Short abstract
The trigeminal nerve (V) is the fifth and largest of all cranial nerves, and it is responsible for detecting sensory stimuli that arise from the craniofacial area. The nerve is divided into three branches: ophthalmic (V1), maxillary (V2), and mandibular (V3); their cell bodies are located in the trigeminal ganglia and they make connections with second-order neurons in the trigeminal brainstem sensory nuclear complex. Ascending projections via the trigeminothalamic tract transmit information to the thalamus and other brain regions responsible for interpreting sensory information. One of the most common forms of craniofacial pain is trigeminal neuralgia. Trigeminal neuralgia is characterized by sudden, brief, and excruciating facial pain attacks in one or more of the V branches, leading to a severe reduction in the quality of life of affected patients. Trigeminal neuralgia etiology can be classified into idiopathic, classic, and secondary. Classic trigeminal neuralgia is associated with neurovascular compression in the trigeminal root entry zone, which can lead to demyelination and a dysregulation of voltage-gated sodium channel expression in the membrane. These alterations may be responsible for pain attacks in trigeminal neuralgia patients. The antiepileptic drugs carbamazepine and oxcarbazepine are the first-line pharmacological treatment for trigeminal neuralgia. Their mechanism of action is a modulation of voltage-gated sodium channels, leading to a decrease in neuronal activity. Although carbamazepine and oxcarbazepine are the first-line treatment, other drugs may be useful for pain control in trigeminal neuralgia. Among them, the anticonvulsants gabapentin, pregabalin, lamotrigine and phenytoin, baclofen, and botulinum toxin type A can be coadministered with carbamazepine or oxcarbazepine for a synergistic approach. New pharmacological alternatives are being explored such as the active metabolite of oxcarbazepine, eslicarbazepine, and the new Nav1.7 blocker vixotrigine. The pharmacological profiles of these drugs are addressed in this review.
Keywords: Facial pain, carbamazepine, oxcarbazepine, sodium channel, Nav1.3, Kv7.2
Basic organization of the trigeminal system
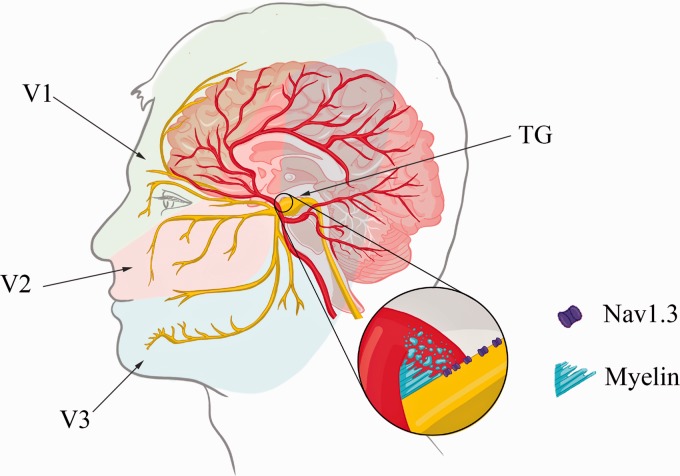
Sensory information from the craniofacial region is conveyed by the trigeminal sensory system, which is composed of peripheral structures, such as the trigeminal nerve (V) and trigeminal ganglia (TG), and central structures, such as the trigeminal brainstem sensory nuclear complex (VBSNC).1 The trigeminal nerve is divided into three branches: ophthalmic (V1), maxillary (V2), and mandibular (V3) (Figure 1). The superior region of the head, that is, meninges and cornea are innervated mainly by the ophthalmic branch. The upper lip, maxillary teeth, and mucosa are innervated by the maxillary branch, while the mandibular branch innervates mainly the mandibula, lower lip, mucosa, and mandibular teeth. The V1 and V2 branches are purely sensory, whereas V3 has motor fibers which are responsible for innervation of the jaw muscles.1 The fibers that form the trigeminal nerve are classified into nociceptive fibers (Aδ and C fibers) and low-threshold mechanoreceptors (LTMs; Aα and Aβ fibers).
Figure 1.
Representation of the trigeminal system and classical trigeminal neuralgia etiology. The area of innervation for the ophthalmic (V1), maxillary (V2), and mandibular (V3) branches is indicated. Neurovascular compression by the superior cerebellar arteries observed at the root entry zone of the trigeminal nerve is highlighted. This compression leads to demyelination and an upregulation of the voltage-gated sodium channels Nav1.3, as demonstrated in the magnification. TG: trigeminal ganglia.
Within the nociceptive fibers, the C fibers are nonmyelinated with a slow conductance velocity and small diameter, while the Aδ fibers are myelinated and have an intermediate diameter and conductance velocity, and both fibers can be activated by noxious triggers, such as mechanical, thermal, and chemical stimuli. The proprioceptive Aα and Aβ fibers are myelinated and display fast conductance and have a larger diameter, and they are responsible for innocuous and proprioceptive stimuli.2 In the trigeminal nerve, the proportion of unmyelinated/myelinated fibers is much lower compared to spinal nerves.3,4 The cell bodies of those fibers are localized in the TG, while the cell bodies from the proprioceptive fibers (Aα) are localized in the mesencephalic trigeminal nucleus. They make connections with second-order neurons in the VBSNC.
The primary function of the trigeminal nucleus is to carry temperature, touch, and pain inputs from the ipsilateral side of the face to the contralateral thalamus via the ventral trigeminothalamic tract.5 The VBSNC is divided into the main/principal sensory nucleus and spinal tract nucleus; the latter being composed by the subnucleus oralis (Sp5O), subnucleus interpolaris (Sp5I), and subnucleus caudalis (Sp5C). The subnucleus caudalis is also denominated as the medullary dorsal horn since it has a laminated structure and C- and Aδ fibers project to laminae I, II, V, and VI, analogous to what occurs in the spinal dorsal horn.4,6–8 It receives major inputs from nociceptive afferents in addition to inputs from other cranial nerves, such as the facial, glossopharyngeal, and vagus nerves (for review, see Sessle3). Beside this similarity between the VBSNC and the spinal dorsal horn, there are some differences, such as the transition zone Sp5I/Sp5C which is involved in the processing of nociceptive stimuli from facial deep tissues, but not in nociceptive stimuli arising from the skin.9,10 Moreover, a group of nociceptive fibers activated from the orofacial region can also be observed within Sp5O.11 Although both structures receive nociceptive inputs, there are some well-described differences, such as the presence and absence of a group of small interneurons (substantia gelatinosa) within the Sp5C and Sp5O, respectively.11 Moreover, intrinsic fibers in the VBSNC representing the collateral incoming primary afferents can make connections between the Sp5O and Sp5C (for review, see Sessle3 and Woda11). The output from these nuclei (i.e., second-order neurons) can be classified as nociceptive specific (NS), wide dynamic range (WDR), and LTMs.12,13 The NS neurons are exclusively activated by noxious stimuli, while WDR neurons, due to their wide range of recognition, are responsive to innocuous and noxious stimuli.14 The second-order neurons redirect the sensory information to different regions of the thalamus where sensory stimuli are processed. The thalamus sends third-order neuronal projections to the primary and secondary somatosensory cortex and insula—regions responsible for interpreting sensory information in terms of location, intensity, and duration. In addition, outputs from the thalamus can be directed to other cortical and limbic structures that are responsible for processing the cognitive, affective, and emotional components of pain.1,12,13
In addition, the activation of mesencephalic and bulbar structures can modulate nociceptive processing. The main inhibitory descending pathway includes structures such as the periaqueductal gray matter (GM) and the rostral ventromedial medulla (RVM), which projects to the VBSNC where the nociceptive responses are modulated.15–17There is growing evidence of differences between the RVM projection to the VBSNC and to the spinal dorsal horn.18 In patients with trigeminal neuropathic pain, an increase in connectivity between the RVM and the Sp5C was reported, in addition to increased connectivity to other brain regions involved in the descending pathways, such as the anterior cingulate cortex (ACC).19 Additionally, it has been demonstrated that there is a functional connection between the Sp5I/Sp5C zone and the RVM, and the result of a lesion of either region is attenuation of facial hyperalgesia.20 Furthermore, it was shown that corticotrigeminal pathways can regulate facial pain perception.21,22 Projections from the somatosensory cortices (SI and SII) to Sp5C target the primary nociceptive afferents from the facial region.23–25 Corticotrigeminal inhibitory effects can also be achieved through presynaptic and postsynaptic mechanisms.26 Indeed, Castro et al.27 demonstrated that corticotrigeminal stimulation can produce analgesia via feed-forward inhibition in the Sp5C.27
The prevalence of pain syndromes that affect the territories innervated by the trigeminal nerve, such as migraines and headaches, is one of the highest and ranks second only to low back pain.
Trigeminal neuralgia: Definition and classification
Trigeminal neuralgia (TN) is the most common form of craniofacial neuropathic pain and is considered the cause of one of the most severe types of pain that a person can experience. The incidence is estimated at 4 to 13 people per 100,000/year.28–31 The International Association for the Study of Pain describes TN as “a sudden usually unilateral severe brief stabbing recurrent episodes of pain in the distribution of one or more branches of the trigeminal nerve.”32 Pain is usually described as stabbing, paroxysmal, reminiscent of electric shock, or burning and is limited to the area innervated by one or more branches of the trigeminal nerve. In approximately 60% of the cases, there is an involvement of only one branch, the maxillary or mandibular branch, whereas in approximately 35% of the cases, both are involved. On the other hand, the ophthalmic branch is rarely affected (i.e., in fewer than 4% of patients).33 Aging is a risk factor for the development of trigeminal pain, commonly occurring in patients over 50 years old.34 The incidence in woman is higher, with a female–male ratio of approximately 2–3:1.31,35 Pain attacks usually occur by stimulating trigger points, usually located in the territory innervated by the trigeminal nerve. Examples of stimuli that trigger attacks of pain include a slight touch of the face, tooth brushing, and activation of the masticatory and facial muscles during speech and feeding. Each episode of pain is followed by a refractory period that can last from a few seconds to several minutes. When attacks of pain become very frequent, patients become unable to perform their daily activities, and even avoid eating and communicating for fear of triggering a new crisis. This, in turn, can lead to a severe impairment of life quality and mental health in these patients.36,37
The etiology of TN and the underlying mechanisms of this condition are still poorly understood and based on the etiology, TN is classified into idiopathic TN, classic TN, and secondary TN. The first is characterized by unknown causes, and in approximately 10% of patients, even after surgical procedures or magnetic resonance imaging, the disease remains without a diagnosed cause.38 Classic TN is associated with neurovascular compression (NVC) in the trigeminal root entry zone, which causes nerve root atrophy or displacement.38–40 Secondary TN may be caused by an underlying disease such as tumors or artery malformations and has been associated with multiple sclerosis (multiple sclerosis patients show a 20-fold high prevalence of TN).41,42 Classical TN has distinct features regarding both pathophysiology and therapeutic approaches, which will be covered in the ensuing section.
Pathophysiology of classical TN
According to the International Classification of Headache Disorder-3 Classical TN is caused by NVC, most frequently by the superior cerebellar artery of the trigeminal nerve roots into the pons.31,34,40 This compression usually results in the demyelination of nerve fibers, which then start firing ectopically (Figure 1).40,43,44 The NVC hypothesis is supported by evidence that after surgical procedures that lead to microvascular decompression, the majority of patients achieve sustained pain relief.45–47 Notwithstanding this evidence, NVC can also be observed in asymptomatic patients.48,49 Several alterations have been described as a result of the vascular compression, including focal demyelination at the entry zone of the trigeminal nerve, atrophy or hypertrophy of peripheral axons, and damage to Schwann cells as well as to peripheral myelin.50–53 The “ignition hypothesis,” proposed by Devor et al., 54 aims to correlate the structural changes with paroxysmal pain attacks, which are characteristic of the condition. It states that after trigeminal root damage, partially damaged neurons trigger a stimulus induced burst of activity, making them hyperexcitable and susceptible to cross excitation as a result from the physical proximity of the neurons to the root compression site.55 Therefore, the drastic increase in posttrigger neuronal activity recruits additional neighboring neurons leading to a rapid accumulation of electrical activity, which can be amplified by ephaptic interaction among neurons, since the myelin sheath is damaged and nerve fibers maintain close contact among them.
There is accumulating evidence that voltage-gated sodium channels (VGSCs) play a crucial role in the generation of ectopic activity in trigeminal afferents. Several preclinical studies using a model of TN by constriction of the infraorbital nerve already demonstrated a dysregulation in VGSCs, which includes an upregulation of Nav1.3 and a downregulation of Nav1.7.56,57 These findings are in accordance with clinical studies which have shown that patients with TN present the same profile of dysregulation in VGSCs.58,59 In addition, there is evidence of a mutation in the SCN8A gene (which encodes Nav1.6) in a TN patient.60 The alteration of a methionine in the position 136 to a valine (M136V) led to an increase in the peak current, without changing any biophysical properties of the channel. As the Nav1.6 channel is important for the activation of resurgent currents, this gain of function mutation facilitates the repetitive firing of action potential in neurons. Thus, VGSCs have been considered the main target for pain control in TN.
In a preclinical model of TN (i.e., constriction of the infraorbital nerve), a dysregulation of the voltage-gated potassium channel Kv7.2 has been reported and this appears to be a key factor in cold allodynia/hyperalgesia associated with this model.61,62 Specifically, an upregulation of the Kv7.2 channel was observed in the infraorbital nerve of constricted animals,62 and the authors suggested that this may act as a compensatory mechanism to dampen the excitability of neurons after nerve injury. It is important to point out that this channel is responsible to generate M-currents (i.e., muscarinic currents), that are slowly activating, noninactivating voltage-gated potassium currents, which are activated at subthreshold potentials.63 M-currents help to modulate the firing properties of neurons since they serve to stabilize the membrane potential and control neuronal excitability.63,64
Neuroimaging studies have shown that patients with TN have alterations in brain structure, function, and connectivity, which were demonstrated by different approaches.65–69 Resting-state functional magnetic resonance imaging (rs-fMRI) is a tool that can acquire data in the absence of stimulus and is based on blood-oxygen-level-dependent (BOLD) blood flow signals.70,71 rs-fMRI can yield a wealth of data and measurements, such as amplitude of low-frequency fluctuation (ALFF) and regional homogeneity (ReHo), which can provide information about brain activity and synchrony, respectively.72,73 White matter volume changes were observed in the brainstem, corpus callosum, cingulum, corona radiata, and superior longitudinal fasciculus.65 Moreover, it was also demonstrated that GM undergoes volume changes. A decrease in GM was detected in both primary and secondary somatosensory cortices, ACC, dorsolateral prefrontal cortex, ventral orbitofrontal cortex, insula, and thalamus.65,67 In addition to structural changes, fMRI studies demonstrated different patterns of brain activation in patients with TN when compared to healthy controls.66,68,69,74–76 A different pattern of activation was also observed in TN patients who report pain after stimulation of trigger zones versus patients who do not.68 Patients with pain evoked by light tactile stimulation showed a bilateral activation of the primary and secondary somatosensory cortices, the ACC, and the prefrontal cortex, contralateral activation of insula and thalamus, ipsilateral activation of the medial cingulate cortex and spinal trigeminal nucleus, and activation of the medial brainstem, including the periaqueductal gray.68 Conversely, TN patients without pain present a different pattern of brain activation, including the bilateral activation of the precentral cortex, contralateral supplementary motor area, prefrontal cortex, thalamus, and insula activation, and ipsilateral activation of medial cingulate cortex.68 Analysis of local spontaneous brain activity using ALFF and ReHo demonstrated that TN has a characteristic spatiotemporal BOLD signal property. Wang et al.66 demonstrated a bilateral increase in ALFF in the temporal and occipital cortices, and in the left middle frontal regions and middle cingulate gyrus; and a decrease in the right inferior temporal gyrus and medial prefrontal cortex. Similar ALFF analysis revealed an increase of ALFF bilaterally in the inferior cerebellum and fusiform gyrus74 and a reduction in the posterior cingulate cortex, dorsolateral prefrontal cortex, insula, and lateral temporal region.69 Moreover, the posterior cingulate cortex-medial prefrontal cortex circuit and the dorsolateral prefrontal cortex-hippocampal region circuit presented an abnormal interaction in the default mode network in TN patients.69 For the ReHo analysis which reflects temporal synchronization of the BOLD signal, TN patients showed an increase in ReHo in the anterior cingulate, middle and inferior temporal gyrus, medial and superior frontal gyrus, right fusiform gyrus, and right thalamus and a decrease in the left amygdala, the parahippocampal gyrus, cerebellum, and insula.75 Collectively, these studies demonstrated that TN may be associated with brain alterations with a complex spatiotemporal pattern activity, highlighting impairments in major brain areas that are part of the “pain matrix.” It has been suggested that changes in brain structure in TN patients have a correlation with disease duration and is related to a worse prognosis. Thus, effective pain control in the initial state of the disease may have implications in the course of TN.
Pharmacological treatment: Carbamazepine mechanisms of action and effectiveness
TN treatment is initially pharmacological in the form of monotherapy; however, combined therapy with different drugs may be used when the efficacy of monotherapy is low.77,78 Patients not responsive to pharmacological treatment or those who present with severe side effects are candidates for more invasive strategies such as nerve block or surgery.30
Studies on heterologously expressed channels
The main pharmacological class used to control pain in patients with TN is anticonvulsants. Although carbamazepine (CBZ) and oxcarbazepine (OXC) are recommended as first-line therapy,79 their comparative efficacy lacks evidence in TN. Either one may be switched or combined with pregabalin, gabapentin, topiramate, and/or baclofen.30 CBZ is currently the only drug approved by the Food and Drug Administration (FDA) for TN treatment, with 70% effectiveness in reducing pain.80,81 Moreover, for long-term treatment, CBZ and OXC are recommended as the first choice.79 CBZ blocks VGSCs resulting in inhibition of action potentials, reduction of synaptic transmission, and stabilization of the membrane potential in hyperexcitable neurons.77,82,83 This effect is achieved in a use- and voltage-dependent manner because CBZ binds to the inactivated channels with higher affinity than to channels in the open or resting state.84–86 Several studies demonstrated that anticonvulsants share a common binding site with local anesthetics in the alpha subunit of the VGSC.86–89 Residues W1716 and F1764 (which comprise the external pore loop and the pore-lining part of the S6 segment of domain IV, respectively) interact to form the binding pocket for CBZ and local anesthetics.86,90 It was proposed that the CBZ binding site is located at the junction of the widened external vestibule and the narrow part of the channel pore, which is able to recognize the two phenyl groups in the structure of the drug.85,90 When CBZ binds to this region, the channel gating property is modified and the channel is stabilized in its inactivated state to prevent Na+ ion influx.86 Furthermore, external application of CBZ effectively blocked the sodium current, while internal application had no effect, suggesting that the region for binding and unbinding could be different than for local anesthetics.85 Studies using whole-cell patch clamp in mouse neuroblastoma cells (N1E-115) showed that CBZ and OXC shift the voltage dependence of fast inactivation of endogenously expressed VGSC.91,92
It was previously demonstrated in HEK293 cells expressing the Nav1.3 channel that CBZ is able to modulate persistent Na+ current. Moreover, a hyperpolarizing shift in the steady-state inactivation curve was observed in a concentration-dependent manner.93 Fast and slow inactivation of sodium currents was evaluated in Chinese Hamster Ovary cells expressing Nav1.3, and both CBZ and OXC shifted the voltage dependence of slow inactivation toward more hyperpolarizing potentials.91 Mutations in the pore region of the Nav1.3 can lead to pharmacoresistance to CBZ and OXC, as observed in patients with cryptogenic pediatric partial epilepsy.94 The alteration of lysine at position 354 to a glutamine (K354Q) led to nonresponsiveness to CBZ and OXC, suggesting that this is a crucial region for the effects of both drugs.94 As noted earlier, TN patients exhibit an upregulation of Nav1.3, which raises the question whether genomic differences in SCN3A gene may influence the effectiveness of CBZ in these patients.
When the effect of CBZ on half voltage for inactivation on different alpha subunits VGSCs was examined, a strong modulation was observed in the Nav1.6 channel, while a weak modulation in the Nav1.3 channel was seen.95 It is worth noting that the Nav1.6 subunit is highly expressed in the axonal initial segments and nodes of Ranvier, and due to its fast recovery from inactivation may facilitate action potential initiation and propagation.96,97 Moreover, it was found that CBZ blockade depends only on the availability of inactivated channels95 and produced a greater degree of inhibition of Nav1.6 compared to OXC (70.4% vs. 46.7%, respectively).98
Jo and Bean evaluated the effect of internal and external application of CBZ on HEK293 cells expressing hNav1.7. These authors found that external, but not internal, application of CBZ was able to block Na+ currents in whole-cell recordings.99 They observed a difference in inactivation behavior of the channels when examined in the whole-cell versus an inside-out configuration, and in such inside-out recordings, application of CBZ was able to block sodium current.99 A whole-cell patch clamp study demonstrated that application of CBZ at 100 µM was unable to produce inhibition of resurgent sodium current in HEK293 cells expressing Nav1.7; however, a higher concentration (200 µM) did block these currents.100 Furthermore, OXC was also able to inhibit sodium currents of hNav1.7 and caused a hyperpolarizing shift in the activation and inactivation properties of Nav1.7 channels.101 Thus, although both CBZ and OXC are able to block Nav current, the effect can be achieved with a lower concentration with OXC.102 It should be noted that the differences in the analgesic properties of CBZ and OXC may in part be related to actions on other voltage-gated ion channels, such as voltage-gated calcium channels (VGCCs),103,104 and thus, their ability to modulate several neurotransmitter systems involved in pain modulation (for review, see Tomić et al.105).
Point mutations in the Nav1.7 channel in patients with chronic pain disorders often show a hyperpolarizing shift in the voltage dependence of activation.106–108 Interestingly, a family with inherited erythromelalgia (IEM) that carried the Nav1.7 V400M mutant was responsive to CBZ109 because a shift in the voltage dependence of activation and in the steady-state inactivation in the V400M mutant were both normalized by CBZ.109
Effects in isolated neurons
In neurons, CBZ was able to reduce tetrodotoxin-resistant (TTX-R) sodium currents in dorsal root ganglion neurons, including TTX-R Nav1.8 currents, an important component for pain transmission.110–114 Furthermore, CBZ was more effective toward blocking Nav1.8 currents in a use-dependent manner, suggesting an interaction with the inactive state of Nav1.8.110,115 Whole-cell recordings in TG neurons demonstrated that CBZ treatment was able to reduce the peak amplitude of TTX-R sodium current in a concentration-dependent manner, without affecting the half voltage for activation.116 Meanwhile, the same study observed that CBZ was able to shift the half voltage for slow inactivation to more hyperpolarizing potentials, suggesting a decrease in channel availability during repetitive depolarization.116 This fits with the idea that CBZ appears to exhibit a higher affinity for the inactivated conformation of the VGSC than the resting conformation.84,117 Along these lines, studies investigating the effects on TTX-sensitive currents suggest that CBZ binds and stabilizes the channels in the inactivate state.115 This suggests a shift in the voltage dependence, reducing the population of channels that is available for opening.
It is noteworthy that VGSCs also contain ancillary beta subunits (β1–β4), which can modulate the biophysical properties of the channel. CBZ reduced the amplitude of fast transient sodium currents (INaT) in both β1−/−and β2−/− knockout mice, with no difference from their wild-type littermates.118 Moreover, in β2−/− animals but not in β1−/−mice CBZ was able to shift the voltage dependence of activation of INaT, suggesting that the presence of β2 subunit can modulate the effect of CBZ.118 In addition to effects on INaT, the authors also evaluated the effect of CBZ on persistent sodium currents (INaP), a type of current that does not inactivate during prolonged depolarization. CBZ was able to reduce the INaP independently of the expression of β subunits and to induce a strong shift in the voltage dependence of INaP to more hyperpolarizing potentials in β1−/− animals. An intriguing finding obtained in this study was that CBZ produces a paradoxical effect in β1−/− mice, increasing the INaP at more hyperpolarizing potentials and reducing it at more depolarizing potentials which results in an ineffective block of repetitive firing in β1−/− mice.118 These data suggest that the loss of beta subunits could affect the cellular response to CBZ. Altogether, these data point out a number of peculiarities in the mechanism of action of CBZ, and perhaps this is responsible for its superior efficacy in TN pain control.
In vivo effects
Along with in vitro studies, several in vivo studies already demonstrated the effectiveness of CBZ and OXC in animal models of pain. In an animal model of TN induced by constriction of the infraorbital nerve, treatment with CBZ was able to reduce spontaneous pain119 and thermal hyperalgesia, without changing the mechanical threshold in doses that do not cause motor deficit.120,121 Likewise, in a TN model consisting of compression of the trigeminal nerve root, CBZ treatment was able to reduce facial mechanical allodynia.122,123 These studies demonstrated the effectiveness of CBZ in reducing evoked and spontaneous pain in different animal models of TN.
The main pharmacokinetic characteristics of CBZ and OXC are an important point of consideration, since they may influence treatment response. After oral administration CBZ presents a lower time to reach the peak concentration (0.5 h) with a peak concentration of 37.8 µg/mL and an elimination half-life of 3.38 h, whereas OXC takes 1 h to reach the peak concentration with a maximum concentration of 30.6 µg/mL and 8.99 h of half-life.124,125 Although the metabolites of CBZ and OXC are very similar, metabolic pathways are quite different. CBZ is metabolized by cytochrome P450 oxidative processes and leads to autoinduction, which results in changes in elimination over time. On the other hand, OXC is metabolized by cytosolic enzymes, presenting a lower potential for drug interactions.126,127 While CBZ and OXC are the first-line drugs recommended for TN, there is not much known about their comparative efficacy in TN.79 Furthermore, both drugs are associated with frequent and/or severe side effects, but the latter is suggested to have greater tolerability.128–133 A systematic review of the effectiveness and safety of CBZ in different pain conditions concluded that 40% to 60% of the patients would exhibit adverse events, mainly impaired cognition, somnolence, dizziness, gastrointestinal symptoms, headaches, dry mouth or taste change, and mood changes. Severe side effects had a very low incidence and included upper gastrointestinal bleeding and cutaneous rashes, which may be considered serious because of association with CBZ-induced Stevens–Johnson syndrome.134 These observations were corroborated by a recent study evaluating the side effects of antiepileptic drugs in TN. It was reported that impact on memory and cognition were the most common complaints. OXC showed a less negative impact on memory and induced less fatigue. Additionally, at low doses, OXC seemed to be better tolerated than CBZ, but an increase in OXC dosage was less tolerated and correlated with higher side effect scores.135 Table 1 summarizes the mechanism of action and pharmacokinetic profile of CBZ and OXC.
Table 1.
Comparison of mechanism of action and pharmacokinetic profile between carbamazepine and oxcarbazepine.
| Drug | Mechanisms of action | Metabolism | Half-life | Therapeutic indication |
|---|---|---|---|---|
| Carbamazepine | VGSC blocker, L-type VGCC blocker | Cytochrome P450 | 0.5 h | Epilepsy, trigeminal neuralgia |
| Oxcarbazepine | VGSC blocker; N-P- and R-type VGCC blocker | Cytosolic enzymes | 9 h | Epilepsy |
VGSC: voltage-gated sodium channel; VGCC: voltage-gated calcium channel.
Pharmacological alternatives to CBZ
CBZ and OXC represent the only first-line recommendation for long-term treatment of TN. However, when necessary (i.e., due to failure or toxicity) other drugs may be combined with either one of them or used instead. The alternatives include other anticonvulsants (e.g., pregabalin, gabapentin, lamotrigine, and phenytoin), baclofen, and botulinum toxin type A (BoNT-A). So far, the quality of evidence for their use in TN management is low to very low.79
Gabapentinoids (gabapentin and pregabalin) have become the mainstay of treatment for various pain syndromes, including fibromyalgia, diabetic neuropathy, and postherpetic neuralgia.136 These anticonvulsants have been consistently shown to induce analgesia by targeting the α2δ auxiliary subunit of VGCCs,137–139 which results in impaired trafficking of VGCCs to the plasma membrane and subsequent reduction of neurotransmitter release and neuronal excitability.140,141 This mechanism may account for their analgesic activity in TN, but in addition, they have been shown to suppress subthreshold oscillations and peripheral ectopia.142,143 It has been suggested that the membrane stabilizing action is due to a selective effect on the slow component of Na+ conductance,143 but it remains to be clarified whether this is a direct effect on Na+ channels or indirect via α2δ binding. In any case, this effect may contribute to its analgesic effect in TN. Several other mechanisms have been proposed to underlie gabapentinoid analgesia, such as suppression of spinal N-methyl-d-aspartic acid receptors via a coagonist binding site, activation of potassium channels leading to neuronal hyperpolarization and inhibition of descending serotonergic facilitation of nociceptive processing, and finally, activation of descending inhibitory noradrenergic input to the dorsal horn105 (for review, see Alles and Smith144 and Kremer et al.145). Since most of these effects, if not all, are related to gabapentinoid interaction with multifunctional α2δ proteins, it would be relevant to determine whether TN patients present changes in α2δ protein expression. Indeed, an upregulation has already been reported in certain experimental conditions146 and may predict a better response to these drugs.
Lamotrigine and phenytoin are effective in pain states by virtue of the same selective blocking properties of high-frequency action potential firing that account for their antiseizure activity.147 Their main mechanism of action is comparable to CBZ, as they induce voltage-dependent and frequency-dependent blockade of VGSCs. In fact, phenytoin was the first drug introduced for the treatment of TN in 1942,148 and in the following decades evidence accumulated regarding its effectiveness in TN pain management (for review, see Keppel Hesselink and Schatman149). Its use, as well of its prodrug fosphenytoin, is still recommended in the treatment of refractory TN or in acute exacerbations of pain.79,150 Phenytoin is considered a weak blocker of VGSCs at hyperpolarized membrane potentials, but its inhibitory action is greatly enhanced by sustained membrane depolarization and during high-frequency channel activity. Compared with CBZ, it has three-fold higher affinity for depolarized channels, but CBZ binds to them at a five-fold faster rate (for review, see Mantegazza et al.151).
It has been suggested that all three anticonvulsants (i.e., CBZ, phenytoin, and lamotrigine) bind at a common site in the inner pore of the sodium channel causing its occlusion.152,153 However, in contrast to CBZ and phenytoin, several additional mechanisms have been proposed for lamotrigine, including inhibition of N-type and P-type high-voltage-activated calcium channels and enhancement of potassium repolarizing currents.154–156 These mechanisms may account for the differential effect of lamotrigine in epileptic states, as it is the only anticonvulsant among the three to be effective in absence seizures (for review, see Mantegazza et al.151). There is currently a weak recommendation for lamotrigine as add-on or monotherapy for TN pain management, but unlike phenytoin, it is not recommended for acute pain exacerbations, since doses must be escalated slowly in order to avoid rashes.79 It is noteworthy that both phenytoin and lamotrigine may interact with CBZ when used as add-on therapies. Phenytoin is reported to reduce plasma CBZ concentrations to a clinically significant extent probably by stimulating CYP3A4, while lamotrigine metabolism is accelerated by CBZ due to its ability to induce the same isoform of cytochrome P enzyme.157
Preclinical observations suggest that baclofen resembles CBZ and phenytoin in its ability to depress excitatory transmission and facilitate segmental inhibition in the trigeminal nucleus.158,159 These data are corroborated by clinical evidence that baclofen is effective as monotherapy or combined with CBZ in the management of TN pain.160–165 Baclofen is a GABAB receptor agonist acting on the β subunit of receptors expressed on neurons at the spinal cord level and brain. It has long been a mainstay in the management of spasticity of several origins.166 The off-label use includes treatment of alcoholic liver disease, maintenance of alcohol abstinence by decreasing alcohol cravings, alcohol-related anxiety, gastroesophageal reflux disease, hiccups, and TN. It induces analgesia possibly by presynaptic inhibition of neurotransmitter release from the central endings of primary nociceptors in the spinal cord and by stimulating inhibitory neuronal signals in the postsynaptic neurons.167 Moreover, it has been demonstrated to have affinity for VGSCs, with a potential to eliminate both persistent sodium currents and, indirectly, sodium-activated potassium currents.168 The relevance of this finding to baclofen-induced analgesia remains to be investigated. Oral use of baclofen is limited by adverse effects, which affect between 25% and 75% of patients and include mainly muscle weakness, nausea, somnolence, and paraesthesia. As baclofen does not easily penetrate the blood-barrier, its intrathecal use has been indicated to control spasticity in refractory patients or those who experience intolerable side effects with oral use.166 To our knowledge, there is no evidence whether this alternative would benefit TN patients.
There is growing evidence that BoNT-A may represent a safe and effective alternative for TN management.150,169–171 However, based on the low quality of evidence, the recommendation given for BoNT-A is weak and restricted to an add-on therapy for medium-term treatment of TN.79 It is now widely accepted that BoNT-A may induced analgesia independently of muscle relaxation but still involves its interaction with the SNAP receptor complex and consequent blockade of synaptic vesicle fusion.172 Through this mechanism, it has been shown to inhibit the release of various pain-modulating neurotransmitters, such as glutamate, substance P, and calcitonin gene-related peptide (CGRP), as well as to reduce the expression of Transient Receptor Potential V1 (TRPV1) channels by inhibiting the exocytosis of TRPV1-harboring vesicles, leading to the proteosomal degradation of TRPV1.172–175 The mechanisms that contribute to the analgesic effects of BoNT-A in TN patients remain unclear, but data from preclinical studies have pointed out that axonal transport of BoNT-A from the periphery to the spinal trigeminal nucleus is a determinant for BoNT-A-mediated analgesia.176,177 Table 2 summarizes the mechanisms of action and therapeutic indications for alternative drugs to CBZ.
Table 2.
Mechanism of action of other therapeutic treatments used for trigeminal neuralgia.
| Drug | Mechanisms of action | Therapeutic indication |
|---|---|---|
| Gabapentinoids | Cavα2δ subunit | Epilepsy, pain |
| Lamotrigine | VGSC blocker, N-P-type VGCC blocker | Epilepsy |
| Phenytoin | VGSC blocker | Epilepsy |
| Baclofen | GABAB receptor agonist | Spasticity |
| BoNT-A | SNARE complex | Spasticity |
VGSC: voltage-gated sodium channel; VGCC: voltage-gated calcium channel; GABA: gamma-aminobutyric acid; BoNT-A: botulinum toxin type A; SNARE: SNAP receptor.
Surgical procedures are indicated for patients with incapacitating symptoms of TN, refractory or recurrent TN or in case of intolerable adverse effects related to medication. These procedures include microvascular decompression, gamma knife radiosurgery, and percutaneous techniques, such as glycerol rhizotomy, radiofrequency thermocoagulation, and percutaneous balloon compression.83 Among these procedures, microvascular decompression is the most invasive, requiring retrosigmoid craniotomy and microsurgical exploration in the posterior fossa, but it offers the higher success rate in pain relief.127,178 Indications and details on the surgical procedures employed for TN treatment have been reviewed by others and are outside the scope of this review.127,179–182 Despite these avenues, there remains a critical need for new therapies, and this requires an in-depth understanding of the underlying pathways and molecular mechanisms of TN.
Additional pharmacological perspectives
Advances in the pharmacological treatment of TN include the assessment of an extended-release formulation of OXC (termed eslicarbazepine) and evaluation of a new selective Nav1.7 channel blocker (BIIB074 or Vixotrigine), which are currently in progress (ClinicalTrials.gov, Identifiers # NCT03374709 and NCT03637387, respectively).
Eslicarbazepine is the active metabolite of OXC and was approved in Europe in 2009 and in 2013 by the FDA and Health Canada as an adjunctive therapy in adults with partial-onset seizures.91 Efficacy and safety of eslicarbazepine in TN patients was first assessed in 2018.183 The results of this open-label study that included 15 patients suggested that eslicarbazepine is an effective, safe, and well-tolerated treatment for TN. It is noteworthy that around 60% of the patients presented adverse events, mainly lightheadedness, severe dizziness, and hyponatremia and 4 of the 15 patients discontinued treatment for this reason.183 Although the adverse events induced by eslicarbazepine are similar to CBZ and OXC, they are considered less frequent.184,185 Eslicarbazepine is contraindicated in patients with hypersensitivity reactions to CBZ (European Medicines Agency, London, UK), but some studies have suggested that severe skin reactions occur less frequently with eslicarbazepine compared to other anticonvulsants, leading to treatment discontinuation in only 0.1% of the cases.186 As data are scarce and contradictory,187–189 further studies are clearly needed before definitive conclusions may be drawn.
Eslicarbazepine has been shown to interact selectively with the inactive state of VGSCs through altered slow inactivation, as opposed to the effects on fast inactivation associated with CBZ and OXC. In addition, eslicarbazepine effectively inhibited Cav3.2 calcium channels with greater affinity than CBZ.91 Eslicarbazepine also failed to cause a paradoxical upregulation of sodium currents, as described for CBZ, indicating a potential to more effectively decrease neuronal firing.91,118 Thus, the pharmacodynamic profile makes eslicarbazepine an interesting alternative to be evaluated for TN pain control. Other advantages of eslicarbazepine include better safety profile, a reduced potential to act on cytochrome P450 enzymes and a longer elimination half-life of 20 to 24 h, which allows single daily dosing.190 However, current evidence is considered insufficient to recommend eslicarbazepine for the treatment of neuropathic pain and cranial neuralgias, since available data come from open observational studies with no control group and a small number of patients.191 Therefore, randomized clinical trials with greater numbers of patients are clearly warranted to support this recommendation.
Vixotrigine was first discovered in 2006 and the target indications included depression, bipolar mood disorder, and substance disorders. It was formerly named raxatrigine and was considered a selective Nav1.3 channel blocker. Subsequently, the compound was stated to be a selective Nav1.7 channel blocker and a lead neuropathic pain candidate with two main indications: TN and lumbar radiculopathy (for review, see Keppel Hesselink192). Vixotrigine has been considered a state- and use-dependent Nav1.7 channel blocker, but its selectivity lacks validation and available data are controversial. In a clinical paper that discusses the experimental design to assess vixotrigine efficacy in TN patients, its preclinical profile is described as the compound having selectivity for Nav1.7 channels over the other Nav isoforms for both the resting and depolarized states.193 In sharp contrast, in a model of Nav1.7-mediated pain, Deuis et al. showed that vixotrigine inhibited Nav channels state dependently but nonselectively.194 In addition, vixotrigine showed analgesic effects when delivered systemically,194 which is in line with more recent data showing that oral administration of vixotrigine fully reversed paw mechanical allodynia in a mouse model of postsurgical pain.195 Despite the controversy regarding the selectivity of vixotrigine, it is plausible that blockade of Nav1.7 channels contributes to its analgesic effect, since data from electrophysiology studies indicated that vixotrigine is able to cause a dramatic hyperpolarizing shift of channel inactivation (without effects on activation) in HEK293 cells transfected with Nav1.7 channels. This enhanced ability of a sodium channel blocker to discriminate between the resting and inactivated channels compared to CBZ may provide a better safety profile and tolerability during systematic administration.196
Results from a phase 2a study indicated that vixotrigine can be administered at therapeutic doses without titration and has shown good tolerability.197 The study first enrolled 67 patients which received vixotrigine (150 mg, three times per day, orally) for 21 days. During this open-label phase, 23 patients withdrew mainly due to lack of efficacy (18 patients). Thus, 44 patients were eligible for the open-label phase which showed that treatment failure was not significantly different between placebo- and vixotrigine-treated groups, but significant treatment differences were found in time to treatment failure, number of paroxysms, average daily pain score, and assessments of overall function and quality of life. The drug was well tolerated, headache, and dizziness being the most frequent adverse events.197 There was a suspicion that vixotrigine might lead to an increase in blood pressure, but a clinical study in healthy patients receiving vixotrigine 300 to 400 mg twice daily for 36 days failed to show a clinically important increase in blood pressure.198
The conclusion regarding efficacy is limited by challenges in the realization of TN clinical studies,199 but the results encourage moving forward to a phase 3 study, which is currently in progress (ClinicalTrials.gov, Identifier # NCT03070132). The drug has been also evaluated in individuals with IEM, the first Nav1.7 channelopathy identified, as well as in small fiber neuropathy (ClinicalTrials.gov), and it failed phase II trial in patients with in painful lumbosacral radiculopathy.200
It is noteworthy that other small molecules and peptide-derived Nav channels blockers are under development by different pharmaceutical companies and may represent perspectives for TN pain control. However, there are several challenges for advancing in this area, including high structural similarity of the Nav subtypes and species-specific differences in the levels of expression and/or biophysical properties of Nav channels (for review, see Kingwell200 and Dib-Hajj and Waxman201).
Another potential target to be explored in TN is the Kv7.2 channel. A study conducted in the rat model of infraorbital nerve constriction showed that Kv7.2 channels were expressed on cold-sensing trigeminal ganglion neurons and that retigabine treatment reduced the excitability of nociceptive cold-sensing neurons and alleviated cold allodynia and hyperalgesia.61,62 Retigabine is a selective Kv7.2 channel opener, which shifts the voltage dependence of Kv7.2 channels to more hyperpolarized potentials, thereby decreasing neuronal hyperexcitability.63 Since the 1980s, an analog of retigabine (i.e., flupirtine) has been used in Europe for treatment of acute and chronic pain. Small clinical studies suggest that flupirtine effectively reduces chronic musculoskeletal pain, migraine and neuralgias, among other types of pain, but, to our knowledge, its efficacy in TN patients has never been investigated.202
Finally, it is tempting to speculate whether CGRP antagonists would provide pain control in TN. This assumption is based mainly in preclinical data showing that CGRP plays a role in trigeminal afferent sensitization and CGRP receptor blockade results in antinociceptive effects in different models of trigeminal neuropathic pain.203–205 Interestingly, blockade of CGRP receptors significantly reduced mechanical allodynia in a model of trigeminal neuropathic pain (i.e., infraorbital nerve constriction) but not in a model of spinal nerve injury (i.e., sciatic nerve ligation).206 In humans, seminal work by Goadsby et al. showed that CGRP is released in the extracerebral circulation during activation of the trigeminovascular system, which was further corroborated by the observation that CGRP was the only neuropeptide released during the headache phase of migraine attacks.207,208 This evidence has contributed to the development of antimigraine therapies that target multiple components of CGRP transmission (for review, see Edvinsson et al.209). Likewise, there are some reports indicating elevated CGRP levels in blood, cerebrospinal fluid, and plasma of TN patients.210,211 These observations allude to the fact that an antibody toward the CGRP receptor is clinically available (for review, see Edvinsson et al.209) and may encourage studies assessing this therapeutic intervention in TN patients. A summary of pharmacological profiles and therapeutic indications of the drugs mentioned earlier are presented in Table 3.
Table 3.
Mechanism of action and half-life profile for new pharmacological approaches to trigeminal neuralgia.
| Drug | Mechanisms of action | Half-life | Therapeutic indication |
|---|---|---|---|
| Eslicarbazepine | VGSC blocker, T-type VGCC blocker | 20–24 h | Epilepsy, pain |
| Vixotrigine | VGSC blocker | 9 h | Depression, bipolar disorder, pain |
| – | CGRP antagonists | – | Antimigraine |
| Flupirtine | Potassium channel opener | – | Acute pain |
VGSC: voltage-gated sodium channel; VGCC: voltage-gated calcium channel; CGRP: calcitonin gene-related peptide.
Concluding remarks
Although TN is considered a rare condition, it dramatically reduces the quality of life of affected individuals not only due to pain attacks but also to other disease-associated comorbidities, such as anxiety and depression. In fact, it is probable that the prevalence of TN in the general populations is underestimated, as studies in this condition are very challenging and population aging is increasing. Likewise, the two main TN-related comorbidities, that is, anxiety and depression, are often underdiagnosed and undertreated and just recently have gained attention. Thus, a better understanding of the pathophysiology is necessary for the improvement of current therapies or development of innovative pharmacological treatments.
Acknowledgments
The authors would like to acknowledge Brooke Belanger (Hotchkiss Brain Institute, University of Calgary) for her contribution to the production and graphic design of Figure 1.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: EG is funded by a studentship from the Cumming School of Medicine. GWZ is supported by the Canadian Institutes of Health Research, and by the Canada-Israel Health Research Initiative, a grant from Alberta Innovates and holds a Canada Research Chair.
ORCID iD
Gerald W. Zamponi https://orcid.org/0000-0002-0644-9066
References
- 1.Sessle BJ. Mechanisms of oral somatosensory and motor functions and their clinical correlates. J Oral Rehabil 2006; 33: 243–261. [DOI] [PubMed] [Google Scholar]
- 2.Dubin AE, Patapoutian A. Nociceptors: the sensors of the pain pathway. J Clin Invest 2010; 120: 3760–3772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sessle BJ. Acute and chronic craniofacial pain: brainstem mechanisms of nociceptive transmission and neuroplasticity, and their clinical correlates. Crit Rev Oral Biol Med 2000; 11: 57–91. [DOI] [PubMed] [Google Scholar]
- 4.Dubner R, Sessle BJ, Storey AT. The neural basis of oral and facial function. London: Plenum, 1978. [Google Scholar]
- 5.Patel NM, Das JM. Neuroanatomy, spinal trigeminal nucleus Treasure Island: StatPearls, 2019. [PubMed]
- 6.Gobel S, Hockfield S, Ma R. Anatomical similarities between medullary and spinal dorsal horns In: Kawamura Y, Dubner R. (eds) Oral-facial sensory and motor functions. Tokyo: Quintessence Publishing, 1981, pp. 221–223. [Google Scholar]
- 7.Dubner R, Bennett GJ. Spinal and trigeminal mechanisms of nociception. Annu Rev Neurosci 1983; 6: 381–418. [DOI] [PubMed] [Google Scholar]
- 8.Sessle BJ. The neurobiology of facial and dental pain: present knowledge, future directions. J Dent Res 1987; 66: 962–981. [DOI] [PubMed] [Google Scholar]
- 9.Wang H, Wei F, Dubner R, Ren K. Selective distribution and function of primary afferent nociceptive inputs from deep muscle tissue to the brainstem trigeminal transition zone. J Comp Neurol 2006; 498: 390–402. [DOI] [PubMed] [Google Scholar]
- 10.Shimizu K, Guo W, Wang H, Zou S, LaGraize SC, Iwata K, Wei F, Dubner R, Ren K. Differential involvement of trigeminal transition zone and laminated subnucleus caudalis in orofacial deep and cutaneous hyperalgesia: the effects of interleukin-10 and glial inhibitors. Mol Pain 2009; 5: 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Woda A. Pain in the trigeminal system: from orofacial nociception to neural network modeling. J Dent Res 2003; 82: 764–768. [DOI] [PubMed] [Google Scholar]
- 12.Ossipov MH, Dussor GO, Porreca F. Central modulation of pain. J Clin Invest 2010; 120: 3779–3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chichorro JG, Porreca F, Sessle B. Mechanisms of craniofacial pain. Cephalalgia 2017; 37: 613–626. [DOI] [PubMed] [Google Scholar]
- 14.Sessle BJ. Peripheral and central mechanisms of orofacial pain and their clinical correlates. Minerva Anestesiol 2005; 71: 117–136. [PubMed] [Google Scholar]
- 15.Basbaum AI, Fields HL. Endogenous pain control systems: brainstem spinal pathways and endorphin circuitry. Annu Rev Neurosci 1984; 7: 309–338. [DOI] [PubMed] [Google Scholar]
- 16.Mason P. Medullary circuits for nociceptive modulation. Curr Opin Neurobiol 2012; 22: 640–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ossipov MH, Morimura K, Porreca F. Descending pain modulation and chronification of pain. Curr Opin Support Palliat Care 2014; 8: 143–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aicher SA, Hermes SM, Whittier KL, Hegarty DM. Descending projections from the rostral ventromedial medulla (RVM) to trigeminal and spinal dorsal horns are morphologically and neurochemically distinct. J Chem Neuroanat 2012; 43: 103–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mills EP, Di Pietro F, Alshelh Z, Peck CC, Murray GM, Vickers ER, Henderson LA. Brainstem pain-control circuitry connectivity in chronic neuropathic pain. J Neurosci 2018; 38: 465–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sugiyo S, Takemura M, Dubner R, Ren K. Trigeminal transition zone/rostral ventromedial medulla connections and facilitation of orofacial hyperalgesia after masseter inflammation in rats. J Comp Neurol 2005; 493: 510–523. [DOI] [PubMed] [Google Scholar]
- 21.Wang J, Li ZH, Feng B, Zhang T, Zhang H, Li H, Chen T, Cui J, Zang WD, Li YQ. Corticotrigeminal projections from the insular cortex to the trigeminal caudal subnucleus regulate orofacial pain after nerve injury via extracellular signal-regulated kinase activation in insular cortex neurons. Front Cell Neurosci 2015; 9: 493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Noseda R, Constandil L, Bourgeais L, Chalus M, Villanueva L. Changes of meningeal excitability mediated by corticotrigeminal networks: a link for the endogenous modulation of migraine pain. J Neurosci 2010; 30: 14420–14429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dunn RC, Jr, Tolbert DL. The corticotrigeminal projection in the cat. A study of the organization of cortical projections to the spinal trigeminal nucleus. Brain Res 1982; 240: 13–25. [DOI] [PubMed] [Google Scholar]
- 24.Tashiro T, Matsuyama T, Higo S. Distribution of cells of origin of the corticotrigeminal projections to the nucleus caudalis of the spinal trigeminal complex in the cat. A horseradish peroxidase (HRP) study. Exp Neurol 1983; 80: 178–185. [DOI] [PubMed] [Google Scholar]
- 25.Dubner R, Ren K. Brainstem mechanisms of persistent pain following injury. J Orofac Pain 2004; 18: 299–305. [PubMed] [Google Scholar]
- 26.Darian-Smith I, Yokota T. Corticofugal effects on different neuron types within the cat’s brain stem activated by tactile stimulation of the face. J Neurophysiol 1966; 29: 185–206. [DOI] [PubMed] [Google Scholar]
- 27.Castro A, Raver C, Li Y, Uddin O, Rubin D, Ji Y, Masri R, Keller A. Cortical regulation of nociception of the trigeminal nucleus caudalis. J Neurosci 2017; 37: 11431–11440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yoshimasu F, Kurland LT, Elveback LR. Tic douloureux in Rochester, Minnesota, 1945-1969. Neurology 1972; 22: 952–956. [DOI] [PubMed] [Google Scholar]
- 29.Katusic S, Beard CM, Bergstralh E, Kurland LT. Incidence and clinical features of trigeminal neuralgia, Rochester, Minnesota, 1945-1984. Ann Neurol 1990; 27: 89–95. [DOI] [PubMed] [Google Scholar]
- 30.Obermann M. Treatment options in trigeminal neuralgia. Ther Adv Neurol Disord 2010; 3: 107–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jones MR, Urits I, Ehrhardt KP, Cefalu JN, Kendrick JB, Park DJ, Cornett EM, Kaye AD, Viswanath O. A comprehensive review of trigeminal neuralgia. Curr Pain Headache Rep 2019; 23: 74. [DOI] [PubMed] [Google Scholar]
- 32.Merskey H, Bogduk N. Classification of chronic pain. 2nd ed Seattle: IASP Press, 2011. [Google Scholar]
- 33.Maarbjerg S, Gozalov A, Olesen J, Bendtsen L. Trigeminal neuralgia – a prospective systematic study of clinical characteristics in 158 patients. Headache 2014; 54: 1574–1582. [DOI] [PubMed] [Google Scholar]
- 34.Montano N, Conforti G, Di Bonaventura R, Meglio M, Fernandez E, Papacci F. Advances in diagnosis and treatment of trigeminal neuralgia. Ther Clin Risk Manag 2015; 11: 289–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bangash TH. Trigeminal neuralgia: frequency of occurrence in different nerve branches. Anesth Pain Med 2011; 1: 70–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Turp JC, Gobetti JP. Trigeminal neuralgia versus atypical facial pain. A review of the literature and case report. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1996; 81: 424–432. [DOI] [PubMed] [Google Scholar]
- 37.Zakrzewska JM, Wu J, Mon-Williams M, Phillips N, Pavitt SH. Evaluating the impact of trigeminal neuralgia. Pain 2017; 158: 1166–1174. [DOI] [PubMed] [Google Scholar]
- 38.Leal PR, Hermier M, Froment JC, Souza MA, Cristino-Filho G, Sindou M. Preoperative demonstration of the neurovascular compression characteristics with special emphasis on the degree of compression, using high-resolution magnetic resonance imaging: a prospective study, with comparison to surgical findings, in 100 consecutive patients who underwent microvascular decompression for trigeminal neuralgia. Acta Neurochir (Wien) 2010; 152: 817–825. [DOI] [PubMed] [Google Scholar]
- 39.Cruccu G. Trigeminal neuralgia. Continuum (Minneap Minn) 2017; 23: 396–420. [DOI] [PubMed] [Google Scholar]
- 40.Arnold M. Headache Classification Committee of the International Headache Society (IHS) the international classification of headache disorders, 3rd edition. Cephalalgia 2018; 38: 1–211. [DOI] [PubMed] [Google Scholar]
- 41.Godazandeh K, Martinez Sosa S, Wu J, Zakrzewska JM. Trigeminal neuralgia: comparison of characteristics and impact in patients with or without multiple sclerosis. Mult Scler Relat Disord 2019; 34: 41–46. [DOI] [PubMed] [Google Scholar]
- 42.Zakrzewska JM, Wu J, Brathwaite TS. A systematic review of the management of trigeminal neuralgia in patients with multiple sclerosis. World Neurosurg 2018; 111: 291–306. [DOI] [PubMed] [Google Scholar]
- 43.Kerr FW. Pathology of trigeminal neuralgia: light and electron microscopic observations. J Neurosurg 1967; 26: 151–156. [DOI] [PubMed] [Google Scholar]
- 44.Jannetta PJ. Gross (mesoscopic) description of the human trigeminal nerve and ganglion. J Neurosurg 1967; 26: 109–111. [DOI] [PubMed] [Google Scholar]
- 45.Jannetta PJ. Arterial compression of the trigeminal nerve at the pons in patients with trigeminal neuralgia. J Neurosurg 1967; 26: 159–162. [DOI] [PubMed] [Google Scholar]
- 46.Zakrzewska JM, Akram H. Neurosurgical interventions for the treatment of classical trigeminal neuralgia. Cochrane Database Syst Rev 2011; 9: CD007312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ferguson GG, Brett DC, Peerless SJ, Barr HW, Girvin JP. Trigeminal neuralgia: a comparison of the results of percutaneous rhizotomy and microvascular decompression. Can J Neurol Sci 1981; 8: 207–214. [DOI] [PubMed] [Google Scholar]
- 48.DeSouza DD, Hodaie M, Davis KD. Structural magnetic resonance imaging can identify trigeminal system abnormalities in classical trigeminal neuralgia. Front Neuroanat 2016; 10: 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Antonini G, Di Pasquale A, Cruccu G, Truini A, Morino S, Saltelli G, Romano A, Trasimeni G, Vanacore N, Bozzao A. Magnetic resonance imaging contribution for diagnosing symptomatic neurovascular contact in classical trigeminal neuralgia: a blinded case-control study and meta-analysis. Pain 2014; 155: 1464–1471. [DOI] [PubMed] [Google Scholar]
- 50.Hilton DA, Love S, Gradidge T, Coakham HB. Pathological findings associated with trigeminal neuralgia caused by vascular compression. Neurosurgery 1994; 35: 299–303, discussion 303. [DOI] [PubMed] [Google Scholar]
- 51.Love S, Hilton DA, Coakham HB. Central demyelination of the Vth nerve root in trigeminal neuralgia associated with vascular compression. Brain Pathol 1998; 8: 1–11, discussion 11–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Devor M, Govrin-Lippmann R, Rappaport ZH. Mechanism of trigeminal neuralgia: an ultrastructural analysis of trigeminal root specimens obtained during microvascular decompression surgery. J Neurosurg 2002; 96: 532–543. [DOI] [PubMed] [Google Scholar]
- 53.Marinkovic S, Gibo H, Todorovic V, Antic B, Kovacevic D, Milisavljevic M, Cetkovic M. Ultrastructure and immunohistochemistry of the trigeminal peripheral myelinated axons in patients with neuralgia. Clin Neurol Neurosurg 2009; 111: 795–800. [DOI] [PubMed] [Google Scholar]
- 54.Devor M, Amir R, Rappaport ZH. Pathophysiology of trigeminal neuralgia: the ignition hypothesis. Clin J Pain 2002; 18: 4–13. [DOI] [PubMed] [Google Scholar]
- 55.Rappaport ZH, Devor M. Trigeminal neuralgia: the role of self-sustaining discharge in the trigeminal ganglion. Pain 1994; 56: 127–138. [DOI] [PubMed] [Google Scholar]
- 56.Xu W, Zhang J, Wang Y, Wang L, Wang X. Changes in the expression of voltage-gated sodium channels Nav1.3, Nav1.7, Nav1.8, and Nav1.9 in rat trigeminal ganglia following chronic constriction injury. Neuroreport 2016; 27: 929–934. [DOI] [PubMed] [Google Scholar]
- 57.Liu M, Zhong J, Xia L, Dou N, Li S. The expression of voltage-gated sodium channels in trigeminal nerve following chronic constriction injury in rats. Int J Neurosci 2019; 129: 955–962. [DOI] [PubMed] [Google Scholar]
- 58.Siqueira SR, Alves B, Malpartida HM, Teixeira MJ, Siqueira JT. Abnormal expression of voltage-gated sodium channels Nav1.7, Nav1.3 and Nav1.8 in trigeminal neuralgia. Neuroscience 2009; 164: 573–577. [DOI] [PubMed] [Google Scholar]
- 59.Costa GMF, Rocha LPC, Siqueira S, Moreira PR, Almeida-Leite CM. No association of polymorphisms in Nav1.7 or nerve growth factor receptor genes with trigeminal neuralgia. Pain Med 2019; 20: 1362–1369. [DOI] [PubMed] [Google Scholar]
- 60.Tanaka BS, Zhao P, Dib-Hajj FB, Morisset V, Tate S, Waxman SG, Dib-Hajj SD. A gain-of-function mutation in Nav1.6 in a case of trigeminal neuralgia. Mol Med 2016; 22: 338–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Abd-Elsayed AA, Ikeda R, Jia Z, Ling J, Zuo X, Li M, Gu JG. KCNQ channels in nociceptive cold-sensing trigeminal ganglion neurons as therapeutic targets for treating orofacial cold hyperalgesia. Mol Pain 2015; 11: 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ling J, Erol F, Gu JG. Role of KCNQ2 channels in orofacial cold sensitivity: KCNQ2 upregulation in trigeminal ganglion neurons after infraorbital nerve chronic constrictive injury. Neurosci Lett 2018; 664: 84–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tatulian L, Delmas P, Abogadie FC, Brown DA. Activation of expressed KCNQ potassium currents and native neuronal M-type potassium currents by the anti-convulsant drug retigabine. J Neurosci 2001; 21: 5535–5545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.White HS, Rho JM. Potassium channels. Mechanisms of action of antiepileptic drugs. 1st ed. West Islip: Professional Communications, Inc, 2010, pp. 109–119. [Google Scholar]
- 65.Wang Y, Cao DY, Remeniuk B, Krimmel S, Seminowicz DA, Zhang M. Altered brain structure and function associated with sensory and affective components of classic trigeminal neuralgia. Pain 2017; 158: 1561–1570. [DOI] [PubMed] [Google Scholar]
- 66.Wang Y, Xu C, Zhai L, Lu X, Wu X, Yi Y, Liu Z, Guan Q, Zhang X. Spatial-temporal signature of resting-state BOLD signals in classic trigeminal neuralgia. J Pain Res 2017; 10: 2741–2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Obermann M, Rodriguez-Raecke R, Naegel S, Holle D, Mueller D, Yoon MS, Theysohn N, Blex S, Diener HC, Katsarava Z. Gray matter volume reduction reflects chronic pain in trigeminal neuralgia. Neuroimage 2013; 74: 352–358. [DOI] [PubMed] [Google Scholar]
- 68.Moisset X, Villain N, Ducreux D, Serrie A, Cunin G, Valade D, Calvino B, Bouhassira D. Functional brain imaging of trigeminal neuralgia. Eur J Pain 2011; 15: 124–131. [DOI] [PubMed] [Google Scholar]
- 69.Zhang Y, Mao Z, Pan L, Ling Z, Liu X, Zhang J, Yu X. Frequency-specific alterations in cortical rhythms and functional connectivity in trigeminal neuralgia. Brain Imaging Behav 2019; 13: 1497–1509. [DOI] [PubMed] [Google Scholar]
- 70.Biswal BB. Resting state fMRI: a personal history. Neuroimage 2012; 62: 938–944. [DOI] [PubMed] [Google Scholar]
- 71.Logothetis NK. What we can do and what we cannot do with fMRI. Nature 2008; 453: 869–878. [DOI] [PubMed] [Google Scholar]
- 72.Zou QH, Zhu CZ, Yang Y, Zuo XN, Long XY, Cao QJ, Wang YF, Zang YF. An improved approach to detection of amplitude of low-frequency fluctuation (ALFF) for resting-state fMRI: fractional ALFF. J Neurosci Methods 2008; 172: 137–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jiang L, Zuo XN. Regional homogeneity: a multimodal, multiscale neuroimaging marker of the human connectome. Neuroscientist 2016; 22: 486–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chen Y, Xiang CQ, Liu WF, Jiang N, Zhu PW, Ye L, Li B, Lin Q, Min YL, Su T, He LC, Shao Y. Application of amplitude of lowfrequency fluctuation to altered spontaneous neuronal activity in classical trigeminal neuralgia patients: a restingstate functional MRI study. Mol Med Rep 2019; 20: 1707–1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Xiang CQ, Liu WF, Xu QH, Su T, Yong-Qiang S, Min YL, Yuan Q, Zhu PW, Liu KC, Jiang N, Ye L, Shao Y. Altered spontaneous brain activity in patients with classical trigeminal neuralgia using regional homogeneity: a resting-state functional MRI study. Pain Pract 2019; 19: 397–406. [DOI] [PubMed] [Google Scholar]
- 76.Zhu PW, Chen Y, Gong YX, Jiang N, Liu WF, Su T, Ye L, Min YL, Yuan Q, He LC, Shao Y. Altered brain network centrality in patients with trigeminal neuralgia: a resting-state fMRI study. Acta Radiol 2020; 61: 67–75. [DOI] [PubMed] [Google Scholar]
- 77.Cheshire WP. Trigeminal neuralgia: for one nerve a multitude of treatments. Expert Rev Neurother 2007; 7: 1565–1579. [DOI] [PubMed] [Google Scholar]
- 78.Maarbjerg S, Di Stefano G, Bendtsen L, Cruccu G. Trigeminal neuralgia – diagnosis and treatment. Cephalalgia 2017; 37: 648–657. [DOI] [PubMed] [Google Scholar]
- 79.Bendtsen L, Zakrzewska JM, Abbott J, Braschinsky M, Di Stefano G, Donnet A, Eide PK, Leal PRL, Maarbjerg S, May A, Nurmikko T, Obermann M, Jensen TS, Cruccu G. European Academy of Neurology guideline on trigeminal neuralgia. Eur J Neurol 2019; 26: 831–849. [DOI] [PubMed] [Google Scholar]
- 80.Food and Drug Administration. Public meeting on neuropathic pain associated with peripheral neuropathy patient-focused drug development, www.fda.gov (2016, accessed 9 January 2019).
- 81.van Kleef M, Lataster A, Narouze S, Mekhail N, Geurts JW, van Zundert J. Evidence-based interventional pain medicine according to clinical diagnoses. 2. Cluster headache. Pain Pract 2009; 9: 435–442. [DOI] [PubMed] [Google Scholar]
- 82.Maan JS, Saadabadi A. Carbamazepine. StatPearls Treasure Island (FL), 2018.
- 83.Al-Quliti KW. Update on neuropathic pain treatment for trigeminal neuralgia. The pharmacological and surgical options. Neurosciences (Riyadh) 2015; 20: 107–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kuo CC, Chen RS, Lu L, Chen RC. Carbamazepine inhibition of neuronal Na+ currents: quantitative distinction from phenytoin and possible therapeutic implications. Mol Pharmacol 1997; 51: 1077–1083. [DOI] [PubMed] [Google Scholar]
- 85.Kuo CC. A common anticonvulsant binding site for phenytoin, carbamazepine, and lamotrigine in neuronal Na+ channels. Mol Pharmacol 1998; 54: 712–721. [PubMed] [Google Scholar]
- 86.Yang YC, Huang CS, Kuo CC. Lidocaine, carbamazepine, and imipramine have partially overlapping binding sites and additive inhibitory effect on neuronal Na+ channels. Anesthesiology 2010; 113: 160–174. [DOI] [PubMed] [Google Scholar]
- 87.Yarov-Yarovoy V, Brown J, Sharp EM, Clare JJ, Scheuer T, Catterall WA. Molecular determinants of voltage-dependent gating and binding of pore-blocking drugs in transmembrane segment IIIS6 of the Na(+) channel alpha subunit. J Biol Chem 2001; 276: 20–27. [DOI] [PubMed] [Google Scholar]
- 88.Yarov-Yarovoy V, McPhee JC, Idsvoog D, Pate C, Scheuer T, Catterall WA. Role of amino acid residues in transmembrane segments IS6 and IIS6 of the Na+ channel alpha subunit in voltage-dependent gating and drug block. J Biol Chem 2002; 277: 35393–35401. [DOI] [PubMed] [Google Scholar]
- 89.Ragsdale DS, McPhee JC, Scheuer T, Catterall WA. Common molecular determinants of local anesthetic, antiarrhythmic, and anticonvulsant block of voltage-gated Na+ channels. Proc Natl Acad Sci USA 1996; 93: 9270–9275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yang YC, Hsieh JY, Kuo CC. The external pore loop interacts with S6 and S3-S4 linker in domain 4 to assume an essential role in gating control and anticonvulsant action in the Na(+) channel. J Gen Physiol 2009; 134: 95–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Soares-da-Silva P, Pires N, Bonifácio MJ, Loureiro AI, Palma N, Wright LC. Eslicarbazepine acetate for the treatment of focal epilepsy: an update on its proposed mechanisms of action. Pharmacol Res Perspect 2015; 3: e00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hebeisen S, Pires N, Loureiro AI, Bonifacio MJ, Palma N, Whyment A, Spanswick D, Soares-da-Silva P. Eslicarbazepine and the enhancement of slow inactivation of voltage-gated sodium channels: a comparison with carbamazepine, oxcarbazepine and lacosamide. Neuropharmacology 2015; 89: 122–135. [DOI] [PubMed] [Google Scholar]
- 93.Sun GC, Werkman TR, Battefeld A, Clare JJ, Wadman WJ. Carbamazepine and topiramate modulation of transient and persistent sodium currents studied in HEK293 cells expressing the Na(v)1.3 alpha-subunit. Epilepsia 2007; 48: 774–782. [DOI] [PubMed] [Google Scholar]
- 94.Holland KD, Kearney JA, Glauser TA, Buck G, Keddache M, Blankston JR, Glaaser IW, Kass RS, Meisler MH. Mutation of sodium channel SCN3A in a patient with cryptogenic pediatric partial epilepsy. Neurosci Lett 2008; 433: 65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Qiao X, Sun G, Clare JJ, Werkman TR, Wadman WJ. Properties of human brain sodium channel alpha-subunits expressed in HEK293 cells and their modulation by carbamazepine, phenytoin and lamotrigine. Br J Pharmacol 2014; 171: 1054–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Boiko T, Rasband MN, Levinson SR, Caldwell JH, Mandel G, Trimmer JS, Matthews G. Compact myelin dictates the differential targeting of two sodium channel isoforms in the same axon. Neuron 2001; 30: 91–104. [DOI] [PubMed] [Google Scholar]
- 97.Lorincz A, Nusser Z. Cell-type-dependent molecular composition of the axon initial segment. J Neurosci 2008; 28: 14329–14340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Atkin TA, Maher CM, Gerlach AC, Gay BC, Antonio BM, Santos SC, Padilla KM, Rader J, Krafte DS, Fox MA, Stewart GR, Petrovski S, Devinsky O, Might M, Petrou S, Goldstein DB. A comprehensive approach to identifying repurposed drugs to treat SCN8A epilepsy. Epilepsia 2018; 59: 802–813. [DOI] [PubMed] [Google Scholar]
- 99.Jo S, Bean BP. Sidedness of carbamazepine accessibility to voltage-gated sodium channels. Mol Pharmacol 2014; 85: 381–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Theile JW, Cummins TR. Inhibition of Navbeta4 peptide-mediated resurgent sodium currents in Nav1.7 channels by carbamazepine, riluzole, and anandamide. Mol Pharmacol 2011; 80: 724–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhang S, Zhang Z, Shen Y, Zhu Y, Du K, Guo J, Ji Y, Tao J. SCN9A epileptic encephalopathy mutations display a gain-of-function phenotype and distinct sensitivity to oxcarbazepine. Neurosci Bull 2019; 36: 11–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.McLean MJ, Schmutz M, Wamil AW, Olpe HR, Portet C, Feldmann KF. Oxcarbazepine: mechanisms of action. Epilepsia 1994; 35: S5–S9. [DOI] [PubMed] [Google Scholar]
- 103.Schmidt D, Elger CE. What is the evidence that oxcarbazepine and carbamazepine are distinctly different antiepileptic drugs? Epilepsy Behav 2004; 5: 627–635. [DOI] [PubMed] [Google Scholar]
- 104.Abou-Khalil BW. Oxcarbazepine and carbamazepine: expected and unexpected differences and similarities. Epilepsy Curr 2007; 7: 74–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tomić M, Pecikoza U, Micov A, Vučković S, Stepanović-Petrović R. Antiepileptic drugs as analgesics/adjuvants in inflammatory pain: current preclinical evidence. Pharmacol Ther 2018; 192: 42–64. [DOI] [PubMed] [Google Scholar]
- 106.Cummins TR, Dib-Hajj SD, Waxman SG. Electrophysiological properties of mutant Nav1.7 sodium channels in a painful inherited neuropathy. J Neurosci 2004; 24: 8232–8236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Dib-Hajj SD, Rush AM, Cummins TR, Hisama FM, Novella S, Tyrrell L, Marshall L, Waxman SG. Gain-of-function mutation in Nav1.7 in familial erythromelalgia induces bursting of sensory neurons. Brain 2005; 128: 1847–1854. [DOI] [PubMed] [Google Scholar]
- 108.Faber CG, Hoeijmakers JG, Ahn HS, Cheng X, Han C, Choi JS, Estacion M, Lauria G, Vanhoutte EK, Gerrits MM, Dib-Hajj S, Drenth JP, Waxman SG, Merkies IS. Gain of function NaV1.7 mutations in idiopathic small fiber neuropathy. Ann Neurol 2012; 71: 26–39. [DOI] [PubMed] [Google Scholar]
- 109.Fischer TZ, Gilmore ES, Estacion M, Eastman E, Taylor S, Melanson M, Dib-Hajj SD, Waxman SG. A novel Nav1.7 mutation producing carbamazepine-responsive erythromelalgia. Ann Neurol 2009; 65: 733–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Rush AM, Elliott JR. Phenytoin and carbamazepine: differential inhibition of sodium currents in small cells from adult rat dorsal root ganglia. Neurosci Lett 1997; 226: 95–98. [DOI] [PubMed] [Google Scholar]
- 111.John VH, Main MJ, Powell AJ, Gladwell ZM, Hick C, Sidhu HS, Clare JJ, Tate S, Trezise DJ. Heterologous expression and functional analysis of rat Nav1.8 (SNS) voltage-gated sodium channels in the dorsal root ganglion neuroblastoma cell line ND7-23. Neuropharmacology 2004; 46: 425–438. [DOI] [PubMed] [Google Scholar]
- 112.Stummann TC, Salvati P, Fariello RG, Faravelli L. The anti-nociceptive agent ralfinamide inhibits tetrodotoxin-resistant and tetrodotoxin-sensitive Na+ currents in dorsal root ganglion neurons. Eur J Pharmacol 2005; 510: 197–208. [DOI] [PubMed] [Google Scholar]
- 113.Akopian AN, Sivilotti L, Wood JN. A tetrodotoxin-resistant voltage-gated sodium channel expressed by sensory neurons. Nature 1996; 379: 257–262. [DOI] [PubMed] [Google Scholar]
- 114.Brau ME, Dreimann M, Olschewski A, Vogel W, Hempelmann G. Effect of drugs used for neuropathic pain management on tetrodotoxin-resistant Na(+) currents in rat sensory neurons. Anesthesiology 2001; 94: 137–144. [DOI] [PubMed] [Google Scholar]
- 115.Cardenas CA, Cardenas CG, de Armendi AJ, Scroggs RS. Carbamazepine interacts with a slow inactivation state of NaV1.8-like sodium channels. Neurosci Lett 2006; 408: 129–134. [DOI] [PubMed] [Google Scholar]
- 116.Han JE, Cho JH, Nakamura M, Lee MG, Jang IS. Effect of carbamazepine on tetrodotoxin-resistant Na(+) channels in trigeminal ganglion neurons innervating to the dura. Korean J Physiol Pharmacol 2018; 22: 649–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Courtney KR, Etter EF. Modulated anticonvulsant block of sodium channels in nerve and muscle. Eur J Pharmacol 1983; 88: 1–9. [DOI] [PubMed] [Google Scholar]
- 118.Uebachs M, Opitz T, Royeck M, Dickhof G, Horstmann MT, Isom LL, Beck H. Efficacy loss of the anticonvulsant carbamazepine in mice lacking sodium channel beta subunits via paradoxical effects on persistent sodium currents. J Neurosci 2010; 30: 8489–8501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Deseure K, Hans GH. Differential drug effects on spontaneous and evoked pain behavior in a model of trigeminal neuropathic pain. J Pain Res 2017; 10: 279–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kopruszinski CM, Reis RC, Chichorro JG. B vitamins relieve neuropathic pain behaviors induced by infraorbital nerve constriction in rats. Life Sci 2012; 91: 1187–1195. [DOI] [PubMed] [Google Scholar]
- 121.Idänpään-Heikkilä JJ, Guilbaud G. Pharmacological studies on a rat model of trigeminal neuropathic pain: baclofen, but not carbamazepine, morphine or tricyclic antidepressants, attenuates the allodynia-like behaviour. Pain 1999; 79: 281–290. [DOI] [PubMed] [Google Scholar]
- 122.Ahn DK, Lim EJ, Kim BC, Yang GY, Lee MK, Ju JS, Han SR, Bae YC. Compression of the trigeminal ganglion produces prolonged nociceptive behavior in rats. Eur J Pain 2009; 13: 568–575. [DOI] [PubMed] [Google Scholar]
- 123.Jeon HJ, Han SR, Park MK, Yang KY, Bae YC, Ahn DK. A novel trigeminal neuropathic pain model: compression of the trigeminal nerve root produces prolonged nociception in rats. Prog Neuropsychopharmacol Biol Psychiatry 2012; 38: 149–158. [DOI] [PubMed] [Google Scholar]
- 124.Fortuna A, Alves G, Soares-da-Silva P, Falcão A. Pharmacokinetics, brain distribution and plasma protein binding of carbamazepine and nine derivatives: new set of data for predictive in silico ADME models. Epilepsy Res 2013; 107: 37–50. [DOI] [PubMed] [Google Scholar]
- 125.Farrokh S, Tahsili-Fahadan P, Ritzl EK, Lewin JJ, 3rd, Mirski MA. Antiepileptic drugs in critically ill patients. Crit Care 2018; 22: 153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Beydoun S, Alarcon F, Mangat S, Wan Y. Long-term safety and tolerability of oxcarbazepine in painful diabetic neuropathy. Acta Neurol Scand 2007; 115: 284–288. [DOI] [PubMed] [Google Scholar]
- 127.Cruccu G, Gronseth G, Alksne J, Argoff C, Brainin M, Burchiel K, Nurmikko T, Zakrzewska JM. AAN-EFNS guidelines on trigeminal neuralgia management. Eur J Neurol 2008; 15: 1013–1028. [DOI] [PubMed] [Google Scholar]
- 128.Rockliff BW, Davis EH. Controlled sequential trials of carbamazepine in trigeminal neuralgia. Arch Neurol 1966; 15: 129–136. [DOI] [PubMed] [Google Scholar]
- 129.Campbell FG, Graham JG, Zilkha KJ. Clinical trial of carbazepine (tegretol) in trigeminal neuralgia. J Neurol Neurosurg Psychiatry 1966; 29: 265–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wiffen PJ, Derry S, Moore RA, Kalso EA. Carbamazepine for chronic neuropathic pain and fibromyalgia in adults. Cochrane Database Syst Rev 2014; 4: CD005451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Di Stefano G, Truini A. Pharmacological treatment of trigeminal neuralgia. Expert Rev Neurother 2017; 17: 1003–1011. [DOI] [PubMed] [Google Scholar]
- 132.Beydoun A. Safety and efficacy of oxcarbazepine: results of randomized, double-blind trials. Pharmacotherapy 2000; 20: 152S–158S. [DOI] [PubMed] [Google Scholar]
- 133.Di Stefano G, La Cesa S, Truini A, Cruccu G. Natural history and outcome of 200 outpatients with classical trigeminal neuralgia treated with carbamazepine or oxcarbazepine in a tertiary centre for neuropathic pain. J Headache Pain 2014; 15: 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wiffen PJ, Derry S, Moore RA, McQuay HJ. Carbamazepine for acute and chronic pain in adults. Cochrane Database Syst Rev 2011; 1: CD005451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Tentolouris-Piperas V, Lee G, Reading J, O’Keeffe AG, Zakrzewska JM, Cregg R. Adverse effects of anti-epileptics in trigeminal neuralgiform pain. Acta Neurol Scand 2018; 137: 566–574. [DOI] [PubMed] [Google Scholar]
- 136.Bialer M. Why are antiepileptic drugs used for nonepileptic conditions? Epilepsia 2012; 53: 26–33. [DOI] [PubMed] [Google Scholar]
- 137.Sills GJ. The mechanisms of action of gabapentin and pregabalin. Curr Opin Pharmacol 2006; 6: 108–113. [DOI] [PubMed] [Google Scholar]
- 138.Patel R, Dickenson AH. Mechanisms of the gabapentinoids and alpha 2 delta-1 calcium channel subunit in neuropathic pain. Pharmacol Res Perspect 2016; 4: e00205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Field MJ, Cox PJ, Stott E, Melrose H, Offord J, Su TZ, Bramwell S, Corradini L, England S, Winks J, Kinloch RA, Hendrich J, Dolphin AC, Webb T, Williams D. Identification of the alpha2-delta-1 subunit of voltage-dependent calcium channels as a molecular target for pain mediating the analgesic actions of pregabalin. Proc Natl Acad Sci USA 2006; 103: 17537–17542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Taylor CP, Angelotti T, Fauman E. Pharmacology and mechanism of action of pregabalin: the calcium channel alpha2-delta (alpha2-delta) subunit as a target for antiepileptic drug discovery. Epilepsy Res 2007; 73: 137–150. [DOI] [PubMed] [Google Scholar]
- 141.Bauer CS, Nieto-Rostro M, Rahman W, Tran-Van-Minh A, Ferron L, Douglas L, Kadurin I, Sri Ranjan Y, Fernandez-Alacid L, Millar NS, Dickenson AH, Lujan R, Dolphin AC. The increased trafficking of the calcium channel subunit alpha2delta-1 to presynaptic terminals in neuropathic pain is inhibited by the alpha2delta ligand pregabalin. J Neurosci 2009; 29: 4076–4088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Pan HL, Eisenach JC, Chen SR. Gabapentin suppresses ectopic nerve discharges and reverses allodynia in neuropathic rats. J Pharmacol Exp Ther 1999; 288: 1026–1030. [PubMed] [Google Scholar]
- 143.Yang RH, Wang WT, Chen JY, Xie RG, Hu SJ. Gabapentin selectively reduces persistent sodium current in injured type-A dorsal root ganglion neurons. Pain 2009; 143: 48–55. [DOI] [PubMed] [Google Scholar]
- 144.Alles SRA, Smith PA. The anti-allodynic gabapentinoids: myths, paradoxes, and acute effects. Neuroscientist 2017; 23: 40–55. [DOI] [PubMed] [Google Scholar]
- 145.Kremer M, Salvat E, Muller A, Yalcin I, Barrot M. Antidepressants and gabapentinoids in neuropathic pain: mechanistic insights. Neuroscience 2016; 338: 183–206. [DOI] [PubMed] [Google Scholar]
- 146.Li KW, Yu YP, Zhou C, Kim DS, Lin B, Sharp K, Steward O, Luo ZD. Calcium channel alpha2delta1 proteins mediate trigeminal neuropathic pain states associated with aberrant excitatory synaptogenesis. J Biol Chem 2014; 289: 7025–7037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Rogawski MA, Loscher W. The neurobiology of antiepileptic drugs. Nat Rev Neurosci 2004; 5: 553–564. [DOI] [PubMed] [Google Scholar]
- 148.Bergouignan M. [Fifteen years of trial therapy of essential trigeminal neuralgia: the place of diphenylhydantoin and its derivatives]. Rev Neurol (Paris) 1958; 98: 414–416. [PubMed] [Google Scholar]
- 149.Keppel Hesselink JM, Schatman ME. Phenytoin and carbamazepine in trigeminal neuralgia: marketing-based versus evidence-based treatment. J Pain Res 2017; 10: 1663–1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Moore D, Chong MS, Shetty A, Zakrzewska JM. A systematic review of rescue analgesic strategies in acute exacerbations of primary trigeminal neuralgia. Br J Anaesth 2019; 123: e385–e396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Mantegazza M, Curia G, Biagini G, Ragsdale DS, Avoli M. Voltage-gated sodium channels as therapeutic targets in epilepsy and other neurological disorders. Lancet Neurol 2010; 9: 413–424. [DOI] [PubMed] [Google Scholar]
- 152.Lipkind GM, Fozzard HA. Molecular model of anticonvulsant drug binding to the voltage-gated sodium channel inner pore. Mol Pharmacol 2010; 78: 631–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Tikhonov DB, Zhorov BS. Mechanism of sodium channel block by local anesthetics, antiarrhythmics, and anticonvulsants. J Gen Physiol 2017; 149: 465–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Stefani A, Spadoni F, Siniscalchi A, Bernardi G. Lamotrigine inhibits Ca2+ currents in cortical neurons: functional implications. Eur J Pharmacol 1996; 307: 113–116. [DOI] [PubMed] [Google Scholar]
- 155.Dibue-Adjei M, Kamp MA, Alpdogan S, Tevoufouet EE, Neiss WF, Hescheler J, Schneider T. Cav2.3 (R-type) calcium channels are critical for mediating anticonvulsive and neuroprotective properties of lamotrigine in vivo. Cell Physiol Biochem 2017; 44: 935–947. [DOI] [PubMed] [Google Scholar]
- 156.Zona C, Tancredi V, Longone P, D’Arcangelo G, D’Antuono M, Manfredi M, Avoli M. Neocortical potassium currents are enhanced by the antiepileptic drug lamotrigine. Epilepsia 2002; 43: 685–690. [DOI] [PubMed] [Google Scholar]
- 157.Spina E, Pisani F, Perucca E. Clinically significant pharmacokinetic drug interactions with carbamazepine. An update. Clin Pharmacokinet 1996; 31: 198–214. [DOI] [PubMed] [Google Scholar]
- 158.Fromm GH, Chattha AS, Terrence CF, Glass JD. Role of inhibitory mechanisms in trigeminal neuralgia. Neurology 1981; 31: 683–687. [DOI] [PubMed] [Google Scholar]
- 159.Terrence CF, Sax M, Fromm GH, Chang CH, Yoo CS. Effect of baclofen enantiomorphs on the spinal trigeminal nucleus and steric similarities of carbamazepine. Pharmacology 1983; 27: 85–94. [DOI] [PubMed] [Google Scholar]
- 160.Fromm GH, Terrence CF, Chattha AS. Baclofen in the treatment of trigeminal neuralgia: double-blind study and long-term follow-up. Ann Neurol 1984; 15: 240–244. [DOI] [PubMed] [Google Scholar]
- 161.Fromm GH, Terrence CF, Chattha AS, Glass JD. Baclofen in trigeminal neuralgia: its effect on the spinal trigeminal nucleus: a pilot study. Arch Neurol 1980; 37: 768–771. [DOI] [PubMed] [Google Scholar]
- 162.Steardo L, Leo A, Marano E. Efficacy of baclofen in trigeminal neuralgia and some other painful conditions. A clinical trial. Eur Neurol 1984; 23: 51–55. [DOI] [PubMed] [Google Scholar]
- 163.Baker KA, Taylor JW, Lilly GE. Treatment of trigeminal neuralgia: use of baclofen in combination with carbamazepine. Clin Pharm 1985; 4: 93–96. [PubMed] [Google Scholar]
- 164.Parmar BS, Shah KH, Gandhi IC. Baclofen in trigeminal neuralgia – a clinical trial. Indian J Dent Res 1989; 1: 109–113. [PubMed] [Google Scholar]
- 165.Puri N, Rathore A, Dharmdeep G, Vairagare S, Prasad BR, Priyadarshini R, Singh HP. A clinical study on comparative evaluation of the effectiveness of carbamazepine and combination of carbamazepine with baclofen or capsaicin in the management of trigeminal neuralgia. Niger J Surg 2018; 24: 95–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Ertzgaard P, Campo C, Calabrese A. Efficacy and safety of oral baclofen in the management of spasticity: a rationale for intrathecal baclofen. J Rehabil Med 2017; 49: 193–203. [DOI] [PubMed] [Google Scholar]
- 167.Ghanavatian S, Derian A. Baclofen. Treasure Island: StatPearls, 2019. [Google Scholar]
- 168.Li P, Stewart R, Butler A, Gonzalez-Cota A L, Harmon S, Salkoff L, Li P, Stewart R, Butler A, Gonzalez-Cota AL, Harmon S, Salkoff L. GABA-B controls persistent Na+ current and coupled Na+-activated K+ current. eNeuro 2017; 4: ENEURO.0114-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Chen YW, Chuang SK. Botulinum toxin A might be an alternative or adjunct therapy for the treatment of trigeminal and postherpetic neuralgia. J Evid Based Dent Pract 2017; 17: 259–261. [DOI] [PubMed] [Google Scholar]
- 170.Meng F, Peng K, Yang JP, Ji FH, Xia F, Meng XW. Botulinum toxin-A for the treatment of neuralgia: a systematic review and meta-analysis. J Pain Res 2018; 11: 2343–2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Ostrowski H, Roszak J, Komisarek O. Botulinum toxin type A as an alternative way to treat trigeminal neuralgia: a systematic review. Neurol Neurochir Pol 2019; 53: 327–334. [DOI] [PubMed] [Google Scholar]
- 172.Kim DW, Lee SK, Ahnn J. Botulinum toxin as a pain killer: players and actions in antinociception. Toxins (Basel) 2015; 7: 2435–2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Durham PL, Cady R, Cady R. Regulation of calcitonin gene-related peptide secretion from trigeminal nerve cells by botulinum toxin type A: implications for migraine therapy. Headache 2004; 44: 35–42, discussion 42–33. [DOI] [PubMed] [Google Scholar]
- 174.Meng J, Ovsepian SV, Wang J, Pickering M, Sasse A, Aoki KR, Lawrence GW, Dolly JO. Activation of TRPV1 mediates calcitonin gene-related peptide release, which excites trigeminal sensory neurons and is attenuated by a retargeted botulinum toxin with anti-nociceptive potential. J Neurosci 2009; 29: 4981–4992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Shimizu T, Shibata M, Toriumi H, Iwashita T, Funakubo M, Sato H, Kuroi T, Ebine T, Koizumi K, Suzuki N. Reduction of TRPV1 expression in the trigeminal system by botulinum neurotoxin type-A. Neurobiol Dis 2012; 48: 367–378. [DOI] [PubMed] [Google Scholar]
- 176.Matak I, Bach-Rojecky L, Filipović B, Lacković Z. Behavioral and immunohistochemical evidence for central antinociceptive activity of botulinum toxin A. Neuroscience 2011; 186: 201–207. [DOI] [PubMed] [Google Scholar]
- 177.Wu C, Xie N, Lian Y, Xu H, Chen C, Zheng Y, Chen Y, Zhang H. Central antinociceptive activity of peripherally applied botulinum toxin type A in lab rat model of trigeminal neuralgia. Springerplus 2016; 5: 431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Gronseth G, Cruccu G, Alksne J, Argoff C, Brainin M, Burchiel K, Nurmikko T, Zakrzewska JM. Practice parameter: the diagnostic evaluation and treatment of trigeminal neuralgia (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology and the European Federation of Neurological Societies. Neurology 2008; 71: 1183–1190. [DOI] [PubMed] [Google Scholar]
- 179.Bohman LE, Pierce J, Stephen JH, Sandhu S, Lee JY. Fully endoscopic microvascular decompression for trigeminal neuralgia: technique review and early outcomes. Neurosurg Focus 2014; 37: E18. [DOI] [PubMed] [Google Scholar]
- 180.Missios S, Mohammadi AM, Barnett GH. Percutaneous treatments for trigeminal neuralgia. Neurosurg Clin N Am 2014; 25: 751–762. [DOI] [PubMed] [Google Scholar]
- 181.Sade B, Lee JH. Microvascular decompression for trigeminal neuralgia. Neurosurg Clin N Am 2014; 25: 743–749. [DOI] [PubMed] [Google Scholar]
- 182.Bescos A, Pascual V, Escosa-Bage M, Malaga X. [ Treatment of trigeminal neuralgia: an update and future prospects of percutaneous techniques]. Rev Neurol 2015; 61: 114–124. [PubMed] [Google Scholar]
- 183.Sanchez-Larsen A, Sopelana D, Diaz-Maroto I, Perona-Moratalla A B, Gracia-Gil J, García-Muñozguren S, Palazón-García E, Segura T. Assessment of efficacy and safety of eslicarbazepine acetate for the treatment of trigeminal neuralgia. Eur J Pain 2018; 22: 1080–1087. [DOI] [PubMed] [Google Scholar]
- 184.Gil-Nagel A, Elger C, Ben-Menachem E, Halász P, Lopes-Lima J, Gabbai AA, Nunes T, Falcão A, Almeida L, da-Silva P S. Efficacy and safety of eslicarbazepine acetate as add-on treatment in patients with focal-onset seizures: integrated analysis of pooled data from double-blind phase III clinical studies. Epilepsia 2013; 54: 98–107. [DOI] [PubMed] [Google Scholar]
- 185.Villanueva V, Serratosa JM, Guillamón E, Garcés M, Giráldez BG, Toledo M, Salas-Puig J, López González FJ, Flores J, Rodríguez-Uranga J, Castillo A, Mauri JA, Camacho JL, López-Gomáriz E, Giner P, Torres N, Palau J, Molins A. Long-term safety and efficacy of eslicarbazepine acetate in patients with focal seizures: results of the 1-year ESLIBASE retrospective study. Epilepsy Res 2014; 108: 1243–1252. [DOI] [PubMed] [Google Scholar]
- 186.Owen RT. Eslicarbazepine acetate: a novel agent for the adjunctive treatment of epilepsy. Drugs Today 2010; 46: 23–31. [DOI] [PubMed] [Google Scholar]
- 187.Massot A, Gimenez-Arnau A. Cutaneous adverse drug reaction type erythema multiforme major induced by eslicarbazepine. J Pharmacol Pharmacother 2014; 5: 271–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Kay L, Willems LM, Zollner JP, Reif PS, Klein KM, Rosenow F, Strzelczyk A. Eslicarbazepine acetate as a therapeutic option in a patient with carbamazepine-induced rash and HLA-A*31:01. Seizure 2017; 47: 81–82. [DOI] [PubMed] [Google Scholar]
- 189.Finelli E, Custodio P, Porovska O, Prates S, Leiria-Pinto P. Patch testing in a case of eslicarbazepine and carbamazepine induced cutaneous reaction. Eur Ann Allergy Clin Immunol 2018; 50: 229–231. [DOI] [PubMed] [Google Scholar]
- 190.Bialer M, Soares-da-Silva P. Pharmacokinetics and drug interactions of eslicarbazepine acetate. Epilepsia 2012; 53: 935–946. [DOI] [PubMed] [Google Scholar]
- 191.Alcantara Montero A, Sanchez Carnerero CI. Eslicarbazepine acetate for neuropathic pain, headache, and cranial neuralgia: evidence and experience. Neurologia 2019; 34: 386–395. [DOI] [PubMed] [Google Scholar]
- 192.Keppel Hesselink JM. Moving targets in sodium channel blocker development: the case of raxatrigine: from a central NaV1.3 blocker via a peripheral NaV1.7 blocker to a less selective sodium channel blocker. J Med Therap 2017; 1: 1–3. [Google Scholar]
- 193.Zakrzewska JM, Palmer J, Ettlin DA, Obermann M, Giblin GM, Morisset V, Tate S, Gunn K. Novel design for a phase IIa placebo-controlled, double-blind randomized withdrawal study to evaluate the safety and efficacy of CNV1014802 in patients with trigeminal neuralgia. Trials 2013; 14: 402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Deuis JR, Wingerd JS, Winter Z, Durek T, Dekan Z, Sousa SR, Zimmermann K, Hoffmann T, Weidner C, Nassar MA, Alewood PF, Lewis RJ, Vetter I. Analgesic effects of GpTx-1, PF-04856264 and CNV1014802 in a mouse model of NaV1.7-mediated pain. Toxins (Basel) 2016; 8: 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Mueller A, Starobova H, Morgan M, Dekan Z, Cheneval O, Schroeder CI, Alewood PF, Deuis JR, Vetter I. Anti-allodynic effects of the selective NaV1.7 inhibitor Pn3a in a mouse model of acute post-surgical pain: evidence for analgesic synergy with opioids and baclofen. Pain 2019; 160: 1766–1780. [DOI] [PubMed] [Google Scholar]
- 196.Zheng YM, Wang WF, Li YF, Yu Y, Gao ZB. Enhancing inactivation rather than reducing activation of Nav1.7 channels by a clinically effective analgesic CNV1014802. Acta Pharmacol Sin 2018; 39: 587–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Zakrzewska JM, Palmer J, Morisset V, Giblin GM, Obermann M, Ettlin DA, Cruccu G, Bendtsen L, Estacion M, Derjean D, Waxman SG, Layton G, Gunn K, Tate S, Study Investigators. Safety and efficacy of a Nav1.7 selective sodium channel blocker in patients with trigeminal neuralgia: a double-blind, placebo-controlled, randomised withdrawal phase 2a trial. Lancet Neurol 2017; 16: 291–300. [DOI] [PubMed] [Google Scholar]
- 198.Fong R, Ballow CH, Naik H, Steiner D, Palmer J, White WB. Effects of a state- and use-dependent Nav1.7 channel blocker on ambulatory blood pressure: a randomized, controlled crossover study. J Clin Pharmacol 2019; 59: 90–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Zakrzewska JM, Palmer J, Bendtsen L, Di Stefano G, Ettlin DA, Maarbjerg S, Obermann M, Morisset V, Steiner D, Tate S, Cruccu G. Challenges recruiting to a proof-of-concept pharmaceutical trial for a rare disease: the trigeminal neuralgia experience. Trials 2018; 19: 704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Kingwell K. Nav1.7 withholds its pain potential. Nat Rev Drug Discov 2019; 18: 321–323. doi: 10.1038/d41573-019-00065-0. [DOI] [PubMed] [Google Scholar]
- 201.Dib-Hajj SD, Waxman SG. Sodium channels in human pain disorders: genetics and pharmacogenomics. Annu Rev Neurosci 2019; 42: 87–106. [DOI] [PubMed] [Google Scholar]
- 202.Devulder J. Flupirtine in pain management: pharmacological properties and clinical use. CNS Drugs 2010; 24: 867–881. [DOI] [PubMed] [Google Scholar]
- 203.Mikuzuki L, Saito H, Katagiri A, Okada S, Sugawara S, Kubo A, Ohara K, Lee J, Toyofuku A, Iwata K. Phenotypic change in trigeminal ganglion neurons associated with satellite cell activation via extracellular signal-regulated kinase phosphorylation is involved in lingual neuropathic pain. Eur J Neurosci 2017; 46: 2190–2202. [DOI] [PubMed] [Google Scholar]
- 204.Sugawara S, Okada S, Katagiri A, Saito H, Suzuki T, Komiya H, Kanno K, Ohara K, Iinuma T, Toyofuku A, Iwata K. Interaction between calcitonin gene-related peptide-immunoreactive neurons and satellite cells via P2Y12 R in the trigeminal ganglion is involved in neuropathic tongue pain in rats. Eur J Oral Sci 2017; 125: 444–452. [DOI] [PubMed] [Google Scholar]
- 205.Michot B, Kayser V, Hamon M, Bourgoin S. CGRP receptor blockade by MK-8825 alleviates allodynia in infraorbital nerve-ligated rats. Eur J Pain 2015; 19: 281–290. [DOI] [PubMed] [Google Scholar]
- 206.Michot B, Bourgoin S, Viguier F, Hamon M, Kayser V. Differential effects of calcitonin gene-related peptide receptor blockade by olcegepant on mechanical allodynia induced by ligation of the infraorbital nerve vs the sciatic nerve in the rat. Pain 2012; 153: 1939–1948. [DOI] [PubMed] [Google Scholar]
- 207.Goadsby PJ, Edvinsson L, Ekman R. Release of vasoactive peptides in the extracerebral circulation of humans and the cat during activation of the trigeminovascular system. Ann Neurol 1988; 23: 193–196. [DOI] [PubMed] [Google Scholar]
- 208.Goadsby PJ, Edvinsson L, Ekman R. Vasoactive peptide release in the extracerebral circulation of humans during migraine headache. Ann Neurol 1990; 28: 183–187. [DOI] [PubMed] [Google Scholar]
- 209.Edvinsson L, Haanes KA, Warfvinge K, Krause DN. CGRP as the target of new migraine therapies – successful translation from bench to clinic. Nat Rev Neurol 2018; 14: 338–350. [DOI] [PubMed] [Google Scholar]
- 210.Qin ZL, Yang LQ, Li N, Yue JN, Wu BS, Tang YZ, Guo YN, Lai GH, Ni JX. Clinical study of cerebrospinal fluid neuropeptides in patients with primary trigeminal neuralgia. Clin Neurol Neurosurg 2016; 143: 111–115. [DOI] [PubMed] [Google Scholar]
- 211.Farajzadeh A, Bathaie SZ, Arabkheradmand J, Ghodsi SM, Faghihzadeh S. Different pain states of trigeminal neuralgia make significant changes in the plasma proteome and some biochemical parameters: a preliminary cohort study. J Mol Neurosci 2018; 66: 524–534. [DOI] [PubMed] [Google Scholar]