Abstract
Background:
Intra-articular hyaluronic acid (HA) injections and oral nonsteroidal anti-inflammatory drugs (NSAIDs) are common treatments for symptomatic knee osteoarthritis (OA). However, the comparative effects of these treatments are unclear.
Purpose:
To compare the efficacy and safety of intra-articular HA injections compared with oral NSAIDs for the treatment of knee OA.
Study Design:
Systematic review; Level of evidence, 1.
Methods:
We systematically searched Medline, Embase, and the Cochrane Central Register of Controlled Trials for randomized trials of knee OA treatment with HA injections compared with oral NSAIDs. The main outcomes were knee pain, knee function, adverse events (AEs), serious AEs, study withdrawals, and study withdrawals because of AEs. Pooled effect sizes were reported at the final follow-up with standardized mean difference (SMD) for efficacy outcomes and risk ratio (RR) for safety outcomes.
Results:
In 6 randomized trials of 831 patients (414 HA, 417 NSAIDs), with follow-up ranging from 5 to 26 weeks, HA injections were associated with small, statistically significant improvements in knee pain (SMD, 0.15; P = .04) and knee function (SMD, 0.23; P = .01) compared with oral NSAIDs. The risk of AEs was lower with HA compared with NSAIDs (19.8% vs 29.0%; RR, 0.74; P = .01). The risk of a serious AE (RR, 1.37; P = .71), study withdrawal (RR, 1.05; P = .68), or study withdrawal because of an AE (RR, 0.65; P = .22) was comparable between groups. Gastrointestinal concerns were the most frequent AE reported, occurring more often with NSAIDs (23.4% vs 14.1%; P = .001). AEs reported more frequently with HA injections were injection site pain (11.7% vs 4.7%; P < .001), headache (8.4% vs 4.4%; P = .03), and arthralgia (8.1% vs 2.9%; P = .001). Significant heterogeneity or publication bias was not observed for any outcome.
Conclusion:
Comparing short-term outcomes of HA injections with oral NSAIDs for treatment of knee OA, HA injections provided statistically significant but not clinically important improvements in knee pain and function, along with a lower overall risk of AEs.
Keywords: knee function, knee pain, nonsteroidal anti-inflammatory drug, safety, viscosupplementation
Osteoarthritis (OA) is a degenerative disease of the synovial joints that is characterized by progressive articular destruction and manifests clinically as joint pain and dysfunction. Knee OA is the leading cause of disability in older adults,11 and the prevalence of this disease is anticipated to increase in the coming decades.32 More than 1 in 3 Americans over 60 years of age have radiographic evidence of knee OA, with 35%-40% of affected patients reporting bothersome symptoms.17
Numerous pharmacologic and nonpharmacologic treatment options are available to individuals with symptomatic knee OA. In the clinical practice guidelines for knee OA treatment released by the American Academy of Orthopaedic Surgeons,4 the only recommended nonsurgical treatment appropriate for all patients was nonsteroidal anti-inflammatory drugs (NSAIDs). Oral NSAIDs are prescribed to approximately 65% of patients with knee OA19 despite significant limitations and health risks. Most patients who chronically consume NSAIDs continue to report persistent pain and disability, and over half will discontinue use within 1 year of receiving a prescription.36 Compared with placebo, ibuprofen increases the risk of stroke by over 3-fold.39 Diclofenac, which is the most effective NSAID available for OA treatment,14 increases the risks of stroke by 3-fold, cardiovascular death by 4-fold, and all-cause death by 2-fold.39 Further, NSAIDs as a class increase the risk of gastrointestinal complications with long-term use.36 For these reasons, oral NSAIDs are generally recommended for intermittent or cyclic use. Yet, this recommendation poses a dilemma to individuals with knee OA—continue with a therapy that may provide partial symptom relief at the expense of systemic complication risks or utilize an intermittent or cyclic medication regimen but with insufficient symptom control during periods of NSAID abstinence. In the typically older patient population with knee OA, an ideal nonsurgical treatment would provide clinically important improvements in OA-related symptoms without systemic complication risks.
Intra-articular injection of hyaluronic acid (HA) is intended to alleviate knee symptoms by improving the viscoelastic properties of synovial fluid and reversing OA-induced proinflammatory pathways.15 HA injections have been demonstrated to be safe for the treatment of knee OA,37 but efficacy results have been mixed.5–7,13,31,34,35,37,40 Because HA injections and oral NSAIDs are among the most commonly utilized nonoperative knee OA treatments,10 evaluation of their comparative efficacy and safety is warranted. Bannuru et al7 performed the only known direct-evidence meta-analysis of randomized trials comparing HA injections with oral NSAIDs for knee OA. The review included randomized trials published through 2013 and evaluated efficacy outcomes through 12-week follow-up. Yet, HA injections may provide symptom relief for longer periods,37 and oral NSAID use over longer durations is prevalent.21 Therefore, the objective of the current study was to extend these findings by performing a contemporary systematic review and meta-analysis comparing the efficacy and safety of HA injections and oral NSAIDs for treating knee OA through 26-week follow-up.
Methods
The methodology, analysis, and reporting of this systematic review adhered to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines.28 The review was prospectively registered in the International Prospective Register of Systematic Reviews (PROSPERO) public database (CRD42019128797; http://www.crd.york.ac.uk/PROSPERO).
Eligibility Criteria and Search Strategy
Eligible studies were randomized trials comparing HA injections with oral NSAIDs for treatment of knee OA. We systematically searched Medline, Embase, and the Cochrane Central Register of Controlled Trials for potentially eligible studies, with supplemental searches conducted in the Directory of Open Access Journals and Google Scholar. Manual searches of the reference lists of included papers and relevant meta-analyses were performed. No date, language, or sample size restrictions were applied to the searches. The search strategy included a combination of study design–, diagnosis-, and treatment-specific keywords (see Appendix Table A1). Two researchers (L.M.), both with expertise in systematic review searching, independently screened titles and abstracts for eligibility. Full-text manuscripts were obtained for all eligible studies and for those where eligibility was uncertain. Disagreements related to study eligibility were resolved by discussion. The final searches were performed on March 31, 2019.
Data Extraction
Data were extracted independently from eligible studies by 2 researchers (L.M.) using standardized data collection forms developed a priori. Data extraction discrepancies between the 2 researchers were resolved by discussion. Data items included metadata, patient characteristics, study characteristics, treatment regimens, efficacy outcomes, and safety outcomes. Efficacy outcomes included knee pain and knee function, which were extracted from papers in a nonbiased manner using the knee OA outcome meta-analysis hierarchy of Juhl et al.25 We extracted efficacy data at 3 distinct intervals—4 ± 1 weeks, 12 ± 1 weeks, and 26 ± 1 weeks—which align with typical reporting standards in the HA injection literature. The 26-week follow-up duration was chosen to approximate the 6-month stated duration of the effect on product labeling of HA products. Safety outcomes included adverse events (AEs), serious AEs, study withdrawals, and study withdrawals because of AEs. We also extracted and analyzed individual AEs reported in each study. Safety data were reported through the final follow-up in each trial. The frequency of AEs was categorized as very common (≥10%), common (1% to 10%), or uncommon (0.1% to 1%).38 We used the Cochrane Collaboration tool to assess the risk of bias in individual studies.22 Industry funding was considered as high risk of bias under the “other sources of bias” domain.
Data Analysis
Pooled efficacy outcomes were reported using the standardized mean difference (SMD) statistic. The SMD is used as a summary statistic when studies assess the same outcome using different scales, and, therefore, it is necessary to standardize the results to a uniform scale for analysis purposes. The SMD expresses the size of the treatment effect relative to the variability observed. For reference, SMD values of 0.2, 0.5, 0.8, and 1.0 are defined as small, medium, large, and very large effect sizes, respectively.12 Positive SMD values favored the HA group and negative values favored the NSAID group. The primary efficacy analysis was performed at the final follow-up in each trial, with temporal trends at each interval evaluated in a sensitivity analysis. Pooled safety outcomes were reported using the risk ratio (RR); an RR < 1 indicated lower risk with HA injections, and an RR > 1 indicated higher risk with HA injections. For each outcome, the effect size and 95% CI were calculated for each study, and the overall pooled results were visually displayed using forest plots. We used the I 2 statistic to estimate the heterogeneity of outcomes among studies, where a value of 0% represented no heterogeneity and larger values represented increasing heterogeneity.23 Significant heterogeneity was defined by a Cochran Q test (P < .1 or I 2 > 50%). When significant heterogeneity existed, a random-effects model was planned; otherwise, a fixed-effects model was planned.27 Publication bias was assessed by visual inspection of funnel plots and with Egger regression test.18 In accordance with Cochrane Collaboration recommendations, we planned to perform a meta-regression on the association of study-level factors with any efficacy or safety outcome reported in at least 10 studies; otherwise, these associations would be reported descriptively only. We analyzed the frequency of individual AEs in each group using counts and frequencies and compared the groups using the Fisher exact test. P values were 2-sided with a significance level <.05. Analyses were performed using Stata v 14.2 (StataCorp) and Review Manager v 5.3 (The Cochrane Collaboration).
Results
Study Selection
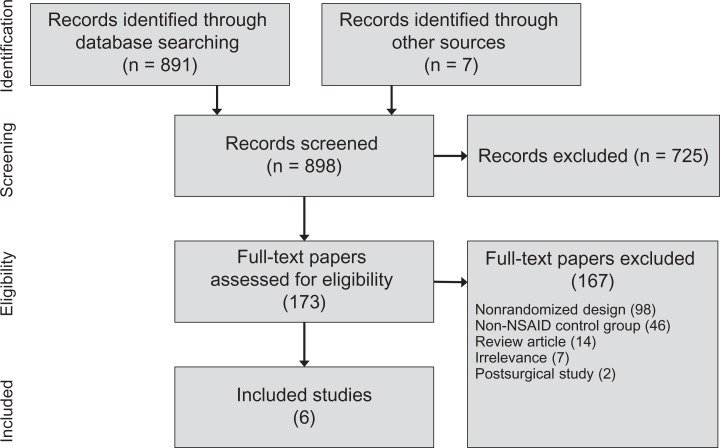
Among 898 records identified in our searches, we reviewed the full text of 173 potentially relevant papers. After review, 167 papers were excluded from further consideration, most commonly because of nonrandomized controlled study design or use of a non-NSAID control group. Ultimately, 6 randomized controlled trials comprising 831 patients (414 HA, 417 NSAIDs) were included in this review. A PRISMA flow diagram depicting the study identification and selection process is provided in Figure 1.
Figure 1.
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) study flow diagram. NSAID, nonsteroidal anti-inflammatory drug.
Patient and Study Characteristics
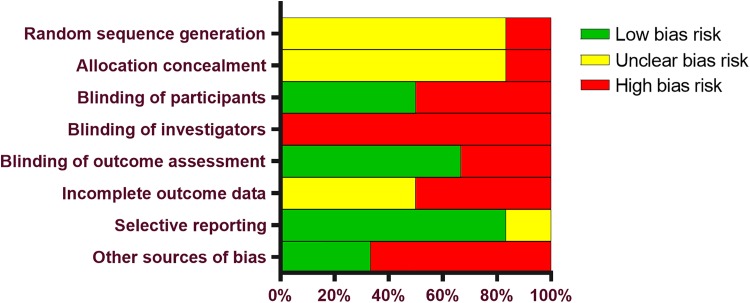
Baseline patient and study characteristics in each group are reported in Table 1. The mean patient age in each study ranged from 57 to 69 years, and there was a slight female predominance in most studies. Studies generally enrolled patients with mild-to-moderate radiographic evidence of knee OA; no study enrolled patients with a Kellgren-Lawrence grade 4 diagnosis. The total sample size in each trial ranged from 60 to 327, and follow-up durations ranged from 5 to 26 weeks. The study of Buendía-López et al8 included follow-up data through 52 weeks, but only outcomes through 26 weeks were extracted for meta-analysis. HA injections and oral NSAID treatment protocols varied among studies. HA injection treatment regimens included a single course of 1- (1 study), 3- (3 studies), or 5- (2 studies) weekly injections, and 3 studies facilitated patient blinding via use of oral placebos. The oral NSAID group consumed diclofenac in 2 studies and loxoprofen, etoricoxib, naproxen, or unspecified NSAIDs in 1 study each. Patient blinding in the oral NSAID group was conducted using additional arthrocentesis or saline injections in 4 studies. The most common potential sources of bias were lack of reports specifying investigator or participant blinding, industry bias, and incomplete outcome data (Appendix Table A2, Figure A1).
Table 1.
Patient and Study Characteristics in Randomized Controlled Trials Included in the Meta-analysisa
| Study | No. Randomized (HA, NSAID) | Age, y (HA, NSAID) | Female, % (HA, NSAID) | BMI, kg/m2 (HA, NSAID) | Symptom Duration, y (HA, NSAID) | K-L Grade | Treatment Regimen | Effectiveness Outcomes | Follow-up, wk |
|---|---|---|---|---|---|---|---|---|---|
| Adams, 19951 | 31, 34 | 61, 63 | 68, 68 | 27, 24 | 5, 8 | 1-3 | HA: 3-weekly Synvisc injections NSAID: Usual NSAIDs and 3-weekly arthrocentesis |
Pain: Pain with motion VAS Function: Restriction of activity VAS |
26 |
| Altman, 19982 | 164, 163 | 62, 63 | 61, 57 | 32, 32 | ≥1, ≥1 | 2-3 | HA: 5-weekly Hyalgan injections and oral placebo bid NSAID: Naproxen 500 mg bid and 5-weekly sham injections |
Pain: Pain on 50-foot walk VAS Function: Not reported |
26 |
| Dickson, 200116 | 53, 55 | 65, 64 | 57, 55 | 29, 29 | — | — | HA: 3-weekly Synvisc injections and oral placebo qd NSAID: Diclofenac 100 mg qd and 3-weekly arthrocentesis |
Pain: WOMAC Pain Function: WOMAC Function |
12 |
| Petrella, 200233 | 30, 30 | 67, 66 | 36, 42 | 30, 29 | — | 1-3 | HA: 3-weekly Suplasyn injections and oral placebo bid NSAID: Diclofenac 75 mg bid and 3-weekly saline injections |
Pain: WOMAC Pain Function: WOMAC Function |
12 |
| Ishijima, 201424 | 100, 100 | 68, 69 | 72, 71 | 24, 24 | — | 1-3 | HA: 5-weekly Suvenyl injections NSAID: Loxoprofen 60 mg tid |
Pain: Pain VAS Function: JKOM |
5 |
| Buendía-López, 20188 | 36, 35 | 57, 57 | 53, 52 | 25, 25 | — | 1-2 | HA: 1 Durolane injection NSAID: Etoricoxib 60 mg qd |
Pain: WOMAC Pain Function: WOMAC Function |
26b |
abid, twice a day; BMI, body mass index; HA, hyaluronic acid; JKOM, Japanese Knee Osteoarthritis Measure; K-L, Kellgren-Lawrence; NSAID, nonsteroidal anti-inflammatory drug; qd, four times a day; tid, three times a day; VAS, visual analog scale; WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index.
bStudy follow-up was through 52 wk, but data for meta-analysis were extracted through 26 wk only.
Efficacy Outcomes
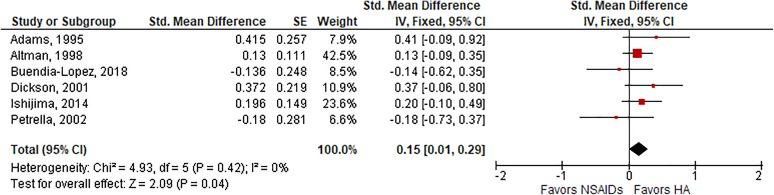
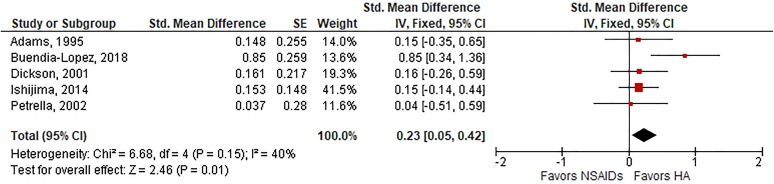
Among 6 trials with follow-up ranging from 5 to 26 weeks, HA injections were associated with a small, statistically significant improvement in knee pain compared with oral NSAIDs (SMD, 0.15; P = .04) (Figure 2). Heterogeneity among studies was low (I 2 = 0%; P = .42), and publication bias was not observed (Egger P = .84). Similar results were observed for knee function, in which a small effect size favoring HA injections was observed in 5 trials (SMD, 0.23; P = .01) (Figure 3), with low-to-moderate heterogeneity (I 2 = 40%; P = .15) and no evidence of publication bias (Egger P = .62). In a sensitivity analysis of temporal trends, there were no statistically significant differences between groups for knee pain or knee function at 4-week or 12-week follow-up. At 26-week follow-up, HA injections were associated with small but not statistically significant improvements in knee pain (SMD, 0.17; P = .07) and moderate improvements in knee function (SMD, 0.49; P < .01) relative to oral NSAIDs (Table 2).
Figure 2.
Standardized mean difference (SMD) for knee pain with hyaluronic acid (HA) injection vs oral nonsteroidal anti-inflammatory drugs (NSAIDs). SMD = 0.15 (P = .04), favoring HA injections, in fixed-effects model. Heterogeneity: I 2 = 0%, P = .42. Publication bias: Egger P value = .84. IV, inverse variance; SE, standard error.
Figure 3.
Standardized mean difference (SMD) for knee function with hyaluronic acid (HA) injection vs oral nonsteroidal anti-inflammatory drugs (NSAIDs). SMD = 0.23 (P = .01), favoring HA injections, in fixed-effects model. Heterogeneity: I 2 = 40%, P = .15. Publication bias: Egger P value = .62. IV, inverse variance; SE, standard error.
Table 2.
Sensitivity Analysis of Temporal Trends in Efficacy Outcomes With Hyaluronic Acid Injection vs Oral Nonsteroidal Anti-Inflammatory Drugsa
| Follow-Up | Knee Pain | Knee Function |
|---|---|---|
| 4-5 wk | SMD = 0.00, P = .99 (3 studies) | SMD = 0.13, P = .33 (2 studies) |
| 12 wk | SMD = –0.03, P = .78 (3 studies) | SMD = 0.01, P = .97 (2 studies) |
| 26 wk | SMD = 0.17, P = .07 (3 studies) | SMD = 0.49, P < .01 (2 studies) |
| Final (≥4 wk)b | SMD = 0.15, P = .04 (6 studies) | SMD = 0.23, P = .01 (5 studies) |
| Final (≥12 wk)c | SMD = 0.17, P = .05 (4 studies) | SMD = 0.36, P = .01 (3 studies) |
aSMD, standardized mean difference.
bFinal follow-up interval in each study between 4 and 26 wk used for analysis.
cFinal follow-up interval in each study between 12 and 26 wk used for analysis.
Safety Outcomes
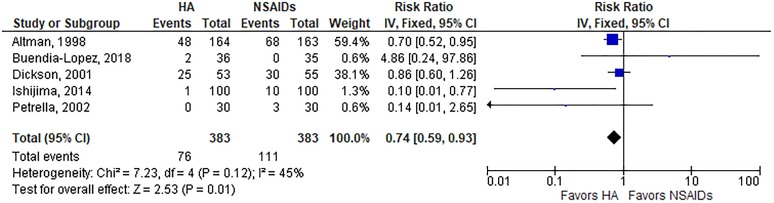
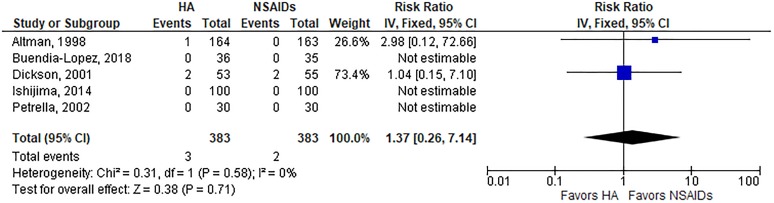
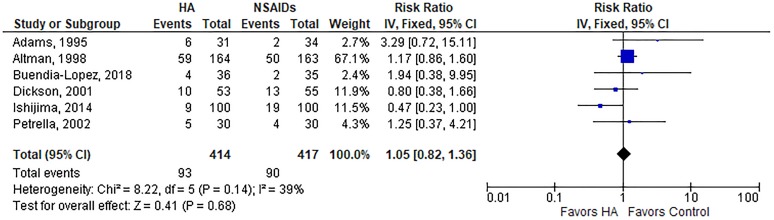
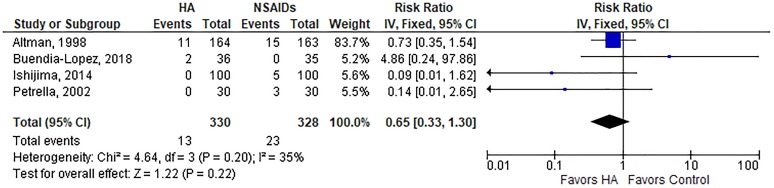
A summary of safety outcomes is provided in Table 3. The risk of AEs was lower with HA injections than oral NSAIDs (19.8% vs 29.0%; RR, 0.74; P = .01) (Appendix Figure A2). The risk of a serious AE (RR, 1.37; P = .71) (Appendix Figure A3), study withdrawal (RR, 1.05; P = .68) (Appendix Figure A4), or study withdrawal because of an AE (RR, 0.65; P = .22) (Appendix Figure A5) was comparable between groups. Significant heterogeneity or publication bias was not observed for any safety outcome. While the overall risk of AEs was lower with HA injections, analysis of individual AEs revealed distinct risks associated with each treatment. Gastrointestinal concerns were the most frequent AE reported, occurring more often in patients treated with NSAIDs (23.4% vs 14.1%; P = .001). AEs reported more often in the HA group included injection site pain (11.7% vs 4.7%; P < .001), headache (8.4% vs 4.4%; P = .03), and arthralgia (8.1% vs 2.9%; P = .001) (Table 4).
Table 3.
Summary of Safety Outcomes With HA Injection vs Oral NSAIDsa
| Outcome | No. Studies | Frequency, % | Meta-analysis Results | |||||
|---|---|---|---|---|---|---|---|---|
| HA | NSAID | Model | Risk Ratio (95% CI)b | P | Heterogeneity (I 2) | Publication Bias (Egger P Value) | ||
| AE | 5 | 19.8 | 29.0 | Fixed | 0.74 (0.59, 0.94) | .01 | 45%, P = .12 | .58 |
| Serious AE | 5 | 1.2 | 0.9 | Fixed | 1.37 (0.26, 7.14) | .71 | 0%, P = .58 | .83 |
| Study withdrawals | 6 | 22.5 | 21.6 | Fixed | 1.05 (0.82, 1.36) | .68 | 39%, P = .14 | .88 |
| AE-related study withdrawals | 4 | 3.9 | 7.0 | Fixed | 0.65 (0.33, 1.30) | .22 | 36%, P = .20 | .70 |
aAE, adverse event; HA, hyaluronic acid; NSAID, nonsteroidal anti-inflammatory drug.
bRisk ratio values <1 indicate lower risk and values >1 indicate higher risk for hyaluronic acid injections.
Table 4.
Frequency of Individual AEs With HA Injection vs Oral NSAIDsa
| AEb | HA (n = 383) | NSAID (n = 385) | P |
|---|---|---|---|
| Very common | |||
| Gastrointestinal | 54 (14.1) | 90 (23.4) | .001 |
| Injection site pain | 45 (11.7) | 18 (4.7) | <.001 |
| Common | |||
| Headache | 32 (8.4) | 17 (4.4) | .03 |
| Arthralgia | 31 (8.1) | 11 (2.9) | .001 |
| Local skin reaction | 23 (6.0) | 29 (7.5) | .47 |
| Pruritis | 12 (3.1) | 7 (1.8) | .26 |
| Uncommon | |||
| Drug allergy/intolerance | 0 | 4 (1.0) | .12 |
| Lower leg effusion | 1 (0.3) | 2 (0.5) | >.99 |
| Unspecified | 2 (0.5) | 2 (0.5) | >.99 |
| Cardiac | 1 (0.3) | 0 | .50 |
aData are reported as count (%). AE, adverse event; HA, hyaluronic acid; NSAID, nonsteroidal anti-inflammatory drug.
bFrequency of adverse events categorized according to guidelines set forth by the Council for International Organizations of Medical Sciences Working Group.38
Post-Hoc Meta-Regression Results
Because <10 trials were included in this meta-analysis, meta-regression results were reported descriptively only. For each efficacy and safety outcome, we evaluated the influence of double blinding, industry funding, sample size, number of HA injections, and oral NSAID type. The most consistent finding of this analysis was related to the risk of AEs among influential confounders. Specifically, the overall risk of AEs remained lower with HA injections than oral NSAIDs among double-blind trials (RR, 0.75; 95% CI, 0.60, 0.95), trials without industry funding (RR, 0.75; 95% CI, 0.60, 0.95), and trials with sample sizes of at least 100 per group (RR, 0.67; 95% CI, 0.50, 0.91) (Appendix Table A3).
Discussion
HA injections and oral NSAIDs are common treatments for knee OA, yet there is a paucity of high-quality evidence directly comparing these treatments. In this systematic review and meta-analysis of randomized trials comparing HA injections with oral NSAIDs, we identified several main findings. Regarding efficacy, statistically significant improvements in knee pain and knee function were observed with HA injections relative to oral NSAIDs, but the differences were not clinically important. Regarding safety, the overall risk of AEs was lower with HA injections, yet each treatment was associated with a distinct risk profile where oral NSAIDs increased the risk of gastrointestinal concerns and HA injections increased the risk of local reactions and headache. Finally, because only 6 trials were included in this review with only 3 providing follow-up results through 26 weeks, additional studies are warranted to provide more stable effect size estimates and to facilitate more robust analyses regarding potential sources of heterogeneity.
That HA injections yielded statistically significant improvements in knee OA symptoms relative to oral NSAIDs was a novel finding and warrants further discussion. The only previous systematic review and meta-analysis directly comparing HA injections with oral NSAIDs for knee OA was performed by Bannuru et al,7 who reported no differences between groups for knee pain. Our results were nearly identical to those of Bannuru et al for knee pain at 4 weeks (SMD, 0.00 vs SMD, 0.01) and 12 weeks (SMD, –0.03 vs SMD, –0.05). However, our study additionally evaluated pain at 26-week follow-up, whereas Bannuru et al's study utilized 12 weeks of follow-up. Our study showed that the efficacy benefits of HA injections were mainly realized with longer follow-up duration, which explains the discrepant conclusions between the 2 meta-analyses. Collectively, the results of these meta-analyses suggest that the efficacy of HA injections and oral NSAIDs is generally comparable through 12 weeks, with slightly greater symptomatic relief with HA injections through 26 weeks. However, the magnitude of the treatment effect was small, and, therefore, the group differences observed in efficacy outcomes were not clinically important.
The efficacy data presented here should be balanced against treatment safety considerations, particularly because patients with knee OA are often elderly with polypharmacy.9 Oral NSAIDs increased the risk of gastrointestinal concerns, whereas HA injections were associated with higher risk for local events and headache. Gastrointestinal complications associated with NSAID use can be mitigated by using Cox-selective NSAIDs, with further risk reductions with the addition of a proton-pump inhibitor.3 Strategies to prevent local events with HA injections are less clear, but it appears that younger age, longer time since diagnosis, and previous HA injections increase these risks.26 Serious AEs were rare, occurring in approximately 1% of patients, and none were related to treatment. Unfortunately, we were unable to determine long-term risks with either treatment, which would have more relevance to the typical patient with knee OA who attempts nonsurgical treatments over a period of years.29 For example, the increased cardiac risks associated with NSAIDs may not be detectable for at least 12 to 18 months,20 a duration that greatly exceeds the follow-up duration of studies in the current review. Ultimately, the results of this meta-analysis suggest that the risk-to-benefit profile may slightly favor HA injections over oral NSAIDs in the short term, but longer term data are needed to better replicate real-world conditions and are encouraged to provide higher quality evidence to assist in clinical decision making.
An important consideration that was not formally assessed in this meta-analysis, yet strongly influences treatment selection, is the cost of therapy. The cost of a 6-month course of oral NSAIDs is highly variable, ranging from $130 for over-the-counter naproxen, to $440 for generic celecoxib, to $1750 for brand name celecoxib (Celebrex),30 while a course of HA injections that is anticipated to provide 6 months of symptom relief costs approximately $1100.41 In previous studies, cost-effectiveness appears to have been comparable with HA injections and nonselective oral NSAIDs (each approximately $15,000 per quality-adjusted life year).29 Cox-selective NSAIDs, on the other hand, were not cost-effective for treatment of knee OA.29,30 Because HA injections resulted in slightly better clinical outcomes than oral NSAIDs in the current study, but likely at a higher cost, contemporary cost-effectiveness analyses that compare these 2 treatments are warranted.
Strengths of this meta-analysis included reporting of knee pain, knee function, and detailed safety data through 26-week follow-up, none of which have been compared previously with these treatments. There were also several limitations pertaining to the quality and quantity of the underlying studies included in this review. First, only 6 trials comparing HA injections with oral NSAIDs for knee OA treatment have been conducted, with only 3 providing follow-up results through 26 weeks. This limited number of studies provided low statistical power to detect publication bias and temporal trends in efficacy and hindered adequate exploration of sources of heterogeneity, such as HA type, oral NSAID type, blinding techniques, and industry funding. Second, none of the trials enrolled patients with severe knee OA, and, therefore, the comparative efficacy of HA injections and oral NSAIDs in this patient population remains unclear. Third, patient-reported efficacy outcomes were susceptible to bias because patients were aware of their treatment assignment in 3 of 6 studies. Fourth, while some studies used sham injections to reduce bias in patient-reported outcomes, the AEs reported in this study may differ from real-world practice because events, such as injection site pain and local skin reaction, would not apply to patients taking oral NSAIDs only. Finally, because few patients with knee OA ultimately receive knee arthroplasty29 and must resort to chronic use of nonoperative treatments, the short-term outcomes of this meta-analysis may not be reflective of the results associated with longer term HA and oral NSAID use.
Conclusion
Comparing short-term outcomes of HA injections and oral NSAIDs for treatment of knee OA, HA injections provided statistically significant but not clinically important improvements in knee pain and function, along with a lower overall risk of AEs.
Acknowledgment
The authors thank David Fay, PhD, for assistance with systematic review and data extraction.
Appendix
Table A1.
Medline Search Strategya
| Study design |
| 1. clinical trial.pt |
| 2. clinical trial, phase I.pt |
| 3. clinical trial, phase II.pt |
| 4. clinical trial, phase III.pt |
| 5. clinical trial, phase IV.pt |
| 6. controlled clinical trial.pt |
| 7. randomized controlled trial.pt |
| 8. random* |
| General diagnosis |
| 9. arthriti**, ti, ab |
| 10. arthro**, ti, ab |
| 11. gonarthriti**, ti, ab |
| 12. gonarthro**, ti, ab |
| 13. osteoarthriti*, ti, ab |
| 14. osteoarthritis, majr |
| 15. osteoarthro**, ti, ab |
| Diagnosis location |
| 16. knee, mp |
| Hyaluronic acid |
| 17. adant, mp |
| 18. arthrum, mp |
| 19. artz*, mp |
| 20. biohy, mp |
| 21. durolane, mp |
| 22. euflexxa, mp |
| 23. gel-one, mp |
| 24. gelsyn*, mp |
| 25. healon, mp |
| 26. hya-ject, mp |
| 27. hyalectin, mp |
| 28. hyalgan, mp |
| 29. hyaluron*, mp |
| 30. hylan, mp |
| 31. hymovis, mp |
| 32. monovisc, mp |
| 33. nrd101, mp |
| 34. nuflexxa, mp |
| 35. orthovisc, mp |
| 36. ostenil, mp |
| 37. supartz, mp |
| 38. suplasyn, mp |
| 39. synojoint, mp |
| 40. synvisc*, mp |
| 41. trivisc, mp |
| 42. viscosupplement*, mp |
| 43. visco-3, mp |
| Nonsteroidal anti-inflammatory drugs |
| 44. aspirin |
| 45. celebrex |
| 46. celecoxib |
| 47. cox-2 |
| 48. diclofenac |
| 49. diflunisal |
| 50. etodolac |
| 51. fenoprofen |
| 52. ibuprofen |
| 53. indomethacin |
| 54. ketoprofen |
| 55. meclofenamate |
| 56. mefanamic |
| 57. meloxicam |
| 58. nabumetone |
| 59. naproxen |
| 60. nonsteroidal anti-inflammatory drug |
| 61. NSAID |
| 62. oxaprozin |
| 63. piroxicam |
| 64. sulindac |
| 65. tolmetin |
| Combination terms |
| 66. or/1-8 |
| 67. or/9-15 |
| 68. or/16 |
| 69. or/17-43 |
| 70. or/44-65 |
| 71. and/66-70 |
aAn asterisk represents wildcard end-truncation.
Table A2.
Cochrane Risk of Bias Assessment Among Individual Studies
| Study | Random Sequence Generation | Allocation Concealment | Blinding of Participants | Blinding of Personnel | Blinding of Outcome Assessment | Incomplete Outcome Data | Selective Outcome Reporting | Other Sources of Bias |
|---|---|---|---|---|---|---|---|---|
| Adams, 19951 |

|

|

|

|

|

|

|

|
| Altman, 19982 |

|

|

|

|

|

|

|

|
| Dickson, 200116 |

|

|

|

|

|

|

|

|
| Petrella, 200233 |

|

|

|

|

|

|

|

|
| Ishijima, 201424 |

|

|

|

|

|

|

|

|
| Buendía-López, 20188 |

|

|

|

|

|

|

|

|
 low bias risk;
low bias risk;  uncertain bias risk;
uncertain bias risk;  high bias risk.
high bias risk.
Table A3.
Post Hoc Subgroup Analysis of the Association of Study-Level Factors With Treatment Effects With HA Injection vs Oral NSAIDsa
| Variable | SMD (95% CI)b | Risk Ratio (95% CI)c | ||||
|---|---|---|---|---|---|---|
| Knee Pain | Knee Function | AE | Serious AE | Study Withdrawal | Study Withdrawal Because of AE | |
| Double-blinding | ||||||
| Yes | 0.14 (–0.04, 0.32) | 0.11 (−0.22, 0.45) | 0.75 (0.60, 0.95) | 1.31 (0.29, 5.97) | 1.11 (0.85, 1.47) | 0.66 (0.32, 1.36) |
| No | 0.17 (–0.06, 0.39) | 0.29 (0.06, 0.51) | 0.34 (0.06, 1.84) | 0.99 (0.06, 15.6) | 0.80 (0.43, 1.48) | 0.61 (0.08, 4.90) |
| Industry funding | ||||||
| Yes | 0.17 (0.00, 0.34) | 0.13 (−0.15, 0.40) | 0.34 (0.06, 1.84) | 0.99 (0.06, 15.6) | 0.60 (0.31, 1.19) | 0.61 (0.08, 4.90) |
| No | 0.11 (–0.14, 0.36) | 0.32 (0.07, 0.58) | 0.75 (0.60, 0.95) | 1.31 (0.29, 5.97) | 1.15 (0.88, 1.51) | 0.66 (0.32, 1.36) |
| Sample size | ||||||
| ≥100/group | 0.15 (–0.02, 0.33) | 0.15 (−0.14, 0.44) | 0.67 (0.50, 0.91) | 1.93 (0.16, 22.8) | 1.03 (0.77, 1.37) | 0.64 (0.31, 1.32) |
| <100/group | 0.15 (–0.10, 0.39) | 0.29 (0.05, 0.54) | 0.86 (0.60, 1.25) | 1.02 (0.21, 4.94) | 1.16 (0.67, 2.00) | 0.80 (0.10, 6.45) |
| No. of HA injections | ||||||
| 1 | d | d | d | d | d | d |
| 3 | 0.24 (−0.04, 0.52) | 0.13 (−0.15, 0.40) | 0.84 (0.58, 1.22) | 1.03 (0.18, 5.78) | 1.09 (0.61, 1.94) | 0.14 (0.01, 2.65) |
| 5 | 0.15 (−0.02, 0.33) | 0.15 (−0.14, 0.44) | 0.67 (0.50, 0.91) | 1.93 (0.16, 22.8) | 1.03 (0.77, 1.37) | 0.64 (0.31, 1.32) |
| Oral NSAID type | ||||||
| Cox-selective | d | d | d | d | d | d |
| Nonselectiveb | 0.18 (0.03, 0.33) | 0.14 (−0.06, 0.34) | 0.73 (0.58, 0.93) | 1.26 (0.31, 5.20) | 1.04 (0.80, 1.34) | 0.59 (0.29, 1.18) |
aAE, adverse event; HA, hyaluronic acid; NSAID, nonsteroidal anti-inflammatory drug; SMD, standardized mean difference.
bBolded values indicate a statistically significant difference between groups because the 95% CI of the SMD does not include 0.
cBolded values indicate a statistically significant difference between groups because the 95% CI of the risk ratio does not include 1.
dData reported in a single trial; therefore, pooled estimates are not available.
Figure A1.
Cochrane risk of bias assessment among possible sources of bias.
Figure A2.
Risk of adverse event with hyaluronic acid (HA) injection vs oral nonsteroidal anti-inflammatory drugs (NSAIDs). Risk ratio = 0.74 (P = .01), favoring hyaluronic acid injections, in fixed-effects model. Heterogeneity: I 2 = 45%, P = .12. Publication bias: Egger P value = .58. IV, inverse variance.
Figure A3.
Risk of serious adverse event with hyaluronic acid (HA) injection vs oral nonsteroidal anti-inflammatory drugs (NSAIDs). Risk ratio = 1.37 (P = .71), indicating no group differences, in fixed-effects model. Heterogeneity: I 2 = 0%, P = .58. Publication bias: Egger P value = .83. IV, inverse variance.
Figure A4.
Risk of study withdrawal with hyaluronic acid (HA) injection vs oral nonsteroidal anti-inflammatory drugs (NSAIDs). Risk ratio = 1.05 (P = .68), indicating no group differences, in fixed-effects model. Heterogeneity: I 2 = 39%, P = .14. Publication bias: Egger P value = .88. IV, inverse variance.
Figure A5.
Risk of study withdrawal because of adverse event with hyaluronic acid (HA) injection vs oral nonsteroidal anti-inflammatory drugs (NSAIDs). Risk ratio = 0.65 (P = .22), indicating no group differences, in fixed-effects model. Heterogeneity: I 2 = 35%, P = .20. Publication bias: Egger P value = .70. IV, inverse variance.
Footnotes
Final revision submitted September 25, 2019; accepted October 10, 2019.
One or more of the authors has declared the following potential conflict of interest or source of funding: L.E.M. has consulting affiliations with DePuy Synthes and OsteoArthritis Centers of America. M.F. has received honoraria from Fidia Pharma and hospitality payments from DePuy and Ipsen Innovation. R.D.A. has received speaking fees from Genzyme and Iroko Pharmaceuticals; hospitality payments from Bioventus; honoraria from Teva Pharmaceuticals; and consulting fees from Meda Pharmaceuticals, Insys Therapeutics, Ferring Pharmaceuticals, Pfizer, Genzyme, Iroko Pharmaceuticals, Novartis, Merck Sharp & Dohme, and Sanofi-Aventis. He also reports affiliations with GlaxoSmithKline, Sorrento, and Theralogix. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
References
- 1. Adams ME, Atkinson MH, Lussier AJ, et al. The role of viscosupplementation with hylan G-F 20 (Synvisc) in the treatment of osteoarthritis of the knee: a Canadian multicenter trial comparing hylan G-F 20 alone, hylan G-F 20 with non-steroidal anti-inflammatory drugs (NSAIDs) and NSAIDs alone. Osteoarthritis Cartilage. 1995;3(4):213–225. [DOI] [PubMed] [Google Scholar]
- 2. Altman RD, Moskowitz R. Intraarticular sodium hyaluronate (Hyalgan) in the treatment of patients with osteoarthritis of the knee: a randomized clinical trial. Hyalgan Study Group. J Rheumatol. 1998;25(11):2203–2212. [PubMed] [Google Scholar]
- 3. Bakhriansyah M, Souverein PC, de Boer A, Klungel OH. Gastrointestinal toxicity among patients taking selective COX-2 inhibitors or conventional NSAIDs, alone or combined with proton pump inhibitors: a case-control study. Pharmacoepidemiol Drug Saf. 2017;26(10):1141–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. American Academy of Orthopaedic Surgeons. Treatment of osteoarthritis of the knee: evidence-based guideline. 2nd ed Rosemont, IL: American Academy of Orthopaedic Surgeons; 2013. [DOI] [PubMed] [Google Scholar]
- 5. Bannuru RR, Natov NS, Dasi UR, Schmid CH, McAlindon TE. Therapeutic trajectory following intra-articular hyaluronic acid injection in knee osteoarthritis--meta-analysis. Osteoarthritis Cartilage. 2011;19(6):611–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bannuru RR, Natov NS, Obadan IE, Price LL, Schmid CH, McAlindon TE. Therapeutic trajectory of hyaluronic acid versus corticosteroids in the treatment of knee osteoarthritis: a systematic review and meta-analysis. Arthritis Rheum. 2009;61(12):1704–1711. [DOI] [PubMed] [Google Scholar]
- 7. Bannuru RR, Vaysbrot EE, Sullivan MC, McAlindon TE. Relative efficacy of hyaluronic acid in comparison with NSAIDs for knee osteoarthritis: a systematic review and meta-analysis. Semin Arthritis Rheum. 2014;43(5):593–599. [DOI] [PubMed] [Google Scholar]
- 8. Buendía-López D, Medina-Quiros M, Fernandez-Villacanas Marin MA. Clinical and radiographic comparison of a single LP-PRP injection, a single hyaluronic acid injection and daily NSAID administration with a 52-week follow-up: a randomized controlled trial. J Orthop Traumatol. 2018;19(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cahir C, Fahey T, Teeling M, Teljeur C, Feely J, Bennett K. Potentially inappropriate prescribing and cost outcomes for older people: a national population study. Br J Clin Pharmacol. 2010;69(5):543–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Carlson VR, Ong AC, Orozco FR, Hernandez VH, Lutz RW, Post ZD. Compliance with the AAOS guidelines for treatment of osteoarthritis of the knee: a survey of the American Association of Hip and Knee Surgeons. J Am Acad Orthop Surg. 2018;26(3):103–107. [DOI] [PubMed] [Google Scholar]
- 11. Centers for Disease Control and Prevention. Prevalence and most common causes of disability among adults--United States, 2005. MMWR Morb Mortal Wkly Rep. 2009;58(16):421–426. [PubMed] [Google Scholar]
- 12. Cohen J. Statistical Power Analysis for the Behavioral Sciences. Hillsdale, NJ: Lawrence Erlbaum Associates; 1987. [Google Scholar]
- 13. Colen S, van den Bekerom MP, Mulier M, Haverkamp D. Hyaluronic acid in the treatment of knee osteoarthritis: a systematic review and meta-analysis with emphasis on the efficacy of different products. BioDrugs. 2012;26(4):257–268. [DOI] [PubMed] [Google Scholar]
- 14. da Costa BR, Reichenbach S, Keller N, et al. Effectiveness of non-steroidal anti-inflammatory drugs for the treatment of pain in knee and hip osteoarthritis: a network meta-analysis. Lancet. 2017;390(10090):e21–e33. [DOI] [PubMed] [Google Scholar]
- 15. Dahl LB, Dahl IM, Engstrom-Laurent A, Granath K. Concentration and molecular weight of sodium hyaluronate in synovial fluid from patients with rheumatoid arthritis and other arthropathies. Ann Rheum Dis. 1985;44(12):817–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dickson DJ, Hosie G, English JR. A double-blind, placebo-controlled comparison of hylan G-F 20 against diclofenac in knee osteoarthritis. J Clin Res. 2001;4:41–52. [Google Scholar]
- 17. Dillon CF, Rasch EK, Gu Q, Hirsch R. Prevalence of knee osteoarthritis in the United States: arthritis data from the Third National Health and Nutrition Examination Survey 1991-94. J Rheumatol. 2006;33(11):2271–2279. [PubMed] [Google Scholar]
- 18. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gore M, Tai KS, Sadosky A, Leslie D, Stacey BR. Use and costs of prescription medications and alternative treatments in patients with osteoarthritis and chronic low back pain in community-based settings. Pain Pract. 2012;12(7):550–560. [DOI] [PubMed] [Google Scholar]
- 20. Graham DJ, Campen D, Hui R, et al. Risk of acute myocardial infarction and sudden cardiac death in patients treated with cyclo-oxygenase 2 selective and non-selective non-steroidal anti-inflammatory drugs: nested case-control study. Lancet. 2005;365(9458):475–481. [DOI] [PubMed] [Google Scholar]
- 21. Gregori D, Giacovelli G, Minto C, et al. Association of pharmacological treatments with long-term pain control in patients with knee osteoarthritis: a systematic review and meta-analysis. JAMA. 2018;320(24):2564–2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:D5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ishijima M, Nakamura T, Shimizu K, et al. Intra-articular hyaluronic acid injection versus oral non-steroidal anti-inflammatory drug for the treatment of knee osteoarthritis: a multi-center, randomized, open-label, non-inferiority trial. Arthritis Res Ther. 2014;16(1):R18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Juhl C, Lund H, Roos EM, Zhang W, Christensen R. A hierarchy of patient-reported outcomes for meta-analysis of knee osteoarthritis trials: empirical evidence from a survey of high impact journals. Arthritis. 2012;2012:136245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kemper F, Gebhardt U, Meng T, Murray C. Tolerability and short-term effectiveness of hylan G-F 20 in 4253 patients with osteoarthritis of the knee in clinical practice. Curr Med Res Opin. 2005;21(8):1261–1269. [DOI] [PubMed] [Google Scholar]
- 27. Lau J, Ioannidis JP, Schmid CH. Quantitative synthesis in systematic reviews. Ann Intern Med. 1997;127(9):820–826. [DOI] [PubMed] [Google Scholar]
- 28. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. 2009;151(4):W65–W94. [DOI] [PubMed] [Google Scholar]
- 29. London NJ, Miller LE, Block JE. Clinical and economic consequences of the treatment gap in knee osteoarthritis management. Med Hypotheses. 2011;76(6):887–892. [DOI] [PubMed] [Google Scholar]
- 30. Losina E, Usiskin IM, Smith SR, et al. Cost-effectiveness of generic celecoxib in knee osteoarthritis for average-risk patients: a model-based evaluation. Osteoarthritis Cartilage. 2018;26(5):641–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Miller LE, Block JE. US-approved intra-articular hyaluronic acid injections are safe and effective in patients with knee osteoarthritis: systematic review and meta-analysis of randomized, saline-controlled trials. Clin Med Insights Arthritis Musculoskelet Disord. 2013;6:57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Palazzo C, Ravaud JF, Papelard A, Ravaud P, Poiraudeau S. The burden of musculoskeletal conditions. PLoS One. 2014;9(3):e90633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Petrella RJ, DiSilvestro MD, Hildebrand C. Effects of hyaluronate sodium on pain and physical functioning in osteoarthritis of the knee: a randomized, double-blind, placebo-controlled clinical trial. Arch Intern Med. 2002;162(3):292–298. [DOI] [PubMed] [Google Scholar]
- 34. Rutjes AW, Juni P, da Costa BR, Trelle S, Nuesch E, Reichenbach S. Viscosupplementation for osteoarthritis of the knee: a systematic review and meta-analysis. Ann Intern Med. 2012;157(3):180–191. [DOI] [PubMed] [Google Scholar]
- 35. Sadabad HN, Behzadifar M, Arasteh F, Behzadifar M, Dehghan HR. Efficacy of platelet-rich plasma versus hyaluronic acid for treatment of knee osteoarthritis: a systematic review and meta-analysis. Electron Physician. 2016;8(3):2115–2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Scott DL, Berry H, Capell H, et al. The long-term effects of non-steroidal anti-inflammatory drugs in osteoarthritis of the knee: a randomized placebo-controlled trial. Rheumatology (Oxford). 2000;39(10):1095–1101. [DOI] [PubMed] [Google Scholar]
- 37. Strand V, McIntyre LF, Beach WR, Miller LE, Block JE. Safety and efficacy of US-approved viscosupplements for knee osteoarthritis: a systematic review and meta-analysis of randomized, saline-controlled trials. J Pain Res. 2015;8:217–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. The Council for International Organizations of Medical Sciences. Guidelines for Preparing Core Clinical-Safety Information on Drugs Second Edition—Report of CIOMS Working Groups III and V. Geneva, Switzerland: CIOMS Working Group; 1999. [Google Scholar]
- 39. Trelle S, Reichenbach S, Wandel S, et al. Cardiovascular safety of non-steroidal anti-inflammatory drugs: network meta-analysis. BMJ. 2011;342:C7086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wang F, He X. Intra-articular hyaluronic acid and corticosteroids in the treatment of knee osteoarthritis: a meta-analysis. Exp Ther Med. 2015;9(2):493–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Weick JW, Bawa HS, Dirschl DR. Hyaluronic acid injections for treatment of advanced osteoarthritis of the knee: utilization and cost in a national population sample. J Bone Joint Surg Am. 2016;98(17):1429–1435. [DOI] [PubMed] [Google Scholar]