Abstract
Recurrent vulvovaginal infections (RVVI) has not only become an epidemiological and clinical problem but also include large social and psychological consequences. Understanding the mechanisms of both commensalism and pathogenesis are necessary for the development of efficient diagnosis and treatment strategies for these enigmatic vaginal infections. Through this review, an attempt has been made to analyze vaginal microbiota (VMB) from scratch and to provide an update on its current understanding in relation to health and common RVVI i.e. bacterial vaginosis, vulvovaginal candidiaisis and Trichomoniasis, making the present review first of its kind. For this, potentially relevant studies were retrieved from data sources and critical analysis of the literature was made. Though, culture-independent methods have greatly unfolded the mystery regarding vaginal bacterial microbiome, there are only a few studies regarding the composition and diversity of vaginal mycobiome and different Trichomonas vaginalis strains. This scenario suggests a need of further studies based on comparative genomics of RVVI pathogens to improve our perceptive of RVVI pathogenesis that is still not clear (Fig. 5). Besides this, the review details the rationale for Lactobacilli dominance and changes that occur in healthy VMB throughout a women’s life. Moreover, the list of possible agents continues to expand and new species recognised in both health and VVI are updated in this review. The review concludes with the controversies challenging the widely accepted dogma i.e. “VMB dominated with Lactobacilli is healthier than a diverse VMB”. These controversies, over the past decade, have complicated the definition of vaginal health and vaginal infections with no definite conclusion. Thus, further studies on newly recognised microbial agents may reveal answers to these controversies. Conversely, VMB of women could be an answer but it is not enough to just look at the microbiology. We have to look at the woman itself, as VMB which is fine for one woman may be troublesome for others. These differences in women’s response to the same VMB may be determined by a permutation of behavioural, cultural, genetic and various other anonymous factors, exploration of which may lead to proper definition of vaginal health and disease.
Keywords: Bacteriobiota, Community state types (CSTs), Mixed infections, Mycobiota, Menopause, Non-albicans Candida (NAC) Species, Vaginal Mycobiome, Vaginal ecosystem and vaginal dysbiosis
Background
Vaginal symptoms, such as discharge, odor and itching are frequently known causes of suffering and discomfiture in reproductive age women. These symptoms can be attributed to recurrent vulvovaginal infections (RVVI), that have not only became an epidemiological and clinical problem, but also include larger social and psychological consequences. The untreated RVVI can lead to complications like infertility, pre-term birth, miscarriages and other infectious diseases [1]. Due to these adverse effects on the reproductive health and well-being of women, vaginal infections have become a major public health concern all over the globe. The emergence and spread of human immunodeficiency virus (HIV) have made the prevention and management of RVVI even more important and urgent. World Health Organization (WHO), IUSTI/WHO and centres for diseases control (CDC) have provided guidelines on vaginal discharge management [2–4]. However, despite of these efforts, the cases of RVVI are still persisting and emerging, probably owing to misdiagnosis and wrong treatment. Literature has suggested fall in Lactobacilli predominance and overgrowth of opportunistic pathogens as the reason behind RVVI pathogenesis. Lactobacilli have been shown to constitute first line of defence against these pathogens, which by competing with them maintains their low number in vagina, hence suggested to be associated with vaginal health. In spite of this natural primary defence, vaginal infections occur repeatedly. This recommended the need to understand the factors that affect the dynamics and thus composition of microbial communities in vagina in both health and disease conditions, which further will be instrumental for the development of efficient diagnostic and treatment strategies. Over the past few decades, studies have provided some insights into the role of microbial communities inhabiting the vaginal cavity. However, the focus of most of these studies remains the bacterial part of vaginal microbiota both in term of vaginal health and vaginal disease. These studies undervalued the fact that human microbiota also constitutes the fungal part that may equally affect the human health, and other RVVI i.e. vulvovaginal candidiaisis (VVC) and trichomoniasis (TV) are also commonly occurring and clinically important similar to bacterial vaginosis (BV). Thus, through this review, an attempt has been made to analyze vaginal microbiota (VMB) from scratch and to provide an update on its current understanding in relation to health and common RVVI, making the present review first of its kind.
Selection of literature for review
The potentially relevant studies were retrieved from the Medline/PubMed and Google Scholar. Multiple keywords were used for the literature search both alone as well as in combination. Some of the important keywords used for literature search were microbiota, microbiome, microbial communities, healthy vagina, common vaginal infections, vulvovaginal infections, vaginal ecosystem, vaginal dysbiosis, bacterial vaginosis, vulvovaginal candidiasis, trichomoniasis et cetera. Only articles with English language were considered in the present study. The reference lists of retrieved articles were also screened to find relevant articles that were not identified by the initial search strategy.
Vaginal microbiota in health
Characteristics and composition of healthy vaginal microbiota
The community of microorganisms that lives interior or on the outer surface of human body forms human microflora/microbiota and their genomic constitution is referred to as human microbiome. The human microbiota usually involves symbionts that are benefitting from the host, but in turn may not affect (commensalism) or may affect positively (mutuality) and negatively (Pathogenic) the functioning, immunity and nourishment of host [5]. Individual microbiome achieved during birth changes throughout life, indicating microbiome specificity. In vagina, the sharp co-operative relationship of microbes with the host provides first line of defence against the migration of opportunistic pathogens. This healthy balance is referred to as eubiosis. However, outweighing of opportunistic pathogens, disrupt this symbiotic balance referred to as dysbiosis that further leads to inflammation. A mutual relationship exists between woman reproductive physiology and vaginal microbiota (VMB) i.e. not only the physiological changes that starts from birth and continues till post-menopause, can affect VMB, but in turn VMB can also affect reproductive physiology [6].
The VMB composition and structure have been described adequately in the literature starting from analyses using light microscopy to high-throughput sequencing techniques [reviewed in 5]. From the very past, the VMB of a healthy reproductive aged women is defined as Lactobacillus dominated microflora, producing ample quantity of lactic acid with pH < 4.5 [reviewed in 7]. However, molecular based techniques facilitated the detection of uncultivated and fastidious bacteria that were not previously recognized with conventional techniques, resulting in establishment of unique microbial community state types (CSTs). Based on the abundance and composition of vaginal bacterial species in reproductive age women, these CSTs have been classified into five major types [8, 9]. CST-I, CST-II, CST-III and CST-V are characterised by abundance of Lactobacillus crispatus, L. gasseri, L. iners and L. jensenii respectively. However, CST-IV is characterised by blend of diverse facultative anaerobes with low levels of Lactobacilli. This CST-IV has been further divided into two sub-states CST IV-A and CST IV-B. CST IV-A contains species of genera Anaerococus, Peptoniphilus, Corynebacterium, Prevotella, Finegoldia and Streptococcus. CST IV-B is characterised by Atopobium, Gardnerella, Sneathia, Mobiluncus, Megasphera and other taxa of order Clostridiales [8, 9]. Based on Nugent score, CST-IV represents the most common dysbiosis state i.e. bacterial vaginosis (discussed later in review). However, this state was also found to be reported in various healthy individuals, predominantly (40%) in black and Hispanic women [8]. Therefore, it is still debateable whether this CST represents a healthy state or an asymptomatic state of BV; further giving emphasis to redefine and distinguish between what is healthy and asymptomatic. Recently, a new genus Vaginella massiliensis, a rod-shaped, non-motile; non-sporulous obligate aerobic gram-negative bacteria was cultured from vaginal sample of healthy women [10]. Marseille P2517 is the representative strain of this new genus belonging to the family Flavobacteriaceae and phylum Bacteroidetes.
Rationales for Lactobacilli dominance in healthy VMB
The production of various antimicrobial substances by Lactobacilli has been documented as the major rationale behind its predominance in healthy human VMB. These antimicrobial substances include lactic acid, narrow-ranging bacteriocins and wide-ranging hydrogen peroxide (H2O2). These are suggested to play various important roles in host defence.
Lactic acid
Lactobacilli produce lactic acid as a result of fermentation of carbohydrates, mainly glycogen, present in the vaginal epithelium of menarchal women. This acidic environment provides protection against infectious diseases by preventing vaginal colonization of potential pathogens. Studies have suggested that Lactobacilli abundance acidify the vaginal medium to mean pH, 3.5 ± 0.2, mainly attributed to lactic acid accumulation [11]. The sufficiently protective levels of lactic acid in vagina are mainly dependent on VMB, as host epithelial cells contribute only 4–30% of the whole vaginal lactic acid [12]. Lactic acid is present in vagina in two different isomeric forms i.e. D(–) and L(+). It was suggested that glycogen availability into the vaginal lumen increases due to exfoliation of glycogen-rich epithelial cells by hyaluronidase-1 and matrix metalloproteinase activity [13]. This further causes α-amylase to degrade this available glycogen, that subsequently gets converted into D(–) lactic acid by Lactobacilli. However, no correlation of L(+) lactic acid was observed with α-amylase, suggesting that D(–) lactic acid is responsible for maintaining the vaginal pH at ≤ 4.5 and prevents growth of other bacteria. In addition, a recent study indicated that lactic acid, particularly in its L(+) form, suppress HIV-1 infection independent of its pH lowering property [14]. Another study suggested that lactic acid in its both D(–) and L(+) forms induce an anti-inflammatory response of human cervico-vaginal epithelial cells against HIV [15]. Also, both D(–) and L(+) lactic acid were found to inhibit histone deacetylases, thereby increasing DNA repair by regulating transcription of genes associated with it [16].
In addition, different studies have suggested the role of lactic acid in inhibiting wide variety of infections including Chlamydia trachomatis infection, Herpes simplex virus type 2 (HSV-2), HIV, HIV-1 and broad range of BV-associated microbes [14, 17–19]. Lactic acid have been shown to affect host immune responses through different mechanisms including eliciting significant increases in the production of the anti-inflammatory mediator i.e. interleukin-1 receptor antagonist (IL-1RA) from vaginal epithelial cells, inhibiting production of pro-inflammatory mediators i.e. IL-6, IL-8, tumor necrosis factor alpha (TNFα), RANTES (regulated on activation, normal T cell expressed and secreted) and macrophage inflammatory protein-3 alpha (MIP3α) [15], releasing transforming growth factor beta (TGF-β) to stimulate antiviral response [20], stimulating the T helper 17 (Th17) T lymphocyte pathway via IL-23 production on exposure to bacterial lipopolysaccharide [21] and building up cytosolic lactic acid that blocks the production of cyclic adenosine monophosphate (cAMP) leading to increased autophagy in epithelial cells for the degradation of intracellular microbes and upholding homeostasis [22]. Overall, these studies suggested different defensive properties of lactic acid that further depends on its isomeric state. These properties either independently or together determine the host susceptibility and consequently host–microbiota relationship.
Bacteriocins
Bacteriocins including Bacteriocins IIa, IIc, J46, acidocin lF221A, gassericin T and type-A lantibiotic are proteinaceous substances with bactericidal activity, synthesized by Lactobacilli particularly L. crispatus and L. gasseri [23]. Bacteriocins permeablize the cell membrane of non-indigenous pathogenic organisms i.e. S. aureus, Klebsiella spp., E. faecalis, E. coli and plays a major role in preventing their growth [23].
Hydrogen peroxide (H2O2)
H2O2 is another antimicrobial substance that is known to be produced in vitro by many Lactobacillus species in the presence of oxygen (O2) [24]. However, the levels of dissolved O2 are low in vagina maintaining an anaerobic atmosphere. So, the accumulation of H2O2 to a level sufficient to provide toxicity to pathogens is doubtful in vagina. O’Hanlon et al. [17] further complemented this presumption and showed that H2O2 production is not a considerable factor in preventing pathogens. The study demonstrated that under low levels of O2 i.e. anaerobic conditions of vagina, H2O2 had no noticeable effect on bacteria associated with BV [17]. Additionally, high levels of H2O2 were found to be more harmful to Lactobacilli responsible for eubiosis than bacteria responsible for dysbiosis [17]. In contrast to this study, some earlier studies have shown that H2O2 producing vaginal Lactobacilli are more likely to be protective against BV and suppressing Candida overgrowth and invasive hyphal formation [25]. Overall, suggesting that lactic acid and bacteriocins production mainly contributes to the protective role of Lactobacilli in VMB. However, the role of H2O2 in VMB protection is still debatable signifying its role as a substitute marker for other, yet unknown, physiological factors.
A recent study has suggested the mechanisms by which Lactobacillus suppress HIV type-1 (HIV-1) infection [14]. The acidification provided by Lactobacilli abundance was found to be efficiently mimicked by culture medium with Hydrochloric acid (HCl) of same mean pH (between 3.8 and 4.6) in preventing HIV-1 replication. However, the pH of both medium i.e. Lactobacillus conditioned medium and culture medium with HCl was diluted fivefold to reach pH 6.9, the effect was not mimicked by diluted HCl medium. As fivefold diluted Lactobacillus medium was still suppressive for HIV-1 infection, acting as a sink diminishing the amount of free virions, while diluted HCl medium was not. This suggested the possibility of other multiple factors and mechanisms responsible for the virucidal effect of Lactobacilli and its predominance in VMB that are yet to be discovered.
Candida live as commensal in healthy vagina
The VMB of healthy reproductive aged women also constitute fungal part designated as the “vaginal mycobiota” and its genomic constitution as “vaginal mycobiome” [26]. Researchers using culture-dependent older techniques recovered vaginal fungi in ≈ 20% of the asymptomatic women. Certainly, the predominant part of this mycobiota was occupied by C. albicans (72–91%) followed by non-albicans Candida (NAC) species including C. glabrata, C. tropicalis and C. parapsilosis [27]. However, the complexity of vaginal mycobiota remains unrecognized due to the limitations of conventional approaches. Only few studies regarding the composition and diversity of vaginal mycobiome were based on high-throughput sequencing techniques. In fact, the first study based on pyrosequencing revealing the vaginal myco-communities in asymptomatic women of Estonia came in print in 2013 [26]. The study recovered Candida species in 64.5% of the participants, which is significantly higher than the frequency (20%) found in asymptomatic healthy women, investigated with earlier culture based techniques [26, 27]. Also in full agreement with aforementioned earlier studies, the predominant part of this mycobiota, as predicted, was C. albicans (82%). Moreover, the NAC species detected in this study were C. dubliniensis, C. parapsilosis, C. krusei and Candida sp. VI04616. Other than Candida species, this study also found 38% of undesignated operational taxonomic units for which no taxonomic assignment were available, emphasizing to a serious dilemma of molecular based fungal taxonomy. Though recent studies have uncovered the underestimated diversity of vaginal mycobiota, C. albicans again remain the ultimate performer, further proposing future follow up studies [26, 28].
Candida species, though present in healthy women, still designated as opportunistic pathogen, due to their high prevalence (85–95%) in patients suffering from VVC, which is the second most prevalent dysbiosis after BV [1]. Thus, it remains to be determined whether the presence of C. species conveys a benefit to the host by maintaining balanced microbiota (eubiosis) or is responsible for causing VVC (dysbiosis). The answer to this may lie in the unique characteristic of Candida i.e. dimorphic transition. This feature allows Candida to undergo a morphological change from a round-ovoid yeast cell to a hyphal mycelial growing organism, further permitting it to live a dual lifestyle both as commensal as well as pathogen. The yeast form is usually found in healthy asymptomatic women in contrast to hyphal form, which has consistently been isolated from cases of severe VVC. This further supports the association of yeast form with commensalism and hyphal form with pathogenicity [29]. There are many factors that are responsible for Candida commensalism and one main factor is nutrition. Different studies have indicated the effect of carbon source on cell wall structure, which further affect the virulence of Candida and its interaction with host immune cells [30, 31]. Candida can utilize different carbon sources including glycogen, its breakdown products and even lactic acid as an alternative carbon source, which makes Candida highly flexible to dietary shifts in vagina [32]. Recently, a study suggested that growing Candida on lactate as its only nutrient source lead to modulation of its interactions with immune cells dampens phagocytosis, mount IL-10 and decline IL-17 production making it less vulnerable to immune responses [33]. Other studies have confirmed these findings, recommending that as Candida grows, it reduces the immune responses generated against it and thus endorse its own existence, establishing commensalism [34, 35].
Candida-bacteria interactions
Candida and bacteria plays a major role in sustaining Candida commensal form through five main clinically important bacteria-fungal interactions that commonly occur in human microbiota as reviewed by Peleg et al. [36]. These interactions also take place in VMB and include physical interactions, exchanges of chemicals, use of metabolic by-products, milieu variations and modification of the host immune response [25, 37, 38]. Studies based on co-culturing of vaginal yeast and bacteria suggested that bacteria inhibit Candida yeast-to-hyphae switch, maintaining its low number in VMB and compete with Candida cells for adhesion sites on epithelial receptors owing to its higher affinity [25, 37]. Also, Low pH and bactericidal compounds secreted by Lactobacilli tends to suppress Candida overgrowth and its transition from avirulent yeast form to virulent hyphal form [25, 39]. Recently, a study showed that L. crispatus, one of the dominant member of VMB, can diminish C. albicans virulence and enhance the local immune response of vaginal epithelial cells by modulating immune cytokines and chemokines profile i.e. upregulating IL-2, IL-6, IL-17 and downregulating IL-8 [38]. Overall, these studies suggested that bacteriobiota of VMB arbitrate tolerance to C. albicans in vagina. To complement this, different studies have linked the scarcity of Lactobacilli with susceptibility to symptomatic VVC and other lower genital tract infections including BV and HIV [40, 41]. All these findings suggests that Lactobacillus abundance and low Candida number along with their interactions play an important role in maintaining microbiota balance and disturbance in this may lead to vaginal infections.
Vaginal mucosa
The vaginal mucosa as a caretaker protects the lower genital tract from harmful pathogens including HIV [42]. Surfactant protein A (SP-A), produced in vaginal mucosa provides host defence by opsonizing pathogens, altering levels of pro-inflammatory cytokines, stimulating oxidative burst by phagocytic cells and promoting differentiation of antigen presenting cells, there by linking innate with adaptive immunity [43]. In addition, studies have shown that VMB composition also determines vaginal mucosa defence property which is shown to be strengthening by L. crispatus dominated VMB and weaken by L. iners dominated VMB [42, 44]. Other than this, most of the Candida-bacteria interactions likely to take place in vaginal mucosa where C. albicans property of biofilm formation was shown to be inhibited by Lactobacilli [45] though, only recently no in vivo biofilm formation by vaginal Candida species has been claimed based on histopathogical evidences [46]. Thus, change in quality and quantity of standard SP-A and vaginal microbial community composition can affect the gate keeping property of vaginal mucosa and therefore susceptibility to vaginal infections.
VMB throughout the women’s life
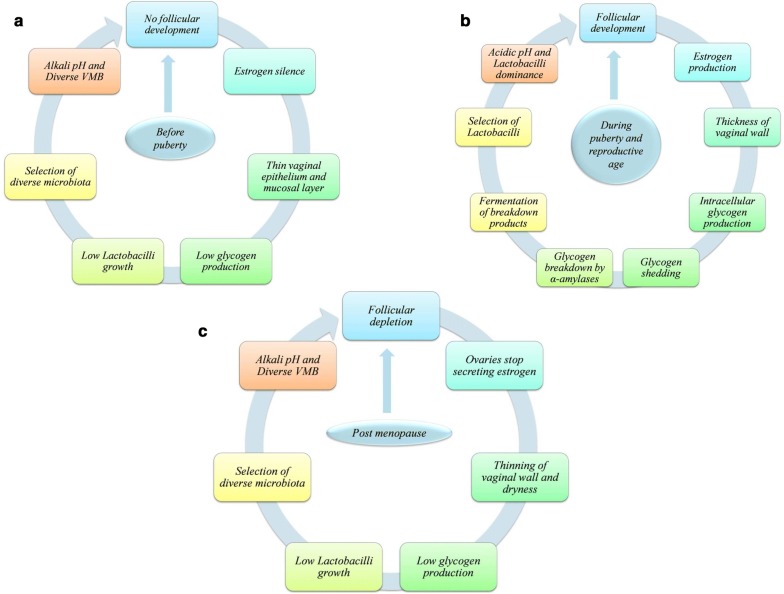
As women ages, its VMB composition changes significantly together with vaginal epithelium structure, and hormones have been suggested as main players (Fig. 1) [6, 9, 47].
Fig. 1.
Host physiology effects VMB throughout a women’s life i.e. a Before puberty, b during puberty and reproductive age and c post-menopause
At birth
The initial colonization of microbes takes place from mother vagina, if delivered normally, or from the hands of person that first hold the infant after caesarean [48]. Though little information is available, it is suggested that within weeks these microbes differentiate into different communities and establish in skin, gut and vagina [48]. There is evidence that infants have residual amount of circulating estrogen of mother which may be consumed by some Lactobacillus species for metabolism [49].
Before puberty
The vagina of child has CST-IV of VMB, neutral pH and thin stratified squamous epithelium roofed with thin mucosal layer [50]. Till 10 years of age, ovaries do not produce hormones (estrogen silence), resulting in low glycogen levels, consequently low growth of Lactobacilli and thus alkaline vaginal milieu. However, after 13 years of age, adrenal and gonadal maturation leads to pubertal changes in the vulva and vagina.
During puberty and reproductive age
Follicular development in puberty results in estrogen production. High estrogen promotes the thickness of vaginal epithelium along with mucosal layer and intracellular glycogen production [6]. Shedding of the glycogen rich epithelial cells in response to reproductive hormones leads to availability of free glycogen, which is further processed by α-amylases [32]. Available glycogen and its breakdown products i.e. maltose and maltotriose causes the selection of Lactobacilli capable of fermenting glycogen to lactic acid resulting in vaginal acidification and Lactobacilli dominance in VMB (CST I, II, II and V), with exception of CST IV microbial diversity found in some healthy women [6, 11]. Both the culture dependent and independent studies tried to uncover the complex and dynamic nature of vgainal microbial communities. The culture dependent studies have shown day to day variations in VMB composition [51, 52]. These findings were replicated by culture-independent studies based on longitudinal analyses of women who have done self sampling for weeks [9, 47, 53]. These studies have shown that some women showed switching between different CSTs over a short span, while others remain relatively consistent particularly the CST dominated by L. crispatus [9, 53]. These CST conversions were shown to be eliciting by menstruation [9, 47]. In 81% of menstrual cycles, the levels of G. vaginalis along with L. iners was shown to boost significantly with menses and decreases toward its end, while vice versa occurs with levels of the other two Lactobacilli namely L. crispatus and L. jensenii [47]. This can be attributed to the availability of iron during menses that improves the growth of G. vaginalis and L. iners, as easy culturing of both have been observed in blood agar medium [54]. Throughout a woman’s reproductive age, glycogen levels remain high, which steadily turn down when reaching menopause [55].
Post-menopause
Post-menopause leads to number of changes in the vaginal milieu that includes lowering of estrogen and glycogen levels, thinning of vaginal epithelium as that of pre-puberty stage, VMB shifting from Lactobacilli to microbial diversity (CST-IV), rise in pH, reduced vaginal secretions, dryness and dyspareunia [56]. Thus, VMB of girls at pre-puberty stage resembles with VMB of menopausal and post-menopausal women suggesting that reproductive physiology plays a major role in determination of women VMB [57]. However, vaginal milieu in postmenopausal women was found to be restored by application of estrogen cream, similar to that of reproductive age women [58].
Overall, the work highlighted the vibrant nature of VMB. However, the women considered in these studies were healthy indicating that CST’s compositions are not the sole indicator of dysbiosis, rather there are some uncharacterized factors that are needed to be resolve immedicately [9].
Vaginal microbiota in recurrent vulvovaginal infections (RVVI)
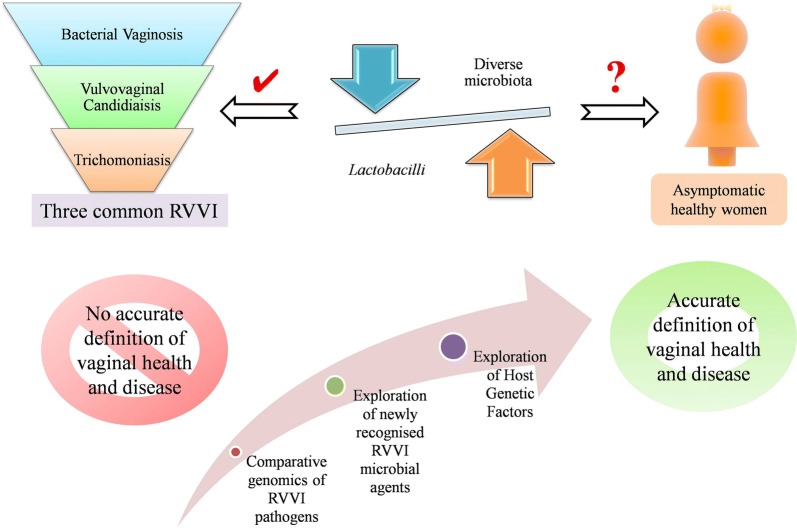
Diversity in VMB causes dysbiosis, i.e. different communities of VMB compete with each other leading to the selection of community capable of tolerating adverse conditions. This process may lead to damage or fall in number of beneficial Lactobacillus species, thus increasing host susceptibility to opportunistic pathogens either normally present in human VMB in lower quantity or are sexually transmitted. As this nasty cycle remains unchecked for long period, it may magnify the effect of dysbiosis leading to persistent imbalance with chronic inflammation i.e. vaginitis or vulvovaginal infections (VVI). These VVI are usually named on the predominant community or infectious agent present in vagina responsible for causing severe symptoms, like excessive vaginal discharge (offensive/non-offensive), vulval itching, fissuring, soreness, erythema, oedema, dysuria, dyspareunia and skin lesions. Of all these symptoms, an abnormal vaginal discharge is the key trait of these infections. Bacterial Vaginosis (BV), Vulvovaginal Candidiasis (VVC) and Trichomoniasis (TV) are the three main commonly prevalent VVI [59–62] and the centre point of this review. Moreover, recurrent VVI (RVVI) i.e. repeated experiences of common vaginal infections is emerging and is the major concern for researchers these days. For example, the repetition rates as high as 30–50% within 3 months is designated as recurrent BV (RBV) while ≥ 4 repetitive episodes of VVC in 12-months are referred as recurrent VVC (RVVC) [1, 63]. Similarly, cases of recurring TV (RTV) have also been reported with recurrence rates as high as 5–8% within 2 months of initial diagnosis [64].
Bacterial vaginosis (BV)
VMB composition in BV
As aforementioned, BV is a dysbiotic state characterised by deficiency of lactic acid producing bacteria and sufficiency of anaerobic bacterial diversity. This anaerobic diversity includes species of Anaerococcus, Atopobium, Bacteroides, Bacterial vaginosis-associated bacteria type 1 (BVAB1), BVAB2, BVAB3, Gardnerella, Leptotrichia, Mobiluncus, Mycoplasma, Mobiluncus, Peptostreptococcus, Peptoniphilus, Prevotella and Sneathia. Advancement in molecular based technology has been continually expanding the list of potential agents associated with BV, including the non-cultivable bacteriobiota that has limited the use of earlier culture based techniques [65]. For example, very recently three strains namely “Olegusella massiliensis” strain KHD7T, “Ezakiella massiliensis” strain Marseille P2951T and “Corynebacterium fournierii” strain Marseille P2948T were isolated from the VMB of woman with BV [66–68]. All these three strains were gram-positive with strain KHD7T and strain Marseille P2951T being strictly anaerobic, while strain Marseille P2948T was a facultative anaerobe, that grows very well under aerobic conditions and feeble under anaerobic conditions. Furthermore, scarcity of both isomeric forms of lactic acid were found in women diagnosed with BV, resulting in vaginal pH > 4.5 [69]. The latter is one of the four clinical symptoms of BV described by Amsel’s criteria for clinical diagnosis of BV (Fig. 2) [70].
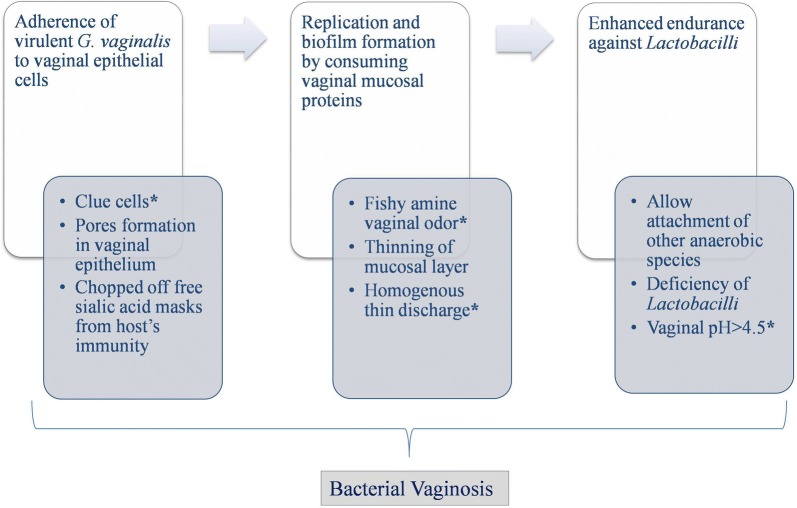
Fig. 2.
Pathway implicated in the pathogenesis of bacterial vaginosis, ‘*’ indicates four clinical symptoms of BV described by Amsel’s criteria
VMB’s energy source and G. vaginalis as a key component
In addition, metabolites assessment has suggested the anaerobic diversity in BV patients was found to be dependent on nitrogen source rather than on carbon source for their energy requirement. Catabolism of amino acids results in amines that is responsible for fishy amine vaginal odor observed in BV women, which is one of the four symptoms of BV described by Amsel’s criteria [71]. Not only this, catabolism of mucosal proteins by bacterial enzymes leads to the thinning of mucosal layer, making it less defensive and results in production of thin homogenous discharge lining the vaginal walls, another symptom of BV described by Amsel’s criteria [71]. Recently, G. vaginalis was suggested to be the key component of anaerobic diversity responsible for symptoms found in BV patients [72, 73]. G. vaginalis adhere to the surface of vaginal epithelial cells leading to the formation of clue cells, the fourth symptom of BV described by Amsel’s criteria [74]. Afterwards, G. vaginalis starts replicating in vaginal mucosa by consuming it and developing a thin biofilm that allow the attachment of other species [75]. This biofilm formation enhances the endurance of G. vaginalis against beneficial bacteria of VMB and antibacterial regimens [76].
Virulent forms and factors of G. vaginalis
As aforesaid, G. vaginalis is also a part of CST-IV of healthy VMB that challenges it as a cause for BV. This inconsistency led the researchers hypothesised that G. vaginalis might exists in different virulent forms and found that genotypic and phenotypic diversity actually exist in G. vaginalis [77, 78]. This has been further affirmed by a recent study, proposing the existence of ten strains of G. vaginalis (β-galactosidase positive) [79]. Apart from this, G. vaginalis strains expressing sialidase A gene has shown to be associated with BV and biofilm formation [73]. Sialidase A gene codes for enzyme sialidases that chop off sialic acid from glycoproteins of vaginal mucosal layer leaving behind basic glycan structure that serves as adherent for G. vaginalis [80]. Additionally, the free sialic acid residues provide nutrition to the bacteria as well as incorporate in surface, masking it from host’s immune responses [81]. Adherence, nutrition and masking allow proliferation of G. vaginalis resulting in continuation of this process subsequently leading to degradation and thinning of protective mucosal barrier of vagina [80]. Besides this, G. vaginalis also produces vaginolysin and prolidase, other characterized virulence factors of this bacterium. Vaginolysin is a cholesterol-binding cytolysin, primarily known as hemolysin, which encourages cell lysis through a colloid osmotic mechanism that leads to pores formation in the vaginal epithelium, contributing to BV signs and symptoms including vaginal fissures or sores [82]. Another virulence factor, prolidase, is a dipeptidase that degrades proline specific dipeptides contributing in amine odor observed in BV as mentioned above [83]. Apart from this, a new mucolytic enzyme, i.e. glycosulfatase, that chop off sulphate from glycosulphates, was found to be associated with BV [84]. The theory behind the removal of targeted residues is same in both glycosulfatases and sialidases.
G. vaginalis is not sole responsible for BV
Moreover other bacteria, including Bacteroides and Prevotella species, from anaerobic diversity of BV also produces sialidases and glycosulphates [85]. In addition, recently a culture-independent study based on longitudinal analyses has linked increased vaginal bacterial diversity in BV to vaginal inflammation [86]. The study described the longitudinal changes in VMB and immune mediators of African women with and without BV for 8 weeks and found that women with BV had significantly high concentrations of bacterial diversity and of pro-inflammatory interleukins including IL-1β and IL-12 (p70), that can cause inflammation and thus health damage. Also, a recent study has shown the occurrence of localised inflammation due to RBV [87]. Overall, these studies suggest that G. vaginalis is not solely responsible for BV. In fact, BV is a multimicrobial disease caused by a variety of communities formed from diverse microbes that synergistically lead to same physiological symptoms. As per the definition of syndrome, all these symptoms together leads to clinical diagnosis of BV [Amsel’s criteria, 70]. However, one statement i.e. same microbial diversity also exists in healthy/asymptomatic women (i.e. CST-IV of VMB) makes all the important findings looks vague. No definite conclusion could be drawn regarding anaerobic diversity acting as pathogen or opportunistic organisms, suggesting the presence of additional factors that are yet to be determined.
Another bewildered bacterial dysbiosis
Additionally, another dysbiotic condition known as aerobic vaginitis (AV) is repeatedly baffled with BV. This is because, both resembles with CST-IV of VMB i.e. lacking significant number of Lactobacilli and hence pH > 4.5. However, literature differ these two conditions based on the type of microbial diversity and inflammation. While, BV is designated by the presence of strictly anaerobic bacteria and as a non-inflammatory condition, AV is designated by the presence of aerobic intestinal bacteria including Escherichia coli, Staphylococcus aureus, group B Streptococcus (Streptococcus agalactiae) or Enterococcus and as inflammatory condition [88]. Infact, a severe AV is the same as desquamative inflammatory vaginitis (DIV), which is an unprecedented serious type of chronic purulent vaginitis of unknown aetiology causing non-specific signs and symptoms with abnormal vaginal flora, elevated pH and increased inflammation [89]. Although, nearly always high numbers of Streptococcus agalactiae and others species are found, AV and DIV are probably immunological disorders of the vagina and not infections owing to their positive response to anti-inflammtory agents. Apart from these, Group A streptococcal vaginitis is a recognized cause of vaginitis among prepubertal girls and an emerging disease entity in adult women as well, therefore should be considered a diagnosis when more common causes of vaginitis have been precluded [reviewed in 90].
Vulvovaginal candidiasis (VVC)
Debabtable VMB composition in VVC
Literature has reported normal vaginal pH (pH ~ 4.5) during VVC, indicating the presence of sufficient number of Lactobacilli to maintain the acidification of vagina [3]. In accordance to this observation, a study has reported elevated L(+) lactic acid levels in women with VVC [69]. Studies by different groups found no difference in VMB of women with and without VVC and were found to be dominated by Lactobacilli [91, 92]. A recent study by Wu et al. [93] found Lactobacilli dominant VMB in patients with severe VVC. All these findings support the presence of Lactobacilli dominance in women with VVC, suggesting their futile role in providing defence against VVC. Indeed, a study suggested that the probability of getting VVC increases 4 times with Lactobacilli colonization [94]. However, in contrast to these findings, a study found low density of Lactobacilli in RVVC women relative to controls [95]. This inconsistency in results provided by culture based studies was confirmed by the only study based on next generation sequencing. This study revealed diverse microbial community patterns in VMB of women with VVC and found that VMB of VVC did not show any typical pattern as found in VMB of healthy women and of BV [96]. In fact, it ranges from a CST dominated with Lactobacillus (healthy women) to CST with anaerobic diversity (BV). Thus, the part of VMB in VVC is debatable depicting its complex nature in VVC.
Co-ordination of stimuli for VVC pathogenesis
VVC is dysbiotic state typified by the excessive growth of Candida species, whose morphogenetic transitions to mycelial form is an opening step in pathogenesis [97]. Just like in BV, pathogenesis of VVC involves three steps i.e. adherence followed by invasion to epithelial cells, biofilm formation and secretion of virulence factors (Fig. 3) [29]. Studies have shown co-ordination of stimuli i.e. starvation, pH ≥ neutrality, 37 °C temperature and low Candida cell densities (< 107cells/mL) in modulating Candida gene expression profile to trigger hyphae formation, followed by adherence to vaginal epithelial cells and further pathogenesis [39, 98]. These genes encode for the proteins including agglutinin-like sequence protein (AlS3), secreted aspartic proteases (SAP 4 to 6), hyphal wall protein (Hwp1) and the hypha associated proteins (HGC1, Ece1 and Hyr1) [39, 99]. Out of these, two proteins i.e. AlS3 and Hwp1 act as ‘adhesins’, facilitating the adherence of C. albicans to host epithelial cells [100]. Following adhesion, Candida invades the host cells with the help of two proteins namely AlS3 and Ssa1, acting as ‘invasins’ [101, 102].
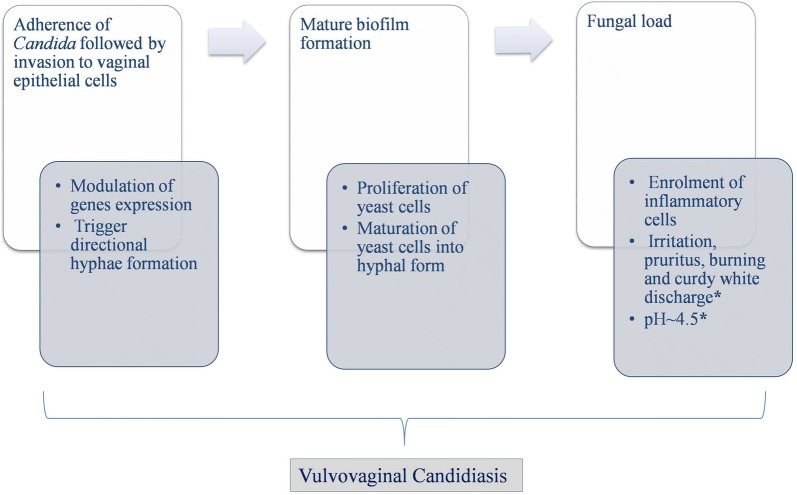
Fig. 3.
Pathway implicated in the pathogenesis of vulvovaginal candidiasis, ‘*’ indicates clinical symptoms of VVC
Effect of nutrient starvation and surrounding pH on Candida
Phagocytes create an environment of nutrient starvation for Candida [103]. In response of which Candida activate glyoxylate pathways for the synthesis of carbohydrates that plays a role in gluconeogensis [104]. Inspite of this, if C. albicans sense lack of carbohydrates, it starts using alternative metabolic pathways to consume proteins and lipids. Consumption of proteins liberates ammonia, resulting in increased pH, that auto-induce hyphae formation [92]. This mechanism not only allows Candida to grow under starvation but also cause hyphae formation, external pH modulation and thus increasing its virulence [31]. This property allows Candida to survive and escape phagocytes by causing host cell death by two different mechanisms including pyroptosis and membrane rupturing of phagocytes [105]. C. albicans can not only modulate the external pH, indeed, it can also sense and adapt to surrounding pH with the help of its pH sensitive proteins including Phr1 and Phr2 [106]. However, these proteins along with nutrient gaining property of Candida yeast get impaired under stress provided by neutral to alkaline pH [106]. This shows the effect of surrounding pH on Candida morphology with acidic pH maintaining yeast cell growth and neutral to alkaline pH leading to hyphal growth [106].
Contact sensing and biofilm formation
Additionally, contact sensing is also considered as crucial factor in triggering thigmotropism, i.e. directional hyphae growth and mature biofilm formation [107, 108]. A study has shown importance of directional growth of C. albicans for normal virulence, active penetration and complete damage of epithelial cells [109]. Just like BV associated bacteria, Candida is competent in making mature biofilms on mucosal epithelial surface after adherence [110, 111]. It involves a chronological process of yeast cells proliferation, followed by maturation of upper yeast cells into hyphal form and subsequently building of surface milieu [108]. This biofilm formation is under the control of certain transcription regulators namely Efg1, Tec1, Bcr1, Rob1, Ndt80 and Brg1 [108, 112]. Eliminating any of these factors may lead to faulty biofilm formation in vivo [113]. Additionally, AlS3 and Hwp1 also play a complementary role in biofilm formation [112]. This illustrates the underlying role of AlS3 in Candida pathogenesis performing three roles as adhesins, invasins and in biofilm formation. Above a certain threshold of fungal load, enrolment of inflammatory cells starts, that consequently leads to vaginal symptoms including irritation, pruritus, burning and curdy white discharge [114]. The later involves the detachment of hyphae along with recruited inflammatory cells, lysed cells debris and vaginal liquid making the curdy white vaginal discharge, which is one of the characteristic clinical symptoms of VVC.
Virulence factors and contradiction in view of Candida’s biofilm formation
The biofilm formation by Candida is a big issue, as it provides heightened virulence and resistance against the host immune responses, antifungal agents, consequently leading to recurrent infections and antifungal treatment failures [115]. Metabolic flexibility, compound biofilm structure, surface milieu of biofilm is some of the factors responsible for biofilm resistance, while diffusion of yeast cells from biofilm contributes to virulence [116]. Though, Candida-biofilms have been shown in a myriad of experiments in vitro as well as on catheters and prosthetic surfaces in vivo, no proof of in situ, in vivo Candida biofilm presence has been reported on the vaginal surface, regardless of the likelihood that Candida biofilms are a crucial factor in VVC pathogenesis. Paradoxically, a recent study contradicts this well-known supposition, suggesting no resemblance of in vivo histopathological lesions in vagina with biofilms in VVC [46]. This is the reason an alternate understanding what a Candida-biofilm is required. The other known virulence factors include secreted hydrolases namely protease and phospholipase that facilitate invasion and nutrients acquisition [117, 118]. The well-studied proteolytic enzymes employed by Candida include family of SAPs that is known to cause epithelial cell damage and systemic infections [118]. Recently, a new cytolytic peptide toxin namely Candidalysin has been proposed to be secreted by C. albicans, that leads to epithelial cell damage and triggers host immune responses [119]. The strains lacking this toxin were found to be avirulent, suggesting it as a causal determinant of virulent strains. Overall, researchers are still not able to fully uncover the mechanisms behind Candida pathogenesis leading to VVC. The factors responsible for pathogenic morphology transitions are still difficult to understand and the system behind immune-protection are still debatable.
Genetic variability in Candida species
However, efforts are being made for better understanding of these factors based on comparative genomics. As, there are strains of Candida that are avirulent and do not have the capacity to undergo dimorphic transition [120]. Earlier study based on longitudinal karyotyping suggested the persistent presence of single Candida strain in VMB, experiencing genetic microevolution and was responsible for repeated episodes of VVC [121]. This fungal persistence and resistance was found to be associated with variety of genetic variations found in Candida species including Single nucleotide polymorphisms (SNPs), reshuffling, heterozygosity loss and copy number variations (CNVs) [122]. There are two studies that are based on whole genome sequence analysis of Candida species isolated from women with VVC [123, 124]. These studies recommended the modulation of genetic system under the influence of unique host environment that affects the growth of Candida species. Without a doubt, these comparative genomics analyses of Candida will reveal the various anonymous factors and genomic variations responsible for dimorphic transitions that occur in response to the host dynamic vaginal milieu. However, no VMB differences were found in women with and without VVC suggests the exploration of host itself that will improve our understanding behind the pathogenesis of VVC.
Trichomoniasis/Trichomonas vaginitis (TV)
Initiation of pathogenesis by parasite
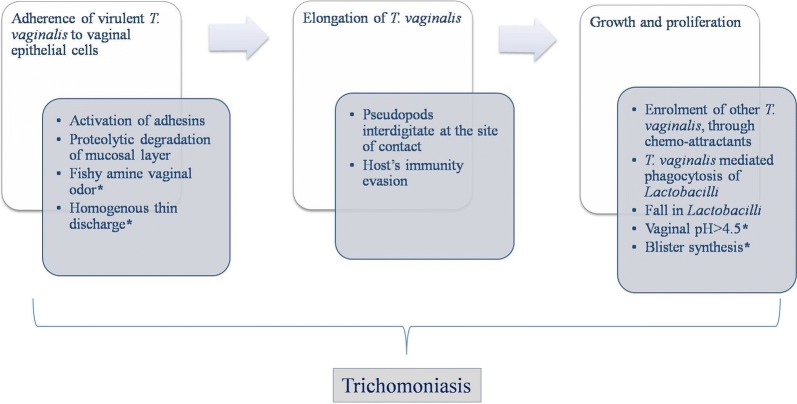
Another common type of RVVI is Trichomoniasis. As the name suggest, it is caused by Trichomonas vaginalis, the prevalent pathogen present in vagina during infection. T. vaginalis is an anaerobic flagellated parasite which normally exists as trophozoite but sporadically in amoeboid form. T. vaginalis pass on sexually amongst human, its only reported host [125, 126]. The first host surface that came across by trichomonads is the host vaginal mucosal layer. Network of Mucin, a key protein component of mucosal layer, serve as a gate keeper to pathogen invasion. However, this barrier was broken down by proteolytic degradation by mucinases (cysteine-like peptidases) found in T. vaginalis [127]. This further permits the contact of T. vaginalis to the underlying vaginal epithelium and its components including phosphoglucomutase, enolase, lamimin binding, fibronectin and α-actinin that equivalently plays a role in its cytoadherence [128]. Just like first two dysbiotic states, the initial step in pathogenesis of trichomoniasis is adhesion to vaginal epithelial cells via opposite site of undulating membrane (Fig. 4) [129]. A lipophosphoglycan (LPG) dominated dense glycocalyx is anchored to the plasma membrane of T. vaginalis via inositol phosphoceramide [130]. This LPG supports T. vaginalis adherence to the vaginal epithelium [131]. The process of adhesion involves two kinds of proteins namely adhesins and cysteine proteases. The former includes five types namely AP23, AP33, AP51, AP65 and AP120, while cysteine proteases involve 11 to 23 different proteins [132, 133]. Adhesins are usually present on the surface of trichomonds in inactive form [134]. The activation process requires unmasking of adhesins from the specific proteins covering it, with the help of cysteine proteases [126]. The activated adhesins interact with vaginal epithelial cells and mediate attachment of trichomonads with it [134]. This binding leads to the elongation of T. vaginalis making it amoeboid with the formation of pseudopods that interdigitate with target cells at the site of contact [135]. Attachment of one trichomonad is shown to facilitate the enrolment of others, through the secretion of chemo-attractant [136]. A recent study reported the release of membrane vesicles from T. vaginalis surface that interact with target host cells through receptor or through fusion with target cell membrane to deliver certain signals important for this parasite pathogenesis [137].
Fig. 4.
Pathway implicated in the pathogenesis of trichomoniasis, ‘*’ indicates clinical symptoms of TV
Important nutrients as a regulatory source of virulence
Just like other microbes, the T. vaginalis use carbohydrates as its favoured carbon source for growth and nutrition, but in the absence of sugars, its switches to lipids and amino acid metabolism [138]. However, T. vaginalis do not have the ability to synthesise lipids, they mediate lysis of host erythrocytes (similar to the hameolysis of BV), which is shown to be maximum at normal vaginal pH of 4.5 and use it as a source of fatty acids [139]. Moreover, T. vaginalis also acquire CD59 from erythrocytes; thereby evading itself from host complement mediated killing [140]. This hameolysis is facilitated by the cysteine proteases; another virulent property of T. vaginalis, which also helps in iron acquisition by T. vaginalis [141, 142]. Iron is considered to be an important nutrient of T. vaginalis that upregulates the levels of various adhesins and proteases, thereby amplifying the loads of infection [141]. Recently, a 120 kDa protein named pyruvate ferredoxin oxidoreductase (PFOR) has been shown to influence pathogenicity of T. vaginalis by modulating adhesion, proliferation and blister synthesis [143]. Moreover, this PFOR has been verified to show structural and functional homology with AP120, a novel adhesin of T. vaginalis induced by iron [133]. Other than this, adhesins including AP51 and AP65 of T. vaginalis were also shown to serve as heme and haemoglobin binding proteins, validating their multifunctional role in T. vaginalis [144]. A part from this, iron up-regulated cysteine proteases help in evasion of host’s immune responses by degrading its various components that includes, subclasses of host antibodies (IgG and IgA), C3 opsonin, secretary leukocyte protease inhibitor (SLPI), an antimicrobial peptide and B cells [142, 145, 146]. In addition to iron, zinc also plays an important role in regulation of T. vaginalis proteins. Total 27 proteins of T. vaginalis showed differential expression in the presence of zinc. Some of these proteins showed up-regulation while others showed down-regulation that was subsequently associated with modulation of some virulence properties e.g. cytotoxicity [147]. It was suggested that T. vaginalis can survive the highly dynamic vaginal milieu even during the extreme changes of menstruation. Recently, a study characterized a new virulence factor of T. vaginalis, named as TvMP50, a metalloproteinase mediated by zinc that contributes to cytotoxicity against DU145 prostatic cells [148].
T. vaginalis interaction with Lactobacilli in host VMB
Furthermore, adhesion of this parasite was shown to be increased by lactic acid [149]. Another study by Petrin et al. [125] showed that the parasite grows well at pH > 4.5, suggesting that normal acidic vaginal milieu, due to lactic acid, are important for the colonization of this parasite. After colonization, T. vaginalis modify the vaginal environment including fall in Lactobacilli and pH > 4.5, that supports its further growth and proliferation. This might be due to T. vaginalis mediated Lactobacilli phagocytosis that destabilize and challenges the host protective environment [150]. However, the exact mechanism behind this is still puzzling and has not been clearly elucidated. For this, better understanding of interaction between T. vaginalis and host VMB is needed. Recent studies have confirmed that vaginal microbiome changes during TV [151, 152]. L. acidophilus leads to increase in adherence of T. vaginalis to vaginal epithelial cells, during the early stages of infection [149]. As the number of L. acidophilus increases, the T. vaginalis become incapable of growing, while, vice versa occurs with decrease in number of L. acidophilus at the end of menses and during menopause, consequently leading to increase in TV symptoms [153]. This parasite has also been shown to have damaging effects on L. acidophilus, either by phagocytosis or via proteases [125]. Other than this, L. gasseri has been shown to inhibit adhesion of various T. vaginalis strains, while L. plantarum has been shown to enhance T. vaginalis adhesion by modifying their adhesive properties [154]. A study showed that TV transform the VMB of women by repressing bacteria associated with BV and increasing the growth of mycoplasma that may be the cause of its high prevalence found in women with intermediate BV [151]. A study based on pyrosequencing of barcoded 16S rRNA genes found an association of T. vaginalis with CST-IV of VMB including anaerobes Sneathia, Parvimonas, Mycoplasma and low levels of Lactobacilli [155].
Symbiotic associations of T. vaginalis and increased virulence
Moreover, a new species of Mycoplamsa i.e. “Candidatus Mycoplasma girerdii” primarily known as Mnola was found to be strongly associated with TV and was rarely found in healthy women [151, 156]. This species is shown to be symbiotically associated with T. vaginalis [151]. However, Fettweis et al. [156] suggest that “Ca. M. girerdii” is not an obligate symbiont of the parasite, contrary to M. Hominis and M. Penetrans, that are the obligatory symbionts and can penetrate as well as replicate inside T. vaginalis and upregulate energy production, cytolysis, growth rate and proinflammatory response of T. vaginalis [157, 158]. However, more studies are required to determine the symbiotic and synergistic relationship between “Ca. M. girerdii” and T. vaginalis. Additionally four intracellular dsRNA viruses were identified in T. vaginalis [159]. Recent studies have suggested that these viruses, along with other symbionts, enhance the expression of T. vaginalis virulence genes, consequently leading to increased adhesion to vaginal epithelial cells and cytotoxic activity against Lactobacilli, thus modify its growth rate and disease outcome [160, 161]. Other collaborative means by which different microbes including Mollicutes and dsRNA viruses, exibit symbiosis with T. vaginalis has been recently reviewed by Fichorova et al. [162]. Apart from these, Leptothrix vaginalis, a non-sporing, anaerobic, non-pathogenic, gram positive organism, is frequently found in association with T. vaginalis. However, its role in modulation of T. vaginalis‘s virulence remains elusive [163].
Unexplored role of genetic variability of T. vaginalis
Overall these studies confirm the potential role of TV in modulating vaginal milieu and importance of its interactions with VMB. However, similar to BV and VVC, asymptomatic cases of TV also exist [149, 164]. This suggests the presence of different virulent strains of T. vaginalis, as reported by some earlier studies [165, 166]. However, no studies based on whole genome comparison of different T. vaginalis strains are available. In fact, the first draft genome sequence of the T. vaginalis was reported in 2007 [167]. Although, effort was made by a study that has tried to identify epigenetic variations in conserved region of different T. vaginalis strains isolated from asymptomatic women using nested polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) technique [164]. This suggests that more studies based on comparative genomics of T. vaginalis strains are needed. Exploration of host itself will also reveal the various indefinite factors and improve our understanding of TV pathogenesis that is still not clear.
Dysbiosis leading to other dysbiosis (mixed infections) and foster infections
As mentioned above, milieu conditions of vagina during BV are the same as required for the transition of commensal Candida yeast to pathogenic hyphae and for the pathogenesis of TV. These similar conditions for pathogen prevalence are the rationale behind co-occurrence of many pathogens together leading to mixed infections and co-infections. A mixed infection is a condition in which there is more than one pathogen that is responsible for causing symptoms. Mostly these cases are of BV with VVC, BV with TV or combination of three [59]. This condition is mostly puzzled with cases of co-infections, where also more than one pathogen is present but only one pathogen is responsible for causing signs and symptoms. Additionally, if the three common VVI are left untreated, will not only effect the female reproductive health but may also result in many foster infections/diseases and adverse pregnancy outcomes. These vaginal infections effect reproductive health leading to infertility because of fallopian tube occlusion [168]. The most common examples of foster infections include bacterial and viral sexually transmitted infections (STIs). Other than T. vaginalis, C. trachomatis and Neisseria Gonorrhoeae are the most common sexually transmitted bacteria that can occur as advance infections, if previous infections left untreated [169]. The best depiction of it is, BV leading to foster C. trachomatis infection. Tryptophan is an important component of C. trachomatis nutrition and thus relies on pool of host tryptophan for establishment and pathogenesis. This advantage is taken by host by producing IFN-γ that activates the human indoleamine-2,3-dioxygenase (IDO1) enzyme, for the degradation of tryptophan, eventually leading to tryptophan deficiency and C. trachomatis clearance. However, indole produced by anaerobes present in VMB of BV (also in CST-IV) is used by C. trachomatis as an alternative substrate for tryptophan synthesis by their own tryptophan synthase enzyme, thus allowing its establishment and pathogenesis by avoiding host immune responses [170].
Moreover, Lactobacilli, the dominant flora of healthy VMB, have been shown to reduced risk of sexually transmitted N. Gonorrhoeae infection [7, 171]. L. jensenii and L. gasseri, have been shown to inhibit both adherence and invasion of N. Gonorrhoeae, the two main steps required for establishment of an infection. Furthermore, these Lactobacilli have also been shown to act as post exposure prophylactic, because of their ability to displace already adhered gonococci from vaginal epithelial cells [171]. However, absence of Lactobacilli, during common vaginal infections may lead to N. Gonorrhoeae infection by allowing its establishment and pathogenesis. In addition, VMB during VVIs mainly BV, were found to be associated with many viral STIs including HIV, human papilloma virus (HPV), HSV and precancerous cervical lesions [reviewed in 172]. Additionally, dysbiosis during vaginal infections also leads to pelvic inflammatory disease [173]. Though, the data is not entirely consistent with these associations, but all are directed to increased anaerobic diversity, leading to foster infections. Recently, a study suggested the increased risk of HIV acquisition with RBV (87). Not only this, studies have linked the ascending of vaginal infections to mother womb leading to adverse pregnancy outcomes [174, 175]. BV was found to be associated with increased risks of preterm birth, early pregnancy loss; spontaneous abortion, low birth weight, increased neonatal morbidity and higher rates of postpartum endometritis [168, 176, 177]. Thus, treatment of vaginal infections is mandatory for female reproductive health and to prevent adverse foster infections leading to morbidity. Finding the key reason behind these infections is important to develop the new preventive strategies.
Controversies
“A VMB dominated with Lactobacilli is healthier than a diverse VMB” was indeed a widely recognized dogma at an earlier time, due to the health benefits of Lactobacilli and dominance in approximately 70% of healthy women. However, this statement seemed to be challenged by two disputes uncovered by high throughput sequencing approaches. First, the term beneficial and protective is not fully applicable on all the Lactobacillus species. For example, L. iners is considered to be pathogenic due to its pore forming ability in vaginal epithelial cells, presence of virulence factors such as inerolysin and cytolysin, absence of protective factors like H2O2, D(–) lactic acid, very less production of L(+) lactic acid and due to its reported associations with preterm birth as well as BV [178–180]. Similarity, L. jensenii produces only D(–) lactic acid and both L. gasseri and L. iners were found to be associated with VMB instability in pregnant women [178, 179]. It is only L. crispatus that is so far consistently been linked with good vaginal health, VMB stability and lower risk of developing VVI [181]. This suggests the species specific effects of Lactobacilli on host.
Moreover, as aforementioned, there is increased evidence regarding the presence of diverse VMB in 20–30% of healthy women that are also designated as asymptomatic [8]. Some studies have suggested ethnic and regional differences in stipulations of what is actually healthy, as high percentage of these asymptomatic cases belongs to Black and Hispanic women [8, 182]. But again the biggest puzzle is, small but definite proportion of these asymptomatic cases is also found in women of other ethnic groups having high proportion of CSTs dominated by Lactobacilli [183]. Moreover, a recent study using both culture dependent and independent approaches claimed of no ethnic difference in VMB of white and black asymptomatic women [184]. In addition, other studies have suggested that diverse VMB in asymptomatic women is healthy, as these species e.g. Atopobium, Megasphaera and Leptotrichia are capable of producing lactic acid and other antimicrobial substances [8, 9]. This is again mystifying due to the presence of these species in BV women and their contribution in increasing pH and BV pathogenesis. This is due to these controversies, that in spite of doing decades of research, we are not being able to find a single pathogen or etiological agent responsible for causing RVVI. Since, these microbes could not satisfy the Koch’s postulates, according to which the causative agent is essential for causing disease and should not be present in healthy population [185]. Owing to these reasons, studies over the past decade on VMB have complicated the definition of vaginal health and vaginal infections with no definite conclusion.
Conclusions
Though, culture-independent methods have greatly unfolded the mystery regarding vaginal bacterial microbiome, there are only a few studies regarding the composition and diversity of vaginal mycobiome and different Trichomonas vaginalis strains. This scenario suggests a need of further studies based on comparative genomics of RVVI pathogens to improve our perceptive of RVVI pathogenesis that is still not clear (Fig. 5). Moreover, the list of possible agents continues to expand, further studies on newly recognised microbial agents, may reveal answers to the controversies. Conversely, VMB of women could be an answer but it is not enough to just look at the microbiology. We have to look at the woman itself, as VMB which is fine for one woman may be troublesome for others. These differences in women’s response to the same VMB may be determined by a permutation of behavioural, cultural, genetic and various other anonymous factors. Exploration of these factors may lead to proper definition of vaginal health and disease.
Fig. 5.
Controversial nature of vaginal microbiota in health as well as disease and future directions
Acknowledgements
The authors express their sincere gratitude to University Grants Commission, New Delhi, Govt. of India for providing Ph.D. fellowship to NK.
Abbreviations
- RVVI
Recurrent vulvovaginal infections
- HIV
Human immunodeficiency virus
- WHO
World Health Organization
- CDC
Centres for diseases control
- VVC
Vulvovaginal Candidiaisis
- TV
Trichomoniasis
- BV
Bacterial vaginosis
- VMB
Vaginal microbiota
- CSTs
Community state types
- HSV-2
Herpes simplex virus type 2
- IL-1RA
Interleukin-1 receptor antagonist
- TNFα
Tumor necrosis factor α
- RANTES
Regulated on activation, normal T cell expressed and secreted
- MIP3α
Macrophage inflammatory protein-3 alpha
- TGF-β
Transforming growth factor beta
- Th17
T helper 17
- cAMP
Cyclic adenosine monophosphate
- O2
Oxygen
- HIV-1
HIV type-1
- HCl
Hydrochloric acid
- NAC
Non-albicans Candida
- SP-A
Surfactant protein A
- VVI
Vulvovaginal infections
- RBV
Recurrent BV
- RVVC
Recurrent VVC
- RTV
Recurrent TV
- BVAB1
Bacterial vaginosis-associated bacteria type 1
- AV
Aerobic vaginitis
- AlS3
Agglutinin-like sequence protein
- SAP
Secreted aspartic proteases
- Hwp
Hyphal wall protein
- SNPs
Single nucleotide polymorphisms
- CNVs
Copy number variations
- PFOR
Pyruvate ferredoxin oxidoreductase
- Ig
Antibodies
- SLPI
Secretary leukocyte protease inhibitor
- PBMCs
Peripheral blood mononuclear cells
- RBCs
Red blood cells
- PCR–RFLP
Polymerase chain reaction-restriction fragment length polymorphism
- STIs
Sexually transmitted infections
- IDO1
Indoleamine-2,3-dioxygenase
- HPV
Human papilloma virus
- H2O2
Hydrogen peroxide
Authors’ contributions
NK collected the literature and written the manuscript. JS and MK designed and critically checked the manuscript. All authors read and approved the final manuscript.
Funding
Not applicable
Availability of data and materials
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Namarta Kalia, Email: kalianamarta62@gmail.com.
Jatinder Singh, Email: jatinderarora2009@gmail.com.
Manpreet Kaur, Email: dr.manpreetdhuna@gmail.com.
References
- 1.Powell AM, Nyirjesy P. Recurrent vulvovaginitis. Best Pract Res Clin Obstet Gynaecol. 2014;28(7):967–976. doi: 10.1016/j.bpobgyn.2014.07.006. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization . Guidelines for the management of sexually transmitted infections. Geneva: WHO; 2003. [PubMed] [Google Scholar]
- 3.Workowski KA, Berman S. Sexually transmitted diseases treatment guidelines, 2010. Atlanta: Centers for Disease Control and Prevention; 2010. [DOI] [PubMed] [Google Scholar]
- 4.Sherrard J, Wilson J, Donders G, Mendling W, Jensen JS. 2018 European (IUSTI/WHO) International Union against sexually transmitted infections (IUSTI) World Health Organisation (WHO) guideline on the management of vaginal discharge. Int J STD AIDS. 2018;29(13):1258–1272. doi: 10.1177/0956462418785451. [DOI] [PubMed] [Google Scholar]
- 5.Martin DH. The microbiota of the vagina and its influence on women’s health and disease. Am J Med Sci. 2012;343(1):2–9. doi: 10.1097/MAJ.0b013e31823ea228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Farage M, Maibach H. Lifetime changes in the vulva and vagina. Arch Gynecol Obstet. 2006;273(4):195–202. doi: 10.1007/s00404-005-0079-x. [DOI] [PubMed] [Google Scholar]
- 7.Petrova MI, Lievens E, Malik S, Imholz N, Lebeer S. Lactobacillus species as biomarkers and agents that can promote various aspects of vaginal health. Front Physiol. 2015;6:81. doi: 10.3389/fphys.2015.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ravel J, Gajer P, Abdo Z, Schneider GM, Koenig SS, McCulle SL, Karlebach S, Gorle R, Russell J, Tacket CO, Brotman RM. Vaginal microbiome of reproductive-age women. Proc Natl Acad Sci. 2011;108(Supplement 1):4680–4687. doi: 10.1073/pnas.1002611107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gajer P, Brotman RM, Bai G, Sakamoto J, Schütte UM, Zhong X, Koenig SS, Fu L, Ma ZS, Zhou X, Abdo Z. Temporal dynamics of the human vaginal microbiota. Sci Transl Med. 2012;4(132):132ra52. doi: 10.1126/scitranslmed.3003605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diop K, Mediannikov O, Raoult D, Bretelle F, Fenollar F. “Vaginella massiliensis” gen. nov., sp. nov., a new genus cultivated from human female genital tract. New Microbes New infect. 2017;15:18–20. doi: 10.1016/j.nmni.2016.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O’Hanlon DE, Moench TR, Cone RA. Vaginal pH and microbicidal lactic acid when Lactobacilli dominate the microbiota. PLoS ONE. 2013;8(11):e80074. doi: 10.1371/journal.pone.0080074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boskey ER, Cone RA, Whaley KJ, Moench TR. Origins of vaginal acidity: high D/L lactate ratio is consistent with bacteria being the primary source. Hum Reprod. 2001;16(9):1809–1813. doi: 10.1093/humrep/16.9.1809. [DOI] [PubMed] [Google Scholar]
- 13.Nasioudis D, Beghini J, Bongiovanni AM, Giraldo PC, Linhares IM, Witkin SS. α-Amylase in vaginal fluid: association with conditions favorable to dominance of Lactobacillus. Reprod Sci. 2015;22(11):1393–1398. doi: 10.1177/1933719115581000. [DOI] [PubMed] [Google Scholar]
- 14.Palomino Ñ, Rogers A, Zicari S, Vanpouille C, Vitali B, Margolis L. Vaginal Lactobacillus inhibits HIV-1 replication in human tissues ex vivo. Front Microbiol. 2017;8:906. doi: 10.3389/fmicb.2017.00906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hearps AC, Tyssen D, Srbinovski D, Bayigga L, Diaz DJD, Aldunate M, Cone RA, Gugasyan R, Anderson DJ, Tachedjian G. Vaginal lactic acid elicits an anti-inflammatory response from human cervicovaginal epithelial cells and inhibits production of pro-inflammatory mediators associated with HIV acquisition. Mucosal Immunol. 2017;10(6):1480. doi: 10.1038/mi.2017.27. [DOI] [PubMed] [Google Scholar]
- 16.Wagner W, Ciszewski WM, Kania KD. L-and D-lactate enhance DNA repair and modulate the resistance of cervical carcinoma cells to anticancer drugs via histone deacetylase inhibition and hydroxycarboxylic acid receptor 1 activation. Cell Commun Signal. 2015;13(1):36. doi: 10.1186/s12964-015-0114-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O’Hanlon DE, Moench TR, Cone RA. In vaginal fluid, bacteria associated with bacterial vaginosis can be suppressed with lactic acid but not hydrogen peroxide. BMC Infect Dis. 2011;11(1):200. doi: 10.1186/1471-2334-11-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Isaacs CE, Xu W. Theaflavin-3, 3′-digallate and lactic acid combinations reduce herpes simplex virus infectivity. Antimicrob Agents Chemother. 2013;57(8):3806–3814. doi: 10.1128/AAC.00659-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gong Z, Luna Y, Yu P, Fan H. Lactobacilli inactivate Chlamydia trachomatis through lactic acid but not H2O2. PLoS ONE. 2014;9(9):e107758. doi: 10.1371/journal.pone.0107758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mossop H, Linhares IM, Bongiovanni AM, Ledger WJ, Witkin SS. Influence of lactic acid on endogenous and viral RNA-induced immune mediator production by vaginal epithelial cells. Obstet Gynecol. 2011;118(4):840–846. doi: 10.1097/AOG.0b013e31822da9e9. [DOI] [PubMed] [Google Scholar]
- 21.Witkin SS, Alvi S, Bongiovanni AM, Linhares IM, Ledger WJ. Lactic acid stimulates interleukin-23 production by peripheral blood mononuclear cells exposed to bacterial lipopolysaccharide. FEMS Immunol Med Microbiol. 2010;61(2):153–158. doi: 10.1111/j.1574-695X.2010.00757.x. [DOI] [PubMed] [Google Scholar]
- 22.Ghadimi D, de Vrese M, Heller KJ, Schrezenmeir J. Lactic acid bacteria enhance autophagic ability of mononuclear phagocytes by increasing Th1 autophagy-promoting cytokine (IFN-γ) and nitric oxide (NO) levels and reducing Th2 autophagy-restraining cytokines (IL-4 and IL-13) in response to Mycobacterium tuberculosis antigen. Int Immunopharmacol. 2010;10(6):694–706. doi: 10.1016/j.intimp.2010.03.014. [DOI] [PubMed] [Google Scholar]
- 23.Stoyancheva G, Marzotto M, Dellaglio F, Torriani S. Bacteriocin production and gene sequencing analysis from vaginal Lactobacillus strains. Arch Microbiol. 2014;196(9):645–653. doi: 10.1007/s00203-014-1003-1. [DOI] [PubMed] [Google Scholar]
- 24.Vallor AC, Antonio MA, Hawes SE, Hillier SL. Factors associated with acquisition of, or persistent colonization by, vaginal lactobacilli: role of hydrogen peroxide production. J Infect Dis. 2001;184(11):1431–1436. doi: 10.1086/324445. [DOI] [PubMed] [Google Scholar]
- 25.Boris S, Barbés C. Role played by Lactobacilli in controlling the population of vaginal pathogens. Microbes Infect. 2000;2(5):543–546. doi: 10.1016/S1286-4579(00)00313-0. [DOI] [PubMed] [Google Scholar]
- 26.Drell T, Lillsaar T, Tummeleht L, Simm J, Aaspõllu A, Väin E, Saarma I, Salumets A, Donders GG, Metsis M. Characterization of the vaginal micro-and mycobiome in asymptomatic reproductive-age estonian women. PLoS ONE. 2013;8(1):e54379. doi: 10.1371/journal.pone.0054379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barousse MM, Van Der Pol BJ, Fortenberry D, Orr D, Fidel PL. Vaginal yeast colonisation, prevalence of vaginitis, and associated local immunity in adolescents. Sex Transm Infect. 2004;80(1):48–53. doi: 10.1136/sti.2002.003855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guo R, Zheng N, Lu H, Yin H, Yao J, Chen Y. Increased diversity of fungal flora in the vagina of patients with recurrent vaginal candidiasis and allergic rhinitis. Microb Ecol. 2012;64(4):918–927. doi: 10.1007/s00248-012-0084-0. [DOI] [PubMed] [Google Scholar]
- 29.Sobel JD. Current topics in medical mycology. New York: Springer; 1989. Pathogenesis of Candida vulvovaginitis; pp. 86–108. [DOI] [PubMed] [Google Scholar]
- 30.Sosinska GJ, de Groot PW, de Mattos MJT, Dekker HL, De Koster CG, Hellingwerf KJ, Klis FM. Hypoxic conditions and iron restriction affect the cell-wall proteome of Candida albicans grown under vagina-simulative conditions. Microbiol. 2008;154(2):510–520. doi: 10.1099/mic.0.2007/012617-0. [DOI] [PubMed] [Google Scholar]
- 31.Ene IV, Adya AK, Wehmeier S, Brand AC, MacCallum DM, Gow NA, Brown AJ. Host carbon sources modulate cell wall architecture, drug resistance and virulence in a fungal pathogen. Cell Microbiol. 2012;14(9):1319–1335. doi: 10.1111/j.1462-5822.2012.01813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spear GT, French AL, Gilbert D, Zariffard MR, Mirmonsef P, Sullivan TH, Spear WW, Landay A, Micci S, Lee BH, Hamaker BR. Human α-amylase present in lower-genital-tract mucosal fluid processes glycogen to support vaginal colonization by Lactobacillus. J Infect Dis. 2014;210(7):1019–1028. doi: 10.1093/infdis/jiu231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ene IV, Cheng SC, Netea MG, Brown AJ. Growth of Candida albicans cells on the physiologically relevant carbon source lactate affects their recognition and phagocytosis by immune cells. Infect Immun. 2013;81(1):238–248. doi: 10.1128/IAI.01092-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Noverr MC, Phare SM, Toews GB, Coffey MJ, Huffnagle GB. Pathogenic yeasts Cryptococcus neoformans and Candida albicans produce immunomodulatory prostaglandins. Infect Immun. 2001;69(5):2957–2963. doi: 10.1128/IAI.69.5.2957-2963.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Masson L, Salkinder AL, Olivier AJ, McKinnon LR, Gamieldien H, Mlisana K, Scriba TJ, Lewis DA, Little F, Jaspan HB, Ronacher K. Relationship between female genital tract infections, mucosal interleukin-17 production and local T helper type 17 cells. Immunology. 2015;146(4):557–567. doi: 10.1111/imm.12527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peleg AY, Hogan DA, Mylonakis E. Medically important bacterial–fungal interactions. Nat Rev Microbiol. 2010;8(5):340. doi: 10.1038/nrmicro2313. [DOI] [PubMed] [Google Scholar]
- 37.Noverr MC, Huffnagle GB. Regulation of Candida albicans morphogenesis by fatty acid metabolites. Infect Immun. 2004;72(11):6206–6210. doi: 10.1128/IAI.72.11.6206-6210.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Niu XX, Li T, Zhang X, Wang SX, Liu ZH. Lactobacillus crispatus modulates vaginal epithelial cell innate response to Candida albicans. Chin Med J. 2017;130(3):273. doi: 10.4103/0366-6999.198927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mayer FL, Wilson D, Hube B. Candida albicans pathogenicity mechanisms. Virulence. 2013;4(2):119–128. doi: 10.4161/viru.22913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Donders GG, Bosmans E, Dekeersmaeckerb A, Vereecken A, Van Bulck B, Spitz B. Pathogenesis of abnormal vaginal bacterial flora. Am J Obstet Gynecol. 2000;182(4):872–878. doi: 10.1016/S0002-9378(00)70338-3. [DOI] [PubMed] [Google Scholar]
- 41.Lai SK, Hida K, Shukair S, Wang YY, Figueiredo A, Cone R, Hope TJ, Hanes J. Human immunodeficiency virus type 1 is trapped by acidic but not by neutralized human cervicovaginal mucus. J Virol. 2009;83(21):11196–11200. doi: 10.1128/JVI.01899-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nunn KL, Wang YY, Harit D, Humphrys MS, Ma B, Cone R, Ravel J, Lai SK. Enhanced trapping of HIV-1 by human cervicovaginal mucus is associated with Lactobacillus crispatus-dominant microbiota. MBio. 2015;6(5):e01084. doi: 10.1128/mBio.01084-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.MacNeill C, Umstead TM, Phelps DS, Lin Z, Floros J, Shearer DA, Weisz J. Surfactant protein A, an innate immune factor, is expressed in the vaginal mucosa and is present in vaginal lavage fluid. Immunology. 2004;111(1):91–99. doi: 10.1111/j.1365-2567.2004.01782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Arnold K, Birse K, Mckinnon L, Lingappa J, Novak R, Westmacott G, Ball TB, Lauffenburger D, Burgener A. Mucosal integrity factors are perturbed during bacterial vaginosis: a proteomic analysis. AIDS Res Hum Retroviru. 2014;30(S1):A30. doi: 10.1089/aid.2014.5046.abstract. [DOI] [Google Scholar]
- 45.Park SJ, Han KH, Park JY, Choi SJ, Lee KH. Influence of bacterial presence on biofilm formation of Candida albicans. Yonsei Med J. 2014;55(2):449–458. doi: 10.3349/ymj.2014.55.2.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Swidsinski A, Guschin A, Tang Q, Dörffel Y, Verstraelen H, Tertychnyy A, Khayrullina G, Luo X, Sobel JD, Jiang X. Vulvovaginal candidiasis: histologic lesions are primarily polymicrobial and invasive and do not contain biofilms. Am J Obstet Gynecol. 2019;220(1):91. doi: 10.1016/j.ajog.2018.10.023. [DOI] [PubMed] [Google Scholar]
- 47.Srinivasan S, Liu C, Mitchell CM, Fiedler TL, Thomas KK, Agnew KJ, Marrazzo JM, Fredricks DN. Temporal variability of human vaginal bacteria and relationship with bacterial vaginosis. PLoS ONE. 2010;5(4):e10197. doi: 10.1371/journal.pone.0010197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dominguez-Bello MG, Costello EK, Contreras M, Magris M, Hidalgo G, Fierer N, Knight R. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci. 2010;107(26):11971–11975. doi: 10.1073/pnas.1002601107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bernbaum JC, Umbach DM, Ragan NB, Ballard JL, Archer JI, Schmidt-Davis H, Rogan WJ. Pilot studies of estrogen-related physical findings in infants. Environ Health Perspect. 2008;116(3):416. doi: 10.1289/ehp.10409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Alvarez-Olmos MI, Barousse MM, Rajan L, Van Der Pol BJ, Fortenberry D, Orr D, Fidel PL., Jr Vaginal Lactobacilli in adolescents: presence and relationship to local and systemic immunity, and to bacterial vaginosis. Sex Transm Dis. 2004;31(7):393–400. doi: 10.1097/01.OLQ.0000130454.83883.E9. [DOI] [PubMed] [Google Scholar]
- 51.Keane FEA, Ison CA, Taylor-Robinson D. A longitudinal study of the vaginal flora over a menstrual cycle. Int J STD AIDS. 1997;8(8):489–494. doi: 10.1258/0956462971920631. [DOI] [PubMed] [Google Scholar]
- 52.Schwebke JR, Richey CM, Weiss HL. Correlation of behaviors with microbiological changes in vaginal flora. J Infect Dis. 1999;180(5):1632–1636. doi: 10.1086/315065. [DOI] [PubMed] [Google Scholar]
- 53.Ravel J, Brotman RM, Gajer P, Ma B, Nandy M, Fadrosh DW, Sakamoto J, Koenig SS, Fu L, Zhou X, Hickey RJ. Daily temporal dynamics of vaginal microbiota before, during and after episodes of bacterial vaginosis. Microbiome. 2013;1(1):29. doi: 10.1186/2049-2618-1-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Weinberg ED. Iron availability and infection. Biochimica et Biophysica Acta (BBA)-General Subjects. 2009;1790(7):600–605. doi: 10.1016/j.bbagen.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 55.Eschenbach DA, Thwin SS, Patton DL, Hooton TM, Stapleton AE, Agnew K, Winter C, Meier A, Stamm WE. Influence of the normal menstrual cycle on vaginal tissue, discharge, and microflora. Clin Infect Dis. 2000;30(6):901–907. doi: 10.1086/313818. [DOI] [PubMed] [Google Scholar]
- 56.Brotman RM, Shardell MD, Gajer P, Fadrosh D, Chang K, Silver M, Viscidi RP, Burke AE, Ravel J, Gravitt PE. Association between the vaginal microbiota, menopause status and signs of vulvovaginal atrophy. Menopause. 2014;21(5):450. doi: 10.1097/GME.0b013e3182a4690b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hickey RJ, Zhou X, Settles ML, Erb J, Malone K, Hansmann MA, Shew ML, Van Der Pol B, Fortenberry JD, Forney LJ. Vaginal microbiota of adolescent girls prior to the onset of menarche resemble those of reproductive-age women. MBio. 2015;6(2):e00097. doi: 10.1128/mBio.00097-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nilsson K, Risberg B, Heimer G. The vaginal epithelium in the post-menopause—cytology, histology and pH as methods of assessment. Maturitas. 1995;21(1):51–56. doi: 10.1016/0378-5122(94)00863-3. [DOI] [PubMed] [Google Scholar]
- 59.Kalia N, Singh J, Sharma S, Kamboj SS, Arora H, Kaur M. Prevalence of vulvovaginal infections and species specific distribution of vulvovaginal candidiasis in married women of north india. Int J Curr Microbiol App Sci. 2015;4(8):253–266. [Google Scholar]
- 60.Kalia N, Singh J, Sharma S, Arora H, Kaur M. Genetic and phenotypic screening of Mannose-Binding Lectin in relation to risk of recurrent vulvovaginal infections in women of north India: a prospective cohort study. Front Microbiol. 2017;8:75. doi: 10.3389/fmicb.2017.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kalia N, Kaur M, Sharma S, Singh J. A comprehensive in silico analysis of regulatory SNPs of human CLEC7A gene and its validation as genotypic and phenotypic disease marker in recurrent vulvovaginal infections. Front Cell Infec Microbiol. 2018;8:65. doi: 10.3389/fcimb.2018.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kalia N, Singh J, Sharma S, Kaur M. SNPs in 3′-UTR region of MBL2 increases susceptibility to recurrent vulvovaginal infections by altering sMBL levels. Immunobiology. 2019;224(1):42–49. doi: 10.1016/j.imbio.2018.10.009. [DOI] [PubMed] [Google Scholar]
- 63.Belayneh M, Sehn E, Korownyk C. Recurrent vulvovaginal candidiasis. Can Fam Physician. 2017;63(6):455. [PMC free article] [PubMed] [Google Scholar]
- 64.Kissinger P, Secor WE, Leichliter JS, Clark RA, Schmidt N, Curtin E, Martin DH. Early repeated infections with Trichomonas vaginalis among HIV-positive and HIV-negative women. Clin Infect Dis. 2008;46(7):994–999. doi: 10.1086/529149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lamont RF, Sobel JD, Akins RA, Hassan SS, Chaiworapongsa T, Kusanovic JP, Romero R. The vaginal microbiome: new information about genital tract flora using molecular based techniques. BJOG. 2011;118(5):533–549. doi: 10.1111/j.1471-0528.2010.02840.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Diop K, Diop A, Bretelle F, Cadoret F, Michelle C, Richez M, Cocallemen JF, Raoult D, Fournier PE, Fenollar F. Olegusella massiliensis gen. nov., sp. nov., strain KHD7T, a new bacterial genus isolated from the female genital tract of a patient with bacterial vaginosis. Anaerobe. 2017;44:87–95. doi: 10.1016/j.anaerobe.2017.02.012. [DOI] [PubMed] [Google Scholar]
- 67.Diop K, Raoult D, Bretelle F, Fenollar F. “Ezakiella massiliensis” sp. nov., a new bacterial species isolated from human female genital tract. New Microbes New Infect. 2017;15:16–17. doi: 10.1016/j.nmni.2016.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Diop K, Bretelle F, Raoult D, Fenollar F. ‘Corynebacterium fournierii’, a new bacterial species isolated from the vaginal sample of a patient with bacterial vaginosis. New Microbes New Infect. 2017;18:6–7. doi: 10.1016/j.nmni.2017.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Beghini J, Linhares IM, Giraldo PC, Ledger WJ, Witkin SS. Differential expression of lactic acid isomers, extracellular matrix metalloproteinase inducer, and matrix metalloproteinase-8 in vaginal fluid from women with vaginal disorders. BJOG. 2015;122(12):1580–1585. doi: 10.1111/1471-0528.13072. [DOI] [PubMed] [Google Scholar]
- 70.Amsel R, Totten PA, Spiegel CA, Chen KC, Eschenbach D, Holmes KK. Nonspecific vaginitis: diagnostic criteria and microbial and epidemiologic associations. Am J Med. 1983;74(1):14–22. doi: 10.1016/0002-9343(83)91112-9. [DOI] [PubMed] [Google Scholar]
- 71.Srinivasan S, Morgan MT, Fiedler TL, Djukovic D, Hoffman NG, Raftery D, Marrazzo JM, Fredricks DN. Metabolic signatures of bacterial vaginosis. MBio. 2015;6(2):e00204–e00215. doi: 10.1128/mBio.00204-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schellenberg JJ, Patterson MH, Hill JE. Gardnerella vaginalis diversity and ecology in relation to vaginal symptoms. Res Microbiol. 2017;168(9–10):837–844. doi: 10.1016/j.resmic.2017.02.011. [DOI] [PubMed] [Google Scholar]
- 73.Hardy L, Jespers V, Van den Bulck M, Buyze J, Mwambarangwe L, Musengamana V, Vaneechoutte M, Crucitti T. The presence of the putative Gardnerella vaginalis sialidase A gene in vaginal specimens is associated with bacterial vaginosis biofilm. PLoS ONE. 2017;12(2):e0172522. doi: 10.1371/journal.pone.0172522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Patterson JL, Stull-Lane A, Girerd PH, Jefferson KK. Analysis of adherence, biofilm formation and cytotoxicity suggests a greater virulence potential of Gardnerella vaginalis relative to other bacterial-vaginosis-associated anaerobes. Microbiology. 2010;156(2):392–399. doi: 10.1099/mic.0.034280-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hardy L, Jespers V, Dahchour N, Mwambarangwe L, Musengamana V, Vaneechoutte M, Crucitti T. Unravelling the bacterial vaginosis-associated biofilm: a multiplex Gardnerella vaginalis and Atopobium vaginae fluorescence in situ hybridization assay using peptide nucleic acid probes. PLoS ONE. 2015;10(8):e0136658. doi: 10.1371/journal.pone.0136658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Van Acker H, Van Dijck P, Coenye T. Molecular mechanisms of antimicrobial tolerance and resistance in bacterial and fungal biofilms. Trends Microbiol. 2014;22(6):326–333. doi: 10.1016/j.tim.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 77.Ahmed A, Earl J, Retchless A, Hillier SL, Rabe LK, Cherpes TL, Powell E, Janto B, Eutsey R, Hiller NL, Boissy R. Comparative genomic analyses of seventeen clinical isolates of Gardnerella vaginalis provides evidence of multiple genetically isolated clades consistent with sub-speciation into genovars. J Bacteriol. 2012;194(15):3922–3937. doi: 10.1128/JB.00056-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schellenberg JJ, Jayaprakash TP, Gamage NW, Patterson MH, Vaneechoutte M, Hill JE. Gardnerella vaginalis subgroups defined by cpn60 sequencing and sialidase activity in isolates from Canada. Belgium and Kenya. PLoS One. 2016;11(1):e0146510. doi: 10.1371/journal.pone.0146510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vaneechoutte M, Guschin A, Van Simaey L, Gansemans Y, Van Nieuwerburgh F, Cools P. Emended description of Gardnerella vaginalis and description of Gardnerella leopoldii sp. nov., Gardnerella piotii sp. nov. and Gardnerella swidsinskii sp. nov., with delineation of 13 genomic species within the genus Gardnerella. Int J Syst Evol Microbiol. 2019;69(3):679–687. doi: 10.1099/ijsem.0.003200. [DOI] [PubMed] [Google Scholar]
- 80.Lewis WG, Robinson LS, Gilbert NM, Perry JC, Lewis AL. Degradation, foraging, and depletion of mucus sialoglycans by the vagina-adapted Actinobacterium Gardnerella vaginalis. J Biol Chem. 2013;288(17):12067–12079. doi: 10.1074/jbc.M113.453654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Severi E, Hood DW, Thomas GH. Sialic acid utilization by bacterial pathogens. Microbiology. 2007;153(9):2817–2822. doi: 10.1099/mic.0.2007/009480-0. [DOI] [PubMed] [Google Scholar]
- 82.Zilnyte M, Venclovas Č, Zvirbliene A, Pleckaityte M. The cytolytic activity of vaginolysin strictly depends on cholesterol and is potentiated by human CD59. Toxins. 2015;7(1):110–128. doi: 10.3390/toxins7010110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cauci S, Thorsen P, Schendel DE, Bremmelgaard A, Quadrifoglio F, Guaschino S. Determination of immunoglobulin A against Gardnerella vaginalis hemolysin, sialidase, and prolidase activities in vaginal fluid: implications for adverse pregnancy outcomes. J Clin Microbiol. 2003;41(1):435–438. doi: 10.1128/JCM.41.1.435-438.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Roberton AM, Wiggins R, Horner PJ, Greenwood R, Crowley T, Fernandes A, Berry M, Corfield AP. A novel bacterial mucinase, glycosulfatase, is associated with bacterial vaginosis. J Clin Microbiol. 2005;43(11):5504–5508. doi: 10.1128/JCM.43.11.5504-5508.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Briselden AM, Moncla BJ, Stevens CE, Hillier SL. Sialidases (neuraminidases) in bacterial vaginosis and bacterial vaginosis-associated microflora. J Clin Microbiol. 1992;30(3):663–666. doi: 10.1128/JCM.30.3.663-666.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jespers V, Kyongo J, Joseph S, Hardy L, Cools P, Crucitti T, Mwaura M, Ndayisaba G, Delany-Moretlwe S, Buyze J, Vanham G. A longitudinal analysis of the vaginal microbiota and vaginal immune mediators in women from sub-Saharan Africa. Sci Rep. 2017;7(1):11974. doi: 10.1038/s41598-017-12198-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lennard K, Dabee S, Barnabas SL, Havyarimana E, Blakney A, Jaumdally SZ, Botha G, Mkhize NN, Bekker LG, Lewis DA, Gray G. Microbial composition predicts genital tract inflammation and persistent bacterial vaginosis in South African adolescent females. Infect Immun. 2018;86(1):e00410–e00417. doi: 10.1128/IAI.00410-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Donders GG, Ruban K, Bellen G. Selecting anti-microbial treatment of aerobic vaginitis. Curr Infect Dis Rep. 2015;17(5):24. doi: 10.1007/s11908-015-0477-6. [DOI] [PubMed] [Google Scholar]
- 89.Reichman O, Sobel J. Desquamative inflammatory vaginitis. Best Pract Res Clin Obstet Gynaecol. 2014;28(7):1042–1050. doi: 10.1016/j.bpobgyn.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 90.Verstraelen H, Verhelst R, Vaneechoutte M, Temmerman M. Group A streptococcal vaginitis: an unrecognized cause of vaginal symptoms in adult women. Arch Gynecol Obstet. 2011;284(1):95–98. doi: 10.1007/s00404-011-1861-6. [DOI] [PubMed] [Google Scholar]
- 91.Zhou X, Westman R, Hickey R, Hansmann MA, Kennedy C, Osborn TW, Forney LJ. Vaginal microbiota of women with frequent vulvovaginal candidiasis. Infect Immun. 2009;77(9):4130–4135. doi: 10.1128/IAI.00436-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Vylkova S, Carman AJ, Danhof HA, Collette JR, Zhou H, Lorenz MC. The fungal pathogen Candida albicans autoinduces hyphal morphogenesis by raising extracellular pH. MBio. 2011;2(3):e00055. doi: 10.1128/mBio.00055-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wu W, Liao Q, Liu Z. Analysis of the vaginal microecology in patients with severe vulvovaginal candidiasis. Biomed Res. 2017;28(1):118–121. [Google Scholar]
- 94.McClelland RS, Richardson BA, Hassan WM, Graham SM, Kiarie J, Baeten JM, Mandaliya K, Jaoko W, Ndinya-Achola JO, Holmes KK. Prospective study of vaginal bacterial flora and other risk factors for vulvovaginal candidiasis. J Infect Dis. 2009;199(12):1883–1890. doi: 10.1086/599213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jian SH, Su MJ. Features of vaginal bacteria community in women with recurrent vulvovaginal candidiasis. J Reprod Contracept. 2015;26(4):229–238. [Google Scholar]
- 96.Liu MB, Xu SR, He Y, Deng GH, Sheng HF, Huang XM, Ouyang CY, Zhou HW. Diverse vaginal microbiomes in reproductive-age women with vulvovaginal candidiasis. PLoS ONE. 2013;8(11):e79812. doi: 10.1371/journal.pone.0079812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Iliev ID, Underhill DM. Striking a balance: fungal commensalism versus pathogenesis. Curr Opin Microbiol. 2013;16(3):366–373. doi: 10.1016/j.mib.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Biswas S, Van Dijck P, Datta A. Environmental sensing and signal transduction pathways regulating morphopathogenic determinants of Candida albicans. Microbiol Mol Biol Rev. 2007;71(2):348–376. doi: 10.1128/MMBR.00009-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Moyes DL, Richardson JP, Naglik JR. Candida albicans-epithelial interactions and pathogenicity mechanisms: scratching the surface. Virulence. 2015;6(4):338–346. doi: 10.1080/21505594.2015.1012981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zordan R, Cormack B. Candida and Candidiasis. 2. Washington: American Society of Microbiology; 2012. Adhesins in opportunistic fungal pathogens; pp. 243–259. [Google Scholar]
- 101.Phan QT, Myers CL, Fu Y, Sheppard DC, Yeaman MR, Welch WH, Ibrahim AS, Edwards JE, Jr, Filler SG. Als3 is a Candida albicans invasin that binds to cadherins and induces endocytosis by host cells. PLoS Biol. 2007;5(3):e64. doi: 10.1371/journal.pbio.0050064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sun JN, Solis NV, Phan QT, Bajwa JS, Kashleva H, Thompson A, Liu Y, Dongari-Bagtzoglou A, Edgerton M, Filler SG. Host cell invasion and virulence mediated by Candida albicans Ssa1. PLoS Pathog. 2010;6(11):e1001181. doi: 10.1371/journal.ppat.1001181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Frohner IE, Bourgeois C, Yatsyk K, Majer O, Kuchler K. Candida albicans cell surface superoxide dismutases degrade host-derived reactive oxygen species to escape innate immune surveillance. Mol Microbiol. 2009;71(1):240–252. doi: 10.1111/j.1365-2958.2008.06528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lorenz MC, Bender JA, Fink GR. Transcriptional response of Candida albicans upon internalization by macrophages. Eukaryot Cell. 2004;3(5):1076–1087. doi: 10.1128/EC.3.5.1076-1087.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Uwamahoro N, Verma-Gaur J, Shen HH, Qu Y, Lewis R, Lu J, Bambery K, Masters SL, Vince JE, Naderer T, Traven A. The pathogen Candida albicans hijacks pyroptosis for escape from macrophages. MBio. 2014;5(2):e00003–e00014. doi: 10.1128/mBio.00003-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Davis DA. How human pathogenic fungi sense and adapt to pH: the link to virulence. Curr Opin Microbiol. 2009;12(4):365–370. doi: 10.1016/j.mib.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 107.Kumamoto CA. Molecular mechanisms of mechanosensing and their roles in fungal contact sensing. Nat Rev Microbiol. 2008;6(9):667. doi: 10.1038/nrmicro1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Finkel JS, Mitchell AP. Genetic control of Candida albicans biofilm development. Nat Rev Microbiol. 2011;9(2):109. doi: 10.1038/nrmicro2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wachtler B, Wilson D, Haedicke K, Dalle F, Hube B. From attachment to damage: defined genes of Candida albicans mediate adhesion, invasion and damage during interaction with oral epithelial cells. PLoS ONE. 2011;6(2):e17046. doi: 10.1371/journal.pone.0017046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Harriott MM, Lilly EA, Rodriguez TE, Fidel PL, Jr, Noverr MC. Candida albicans forms biofilms on the vaginal mucosa. Microbiology. 2010;156(12):3635–3644. doi: 10.1099/mic.0.039354-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Paiva LC, Vidigal PG, Donatti L, Svidzinski TI, Consolaro ME. Assessment of in vitro biofilm formation by Candida species isolates from vulvovaginal candidiasis and ultrastructural characteristics. Micron. 2012;43(2–3):497–502. doi: 10.1016/j.micron.2011.09.013. [DOI] [PubMed] [Google Scholar]
- 112.Nobile CJ, Schneider HA, Nett JE, Sheppard DC, Filler SG, Andes DR, Mitchell AP. Complementary adhesin function in C. albicans biofilm formation. Curr Biol. 2008;18(14):1017–1024. doi: 10.1016/j.cub.2008.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Nobile CJ, Fox EP, Nett JE, Sorrells TR, Mitrovich QM, Hernday AD, Tuch BB, Andes DR, Johnson AD. A recently evolved transcriptional network controls biofilm development in Candida albicans. Cell. 2012;148(1):126–138. doi: 10.1016/j.cell.2011.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Fidel PL., Jr Immunity in vaginal candidiasis. Curr Opin Infect Dis. 2005;18(2):107–111. doi: 10.1097/01.qco.0000160897.74492.a3. [DOI] [PubMed] [Google Scholar]
- 115.Muzny CA, Schwebke JR. Biofilms: an underappreciated mechanism of treatment failure and recurrence in vaginal infections. Clin Infect Dis. 2015;61(4):601–606. doi: 10.1093/cid/civ353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Uppuluri P, Chaturvedi AK, Srinivasan A, Banerjee M, Ramasubramaniam AK, Köhler JR, Kadosh D, Lopez-Ribot JL. Dispersion as an important step in the Candida albicans biofilm developmental cycle. PLoS Pathog. 2010;6(3):e1000828. doi: 10.1371/journal.ppat.1000828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Naglik JR, Rodgers CA, Shirlaw PJ, Dobbie JL, Fernandes-Naglik LL, Greenspan D, Agabian N, Challacombe SJ. Differential expression of Candida albicans secreted aspartyl proteinase and phospholipase B genes in humans correlates with active oral and vaginal infections. J Infect Dis. 2003;188(3):469–479. doi: 10.1086/376536. [DOI] [PubMed] [Google Scholar]
- 118.Schaller M, Bein M, Korting HC, Baur S, Hamm G, Monod M, Beinhauer S, Hube B. The secreted aspartyl proteinases Sap1 and Sap2 cause tissue damage in an in vitro model of vaginal candidiasis based on reconstituted human vaginal epithelium. Infect Immun. 2003;71(6):3227–3234. doi: 10.1128/IAI.71.6.3227-3234.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Moyes DL, Wilson D, Richardson JP, Mogavero S, Tang SX, Wernecke J, Höfs S, Gratacap RL, Robbins J, Runglall M, Murciano C. Candidalysin is a fungal peptide toxin critical for mucosal infection. Nature. 2016;532(7597):64. doi: 10.1038/nature17625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Peters BM, Palmer GE, Nash AK, Lilly EA, Fidel PL, Noverr MC. Fungal morphogenetic pathways are required for the hallmark inflammatory response during Candida albicans vaginitis. Infect Immun. 2014;82(2):532–543. doi: 10.1128/IAI.01417-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Vazquez JA, Sobel JD, Demitriou R, Vaishampayan J, Lynch M, Zervos MJ. Karyotyping of Candida albicans isolates obtained longitudinally in women with recurrent vulvovaginal candidiasis. J Infect Dis. 1994;170(6):1566–1569. doi: 10.1093/infdis/170.6.1566. [DOI] [PubMed] [Google Scholar]
- 122.Diogo D, Bouchier C, d’Enfert C, Bougnoux ME. Loss of heterozygosity in commensal isolates of the asexual diploid yeast Candida albicans. Fungal Genet Biol. 2009;46(2):159–168. doi: 10.1016/j.fgb.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 123.Hirakawa MP, Martinez DA, Sakthikumar S, Anderson MZ, Berlin A, Gujja S, Zeng Q, Zisson E, Wang JM, Greenberg JM, Berman J. Genetic and phenotypic intra-species variation in Candida albicans. Genome Res. 2015;25(3):413–425. doi: 10.1101/gr.174623.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bradford LL, Chibucos MC, Ma B, Bruno V, Ravel J. Vaginal Candida spp. genomes from women with vulvovaginal candidiasis. Pathog Dis. 2017;75(6):ftx061. doi: 10.1093/femspd/ftx061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Petrin D, Delgaty K, Bhatt R, Garber G. Clinical and microbiological aspects ofTrichomonas vaginalis. Clin Microbiol Rev. 1998;11(2):300–317. doi: 10.1128/CMR.11.2.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Marquardt WC, Demaree RS, Grieve RB. Parasitology and vector biology. 2. San Diego: Harcourt Academic Press; 2003. pp. 73–87. [Google Scholar]
- 127.Lehker MW, Sweeney D. Trichomonad invasion of the mucous layer requires adhesins, mucinases, and motility. Sex Transm Infect. 1999;75(4):231–238. doi: 10.1136/sti.75.4.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kucknoor AS, Mundodi V, Alderete JF. The proteins secreted by Trichomonas vaginalis and vaginal epithelial cell response to secreted and episomally expressed AP65. Cell Microbiol. 2007;9(11):2586–2597. doi: 10.1111/j.1462-5822.2007.00979.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Alderete JF, Garza GE. Identification and properties of Trichomonas vaginalis proteins involved in cytadherence. Infect Immun. 1988;56(1):28–33. doi: 10.1128/IAI.56.1.28-33.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Singh BN, Beach DH, Lindmark DG, Costello CE. Identification of the lipid moiety and further characterization of the novel lipophosphoglycan-like glycoconjugates of Trichomonas vaginalis and Trichomonas foetus. Arch Biochem Biophys. 1994;309(2):273–280. doi: 10.1006/abbi.1994.1113. [DOI] [PubMed] [Google Scholar]
- 131.Fichorova RN, Trifonova RT, Gilbert RO, Costello CE, Hayes GR, Lucas JJ, Singh BN. Trichomonas vaginalis lipophosphoglycan triggers a selective upregulation of cytokines by human female reproductive tract epithelial cells. Infect Immun. 2006;74(10):5773–5779. doi: 10.1128/IAI.00631-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Arroyo R, Engbring J, Alderete JF. Molecular basis of host epithelial cell recognition by Trichomonas vaginalis. Mol Microbiol. 1992;6(7):853–862. doi: 10.1111/j.1365-2958.1992.tb01536.x. [DOI] [PubMed] [Google Scholar]
- 133.Moreno-Brito V, Yáñez-Gómez C, Meza-Cervantez P, Ávila-González L, Rodríguez MA, Ortega-López J, González-Robles A, Arroyo R. A Trichomonas vaginalis 120 kDa protein with identity to hydrogenosome pyruvate: ferredoxin oxidoreductase is a surface adhesin induced by iron. Cell Microbiol. 2005;7(2):245–258. doi: 10.1111/j.1462-5822.2004.00455.x. [DOI] [PubMed] [Google Scholar]
- 134.Alderete JF, Lehker MW, Arroyo R. The mechanisms and molecules involved in cytoadherence and pathogenesis of Trichomonas vaginalis. Parasitol Today. 1995;11(2):70–74. doi: 10.1016/0169-4758(95)80122-7. [DOI] [Google Scholar]
- 135.Benchimol M. Trichomonads under microscopy. Microsc Microanal. 2004;10(5):528–550. doi: 10.1017/S1431927604040905. [DOI] [PubMed] [Google Scholar]
- 136.Rasmussen SE, Nielsen MH, Lind I, Rhodes JM. Morphological studies of the cytotoxicity of Trichomonas vaginalis to normal human vaginal epithelial cells in vitro. Sex Transm Infect. 1986;62(4):240–246. doi: 10.1136/sti.62.4.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Nievas YR, Coceres VM, Midlej V, de Souza W, Benchimol M, Pereira-Neves A, Vashisht AA, Wohlschlegel JA, Johnson PJ, de Miguel N. Membrane-shed vesicles from the parasite Trichomonas vaginalis: characterization and their association with cell interaction. Cell Mol Life Sci. 2017;75(12):2211–2226. doi: 10.1007/s00018-017-2726-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Yoon K, Ryu JS, Min DY. Cytotoxicity of lymphokine activated peritoneal macrophages against Trichomonas vaginalis. Korean J Parasitol. 1991;29(4):381–388. doi: 10.3347/kjp.1991.29.4.381. [DOI] [PubMed] [Google Scholar]
- 139.Fiori PL, Rappelli P, Addis MF, Sechi A, Cappuccinelli P. Trichomonas vaginalishaemolysis: pH regulates a contact-independent mechanism based on pore-forming proteins. Microb Pathog. 1996;20(2):109–118. doi: 10.1006/mpat.1996.0010. [DOI] [PubMed] [Google Scholar]
- 140.Ibanez-Escribano A, Nogal-Ruiz JJ, Pérez-Serrano J, Gómez-Barrio A, Escario JA, Alderete JF. Sequestration of host-CD59 as potential immune evasion strategy of Trichomonas vaginalis. Acta Trop. 2015;149:1–7. doi: 10.1016/j.actatropica.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 141.Lehker MW, Chang TH, Dailey DC, Alderete JF. Specific erythrocyte binding is an additional nutrient acquisition system for Trichomonas vaginalis. J Exp Med. 1990;171(6):2165–2170. doi: 10.1084/jem.171.6.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Min DY, Hyun KH, Ryu JS, Ahn MH, Cho MH. Degradations of human immunoglobulins and hemoglobin by a 60 kDa cysteine proteinase of Trichomonas vaginalis. Korean J Parasitol. 1998;36(4):261. doi: 10.3347/kjp.1998.36.4.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Song HO. Influence of 120 kDa Pyruvate: ferredoxin Oxidoreductase on Pathogenicity of Trichomonas vaginalis. Korean J Parasitol. 2016;54(1):71. doi: 10.3347/kjp.2016.54.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ardalan S, Lee BC, Garber GE. Trichomonas vaginalis: the adhesins AP51 and AP65 bind heme and hemoglobin. Exp Parasitol. 2009;121(4):300–306. doi: 10.1016/j.exppara.2008.11.012. [DOI] [PubMed] [Google Scholar]
- 145.Mercer F, Diala FGI, Chen YP, Molgora BM, Ng SH, Johnson PJ. Leukocyte lysis and cytokine induction by the human sexually transmitted parasite Trichomonas vaginalis. PLoS Negl Trop Dis. 2016;10(8):e0004913. doi: 10.1371/journal.pntd.0004913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Nemati M, Malla N, Yadav M, Khorramdelazad H, Jafarzadeh A. Humoral and T cell-mediated immune response against trichomoniasis. Parasite Immunol. 2017;40:e12510. doi: 10.1111/pim.12510. [DOI] [PubMed] [Google Scholar]
- 147.Vazquez Carrillo LI, Quintas Granados LI, Arroyo R, Mendoza Hernández G, González Robles A, Carvajal Gamez BI, Alvarez Sánchez ME. The effect of Zn2 + on prostatic cell cytotoxicity caused by Trichomonas vaginalis. J integr OMICS. 2011;1(2):198–210. [Google Scholar]
- 148.Puente-Rivera J, Villalpando JL, Villalobos-Osnaya A, Vázquez-Carrillo LI, León-Ávila G, Ponce-Regalado MD, López-Camarillo C, et al. The 50 kDa metalloproteinase TvMP50 is a zinc-mediated Trichomonas vaginalis virulence factor. Mol Biochem Parasitol. 2017;217:32–41. doi: 10.1016/j.molbiopara.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 149.Valadkhani Z. Role of pH on adhesion of Trichomonas vaginalis isolated from symptomatic and asymptomatic women to vaginal epithelial cells in vitro. Iran J Med Sci. 2015;29(3):134–139. [Google Scholar]
- 150.Rendon-Maldonado JG, Espinosa-Cantellano M, González-Robles A, Martínez-Palomo A. Trichomonas vaginalis: in vitro phagocytosis of lactobacilli, vaginal epithelial cells, leukocytes, and erythrocytes. Exp Parasitol. 1998;89(2):241–250. doi: 10.1006/expr.1998.4297. [DOI] [PubMed] [Google Scholar]
- 151.Martin DH, Zozaya M, Lillis RA, Myers L, Nsuami MJ, Ferris MJ. Unique vaginal microbiota that includes an unknown Mycoplasma-like organism is associated with Trichomonas vaginalis infection. J Infect Dis. 2013;207(12):1922–1931. doi: 10.1093/infdis/jit100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Hirt RP, Sherrard J. Trichomonas vaginalis origins, molecular pathobiology and clinical considerations. Curr Opin Infect Dis. 2015;28(1):72–79. doi: 10.1097/QCO.0000000000000128. [DOI] [PubMed] [Google Scholar]
- 153.Abraham MC, Desjardins M, Filion LG, Garber GE. Inducible immunity to Trichomonas vaginalis in a mouse model of vaginal infection. Infect Immun. 1996;64(9):3571–3575. doi: 10.1128/IAI.64.9.3571-3575.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Phukan N, Parsamand T, Brooks AE, Nguyen TN, Simoes-Barbosa A. The adherence of Trichomonas vaginalis to host ectocervical cells is influenced by lactobacilli. Sex Transm Infect. 2013;89:455–459. doi: 10.1136/sextrans-2013-051039. [DOI] [PubMed] [Google Scholar]
- 155.Brotman RM, Bradford LL, Conrad M, Gajer P, Ault K, Peralta L, Forney LJ, Carlton JM, Abdo Z, Ravel J. Association between Trichomonas vaginalis and vaginal bacterial community composition among reproductive-age women. Sex Transm Dis. 2012;39(10):807. doi: 10.1097/OLQ.0b013e3182631c79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Fettweis JM, Serrano MG, Huang B, Brooks JP, Glascock AL, Sheth NU, Strauss JF, III, Jefferson KK, Buck GA, Vaginal Microbiome Consortium An emerging mycoplasma associated with trichomoniasis, vaginal infection and disease. PLoS ONE. 2014;9(10):e110943. doi: 10.1371/journal.pone.0110943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Lo SC, Hayes MM, Tully JG, Wang RYH, Kotani H, Pierce PF, Rose DL, Shih JWK. Mycoplasma penetrans sp. nov., from the urogenital tract of patients with AIDS. Int J Syst Evol Microbiol. 1992;42(3):357–364. doi: 10.1099/00207713-42-3-357. [DOI] [PubMed] [Google Scholar]
- 158.Margarita V, Rappelli P, Dessì D, Pintus G, Hirt RP, Fiori PL. Symbiotic association with Mycoplasma hominis can influence growth rate, ATP production, cytolysis and inflammatory response of Trichomonas vaginalis. Front Microbiol. 2016;7:953. doi: 10.3389/fmicb.2016.00953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Khanaliha K, Masoumi-Asl H, Bokharaei-Salim F, Tabatabaei A, Naghdalipoor M. Double-stranded RNA viral infection of Trichomonas vaginalis (TVV1) in Iranian isolates. Microb Pathog. 2017;109:56–60. doi: 10.1016/j.micpath.2017.04.032. [DOI] [PubMed] [Google Scholar]
- 160.Fichorova RN, Lee Y, Yamamoto HS, Takagi Y, Hayes GR, Goodman RP, Chepa-Lotrea X, Buck OR, Murray R, Kula T, Beach DH. Endobiont viruses sensed by the human host–beyond conventional antiparasitic therapy. PLoS ONE. 2012;7(11):e48418. doi: 10.1371/journal.pone.0048418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Fichorova RN, Buck OR, Yamamoto HS, Fashemi T, Dawood HY, Fashemi B, Hayes GR, Beach DH, Takagi Y, Delaney ML, Nibert ML. The villain team-up or how Trichomonas vaginalis and bacterial vaginosis alter innate immunity in concert. Sex Transm Infect. 2013;2013(89):460–466. doi: 10.1136/sextrans-2013-051052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Fichorova R, Fraga J, Rappelli P, Fiori PL. Trichomonas vaginalis infection in symbiosis with Trichomonasvirus and Mycoplasma. Res Microbiol. 2017;168(9–10):882–891. doi: 10.1016/j.resmic.2017.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Philip J. Leptothrix vaginalis. PathologyOutlines.com Revised: 21 December 2018 https://www.pathologyoutlines.com/topic/cervixleptothrix.html.
- 164.Spotin A, Eghtedar ST, Shahbazi A, Salehpour A, Sarafraz S, Shariatzadeh SA, Mahami-Oskouei M. Molecular Characterization of Trichomonas vaginalis strains based on identifying their probable variations in asymptomatic patients. Iran J Parasitol. 2016;11(4):507. [PMC free article] [PubMed] [Google Scholar]
- 165.Małyszko E, Januszko T, Smorczewska-Czupryńska B, Ustymowicz-Farbiszewska J. Virulence of Trichomonas vaginalis strains in relation to clinical forms of Trichomonas vaginitis. Wiad Parazytol. 1991;37(2):219–223. [PubMed] [Google Scholar]
- 166.Gold D. Trichomonas vaginalis: strain differences in adhesion to plastic and virulence in vitro and in vivo. Parasitol Res. 1993;79(4):309–315. doi: 10.1007/BF00932187. [DOI] [PubMed] [Google Scholar]
- 167.Carlton JM, Hirt RP, Silva JC, Delcher AL, Schatz M, Zhao Q, Wortman JR, Bidwell SL, Alsmark UCM, Besteiro S, Sicheritz-Ponten T. Draft genome sequence of the sexually transmitted pathogen Trichomonas vaginalis. Science. 2007;315(5809):207–212. doi: 10.1126/science.1132894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.van Oostrum N, De Sutter P, Meys J, Verstraelen H. Risks associated with bacterial vaginosis in infertility patients: a systematic review and meta-analysis. Hum Reprod. 2013;28(7):1809–1815. doi: 10.1093/humrep/det096. [DOI] [PubMed] [Google Scholar]
- 169.Gallo MF, Macaluso M, Warner L, Fleenor ME, Hook EW, Brill I, Weaver MA. Bacterial vaginosis, gonorrhea, and chlamydial infection among women attending a sexually transmitted disease clinic: a longitudinal analysis of possible causal links. Ann Epidemiol. 2012;22(3):213–220. doi: 10.1016/j.annepidem.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 170.Ziklo N, Vidgen ME, Taing K, Huston WM, Timms P. Dysbiosis of the vaginal microbiota and higher vaginal kynurenine/tryptophan ratio reveals an association with Chlamydia trachomatis genital infections. Front Cell Infect Microbiol. 2018;8:1. doi: 10.3389/fcimb.2018.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Spurbeck RR, Arvidson CG. Inhibition of Neisseria gonorrhoeae epithelial cell interactions by vaginal Lactobacillus species. Infect Immun. 2008;76(7):3124–3130. doi: 10.1128/IAI.00101-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Nardis C, Mosca L, Mastromarino P. Vaginal microbiota and viral sexually transmitted diseases. Ann Ig. 2013;25(5):443–456. doi: 10.7416/ai.2013.1946. [DOI] [PubMed] [Google Scholar]
- 173.Sharma H, Tal R, Clark NA, Segars JH. Microbiota and pelvic inflammatory disease. Bethesda: NIH; 2014. p. 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.McClure EM, Dudley DJ, Reddy U, Goldenberg RL. Infectious causes of stillbirth: a clinical perspective. Clin Obstet Gynecol. 2010;53(3):635. doi: 10.1097/GRF.0b013e3181eb6620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Waldorf KMA, McAdams RM. Influence of infection during pregnancy on fetal development. Reproduction. 2013;146(5):R151–R162. doi: 10.1530/REP-13-0232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Giakoumelou S, Wheelhouse N, Cuschieri K, Entrican G, Howie SE, Horne AW. The role of infection in miscarriage. Human reproduction update. 2015;22(1):116–133. doi: 10.1093/humupd/dmv041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Tellapragada C, Eshwara VK, Bhat P, Acharya S, Kamath A, Bhat S, Rao C, Nayak S, Mukhopadhyay C. Risk factors for preterm birth and low birth weight among pregnant Indian women: a hospital-based prospective study. J Prev Med Public Health. 2016;49(3):165. doi: 10.3961/jpmph.16.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Macklaim JM, Fernandes AD, Di Bella JM, Hammond JA, Reid G, Gloor GB. Comparative meta-RNA-seq of the vaginal microbiota and differential expression by Lactobacillus iners in health and dysbiosis. Microbiome. 2013;1(1):12. doi: 10.1186/2049-2618-1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Witkin SS, Mendes-Soares H, Linhares IM, Jayaram A, Ledger WJ, Forney LJ. Influence of vaginal bacteria and D-and L-lactic acid isomers on vaginal extracellular matrix metalloproteinase inducer: implications for protection against upper genital tract infections. MBio. 2013;4(4):e00460. doi: 10.1128/mBio.00460-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Petricevic L, Domig KJ, Nierscher FJ, Sandhofer MJ, Fidesser M, Krondorfer I, Husslein P, Kneifel W, Kiss H. Characterisation of the vaginal Lactobacillus microbiota associated with preterm delivery. Sci Rep. 2014;4:5136. doi: 10.1038/srep05136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Leizer J, Nasioudis D, Forney L, Schneider GM, Gliniewicz K, Boester A, Witkin SS. 447: large differences in the composition of vaginal secretions in pregnant women in the presence of Lactobacillus crispatus and L. iners. Am J Obstet Gynecol. 2017;216(1):S264. doi: 10.1016/j.ajog.2016.11.705. [DOI] [Google Scholar]
- 182.Zhou X, Brown CJ, Abdo Z, Davis CC, Hansmann MA, Joyce P, Foster JA, Forney LJ. Differences in the composition of vaginal microbial communities found in healthy Caucasian and black women. The ISME J. 2007;1(2):121. doi: 10.1038/ismej.2007.12. [DOI] [PubMed] [Google Scholar]
- 183.Zhou X, Hansmann MA, Davis CC, Suzuki H, Brown CJ, Schütte U, Pierson JD, Forney LJ. The vaginal bacterial communities of Japanese women resemble those of women in other racial groups. FEMS Immunol Med Microbiol. 2010;58(2):169–181. doi: 10.1111/j.1574-695X.2009.00618.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Beamer MA, Austin MN, Avolia HA, Meyn LA, Bunge KE, Hillier SL. Bacterial species colonizing the vagina of healthy women are not associated with race. Anaerobe. 2017;45:40–43. doi: 10.1016/j.anaerobe.2017.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Fredericks DN, Relman DA. Sequence-based identification of microbial pathogens: a reconsideration of Koch’s postulates. Clin Microbiol Rev. 1996;9(1):18–33. doi: 10.1128/CMR.9.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.