Abstract
Purpose of Review
Pancreatic β-cells play a critical role in whole-body glucose homeostasis by regulating the release of insulin in response to minute by minute alterations in metabolic demand. As such, β-cells are staunchly resilient but there are circumstances where they can become functionally compromised or physically lost due to pathophysiological changes which culminate in overt hyperglycemia and diabetes.
Recent Findings
In humans, β-cells mass appears to be largely defined in the postnatal period and this early replicative and generative phase is followed by a refractory state which persists throughout life. Despite this, efforts to identify physiological and pharmacological factors which might re-initiate β-cells replication (or cause the replenishment of β-cells by neogenesis or transdifferentiation) are beginning to bear fruit.
Keywords: Proliferation, Diabetes, Transdifferentiation, Islets of Langerhans, β-Cell mass, Ki67
Summary
Controlled manipulation of β-cells mass in humans still represents a holy grail for therapeutic intervention in diabetes, but progress is being made which may lead to ultimate success.
Introduction: Background and Rationale
The β-cells plays a unique role in human physiology being the only cell capable of elaborating a hormone (insulin) which can lower blood glucose by promoting the uptake and metabolism of sugar in peripheral tissues. As a result, physiological glucose homeostasis becomes dysregulated when β-cells function is compromised, or the cells are lost, leading to diabetes mellitus. In normal human physiology, β-cells growth and maturation occur in parallel with fetal development meaning that, during the final trimester of pregnancy, human fetal β-cells are glucose-responsive and become involved intimately in the control of fetal growth [1, 2]. Thus, fetal insulin is correspondingly increased and macrosomia results under conditions when maternal glucose is elevated. Conversely, low birth weight is often associated with reduced fetal insulin secretion [2]. Such scenarios are not evident in most rodent models, where fetal β-cells remain functionally immature until early in postnatal period.
It is clear from this that the growth and development of β-cells represent critical aspects of early life in humans and it is believed that the cells may arise and develop either by neogenesis from stem cell precursors or by replication of existing cells [3]. β-cells replication has been studied most extensively in early postnatal life and much less is known about the precise timing of fetal β-cells proliferation. Indeed, the rate of β-cells mitosis is high in the immediate postnatal period but then declines progressively, and increasingly quickly, to reach a stable but still low rate by about the 2nd year of life [4]. Consequently, the number of β-cells found in children during their early years is probably close to the full complement that is then retained for the remainder of life. Studies of lipofuscin accumulation coupled with mathematical modeling of cell turnover in adult β-cells have argued that the cells are very long-lived with minimal rates of division [5].
There is, though, at least one notable exception to this general rule, which applies to women during pregnancy. Over the course of pregnancy, insulin sensitivity declines and must be compensated by an increase in β-cells mass to provide sufficient insulin to meet the increased metabolic demand [6]. In rodents, there is firm evidence that increased β-cells proliferation provides the mechanism by which this requirement is fulfilled while, in humans, the evidence remains equivocal [7]. In rodents, lactogenic hormones such as prolactin promote β-cells proliferation via STAT5-dependent mechanisms, but such responses seem less effective in human islet cells despite the expression of relevant signaling components. It remains unclear whether different factors and/or molecular pathways are involved in promoting β-cells proliferation during pregnancy in humans. Nevertheless, there seems little doubt that β-cells numbers are increased in pregnant women although, in the few studies where this aspect has been addressed, rates of proliferation of existing β-cells appear relatively stable [6]. Thus, the increase may derive mainly from enhanced neogenesis rather than from increased proliferation. Indeed, this paradigm may represent the more general situation in humans where physiologically relevant increases in β-cells proliferation are rarely detected beyond the earliest years of life.
If this is true, then two factors can be considered as critical in determining the number of β-cells present in the human pancreas at different stages of life: (1) the absolute number of β-cells generated during late fetal and early postnatal development and (2) the net rate of β-cells loss which can occur over time. The total number of mature β-cells varies dramatically among individuals [8]. As a result, there is no consensus as to the “typical” β-cells mass at baseline in humans and it is probable that this encompasses a very broad continuum. Consequently, some individuals will have a much greater β-cells reserve than others and this will influence the outcomes to events that place an additional workload on these cells (e.g., in response to obesity-induced insulin resistance). It will also affect the rate at which whole-body glucose homeostasis declines when β-cells are lost, as may happen in either type 1 (T1D) or type 2 diabetes (T2D).
The reduction of functional β-cells mass which leads to hyperglycemia in both T1D and T2D is due to targeted deletion of existing β-cells or, perhaps, by transdifferentiation of mature β-cells, which causes a loss of β-cells identity (i.e., the expression of typical β-cells genes such as insulin or pancreatic and duodenal homeobox 1 (Pdx-1)), as well as a reduced capacity to secrete insulin in response to glucose [9–11]. In T1D, loss of β-cells is the most prevalent mechanism (at least in younger children) and occurs by an immune-mediated process in which islets are infiltrated by specific immune cell subsets that promote selective β-cells death [12]. In this scenario, it might be imagined that a compensatory drive to promote β-cells proliferation would be heightened. Some investigators have failed to find any evidence of increased β-cells proliferation in pancreas samples collected from T1D patients [13], although these investigations involved largely patients with diabetes either in a well-advanced stage or beyond the immediate post-diagnosis period. By contrast, others have used autopsy samples recovered from individuals much closer to the diagnosis of T1D and reported a tenfold increase in 13cell proliferation [14, 15]. These data importantly imply that human β-cells are not entirely refractory to pro-proliferative stimuli and suggest that targeted induction of proliferation might be an attainable goal. Increased β-cells proliferation was detected using two independent markers of mitosis (i.e., Ki67 and Mcm-2) and positively correlated with the extent of islet inflammation. Indeed, β-cells mitosis was rarely seen in the uninflamed islets of individuals with T1D [14]. Furthermore, expansion of β-cells mass driven by soluble factors secreted by T cells consequently to islet infiltration has been reported in animal models of T1D [16]. This may imply that molecules released during the autoimmune attack are able to initiate cell cycle entry in both rodent and human β-cells and it is important that these are identified and characterized as they may offer therapeutic potential.
Irrespective of this evidence, it remains clear that human β-cells are extremely resistant to factors promoting their re-entry into the cell cycle. An obvious explanation for this might be that their complement of cyclins, associated kinases, and other regulatory molecules becomes depleted as they mature such that they lack the capacity to replicate. In a series of elegant studies, Stewart and colleagues have addressed this and shown that this explanation is unlikely [17, 18]. Rather, the authors report that, in human β-cells, many of the critical proteins regulating mitosis are displaced from the nucleus to occupy a primarily cytosolic localization. Thus, it may be the subcellular disposition of these molecules rather than their absolute expression which determines the refractory status of β-cells. However, in exploring this situation further by analysis of islets retained in pancreas sections in situ, it was noted that the subcellular distribution of cyclins and related molecules may be influenced significantly by the handling of tissue [19]. In pancreas sections that had been fixed during lengthy autopsy procedures, cyclins were either completely lost or located mainly in the cytosol of β-cells, consistent with observations made in isolated islets. By contrast, where pancreas samples had been processed and fixed more rapidly after death, proteins belonging to the cell cycle machineries tended to adopt a nuclear localization. Thus, it remains unclear whether the changes in the cellular distribution of key cell cycle regulators in comparison with rodents are either a genuine characteristic of human β-cells or are a consequence of islet isolation procedures and sample manipulations during the post mortem period. A related point that is debated is the possibility of underestimating the rate of turnover of human β-cells, when using Ki67 labeling measuring cell proliferation. Long-term storage of human pancreases obtained from autopsies or organ donors has been suggested to underlie a decline in Ki67 staining, leading to the erroneous assumption that human β-cells are resistant to division [20, 21•]. Nevertheless, it seems clear that human β-cells express the requisite machinery to induce proliferation if appropriate stimuli are encountered.
Therapeutic Effects of β-Cell Division
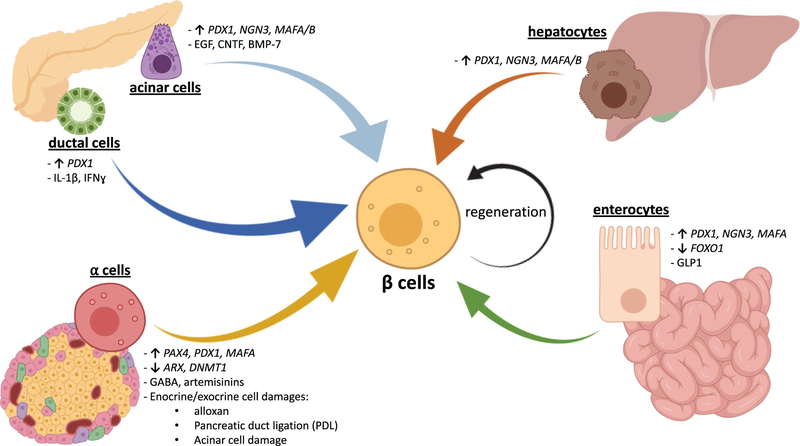
As noted above, a characteristic feature that is common to both T1D and T2D is the progressive loss of functional β-cells mass leading to poor glycemic control. Therefore, one of the challenging strategies has been to consider compensating for the lack of insulin, improve glucose homeostasis, and treat diabetes by expanding the population of functional β-cells. To increase the number of functional β-cells in patients with diabetes, insulin-producing cells can be generated either from non-pancreatic somatic cells (e.g., hepatocytes or intestinal cells), pancreatic exocrine cells (e.g., acinar and ductal cells), or pancreatic islet cells (e.g., α-cells), by inducing cell identity switches termed transdifferentiation. The cell types that can be used as a source for generating new β-cells, including the mechanisms that regulate such transdifferentiation processes, are listed in Table 1 and represented in Fig. 1. An alternative approach considered by many groups is the identification of molecules capable of stimulating self-replication of preexisting human β-cells.
Table 1.
List of somatic cell types as a potential source for generating new β-cells
| Cell type | Treatment/modification | Species | Reference |
|---|---|---|---|
| Hepatocytes | Overexpression of Pdx1, NeuroD1, Ngn-3, MafA/B | Mouse | [22–26] |
| Adult and fetal hepatocytes | Overexpression of PDX-1 | Human | [27–29] |
| Enterocytes | Overexpression of Pdx1, Ngn-3, MafA - GLP1 treatment | Mouse | [30,31] |
| Enteroendocrine progenitors | Downregulation of FoxO1 | Mouse | [32] |
| Enterocytes | Downregulation of FOXO1 - GLP1 treatment | Human | [31, 33] |
| Acinar cells | Overexpression of Pdx1, Ngn-3, MafA - treatment with cytokines, EGF or CNTF | Mouse | [34–37] |
| Treatment with BMP-7 | Human | [38] | |
| Ductal cells | Transduction of Pdx1 | Rat | [39] |
| Treatment with cytokines | Mouse/human | [40] | |
| α-cells | Overexpression of Pax4 - downregulation of Arx and Dnmt1 - treatment with alloxan, PDL, or acinar damage | Mouse | [41–46] |
| Treatment with GABA, artemisinins | Mouse/human | [47, 48•] | |
| Overexpression of PDX-1 and MAFA | Human | [49, 50] |
Fig. 1.
Generation of human β-like cells by inducing transdifferentiation from adult somatic cells. Representative scheme summarizing the stimuli and/or genetic manipulations that regulate cell-to-cell conversion into human insulin-positive cells with a β-like phenotype. In particular, β-like cells can be generated by hepatocytes (orange arrow), enterocytes (green arrow), α-cells (yellow arrow), ductal cells (dark blue arrow), acinar cells (light blue arrow), or by self-replication of pre-existing β-cells (circular black arrow). Up arrows indicate gene overexpressions; down arrows indicate gene downregulations. PDX-1, pancreatic and duodenal homeobox 1; NGN3, neurogenin 3; MAFA/B, MAF BZIP transcription factor A/B; FOXO1, forkhead box O1; GLP1, glucagonlike peptide 1; PAX4, paired box 4; ARX, aristaless-related homeobox; DNMT1, DNA methyltransferase 1; GABA, gamma-aminobutyric acid; PDL, pancreatic duct ligation; IL-1β, interleukin 1β; IFN-ɣ, interferon-ɣ; EGF, epidermal growth factor; CNTF, ciliary neurotrophic factor; BMP-7, bone morphogenetic protein 7 (Created with BioRender)
Generation of β-Cells from Non-pancreatic Somatic Cells
Transdifferentiation generally involves the conversion of one differentiated cell type into another mature type of cell, via an intermediate with a dual-phenotype or a dedifferentiation step (Fig. 1, Table 1). Hepatocytes and gastrointestinal cells represent a viable endogenous source for generating insulin-producing cells, since both cell types are derived from the primitive foregut endoderm and share early developmental stages. Many studies have established robust approaches to obtain β-like cells from hepatocytes by overexpression of pancreatic transcription factors that are important for β-cells lineage, such as Pdx-1 [22, 23] and/or neuronal differentiation 1 (NeuroD1; [24]), neurogenin 3 (Ngn-3; [25]), and MAF BZIP transcription factor A/B (MafA/B; [26]) in murine hepatocytes by adenoviral delivery, leading to an increase in the expression of bioactive insulin and restoration of normoglycemia in multiple diabetic animal models. Importantly for clinical translation, several groups were able to engineer either fetal [27, 28] or adult [29] human liver cells overexpressing PDX-1 with supplementation of soluble factors, resulting in the activation of insulin promoter and the resolution of the diabetic phenotype after transplantation into streptozotocin (STZ)-treated mice.
Similarly, insulin expression can be induced in gastrointestinal cells via transient transgenic expression of Pdx-1, MafA, and Ngn-3 in vivo [30] (Fig. 1, Table 1). Moreover, downregulation of forkhead box O1 (FoxO1) expression in murine enteroendocrine progenitors [32] and human gut organoids [33] increased insulin production, suggesting a new mechanism in the approach to create β-like cells. Interestingly enough, Suzuki et al. demonstrated that the inactive full-length form of glucagon-like peptide 1 (GLP-1) mediated the conversion of rodent and human intestinal epithelial cells into insulin-producing cells by upregulating hepatic nuclear factor 6 (HNF-6)–induced expression of Ngn-3 [31]. However, most studies in this field of research are based on observations in rodents and in vitro, proof-of-concept experiments, on human cells. Thus, whether the β-cells reprogramming processes from extra-pancreatic cells can be induced in human patients in vivo with the ultimate goal of increasing β-cells mass and treating diabetes continues to be a significant challenge.
Generation of β-Cells from Exocrine Pancreatic Cells
Acinar cells represent a reasonable source for generating large numbers of β-cells, considering their abundance, close proximity and shared developmental origin with endocrine cells (Fig. 1, Table 1). Acinar-to-β-cells transdifferentiation was not only obtained by overexpressing Pdx-1, Ngn-3, and MafA [34] but also by treatments with cytokines [35] or growth factors [36] as the epidermal growth factor (EGF) and ciliary neurotrophic factor (CNTF) [37] without genetic manipulations in rodent acinar cells in vitro and in vivo. Curiously, a β-like phenotype was induced in acinar cells after treatment with bone morphogenetic protein 7 (BMP-7) in humans [38]. Despite these events having been well described in animal models, translation of the findings to human acinar cells in vitro represents a major task, since in vitro cultured acinar cells display a high tendency to transdifferentiate spontaneously into ductal cells [51, 52]. However, many reports showed similar versatile properties of acinar cells in human pancreases. Single-cell RNA-se-quencing and immunohistochemistry experiments had revealed that subpopulations of acinar cells expressed high levels of the transcription factor SRY-Box 9 (SOX9), a marker of pancreatic progenitor cells, suggesting the presence of acinar cells in a dedifferentiated stage [53]. The plasticity of human acinar cells in vivo was recently shown by Masini and coworkers, by identifying cells simultaneously expressing insulin and acinar markers within the human pancreases, where they demonstrated a higher prevalence in T2D patients [54]. However, it is worth noting that acinar cell dedifferentiation or genetic reprogramming has the potential to cause adverse effects, including an increased risk of developing tumors such as pancreatic ductal adenocarcinoma [55]. In conclusion, further investigations about the safeness and stability of acinar-to-β reprogramming are necessary to consider efficiently and safely translating these approaches as therapeutic modalities for patients with diabetes.
During the early stages of pancreas development, ductal cells initiate the transdifferentiation process towards the endocrine lineage in mice, acting as an islet cell progenitor. This process occurs spontaneously in young mice during embryogenesis but not after birth [56]. However, identity transitions from ductal to β-cells were induced in mature cells by activating insulin gene promoter following transduction of PDX-1 protein into rat ductal cells [39]. Furthermore, Valdez et al. reported that pro-inflammatory cytokines increased Ngn-3 expression in murine and human ductal cells and enabled epithelial-mesenchymal transition (EMT), an essential step for initiating differentiation towards endocrine cells, independently of hyperglycemia [40]. Neogenesis of insulin-producing cells from ductal cells has also been reported to occur in humans. Ductal cells obtained from donors with <10 years of age exhibited insulin-positive cells when transplanted under the kidney capsule of nude mice [57]. In addition, Meier and colleagues reported increased levels of insulin-positive ductal cells in obese patients compared with those in non-obese subjects [58]. These findings suggest that duct-to-β transdifferentiation is one of the potential mechanisms by which new β-cells are generated to compensate for systemic insulin resistance due to obesity [59, 60]. Interestingly, there is evidence from in vitro studies that the establishment of persistent enteroviral infection in pancreatic ductal cells may reduce their capacity to differentiate into β-cells [61]. Since enteroviral infection of pancreatic cells has been implicated as a potential underlying cause of T1D [62], this might compromise the ability of β-cells to be replenished in T1D. In addition, insulin-positive ductal cells were observed in transplanted pancreas of T1D patients who received a simultaneous pancreas-kidney transplant (SPK) [63]. Remarkably, Dirice et al. were able to detect ductal cells positive for immature β-cells markers in pregnant women and individuals with T2D, highlighting neogenesis as a compensatory mechanism to expand β-cells mass in physiological and pathophysiological conditions characterized by high insulin demands such as pregnancy or T2D in humans [64].
Generation of β-Cells from Endocrine Pancreatic Cells
Other efforts in parallel have focused on targeting α-cells as a pool for expanding β-cells mass (Fig. 1, Table 1). The rationale for this approach is based on several studies which suggest that β-cells originate from α-cells during islet embryogenesis in mice [65–68]. Furthermore, the close lineage relationships and the large overlap of transcriptomes between α and β-cells, in addition to the apparent high resistance of α-cells to metabolic stressors, represent some of the advantages for considering α-to-β transdifferentiation as an approach with therapeutic potential. However, other reports caution that differentiation of α-to-β-cells with the consequent depletion of α-cells and the resultant lower circulating glucagon levels could increase the risks of hypoglycemic events [69]. Notwithstanding these concerns, studies in rodents showed that loss of α-cells did not affect overall health or lifespan and glucagon signaling was still preserved by a small number of α-cells [41, 70].
To further explore the use of α-cells as a practical tool to expand β-cells and treat diabetes, Collombat and colleagues reported that ectopic overexpression of Paired Box 4 (Pax4) mediates the differentiation of murine progenitor endocrine cells into β-cells after generating an intermediate α-like cell stage [41]. Independent studies have confirmed that upregulation of Pax4, induced by inhibition of aristaless-related homeobox (Arx), converted α-cells into insulin-producing cells [42]. In addition, downregulation of Arx in combination with the silencing of DNA methyltransferase 1 (Dnmt1) gene expression, specifically in α-cells, promoted rapid changes in the whole transcriptome towards a phenotype typical of native β-cells [43]. Some parallels in this context have been reported in T1D in humans. For example, α-cells have been shown to express reduced levels of DNMT1 and ARX genes in T1D patients [43]. Moreover, transdifferentiation α-to-β is induced in mice after almost complete β-cells depletion using β-cells toxins [44] in combination with pancreatic duct ligation (PDL) [45] or acinar tissue damages [46].
Recently, gamma-aminobutyric acid (GABA) signaling has been a matter of much attention, after the discovery of its role in mediating α-to-β transdifferentiation. In particular, in vivo treatment with GABA caused downregulation of Arx specifically in α-cells, generating cells expressing Ngn-3 and insulin with a high proliferation rate, able to restore euglycemia in diabetic mice [47]. Independently, Li et al. reported similar results by treating human islets with artemisinins, a group of antimalarial drugs, able to modulate gephyrin, a binding protein of the GABA receptor [48•]. However, Ackermann and colleagues failed to reproduce these findings, using a tamoxifen-inducible Glucagon-Cre YFP mouse model to track α-cell fate upon GABA or artenusate treatments [71•]. Whether the protocols used in the two studies play a role in the differences in the outcome is unclear [72], and further investigations are necessary to better define the role of GABA in inducing α-to-β transition. Finally, gene therapy approaches have been touted as a more efficient way to convert α-cells into β-like cells. In particular, adenovirus delivery of transgenic constructs carrying PDX-1 and MAFA genes into α-cells via the pancreatic duct has been shown to expand the β-cells population and to counteract diabetes in STZ-treated and NOD mice [49]. α-to-β identity switches were shown to be induced also in human islets upon PDX-1-MAFA combined overexpression. Additionally, these genetically derived β-like cells were able to ameliorate glucose intolerance in diabetic immunodeficient mice and enabled maintenance of euglycemia for 6 months after transplantation, highlighting the potential therapeutic properties of such applications [50].
A Role for Endogenous/Exogenous Factors That Can Stimulate Human (β-cells Proliferation
As we have explained, it is generally accepted that adult human β-cells have a very low capacity to divide. Nevertheless, it is also evident that β-cells have an intrinsic flexibility to adapt to environmental changes which require expansion of insulin-producing cells in mammals. This flexibility has been mostly studied in rodent β-cells. For example, several studies have shown that β-cells mass is maintained by duplication of pre-existing β-cells, rather than differentiation from pancreatic progenitors or other cell types [73–75]. In this context, there has been considerable effort to characterize the molecular signaling pathways that can regulate or trigger β-cells replication (accurately reviewed in [76–79]). A challenge in translating the findings in rodents to human β-cells is largely due to a dissimilarity of the pathways that regulate cell replication between the two species [80–82]. An attractive unbiased approach that has garnered attention is the use of high-throughput screenings to identify small molecules or to identify endogenous factors capable of specifically activating the proliferative machinery in human β-cells (Fig. 2).
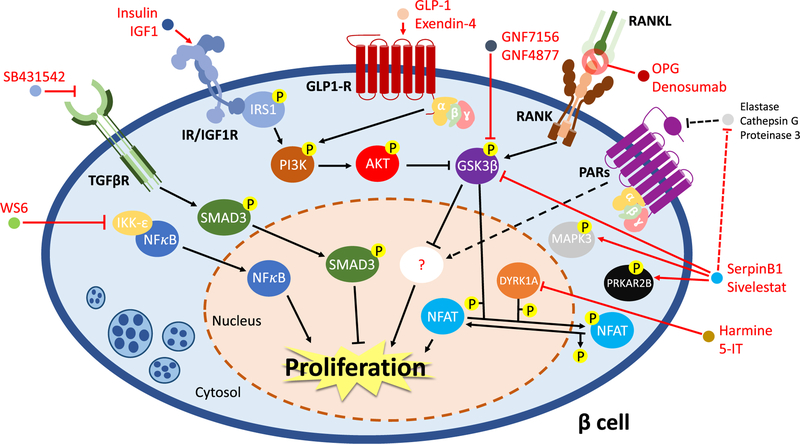
Fig. 2.
Molecular mechanism(s) regulating human β-cell proliferation. The nuclear factor kappa-B (NFκB) is retained in the cytosol and its function is repressed by inhibitor of nuclear factor kappa-B kinase subunit epsilon (IKK-ε). WS6 blocks IKK-ε inhibition on NFκB, which can translocate into the nucleus and promote cell growth. TGF-β pathway impacts β-cell proliferation via the activation of SMAD3. SB431542, a TGF-βR inhibitor, promotes cell growth by preventing SMAD3 activation. Insulin receptor (IR)/insulin-like growth factor (IGF1R) and glucagon-like peptide 1 receptor (GLP-1R) signaling pathways trigger β-cell regeneration via modulating PI3K-AKT axis, resulting in the inhibition of glycogen synthase kinase-3β (GSK3β) activity. In addition, inactivation of GSK3β is also obtained by treatments with GNF7156 and GNF4877, GSK3β inhibitors, or osteoprotegerin (OPG) or denosumab. In particular, OPG and denosumab act as mimics of receptor activator of nuclear factor kappa-B ligand (RANKL), preventing its interaction with receptor activator of nuclear factor kappa-B (RANK) and avoiding the activation of the extrinsic apoptotic pathways. Moreover, the hepatokine SerpinB1 and the elastase inhibitor sivelestat stimulate human β-cell proliferation increasing the phosphorylation levels of mitogen-activated protein kinase 3 (MAPK3), protein kinase cAMP-dependent type II regulatory subunit beta (PRKAR2B) and GSK3β, likely following inhibition of proteases as elastase, cathepsin G, or proteinase 3. These effects might involve the protease-activated receptor (PARs) signaling, but such a hypothesis requires further investigations (dotted lines and arrows). The dual-specificity tyrosine-regulated kinase-1a (DYRK1A) represses β-cell proliferation by phosphorylating and retaining into the cytosol the nuclear factor of activated T cells (NFAT). The inhibition of DYRK1A, using small molecules as harmine or 5-iodotubercidin (5-IT), results in the decrease of the phosphorylation state of NFAT, which translocate into the nucleus and activate the mitogenic pathways in human β-cells
The versatility and ability to optimize high-throughput screening (HTS) assays have provided an important platform in this field. Among the initial HTS studies, focused on the identification of compounds to activate β-cells proliferation, was the discovery of diarylurea WS1, which could induce proliferation of R7T1 cells, a quiescent rat β-cells line [83]. The analogue WS6, a diarylamide, was also able to stimulate proliferation in human β-cells [83], as well as α-cells [84], mainly by repressing the function of the inhibitor of nuclear factor kappa-B kinase subunit epsilon (IKK-ε), a negative regulator of the nuclear factor kappa-B (NFκB), and by regulating the cell replication suppressor ErbB3-binding protein 1 (EBP1), thereby stimulating E2F1-mediated cell proliferation (Fig. 2) [83]. Wang et al. found harmine, a plant-derived alkaloid drug, as an inducer of the MYC promoter and cell proliferation in HepG2 cells using a HTS. The authors confirmed that mitogenic pathways were also induced in cultured and transplanted human β-cells [85••]. The major target of harmine is the dual-specificity tyrosine phosphorylation regulated kinase 1A (DYRK1A), which regulates phosphorylation and activity of the nuclear factor of activated T cell (NFAT). NFAT is usually localized in the cytosol in a phosphorylated state, but when DYRK1A function is inhibited by harmine, the phosphorylation levels are reduced and it translocates into the nucleus to promote its mitogenic function [85••]. Recently, another DYRK1A inhibitor, 5-iodotubercidin (5-IT), was also identified from an HTS using dissociated human islet cells [86••, 87]. The discovery that 5-IT is able to induce β-cells regeneration also in transplanted human islets provides another example of targeting the DYRK1A-NFAT axis to expand β-cell mass with implications for therapeutics in patients with diabetes (Fig. 2).
Other pathways explored to regulate β-cells division include inhibitors of TGF-β. The signals transduced intracellularly by TGF-β receptors results in the activation of SMAD proteins that translocate into the nucleus to modulate gene expression, together with the stimulation of non-canonical effectors such as ERK, p38, JNK, and AKT [88, 89]. Recently, Dhawan et al. observed that the compound SB431542, a TGF-β signaling inhibitor, promotes β-cells proliferation in human islets in vitro and in vivo by repressing the expression of p16INK4a, an inhibitor of cell cycle machinery, induced by the activation of SMAD3 (Fig. 2) [90]. Interestingly, proliferation levels of human β-cells can be further increased by up to 5–8% of the total β-cells population by the combined pharmacological blockade of DYRK1A activity and TGF-β pathway. The simultaneous treatment of human islets with harmine and a TGF-β signaling inhibitor resulted in the activation of cyclins and cyclin-dependent kinases, on the one hand, and inhibition of key cell cycle regulators, on the other, in a synergistic fashion [91••].
Insulin/IGF-1 receptors and proteins in their signaling pathways are known to induce proliferation in virtually all mammalian cells by activating the PI3K-AKT signaling pathway and subsequent activation of MAPK signaling, and through inhibition of GSK3β activity (Fig. 2). GSK3β has been characterized as an inhibitor of replication in both rodent β-cells [92–94] and human islets [95]. Thus, the identification of GSK3β inhibitors as stimulators of human β-cells proliferation has been exploited for potential clinical therapeutics. In particular, two small molecules synthetized from a pyrazine scaffold, GNF7156 and GNF4877, were identified as GSK3β inhibitors and potent regulators of β-cells proliferation in cultured and engrafted human islets [96]. Although the application of chemical compounds to target stimulators/repressors of cell division selectively in human β-cells has been demonstrated to be efficient and accurate, it continues to raise concerns about the safety and the possibility of off-target, undesired effects when administered systemically in patients with diabetes. One approach to circumvent the side effects of exogenous compounds is the identification of endogenous factors that can expand β-cells mass in physiological conditions.
In this context, pregnancy has been widely studied as a physiological state characterized by a compensatory increase in β-cells in response to the high insulin demand. As mentioned earlier, the dramatic increase in rodent β-cells proliferation and mass during pregnancy was linked to the activation of FoxM1, menin, and serotonin pathways mediated by prolactin (PRL) and placental lactogen (PL) [97–101]. While such a mechanism has not been confirmed in humans, osteoprotegerin (OPG), one of the upregulated proteins in mice during pregnancy [102], is expressed in human β-cells in response to cytokine treatment [103]. OPG is a cytokine receptor belonging to the superfamily of tumor necrosis factor (TNF) receptors and was initially discovered as a soluble decoy receptor for the receptor activator of nuclear factor kappa-B ligand (RANKL) and the TNF-related apoptosis-inducing ligand (TRAIL) [104]. When bound by OPG, RANKL and TRAIL are prevented from interacting with their cognate receptors, receptor activator of nuclear factor kappa-B (RANK), and death receptor (DR), respectively, and are unable to induce cell death programs. Of relevance to human β-cells, OPG blocked RANKL/TRAIL-mediated apoptotic pathways by preventing phosphorylation of p38 MAPK to protect β-cells from cytokine-induced death [103]. OPG or denosumab an FDA-approved monoclonal antibody which mimics th functions of OPG, promoted human β-cell proliferation b; inhibiting RANKL-RANK association and stimulating phos phorylation and inactivation of GSK3β (Fig. 2) [105].
As mentioned in the previous paragraphs, the differences in the molecular pathways that control β-cell pro liferation between mice and humans are one of the challenges in translating animal research into the clinic. A case in point is the incretin hormone GLP-1. GLP-1, or its analogue exendin-4, is well-known potentiator of glucose-stimulated insulin secretion in both rodent an human islets [106]. The incretins have also been reported to induce replication of rodent β-cells by regulating multiple signaling pathways [107]. For example, GLP-1 or evendin-4 activates the GLP-1 receptor (GLP-1R) a G protein–coupled receptor, to stimulate the PI3K-AKT cascade, the cAMP-PKA-CREB axis, and PKCζ in a non-canonical fashion (Fig. 2) [108]. Furthermore, exendin-4 activates proliferative processes via the mTORC1 signaling [109]. GLP-1 has also been reported to upregulate the cell cycle proteins including cyclin D1 and cyclin A2, in combination with the degradation of the cell cycle inhibitor p27 [110, 111]. While these data generated much excitement in the field, none of these findings have been replicated in human islet/β-cells to date, revealing either the poor similarity of GLP-1 signaling effectors among different mammalian species or the lack of a precise protocol. One notable exception is the ability of exendin-4 treatment to stimulate β-cells proliferation in transplanted human islets isolated from young donors (~ 18 years old) [112]. Whether GLP-1 and its agonists can be therapeutically adapted for treating younger patients with diabetes awaits further investigation.
One observation appreciated over several decades is the ability of β-cells to compensate for states of insulin resistance both in the presence and in the absence of diabetes. Thus, obese individuals display a remarkable ability to increase their β-cell mass as a form of compensation to delay the onset of diabetes [113]. The signals that contribute to the expansion of β-cell mass could be multiple and may arise from multiple metabolic organs that are insulin resistant. Studies aimed at understanding inter-organ cross talk have begun to yield some important insights in this area [114]. One such example is the identification of SerpinB1, a hepatokine produced by the insulin-resistant liver involving FoxO1 regulation [115, 116]. The levels of circulating SerpinB1 have been shown to be altered in many models of diabetes both in rodents and humans [115–118]. SerpinB1 belongs to the serine protease inhibitor family and targets elastase, proteinase 3, and cathepsin G, among other proteases (Fig. 2) [119]. The protease inhibitor has been reported to induce a twofold increase in the proliferation in zebrafish, mouse, and human β-cells [115]. Using a phosphoproteomic analysis, El Ouaamari and colleagues observed that SerpinB1 treatment of mouse islets led to an increase in phosphorylation of the protein kinase cAMP-dependent type II regulatory subunit β (PRKAR2B), GSK3, and MAPK3 (Fig. 2). To begin to explore the mechanism of action of SerpinB1, El Ouaamari et al. focused on elastase inhibition and reported that mouse and human islets treated with established chemical elastase inhibitors, such as sivelestat or GW311616A, showed similar results (Fig. 2) [115]. Thus, one pathway that may be modulated by SerpinB 1 is elastase inhibition in the islet extracellular environment that leads to the activation of the proliferative pathways within β-cells. Whether SerpinB1 modulates alternative pathways including the inhibition of other proteases and/or the regulation of protease-activated receptors (PARs) requires further research (Fig. 2).
β-Cell Mass and Functional Responses
An important insight which is now gaining widespread acceptance is that, even in patients with long-standing T1D, there is a functional reserve of β-cells. This is a surprising finding which has challenged the long-held dogma that T1D only develops when 80–90% of β-cells are lost. This is probably the case in children who develop T1D within the first 7 years of life but, in those who are older at onset (teens and beyond), β-cell loss is much less extensive and residual β-cells may persist for many years [120]. Indeed, apparently, normal β-cells can still be found in the islets of some of the cohort of Joslin medalists who had lived with T1D for more than 50 years prior to their death [121]. Moreover, analysis of C-peptide levels in patients living with T1D for increasing periods has provoked the surprising conclusion that the rate of β-cell loss is initially exponential but then declines dramatically at about 7 years post-diagnosis such that β-cell mass remains stable thereafter [122•]. This could imply that autoimmunity is halted at this point (which seems inherently unlikely) or that a population of β-cells is retained which, for reasons that remain unexplored, are not visible to the immune system and can persist for long periods.
A key question then arises as to whether these persistent cells are functionally competent or if they are compromised. This issue has not been resolved although it has been reported that many patients are insulin “microsecretors” and that their circulating C-peptide levels increase after a meal [123]. This implies that at least a proportion of the residual β-cells must retain functional competence although it is uncertain whether this is true for the majority. In this context, studies of islets isolated from newly diagnosed patients imply that they may be refractory to stimulation upon initial isolation but that glucose-induced insulin release can be restored during a period of ex vivo culture [124]. Irrespective of the precise number of functional β-cells at any specific point in the disease process, these results suggest two important conclusions. Firstly, that absolute β-cell mass may not equate directly to functional β-cell mass in people with T1D and, secondly, that β-cell functionality can be retained (or restored) in the face of a long-term autoimmune state. This raises the hope that a targeted expansion of endogenous β-cell mass, stimulated by using combinations of the methods and factors outlined above or by new approaches still to be discovered, could become a realistic possibility in the future, even among patients who have lived with T1D for many years. This is an exciting prospect but the challenges to fulfill this potential should not be overlooked. Importantly, for example, the use of targeted agents such as the DYRK1A inhibitor, harmine, is not selective in their actions and causes the expansion of both β and other islet endocrine cells [87]. Similarly, in patients with recent onset T1D where β-cell proliferation is increased in inflamed islets, an expansion of α-cell mass is also detected [14]. Thus, methods must be established which can both target the β-cells selectively and are sufficiently well regulated that they do not lead to uncontrolled proliferation.
Conclusions
Taken together, the work summarized in this review gives cause for optimism that approaches which enhance endogenous β-cell mass are within reach. The physiological resilience of the cells and their natural longevity may be factors which act in their favor as technologies become available which allow their selective expansion in periods of life when they would normally exist in a post-mitotic state.
Acknowledgments
Funding Information R.N.K. acknowledges support from the JDRF, and the National Institutes of Health Grants R01 DK067536, UC4 DK116278, and UC4 DK116255. N.G.M. is grateful for the support from Diabetes UK (project grants 15/0005156 and 16/0005480) and from JDRF (nPOD-V collaborative award 3-SRA-2017–492-A-N and strategic research award 2-SRA-2018–474-S-B).
Footnotes
Compliance with Ethical Standards
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors.
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Conflict of Interest The authors declare that they have no conflict of interest.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Shields BM, Freathy RM, Hattersley AT. Genetic influences on the association between fetal growth and susceptibility to type 2 diabetes. J Dev Orig Health Dis. 2010;1(2):96–105. [DOI] [PubMed] [Google Scholar]
- 2.Spyer G, Macleod KM, Shepherd M, Ellard S, Hattersley AT. Pregnancy outcome in patients with raised blood glucose due to a heterozygous glucokinase gene mutation. Diabet Med. 2009;26(1):14–8. [DOI] [PubMed] [Google Scholar]
- 3.Jennings RE, Berry AA, Strutt JP, Gerrard DT, Hanley NA. Human pancreas development. Development. 2015;142(18): 3126–37. [DOI] [PubMed] [Google Scholar]
- 4.Bonner-Weir S, Aguayo-Mazzucato C, Weir GC. Dynamic development of the pancreas from birth to adulthood. Ups J Med Sci. 2016;121(2):155–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cnop M, Igoillo-Esteve M, Hughes SJ, Walker JN, Cnop I, Clark A. Longevity of human islet alpha and beta cells. Diabetes Obes Metab. 2011;13(Suppl 1):39–46. [DOI] [PubMed] [Google Scholar]
- 6.Butler AE, Cao-Minh L, Galasso R, Rizza RA, Corradin A, Cobelli C, et al. Adaptive changes in pancreatic beta cell fractional area and beta cell turnover in human pregnancy. Diabetologia. 2010;53(10):2167–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Genevay M, Pontes H, Meda P. Beta cell adaptation in pregnancy: a major difference between humans and rodents? Diabetologia. 2010;53(10):2089–92. [DOI] [PubMed] [Google Scholar]
- 8.Wang YJ, Golson ML, Schug J, Traum D, Liu C, Vivek K, et al. Single-cell mass cytometry analysis of the human endocrine pancreas. Cell Metab. 2016;24(4):616–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Talchai C, Xuan S, Lin HV, Sussel L, Accili D. Pancreatic beta cell dedifferentiation as a mechanism of diabetic beta cell failure. Cell. 2012;150(6):1223–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cinti F, Bouchi R, Kim-Muller JY, Ohmura Y, Sandoval PR, Masini M, et al. Evidence of beta-cell dedifferentiation in human type 2 diabetes. J Clin Endocrinol Metab. 2016;101(3):1044–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aguayo-Mazzucato C, Bonner-Weir S. Pancreatic beta cell regeneration as a possible therapy for diabetes. Cell Metab. 2018;27(1): 57–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morgan NG, Richardson SJ. Fifty years of pancreatic islet pathology in human type 1 diabetes: insights gained and progress made. Diabetologia. 2018;61(12):2499–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lam CJ, Jacobson DR, Rankin MM, Cox AR, Kushner JA. Beta cells persist in T1D pancreata without evidence of ongoing β-cell turnover or neogenesis. J Clin Endocrinol Metab. 2017;102(8): 2647–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Willcox A, Richardson SJ, Bone AJ, Foulis AK, Morgan NG. Evidence of increased islet cell proliferation in patients with recent-onset type 1 diabetes. Diabetologia. 2010;53(9):2020–8. [DOI] [PubMed] [Google Scholar]
- 15.Willcox A, Richardson SJ, Bone AJ, Foulis AK, Morgan NG. Immunohistochemical analysis of the relationship between islet cell proliferation and the production of the enteroviral capsid protein, VP1, in the islets of patients with recent-onset type 1 diabetes. Diabetologia. 2011;54(9):2417–20. [DOI] [PubMed] [Google Scholar]
- 16.Dirice E, Kahraman S, Jiang W, El Ouaamari A, De Jesus DF, Teo AK, et al. Soluble factors secreted by T cells promote beta-cell proliferation. Diabetes. 2014;63(1):188–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fiaschi-Taesch NM, Kleinberger JW, Salim FG, Troxell R, Wills R, Tanwir M, et al. Human pancreatic beta-cell G1/S molecule cell cycle atlas. Diabetes. 2013;62(7):2450–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fiaschi-Taesch NM, Kleinberger JW, Salim FG, Troxell R, Wills R, Tanwir M, et al. Cytoplasmic-nuclear trafficking of G1/S cell cycle molecules and adult human beta-cell replication: a revised model of human beta-cell G1/S control. Diabetes. 2013;62(7): 2460–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taniguchi K, Russell MA, Richardson SJ, Morgan NG. The subcellular distribution of cyclin-D1 and cyclin-D3 within human islet cells varies according to the status of the pancreas donor. Diabetologia. 2015;58(9):2056–63. [DOI] [PubMed] [Google Scholar]
- 20.Caballero F, Siniakowicz K, Hollister-Lock J, Duran L, Katsuta H, Yamada T, et al. Birth and death of human beta-cells in pancreases from cadaver donors, autopsies, surgical specimens, and islets transplanted into mice. Cell Transplant. 2014;23(2):139–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sullivan BA, Hollister-Lock J, Bonner-Weir S, Weir GC. Reduced Ki67 staining in the postmortem state calls into question past conclusions about the lack of turnover of adult human betα-cells. Diabetes. 2015;64(5):1698–702.• An important study which suggests that estimates of Ki67 immunopositivity may not correlate fully with beta cell replication in post mortem tissues.
- 22.Ferber S, Halkin A, Cohen H, Ber I, Einav Y, Goldberg I, et al. Pancreatic and duodenal homeobox gene 1 induces expression of insulin genes in liver and ameliorates streptozotocin-induced hyperglycemia. Nat Med. 2000;6(5):568–72. [DOI] [PubMed] [Google Scholar]
- 23.Ber I, Shternhall K, Perl S, Ohanuna Z, Goldberg I, Barshack I, et al. Functional, persistent, and extended liver to pancreas transdifferentiation. JBiol Chem. 2003;278(34):31950–7. [DOI] [PubMed] [Google Scholar]
- 24.Kojima H, Fujimiya M, Matsumura K, Younan P, Imaeda H, Maeda M, et al. NeuroD-betacellulin gene therapy induces islet neogenesis in the liver and reverses diabetes in mice. Nat Med. 2003;9(5):596–603. [DOI] [PubMed] [Google Scholar]
- 25.Tang DQ, Shun L, Koya V, Sun Y, Wang Q, Wang H, et al. Genetically reprogrammed, liver-derived insulin-producing cells are glucose-responsive, but susceptible to autoimmune destruction in settings of murine model of type 1 diabetes. Am J Transl Res. 2013;5(2):184–99. [PMC free article] [PubMed] [Google Scholar]
- 26.Nagasaki H, Katsumata T, Oishi H, Tai PH, Sekiguchi Y, Koshida R, et al. Generation of insulin-producing cells from the mouse liver using beta cell-related gene transfer including Mafa and Mafb. PLoS One. 2014;14;9(11):e113022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zalzman M, Gupta S, Giri RK, Berkovich I, Sappal BS, Karnieli O, et al. Reversal of hyperglycemia in mice by using human expandable insulin-producing cells differentiated from fetal liver progenitor cells. Proc Natl Acad SciU S A. 2003;100(12):7253–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zalzman M, Anker-Kitai L, Efrat S. Differentiation of human liver-derived, insulin-producing cells toward the beta-cell phenotype. Diabetes. 2005;54(9):2568–75. [DOI] [PubMed] [Google Scholar]
- 29.Sapir T, Shternhall K, Meivar-Levy I, Blumenfeld T, Cohen H, Skutelsky E, et al. Cell-replacement therapy for diabetes: generating functional insulin-producing tissue from adult human liver cells. Proc Natl Acad Sci USA. 2005;102(22):7964–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen YJ, Finkbeiner SR Weinblatt D, Emmett MJ, Tameire F, Yousefi M, et al. De novo formation of insulin-producing “neo-β cell islets” from intestinal crypts. Cell Rep. 2014;6(6):1046–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Suzuki A, Nakauchi H, Taniguchi H. Glucagon-like peptide 1 (137) converts intestinal epithelial cells into insulin-producing cells. Proc Natl Acad Sci USA. 2003;100(9):5034–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Talchai C, Xuan S, Kitamura T, DePinho RA, Accili D. Generation of functional insulin-producing cells in the gut by Foxo1 ablation. Nat Genet 2012; 44(4):406–12, S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bouchi R, Foo KS, Hua H, Tsuchiya K, Ohmura Y, Sandoval PR, et al. FOXO1 inhibition yields functional insulin-producing cells in human gut organoid cultures. Nat Commun. 2014;5:4242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou Q, Brown J, Kanarek A, Rajagopal J, Melton DA. In vivo reprogramming of adult pancreatic exocrine cells to beta-cells. Nature. 2008;455(7213):627–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baeyens L, Lemper M, Leuckx G, De Groef S, Bonfanti P, Stangà G, Shemer R, Nord C, Scheel DW, Pan FC, Ahlgren U, Gu G, Stoffers DA, Dor Y, Ferrer J, Gradwohl G, Wright CV, Van de Casteele M, German MS, Bouwens L, Heimberg H. Transient cytokine treatment induces acinar cell reprogramming and regenerates functional beta cell mass in diabetic mice. Nat Biotechnol 2014; 32(1):76–83. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 36.Rooman I, Bouwens L. Combined gastrin and epidermal growth factor treatment induces islet regeneration and restores normoglycaemia in C57Bl6/J mice treated with alloxan. Diabetologia. 2004;47(2):259–65. [DOI] [PubMed] [Google Scholar]
- 37.Lemper M, De Groef S, Stangé G, Baeyens L, Heimberg H. A combination of cytokines EGF and CNTF protects the functional beta cell mass in mice with short-term hyperglycaemia. Diabetologia. 2016;59(9):1948–58. [DOI] [PubMed] [Google Scholar]
- 38.Klein D, Álvarez-Cubela S, Lanzoni G, Vargas N, Prabakar KR, Boulina M, et al. BMP-7 induces adult human pancreatic exocrine-to-endocrine conversion. Diabetes. 2015;64(12):4123–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Noguchi H, Kaneto H, Weir GC, Bonner-Weir S. PDX-1 protein containing its own antennapedia-like protein transduction domain can transduce pancreatic duct and islet cells. Diabetes. 2003;52(7): 1732–7. [DOI] [PubMed] [Google Scholar]
- 40.Valdez IA, Dirice E, Gupta MK, Shirakawa J, Teo AKK, Kulkarni RN. Proinflammatory cytokines induce endocrine differentiation in pancreatic ductal cells via STAT3-dependent NGN3 activation. Cell Rep. 2016;15(3):460–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Collombat P, Xu X, Ravassard P, Sosa-Pineda B, Dussaud S, Billestrup N, et al. The ectopic expression of Pax4 in the mouse pancreas converts progenitor cells into alpha and subsequently beta cells. Cell. 2009;138(3):449–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Courtney M, Gjernes E, Druelle N, Ravaud C, Vieira A, Ben-Othman N, et al. The inactivation of Arx in pancreatic α-cells triggers their neogenesis and conversion into functional beta-like cells. PLoS Genet. 2013;9(10):e1003934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chakravarthy H, Gu X, Enge M, Dai X, Wang Y, Damond N, et al. Converting adult pancreatic islet α cells into β cells by targeting both Dnmt1 and Arx. Cell Metab. 2017;25(3):622–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thorel F, Napote V, Avril I, Kohno K, Desgraz R, Chera S, et al. Conversion of adult pancreatic alpha-cells to beta-cells after extreme beta-cell loss. Nature. 2010;464(7292):1149–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chung CH, Hao E, Piran R, Keinan E, Levine F. Pancreatic beta-cell neogenesis by direct conversion from mature alpha-cells. Stem Cells. 2010;28:1630–8. [DOI] [PubMed] [Google Scholar]
- 46.Piran R, Lee SH, Kuss P, Hao E, Newlin R, Millan JL, et al. PAR2 regulates regeneration, transdifferentiation, and death. Cell Death Dis. 2016;7(11):e2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ben-Othman N, Vieira A, Courtney M, Record F, Gjernes E, Avolio F, et al. Long-term GABA administration Induces alpha cell-mediated beta-like cell neogenesis. Cell. 2017;168(1–2):73–85.e11. [DOI] [PubMed] [Google Scholar]
- 48.Li J, Casteels T, Frogne T, Ingvorsen C, Honora C, Courtney M, Huber KVM, Schmitner N, Kimmel RA, Romanov RA, Sturtzel C, Lardeau CH, Klughammer J, Farlik M, Sdelci S, Vieira A, Avolio F, Briand F, Baburin I, Májek P, Pauler FM, Penz T, Stukalov A, Gridling M, Parapatics K, Barbieux C, Berishvili E, Spittler A, Colinge J, Bennett KL, Hering S, Sulpice T, Bock C, Distel M, Harkany T, Meyer D, Superti-Furga G, Collombat P, Hecksher S, Rensen J, Kubicek S. Artemisinins target GABA(A) receptor signaling and impair alpha cell identity. Cell. 2017; 168(1–2):86–100.e15.• Presents opposing sides in the important debate about the role of artemisinins as regulators of islet cell transdifferentation
- 49.Xiao X, Guo P, Shiota C, Zhang T, Coudriet GM, Fischbach S, et al. Endogenous reprogramming of alpha cells into beta cells, induced by viral gene therapy, reverses autoimmune diabetes. Cell Stem Cell. 2018;22(1):78–90.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Furuyama K, Chera S, van Gurp L, Oropeza D, Ghila L, Damond N, Vethe H, Paulo JA, Joosten AM, Berney T, Bosco D, Dorrell C, Grompe M, Ræder H, Roep BO, Thorel F, Herrera PL. Diabetes relief in mice by glucose-sensing insulin-secreting human beta-cells. Nature. 2019; 567(7746):43–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.De Lisle RC, Logsdon CD. Pancreatic acinar cells in culture: expression of acinar and ductal antigens in a growth-related manner. Eur J Cell Biol. 1990;51(1):64–75. [PubMed] [Google Scholar]
- 52.Hall PA, Lemoine NR. Rapid acinar to ductal transdifferentiation in cultured human exocrine pancreas. J Pathol. 1992;166(2):97–103. [DOI] [PubMed] [Google Scholar]
- 53.Furuyama K, Kawaguchi Y, Akiyama H, Horiguchi M, Kodama S, Kuhara T, et al. Continuous cell supply from a Sox9-expressing progenitor zone in adult liver, exocrine pancreas and intestine. Nat Genet. 2011;43(1):34–41. [DOI] [PubMed] [Google Scholar]
- 54.Masini M, Marselli L, Himpe E, Martino L, Bugliani M, Suleiman M, et al. Co-localization of acinar markers and insulin in pancreatic cells of subjects with type 2 diabetes. PLoS One. 2017;12(6): e0179398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Roy N, Hebrok M. Regulation of cellular identity in cancer. Dev Cell. 2015;35(6):674–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Solar M, Cardalda C, Houbracken I, Martan M, Maestro MA, De Medts N, et al. Pancreatic exocrine duct cells give rise to insulin-producing beta cells during embryogenesis but not after birth. Dev Cell. 2017;17(6):849–60. [DOI] [PubMed] [Google Scholar]
- 57.Bogdani M, Lefebvre V, Buelens N, Bock T, Pipeleers-Marichal M, In’t Veld P, et al. Formation of insulin-positive cells in implants of human pancreatic duct cell preparations from young donors. Diabetologia. 2003;46(6):830–8. [DOI] [PubMed] [Google Scholar]
- 58.Meier JJ, Butler AE, Galasso R, Butler PC. Hyperinsulinemic hypoglycemia after gastric bypass surgery is not accompanied by islet hyperplasia or increased beta-cell turnover. Diabetes Care. 2006;29(7):1554–9. [DOI] [PubMed] [Google Scholar]
- 59.Bonner-Weir S, Toschi E, Inada A, Reitz P, Fonseca SY, Aye T, et al. The pancreatic ductal epithelium serves as a potential pool of progenitor cells. Pediatr Diabetes. 2004;5(Suppl 2):16–22. [DOI] [PubMed] [Google Scholar]
- 60.Bonner-Weir S, Guo L, Li WC, Ouziel-Yahalom L, Lysy PA, Weir GC, et al. Islet neogenesis: a possible pathway for beta-cell replenishment. Rev Diabet Stud. 2012;9(4):407–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Alidjinou EK, Sana F, Bertin A, Caloone D, Hober D. Persistent infection of human pancreatic cells with Coxsackievirus B4 is cured by fluoxetine. Antivir Res. 2015;116:51–4. [DOI] [PubMed] [Google Scholar]
- 62.Dunne JL, Richardson SJ, Atkinson MA, Craig ME, DahlJorgensen K, Flodstrom-Tullberg M, et al. Rationale for enteroviral vaccination and antiviral therapies in human type 1 diabetes. Diabetologia. 2019. January 23;62:744–53. 10.1007/s00125-019-4811-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Martin-Pagola A, Sisino G, Allende G, Dominguez-Bendala J, Gianani R, Reijonen H, et al. Insulin protein and proliferation in ductal cells in the transplanted pancreas of patients with type 1 diabetes and recurrence of autoimmunity. Diabetologia. 2008;51(10):1803–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dirice E, De Jesus DF, Kahraman S, Basile G, Ng RW, El Ouaamari A, Teo AKK, Bhatt S, Hu J, Kulkarni RN. Human duct cells contribute to β cell compensation in insulin resistance. JCI Insight. 2019; 4(8): pii: 99576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rall LB, Pictet RL, Williams RH, Rutter WJ. Early differentiation of glucagon-producing cells in embryonic pancreas: a possible developmental role for glucagon. Proc Natl Acad Sci USA. 1973;70(12):3478–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Teitelman G, Alpert S, Polak JM, Martinez A, Hanahan D. Precursor cells of mouse endocrine pancreas coexpress insulin, glucagon and the neuronal proteins tyrosine hydroxylase and neuropeptide Y, but not pancreatic polypeptide. Development. 1993;118(4):1031–9. [DOI] [PubMed] [Google Scholar]
- 67.Johansson KA, Dursun U, Jordan N, Gu G, Beermann F, Gradwohl G, et al. Temporal control of neurogenin3 activity in pancreas progenitors reveals competence windows for the generation of different endocrine cell types. Dev Cell. 2007;12(3):457–65. [DOI] [PubMed] [Google Scholar]
- 68.Sharon N, Chawla R, Mueller J, Vanderhooft J, Whitehorn LJ, Rosenthal B, et al. A peninsular structure coordinates asynchronous differentiation with morphogenesis to generate pancreatic islets. Cell. 2019;176(4):790–804.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hancock AS, Du A, Liu J, Miller M, May CL. Glucagon deficiency reduces hepatic glucose production and improves glucose tolerance in adult mice. Mol Endocrinol. 2010;24(8):1605–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Thorel F, Damond N, Chera S, Wiederkehr A, Thorens B, Meda P, et al. Normal glucagon signaling and β-cell function after neartotal α-cell ablation in adult mice. Diabetes. 2011;60(11):2872–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ackermann AM, Moss NG, Kaestner KH. GABA and artesunate do not induce pancreatic alpha-to-beta cell transdifferentiation in vivo. Cell Metab. 2018;28(5):787–792.e3.• Presents opposing sides in the important debate about the role of artemisinins as regulators of islet cell transdifferentation.
- 72.Eizirik DL, Gurzov EN. Can GABA turn pancreatic alpha-cells into beta-cells? Nat Rev Endocrinol. 2018;14(11):629–30. [DOI] [PubMed] [Google Scholar]
- 73.Dor Y, Brown J, Martinez OI, Melton DA. Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature. 2004;429(6987):41–6. [DOI] [PubMed] [Google Scholar]
- 74.Georgia S, Bhushan A. Beta cell replication is the primary mechanism for maintaining postnatal beta cell mass. J Clin Invest. 2004;114(7):963–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kulkarni RN, Jhala US, Winnay JN, Krajewski S, Montminy M, Kahn CR. PDX-1 haploinsufficiency limits the compensatory islet hyperplasia that occurs in response to insulin resistance. J Clin Invest. 2004;114(6):828–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Vasavada RC, Gonzalez-Pertusa JA, Fujinaka Y, Fiaschi-Taesch N, Cozar-Castellano I, Garcia-Ocaña A. Growth factors and beta cell replication. Int J Biochem Cell Biol 2006; 38(5–6):931–950. [DOI] [PubMed] [Google Scholar]
- 77.Heit JJ, Karnik SK, Kim SK. Intrinsic regulators of pancreatic beta-cell proliferation. Annu Rev Cell Dev Biol. 2006;22:311–38. [DOI] [PubMed] [Google Scholar]
- 78.Assmann A, Hinault C, Kulkarni RN. Growth factor control of pancreatic islet regeneration and function. Pediatr Diabetes. 2009;10(1):14–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jiang WJ, Peng YC, Yang KM. Cellular signaling pathways regulating beta-cell proliferation as a promising therapeutic target in the treatment of diabetes. Exp Ther Med. 2018;16(4):3275–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kulkarni RN, Mizrachi EB, Ocana AG, Stewart AF. Human beta-cell proliferation and intracellular signaling: driving in the dark without a road map. Diabetes. 2012;61(9):2205–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bernal-Mizrachi E, Kulkarni RN, Scott DK, Mauvais-Jarvis F, Stewart AF, Garcia-Ocaña A. Human beta-cell proliferation and intracellular signaling part 2: still driving in the dark without a roadmap. Diabetes. 2014;63(3):819–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Stewart AF, Hussain MA, Garcia-Ocaña A, Vasavada RC, Bhushan A, Bernal-Mizrachi E, et al. Human beta-cell proliferation and intracellular signaling: part 3. Diabetes. 2015;64(6): 1872–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shen W, Tremblay MS, Deshmukh VA, Wang W, Filippi CM, Harb G, et al. Small-molecule inducer of beta cell proliferation identified by high-throughput screening. J Am Chem Soc. 2013;135(5):1669–72. [DOI] [PubMed] [Google Scholar]
- 84.Boerner BP, George NM, Mir SU, Sarvetnick NE. WS6 induces both alpha and beta cell proliferation without affecting differentiation or viability. Endocr J. 2015;62(4):379–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang P, Alvarez-Perez JC, Felsenfeld DP, Liu H, Sivendran S, Bender A, et al. A high-throughput chemical screen reveals that harmine-mediated inhibition of DYRK1A increases human pancreatic beta cell replication. Nat Med. 2015;21(4):383–8.•• Provides convincing evidence that harmine may be capable of promoting beta cell profilferation via its ability to inhibit DYRK1A.
- 86.Dirice E, Walpita D, Vetere A, Meier BC, Kahraman S, Hu J, et al. Inhibition of DYRK1A stimulates human beta-cell proliferation. Diabetes. 2016;65(6):1660–71.•• Critical evidence that inhibitors of a key kinase may be mediators of beta cell proliferation.
- 87.Walpita D, Hasaka T, Spoonamore J, Vetere A, Takane KK, Fomina-Yadlin D, et al. A human islet cell culture system for high-throughput screening. J Biomol Screen. 2012;17(4):509–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature. 2003;425(6958): 577–84. [DOI] [PubMed] [Google Scholar]
- 89.Massague J TGF-beta signalling in context. Nat Rev Mol Cell Biol. 2012;13(10):616–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dhawan S, Dirice E, Kulkarni RN, Bhushan A. Inhibition of TGF-beta signaling promotes human pancreatic beta-cell replication. Diabetes. 2016;65(5): 1208–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang P, Karakose E, Liu H, Swartz E, Ackeifi C, Zlatanic V, et al. Combined inhibition of DYRK1A, SMAD, and trithorax pathways synergizes to induce robust replication in adult human beta cells. Cell Metab. 2019;29(3):638–652.e5.•• Offers a new therapeutic route to achieve beta cell proliferation by application of exogenous small molecules.
- 92.Liu Z, Tanabe K, Bernal-Mizrachi E, Permutt MA. Mice with beta cell overexpression of glycogen synthase kinase-3beta have reduced beta cell mass and proliferation. Diabetologia. 2008;51(4):623–31. [DOI] [PubMed] [Google Scholar]
- 93.Tanabe K, Liu Z, Patel S, Doble BW, Li L, Cras-Meneur C, et al. Genetic deficiency of glycogen synthase kinase-3beta corrects diabetes in mouse models of insulin resistance. PLoS Biol. 2008;6(2):e37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Liu Y, Tanabe K, Baronnier D, Patel S, Woodgett J, Cras-Meneur C, Permutt MA. Conditional ablation of Gsk-β in islet beta cells results in expanded mass and resistance to fat feeding-induced diabetes in mice. Diabetologia. 2010; 53(12):2600–2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Liu H, Remedi MS, Pappan KL, Kwon G, Rohatgi N, Marshall CA, et al. Glycogen synthase kinase-3 and mammalian target of rapamycin pathways contribute to DNA synthesis, cell cycle progression, and proliferation in human islets. Diabetes. 2009;58(3): 663–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Shen W, Taylor B, Jin Q, Nguyen-Tran V, Meeusen S, Zhang YQ, et al. Inhibition of DYRK1A and GSK3B induces human betacell proliferation. Nat Commun. 2015;6:8372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rieck S, Kaestner KH. Expansion of beta-cell mass in response to pregnancy. Trends Endocrinol Metab. 2010;21:151–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhang H, Zhang J, Pope CF, Crawford LA, Vasavada RC, Jagasia SM, et al. Gestational diabetes mellitus resulting from impaired beta-cell compensation in the absence of FoxM1, a novel downstream effector of placental lactogen. Diabetes. 2010;59(1):143–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Karnik SK, Chen H, McLean GW, Heit JJ, Gu X, Zhang AY, et al. Menin controls growth of pancreatic beta-cells in pregnant mice and promotes gestational diabetes mellitus. Science. 2007;318(5851):806–9. [DOI] [PubMed] [Google Scholar]
- 100.Kim H, Toyofuku Y, Lynn FC, Chak E, Uchida T, Mizukami H, et al. Serotonin regulates pancreatic beta cell mass during pregnancy. Nat Med. 2010;16(7):804–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shirakawa J, Fernandez M, Takatani T, El Ouaamari A, Jungtrakoon P, Okawa ER, et al. Insulin signaling regulates the FoxM1/PLK1/CENP-A pathway to promote adaptive pancreatic β cell proliferation. Cell Metab. 2017;25(4):868–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rieck S, White P, Schug J, Fox AJ, Smirnova O, Gao N, et al. The transcriptional response of the islet to pregnancy in mice. Mol Endocrinol. 2009;23(10):1702–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Schrader J, Rennekamp W, Niebergall U, Schoppet M, Jahr H, Brendel MD, et al. Cytokine-induced osteoprotegerin expression protects pancreatic beta cells through p38 mitogen-activated protein kinase signalling against cell death. Diabetologia. 2007;50(6): 1243–7. [DOI] [PubMed] [Google Scholar]
- 104.Walsh MC, Choi Y. Biology of the RANKL-RANK-OPG system in immunity, Bone, and beyond. Front Immunol. 2014;5:511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kondegowda NG, Fenutria R, Pollack IR, Orthofer M, Garcia-Ocaña A, Penninger JM, et al. Osteoprotegerin and denosumab stimulate human beta cell proliferation through inhibition of the receptor activator of NF-KB ligand pathway. Cell Metab. 2015;22(1):77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Jones B, Bloom SR, Buenaventura T, Tomas A, Rutter GA. Control ofinsulin secretion by GLP-1. Peptides. 2018;100:75–84. [DOI] [PubMed] [Google Scholar]
- 107.Lavine JA, Attie AD. Gastrointestinal hormones and the regulation of beta-cell mass. Ann N Y Acad Sci. 2010;1212:41–58. [DOI] [PubMed] [Google Scholar]
- 108.Campbell JE, Drucker DJ. Pharmacology, physiology, and mechanisms of incretin hormone action. Cell Metab. 2013;17(6):819–37. [DOI] [PubMed] [Google Scholar]
- 109.Xie J, El Sayed NM, Qi C, Zhao X, Moore CE, Herbert TP. Exendin-4 stimulates islet cell replication via the IGF1 receptor activation of mTORC1/S6K1. J Mol Endocrinol. 2014;53(1): 105–15. [DOI] [PubMed] [Google Scholar]
- 110.Friedrichsen BN, Neubauer N, Lee YC, Gram VK, Blume N, Petersen JS, et al. Stimulation of pancreatic beta-cell replication by incretins involves transcriptional induction of cyclin D1 via multiple signalling pathways. J Endocrinol. 2006;188(3):481–92. [DOI] [PubMed] [Google Scholar]
- 111.Tschen SI, Georgia S, Dhawan S, Bhushan A. Skp2 is required for incretin hormone-mediated beta-cell proliferation. Mol Endocrinol. 2011;25 (12):2134–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tian L, Gao J, Weng G, Yi H, Tian B, O’Brien TD, et al. Comparison of exendin-4 on beta-cell replication in mouse and human islet grafts. Transpl Int. 2011;24(8):856–64. [DOI] [PubMed] [Google Scholar]
- 113.Saisho Y, Butler AE, Manesso E, Elashoff D, Rizza RA, Butler PC. Beta-cell mass and turnover in humans: effects of obesity and aging. Diabetes Care. 2013;36(1):111–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Shirakawa J, Kulkarni RN. Novel factors modulating human betacell proliferation. Diabetes Obes Metab. 2016;18(Suppl 1):71–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.El Ouaamari A, Dirice E, Gedeon N, Hu J, Zhou JY, Shirakawa J, et al. SerpinB1 promotes pancreatic beta cell proliferation. Cell Metab. 2016;23(1):194–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.El Ouaamari A, O-Sullivan I, Shirakawa J, Basile G, Zhang W, Roger S, et al. Forkhead box protein O1 (FoxO1) regulates hepatic serine protease inhibitor B1 (serpinB1) expression in a non-cell-autonomous fashion. J Biol Chem. 2019;294(3):1059–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Michael MD, Kulkarni RN, Postic C, Previs SF, Shulman GI, Magnuson MA, et al. Loss of insulin signaling in hepatocytes leads to severe insulin resistance and progressive hepatic dysfunction. Mol Cell. 2000. l;6(1):87–97. [PubMed] [Google Scholar]
- 118.Takebayashi K, Hara K, Terasawa T, Naruse R, Suetsugu M, Tsuchiya T, et al. Circulating SerpinB1 levels and clinical features in patients with type 2 diabetes. BMJ Open Diabetes Res Care. 2016;4(1):e000274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sugimori T, Cooley J, Hoidal JR, Remold-O’Donnell E. Inhibitory properties of recombinant human monocyte/neutrophil elastase inhibitor. Am J Respir Cell Mol Biol. 1995;13(3):314–22. [DOI] [PubMed] [Google Scholar]
- 120.Leete P, Willcox A, Krogvold L, Dahl-Jorgensen K Foulis AK, Richardson SJ, Morgan NG. Differential insulitic profiles determine the extent of beta-cell destruction and the age at onset of type 1 diabetes. Diabetes. 2016; 65(5):1362–1369. [DOI] [PubMed] [Google Scholar]
- 121.Keenan HA, Sun JK, Levine J, Doria A, Aiello LP, Eisenbarth G, Bonner-Weir S, King GL. Residual insulin production and pancreatic β-cells turnover after 50 years of diabetes: Joslin Medalist Study Diabetes. 2010; 59(11):2846–2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Shields BM, TJ MD, Oram R, Hill A, Hudson M, Leete P, et al. TIGI Consortium. C-Peptide decline in type 1 diabetes has two phases: an initial exponential fall and a subsequent stable phase. Diabetes Care. 2018;41(7):1486–92.• Provides important evidence that beta-cell death may be arrested after the initial phase of loss during the normal progression of type 1 diabetes.
- 123.Oram RA, Jones AG, Besser RE, Knight BA, Shields BM, Brown RJ, et al. The majority of patients with long-duration type 1 diabetes are insulin microsecretors and have functioning beta cells. Diabetologia. 2014;57(1):187–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Krogvold L, Skog O, Sundstrom G, Edwin B, Buanes T, Hanssen KF, et al. Function of isolated pancreatic islets from patients at onset of type 1 diabetes: insulin secretion can be restored after some days in a nondiabetogenic environment in vitro: results from the DiViD study. Diabetes. 2015;64(7):2506–12. [DOI] [PubMed] [Google Scholar]