Abstract
Introduction:
Psoriasis is an autoimmune disease. The important role of oxidative stress in the pathogenesis of psoriasis had been investigated in different studies. Thioredoxin reductase (TrxR) is a selenocysteine-containing enzyme which is involved in the protection of cells against oxidative stress. Here, we investigated the TrxR activity in skin lesions of psoriatic patients and the possible correlation between this activity and the severity of the disease that was scored based on the Psoriasis Area and Severity Index (PASI).
Materials and Methods:
TrxR activity was determined using TrxR colorimetric method based on the reduction of 5,5'-dithiobis-(2-nitrobenzoic acid) to 5-thio-2-nitrobenzoic acid by TrxR using nicotinamide adenine dinucleotide phosphate in 20 psoriatic patients (11 men and 9 women) aged 38.9 ± 12.6 years. For evaluating the disease severity, PASI score system (mild [PASI <10], moderate [PASI 10–20], or severe [PASI >20]) was utilized that was based on three factors including thickness, erythema, and scaling of lesions.
Results:
Our results revealed that the TrxR activity between different groups of psoriatic patients (according to the PASI score) was statistically significant and it was higher in psoriatic patients with mild disease (correlation coefficient = −0.85).
Conclusion:
These results further strengthen the association between psoriasis and oxidative stress. The increased level of TrxR could be due to the protective effect of this enzyme against the inflammatory process and oxidative stress. Moreover, TrxR could be used as a novel marker for evaluating psoriasis severity.
KEY WORDS: Oxidative stress, psoriasis, psoriasis area and severity index, thioredoxin reductase activity
Introduction
Psoriasis is a chronic autoimmune disease, with a global prevalence of between 2% and 3%.[1] Psoriatic patients exhibit typical features including epidermal hyperproliferation, immunological dysfunction, and angiogenesis.[2] Different mechanisms have been suggested to be involved in its pathogenesis, such as induction of interleukin-6 (IL-6), IL-7, IL-8, IL-12, IL-15, and IL-18 by tumor necrosis factor-alpha (TNF-α) and interferon-gamma and production of macrophage chemokines.[3,4] Recently, the important role of oxidative stress in the pathogenesis of psoriasis had been investigated.[5]
Oxidative stress, as a consequence of an imbalance between oxidants and antioxidants in favor of the former, has an important role in the pathogenesis of many diseases, such as cancer, cardiovascular disease, and neurodegenerative disorder.[2,6,7] The role of oxidative stress in the pathogenesis of several mucocutaneous diseases including basal cell carcinoma, pemphigus vulgaris, and lichen planus has also been reported. The disruption in oxidant/antioxidant homeostasis results in oxidation and subsequent damage to biomolecules such as proteins, lipids, and DNA.[8,9]
Thioredoxin reductase (TrxR) is a selenocysteine-containing enzyme which is involved in different intracellular processes, especially in the protection of cells against oxidative stress.[10] This nicotinamide adenine dinucleotide phosphate (NADPH)-dependent enzyme catalyzes the reduction of oxidized Trx to its reduced form that is essential for providing and maintaining the reduced state of the intracellular environment.[11] A number of studies had shown an increase in the serum level of TrxR in several diseases including inflammation, infection, and cancer.[12]
With respect to the importance of oxidative stress in the pathogenesis of psoriasis, the present study was planned to evaluate the TrxR activity in the dermis of psoriasis lesions, and we also investigated the possible correlation between this activity and the severity of the disease that was scored based on the Psoriasis Area and Severity Index (PASI) scoring system.[13]
Materials and Methods
In this study, 20 (11 men and 9 women) newly diagnosed psoriatic patients aged 38.85±12.57 years (mean ± standard deviation) were recruited who had received no specific treatment. Moreover, patients who had a history of consumption of antioxidant supplements, immunosuppressive agents, and anti-inflammatory drugs and also patients with systemic disorders such as diabetes or cardiovascular diseases were excluded from the study. This study was ethically approved by the Research Council of Mashhad University of Medical Sciences, and a written informed consent was obtained from each participant.
Skin biopsy samples with a thickness of 3–4 mm were obtained from psoriatic lesions and were washed with phosphate-buffered saline, pH 7.4, to remove blood and clots. Thereafter, samples were resuspended in cold buffer (5 ml of 50 mmol/L KPO4 and 1 mmol/L ethylenediaminetetraacetic acid per gram of biopsy sample) and homogenized. Then, samples were centrifuged at 10,000 rpm for 15 min at 4°C, and supernatants were collected and stored at −80°C until laboratory analysis.
Thioredoxin reductase activity measurement
TrxR activity was determined using the TrxR Colorimetric Assay Kit (Caymans Chemical, USA, Item No. 10007892). The assay was performed as described by the manufacturer that was based on the reduction of 5,5'-dithiobis-(2-nitrobenzoic acid) to 5-thio-2-nitrobenzoic acid by TrxR using NADPH. The absorbance changes were followed at 414 nm and were utilized for calculating TrxR activity.
Evaluating the disease severity
For evaluating the disease severity, PASI scoring system was utilized that was based on three factors including thickness, erythema, and scaling of lesions.[13] For determining the severity of skin lesions, we assigned a score between 0 and 4, and for total body involvement, we considered a score between 0 and 6. Then, the final PASI score was calculated by multiplying the severity scores of lesions by the area score. Then, patients were grouped based on having mild (PASI <10), moderate (PASI 10–20), or severe (PASI >20) psoriasis.
Statistical analysis
All statistical analyses were performed using the SPSS statistical software package (version 11.5, SPSS Inc., Chicago, IL) Chi-square test, independent t-test, and its nonparametric equivalent were used for data assessment. P<0.05 was considered statistically significant.
Results
In this study, a total of 20 participants (11 men and 9 women) with the average age of 38.85±12.57 years were enrolled. The characteristics of the participants are shown in Table 1.
Table 1.
Descriptive characteristics of the study participants
| Parameter | Frequency | Average | Minimum | Maximum | Difference range | SD |
|---|---|---|---|---|---|---|
| Age | 20 | 38.8500 | 23.00 | 64.00 | 41.00 | 12.57096 |
| Redness | 20 | 3.1500 | 0.80 | 7.20 | 6.40 | 1.64076 |
| Thickness | 20 | 3.0450 | 0.60 | 6.40 | 5.80 | 1.69968 |
| Scaling | 20 | 3.1150 | 0.60 | 5.40 | 4.80 | 1.53804 |
| PASI score | 20 | 9.3000 | 2.00 | 19.00 | 17.00 | 4.61234 |
| TrxR activity | 20 | 0.3995 | 0.18 | 0.54 | 0.36 | 0.10344 |
PASI: Psoriasis Area and Severity Index, TrxR: Thioredoxin reductase, SD: Standard deviation
Clinical severity of psoriasis based on the Psoriasis Area and Severity Index scoring system
According to the PASI scoring system, the clinical severity of psoriasis in patients ranged from 2 to 19. Among the 20 participants, 12 had a score ≤ 10 and were grouped as a mild disease, while 8 had a score of 10–20 (moderate disease). No patient had severe disease.
Relationship between thioredoxin reductase activity and psoriasis severity
As shown in Figure 1, the correlation analysis showed that there was a significant inverse association between disease severity and TrxR activity (correlation coefficient = −0.85) (P<0.01).
Figure 1.
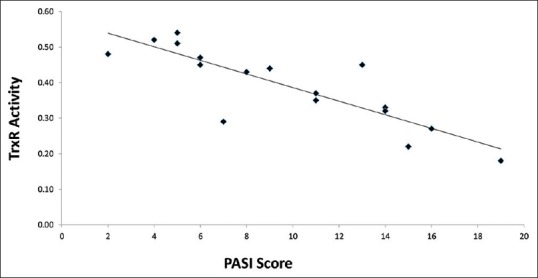
Dispersion diagram showing the relationship between psoriasis severity and tissue thioredoxin reductase activity
Discussion
In the present study, we observed a higher activity of TrxR enzyme in psoriatic patients. However, with increase of the disease severity according to the PASI score, the activity of TrxR was decreased. Our findings suggested that TrxR, which was an antioxidant enzyme, was associated with psoriasis in a negative way.
Psoriasis is an inflammatory skin disease, and reactive oxygen species (ROS) generation is associated with disease progression.[14] Evaluating the level of oxidative stress is useful in diagnosing the state of the disease, assessing the progression of the disease, and developing new guidelines to evaluate the response to treatment.[15]
The cell response to oxidative stress condition includes an increase in antioxidant capacity and upregulation in signaling pathways which contribute to defense against oxidative stress and ultimately protect the cell against damages. After producing free radicals inside the cytoplasm, signaling cascade of thioredoxin (Txr) initiates in response to oxidative stress.[16]
On the other hand, studies had shown an increase in pro-angiogenic factors, such as, IL-8, vascular endothelial growth factor, basic fibroblast growth factor, hypoxia-inducible factor-1, TNF, and transforming growth factor-alpha (TGF-α) in psoriasis. These factors play a key role in the development of new vessels with higher permeability and ultimately the recruitment of inflammatory cells. Furthermore, studies had shown the involvement of growth factors and cytokines in ROS production.[17]
Moreover, data from different studies revealed that the Trx/TrxR system has a key role in stimulating angiogenesis and cell proliferation.[18] Taken together, these data indicate the contradictory role of the Trx/TrxR system in the pathogenesis of psoriasis.
In this study, we found a statistically significant decrease in the level of TrxR from mild to moderate psoriatic patients. There are a number of studies suggesting that there is an imbalance in the antioxidant system in psoriatic patients. Similar to our result, other researchers observed that increase in psoriasis severity as defined by PASI was associated with oxidative stress.[19,20]
A recent study conducted by Hashemi et al. had shown a low total level of antioxidants in psoriatic patients. They concluded that this could be due to excess generation of ROS and can be attributed to a less efficient antioxidant system.[19]
Nemati et al. had found that the levels of superoxide dismutase, paraoxonase 1, and catalase, the antioxidant agents, were significantly decreased in psoriatic patients. It has been hypothesized that there is a relationship between superoxide dismutase and catalase activity and epidermal hyperproliferation,[20] these findings are in agreement with our results.
Conclusion
These results further strengthen the linkage between psoriasis and oxidative stress. Moreover, TrxR could be used as a marker to evaluate psoriasis severity, as it is not based on an evaluation of a disease state with the PASI scoring system. Furthermore, these data could provide information for the development of therapies targeting psoriasis disease.
Financial support and sponsorship
This work was supported by a grant from Mashhad University of Medical Sciences (grant number: 910532).
Conflicts of interest
There are no conflicts of interest.
References
- 1.Gu X, Nylander E, Coates PJ, Nylander K. Oxidation reduction is a key process for successful treatment of psoriasis by narrow-band UVB phototherapy. Acta Derm Venereol. 2015;95:140–6. doi: 10.2340/00015555-1905. [DOI] [PubMed] [Google Scholar]
- 2.Gerkowicz A, Pietrzak A, Szepietowski JC, Radej S, Chodorowska G. Biochemical markers of psoriasis as a metabolic disease. Folia Histochem Cytobiol. 2012;50:155–70. doi: 10.5603/fhc.2012.0025. [DOI] [PubMed] [Google Scholar]
- 3.Gudjonsson JE, Johnston A, Sigmundsdottir H, Valdimarsson H. Immunopathogenic mechanisms in psoriasis. Clin Exp Immunol. 2004;135:1–8. doi: 10.1111/j.1365-2249.2004.02310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Späh F. Inflammation in atherosclerosis and psoriasis: Common pathogenic mechanisms and the potential for an integrated treatment approach. Br J Dermatol. 2008;159(Suppl 2):10–7. doi: 10.1111/j.1365-2133.2008.08780.x. [DOI] [PubMed] [Google Scholar]
- 5.Gupta M, Chari S, Borkar M, Ch M. Dyslipidemia and oxidative stress in patients of psoriasis. Biomed Res. 2011;22:222–5. [Google Scholar]
- 6.Boaz M, Smetana S, Weinstein T, Matas Z, Gafter U, Iaina A, et al. Secondary prevention with antioxidants of cardiovascular disease in endstage renal disease (SPACE): Randomised placebo-controlled trial. Lancet. 2000;356:1213–8. doi: 10.1016/s0140-6736(00)02783-5. [DOI] [PubMed] [Google Scholar]
- 7.Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J, et al. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 8.Birben E, Sahiner UM, Sackesen C, Erzurum S, Kalayci O. Oxidative stress and antioxidant defense. World Allergy Organ J. 2012;5:9–19. doi: 10.1097/WOX.0b013e3182439613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duracková Z. Some current insights into oxidative stress. Physiol Res. 2010;59:459–69. doi: 10.33549/physiolres.931844. [DOI] [PubMed] [Google Scholar]
- 10.Hashemy SI, Holmgren A. Regulation of the catalytic activity and structure of human thioredoxin 1 via oxidation and S-nitrosylation of cysteine residues. J Biol Chem. 2008;283:21890–8. doi: 10.1074/jbc.M801047200. [DOI] [PubMed] [Google Scholar]
- 11.Mustacich D, Powis G. Thioredoxin reductase. Biochem J. 2000;346(Pt 1):1–8. [PMC free article] [PubMed] [Google Scholar]
- 12.Holmgren A, Lu J. Thioredoxin and thioredoxin reductase: Current research with special reference to human disease. Biochem Biophys Res Commun. 2010;396:120–4. doi: 10.1016/j.bbrc.2010.03.083. [DOI] [PubMed] [Google Scholar]
- 13.Langley RG, Ellis CN. Evaluating psoriasis with psoriasis area and severity index, psoriasis global assessment, and lattice system physician's global assessment. J Am Acad Dermatol. 2004;51:563–9. doi: 10.1016/j.jaad.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 14.Kadam DP, Suryakar AN, Ankush RD, Kadam CY, Deshpande KH. Role of oxidative stress in various stages of psoriasis. Indian J Clin Biochem. 2010;25:388–92. doi: 10.1007/s12291-010-0043-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matsuo Y, Yodoi J. Extracellular thioredoxin: A therapeutic tool to combat inflammation. Cytokine Growth Factor Rev. 2013;24:345–53. doi: 10.1016/j.cytogfr.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 16.Nguyen P, Awwad RT, Smart DD, Spitz DR, Gius D. Thioredoxin reductase as a novel molecular target for cancer therapy. Cancer Lett. 2006;236:164–74. doi: 10.1016/j.canlet.2005.04.028. [DOI] [PubMed] [Google Scholar]
- 17.Armstrong AW, Voyles SV, Armstrong EJ, Fuller EN, Rutledge JC. Angiogenesis and oxidative stress: Common mechanisms linking psoriasis with atherosclerosis. J Dermatol Sci. 2011;63:1–9. doi: 10.1016/j.jdermsci.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 18.Creamer D, Sullivan D, Bicknell R, Barker J. Angiogenesis in psoriasis. Angiogenesis. 2002;5:231–6. doi: 10.1023/a:1024515517623. [DOI] [PubMed] [Google Scholar]
- 19.Hashemi M, Mehrabifar H, Daliri M, Ghavami S. Adenosine deaminase activity, trypsin inhibitory capacity and total antioxidant capacity in psoriasis. J Eur Acad Dermatol Venereol. 2010;24:329–34. doi: 10.1111/j.1468-3083.2009.03416.x. [DOI] [PubMed] [Google Scholar]
- 20.Nemati H, Khodarahmi R, Sadeghi M, Ebrahimi A, Rezaei M, Vaisi-Raygani A, et al. Antioxidant status in patients with psoriasis. Cell Biochem Funct. 2014;32:268–73. doi: 10.1002/cbf.3011. [DOI] [PubMed] [Google Scholar]


