Abstract
Glibenclamide (GBC) improves cerebral outcome after cardiac arrest (CA) in rats. We aim to investigate the effect of GBC on electrophysiological recovery and to explore the mechanism of neuroprotective effect of GBC in the acute stage of brain injury after the return of spontaneous circulation (ROSC) in a rodent model of CA. 16 anesthetized male Wistar rats subjected to 8-min asphyxia-CA were randomly assigned to the GBC or control group (N=8 each group). GBC was administered with a loading dose of 10ug/kg via i. p. injection 10 min after ROSC and was followed by a maintenance dose of 1.6ug/kg per 8 hours throughout the first 24 hours. Quantitative measures of EEG-information quantity (qEEG-IQ) and neurological deficit score (NDS) were used to predict and evaluate the functional outcome. There was a significant improvement of NDS in rats treated with GBC compared with the control group (p < 0.01). Compared to the control group, the rats treated with GBC showed qEEG-IQ scores that indicated better recovery (p < 0.001). Meanwhile, early qEEG-IQ was significantly correlated with 72-hr NDS as early as 45min after ROSC. Furthermore, on the molecular basis, the NLRP3 inflammasome was strongly activated in the hippocampal CA1 area 3 days after CA in control rats, but was suppressed with GBC treatment. Taken together, GBC treatment markedly improved electrophysiological and neurologic outcomes of the acute brain injury after CA. These neuroprotective effects may be associated with the attenuation of inflammatory response via down-regulation of NLRP3 inflammasome signal.
I. Introduction
Cardiac arrest (CA) is one of the leading causes of death in the United States, with an incidence of 55 per 100 000 person-years [1]. Although some progress has been made in the field of first aid and treatment of CA in the past ten years, sudden cardiac death still accounts for nearly 300,000 deaths among adults each year in the U.S. [2]. In addition, 92.6% of CA survivors were discharged with neurological impairment due to irreversible brain injury [3]. Ischemic brain injury after CA occurs mostly in the hippocampus and leads to poor neurological function. Therefore, neuroprotective therapy and cerebral resuscitation are very important after cardiopulmonary resuscitation (CPR). The complex and diverse mechanisms involved in the brain injury after CA include inflammatory response, reactive oxygen species, autophagy and apoptosis. Current clinical treatments include many mechanism-driven therapies, like hypothermia, that aim to improve the survival rate and neurological recovery after CA. We have previously developed multimodal monitoring including neurophysiological signal and CBF recording, to evaluate the hypoxia-induced cerebral damage, and to track brain injury and recovery after CA [4–7]. Electroencephalography (EEG) is an important method to predict the prognosis of hypoxic-ischemic brain damage based on its real-time evaluation of neurological function. We have also shown that quantitative EEG (qEEG) is a powerful prognostic marker for brain recovery after CA [5, 7–11].
The nucleotide-binding oligomerization domain, leucine rich, repeat and, pyrin domain-containing 3 (NLRP3) inflammasome, is a subcellular multiprotein complex that is abundantly expressed in the central nervous system (CNS) [12]. Cryopyrin/NALP3/NLRP3 can sense and respond to a wide range of exogenous and endogenous stimuli, which results in the activation of caspase-1. Activated caspase-1 and other pro-inflammatory cytokines drive inflammatory responses through diverse downstream signaling pathways, leading to neuronal damage and possibly cell death [13]. We have previously shown that by inhibiting the NLRP3 inflammasome, fimasartan can protect against secondary brain damage in an intracerebral hemorrhage model [12].
Glibenclamide (GBC) has protective effects in several brain disorders such as stroke and traumatic brain injury in animal models [14, 15]. GBC can pass the blood-brain barrier (BBB) into the brain and improve neurological outcome after CA in rats [16]. Although the exact mechanisms remain unknown, GBC may inhibit NLRP3 inflammasome activation by inhibiting the blockade of the P2X7 receptor, which then suppresses the activation of caspase-1 [17].
In this study, we sought to evaluate the neuroprotective effects of GBC against the acute injury of global brain ischemia after CA and to explore the involved mechanism. We hypothesize that the neuroprotective effects of GBC on CA may be due to the attenuation of the activation of the NLRP3 inflammasome.
II. Material and methods
Sixteen adult Wistar rats (300–325g, Charles River, Wilmington MA) were randomly divided into the GBC treatment group (N=8) and control group (N=8). All rats were housed with free access to water and food under 12 hours light-dark cycle. The experimental protocol was approved by the University of Maryland School of Medicine Animal Care and Use Committee.
A. Experimental asphyxia-CA model
The asphyxia CA and cardiopulmonary resuscitation (CPR) model used our well-validated protocols [8–11]. Rats were anesthetized with 4.5% isoflurane and maintained with 1.5% isoflurane after tracheal intubation. The rat was ventilated by a rodent ventilator (Harvard Apparatus Model 683) setting the parameters of tidal volume as 8ml/Kg and ventilation frequency around 50 bpm. The ventilator gas consisted of 50%N2 /50% O2. Arterial pressure (including mean arterial pressure, MAP), body temperature, heart rate, and ECG were continuously monitored. Isoflurane washout was performed for 5 minutes after 5 minutes of EEG baseline recording. The muscle relaxant vecuronium (2 mg/ml) was injected into the rats and the dose (2 ml/kg) was adjusted to the animals’ weight [4]. Global asphyxia began with the stop of the ventilator and the clamping of the respiratory circuit for 8 min. CA is defined as when the pulse pressure<10mmHg. After 8 minutes of asphyxia CA, standard CPR with chest compression (200 bpm) was performed. Ventilation with 100% oxygen, intravenous injection of epinephrine and sodium bicarbonate were administrated through the pre-cannulated femoral vein to resuscitate the animal and rectify metabolic acidosis. The threshold of ROSC is MAP>60 mmHg. The arterial blood gas analyses (ABG) were serially tested at baseline, 20 min, and 40 min after ROSC.
B. Glibenclamide Treatment
The GBC (#G2539; Sigma, St. Louis, MO) was dissolved in dimethyl sulfoxide (DMSO) and diluted in saline as we described [15]. GBC was administrated intraperitoneally with a loading dose of 10 ug/kg at 10 min after ROSC and three maintenance doses of 1.6 ug at 8, 16, and 24h after ROSC. The dose selected has not resulted in hypoglycemia to rats per previous studies [16]
C. Neurological Recovery Evaluation
The evaluation of neurological recovery was assessed using the neurological deficit scores (NDS) and tail suspension test. The NDS, which ranges from 0 to 80, was assessed at 6, 24, 48 and 72hr after ROSC as we well-validated [8–11]. The primary outcome was defined by the 72hr score. The tail suspension test [18] was assessed at baseline and 72hr after CA.
D. EEG Recording and Data Analysis
Two channel EEG electrodes were implanted 24h before surgery. EEG recording (Tucker-Davis Technologies, USA) consists of two parts: 5 min baseline EEG prior to CA and followed by 360 min continuous recording post-ROSC. Using the core algorithm of Matlab (MathWorks, Natick, MA), all the EEG data was calculated basing on modified entropy and the output was in the form of qEEG information Quantify (qEEG-IQ) value, which was published in our previous studies [8–11].
E. Immunofluorescence staining
The rats were transcardially perfused with phosphate buffered saline and fixed with 4% paraformaldehyde. The cryopreserved brain samples were sectioned (20μm) in the coronal plane and then incubated with primary antibody NLRP3 (Abcam, 1:200) and Caspase-1 (proteintech™, 1:100) overnight at 4°C. Bright field and fluorescence micrographs were obtained using a Leica DMi8 microscope (Leica Microsystems).
F. Statistical Methods
The results of parametric tests (qEEG-IQ) were presented as mean ± S.E.M. and the non-parametric test results (NDS) were shown as median (25th–75th interquartile). The univariate analyses are used to evaluate the difference of qEEG-IQ between groups. The bivariate analyses are used to analyze the association between qEEG-IQ and 72hr NDS. p < 0.05 was considered significant. All statistical analyses were performed using SPSS (IBM SPSS Statistics, version 22).
III. RESULTS
A. NDS Analysis, survival rate, and functional recovery
Aggregate analysis of NDS shows a significant improvement in the GBC group (median [interquartile range], 51 [44–61]) compared to the Control group (46 [0–51]), (p<0.01). GBC treatment led to the consistently improved neurological outcome, compared with the control group at all time periods during the 72-hr experiment (Fig 1A).
Fig. 1A.
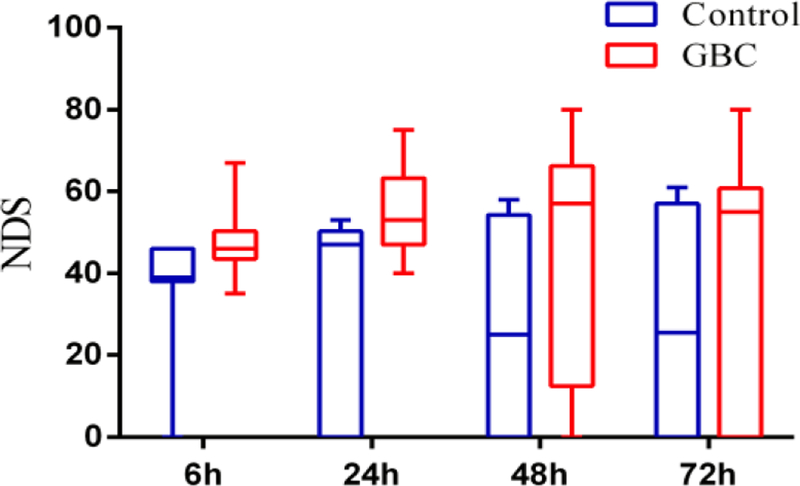
NDS of control and GBC group at 6, 24, 48 and 72hr after ROSC
Kaplan-Meier analysis shows trends of improvement in survival for the GBC group. The survival rate at 72hrs among GBC treated rats (2/8, 75%) is greater than the control group (4/8, 50%), (Fig1B).
Fig 1B.
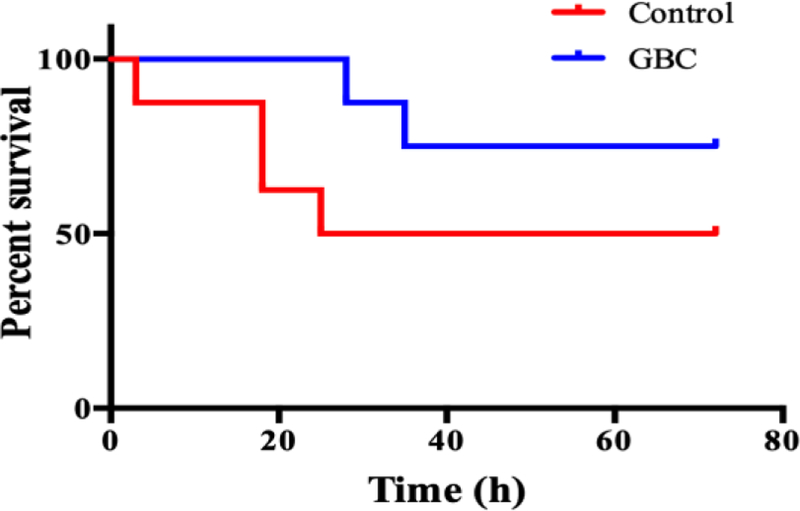
Cum survival of control and GBC treated groups. The tail suspension test was significantly improved in the GBC group on 72hr after ROSC compared with the control group (Fig 2, p < 0.01).
B. Quantitative EEG (qEEG) analysis
These signals decrease from the baseline waveform to the flat (isoelectric) EEG soon after CA and gradually recover after ROSC following with the time periods (qEEG-IQ started from baseline 1, to CA 0, and gradually increased until 150min qEEG-IQ, GBC VS. Control, 1.08 ± 0.10 VS. 0.92 ± 0.1). The aggregate analysis of qEEG-IQ presents better recovery in the GBC treatment group (0.77 ± 0.06) than in the control group (0.63 ± 0.04), (Fig. 3, p < 0.001).
Figure 3.
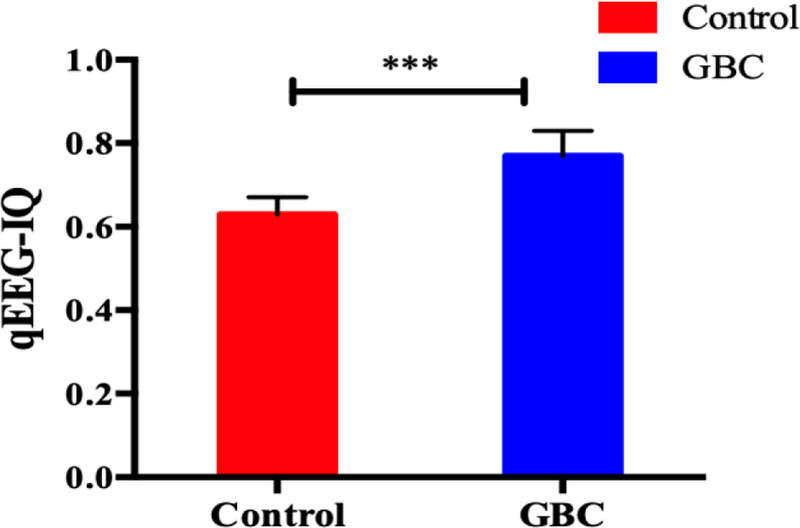
Comparison of aggregate qEEG-IQ (mean ± S.E.M) between GBC and control group (***, p <0.001).
C. Association between qEEG-IQ values and 72-hr NDS
Bivariate analyses showed that the qEEG-IQ values correlate well with the 72-hr NDS as early as 45 mins after ROSC. The correlations are significant at 60 mins (Pearson’s correlation, 0.600, p =0.02), 90 mins (Pearson’s correlation, 0.621, p =0.02) and 120 mins (Pearson’s correlation, 0.583, p =0.02).
D. Immunofluorescence staining for NLRP3 and Caspase 1
Immunofluorescence staining was performed on the brain tissue to reveal the mechanism of the neuroprotective effects of GBC on the immune response after CA. NLRP3 inflammasome was upregulated in the area around the hippocampus and cortex, compared to the control group. However, these activations of NLRP3 and Caspase-1 are markedly inhibited after GBC treatment (Fig.4).
Figure 4.
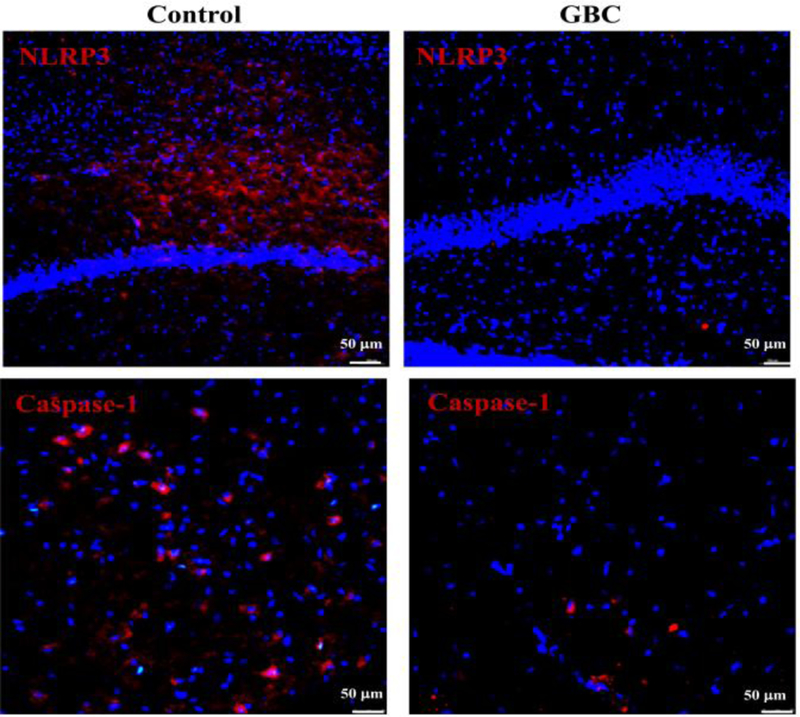
Immunofluorescence staining for NLRP3 and Caspase-1. The expression of NLRP3 and Caspase-1 were inhibited after GBC treatment.
IV. DISCUSSION
Asphyxia-induced CA is a complex system of ischemia in both human and animal models. In vivo, systemic hypoxia not only causes the obstruction of oxygen supply but also leads to a disorder of metabolite elimination. It is particularly pronounced in the brain because of the brain’s low tolerance to ischemia and hypoxia. As a result, the treatments of brain injury after CA remain suboptimal. Several studies suggest that neuroprotective drug therapy has a potentially broad therapeutic prospect [19]. Our study indicates that early GBC administration improves subsequent electrophysiological recovery (qEEG-IQ) and neurological functional outcome in rats with attenuation of the NLRP3 inflammasome signal.
Neuroinflammation has been proven to be significantly related to the outcome of many neurological diseases and an important molecular mechanism of brain injury after CA. Immediate cerebral ischemia after CA and ischemia-reperfusion injury after ROSC activate inflammation in the brain [20]. NLRP3 inflammasome, a multiprotein complex expressed in the central nervous system (CNS), especially in microglia and astrocytes, was first characterized in Muckle-Wells Autoinflammatory Disorder. In the previous research, NLRP3 inflammasome is considered to be of crucial importance in the development of both acute and chronic inflammatory responses[21]. Based on the above research, we hypothesize that NLRP3 inflammasome may be a potential therapeutic target for CA. In our study, we found inhibition of NLRP3 inflammasome activation in rats treated with GBC led to improved neurological recovery after CA.
Glibenclamide, a diabetic drug, has been found to have therapeutic effects in various neurological disorders such as ischemic stroke, traumatic brain injury, and subarachnoid hemorrhage in animal models [14, 15, 22]. When cerebral ischemia occured, the activated microglia released inflammatory cytokines and initiated downstream signaling pathways, resulting in neuronal cell loss [23]. In the ischemic stroke model, IL-1b and IL-18 were expressed at a high level with consistent activation of NLRP3 [24]. By modulating the activation of NLRP3, the infarct volume of ischemic stroke can be reduced, vascular injury can be alleviated, and neurological function can be improved [25]. In view of this accumulated evidence, this study compared the differences in EEG recovery and neurological outcome between GBC and control groups targeting post-CA immune response. We found that GBC could not only improve the electrophysiological recovery in the early stage (within 6 hours), but also promote the survival rate and neurological functions in the acute stage of CA.
We focused on the neuroprotective effect of GBC without affecting blood sugar in this research. The relationship between GBC dosage and neurological outcomes in CA models may need to be further studied. In addition, as a potential neuroprotective drug, GBC may have a variety of neuroprotective mechanisms because its molecular targets may not be unique. For example, GBC alleviates cerebral edema and neurological injury by inhibiting the upregulation of SUR1-TRPM4 channel [26]. Other pathophysiological mechanisms of inflammation-related brain injury after CA, even systemic inflammatory response syndrome (SIRS) may be valuable to further explore.
V. Conclusion
In conclusion, we investigated the therapeutic effects of GBC on a rat CA model and demonstrated that GBC treatment improved both electrophysiological and neurologic outcomes after CA. These neuroprotective effects of GBC may be associated with the inhibition of NLRP3 inflammasome on acute brain injury, which is involved in the inflammatory response after CA.
Fig 2.
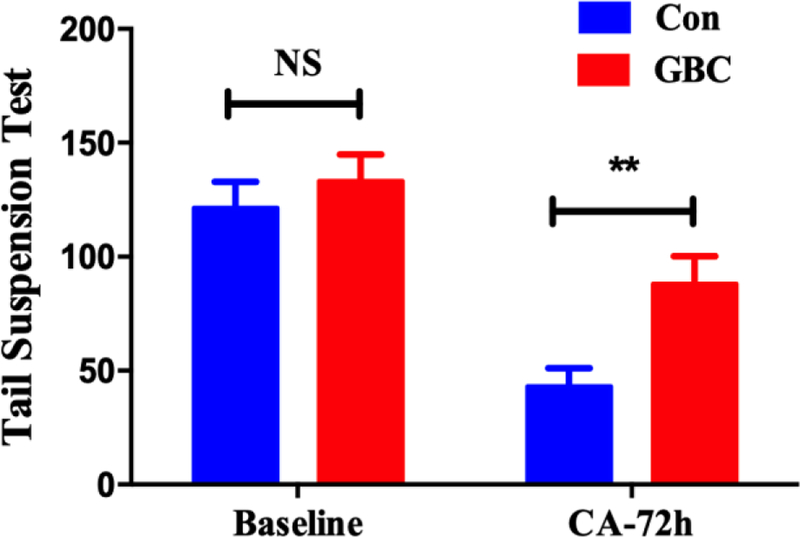
Tail suspension test on 72hr after ROSC (**p < 0.01)
Table 1.
NDS by Control and GBC groups (median [25–75th percentile]), N = 8.
| GROUP | 6h | 24h | 48h | 72h |
|---|---|---|---|---|
| Control | 39.0 (38.0–46.0) | 47.0 (0–50.3) | 25.0 (0–54.3) | 25.5 (0–57.0) |
| GBC | 46.0 (43.5–50.3) | 53.0 (47.0–63.3) | 57.0 (12.5–66.3) | 55.0 (0–60.8) |
Acknowledgments
Research supported by R01HL118084 and R01NS110387 from United States National Institutes of Health (NIH) (both to XJ).
Contributor Information
Xiuli Yang, Department of Neurosurgery, University of Maryland School of Medicine, Baltimore, MD, USA 21201..
Zhuoran Wang, Department of Neurosurgery, University of Maryland School of Medicine, Baltimore, MD, USA 21201..
Xiaofeng Jia, Department of Neurosurgery, Anatomy Neurobiology, Orthopaedics, University of Maryland School of Medicine, Baltimore, MD, USA 21201, and Department of Biomedical Engineering, Anesthesiology and Critical Care Medicine Johns Hopkins University, Baltimore, MD, USA21205.
References
- [1].Rea TD, Eisenberg MS, Sinibaldi G, and White RD, “Incidence of EMS-treated out-of-hospital cardiac arrest in the United States,” Resuscitation, vol. 63, no. 1, pp. 17–24, October 2004. [DOI] [PubMed] [Google Scholar]
- [2].Go AS et al. , “Executive summary: heart disease and stroke statistics--2014 update: a report from the American Heart Association,” Circulation, vol. 129, no. 3, pp. 399–410, January 21 2014. [DOI] [PubMed] [Google Scholar]
- [3].Girotra S et al. , “Regional Variation in Out-of-Hospital Cardiac Arrest Survival in the United States,” Circulation, vol. 133, no. 22, pp. 2159–68, May 31 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Deng R, Young LM, and Jia X, “Quantitative EEG markers in severe post-resuscitation brain injury with therapeutic hypothermia,” Conference proceedings : … Annual International Conference of the IEEE Engineering in Medicine and Biology Society. IEEE Engineering in Medicine and Biology Society. Annual Conference, vol. 2015, pp. 6598–601, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].He J et al. , “Real-time quantitative monitoring of cerebral blood flow by laser speckle contrast imaging after cardiac arrest with targeted temperature management,” J Cereb Blood Flow Metab, p. 271678X17748787, December 28 2017. [DOI] [PMC free article] [PubMed]
- [6].Jones S, Schwartzbauer G, and Jia X, “Brain Monitoring in Critically Neurologically Impaired Patients,” International journal of molecular sciences, vol. 18, no. 1, December 27 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Deng R, Koenig MA, Young LM, and Jia X, “Early Quantitative Gamma-Band EEG Marker is Associated with Outcomes After Cardiac Arrest and Targeted Temperature Management,” Neurocritical care, vol. 23, no. 2, pp. 262–73, October 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Jia X et al. , “Quantitative EEG and neurological recovery with therapeutic hypothermia after asphyxial cardiac arrest in rats,” (in eng), Brain research, vol. 1111, no. 1, pp. 166–75, September 21 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Jia X, Koenig MA, Nickl R, Zhen G, Thakor NV, and Geocadin RG, “Early electrophysiologic markers predict functional outcome associated with temperature manipulation after cardiac arrest in rats,” Critical care medicine, vol. 36, no. 6, pp. 1909–16, June 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Jia X et al. , “Improving neurological outcomes post-cardiac arrest in a rat model: immediate hypothermia and quantitative EEG monitoring,” (in eng), Resuscitation, vol. 76, no. 3, pp. 431–42, March 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Shin HC, Tong S, Yamashita S, Jia X, Geocadin RG, and Thakor NV, “Quantitative EEG and effect of hypothermia on brain recovery after cardiac arrest,” (in eng), IEEE transactions on bio-medical engineering, vol. 53, no. 6, pp. 1016–23, June 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Yang X et al. , “Pretreatment with low-dose fimasartan ameliorates NLRP3 inflammasome-mediated neuroinflammation and brain injury after intracerebral hemorrhage,” Exp Neurol, vol. 310, pp. 22–32, December 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].de Rivero Vaccari JP, Dietrich WD, and Keane RW, “Activation and regulation of cellular inflammasomes: gaps in our knowledge for central nervous system injury,” J Cereb Blood Flow Metab, vol. 34, no. 3, pp. 369–75, March 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Zweckberger K et al. , “Glibenclamide reduces secondary brain damage after experimental traumatic brain injury,” Neuroscience, vol. 272, pp. 199–206, July 11 2014. [DOI] [PubMed] [Google Scholar]
- [15].Simard JM et al. , “Glibenclamide-10-h Treatment Window in a Clinically Relevant Model of Stroke,” Transl Stroke Res, vol. 3, no. 2, pp. 286–95, June 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Huang K et al. , “Glibenclamide Prevents Water Diffusion Abnormality in the Brain After Cardiac Arrest in Rats,” Neurocritical care, vol. 29, no. 1, pp. 128–135, August 2018. [DOI] [PubMed] [Google Scholar]
- [17].Lamkanfi M et al. , “Glyburide inhibits the Cryopyrin/Nalp3 inflammasome,” The Journal of cell biology, vol. 187, no. 1, pp. 61–70, October 5 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Can A, Dao DT, Terrillion CE, Piantadosi SC, Bhat S, and Gould TD, “The tail suspension test,” J Vis Exp, no. 59, p. e3769, January 28 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Athanasios Chalkias TX, “Post-cardiac arrest brain injury: Pathophysiology and treatment,” J Neurol Sci, vol. 315, no. 1–2, pp. 1–8, 2011. [DOI] [PubMed] [Google Scholar]
- [20].Adrie C et al. , “Successful Cardiopulmonary Resuscitation After Cardiac Arrest as a “Sepsis-Like” Syndrome,” Circulation, vol. 106, no. 5, pp. 562–568, 2002. [DOI] [PubMed] [Google Scholar]
- [21].Agostini L MF, Burns K, McDermott MF, Hawkins PN, Tschopp J, “NALP3 forms an IL-1beta-processing inflammasome with increased activity in Muckle-Wells autoinflammatory disorder,” Immunity, vol. 20, no. 3, pp. 319–325, 2004. [DOI] [PubMed] [Google Scholar]
- [22].Simard JM et al. , “Glibenclamide reduces inflammation, vasogenic edema, and caspase-3 activation after subarachnoid hemorrhage,” Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism, vol. 29, no. 2, pp. 317–30, February 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Harari OA and Liao JK, “NF-kappaB and innate immunity in ischemic stroke,” (in eng), Ann N Y Acad Sci, vol. 1207, pp. 32–40, October 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Lammerding L SA, Johann S, Beyer C, Zendedel A, “Poststroke Inflammasome Expression and Regulation in the Peri-Infarct Area by Gonadal Steroids after Transient Focal Ischemia in the Rat Brain,” Neuroendocrinology, vol. 103, no. 5, pp. 460–75, 2016. [DOI] [PubMed] [Google Scholar]
- [25].Fann DY et al. , “Intravenous immunoglobulin suppresses NLRP1 and NLRP3 inflammasome-mediated neuronal death in ischemic stroke,” Cell death & disease, vol. 4, p. e790, September 5 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Lin Z et al. , “Glibenclamide ameliorates cerebral edema and improves outcomes in a rat model of status epilepticus,” Neuropharmacology, vol. 121, pp. 1–11, July 15 2017. [DOI] [PubMed] [Google Scholar]


