Abstract
Background
Compared with open gastrectomy (OG), laparoscopic gastrectomy (LG) for gastric cancer has achieved rapid development and popularities in the past decades. However, lack of comprehensive analysis in long-term oncological outcomes such as recurrence and mortality hinder its full support as a valid procedure. Therefore, there are still debates on whether one of these options is superior.
Aim
To evaluate the primary and secondary outcomes of laparoscopic versus open gastrectomy for gastric cancer patients
Methods
Two authors independently extracted study data. Risk ratio (RR) with 95% confidence interval (CI) was calculated for binary outcomes, mean difference (MD) or the standardized mean difference (SMD) with 95% CI for continuous outcomes, and the hazard ratio (HR) for time-to-event outcomes. Review Manager 5.3 and STATA software were used for the meta-analysis.
Results
Seventeen randomized controlled trials (RCTs) involving 5204 participants were included in this meta-analysis. There were no differences in the primary outcomes including the number of lymph nodes harvested during operation, severe complications, short-term and long-term recurrence, and mortality. As for secondary outcomes, compared with the OG group, longer operative time was required for patients in the LG group (MD = 58.80 min, 95% CI = [45.80, 71.81], P < 0.001), but there were less intraoperative blood loss (MD = − 54.93 ml, 95% CI = [− 81.60, − 28.26], P < 0.001), less analgesic administration (frequency: MD = − 1.73, 95% CI = [− 2.21, − 1.24], P < 0.001; duration: MD = − 1.26 days, 95% CI = [− 1.40, − 1.12], P < 0.001), shorter hospital stay (MD = − 1.37 days, 95% CI = [− 2.05, − 0.70], P < 0.001), shorter time to first flatus (MD = − 0.58 days, 95% CI = [− 0.79, − 0.37], P < 0.001), ambulation (MD = − 0.50 days, 95% CI = [− 0.90, − 0.09], P = 0.02) and oral intake (MD = − 0.64 days, 95% CI = [− 1.24, − 0.03], P < 0.04), and less total complications (RR = 0.81, 95% CI = [0.71, 0.93], P = 0.003) in the OG group. There was no difference in blood transfusions (number, quantity) between these two groups. Subgroup analysis, sensitivity analysis, and the adjustment of Duval’s trim and fill methods for publication bias did not change the conclusions.
Conclusion
LG was comparable to OG in the primary outcomes and had some advantages in secondary outcomes for gastric cancer patients. LG is superior to OG for gastric cancer patients.
Keywords: Gastric cancer, Meta-analysis, Recurrence, Mortality, Laparoscopic gastrectomy (LG), Open gastrectomy (OG)
Introduction
Gastric cancer is the third leading cause of cancer death and the fifth most common cancer worldwide [1–3]. Even though there is a steady decline in its incidence and mortality in recent years, an estimated 1,000,000 patients were newly diagnosed and more than 783,000 patients died from gastric cancer in 2018 [1]. More seriously, this trend has shown signs of change. A recent study demonstrated that the increasing rates of gastric cancer among people less than 50 years old might reverse the overall decline in the incidence of gastric cancer [4, 5].
Open gastrectomy (OG) remains the mainstay of curative approach for gastric cancer for a long time. Until 1994, Kitano firstly described the efficacy of laparoscopy gastrectomy (LG) in the case of early stage carcinoma in the antrum of the stomach [6]. Then, the employment of LG for gastric cancer has achieved rapid development and popularities in past decades due to minimal invasion, less blood loss, less time of using analgesic requirement and quicker recovery [7–10]. Another benefit of laparoscopic surgery is the capacity to observe the surgical field in a magnified view, which could help surgeons with more meticulous dissection of lymph nodes which is important to patient’s prognosis [11]. However, previous studies showed decreased number of harvested lymph nodes for gastric patients during LG compared with OG [12, 13]. Besides, like all the laparoscopic procedure, port site metastases and seeding during LG were inevitable because of intra-abdominal hyperpressure and adherence of laparoscopic instrument [14–17]. What is more, though there are some studies comparing the secondary outcomes between the LG and OG groups, lack of long-term oncological outcomes such as recurrence and mortality hinders its full support as a valid procedure [18–20]. Therefore, debates still exist whether LG is superior to OG for gastric cancer patients.
The aim of this meta-analysis was to identify and analyze random controlled trials (RCTs) in order to compare the primary and secondary outcomes of LG versus OG. Subgroup analyses were conducted to evaluate the primary outcomes which are key surgical and prognostic outcomes and may be influenced by the tumor stage and the gastrectomy type. Sensitivity analysis was implemented to validate the stability of the conclusion based on different effect models.
Methods
Search strategy
Two authors independently searched Pubmed, Embase, Cochrane Library, WANFANG, and China National Knowledge Internet until Nov. 25, 2018. The following combined search terms were used: (“Abdominal neoplasms” OR “Intestinal neoplasms” OR “Stomach neoplasms”) AND “Laparoscopy” AND “Gastrectomy” AND “Clinical trials” [21]. Details of the search strategies can be found in Additional file 1: Table S1.
Selection criteria
Studies were selected based on the following inclusion criteria: (1) study design, RCT in English or Chinese (animal studies, observational studies, basic research, retrospective studies, case-control studies, quasi-randomized studies, case reports, and cohort studies were excluded); (2) participants, gastric cancer patients undergoing gastrectomy; (3) interventions, surgical operation comparing LG with OG; and (4) outcomes, primary outcomes and secondary outcomes. Primary outcomes are (1) number of lymph nodes harvested during surgery, (2) severe complications, (3) short-term and long-term recurrence, and (4) short-term and long-term mortality. Secondary outcomes are (5) operative time, (6) intraoperative blood loss, (7) measures of earlier postoperative recovery (analgesic administration, time to first flatus, first ambulation and first oral intake, hospital stay), (8) blood transfusion (number, quantity), and (9) total complications. If there were two or more studies from the same authors or institutions, only the study with the largest sample size was chosen. Studies were excluded if full text of the trial was not available or they did not fulfill the inclusion criteria.
Data extraction and quality assessment
The records from the initial search were scanned by two authors to exclude any duplicate and irrelevant studies. The following data were extracted: first authors, publication date, country of origin, study period, tumor stage, gastrectomy type, lymph-node dissection, number of OG and LG cases, characteristics of the study population (including sex, age), follow-up, and primary and secondary outcomes (number of lymph nodes harvested during surgery, severe complications, recurrence and mortality; operative time, blood loss, indictors of earlier postoperative recovery (analgesic administration, first flatus, first ambulation, oral intake, hospital stay), blood transfusion (number, quantity), and total complications). Any discrepancies were resolved by discussion. Study quality was estimated using an adaptation of the Cochrane Handbook for Systematic Reviews of Interventions via the following characteristics: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective data, and other bias.
Statistical analysis
I2 and P value were used to evaluate the statistical heterogeneity. A fixed effects model was adopted with significant heterogeneity (I2 ≤ 50% and P ≥ 0.1), while a random effects model was employed in all other instances (I2 > 50% or P < 0.1) [22–24]. Risk ratio (RR) with 95% confidence interval (CI) was calculated for binary outcomes, mean difference (MD), or the standardized mean difference (SMD) with 95% CI for continuous outcomes and the hazard ratio (HR) for time-to-event outcomes. Subgroup analyses based on tumor stage and the type of gastrectomy were performed to evaluate the primary outcomes. Sensitivity analysis was used to explore the consistence of the conclusion based on fixed/random-effect models. Publication bias was evaluated by Egger’s test. If publication bias was conformed, the Duval’s trim and fill method was implemented to adjust for this bias. All statistical calculations were performed by Review Manager 5.3 (Cochrane collaboration. Copenhagen) and STATA software (Version 12.0; STATA Corporation, College Station, TX, USA). P value less than 0.05 was considered statistically significant.
Results
Search results and studies characteristics
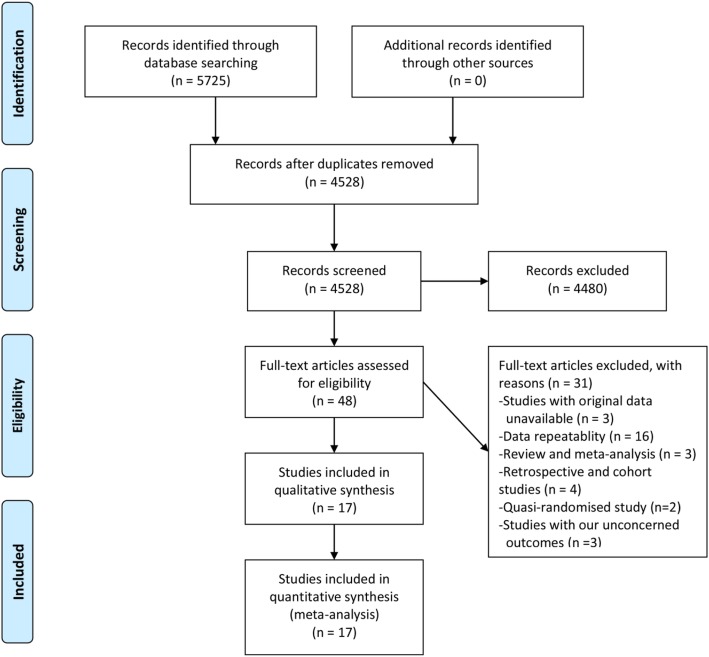
Our search initially yielded 5725 studies with 1197 studies subsequently excluded due to duplication. After a review of the titles and abstracts, we obtained 48 studies by excluding an additional 4480 studies. We further excluded 31 studies by scanning the full text (original data unavailable [n = 3], data repeatability [n = 8], review and meta-analysis [n = 11], retrospective and cohort studies [n = 4], quasi-randomized studies [n = 2], and studies with our unconcerned outcomes [n = 3]). Finally, seventeen RCTs were included in our analysis [11, 25–40] (Fig. 1).
Fig. 1.
Flowchart of literature search and study selection process
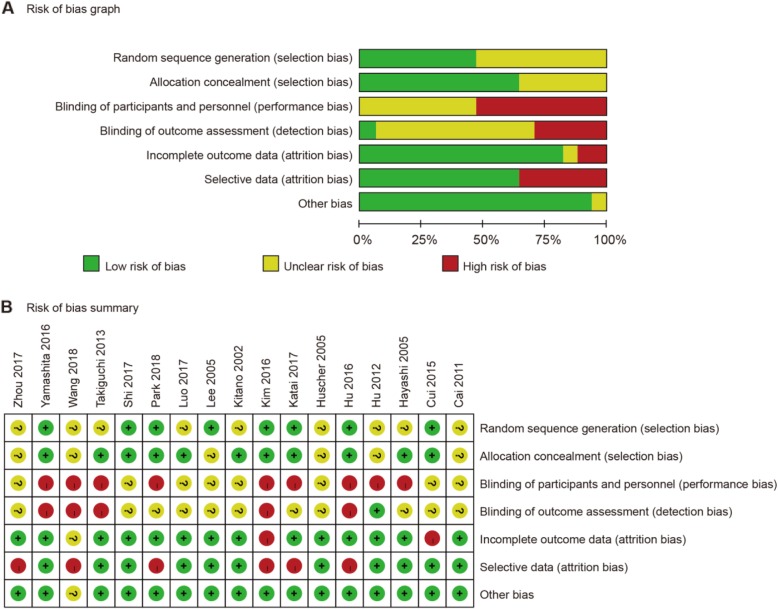
Characteristics of seventeen eligible RCTs were presented in Table 1. These RCTs were published between 2002 and 2018, involving 5204 patients (50.3% patients with LG). There were no differences in the demographics and clinicopathological characteristics of patients in the LG and OG group for each study. Eight trials were conducted in China [25–27, 29, 35, 37, 39, 40], five studies in Japan [28, 31, 32, 36, 38], three in Korea [11, 33, 34], and one in Italy [30]. Early gastric cancer (EGC) patients were included in six studies [28, 32, 33, 36, 38, 39], and advanced gastric cancer (AGC) patients were enrolled in another six trials [25, 29, 34, 35, 37, 40]. Distal gastrectomy was adopted in nine trials [26, 28, 30, 32–34, 36, 38, 40]. The results of methodological quality assessment about each risk of bias item for each included trial were shown in Fig. 2.
Table 1.
Baseline characteristics of studies included in the meta-analysis
| Author year | Country | Study period | Tumor stage | Gastrectomy type | LND | Group | Cases | Age | M/F | Follow-up (months) |
|---|---|---|---|---|---|---|---|---|---|---|
| Kitano 2002 [32] | Japan | 1998.11–2001.3 | EGC | DG | NA | LG OG | 14 14 | 63.2 60.1 | 9/5 8/4 | 24.3 18.8 |
| Hayashi 2005 [28] | Japan | 1999.12–2001.11 | EGC | DG | D1 | LG OG | 14 14 | 56 62 | 9/4 13/1 | 39 45 |
| Huscher 2005 [30] | Italy | 1992.11–1996.2 | EGC, AGC | DG | D1, D2 | LG OG | 30 29 | 63.2 63.6 | 18/12 21/8 | 52.2 49.7 |
| Lee 2005 [33] | Korea | 2001.11–2003.8 | EGC | DG | D2, DSL | LG OG | 24 23 | 56.6 59.5 | 11/13 15/8 | 14 14 |
| Cai 2011 [25] | China | 2008.3–2009.12 | AGC | PG, DG, TG | D2 | LG OG | 49 47 | 60.2 60.3 | 39/10 37/10 | 22.1 22.1 |
| Hu 2012 [26] | China | 2009.1–2011.5 | EGC, AGC | DG | NA | LG OG | 41 41 | 60.9 64.3 | 20/21 21/20 | 1 1 |
| Takiguchi 2013 [36] | Japan | 2003.7–2006.1 | EGC | DG | D1 | LG OG | 20 20 | 61.5 62.5 | 12/8 13/7 | 60 60 |
| Cui 2015 [27] | China | 2010.11–2012.9 | EGC, AGC | PG, DG, TG | D2 | LG OG | 128 142 | 60.1 57.5 | 88/40 98/44 | 1 1 |
| Hu 2016 [29] | China | 2012.9–2014.12 | AGC | DG, TG | D2 | LG OG | 519 520 | 56.5 55.8 | 380/139 346/174 | 1 1 |
| Kim 2016 [11] | Korea | 2006.2–2010.8 | EGC, AGC | DG, TG | D1, D2 | LG OG | 644 612 | 56.8 57.8 | 425/219 412/200 | 1 1 |
| Yamashita 2016 [38] | Japan | 2005.11–2008.2 | EGC | DG | DSL | LG OG | 31 32 | 58 61 | 17/14 25/7 | 63 63 |
| Luo 2017 [40] | China | 2008.5–2012.4 | AGC | DG | D2 | LG OG | 62 62 | 64.0 64.0 | 42/20 43/19 | 36 36 |
| Zhou 2017 [39] | China | 2012–2015 | EGC | PG, DG, TG | D1, D2 | LG OG | 100 100 | 53.2 53.1 | 50/50 50/50 | 60 60 |
| Shi 2017 [35] | China | 2010.1–2012.6 | AGC | PG, DG, TG | D2 | LG OG | 162 160 | 55.2 55 | 122/40 105/55 | 1 1 |
| Katai 2017 [31] | Japan | 2010.3–2013.11 | EGC, AGC | DG, PPG | D1, D2 | LG OG | 457 455 | 63 64 | 289/173 275/184 | 1 1 |
| Wang 2018 [37] | China | 2014.3–2017.8 | AGC | DG, TG | D2 | LG OG | 222 220 | 59.4 60.6 | 144/78 133/87 | 1 1 |
| Park 2018 [34] | Korea | 2010.6–2011.11 | AGC | DG | D2 | LG OG | 100 96 | 58.6 60.1 | 69/31 65/31 | 38.2 38.2 |
EGC early gastric cancer, AGC advanced gastric cancer, LND lymph node dissection, PG proximal gastrectomy, DG distal gastrectomy, TG total gastrectomy, PPG pylorus preserving gastrectomy, DSL dissecting selected lymph nodes, M male, F female
Fig. 2.
Risk of bias. a Risk of bias graph. b Risk of bias summary
Primary outcomes
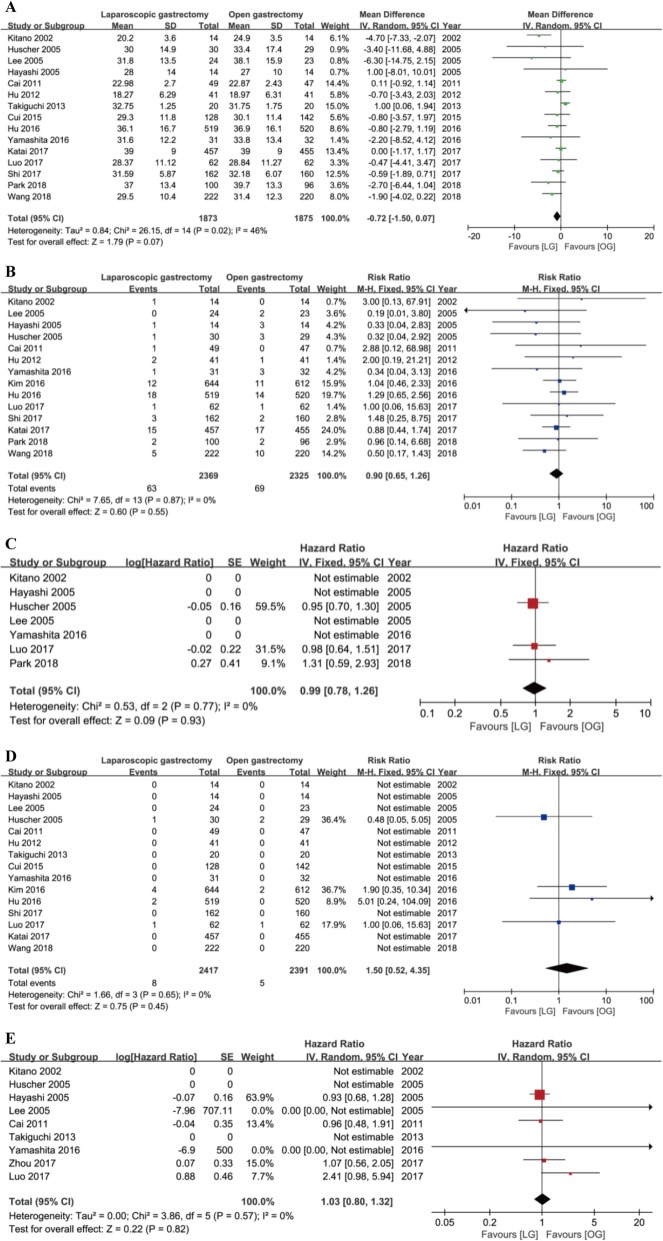
Sixteen trials reported the number of lymph nodes harvested during surgery. However, in Kim’s trial, the baseline was statistically significant in the extent of lymphadenectomy (P = 0.002). More patients suffered from D2 lymphadenectomy in the OG group than the LG group, which could cause a significant bias in the number of lymph nodes harvested during surgery [11]. Therefore, we excluded this trial in our analysis. Plotted data showed that there was no difference between these two groups in the number of lymph nodes harvested during surgery with a modern heterogeneity using the random model (MD = − 0.72, 95% CI = [− 1.50, 0.07], P = 0.07) (Fig. 3a).
Fig. 3.
Forest plot between laparoscopy gastrectomy (LG) and open gastrectomy (OG) group on primary outcomes. a The number of lymph nodes harvested during surgery. b Severe complications. c Long-term recurrence. d Short-term mortality. e Long-term mortality
Severe complications were defined when the extent of complications was up to grade III or more based on the Common Terminology Criteria for Adverse Events (CTCAE) ver. 4.0 or the Clavien-Dindo classification. Fourteen trials reported the severe complications. Fixed model showed no difference in these two groups without statistically significant heterogeneity (RR = 0.90, 95% CI = [0.65, 1.26], P = 0.55) (Fig. 3b).
Short-term recurrence was described as local recurrence, surgical recurrence, or distal metastases that existed within 6 months after surgery. Four trials reported the short-term recurrence while no patients were recurrent in the two groups. Therefore, we could conclude that there was no difference in the short-term recurrence between the LG and OG groups though we could not calculate the effect estimate. Seven trials reported the long-term recurrence which was defined as recurrence beyond 6 months after surgery. Fixed model showed no difference in these two groups without heterogeneity (HR = 0.99, 95% CI = [0.78, 1.26], P = 0.93) (Fig. 3c).
Fifteen trials reported short-term mortality which was regarded as death in hospital or within 1 month after surgery. Fixed model showed no difference in these two groups without statistically significant heterogeneity (RR = 1.50, 95% CI = [0.52, 4.35], P = 0.45) (Fig. 3d). Nine trials reported long-term mortality which was described as death out of hospital and beyond 1 month after operation. Fixed model showed no difference in these two groups without heterogeneity (HR = 1.03, 95% CI = [0.80, 1.32], P = 0.82) (Fig. 3e).
Secondary outcomes
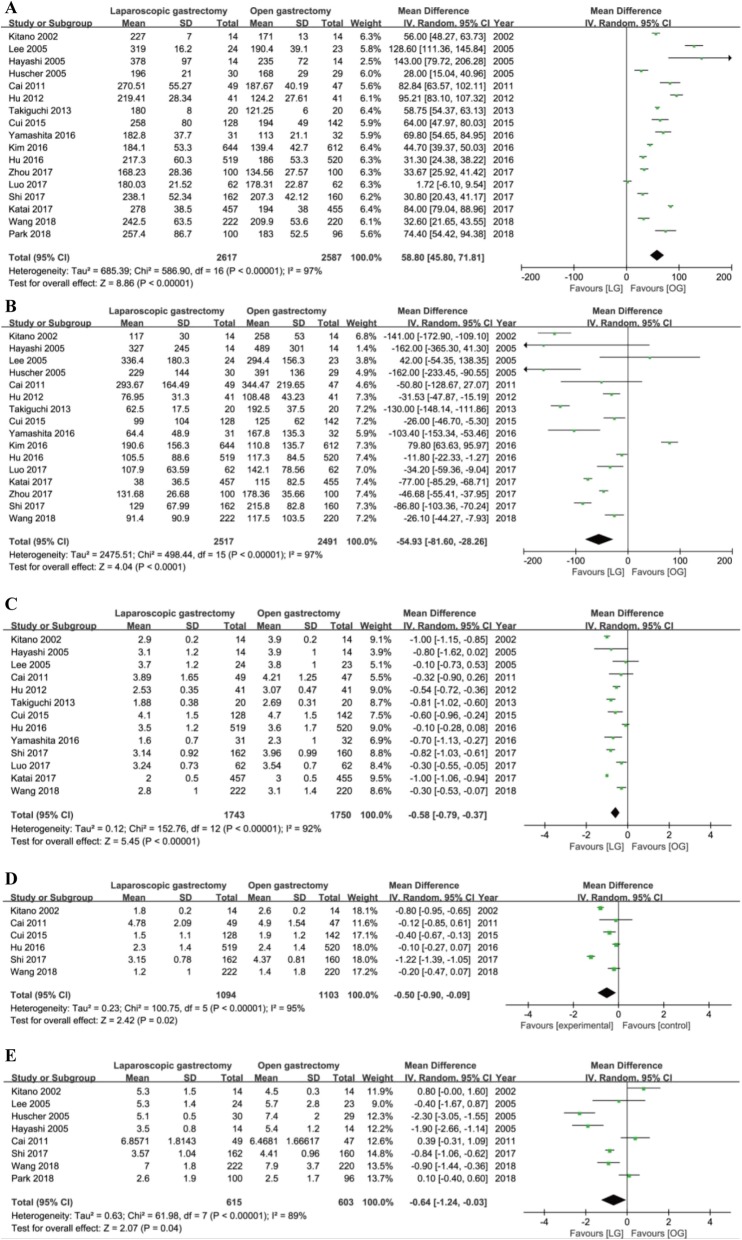
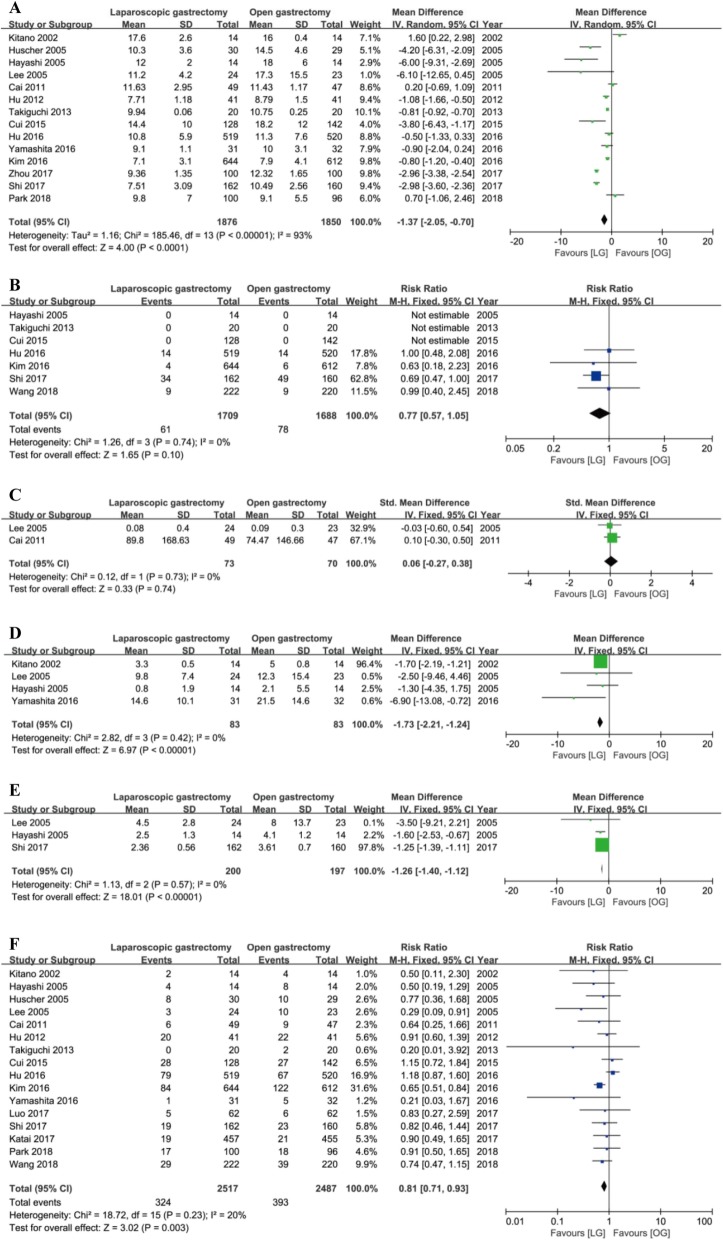
There were longer operative time (MD = 58.80 min, 95% CI = [45.80, 71.81], P < 0.001), less intraoperative blood loss (MD = − 54.93 ml, 95% CI = [− 81.60, − 28.26], P < 0.001), less time to first flatus (MD = − 0.58 days, 95% CI = [− 0.79, − 0.37], P < 0.001), first ambulation (MD = − 0.50 days, 95% CI = [− 0.90, − 0.09], P = 0.02) and first oral intake (MD = − 0.64 days, 95% CI = [− 1.24, − 0.03], P < 0.04), and less hospital stay (MD = − 1.37 days, 95% CI = [− 2.05, − 0.70], P < 0.001) in the LG group versus the OG group with significant heterogeneity using random models (Fig. 4a–e, Fig. 5a).
Fig. 4.
Forest plot between the LG and OG group on secondary outcomes. a Operative time. b Intraoperative blood loss on secondary outcomes. c Time to first flatus. d Time to first ambulation. e Time to first oral intake
Fig. 5.
Forest plot between the LG and OG group on secondary outcomes. a Hospital stay. b The number of patients who need blood transfusion. c The quantity of blood transfusion. d The frequency of analgesic administration. e The duration of analgesic administration. f Total complications
There were no differences in the number of patients who need blood transfusion (RR = 0.77, 95% CI = [0.57, 1.05], P = 0.1) and the quantity of blood transfusion (SMD = 0.06, 95% CI = [− 0.27, 0. 38], P = 0.74) using a fixed model with no heterogeneity (Fig. 5b, c). Also, the fixed models showed that the frequency and the duration of analgesic administration was less and shorter in the LG group than the OG group with no heterogeneity (frequency: MD = − 1.73, 95% CI = [− 2.21, − 1.24], P < 0.001; I2 = 0, P = 0.42; duration: MD = − 1.26, 95% CI = [− 1.40, − 1.12], P < 0.001; I2 = 0, P = 0.57) (Fig. 5d, e).
Total complications were defined as complications that occurred during the same hospitalization or within 30 days after the operation. Sixteen trials reported the total complications. Fixed model showed that patients in the LG group underwent fewer total complications after surgery than the OG group (RR = 0.81, 95% CI = [0.71, 0.93], P = 0.003) without statistically significant heterogeneity (Fig. 5f).
Subgroup analysis
Primary outcomes consist of lymph nodes harvested during surgery, severe complications, short and long-term recurrence, and mortality. Considering that primary outcomes are the key surgical and prognostic markers, we conducted the subgroup analysis about these indicators. Subgroup analysis was stratified based on the different cancer stages (early gastric cancer and advanced gastric cancer) and different types of gastrectomy (distal gastrectomy). Subgroup analysis showed no difference in lymph nodes harvested during surgery, severe complications, recurrence, and mortality between these two groups. Detailed results were shown in Tables 2 and 3.
Table 2.
Subgroup analysis of laparoscopic versus open gastrectomy stratified by different tumor stage
| Outcome | Studies | Participants | Heterogeneity | Model | WMD, RR, or HR | 95% CI | P | |
|---|---|---|---|---|---|---|---|---|
| I2 | P | |||||||
| Lymph nodes harvested | ||||||||
| 1. EGC | 5 | 206 | 79% | < 0.001 | Random | − 2.02 | [− 5.76, 1.72] | 0.29 |
| 2. AGC | 6 | 2219 | 0 | 0.49 | Fixed | − 0.51 | [− 1.19, 0.18] | 0.15 |
| Severe adverse complications | ||||||||
| 1. EGC | 4 | 166 | 0 | 0.60 | Fixed | 0.44 | [0.14, 1.39] | 0.16 |
| 2. AGC | 6 | 2219 | 0 | 0.73 | Fixed | 1.03 | [0.62, 1.69] | 0.92 |
| Short-term recurrence | ||||||||
| 1. EGC | 4 | 166 | Totals not selected | |||||
| 2. AGC | 0 | 0 | ||||||
| Long-term recurrence | ||||||||
| 1. EGC | 4 | 166 | Totals not selected | |||||
| 2. AGC | 2 | 320 | 0 | 0.53 | Fixed | 1.05 | [0.72, 1.53] | 0.82 |
| Short-term mortality | ||||||||
| 1. EGC | 5 | 206 | Totals not selected | |||||
| 2. AGC | 5 | 2023 | 0 | 0.44 | Fixed | 2.34 | [0.35, 15.70] | 0.38 |
| Long-term mortality | ||||||||
| 1. EGC | 6 | 406 | 0 | 0.99 | Fixed | 0.96 | [0.72, 1.27] | 0.76 |
| 2. AGC | 2 | 220 | 61% | 0.11 | Random | 1.45 | [0.59, 3.55] | 0.42 |
Table 3.
Subgroup analysis of laparoscopic versus open gastrectomy stratified by different type of gastrectomy
| Outcome | Studies | Participants | Heterogeneity | Model | WMD, RR, or HR | 95% CI | P | |
|---|---|---|---|---|---|---|---|---|
| I2 | P | |||||||
| Lymph nodes harvested | ||||||||
| 1. Distal gastrectomy | 9 | 667 | 64% | 0.005 | Random | −1.64 | [−3.76, 0.39] | 0.11 |
| Severe adverse complications | ||||||||
| 1. Distal gastrectomy | 8 | 627 | 0 | 0.81 | Fixed | 0.62 | [0.29, 1.34] | 0.22 |
| Short-term recurrence | ||||||||
| 1. Distal gastrectomy | 4 | 166 | Totals not selected | |||||
| Long-term recurrence | ||||||||
| 1. Distal gastrectomy | 7 | 545 | 0 | 0.77 | Fixed | 0.99 | [0.78, 1.26] | 0.93 |
| Short-term mortality | ||||||||
| 1. Distal gastrectomy | 8 | 471 | 0 | 0.69 | Fixed | 0.65 | [0.11, 3.79] | 0.64 |
| Long-term mortality | ||||||||
| 1. Distal gastrectomy | 7 | 389 | 0 | 0.28 | Fixed | 1.22 | [0.68, 2.17] | 0.50 |
Sensitivity analysis and publication bias
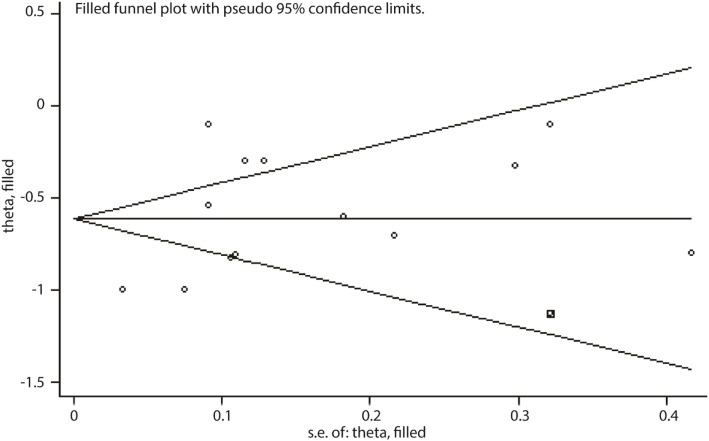
Sensitivity analysis is an analytic procedure which could be used to explore the source of uncertainty in the pooled results. We used fixed/random-effect models to test each comparison and arrived at a consistent conclusion (data not shown). Egger’s test was conducted for each comparison to evaluate the publication bias. There exists publication bias in the number of lymph nodes harvested during surgery, the duration of analgesic administration and the time to first flatus (Table 4); however, when applying the trim-and-fill method, there were not any trials trimmed in the number of lymph nodes harvested and the duration of analgesic administration. About the time to first flatus, after filling one trial, the revised result was still consistent using random model (MD = − 0.61 days, 95% CI = [− 0.82, − 0.41], P < 0.001) or fixed model (MD = − 0.81 days, 95% CI = [− 0.86, − 0.76], P < 0.001), indicating no publication bias in the comparison. The filled plot was shown in Fig. 6.
Table 4.
Publication bias by Egger’s test
| Outcome | Studies | P (Egger’s test) |
|---|---|---|
| Operative time | 17 | 0.75 |
| Blood loss | 16 | 0.82 |
| Blood transfusion | ||
| 1. Number | 7 | 0.49 |
| 2. Quantity | 2 | – |
| Lymph nodes harvested | 16 | 0.02 |
| Analgesic administration | ||
| 1. Frequency | 4 | 0.42 |
| 2. Duration | 3 | 0.03 |
| Hospital stay | 14 | 0.30 |
| Time to first flatus | 13 | 0.03 |
| Time to first ambulation | 6 | 0.53 |
| Time to first oral intake | 8 | 0.75 |
| Adverse complications | ||
| 1. Total | 16 | 0.10 |
| 2. Severe | 14 | 0.52 |
| Recurrence | ||
| 1. Short-term | 4 | – |
| 2. Long-term | 7 | 0.15 |
| Mortality | ||
| 1. Short-term | 15 | 0.97 |
| 2. Long-term | 9 | 0.27 |
Fig. 6.
Filled funnel plot with pseudo 95% confidence limits on time to first flatus
Discussion
Though there are some meta-analyses comparing the safety and efficacy of the LG and OG for gastric cancer patients, there still exist some concerns about the number of lymph nodes harvested during the surgery and the long-term outcomes [12, 13, 18–20]. In our meta-analysis, we summarized the primary and secondary outcomes of LG versus OG for gastric cancer patients. After an extensive search of the literature, 17 RCTs were identified and included.
Of the primary outcomes, they are key surgical and prognostic indictors including the number of lymph nodes harvest during surgery, severe complications, recurrence, and mortality. As for the number of lymph nodes harvested during surgery, we excluded Kim’s trail because there was statistical significance in the extent of lymphadenectomy. There are 390 patients with D2 lymphadenectomy and 216 patients with D1 lymphadenectomy in the OG group while 360 and 284 patients suffered from D2 and D1 lymphadenectomy in the LG group, separately (P = 0.004). Kim et al. also admitted that this bias could be the reason that more lymph nodes were dissected in the OG group than in the LG group [11]. Therefore, it is necessary to exclude the trial in the pooled analysis of the number of lymph nodes dissection during surgery. Through the meta-analysis, the plotted data demonstrated that there were no statistically significant differences in primary outcomes between the LG and OG groups. Stratified by the different cancer stage and different types of gastrectomy, subgroup analysis was conducted to check the sensitivity and stability of the results. The conclusion was consistent, which suggested that LG has a comparable efficacy compared with OG for gastric cancer patients.
As for the secondary outcomes, they consist of operative time, intraoperative blood loss, blood transfusion (number, quantity), measures of earlier postoperative recovery (analgesic administration, time to first flatus, first ambulation and first oral intake, and hospital stay), and total complications. Plotted data showed that there were no differences between the two groups in the number of patients who need transfusions and the quantity of blood transfusions. Longer operative time was required for patients in the LG group than the OG group. However, compared with patients in OG group, patients in LG group lost less blood during operation, achieved lower total complications; required less analgesic administration; shorter time to first flatus, first ambulation, and first oral intake; and shorter hospital stay. That means LG has an advantage over OG in the safety for gastric cancer patients.
In order to check the stability of our results, we conducted sensitivity analysis. We used fixed/random models to test each comparison and the conclusions were unchanged. Egger’s test showed that publication bias existed in the number of lymph nodes harvested during surgery, the duration of analgesic administration and the time to first flatus. Conclusions were consistent by the Duval’s trim and fill method, which means our results were stable and reliable.
Despite all this, this meta-analysis has some limitations. Firstly, all these RCTs have high or unclear risk in blinding due to medical ethics. Secondly, heterogeneity exists in operative time, blood loss, analgesic administration, hospital stay, and time to first flatus, ambulation, and oral intake. Finally, limited data were available to compare the hospital costs and health-related quality of life which are also important for patients to choose the method of operation [26, 39, 40].
Conclusion
In our analysis, we could conclude that LG was comparable to OG in the primary outcomes and had some advantages in secondary outcomes. That means LG is superior to OG for gastric cancer patients.
Supplementary information
Additional file 1: Table S1. The detailed search strategies.
Acknowledgements
None
Abbreviations
- AGC
Advanced gastric cancer
- CI
Confidence interval
- EGC
Early gastric cancer
- HR
Hazard ratio
- LG
Laparoscopic gastrectomy
- MD
Mean difference
- OG
Open gastrectomy
- RCT
Randomized controlled trials
- RR
Risk ratio
- SMD
Standardized mean difference
Authors’ contributions
All authors participated in the study. WW and GTD contributed to the conception and design of the research, FRZ and LC contributed to the acquisition of the data, FRZ and GTD contributed to the analysis and interpretation of the data, BC, MTL, JL, and GTD contributed to the statistical analysis. GTD and WW contributed to the drafting of the manuscript. FRZ and MTL contributed to the revision of the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by National Natural Science Foundation of China (grant number 81803373).
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Furong Zeng and Lang Chen contributed equally to this work.
Contributor Information
Wei Wu, Email: wwtw1972@126.com.
Guangtong Deng, Email: dengguangtong@outlook.com.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s12957-020-1795-1.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Collaborators GBDPC. The global, regional, and national burden of pancreatic cancer and its attributable risk factors in 195 countries and territories, 1990-2017: a systematic analysis for the global burden of disease study 2017. Lancet Gastroenterol Hepatol. 2019;4(12):934–947. doi: 10.1016/S2468-1253(19)30347-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-Years for 29 Cancer Groups, 1990 to 2017: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2019. 10.1001/jamaoncol.2019.2996. [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- 4.Anderson WF, Rabkin CS, Turner N, Fraumeni JF, Jr, Rosenberg PS, Camargo MC. The changing face of noncardia gastric cancer incidence among US non-Hispanic whites. J Natl Cancer Inst. 2018;110(6):608–615. doi: 10.1093/jnci/djx262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Collaborators GBDSC The global, regional, and national burden of stomach cancer in 195 countries, 1990-2017: a systematic analysis for the global burden of disease study 2017. Lancet Gastroenterol Hepatol. 2020;5(1):42–54. doi: 10.1016/S2468-1253(19)30328-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kitano S, Iso Y, Moriyama M, Sugimachi K. Laparoscopy-assisted Billroth I gastrectomy. Surg Laparosc Endosc. 1994;4(2):146–148. [PubMed] [Google Scholar]
- 7.Ding J, Liao G, Yan Z, et al. Meta-analysis of proximal gastrectomy and total gastrectomy for cancer of cardia and fundus. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2011;36(6):570–575. doi: 10.3969/j.issn.1672-7347.2011.06.016. [DOI] [PubMed] [Google Scholar]
- 8.Ding J, Liao GQ, Liu HL, Liu S, Tang J. Meta-analysis of laparoscopy-assisted distal gastrectomy with D2 lymph node dissection for gastric cancer. J Surg Oncol. 2012;105(3):297–303. doi: 10.1002/jso.22098. [DOI] [PubMed] [Google Scholar]
- 9.Basilar artery thrombectomy: assessment of outcome and identification of prognostic factors. Acta Neurol Belg. 2019. 10.1007/s13760-019-01223-2. [Epub ahead of print]. [DOI] [PubMed]
- 10.Vu LN, Nghia NQ, Thanh DT, et al. Laparoscopic living donor right nephrectomy: assessment of outcome and association of BMI to length of right renal vein. Actas Urol Esp. 2019;43(10):536–542. doi: 10.1016/j.acuro.2019.05.005. [DOI] [PubMed] [Google Scholar]
- 11.Kim W, Kim HH, Han SU, et al. Decreased morbidity of laparoscopic distal gastrectomy compared with open distal gastrectomy for stage I gastric cancer: short-term outcomes from a multicenter randomized controlled trial (KLASS-01) Ann Surg. 2016;263(1):28–35. doi: 10.1097/SLA.0000000000001346. [DOI] [PubMed] [Google Scholar]
- 12.Deng Y, Zhang Y, Guo TK. Laparoscopy-assisted versus open distal gastrectomy for early gastric cancer: a meta-analysis based on seven randomized controlled trials. Surg Oncol. 2015;24(2):71–77. doi: 10.1016/j.suronc.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 13.Vinuela EF, Gonen M, Brennan MF, Coit DG, Strong VE. Laparoscopic versus open distal gastrectomy for gastric cancer: a meta-analysis of randomized controlled trials and high-quality nonrandomized studies. Ann Surg. 2012;255(3):446–456. doi: 10.1097/SLA.0b013e31824682f4. [DOI] [PubMed] [Google Scholar]
- 14.Greco F, Wagner S, Reichelt O, et al. Huge isolated port-site recurrence after laparoscopic partial nephrectomy: a case report. Eur Urol. 2009;56(4):737–739. doi: 10.1016/j.eururo.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 15.Lago V, Gimenez L, Matute L, et al. Port site resection after laparoscopy in advance ovarian cancer surgery: time to abandon? Surg Oncol. 2019;29:1–6. doi: 10.1016/j.suronc.2019.01.007. [DOI] [PubMed] [Google Scholar]
- 16.Palomba S, Mandato VD, La Sala GB. Isolated port-site metastasis after robotic hysterectomy for stage IA endometrial adenocarcinoma. Obstet Gynecol. 2014;123(3):664. doi: 10.1097/AOG.0000000000000155. [DOI] [PubMed] [Google Scholar]
- 17.Song J, Kim E, Mobley J, et al. Port site metastasis after surgery for renal cell carcinoma: harbinger of future metastasis. J Urol. 2014;192(2):364–368. doi: 10.1016/j.juro.2014.02.089. [DOI] [PubMed] [Google Scholar]
- 18.Chen G, Xu X, Gong J, Zhang G, Cao Y, Zhang L. Safety and efficacy of hand-assisted laparoscopic versus open distal gastrectomy for gastric cancer: a systematic review and meta-analysis. Zhonghua Wei Chang Wai Ke Za Zhi. 2017;20(3):320–325. [PubMed] [Google Scholar]
- 19.Zhang CD, Yamashita H, Zhang S, Seto Y. Reevaluation of laparoscopic versus open distal gastrectomy for early gastric cancer in Asia: a meta-analysis of randomized controlled trials. Int J Surg. 2018;56:31–43. doi: 10.1016/j.ijsu.2018.05.733. [DOI] [PubMed] [Google Scholar]
- 20.Zheng XY, Pan Y, Chen K, Gao JQ, Cai XJ. Comparison of intracorporeal and extracorporeal esophagojejunostomy after laparoscopic total gastrectomy for gastric cancer: a meta-analysis based on short-term outcomes. Chin Med J. 2018;131(6):713–720. doi: 10.4103/0366-6999.226899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Best LM, Mughal M, Gurusamy KS. Laparoscopic versus open gastrectomy for gastric cancer. Cochrane Database Syst Rev. 2016;3:CD011389. doi: 10.1002/14651858.CD011389.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li H, Jiang C, Wu D, et al. The prognostic and clinicopathologic characteristics of CD147 and esophagus cancer: a meta-analysis. PLoS One. 2017;12(7):e0180271. doi: 10.1371/journal.pone.0180271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li H, Xi Z, Dai X, et al. CD147 and glioma: a meta-analysis. J Neuro-Oncol. 2017;134(1):145–156. doi: 10.1007/s11060-017-2499-4. [DOI] [PubMed] [Google Scholar]
- 24.Zeng F, Chen B, Zeng J, Wang Z, Xiao L, Deng G. Preoperative neutrophil-lymphocyte ratio predicts the risk of microvascular invasion in hepatocellular carcinoma: a meta-analysis. Int J Biol Markers. 2019;34(3):213–220. doi: 10.1177/1724600819874487. [DOI] [PubMed] [Google Scholar]
- 25.Cai J, Wei D, Gao CF, Zhang CS, Zhang H, Zhao T. A prospective randomized study comparing open versus laparoscopy-assisted D2 radical gastrectomy in advanced gastric cancer. Dig Surg. 2011;28(5–6):331–337. doi: 10.1159/000330782. [DOI] [PubMed] [Google Scholar]
- 26.Chen Hu J, Xin Jiang L, Cai L, et al. Preliminary experience of fast-track surgery combined with laparoscopy-assisted radical distal gastrectomy for gastric cancer. J Gastrointest Surg. 2012;16(10):1830–1839. doi: 10.1007/s11605-012-1969-4. [DOI] [PubMed] [Google Scholar]
- 27.Cui M, Li Z, Xing J, et al. A prospective randomized clinical trial comparing D2 dissection in laparoscopic and open gastrectomy for gastric cancer. Med Oncol. 2015;32(10):241. doi: 10.1007/s12032-015-0680-1. [DOI] [PubMed] [Google Scholar]
- 28.Hayashi H, Ochiai T, Shimada H, Gunji Y. Prospective randomized study of open versus laparoscopy-assisted distal gastrectomy with extraperigastric lymph node dissection for early gastric cancer. Surg Endosc. 2005;19(9):1172–1176. doi: 10.1007/s00464-004-8207-4. [DOI] [PubMed] [Google Scholar]
- 29.Hu Y, Huang C, Sun Y, et al. Morbidity and mortality of laparoscopic versus open D2 distal gastrectomy for advanced gastric cancer: a randomized controlled trial. J Clin Oncol. 2016;34(12):1350–1357. doi: 10.1200/JCO.2015.63.7215. [DOI] [PubMed] [Google Scholar]
- 30.Huscher CG, Mingoli A, Sgarzini G, et al. Laparoscopic versus open subtotal gastrectomy for distal gastric cancer: five-year results of a randomized prospective trial. Ann Surg. 2005;241(2):232–237. doi: 10.1097/01.sla.0000151892.35922.f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Katai H, Mizusawa J, Katayama H, et al. Short-term surgical outcomes from a phase III study of laparoscopy-assisted versus open distal gastrectomy with nodal dissection for clinical stage IA/IB gastric cancer: Japan Clinical Oncology Group Study JCOG0912. Gastric Cancer. 2017;20(4):699–708. doi: 10.1007/s10120-016-0646-9. [DOI] [PubMed] [Google Scholar]
- 32.Kitano S, Shiraishi N, Fujii K, Yasuda K, Inomata M, Adachi Y. A randomized controlled trial comparing open vs laparoscopy-assisted distal gastrectomy for the treatment of early gastric cancer: an interim report. Surgery. 2002;131(1 Suppl):S306–S311. doi: 10.1067/msy.2002.120115. [DOI] [PubMed] [Google Scholar]
- 33.Lee JH, Han HS, Lee JH. A prospective randomized study comparing open vs laparoscopy-assisted distal gastrectomy in early gastric cancer: early results. Surg Endosc. 2005;19(2):168–173. doi: 10.1007/s00464-004-8808-y. [DOI] [PubMed] [Google Scholar]
- 34.Park YK, Yoon HM, Kim YW, et al. Laparoscopy-assisted versus open D2 distal gastrectomy for advanced gastric cancer: results from a randomized phase II multicenter clinical trial (COACT 1001) Ann Surg. 2018;267(4):638–645. doi: 10.1097/SLA.0000000000002168. [DOI] [PubMed] [Google Scholar]
- 35.Shi Y, Xu X, Zhao Y, et al. Short-term surgical outcomes of a randomized controlled trial comparing laparoscopic versus open gastrectomy with D2 lymph node dissection for advanced gastric cancer. Surg Endosc. 2018;32(5):2427–2433. doi: 10.1007/s00464-017-5942-x. [DOI] [PubMed] [Google Scholar]
- 36.Takiguchi S, Fujiwara Y, Yamasaki M, et al. Laparoscopy-assisted distal gastrectomy versus open distal gastrectomy. A prospective randomized single-blind study. World J Surg. 2013;37(10):2379–2386. doi: 10.1007/s00268-013-2121-7. [DOI] [PubMed] [Google Scholar]
- 37.Wang Z, Xing J, Cai J, et al. Short-term surgical outcomes of laparoscopy-assisted versus open D2 distal gastrectomy for locally advanced gastric cancer in North China: a multicenter randomized controlled trial. Surg Endosc. 2019;33(1):33–45. doi: 10.1007/s00464-018-6391-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yamashita K, Hosoda K, Moriya H, Mieno H, Katada N, Watanabe M. Long-term prognostic outcome of cT1 gastric cancer patients who underwent laparoscopic gastrectomy after 5-year follow-up. Langenbeck’s Arch Surg. 2016;401(3):333–339. doi: 10.1007/s00423-016-1402-7. [DOI] [PubMed] [Google Scholar]
- 39.Lei Zhou GZ, Liu J, Liu H, Li C. Comparison among the early gastric cancer patients receiving laparoscopy radical gastrectomy and those receiving open radical gast. Biomed Res. 2017;28(22):10092–10095. [Google Scholar]
- 40.Guode Luo YC, Gong J, Wang X, Wang B, Zhou J, Li Y. Hand-assisted laparoscopic versus open surgery radical gastrectomy for advanced distal gastric cancer: a prospective randomized study. Int J Clin Exp Med. 2017;10(3):5001–5010. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. The detailed search strategies.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.