Abstract
Purpose
Triple-negative breast cancer (TNBC) is a refractory type of breast cancer with poor prognosis and limited choice for treatment. Previous studies had shown that TNBC has high expressions of transmembrane prostate androgen-induced protein (TMEPAI). TMEPAI was known to be induced by TGF-β/Smad signaling and have tumorigenic functions that converting TGF-β from tumor suppressor to tumor promoter and inducing epithelial–mesenchymal transition (EMT). Therefore, we aimed to define the role of TMEPAI in triple-negative breast cancer cells treatment using several anti-cancers in the presence of TGF-β.
Methods
TMEPAI-knock out (KO) was carried out in a triple-negative breast cancer cell, BT549. TMEPAI editing was developed using the CRISPR-Cas9 system using two combinations of sgRNA to remove exon 4 of the TMEPAI gene entirely. Genotyping and proteomic analysis were performed to check the establishment of the TMEPAI-KO cells. Wild type (WT) and KO cells were used to determine inhibitory concentration 50% (IC50) of several anti-cancers: doxorubicin, cisplatin, paclitaxel, and bicalutamide in the presence of TGF-β treatment.
Results
KO cells were successfully established by completely removing the TMEPAI gene, which was proven in genomic and proteomic analysis. Further, in TMEPAI-KO cells, we found a significant reduction of IC50 for doxorubicin and paclitaxel, and minimal effects were seen for cisplatin and bicalutamide. Our findings suggest that TGF-β-induced TMEPAI attenuates the response of TNBC to doxorubicin and paclitaxel, but not to cisplatin and bicalutamide.
Conclusion
TGF-β induced TMEPAI contributes to the reduced response of TNBC treatment to doxorubicin and paclitaxel, but minimal on cisplatin and bicalutamide. Further study is needed to confirm our findings in other growth factor-induced cells, as well as in in vivo model.
Keywords: TMEPAI, TGF-β, doxorubicin, paclitaxel
Introduction
Transmembrane prostate androgen-induced protein (TMEPAI), also known as PMEPA1 (prostate transmembrane protein androgen induced-1) or STAG1 (solid tumor-associated gene-1) is a type 1b transmembrane proteins, originally known to be androgen-induced. Later on, TMEPAI was discovered to be induced by multiple signaling pathways which resulted in an increase in tumorigenic activities.1–3 TMEPAI was reported to be highly expressed in various types of cancers such as lung,4 breast,5,6 colon,7 pancreas,8 and renal.9 TMEPAI is also strongly induced by TGF-β (tumor growth factor-β) and multiple intracellular signaling pathways such as EGF (epidermal growth factor) and Wnt signaling further enhance TGF-β-induced TMEPAI expression.10 In the TGF-β signaling pathway, TMEPAI is a target gene, as well as a negative regulator. TMEPAI competes with SARA (Smad Anchor for Receptor Activation) for its binding to R-Smads.11 TMEPAI was also reported to cause degradation of the TGF-β type I receptor by its recruiting Nedd4.12 In addition, TMEPAI was reported to act as a converter of TGF-β from tumor suppressor to tumor promoter.13 All of the above functions are mediated by its PY and SIM domain, encoded in exon 4 of the TMEPAI gene.11
Studies in triple-negative breast cancer showed that TMEPAI is not only involved in Smad-dependent (canonical) TGF-β signaling pathway but also Smad-independent (non-canonical) pathway. In TGF-β non-canonical pathway, TMEPAI promotes activation of PI3K/Akt signaling, which leads to cell proliferation by suppressing PTEN (Phosphatase and Tensin Homolog). The chain of events resulted in increased tumor proliferation in a xenograft model of triple-negative breast cancer.6
Triple-negative breast cancer (TNBC) is a highly aggressive type of breast cancer lacking estrogen receptor (ER), progesterone receptor (PR) and human epidermal growth factor2 (HER2), which resulted in the lack of target for treatment.14 Up to date, drug treatment for triple-negative breast cancer still limited to cytotoxic agents such as taxanes (paclitaxel), anthracycline (doxorubicin) and platinum (cisplatin).15,16 Recently, androgen antagonist such as bicalutamide or enzalutamide is also used in TNBC, since about 21% of TNBC patients have a high expression of androgen receptor.17,18 Unfortunately, the use of cytotoxics and androgen antagonist leads to resistance problems in a short period, followed by disease relapse and metastatic.14,15,19,20 To the best of our knowledge, resistance of cancer cells to drug treatment is eventually unavoidable. Therefore, a profound understanding of the mechanism of drug resistance is needed to improve the treatment regimen in TNBC.
In our effort to understand the mechanism of anticancer resistance in triple-negative breast cancer, we highlighted TMEPAI as our protein of interest. TMEPAI was shown to be involved in the development of lung cancer resistance to anticancer.21 While another study in triple-negative breast cancer reported that amplified TMEPAI caused increased expression of Snail, in favor of cells’ metastatic behavior.22,23 We hypothesized that TMEPAI might be related to how triple-negative breast cancer cells respond to anticancer. The present study was aimed to investigate the role of TMEPAI to the triple-negative breast cancer cells’ sensitivity to several anticancer.
Materials and Methods
Cell Culture
BT549, a mesenchymal subtype of triple-negative breast cancer cell line, and HEK293T, a human embryonic kidney cell line, were obtained from ATCC or Riken BioResource center. Both cell lines were cultured in Dulbecco’s modified Eagle medium (DMEM, Invitrogen) supplemented with 10% Fetal Bovine Serum (FBS, Gibco), 10 µg/mL insulin, 100 units/mL of penicillin G and 0.1 mg/mL of streptomycin sulfate (Wako).
Plasmid Construction
Six gRNA against the region surrounding TMEPAI exon4 cording locus were designed in silico using CRISPR Design Online Tool (https://crispr.dbcls.jp). Then, the two sets of oligonucleotide primers were designed as forward:
TTTCTTGGCTTTATATATCTTGTGGAAAGGACGAAACACCGnnnnnnnnnnnnnnnnnnn and reverse:
GACTAGCCTTATTTTAACTTGCTATTTCTAGCTCTAAAACnnnnnnnnnnnnnnnnnnnC.
The sequence, which is mentioned as “nnn” was replaced by the designed each gRNA sequence. Those complete oligonucleotides for gRNA are shown in Table 1.
Table 1.
Oligonucleotide for gRNA
| gRNA#1 | Forward | TTTCTTGGCTTTATATATCTTGTGGAAAGGACGAAACACCGGAGGCAGATGATCTCCGGCA |
| Reverse | GACTAGCCTTATTTTAACTTGCTATTTCTAGCTCTAAAACTGCCGGAGATCATCTGCTCC | |
| gRNA#2 | Forward | TTTCTTGGCTTTATATATCTTGTGGAAAGGACGAAACACCGCCAGACCTGATCCCGCGG |
| Reverse | GACTAGCCTTATTTTAACTTGCTATTTCTAGCTCTAAAACCCGCGGGATCAGGCTCTGGC | |
| gRNA#3 | Forward | TTTCTTGGCTTTATATATCTTGTGGAAAGGACGAAACACCGCCAGAAGCGATCCTGAGAC |
| Reverse | GACTAGCCTTATTTTAACTTGCTATTTCTAGCTCTAAAACGTCTCAGGATCGCTTCTGGC | |
| gRNA#4 | Forward | TTCTTGGCTTTATATATCTTGTGGAAAGGACGAAACACCGGCGCAGCAACGCATCGTG |
| Reverse | GACTAGCCTTATTTTAACTTGCTATTTCTAGCTCTAAAACCACGATGCGTTGCTGCGCCC | |
| gRNA#5 | Forward | TTTCTTGGCTTTATATATCTTGTGGAAAGGACGAAACACCGAGAGAAACTGTATGTGCGA |
| Reverse | GACTAGCCTTATTTTAACTTGCTATTTCTAGCTCTAAAACTCGCACATACAGTTTCTCTC | |
| gRNA#6 | Forward | TTTCTTGGCTTTATATATCTTGTGGAAAGGACGAAACACCGGGTGCGGTAACTGCCGCAC |
| Reverse | GACTAGCCTTATTTTAACTTGCTATTTCTAGCTCTAAAACGTGCGGCAGTTACCGCACCC |
Abbreviations: TMEPAI, Transmembrane Prostate Androgen-Induced; TNBC, triple-negative breast cancer; CC50, cytotoxic concentration 50%; KO, knock-out; WT, wild-type.
Forward and reverse oligonucleotide was annealed using standard method followed by extension using PrimeSTAR DNA polymerase (Takara Bio). Extended 100bps of double-strand DNA was purified using the PCR purification kit (Qiagen) and inserted into Afl II-cleaved-gRNA_Cloning Vector (Addgene plasmid #41824), which was a gift from Dr. George Church, using Gibson assembly system (Biolabs).
Cleavage Efficiency Test
Since each gRNA gives different activities on the DNA target site, we checked the cleavage efficiency of each gRNA using EGxxFP system.24 First, three DNA target sites, named target #1-#3, were amplified by PCR and inserted into pCAG-EGxxFP vector which is a gift from Dr. Masahito Ikawa. Target #1 contains target sequences of gRNA#1, #2, and #3. Target #2 contains target sequences of gRNA#4 and #5. Target#3 contains target sequences of gRNA#6. In order to test the gRNAs efficiency, 1 x 105 HEK293T cells were plated on a 6-well microplate in 2 mL complete medium. After 24 hrs, these cells were transfected with 500 ng of each pCAG-EGxxFP vectors including target gRNA sequence, 500 ng of each related gRNA expression vectors, and 500 ng hCas9 plasmids (Addgene #41815, a gift from Dr. George Church) plasmids using a PEI (polyethyleneimine) transfection reagent. In brief, after successful DNA cleavage occurred by Crispr-Cas9 on target site in EGxxFP vector, the full-length EGFP sequence could be restored and generate EGFP protein. So, we performed Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and Western blotting using the lysates of transfected cells to check the quantification of EGFP protein. Negative control used in this experiment was transfection using a plasmid mix without gRNA expression vectors. Normal cells were used as untransfected cells.
Establishment of TMEPAI Knock-Out (KO) Cells
We deliver two best gRNA combination (300 ng each gRNA cloning vector for #3 and #5), 500 ng pCas9_GFP plasmid (Addgene #44719 was a gift from Kiran Musunuru), and 300 ng Puromycin resistant plasmid using PEI transfection reagent in TNBC cell line, BT-549. Transfected cells were selected by Puromycin 1 µg/mL for 3 days after 24 hrs following transfection, and each transfectant was cloned.
DNA Isolation and PCR
Cells were lysed using cell 500 µL lysis buffer (50 mM Tris pH 8.0, 100 mM NaCl, 100 mM EDTA pH 8.0, 1% SDS) which is added 20 µL proteinase K 20 mg/mL immediately before use. After 60°C overnight incubation, lysates were treated with 167 µL of 5M NaCl to precipitate protein and lipid. After centrifugation, the supernatant was collected into a new tube and mixed with an equal volume of isopropanol to precipitate nucleotide. Precipitated nucleotide was washed with 70% EtOH then solved in TE buffer (10 mM Tris-HCl pH 8.0, 1 mM EDTA). PCR was performed using ExTaq (Takara Bio) by GeneAmp®PCR System 9700 (Applied Biosystems). Primers were designed as follows: 5ʹ-TCTCTGCTCTCTGCACACGG-3ʹ (forward: F1), 5ʹ- GCCTGGCACTATCCATCAGG-3ʹ (reverse-1: R1), and 5ʹ-TGACTTGGCACTCAAAAATTGCC-3ʹ (reverse-2: R2).
SDS-PAGE and Western Blotting
Cells were collected with Tris buffer pH 7.5 then lysed with SDS-sample buffer (4% SDS, 20% glycerol, 1.44 M β-mercaptoethanol, 125 mM Tris pH 7.4, and 0.01% bromophenol blue) in equal volume ratio. SDS-PAGE was done on cell lysates using the method as mentioned previously.25 The primary antibodies were rabbit anti-TMEPAI antibodies (homemade, 1:250),4 rabbit anti-GFP antibodies (Santa Cruz Biotechnology, 1:2000), and mouse anti-β-actin antibody (Sigma, 1:5000). Primary antibodies were bounded with horseradish peroxidase-conjugated anti-rabbit IgG (GE Healthcare, 1:10,000) and anti-mouse IgG (GE Healthcare, 1:10,000). Reacted antibodies were detected by chemiluminescence using enhanced chemiluminescent reagent, Immuno Star Zeta (Wako) and EZ capture MG (ATTO).
Inhibitory Concentration
Inhibitory concentration was measured based on cell viability using MTS [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium] assay by following the protocol in CellTiter96® Aqueous One Solution Cell Proliferation Assay (Promega). Cells (1×104) were seed in 96-microplate and grown until about 70% confluency. Afterward, we cultured cells with DMEM supplemented with 1% FBS medium and 2 ng/mL TGF-β, simultaneously with anticancer drugs. After 24 hrs of incubation, we performed an MTS assay. Doxorubicin, paclitaxel, cisplatin, and bicalutamide (Wako) were dissolved in DMSO and were diluted until the required concentrations. DMSO used in final concentrations were not exceeding 0.01%. DMSO was used as a control in this experiment. IC50 of the three drugs was calculated from extrapolation from the log concentration of drugs VS cell viability equations. The experiments were done three times in duplicate.
Results
Selection of gRNA for Knocking Out of TMEPAI Gene
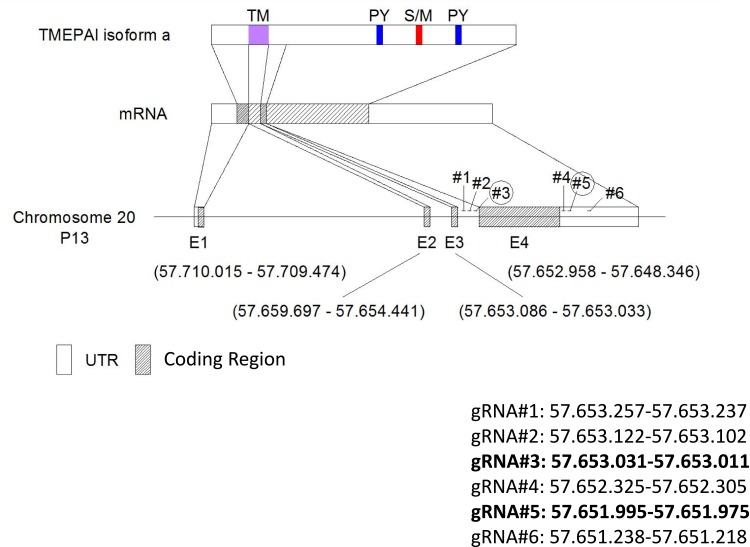
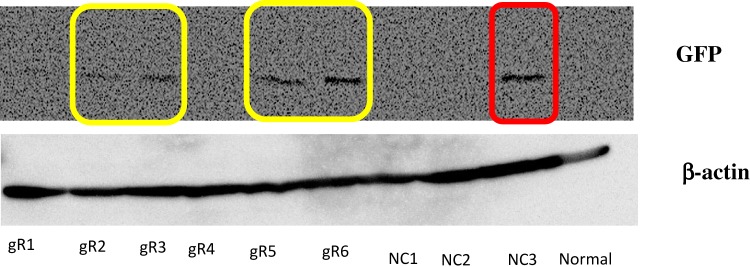
We designed six gRNAs to cut out the exon 4 of TMEPAI gene in genomic DNA (Figure 1) and first performed cleavage efficiency test of each gRNA using EGxxFP system which indicates cleavage efficiency as showing expression of EGFP protein. As a result, the gRNA #2, #3, #5 and #6 transfected cells showed induction of EGFP (as shown in the yellow box in Figure 2). Unfortunately, the negative control for gRNA#6 (NC3; red box in Figure 2) gave a clear EGFP band. Hence, we decided to use gRNA #2 and #3, targeting upstream of TMEPAI exon 4, and gRNA#5, targeting 3ʹ UTR of TMEPAI gene, for a subsequent experiment.
Figure 1.
Schematic targeted sequence of TMEPAI. CRISPR-Cas9 mediated gene editing using two designed gRNA for completely removing exon 4 of TMEPAI gene. We design 3 gRNA before exon 4 (gRNA#1-#3) and 3 after stop codon (gRNA#4-#6).
Abbreviations: UTR, untranslated region; gRNA, guide-RNA.
Figure 2.
Cleavage activity test was performed in HEK 293T cells. Cells were transfected by gRNA, human-cas9, and checking vector except negative control and normal cells. Negative controls were transfected by human-cas9 and checking vector. Normal cells were not transfected-cells. The gRNA worked when human-cas9 can cleavage the target sequences, then the cells could express green fluorescence protein (GFP). GFP was detected for checking cleavage test by Western blot analysis. β-Actin was used as a loading control. Yellow box: expected GFP band which showed that gRNA worked well; red box: unexpected GFP band.
Abbreviations: gR, guide RNA; NC, negative control.
Establish Knocked-Out TMEPAI in TNBC Cell Line Using the CRISPR-Cas9 System
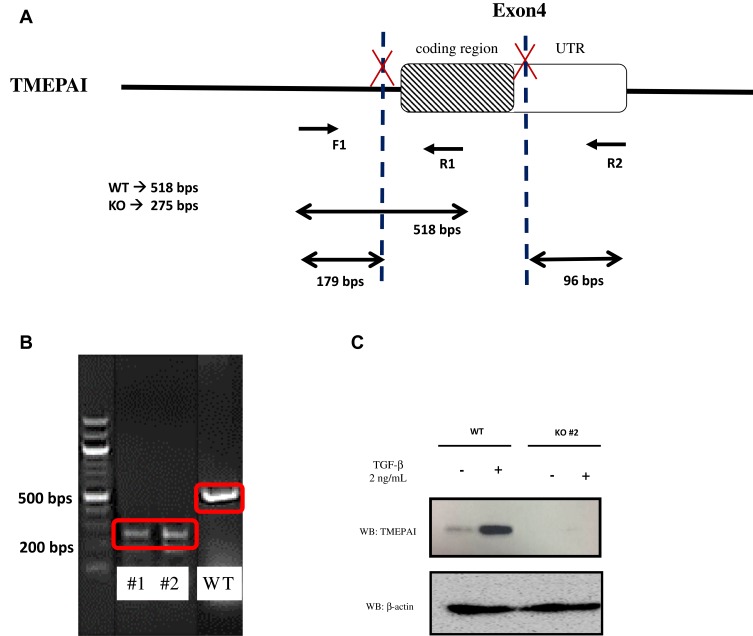
We used two best combinations of gRNA (gRNA#2 and #5; gRNA#3 and #5) to cut out TMEPAI exon 4 from the genomic DNA in TNBC cell line, BT-549. After selecting transfected cells with Puromycin for 72 hrs, we isolated clones and then performed the genotyping analysis by PCR using the primer as shown in Figure 3A. The result suggested that clone #1 and #2 showed only the PCR product from KO allele which lacks exon 4 cording region of TMEPAI gene, unlike the PCR product from wild type (WT) cells (Figure 3B). Furthermore, we checked the protein expression of TMEPAI using Western blot analysis. According to previous reports, TMEPAI was induced by TGF-β stimulation. Thus, we stimulated cells with TGF-β in our system. As expected, TMEPAI KO cells do not express TMEPAI protein even after stimulation of TGF-β (Figure 3C). Our data indicate that we successfully establish the TMEPAI KO TNBC cell line using the CRISPR-Cas9 system. However, we successfully obtained cloned cells eventually which had been edited by gRNA#3 and #5.
Figure 3.
Knock-out TMEPAI in the TNBC cell line using the CRISPR-Cas9 system. (A) The scheme of primers designed to detect WT and KO clone. (B) Genotyping of KO cells using PCR. (C) The expression of TMEPAI in WT and KO BT549 cells, as shown by Western blot analysis after 2 ng/mL TGF-β stimulation for 8 hrs, β-actin was used as a loading control.
Abbreviation: WT, wildtype; KO, knock-out (clone was shown as #1 and #2); UTR, untranslated region.
TMEPAI KO Cells Shows Higher Sensitivity for Doxorubicin and Paclitaxel
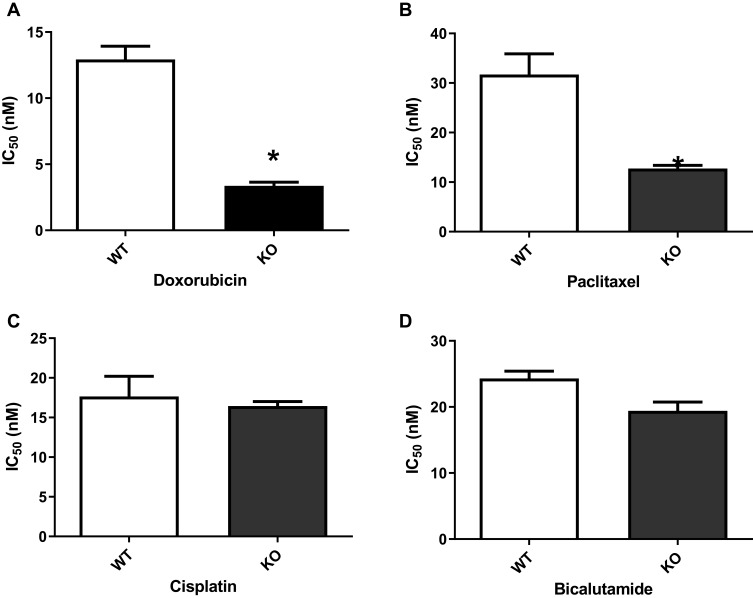
TMEPAI is known to be implicated in many intracellular signaling and following cellular events such as cell proliferation, migration and EMT. Collaterally, these signaling pathways and cellular events are also well known to be involved in drug resistance in cancer cells. So, we investigated the anti-cancer drug sensitivity in TMEPAI KO cells. Our results indicated that knock-out of TMEPAI significantly decreased IC50 for doxorubicin and paclitaxel compared with WT parental cells. However, there are no significant changes in IC50 for cisplatin and bicalutamide in WT and TMEPAI-KO cells (Figure 4).
Figure 4.
TGF-β induced TMEPAI WT showed less response as compared to TMEPAI-KO cells to (A) Doxorubicin, (B) Paclitaxel, (C) Cisplatin, (D) Bicalutamide. BT549 WT and TMEPAI-KO cells’ response to treatment was determined by IC50 after 24 hrs of anti-cancer treatment and TGF-β induction. All experiments were done three-time in duplicate. Statistical analyses were performed using the independent t-test. *p<0.05 compared to WT.
Abbreviation: WT, wild type; KO, knock-out; IC, inhibitory concentration.
Discussion
Triple-negative breast cancer (TNBC) is a type of breast cancer with refractory characteristics. Limited choice of treatment is available for TNBC, which includes cytotoxic drugs such as doxorubicin, paclitaxel, or cisplatin. Recently, bicalutamide, an androgen receptor antagonist, is also given as part of TNBC treatment.14 The previous study showed that 69% TNBC patients have TMEPAI overexpression, which correlates with poor prognosis and survival.6 In addition to having tumorigenic activity,4 TMEPAI is reported to act as a converter for TGF-β in TNBC, from tumor promotor to tumor suppressor, via its activity in PI3K/Akt signaling.6 PI3K/Akt pathway is also crucial to the anticancer mechanism to inhibit cell proliferation and survival.26 Moreover, TMEPAI is both a direct target gene and a negative regulator in TGF-β signaling.4,11 TMEPAI is also shown to trigger EMT and increase metastasis in lung and breast cancer.27,28 Based on the above findings, we presumed that TGF-β induced TMEPAI might play a critical point in determining the response of cells to anticancer.
In this study, we used BT-549, a mesenchymal subtype of TNBC cell line. Lehman et al stated that the most appropriate subtype to study TNBC in relation to TGF-β signaling is a mesenchymal subtype.29 Moreover, Tseng et al30 reported the most common subtype of TNBC in the Taiwanese population is mesenchymal-like, while in Taiwanese population is mesenchymal type. Therefore, we chose mesenchymal TNBC cell line in our experiments. One of the available TNBC of mesenchymal subtypes is BT-549.29,31
We developed a knock-out cell line using CRISPR-Cas9 gene editing. We designed two combinations of sgRNA to entirely remove exon 4 of TMEPAI in the TNBC cell line, BT549. The previous study demonstrated that TMEPAI has two relevant domains, PY domain, and SIM domain, located in the cytosolic part of the protein. PY domain is the part of TMEPAI, which interacts with NEDD4 to increase PTEN degradation. As a result, PTEN fails to regulate the PI3K/Akt pathway, so that this pathway continues to be activated in TNBC.6 While the SIM domain interacts antagonistically with SARA (Smad Anchor for Receptor Activation), thus inhibiting the activation of the Smad complex. Consequently, TMEPAI acts as a negative regulator in the Smad-dependent TGF-β signaling pathway.11 Both domains are encoded in Exon 4 of the TMEPAI gene.6,11 Therefore, we target exon 4 to establish TMEPAI knock-out cells.
In order to investigate the role of TMEPAI in TNBC, we performed gene editing of TEMPAI. Exon 4 of TMEPAI gene was removed entirely using the CRISPR-Cas9 system, using a pair of sgRNA. One sgRNA was designed ahead of exon 4 and the other one after the stop codon. Afterward, we tested the efficiency of sgRNA cutting on HEK293-T cells. The efficiency result showed that our sgRNA removed all exon 4. After application in TNBC, BT549, we successfully obtained cell clones that do not have exon 4. Another study was done by Abdelaziz et al also applied the CRISPR-Cas9 system to cut TMEPAI in exon 2 in TNBC, MDA-MB-231.28
It is uncertain how exon difference might affect the phenotype result after TMEPAI gene editing. Besides, the off-target effect after gene editing could not be observed. Rodriguez et al,32 mentioned that several bioinformatic strategies and modifications of cas9 into nickase-cas9 are developed to minimize off target cuts and increase on-target specificity. We used a software that was developed by Naito et al,33 to minimize it. However, we performed Western blot for TMEPAI repetitively and got a constant band.
Amalia et al used the KO cell clone that was edited at exon 2 and exon 4 of TMEPAI using CRISPR-Cas9 on TNBC cells.34 Amalia et al reported that Wnt signaling was increased without being affected by the removed exons. Multiple studies done by Watanabe et al,11 Vo Nguyen et al,4 Singha et al,6 and Lou et al21 have also demonstrated TMEPAI gene silencing using shRNA to knockdown TMEPAI expression on breast cancer and lung cancer cells. However, gene silencing using shRNA is temporal, while CRISPR-Cas9 allows stable mutant cell clones.
Modulation of TMEPAI expression was carried out to investigate the role of TMEPAI in carcinogenesis,4,11 both stemness, and angiogenesis.21,28,34 However, limited data were available on the role of TMEPAI on the response of TNBC to anticancer. Lou et al have reported that in TMEPAI knocked down cells, there was increased sensitivity of cancer cells to several chemotherapeutics.21
Our selection of drugs were based on the latest available guideline for TNBC treatment, which is anthracycline-based (doxorubicin, liposomal doxorubicin), or taxane-based (paclitaxel) or platinum-based chemotherapy.35 Although antiandrogen (bicalutamide) is not standard therapy for TNBC, this drug showed promising results in TNBC patients and currently is in the clinical trial stage.18,36,37
In our previous study, we reported that in TNBC-KO, a higher dose of doxorubicin, paclitaxel, and cisplatin as compared to wild type, which is in contrast with our present result.38 TMEPAI-KO cells showed decreased sensitivity to several chemotherapy drugs. In this system, TGF-β was not used to induce TMEPAI. Therefore, the effect of TMEPAI was not clearly demonstrated. Singha et al,6 Watanabe et al,11 Azami et al,10 Vo Nguyen et al,4 and Xu et al39 showed that TMEPAI needs to be induced by TGF-β, EGF, or DHT.
The difference with our present experimental design, TGF-β was not used to increase TMEPAI expression. In the absence of TGF-β, wild-type TNBC cells were more sensitive to cytotoxic than the KO-cells, followed by a decrease in drug efflux transporters, P-gp, and BCRP expressions.38 Therefore, we suggest that the effect of TMEPAI on the change in cell response to anticancer is highly dependent on TGF-β.
In the present study, we add TGF-β to increase TMEPAI expression (Figure 1E). This time, we demonstrated that in TGF-β induced TMEPAI in TNBC, a higher dose of doxorubicin and paclitaxel is needed to suppress cell proliferation. We also showed that TGF-β induced TMEPAI to have a minimum effect on the response of cells to cisplatin and bicalutamide.
Doxorubicin, paclitaxel, and cisplatin are the backbone of TNBC treatment. Doxorubicin is used in about 78% TNBC patients, while paclitaxel in 49% of the patients.40 The three drugs are general cytotoxic with an almost similar mechanism of action. The drugs kill cancer cells by inducing DNA damage, promoting apoptosis, and disrupting cell cycle.41–43 However, the effect of TGF-β induced TMEPAI is more pronounced when TNBC is treated with doxorubicin and paclitaxel. Doxorubicin is known to kill cancer cells through its intercalation with DNA, disruption of topoisomerase II, and increase free radicals.44 While paclitaxel acts as an anticancer by inducing mitotic arrest that leads to cell death.42 PY domain in TMEPAI gene interacts with the WW NEDD4 domain which leads to PTEN degradation followed by continuous activation of PI3K/Akt.6 Through that mechanism, high TMEPAI expressions (induced by TGF-β) inhibit cyclin-dependent kinase (CDKIs), p27 and p21,6,11,45 so that cell cycle can continue, thus, attenuating the effect of doxorubicin and paclitaxel to TNBC.46
In agreement with our result, Luo et al have also reported that TMEPAI depletion increases cancer cell sensitivity to cytostatic (doxorubicin, paclitaxel, mitomycin, and chloroquine). Lou et al use luminal breast cancer cells, lung cancer cells, and gastric cancer cells. Lou et al showed that in TMEPAI knock-down cell model, reduce autophagosome formation followed by inhibition of autophagy and suppression of beclin-1 expression.21 Cancer cells can eliminate the damage caused by cytostatic via the autophagic process.42,44 Inhibition of autophagy in TMEPAI knock-down cells then promotes an increased toe the response of cancer cells to the cytostatics.21
Our result demonstrates the lack of TGF-β induced TMEPAI on the TNBC response to cisplatin. This might be due to the overexpression of Islet-1 in TNBC patients.47 Islet-1 overexpression was reported to reduce TNBC cell response to cisplatin.47,48 In the cells that have limited response to cisplatin, the role of TMEPAI is not prominent.
TGF-β induced TMEPAI is also not affecting the response of TNBC to bicalutamide. Bicalutamide is recently used in the treatment of TNBC with positive expression of the androgen receptor. Bicalutamide is an androgen antagonist, that exerts its effect by inhibiting β-catenin and androgen receptor, thus suppressing tumor growth in TNBC.49 Other than TGF-β, androgen also regulates TMEPAI in prostate cancer.2 In the present study, we use TGF-β to induce TMEPAI, so we assume that the role of TMEPAI on androgen signaling pathway is not apparent. As our previous findings, when we did not induce TGF-β, we could not get a confident result on the cell’s response to drugs.38
We are aware that we only use one cell line in our experiment, which is a mesenchymal type of TNBC. Hence, our results need to be confirmed in another subtype of TNBC.
Conclusion
TGF-β induced TMEPAI attenuates the response of mesenchymal type TNBC to doxorubicin and paclitaxel, but a minimal effect on cisplatin and bicalutamide. This is an initial finding to continue a more comprehensive research of TMEPAI as a marker or target therapy in TNBC.
Acknowledgment
This research was supported by QQ Research Grant from Directorate for Research and Community Engagement, Universitas Indonesia.
Disclosure
All the authors declare no conflicts of interest in this work.
References
- 1.Wu G, Chen Y-G, Ozdamar B, et al. Structural basis of Smad2 recognition by the Smad anchor for receptor activation. Science. 2000;287(5450):92–97. doi: 10.1126/science.287.5450.92 [DOI] [PubMed] [Google Scholar]
- 2.Xu LL, Shanmugam N, Segawa T, et al. A novel androgen-regulated gene, PMEPA1, located on chromosome 20q13 exhibits high level expression in prostate. Genomics. 2000;66(3):257–263. doi: 10.1006/geno.2000.6214 [DOI] [PubMed] [Google Scholar]
- 3.Xu LL, Shi Y, Petrovics G, et al. PMEPA1, an androgen-regulated NEDD4-binding protein, exhibits cell growth inhibitory function and decreased expression during prostate cancer progression. Cancer Res. 2003;63(15):4299–4304. [PubMed] [Google Scholar]
- 4.Vo Nguyen TT, Watanabe Y, Shiba A, Noguchi M, Itoh S, Kato M. TMEPAI/PMEPA1 enhances tumorigenic activities in lung cancer cells. Cancer Sci. 2014;105(3):334–341. doi: 10.1111/cas.12355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tanner MM, Tirkkonen M, Kallioniemi A, et al. Increased copy number at 20q13 in breast cancer: defining the critical region and exclusion of candidate genes. Cancer Res. 1994;54(16):4257–4260. [PubMed] [Google Scholar]
- 6.Singha PK, Pandeswara S, Geng H, Lan R, Venkatachalam MA, Saikumar P. TGF-β induced TMEPAI/PMEPA1 inhibits canonical Smad signaling through R-Smad sequestration and promotes non-canonical PI3K/Akt signaling by reducing PTEN in triple negative breast cancer. Genes Cancer. 2014;5(9–10):320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brunschwig EB, Wilson K, Mack D, et al. PMEPA1, a transforming growth factor-β-induced marker of terminal colonocyte differentiation whose expression is maintained in primary and metastatic colon cancer. Cancer Res. 2003;63(7):1568–1575. [PubMed] [Google Scholar]
- 8.Ishkanian AS, Mallof CA, Ho J, et al. High‐resolution array CGH identifies novel regions of genomic alteration in intermediate‐risk prostate cancer. Prostate. 2009;69(10):1091–1100. doi: 10.1002/pros.20959 [DOI] [PubMed] [Google Scholar]
- 9.Rae FK, Hooper JD, Nicol DL, Clements JA. Characterization of a novel gene, STAG1/PMEPA1, upregulated in renal cell carcinoma and other solid tumors. Mol Carcinog. 2001;32(1):44–53. doi: 10.1002/(ISSN)1098-2744 [DOI] [PubMed] [Google Scholar]
- 10.Azami S, Nguyen TTV, Watanabe Y, Kato M. Cooperative induction of transmembrane prostate androgen induced protein TMEPAI/PMEPA1 by transforming growth factor-β and epidermal growth factor signaling. Biochem Biophys Res Commun. 2015;456(2):580–585. doi: 10.1016/j.bbrc.2014.11.107 [DOI] [PubMed] [Google Scholar]
- 11.Watanabe Y, Itoh S, Goto T, et al. TMEPAI, a transmembrane TGF-β-inducible protein, sequesters Smad proteins from active participation in TGF-β signaling. Mol Cell. 2010;37(1):123–134. doi: 10.1016/j.molcel.2009.10.028 [DOI] [PubMed] [Google Scholar]
- 12.Bai X, Jing L, Li Y, et al. TMEPAI inhibits TGF-β signaling by promoting lysosome degradation of TGF-β receptor and contributes to lung cancer development. Cell Signal. 2014;26(9):2030–2039. doi: 10.1016/j.cellsig.2014.06.001 [DOI] [PubMed] [Google Scholar]
- 13.Singha PK, Yeh I-T, Venkatachalam MA, Saikumar P. Transforming growth factor-β (TGF-β)–inducible gene TMEPAI converts TGF-β from a tumor suppressor to a tumor promoter in breast cancer. Cancer Res. 2010;70(15):6377–6383. doi: 10.1158/0008-5472.CAN-10-1180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sharma P. Biology and management of patients with triple-negative breast cancer. Oncologist. 2016;21(9):1050–1062. doi: 10.1634/theoncologist.2016-0067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anders CK, Abramson V, Tan T, Dent R. The evolution of triple-negative breast cancer: from biology to novel therapeutics. Am Soc Clin Oncol Educ Book. 2016;36:34–42. doi: 10.14694/EDBK_159135 [DOI] [PubMed] [Google Scholar]
- 16.Hudis CA, Gianni L. Triple-negative breast cancer: an unmet medical need. Oncologist. 2011;16(Supplement 1):1–11. doi: 10.1634/theoncologist.2011-S1-01 [DOI] [PubMed] [Google Scholar]
- 17.Liu Y-X, Zhang K-J, Tang -L-L. Clinical significance of androgen receptor expression in triple negative breast cancer-an immunohistochemistry study. Oncol Lett. 2018;15(6):10008–10016. doi: 10.3892/ol.2018.8548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mina A, Yoder R, Sharma P. Targeting the androgen receptor in triple-negative breast cancer: current perspectives. OncoTargets Ther. 2017;10:4675. doi: 10.2147/OTT [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giovannelli P, Di Donato M, Galasso G, Di Zazzo E, Bilancio A, Migliaccio A. The androgen receptor in breast cancer. Front Endocrinol. 2018;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harvell DM, Spoelstra NS, Singh M, et al. Molecular signatures of neoadjuvant endocrine therapy for breast cancer: characteristics of response or intrinsic resistance. Breast Cancer Res Treat. 2008;112(3):475. doi: 10.1007/s10549-008-9897-4 [DOI] [PubMed] [Google Scholar]
- 21.Luo S, Yang M, Lv D, et al. TMEPAI increases lysosome stability and promotes autophagy. Int J Biochem Cell Biol. 2016;76:98–106. doi: 10.1016/j.biocel.2016.05.004 [DOI] [PubMed] [Google Scholar]
- 22.Zheng X, Carstens JL, Kim J, et al. Epithelial-to-mesenchymal transition is dispensable for metastasis but induces chemoresistance in pancreatic cancer. Nature. 2015;527(7579):525. doi: 10.1038/nature16064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fischer KR, Durrans A, Lee S, et al. Epithelial-to-mesenchymal transition is not required for lung metastasis but contributes to chemoresistance. Nature. 2015;527(7579):472. doi: 10.1038/nature15748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mashiko D, Fujihara Y, Satouh Y, Miyata H, Isotani A, Ikawa M. Generation of mutant mice by pronuclear injection of circular plasmid expressing Cas9 and single guided RNA. Sci Rep. 2013;3:3355. doi: 10.1038/srep03355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wardhani BWK, Puteri MU, Watanabe Y, Louisa M, Setiabudy R, Kato M. TMEPAI genome editing in triple negative breast cancer cells. Med J Indones. 2017;26(1):14–18. doi: 10.13181/mji.v26i1.1871 [DOI] [Google Scholar]
- 26.Lee A, Djamgoz MB. Triple negative breast cancer: emerging therapeutic modalities and novel combination therapies. Cancer Treat Rev. 2018;62:110–122. doi: 10.1016/j.ctrv.2017.11.003 [DOI] [PubMed] [Google Scholar]
- 27.Hu Y, He K, Wang D, et al. TMEPAI regulates EMT in lung cancer cells by modulating the ROS and IRS-1 signaling pathways. Carcinogenesis. 2013;34(8):1764–1772. doi: 10.1093/carcin/bgt132 [DOI] [PubMed] [Google Scholar]
- 28.Abdelaziz M, Watanabe Y, Kato M. PMEPA1/TMEPAI knockout impairs tumour growth and lung metastasis in MDA-MB-231 cells without changing monolayer culture cell growth. J Biochem. 2019;165(5):411–414. doi: 10.1093/jb/mvz022 [DOI] [PubMed] [Google Scholar]
- 29.Lehmann BD, Bauer JA, Chen X, et al. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest. 2011;121(7):2750–2767. doi: 10.1172/JCI45014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tseng L-M, Chiu J-H, Liu C-Y, et al. A comparison of the molecular subtypes of triple-negative breast cancer among non-Asian and Taiwanese women. Breast Cancer Res Treat. 2017;163(2):241–254. doi: 10.1007/s10549-017-4195-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dai X, Cheng H, Bai Z, Li J. Breast cancer cell line classification and its relevance with breast tumor subtyping. J Cancer. 2017;8(16):3131. doi: 10.7150/jca.18457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rodríguez‑Rodríguez DR, Ramírez‑Solís R, Garza‑Elizondo MA, Garza‑Rodríguez MDL, Barrera‑Saldaña HA. Genome editing: a perspective on the application of CRISPR/Cas9 to study human diseases. Int J Mol Med. 2019;43(4):1559–1574. doi: 10.3892/ijmm.2019.4112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Naito Y, Hino K, Bono H, Ui-Tei K. CRISPRdirect: software for designing CRISPR/Cas guide RNA with reduced off-target sites. Bioinformatics. 2014;31(7):1120–1123. doi: 10.1093/bioinformatics/btu743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Amalia R, Abdelaziz M, Puteri MU, et al. TMEPAI/PMEPA1 inhibits Wnt signaling by regulating β-catenin stability and nuclear accumulation in triple negative breast cancer cells. Cell Signal. 2019;59:24–33. doi: 10.1016/j.cellsig.2019.03.016 [DOI] [PubMed] [Google Scholar]
- 35.NCCN. National comprehensive cancer network guidelines: breast cancer version 1; March 14, 2019. Available from: www.nccn.org/professionals/physicians_gls/pdf.
- 36.Mehanna J, Haddad FG, Eid R, Lambertini M, Kourie HR. Triple-negative breast cancer: current perspective on the evolving therapeutic landscape. Int J Women’s Health. 2019;11:431. doi: 10.2147/IJWH.S178349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barton VN, Gordon MA, Richer JK, Elias A. Anti-androgen therapy in triple-negative breast cancer. Ther Adv Med Oncol. 2016;8(4):305–308. doi: 10.1177/1758834016646735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wardhani BWK, Puteri MU, Watanabe Y, Louisa M, Setiabudy R, Kato M. Decreased sensitivity of several anticancer drugs in TMEPAI knockout triple-negative breast cancer cells. Med J Indonesia. 2019;28(2):110–115. doi: 10.13181/mji.v28i2.2687 [DOI] [Google Scholar]
- 39.Xu Y, Chen S-Y, Ross KN, Balk SP. Androgens induce prostate cancer cell proliferation through mammalian target of rapamycin activation and post-transcriptional increases in cyclin D proteins. Cancer Res. 2006;66(15):7783–7792. doi: 10.1158/0008-5472.CAN-05-4472 [DOI] [PubMed] [Google Scholar]
- 40.James M, Dixit A, Robinson B, Frampton C, Davey V. Outcomes for patients with non-metastatic triple-negative breast cancer in New Zealand. Clin Oncol. 2019;31(1):17–24. doi: 10.1016/j.clon.2018.09.006 [DOI] [PubMed] [Google Scholar]
- 41.Dasari S, Tchounwou PB. Cisplatin in cancer therapy: molecular mechanisms of action. Eur J Pharmacol. 2014;740:364–378. doi: 10.1016/j.ejphar.2014.07.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weaver BA. How Taxol/paclitaxel kills cancer cells. Mol Biol Cell. 2014;25(18):2677–2681. doi: 10.1091/mbc.e14-04-0916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lovitt CJ, Shelper TB, Avery VM. Doxorubicin resistance in breast cancer cells is mediated by extracellular matrix proteins. BMC Cancer. 2018;18(1):41. doi: 10.1186/s12885-017-3953-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tacar O, Sriamornsak P, Dass CR. Doxorubicin: an update on anticancer molecular action, toxicity and novel drug delivery systems. J Pharm Pharmacol. 2013;65(2):157–170. doi: 10.1111/j.2042-7158.2012.01567.x [DOI] [PubMed] [Google Scholar]
- 45.Singha PK, Pandeswara S, Geng H, et al. Increased Smad3 and reduced Smad2 levels mediate the functional switch of TGF-β from growth suppressor to growth and metastasis promoter through TMEPAI/PMEPA1 in triple negative breast cancer. Genes Cancer. 2019;10:5–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zheng K, He Z, Kitazato K, Wang Y. Selective autophagy regulates cell cycle in cancer therapy. Theranostics. 2019;9(1):104. doi: 10.7150/thno.30308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li L, Sun F, Chen X, Zhang M. ISL1 is upregulated in breast cancer and promotes cell proliferation, invasion, and angiogenesis. OncoTargets Ther. 2018;11:781. doi: 10.2147/OTT [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang Y, Wang L, Gao P, et al. ISL1 promotes cancer progression and inhibits cisplatin sensitivity in triple-negative breast cancer cells. Int J Mol Med. 2018;42(5):2343–2352. doi: 10.3892/ijmm.2018.3842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huang R, Han J, Liang X, et al. Androgen receptor expression and bicalutamide antagonize androgen receptor inhibit β-catenin transcription complex in estrogen receptor-negative breast cancer. Cell Physiol Biochem. 2017;43(6):2212–2225. doi: 10.1159/000484300 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- NCCN. National comprehensive cancer network guidelines: breast cancer version 1; March 14, 2019. Available from: www.nccn.org/professionals/physicians_gls/pdf.