudinal changes in growth parameters are correlated with changes in pulmonary function in children with cystic fibrosis. Pediatrics 2003; 112(3 Pt 1): 588–592. [DOI] [PubMed] [Google Scholar]
- 27. Shoff SM, Tluczek A, Laxova A, et al. Nutritional status is associated with health-related quality of life in children with cystic fibrosis aged 9-19 years. J Cyst Fibros 2013; 12(6): 746–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Konstan MW, Butler SM, Wohl ME, et al. Growth and nutritional indexes in early life predict pulmonary function in cystic fibrosis. J Pediatr 2003; 142(6): 624–630. [DOI] [PubMed] [Google Scholar]
- 29. Kerem E, Viviani L, Zolin A, et al. Factors associated with FEV1 decline in cystic fibrosis: analysis of the ECFS patient registry. Eur Respir J 2014; 43(1): 125–133. [DOI] [PubMed] [Google Scholar]
- 30. Stephenson A, Hux J, Tullis E, et al. Higher risk of hospitalization among females with cystic fibrosis. J Cyst Fibros 2011; 10(2): 93–99. [DOI] [PubMed] [Google Scholar]
- 31. DeLambo KE, Ievers-Landis CE, Drotar D, et al. Association of observed family relationship quality and problem-solving skills with treatment adherence in older children and adolescents with cystic fibrosis. J Pediatr Psychol 2004; 29(5): 343–353. [DOI] [PubMed] [Google Scholar]
- 32. Patterson JM, Wall M, Berge J, et al. Gender differences in treatment adherence among youth with cystic fibrosis: development of a new questionnaire. J Cyst Fibros 2008; 7(2): 154–164. [DOI] [PubMed] [Google Scholar]
- 33. Abbott J, Morton AM, Musson H, et al. Nutritional status, perceived body image and eating behaviours in adults with cystic fibrosis. Clin Nutr 2007; 26(1): 91–99. [DOI] [PubMed] [Google Scholar]
- 34. Simon SL, Duncan CL, Horky SC, et al. Body satisfaction, nutritional adherence, and quality of life in youth with cystic fibrosis. Pediatr Pulmonol 2011; 46(11): 1085–1092. [DOI] [PubMed] [Google Scholar]