Abstract
Nightmares are a highly prevalent and distressing feature of post-traumatic stress disorder (PTSD). Previous studies have reached mixed conclusions regarding the effects of prazosin on nightmares, sleep quality and overall PTSD symptoms in patients with PTSD. MEDLINE, EMBASE, all EBM databases, PsycIFNO and CINAHL were systematically searched from inception date to October 2018 for randomized clinical trials that included reporting of nightmares, sleep quality or overall PTSD symptoms. The analysis included data from eight trials involving 286 PTSD patients in the prazosin group and 289 PTSD patients in the placebo group. In our meta-analysis, prazosin resulted in a statistically significant improvement in nightmares (standardized mean difference (SMD) = −1.13, 95% confidence interval (CI) = −1.91 to −0.36), but was not more beneficial than placebo for overall PTSD symptoms (SMD = −0.45, 95% CI = −0.95 to 0.05) and sleep quality (SMD = −0.44, 95%CI = −1.44 to 0.55). In terms of acceptability, there was no significant difference between the prazosin group and the placebo group with respect to discontinuation for all causes (odds ratio (OR) = 1.00, 95% CI = 0.62–1.62). In conclusion, the use of prazosin was associated with an improvement of nightmare symptoms. Our findings indicate that additional studies are needed before considering downgrading the use of prazosin in the treatment of nightmares in patients with PTSD.
Keywords: Prazosin, Nightmare, Post-traumatic stress disorder, Meta-analysis
1. Introduction
Post-traumatic stress disorder (PTSD) is a maladaptive response to a traumatic event and is characterized by intrusive thoughts related to the event, negative mood and cognitions, avoidance of reminders of the event, and heightened arousal and reactivity [1]. Nightmares are a highly prevalent and distressing feature of PTSD [2]. Recurrent nightmares have been associated with poor overall sleep quality [2,3], depression, and heightened risk of suicide [4–6].
Nightmares and other sleep disturbances often persist at clinically significant levels following PTSD treatment [7–9], and require additional targeted treatment interventions [2,10]. In recent years, some non-pharmacological treatment options, such as progressive deep muscle relaxation training, hypnosis, eye movement desensitization and reprocessing, have been suggested as options for the treatment of nightmares [11,12]. Some variants of cognitive behavioral therapy (CBT) including sleep dynamic therapy, lucid dreaming therapy, and systematic desensitization, exposure, relaxation and rescripting therapy, and image rehearsal therapy (IRT) have received attention as potential treatments for nightmares [11,12]. Of these, IRT, a well-supported cognitive behavioral intervention, was given a level-A recommendation for the treatment of PTSD-associated nightmares by the American Academy of Sleep Medicine (AASM) in 2010 [11], and is still recommended in a recent AASM position paper [12].
With respect to pharmacological treatment, the effects of various drugs (i.e. antidepressants, antipsychotics, and benzodiazepine) on sleep disturbances in PTSD, are still a matter of controversy [13]. Beginning in 2010, the AASM gave only one pharmacological agent, prazosin, a level-A recommendation for the treatment of nightmares in adults [11]. Prazosin, a centrally acting α1-adrenergic receptor antagonist, was hypothesized to counteract noradrenergic stimulation and, therefore, potentially reduce the startle response and primitive fear response [14]. Several randomized controlled trials (RCTs) [15–17] have reported the effects of prazosin on nightmares in patients with PTSD, but individual trial results have been imprecise, and there remains uncertainty about the associations of prazosin with overall sleep quality and overall PTSD symptoms. Although prior meta-analyses [18–22] exist, none included the large Prazosin and Combat Trauma PTSD (PACT) trial [15], which adds substantive new data and insights, and raises questions, regarding the likely effects of prazosin on nightmares, sleep quality, PTSD symptoms. Furthermore, due to contradictory evidence regarding the efficacy of prazosin, the AASM downgraded its recommendation regarding its use in a recent position paper [12]. The significant questions regarding the use of prazosin in PTSD thus indicate the need for a new meta-analysis study designed to evaluate the best current evidence regarding its effects. In addition, to our knowledge, acceptability and adverse events of prazosin in patients with PTSD have not been explored using a meta-analytic approach.
This systematic review and meta-analysis examined RCTs, evaluated the effects of prazosin on nightmares, sleep quality, and overall PTSD symptoms, and assessed its acceptability and frequency of adverse events in adult patients with PTSD.
2. Methods
The methodology for this study follows PROSPERO protocol CRD42018110986 in accordance with the preferred reporting items for systematic reviews and meta-analyses statement [23].
2.1. Inclusion and Exclusion Criteria
The inclusion criteria were constructed (assembled, put together) using the PICOS acronym: Participants (P) were patients with PTSD according to Diagnostic and Statistical Manual of Mental Disorders criteria. Intervention (I) was prazosin treatment. Comparison (C) was participants who were randomized to placebo control group. Outcomes (O) were pre- and post-treatment data for both intervention and controls, including sleep quality, severity of nightmare and PTSD symptoms, and the rates of treatment discontinuation (acceptability) and adverse events. Study design (S) was RCT. Other inclusion criteria required that the studies were published in English and were obtained from peer-reviewed journals. Studies were excluded if they did not include an intervention with prazosin or outcomes of interest.
2.2. Information sources
MEDLINE via Ovid (up to Oct 13, 2018); Embase via Ovid (up to 13 October 2018); All EBM databases via Ovid (up to Oct 13, 2018); PsycINFO via EBSCO (up to 13 October 2018); CINAHL via EBSCO (up to 13 October 2018).
2.3. Search
The search strategies for all databases are included in Supplementary Tables S1–S5. The reference lists of all primary studies and review articles were checked for additional references.
2.4. Study Selection
Two investigators (Y.Z. and R.R.) selected relevant publications independently according to the eligibility criteria. Any disagreement was resolved by thorough discussion and consultation with another author (X.D.T.). When the same group of authors published more than one article using data from the same group of subjects, we considered it as one set of comparisons and used the most comprehensive dataset that was available.
2.5. Data Extraction
Two investigators (Y.Z. and R.R.) extracted the data independently using a pre-designed form. Disagreements were resolved by thorough discussion and consultation with the senior author (X.D.T.). The data were entered by a single author (Y.Z.) and verified by both reviewers (Y.Z. and R.R.). Data were obtained from the original articles and by contacting the authors when necessary.
2.6. Outcomes
We considered the mean overall change in nightmare symptoms from baseline to endpoint and the proportion of patients who terminated the study for any reason (acceptability) for our primary analyses. To measure improvement in nightmare symptoms, if the Clinician-Administered PTSD Scale (CAPS) ‘recurrent distressing dreams’ (item #2), or other items which could reflect nightmare frequency/distress were used in the included studies, we extracted the score data. Secondary outcomes included the mean change in overall PTSD symptoms, sleep quality, blood pressure (supine systolic, supine diastolic, standing systolic, and standing diastolic) from baseline to endpoint, and the number of patients who had adverse events (i.e. headache, dizziness postural changes, nausea, dry mouth, etc.). To measure improvement in overall PTSD symptoms and sleep quality, if the validated tools for PTSD (i.e. CAPS, or PTSD Checklist-Civilian or PTSD symptom scale-interview version) and the Pittsburgh Sleep Quality Index (PSQI) were used, respectively, in the included studies, we extracted the score data.
2.7. Quality Assessment
We evaluated the quality of our included studies using Cochrane’s risks of bias assessment [24], which has seven domains: random sequence generation, blinding of participants and personnel, allocation concealment, blinding of outcome assessments, complete collection and reporting of outcome data, selective outcome reporting and other sources of bias. We defined other bias as studies sponsored by drug companies and studies in which baseline characteristics were not similar between different intervention groups. Each domain was rated as ‘high risk’, ‘low risk’ or ‘unclear’. The included studies were graded as high quality, low quality, or moderate quality based on the following criteria: (1) studies were considered high quality when both allocation concealment and randomization were assessed as a low risk of bias, and other items were assessed as unclear or low risk of bias; (2) studies were considered low quality if either allocation concealment or randomization was assessed as a high risk of bias, regardless of the risk of other items; (3) studies were considered moderate quality if they did not meet criteria for low or high risk.
2.8. Statistical Analysis
All analyses were performed using RevMan 5.3 and Comprehensive Meta-Analysis software. Efficacy was evaluated by change values from baseline to end point data on each outcome in this meta-analysis [25]. When standard deviation (SD) was missing in an article, it was calculated from reported t-values, p-values, confidence intervals (CIs) or standard errors (SEs) [26]. A random-effects model was applied in this meta-analysis in order to get relatively robust results. Standardized mean differences (SMDs) in continuous measure (severity of nightmare, sleep quality and overall PTSD symptoms), and the odds ratios (ORs) in dichotomous measure (i.e. the number of discontinuation patients and adverse events) were estimated by inverse variance models. Heterogeneity was evaluated with the test of inconsistency (I2). I2 values of 75%, 50%, and 25% are considered to indicate high, moderate, and low heterogeneity [27]. We used subgroup analyses and meta-regression analyses to find possible sources of the heterogeneity. Publication bias was tested using the Egger regression method [28], with p-values of <0.05 suggesting the presence of bias.
3. Results
3.1. Study Selection
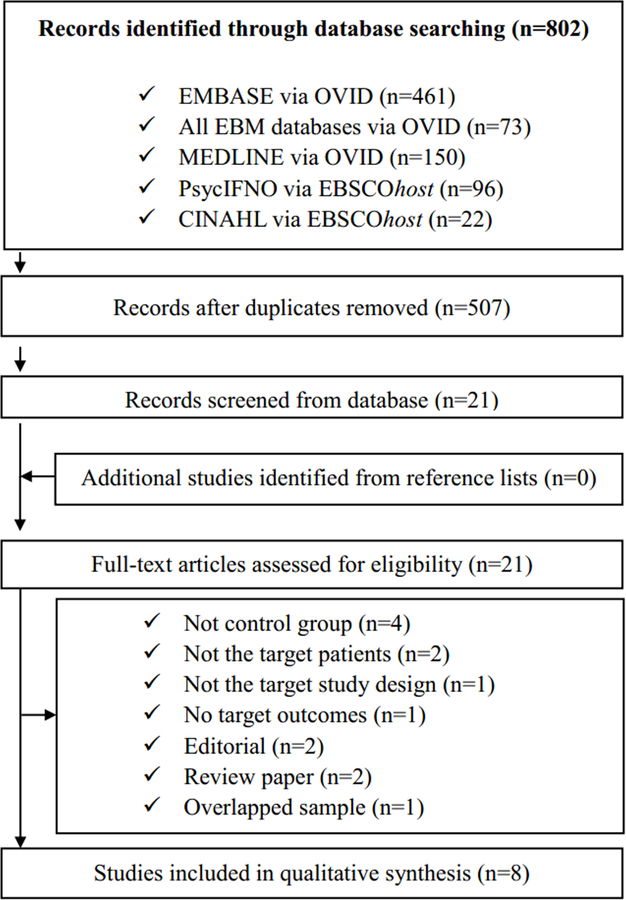
Our search yielded 802 publications (Fig. 1). After removing the duplicates, we screened the titles and abstracts of the remaining 507 articles. A total of 21 articles were selected for full paper review. Of these, eight articles [15–17, 29–33] were found to meet inclusion criteria for the meta-analysis (Table 1). The excluded studies and reasons for their exclusion are provided in Supplementary Table S6.
Fig. 1.
Flow chart used for the identification of eligible studies.
Table 1.
Study characteristics.
| Study | Study design | Sample size | PTSD criteria | Male % | Mean age (years) | Prazosin dosage (mg) | Treatment duration (weeks) | Outcomes |
|---|---|---|---|---|---|---|---|---|
| Raskind et al., 2003 [16] | Crossover | Prazosin (10) | DSM-IV | 1 | 53 | 9.5 | 9 | Overall PTSD symptoms; nightmares |
| Placebo (10) | 1 | 53 | 9 | |||||
| Raskind et al., 2007 [17] | Parallel | Prazosin (17) | DSM-IV | NR | 56 | 13 | 8 | Overall PTSD symptoms; sleep quality; nightmares; adverse events |
| Placebo (17) | NR | 56 | 8 | |||||
| Taylor et al., 2008 [29] | Crossover | Prazosin (13) | DSM-IV | 0.154 | 49 | 3.1 | 3 | Overall PTSD symptoms; acceptability |
| Placebo (13) | 0.154 | 49 | 3 | |||||
| Raskind et al., 2013 [33] | Parallel | Prazosin (32) | DSM-IV | 0.813 | 30.0 | 15.6 | 15 | Overall PTSD symptoms; sleep quality; nightmares; adverse events |
| Placebo (35) | 0.886 | 30.8 | 15 | |||||
| Ahmadpanah et al., 2014 [30] | Parallel | Prazosin (33) | DSM-IV | 0.758 | 36.18 | 15 | 8 | Overall PTSD symptoms; sleep quality; nightmares; adverse events |
| Placebo (33) | 0.667 | 34.21 | 8 | |||||
| Simpson et al., 2015 [31] | Parallel | Prazosin (9) | DSM-IV | 0.60 | 43.5 | 6 | 8 | Overall PTSD symptoms; acceptability; adverse events |
| Placebo (12) | 0.667 | 43.1 | 8 | |||||
| Petrakis et al., 2016 [32] | Parallel | Prazosin (50) | DSM-IV | 0.92 | 44.5 | 16 | 13 | Overall PTSD symptoms; sleep quality; nightmares; acceptability |
| Placebo (46) | 0.956 | 43.4 | 13 | |||||
| Raskind et al., 2018 [15] | Parallel | Prazosin (122) | DSM-IV | 0.961 | 52.3 | 14.8 | 26 | Overall PTSD symptoms; sleep quality; nightmares; acceptability; adverse events |
| Placebo (123) | 0.993 | 51.4 | 26 |
DSM-IV, Diagnostic and Statistical Manual of Mental Disorders, fourth edition; NR, not recorded; PTSD, post-traumatic stress disorder.
3.2. Description of the Included Studies
The eight included studies reported on a total of 286 PTSD patients in the prazosin group and 289 PTSD patients in the placebo group (Table 1). Mean age of PTSD patients ranged from 30 to 56 years in the prazosin group and from 30.8 to 56 years in the placebo group. Males as percentages of PTSD patients ranged from 15.4 to 100% in the prazosin group and in the placebo group. Six trials [15,17,30–33] were parallel RCTs, and two trials [16,29] were crossover RCTs. The mean dosage of prazosin ranged from 3.1 to 16 mg per day, and the treatment duration of prazosin ranged from 3 to 26 weeks.
3.3. Risk of Bias
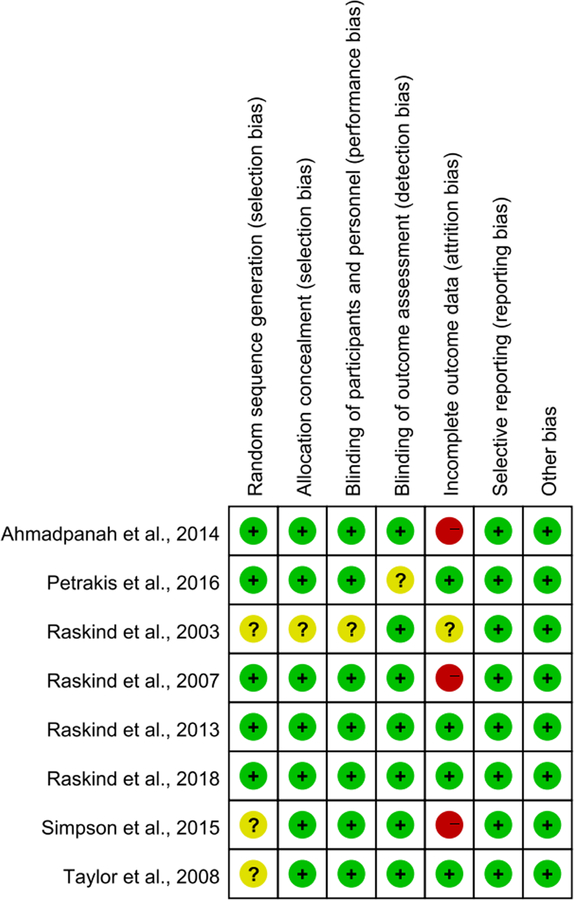
As shown in Fig. 2, all of the included trials indicated that assignment of patients was random, but only five trials [15,17,30,32,33] with ‘low bias’ ratings reported detailed instruments for random sequence generation. Allocation concealment was described in seven trials [15,17,29–33] that were rated as having ‘low bias.’ Seven trials [15,17,29–33] performed blinding of participants and personnel. Seven trials [15–17,29–31,33] performed blinding of outcome assessments. In the domain of incomplete data, four trials [15,29,32,33] reported discontinuation rates and reasons for discontinuation and were rated as ‘low bias.’ All included trials also received a ‘low bias’ rating based on minimal or no evidence of selective reporting, similar baselines for drug and placebo groups, and for not being sponsored by drug companies. Overall, the summary quality assessment of all included trials was moderate to high.
Fig. 2.
Risk of bias for the included studies. (+) Low risk of bias, (?) unclear risk of bias, (−) high risk of bias.
3.4. Efficacy Outcomes
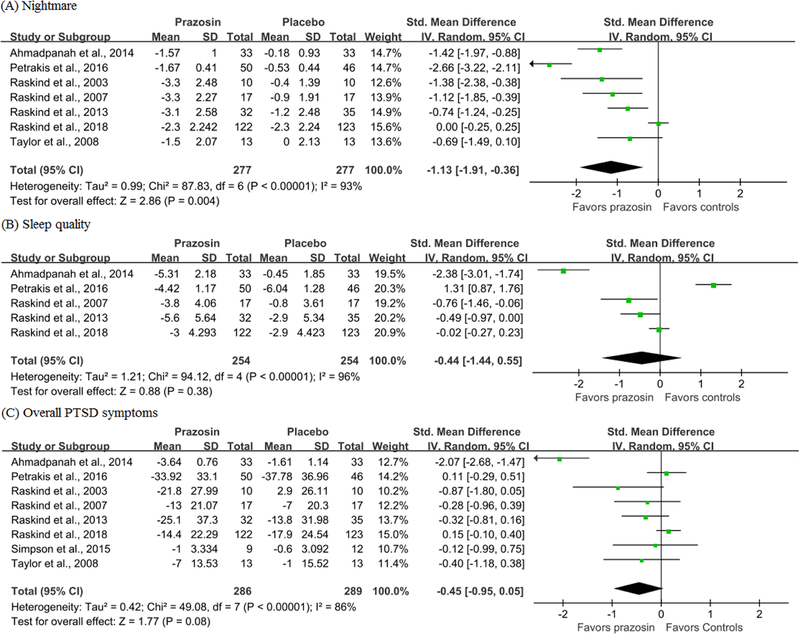
In PTSD patients, prazosin resulted in a statistically significant improvement in nightmare symptoms (SMD = −1.13, 95% CI = −1.91 to −0.36, I2 = 93%; Fig. 3), but was not more beneficial than placebo for overall PTSD symptoms (SMD = −0.45, 95% CI = −0.95 to 0.05, I2 = 86%) and sleep quality (SMD = −0.44, 95% CI = −1.44 to 0.55, I2=96%). Age, male percentage, prazosin dosage and treatment duration of PTSD patients were not associated with the differences in nightmare, sleep quality and overall PTSD symptoms between groups (p>0.05). No publication bias was found by the Egger test (p>0.05).
Fig. 3.
Meta-analysis results for efficacy outcomes of prazosin compared with placebo.
3.5. Acceptability and Adverse Events
In terms of acceptability, there was no significant difference between the prazosin group and the placebo group in discontinuation arising from all causes (OR = 1.00, 95% CI = 0.62–1.62, I2 = 7%; Fig. 4). In terms of adverse events, patients in the prazosin group were more likely to have dry mouth compared with individuals in the placebo group (OR = 3.90, 95% CI = 1.18–12.91, I2 = 37%; Supplementary Fig. S1). There were no significant differences in other adverse events, including changes in supine systolic, supine diastolic, standing systolic, and standing diastolic measures pre- and post-intervention. There also were no significant group differences in symptoms of dizziness, dizziness postural changes, headache, nausea, lack of energy, muscle weakness/asthenia, drowsiness/somnolence, syncope, nasal congestion, or palpitations during treatment (p>0.05).
Fig. 4.
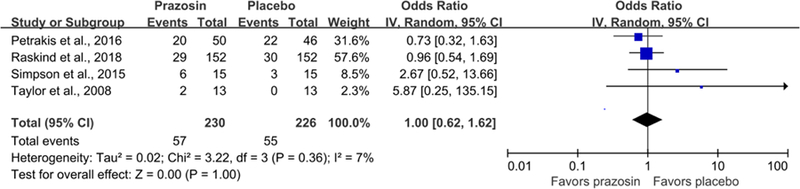
Acceptability (discontinuation for all causes) of prazosin compared with placebo.
4. Discussion
This meta-analysis showed that prazosin was significantly associated with an improvement of nightmare symptoms, but not with overall PTSD symptoms or sleep quality in PTSD patients. These results were generally consistent regardless of age, gender, dose of prazosin, or treatment duration. Dry mouth is a common adverse event for prazosin; however, prazosin was well accepted as evidenced by the lack of significant differences between prazosin and placebo in discontinuation.
Prazosin has been recommended for treatment of PTSD-associated nightmares (Level A) [11], but the recent highly recognized PACT trial reported that prazosin did not alleviate distressing dreams nor improve sleep quality in military veterans with chronic PTSD [15]. After the PACT trial was published, a Task Force commissioned by the AASM to evaluate nightmare disorder treatment in adults agreed unanimously in their position paper that it was appropriate to downgrade the recommendation regarding prazosin use due to contradictory evidence regarding its efficacy [12]. However, the AASM position paper emphasized that it is clearly apparent to clinicians that many PTSD patients respond very well to prazosin and this agent remains the first choice for pharmacologic therapy [12]. After including the PACT trial and other newly published data, our findings still indicate a beneficial effect of prazosin in improving nightmare symptoms in PTSD, which is consistent with previous meta-analysis papers [18,20,22]. It should be noted that a previous meta-analysis by Seda et al. [18] reported that prazosin and another AASM Level A recommended intervention [11], IRT, yield overall comparable effect sizes for treatment of nightmares. It would now be valuable to evaluate new published data including the PACT trial and studies pertaining to the efficiency of IRT on nightmares in PTSD to explore whether the efficiency of prazosin remains comparable with that of IRT via a meta-analytic approach. This comparison would be useful for deciding whether prazosin should be downgraded for the treatment of nightmares in PTSD.
Compared with our findings regarding nightmares, we did not find that prazosin significantly improved sleep quality based on PSQI scores or that it produced an improvement in overall PTSD symptoms. By comparison, Khachatryan et al. [22] reported that prazosin was significantly more effective than placebo in improving sleep quality (g = 0.987, 95% CI: 0.324–1.651) and in reducing overall PTSD symptoms (g = 0.699, 95% CI: 0.139–1.260). Seda et al. [18] reported that treatment effects of prazosin and IRT on sleep quality and post-traumatic stress symptoms are comparable and of moderate magnitude. Our conflicting findings with prior meta-analyses [18,22] may involve the following factors. First, antidepressants used by PTSD patients in the included studies may interact with the effects of prazosin and impact sleep quality and PTSD symptoms. Selective serotonin reuptake inhibitors (SSRIs) can increase the number of arousals, decrease total sleep time and suppress rapid eye movement (REM) sleep [13,34], which are opposite to the effects of prazosin on sleep [29]. It has been reported that SSRIs may decrease the efficiency of prazosin on sleep quality and post-traumatic stress symptoms in PTSD patients [33]. In our included studies, the use of antidepressants was common. For example, in the large PACT study, over 70% of patients in the prazosin and in the placebo groups were receiving a maintenance dose of some antidepressants [15], which could potentially mask the effects of prazosin. Second, PTSD patients may overestimate their sleep disturbances in subjective sleep assessments [35–37]; thus, it is possible that the lack of the effect of prazosin on sleep quality might be due to the lack of objective assessment in our meta-analysis. Indeed, there was only one included study that evaluated objective sleep parameters. It found that prazosin significantly increased total sleep time and REM sleep time compared with placebo [29]. Due to the limited available data, performing a meta-analysis for possible changes in objective sleep parameters pre- and post-prazosin treatment compared with placebo was not possible. To clarify whether prazosin differentially impacts objective and subjective sleep parameters, more RCTs are needed that concurrently evaluate objective (i.e. polysomnographic or actigraphic data) and subjective sleep parameters before and after treatment in PTSD patients. Third, the effects of differences in the included studies across meta-analysis studies should also be considered. For instance, our meta-analysis only included the studies that used the PSQI to evaluate sleep quality. Thus, two included studies [16,29] in the meta-analysis by Khachatryan et al. [22] that did not use the PSQI were excluded from our meta-analysis. Furthermore, in another RCT [38] included in previous meta-analysis papers [19,22], individuals with and without PTSD were included as participants. Due to our strict inclusion criteria for participants in our study, this study was excluded. In addition, some recent published studies [15,31,32], especially the PACT trial which had a large sample size [15], add substantive new data and insights into the likely effects of prazosin, yielding a different pooled effect size compared with previous meta-analysis contributions [19,20,22]. Fourth, another possible reason for the lack of significant differences in sleep quality and overall PTSD symptoms in our meta-analysis was a potential lack of statistical power due to the limited number of included studies.
Given our negative findings for the effects of prazosin on sleep quality and overall PTSD symptoms, it should be considered whether adding another sleep intervention would be helpful. Cognitive behavioral treatment for insomnia (CBT-I), the first-line treatment of insomnia [39], which is recommended for veterans with PTSD [40], could be a potential choice for additional therapy. Previous meta-analytic studies indicated that adding CBT-I to IRT appeared to improve treatment outcomes regarding sleep quality and post-traumatic stress symptoms [18]. Given that IRT and prazosin may yield comparable effect sizes for sleep quality and post-traumatic stress symptoms [18], adding CBT-I to prazosin may also have potential for improving the treatment outcomes for sleep quality and post-traumatic stress symptoms. This may be an important direction for future studies.
With the exception of dry mouth, discontinuation rates for all causes as well as adverse events were not significantly higher in the prazosin group than in the placebo group, which indicated that prazosin was well tolerated for the treatment of nightmares in PTSD. Furthermore, it is known that prazosin could impact blood pressure, and the PACT study indicated that there were significant decreases in supine systolic, supine diastolic, standing systolic, and standing diastolic measurements in the prazosin group compared to the placebo group [15]. By comparison, our meta-analysis derived from three [15,17,33] included studies revealed that there were no significant differences between groups in these measures pre- and post-intervention. This finding suggests that prazosin likely does not adversely affect blood pressure in PTSD patients. However, due to the small sample sizes and short-term follow-up, the safety of prazosin, especially regarding blood pressure, requires further evaluation.
There are some limitations in the current study. First, a meta-analysis indicated that the pooled prevalence rates of obstructive sleep apnea (OSA) based on different apnea–hypopnea index (AHI) criteria in PTSD patients was 75.7% for AHI≥5 and 43.6% for AHI≥10 [41]. Fragmentation of sleep caused by recurrent episodes of upper-airway obstruction and episodic desaturations in OSA may provoke nightmares and worsen PTSD symptoms [42–44]. In fact, none of the included studies screened for OSA in patients with PTSD. Thus, comorbidity of OSA in PTSD may bias the treatment effects of prazosin [45], suggesting that the pooled effect size in our meta-analysis should be interpreted with caution. Second, the effects of antidepressant medications in the included studies could not be explored due to a lack of individual patient-level data. This potential effect needs to be examined in the future. Third, the lack of the effect of prazosin on sleep quality might be associated with the lack of objective assessment in our meta-analysis study. Further studies with objective sleep data are needed which could allow clarification as to whether prazosin differentially impacts objective and subjective sleep parameters in patients with PTSD. Fourth, although the PACT trial [15] with its large sample was included in our meta-analysis, the low number of participants in the majority of our included studies resulted in limited total numbers of participants for review.
Even with these limitations, given the contradictory position regarding prazosin for improving nightmares and sleep quality in PTSD, the results of the present study are of clinical relevance and concern for treating patients with PTSD.
5. Conclusion
In this meta-analysis of RCTs, the use of prazosin was associated with an improvement of nightmare symptoms, but not with sleep quality and overall PTSD symptoms in patients with PTSD. Thus, our findings support the use of prazosin in the treatment of nightmares in patients with PTSD and suggest that more studies are needed before considering downgrading its recommendation as a treatment option.
Supplementary Material
Highlights.
Prazosin was significantly associated with an improvement of nightmare symptoms, but not with overall PTSD symptoms or sleep quality in PTSD patients.
Prazosin was well accepted as evidenced by the lack of significant differences between prazosin and placebo in discontinuation.
Additional studies are needed before considering downgrading the use of prazosin in the treatment of nightmares in PTSD patients.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (81530002, 81629002, 81800093), National Basic Research Program of China (2015CB856406), and the National Institutes of Health of the United States research grant (MH64827).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest
All authors report no biomedical financial interests or potential conflicts of interest.
Supplementary material
Supplementary material related to this article can be found in the online version at:
References
- [1].American Psychiatric Association. Diagnostic and statistical manual of mental disorders Washington, DC; 2013. [Google Scholar]
- [2].Brownlow JA, Harb GC, Ross RJ. Treatment of sleep disturbances in post-traumatic stress disorder: A review of the literature. Curr Psychiatry Rep 2015; 17(6): 41. [DOI] [PubMed] [Google Scholar]
- [3].Levin R, Fireman G. Nightmare prevalence, nightmare distress, and self-reported psychological disturbance. Sleep 2002; 25(2): 205–12. [PubMed] [Google Scholar]
- [4].Nadorff MR, Nazem S, Fiske A. Insomnia symptoms, nightmares, and suicidal ideation in a college student sample. Sleep 2011; 34(1): 93–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Bernert RA, Joiner TE Jr., Cukrowicz KC, et al. Suicidality and sleep disturbances. Sleep 2005; 28(9): 1135–41. [DOI] [PubMed] [Google Scholar]
- [6].Sjostrom N, Waern M, Hetta J. Nightmares and sleep disturbances in relation to suicidality in suicide attempters. Sleep 2007; 30(1): 91–5. [DOI] [PubMed] [Google Scholar]
- [7].Belleville G, Guay S, Marchand A. Persistence of sleep disturbances following cognitive-behavior therapy for posttraumatic stress disorder. J Psychosom Res 2011; 70(4): 318–27. [DOI] [PubMed] [Google Scholar]
- [8].Larsen SE, Fleming CJE, Resick PA. Residual symptoms following empirically supported treatment for PTSD. Psychol Trauma 2019;11(2): 207–15. [DOI] [PubMed] [Google Scholar]
- [9].Koffel E, Khawaja IS, Germain A. Sleep disturbances in posttraumatic stress disorder: updated review and implications for treatment. Psychiatr Ann 2016; 46(3): 173–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Espie CA, Morin CM. The Oxford Handbook of Sleep and Sleep Disorder. Oxford University Press 2012.
- [11].Aurora RN, Zak RS, Auerbach SH, et al. Best practice guide for the treatment of nightmare disorder in adults. J Clin Sleep Med 2010; 6(4): 389–401. [PMC free article] [PubMed] [Google Scholar]
- [12].Morgenthaler TI, Auerbach S, Casey KR, et al. Position paper for the treatment of nightmare disorder in adults: an American Academy of Sleep Medicine position paper. J Clin Sleep Med 2018; 14(6): 1041–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Maher MJ, Rego SA, Asnis GM. Sleep disturbances in patients with post-traumatic stress disorder: epidemiology, impact and approaches to management. CNS Drugs 2006; 20(7): 567–90. [DOI] [PubMed] [Google Scholar]
- [14].Mellman TA, Bustamante V, Fins AI, et al. REM sleep and the early development of posttraumatic stress disorder. Am J Psychiatry 2002; 159(10): 1696–701. [DOI] [PubMed] [Google Scholar]
- [15].Raskind MA, Peskind ER, Chow B, et al. Trial of prazosin for post-traumatic stress disorder in military veterans. N Engl J Med 2018; 378(6): 507–17. [DOI] [PubMed] [Google Scholar]
- [16].Raskind MA, Peskind ER, Kanter ED, et al. Reduction of nightmares and other PTSD symptoms in combat veterans by prazosin: a placebo-controlled study. Am J Psychiatry 2003; 160(2): 371–3. [DOI] [PubMed] [Google Scholar]
- [17].Raskind MA, Peskind ER, Hoff DJ, et al. A parallel group placebo controlled study of prazosin for trauma nightmares and sleep disturbance in combat veterans with post-traumatic stress disorder. Biol Psychiatry 2007; 61(8): 928–34. [DOI] [PubMed] [Google Scholar]
- [18].Seda G, Sanchez-Ortuno MM, Welsh CH, et al. Comparative meta-analysis of prazosin and imagery rehearsal therapy for nightmare frequency, sleep quality, and posttraumatic stress. J Clin Sleep Med 2015; 11(1): 11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].George KC, Kebejian L, Ruth LJ, et al. Meta-analysis of the efficacy and safety of prazosin versus placebo for the treatment of nightmares and sleep disturbances in adults with posttraumatic stress disorder. J Trauma Dissociation 2016; 17(4): 494–510. [DOI] [PubMed] [Google Scholar]
- [20].De Berardis D, Marini S, Serroni N, et al. Targeting the noradrenergic system in posttraumatic stress disorder: A systematic review and meta-analysis of prazosin trials. Curr Drug Targets 2015; 16(10): 1094–106. [DOI] [PubMed] [Google Scholar]
- [21].Singh B, Hughes AJ, Mehta G, et al. Efficacy of prazosin in posttraumatic stress disorder: A systematic review and meta-analysis. Prim Care Companion CNS Disord 2016; 18(4). doi: 10.4088/PCC.16r01943 [DOI] [PubMed] [Google Scholar]
- [22].Khachatryan D, Groll D, Booij L, et al. Prazosin for treating sleep disturbances in adults with posttraumatic stress disorder: a systematic review and meta-analysis of randomized controlled trials. Gen Hosp Psychiatry 2016; 39: 46–52. [DOI] [PubMed] [Google Scholar]
- [23].Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009; 6(7): e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Higgins JP, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions, version 5.1.0 (updated March 2011). [Google Scholar]
- [25].da Costa BR, Nuesch E, Rutjes AW, et al. Combining follow-up and change data is valid in meta-analyses of continuous outcomes: a meta-epidemiological study. J Clin Epidemiol 2013; 66(8): 847–55. [DOI] [PubMed] [Google Scholar]
- [26].Furukawa TA, Barbui C, Cipriani A, et al. Imputing missing standard deviations in meta-analyses can provide accurate results. J Clin Epidemiol 2006; 59(1): 7–10. [DOI] [PubMed] [Google Scholar]
- [27].Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003; 327(7414): 557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997; 315(7109): 629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Taylor FB, Martin P, Thompson C, et al. Prazosin effects on objective sleep measures and clinical symptoms in civilian trauma posttraumatic stress disorder: a placebo-controlled study. Biol Psychiatry 2008; 63(6): 629–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Ahmadpanah M, Sabzeiee P, Hosseini SM, et al. Comparing the effect of prazosin and hydroxyzine on sleep quality in patients suffering from posttraumatic stress disorder. Neuropsychobiology 2014; 69(4): 235–42. [DOI] [PubMed] [Google Scholar]
- [31].Simpson TL, Malte CA, Dietel B, et al. A pilot trial of prazosin, an alpha-1 adrenergic antagonist, for comorbid alcohol dependence and posttraumatic stress disorder. Alcohol Clin Exp Res 2015; 39(5): 808–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Petrakis IL, Desai N, Gueorguieva R, et al. Prazosin for veterans with posttraumatic stress disorder and comorbid alcohol dependence: A clinical trial. Alcohol Clin Exp Res 2016; 40(1): 178–86. [DOI] [PubMed] [Google Scholar]
- [33].Raskind MA, Peterson K, Williams T, et al. A trial of prazosin for combat trauma PTSD with nightmares in active-duty soldiers returned from Iraq and Afghanistan. Am J Psychiatry 2013; 170(9): 1003–10. [DOI] [PubMed] [Google Scholar]
- [34].Singareddy RK, Balon R. Sleep in posttraumatic stress disorder. Ann Clin Psychiatry 2002; 14(3): 183–90. [DOI] [PubMed] [Google Scholar]
- [35].Woodward SH, Bliwise DL, Friedman MJ, et al. Subjective versus objective sleep in Vietnam combat veterans hospitalized for PTSD. J Trauma Stress 1996; 9(1): 137–43. [DOI] [PubMed] [Google Scholar]
- [36].Kobayashi I, Huntley E, Lavela J, et al. Subjectively and objectively measured sleep with and without posttraumatic stress disorder and trauma exposure. Sleep 2012; 35(7): 957–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Hurwitz TD, Mahowald MW, Kuskowski M, et al. Polysomnographic sleep is not clinically impaired in Vietnam combat veterans with chronic posttraumatic stress disorder. Biol Psychiatry 1998; 44(10): 1066–73. [DOI] [PubMed] [Google Scholar]
- [38].Germain A, Richardson R, Moul DE, et al. Placebo-controlled comparison of prazosin and cognitive-behavioral treatments for sleep disturbances in US Military Veterans. J Psychosom Res 2012; 72(2): 89–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Wilson SJ, Nutt DJ, Alford C, et al. British Association for Psychopharmacology consensus statement on evidence-based treatment of insomnia, parasomnias and circadian rhythm disorders. J Psychopharmacol 2010; 24(11): 1577–601. [DOI] [PubMed] [Google Scholar]
- [40].Veterans Affairs/Department of Defense. VA/DOD Clinical Practice Guideline for Management of Post-traumatic Stress Washington, DC; 2016. [Google Scholar]
- [41].Zhang Y, Weed JG, Ren R, et al. Prevalence of obstructive sleep apnea in patients with posttraumatic stress disorder and its impact on adherence to continuous positive airway pressure therapy: a meta-analysis. Sleep Med 2017; 36: 125–32. [DOI] [PubMed] [Google Scholar]
- [42].BaHammam AS, Al-Shimemeri SA, Salama RI, et al. Clinical and polysomnographic characteristics and response to continuous positive airway pressure therapy in obstructive sleep apnea patients with nightmares. Sleep Med 2013; 14(2): 149–54. [DOI] [PubMed] [Google Scholar]
- [43].Schredl M Dreams in patients with sleep disorders. Sleep Med Rev 2009; 13(3): 215–21. [DOI] [PubMed] [Google Scholar]
- [44].Krakow BJ, Ulibarri VA, Moore BA, et al. Posttraumatic stress disorder and sleep-disordered breathing: a review of comorbidity research. Sleep Med Rev 2015; 24: 37–45. [DOI] [PubMed] [Google Scholar]
- [45].Mysliwiec V, Brock MS. Prazosin for post-traumatic stress disorder. N Engl J Med 2018; 378(17): 1649. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.