Abstract
Background
Neuromuscular blocking agent (NMBA) has been proposed by medical guidelines for early severe acute respiratory distress syndrome (ARDS) because of its survival benefits. However, new studies have provided evidence contradicting these results.
Method
A search was performed of the Pubmed, Scopus, Clinicaltrials.gov, and Virtual Health Library databases for randomized controlled trials (RCT) evaluating 28-day mortality in ARDS patients treated with NMBA within 48 h. An English language restriction was applied. Relevant data were extracted and pooled into risk ratios (RR), mean differences (MD), and corresponding 95% confidence intervals (CI) using random-effect model. Sensitivity and meta-regression analysis were performed.
Results
From 2675 studies, we included five RCTs in the analysis, for a total of 1461 patients with a mean PaO2/FIO2 of 104 ± 35 mmHg. The cisatracurium group had the same risk of death at 28 days (RR, 0.90; 95% CI, 0.78–1.03; I2 = 50%, p = 0.12) and 90 days (RR, 0.81; 95% CI, 0.62–1.06; I2 = 56%, p = 0.06) as the control group (no cisatracurium). The secondary outcomes of mechanical ventilation duration and ventilator-free days were not different between the two groups. Cisatracurium had a significantly lower risk of barotrauma than the control group with no difference in intensive care unit (ICU)–induced weakness. The PaO2/FIO2 ratio was higher in the cisatracurium group but not until 48 h. Meta-regression analysis of the baseline PaO2/FIO2 ratio, positive end-expiratory pressure (PEEP) revealed no heterogeneity. Subgroup analysis excluding the trial using high PEEP and light sedation strategy yielded an improvement in all mortality outcomes.
Conclusion
NMBA improves oxygenation only after 48 h in moderate, severe ARDS patients and has a lower barotrauma risk without affecting ICU weakness. However, NMBA does not reduce ventilator-free days, duration of mechanical ventilation or, most importantly, the mortality risk regardless of the severity of ARDS.
Keywords: Neuromuscular blocking agent, Cisatracurium, Acute respiratory distress syndrome
Background
Acute respiratory distress syndrome (ARDS) is a severe diffuse lung injury characterized by significantly impaired oxygenation and lung compliance that frequently leads to mechanical ventilation [1–3]. ARDS is common, accounting for 10 to 15% of intensive care unit admissions and 200,000 patients a year in the US [4, 5]. In a large observational study, the mortality rate of moderate to severe ARDS was up to 40% [4]. Neuromuscular blocking agents (NMBA) have been proposed during the early course of moderate to severe ARDS especially in those with PaO2/FIO2 < 120 mmHg to improve oxygenation and reduce patient-ventilator dyssynchrony [6–8]. NMBA facilitates oxygenation in various studies [9–11] by various mechanisms, including reducing ventilator-induced lung injuries and inhibiting inflammatory cytokines [12, 13]. An early, short-term continuous infusion of cisatracurium, an NMBA, was evaluated in a large multicenter randomized study (ACURASYS trial), and evidence of its survival benefits in ARDS was found in an anticipated post hoc analysis of patients with PaO2/FIO2 < 120 mmHg. NMBA, however, could be associated with muscular weakness and the need of more sedatives [6, 14, 15]. Of note, besides NMBA use, increasing evidence of other supportive treatments for ARDS patients such as sedation, positive end-expiratory pressure (PEEP) strategy, and prone positioning has been developed since major data on NMBA were published [16–18]. The question of whether NMBA is effective is still unknown. Recently, the largest randomized controlled trial on NMBA in early ARDS patients was published to reassess the effect of an early, short-term continuous infusion of cisatracurium (ROSE trial), and no survival benefit was found [19]. This result was different from major studies published previously on the same topic [9–11].
Therefore, the objective of this study was to provide a comprehensive meta-analysis of data from all randomized controlled trials on neuromuscular blocking agent use in ARDS patients. We clarify the effects of NMBA on patient survival, oxygenation, and adverse events to potentially guide decisions among medical professionals treating ARDS patients.
Methods
Search strategy and study identification
We used the following electronic databases: Pubmed (1970 to present), Scopus (1970 to present), Clinicaltrials.gov (1970 to present), and Virtual health library (1970 to present) to identify relevant articles in July 2019. Search terms: (((((((ARDS[Title/Abstract]) OR acute respiratory distress syndrome[Title/Abstract]) OR respiratory distress syndrome, adult[MeSH Terms]) OR adult respiratory distress syndrome[MeSH Terms]) OR acute respiratory distress syndrome[MeSH Terms]) OR respiratory failure[Title/Abstract])) AND ((((((cisatracurium[Title/Abstract]) OR Neuromuscular blockade[Title/Abstract]) OR Neuromuscular blocking agent[Title/Abstract]) OR NMBA[Title/Abstract]) OR paraly*[Title/Abstract]) OR Nimbex[Title/Abstract]) were used. The study protocol followed the recommendations of the Preferred Reporting item for Systematic Review and Meta-analysis (PRISMA) statement [20]. A 2-level literature search was performed. A secondary search included manual scrutiny of the reference list of relevant articles received in the initial search after importing all searched studies from the databases into Endnote. English language restriction was applied.
Study selection and data collection
We used the following inclusion criteria: randomized control trials (RCTs) in patients with adult respiratory distress syndrome (ARDS) who were randomized to receive neuromuscular blocker within the first 48 h of the diagnosis and who have evaluated 28-day mortality rate between the neuromuscular blocker group with the conventional treatment group. Studies were excluded if they were: (1) non-randomized controlled trials, (2) conference posters, and (3) did not measure mortality outcomes. Any difference in opinion about the eligibility was solved by discussion and consensus.
Full-text screening and data extraction
Two reviewers extracted the data independently into a spreadsheet. The data sought included (1) study characteristics (title, authors, year of publication, study location, number of patients, age, important exclusion criteria); (2) baseline patients demographic and clinical characteristics (age, sex, baseline fractional inspiratory oxygen (FIO2), PEEP, PaO2/FIO2 ratio, tidal volume, causes of ARDS, sepsis scores); (3) mortality at 28 days, 90 days, ICU mortality, mechanical ventilation duration, ventilator-free days at day 28, days not in ICU at day 28; (4) adverse events (Medical Research Council scale, ICU weakness at day 28, barotrauma). Barotrauma was defined as either newly developed pneumothorax, pneumomediastinum, or subcutaneous emphysema. Any disagreement between the two reviewers was resolved by consensus.
Endpoints
The primary endpoint of this meta-analysis was the 28-day mortality relative risk between the NMBA interventional group and the control group. The secondary endpoint was the 90-day ICU mortality relative risks, the difference in mechanical ventilation duration, ventilator-free days at day 28, days not in ICU at day 28, and the adverse events.
Risk of bias and evidence profile
We used the Cochrane Risk of Bias tool for a randomized control trial to assess the bias profile of individual trials [21]. The tool includes 7 domains: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other possible bias. Risks were classified as low, high risk, or unclear. Publication bias was analyzed using Egger’s regression test and funnel plot. The quality of the evidence was assessed for the primary endpoint in agreement with the Grading of Recommendations Assessment Development and Evaluation system [22].
Statistical analysis
Statistical analyses were performed using the Review Manager 5.3 software (Cochrane Collaborative Oxford, UK) and Comprehensive Meta-Analysis 2.0 software. Individual study relative risks (RRs) and 95% CI were calculated for dichotomous outcomes and mean differences (MDs) with 95% CI were estimated for continuous outcomes. Summary estimates of RRs were determined using the DerSimonian and Laird random-effects models. The variability across studies not by chance was tested using the I2 statistics and p > 0.05 was considered not statistically significant. Inconsistency across the studies was classified as low if 25% < I2 ≤ 50%, moderate if 50% < I2 ≤ 75%, and high if I2 > 75 [23]. Additional sensitivity analyses were performed to evaluate the effect of key assumptions on the overall results or the robustness of the overall results. We further form meta-regression models to assess for potential heterogeneity for the following factors: PaO2/FIO2 ratio (P/F ratio), baseline PEEP, predicted mortality, PEEP strategy, and prone positioning. Predicted mortality was based on available studies on SOFA score (first in full text) and SAPS II (first in full text) score [24–26]. Publication bias was analyzed as previously described by the funnel plot and regression test for funnel plot asymmetry. A p value of < 0.05 was considered a statistically significant publication bias.
Results
Literature search results and study characteristics
Using the search terms mentioned above, we identified 2675 studies. After screening the study abstracts of the above studies, five studies met the inclusion criteria for comparing mortality in patients with ARDS treated with an additional NMBA vs conventional treatment groups. Five studies were included for a full-text review [9–11, 19, 27]. The flow diagram of the study selection process is shown in Fig. 1.
Fig. 1.

Screening and selection process flow diagram
A description of the studies included in our analysis is shown in Table 1. A total of 1462 patients were included in the five trials. All of the studies included patients within 48 h of the ARDS diagnosis with a cut-off PaO2/FIO2 ratio mostly below 150 mmHg with the exception of Forel et al. (cut-off below 200). Guervilly et al. only randomized patients with moderate ARDS and PaO2/FIO2 between 100 and 150 mmHg. The pooled data for the baseline respiratory parameters had a PEEP (cmH20) of 11.68 ± 3.74 and a P/F ratio of 104 ± 32.50. The baseline demographic and respiratory parameters (PEEP, PaO2/FIO2 ratio) were not statistically significantly different between the NMBA group and the control group. The ARDS causes mostly include pneumonia (59%), followed by aspiration, extrapulmonary sepsis, other causes, and lung contusion (17%, 14%, 9%, and 1% respectively).
Table 1.
Characteristics of included trials
| Trial author or trial name | Gainnier et al. [10] | Forel et al. [9] | ACURASYS trial [11] | Guervilly et al. [27] | ROSE trial [19] |
|---|---|---|---|---|---|
| Year | 2004 | 2006 | 2010 | 2017 | 2019 |
| Country | France | France | France | France | USA |
| NBMA vs control (patients) | 28 vs 28 | 18 vs 18 | 177 vs 162 | 13 vs 11 | 501 vs 505 |
| PaO2/FIO2 ratio and PEEP for inclusion (cm H2O) |
< 150 > 5 |
< 200 |
< 150 > 5 |
100–150 > 5 |
< 150 > 8 |
| Important exclusion criteria | NMBA use within 2 weeks | NMBA and steroids use within 2 weeks | Current continuous infusion of NMBA | Current continuous infusion of NMBA | Current continuous infusion of NMBA |
| Ventilator management strategy | Lung protective ARDS net protocol | Lung protective ARDS net protocol | Lung protective ARDS net protocol | Lung protective ARDS net protocol | Lung protective high PEEP strategy |
| Cisatracurium dose | Bolus of 50 mg, 5 μg/kg/min | Bolus 0.2 mg/kg, 5 μg/kg/min | Bolus of 15 mg, 37.5 mg/hour | Bolus of 15 mg, 37.5 mg/hour | Bolus 15 mg, 37.5 mg/hour |
| Assessment for an extra dose | Train-of-four | Train-of-four | Plateau pressure > 32 | Train-of-four | No adjustment |
| Sedation goal | Ramsay 6 | Ramsay 6b | Ramsay 6 | Ramsay 6 |
RASS − 4, − 5 in the intervention group RASS 0 to − 1 in the control group)c |
| Risk of bias | |||||
| Sequence generation | Low | low | Low | Low | Low |
| Allocation concealment | Low | Low | Low | Low | Low |
| Blinding | Higha | Higha | Low | Higha | Higha |
| Withdrawal | Low | Low | Low | Low | Low |
| Selective outcome | Low | Low | Low | Low | Low |
| Free of other bias | Low | Low | Low | Low | Low |
| Overall | High | High | Low | High | High |
All of the studies followed the ARDS net protocol lung protective strategy for ventilator management. Cisatracurium bolus and infusion was used as the NBMA agent with the bolus and continuous infusion doses varying across studies. The most recent two large RCT by Moss and Papazian did not use train-of-four nerve stimulation for additional boluses and cisatracurium was given as a fixed-dose continuous infusion. All of the studies were considered high risk for bias because of the lack of blinding to the clinical nurses or other healthcare professionals, except for the trial by Papazian et al.
Mortality outcomes
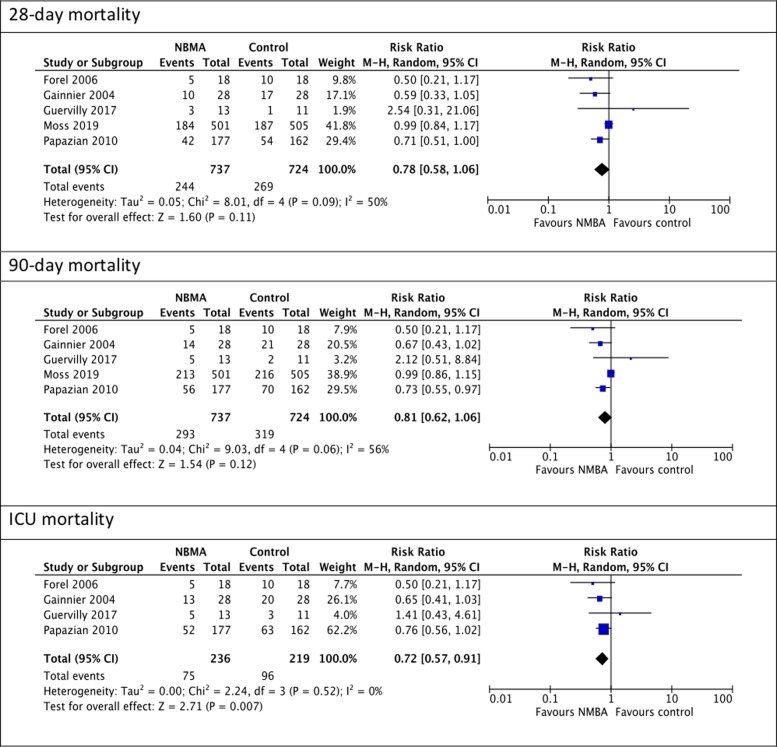
The mortality outcomes are presented in Fig. 2. The cisatracurium group had the same risk of death at 28 days (RR, 0.90; 95% CI, 0.78–1.03; I2 = 50%, p = 0.12) and 90 days (RR, 0.81; 95% CI, 0.62–1.06; I2= 56%, p = 0.06) but had a significantly lower ICU mortality compared to the control group (RR, 0.72; 95% CI, 0.57–0.91; I2 = 0%; p = 0.007). It was noted that the ICU mortality pooled RR did not include the ROSE trial.
Fig. 2.
Mortality outcomes
Other outcomes
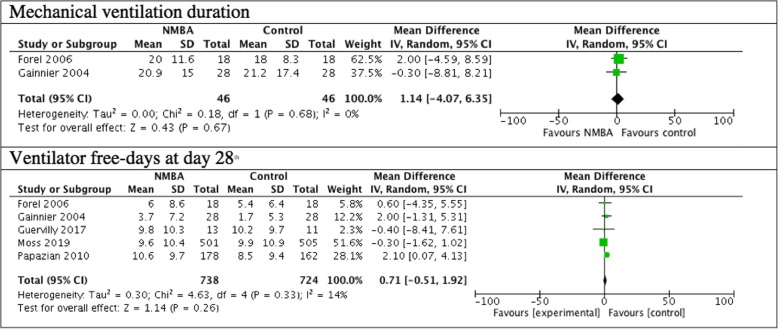
The mechanical ventilation duration was measured in two studies totaling 92 patients and ventilator-free days at day 28 were measured by five trials totalizing 1462 patients. The secondary outcomes of mechanical ventilation duration and ventilator-free days were not different between the cisatracurium group and the control group (Fig. 3).
Fig. 3.
Mechanical ventilation duration and ventilator-free days truncated at day 28th outcomes
Adverse events
The cisatracurium group had a statistically significant lower risk of barotrauma than the control group (RR, 0.55; 95% CI 0.35–0.85, I2 = 0%, p = 0.007). Four trials reported ICU weakness. There was no difference in the risk of ICU weakness at day 28 between the two groups (RR, 1.23; 95% CI, 0.81–1.88; I2 = 21%; p = 0.33). The Medical Research Council (MRC) scale was used in 2 studies to evaluate ICU weakness and was not significantly different between the two groups (see Fig. 4).
Fig. 4.
Adverse events
Oxygenation and plateau pressure
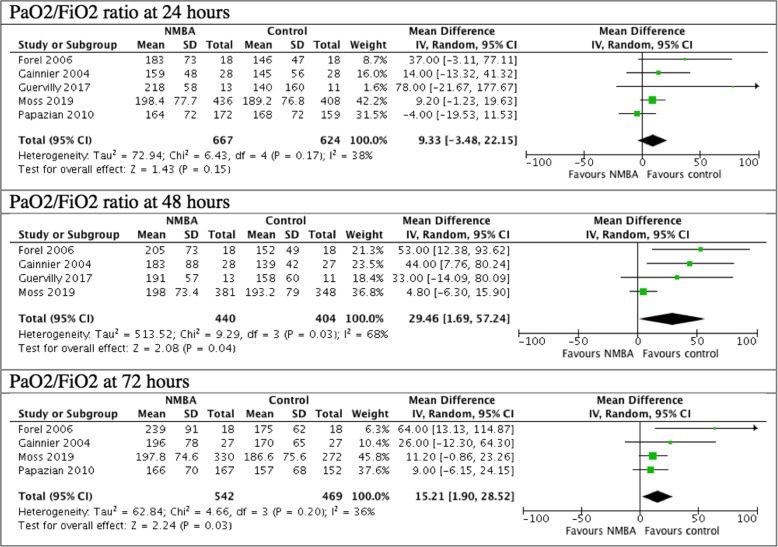
The comparison of the PaO2/FIO2 ratio between the NMBA group and the control group showed a statistically significant difference at 48 h and at 72 h ((MD, 29.46; 95% CI 1.69–57.24, I2 = 68%, p = 0.04; MD, 15.21; 95% CI, 1.9–28.52, I2 = 36%, p = 0.03). From 0 to 24 h, there was no difference noted between the NMBA group and the control group (p > 0.05) (Fig. 5). There were only two studies, totaling 596 patients, reporting PaO2/FIO2 ratio on day 7 [11, 19]. PaO2/FIO2 ratio was higher in the intervention group (MD, 12.97; 95% CI, 0.26, 25.68, I2 = 0%, p = 0.05).
Fig. 5.
P/F ratio
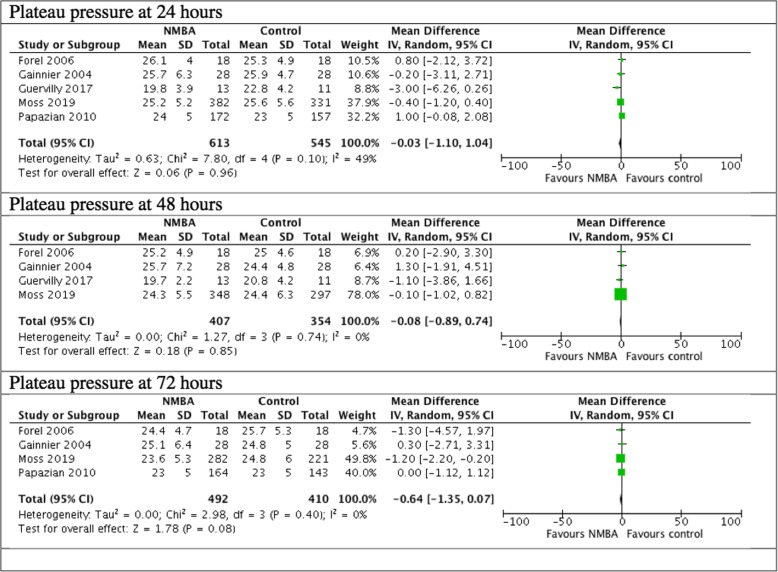
There was no difference in the plateau pressure between the NMBA group and the control group in 24 h, 48 h, or 72 h (Fig. 6).
Fig. 6.
Plateau pressure
Sensitivity analysis, subgroup analysis, and meta-regression
A sensitivity analysis was performed for all outcomes by changing the statistical measure to odds ratios or risk differences did not show a change in results. A meta-regression analysis investigating the potential effects of baseline PaO2/FIO2, PEEP, prone positioning rate, and overall mortality of the control group revealed no significant relationship between those factors and 28-day mortality (p values = 0.42, 0.58, 0.55, 0.19).
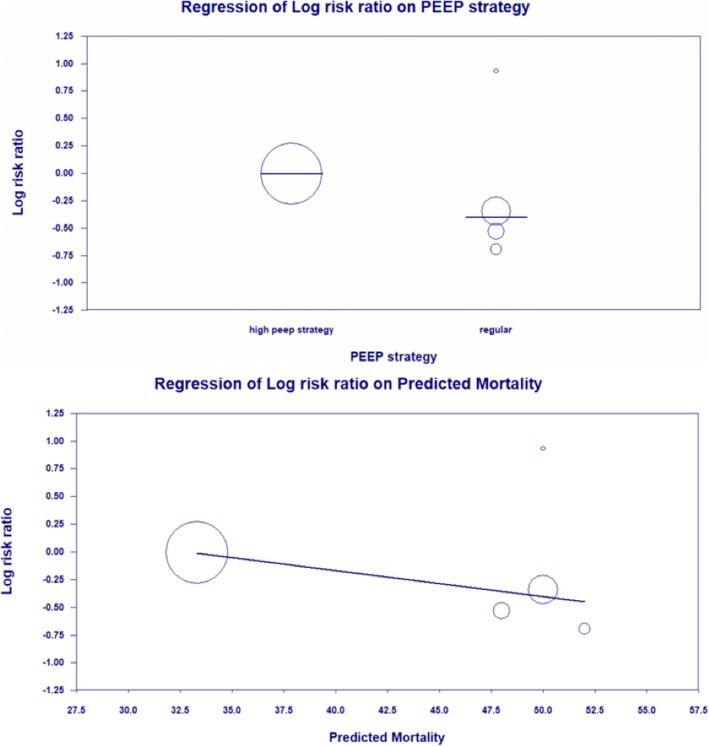
Similarly, a meta-regression analysis was performed to evaluate the changes in predicted mortality on day 1 based on the disease severity grading system (SAPS II and SOFA score). This analysis found a significant effect of predicted mortality on the relationship between 28-day mortality and NMBA use (p = 0.0197, see Fig. 7).
Fig. 7.
Regression of Log risk ratio on PEEP strategy and predicted mortality
A subgroup analysis of PEEP strategy that excluded the study using the high PEEP strategy by Moss et al. yielded a difference in all mortality outcomes with the NMBA group having significantly lower mortality at 28 days, at 90 days, and in the ICU. The statistical measure for 28-day mortality was RR 0.66, 95% CI 0.50–0.87, I2 = 58%, and p = 0.003; for 90-day mortality was RR 0.69, 95% CI 0.55–0.87, I2 = 0%, and p = 0.001; and for ICU mortality was RR 0.72, 95% CI 0.57–0.91, I2 = 0%, and p = 0.006.
Publication bias
We carried out Egger’s regression test and funnel plots to assess the presence of publication bias. The funnel plots showed no clear evidence of asymmetry and Egger’s regression test indicated no evidence of publication bias (data not shown).
Quality of evidence
The quality of evidence is found in Table 2.
Table 2.
Quality of evidence assessment
| Question | Quality assessment | Quality of evidence | |||
|---|---|---|---|---|---|
| Bias | Inconsistency | Indirectness | Imprecision | ||
| 28-day mortality | Seriousa | Moderateb | No serious indirectness | No serious imprecision | Moderate |
| 90-day mortality | Seriousa | Moderateb | No serious indirectness | Undetected | Moderate |
| ICU mortality | Seriousa | Undetected | Undetected | Detectedc | Low |
| Mechanical ventilation duration | Seriousa | Undetected | No serious indirectness | No serious imprecision | Moderate |
| Ventilator-free days at day 28th | Seriousa | Undetected | No serious indirectness | No serious imprecision | Moderate |
| ICU weakness | Seriousa | Undetected | No serious indirectness | No serious imprecision | Moderate |
| Barotrauma | Seriousa | Undetected | No serious indirectness | No serious imprecision | Moderate |
aBias was rated high because of no blinding in four out of five studies (see Table 1)
bInconsistency across studies in these two outcomes based on I2 was moderate
cImprecision was detected for this outcome because there was no value for this outcome available for 1 out of 5 studies
Discussion
In our study, we performed a comprehensive analysis of the most current available data on the effects of neuromuscular blockers on outcomes in patients with ARDS. We reviewed all good-quality randomized controlled trials performed over the past 15 years. The main finding of our study was that neuromuscular blocker, specifically cisatracurium did not lead to a reduction in the mortality risk at 28 days and 90 days for ARDS patients. Additionally, there was no reduction of ventilator-free days or duration of mechanical ventilation. NMBA improved oxygenation but not until 48 h and was associated with lower barotrauma but did not increase the risk of ICU weakness. The mortality risk at 28 days and 90 days was not affected by prone positioning rate, baseline P/F ratio, or PEEP but was affected by the PEEP strategy and predicted mortality risk before randomization.
Neuromuscular blockers have long been used for severe ARDS to control patient-ventilator dyssynchrony and allow a tolerance of permissive hypercapnia [30]. Since the three randomized controlled trials on the effects of neuromuscular blocker totaling 431 showed survival benefits dating back before 2010, the clinical guidelines have recommended NMBA as supportive treatment of severe ARDS. The recommendation strength was only weak to moderate due to the lack of blinding and the risk of indirectness in the contributing studies [6, 8, 31]. There are also still concerns about ICU-related weakness and other risks associated with NMBA, such as atelectasis, diaphragm paralysis, and anaphylaxis.
A meta-analysis of the mortality outcomes at 28 and 90 days did not show a statistically significant different risk between the group given NMBA and control group. Subgroup analysis of all studies excluding the ROSE trial by Moss et al. showed a reduction in all mortality outcomes in the NMBA group. ROSE was the first trial on the topic performed not in France but in the US. The authors used a few different methods compared to other trials. For example, the control arm of Moss et al. included 84 (17%) patients receiving NMBA which was different than other studies and may have reduced the mortality of the control arm more than expected. A meta-regression analysis also showed that the heterogeneity in the outcome measure was due to the predicted mortality, sedation strategy and PEEP strategy which were the major differences between ROSE and other studies.
Furthermore, Moss et al. used a modified PEEP table from the ALVEOLI trial while the other four trials used the protocol proposed by the National Institutes of Health for the ARDS group [32]. The high PEEP strategy in the ROSE study applied higher PEEP than the regular strategy used in other trials (see Additional file 1 for the PEEP strategies). It was noted that regardless of the implementation of high peep, the barotrauma incidence in the ROSE trial was the same as the average barotrauma incidence in the remaining studies (5.1% vs 6.9%, p = 0.2). In addition, Moss et al. used a light sedation strategy (RASS score of 0 to − 1, alert and calm to drowsy) for the control group while other trials aimed for a RAMSAY score of 6 (no response) for both the intervention and control group. This light sedation strategy in the control group could explain the primary outcome difference between the ROSE study and other trials. Light sedation was associated with less use of vasopressor, shorter time to extubation [33], and lower mortality in mechanically ventilated patients [17, 18].
The meta-regression showed that the baseline PaO2/FIO2 ratio and baseline PEEP variables had no effect on the 28-day mortality rate and NMBA. This finding is a new concept because the ACURASYS trial showed a mortality benefit for NMBA use in ARDS patients with a P/F ratio less than 120 [11]. Prone positioning was shown to improve mortality in severe ARDS in available literature [16, 34, 35]. In this analysis, we do not have sufficient data to draw the conclusion on whether or not the cisatracurium besylate infusion was more effective when combined with prone positioning. However, the percentage of patients who were proned does not affect the relationship between mortality and NMBA.
Our study showed a positive effect of NMBA on oxygenation with a decreased PaO2/FIO2 ratio observed after 48 h, which was consistent with earlier evidence [36]. NMBA’s positive effect on oxygenation could be related to the decrease in ventilator-induced lung injury associated with barotrauma. The risk of barotrauma was lower in the NMBA group compared to the control group when we combined the effects of the four studies reporting this outcome. Moreover, NMBA also had an anti-inflammatory effect and decreased oxygen consumption by active muscle groups [9, 30]. However, NMBA had this positive effect on oxygenation only after 48 h and did not improve plateau pressure at 24 to 72 h.
Cisatracurium was not associated with the risk of ICU-acquired weakness. Although neuromuscular blocker has been widely used, there is still a concern regarding their use in clinical practice [37], especially when concomitant use of corticosteroids for severe septic shock could have an additive effect with NMBA [14]. Our finding of no risk of ICU-acquired weakness is likely because of the short duration of neuromuscular blocker of only 48 h in all the studies. Of note, only one study by Gainnier et al. did not use corticosteroids in the first 5 days. Other studies used corticosteroids in 5 to 45% for septic shock but there is no difference between the corticosteroids use when we compare between the NMBA group and the control group (OR 1.13; 95% CI, 0.89–1.44; p = 0.14).
The limitation of the quality of the evidence in this analysis included the bias from the unblind healthcare professionals in almost all studies. The largest trial also gave cisatracurium to a percentage of patients in the control group and was stopped early due to futility before a sample size with an adequate power was reached. Regardless, trials that stopped early for benefit do not have strong evidence of causing a substantial bias in the evaluation of the treatment effects [38]. Because of these limitations, the quality of evidence was judged to be only moderate. The second limitation was that we could not obtain the ICU mortality in the study of Moss et al. so we could not draw a conclusion about this particular outcome.
Despite these limitations, our study comprehensively reviewed all available randomized controlled trials for evidence of the use of neuromuscular blockers in ARDS patients. The clinical management of ARDS patients is still a complicated field that considers multiple other factors. In clinical practice, NMBA still has a benefit of improving oxygenation and reducing barotrauma without increasing ICU weakness. However, it is noted that this benefit of oxygenation is not observed until 48 h. In fact, NMBA may not be necessary for the purpose of mortality reduction, when there is an implementation of high PEEP with appropriate protocols to decrease PEEP, light sedation in combination with prone positioning and low tidal volume ventilation.
Conclusion
NMBA did not lead to a reduction in the mortality risk of 28 days and 90 days, ventilator-free day, duration of mechanical ventilation for moderate/severe ARDS patients. NMBA improved oxygenation but not until 48 h and was associated with lower barotrauma without affecting ICU weakness. The mortality risk of 28-day with NMBA was not affected by the baseline severity of ARDS.
Supplementary information
Acknowledgements
No acknowledgments
Abbreviations
- ARDS
Acute respiratory distress syndrome
- NMBA
Neuromuscular blocking agents
- PEEP
Positive end-expiratory pressure
- RCT
Randomized controlled trial
- RR
Relative risk
Authors’ contributions
All authors contributed to the manuscript. ATNH collected the data, performed the analysis, and wrote the manuscript. SP came up with the topic, collected, reviewed the data, helped to perform the analysis, and proofread the manuscript. CG provided the data for one study for the analysis and proofread the manuscript. All authors read and approved the final manuscript.
Funding
There has been no financial support for this work that could have influenced its outcome. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Availability of data and materials
The datasets used for the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
Not applicable. This is a review article only.
Consent for publication
Not applicable. We do not use individual patient’s data.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s40560-020-0431-z.
References
- 1.Piantadosi CA, Schwartz DA. The acute respiratory distress syndrome. Ann Intern Med. 2004;141(6):460–470. doi: 10.7326/0003-4819-141-6-200409210-00012. [DOI] [PubMed] [Google Scholar]
- 2.Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, et al. Acute respiratory distress syndrome: the Berlin definition. JAMA. 2012;307(23):2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 3.Thille AW, Contou D, Fragnoli C, Cordoba-Izquierdo A, Boissier F, Brun-Buisson C. Non-invasive ventilation for acute hypoxemic respiratory failure: intubation rate and risk factors. Crit Care. 2013;17(6):R269. doi: 10.1186/cc13103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bellani G, Laffey JG, Pham T, Fan E, Brochard L, Esteban A, et al. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA. 2016;315(8):788–800. doi: 10.1001/jama.2016.0291. [DOI] [PubMed] [Google Scholar]
- 5.Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, et al. Incidence and outcomes of acute lung injury. N Engl J Med. 2005;353(16):1685–1693. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- 6.Murray MJ, DeBlock H, Erstad B, Gray A, Jacobi J, Jordan C, et al. Clinical practice guidelines for sustained neuromuscular blockade in the adult critically ill patient. Crit Care Med. 2016;44(11):2079–2103. doi: 10.1097/CCM.0000000000002027. [DOI] [PubMed] [Google Scholar]
- 7.Murray MJ, DeBlock HF, Erstad BL, Gray AW, Jr, Jacobi J, Jordan CJ, et al. Clinical practice guidelines for sustained neuromuscular blockade in the adult critically ill patient: 2016 update-executive summary. Am J Health Syst Pharm. 2017;74(2):76–78. doi: 10.2146/ajhp160803. [DOI] [PubMed] [Google Scholar]
- 8.Griffiths MJD, McAuley DF, Perkins GD, Barrett N, Blackwood B, Boyle A, et al. Guidelines on the management of acute respiratory distress syndrome. BMJ Open Respir Res. 2019;6(1):e000420. doi: 10.1136/bmjresp-2019-000420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Forel JM, Roch A, Marin V, Michelet P, Demory D, Blache JL, et al. Neuromuscular blocking agents decrease inflammatory response in patients presenting with acute respiratory distress syndrome. Crit Care Med. 2006;34(11):2749–2757. doi: 10.1097/01.CCM.0000239435.87433.0D. [DOI] [PubMed] [Google Scholar]
- 10.Gainnier M, Roch A, Forel JM, Thirion X, Arnal JM, Donati S, et al. Effect of neuromuscular blocking agents on gas exchange in patients presenting with acute respiratory distress syndrome. Crit Care Med. 2004;32(1):113–119. doi: 10.1097/01.CCM.0000104114.72614.BC. [DOI] [PubMed] [Google Scholar]
- 11.Papazian L, Forel JM, Gacouin A, Penot-Ragon C, Perrin G, Loundou A, et al. Neuromuscular blockers in early acute respiratory distress syndrome. N Engl J Med. 2010;363(12):1107–1116. doi: 10.1056/NEJMoa1005372. [DOI] [PubMed] [Google Scholar]
- 12.Slutsky AS, Tremblay LN. Multiple system organ failure. Is mechanical ventilation a contributing factor? Am J Respir Crit Care Med. 1998;157(6 Pt 1):1721–1725. doi: 10.1164/ajrccm.157.6.9709092. [DOI] [PubMed] [Google Scholar]
- 13.Tremblay LN, Slutsky AS. Ventilator-induced lung injury: from the bench to the bedside. Intensive Care Med. 2006;32(1):24–33. doi: 10.1007/s00134-005-2817-8. [DOI] [PubMed] [Google Scholar]
- 14.Kindler CH, Verotta D, Gray AT, Gropper MA, Yost CS. Additive inhibition of nicotinic acetylcholine receptors by corticosteroids and the neuromuscular blocking drug vecuronium. Anesthesiology. 2000;92(3):821–832. doi: 10.1097/00000542-200003000-00026. [DOI] [PubMed] [Google Scholar]
- 15.Latronico N, Bolton CF. Critical illness polyneuropathy and myopathy: a major cause of muscle weakness and paralysis. Lancet Neurol. 2011;10(10):931–941. doi: 10.1016/S1474-4422(11)70178-8. [DOI] [PubMed] [Google Scholar]
- 16.Munshi L, Del Sorbo L, Adhikari NKJ, Hodgson CL, Wunsch H, Meade MO, et al. Prone position for acute respiratory distress syndrome: a systematic review and meta-analysis. Ann Am Thorac Soc. 2017;14:S280–S2S8. doi: 10.1513/AnnalsATS.201704-343OT. [DOI] [PubMed] [Google Scholar]
- 17.Brower RG, Lanken PN, MacIntyre N, Matthay MA, Morris A, Ancukiewicz M, et al. Higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. N Engl J Med. 2004;351(4):327–336. doi: 10.1056/NEJMoa032193. [DOI] [PubMed] [Google Scholar]
- 18.Boncyk C, Nahrwold DA, Hughes CG. Targeting light versus deep sedation for patients receiving mechanical ventilation. J Emerg Crit Care Med. 2018;2:79. doi: 10.21037/jeccm.2018.10.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moss M, Huang DT, Brower RG, Ferguson ND, Ginde AA, Gong MN, et al. Early neuromuscular blockade in the acute respiratory distress syndrome. N Engl J Med. 2019;380(21):1997–2008. doi: 10.1056/NEJMoa1901686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. 2009;151(4):W65–W94. doi: 10.7326/0003-4819-151-4-200908180-00136. [DOI] [PubMed] [Google Scholar]
- 21.Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, et al. The Cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64(4):383–394. doi: 10.1016/j.jclinepi.2010.04.026. [DOI] [PubMed] [Google Scholar]
- 23.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 24.Ferreira FL, Bota DP, Bross A, Melot C, Vincent JL. Serial evaluation of the SOFA score to predict outcome in critically ill patients. JAMA. 2001;286(14):1754–1758. doi: 10.1001/jama.286.14.1754. [DOI] [PubMed] [Google Scholar]
- 25.Le Gall JR, Lemeshow S, Saulnier F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA. 1993;270(24):2957–2963. doi: 10.1001/jama.270.24.2957. [DOI] [PubMed] [Google Scholar]
- 26.Vincent JL, Moreno R, Takala J, Willatts S, De Mendonca A, Bruining H, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22(7):707–710. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 27.Guervilly C, Bisbal M, Forel JM, Mechati M, Lehingue S, Bourenne J, et al. Effects of neuromuscular blockers on transpulmonary pressures in moderate to severe acute respiratory distress syndrome. Intensive Care Med. 2017;43(3):408–418. doi: 10.1007/s00134-016-4653-4. [DOI] [PubMed] [Google Scholar]
- 28.Dawson R, von Fintel N, Nairn S. Sedation assessment using the Ramsay scale. Emerg Nurse. 2010;18(3):18–20. doi: 10.7748/en2010.06.18.3.18.c7825. [DOI] [PubMed] [Google Scholar]
- 29.Sessler CN, Gosnell MS, Grap MJ, Brophy GM, O'Neal PV, Keane KA, et al. The Richmond agitation-sedation scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002;166(10):1338–1344. doi: 10.1164/rccm.2107138. [DOI] [PubMed] [Google Scholar]
- 30.Slutsky AS. Neuromuscular blocking agents in ARDS. N Engl J Med. 2010;363(12):1176–1180. doi: 10.1056/NEJMe1007136. [DOI] [PubMed] [Google Scholar]
- 31.Papazian L, Aubron C, Brochard L, Chiche JD, Combes A, Dreyfuss D, et al. Formal guidelines: management of acute respiratory distress syndrome. Ann Intensive Care. 2019;9(1):69. doi: 10.1186/s13613-019-0540-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brower RG, Matthay MA, Morris A. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342(18):1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 33.Arroliga AC, Thompson BT, Ancukiewicz M, Gonzales JP, Guntupalli KK, Park PK, et al. Use of sedatives, opioids, and neuromuscular blocking agents in patients with acute lung injury and acute respiratory distress syndrome. Crit Care Med. 2008;36(4):1083–1088. doi: 10.1097/CCM.0B013E3181653895. [DOI] [PubMed] [Google Scholar]
- 34.Fan E, Brodie D, Slutsky AS. Acute respiratory distress syndrome advances in diagnosis and treatment. JAMA. 2018;319(7):698–710. doi: 10.1001/jama.2017.21907. [DOI] [PubMed] [Google Scholar]
- 35.Guérin C, Reignier J, Richard JC, Beuret P, Gacouin A, Boulain T, et al. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med. 2013;368(23):2159–2168. doi: 10.1056/NEJMoa1214103. [DOI] [PubMed] [Google Scholar]
- 36.Alhazzani W, Alshahrani M, Jaeschke R, Forel JM, Papazian L, Sevransky J, et al. Neuromuscular blocking agents in acute respiratory distress syndrome: a systematic review and meta-analysis of randomized controlled trials. Crit Care. 2013;17(2):R43. doi: 10.1186/cc12557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Price DR, Mikkelsen ME, Umscheid CA, Armstrong EJ. Neuromuscular blocking agents and neuromuscular dysfunction acquired in critical illness: a systematic review and meta-analysis. Crit Care Med. 2016;44(11):2070–2078. doi: 10.1097/CCM.0000000000001839. [DOI] [PubMed] [Google Scholar]
- 38.Berry SM, Carlin BP, Connor J. Bias and trials stopped early for benefit. JAMA. 2010;304(2):156–159. doi: 10.1001/jama.2010.930. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used for the current study are available from the corresponding author on reasonable request.